Abstract
Members of the mammalian AlkB family are known to mediate nucleic acid demethylation1,2. ALKBH7, a mammalian AlkB homologue, localizes in mitochondria and affects metabolism3, but its function and mechanism of action are unknown. Here we report an approach to site-specifically detect N1-methyladenosine (m1A), N3-methylcytidine (m3C), N1-methylguanosine (m1G) and N2,N2-dimethylguanosine (m22G) modifications simultaneously within all cellular RNAs, and discovered that human ALKBH7 demethylates m22G and m1A within mitochondrial Ile and Leu1 pre-tRNA regions, respectively, in nascent polycistronic mitochondrial RNA4-6. We further show that ALKBH7 regulates the processing and structural dynamics of polycistronic mitochondrial RNAs. Depletion of ALKBH7 leads to increased polycistronic mitochondrial RNA processing, reduced steady-state mitochondria-encoded tRNA levels and protein translation, and notably decreased mitochondrial activity. Thus, we identify ALKBH7 as an RNA demethylase that controls nascent mitochondrial RNA processing and mitochondrial activity.
The AlkB family proteins catalyse oxidative demethylation of nucleic acid substrates7-10. We previously identified two mammalian AlkB homologues, ALKBH5 and FTO, as RNA demethylases that demethylate N6-methyladenosine (m6A) in polyA-tailed RNA1,2,11-13. The m6A modification regulates diverse functions, including translation, mRNA stability, pre-mRNA processing and transcription14,15. ALKBH1, another mammalian AlkB homologue, affects translation via demethylation of m1A in tRNA16.
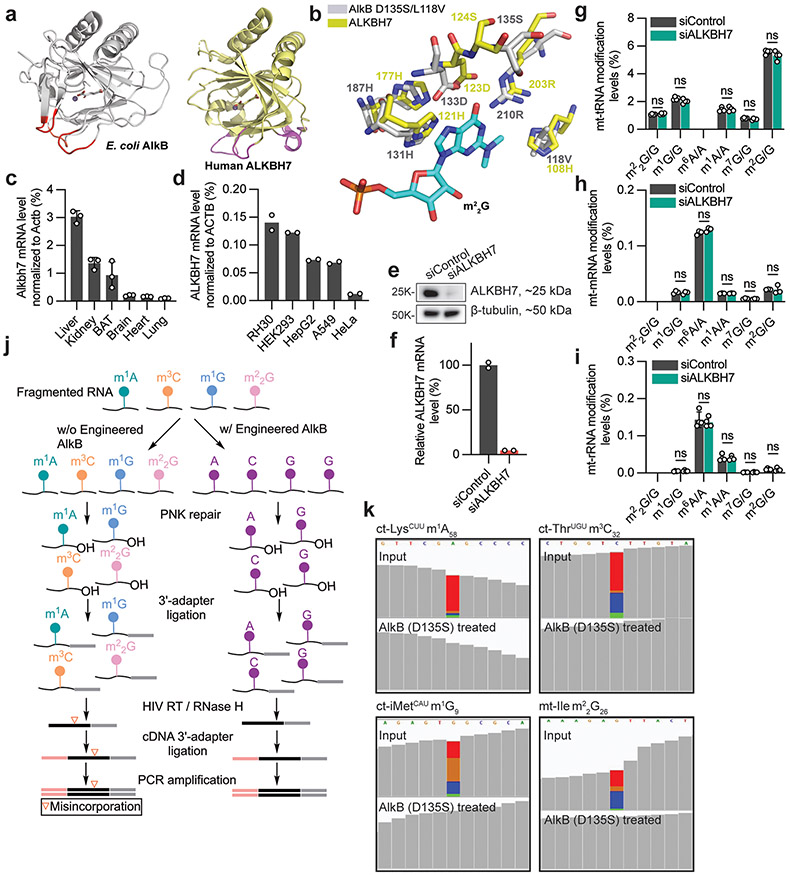
Knockouts of FTO, ALKBH5, ALKBH1 (ref. 17), ALKBH4 (ref. 18) and ALKBH7 (ref. 3), genes encoding mammalian AlkB homologues, display notable phenotypes. For instance, Alkbh7-knockout mice exhibit obesity3. ALKBH7 is nuclear encoded, but displays a predominantly mitochondrial localization in mammalian cells3,19,20. We examined the crystal structures of ALKBH7 (ref. 21) and DNA-bound Escherichia coli AlkB (ref. 10) and observed conservation of the nucleic acid–binding loops (Extended Data Fig. 1a). In addition, the ALKBH7 active site contains two residues (S124 and H108) that are similar to those within an AlkB mutant protein (D135S/L118V; Extended Data Fig. 1b) that we previously engineered to convert AlkB into an m22G demethylase22. This finding suggests that ALKBH7 could be a naturally existing m22G demethylase in mammalian mitochondria. Human cytoplasmic and mitochondrial (mt) tRNAs are modified with m22G at position 26 (m22G26)4, which influences the stability and structure of certain tRNAs23,24.
In mice, ALKBH7 is expressed at high levels in liver, kidney, fat, brain and heart3 (Extended Data Fig. 1c). Accordingly, the human cell lines HepG2 and HEK293 displayed high levels of ALKBH7 mRNA, whereas the cervical cancer cell line HeLa showed relatively low levels of ALKBH7 mRNA (Extended Data Fig. 1d). We used RNA-mediated interference to deplete ALKBH7 in HepG2 cells (Extended Data Fig. 1e,f), and performed liquid chromatography–tandem mass spectrometry (LC-MS/MS) to quantify modification level changes for m22G, m1G, m6A, m1A, m7G and m2G in mature mt-RNAs. However, we did not observe altered levels of these modifications in mature mt-tRNAs, mt-mRNAs or mt-rRNAs (Extended Data Fig. 1g-i).
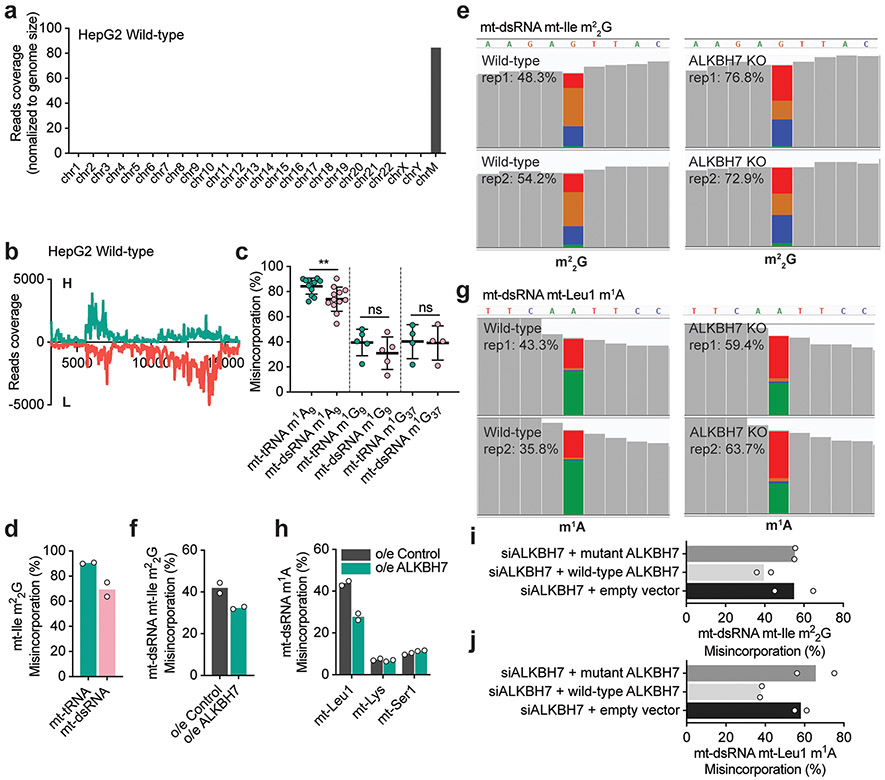
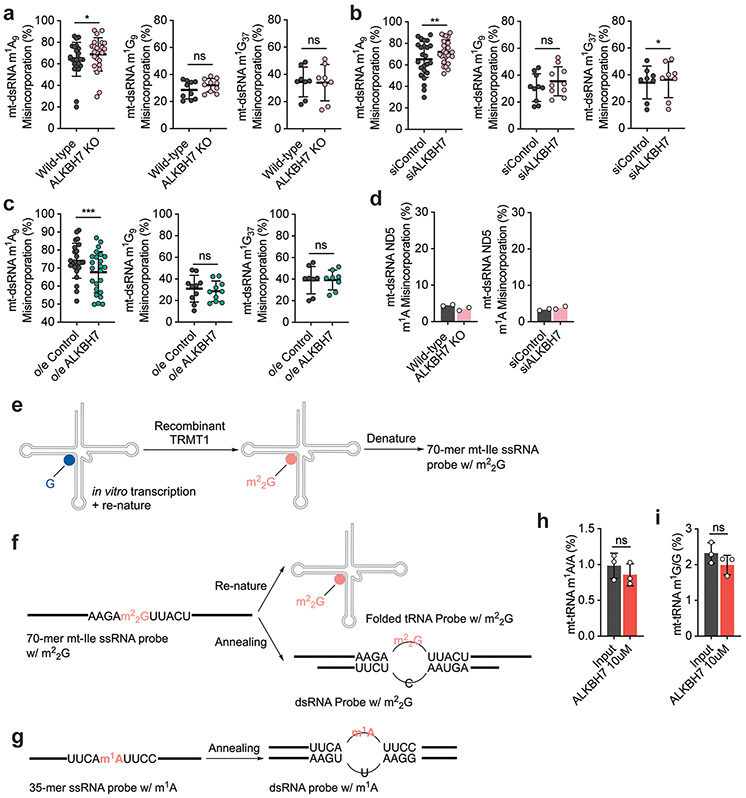
We hypothesized that ALKBH7 targets specific sites within mt-RNAs. To test this hypothesis, we developed a sequencing approach that can simultaneously detect and monitor methylation levels of m1A, m3C, m1G and m22G within RNA. These four modifications disrupt Watson–Crick base pairing, and generate mutations during reverse transcription (RT) by the human immunodeficiency virus (HIV) reverse transcriptase. To detect modified sites, we also optimized the conditions using an engineered AlkB (D135S)25 to eliminate all four modifications in one demethylation treatment (Extended Data Fig. 1j,k). We then can treat fragmented cellular RNA with or without AlkB-D135S, and detect modified positions as those with an almost complete elimination of mutations in the treated sample. We term this approach demethylation-assisted multiple methylation sequencing (DAMM-seq). This method allows us to monitor the modification level based on the mutation rate at each modified site for these four methylation types26,27. To validate DAMM-seq, we analysed HepG2 mt-tRNAs. We confirmed fourteen mt-tRNAs possessing m1A9, three with m1A58, two with m3C32, five with m1G9, and four with m1G37 (Fig. 1a-d and Extended Data Fig. 2a-e).
Fig. 1 ∣. Human ALKBH7 regulates mitochondrial m22G methylation in mt-dsRNA.
a–e, RT misincorporation frequency (input versus engineered AlkB treatment) at m1A in mt-tRNAs (fourteen m1A9 and three m1A58 sites; ****P < 0.0001; paired, two-tailed t-test; n = 17 independent m1A sites; a), m3C32 in mt-tRNAs and cytoplasmic (ct) tRNAs (eight m3C32 sites; ****P < 0.0001; paired, two-tailed t-test; n = 8 independent m3C sites; b), m1G in mt-tRNAs (five m1G9 and four m1G37 sites; ****P < 0.0001; paired, two-tailed t-test; n = 9 independent m1G sites; c), m1A58 in 3 mt-tRNAs that contain m1A58 (d) and G26 in 6 mt-tRNAs containing G26 (e). For d,e, n = 2 biologically independent samples. f, A9, G9 and G37 in mature mt-tRNA compared with those in enriched mt-dsRNA. A9: **P = 0.0082; paired, two-tailed t-test; n = 12 independent m1A sites. G9: P = 0.1502; paired, two-tailed t-test; n = 5 independent m1G sites. G37: P = 0.0795; paired, two-tailed t-test; n = 4 independent m1G sites. For a–c and f, each data point represents one methylation site, with the average misincorporation from two biologically independent samples; data are presented as mean values ± s.d. HepG2 cells were used for a–f. g, mt-Ile m22G in mature mt-tRNA compared with that in enriched mt-dsRNA in HepG2 and HeLa cells. WT, wild type; o/e, overexpression. h, m22G in mt-dsRNA (mt-Ile region) in TRMT1-depleted HeLa cells versus the control. i, mt-Leu1 m1A in mature mt-tRNA compared with that in enriched mt-dsRNA in HepG2 and HeLa cells. j, m22G in mt-dsRNA (mt-Ile region) in siALKBH7 HepG2 cells versus siControl. k, m22G in mt-dsRNA (mt-Ile region) in ALKBH7-knockout HepG2 cells versus the wild type. l, m1A in mt-dsRNA (mt-Leu1 region) in siALKBH7 HepG2 cells versus siControl. m, m1A in mt-dsRNA (mt-Leu1 region) in ALKBH7-knockout HepG2 cells versus the wild type. For g–m, n = 2, biologically independent samples. n, A schematic of four types of methylated bases detected within mt-dsRNA: m22G (yellow diamond), m1A (green circle for m1A58), m1A (light blue square for m1A9) and m1G (both m1G9 and m1G37, red ring). The junction sites monitored in this study are marked with blue arrows.
