Abstract
Coronary artery disease (CAD) remains the leading cause of death worldwide. Expanding patients' metabolic phenotyping beyond clinical chemistry investigations could lead to earlier recognition of disease onset and better prevention strategies. Additionally, metabolic phenotyping, at the molecular species level, contributes to unravel the roles of metabolites in disease development. In this cross-sectional study, we investigated clinically healthy individuals (n = 116, 65% male, 70.8 ± 8.7 years) and patients with CAD (n = 54, 91% male, 67.0 ± 11.5 years) of the COmPLETE study. We applied a high-coverage quantitative liquid chromatography-mass spectrometry approach to acquire a comprehensive profile of serum acylcarnitines, free carnitine and branched-chain amino acids (BCAAs), as markers of mitochondrial health and energy homeostasis. Multivariable linear regression analyses, adjusted for confounders, were conducted to assess associations between metabolites and CAD phenotype. In total, 20 short-, medium- and long-chain acylcarnitine species, along with L-carnitine, valine and isoleucine were found to be significantly (adjusted p ≤ 0.05) and positively associated with CAD. For 17 acylcarnitine species, associations became stronger as the number of affected coronary arteries increased. This implies that circulating acylcarnitine levels reflect CAD severity and might play a role in future patients' stratification strategies. Altogether, CAD is characterized by elevated serum acylcarnitine and BCAA levels, which indicates mitochondrial imbalance between fatty acid and glucose oxidation.
Keywords: metabolomics, coronary artery disease, carnitine, acylcarnitine, branched-chain amino acids, fatty acid oxidation (FAO), mitochondria
Introduction
Coronary artery disease (CAD) remains the leading cause of death worldwide (1). In spite of this, in clinical practice, patients' biochemical stratification is still mainly limited to total cholesterol and triglyceride quantification (2). Following recent advances in mass spectrometry and bioinformatics, high-throughput and high-coverage metabolomic approaches now provide more precise metabolic profiling at the molecular species level (3). Upgrading patients' metabolic phenotyping could lead to earlier recognition of disease onset, optimization of prevention strategies and health monitoring, ultimately reducing CAD-related burden (4, 5). In addition to biomarker discovery, patients' metabolic phenotyping is of utmost importance to decipher the roles of metabolites in disease development (6).
Metabolites, including acylcarnitines and amino acids, have long been used to diagnose inborn errors of metabolism (7, 8). Recently, acylcarnitines have been suggested as potential biomarkers of cardiometabolic diseases (9). Specifically, elevated acylcarnitines levels were observed in patients with type 2 diabetes (10, 11), heart failure (12–14), and in middle-aged adults and elderly with a combination of different cardiovascular diseases (15, 16). Additionally, circulating acylcarnitines were associated with the risk of myocardial infarction and cardiovascular death in individuals with stable angina pectoris (17) as well as with the risk of cardiovascular events at 3-year follow-up in patients with CAD (18, 19). Similarly, elevated serum concentration of branched-chain amino acids (BCAAs), whose metabolism is tightly related to that of short-chain acylcarnitines, was observed in patients with insulin resistance (20–22), obesity (23, 24), diabetes (25–27), dyslipidemia, and CAD (19, 28, 29). These studies, however, investigated patients already suffering from cardiometabolic diseases without including healthy controls (12, 13, 15, 17–19, 23–28) or with poorly characterized controls (14, 16, 20, 22, 29). Comparing diseased against healthy metabolic signature is essential to reveal disease-associated alterations and to improve our understanding of metabolites' roles in pathophysiological processes (30, 31). Furthermore, these studies investigating a limited number of acylcarnitines with a maximum of 12 species detected (19). This number can now be outranged due to technological advancements (32).
Acylcarnitines play a key role in the transport of fatty acids longer than 10 carbon atoms (C) across mitochondrial membranes for oxidation (Figure 5) (37, 46). Reduced oxygen availability impairs fatty acid oxidation, with consequent accumulation of acyl-CoA and acylcarnitines in mitochondria (40, 47, 48). Accumulation of long-chain acylcarnitines further disrupts membrane function and energy metabolism, which ultimately leads to cellular stress, inflammation, insulin resistance and cardiac arrhythmias (32, 44, 45). In contrast to medium- and long-chain acylcarnitines (C6–C22), whose accumulation is mainly related to impaired fatty acid oxidation, short- and odd-chained acylcarnitines (C3 and C5) usually derive from disrupted BCAA catabolism (Figure 5) (43–45). Thus, acylcarnitines and BCAAs, which appear to be promising indicators of mitochondrial function and cardiometabolic health, could be used to improve patients' metabolic phenotyping.
This cross-sectional population-based study quantified a large panel of circulating short-, medium- and long-chain acylcarnitines as well as BCAAs within well-characterized clinically healthy individuals and patients with CAD of the COmPLETE study (49). Our approach applied a high-coverage targeted hydrophilic interaction liquid chromatography coupled with high resolution mass spectrometry (HILIC-HRMS) (32). The acquired comprehensive metabolic profiles allowed us to identify a set of acylcarnitines and BCAAs associated with CAD.
Materials and Methods
Study Design and Participants
Subsets of the COmPLETE-Health (n = 116, mean age 70.8 ± 8.7 years old, 65% male) and COmPLETE-Heart samples (n = 54, mean age 67.0 ± 11.5 years old, 91% male) were investigated. As reported in the study protocol, only clinically healthy participants from the Basel area (Switzerland), who had no exercise-limiting chronic diseases and were non-smokers (or had quit at least 10 years previously) were included in the COmPLETE-Health sample. This excluded participants with a history of CAD, stroke, heart failure, lower-extremity artery disease, any kind of malignant tumor, diabetes, obesity, clinically apparent kidney failure, severe liver disease, chronic obstructive pulmonary disease GOLD stages two to four, arterial hypertension grades two and three, drug or alcohol abuse, exercise-limiting osteoporosis or musculoskeletal conditions and clinically manifest Alzheimer's disease or dementia. The investigated subset of the COmPLETE-Heart sample consisted exclusively of patients suffering from CAD, diagnosis of which was confirmed by senior cardiologists. Patients with unstable angina pectoris, uncontrolled brady- or tachyarrythmia, permanent atrial fibrillation, severe valvular disease, acute myocardial infarction, transient ischemic attack, or stroke in the last 3 months were excluded. The exact recruitment procedure and the full list of inclusion and exclusion criteria can be found in the COmPLETE study protocol (49). The COmPLETE study was funded by the Swiss National Science Foundation (Grant No. 182815) and approved by the Ethics Committee of North-Western and Central Switzerland (EKNZ 2017-01451). A written informed consent document was obtained from all participants prior to inclusion.
Data Sources
Data collection was carried out between January 2018 and June 2019. Medical history and medication were reviewed by a physician using a standardized questionnaire. Participants were randomly allocated to one of five time slots (08:00, 10:00, 12:00, 14:00 and 16:00) for the measurements, which took around 4 h in total. They were instructed not to diverge from habitual eating behavior (for the previous 72 h), to avoid exercising, drinking alcohol (for the previous 24 h) and drinking caffeinated beverages (for the previous 4 h). On the day of sample collection, participants took the prescribed medication as usual. Fasting blood samples (at least 3 h fasting time) were collected before any kind of measurements involving physical activity. Trained medical staff collected serum samples (2 × 7.5 mL serum-gel, Monovette®, Sarstedt, Nümbrecht, Germany) by venipuncture in the cubital fossa. Serum samples were slightly shaken for 30 min, centrifuged (3,000 rpm; 10 min; 20–23°C) and aliquoted before being frozen at −80°C.
Anthropometric Values
Height and body mass were measured to the nearest 0.5 cm and 0.1 kg, respectively. Body mass index was calculated as kg/m2. Body composition was analyzed using a four-segment bioelectrical impedance analysis (Inbody 720, Inbody Co. Ltd., Seoul, South Korea). Using InBody 720, -as opposed to dual-energy x-ray absorptiometry analysis, to measure appendicular muscle mass was judged to be acceptable (50). After a rest phase of 10 min, resting systolic and diastolic blood pressures and resting heart rate were measured in supine position using a non-invasive vascular screening system (VaSera VS-1500 N; Fukuda Denshi, Tokyo, Japan). The peak oxygen uptake (VO2 peak) was used as marker for the cardiorespiratory fitness and was determined during an exercise test until maximal exertion (i.e., volitional exertion, dyspnea, or fatigue) using an electromagnetically braked cycle ergometer (Ergoselect 200; Ergoline, Bitz, Germany) and a computer-based system (MetaMax 3B; Cortex Biophysik GmbH, Leipzig, Germany). VO2 peak was defined as the highest 30 s average of VO2 at any point of the test.
Biochemical Analysis
Serum samples were analyzed for triglyceride, total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol concentrations using an Olympus AU680 automatic analyzer (Beckman Coulter, Brea, CA, USA), enzymatic reagents (DiaSys, Holzheim, Germany) and secondary standards (Roche Diagnostics, Mannheim, Germany). For glycated hemoglobin (HbA1c), whole blood was analyzed by high pressure liquid chromatography (HPLC) using D-10 (Bio-Rad, Hercules, CA, USA). NT-proBNP was determined using a chemiluminescent microparticle immunoassay (Architect, Abott, IL, United States).
Metabolic Profiling
A large panel of carnitine related metabolites, including free carnitine, deoxycarnitine and 36 acylcarnitine species (Supplementary Table 1) were targeted in serum samples. BCAAs were also measured, as C3- and C5-acylcarnitine species are byproducts of the BCAA catabolism (43–45). Analysis was conducted at the Metabolomics Platform, Faculty of Biology and Medicine, University of Lausanne (Switzerland). A detailed description of the method used is available (32).
Sample Preparation
For absolute quantification of acylcarnitines and BCAAs, samples were prepared by mixing 20 μL of serum with 250 μL of ice-cold methanol spiked with internal standard (IS) solution of corresponding isotopically labeled acylcarnitines and BCAAs (Supplementary Table 1), which was completed to 300 μL with 0.1% formic acid in water. Samples were then mixed by shaking and centrifuged for 15 min at 4°C and 2,700 g. The resulting supernatants were transferred to LC-MS vials prior to injection.
