Abstract
Osseointegration of dental, craniofacial, and orthopedic implants is critical for their long-term success. Multifunctional surface treatment of implants was found to significantly improve cell adhesion and induce osteogenic differentiation of dental-derived stem cells in vitro. Moreover, local and sustained release of antibiotics via nanolayers from the surface of implants can present unparalleled therapeutic benefits in implant dentistry. Here, we present a layer-by-layer surface treatment of titanium implants capable of incorporating BMP-2–mimicking short peptides and gentamicin to improve their osseointegration and antibacterial features. Additionally, instead of conventional surface treatments, we employed polydopamine coating before layer-by-layer assembly to initiate the formation of the nanolayers on rough titanium surfaces. Cytocompatibility analysis demonstrated that modifying the titanium implant surface with layer-by-layer assembly did not have adverse effects on cellular viability. The implemented nanoscale coating provided sustained release of osteoinductive peptides with an antibacterial drug. The surface-functionalized implants showed successful osteogenic differentiation of periodontal ligament stem cells and antimicrobial activity in vitro and increased osseointegration in a rodent animal model 4 wk postsurgery as compared with untreated implants. Altogether, our in vitro and in vivo studies suggest that this approach can be extended to other dental and orthopedic implants since this surface functionalization showed improved osseointegration and an enhanced success rate.
Keywords: titanium implants, osteogenesis, stem cells, nanolayers, growth factors, antibiotic
Introduction
The success of dental implants relies on successful osseointegration, which is governed by the level of direct contact between the surface of the implant and the surrounding bone. The presence of microbial biofilms is known to initiate or accelerate implant failure pathways even in cases where the infection was not the primary reason for failure (Raphel et al. 2016). Aseptic loosening and infection are among the major challenges associated with implant failure, representing up to 18% and 20% of failures, respectively (Raphel et al. 2016). Therefore, modifying the implant surface to improve osseointegration while preventing microbial accumulation is critical for successful outcomes.
Roughening the implant surfaces to increase bone-implant interlocking has been introduced as a strategy to promote osseointegration (Fouziya et al. 2016). Additionally, the combination of surface modification with physicochemical methods and incorporation of biomaterials can improve the outcome. Using biomaterials to provide prolonged release of bioactive agents (e.g., small molecule drugs and protein therapeutics) from the implant surface is a promising modality to achieve improved outcomes (Meng et al. 2016).
Polydopamine (PDA)–assisted surface modification has gained considerable attention over the past few years. In addition to its potent bioadhesive properties, PDA can be employed as a surface modifier to enhance the interfacial properties of the implants (Huang et al. 2016; Hasani-Sadrabadi et al. 2020). PDA coating can be modified to produce a multifunctional surface by acting as an anchorage point to immobilize antimicrobial agents or nucleophilic factors such as peptides (Jia et al. 2019).
Implant surface with microfeatures is known to increase bone-implant interaction leading to better osseointegration, while nanofeatures are known to induce osteoblast maturation (Mendonça et al. 2009; Gittens et al. 2013). Therefore, combining the micro- and nanofeatures on implant surfaces could improve the overall function of the implants (Mendonça et al. 2010). In this context, a promising approach is to create a conformal nanoscale with a layer-by-layer (LbL) assembly technique to stack alternating coatings of polyanionic and polycationic coatings on metal implants (Yost et al. 2015). Charged therapeutic agents such as antibiotics can be loaded within the coating during the formation of the layers and subsequently be released upon dissolution or degradation of the layers or by diffusion through the layers (Guzmán et al. 2017). LbL nanolayers can be used to encapsulate small molecule drugs in substantial amounts (Min et al. 2016). These coatings can be formed without harsh processing conditions, which could adversely affect the activity of the therapeutic agents.
In this study, we propose a scalable coating platform by constructing nanolayers on microroughened titanium (Ti) dental implants to boost the osteogenic and antimicrobial activity of the implant simultaneously by sustained delivery of an antibiotic and an osteoinductive growth factor. To the best of our knowledge, this combination has never been reported specifically for implant dentistry.
Materials and Methods
Preparation and Characterization of Surface-Modified Ti Disks and Implants
Ti disks (20-mm diameter × 1-mm thickness) and commercially pure Ti grade 2 implants were used. Acid-etched Ti discs were incubated in dopamine hydrochloride solution for 16 h, followed by manually constructing alternating layers of poly-L-lysine (PLL) and hyaluronan (HA). Refer to the Appendix for details on the preparation of acid-etched Ti samples and surface modification (Picart et al. 2001; Ueno et al. 2016; Jia et al. 2019).