G26 in mt-tRNAs is methylated to m2G and m22G by the methyltransferase TRMT1 (refs. 4,23,24). As m2G only slightly hinders Watson–Crick base pairing, this modification does not induce noticeable mutations when using mutation-based sequencing such as DAMM-seq. Among the human mt-tRNAs we analysed, only mt-Ile showed a high misincorporation rate at G26, which was substantially reduced by AlkB-mediated demethylation (Fig. 1e). As expected, TRMT1 knockdown in HeLa cells resulted in a decreased RT misincorporation at mt-Ile G26 (Extended Data Fig. 2f-h), confirming TRMT1 as an m22G ‘writer’ at position 26 of mt-Ile.
To determine whether ALKBH7 regulates the methylation of these positions in mature mt-tRNAs, we altered ALKBH7 levels. Specifically, we depleted ALKBH7 in HepG2 cells, and overexpressed ALKBH7 in HeLa cells. However, neither treatment altered the mutation frequency at these positions in mature mt-tRNAs (Extended Data Fig. 2i-t), consistent with our mass spectrometry observations (Extended Data Fig. 1g). Our data suggest that ALKBH7 does not demethylate mature mt-tRNAs in these cells.
Mitochondria also contain unprocessed nascent RNA transcribed from the mitochondrial genome. Nascent mt-RNA represents a small fraction of the total mt-RNA. Mitochondrial transcription generates two complementary polycistronic mt-RNAs that can form inter- and/or intra-molecular double-stranded RNA (dsRNA) in vivo5,6,28. Processing of the nascent mt-RNA generates 2 mt-rRNAs, 22 mt-tRNAs and 13 mt-mRNAs5,6. Most human mt-mRNAs and mt-rRNAs are flanked by mt-tRNAs in the nascent mt-RNA, and are released via endonucleolytic cleavage of the mt-tRNAs, referred to as the tRNA punctuation model29.
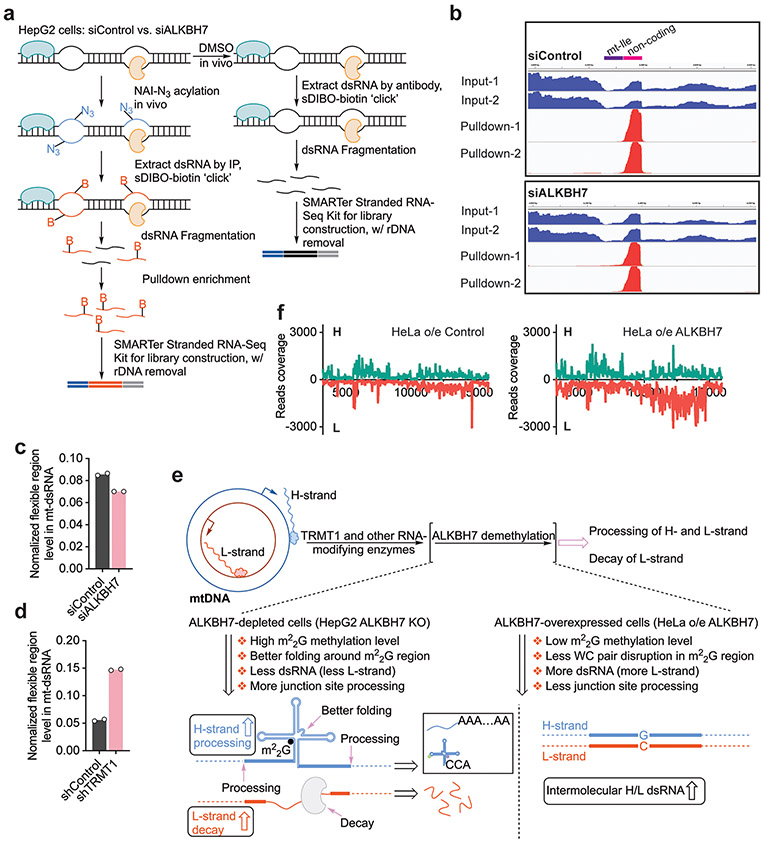
To investigate the methylation status of nascent mt-RNAs, we enriched nascent mt-dsRNA from cells by immunoprecipitation (IP) using an antibody that recognizes dsRNA, including mt-dsRNA28,30. We confirmed that a majority of reads from the IP-enriched dsRNA aligned to the mitochondrial genome (Extended Data Fig. 3a), with high coverage of both the heavy (H) and light (L) strands as well as unprocessed junctions within nascent mt-dsRNA28 (Extended Data Fig. 3b). The RT mutation frequencies at positions A9, G9 and G37 were only slightly reduced in mt-dsRNA compared to the mature mt-tRNAs in HepG2 (Fig. 1f) and HeLa (Extended Data Fig. 3c) cells. However, the RT mutation frequencies observed at mt-Ile m22G26 were substantially lower within mt-dsRNA (~40–50%) than within the mature mt-tRNA (~90–95%; Fig. 1g). TRMT1 knockdown in HeLa cells reduced the RT mutation rate at the mt-Ile m22G site in mt-dsRNA, indicating that TRMT1 methylates nascent mt-RNA at this site (Fig. 1h,n and Extended Data Fig. 3d). Additionally, mt-Leu1 m1A58 had an RT mutation frequency of ~40% within mt-dsRNA compared to ~90% in mature mt-tRNA (Fig. 1i). These observations show that mt-Ile m22G and mt-Leu1 m1A58 sites are partially methylated in mt-dsRNA.
To determine whether ALKBH7 mediates demethylation at these sites on nascent mt-RNA, we examined our knockdown and overexpression cells. Strikingly, ALKBH7 knockdown in HepG2 cells resulted in an ~21% increase in the RT mutation rate at the mt-Ile m22G site in mt-dsRNA (Fig. 1j). Similarly, we observed an ~24% increase in the RT mutation rate at the mt-Ile m22G site within mt-dsRNA of ALKBH7-knockout cells versus controls (Fig. 1k and IGV visualization in Extended Data Fig. 3e). Moreover, overexpression of ALKBH7 in HeLa cells decreased the RT mutation rate at the mt-Ile m22G site in mt-dsRNA (Extended Data Fig. 3f). We also observed an ~22% increase in the RT mutation rate at the mt-Leu1 m1A58 site (Fig. 1n) in mt-dsRNA of ALKBH7-depleted and ALKBH7-knockout HepG2 cells (Fig. 1l,m and IGV visualization in Extended Data Fig. 3g), and a reduced RT mutation rate at the same site in HeLa cells overexpressing ALKBH7 (Extended Data Fig. 3h). Both mt-Ile m22G and mt-Leu1 m1A demethylation can be rescued with the wild-type ALKBH7 protein but not by an ALKBH7 mutant without the mitochondrial targeting sequence (Extended Data Fig. 3i,j). Compared with mt-Ile m22G and mt-Leu1 m1A, altering the expression of ALKBH7 shows a weaker effect on known m1A9 sites within mt-dsRNA (Extended Data Fig. 4a-c). Other m1G9 and m1G37 sites in mt-dsRNA were not affected by ALKBH7 (Fig. 1n and Extended Data Fig. 4a-c). For instance, the m1A site within MT-ND5 mt-mRNA27 is methylated at a low level in mt-dsRNA and did not respond to ALKBH7 depletion (Extended Data Fig. 4d). Overall, these data suggest that ALKBH7 demethylates mt-Ile m22G26 and mt-Leu1 m1A58 within mt-dsRNA.
To further investigate the potential demethylase activity of ALKBH7, we in vitro transcribed an RNA probe comprising the whole human mt-Ile sequence and treated the renatured mt-Ile tRNA with recombinant human TRMT1 protein4,23,24 in vitro to methylate G26 (Extended Data Fig. 4e,f). We used LC–MS/MS to confirm the G26 modification, as revealed by an m22G/A ratio of ~1.1% (Fig. 2a), whereas those for m2G/A and m1G/A were lower than 0.02%. We treated both denatured and refolded m22G-modified mt-Ile tRNA probes with recombinant human ALKBH7. In both cases, ALKBH7 reduced the m22G/A ratio from ~1.1% to <0.05% (Fig. 2b), revealing a high m22G demethylase activity of ALKBH7. ALKBH7 treatment resulted in only a small increase in the m2G/A ratio from ~0.01% to ~0.04%, indicating that ALKBH7 converts almost all m22G into unmethylated G (Fig. 2c). In addition, we annealed a 35-base complementary RNA to the mt-Ile m22G RNA probe (Extended Data Fig. 4f) and found that ALKBH7 exhibited high demethylase activity towards m22G within the dsRNA (Fig. 2d). Overall, these findings show that human ALKBH7 can indeed demethylate mt-Ile m22G26 in vitro (Fig. 2e). Considering that ALKBH7 also affects m1A methylation of mt-Leu1 in cells, we synthesized a 35-base RNA probe consisting of a single m1A flanked by the mt-Leu1 sequence (Extended Data Fig. 4g). ALKBH7 demethylated this probe in vitro, with slightly lower activity towards dsRNA than single-stranded RNA (ssRNA; Fig. 2f,g). By incubating recombinant ALKBH7 protein with purified mt-tRNA from HepG2 cells, we further demonstrated that ALKBH7 does not work on other known mt-tRNA m1A9-, m1G9- and m1G37-modified sites in vitro, through monitoring m1A/A and m1G/G ratios (Extended Data Fig. 4h,i).
Fig. 2 ∣. ALKBH7 catalyses m22G demethylation.
a, Modification levels of m22G/A, m2G/A and m1G/A by LC–MS/MS for an RNA probe mimicking mt-Ile after in vitro treatment with recombinant human TRMT1. b, Modification levels of m22G/A in RNA before and after treatment with recombinant human ALKBH7. Left: mt-Ile ssRNA probe; **P = 0.0061 and **P = 0.0061 for ALKBH7 5 μM and 10 μM versus input, respectively; unpaired, two-tailed t-test. Right: folded mt-Ile tRNA probe; **P = 0.0034 and **P = 0.0035 for ALKBH7 5 μM and 10 μM versus input, respectively; unpaired, two-tailed t-test. c, Modification levels of m2G/A in RNA before and after treatment with recombinant human ALKBH7. Left: mt-Ile ssRNA probe; *P = 0.0148 and ***P = 0.0004 for ALKBH7 5 μM and 10 μM versus input, respectively; unpaired, two-tailed t-test. Right: folded mt-Ile tRNA probe; **P = 0.0016 and **P = 0.0018 for ALKBH7 5 μM and 10 μM versus input, respectively; unpaired, two-tailed t-test. Most m22G modifications were converted to G. d, Modification levels of m22G/A in a dsRNA probe mimicking the mt-Ile region in mt-dsRNA before and after treatment with recombinant human ALKBH7. ***P = 0.0002 and ***P = 0.0002 for ALKBH7 5 μM and 10 μM versus input, respectively; unpaired, two-tailed t-test. e, ALKBH7 mediates RNA m22G demethylation. f, Modification levels of m1A/A in a ssRNA probe mimicking the mt-Leu1 region in mt-dsRNA before and after treatment with recombinant human ALKBH7. ***P = 0.0005 and ***P = 0.0005 for ALKBH7 5 μM and 10 μM versus input, respectively; unpaired, two-tailed t-test. g, Modification levels of m1A/A in a dsRNA probe mimicking the mt-Leu1 region in mt-dsRNA before and after treatment with recombinant human ALKBH7. ***P = 0.0005 and ***P = 0.0008 for ALKBH7 5 μM and 10 μM versus input, respectively; unpaired, two-tailed t-test. For a–d,f,g, n = 3 biologically independent samples; data are presented as mean values ± s.d.
Nascent, polycistronic mt-RNAs are cleaved at the junction sites flanking each mt-tRNA to produce mature mt-tRNAs, mt-rRNAs and mt-mRNAs. The folding of pre-tRNAs is known to be critical for their processing by RNase P and RNase Z (refs. 31,32). On the basis of our finding that ALKBH7 regulates mt-Ile m22G26 levels within nascent, polycistronic mt-RNA, we propose that this modification affects processing of nascent mt-RNAs. To test this hypothesis, we performed an in vitro experiment to analyse maturation of the nascent mt-RNAs.