Metabolite Quantification
Extracted samples were analyzed by HILIC-HRMS in full scan MS mode using a Q Exactive™ Hybrid Quadrupole-Orbitrap interfaced with the ultra-high-performance liquid chromatography (UHPLC) Vanquish Horizon (Thermo Fisher Scientific) as previously described (32). Metabolites were separated using an ethylene bridged hybrid (BEH) amide column (1.7 μm, 100 mm × 2.1 mm I.D.) (Waters, MA, US) in positive ionization mode. The mobile phase was composed of A = 20 mM ammonium formate and 0.1% formic acid (FA) in water and B = 0.1% FA in acetonitrile (ACN). The gradient elution started at 95% B (0–2 min) decreasing to 65% B (2–14 min), reaching 50% B at 16 min and was followed by an isocratic step (16–18 min) before a 4 min post-run for column re-equilibration. The flow rate was 400 μL/min, column compartment 25°C and the sample injection volume was 2 μl. Heated electrospray ionization (HESI) source conditions were set as follows; sheath gas flow at 60, aux gas flow rate at 20, sweep gas flow rate at 2, spray voltage at +3 kV, capillary temperature at 300°C, s-lens RF level at 60 and aux gas heater temperature at 300°C. Full scan HRMS data was acquired over the m/z range 50–750, with the following MS acquisition parameters; mass resolving power at 70,000 full width at half maximum (FWHM), 1 microscan, 1e6 automatic gain control (AGC) and maximum inject time at 100 ms.
Data Processing and Analysis
Raw data files were processed using Xcalibur 4.1 (Thermo Fisher Scientific). Peak was manually curated and corrected if necessary. For absolute quantification, calibration curves and the stable isotope spike (or internal standard spike) at known concentration were used to report the concentrations quantified in each serum sample. Linearity of the standard curves was evaluated for each metabolite using 11-point range. A human plasma standard reference material (Certificate of Analysis, NIST 1950) was analyzed within each batch of samples and used as a quality control for the validation of measurement accuracy.
Statistical Methods
Metabolite concentrations were log2-transformed and z-standardized prior to statistical analysis. Multiple linear regressions were run to assess associations between metabolites and CAD phenotype. To determine which confounders required adjustment for regressions, directed acyclic graph (DAG) were drawn (Supplementary Figure 1) (51, 52). Metabolites were used as dependent variables, while CAD phenotype and confounders served as independent variables. Regressions using acylcarnitines or carnitine as dependent variable were adjusted for the following confounders age (53, 54), sex (55), HbA1c (%) (11, 56, 57), body fat (%) (11, 58, 59), smoking habits (60), antihypertensive and lipid lowering medication (61–64) as well as fasting and sampling time (Supplementary Figure 1) (65–67). Regressions using BCAAs as dependent variable were adjusted for the following confounders: age, sex (67–70), skeletal muscle mass (71, 72), smoking habits (60, 73), sampling time and fasting time (Supplementary Figure 2) (65–67).
Two sets of multiple linear regressions were run. In the first set, CAD was defined as a categorical two-level variable opposing sickness vs. health. In the second set, CAD was defined as the number of stenosed coronary arteries (0, 1, 2, or 3). As the concentrations of deoxycarnitine, hydroxyhexanoylcarnitine (C6:0-OH), hydroxydodecanoylcarnitine (C12:0-OH) and arachidonylcarnitine (C20:4) were below the quantification limit (0.003 μM) of the HILIC-HRMS method employed for some participants (deoxycarnitine n=1, hydroxyhexanoylcarnitine n = 2, hydroxydodecanoylcarnitine n = 1, arachidonylcarnitine n = 1), a Tobit regression using the CensReg R package was applied for these metabolites to estimate regression coefficients in the presence of left censored values (74).
Graphical methods were used to assess linearity, normal distribution, and homoscedasticity of data. P-values were adjusted using the Benjamini-Hochberg (BH) method, separately within each set of multiple linear regression (75). Adjusted p ≤ 0.05 were considered as significant. Statistical analyses were carried out using R (version 4.0.2) (76). Rain plots were computed using a previously published R-code (77).
Results
Participants' Characteristics
The investigated subjects consisted of 116 healthy individuals (70.8 ± 8.7 years, 65% male) and 54 patients with confirmed CAD (67 ± 11.5 years, 91% male) (Table 1). The clinically healthy individuals had normal body mass index (BMI) and HbA1c values. (78). Mean blood pressure values were in the high normal range, with 20% of participants treated for arterial hypertension grade one (79). Sixty-six percent of the clinically healthy individuals had never smoked, whereas 34% had quit smoking more than 10 years previously. Both clinically healthy controls and CAD patients were characterized by elevated LDL-cholesterol according to the 2019 ESC/EAS guidelines on primary (clinically healthy participants) and secondary (CAD patients) prevention (2). Eighty nine percentage of the CAD patients were under statin, compared to only 8% of the clinically healthy participants, which could explain the lower levels of LDL-cholesterol in the CAD patients. CAD patients displayed elevated NT-proBNP levels and a mean BMI value in the overweight range, while HbA1c, triglyceride and HDL-cholesterol levels as well as systolic and diastolic blood pressure values were normal (2, 78–80). It is worth noting that all CAD patients were on antihypertensive medications. Additionally, 50% of CAD patients were non-smokers, while 20% ceased smoking at least 10 years ago. Fasting duration was of at least 3 h with mean of 8.5 ± 5.3 h for those with CAD and 6.7 ± 3.0 h for clinically healthy individuals.
Table 1.
Participants' characteristics.
| Clinically healthy | CAD | P-value (t-test or Mann Whitney U test) | P-value (chi-squared test | |
|---|---|---|---|---|
| Participants, n (%) | 116 (68) | 54 (32) | ||
| Anthropometry, mean ± SD | ||||
| Age (years) | 70.8 ± 8.7 | 67.0 ± 11.5 | 0.034 | |
| Male (%) | 64.7% | 90.7% | <0.001 | |
| Body mass (kg) | 67.0 ± 10.4 | 84.0 ± 14.9 | <0.001 | |
| Body fat (%) | 27.9 ± 6.9 | 30.2 ± 6.8 | 0.043 | |
| Lean mass (kg) | 26.3 ± 5.1 | 32.2 ± 5.5 | <0.001 | |
| BMI (kg/m2) | 24.0 ± 2.8 | 27.8 ± 4.1 | <0.001 | |
| Systolic blood pressure (mmHg) | 131.8 ± 13.2 | 126.7 ± 15.4 | 0.029 | |
| Diastolic blood pressure (mmHg) | 80.8 ± 8.3 | 77.3 ± 10.8 | 0.024 | |
| VO2peak (L/min) | 1.86 ± 0.53 | 1.82 ± 0.60 | 0.629 | |
| Smoking status, n (%) | ||||
| Never smoked | 76 (66) | 27 (50) | 0.019 | |
| Smokers | 0 (0) | 6 (11) | <0.001 | |
| Ex-smokers (quit <10 years ago) | 40 (34) | 11 (20) | <0.001 | |
| Ex-smokers (quit >10 years ago) | 0 (0) | 10 (19) | 0.110 | |
| Biochemical parameters, mean ± SD | ||||
| Fasting duration prior to blood sampling (h) | 6.7 ± 3.0 | 8.5 ± 5.3 | 0.522 | |
| Total cholesterol (mmol/L) | 6.17 ± 1.04 | 4.12 ± 0.82 | <0.001 | |
| LDL-C (mmol/L) | 3.46 ± 0.68 | 2.16 ± 0.52 | <0.001 | |
| HDL-C (mmol/L) | 3.47 ± 0.68 | 2.16 ± 0.52 | <0.001 | |
| Triglycerides (mmol/L) | 1.37 ± 0.76 | 1.50 ± 0.96 | 0.611 | |
| HbA1c (%) | 5.4 ± 0.3 | 6.1 ± 0.7 | <0.001 | |
| NT-ProBNP (pg/ml) | 145.5 ± 110.4 | 603.0 ± 651.4 | <0.001 | |
| Comorbidities | ||||
| Hypertension | 23 (20) | 54 (100) | <0.001 | |
| No of coronary artery with stenosis, n (%) | ||||
| 0 | 116 (100) | 0 (0) | ||
| 1 | 0 (0) | 12 (22) | ||
| 2 | 0 (0) | 15 (28) | ||
| 3 | 0 (0) | 24 (44) | ||
| Not known | 0 (0) | 3 (6) | ||
| Diabetes mellitus | 0 (0) | 10 (19) | <0.001 | |
| Cardiovascular medications, n (%) | ||||
| Antihypertensive | 23 (20) | 54 (100) | <0.001 | |
| ACE inhibitors | 3 (3) | 32 (59) | <0.001 | |
| Angiotensin receptor blockers (ARBs) | 19 (16) | 18 (33) | <0.001 | |
| Amlodipin | 6 (5) | 7 (13) | 0.008 | |
| Beta-blockers | 4 (3) | 43 (80) | <0.001 | |
| Statins | 9 (8) | 48 (89) | <0.001 | |
| Diabetes medications, n (%) | ||||
| Oral antidiabetic drugs | 0 (0) | 9 (17) | <0.001 | |
| Insulin | 0 (0) | 6 (11) | <0.001 | |
| Other medications, n (%) | 52 (45) | 52 (96) | ||
A Student's t-test was performed to compare body fat, lean mass, BMI, systolic and diastolic blood pressure between clinically healthy and sick individuals. Other continuous variables were compared using a Mann-Whitney U test. A chi-squared. BMI, body mass index; LDL-C, low density lipoprotein cholesterol; HDL-C, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; NT-proBNT, N-terminal (NT)-pro hormone B-type natriuretic peptide. Other drugs include: Acetylsalicylic acid (57), diuretics (36), anticoagulants/antiplatelets (32), vitamins (32), proton-pump inhibitors (30), chondroitinsulfat (13), lipid-lowering drugs except statins (11), non-steroidal anti-inflammatory drugs (11), thyroid hormones (11), topical ophthalmic drugs (11), estrogen/hormone replacement therapy (9), 5α-reductase inhibitors (7), paracetamol (5), uricostatic drugs (5), antidepressants (3), antihistamines (3), bisphosphonate (3), ginkgo (3), non-benzodiazepine benzodiazepine receptor agonists (3), fluticasone/salmeterol (2), prednisolone (2), pregabalin (2), benzodiazepine (1), febuxostatum (1), fluticasone/vilanterol (1), gabapentin (1), L-dopa/benserazid (1), melatonin (1), mesalazine (1), molsidomin (1), mometasone (1), polystyrene sulfonate (1), tamsulosin (1), topic fluticasone (1), tiotropium (1), rifamycin (1).