The number of layer pairs in all the experiments was set to 20 unless otherwise stated. Nanolayers were crosslinked with an aqueous solution of EDC (100 mg·mL−1) and N-hydrosulfosuccinimide (20 mg·mL−1). Only HA-ending nanolayers were studied due to the intrinsic affinity of stem cells to HA via their CD44 receptor (Yang et al. 2010; Kim and Kumar 2014). Surface-modified Ti discs were characterized by scanning electron microscopy (SEM), atomic force microscope, and x-ray photoelectron spectroscopy (XPS), as explained in the Appendix. Enzymatic degradation of the layers is demonstrated in Appendix Figure 1 (Wang et al. 2017).
Therapeutic Peptide and Antibiotic-Loaded Nanolayers
Bioactive nanolayers were formed by incorporating bone morphogenetic protein 2 (BMP-2)–mimicking peptide. For more information on the BMP-2 short peptide loading and release procedures, refer to the Appendix (Cui et al. 2018). Incorporation and release studies were performed on (PLL/HA) n films constructed in 24-well plates in phosphate-buffered saline at 37 °C.
Gentamicin as a model antibiotic drug was mixed with PLL (1:1 mass ratio) and used during the LbL coating process. Detailed information on gentamicin loading and release procedure are in Appendix (Zarubova et al. 2020). The release was evaluated for 3 initial loading levels of 5-, 10-, and 75-µg gentamicin per implant.
Viability and Osteogenic Differentiation of the Human Periodontal Ligament Stem Cells In Vitro
Isolation of periodontal ligament stem cells (PDLSCs) is discussed in Appendix (Park et al. 2011; Ansari et al. 2017). Cytocompatibility of the surfaces was studied on modified and nonmodified 100-mm tissue culture plates for 1 wk with a Viability/Cytotoxicity Kit (Invitrogen). Tissue culture plates were modified with the assembly of nanolayers with a similar process already utilized to modify the Ti implants, as mentioned earlier. The percentage of live cells was quantified by ImageJ software (National Institutes of Health).
The cytocompatibility of the antibiotic-loaded layers was assessed by incorporating 75-µg/mL gentamicin within the layers, the highest concentration used for the antibacterial studies. The morphology of the PDLSCs cultured on Ti implants was imaged with immunofluorescence staining via FITC-Phalloidin and DAPI (Vector Laboratories) as previously described (Hasani-Sadrabadi et al. 2019).
To examine the osteogenic potential of the modified surfaces, PDLSCs were cultured on the modified Ti implants and incubated for 4 wk in osteogenic media. Mineralization and gene expression procedures are discussed in the Appendix (Moshaverinia et al. 2013; Sevari et al. 2020).
Antibacterial Analyses
We tested the antibacterial properties of gentamicin-incorporated LbL surface-modified Ti implants against 2 bacterial strains, Aggregatibacter actinomycetemcomitans and Escherichia coli, that often contribute to poor oral health, as discussed in the Appendix.
Animal Model
All experimental protocols followed the ARRIVE guidelines (Animal Research Reporting of In Vivo Experiments). All experiments were performed following protocols approved by the Chancellor’s Animal Research Committee at the University of California, Los Angeles (2005-175-41G), and the Public Health Service Policy for the Humane Care and Use of Laboratory Animals. Sixteen male and female Sprague-Dawley rats were used to study the effect of surface modification on the osseointegration of the Ti implants with a biomechanical push-in test as reported previously (Ogawa et al. 2000).
Statistical Analysis
The Kruskal-Wallis rank sum test, 1-way analysis of variance with Holm-Sidak post hoc analysis, and 2-tailed Student’s t test were utilized as appropriate to analyze the data at a significance of α or P < 0.05. Quantitative data were expressed as mean ± SD. Experiments were performed at least in triplicate, with 3 independent samples per condition in each experiment. To determine the number of specimens for the proposed experiments, a power analysis was conducted on the basis of our preliminary data.
Results
Characterization of Surface-Modified Ti Implants
Previous studies reported that a rough surface may result in the better assembly of the layers on the substrate (Ghiasi et al. 2019). Here, we utilized an acid-etching method to roughen the surfaces of Ti implants, as visualized with SEM before applying the PDA coating and commencing LbL assembly (Fig. 1A–C).
Figure 1.
Modification of titanium surface with an assembly of bioactive nanolayers. (A) Schematic of polydopamine-coating process that can be applied on titanium discs or implants without affecting their microstructures, (B, C) as demonstrated via scanning electron microscopy images. Inserts show surfaces with higher magnification. (D) Layer-by-layer deposition of poly-L-lysine and hyaluronic acid polymers in the presence and absence of BMP-2–mimicking short peptide.