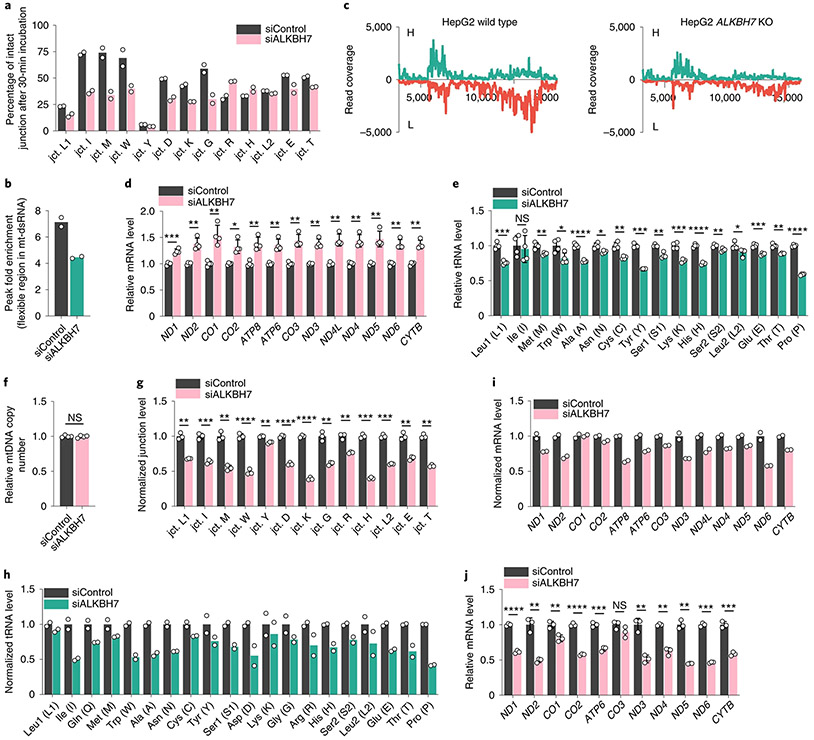
We extracted mt-dsRNAs from HepG2 cells transfected with control short interfering RNA (siRNA; siControl) or ALKBH7 siRNA (siALKBH7) and treated them with freshly prepared HepG2 mitochondrial lysate in vitro to examine junction processing over time. Specifically, we used quantitative PCR with RT (RT-qPCR) to quantify the extent of cleavage at mt-tRNA/mt-mRNA junctions (Fig. 1n). Notably, after a 30-min reaction, the level of the mt-ND1-mRNA/mt-Ile-tRNA junction site (I in Fig. 1n) was reduced by ~63% for mt-dsRNA isolated from siALKBH7 cells but only by ~27% in mt-dsRNA isolated from controls (Fig. 3a). These data suggest that the presence of m22G enhances cleavage of the mt-ND1-mRNA/mt-Ile-tRNA junction site within nascent mt-RNA. Several other junctions within the nascent mt-RNA behaved similarly, in particular those near the mt-Ile-tRNA region.
Fig. 3 ∣. ALKBH7-mediated m22G demethylation affects polycistronic mt-RNA processing and alters steady-state mt-RNA levels.
a, In vitro assay to monitor tRNA/mRNA junction site processing. The percentage of intact junction site after incubation for 30 min is shown. b, Fold enrichment calculated from the enriched mt-dsRNA flexible region (icSHAPE peak region) in siALKBH7 HepG2 cells versus siControl. c, DAMM-seq read coverage of IP-enriched dsRNA across the mitochondrial genome (protein-coding region, −3.5–16 kb). The H and L strands are shown in green and red, respectively (both normalized to 18S rRNA). d, Relative mRNA levels of 13 mt-mRNAs (normalized to ACTB) in ALKBH7-depleted HepG2 cells versus the control. P = 0.0006, 0.0053, 0.0088, 0.0139, 0.0046, 0.0069, 0.0035, 0.0027, 0.003, 0.0031, 0.0074, 0.0016 and 0.0014, respectively; unpaired, two-tailed t-test. e, Relative levels of 16 mt-tRNAs (normalized to U6 small nuclear RNA (snRNA)) in ALKBH7-depleted HepG2 cells versus the control. P = 0.0003, 0.7187, 0.0069, 0.0235, <0.0001, 0.0109, 0.003, 0.0001, 0.0028, 0.0005, <0.0001, 0.0085, 0.0356, 0.0004, 0.002 and <0.0001, respectively; unpaired, two-tailed t-test. f, Relative level of mtDNA (normalized to gDNA) in siALKBH7 versus siControl. P value = 0.7822; unpaired, two-tailed t-test. g, Normalized tRNA/mRNA junction levels in 10-min EU-labelled nascent mt-RNA extracted from siALKBH7 HepG2 cells versus siControl. P = 0.006, 0.0005, 0.0011, <0.0001, 0.0014, <0.0001, <0.0001, 0.0049, 0.0064, 0.0001, 0.0005, 0.0022 and 0.002, respectively; unpaired, two-tailed t-test. h, Normalized DAMM-seq read coverage of nascent mt-tRNA in 10-min EU-labelled mt-RNA extracted from siALKBH7 HepG2 cells versus siControl. i, Normalized DAMM-seq read coverage of nascent mt-mRNA in 10-min EU-labelled mt-RNA extracted from siALKBH7 HepG2 cells versus siControl. j, Relative mt-mRNA levels in 10-min EU-labelled nascent mt-RNA extracted from siALKBH7 HepG2 cells versus siControl. P = <0.0001, 0.0076, 0.0081, <0.0001, 0.0002, 0.136, 0.0032, 0.0041, 0.0039, 0.0001 and 0.0006, respectively; unpaired, two-tailed t-test. For a,b,h,i, n = 2 biologically independent samples. For d–f, n = 4 biologically independent samples; data are presented as mean values ± s.d. For g,j, n = 3 biologically independent samples; data are presented as mean values ± s.d. For d–g and j, NS, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
m22G disrupts Watson–Crick base pairing, which could alter interactions both within and between nascent mt-RNAs, as well as RNA–protein interactions. We employed icSHAPE33 to investigate how m22G affects the dynamics and solvent exposure of mt-dsRNA (Extended Data Fig. 5a). Briefly, we used NAI-N3 to modify accessible 2′-hydroxyl groups in IP-enriched mt-dsRNA, and captured NAI-N3-modified regions in mt-dsRNA. The icSHAPE33 data revealed that, within the H strand, only one region is notably accessible to NAI-N3 modification. This noncoding ~70-nt region is ~40–50 nt downstream of the m22G site in mt-Ile tRNA on the H strand (Extended Data Fig. 5b). Depletion of ALKBH7, which increased mt-Ile m22G methylation in mt-dsRNA, noticeably decreased the icSHAPE signal at this noncoding region (Fig. 3b and Extended Data Fig. 5b). To confirm this observation, we used targeted primers to quantify the pulldown of this flexible region by RT–qPCR. Again, we observed an ~20% reduction of the normalized pulldown signal in siALKBH7 cells compared to controls (Extended Data Fig. 5c). Importantly, we also observed an ~1.5-fold increase of the pulldown RNA in icSHAPE using TRMT1-depleted cells compared to controls (Extended Data Fig. 5d). These data suggest that m22G alters the local structure of the H strand in mt-dsRNA.
Both the H and L strands of nascent mt-RNAs are modified and processed after transcription, and a large fraction of the L strand is degraded6. Using anti-dsRNA antibody, we could pull down mt-dsRNA and measure the levels of the H and L strands with normalization to 18S rRNA. Loss of ALKBH7-mediated demethylation of m22G on the H strand is expected to promote tRNA folding and inhibit pairing with the L strand (Extended Data Fig. 5e). Indeed, ALKBH7-depleted cells displayed reduced levels of mt-dsRNA, in particular reduced L strand in mt-dsRNA (Fig. 3c). As expected, HeLa cells overexpressing ALKBH7 had a higher amount of L-strand dsRNA compared with the control (Extended Data Fig. 5f). Overall, our results suggest that m22G promotes local folding of the H strand, inhibits pairing with the L strand and increases nascent mt-RNA processing.
Given our observation that ALKBH7 suppresses processing of nascent mt-RNA, we next asked whether ALKBH7 regulates the steady-state levels of different mature mt-RNAs. Indeed, ALKBH7 knockdown in HepG2 and HEK293 cells increased the steady-state levels of all mt-mRNAs by 25–50% (Fig. 3d and Extended Data Fig. 6a). On the other hand, 11 out of 13 mt-mRNAs exhibited reduced steady-state levels in HeLa cells overexpressing ALKBH7 (Extended Data Fig. 6b). The mt-rRNA level exhibited a slight decrease in ALKBH7-depleted cells (Extended Data Fig. 6c). However, the steady-state levels of most mt-tRNAs were notably reduced upon knockdown of ALKBH7 (Fig. 3e). Therefore, the absence of ALKBH7 reduces the steady-state levels of most mt-tRNAs but elevates the steady-state levels of mt-mRNAs.
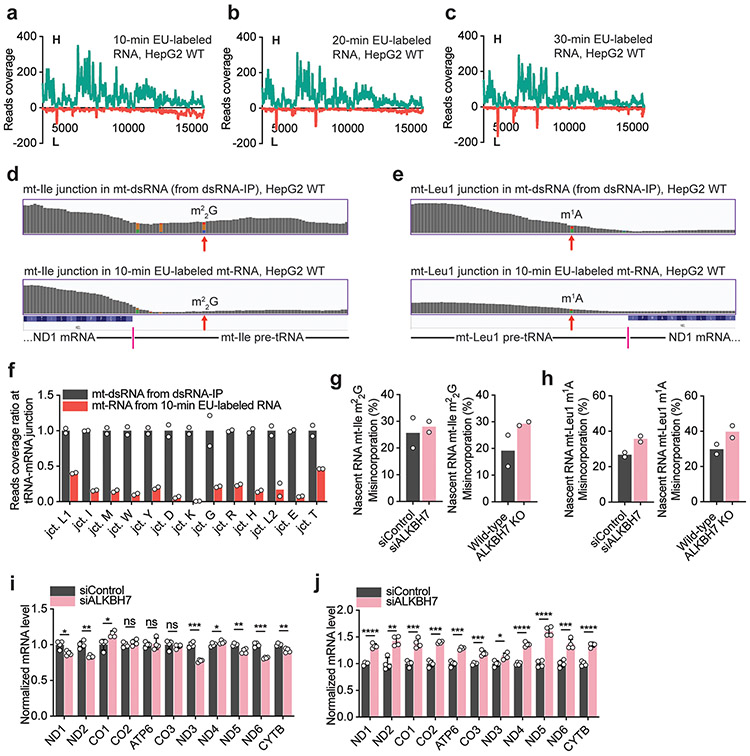
On the basis of the in vitro evidence that ALKBH7 depletion accelerates polycistronic mt-RNA processing (Fig. 3a), we next investigated the overall nascent mt-RNA processing inside cells in an unbiased manner. We fed siControl and siALKBH7 HepG2 cells with 5-ethynyl uridine (EU) for 1–5 min, captured nascently transcribed, EU-labelled RNA via click reaction and performed streptavidin pulldown. Using RT–qPCR, we examined the mt-mRNA regions at the very early stage of nascent mt-RNA and largely excluded potential effects by ALKBH7 depletion on mitochondrial transcription (Extended Data Fig. 6d). The mtDNA copy number was also not affected by ALKBH7 loss (Fig. 3f). However, when inspecting the tRNA/mRNA junction level in nascent mt-RNA from 1–5-min EU labelling, an observable faster processing started to appear at several junctions (Extended Data Fig. 6e), implying that ALKBH7 could impact early-stage mt-RNA processing inside cells.
We further monitored tRNA/mRNA junction levels in 10-min EU-labelled mt-RNA by RT–qPCR and found that the loss of ALKBH7 led to significantly elevated processing at most junctions (Fig. 3g), indicating that the ALKBH7-mediated demethylation markedly suppresses cleavage at these junctions and could impact the subsequent mt-tRNA and mt-mRNA processing before the 10-min time point.
With 10 min as the earliest time point we could collect enough materials for DAMM-seq, we constructed nascent RNA libraries from EU-labelled RNA isolated at 10, 20 and 30 min after EU addition. At the 10-min time point, we found that the L strand of EU-labelled mt-RNA was already mostly degraded (Fig. 3c and Extended Data Fig. 7a-c), suggesting that most mt-dsRNA exists in the early-stage nascent mt-RNA (within 10 min of transcription). By examining each tRNA/mRNA junction in nascent mt-RNA from 10-min EU labelling, the tRNA/mRNA read coverage ratio was found to be much lower than the ratio at the same junctions in mt-dsRNA from the dsRNA-IP results (Extended Data Fig. 7d-f). This observation indicates that most junction regions largely decayed within 10 min after their transcription. We then monitored the misincorporation at m1A and m22G sites in mt-Leu1 and mt-Ile regions (Extended Data Fig. 7d,e), respectively, at the 10-min time point, from the leftover read coverage, and observed slight increases after ALKBH7 depletion, although not as notable as those in mt-dsRNA (Extended Data Fig. 7g,h and Fig. 1j-m).
We next compared the read coverage specifically within mt-tRNA regions, which includes the overall level of both the tRNA region within unprocessed junction and the released immature pre-tRNA. ALKBH7 depletion led to decreased read coverage in most mt-tRNA regions (Fig. 3h) compared with the control, indicating an overall reduced tRNA level at the nascent mt-tRNA stage with ALKBH7 depletion. Similarly, for the overall level of nascent mt-mRNA, ALKBH7 knockdown also showed the same trend of reduced mRNA levels at the 10-min time point (Fig. 3i), suggesting that ALKBH7 depletion causes altered pre-RNA processing that reduces the overall nascent mt-tRNA and mt-mRNA levels. While consistent with the reduced overall mt-tRNA levels observed (Fig. 3e), these results cannot explain the higher steady-state mt-mRNA levels we observed (Fig. 3d).
We next probed nascent mt-mRNA level changes using EU labelling for 10–60 min. Interestingly, the mt-mRNAs from ALKBH7-depleted cells were more stable compared to mt-mRNA from control cells. At the 10-min time point after transcription (Fig. 3j), ALKBH7-depleted cells showed mt-mRNA levels consistently lower than those from control cells, but they caught up at 30 min after transcription, and became higher at 60 min after transcription (Extended Data Fig. 7i,j). Therefore, depletion of ALKBH7 caused altered processing of polycistronic mt-RNA, leading to reduced levels of nascent mt-tRNA and reduced levels of nascent mt-mRNA, which was also more stable. Future mechanistic studies are required to understand the underlying mechanisms.