Associations Between Circulating Acylcarnitines and CAD
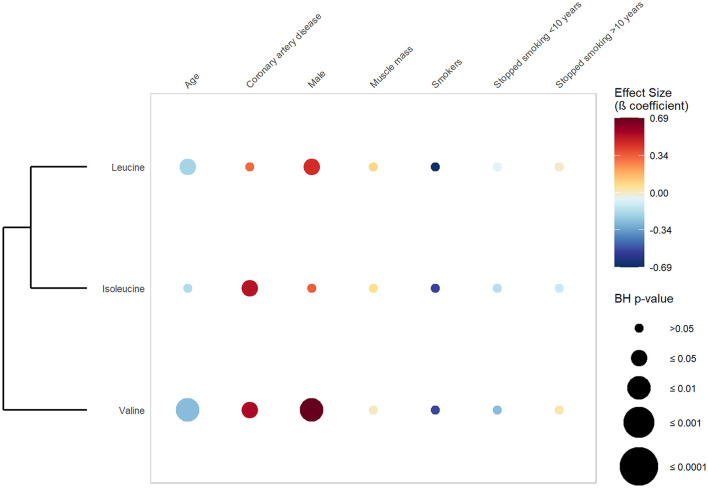
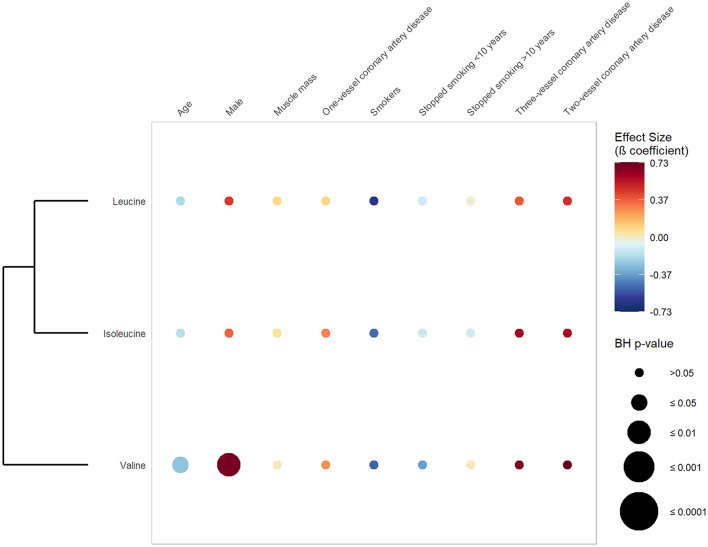
Figure 1 exhibits the results of the first set of regression, in which CAD was defined as a dichotomous variable opposing CAD vs. absence of CAD. It shows that 20 out of 30 quantified carnitines (seven short-chain, eight medium-chain and five long-chain acylcarnitines) were significantly and positively associated with CAD phenotype after adjustment for confounders (Table 2, Supplementary Table 2, Supplementary Figure 3). Hexanoylcarnitine (C6:0) showed the strongest positive association with CAD (β-coefficient 1.02; BH p ≤ 0.004) followed by palmitoylcarnitine (C16:0) (β-coefficient 1.02; BH p-value 0.002), and hexadecenoylcarnitine (C16:1) (β-coefficient 0.96; BH p-value 0.003) (Supplementary Table 2). Figure 2 represents the results of the second set of regressions, in which CAD was defined as the number of stenosed coronary arteries (zero-, one-, two- and three-vessel coronary artery disease). It shows that the strength of association (β-coefficient) increased with increasing number of affected coronary arteries for 17 acylcarnitines (3 short-, 8 medium- and 6 long-chain acylcarnitine species) (Supplementary Table 2). Besides, individuals with CAD had significant elevated levels of two short-chain hydroxylated acylcarnitines (C4:0-OH and C5:0-OH) and dicarboxylic acylcarnitine suberoylcarnitine (C8:0-DC) (Supplementary Table 2).
Figure 1.
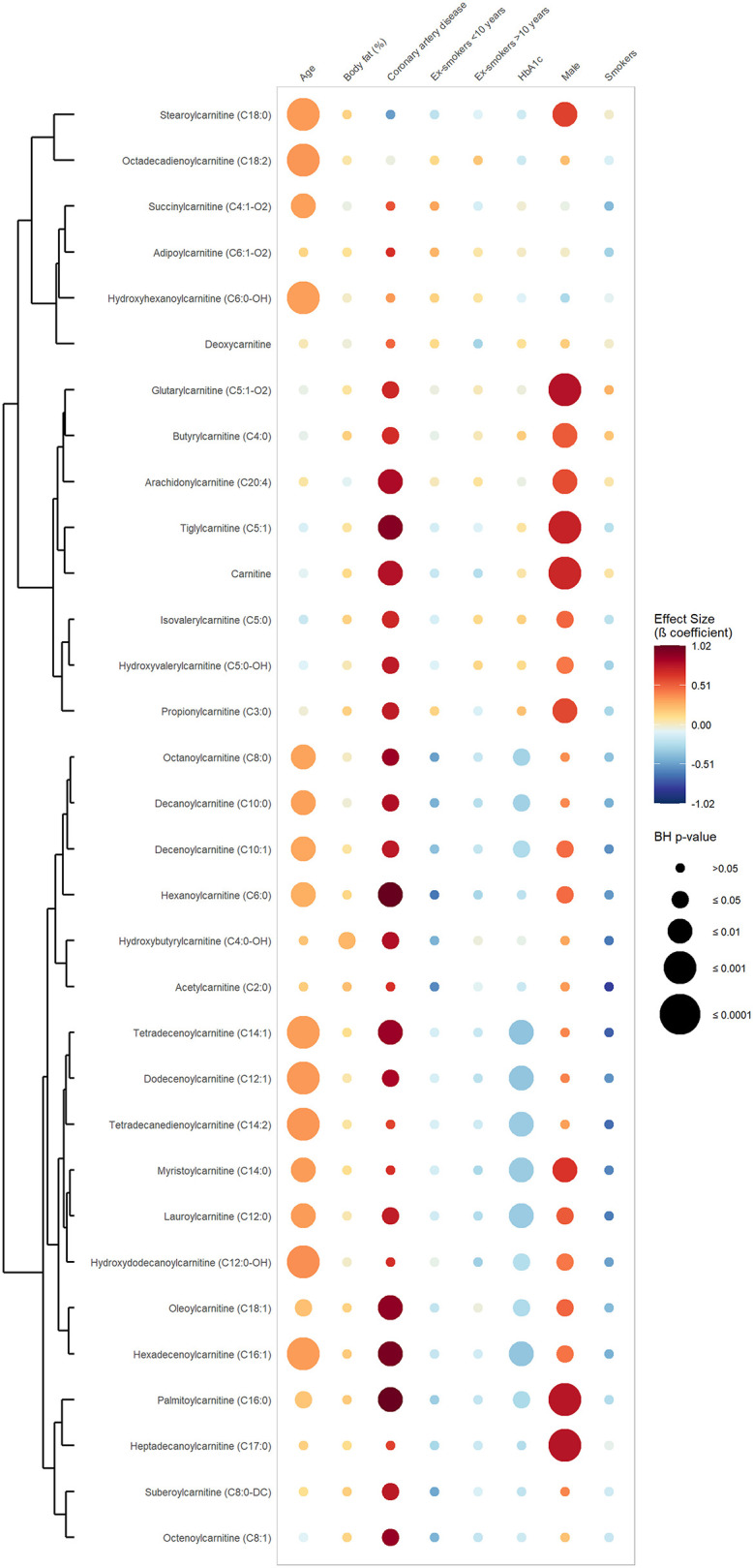
Associations between serum carnitine/acylcarnitine species, coronary artery disease and selected cardiovascular risk factors. This rainplot represents the results of the first set of regression, in which metabolites were used as dependent variables (vertical axis), while CAD phenotype (two-level variable opposing sickness vs. health) and confounders served as independent variables (horizontal axis). The redder the dots the higher the beta coefficient and the bigger the dot the smaller the adjusted p-value. A clustering has been done regrouping the metabolites with similar beta-coefficients and adjusted p-values. BH, Benjamini-Hochberg; HbA1c, glycated hemoglobin.
Table 2.
Quantified metabolites.
| Abbreviation | Targeted metabolites | |
|---|---|---|
| C0 | Carnitine | |
| Deoxycarnitine | ||
| Short-chain (n = 9) | C2:0 | Acetylcarnitine |
| C3:0 | Propionylcarnitine | |
| C4:0 | Butyrylcarnitine | |
| C4:0-OH | Hydroxybutyrylcarnitine | |
| C4:1-O2 | O-succinylcarnitine | |
| C5:0 | Isovalerylcarnitine | |
| C5:0-OH | 3-Hydroxyvalerylcarnitine | |
| C5:1 | Tiglylcarnitine | |
| C5:1-O2 | Glutarylcarnitine | |
| Medium-chain (n = 11) | C6:0 | Hexanoylcarnitine |
| C6:0-OH | 3-Hydroxyhexanoylcarnitine | |
| C6:1-O2 | Adipoylcarnitine | |
| C8:0 | Octanoylcarnitine | |
| C8:0-DC | Suberoylcarnitine | |
| C8:1 | 2-Octenoylcarnitine | |
| C10:0 | Decanoylcarnitine | |
| C10:1 | Trans-2-decenoylcarnitine | |
| C12:0 | Lauroylcarnitine (dodecanoylcarnitine) | |
| C12:0-OH | 3-Hydroxydodecanoylcarnitine | |
| C12:1 | Trans-2-dodecenoylcarnitne | |
| Long-chain (n = 10) | C14:0 | Myristoylcarnitne (tetradecanoylcarnitine) |
| C14:1 | Trans-2-tetradecenoylcarnitine | |
| C14:2 | Cis, cis-5,8-tetradecanedienoylcarnitine | |
| C16:0 | Palmitoylcarnitine (hexadecanoylcarnitine) | |
| C16:1 | Trans-2-hexadecenoylcarnitine | |
| C17:0 | Heptadecanoylcarnitine | |
| C18:0 | Stearoylcarnitine (octadecanoylcarnitine) | |
| C18:1 | Oleoylcarnitine (octadecenoylcarnitine) | |
| C18:2 | Cis, cis-9,12- octadecadienoylcarnitine | |
| C20:4 | Arachidonylcarnitine | |
| BCAA | Leucine | |
| Isoleucine | ||
| Valine |
Acylcarnitines can be categorized depending on the number of carbon atoms of their acyl-group into short-chain (C2–C5), medium-chain (C6–C13), and long-chain (C14–C21) acylcarnitines (34, 80). C, number of carbon atoms of the acyl-group; DC, dicarboxyl; OH, Hydroxy; BCAA, branched-chain amino acid.
