Summary
Background
Lung cancer is a major health problem. CT lung screening can reduce lung cancer mortality through early diagnosis by at least 20%. Screening high-risk individuals is most effective. Retrospective analyses suggest that identifying individuals for screening by accurate prediction models is more efficient than using categorical age-smoking criteria, such as the US Preventive Services Task Force (USPSTF) criteria. This study prospectively compared the effectiveness of the USPSTF2013 and PLCOm2012 model eligibility criteria.
Methods
In this prospective cohort study, participants from the International Lung Screening Trial (ILST), aged 55–80 years, who were current or former smokers (ie, had ≥30 pack-years smoking history or ≤15 quit-years since last permanently quitting), and who met USPSTF2013 criteria or a PLCOm2012 risk threshold of at least 1·51% within 6 years of screening, were recruited from nine screening sites in Canada, Australia, Hong Kong, and the UK. After enrolment, patients were assessed with the USPSTF2013 criteria and the PLCOm2012 risk model with a threshold of at least 1·70% at 6 years. Data were collected locally and centralised. Main outcomes were the comparison of lung cancer detection rates and cumulative life expectancies in patients with lung cancer between USPSTF2013 criteria and the PLCOm2012 model. In this Article, we present data from an interim analysis. To estimate the incidence of lung cancers in individuals who were USPSTF2013-negative and had PLCOm2012 of less than 1·51% at 6 years, ever-smokers in the Prostate Lung Colorectal and Ovarian Cancer Screening Trial (PLCO) who met these criteria and their lung cancer incidence were applied to the ILST sample size for the mean follow-up occurring in the ILST. This trial is registered at ClinicalTrials.gov, NCT02871856. Study enrolment is almost complete.
Findings
Between June 17, 2015, and Dec 29, 2020, 5819 participants from the International Lung Screening Trial (ILST) were enrolled on the basis of meeting USPSTF2013 criteria or the PLCOm2012 risk threshold of at least 1·51% at 6 years. The same number of individuals was selected for the PLCOm2012 model as for the USPSTF2013 criteria (4540 [78%] of 5819). After a mean follow-up of 2·3 years (SD 1·0), 135 lung cancers occurred in 4540 USPSTF2013-positive participants and 162 in 4540 participants included in the PLCOm2012 of at least 1·70% at 6 years group (cancer sensitivity difference 15·8%, 95% CI 10·7–22·1%; absolute odds ratio 4·00, 95% CI 1·89–9·44; p<0·0001). Compared to USPSTF2013-positive individuals, PLCOm2012-selected participants were older (mean age 65·7 years [SD 5·9] vs 63·3 years [5·7]; p<0·0001), had more comorbidities (median 2 [IQR 1–3] vs 1 [1–2]; p<0·0001), and shorter life expectancy (13·9 years [95% CI 12·8–14·9] vs 14·8 [13·6–16·0] years). Model-based difference in cumulative life expectancies for those diagnosed with lung cancer were higher in those who had PLCOm2012 risk of at least 1·70% at 6 years than individuals who were USPSTF2013-positive (2248·6 years [95% CI 2089·6–2425·9] vs 2000·7 years [1841·2–2160·3]; difference 247·9 years, p=0·015).
Interpretation
PLCOm2012 appears to be more efficient than the USPSTF2013 criteria for selecting individuals to enrol into lung cancer screening programmes and should be used for identifying high-risk individuals who benefit from the inclusion in these programmes.
Funding
Terry Fox Research Institute, The UBC-VGH Hospital Foundation and the BC Cancer Foundation, the Alberta Cancer Foundation, the Australian National Health and Medical Research Council, Cancer Research UK and a consortium of funders, and the Roy Castle Lung Cancer Foundation for the UK Lung Screen Uptake Trial.
Research in context.
Evidence before this study
Lung cancer screening reduces lung cancer mortality through early detection and is most effective when applied to high-risk individuals. Past guidelines have recommended that screening eligibility should be based on categorisations of age, pack-years (number of pack of cigarettes smoked per day multiplied by number of years smoked), and years since last permanently quitting smoking. For example, the US Preventive Services Task Force 2013 (USPSTF2013) guideline defines eligible individuals for screening as those aged 55–80 years, who smoked 30 or more pack-years, and had 15 quit-years or less. Several studies have found that accurate risk prediction models of lung cancer, such as the PLCOm2012, are more sensitive at identifying individuals who develop lung cancer than using categorical age-smoking criteria. However, studies have argued that risk prediction models identify individuals who are older, have more comorbidities, and shorter life expectancies than categorical eligibility approaches, such as the USPSTF criteria, and thus might not optimise life-years gained. Most evidence so far has come from retrospective analyses of trial or survey data and microsimulation modelling, and not from prospective studies. We searched PubMed for studies published in English between Jan 1, 2004, and Dec 31, 2020, related to eligible patients of lung cancer screening, PLCOm2012, and USPSTF, using search terms including “lung cancer screening eligibility”, “lung cancer screening criteria”, “lung cancer screening guidelines”, “lung cancer screening PLCOm2012”, “PLCOm2012”, and “lung cancer screening USPSTF”. All information from peer-reviewed articles, reference lists, books, and websites was considered. In addition, data were retrieved and analysed from datasets (Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, National Lung Screening Trial, and Pan-Canadian Early Detection of Lung Cancer Study).
Added value of this study
This study provides prospective evidence based on a large multinational trial that shows that the PLCOm2012 model is significantly more sensitive at identifying those who will be diagnosed with lung cancer than the USPSTF2013 criteria and demonstrates that the cumulative potential life-years gained in individuals diagnosed with lung cancer was significantly greater in the group assigned to PLCOm2012 threshold of at least 1·70% at 6 years than in the group assigned to USPSTF2013 criteria. In addition, PLCOm2012 identified significantly more lung cancers in women than in men.
Implications of all the available evidence
This new evidence is consistent and supportive of past retrospective and microsimulation studies. These data provide support for continual use and for new uptake of PLCOm2012 for lung cancer screening selection in lung cancer screening programmes.
Introduction
Lung cancer is the leading cause of cancer mortality in the world.1 Although 5-year relative survival in the USA has improved from 10·7% in 1973 to 19·8% in 2010, survival generally remains poor.2 Randomised controlled trials have shown that lung cancer screening of high-risk individuals with low-dose CT can reduce lung cancer mortality by at least 20%.3, 4 Lung cancer screening is most effective when applied to high-risk individuals.5, 6 A major issue remains how to best select these individuals for lung cancer screening. Many trials have used, and guidelines recommend using, non-quantitative approaches based on categorical age, pack-years (number of pack of cigarettes smoked per day multiplied by number of years smoked), and years since last permanently quitting smoking, for which risk is not estimated. Examples include the National Lung Screening Trial (NLST) and the Dutch–Belgian NELSON trial eligibility criteria,3, 4 and the US Preventive Services Task Force (USPSTF) and Centers for Medicaid and Medicare Services guidelines.7, 8
Retrospective and cost-effectiveness analyses have shown that selecting participants by an accurate risk prediction model identifies individuals who develop lung cancer at a significantly higher sensitivity, averts more lung cancer deaths, has smaller number needed to screen to avert one lung cancer death, and is more cost-effective than categorical approaches.6, 9, 10, 11 PLCOm2012 is one of these available risk prediction models.9 The PLCOm2012 model predicts incident lung cancers within 6 years of screening using 11 predictors: age, race or ethnicity, education, body-mass index, history of chronic obstructive pulmonary disease, personal history of cancer, family history of lung cancer, and smoking status, and intensity (mean number of cigarettes smoked per day), duration, and quit-years in patients who used to smoke. A PLCOm2012 calculator with model parameters can be found online. The PLCOm2012 model has been validated by different research teams in the USA, Germany, Australia, the UK, Canada, Brazil, Poland, and in multiple other countries.9, 10, 12, 13, 14, 15, 16, 17, 18 However, no large multinational prospective study to date has compared the effectiveness of a standard categorical criteria versus an accurate risk prediction model for enrolling individuals into lung cancer screening.