To investigate the potential functional relevance of altered mt-RNA levels, we examined their effects on the metabolic potential and energy profile. Compared with the controls, ALKBH7-knockdown cells displayed a diminished baseline activity together with reduced mitochondrial respiration and glycolysis in response to carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) stress (Fig. 4a and Extended Data Fig. 8a,b). Furthermore, ALKBH7 depletion caused a notable change for the OXPHOS complex IV subunit mt-CO1 and complex I, when normalized to β-actin (Fig. 4b), further supporting the observed effect of ALKBH7 loss on mitochondrial activity. We excluded the possibility that the number of mitochondria was affected by ALKBH7 knockdown (Extended Data Fig. 8c). Using commercially available antibodies for other mt-proteins, we observed dramatically or notably decreased protein levels of mt-CO2, mt-ND3, mt-ND4, mt-ATP6 and mt-CYTB in ALKBH7-knockdown cells, whereas the levels of three other proteins did not display a notable reduction (Fig. 4c). A metabolic labelling assay34 confirmed that ALKBH7 depletion led to a decreased protein synthesis rate in mitochondria in general (Extended Data Fig. 8d), which could be caused by the overall reduction in the level of mt-tRNAs; the levels of a few mt-tRNAs were reduced by 30–40% (Fig. 3e).
Fig. 4 ∣. ALKBH7 loss leads to reduced protein translation and decreased mitochondrial activity.
a, A metabolic phenotype plot of oxygen consumption rate (OCR) versus extracellular acidification rate (ECAR). ALKBH7 knockdown decreases mitochondrial activity under both baseline and stress conditions. n = 6 biologically independent samples; data are presented as mean values ± s.d. b, Western blotting showed that ALKBH7 knockdown markedly decreases OXPHOS complex IV assembly by affecting mt-CO1 protein levels (normalized to β-actin). c, Western blotting showed that ALKBH7 depletion leads to a notable decrease in the level of most mt-proteins (normalized to β-tubulin). For b,c, two rounds of immunoblots for biologically independent samples were performed, with similar results obtained. d, A model scheme for the effects of ALKBH7 on mt-RNA processing.
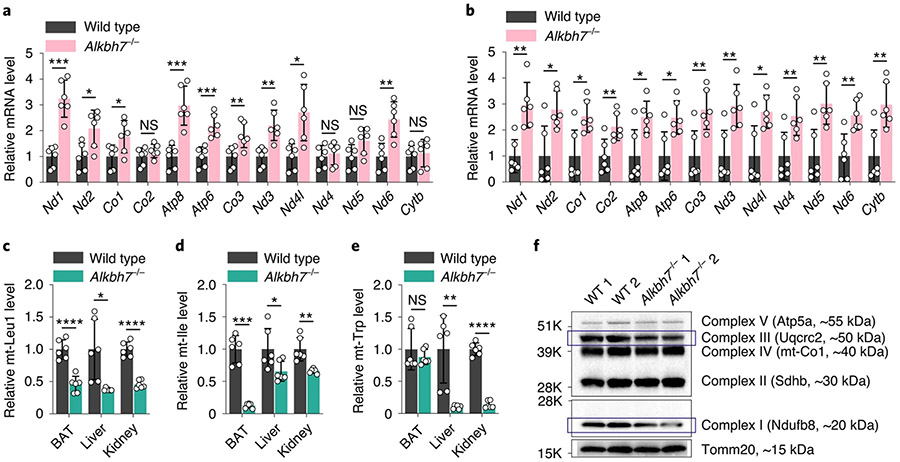
Our studies in cell lines indicate that depletion of ALKBH7 causes faster and probably abnormal processing of mitochondrial polycistronic RNA, which leads to overall reduced mt-tRNA and reduced protein translation in mitochondria (Fig. 4d). Unlike mt-mRNA, mt-tRNA can have quite a long lifetime. The 48-h siRNA knockdown of ALKBH7 in human cell lines may not fully recapitulate the effects of ALKBH7 on mt-tRNA changes. We next turned to investigate the Alkbh7-knockout mouse reported previously by us3, in which we can evaluate steady-state mt-tRNA level changes. With brown adipose tissue (BAT), liver and kidney as tissues with high ALKBH7 expression (Extended Data Fig. 1c), we indeed confirmed accumulation of most mt-mRNAs compared to controls (Fig. 5a,b and Extended Data Fig. 9a,b). As expected, Alkbh7 knockout led to an −55–65% reduction of mt-Leu1 tRNA, −35–90% reduction of mt-Ile tRNA and −85–90% reduction of mt-Trp tRNA in BAT, liver and kidney (Fig. 5c-e). Importantly, these are mt-tRNAs adjacent to the mt-Leu1 and mt-Ile junctions in the polycistronic mt-RNA stage (Fig. 1n). These reductions are much more severe than those observed in the cell lines, probably due to complete Alkbh7 knockout. The reductions in several other mt-tRNAs were also observed in Alkbh7-knockout tissues (Extended Data Fig. 9c-e). Therefore, these mouse tissues of high mitochondrial activity might have a requirement for ALKBH7 expression to maintain proper levels of mt-tRNAs. Of note, Alkbh7 knockout did not affect the number of mitochondria in these tissues (Extended Data Fig. 9f).
Fig. 5 ∣. Depletion of ALKBH7 in mouse tissues led to altered levels of mature mt-mRNAs and tRNAs.
a, Relative mRNA levels of 13 mt-mRNAs (normalized to mouse Actb) in BAT isolated from Alkbh7-knockout mice (Alkbh7−/−) versus wild-type mice. P = 0.0002, 0.0137, 0.0373, 0.2364, 0.0005, 0.0005, 0.0097, 0.0024, 0.0102, 0.6519, 0.0567, 0.0024 and 0.6445, respectively; unpaired, two-tailed t-test. b, Relative mRNA levels of 13 mt-mRNAs (normalized to mouse Actb) in liver isolated from Alkbh7-knockout mice (Alkbh7−/−) versus wild-type mice. P = 0.0016, 0.0116, 0.0123, 0.0085, 0.0141, 0.0108, 0.0059, 0.0045, 0.0143, 0.0099, 0.0037, 0.0047 and 0.0046, respectively; unpaired, two-tailed t-test. c, Relative tRNA levels of mt-Leu1 (normalized to mouse U6 snRNA) in BAT, liver and kidney isolated from Alkbh7-knockout mice (Alkbh7−/− versus wild-type mice. P = <0.0001, 0.0221 and <0.0001, respectively; unpaired, two-tailed t-test. d, Relative tRNA levels of mt-Ile (normalized to mouse U6 snRNA) in BAT, liver and kidney isolated from Alkbh7-knockout mice (Alkbh7−/− versus wild-type mice. P = 0.0001, 0.0485 and 0.0056, respectively; unpaired, two-tailed t-test. e, Relative tRNA levels of mt-Trp (normalized to mouse U6 snRNA) in BAT, liver and kidney isolated from Alkbh7-knockout mice (Alkbh7−/−) versus wild-type mice. P = 0.4192, 0.0086 and <0.0001, respectively; unpaired, two-tailed t-test. For a–e, n = 6 biologically independent samples; data are presented as mean values ± s.d. NS, P ≥ 0.05; *P < 0.05; **P <0.01; ***P < 0.001; and ****P < 0.0001. f, Western blotting showed that Alkbh7 knockout led to decreased levels of OXPHOS complex I and III in BAT (normalized to Tomm20). Two rounds of immunoblots for biologically independent samples were performed, with similar results obtained.
We previously reported that ALKBH7, directly or indirectly, facilitates the utilization of short-chain fatty acids3 and suggested more fatty acid storage in Alkbh7−/− mice as an underlying pathway to explain the obesity phenotype. We conducted fatty acid oxidation (FAO) assays35,36 in mouse brain, BAT, heart, liver and kidney, to measure octanoyl-CoA beta-oxidation37 in mitochondria. ALKBH7 depletion triggers a decreased FAO activity in mitochondria of BAT but not the other tissues (Extended Data Fig. 9g,h); we also observed a concomitant reduction of OXPHOS complex I and III in BAT (Fig. 5f). Growing evidence has shown a close link between mitochondrial FAO and OXPHOS pathways via either direct protein interactions or secondary effects through buildup of metabolic intermediates as these pathways share substrates38-40. Disorders of one of these pathways would inhibit or disturb the other. These results suggested that ALKBH7 loss causes impaired OXPHOS function and decreased beta-oxidation of fatty acids in fat tissue, which correlates to more fat storage in mice.
In summary, we identify ALKBH7 as an RNA demethylase that mediates unprecedented m22G and m1A demethylation of nascent mt-RNA, which regulates nascent polycistronic mt-RNA processing and mitochondrial function. Depletion of ALKBH7 led to reduced mt-tRNA and reduced mt-protein levels, leading to attenuated mitochondrial activity. In mouse fat tissues, loss of ALKBH7 attenuates OXPHOS function and reduces FAO activity, causing fat storage and the obesity phenotype. Future studies will be of interest to determine the dynamics of ALKBH7 in different contexts and the impacts of this regulation on cellular metabolism in diverse biological processes.
Methods
Cell culture.
HepG2, HeLa and HEK 293T cell lines were purchased from the American Type Culture Collection (ATCC). The HeLa cell line was grown in DMEM medium (Gibco, 11965) supplemented with 10% v/v FBS and 1% penicillin/streptomycin (Gibco). The HepG2 and HEK 293T cell lines were maintained in DMEM (Gibco, 11995) with 10% FBS and 1% penicillin/streptomycin. Cells were cultured at 37 °C with 5.0% CO2 in a Heracell VIOS 160i incubator (Thermo Scientific).
Antibodies.
The following antibodies were used in this study: mouse monoclonal anti-dsRNA J2 (refs. 28,30; 10010500, Scions, IP 1:200), rabbit polyclonal anti-TRMT1 (A304-205A, Bethyl, western blot (WB) 1:1,000), rabbit polyclonal anti-ALKBH7 (ref. 20; 15470-1-AP, Proteintech, WB 1:1,000), rabbit monoclonal anti-β-actin (13E5; 4970S, Cell Signaling, WB 1:1,000), rabbit polyclonal anti-β-tubulin (2146S, Cell Signaling, WB 1:1,000), mouse monoclonal GAPDH antibody (0411) HRP (sc-47724 HRP, Santa Cruz, WB 1:1,000), anti-rabbit IgG, HRP-linked antibody (7074S, Cell Signaling, WB 1:2,000), anti-mouse IgG, HRP-linked antibody (7076S, Cell Signaling, WB 1:2,000), HRP–streptavidin antibody (RABHRP3, Sigma-Aldrich, WB 1:2,000), total OXPHOS rodent WB antibody cocktail (ab110413, Abcam, WB 1:1,000), mouse monoclonal anti-MT-CO2 (12C4F12; ab110258, Abcam, WB 1:1,000), rabbit monoclonal anti-MT-ND1 (ab181848, Abcam, WB 1:1,000), rabbit polyclonal anti-MT-ND3 (ab192306, Abcam, WB 1:1,000), mouse monoclonal anti-MT-ND2, clone 9E12-1B3 (MABS2047M, Sigma-Aldrich, WB 1:500), mouse monoclonal anti-MT-ND4, clone 9E4-2D8 (MABS1994M, Sigma-Aldrich, WB 1:1,000), mouse monoclonal anti-MT-ATP6, clone 1G7-1G2 (MABS1995M, Sigma-Aldrich, WB 1:1,000), mouse monoclonal anti-MT-CYTB, clone 5B3-6E3 (MABS2036M, Sigma-Aldrich, WB 1:1,000), rabbit polyclonal anti-MT-ATP8 (26723-1-AP, Proteintech, WB 1:1,000), rabbit monoclonal anti-SDHA, clone D6J9M (11998S, Cell Signaling, WB 1:1,000), rabbit monoclonal anti-HSP60, clone D6F1 (12165S, Cell Signaling, WB 1:1,000), rabbit monoclonal anti-VDAC, clone D73D12 (4661S, Cell Signaling, WB 1:1,000), rabbit monoclonal anti-Tomm20, clone D8T4N (42406S, Cell Signaling, WB 1:1,000) and rabbit polyclonal anti-VDAC1/porin (ab15895, Abcam, WB 1:1,000).
siRNA knockdown and plasmid transfection.
AllStars negative control siRNA (1027281) and human ALKBH7 siRNA (SI00730793) were purchased from Qiagen. ALKBH7-knockdown HepG2 and HEK 293T cells were transfected with Lipofectamine RNAiMAX (Invitrogen) for 48 h. Human ALKBH7 overexpression plasmid was purchased from VectorBuilder (Vector ID: VB180629-1142wpx). ALKBH7-overexpressing HeLa cells were transfected with Lipofectamine LTX Plus (Invitrogen) for 24 h.
shRNA knockdown.
The TRMT1 (TRCN0000038765) and control vectors were purchased from Horizon Discovery. The plasmids pMD2.G and psPAX2 were purchased from Addgene. Plasmids were amplified with the HiSpeed Plasmid Maxi Kit (Qiagen). At 80% confluency, the medium was replaced with Opti-MEM 4 h before transfection. For HEK 293T cells in a 10-cm plate, lentiviral vector and the packaging vectors were prepared in an equimolar ratio with 10 μg in total mixed in 300 μl of Opti-MEM. A 30-μl volume of PEI-MAX (Polysciences, 1 mg ml−1 in water, pH 7.0) was suspended in 300 μl of Opti-MEM. Both were incubated for 5–10 min at room temperature and then mixed for another 5–10 min incubation. Transfection vectors were added to the cells for 4–6 h, after which the medium was changed to regular culture medium. After 2 days, the supernatant was collected and centrifuged at 2,000 r.p.m. for 3 min to remove cell debris. PEG-it Virus Precipitation Solution (System Biosciences) was added to the supernatant. After an overnight incubation at 4 °C, the solution was centrifuged at 1,500g for 30 min and the supernatant was discharged without disturbing the viral pellet. The pellet could be stored at −80 °C until use. The pellet was resuspended in culture medium with the TransDux MAX Lentivirus Transduction Enhancer (System Biosciences) according to the manufacturer’s protocol and added to HeLa cells in a 6-well plate at 70% confluency. The cells were selected by 1 μg ml−1 puromycin.