Figure 2.
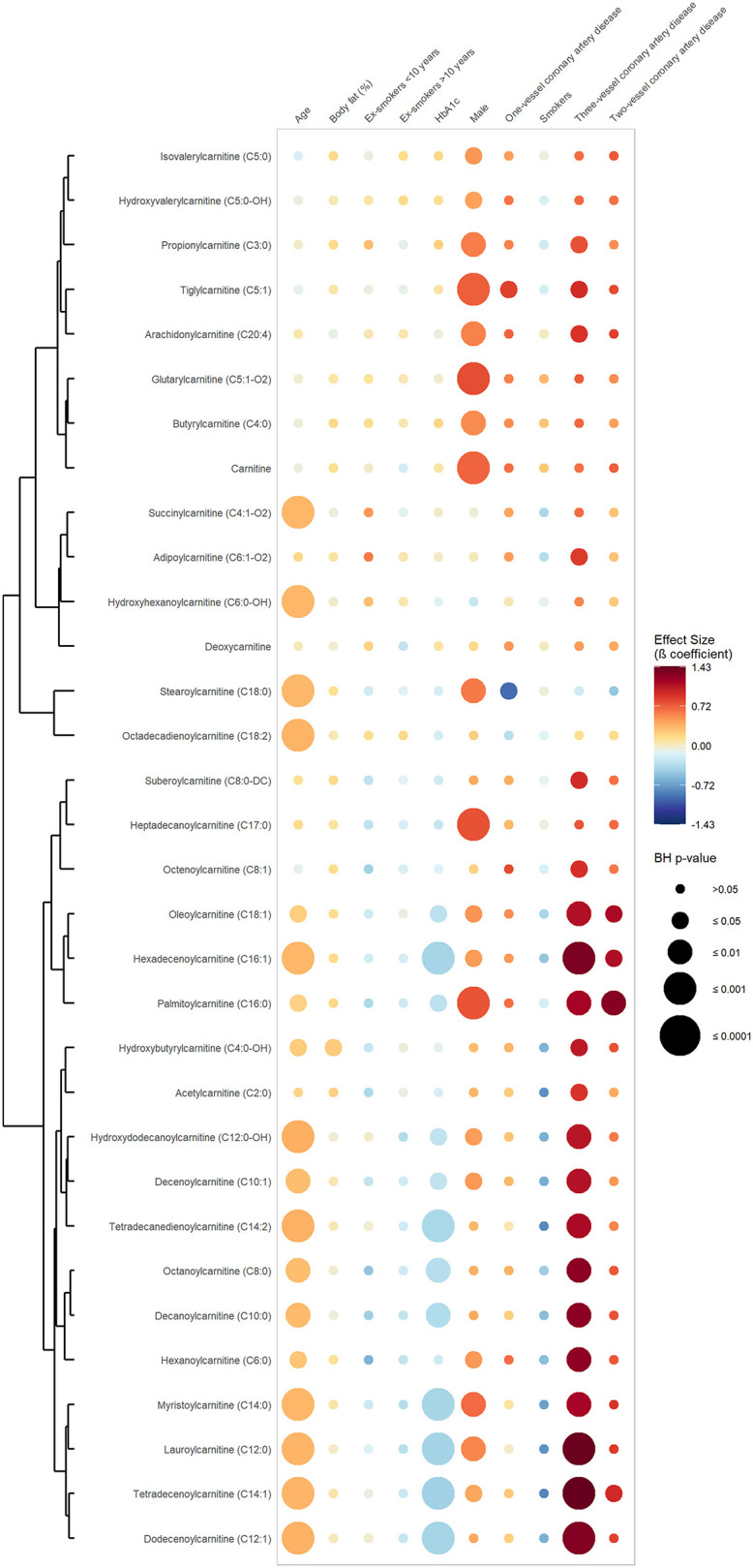
Associations between serum carnitine/acylcarnitine species, number of stenosed coronary arteries and selected cardiovascular risk factors. This rainplot represents the results of the second set of regression, in which metabolites were used as dependent variables (vertical axis), while the number of stenosed coronary arteries (0, 1, 2, or 3) and confounders served as independent variables (horizontal axis). The redder the dots the higher the beta coefficient and the bigger the dot the smaller the adjusted p-value. A clustering has been done regrouping the metabolites with similar beta-coefficients and adjusted p-values. BH, Benjamini-Hochberg; HbA1c, glycated hemoglobin.
Regarding the cardiometabolic risk factors used as confounders, age showed significant and positive associations with more than 50% of the measured acylcarnitine species (one short-, eight medium- and eight long-chain species, including two hydroxylated acylcarnitines). Six medium-chain and six long-chain acylcarnitines were found to be significantly and negatively associated with HbA1c, while no significant association was found between acylcarnitines and smoking status.
The BCAA Signature of CAD
Figure 3 exhibits the results of the first set of regression, in which CAD was defined as a dichotomous variable opposing CAD vs. absence of CAD. It shows that valine and isoleucine were significantly and positively associated with CAD phenotype (valine: β-coefficient 0.55; BH p-value 0.046 and isoleucine: β-coefficient 0.52; BH p-value 0.046) (Supplementary Table 2). Conversely, no significant association was found between BCAAs and the number of stenosed coronary arteries (Figure 4). Concerning the cardiometabolic risk factors used as confounders, valine and leucine showed a significant negative association with age and a significant positive association with the male sex. No significant association was found between muscle mass and BCAAs.
Figure 3.
Associations between serum branched-chain amino acids, coronary artery disease and confounders. This rainplot represents the results of the first set of regression, in which metabolites were used as dependent variables (vertical axis), while CAD phenotype (two-level variable opposing sickness vs. health) and confounders served as independent variables (horizontal axis). The redder the dots the higher the beta coefficient and the bigger the dot the smaller the adjusted p-value. A clustering has been done regrouping the metabolites with similar beta-coefficients and adjusted p-values. BH, Benjamini-Hochberg; HbA1c, glycated hemoglobin.
Figure 4.
Association between serum branched-chain amino acids, number of stenosed coronary arteries and confounders. This rainplot represents the results of the second set of regression, in which metabolites were used as dependent variables (vertical axis), while the number of stenosed coronary arteries (0, 1, 2, or 3) and confounders served as independent variables (horizontal axis). The redder the dots the higher the beta coefficient and the bigger the dot the smaller the adjusted p-value. A clustering has been done regrouping the metabolites with similar beta-coefficients and adjusted p-values. BH, Benjamini-Hochberg; HbA1c, glycated hemoglobin.
Discussion
The present study revealed significant elevated levels of circulating acylcarnitines and BCAAs in patients with CAD compared to clinically healthy individuals. Acylcarnitine species of all chain-length showed positive associations with CAD phenotype. Compared to previous studies, the present work quantified a larger panel of acylcarnitines, which allowed the identification of novel associations between acylcarnitine species and CAD phenotype (17–19). For instance, the short- and medium-chain acylcarnitines C4:0, C4:0-OH, C5:0-OH, C5:1, C5:1-O2, C6:0, and C8:1 were observed for the first time to be associated with CAD. To the authors' best knowledge, this is the first study to have examined associations between the number of stenosed coronary arteries and circulating acylcarnitines and BCAAs. For 17 acylcarnitine species, associations became stronger as the number of affected coronary arteries increased. The number of coronary artery disease has been described as a simple measure of CAD severity (81–84). Globally, the higher the number of affected coronary arteries, the higher the probability that a bigger part of the myocardium could be damaged (85, 86). Therefore, there is a relation between the number of affected coronary arteries, the area of impaired myocardium and heart function. This implicates that circulating acylcarnitine levels might reflect CAD severity.
Elevated Medium- and Long-Chain Acylcarnitine Levels in CAD
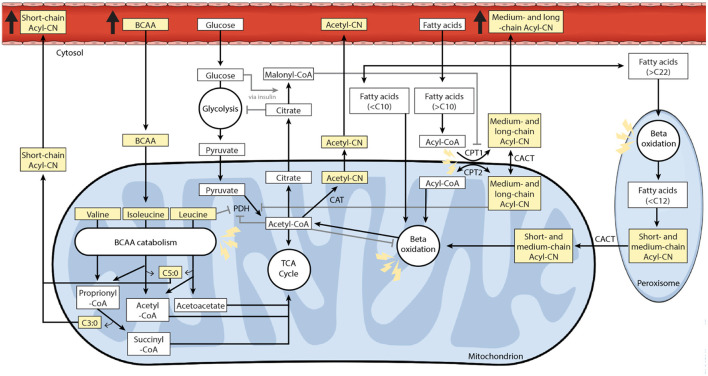
In the present study, circulating medium- and long-chain acylcarnitines, especially C6:0, C8:0, C8:1, C12:1, C14:1, C16:0, C16:1, C18:1, and C20:4, were found to be elevated in CAD patients. This accumulation could be explained by a dysregulation in carnitine shuttle enzymes and by an inefficient beta-oxidation as previously demonstrated (34, 38, 40, 44, 56, 87–90). The main carnitine shuttle enzymes are carnitine palmitoyltransferase 1 (CPT1) and carnitine palmitoyltransferase 2 (CPT2), which are responsible for the conversion of acyl-CoA and carnitine to free CoA and acylcarnitine and the opposite reaction, respectively. The conversion of acyl-CoA to acylcarnitine allow fatty acids longer than 10 carbon atoms to be transported across the mitochondrial membrane for subsequent beta oxidation. In ischemic conditions, CPT1 activity is increased and CPT2 activity decreased, leading to an accumulation of medium- and long-chain acylcarnitines (Figure 5) (87). Furthermore, ischemia leads to an altered beta-oxidation, which may be attributed to impaired function of fatty acid oxidation enzymes or increased fatty acid oxidation relative to tricarboxylic acid (TCA) flux (12, 38), both leading to accumulation of acyl-CoA (12, 34, 47, 88, 91, 92). Excess acyl-CoA can be retroconverted to acylcarnitine, which can then be excreted via blood and urine, thus detoxifying mitochondria of excess carbons (Figure 5) (37, 39, 88, 93).