In the USA, public health policy decisions regarding selection of screened participants (ie, USPSTF guidelines) have been developed on the basis of Cancer Intervention and Surveillance Modeling Network (CISNET) microsimulation modelling that makes assumptions, such as participants of the modelling cohorts enter screening as soon as they reach the eligible age and that these cohorts have 100% participation and adherence.19 Such modelling might not reflect real-world screening practice. The USPSTF has been reluctant to recommend risk models for patient selection because they argue that risk models enrol individuals with shorter life expectancies, which might lead to fewer life-years gained, and evidence supporting risk models have not been based on prospective data.20
The International Lung Screening Trial (ILST) aimed to compare the effectiveness of the USPSTF2013 criteria with that of the PLCOm2012 model by comparing the proportions of lung cancers detected and cumulative life expectancies from study entry of those diagnosed with lung cancer had they not developed lung cancer.
Methods
Study design and participants
This prospective cohort study included participants aged 55–80 years, who were current or former smokers (ie, had ≥30 pack-years smoking history and ≤15 quit-years since last permanently quitting), and who met USPSTF2013 criteria or a PLCOm2012 risk threshold of at least 1·51% within 6 years of baseline screening. A previous study indicated that a PLCOm2012 risk threshold of at least 1·51% within 6 years was a suitable threshold because in the NLST, lung cancer mortality rates in the low-dose CT and chest x-ray comparison group consistently diverged above this threshold.6 Participants were excluded if they had symptoms of lung cancer, lung cancer history, or any serious life-threatening conditions (eg, heart disease). A full list of inclusion and exclusion criteria is available in the protocol.21 Participants were recruited from nine screening sites in four countries: Australia (Brisbane, Sydney, two sites in Melbourne, and Perth), Canada (Vancouver and Alberta), Hong Kong, and the UK. Enrolment characteristics, including number of participants, follow-up time, and dates of enrolment, for each site are described in the appendix (p 2). Alberta (NCT02431962)22 and the UK23 had launched their studies independently and their study protocols differ in some respects from the original ILST protocol, but their enrolment criteria were the same as at other sites.
Written informed consent was obtained from each participant and research ethics board approval was obtained at each site.
Procedures
Initial contact for enrolment was made using the following methods in decreasing order of frequency: social media, contact with primary care provider, advertisement in newspaper or TV, mailings using government or electoral lists, recommendation by friend or family, advertisement through radio or poster, contact with previous study participants, or emails.
At initial encounter, potential participants were risk assessed and questions regarding the following were asked: current age, smoking status, average number of cigarettes smoked per day when smoking, duration of smoking in years, years since quitting in former smokers, height, weight, race or ethnicity, level of education, history of chronic obstructive pulmonary disease, family history of lung cancer, and personal history of cancer. The first five items of information were used to determine eligibility by the USPSTF2013 criteria and all items of information were put into a spreadsheet calculator to determine PLCOm2012 risk score and determine eligibility. Adaption of the PLCOm2012 model for use in different settings is discussed in the appendix (p 3). To allow fair comparison of sensitivities or cancer detection rates while holding specificities similar in both groups, for analysis the PLCOm2012 threshold for eligibility was set at a level that resulted in the same number of individuals being selected for screening as for the USPSTF criteria. This number could not be known and preset a priori. In the analysis, the PLCOm2012 risk threshold of at least 1·70% at 6 years was used because a published CISNET report had demonstrated the equivalence of this threshold and USPSTF 2013 and had been previously used in other studies.12, 24, 25 The PLCOm2012 threshold of at least 1·70% at 6 years is approximately in between existing lung cancer screening programme thresholds; for example, the UK NHS Targeted Lung Health Checks uses a PLCOm2012 threshold of at least 1·51% at 6 years and the Ontario Lung Screening Program uses a PLCOm2012 threshold of at least 2·0% at 6 years.26, 27 In addition to comparative sensitivities, the PLCOm2012 threshold of at least 1·70% at 6 years produces conservative results compared with the PLCOm2012 at least 1·51% at 6 years threshold.
Following baseline scans, individuals entered a pathway leading to a 2-year follow-up planned low-dose CT scan, or a 3-month or 12-month surveillance scan for suspicious nodules, or were sent directly to detailed diagnostic evaluation, depending on the level of malignancy risk determined by the PanCan model estimated risk score.28
Not all sites followed up ineligible individuals to determine their lung cancer outcomes. To estimate the incidence of lung cancers in individuals who were USPSTF2013-negative and had PLCOm2012 of less than 1·51% at 6 years, ever-smokers in the Prostate Lung Colorectal and Ovarian Cancer Screening Trial (PLCO) who met these criteria and their lung cancer incidence were applied to the ILST sample size for the mean follow-up occurring in the ILST. These data allowed estimation of criteria sensitivities at the population level.
Lung cancer histology was classified according to the 2015 WHO Classification of Lung Tumors and staging was by the seventh or eighth editions of Lung Cancer Stage Classification.29, 30
Sociodemographic, medical, including comorbidity, exposure, and outcome data were collected.
Outcomes
The main outcomes of interest in this study were incident lung cancer and estimated life expectancies given baseline participant characteristics, which allowed calculation of criteria sensitivities and cumulative potential life-years gainable in those diagnosed with lung cancer. ILST study objective two is a prospective evaluation of the PanCan or Brock model-based PanCan nodule management protocol.28 The theme of that study is distinct from the current study and will be presented in a separate paper.
Statistical analysis
The original planned sample size was 4000 individuals and the sample size calculations have been detailed elsewhere.21 However, the model threshold for comparison changed from at least 1·51% at 6 years to 1·70% at 6 years, making the selected comparison group smaller and thus requiring a larger sample size, and to obtain more precise estimates for study aim 2 (PanCan Protocol evaluation) we expanded recruitment to 6000 individuals.
In this Article, we present an interim analysis of the ILST. Descriptive statistics describing sociodemographic and exposure characteristics of the study sample were prepared using contingency table analysis for categorical data and applying Fisher's exact test. 95% CIs for proportions were estimated using the binomial exact method. Comparisons of skewed continuous data used non-parametric test of trend and for approximately normally distributed continuous data used Student's t-test not assuming equal variance.