Construction of ALKBH7-knockout cell line.
Sense and antisense oligonucleotides for a guide RNA were computationally designed for human ALKBH7 (http://crispr.mit.edu) and cloned into the vector pX330-mcherry (Addgene, 98750). HepG2 cells were transfected using Lipofectamine 2000. After 24 h, HepG2 cells expressing red fluorescent protein were selected by fluorescence-activated cell sorting (Aria II, BD Bioscience) and plated at a very low density. After 5–8 days of cell culture, ALKBH7 knockout was sequenced with the following primers: ALKBH7_Identify-F: GTGCGAGGCTCGGGCCCTTCCGTGC; ALKBH7_Identify-R: CCCCGCCCCAGCGTGGCCCCGCCC. One strain with successful ALKBH7 knockout was used in the current study. The sequences of wild-type ALKBH7 and its knockout are AGCTGCGCCGCCGCCG----(38 base pairs)---- GCCGGGGGCGAGGGAC and AGCTGCGCCGCCGCCGGCCGGGGGCGAGGGAC, respectively.
Small RNA isolation.
Cellular total RNA was isolated with TRIzol reagent (Invitrogen) following the manufacturer’s protocol by isopropanol precipitation. The small RNA fraction (size < 200 nt) was further extracted from the purified total RNA using the mirVana miRNA Isolation Kit (AM1560, Invitrogen).
Cellular dsRNA isolation.
ALKBH7-overexpressing HeLa cells, and ALKBH7-knockdown and ALKBH7-knockout HepG2 cells were prepared as described previously (two replicates each, five 15-cm plates per replicate, ~70% confluency before cell lysis). For each replicate, 250 μl of Protein G Dynabeads (Invitrogen, 10004D) was washed twice and resuspended in 2.5 ml of IP wash buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM MgCl2 and 0.5% NP-40). A 25-μg quantity of monoclonal anti-dsRNA (J2) antibody28,30 was added to the beads and incubated for 1 h at room temperature, followed by another 1 h at 4 °C. Cultured cells were washed once with cold Dulbecco’s PBS (DPBS). The cells in each plate were soaked in 5 ml cold DPBS and collected by cell lifters, combining 5 plates for each replicate. The cell suspension was spun at 300g at 4 °C for 2 min. Cells were resuspended in 1 ml NP-40 lysis buffer (10 ml solution: 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM EDTA, 10 mM MgCl2 and 0.5% NP-40, plus 50 μl TURBO DNase (2 U μl−1, AM2238, Invitrogen), 500 μl SUPERase•In RNase Inhibitor (20 U μl−1, AM2694, Invitrogen) and a Pierce EDTA-free protease inhibitor tablet) and incubated at 4 °C for 30 min. The lysate was spun at 16,000g at 4 °C for 5 min. The lysate was diluted to 4.75 ml with IP wash buffer. A 250-μl volume of J2–Dynabeads was added to the lysate and incubated at 4 °C for 2 h. The magnetic beads were washed once with 1 ml IP wash buffer and transferred to a new Eppendorf tube. The beads were further washed 4 times by 1 ml of IP wash buffer. J2-bound dsRNA was extracted using TRIzol, with small RNA (<200 nt) removal by the RNA Clean & Concentrator (Zymo Research) protocol.
DAMM-seq for methylated site detection in cellular small RNA.
A 300- to 500-ng quantity of human cellular small RNA (size < 200 nt) was fragmented into 40- to 50-nt lengths using RNA Fragmentation Reagent (AM8740, Invitrogen) following the manufacturer’s protocol and purified by Oligo Clean & Concentrator (Zymo Research). About 50 ng was saved as input and the remainder was subjected to an optimized demethylation treatment (30 mM MES buffer pH 5.5, 100 μM (NH4)2Fe(SO4)2·6H2O 300 μM α-ketoglutarate, 2 mM l-ascorbic acid, 150 mM NaCl, 2 mM MgCl2 and 40 μg ml−1 BSA) with 2.5 μl SUPERase•In RNase Inhibitor and 1.0 μl of engineered AlkB (D135S (ref. 25), 10 mg ml−1) added to the 50-μl reaction mixture, followed by incubation at 25 °C for 2 h and then purification using Oligo Clean & Concentrator. 3′-end repair was conducted for the input and demethylase-treated RNA using T4 Polynucleotide Kinase (EK0032, Thermo Fisher Scientific). RNA was mixed with 5 μl of 10× T4 Polynucleotide Kinase Reaction Buffer (B0201S, NEB) and 5 μl of T4 PNK, diluted to a final volume of 50 μl, and incubated at 37 °C for 1 h followed by Oligo Clean & Concentrator purification. To perform 3′-adapter ligation, repaired RNA fragments were incubated with 1.0 μl of 30 μM RNA 3′-linker (5′rApp-NNNNNAGATCGGAAGAGCGTCGTG-3SpC3) at 70 °C for 2 min and immediately placed on ice. A 2.5-μl volume of 10× T4 RNA Ligase Reaction Buffer (NEB), 7.5 μl of 50% PEG8000, 1 μl of SUPERase•In RNase Inhibitor and 2 μl of T4 RNA ligase 2 truncated KQ (NEB) were added to the RNA–adapter mixture. The reaction was diluted to a final volume of 25 μl and incubated at 25 °C for 2 h followed by 16 °C for 10 h. The reaction was further diluted to 47 μl with nuclease-free water, and the excessive adapters were removed with 2 μl of 5′-deadenylase (NEB) at 30 °C for 1 h followed by adding 1.0 μl of RecJf (NEB) for ssDNA digestion at 37 °C for 1 h. The 3′-end-ligated RNA was extracted by RNA Clean & Concentrator (Zymo Research).
The purified RNA was incubated with 1.0 μl of 2.0 μM RT primer (5′-ACACGACGCTCTTCCGATCT-3′) at 65 °C for 2 min and immediately moved to ice. A 2-μl volume of 10× AMV Reverse Transcriptase Reaction Buffer (NEB), 2 μl of 10 mM dNTP Solution Mix (NEB), 1 μl of RNaseOUT Recombinant Ribonuclease Inhibitor (Thermo Fisher Scientific) and 2 μl of HIV Recombinant Reverse Transcriptase (Worthington) were added to the RNA–primer mixture. The reaction was diluted to 20 μl and incubated at 37 °C for 90 min. A 1.0-μl volume of RNase H (NEB, M0297L) and 2.5 μl 10× RNase H Reaction Buffer were then added and the reaction was incubated at 37 °C for 20 min. The cDNAs were purified and eluted in 7 μl by Oligo Clean & Concentrator (Zymo Research). A mixture of cDNA and 1.0 μl of 50 μM cDNA 3′-linker (5′Phos-NNNNNAGATCGGAAGAGCACACGTCTG-3SpC3) was denatured at 75 °C for 2 min and then left on ice. A 3-μl volume of 10× T4 RNA Ligase Reaction Buffer (NEB), 15 μl of 50% PEG8000, 3 μl of 10 mM ATP and 1.0 μl of T4 RNA ligase 1 (high concentration, NEB) were added to the cDNA–adapter mixture, and the reaction was incubated at 25 °C for 12 h. The libraries were amplified using universal and indexed primers from NEBNext Multiplex Oligos for Illumina (NEB). All libraries were sequenced on an Illumina NovaSeq 6000 with a single-end read length of 100 bp (Supplementary Table 1). Unlike DM-TGIRT-seq25, DAMM-seq achieved a high demethylation efficiency on all of the m1A, m3C, m1G and m22G bases simultaneously. Furthermore, a more robust RT with HIV RT enzyme gives excellent read-through at these methylation sites for methylation fraction information.
DAMM-seq for methylation level quantification.
Around 10–50 ng of cellular small RNA (<200 nt) or cellular dsRNA or EU-labelled RNA was fragmented into 40- to 50-nt lengths using RNA Fragmentation Reagent (AM8740, Invitrogen) and purified with Oligo Clean & Concentrator (Zymo Research). The RNAs can be from diverse cellular treatments such as siALKBH7 versus siControl, control overexpression versus ALKBH7 overexpression, wild type versus ALKBH7 knockout or shTRMT1 versus shControl. The library construction protocol is the same as for DAMM-seq for methylated site detection in cellular small RNA, but without the demethylation step. All libraries were sequenced on an Illumina NovaSeq 6000 with a single-end read length of 100 bp or a paired-end read length of 50 bp (Supplementary Table 1).
Quantification of steady-state mt-mRNAs and mtDNA.
Cellular total RNA was extracted and treated with DNase I (RNase-free, M0303S, NEB) to remove cellular DNA. For RT–qPCR of mt-mRNAs, 200–500 ng of RNAs was transcribed into cDNAs with PrimeScript RT Reagent Kit (Perfect Real Time, Takara, RR037A) and subjected to qPCR analysis with FastStart SYBR Master Mix (Roche) in a LightCycler 96 machine (Roche). Human ACTB and 18S rRNA were used as internal controls. The quantification of HepG2 cell mtDNA was conducted according to a published protocol41. All primer sequences for RT–qPCR can be found in Supplementary Table 2.
Quantification of steady-state mt-tRNAs.
Around 2–4 μg of cellular total RNA (HepG2 cells of siALKBH7 versus siControl, or tissues from wild-type mice versus Alkbh7−/− mice) was extracted and treated with DNase I (RNase-free, M0303S, NEB) to remove cellular DNA. Then the RNA was incubated in 50 mM Tris-HCl pH 9.0 at 37 °C for 1 h. About 800 ng of RNA was denatured at 80 °C for 2 min and immediately moved to ice, followed by the optimized demethylation treatment (described in the section entitled DAMM-seq for methylated site detection in cellular small RNA) at 25 °C for 2 h. About 300 ng of demethylated RNA in 5 μl RNase-free water was heated at 80 °C for 2 min and immediately moved onto ice. The denatured RNA was then mixed with 2 μl 5× PolyA Polymerase Buffer (QuantiMir Kit, RA420A-1, SBI), 1 μl 25 mM MnCl2, 1.5 μl 5 mM ATP and 0.5 μl PolyA Polymerase, followed by an incubation at 37 °C for 30 min and purification using RNA Clean & Concentrator. About 10 μl of eluted RNA was denatured again at 80 °C for 2 min and immediately moved onto ice, followed by adding 4 μl 5× SSIV buffer, 1 μl 100 mM dithiothreitol (DTT), 1 μl RNaseOUT Recombinant Ribonuclease Inhibitor (Thermo Fisher Scientific), 2 μl of 10 mM dNTP Solution Mix (NEB), 1 μl SuperScript IV Reverse Transcriptase (Invitrogen) and 0.5 μl denatured Oligo-dT Adaptor (QuantiMir Kit, RA420A-1, SBI). The RT mixture was incubated at 55 °C for 30 min, followed by qPCR analysis with FastStart SYBR Master Mix (Roche) in a LightCycler 96 machine (Roche). Human and mouse U6 snRNA were used as internal controls (QuantiMir Kit, RA420A-1, SBI). All primer sequences for RT–qPCR can be found in Supplementary Table 2.
Cloning, expression and purification of recombinant proteins.
The cDNA encoding human ALKBH7 (Uniprot ID Q9BT30) was subcloned into a modified PET28a vector with an amino-terminal truncation of 16 amino acids. The truncated ALKBH7 was expressed in BL21 (DE3) E. coli. The transfected bacteria were induced with 2 mM isopropyl 1β-d-1-thiogalactopyranoside for 16 h at 16 °C overnight. The culture was pelleted and resuspended in 1× lysis buffer (20 mM Tris-HCl pH 7.5, 500 mM NaCl, 10% glycerol, 5 mM imidazole, 2 mM DTT and 1 mM phenylmethylsulfonyl fluoride). The recombinant ALKBH7 protein was purified using affinity chromatography with a Ni-NTA super-flow column (QIAGEN) followed by gel-filtration (Superdex 75, Pharmacia) chromatography. Similarly, the cDNA encoding human TRMT1 (Uniprot ID Q9NXH9) was subcloned into a PET22b vector with an N-terminal truncation of 18 amino acids, and then expressed and purified.
In vitro biochemistry assay.
To install an m22G at position 26 of the mt-Ile tRNA probe, a 100-μl reaction mixture containing 50 mM Tris-HCl (pH 8.0), 50 mM KCl, 5 mM MgCl2, 100 μg ml−1 BSA, 2 mM DTT, 100 μM S-adenosyl methionine, 5 μM recombinant TRMT1 protein and 5 μM 69-base mt-Ile probe (AGAAATATGTCTGATAAAAGAGTTACTTTGATAGAGTAAATAATAGGAGCTTAAACCCCCTTATTTCTA, well folded) was incubated at 37 °C for 2 h. The tRNA probe was purified with phenol/chloroform followed by ethanol precipitation.
