Abstract
The retinoblastoma tumor suppressor protein (pRB) negatively regulates early-G1 cell cycle progression, in part, by sequestering E2F transcription factors and repressing E2F-responsive genes. Although pRB is phosphorylated on up to 16 cyclin-dependent kinase (Cdk) sites by multiple G1 cyclin-Cdk complexes, the active form(s) of pRB in vivo remains unknown. pRB is present as an unphosphorylated protein in G0 quiescent cells and becomes hypophosphorylated (∼2 mol of PO4 to 1 mol of pRB) in early G1 and hyperphosphorylated (∼10 mol of PO4 to 1 mol of pRB) in late G1 phase. Here, we report that hypophosphorylated pRB, present in early G1, represents the biologically active form of pRB in vivo that is assembled with E2Fs and E1A but that both unphosphorylated pRB in G0 and hyperphosphorylated pRB in late G1 fail to become assembled with E2Fs and E1A. Furthermore, using transducible dominant-negative TAT fusion proteins that differentially target cyclin D-Cdk4 or cyclin D-Cdk6 (cyclin D-Cdk4/6) and cyclin E-Cdk2 complexes, namely, TAT-p16 and TAT–dominant-negative Cdk2, respectively, we found that, in vivo, cyclin D-Cdk4/6 complexes hypophosphorylate pRB in early G1 and that cyclin E-Cdk2 complexes inactivate pRB by hyperphosphorylation in late G1. Moreover, we found that cycling human tumor cells expressing deregulated cyclin D-Cdk4/6 complexes, due to deletion of the p16INK4a gene, contained hypophosphorylated pRB that was bound to E2Fs in early G1 and that E2F-responsive genes, including those for dihydrofolate reductase and cyclin E, were transcriptionally repressed. Thus, we conclude that, physiologically, pRB is differentially regulated by G1 cyclin-Cdk complexes.
Stimulation by growth factors of resting G0 quiescent cells to enter the early-G1 phase of the cell cycle and to transit across the restriction point into late G1 phase requires the concerted activities of multiple cyclin-dependent kinases (Cdks) that phosphorylate substrates in a cell cycle-specific fashion (for reviews, see references 13, 42, 56, and 64). Activation of cyclin E-Cdk2 at the late G1 restriction point and activation of cyclin A-Cdk2 at the transition from late G1 to S phase suggest the involvement of these cyclin-Cdk complexes at specific cell cycle regulatory checkpoints (42, 56, 57). In contrast, cyclin D-Cdk4 or cyclin D-Cdk6 (cyclin D-Cdk4/6) complexes are inactive in G0 quiescent cells but become activated by growth factor addition in early G1 phase (36, 38). In addition, whereas cyclin E- and A-associated kinase activities remain cell cycle regulated in cycling cells, cyclin D-Cdk4/6 activity is constitutive in cycling cells (5, 17, 35, 44). Importantly, these observations indicate a distinct role for cyclin D-Cdk4/6 complexes in regulating G1 cell cycle progression from that of cyclin E-Cdk2 and cyclin A-Cdk2. Indeed, Datar et al. (7) and Meyer et al. (37) using genetic models of Drosophila melanogaster have recently demonstrated that cyclin D-Cdk4 complexes regulate cellular growth (accumulation of mass) and not cell cycle progression (3, 37). Moreover, consistent with these observations, genetic deletion of the Cdk4 gene in mammalian cells results in a delay in the transition from the G0 to early G1 phase and not an alteration of cell cycle progression (59).
One important substrate of G1 cyclin-Cdk complexes is the retinoblastoma tumor suppressor protein (pRB), a transcriptional negative regulator of G1-phase cell cycle progression (64). pRB regulates transcription by binding cellular transcription factors, such as E2F family members, and remodeling chromatin at targeted genes by interaction with histone deacetylases (HDAC) (for reviews, see references 12, 22, and 46). pRB contains 16 putative Cdk phosphorylation consensus sites spread throughout the protein. In vivo, pRB exists as an unphosphorylated protein in G0 quiescent cells and in two general phosphorylated forms on Cdk sites in cycling cells: hypophosphorylated and hyperphosphorylated. Hypophosphorylated pRB is present in early G1 and contains ∼1 to 2 mol of PO4 per mol of pRB (39; S. A. Ezhevsky and S. F. Dowdy, unpublished observation). Importantly, two-dimensional (2D) phosphopeptide analysis of in vivo hypophosphorylated pRB showed that 13 of the 16 Cdk phosphorylation sites are occupied (40), suggesting that hypophosphorylated pRB may be comprised of a complex mixture of multiple phospho-isoforms. In contrast, hyperphosphorylated pRB first appears at the late G1 restriction point and contains ∼10 mol of PO4 per mol of pRB. Hyperphosphorylated pRB is an inactive form of pRB that fails to assemble with either cellular transcription factors or viral oncoproteins (64).
It is generally accepted that pRB becomes sequentially phosphorylated by the actions of cyclin D-Cdk4/6 and cyclin E-Cdk2 complexes during the G1 phase of the cell cycle (16, 19, 33). However, due to the selection for deregulation of cyclin D-Cdk4/6 activity in the majority of human malignancies (56), it has been assumed that cyclin D-Cdk4/6 phosphorylation of pRB is inactivating. Indeed, earlier studies that used overexpression of cyclin D-Cdk4/6 complexes reported inactivation of pRB by hyperphosphorylation (15, 49, 50). However, we now know that supraphysiologic overexpression of cyclin D-Cdk4/6 complexes can have at least three distinct functions. (i) Cyclin D-Cdk4/6 complexes can phosphorylate pRB, as well as p130 and p107, two related proteins. (ii) The LXCXE domain on D-type cyclins can compete with cellular LXCXE binding proteins, such as HDAC, for binding to pRB, p130, and p107. (iii) Overexpressed cyclin D-Cdk4/6 complexes can inappropriately activate cyclin E-Cdk2 complexes by sequestration of Cdk inhibitors, such as p21 and p27 (57). Thus, in hindsight, supraphysiologic overexpression experiments involving cyclin D-Cdk4/6 complexes are difficult to interpret and it is difficult to compare the functions of these complexes to those of endogenous cyclin D-Cdk4/6 complexes in either primary or tumor cells.