Comorbidity count was missing for 2735 (47%) of 5819 participants and smoking intensity was missing for 805 (14%) of 5819. All other covariates were non-missing or missing only small amounts (<2%). Missing data were handled by multiple imputations using 20 imputed datasets using the mi suite of commands by STATA (version 16.1). The rule of Rubin was used to produce pooled estimates from imputed datasets.31 PLCOm2012 risk scores and USPSTF2013 status data were complete as they were collected for all participants at initial risk assessment to determine entry into the study. For analysis, one site did not provide smoking intensity data, which are required to estimate life expectancies. Multiple imputations allowed estimation of life expectancies in the full dataset.
Differences in the proportions of lung cancers detected by the two eligibility criteria in the analytic sample (comprised of participants USPSTF2013 positive or PLCOm2012 risk threshold ≥1·70% at 6 years) were done by applying McNemar's test comparing marginal proportions and discordant pair odds ratios (OR), along with 95% CIs and p values, as the comparison groups were not mutually exclusive. As part of study aim 1, prespecified comparisons of criteria test accuracies were planned, and included sensitivity, specificity, positive and negative predictive values, and false-negative proportions. Although we did not prespecify stratified analyses by sex, it became evident from study findings published subsequent to our protocol development that evaluation of screening differences by sex could be important. Consequently, in the current analysis, we present some results for aim 1 stratified by sex.
Life expectancies of individuals in the ILST cohort from baseline scan were estimated using a parametric Weibull accelerated failure-time survival model predicting all-cause death, which was developed in PLCO data. Predictors include age, sex, body-mass index, comorbidity count, smoking status, smoking intensity, smoking duration, and quit-years in those who used to smoke. The comorbidity score was 1 if present and zero if absent for heart disease, stroke, hypertension, chronic obstructive pulmonary disease, diabetes, cancer, gastrointestinal disease, liver disease, arthritis, and osteoporosis or osteopenia. Details of the all-cause death survival model are presented in the appendix (p 6). Life expectancy calculations aimed to assess the maximum potential life-years that could be saved by screening. The actual life-years saved is also a function of the probability that screening results in reduction of lung cancer mortality, which depends on stage-shift due to screening and administration of effective treatments.
Statistical analyses were done using STATA MP (version 16.1). All p values are two-sided. When p values were used for hypothesis testing, an α error of less than 0·05 was applied. This is the case for the prespecified tests of whether cancer detection rates or cumulative life expectancies at baseline for those diagnosed with lung cancer differed by eligibility criteria.
This trial is registered at ClinicalTrials.gov, NCT02871856. Study enrolment is almost complete.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between June 17, 2015, and Dec 29, 2020, 5819 participants from the ILST were enrolled who were USPSTF2013 positive or met the PLCOm2012 threshold of at least 1·51% at 6 years, or both. At a risk threshold of at least 1·70% at 6 years, the same number of individuals was selected for the PLCOm2012 model as for the USPSTF2013 criteria (4540 [78%] of 5819). To make fair comparisons, we compared individuals who met the PLCOm2012 risk threshold of at least 1·70% at 6 years with those who met USPSTF2013 criteria (analytic sample).
Participant and tumour characteristics are summarised in table 1. Mean age was 63·3 years (SD 5·7) for the USPSTF2013-positive group and 65·7 years (SD 5·9) for the PLCOm2012 risk of at least 1·70% at 6 years group (p<0·0001). Compared with the USPSTF2013-positive group, the PLCOm2012 risk of at least 1·70% at 6 years group had a smaller proportion who completed high school education or beyond (2211 [48·7%] of 4540 participants vs 2428 [53·5%] of 4439 participants; p<0·0001), lower mean body-mass index (26·9 kg/m2 [SD 4·8] vs 27·6 kg/m2 [SD 5·3]; p<0·0001), a greater incidence of history of coronary obstructive pulmonary disease (1307 [28·8%] vs 1071 [23·6%]; p<0·0001), higher incidence of personal history of cancer (492 [14·7%] vs 342 [10·0%]; p<0·0001), a greater incidence of family history of lung cancer (1310 [28·9%] vs 990 [21·8%]; p<0·0001), and a higher median of comorbidities (2 [IQR 1–3] vs 1 [1–2]; p<0·0001). Comorbidity distributions by criteria are in the appendix (p 5). Individuals meeting PLCOm2012 risk threshold at least 1·70% at 6 years had a similar average smoking intensity to the USPSTF2013-positive group (22·7 [SD 10·3] cigarettes per day vs 23·1 [8·8] cigarettes per day; p=0·13). However, patients in the PLCOm2012 risk of at least 1·70% at 6 years group had a longer smoking duration (43·4 [SD 8·0] years vs 43·0 [7·4] years; p=0·049) and longer mean quit-years (median 8 [IQR 3–16] vs 6 [2–10]; p<0·0001) than those in the USPSTF2013-positive group. Furthermore, participants in the PLCOm2012 of at least 1·70% at 6 years group had a higher median PLCOm2012 risk score than the USPSTF2013 group (3·5 [IQR 2·5–5·8] vs 3·1 [1·8–5·5], p<0·0001).
Table 1.