The ALKBH7 demethylation activity assay was performed in a volume of 100 μl containing 50 mM of Bis-Tris buffer (pH 5.5), 100 mM KCl, 2 mM MgCl2, 0.2 U μl−1 RNase inhibitor, 2 mM l-ascorbic acid, 300 μM α-ketoglutarate, 100 μM (NH4)2Fe(SO4)2·6H2O 5 μM or 10 μM recombinant ALKBH7 protein, and ~500 ng of m22G-methylated mt-Ile probe (AGAAATATGTCTGATAAAAGAm22GTTACTTTGATAGAGTAAATAATA GGAGCTTAAACCCCCTTATTTCTA, ssRNA or well-folded tRNA or dsRNA) or synthesized 35-base mt-Leu1 probe with m1A (CUUUACAGUCAGAGGUUCAm1AUUCCUCUUCUUAACA, ssRNA or dsRNA) as substrates. The reaction was incubated at 37 °C for 2 h. The resulting RNA probes were purified with phenol/chloroform, precipitated in ethanol and then dissolved in a desired amount of RNase-free water for downstream analysis.
Quantitative analysis of modification levels using ultraperformance LC–MS/MS.
A 200- to 300-ng quantity of RNA was treated with Benzonase nuclease, phosphodiesterase I and alkaline phosphatase in a 100-μl reaction volume according to standard procedures. After complete hydrolysis, 10 μl of the solution was injected for ultraperformance LC–MS/MS. The nucleosides were separated on a C18 column (Agilent Zorbax Eclipse Plus C18, 2.1 × 50 mm, 1.8 μm) and detected by a triple-quadruple mass spectrometer (Agilent 6495 QQQ or Sciex 6500 QTRAP) in positive ion multiple reaction monitoring mode. The nucleosides were quantified using the nucleoside-to-base ion mass transitions of 268.1 to 136.2 (A), 284.1 to 152.2 (G), 244.1 to 112.1 (C), 245.0 to 113.1 (U), 312.1 to 180.2 (m22G), 298.1 to 166.1 (m2G), 298.1 to 166.1 (m1G) and 282.1 to 150.2 (m1A). RNA modification levels were quantified using standard curves for pure nucleosides for each methylated base.
Identification of DAMM-seq-induced misincorporation.
The sequencing data were all trimmed with the cutadapt tool to remove adapters and low-quality reads (length shorter than 18 bp). PCR duplicates were removed with the BBMap tool (https://sourceforge.net/projects/bbmap/), random barcodes at reads end were trimmed, and low-quality reads were removed using the cutadapt tool. The remaining reads were aligned to the human genome (hg19) using Tophat2 (version 2.1.1) and bowtie2 (version 2.3.5.1) allowing a maximum of three mismatches. The generated .bam files were split into positive and negative strands and sorted using Samtools. Sequence variants were identified by measuring the base composition at each position using fine-tuned bam-readcount (https://github.com/genome/bam-readcount). The generated bam-readcount output results were parsed and analysed to calculate the misincorporation ratio at each methylated site (m1A, m1G, m22G and m3C) in mt-tRNA or mt-dsRNA, followed by confirmation using direct visualization through IGV software (https://software.broadinstitute.org/software/igv/).
Optimized icSHAPE for ultralow RNA input.
ALKBH7-knockdown HepG2 cells were prepared as described previously (two replicates for input (fed with DMSO), two replicates for pulldown (fed with NAI-N3), five 15-cm plates per replicate, ~70% confluency before cell lysis). On completion of knockdown, cultured cells were washed once with warm DPBS. The cells in each plate were soaked in 5 ml warm DPBS and collected by cell lifters, combining 5 plates for each replicate. The cell suspension was spun at 300g at room temperature for 2 min. For the cell pellet corresponding to each replicate, 500 μl control solution (450 μl DPBS + 50 μl DMSO42) was added to the input samples, and 500 μl NAI-N3 solution (450 μl DPBS + 50 μl 1.0 M NAI-N3 in DMSO42) was added to the pulldown samples. Then the resuspended solution was incubated at 37 °C for 5 min, followed by centrifugation at 300g for 2 min. The supernatant was carefully removed, and the cell pellet was washed with cold DPBS once, followed by centrifugation at 300g for another 2 min.
For all of the cell pellets collected above, J2-bound dsRNA was extracted with TRIzol reagent according to the standard dsRNA isolation procedures. The dsRNA was dissolved in 17 μl RNase-free water (for both DMSO-treated and NAI-N3 treated samples). A 2-μl volume of 2 mM sDIBO–biotin and 1 μl SUPERase•In RNase Inhibitor was added and incubated at 37 °C for 1 h. RNA was purified with Oligo Clean & Concentrator (Zymo Research). Purified RNA was diluted with 16 μl RNase-free water and 2 μl of RNA Fragmentation Buffer (NEBNext Magnesium RNA Fragmentation Module) was added, followed by heating at 95 °C for 3 min. The fragmented RNA was put on ice immediately and 2 μl of Stop Buffer was added. RNA purification was performed for the DMSO-treated samples using Oligo Clean & Concentrator (Zymo Research), to afford input samples. For the NAI-N3-treated samples, the RNA solution was mixed with 20 μl of freshly prepared C1 bead suspension (10 μl Dynabeads MyOne Streptavidin C1 beads washed twice with 200 μl 1× B&W buffer and stored in 20 μl of 2× B&W buffer based on the manufacturer’s protocol). After the 30-min incubation at 4 °C, the beads were washed 4 times with icSHAPE wash buffer (50 mM HEPES pH 7.5, 150 mM NaCl and 0.05% NP-40), followed by proteinase K digestion at 55 °C for 30 min. The collected RNA (pulldown sample; from the NAI-N3-treated samples) was purified by Oligo Clean & Concentrator (Zymo Research) and subjected to library construction together with the input samples using SMARTer Stranded Total RNA-Seq Kit v2 (Takara). All libraries were sequenced on an Illumina NovaSeq 6000 sequencer with a single-end read length of 100 bp (Supplementary Table 1).
RT–qPCR validation of flexible region level.
To 18 μl of the fragmented RNA (with 2 μl Stop Buffer added) from the NAI-N3-treated case (siALKBH7 versus siControl) above, 2 μl of 5 nM biotin-tagged ssDNA spike-in (Supplementary Table 2) was added and 2 μl was kept as input. Then the rest of the RNA solution was mixed with 20 μl of freshly prepared C1 bead suspension and the standard procedures described in the optimized icSHAPE protocol above were conducted. The collected RNA (serving as the pulldown sample, from the NAI-N3-treated samples) and the input RNA were incubated with 1 μl 2 μM RT primer at 65 °C for 2 min and moved onto ice immediately. Then 4 μl 5× First Stranded Buffer (Thermo Fisher Scientific), 2 μl 10 mM dNTP Solution Mix (NEB), 1 μl 0.1 M DTT, 1 μl RNaseOUT Recombinant Ribonuclease Inhibitor (Thermo Fisher Scientific) and 1 μl Superscript III Reverse Transcriptase (Thermo Fisher Scientific) were added to the RNA-primer mixture, followed by incubation of the reaction mixture (diluted into a 20-μl volume) at 42 °C for 5 min, 50 °C for 30 min, and 70 °C for 5 min. Then cDNAs were subjected to qPCR pipelines with FastStart SYBR Master Mix (Roche) in a LightCycler 96 machine (Roche) using appropriate primers for the flexible region and ssDNA spike-in (Supplementary Table 2).
In vitro mt-dsRNA processing assay.
Mitochondria were isolated from four 15-cm plates of HepG2 cells using the Thermo Scientific Mitochondria Isolation Kit for Cultured Cells (Thermo Fisher Scientific, 89874). A 200-μl volume of 1× Mitochondria Lysis Buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 2 mM MgCl2, 10% v/v glycerol, 100 mg digitonin and Pierce EDTA-free protease inhibitor tablet) with 4 μl of DNase I (RNase-free, M0303S, NEB) was added to the pellet. After incubation at 4 °C for 30 min, the solution was spun at 16,000g at 4 °C for 15 min. The supernatant was collected as the mitochondrial lysate.
Fresh dsRNA was isolated from HepG2 cells, with siALKBH7 versus siControl (two replicates for each, ten 15-cm plates per replicate, ~70% confluency before cell lysis). Following its extraction with TRIzol reagent, the dsRNA was dissolved in 20 μl RNase-free water. A 5-μl volume of mitochondrial lysate was diluted with 43 μl of 1× Mitochondria Lysis Buffer and incubated with 2 μl of TURBO DNase (Invitrogen, AM2239) at 37 °C for 15 min. The 50-μl mixture was transferred to ice and further diluted with 200 μl of cold 1× Mitochondria Lysis Buffer. A 4-μl volume of the diluted mitochondrial lysate was combined with 84 μl of cold 1× Mitochondria Lysis Buffer and 8 μl of SUPERase•In RNase Inhibitor, to produce 1× Reaction Stock. To 10 μl of dsRNA from siALKBH7 (or siControl) or 10 μl of RNase-free water, 20 μl of 1× Reaction Stock was added rapidly and mixed well, followed by incubation at 37 °C for 30 min. Then TRIzol reagent and 2 μl of 5 nM ssDNA spike-in were added. These three samples of 30-min treatment were purified by Oligo Clean & Concentrator (Zymo Research). For the input samples of the 0-min time point, 10 μl dsRNA from siALKBH7 or 10 μl dsRNA from siControl was added to TRIzol reagent with 2 μl 5 nM ssDNA spike-in added. All purified RNA samples were denatured at 80 °C for 2 min before conducting RT with HIV Recombinant Reverse Transcriptase (Worthington). Then cDNAs were subjected to qPCR pipelines with FastStart SYBR Master Mix (Roche) in a LightCycler 96 machine (Roche) using appropriate primers for the junction regions and ssDNA spike-in (Supplementary Table 2).
Observation of nascent mt-RNA junction levels inside cells.
HepG2 cells in 10-cm plates were prepared with cellular treatments such as siALKBH7 versus siControl. A 5-ml volume of culture medium with 0.5 mM EU was added to each plate and incubated at 37 °C for 10 min (ref. 43; and also other time points such as 1–5 min or 10–60 min). Cells were washed once with cold DPBS and total RNA was immediately extracted with TRIzol and isopropanol precipitation, and then a biotin-tagged ssDNA spike-in was added. Cellular nascent RNA was enriched by biotin pulldown based on the standard protocol of the Click-iT Nascent RNA Capture Kit (Invitrogen, C10365). All RNA samples were denatured at 80 °C for 2 min before conducting RT with HIV Recombinant Reverse Transcriptase (Worthington). The cDNAs were subjected to qPCR pipelines with FastStart SYBR Master Mix (Roche) in a LightCycler 96 machine (Roche) using appropriate primers for junction regions and ssDNA spike-in (Supplementary Table 2).
Mitochondrial protein synthesis assay.
HepG2 cells in 10-cm plates were subjected to siRNA knockdown (siALKBH7 versus control) for 48 h. Then the cells were washed twice with warm DPBS and 10 ml warm DMEM medium (Gibco, 21013, no methionine) supplemented with 10% v/v FBS and 1% GlutaMAX Supplement (Gibco) was added. The cells were incubated at 37 °C for 1.5 h. Then 200 μl 1.0 M AHA and 100 μl 100 mM emetine dihydrochloride (protein synthesis inhibitor, ab141478, Abcam) were added to each plate. Cell pellets were collected after 3-h incubation34 at 37 °C. The cell lysate concentration was measured by the Pierce BCA Protein Assay Kit (Thermo Scientific). The balanced cell lysate (from siALKBH7 versus control) was treated with 50 μM DBCO–PEG4–biotin at 37 °C for 30 min, followed by western blotting with HRP–streptavidin antibody.
Fatty acid oxidation assay.
We prepared brain, liver, kidney, BAT and heart tissues from Alkbh7−/− versus wild-type mice, with 8 mice per group. The BCA protein assay was used to determine the lysate protein concentration, as the balanced concentration within 1–2 mg ml−1. The enzyme assay was then conducted strictly according to the protocol in the FAO Assay Kit (E-141)35,36, using the difference in the optical density to calculate the enzyme activity.
Cellular bioenergetics analysis using XFe96 extracellular flux analyser.
ALKBH7-knockdown effects on mitochondrial energy activity41 were measured using a Seahorse Bioscience XF96 analyser (Seahorse Bioscience Inc.) and the Seahorse XFp Cell Energy Phenotype Test Kit (Agilent) according to the manufacturer’s instructions. Both siControl and siALKBH7 HepG2 cells were seeded at 2 × 104 cells per well with 7 replicates in Seahorse XF96 cell culture microplates (Agilent). Cells were grown overnight and incubated with XFp medium for around 1 h in a non-CO2 incubator before initiating the cell energy phenotype assay. The 96-well sensor cartridge was hydrated in 200 μl XF calibrant solution (Seahorse Bioscience Inc.) overnight at 37 °C before plate calibration. For the cell energy phenotype assay, cells were treated with 1 μM oligomycin followed by three serial injections of FCCP at final concentrations of 0.5, 1.0 and 2.0 μM. Seahorse Wave software (v2.6) was used for data processing, including detection of outliers and cell number normalization. The final reports for the oxygen consumption rate and extracellular acidification rate signals were generated using the Seahorse XF Cell Energy Phenotype report generator.
Animal culture.
Mice were housed in a virus-free facility at 21 ± 1 °C with a controlled 12-h light cycle (individually ventilated caging system (GM500)). The animals had access to standard chow and water ad libitum. The relative humidity was controlled at 55% ± 10%. The mice (Mus musculus) were fed a standard diet with 5.5% fat content (Rat and Mouse No. 3 breeding diet, Special Diet Services). All mice in this study were C57BL6/N background strain homozygous Alkbh7−/− mice or wild-type mice. All data were obtained from 7-week-old male and female mice.