Catechol and quinone groups in PDA coating can react with thiol- or amine-containing polymers via Michael-type addition or Schiff-base reactions (Yang et al. 2016; Yegappan et al. 2019). Here we used PLL as a cationic and amine-containing polymer to initiate the LbL process (Fig. 1D). HA was selected as a negatively charged polyelectrolyte to form nanolayers. Layers of positively charged PLL alternated with anionic HA to form bilayers, which are designated as [PLL / HA (BMP-2)] X (X = number of bilayers). Chemical structures of lysine, PLL, HA, and PDA are presented in Appendix Figure 2.
Surface characterization with SEM is presented in Figure 2A. Morphologic evaluations with atomic force microscope showed that the root mean square roughness, peak-to-valley roughness, interirregularity space, and surface area were significantly decreased following PDA and LbL coating (Fig. 2B, C) and changed the chemical composition of the surface, as detected by XPS (Fig. 3A–C). The XPS spectra denoted the inclusion of the dopamine and nanolayer elements (carbon, nitrogen, and oxygen). The successful deposition of the PDA coating was indicated by the appearance of the peaks of nitrogen (N 1s peak) at 295 eV and reduction in the atomic percentage of the intrinsic substrate peaks of Ti 2p and oxygen (O 1s peak; Hasani-Sadrabadi et al. 2019). Less nitrogen and more carbon are expected on the surface after LbL coating with PLL and HA due to their chemical composition.
Figure 2.
Characterization of surface modified titanium discs. (A) Scanning electron micrographs and (B) 3-dimensional reconstructed surface roughness images of unmodified, PDA-coated, and PDA-modified LbL-coated titanium surfaces as investigated by AFM. (C) Quantitative measurement of surface roughness parameters of the titanium-based surfaces. Data are presented as mean ± SD. ***P < 0.001. AFM, atomic force microscope; LbL, layer by layer; PDA, polydopamine; Ra, average roughness values; Rz, mean peak to valley roughness.
Figure 3.
Surface chemical composition of (A) unmodified, (B) PDA-coated, and (C) PDA-modified LbL-coated titanium surfaces as investigated by XPS. (D) Cumulative release of BMP-2–mimicking short peptide from LbL coating on titanium surface for 10 d at 37 °C. Data are presented as mean ± SD. (E) Viability and morphology of periodontal ligament stem cells cultured on surface-modified and nonmodified TCPs. (F) Quantification of cellular viability. Data are presented as mean ± SD, n = 4. LbL, layer by layer; NS, not significant; PDA, polydopamine; TCP, tissue culture plate; XPS, x-ray photoelectron spectroscopy.
The peptide diffusion rate can be controlled by the number of the assembled layers and the quality of the LbL assembly, since better-assembled layers result in slower peptide release (Keeney et al. 2015). Figure 3D demonstrates cumulative peptide release profiles from the Ti surface with 5, 10, and 20 assembled bilayers.
Functionality of Surface-Modified Ti In Vitro
In the current study, we selected PDLSCs for studying the functionality of surface-modified Ti implants in vitro, since our group and others have already confirmed their multilineage differentiation capabilities (Moshaverinia et al. 2014; Ansari et al. 2017). Cytocompatibility of the modified surfaces in vitro is demonstrated in Figure 3E. It has been reported that protein adsorption, as well as the formation of semicovalent bonds between thiol or primary amine groups of soluble proteins or cell surface proteins and dopamine residues, can cause strong cell adhesion to PDA-containing surfaces and support the immobilization of therapeutic agents such as BMP-2 on implants (Lee et al. 2018; Hasani-Sadrabadi et al. 2019). As illustrated in Figure 3E, the LbL/PDA-coated Ti surfaces provided similar cell adhesion as compared with traditional tissue culture plates; however, PDA-coated surfaces showed better cell attachment and spreading.
Although results indicated that >80% of the stem cells were live in all the experimental groups without significant differences (P > 0.5), LbL/PDA-coated Ti surfaces demonstrated higher viability (Fig. 3F). It was shown that incorporation of BMP-2 peptide or gentamicin into the surface via LbL assembly of PLL/HA layers did not adversely affect the viability of the cultured cells.
To form an osteoinductive surface on the Ti implants, we first coated the implants with PDA and loaded BMP-2–mimicking peptide in the PLL/HA layers while forming them. This offers the potential for excellent clinical outcomes as the cells are induced to initiate bone formation on the implant, which can lead to improved integration with the host tissue. Actin staining demonstrated adhesion and spreading of the PDLSCs on the different surfaces (Fig. 4A).