Participant and cancer characteristics, overall and by USPSTF and PLCOm2012 criteria positivity
| USPSTF2013 eligible (n=4540) | PLCOm2012 ≥1·70% at 6 years (n=4540) | p value* | Lung cancer cases in the analytic sample (n=171) | ||
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age | 63·3 (5·7) | 65·7 (5·9) | p<0·0001 | 67·3 (5·6) | |
| Sex | .. | .. | pexact=0·17 | .. | |
| Female | 2046 (45·1%) [3·1%] | 2112 (46·5%) [4·0%] | .. | 98 (57·3%) | |
| Male | 2494 (54·9%) [2·5%] | 2428 (53·5%) [2·7%] | .. | 73 (42·7%) | |
| Ethnicity | .. | .. | pexact=0·66† | .. | |
| White | 2989 (65·8%) [2·4%] | 3009 (66·3%) [2·8%] | .. | 100 (58·5%) | |
| East Asian | 271 (6·0%) [2·6%] | 203 (4·5%) [3·9%] | .. | 8 (4·7%) | |
| Other | 144 (3·2%) [1·4%] | 144 (3·2%) [1·4%] | .. | 3 (1·7%) | |
| Missing | 1136 (25·0%) [4·0%] | 1184 (26·1%) [4·7%] | .. | 60 (35·1%) | |
| Education | .. | .. | p<0·0001 | .. | |
| High school completed or less | 2111 (46·5%) [3·6%] | 2329 (51·3%) [4·3%] | .. | 101 (59·1%) | |
| Beyond high school | 2428 (53·5%) [2·0%] | 2211 (48·7%) [2·4%] | .. | 70 (40·9%) | |
| Medical history | |||||
| Body-mass index (kg/m2) | 27·6 (5·3) | 26·9 (4·8) | p<0·0001 | 26·4 (4·7) | |
| COPD | .. | pexact<0·0001 | .. | ||
| No | 3469 (76·4%) [2·5%] | 3233 (71·2%) [3·3%] | .. | 124 (72·5%) | |
| Yes | 1071 (23·6%) [3·5%] | 1307 (28·8%) [3·4%] | .. | 47 (27·5%) | |
| Personal history of cancer | .. | .. | pexact<0·0001 | .. | |
| No | 3062 (89·9%) [2·4%] | 2864 (85·3%) [3·0%] | .. | 100 (90·1%) | |
| Yes | 342 (10·0%) [2·1%] | 492 (14·7%) [2·0%%] | .. | 11 (9·9%) | |
| Family history of lung cancer | .. | .. | pexact<0·0001 | .. | |
| No | 3550 (78·2%) [2·6%] | 3230 (71·1%) [3·3%] | .. | 118 (69·0%) | |
| Yes | 990 (21·8%) [3·3%] | 1310 (28·9%) [3·4%] | .. | 53 (31·0%) | |
| Comorbidity count‡ | 1 (1–2) | 2 (1–3) | pnptrend<0·0001§ | 2 (1–2) | |
| Exposures | |||||
| Smoking status | .. | .. | pexact=0·63 | .. | |
| Former | 2004 (44·1%) [2·5%] | 1981 (43·6%) [3·1%] | .. | 79 (46·2%) | |
| Current | 2536 (55·9%) [3·0%] | 2559 (56·4%) [3·5%] | .. | 92 (53·8%) | |
| Smoking intensity, average cigarettes smoked per day | 23·1 (8·8) | 22·7 (10·3) | p=0·13 | 22·6 (9·6) | |
| Smoking duration, years | 43·0 (7·4) | 43·4 (8·0) | p=0·049 | 45·4 (8·4) | |
| Quit-years in those who used to smoke | 6 (2–10; n=2004) | 8 (3–16; n=1981) | p<0·0001 | 3 (8–14) | |
| Pack-years | 48·8 (18·3) | 47·8 (20·2) | p=0·013 | 49·7 (20·1) | |
| PLCOm2012 score | 3·1 (1·8–5·5) | 3·5 (2·5–5·8) | p<0·0001¶ | 4·8 (2·7–7·9) | |
| Cancer characteristics | |||||
| Stage NSCLC | .. | .. | pexact=0·59 | .. | |
| Early (I, II) | 104/129 (80·6%) | 122/156 (78·2%) | .. | .. | |
| Late (III, IV) | 25/129 (19·4%) | 34/156 (21·8%) | .. | .. | |
| Histology | .. | .. | pexact<1·00 | .. | |
| Adenocarcinoma | 86/135 (63·7%) | 108/162 (66·7%) | .. | .. | |
| Squamous cell | 23/135 (17·0%) | 25/162 (15·4%) | .. | .. | |
| Large cell | 3/135 (2·2%) | 3/162 (1·9%) | .. | .. | |
| Mixed | 4/135 (3·0%) | 6/162 (3·7%) | .. | .. | |
| NSCLC not otherwise specified | 7/135 (5·2%) | 7/162 (4·3%) | .. | .. | |
| SCLC | 9/135 (6·7%) | 9/162 (5·6%) | .. | .. | |
| Carcinoid | 2/135 (1·5%) | 3/162 (1·9%) | .. | .. | |
| Unknown | 1/135 (0·7%) | 1/162 (0·6%) | .. | .. | |
| Follow-up characteristics | |||||
| Survival data | |||||
| Follow-up, years | 2·1 (1·6–2·9) | 2·1 (1·6–2·9) | .. | 1·2 (0·2–2·6) | |
| Incident lung cancers/cumulative follow-up, years‖ | 135/10 445 | 162/10 561 | .. | 171/281 | |
Data are mean (SD), n (%), median (IQR), or n/N (%), unless stated otherwise. Percentages in square brackets represent the proportion of lung cancers found in individuals in the group in that cell. NSCLC=non-small-cell lung cancer. SCLC=small cell lung cancer. USPSTF=US Preventive Services Task Force.
p value testing if the distribution of variable levels differs between criteria.
Ethnicity distribution difference was tested as Whites versus all other categories pooled·
Excludes Alberta site.·
Performed on existing data on ten comorbidities (appendix p 5).
t test performed on natural log transformed PLCOm2012 scores. Three limited-stage and two extensive-stage SCLCs were excluded from these stage calculations.
Used to calculate incidence rate.
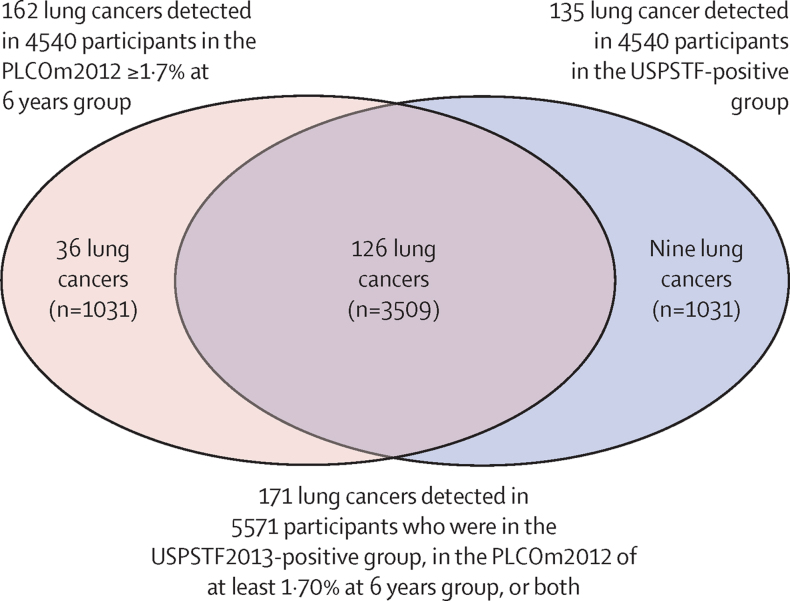
Mean follow-up was 2·3 years (SD 1·0). In the complete cohort of 5819 individuals, 177 lung cancers were diagnosed. Six of these 177 lung cancers were detected in 248 individuals who were in groups PLCOm2012 less than 1·70% at 6 years and USPSTF2013-negative. Of 5571 individuals who were in the USPSTF2013-positive group, in the PLCOm2012 of at least 1·70% at 6 years group, or both (analytic sample), 135 lung cancers were detected in 4540 individuals who were USPSTF2013-positive, 162 in 4540 who had PLCOm2012 risk of at least 1·70% at 6 years, and 126 in 3509 individuals who met both these criteria (figure, table 2). In the analytic sample, the PLCOm2012 threshold of at least 1·70% at 6 years model detected 27 more lung cancers than did USPSTF criteria (162 [94·7%; 95% CI 90·2–97·6] of 171 vs 135 [78·9%; 95% CI 72·1–84·8] of 171; p=0·0001; table 3). This PLCOm2012 threshold gave higher overall cancer detection (15·8% [95% CI 10·7–22·1%]; ORMcNemar's 4·00 [95% CI 1·89–9·44]; p<0·0001).
Figure.