One cornerstone piece of data that supports the notion that cyclin D-Cdk4/6 complexes inactivate pRB is the observation that p16INK4a, a negative regulator of Cdk4/6, when overexpressed in RB−/− human tumor cells or pRB−/− nullizygous mouse embryonic fibroblasts (MEFs) fails to undergo a G1 arrest (25, 31), suggesting a linear p16-cyclin D-pRB pathway (51, 56). However, this notion is directly challenged by the surprising observations of Bruce et al. that demonstrated the inability of p16 to arrest p130 or p107 nullizygous MEFs that remain wild type for pRB, suggesting that p16-mediated arrest requires p130 or p107, as well as pRB (4). In addition, both Jiang et al. (23) and Gius et al. (19) found that prior inactivation of cyclin E-Cdk2 complexes was required for p16INK4a-mediated arrest. Furthermore, some studies have shown that G1 arrest in response to gamma irradiation and cell cycle arrest by treatment with transforming growth factor β results in loss of cyclin E-Cdk2 activity with continued cyclin D-Cdk4/6 activity and the presence of active, hypophosphorylated pRB (5, 44). Moreover, the original in vivo demonstration that pRB binds HDAC was performed with p16INK4a-deleted human tumor cells that contain high levels of deregulated cyclin D-Cdk4/6 activity but retain active pRB (2, 34, 47; P. K. Davis and S. F. Dowdy, unpublished observation). Taken together, these observations challenge the earlier dogma that phosphorylation of pRB by either normal or deregulated cyclin D-Cdk4/6 complexes in vivo is inactivating.
Due to the close overlapping kinase activity profiles of cyclin D-Cdk4/6 and cyclin E-Cdk2 complexes in G1, the physiological consequences of pRB phosphorylation by each kinase remains unclear. Moreover, the biological requirement for pRB hypophosphorylation and the early-G1-pRB-hypophosphorylating kinase(s) remains unknown. Here, having used primary human cells and transducible dominant-negative regulatory proteins that specifically targeted Cdk4/6 or Cdk2, we report that hypophosphorylated pRB represents the biologically active form of pRB in vivo and that it is capable of assembling with both E2F transcription factors and E1A but that both unphosphorylated and hyperphosphorylated pRB remain biologically inactive in the cell. Furthermore, we show that cyclin D-Cdk4/6 complexes are the long sought after early-G1-pRB-hypophosphorylating kinase.
MATERIALS AND METHODS
Cell culture and cell cycle synchronization.
Primary human diploid fibroblasts (Sifts) were maintained as described previously (10). For contact inhibition, 6 × 106 cells per 10-cm-diameter dish were density arrested in Dulbecco's modified Eagle's medium plus 10% serum for 45 h and then replated at 0.6 × 106 to 1 × 106 cells and transduced with 200 to 450 nM TAT–dominant-negative Cdk2 (Cdk2DN) or TAT-green fluorescent protein (GFP) at 2 h postreplating. Primary peripheral blood lymphocytes (PBLs) were isolated from leuko-packs as described previously (18). PBLs (3 × 108) were stimulated with 8 μg of phytohemagglutinin (PHA; Sigma) per ml and transduced with 4 μM TAT-p16 protein (16, 43) for 18 to 24 h. PBLs (5 × 107) at G0 or early G1 (18 to 24 h of PHA stimulation) phase were transduced with 100 nM TAT-E1A (43) for 1.5 h and then subjected to anti-E1A (M73; PharMingen) immunoprecipitation and anti-pRB immunoblotting. Purification of TAT fusion proteins was performed as described previously (43). Synchronization of cycling Jurkat T cells by centrifugal elutriation was performed as previously described (11).
Purification of TAT fusion proteins.
TAT-p16 and TAT-Cdk2DN proteins were purified as previously described (43, 62). Transduction of TAT fusion proteins into fibroblasts and PBLs was confirmed by flow cytometry (fluorescence-activated cell sorting [FACS]; Becton Dickinson) and fluorescence confocal microscopy (62) of fluorescein isothiocyanate-labeled TAT fusion proteins (Pierce). Cell cycle flow cytometry (FACS) was performed as described previously (16). Anti-interleukin-2 receptor (anti-IL-2R; PharMingen) FACS was performed on resting and stimulated PBLs.
Immunoprecipitation, immunoblotting, and kinase assays.
Immunoprecipitations were performed as described previously (16). Briefly, cells were lysed in a solution containing 50 mM HEPES (pH 7.2), 250 mM NaCl, 2 mM EDTA, 0.5% NP-40, 5 μg of aprotinin per μl, and 5 μg of leupeptin per μl. Cell lysates were precleared with 50 μl of zysorbin (Zymed) and subsequently incubated with antibody and 50 μl of protein A beads on a rotating wheel at 4°C for 2 h. Antibodies used were anti-Cdk6 (C-21; Santa Cruz Biotechnology), anti-pRB (14001A; PharMingen), anti-E2F-1 (KH20 and KH95; Upstate Biotechnology), and anti-E2F4 (gift from J. Lees, Massachusetts Institute of Technology). Immunoblot analysis was performed as described previously (16), and blots were probed with anti-pRB (PharMingen), anti-cyclin E, anti-Cdk2, anti-Cdk4, and anti-Cdk6 (Santa Cruz Biotechnology). In vivo [32P]orthophosphate labeling was performed as described previously (16). Reverse transcription-PCR (RT-PCR) for dihydrofolate reductase (DHFR) and cyclin E (29) was performed as described elsewhere (29) from poly(dT)-primed mRNA.