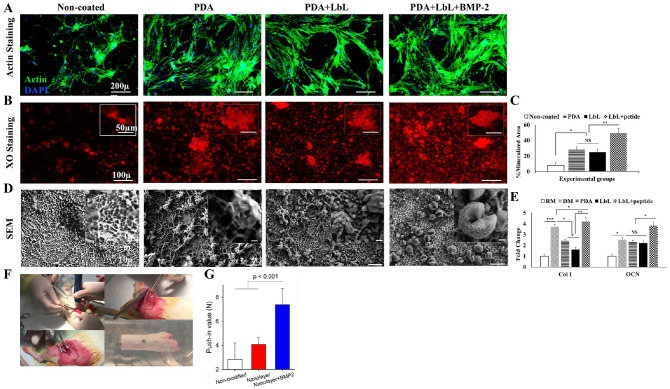
Figure 4.
Osteogenesis of the surface modified titanium implants in vitro and in vivo. (A) Immunofluorescent actin staining shows adhesion of PDLSCs on modified Ti surfaces as compared with the noncoated sample. Scale bars: 200 µm. (B) XO staining demonstrates improved osteogenesis by modifying the Ti surface with LbL assembly. Scale bars: 100 µm. Inserts show higher magnification. Scale bars: 50 µm. (C) Quantitative analyses show the percentage of the mineralized area observed with the XO staining. (D) SEM images show the morphology of the PDLSCs cultured on the coated and non-coated Ti surfaces. Scale bars: 10 µm. Inserts show higher magnification. Scale bars: 1 µm. (E) Reverse transcription polymerase chain reaction analyses evaluate the expression of osteogenic markers Col I and OCN by PDLSCs cultured on Ti implants in RM and DM after 4 wk. Data are expressed as mean ± SD. (F) Surgical procedure for placing the Ti implants. (G) Push-in value of the surface-engineered implants after 4 wk of implantation (n = 5). The P value is shown on the graph as evaluated by 1-way analysis of variance. DM, differentiation medium; LbL, layer by layer; NS, not significant; PDA, polydopamine; PDLSC, periodontal ligament stem cell; RM, regular medium; SEM, scanning electron microscopy; TCP, tissue culture plate; Ti, titanium; XO, xylenol orange; XPS, x-ray photoelectron spectroscopy.
The XO staining demonstrated that the presence of PDA could enhance the mineralization of the cultured cells (Fig. 4B, C). The mineralization was boosted by the inclusion of the BMP-2–mimicking peptide within the LbL assembly.
Morphology of the PDLSCs cultured on the modified Ti surfaces was monitored by SEM after incubation for 4 wk. As shown in Figure 4D, in addition to the deposition of calcium phosphate matrix, cells cultured on the BMP-2 peptide–loaded LbL-modified Ti surface exhibited an extreme transformation in their morphology toward mineralization. These structures are similar to those expected from differentiated/mineralized osteoblasts known as osteocytes, which help to form mineralized structures during new bone formation. To the best of our knowledge, no prior research on biomaterials has reported such a strong osteogenic signal to push the cells to reach this stage.
The upregulation of Col I and OCN by the cultured PDLSCs was studied to evaluate the osteogenic potential of the modified surfaces. The results of reverse transcription polymerase chain reaction studies revealed that the PDA-coated Ti implants could induce osteogenic differentiation of the cultured PDLSCs significantly better than the noncoated ones under regular medium conditions. However, the Ti implant with the BMP-2 peptide incorporated into the LbL assembly induced expression of the relevant genes significantly better than all other conditions, including the differentiation medium (Fig. 4E).
Functionality of the Surface-Modified Ti Implants In Vivo
To examine the functionality of the modified surfaces to improve osteointegration, we studied early-stage bone-implant integration in rodents as described elsewhere (Aita et al. 2009). Four weeks after surgery, femurs containing implants were harvested (Fig. 4F) and embedded into auto-polymerizing resin. The implant integration with the host bone was evaluated with a push-in test. The push-in values were determined by measuring the peak of the load-displacement curve. The strength of osseointegration for the implants with nanolayers delivering BMP-2 peptide showed a significant increase as compared with the untreated implants and the implants with nanolayers but without loaded peptide (Fig. 4G).