All mouse experiments were approved by the Norwegian Animal Research Authority (Norwegian Food Safety Authority) and performed in accordance with institutional guidelines at the Centre for Comparative Medicine at Oslo University Hospital. Animal work was conducted in accordance with the rules and regulations of the Federation of European Laboratory Animal Science Associations.
Statistics and reproducibility.
For DAMM-seq libraries, icSHAPE libraries, validation of the icSHAPE flexible region level and the in vitro mt-dsRNA processing assay, two biological replicates were used in each experiment, with each replicate combining between five and ten 15-cm plates of independently cultured cells; for immunoblots, two rounds of experiments for biologically independent samples were performed, with similar results obtained; for all other experiments in this study, at least three biologically independent replicates were used (data are presented as the mean ± s.d., with two-tailed Student’s t-tests on the statistical significance of differences between groups). All statistical analysis and data graphing were performed in Prism (version 9.1.1) software.
No statistical methods were applied to pre-evaluate sample size. For animal experiments, sample size was determined on the basis of previous experience in literature reports3. For cultured cell data, we collected data from two or three biological replicates. The sample size is sufficient because each replicate combines a large number of independently cultured cell plates. No data were excluded from analysis. Samples in this study were not randomized. We did not set up a control for covariates in the animal experiments because all groups were age and sex matched. Blinding was not used for this study because the cell culture, sample preparation, reagents and experimental settings were kept consistent for each experiment. Furthermore, the key experiments in this paper were conducted by several laboratory members independently and gave similar results.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Extended Data
Extended Data Fig. 1 ∣. Human ALKBH7 effect on mt-RNA methylations by mass spec and the development of DAMM-seq to detect m1A, m3C, m1G and m22G in one sequencing run via misincorporation signature.
a, An overlay of crystal structure of E. coli AlkB and human ALKBH7 on three peripheral loops (two are identical as EcAlkB, one is the ALKBH7 specific loop). b, The unique active site of ALKBH7 resembles an engineered AlkB protein (D135S/L118V) that catalyzes demethylation of m22G. c, Alkbh7 mRNA levels (normalized to mouse Actb) in several mouse tissues. n =3 biologically independent samples; data are presented as mean values ± SD. d, ALKBH7 mRNA levels (normalized to ACTB) in several human cancer cell lines. e, ALKBH7 knockdown efficiency in HepG2 cells by protein level (compared to β-tubulin). f, ALKBH7 knockdown efficiency in HepG2 cells by mRNA level (normalized to β-actin). For d and f, n =2 biologically independent samples. g, Modification levels (m22G/G, m1G/G, m6A/A, m1A/A, m7G/G, m2G/G) by LC-MS/MS for mitochondrial tRNA in ALKBH7-depleted HepG2 cells vs. control. Unpaired, two-tailed t-test; n = 4 biologically independent samples; data are presented as mean values ± SD. h, Modification levels by LC-MS/MS for mitochondrial mRNA in ALKBH7-depleted HepG2 cells vs. control. i, Modification levels by LC-MS/MS for mitochondrial rRNA in ALKBH7-depleted HepG2 cells vs. control. For h-i, unpaired, two-tailed t-test; n = 3 biologically independent samples; data are presented as mean values ± SD. j, A flowchart of DAMM-seq library construction pipeline, detecting four types of methylated bases (m22G, m1G, m1A, m3C) by misincorporation signatures in a “one-pot” manner with or without engineered AlkB treatment. k, IGV plot for visualizing the mutation level drop down to zero after demethylase treatment in DAMM-seq.
Extended Data Fig. 2 ∣. DAMM-seq reveals methylated sites in mitochondrial tRNA, and ALKBH7 does not demethylate m22G or other methylated bases (m1A, m3C, m1G) in mature mt-tRNAs.
a, Modification levels by LC-MS/MS in cellular small RNA fraction (input vs. engineered AlkB treatment) to showcase the demethylation efficiency and selectivity in DAMM-seq. P values = 0.0027, 0.0028, 0.4531, 0.0002, 0.6009, 0.5991, 0.5351, 0.5583 for methylated bases respectively; unpaired, two-tailed t-test. n = 3, biologically independent samples. Data are presented as mean values ± SD. For b-e, RT misincorporation frequency (input vs. engineered AlkB treatment) for: b, m1A9 in 14 mitochondrial tRNAs that contain m1A9; c, m3C32 in 2 mitochondrial tRNAs that contain m3C32; d, m1G9 in 5 mitochondrial tRNAs that contain m1G9; e, m1G37 in 4 mitochondrial tRNAs that contain m1G37. For b-e, n = 2 biologically independent samples. f, An example flowchart of library construction pipeline for DAMM-seq revealing methylation fraction change of specific m1A, m3C, m1G, and m22G sites in vivo, with altered TRMT1. g, TRMT1 knockdown efficiency in HeLa cells by mRNA level (below, normalized to GAPDH. n = 2 biologically independent samples) and protein level (above, compared to GAPDH). h, Mutation levels after RT for m22G26 in mt-tRNA in TRMT1-depleted HeLa cells vs. control. i, Mutation levels after RT for m22G26 in mt-tRNA in ALKBH7-depleted HepG2 cells vs. control. j, Mutation levels after RT for m22G26 in mt-tRNA in ALKBH7-overexpressed HeLa cells vs. control. For k-o, Mutation levels after RT (in ALKBH7-depleted HepG2 cells vs. control) for k, 3 mt-tRNAs with m1A58, l, 2 mt-tRNAs with m3C32, m, 14 mt-tRNAs with m1A9, n, 5 mt-tRNAs with m1G9, o, 4 mt-tRNAs with m1G37. For p-t, Mutation levels after RT (in ALKBH7-overexpressed HeLa cells vs. control) for p, 3 mt-tRNAs with m1A58, q, 2 mt-tRNAs with m3C32, r, 14 mt-tRNAs with m1A9, s, 5 mt-tRNAs with m1G9, t, 4 mt-tRNAs with m1G37. For h-t, n =2 biologically independent samples.
Extended Data Fig. 3 ∣. ALKBH7 regulates demethylation of m22G (mt-Ile region) and m1A (mt-Leu1 region) in mitochondrial dsRNA in vivo.
a, DAMM-seq reads from IP-enriched dsRNA (HepG2 wild-type) were predominantly aligned to the mitochondrial genome (normalized to genome length). b, DAMM-seq reads coverage of IP-enriched dsRNA across the mitochondrial genome spanning entire protein coding region (~3.5–16 kb). H-strand are shown as green color and L-strand as red color (both normalized to 18S rRNA). c, A9, G9 and G37 in HeLa mature mt-tRNA compared with those in enriched mt-dsRNA. A9: **P = 0.0074; paired, two-tailed t-test; n =12 independent m1A sites. G9: P = 0.071; paired, two-tailed t-test; n =5 independent m1G sites. G37: P = 0.5571; paired, two-tailed t-test; n = 4, independent m1G sites. Each data point represents one methylation site, with the average misincorporation from 2 biologically independent samples. Data are presented as mean values ± SD. d, mt-Ile m22G in mature mt-tRNA compared with that in enriched mt-dsRNA in HeLa cells (shControl), where lentivirus infection elevates m22G methylation level in dsRNA stage. e, IGV visualization of mutation ratio at mitochondrial dsRNA m22G (mt-Ile region) in ALKBH7 knockout HepG2 cells vs. wild-type. Mutation ratio values (majorly G→T + G→C) shown in the parenthesis. f, m22G in mt-dsRNA (mt-Ile region) in ALKBH7-overexpressed HeLa cells vs. control. g, IGV visualization of mutation ratio at mitochondrial dsRNA m1A (mt-Leu1 region) in ALKBH7 knockout HepG2 cells vs. wild-type. Mutation ratio values (majorly A→T) shown in the parenthesis. h, m1A in mt-dsRNA (mt-Leu1 region) in ALKBH7-overexpressed HeLa cells vs. control. i, m22G misincorporation in mt-dsRNA (mt-Ile region) in ALKBH7-depleted HepG2 cells (siALKBH7) can be rescued with the wild-type ALKBH7 protein but not by an ALKBH7 mutant without mitochondrial targeting sequence (MTS). j, m1A misincorporation in mt-dsRNA (mt-Leu1 region) in ALKBH7-depleted HepG2 cells (siALKBH7) can be rescued with the wild-type ALKBH7 protein but not by an ALKBH7 mutant without mitochondrial targeting sequence (MTS). For d, f, and h-j, n = 2 biologically independent samples.
Extended Data Fig. 4 ∣. ALKBH7 demethylation effect on other methylations (such as m1A9, m1G9, and m1G37) within mitochondrial dsRNA is lower than that on Ile-m22G and Leu1-m1A inside cells.
a, Mutation levels for m1A9, m1G9, and m1G37 in mt-dsRNA in ALKBH7 knockout HepG2 cells vs. wild-type. m1A9: *P = 0.0398; paired, two-tailed t-test; n = 24, 12 independent m1A sites. m1G9: P = 0.0954; paired, two-tailed t-test; n = 10, 5 independent m1G sites. m1G37: P = 0.38; paired, two-tailed t-test; n = 8, 4 independent m1G sites. b, Mutation levels for m1A9, m1G9, and m1G37 in mt-dsRNA in siALKBH7 HepG2 cells vs. siControl. m1A9: **P = 0.0047; paired, two-tailed t-test; n = 24, 12 independent m1A sites. m1G9: P = 0.1462; paired, two-tailed t-test; n = 10, 5 independent m1G sites. m1G37: *P = 0.0188; paired, two-tailed t-test; n = 8, 4 independent m1G sites. c, Mutation levels for m1A9, m1G9, and m1G37 in mt-dsRNA in ALKBH7-overexpressed HeLa cells vs. control. m1A9: ***P = 0.0002; paired, two-tailed t-test; n = 24, 12 independent m1A sites. m1G9: P = 0.5666; paired, two-tailed t-test; n = 10, 5 independent m1G sites. m1G37: P = 0.4838; paired, two-tailed t-test; n = 8, 4 independent m1G sites. For a-c, each data point represents one methylation site, with the misincorporation from 2 biologically independent samples. Data are presented as mean values ± SD. d, Mutation levels for m1A in mt-ND5 region of mt-dsRNA. Left: ALKBH7 knockout HepG2 cells vs. wild-type; Right: siALKBH7 HepG2 cells vs. siControl. n = 2 biologically independent samples. e, m22G-containing ssRNA probe synthesized by recombinant TRMT1 protein. f, m22G-containing mt-Ile RNA probe in forms of folded tRNA and dsRNA. g, m1A-containing mt-Leu1 RNA probe in forms of ssRNA and dsRNA. h, Modification levels of m1A/A in purified mt-tRNA before and after treatment with recombinant human ALKBH7. P = 0.4048; unpaired, two-tailed t-test. i, Modification levels of m1G/G in purified mt-tRNA before and after treatment with recombinant human ALKBH7. P = 0.2256; unpaired, two-tailed t-test. For h-i, n =3 biologically independent samples. Data are presented as mean values ± SD.
Extended Data Fig. 5 ∣. Optimized icSHAPE detects one flexible region adjacent to mt-Ile region in mitochondrial dsRNA, in which ALKBH7 depletion and overexpression impact the mt-dsRNA level.
a, A flowchart of library construction pipeline for optimized icSHAPE designed for studying secondary structure within mitochondrial dsRNA. b, IGV visualization of icSHAPE peak region (in H-strand) adjacent to mt-Ile region in mitochondrial dsRNA. For the “pull-down” samples we fed the cells with NAI-N3 (dissolved in DMSO) and enriched NAI-N3 labeled RNAs. For the “input” samples we fed the cells with DMSO and then build the libraries without pull-down enrichment. c, The decreased mitochondrial dsRNA flexible region levels (quantified by RT-qPCR, with gene-specific primer during RT and biotin-tagged spike-in) in ALKBH7-depleted HepG2 cells vs. control. d, The increased mitochondrial dsRNA flexible region level (quantified by RT-qPCR, with gene-specific primer during RT and biotin-tagged spike-in) in TRMT1-depleted HeLa cells vs. control. For c-d, n = 2 biologically independent samples. e, A scheme showing proposed effects of m22G demethylation by ALKBH7 on mitochondrial polycistronic RNA processing. f, ALKBH7-overexpressed cells (o/e ALKBH7) display an increased dsRNA level (in particular the L-strand) compared with o/e Control (normalized to 18S rRNA).
Extended Data Fig. 6 ∣. ALKBH7-depleted cells showed altered levels of steady-state mt-RNA and decreased mitochondrial protein synthesis rate, while mitochondrial transcription was not affected.
a, Relative mRNA levels of 13 mt-mRNAs (normalized to ACTB) in ALKBH7-depleted HEK 293T cells vs. control. P values = 0.0001, 0.0002, <0.0001, <0.0001, 0.0004, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001 for 13 mt-mRNAs, respectively; unpaired, two-tailed t-test. b, ALKBH7-overexpressed HeLa cells vs. control. P values = 0.275, 0.0941, 0.0042, 0.0075, 0.0011, 0.0008, 0.013, 0.009, 0.0124, 0.0008, 0.0209, 0.003, 0.0033 for 13 mt-mRNAs, respectively; unpaired, two-tailed t-test. c, Relative levels of 12S and 16S rRNAs (normalized to 18S rRNA) in ALKBH7-depleted HepG2 cells vs. control. P values = 0.0027 and 0.0003 for two rRNAs, respectively; unpaired, two-tailed t-test. For a-c, n=4 biologically independent samples. Data are presented as mean values ± SD. NS, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001. d, The transcription rate for mt-mRNA regions with and without ALKBH7 depletion. RT-qPCR quantification was conducted on isolated EU-labeled RNA at early time points of 1-min, 2-min, 3-min and 5-min, respectively, from siALKBH7 vs. siControl. Normalized levels of mt-mRNA region are shown. e, The normalized level changes of tRNA-mRNA junctions in nascent mt-RNA with and without ALKBH7 depletion. RT-qPCR quantification was conducted on isolated EU-labeled RNA at early time points of 1-min, 2-min, 3-min and 5-min, respectively, from siALKBH7 vs. siControl. Normalized levels of junction site are shown. For d-e, n=3 biologically independent samples. Data are presented as mean values ± SD.