Cdk2 and cyclin E immunoprecipitation kinase assays were done with cell lysates prepared as described above with 2 μg of anti-Cdk2 (M2) and anti-cyclin E (C-19) antibodies (Santa Cruz Biotechnology) for immunoprecipitation. Kinase reactions were performed in 10 mM MgCl2–50 mM HEPES (pH 7.2) with 2 μg of histone H1 (Calbiochem), 50 μM cold ATP, and 1 to 5 μCi of [γ-32P]ATP (Amersham). Reactions were done at 30°C for 30 min. Cdk4 and/or Cdk6 immunoprecipitation kinase assays were done as previously described. Briefly, cells were lysed in a solution containing 50 mM HEPES (pH 7.5), 10 mM MgCl2, 0.1% Tween 20, 1 mM dithiothreitol, 25 μM ATP, 5 μg of aprotinin per μl, and 5 μg of leupeptin per μl. Lysates were precleared with 20 μg of rabbit anti-mouse immunoglobulin G (IgG; Jackson Laboratories) and 100 μl of protein A beads on a rotating wheel at 4°C for 1 h. Two micrograms of anti-cdk4 (C-22) and/or anti-cdk6 (C-21) antibodies were used for immunoprecipitation. Kinase reactions were performed in a solution containing 10 mM MgCl2 and 50 mM HEPES (pH 7.2) with 2 μg of bacterially isolated glutathione S-transferase (GST)–Rb C′ terminus, 50 μM cold ATP, and 10 μCi of [γ-32P]ATP (Amersham). Reactions were done at 30°C for 30 min.
2D-IEF and NH2OH analysis.
2D isoelectric focusing (2D-IEF) was performed as described previously (48) by treating anti-pRB (21C9 monoclonal antibody) or double anti-E2F4–anti-pRB immunoprecipitates from 3 × 109 to 5 × 109 PBLs with 100 μl of 9 M urea–4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate; pH 8.4}, loading them onto the basic end of an equilibrated Immobiline pH 3 to 10 strip (Pharmacia), and electrophoretically separating them with increasing voltage stepwise from 150 to 2,000 V for 11 h. For the second dimension, strips were then equilibrated in 6 M urea–4% sodium dodecyl sulfate (SDS)–30% glycerol–50 mM Tris (pH 8.8) for 3 h, followed by SDS–6% polyacrylamide gel electrophoresis (PAGE) and immunoblotting as described previously (16). For NH2OH analyses, anti-G99 (PharMingen)-hypophosphorylated pRB immunoprecipitates from [32P]orthophosphate-treated cells were separated by SDS–6% PAGE. The pRB band was excised and treated with NH2OH as previously described (52), followed by a second SDS–15% PAGE analysis and phosphorimager analysis.
RESULTS
Only hypophosphorylated pRB is assembled with E2Fs in vivo.
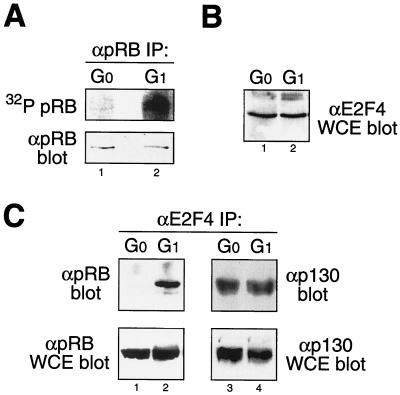
Newly synthesized pRB is unphosphorylated and becomes sequentially phosphorylated to hypophosphorylated forms in early G1 and then to a hyperphosphorylated inactive form in late G1 and S phases by multiple Cdk complexes (16, 33, 39). The active form of pRB has previously been defined by its ability to bind both cellular transcription factors, including members of the E2F family, and viral oncoproteins, such as adenovirus E1A (12, 46, 64). We and others have previously shown that both E2F1 and E1A associate with hypophosphorylated pRB in vivo (16, 40). However, it remains unclear if both unphosphorylated and hypophosphorylated pRB represent the biologically active forms of pRB in vivo. Indeed, due to the comigration of unphosphorylated and hypophosphorylated pRB on SDS-PAGE, in the absence of in vivo [32P]orthophosphate labeling, most studies have potentially misidentified the form of pRB present (Fig. 1A).
FIG. 1.
Hypophosphorylated pRB is the biologically active form of pRB. (A) G0 quiescent human PBLs and PHA-stimulated (24-h) early-G1 PBLs labeled in vivo with [32P]orthophosphate and immunoprecipitated pRB were analyzed by phosphorimaging (top) and immunoblotting (bottom). Note the comigration of unphosphorylated and hypophosphorylated pRB on SDS-PAGE (bottom). (B) Anti-E2F4 immunoblot analysis from G0 and G1 PBLs detects equal levels of E2F4 in both cell types. (C) Anti-E2F4 immunoprecipitation followed by anti-pRB (left) or anti-p130 (right) immunoblot analysis from G0 and G1 PBLs. pRB failed to coimmunoprecipitate with E2F4 from G0 PBLs, while p130 was readily detectable in complex with E2F4. IP, immunoprecipitates; α, antibody; WCE, whole-cell extracts.
To dissect the biological regulation of pRB, we utilized G0 quiescent primary human PBLs that were stimulated with PHA to enter early G1 phase. Treatment of G0 quiescent PBLs with PHA stimulates upregulation of IL-2 and the IL-2R, driving cells into early G1 phase followed by S-phase entry at ∼36 h (8, 18). To assay for the phosphorylation status of pRB, we [32P]orthophosphate labeled in vivo both G0 PBLs and PHA-stimulated (for 18 to 24 h) early-G1 PBLs. Although pRB was present in G0 PBLs (Fig. 1A, bottom panel), it remained unphosphorylated (Fig. 1A, top panel). In contrast, pRB in early-G1 PBLs was present in a hypophosphorylated form after [32P]orthophosphate labeling. Thus, stimulation of G0 quiescent PBLs to enter early G1 results in the appearance of hypophosphorylated pRB.