Figure 5.
Acylcarnitine and branched-chain amino acid (BCAA) metabolism in a cardiac cell. This study found elevated levels of circulating short-, medium- and long-chain acylcarnitine and BCAA species in patients with CAD compared to clinically healthy individuals. Under aerobic conditions, lipids represent the main energetic substrate in cardiac cells (33). The main role of carnitine and acylcarnitines is to transport fatty acids, containing acyl-chain(s) of 10 or more carbon atoms, into the mitochondria for subsequent beta-oxidation. The so-called carnitine shuttle includes several enzymes. The enzyme carnitine palmitoyltransferase 1 (CPT1) located at the outer mitochondrial membrane converts acyl-CoAs into acylcarnitines. These are then transported through the inner mitochondrial membrane by the carrier carnitine/acylcarnitine translocase (CACT). Once inside the mitochondrion, the enzyme carnitine palmitoyltransferase 2 (CPT2) converts acylcarnitines back to their corresponding acyl-CoAs, which will then undergo beta-oxidation to produce acetyl-CoA (34–36). Beyond fuel trafficking, acylcarnitines also defend against mitochondrial stress by buffering the intracellular free CoA to acyl-CoA ratio (37–39). The carnitine shuttle enables mitochondrial export of excess carbons in the form of acylcarnitines, which can then be excreted via blood and urine (37, 39). This process also supports metabolic flexibility by relieving the inhibition of PDH induced by acetyl-CoA accumulation (40). Metabolic flexibility is the ability to switch between substrate for energy production depending on substrate availability (41). Fatty acids and glucose intermediates compete as metabolic substrate for energy production in cardiac mitochondria (Randle cycle) (42). Short- and odd-chain acylcarnitine species, such as propionylcarnitine (C3) and isovalerylcarnitine (C5), are usually derived from BCAA catabolism (43–45). Molecules on a yellow background were measured in this study. Regulatory mechanisms are represented with gray lines, normal arrows for stimulation and arrows to bar for inhibition. Lightning icons represent impairment. Acyl-CN, acylcarnitine; BCAA, branched-chain amino acid; C, number of carbon atoms; CACT, carnitine-acylcarnitine translocase; CAT, carnitine acetyltransferase; CPT1, carnitine palmitoyltransferase 1; CPT2, carnitine palmitoyltransferase 2; PDH, pyruvate dehydrogenase.
Our results are in line with previous findings. Within medium-chain acylcarnitines, hexanoylcarnitine (C6:0) was reported to be able to discriminate patients with cardiovascular diseases from clinically healthy controls (16). Likewise, octanoylcarnitine (C8:0) was associated with cardiovascular mortality and reduced heart function (17, 94). Within long-chain acylcarnitines, palmitoylcarnitine (C16:0) has been associated with heart failure (94), cardiovascular mortality in patients with stable angina pectoris (17) and cardiovascular events in very old individuals with previous history of CAD (15). Similarly, oleoylcarnitine (C18:1) was shown to be able to predict cardiovascular events in elderly individuals with previous history of CAD (15).
The Interconnection of Short-Chain Acylcarnitine and BCAA Metabolism
We found elevated levels of several C3- and C5-acylcarnitine species, as well as of their precursors valine and isoleucine, in patients with CAD. Our results are consistent with those of previous studies, which found that elevated levels of circulating BCAAs (19, 28, 29) and short-chain acylcarnitines (19, 28) were associated with CAD and stroke in a population at high cardiovascular risk (95). BCAAs and acylcarnitines seem to interplay at different levels. First, chronic cardiac ischemia can disrupt the BCAA catabolism, leading to increased BCAA catabolism derivatives such as C3- and C5-acylcarnitines (38, 96, 97). Excess BCAAs can then impair fatty acid oxidation, which results in the accumulation of incompletely oxidized lipid species and acylcarnitines (5, 98).
Acetylcarnitine (C2:0), which is the most abundant circulating acylcarnitine (99), plays a central role in detoxifying mitochondria from excessive acetyl-CoA, the universal degradation product of all metabolic substrates (Figure 5) (93). Interestingly, we found that acetylcarnitine (C2:0) was not significantly associated with CAD phenotype and does not accumulate in CAD patients. As the acetyl-CoA is the main substrate of the TCA cycle, this observation implies that the capacity of the TCA cycle is not necessarily exceeded as previously postulated (12, 38, 98). Therefore, the globally elevated levels of acylcarnitines are likely due to impaired fatty acid oxidation and carnitine shuttle enzymes, rather than to reduced TCA flux.
Hydroxylated and Dicarboxylic Acylcarnitines
The short-chain hydroxylated acylcarnitine hydroxybutyrylcarnitine (C4:0-OH) was found to be elevated in CAD. Accumulation of plasma hydroxybutyrylcarnitine (C4:0-OH) is used for the diagnosis and screening of patients with an inherited defect in the short-chain hydroxyacyl-CoA dehydrogenase (SCHAD), an enzyme of the mitochondrial fatty acid oxidation (100). This finding further supports an impaired beta oxidation in CAD.
Suberoylcarnitine (C8:0-DC) was positively associated with CAD phenotype. This is in line with the findings of Shah et al., which showed that a signature composed of short- and medium-chain dicarboxylic acylcarnitines was predictive of cardiovascular events in individuals with CAD (19). In addition to an alteration in mitochondrial fatty acid oxidation, elevated dicarboxylic acylcarnitine levels in CAD could indicate increased fatty acid omega-oxidation (101). Dicarboxylic acylcarnitines are byproducts of medium-chain dicarboxylic acids. The latter are the final products of microsomal omega-oxidation and of the subsequent peroxisomal beta-oxidation (102). Both mitochondria and peroxisomes perform fatty acid beta-oxidation but with different aims. Short-, medium and long-chain fatty acids are predominantly oxidized in mitochondria, whereas peroxisomes oxidize specific carboxylic acids such as very long-chain fatty acids, branched-chain fatty acids, bile acids, and fatty dicarboxylic acids (DCAs) (103, 104). The carnitine shuttle is then used to transport the end-products (acetyl-CoA, propionyl-CoA, and medium-chain acyl-CoA) from the peroxisome to the mitochondria for complete oxidation via the TCA cycle (Figure 5) (37, 38, 105). While the mitochondrial beta-oxidation is essential for catabolism and energy production, peroxisomal beta-oxidation is mainly involved in biosynthesis pathways (106). Altogether, in addition to impaired mitochondrial beta-oxidation, CAD patients also seem to have an altered peroxisomal and microsomal fatty acid oxidation (105).
Acylcarnitines: Angels or Demons?
An accumulation of medium- and long-chain acylcarnitines can impair several regulatory mechanisms in mitochondria. First, fatty acids and glucose intermediates compete as metabolic substrates for energy production (Randle cycle) (42). An intramitochondrial accumulation of long-chain acylcarnitines inhibits pyruvate and lactate oxidation, leading to metabolic inflexibility (Figure 5) (42) or the incapacity to switch between substrate for energy production depending on their availability (41). Secondly, an excess of long-chain acylcarnitines compromises membrane function, induces electrophysiological alterations through modulation of calcium and potassium channels (contributing to cardiac arrhythmias), promotes insulin resistance and inflammation, inhibits oxidative phosphorylation and stimulates the production of reactive oxygen species (34, 37, 87, 88, 107).
A dysregulation in the BCAA catabolism, with a back-up of BCAAs and their byproducts, also has important consequences. The BCAA catabolism intermediates branched-chain keto acids (BCKAs) can be cytotoxic at high levels, promoting mitochondrial dysfunction, superoxide accumulation, and cardiomyocyte death, eventually leading to heart failure (96). Furthermore, an accumulation of BCAAs sensitizes the heart to ischemic injury (108) and contributes to cardiac dysfunction and remodeling following myocardial ischemia (109, 110). This can be explained by various mechanisms including inhibition of glucose metabolism (108) and activation of the mammalian target of rapamycin (mTOR) (109, 110). Altogether, BCAA catabolism seems to be disrupted in individuals with CAD, provoking an accumulation of BCAAs and their byproducts, favorizing mitochondrial dysfunction.
While an accumulation of BCAAs, medium- and long-chain acylcarnitines have deleterious consequences, research has indicated that some short-chain acylcarnitines could also have positive effects (37). Evidence has suggested that propionylcarnitine (C3) increases cellular carnitine content, stimulates pyruvate dehydrogenase activity and increases TCA cycle efficiency under hypoxia (111, 112). Therefore, supplementation in propionylcarnitine (C3) could be beneficial in the treatment of cardiovascular disorders (112). For L-carnitine, several studies have also reported a protective role on the myocardium by exerting anti-apoptotic effects in cardiomyocytes (94, 113). A protective role of L-carnitine within the myocardium is further supported by studies in individuals with genetic carnitine deficiency developing cardiomyopathies (114). Importantly, in our study, propionylcarnitine and L-carnitine were found to be elevated in CAD patients. Considering the potential beneficial effects of these carnitine species, elevated levels of circulating L-carnitine and proprionylcarnitine observed in CAD patients in our study could suggest an attempt of the body to adapt to chronic ischemia. This hypothesis remains to be further investigated.
Can Acylcarnitines Replace Cholesterol in Clinical Practice?
Mitochondrial dysfunction is a major determinant of metabolic disease such as metabolic syndrome, non-alcoholic fatty liver disease and type 2 diabetes mellitus, conditions which are highly linked to increased risk of cardiovascular disease and myocardial infarction (115, 116). This supports the use of acyclarnitines as markers of mitochondrial function and cardio-metabolic risk. Additionally, understanding the acylcarnitine metabolism in CAD could lead to new treatment targets. Most research on the effects of ischemia on mitochondrial enzymes has been done in acute ischemic situations. This study showed that altered mitochondrial metabolism reflected in high levels of circulating carnitine, acylcarnitines and BCAAs is also a hallmark of CAD, a chronic ischemic situation. These findings should be further investigated at an enzymatic level and in model organisms. There has been emerging evidence that manipulating fuel supply and substrate consumption of the myocardium could have an impact on the development and progression of heart failure (117, 118), which often occurs in CAD. Therapeutic interventions with “metabolic” antianginal agents that suppress fatty acid oxidation and increase the oxidation of pyruvate in the mitochondria could reduce the ischemia-induced accumulation of long-chain fatty acid intermediates and other disruption in cardiac metabolism (119). Hypoxia, anoxia and ischemia might have different effects on the cardiac metabolism and these conditions should be clearly distinguished in future works (33).