Venn diagram describing the distribution of individuals and lung cancer cases by criteria (USPSTF2013 positivity and PLCOm2012 ≥1·7% at 6 years status)
27 (15·8% [95% CI 10·7–22·1%]; p<0·0001) of 171 more lung cancers were detected by PLCOm2012 than USPSTF criteria. The figure excludes six lung cancers detected in 248 individuals who were USPSTF-negative but were enrolled because they had PLCOm2012 risks at least 1·5% at 6 years and less than 1·70% at 6 years.
Table 2.
Distribution of individuals and lung cancer cases by eligibility criteria status observed in ILST data without and with supplementation of PLCO data
| USPSTF2013-negative | USPSTF2013-positive | Total | |
|---|---|---|---|
| ILST data only | |||
| PLCOm2012 <1·70% at 6 years | 6/248 (2·4%) | 9/1031 (0·9%) | 15/1279 (1·2%) |
| PLCOm2012 ≥1·70% at 6 years | 36/1031 (3·5%) | 126/3509 (3·6%) | 162/4540 (3·6%) |
| Total | 42/1279 (3·3%) | 135/4540 (3·0%) | 177/5819 (3·0%) |
| ILST data supplemented with PLCO data | |||
| PLCOm2012 ineligible | *PLCOm2012 ≥1·51% to <1·70% at 6 years: 6/248 (2·4%); † PLCOm2012 <1·51% at 6 years: 13/7106 (0·2%); total 19/7354 (0·3%) | 9/1031 (0·9%) | 28/8385 (0·3%) |
| PLCOm2012 ≥1·70% at 6 years | 36/1031 (3·5%) | 126/3509 (3·6%) | 162/4540 (3·6%) |
| Total | 55/8385 (0·7%) | 135/4540 (3·0%) | 190/12925 (1·5%) |
Data are n/N (%). In the PLCO trial, of 74 207 individuals who had ever smoked, 40 800 (55·0%) individuals were USPSTF2013 negative and had PLCOm2012 risk of less than 1·51% at 6 years. In this group, 189 lung cancers were observed in 6 years of follow-up (0·46% at 6 years). If this proportion and lung cancer rate are applied to the ILST sample, 7106 individuals would be added to the group of patients with USPSTF-negative and PLCOm2012 of less than 1·51% at 6 years and 13 more lung cancers would be expected in them in 2·3 years of follow-up. Supplemented data are PLCOm2012 risk threshold of at least 1·5% to less than 1·70% at 6 years (6/248) from observed ILST data and data estimates of PLCOm2012 less than 1·51% at 6 years (13/7106) from PLCO data. PLCO=Prostate Lung Colorectal and Ovarian Cancer Screening Trial. USPSTF=US Preventive Services Task Force.
Observed ILST data.
Data estimated from PLCO trial. ILST=International Lung Screening Trial.
Table 3.
Accuracy statistics for individuals selected for screening in the ILST by USPSTF2013 criteria and PLCOm2012 risk threshold of at least 1·70% at 6 years
| USPSTF eligible | PLCOm2012 ≥1·70% at 6 years | p value | |
|---|---|---|---|
| ILST data | |||
| Cancer detection rate* | 135/177 (76·3% [69·3–82·3]) | 162/177 (91·5% [86·4–95·2]) | pMcNemar=0·0001 |
| Cancer detection rate† | 135/171 (78·9% [72·1–84·8]) | 162/171 (94·7% [90·2–97·6]) | pMcNemar=0·0001 |
| Positive predictive value† | 135/4540 (2·97% [2·90–3·51]) | 162/4540 (3.57% [3·05–4·15]) | p=0·11 |
| False-negative proportion† | 42/1279 (3·28% [2·38–4·41]) | 15/1279 (1·17% [0·66–1·93]) | p=0·0003 |
| Negative predictive value | 96·72% | 98·83% | .. |
| Supplemented ILST data‡ | |||
| Cancer detection rate† | 135/190 (71·1% [64·0–77·4]) | 162/190 (85·3% [79·4–90·0]) | pMcNemar=0·0001 |
| Specificity† | 8330/12735 (65·4% [64·5–66·2]) | 8357/12735 (65·6% [64·7–66·4]) | p=0·72 |
| False-negative proportion‡ | 55/8385 (0·66% [0·49–0·85]) | 28/8385 (0·33% [0·22–0·48]) | p=0·0030 |
| Negative predictive value | 99·34% | 99·67% | .. |
Data are n/N (% [95% CI]), unless otherwise specified. ILST=International Lung Screening Trial. PLCO=Prostate Lung Colorectal and Ovarian Cancer Screening Trial. USPSTF=US Preventive Services Task Force.
Eligible PLCOm2012 risk threshold of at least 1·51% at 6 years.
Eligible by USPSTF or PLCOm2012 threshold of at least 1·70% at 6 years.
Statistics partly use data supplemented by PLCO trial estimates with PLCO data for USPSTF-negative and PLCOm2012 risk threshold less than 1·51% at 6 years.
Many study sites did not collect outcome data on lung cancer for individuals who were excluded because they did not meet either eligibility criteria. To estimate the number of individuals and lung cancers occurring in this group, we applied statistics obtained from PLCO data. We applied the incidence of lung cancer in participants who were USPSTF negative and met PLCOm2012 threshold of less than 1·51% at 6 years to the ILST study. 7106 individuals and 13 lung cancers in 2·3 years of follow-up were expected in those who were both USPSTF-negative and had PLCOm2021 risks of less than 1·51% at 6 years. These incorporations were in addition to the six lung cancers observed in 248 individuals who were USPSTF-negative and met PLCOm2012 threshold of at least 1·51% at 6 years and less than 1·70% at 6 years. Thus, the adjusted number of lung cancers expected overall was 190 in an estimated 12 925 individuals (table 2). For this quasi-population-estimated sample, the sensitivities (ie, cancer detection rate) of USPSTF2013 criteria and PLCOm2012 threshold of at least 1·70% at 6 years model were 135 (71·1% [95% CI 64·0–77·4]) of 190 individuals and 162 (85·3% [79·4–90·0]; p=0·0001) of 190 individuals, and the specificities were 8330 (65·4% [64·5–66·2]) of 12 735 individuals and 8357 (65·6% [64·7–66·4]; p=0·72; table 3) of 12 735, respectively.
In the analytic sample (n=5571), lung cancer was diagnosed in 98 (3·8%) of 2596 women and 73 (2·5%) of 2975 men (p=0·0050). Of 73 men diagnosed with lung cancer in the analysis sample, 63 (86.3% [95% CI 76·2–93·2%) met USPSTF2013 criteria and 68 (93·2% [95% CI 84·7–97·7%]) met the PLCOm2012 of at least 1·70% at 6 years threshold (ORMcNemar 2·00 [95% CI 0·62–7·46]; p=0·30). Of 98 women diagnosed with lung cancer in the analysis sample, 72 (73·5% [63·6–81·9) were USPSTF2013-positive, and 94 (95·9% [89·9–98·9]) met the PLCOm2012 of at least 1·70% at 6 years threshold (ORMcNemar 6·50 [2·26-25·63]; p<0·0001). The PLCOm2012 at least 1·70% at 6 years threshold was more sensitive at selecting women with lung cancer than the USPSTF2013 criteria.