To ascertain the biologically active form of pRB, we assayed the ability of unphosphorylated pRB from G0 PBLs and hypophosphorylated pRB from early-G1 PBLs to form complexes with endogenous E2Fs. E2F4, a target of pRB (12, 46), is equally expressed in G0 and early-G1 PBLs (Fig. 1B). Therefore, we assayed for the association of pRB and E2F4 in G0 and early-G1 PBLs by coimmunoprecipitation (Fig. 1C, left panel). Surprisingly, unphosphorylated pRB from G0 PBLs failed to associate with endogenous E2F4, though both pRB (Fig. 1A) and E2F4 were present (Fig. 1C). In contrast, hypophosphorylated pRB present in early-G1 PBLs was readily found associated with E2F4 (Fig. 1C). The pRB-related protein p130 has previously been shown to associate with E2F4 in G0 cells (41, 58), and it was used as an internal control. Indeed, p130-E2F4 complexes were readily detectable in G0 PBLs and showed a decreased association by coimmunoprecipitation in early-G1 PBLs (Fig. 1C, right panel). In addition, by in vivo [32P]orthophosphate labeling, p130 was detected as an unphosphorylated protein in G0 PBLs and as a hypophosphorylated form in early-G1 PBLs (data not shown). Thus, endogenous unphosphorylated pRB fails to assemble with E2F4 in vivo whereas hypophosphorylated pRB present in early-G1 PBLs is assembled with E2F4. These observations suggest that, under physiological conditions, hypophosphorylated pRB is the biologically active form of pRB in cells required to assemble with E2F transcription factors.
Hypophosphorylated pRB is phosphorylated on Cdk sites and composed of multiple phospho-isoforms.
pRB contains 16 putative Cdk consensus phosphorylation sites distributed throughout the length of protein, but they are outside of the A and B box pocket domains involved in protein-protein interactions (Fig. 2A, top panel). Surprisingly, by 2D phosphopeptide analysis, hypophosphorylated pRB is phosphorylated on 13 of 16 Cdk phosphorylation sites in vivo (5, 40). However, due to a phosphate-to-pRB molar ratio of ∼2:1 (40; S. A. Ezhevsky and S. F. Dowdy, unpublished observations), hypophosphorylated pRB may be comprised of multiple phospho-isoforms containing a limited number of phosphates per molecule of pRB. Indeed, Brown et al. concluded that pRB is randomly phosphorylated (3).
FIG. 2.
Hypophosphorylated pRB is comprised of multiple phospho-isoforms. (A) pRB contains 16 Cdk consensus phosphorylation sites (top panel). NH2OH cleaves at two specific sites in the N-terminal region of pRB. [32P]orthophosphate-labeled hypophosphorylated pRB was immunoprecipitated, purified by SDS-PAGE (6% polyacrylamide), excised, treated with NH2OH, and then resolved by a second SDS-PAGE (15% polyacrylamide). NH2OH cleavage results in the appearance of four separable cleavage products (A to D) that each contain phosphate groups. Fragment identity was confirmed by anti-pRB immunoblot analysis using N- and C-terminus-specific antibodies. (B) Immunoprecipitated unphosphorylated (Un-PO4) pRB from G0 PBLs separated as a single species (diagonal arrow) by 2D-IEF (top). 2D-IEF of immunoprecipitated pRB from PHA-stimulated (24-h) early-G1 PBLs separated as multiple phospho-isoforms (bracket). Anti-E2F4 antibodies preferentially coimmunoprecipitated a single hypophosphorylated (Hypo-PO4) pRB phospho-isoform (vertical arrow, bottom). pRB was separated by first-dimension IEF (horizontal axis, pH units indicated) and second-dimension SDS-PAGE (vertical axis) and then immunoblotted with anti-pRB antibodies. α, antibody; IP, immunoprecipitates.
To investigate the distribution of phosphates on hypophosphorylated pRB in vivo, we sought to ascertain if hypophosphorylated pRB was phosphorylated throughout the protein or if it was limited to specific regions of pRB. To do so, we treated hypophosphorylated pRB with hydroxylamine (NH2OH), which specifically cleaves proteins between Asn and Gly motifs (52). pRB contains two such motifs at positions 247 and 309 that result in separation of the four N-terminal-most Cdk phosphorylation sites from the C-terminal sites (Fig. 2A). Although NH2OH cleavage is relatively inefficient, treatment of in vivo [32P]orthophosphate-labeled immunoprecipitated hypophosphorylated pRB with NH2OH resulted in the detection of [32P]phosphate groups on all pRB cleavage products, including both N-terminal and C-terminal cleavage products (Fig. 2A). Location of the NH2OH cleavage products were confirmed by anti-pRB immunoblot analysis using both N- and C-terminus-specific antibodies (data not shown). Thus, hypophosphorylated pRB contains phosphorylation sites throughout the length of the protein, including the N terminus.
Although the association of pRB with E2F4 was observed only in early-G1 cells when pRB was hypophosphorylated, we could not definitively exclude the possibility of the binding of unphosphorylated pRB present in the cells. Therefore, to further understand the phosphorylation status of pRB in vivo, we sought to separate hypophosphorylated pRB phospho-isoforms by 2D-IEF (48). By 2D-IEF analysis, unphosphorylated pRB from G0 PBLs was detected principally as a single, unphosphorylated species with a pI of 7.8, close to the predicted pI of 8.3 for unphosphorylated pRB (Fig. 2B, top panel). In contrast, hypophosphorylated pRB from early-G1 PBLs showed both a shift to more acidic isoforms, consistent with the addition of negatively charged phosphate groups, and the presence of multiple pRB isoforms (Fig. 2B, middle panel).