Strengths and Limitations
While previous studies analyzed L-carnitine and/or a limited number of acylcarnitines in patients with cardiometabolic diseases, we examined a large panel of acylcarnitine species and related BCAAs. This resulted in a thorough phenotyping of patients with CAD at the molecular species level (35 metabolites) comparing them to clinically healthy controls, which was rarely the case in previous research. Additionally, we conducted a detailed analysis of possible confounders and adjusted the regressions for those variables. Given the exploratory nature of this study, several limitations should be taken into consideration. First, as multiple organs usually contribute to the circulating pool of metabolites, it is difficult to identify the cellular origin, destination or subcellular localization of these metabolites (120). Therefore, the findings of the present study should be interpreted with caution when it comes to mechanistic explanations. Fortunately, it was recently shown that circulating acylcarnitine levels reflect cardiac tissue content of acylcarnitines (121), whereas elevated plasma levels of BCAAs reflect impaired BCAA catabolism in cardiac cells (122). These two facts support the rationale of the present study. Secondly, the cross-sectional nature of this study only allows for the establishment of associations, and not causality, between metabolites and CAD (123). While it is currently unknown if elevated level of circulating acylcarnitines is a consequence or a cause of ischemia-related cardiac damages, this question is of crucial relevance for the therapy of CAD and should be further investigated. Thirdly, the extent to which the reported associations represent the acylcarnitine and BCAA signature of CAD in females is unclear, as most enrolled patients were male (90.7%). This study likely did not capture the sex-specific metabolic signature of CAD. Fourthly, although serum samples were collected in a fasting state and regression analyses were adjusted for fasting time, the fasting duration might have been too short (6.7 h ± 3.0 h for healthy controls and 8.5 h ± 5.3 h for CAD patients). However, while circulating acylcarnitine levels have been shown to decrease up to 3 h after food intake (124) and increase after 12 h of fasting (125–127), the effect of a fasting time comprised between 3 and 12 h is unknown to the authors' best knowledge. Finally, we did not control for the amount and type of food intake, which is known to influence the metabolome (67).
Conclusion
This study found elevated levels of circulating acylcarnitine and BCAA species in patients with CAD compared to clinically healthy individuals. Acylcarnitine species of all chain-lengths showed positive associations with CAD phenotype. Interestingly, associations between acylcarnitine species and CAD became stronger as the number of affected coronary arteries increased. Thus, circulating acylcarnitine levels might reflect CAD severity and should be considered as potential candidates to improve patients' stratification. Altogether, CAD is characterized, at a molecular species level, by elevated acylcarnitine and BCAA levels, thus implying impaired mitochondrial metabolism in cardiac cells.
Data Availability Statement
All data presented in this study are available within the article and Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of North-Western and Central Switzerland. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JG, JC, HG-A, DI, JW, RK, AS-T, and JI contributed to conception and design of the study. JC, JW, CK, GN, and RK collected the data. HG-A, RB, TT, and JI analyzed the serum samples for the metabolites. JG, JC, and DI performed the statistical analysis. JG and JC wrote the first draft of the manuscript. TT wrote section Biochemical Analysis of the manuscript. HG-A, DI, FC, LS, JW, CK, RK, HH, AS-T, and JI contributed to manuscript revision. JC, JI, and AS-T supervised the study. AS-T was responsible for the funding acquisition. All authors read and approved the submitted version.
Funding
This study was funded by the Swiss National Science Foundation (grant nos. 182815 to AS-T and 316030_183377 to JI). This work was also supported by funds from Faculty of Biology and Medicine (FBM), University of Lausanne (UNIL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Caren Mutschmann (Synlab Analytics, Berlin, Germany) and Winfried März (Synlab Academy, Mannheim, Germany) for the quantification of HbA1c in the serum samples. We thank Hubert Scharnagl (Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Austria) for the analysis of cholesterol and triglycerides in the blood samples. We are grateful to all the Bachelor and Master students (Department of Sport, Exercise and Health, University of Basel, Switzerland) who contributed to the collection of data. Finally, we acknowledge the use of the Mind the Graph platform [www.mindthegraph.com (accessed on 15 July 2021)] to create the Figure 5.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.792350/full#supplementary-material
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 3.Zampieri M, Sekar K, Zamboni N, Sauer U. Frontiers of high-throughput metabolomics. Curr Opin Chem Biol. (2017) 36:15–23. 10.1016/j.cbpa.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 4.Benziger CP, Roth GA, Moran AE. The global burden of disease study and the preventable burden of NCD. Global Heart. (2016) 11:393–7. 10.1016/j.gheart.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 5.Newgard CB. Metabolomics and metabolic diseases: where do we stand? Cell Metab. (2017) 25:43–56. 10.1016/j.cmet.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. (2016) 17:451–9. 10.1038/nrm.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wishart DS. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev. (2019) 99:1819–75. 10.1152/physrev.00035.2018 [DOI] [PubMed] [Google Scholar]
- 8.Wraith JE. Diagnosis and management of inborn errors of metabolism. Arch Dis Child. (1989) 64:1410–5. 10.1136/adc.64.10_Spec_No.1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Canela M, Hruby A, Clish CB, Liang L, Martínez-González MA, Hu FB. Comprehensive metabolomic profiling and incident cardiovascular disease: a systematic review. J Am Heart Assoc. (2017) 6:e005705. 10.1161/JAHA.117.005705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Liang L, Gao X, Zhang H, Yao P, Hu Y, et al. Early prediction of developing type 2 diabetes by plasma acylcarnitines: a population-based study. Diabetes Care. (2016) 39:1563–70. 10.2337/dc16-0232 [DOI] [PubMed] [Google Scholar]
- 11.Mai M, Tönjes A, Kovacs P, Stumvoll M, Fiedler GM, Leichtle AB. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE. (2013) 8:e82459. 10.1371/journal.pone.0082459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter WG, Kelly JP, McGarrah RW, Khouri MG, Craig D, Haynes C, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. (2016) 5:e003190. 10.1161/JAHA.115.003190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad T, Kelly JP, McGarrah RW, Hellkamp AS, Fiuzat M, Testani JM, et al. Long-chain acylcarnitine metabolites are associated with adverse outcomes and reversible with mechanical circulatory support in systolic heart failure. J Am Coll Cardiol. (2016) 67:291–9. 10.1016/j.jacc.2015.10.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Li R, Liu Y, Li Z, Sun Y, Yin P, et al. Characteristics of blood metabolic profile in coronary heart disease, dilated cardiomyopathy and valvular heart disease induced heart failure. Front Cardiovasc Med. (2020) 7:622236. 10.3389/fcvm.2020.622236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizza S, Copetti M, Rossi C, Cianfarani MA, Zucchelli M, Luzi A, et al. Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis. (2014) 232:260–4. 10.1016/j.atherosclerosis.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 16.Kukharenko A, Brito A, Kozhevnikova MV, Moskaleva N, Markin PA, Bochkareva N, et al. Relationship between the plasma acylcarnitine profile and cardiometabolic risk factors in adults diagnosed with cardiovascular diseases. Clin Chim Acta. (2020) 507:250–6. 10.1016/j.cca.2020.04.035 [DOI] [PubMed] [Google Scholar]
- 17.Strand E, Pedersen ER, Svingen GF, Olsen T, Bjørndal B, Karlsson T, et al. Serum acylcarnitines and risk of cardiovascular death and acute myocardial infarction in patients with stable angina pectoris. J Am Heart Assoc. (2017) 6:e003620. 10.1161/JAHA.116.003620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SH, Sun J-L, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. (2012) 163:844–50.e1. 10.1016/j.ahj.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 19.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. (2010) 3:207–14. 10.1161/CIRCGENETICS.109.852814 [DOI] [PubMed] [Google Scholar]
- 20.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. (2009) 9:311–26. 10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. (2009) 32:1678–83. 10.2337/dc08-2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. (2010) 53:757–67. 10.1007/s00125-009-1637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. (2008) 5:e51. 10.1371/journal.pmed.0050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naukkarinen J, Heinonen S, Hakkarainen A, Lundbom J, Vuolteenaho K, Saarinen L, et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. (2014) 57:167–76. 10.1007/s00125-013-3066-y [DOI] [PubMed] [Google Scholar]
- 25.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. (2011) 17:448–53. 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Würtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. (2013) 36:648–55. 10.2337/dc12-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porcu E, Gilardi F, Darrous L, Yengo L, Bararpour N, Gasser M, et al. Triangulating evidence from longitudinal and Mendelian randomization studies of metabolomic biomarkers for type 2 diabetes. Sci Rep. (2021) 11:6197. 10.1038/s41598-021-85684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, Stevens RD, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. (2014) 232:191–6. 10.1016/j.atherosclerosis.2013.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang RY, Wang SM, Sun L, Liu JM, Li HX, Sui XF, et al. Association of branched-chain amino acids with coronary artery disease: a matched-pair case-control study. Nutr Metab Cardiovasc Dis. (2015) 25:937–42. 10.1016/j.numecd.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 30.Bar N, Korem T, Weissbrod O, Zeevi D, Rothschild D, Leviatan S, et al. A reference map of potential determinants for the human serum metabolome. Nature. (2020) 588:135–40. 10.1038/s41586-020-2896-2 [DOI] [PubMed] [Google Scholar]