Self-assembled Nanolayers as an Antibiotic Delivery Vehicle
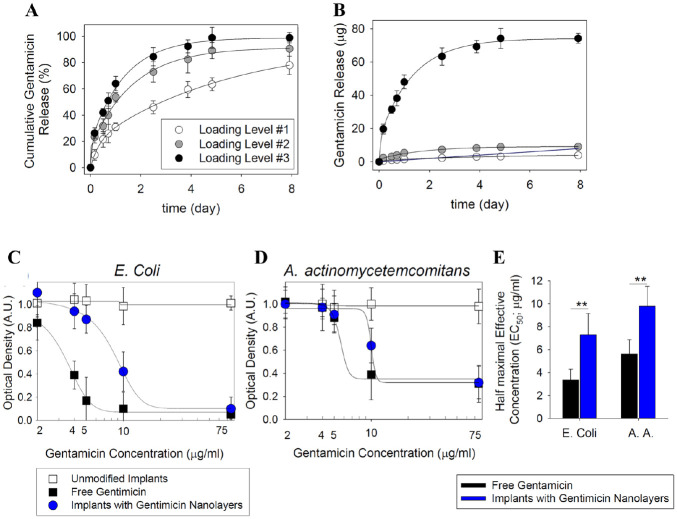
Besides delivering bioactive osteogenic factors, our nanolayers can simultaneously release antibacterial agents to prevent potential bacterial infection (Mombelli 2002; Qian et al. 2012). As a proof of concept for incorporating this type of agent, we studied the release of gentamicin over time (Fig. 5A, B).
Figure 5.
Evaluation of gentamicin release and its antibacterial properties. Release of gentamicin encapsulated in layer-by-layer coated titanium surfaces at 3 loading levels: 5 µg (loading level 1), 10 µg (loading level 2), and 75 µg (loading level 3). Data are presented as mean ± SD (n = 5). Data were evaluated and plotted as (A) cumulative and (B) total released amount. Analysis of the ability of gentamicin-loaded surfaces to inhibit the growth of (C) Escherichia coli and (D) Aggregatibacter actinomycetemcomitans as determined by measurement of absorbance at 600 and 620 nm, respectively. (E) Estimated half-maximal effective concentration (EC50) values for free and nanolayer-encapsulated gentamicin. **P < 0.01, 1-way analysis of variance.
The antibacterial properties of Ti implants with gentamicin-loaded surface coating as compared with soluble gentamicin are demonstrated in Figure 5C and D. Sustained release from the nanolayers reduced the availability of the drug in the medium within 24 h of incubation, which resulted in a reduced half-maximal effective concentration (EC50) of the drug (Fig. 5E). Unmodified surfaces showed no inhibition of bacterial growth, underlining that the effects were caused solely by the gentamicin.
Discussion and Conclusion
The null hypothesis in this study is that there is no difference between the control group and the experimental groups in terms of osteointegration and antimicrobial properties. Based on our results, the null hypothesis was rejected, as assembling the nanolayers on PDA-coated Ti implants increased osteointegration and antimicrobial activity.
Studies have proposed approaches to enhance implant osseointegration by developing an osteogenic surface or to prevent infection by incorporating antibiotic molecules or peptides in the surface coating (Smeets et al. 2016; Escobar et al. 2021). One promising alternative could be the development of implants with multifunctional surfaces capable of interacting with the host tissue to increase osseointegration while reducing bacterial activity (Raphel et al. 2016; Rao et al. 2019). Implant surfaces can be modified with bioactive therapeutics such as BMP-2 to enhance osteogenic potential; however, high costs and complications at high dosages hinder translation into clinical applications. Instead, BMP-2–mimicking short peptides can be used to exert a similar therapeutic effect at lower concentrations.
Here, to respond to the growing demand for alternative therapeutic solutions, we developed an easily processable and scalable platform to produce multifunctional surfaces by employing a nanoscopic LbL surface treatment on PDA-coated Ti discs capable of incorporating BMP-2–mimicking short peptide and gentamicin to simultaneously improve osseointegration and antibacterial activities. Employing PDA as an interfacial coating synergistically boosts the osteogenic potential of the immobilized BMP-2 (Lee et al. 2018).
The presence of PDA can enhance surface mineralization and provide a better substrate for surface modification (Jia et al. 2019). Here, we have used dopamine as a precursor to develop PDA coating. Although the PDA layer is initially buried under HA/PLL layers, the ultimate host tissue integration with Ti implants will happen through the PDA layer as these nanolayers are all biodegradable. As reported here, therapeutics-loaded HA/PLL can accelerate early-stage osteointegration while a supportive PDA layer will provide long-term integrity with the implant.