Extended Data Fig. 7 ∣. The faster nascent mt-RNA processing led to the decreased overall levels of nascent mt-tRNA and mt-mRNA.
For a-c, Reads coverage of 10-min, 20-min and 30-min EU-labeled nascent RNA (from HepG2 WT cells) across the mitochondrial genome spanning entire protein coding region (~3.5-16 kb, normalized to 18S rRNA). H-strand genes are shown in green color and L-strand in red color. d, Above: IGV reads coverage at the mRNA-tRNA junction of mt-ND1/mt-Ile in mt-dsRNA from dsRNA-IP; below: IGV reads coverage at this mt-ND1/mt-Ile junction in nascent mt-RNA from 10-min EU-labeling. e, Above: IGV reads coverage at the mRNA-tRNA junction of mt-Leu1/mt-ND1 in mt-dsRNA from dsRNA-IP; below: IGV reads coverage at this mt-Leu1/mt-ND1 junction in nascent mt-RNA from 10-min EU-labeling. f, Relative reads coverage comparison at each tRNA-mRNA junction in mt-dsRNA (from dsRNA-IP) vs. in nascent mt-RNA from 10-min EU labeled RNA. The tRNA-mRNA junction ratio is defined by pre-tRNA/mRNA reads coverage ratio, which was calculated by the reads sum in pre-tRNA region (~70 nt) compared to the reads sum within its adjacent mt-mRNA region (within a 200-nt window). The ratio of an unprocessed junction is normalized to 1.0. Many regions were already decayed 10 min after transcription. g, Left: m22G in 10-min EU-labeled RNA (mt-Ile region) in siALKBH7 HepG2 cells vs. siControl. Right: m22G in 10-min EU-labeled RNA (mt-Ile region) in ALKBH7 knockout HepG2 cells vs. wild-type. h, Left: m1A in 10-min EU-labeled RNA (mt-Leu1 region) in siALKBH7 HepG2 cells vs. siControl. Right: m1A in 10-min EU-labeled RNA (mt-Leu1 region) in ALKBH7 knockout HepG2 cells vs. wild-type. For f-h, n=2 biologically independent samples. i, Normalized mt-mRNA levels in 30-min EU-labeled mitochondrial nascent RNA extracted from siALKBH7 HepG2 cells vs. siControl. P values = 0.0127, 0.0049, 0.0192, 0.3494, 0.8318, 0.5296, 0.0003, 0.0807, 0.0062, 0.0004, 0.0065 for 11 mt-mRNAs, respectively; unpaired, two-tailed t-test. j, Normalized mt-mRNA levels in 60-min EU-labeled mitochondrial nascent RNA extracted from siALKBH7 HepG2 cells vs. siControl. P values = <0.0001, 0.0013, 0.0006, 0.0004, 0.0004, 0.0004, 0.0135, <0.0001, <0.0001, 0.0009, <0.0001 for 11 mt-mRNAs, respectively; unpaired, two-tailed t-test. For i-j, n = 4 biologically independent samples. NS, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Extended Data Fig. 8 ∣. ALKBH7 loss leads to decreased mitochondrial activity and reduced protein synthesis.
a, Decreased OCR in ALKBH7-depleted HepG2 cells (siALKBH7) vs. siControl. b, Decreased ECAR in ALKBH7-depleted HepG2 cells (siALKBH7) vs. siControl. For a-b, n = 6 biologically independent samples; data are presented as mean values ± SD. c, Western blotting shows protein levels of mitochondrial marker VDAC, SDHA, HSP60, and cellular TRMT1 in siALKBH7 vs. siControl (normalized to β-tubulin), d, The metabolic labeling assay for mitochondrial protein synthesis. Using 3-hour AHA (L-Azidohomoalanine) labeling together with emetine (cytoplasmic translation inhibitor), biotinylated-alkyne-assisted click reaction enables western blotting to reveal mitochondrial nascent protein levels in siALKBH7 vs. siControl. Reduced protein synthesis was observed with ALKBH7 depletion. For c-d, two rounds of immunoblots for biologically independent samples were performed, with similar results obtained.
Extended Data Fig. 9 ∣. Loss of ALKBH7 in mouse tissues led to altered mt-mRNA and mt-tRNA levels, with decreased FAO activity observed in the fat tissue.
a, Relative levels of 13 mt-mRNAs (normalized to Actb) in the kidney isolated from Alkbh7 knockout mice (Alkbh7−/−) vs. wild-type. P values = 0.0408, 0.018, 0.0229, 0.0618, 0.0256, 0.0428, 0.074, 0.0157, 0.0138, 0.0192, 0.0126, 0.0067, 0.0254 for 13 mt-mRNAs, respectively. b, Relative levels of 13 mt-mRNAs (normalized to Actb) in the heart isolated from Alkbh7 knockout mice (Alkbh7−/−) vs. wild-type. P values = 0.0084, 0.0026, 0.0008, 0.025, 0.0048, 0.0056, 0.0043, 0.006, 0.0046, 0.0062, 0.0004, 0.0061, 0.0004 for 13 mt-mRNAs, respectively. c, Relative levels of ten mt-tRNAs in the BAT isolated from Alkbh7 knockout mice (Alkbh7−/−) vs. wild-type. P values = <0.0001, 0.0001, 0.7191, 0.0745, 0.4192, 0.053, 0.1789, 0.0005, 0.4229, 0.4391 for ten mt-tRNAs, respectively. d, Relative levels of ten mt-tRNAs in the liver isolated from Alkbh7 knockout mice (Alkbh7−/−) vs. wild-type. P values = 0.0221, 0.0485, 0.1482, 0.0484, 0.0086, 0.5072, 0.2898, 0.1218, 0.036, 0.1803 for ten mt-tRNAs, respectively. e, Relative levels of ten mt-tRNAs in the kidney isolated from Alkbh7 knockout mice (Alkbh7−/−) vs. wild-type. P values = <0.0001, 0.0056, 0.9016, 0.0086, <0.0001, 0.455, 0.0752, 0.0029, 0.0022, 0.0012 for ten mt-tRNAs, respectively. For c-e, mouse U6 snRNA was used for normalization. For a-e, n = 6 biologically independent samples; unpaired, two-tailed t-test; data are presented as mean values ± SD. f, Western blotting of mitochondrial marker Vdac (normalized to β-tubulin) in the lysate from BAT, liver and kidney isolated from Alkbh7 knockout mice (Alkbh7−/−) vs. wild-type. Two rounds of immunoblots for biologically independent samples were performed, with similar results obtained. g-h, Mitochondrial fatty acid oxidation (FAO) assay on five mouse tissues, where the fresh tissue lysate was incubated with FAO substrate octanoyl-CoA for 60 minutes (g) and 90 minutes (h), which is further oxidized in mitochondria. For g, P values = 0.1519, 0.0196, 0.1916, 0.2827, 0.4914 for 5 tissues, respectively; for h, P values = 0.7372, 0.0068, 0.0879, 0.8232, 0.0389 for 5 tissues, respectively. For g-h, n = 8 biologically independent samples; unpaired, two-tailed t-test; data are presented as mean values ± SD. For a-e and g-h, NS P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Supplementary Material
Acknowledgements
This work was supported by NIH R01 ES030546 (C.H.) and RM1 HG008935 (C.H.). The ALKBH7 biochemistry work was partially supported by The National Key Research and Development Program of China (2020YFA0803400; R.-J.L.) and The National Natural Science Foundation of China (31971230; R.-J.L.). The Mass Spectrometry Facility of the University of Chicago is funded by the National Science Foundation (CHE-1048528). C.H. is an investigator of the Howard Hughes Medical Institute. We thank A. Andersen at Life Science Editors for editorial assistance. We thank X. Dou and X. Cui for help on sequencing data analysis. We thank P. W. Faber, M. Marchuk and L. Scarpitta at the Genomics Facility at the University of Chicago for generous help on high-throughput sequencing.
Footnotes
Competing interests
C.H. is a scientific founder and a member of the scientific advisory board of Accent Therapeutics, Inc. and a shareholder of Epican Genetech.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41556-021-00709-7.
Extended data is available for this paper at https://doi.org/10.1038/s41556-021-00709-7.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41556-021-00709-7.
Data availability
The sequencing data listed in Supplementary Table 1 are available in the Gene Expression Omnibus database under the accession number GSE148202. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
- 1.Jia G et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol 7, 885–887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng G et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solberg A et al. Deletion of mouse Alkbh7 leads to obesity. J. Mol. Cell Biol 5, 194–203 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T et al. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 42, 7346–7357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barchiesi A et al. Transcription, processing, and decay of mitochondrial RNA in health and disease. Int. J. Mol. Sci 20, 2221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotrys AV et al. Mitochondrial gene expression and beyond-novel aspects of cellular physiology. Cells 9, 17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falnes PO et al. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature 419, 178–182 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Trewick SC et al. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 419, 174–178 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Aas PA et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 421, 859–863 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Yang CG et al. Crystal structures of DNA/RNA repair enzymes AlkB and ABH2 bound to dsDNA. Nature 452, 961–965 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z et al. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell 31, 127–141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su R et al. R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell 172, 90–105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31, 591–606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roundtree IA et al. Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao BS et al. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol 18, 31–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell 167, 816–828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordstrand LM et al. Mice lacking Alkbh1 display sex-ratio distortion and unilateral eye defects. PLoS ONE 5, e13827 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M-M et al. ALKBH4-dependent demethylation of actin regulates actomyosin dynamics. Nat. Commun 4, 1832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu D et al. Human ALKBH7 is required for alkylation and oxidation-induced programmed necrosis. Genes Dev. 27, 1089–1100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan JJ et al. ALKBH7 drives a tissue and sex-specific necrotic cell death response following alkylation-induced damage. Cell Death Dis. 8, e2947 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G et al. The atomic resolution structure of human AlkB homolog 7 (ALKBH7), a key protein for programmed necrosis and fat metabolism. J. Biol. Chem 289, 27924–27936 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai Q et al. Selective enzymatic demethylation of N2,N2-dimethylguanosine in RNA and its application in high-throughput tRNA sequencing. Angew. Chem. Int. Ed 56, 5017–5020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewe JM et al. TRMT1-catalyzed tRNA modifications are required for redox homeostasis to ensure proper cellular proliferation and oxidative stress survival. Mol. Cell. Biol 37, e00214–e00217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg S et al. A correlation between N2-dimethylguanosine presence and alternate tRNA conformers. RNA 1, 886–891 (1995). [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng G et al. Efficient and quantitative high-throughput tRNA sequencing. Nat. Methods 12, 835–837 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cozen AE et al. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods 12, 879–884 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou H et al. Evolution of a reverse transcriptase to map N1-methyladenosine in human messenger RNA. Nat. Methods 16, 1281–1288 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhir A et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 560, 238–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojala D et al. tRNA punctuation model of RNA processing in human mitochondria. Nature 290, 470–474 (1981). [DOI] [PubMed] [Google Scholar]
- 30.Kim Y et al. PKR senses nuclear and mitochondrial signals by interacting with endogenous double-stranded RNAs. Mol. Cell 71, 1051–1063 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Rossmanith W et al. Of P and Z: mitochondrial tRNA processing enzymes. Biochim. Biophys. Acta 1819, 1017–1026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daoud R et al. Yeast mitochondrial RNase P, RNase Z and the RNA degradosome are part of a stable supercomplex. Nucleic Acids Res. 40, 1728–1736 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitale RC et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519, 486–490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L et al. Death-associated protein 3 regulates mitochondrial-encoded protein synthesis and mitochondrial dynamics. J. Biol. Chem 290, 24961–24974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwong SC et al. Metabolic role of fatty acid binding protein 7 in mediating triple-negative breast cancer cell death via PPAR-α signaling. J. Lipid Res 60, 1807–1817 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng X et al. Targeting DGAT1 ameliorates glioblastoma by increasing fat catabolism and oxidative stress. Cell Metab. 32, 229–242 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houten SM et al. The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu. Rev. Physiol 78, 23–44 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Nsiah-Sefaa A et al. Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci. Rep 36, e00313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enns GM et al. Mitochondrial respiratory chain complex I deficiency with clinical and biochemical features of long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. J. Pediatr 136, 251–254 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Gargus JJ et al. Respiratory complex II defect in siblings associated with a symptomatic secondary block in fatty acid oxidation. J. Inherit. Metab. Dis 26, 659–670 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Hao Z et al. N6-Deoxyadenosine methylation in mammalian mitochondrial DNA. Mol. Cell 10.1016/j.molcel.2020.02.018 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flynn RA et al. Transcriptome-wide interrogation of RNA secondary structure in living cells with icSHAPE. Nat. Protoc 11, 273–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J et al. N6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data listed in Supplementary Table 1 are available in the Gene Expression Omnibus database under the accession number GSE148202. Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.