We next analyzed the hypophosphorylated form(s) of pRB associated with endogenous E2F4. Anti-E2F4 immunoprecipitates were subjected to 2D-IEF and immunoblotted with anti-pRB antibodies (Fig. 2B, bottom panel). Strikingly, endogenous E2F4 was found preferentially associated with specific pRB phospho-isoforms and, consistent with the data shown in Fig. 1, E2F4 did not coimmunoprecipitate unphosphorylated pRB. These observations suggest that specific hypophosphorylated pRB phospho-isoforms have an enhanced affinity to bind cellular E2F transcription factors but that other hypophosphorylated pRB phospho-isoforms, as well as unphosphorylated pRB, have substantially reduced affinities for endogenous E2Fs. Thus, we conclude that hypophosphorylated pRB is randomly phosphorylated on Cdk consensus sites, resulting in multiple phospho-isoforms, some or all of which represent the biologically active form of pRB in vivo.
Cyclin D-Cdk4/6 complexes hypophosphorylate pRB in early G1.
Sequential phosphorylation of pRB by cyclin D-Cdk4/6 and cyclin E-Cdk2 complexes (16, 33) in G1 is the generally accepted model of pRB phosphorylation (57). However, due to overlapping kinase activity profiles in G1, the exact physiological consequences of pRB phosphorylation by each kinase remain unclear. Moreover, cyclin D-Cdk4 function in Drosophila has recently been linked to cell growth (accumulation of mass) and not to cell cycle progression (7, 37). Therefore, we sought to independently assay for the contribution of cyclin D-Cdk4/6 and cyclin E-Cdk2 complexes in regulating pRB in primary human PBLs. Consistent with previous observations (38), G0 PBLs contained no detectable cyclin D-Cdk6 activity (Fig. 3A). However, PHA stimulation of G0 PBLs resulted in detection of active cyclin D-Cdk6 complexes by 8 to 12 h and maximal activity by 18 to 24 h (Fig. 3A), with no detectable cyclin E-Cdk2 activity until ∼30 h (data not shown). Thus, in vivo, cyclin D-Cdk6 complexes are extensively active during early G1, when pRB is in its active, hypophosphorylated form (Fig. 1). These observations suggested that cyclin D-Cdk4/6 complexes are a candidate for the early-G1-pRB-hypophosphorylating kinase.
FIG. 3.
Cyclin D-Cdk4/6 complexes are active when pRB is hypophosphorylated in early G1. (A) Anti-Cdk6 immunoprecipitation (αCdk6 IP) kinase analysis detected no cyclin D-Cdk6 kinase activity in G0 quiescent human PBLs. PHA-stimulated early-G1 PBLs (24 h) contained substantial cyclin D-Cdk6 activity. αM, anti-mouse IgG negative control; αK6, anti-Cdk6. (B) Anti-p16 immunoblot (αp16 blot) analysis of PBLs treated with increasing concentrations of TAT-p16 protein demonstrated a linear increase of intracellular TAT-p16 protein (top). Anti-Cdk6 immunoprecipitation followed by anti-p16 immunoblot analysis detected transduced TAT-p16 bound to endogenous Cdk6. Note that PBLs do not express endogenous p16. (C) Anti-IL-2R flow cytometry of G0 PBLs and PHA-stimulated early-G1 PBLs with and without TAT-p16 treatment. Upregulation of IL-2R was detected in both PHA-stimulated populations. Unstim., unstimulated; stim., stimulated.
To directly determine if cyclin D-Cdk6 complexes hypophosphorylate pRB in vivo and if hypophosphorylation is required for in vivo association with E2Fs, we used the method of TAT-mediated protein transduction (54, 55) to introduce the p16INK4a tumor suppressor protein, a specific negative regulator of Cdk4/6, into ∼100% of PBLs. Previously, we have demonstrated that TAT-mediated protein transduction occurs in a rapid, concentration-dependent and cell cycle-independent fashion that targets ∼100% of primary and transformed cells in culture and mice (19, 30, 43, 53, 61). We have characterized the ability of transduced TAT-p16 protein to bind and inactivate Cdk4/6 and to elicit G1 cell cycle arrest (16, 29). Consistent with what happens with other cell types, treatment of PBLs with increasing concentrations of TAT-p16 protein resulted in an intracellular concentration-dependent increase of TAT-p16 protein and sequestration of Cdk6 (Fig. 3B). However, treatment of PHA-stimulated PBLs with TAT-p16 did not prevent IL-2R upregulation (Fig. 3C), suggesting that cyclin D-Cdk4/6 activity is not required for IL-2 upregulation. Thus, unlike other methodologies, protein transduction allows for the rapid intracellular introduction of proteins into 100% of cells and thereby allows for dissection of cyclin D-Cdk4/6 function in primary cells.
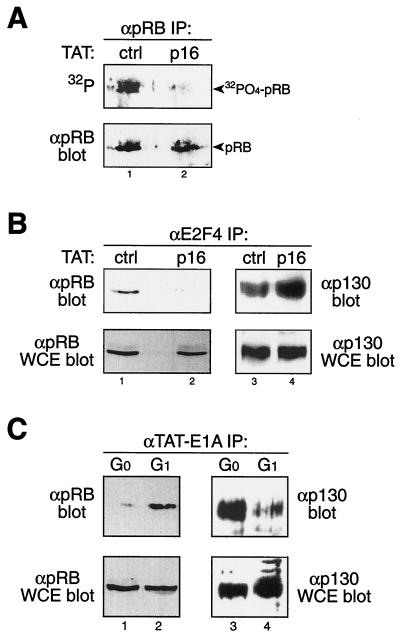
To assay for the contribution of cyclin D-Cdk6 complexes in hypophosphorylating pRB, we treated PHA-stimulated PBLs with TAT-p16 proteins during concomitant in vivo [32P]orthophosphate labeling. Treatment of early-G1 PBLs with TAT-p16 protein resulted in a complete loss of hypophosphorylated pRB and the appearance of unphosphorylated pRB, whereas control PBLs contained hypophosphorylated pRB (Fig. 4A). These observations suggested that, in vivo, cyclin D-Cdk4/6 complexes are the early-G1-pRB-hypophosphorylating kinase. To assay for the in vivo requirement of pRB hypophosphorylation, TAT-p16-treated PBLs were assayed for assembly of pRB-E2F4 complexes. Inactivation of cyclin D-Cdk4/6 complexes in early-G1 PBLs by transduction of TAT-p16 protein and subsequent loss of pRB hypophosphorylation inhibited the ability of pRB to assemble with E2F4 in vivo (Fig. 4B, left panel). In contrast, treatment of PHA-stimulated PBLs with TAT-p16 protein resulted in an enhanced assembly of p130-E2F4 complexes (Fig. 4B, right panel). Importantly, both E2F4 and pRB levels were not altered by TAT-p16 treatment.