- 31.Tebani A, Gummesson A, Zhong W, Koistinen IS, Lakshmikanth T, Olsson LM, et al. Integration of molecular profiles in a longitudinal wellness profiling cohort. Nat Commun. (2020) 11:4487. 10.1038/s41467-020-18148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teav T, Gallart-Ayala H, van der Velpen V, Mehl F, Henry H, Ivanisevic J. Merged targeted quantification and untargeted profiling for comprehensive assessment of acylcarnitine and amino acid metabolism. Anal Chem. (2019) 91:11757–69. 10.1021/acs.analchem.9b02373 [DOI] [PubMed] [Google Scholar]
- 33.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. (1974) 36:413–59. 10.1146/annurev.ph.36.030174.002213 [DOI] [PubMed] [Google Scholar]
- 34.Dambrova M, Zuurbier CJ, Borutaite V, Liepinsh E, Makrecka-Kuka M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic Biol Med. (2021) 165:24–37. 10.1016/j.freeradbiomed.2021.01.036 [DOI] [PubMed] [Google Scholar]
- 35.Taegtmeyer H. Energy metabolism of the heart: From basic concepts to clinical applications applications. Curr Problems Cardiol. (1994) 19:61–86. 10.1016/0146-2806(94)90008-6 [DOI] [PubMed] [Google Scholar]
- 36.Yu Z-R, Ning Y, Yu H, Tang N-J. A HPLC-Q-TOF-MS-based urinary metabolomic approach to identification of potential biomarkers of metabolic syndrome. J Huazhong Univ Sci Technol Med Sci. (2014) 34:276–83. 10.1007/s11596-014-1271-7 [DOI] [PubMed] [Google Scholar]
- 37.Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. (2012) 51:553–72. 10.1007/BF03261931 [DOI] [PubMed] [Google Scholar]
- 38.McCann MR, La George De Rosa MV, Rosania GR, Stringer KA. L-Carnitine and acylcarnitines: mitochondrial biomarkers for precision medicine. Metabolites. (2021) 11:51. 10.3390/metabo11010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoppel C. The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis. (2003) 41:S4–12. 10.1016/S0272-6386(03)00112-4 [DOI] [PubMed] [Google Scholar]
- 40.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. (2001) 33:1065–89. 10.1006/jmcc.2001.1378 [DOI] [PubMed] [Google Scholar]
- 41.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance. Diabetes. (2020) 49:677–83. 10.2337/diabetes.49.5.677 [DOI] [PubMed] [Google Scholar]
- 42.Makrecka M, Kuka J, Volska K, Antone U, Sevostjanovs E, Cirule H, et al. Long-chain acylcarnitine content determines the pattern of energy metabolism in cardiac mitochondria. Mol Cell Biochem. (2014) 395:1–10. 10.1007/s11010-014-2106-3 [DOI] [PubMed] [Google Scholar]
- 43.Platell C, Kong SE, McCauley R, Hall JC. Branched-chain amino acids. J Gastroenterol Hepatol. (2000) 15:706–17. 10.1046/j.1440-1746.2000.02205.x [DOI] [PubMed] [Google Scholar]
- 44.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. (2008) 7:45–56. 10.1016/j.cmet.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 45.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. (2013) 62:1–8. 10.2337/db12-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adeva-Andany MM, Calvo-Castro I, Fernández-Fernández C, Donapetry-García C, Pedre-Piñeiro AM. Significance of l-carnitine for human health. IUBMB Life. (2017) 69:578–94. 10.1002/iub.1646 [DOI] [PubMed] [Google Scholar]
- 47.Lerch R, Tamm C, Papageorgiou I, Benzi RH. Myocardial fatty acid oxidation during ischemia and reperfusion. Mol Cell Biochem. (1992) 116:103–9. 10.1007/BF01270576 [DOI] [PubMed] [Google Scholar]
- 48.Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR. Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem. (1978) 253:4305–9. 10.1016/S0021-9258(17)34720-8 [DOI] [PubMed] [Google Scholar]
- 49.Wagner J, Knaier R, Infanger D, Arbeev K, Briel M, Dieterle T, et al. Functional aging in health and heart failure: the COmPLETE study. BMC Cardiovasc Disord. (2019) 19:180. 10.1186/s12872-019-1164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res. (2012) 32:479–85. 10.1016/j.nutres.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 51.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol. (2016) 45:1887–94. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 52.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. (2008) 8:70. 10.1186/1471-2288-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huynh K, Barlow CK, Jayawardana KS, Weir JM, Mellett NA, Cinel M, et al. High-throughput plasma lipidomics: detailed mapping of the associations with cardiometabolic risk factors. Cell Chem Biol. (2019) 26:71–84. 10.1016/j.chembiol.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 54.Jarrell ZR, Smith MR, He X, Orr M, Jones DP, Go YM. Plasma acylcarnitine levels increase with healthy aging. Aging. (2020) 12:13555–70. 10.18632/aging.103462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. (2011) 7:e1002215. 10.1371/journal.pgen.1002215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. (2009) 139:1073–81. 10.3945/jn.108.103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity. (2010) 18:1695–700. 10.1038/oby.2009.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baek SH, Kim M, Kim M, Kang M, Yoo HJ, Lee NH, et al. Metabolites distinguishing visceral fat obesity and atherogenic traits in individuals with overweight. Obesity. (2017) 25:323–31. 10.1002/oby.21724 [DOI] [PubMed] [Google Scholar]
- 59.Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J, et al. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab. (2015) 309:E736–46. 10.1152/ajpendo.00231.2015 [DOI] [PubMed] [Google Scholar]
- 60.Lacruz ME, Kluttig A, Tiller D, Medenwald D, Giegling I, Rujescu D, et al. Cardiovascular risk factors associated with blood metabolite concentrations and their alterations during a 4-year period in a population-based cohort. Circ Cardiovasc Genet. (2016) 9:487–94. 10.1161/CIRCGENETICS.116.001444 [DOI] [PubMed] [Google Scholar]
- 61.Bhuiyan J, Seccombe DW. The effects of 3-hydroxy-3-methylglutaryl-CoA reductase inhibition on tissue levels of carnitine and carnitine acyltransferase activity in the rabbit. Lipids. (1996) 31:867–70. 10.1007/BF02522982 [DOI] [PubMed] [Google Scholar]
- 62.Iacobazzi V, Convertini P, Infantino V, Scarcia P, Todisco S, Palmieri F. Statins, fibrates and retinoic acid upregulate mitochondrial acylcarnitine carrier gene expression. Biochem Biophys Res Commun. (2009) 388:643–7. 10.1016/j.bbrc.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 63.Panchal AR, Stanley WC, Kerner J, Sabbah HN. Beta-receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Cardiac Fail. (1998) 4:121–6. 10.1016/S1071-9164(98)90252-4 [DOI] [PubMed] [Google Scholar]
- 64.Hiltunen TP, Rimpelä JM, Mohney RP, Stirdivant SM, Kontula KK. Effects of four different antihypertensive drugs on plasma metabolomic profiles in patients with essential hypertension. PLoS ONE. (2017) 12:e0187729. 10.1371/journal.pone.0187729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ang JE, Revell V, Mann A, Mäntele S, Otway DT, Johnston JD, et al. Identification of human plasma metabolites exhibiting time-of-day variation using an untargeted liquid chromatography-mass spectrometry metabolomic approach. Chronobiol Int. (2012) 29:868–81. 10.3109/07420528.2012.699122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feigin RD, Beisel WR, Wannemacher RW. Rhythmicity of plasma amino acids and relation to dietary intake. Am J Clin Nutr. (1971) 24:329–41. 10.1093/ajcn/24.3.329 [DOI] [PubMed] [Google Scholar]
- 67.Tokarz J, Adamski J. Chapter 2-Confounders in metabolomics. In: Adamski J, editor. Metabolomics for Biomedical Research. Academic Press (2020). p. 17–32. 10.1016/B978-0-12-812784-1.00002-5 [DOI] [Google Scholar]
- 68.Foroumandi E, Alizadeh M, Kheirouri S. Age-dependent changes in plasma amino acids contribute to alterations in glycoxidation products. J Med Biochem. (2018) 37:426–33. 10.1515/jomb-2017-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guevara-Cruz M, Vargas-Morales JM, Méndez-García AL, López-Barradas AM, Granados-Portillo O, Ordaz-Nava G, et al. Amino acid profiles of young adults differ by sex, body mass index and insulin resistance. Nutr Metab Cardiovasc Dis. (2018) 28:393–401. 10.1016/j.numecd.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 70.Rist MJ, Roth A, Frommherz L, Weinert CH, Krüger R, Merz B, et al. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS ONE. (2017) 12:e0183228. 10.1371/journal.pone.0183228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jourdan C, Petersen A-K, Gieger C, Döring A, Illig T, Wang-Sattler R, et al. Body fat free mass is associated with the serum metabolite profile in a population-based study. PLoS ONE. (2012) 7:e40009. 10.1371/journal.pone.0040009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy RA, Moore SC, Playdon M, Meirelles O, Newman AB, Milijkovic I, et al. Metabolites associated with lean mass and adiposity in older black men. J Gerontol A Biol Sci Med Sci. (2017) 72:1352–9. 10.1093/gerona/glw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomoda K, Yoshikawa M, Kubo K, Koyama N, Yamamoto Y, Kimura H. Effects of cigarettes smoke on branched chain amino acids (BCAA) levels in plasma and skeletal muscles in rats. J Toxicol Sci. (2014) 39:331–7. 10.2131/jts.39.331 [DOI] [PubMed] [Google Scholar]
- 74.Henningsen A,. censReg: Censored Regression (Tobit) Models. R Package Version 0.5-32. (2020). Available online at: https://CRAN.R-project.org/package=censReg (accessed July 30, 2021).
- 75.Benjamini Y, Hochberg Y. Controlling the false Discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. (1995) 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 76.R Core Team . R: A Language Environment for Statistical Computing. (2020). Available online at: https://www.R-project.org/ (accessed July 30, 2021).