We utilized a BMP-2–mimicking short peptide, which demonstrates similar effects to those of BMP-2 at a lower concentration (10- to 100-fold less), while it can be produced on a large scale at a lower price. The BMP-2–mimicking peptide was mixed with HA polymer and entrapped in the LbL structure.
Entrapment of gentamicin, a cationic drug with a physiologic charge of 5, in LbL-assembled layers of chitosan and alginate was reported recently (He et al. 2020). We investigated the incorporation of a positively charged antibiotic within the LbL assembly. Gentamicin was chosen for its well-known clinical benefits as an antibiotic agent when coencapsulated into the nanolayer coating on Ti implants (Sandros et al. 1994). Since gentamicin strongly interacts with HA, prolonged release can be achieved even in the case of this small molecule drug. The bond strength between the layers can affect their quality and slow the release of loaded therapeutic agents. Another effective means by which the release profile can be tuned is by increasing the number of layers (PLL/HA) as shown in the in vitro release studies: the higher the number of layers, the slower the drug release.
We recently developed PDA-coated polymeric nanofibrous scaffolds that promote osteogenesis of stem cells, enhance mineralization, and induce bone formation in vitro and in vivo (Hasani-Sadrabadi et al. 2019). Here, we enhanced the osteogenic potential of the surface by incorporation of BMP-2–mimicking peptide as confirmed by our in vitro and in vivo studies.
Altogether, the promising findings of the current in vitro and in vivo studies could form the basis for unraveling the capacity of this novel coating method to simultaneously incorporate multiple therapeutic reagents such as growth factors and antibiotics to improve the overall function of implants with an easily processable and scalable manner.
To test the efficacy of the nanolayers in incorporating and releasing antibiotics, we tested gentamicin as a model drug; however, future studies need to include a range of antibiotics to ensure the capability of this LbL assembly as a universal platform for drug delivery. Additionally, as a proof of concept, A. actinomycetemcomitans and E. coli bacteria were utilized to confirm the antimicrobial activity of this antibiotic-incorporated surface-modified Ti implant as the initial building blocks for upcoming studies, which requires investigating more relevant bacterial strains in a clinically relevant disease model such as peri-implantitis. Local and sustained presentation of antibiotics via nanolayers on the surface of implants can present unparalleled therapeutic benefits in implant dentistry. Such an outcome cannot be achieved by the administration of free antibiotics around the implant or systemically.
Finally, in the current study, we did not observe the combined effect of the BMP-2 and gentamicin, and it needs to be observed in future studies. However, based on the previously published studies, it is unlikely to see any inactivation or interference of the ultimate functions of the gentamicin and BMP-2 when incorporated in the PDA-coated Ti implant (Lee et al. 2012). Upcoming studies will detail the clinical applicability, safety, and efficacy of this approach for implant dentistry.
Author Contributions
M.M. Hasani-Sadrabadi, contributed to conception, data acquisition, and analysis, drafted and critically revised the manuscript; S. Pouraghaei, contributed to data analysis, drafted and critically revised the manuscript; E. Zahedi, P. Sarrion, E. Dashtimoghadam, contributed to data acquisition and analysis, drafted and critically revised the manuscript; M. Ishijima, contributed to data acquisition and analysis, critically revised the manuscript; N. Jahedmanesh, contributed to data acquisition, critically revised the manuscript; S. Ansari, A. Moshaverinia, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript; T. Ogawa, contributed to design and data acquisition, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211029185 for Antibacterial and Osteoinductive Implant Surface Using Layer-by-Layer Assembly by M.M. Hasani-Sadrabadi, S. Pouraghaei, E. Zahedi, P. Sarrion, M. Ishijima, E. Dashtimoghadam, N. Jahedmanesh, S. Ansari, T. Ogawa and A. Moshaverinia in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Institute of Dental and Craniofacial Research (DE029876 to A. Moshaverinia).
ORCID iD: S. Pouraghaei  https://orcid.org/0000-0003-0810-2573
https://orcid.org/0000-0003-0810-2573
Data Availability: All data associated with this study are present in the article or the Appendix.