FIG. 4.
Cyclin D-Cdk4/6 complexes generate biologically active, hypophosphorylated pRB. (A) pRB immunoprecipitated from PHA-stimulated PBLs concomitantly labeled in vivo with [32P]orthophosphate and treated with TAT-p16 protein was analyzed by phosphorimaging (top) and anti-pRB immunoblotting (bottom). Inactivation of Cdk4/6 by TAT-p16 resulted in the loss of pRB hypophosphorylation. α, anti-; IP, immunoprecipates; ctrl, control. (B) Anti-E2F4 immunoprecipitation followed by anti-pRB (left) or anti-p130 (right) immunoblot analysis from PHA-stimulated early-G1 PBLs treated with TAT-p16 protein. Inactivation of cyclin D-Cdk4/6 by TAT-p16 prevented assembly of pRB-E2F4 complexes (left) while enhancing p130-E2F4 complex formation (right). pRB (bottom left), p130 (bottom right), and E2F4 (data not shown) levels remained constant in controls and treated cells. WCE, whole-cell extract. (C) G0 and G1 PBLs were treated with TAT-E1A protein (2 h), followed by anti-E1A immunoprecipitation and then anti-pRB (left) or anti-p130 (right) immunoblot analysis. Protein levels in G0 and early-G1 PBLs were controlled by immunoblot analysis of whole-cell extracts with anti-pRB (left) and anti-p130 (right) antibodies.
To measure independently pRB's binding capabilities, we sought to introduce E1A into both G0 and G1 PBLs and assay for pRB binding. We have previously described a transducible TAT-E1A protein (43, 62) that transduces equally efficiently into G0 and G1 PBLs (data not shown). Consistent with above observations, ectopically transduced TAT-E1A protein also preferentially formed complexes with hypophosphorylated pRB from early-G1 PBLs and showed a significantly lower avidity for unphosphorylated pRB from G0 PBLs (Fig. 4C, left panel). In contrast, transduced TAT-E1A readily bound unphosphorylated p130 from G0 PBLs (Fig. 4C, right panel). Taken together, these observations support the notions that hypophosphorylated pRB represents the fully functional form of pRB assembled with both endogenous E2Fs and E1A and that cyclin D-Cdk4/6 complexes are the early-G1-pRB-hypophosphorylating kinase.
Cyclin E-Cdk2 complexes inactivate pRB by hyperphosphorylation in late G1.
As ascertained above, pRB is hypophosphorylated in early G1 by cyclin D-Cdk4/6 complexes and then becomes inactivated by hyperphosphorylation at the late G1 restriction point and remains so throughout late G1, S, G2, and M phases (39). Using synchronized primary human diploid fibroblasts, we next investigated the role of cyclin E-Cdk2 in hyperphosphorylating pRB. Fibroblasts were synchronized by contact inhibition (density arrest) specifically in media containing serum to maintain constant levels of cyclin D-Cdk4/6 activity (9, 36). Replating of arrested fibroblasts (>90% in G1) at low density routinely resulted in 40 to 50% of the cells traversing early G1 and entering S and G2/M phases by 25 h postreplating (data not shown).
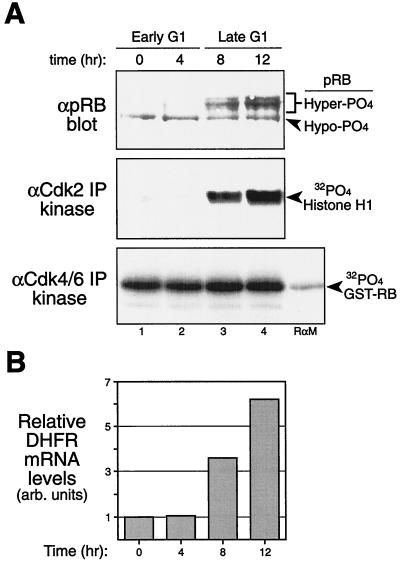
pRB was maintained in a hypophosphorylated form as assayed by in vivo [32P]orthophosphate labeling at the start of the time course and 4 h postreplating (Fig. 5A, top panel; data not shown). The inactive, slower-migrating hyperphosphorylated pRB form first appeared at 8 h postreplating (Fig. 5A, top panel). Significantly, the appearance of hyperphosphorylated pRB correlated with the initial detection of active cyclin E-Cdk2 complexes (Fig. 5A, middle panel). In contrast, cyclin D-Cdk4/6 activity was constant throughout the time course (Fig. 5A, bottom panel), including at the early-G1 time points (0 and 4 h), when pRB remained in the active, hypophosphorylated form (Fig. 5A, top panel) and assembled with E2F4. These observations are consistent with a role for cyclin D-Cdk4/6 complexes in performing the hypophosphorylation of pRB and suggest that cyclin E-Cdk2 may perform the initial physiological inactivating hyperphosphorylation of pRB.
FIG. 5.