- 77.Henglin M, Niiranen T, Watrous JD, Lagerborg KA, Antonelli J, Claggett BL, et al. A single visualization technique for displaying multiple metabolite-phenotype associations. Metabolites. (2019) 9:128. 10.3390/metabo9070128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 79.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. (2018) 36:1953–2041. 10.1097/HJH.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 80.Berry JD, Willis B, Gupta S, Barlow CE, Lakoski SG, Khera A, et al. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The Cooper Center Longitudinal Study. J Am Coll Cardiol. (2011) 57:1604–10. 10.1016/j.jacc.2010.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Özcan C, Deleskog A, Schjerning Olsen A-M, Nordahl Christensen H, Lock Hansen M, Hilmar Gislason G. Coronary artery disease severity and long-term cardiovascular risk in patients with myocardial infarction: a Danish nationwide register-based cohort study. Eur Heart J Cardiovasc Pharmacother. (2018) 4:25–35. 10.1093/ehjcvp/pvx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sponder M, Fritzer-Szekeres M, Marculescu R, Litschauer B, Strametz-Juranek J. A new coronary artery disease grading system correlates with numerous routine parameters that were associated with atherosclerosis: a grading system for coronary artery disease severity. VHRM. (2014) 10:641–7. 10.2147/VHRM.S68919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51:606. 10.1016/S0002-9149(83)80105-2 [DOI] [PubMed] [Google Scholar]
- 84.Mortensen MB, Steffensen FH, Bøtker HE, Jensen JM, Rønnow Sand NP, Kragholm KH, et al. CAD severity on cardiac CTA identifies patients with most benefit of treating LDL-cholesterol to ACC/AHA and ESC/EAS targets. JACC Cardiovasc Imaging. (2020) 13:1961–72. 10.1016/j.jcmg.2020.03.017 [DOI] [PubMed] [Google Scholar]
- 85.Lala A, Desai AS. The role of coronary artery disease in heart failure. Heart Fail Clin. (2014) 10:353–65. 10.1016/j.hfc.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 86.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 87.Liepinsh E, Makrecka-Kuka M, Volska K, Kuka J, Makarova E, Antone U, et al. Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem J. (2016) 473:1191–202. 10.1042/BCJ20160164 [DOI] [PubMed] [Google Scholar]
- 88.McCoin CS, Knotts TA, Adams SH. Acylcarnitines-old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol. (2015) 11:617–25. 10.1038/nrendo.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blair HC, Sepulveda J, Papachristou DJ. Nature and nurture in atherosclerosis: the roles of acylcarnitine and cell membrane-fatty acid intermediates. Vascul Pharmacol. (2016) 78:17–23. 10.1016/j.vph.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bjørndal B, Alterås EK, Lindquist C, Svardal A, Skorve J, Berge RK. Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutr Metab. (2018) 15:10. 10.1186/s12986-018-0241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liepinsh E, Skapare E, Kuka J, Makrecka M, Cirule H, Vavers E, et al. Activated peroxisomal fatty acid metabolism improves cardiac recovery in ischemia-reperfusion. Naunyn Schmiedebergs Arch Pharmacol. (2013) 386:541–50. 10.1007/s00210-013-0849-0 [DOI] [PubMed] [Google Scholar]
- 92.Paulson DJ, Schmidt MJ, Romens J, Shug AL. Metabolic and physiological differences between zero-flow and low-flow myocardial ischemia: effects of L-acetylcarnitine. Basic Res Cardiol. (1984) 79:551–61. 10.1007/BF01910484 [DOI] [PubMed] [Google Scholar]
- 93.Furuichi Y, Goto-Inoue N, Fujii LN. Role of carnitine acetylation in skeletal muscle. JPFSM. (2014) 3:163–8. 10.7600/jpfsm.3.163 [DOI] [Google Scholar]
- 94.Ueland T, Svardal A, Oie E, Askevold ET, Nymoen SH, Bjorndal B, et al. Disturbed carnitine regulation in chronic heart failure — increased plasma levels of palmitoyl-carnitine are associated with poor prognosis. Int J Cardiol. (2013) 167:1892–9. 10.1016/j.ijcard.2012.04.150 [DOI] [PubMed] [Google Scholar]
- 95.Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvadó J, et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem. (2016) 62:582–92. 10.1373/clinchem.2015.251710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du X, You H, Li Y, Wang Y, Hui P, Qiao B, et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Sci Rep. (2018) 8:15809. 10.1038/s41598-018-34245-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. (2016) 311:H1160–9. 10.1152/ajpheart.00114.2016 [DOI] [PubMed] [Google Scholar]
- 98.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. (2012) 15:606–14. 10.1016/j.cmet.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reuter SE Stephanie E, Evans AM, Chace DH, Fornasini G. Determination of the reference range of endogenous plasma carnitines in healthy adults. Ann Clin Biochem. (2008) 45:585–92. 10.1258/acb.2008.008045 [DOI] [PubMed] [Google Scholar]
- 100.Molven A, Matre GE, Duran M, Wanders RJ, Rishaug U, Njølstad PR, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. (2004) 53:221–7. 10.2337/diabetes.53.1.221 [DOI] [PubMed] [Google Scholar]
- 101.Fiamoncini J, Lima TM, Hirabara SM, Ecker J, Gorjão R, Romanatto T, et al. Medium-chain dicarboxylic acylcarnitines as markers of n-3 PUFA-induced peroxisomal oxidation of fatty acids. Mol Nutr Food Res. (2015) 59:1573–83. 10.1002/mnfr.201400743 [DOI] [PubMed] [Google Scholar]
- 102.Houten SM, Denis S, Argmann CA, Jia Y, Ferdinandusse S, Reddy JK, et al. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J Lipid Res. (2012) 53:1296–303. 10.1194/jlr.M024463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. (2006) 75:295–332. 10.1146/annurev.biochem.74.082803.133329 [DOI] [PubMed] [Google Scholar]
- 104.van Veldhoven PP. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res. (2010) 51:2863–95. 10.1194/jlr.R005959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Houten SM, Wanders RJ, Ranea-Robles P. Metabolic interactions between peroxisomes and mitochondria with a special focus on acylcarnitine metabolism. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165720. 10.1016/j.bbadis.2020.165720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Demarquoy J, Le Borgne F. Crosstalk between mitochondria and peroxisomes. WJBC. (2015) 6:301–9. 10.4331/wjbc.v6.i4.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonnet D, Martin D, Pascale DL, Villain E, Jouvet P, Rabier D, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. (1999) 100:2248–53. 10.1161/01.CIR.100.22.2248 [DOI] [PubMed] [Google Scholar]
- 108.Li T, Zhang Z, Kolwicz SC, Abell L, Roe ND, Kim M, et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. (2017) 25:374–85. 10.1016/j.cmet.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu G, Sun H, She P, Youn J-Y, Warburton S, Ping P, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. (2009) 119:1678–87. 10.1172/JCI38151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. (2009) 54:2435–46. 10.1016/j.jacc.2009.08.031 [DOI] [PubMed] [Google Scholar]
- 111.Wiseman LR, Brogden RN. Propionyl-L-carnitine. Drugs Aging. (1998) 12:243–8; discussion 249–50. 10.2165/00002512-199812030-00006 [DOI] [PubMed] [Google Scholar]
- 112.Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C. Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: a review. Ann N Y Acad Sci. (2004) 1033:79–91. 10.1196/annals.1320.007 [DOI] [PubMed] [Google Scholar]
- 113.Andrieu-Abadie N, Jaffrezou JP, Hatem S, Laurent G, Levade T, Mercadier JJ. L-carnitine prevents doxorubicin-induced apoptosis of cardiac myocytes: role of inhibition of ceramide generation. FASEB J. (1999) 13:1501–10. 10.1096/fasebj.13.12.1501 [DOI] [PubMed] [Google Scholar]
- 114.Scholte HR, Luyt-Houwen IE, Vaandrager-Verduin MH. The role of the carnitine system in myocardial fatty acid oxidation: carnitine deficiency, failing mitochondria and cardiomyopathy. Basic Res Cardiol. (1987) 82(Suppl. 1):63–73. 10.1007/978-3-662-08390-1_8 [DOI] [PubMed] [Google Scholar]
- 115.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med. (2010) 88:993–1001. 10.1007/s00109-010-0663-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Serviddio G, Bellanti F, Vendemiale G, Altomare E. Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. (2011) 5:233–44. 10.1586/egh.11.11 [DOI] [PubMed] [Google Scholar]
- 117.Horowitz JD, Chirkov YY, Kennedy JA, Sverdlov AL. Modulation of myocardial metabolism: an emerging therapeutic principle. Curr Opin Cardiol. (2010) 25:329–34. 10.1097/HCO.0b013e328339f191 [DOI] [PubMed] [Google Scholar]
- 118.Revenco D, Morgan JP. Metabolic modulation and cellular therapy of cardiac dysfunction and failure. J Cell Mol Med. (2009) 13:811–25. 10.1111/j.1582-4934.2009.00759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee L, Horowitz J, Frenneaux M. Metabolic manipulation in ischaemic heart disease, a novel approach to treatment. Eur Heart J. (2004) 25:634–41. 10.1016/j.ehj.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 120.Schooneman MG, Achterkamp N, Argmann CA, Soeters MR, Houten SM. Plasma acylcarnitines inadequately reflect tissue acylcarnitine metabolism. Biochim Biophys Acta. (2014) 1841:987–94. 10.1016/j.bbalip.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 121.Makrecka-Kuka M, Sevostjanovs E, Vilks K, Volska K, Antone U, Kuka J, et al. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci Rep. (2017) 7:17528. 10.1038/s41598-017-17797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. (1984) 4:409–54. 10.1146/annurev.nu.04.070184.002205 [DOI] [PubMed] [Google Scholar]
- 123.Chu SH, Huang M, Kelly RS, Benedetti E, Siddiqui JK, Zeleznik OA, et al. Integration of metabolomic and other omics data in population-based study designs: an epidemiological perspective. Metabolites. (2019) 9:117. 10.3390/metabo9060117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shrestha A, Müllner E, Poutanen K, Mykkänen H, Moazzami AA. Metabolic changes in serum metabolome in response to a meal. Eur J Nutr. (2017) 56:671–81. 10.1007/s00394-015-1111-y [DOI] [PubMed] [Google Scholar]
- 125.Hoppel CL, Genuth SM. Carnitine metabolism in normal-weight and obese human subjects during fasting. Am J Physiol. (1980) 238:E409–15. 10.1152/ajpendo.1980.238.5.E409 [DOI] [PubMed] [Google Scholar]
- 126.Costa CC, Almeida IT, de Jakobs C, Poll-The BT, Duran M. Dynamic changes of plasma acylcarnitine levels induced by fasting and sunflower oil challenge test in children. Pediatr Res. (1999) 46:440–4. 10.1203/00006450-199910000-00013 [DOI] [PubMed] [Google Scholar]
- 127.Frohlich J, Seccombe DW, Hahn P, Dodek P, Hynie I. Effect of fasting on free and esterified carnitine levels in human serum and urine: correlation with serum levels of free fatty acids and β-hydroxybutyrate. Metabolism. (1978) 27:555–61. 10.1016/0026-0495(78)90022-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study are available within the article and Supplementary Material.