Histological type distributions were similar between eligibility criteria (p<1·00; table 1). Overall, the most common histological type was adenocarcinoma, followed by squamous cell carcinoma, and small cell lung cancer. The proportion of non-small-cell lung cancers that were early stage (I, II) versus late stage (III, IV) did not differ by eligibility criteria (p=0·59).
In individuals diagnosed with lung cancer the mean life expectancy at study entry was higher in women than in men (16·8 years [95% CI 15·3–18·7] vs 11·7 years [10·6 −13·0]; p<0·0001). For individuals who were diagnosed with lung cancer and who were USPSTF2013-positive, the mean life expectancy at baseline was 14·8 years (13·6–16·0) and for individuals who had PLCOm2012 risk of 1·70% at 6 years or more it was 13·9 years (12·8–14·9). Cumulative estimated life expectancies from baseline for those subsequently diagnosed with lung cancer had they not developed lung cancer were higher in those who had PLCOm2012 risk of at least 1·70% at 6 years than individuals who were USPSTF2013-positive (2248·6 years [2089·6–2425·9] vs 2000·7 years [1841·2–2160·3]; difference 247·9 years, p=0·015).
Discussion
When the PLCOm2012 threshold was set to find the same number of individuals eligible as the USPSTF2013 criteria, cancer detection was significantly higher for PLCOm2012 than USPSTF2013 criteria (162 [94·7%] of 171 individuals vs 135 [78·9%] individuals; p=0·0001). When the ILST sample was supplemented to reflect population estimates of lung cancer rates in individuals who did not meet enrolment criteria, the sensitivity (cancer detection rate) of the USPSTF2013 criteria and the PLCOm2012 threshold of at least 1·70% at 6 years were 71·1% and 85·3%, respectively. It is important to note that the sample and lung cancers added to both the USPSTF2013-negative and PLCOm2012-negative group from estimates made using PLCO trial data do not affect criteria comparisons and conclusions. Study conclusions are based on the findings of the ILST sample alone. These data are presented only to provide magnitude estimates reflective of complete population-based sampling. The CREST study also compared the sensitivity of the two models in a large community-based clinical case series of lung cancers in Chicago (n=883).24 The USPSTF2013 criteria was less sensitive than the PLCOm2012 threshold of more than 1·70% at 6 years (52·3% vs 66·1%; p<0·0001). However, the absolute values of sensitivities are higher in ILST, which is a screening sample.
Although PLCOm2012 criteria detected significantly more lung cancers than did USPSTF criteria, this criterion also selected individuals who were slightly older and had more comorbidities. Consequently, the life expectancies of PLCOm2012 eligible individuals were shorter than USPSTF eligible individuals. Despite the shorter life expectancies in PLCOm2012-positive people, the excess number of lung cancers occurring in PLCOm2012-positive people led to significantly greater cumulative life expectancies in those diagnosed with lung cancer who were PLCOm2012-positive than in those diagnosed with lung cancer who were USPSTF-positive. Life expectancies were estimated using a model that incorporated important study variables of interest in the current study, such as age, sex, and comorbidities, as well as other important predictors.
Comparison of screening effectiveness by sex was not a prespecified hypothesis. However, USPSTF-associated sex disparities in eligibility have been identified.25 This study found that the PLCOm2012 threshold of at least 1·70% at 6 years identified more lung cancers in women than did the USPSTF2013 criteria. This observation is important because the NLST, NELSON, and LUSI trials suggest that women could benefit more from screening than men.3, 4, 32
Pilot studies, such as the Manchester Lung Health Check and the Ontario Health—Cancer Care Ontario pilot, have prospectively recruited individuals for screening using the PLCOm2012 model.16, 27 Eligibility criteria were successful because cancer detection rates were significantly higher than in the NLST or NELSON trials. However, these pilots did not make prospective comparisons with the USPSTF2013 criteria, nor did they compare life expectancies. The Yorkshire Lung Screening Trial33 is an ongoing large (n=62 980) randomised trial investigating the performances of PLCOm2012 (threshold ≥1·51%), Liverpool Lung Project (threshold ≥5%), and USPSTF2013 eligibility criteria for selection of screened participants. The trial will provide additional comparative assessment of the Liverpool Lung Project model that the ILST did not do.
Recently, USPSTF provided updated 2021 guidelines, which have lowered the eligibility age to 50 years and pack-years to at least 20 pack-years and maintained quit-years in those who used to smoke to 15 years or less. Our analysis is still relevant because other jurisdictions are using or considering using categorical age-smoking criteria similar to the USPSTF2013 criteria (eg, the European Union 4-in-the-Lung-Run study and the Korean lung cancer screening programme). In addition, the CREST study analysis of USPSTF2021 versus PLCOm2012 threshold of at least 1·0% at 6 years (equivalent threshold) found that sensitivity differences remained (68·6% vs 79·1%; p<0·0001).19, 20, 34 Expanding the USPSTF criteria by lowering age and pack-year entry thresholds is unlikely to overcome categoirical eligibility design limitations.
ILST recruitment was limited to the USPSTF2013 age criteria of 55–80 years. The PLCOm2012 criteria can additionally identify high-risk individuals and lung cancers outside this age range. The CREST study found that an additional 57 (6·5%) of 883 lung cancers that were USPSTF2013 ineligible because individuals were younger than 55 or older than 80 years would have qualified according to PLCOm2012 threshold of at least 1·70% at 6 years.24 Thus, the differential cancer detection rate presented in this Article might be an underestimate.
Compared with USPSTF criteria, the PLCOm2012 model with equivalent threshold has been shown to reduce Black versus White race disparities in lung cancer participant selection.24, 34 In this study, the number of minority race groups was too small to analyse separately.
This analysis reflects 2·3 years mean follow-up, not 6 years of follow-up for determining lung cancer risk. It is unlikely that with extended follow-up the conclusions would be reversed. Moreover, it has been argued that evaluating criteria differences after 2–3 years of follow-up better represents true differences than evaluation after a longer period of follow-up. The deterioration of prediction with time from predictor measurement occurs because after 6 years of follow-up, important baseline predictor values, such as smoking duration or quit-years, can be out of date, whereas in lung cancer screening programmes, risk assessments can be updated periodically to maintain risk estimate accuracy. In addition, within 2–3 years of enrolment into a screening programme, new information, such as screening results data, can augment risk prediction, thus making 6-year fixed baseline predictions out of date.35, 36
Follow-up data were collected for lung cancer outcomes in ineligible individuals only in some ILST sites. This information would have allowed population-based estimates of criteria sensitivities. To overcome this deficit, we applied statistics from the US multicentred quasi-population-based PLCO trial to estimate missing statistics in the ILST.