Analysis of G1-phase cell cycle progression in human diploid fibroblasts. (A) Human diploid fibroblasts were high density arrested (contact inhibited) in serum for 45 h, released by replating them at low density, and analyzed at the indicated times by anti-pRB immunoblot (top panel), anti-Cdk2 immunoprecipitation kinase (middle panel), and anti-Cdk4/6 immunoprecipitation kinase (bottom panel) assays. Note the presence of the faster-migrating, active, hypophosphorylated pRB form in early-G1 cells (0 and 4 h) and the characteristic slower-migrating, inactive, hyperphosphorylated pRB in late-G1 cells (8 and 12 h). Note that the activation of Cdk2 kinase was detected concomitantly with the appearance of hyperphosphorylated pRB; however, cyclin D-Cdk6 kinase activity remained constant when pRB was present in its active, hypophosphorylated form in early G1 (0 and 4 h). (B) RT-PCR analysis of DHFR mRNA levels during the same time course as in panel A to detect induction of DHFR (an E2F-responsive gene) concomitant with the appearance of hyperphosphorylated pRB and cyclin E-Cdk2 activity at 8 h. DHFR levels were normalized to GAPDH levels. α, anti-; Hyper-PO4, hyperphosphorylated; Hypo-PO4, hypophosphorylated; IP, immunoprecipitates; arb. arbitrary.
pRB represses the transcriptional activities of promoters containing E2F sites, a number of which are involved in DNA synthesis and expressed in late G1, including the DHFR and cyclin E genes (12, 46). Given the requirement for biologically active pRB to repress DHFR transcription, we assayed DHFR mRNA accumulation by RT-PCR analysis. mRNA samples were isolated from replated human fibroblasts over the time course, and RT-PCR was performed for DHFR levels and normalized to GAPDH levels. DHFR mRNA levels remained repressed at the early-G1 time points (0 and 4 h) (Fig. 5B), during which cyclin D-Cdk4/6 complexes were active and pRB was hypophosphorylated (Fig. 5A). In contrast, DHFR levels showed a substantial induction at 8 h postreplating (Fig. 5B), concomitant with both the activation of cyclin E-Cdk2 complexes and the appearance of hyperphosphorylated pRB (Fig. 5A). Similar results were obtained for induction of cyclin E mRNA (data not shown). Thus, contrary to the results of some overexpression studies, these biological observations of normal human diploid fibroblasts suggest that hypophosphorylated pRB remains as both an active and passive repressor of endogenous E2F-responsive genes in the presence of active cyclin D-Cdk4/6 complexes during early G1.
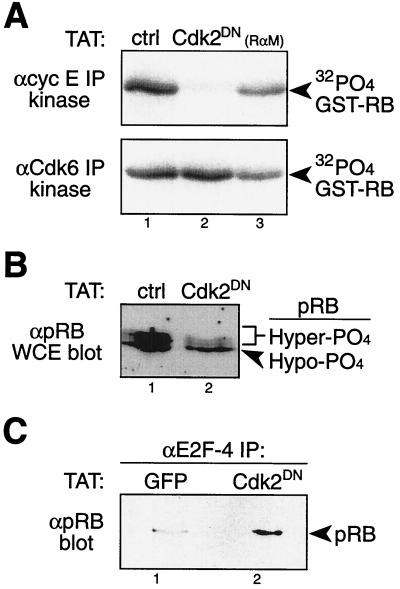
We next sought to specifically inactivate cyclin E-Cdk2 complexes, while maintaining cyclin D-Cdk4/6 activity. Previously, van den Heuvel and Harlow described a Cdk2DN mutant that retains the ability to sequester cyclins E and A but is inactive for kinase activity (60). We made a transducible TAT-Cdk2DN protein that rapidly transduces into ∼100% of cells, sequesters cyclins E and A, and elicits a G1-phase cell cycle arrest (43, 62). Consistent with results obtained by transfection of Cdk2DN into tumor cells (33), transduction of TAT-Cdk2DN protein into replated diploid human fibroblasts elicited an early-G1-phase cell cycle arrest and effectively blocked cyclin E-Cdk2 activity (Fig. 6A). Importantly, TAT-Cdk2DN-treated fibroblasts maintained active cyclin D-Cdk6 complexes (Fig. 6A). Consistent with the above observations, TAT-Cdk2DN-treated fibroblasts retained hypophosphorylated pRB (Fig. 6B) that was biologically active and associated with endogenous E2F4 (Fig. 6C). Control and TAT-GFP-treated fibroblasts contained active Cdk2 (Fig. 6A) and hyperphosphorylated pRB (Fig. 6B) that was not associated with E2F4 (Fig. 6C). Thus, specific and complete inactivation of cyclin E-Cdk2 complexes preserved functional, hypophosphorylated pRB bound to E2Fs in the presence of active cyclin D-Cdk4/6 complexes.
FIG. 6.
Cyclin E-Cdk2 complexes inactivate pRB by hyperphosphorylation at the late-G1 restriction point. (A) Density-arrested and replated human diploid fibroblasts transduced with TAT-Cdk2DN protein for 8 h showed specific loss of cyclin E-Cdk2 kinase activity (top panel) with continued cyclin D-Cdk6 activity (bottom panel). ctrl, control; RαM, anti-mouse IgG negative control; αcyc E IP; anti-cyclin E immunoprecipitates. (B) Replated fibroblasts from the experiment whose results are shown in panel A were treated with TAT-Cdk2DN protein and immunoblotted for pRB-maintained hypophosphorylated (Hypo-PO4) pRB, while control treated fibroblasts contained hyperphosphorylated (Hyper-PO4) pRB. WCE, whole-cell extracts. (C) Replated fibroblasts from the experiment whose results are shown in panel A were treated with TAT-Cdk2DN protein, followed by anti-E2F4 immunoprecipitation and anti-pRB immunoblot analysis. Hypophosphorylated pRB in TAT-Cdk2DN protein-treated fibroblasts remained biologically active and assembled to E2F4, while control TAT-GFP-treated fibroblasts contained inactive, hyperphosphorylated pRB.