References
- Aita H, Hori N, Takeuchi M, Suzuki T, Yamada M, Anpo M, Ogawa T. 2009. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 30(6):1015–1025. [DOI] [PubMed] [Google Scholar]
- Ansari S, Diniz IM, Chen C, Aghaloo T, Wu BM, Shi S, Moshaverinia A. 2017. Alginate/hyaluronic acid hydrogel delivery system characteristics regulate the differentiation of periodontal ligament stem cells toward chondrogenic lineage. J Mater Sci Mater Med. 28(10):162. [DOI] [PubMed] [Google Scholar]
- Cui W, Liu Q, Yang L, Wang K, Sun T, Ji Y, Liu L, Yu W, Qu Y, Wang J, et al. 2018. Sustained delivery of BMP-2-related peptide from the true bone ceramics/hollow mesoporous silica nanoparticles scaffold for bone tissue regeneration. ACS Biomater Sci Eng. 4(1):211–221. [DOI] [PubMed] [Google Scholar]
- Escobar A, Muzzio N, Moya SE. 2021. Antibacterial layer-by-layer coatings for medical implants. Pharmaceutics. 13(1):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouziya B, Uthappa MA, Amara D, Tom N, Byrappa S, Sunny K. 2016. Surface modifications of titanium implants—the new, the old, and the never heard of options. J Adv Clin Res Insights. 3(6):215–219. [Google Scholar]
- Ghiasi S, Behboudi A, Mohammadi T, Khanlari S. 2019. Effect of surface charge and roughness on ultrafiltration membranes performance and polyelectrolyte nanofiltration layer assembly. Colloids Surf A Physicochem Eng Asp. 580:123753. [Google Scholar]
- Gittens RA, Olivares-Navarrete R, Cheng A, Anderson DM, McLachlan T, Stephan I, Geis-Gerstorfer J, Sandhage KH, Fedorov AG, Rupp F, et al. 2013. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 9(4):6268–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán E, Mateos-Maroto A, Ruano M, Ortega F, Rubio RG. 2017. Layer-by-layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv Colloid Interface Sci. 249:290–307. [DOI] [PubMed] [Google Scholar]
- Hasani-Sadrabadi MM, Sarrion P, Nakatsuka N, Young TD, Taghdiri N, Ansari S, Aghaloo T, Li S, Khademhosseini A, Weiss PS, et al. 2019. Hierarchically patterned polydopamine-containing membranes for periodontal tissue engineering. ACS Nano. 13(4):3830–3838. [DOI] [PubMed] [Google Scholar]
- Hasani-Sadrabadi MM, Sarrion P, Pouraghaei S, Chau Y, Ansari S, Li S, Aghaloo T, Moshaverinia A. 2020. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med. 12(534):eaay6853. [DOI] [PubMed] [Google Scholar]
- He LJ, Hao JC, Dai L, Zeng RC, Li SQ. 2020. Layer-by-layer assembly of gentamicin-based antibacterial multilayers on Ti alloy. Mater Lett. 261:127001. [Google Scholar]
- Huang S, Liang N, Hu Y, Zhou X, Abidi N. 2016. Polydopamine-assisted surface modification for bone biosubstitutes. Biomed Res Int. 2016:2389895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Han F, Wang H, Zhu C, Guo Q, Li J, Zhao Z, Zhang Q, Zhu X, Li B. 2019. Polydopamine-assisted surface modification for orthopaedic implants. J Orthop Translat. 17:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney M, Jiang XY, Yamane M, Lee M, Goodman S, Yang F. 2015. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J Mater Chem B. 3(45):8757–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kumar S. 2014. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol Cancer Res. 12(10):1416–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Yun YP, Park K, Kim SE. 2012. Gentamicin and bone morphogenic protein–2 (BMP-2)–delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone. 50(4):974–982. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee J-C, Heo JS. 2018. Polydopamine-assisted BMP-2 immobilization on titanium surface enhances the osteogenic potential of periodontal ligament stem cells via integrin-mediated cell-matrix adhesion. J Cell Commun Signal. 12(4):661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça G, Mendonça DBS, Aragão FJL, Cooper LF. 2010. The combination of micron and nanotopography by H2SO4/H2O2 treatment and its effects on osteoblast-specific gene expression of hMSCs. J Biomed Mater Res A. 94(1):169–179. [DOI] [PubMed] [Google Scholar]
- Mendonça G, Mendonça DBS, Simões LGP, Araújo AL, Leite ER, Duarte WR, Aragão FJL, Cooper LF. 2009. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials. 30(25):4053–4062. [DOI] [PubMed] [Google Scholar]
- Meng HW, Chien EY, Chien HH. 2016. Dental implant bioactive surface modifications and their effects on osseointegration: a review. Biomark Res. 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Choi KY, Dreaden EC, Padera RF, Braatz RD, Spector M, Hammond PT. 2016. Designer dual therapy nanolayered implant coatings eradicate biofilms and accelerate bone tissue repair. ACS Nano. 10(4):4441–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombelli A. 2002. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 28(1):177–189. [DOI] [PubMed] [Google Scholar]
- Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, Shi S. 2013. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A. 20(3–4): 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshaverinia A, Xu X, Chen C, Ansari S, Zadeh HH, Snead ML, Shi S. 2014. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials. 35(9):2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Ozawa S, Shih JH, Ryu KH, Sukotjo C, Yang JM, Nishimura I. 2000. Biomechanical evaluation of osseous implants having different surface topographies in rats. J Dent Res. 79(11):1857–1863. [DOI] [PubMed] [Google Scholar]
- Park JC, Kim JM, Jung IH, Kim JC, Choi SH, Cho KS, Kim CS. 2011. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: in vitro and in vivo evaluations. J Clin Periodontol. 38(8):721–731. [DOI] [PubMed] [Google Scholar]
- Picart C, Lavalle P, Hubert P, Cuisinier FJG, Decher G, Schaaf P, Voegel JC. 2001. Buildup mechanism for poly(L-lysine)/hyaluronic acid films onto a solid surface. Langmuir. 17(23):7414–7424. [Google Scholar]
- Qian J, Wennerberg A, Albrektsson T. 2012. Reasons for marginal bone loss around oral implants. Clin Implant Dent Relat Res. 14(6):792–807. [DOI] [PubMed] [Google Scholar]
- Rao S, Hashemi Astaneh S, Villanueva J, Silva F, Takoudis C, Bijukumar D, Souza JCM, Mathew MT. 2019. Physicochemical and in-vitro biological analysis of bio-functionalized titanium samples in a protein-rich medium. J Mech Behav Biomed Mater. 96:152–164. [DOI] [PubMed] [Google Scholar]
- Raphel J, Holodniy M, Goodman SB, Heilshorn SC. 2016. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 84:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandros J, Papapanou PN, Nannmark U, Dahlén G. 1994. Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontal Res. 29(1):62–69. [DOI] [PubMed] [Google Scholar]
- Sevari SP, Shahnazi F, Chen C, Mitchell JC, Ansari S, Moshaverinia A. 2020. Bioactive glass-containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells. J Biomed Mater Res A. 108(3):557–564. [DOI] [PubMed] [Google Scholar]
- Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O, Precht C, Kloss F, Gröbe A, Heiland M, Ebker T. 2016. Impact of dental implant surface modifications on osseointegration. Biomed Res Int. 2016:6285620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Ikeda T, Tsukimura N, Ishijima M, Minamikawa H, Sugita Y, Yamada M, Wakabayashi N, Ogawa T. 2016. Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials. 108:177–186. [DOI] [PubMed] [Google Scholar]
- Wang B, Liu H, Sun L, Jin Y, Ding X, Li L, Ji J, Chen H. 2017. Construction of high drug loading and enzymatic degradable multilayer films for self-defense drug release and long-term biofilm inhibition. Biomacromolecules. 19(1):85–93. [DOI] [PubMed] [Google Scholar]
- Yang J, Saggiomo V, Velders AH, Stuart MAC, Kamperman M. 2016. Reaction pathways in catechol/primary amine mixtures: a window on crosslinking chemistry. PLoS One. 11(12):e0166490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MC, Chi NH, Chou NK, Huang YY, Chung TW, Chang YL, Liu HC, Shieh MJ, Wang SS. 2010. The influence of rat mesenchymal stem cell CD44 surface markers on cell growth, fibronectin expression, and cardiomyogenic differentiation on silk fibroin–hyaluronic acid cardiac patches. Biomaterials. 31(5):854–862. [DOI] [PubMed] [Google Scholar]
- Yegappan R, Selvaprithiviraj V, Mohandas A, Jayakumar R. 2019. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf B Biointerfaces. 177:41–49. [DOI] [PubMed] [Google Scholar]
- Yost AL, Shahsavari S, Bradwell GM, Polak R, Fachin F, Cohen RE, McKinley GH, Toner M, Rubner MF, Wardle BL. 2015. Layer-by-layer functionalized nanotube arrays: a versatile microfluidic platform for biodetection. Microsyst Nanoeng. 1:15037. [Google Scholar]
- Zarubova J, Hasani-Sadrabadi MM, Bacakova L, Li S. 2020. Nano-in-micro dual delivery platform for chronic wound healing applications. Micromachines. 11(2):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211029185 for Antibacterial and Osteoinductive Implant Surface Using Layer-by-Layer Assembly by M.M. Hasani-Sadrabadi, S. Pouraghaei, E. Zahedi, P. Sarrion, M. Ishijima, E. Dashtimoghadam, N. Jahedmanesh, S. Ansari, T. Ogawa and A. Moshaverinia in Journal of Dental Research