To our knowledge, this study is the first large, multinational trial to prospectively compare USPSTF2013 and PLCOm2012 criteria. Most previous studies have been retrospective, model-based, or microsimulation analyses. The prospective study design allowed for accurate and complete collection of risk model predictor data, which are commonly missing in retrospective studies. This design also enabled real-world collection of ages and comorbidities and thus potentially provided more valid estimates of life expectancies than might have been obtained using other approaches. We found that the mean age of entry into screening for USPSTF2013 eligible individuals was 63·3 years, not 55·6 years as reported for CISNET modelling.37 The younger age for those who are USPSTF eligible in CISNET is a consequence of using a single US birth cohort and individuals becoming eligible for screening from age 55 years onwards provided they met eligibility criteria at that age. Microsimulation analysis considering multiple birth cohorts that start screening in a single calendar year is expected to find average age of entry into screening to be similar to that observed in the ILST. Other studies, especially those promoting selection based on a life-years gained strategy, have estimated median or mean ages for those who are risk-model eligible to be substantially older, reducing their estimated life expectancies much further than observed in the ILST. For example, in the study by Cheung and colleagues,38 the mean age for those who were selected for screening by the risk-based strategy was 75 years. Interpretation of results coming from such studies and planning of future modelling studies should take into consideration ILST findings.
Our study findings indicate that selecting individuals for lung cancer screening on the basis of an accurate lung cancer risk prediction model, PLCOm2012, is superior to using USPSTF2013 criteria. The PLCOm2012 model has been adopted for use in the UK NHS Lung Health Check Programme and is in use or planned for use in multiple Canadian provincial programmes and projects (Ontario, Quebec, and British Columbia); it is recommended for an Australian lung cancer screening programme and is being studied for use in the EU 4-in-the-Lung-Run Study.26, 39, 40 The USPSTF has been reluctant to adopt lung cancer risk prediction models, arguing that there is insufficient evidence to support their use; this study might provide such evidence.
A common argument against using the PLCOm2012 model or a similar risk prediction model for determining screening eligibility is that it is too onerous to implement. A Health Technology Assessment27 carried out by Ontario Health-Cancer Care Ontario found that conducting a high-quality quantitative risk assessment took only slightly longer than NLST-like categorical criteria, and multiple focus groups found that physicians overwhelmingly preferred navigators to conduct risk assessments as well as risk-benefits discussions.27 Many reviewers of the evidence accept that high-quality risk prediction models are superior at identifying high-risk individuals compared with simple categorical age-smoking approaches. Future research should focus efforts on improving such models. Priorities are to develop models that predict risk accurately in Indigenous peoples, Hispanics, and Asians, and in East Asian individuals who have never smoked. Models are currently being developed that include occupation and biomarkers.
Instead of a PLCOm2012 threshold of at least 1·70% at 6 years, a threshold of at least 1·51% at 6 years might be considered for widespread public health screening practice for several reasons: mortality benefits can occur at this level;9 the USPSTF guidelines are shifting to a much more liberal threshold than what is equivalent to the PLCOm2012 threshold of at least 1·51% at 6 years;19 broadening eligibility criteria might improve screening uptake; and screening healthier individuals might increase beneficial outcomes (comorbidities increase and life expectancies decrease with higher scores of PLCOm2012).
Compared with the USPSTF2013 criteria, the PLCOm2012 risk prediction model at equivalent threshold (≥1·70% at 6 years) selected significantly more individuals for screening who were diagnosed with lung cancers and who had significantly more cumulative life expectancy from baseline. For selecting individuals to enrol into lung cancer screening programmes, from a public health and clinical perspective, using the PLCOm2012 model appears to be an evidence-based preferred option.
Data sharing
De-identified individual level data and the data dictionary will be made available to qualified researchers who present study protocols, which will require approval by an International Lung Screening Trial scientific steering committee. These data will only be made available from study sites at which the institution and ethics review board allow such release.
Declaration of interests
MCT developed the PLCOm2012 lung cancer risk prediction models, which is used in this study. The model is open access and is available free of charge to non-commercial users. For commercial users, licensing has been assigned to Brock University. MCT has not received any money for use of the PLCOm2012 model and does not anticipate any payments in the future. AM received travel or accommodation support for meetings by Roche, Olympus, and the International Association for the Study of Lung Cancer. JE received travel or accommodation support for meetings by Terry Fox Research Institute. MR received travel support from Takeda and American Thoracic Society. KF received travel support from various medical or scientific meeting organisers for participating or being a speaker (or both) and received additional grants or contracts from Olympus and Australian MRFF Next Generation Clinical Researchers Program and MeVis Medical Solutions AG/HealthInc; and payment or honoraria for lectures, presentations, and speaker's bureaus from Willey Cochrane Clinical Answers and is the Chair for Lung Cancer Consultative Group (unpaid) and a Council member. AT received consultancy fees from Olympus Respiratory America and additional grants or contracts from Biodesix Inc and Arch Biomedical Inc. CDB received consultancy fees from Mercy BioAnalytics, Grail, and Lucid Diagnostics and participated on a data safety monitoring board or advisory board for the International Lung Screening Trial. MR, DP, and DS received payment or honoraria for lectures, presentations, and speaker's bureaus by AstraZeneca. DS further received payment or honoraria for lectures, presentations, and speaker's bureaus from Bronchus Medical. MR further received additional grants or contracts provided by Grail. SL is an expert advisor and chair for Pan-Canadian Lung Cancer Screening Network and the Canadian Partnership Against Cancer. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The International Lung Screening Trial was funded by the Terry Fox Research Institute (Vancouver and Hong Kong sites), the Vancouver General Hospital and University British Columbia Hospital Foundation and the BC Cancer Foundation (Vancouver site), the Australian National Health and Medical Research Council (Australian Sites), the UK National Awareness and Early Diagnosis Initiative project grant by Cancer Research UK, the Roy Castle Lung Cancer Foundation for the UK Lung Screen Uptake Trial, and the Alberta Cancer Foundation (Alberta site). SLQ is supported by a CRUK postdoctoral fellowship (C50664/A24460). We thank the following individuals for their support in the study: Isaac Streit, Nancy Norton, David Chen, Jessica Luu, Anne Dybuncio, Jennifer Dickson, Helen Hall, Rommy Koetzler, Janelle Pellizzari, Jessica Culling, Gavin Armstrong, Raoul Pereira, Mike Bristow, John Macgregor, Andrew Lee, Tracy Elliot, Carmen Lydell, Lancia Guo, Niloofar Taghizadeh, James A Dickinson, Huiming Yang, Rachael O'Rouke, Katrina Dahl, Anne Williamson, Elizabeth McCaul (Brisbane, Jacqueline Logan, and Siobhan Dormer. We thank the National Cancer Institute for access to its data collected by the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by National Cancer Institute. We thank the PLCO screening centre investigators and the staff from Information Management Services and Westat, and the study participants.
Contributors
MCT, MR, AT, SC, PM, PB, AM, SM, DCLL, CDB, SMJ, KF, and SL conceived the study. MCT, MR, AT, RM, SA-K, RY, SC, JE, EB, PM, PB, SLQ, HM, IY, RB, LP, AM, FB, KPL, LM, SM, BS, MT, RS, YK, RM, LI, DS, MM, DP, PF, ES, DCLL, M-YN, VV, RJH, SMJ, KF, and SL contributed to data curation. MCT, SA-K, and SL verified the raw data. MCT, MR, S-AK, EB, PB, SLQ, HM, KL, LM, LI, SMJ, and SL analysed the data. MCT, MR, AT, JM, JY, SC, EB, PB, RB, AM, DCLL, RJH, SMJ, KF, and SL contributed to funding acquisition. MCT, MR, RM, S-AK, PB, AM, MT, SMJ, KF, and SL wrote the first draft of the manuscript. All authors critically reviewed the manuscript and agreed with the decision to submit for publication. All authors had full access to all the data in the study and MCT, SL, and SA-K take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–953. doi: 10.2147/CMAR.S187317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomised trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 5.Kovalchik SA, Tammemägi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tammemägi M, Church T, Hocking W, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyer VA, US Preventive Services Task Force Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services (CMS) Screening for lung cancer with low dose computed tomography (LDCT). CAG-00439N. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274&NcaName=Screening+for+Lung+Cancer+with+Low+Dose+Computed+Tomography+(LDCT)&MEDCACId=68&IsPopup=y&bc=AAAAAAAAAgAAAA %3d%3d&
- 9.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katki HA, Kovalchik SA, Petito LC, et al. Implications of nine risk prediction models for selecting ever-smokers for computed tomography lung cancer screening. Ann Int Med. 2018;169:10–19. doi: 10.7326/M17-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cressman S, Peacock SJ, Tammemägi MC, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol. 2017;12:1210–1222. doi: 10.1016/j.jtho.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Ten Haaf K, Jeon J, Tammemägi MC, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K, Husing A, Sookthai D, et al. Selecting high-risk individuals for lung cancer screening: a prospective evaluation of existing risk models and eligibility criteria in the German EPIC cohort. Cancer Prev Res. 2015;8:777–785. doi: 10.1158/1940-6207.CAPR-14-0424. [DOI] [PubMed] [Google Scholar]
- 14.Husing A, Kaaks R. Risk prediction models versus simplified selection criteria to determine eligibility for lung cancer screening: an analysis of German federal-wide survey and incidence data. Eur J Epidemiol. 2020;35:899–912. doi: 10.1007/s10654-020-00657-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber M, Yap S, Goldsbury D, et al. Identifying high risk individuals for targeted lung cancer screening: independent validation of the PLCOM2012 risk prediction tool. Int J Cancer. 2017;141:242–253. doi: 10.1002/ijc.30673. [DOI] [PubMed] [Google Scholar]
- 16.Crosbie PA, Balata H, Evison M, et al. Second round results from the Manchester ‘Lung Health Check' community-based targeted lung cancer screening pilot. Thorax. 2018;74:700–704. doi: 10.1136/thoraxjnl-2018-212547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tammemägi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;18:1523–1531. doi: 10.1016/S1470-2045(17)30597-1. [DOI] [PubMed] [Google Scholar]
- 18.Teles G, Macedo A, Chate R, Valente V, Funari M, Szarf G. LDCT lung cancer screening in populations at different risk for lung cancer. BMJ. 2020;7 doi: 10.1136/bmjresp-2019-000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: a collaborative modelling study for the US preventive services task force. JAMA. 2021;325:988–997. doi: 10.1001/jama.2021.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Preventive Services Task Force. Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 21.Lim KP, Marshall H, Tammemägi M, et al. Protocol and rationale for the International Lung Screening trial. Ann Am Thorac Soc. 2020;17:503–512. doi: 10.1513/AnnalsATS.201902-102OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremblay A, Taghizadeh N, MacGregor JH, et al. Application of lung-screening reporting and data system versus pan-Canadian early detection of lung cancer nodule risk calculation in the Alberta Lung Cancer Screening study. J Am Coll Radiol. 2019;16:1425–1432. doi: 10.1016/j.jacr.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Quaife SL, Ruparel M, Beeken RJ, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and ‘hard-to-reach’ patients. BMC Cancer. 2016;16:281. doi: 10.1186/s12885-016-2316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States preventive services task force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15:1738–1747. doi: 10.1016/j.jtho.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Addressing gender disparities in lung cancer screening eligibility: USPSTF versus PLCOm2012 criteria. Chest. 2021 doi: 10.1016/j.chest.2021.06.066. published online July 9. [DOI] [PubMed] [Google Scholar]
- 26.National Health Service England: National Cancer Programme Targeted screening for lung cancer with low radiation dose computed tomography—standard protocol prepared for the targeted lung health checks programme. January 2019. https://www.england.nhs.uk/wp-content/uploads/2019/02/targeted-lung-health-checks-standard-protocol-v1.pdf
- 27.Tammemägi MC, Darling GE, Schmidt H, et al. Selection of individuals for lung cancer screening based on risk prediction model performance and economic factors—the Ontario experience. Lung Cancer. 2021;156:31–40. doi: 10.1016/j.lungcan.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 28.McWilliams A, Tammemägi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumours: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 30.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 32.Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening—results from the randomized German LUSI trial. Int J Cancer. 2020;146:1503–1513. doi: 10.1002/ijc.32486. [DOI] [PubMed] [Google Scholar]
- 33.Crosbie PA, Gabe R, Simmonds I, et al. Yorkshire Lung Screening Trial (YLST): protocol for a randomised controlled trial to evaluate invitation to community-based low-dose CT screening for lung cancer versus usual care in a targeted population at risk. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-037075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Brief report: risk prediction model versus United States Preventive Services Task Force 2020 draft lung cancer screening eligibility criteria—reducing race disparities. J Thorac Oncol. 2021;2 doi: 10.1016/j.jtocrr.2020.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patz EF, Jr, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol. 2016;17:590–599. doi: 10.1016/S1470-2045(15)00621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tammemägi MC, Ten Haaf K, Toumazis I, et al. Development and validation of a multivariable lung cancer risk prediction model that includes low-dose computed tomography screening results: a Secondary analysis of data from the National Lung Screening trial. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ten Haaf K. Cost-effective implementation of lung cancer screening in Europe. IASLC CT Screening Symposium: Forefront Advances in Lung Cancer Screening May 7–8, 2021: International Association for the Study of Lung Cancer. 2021. https://www.iaslc.org/meetings-webinars/iaslc-2021-ct-screening-symposium
- 38.Cheung LC, Berg CD, Castle PE, Katki HA, Chaturvedi AK. Life-gained-based versus risk-based selection of smokers for lung cancer screening. Ann Internal Med. 2019;171:623–632. doi: 10.7326/M19-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cancer Australia Report on the lung cancer screening enquiry. https://www.canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/report-lung-cancer-screening-enquiry
- 40.European Commission: CORDIS EU research results 4-IN THE LUNG RUN: towards INdividually tailored INvitations, screening INtervals, and INtegrated co-morbidity reducing strategies in lung cancer screening. 2020. https://cordis.europa.eu/project/id/848294
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual level data and the data dictionary will be made available to qualified researchers who present study protocols, which will require approval by an International Lung Screening Trial scientific steering committee. These data will only be made available from study sites at which the institution and ethics review board allow such release.