Taken together, these observations made with primary, human diploid cells (fibroblasts and PBLs) support a role for cyclin D-Cdk4/6 complexes as the early-G1-pRB-hypophosphorylating kinase and a role for cyclin E-Cdk2 complexes as the inactivating pRB-hyperphosphorylating kinase at the late-G1 restriction point.
pRB remains hypophosphorylated and bound to E2Fs, and E2F-responsive genes remain repressed in early G1 phase of p16-minus tumor cells.
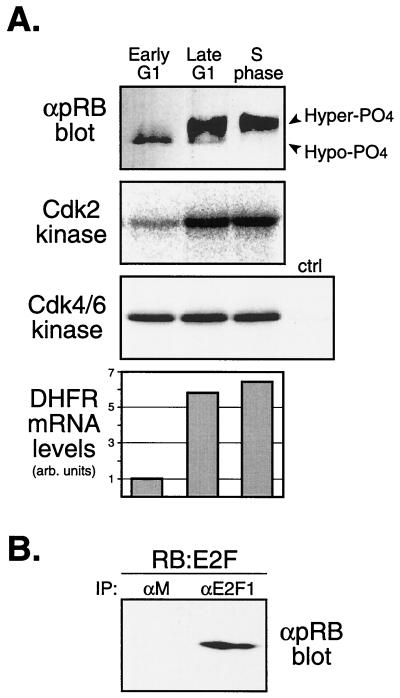
Loss of the p16INK4a gene or amplification of the cyclin D1, Cdk4, or Cdk6 genes occurs in the vast majority of human malignancies (51, 56). Previous work has suggested that deregulation of cyclin D-Cdk4/6 kinases results in phosphorylation of pRB and loss of pRB transcriptional repression (15, 21, 49, 50, 56). Given the role of cyclin D-Cdk4/6 complexes in hypophosphorylating pRB in normal, wild-type PBLs and diploid human fibroblasts (see above), we sought to determine the status of pRB during the early G1 phase in continuously cycling, p16-minus tumor cells. To do so, we synchronized cycling, human Jurkat leukemic T cells, which have both copies of the p16INK4a gene deleted (47), by centrifugal elutriation in medium plus serum (11). Elutriated fractions containing early-G1, late-G1, and S-phase cells were analyzed for pRB phosphorylation status; the activities of cyclin E-Cdk2 and cyclin D-Cdk4/6 complexes; induction of the endogenous DHFR gene, an E2F-responsive gene (12, 46); and association of pRB with E2F1 (Fig. 7). Consistent with the results from the wild-type cells described above, early-G1 tumor cells contained pRB bound to E2F1 (Fig. 7B) in the presence of high levels of cyclin D-Cdk4/6 kinase activity and inactive cyclin E-Cdk2 complexes (Fig. 7A). Importantly, the DHFR gene remained transcriptionally repressed during the early G1 phase (Fig. 7A), as did the cyclin E gene, another E2F-responsive gene (data not shown). In contrast, activation of cyclin E-Cdk2 complexes in late-G1-phase tumor cells resulted in hyperphosphorylation of pRB and induction of both the DHFR and cyclin E genes (Fig. 7 and data not shown). Moreover, these results are consistent with the original detection of pRB-HDAC complexes in p16-deleted Jurkat T cells (2, 34, 47). Thus, in both normal wild-type human cells and human tumor cells in which p16INK4a is deleted, pRB is hypophosphorylated and bound to E2Fs and E2F-responsive genes remain transcriptionally repressed in early G1 phase.
FIG. 7.
Analysis of synchronized p16-minus Jurkat leukemic T cells. (A) Asynchronous Jurkat T cells were synchronized by centrifugal elutriation. Early-G1, late-G1, and S-phase cellular fractions were analyzed for pRB phosphorylation status (immunoblot analysis), cyclin E-Cdk2 and cyclin D-Cdk4/6 kinase activities, and DHFR mRNA levels (RT-PCR analysis). αpRB blot, anti-pRB blotting; Hyper-PO4, hyperphosphorylated; Hypo-PO4, hypophosphorylated; arb., arbitrary. (B) Early-G1-phase elutriated Jurkat cellular fractions from the above-described experiment were immunoprecipitated with anti-E2F1 or control anti-IgG (αM) antibodies, followed by anti-pRB immunoblot analysis. pRB-E2F1 complexes remained assembled in early-G1-phase cells. IP, immunoprecipitates.
DISCUSSION
Previous studies investigating the role of specific G1 cyclin-Cdk complexes in regulating pRB function have generally relied on overexpression systems using tumor cell lines containing multiple genetic alterations (51, 56). In the biological experiments presented here, we specifically focused on two different primary human diploid cells, PBLs and fibroblasts, that were manipulated by the introduction of negative regulatory (dominant-negative) transducible proteins. We find that, under physiological conditions, hypophosphorylated pRB is the biologically active form of pRB that it assembled with transcription factors, such as E2F family members, and with ectopically introduced viral oncoproteins, such as E1A, with high avidity. Surprisingly, in vivo, unphosphorylated pRB failed to form complexes with either endogenous E2F4 or transduced E1A. However, these observations are entirely consistent with previous reports demonstrating that as cells transit from early G1 back into G0 quiescence, E2Fs shift from pRB complexes to binding the pRB-related protein p130 (41, 58). Indeed, our results offer a molecular mechanism to explain the observed switch of E2Fs from p130 to pRB and back again. Thus, cells in G0 select for p130-E2F complexes by maintaining pRB in an unphosphorylated state with consequential low avidity for E2Fs whereas cells in early G1 select for pRB-E2F complexes by increasing pRB's avidity for E2Fs (and E1A) by activating the early-G1-pRB-hypophosphorylating kinase, cyclin D-Cdk4/6.
The complexity of pRB hypophosphorylation has largely been overlooked since its first discovery (26). Indeed, in vivo, hypophosphorylated pRB is phosphorylated on 13 of the 16 Cdk consensus sites that are also used to inactivate pRB by hyperphosphorylation (8, 27, 40). However, hypophosphorylated pRB contains ∼1 to 2 mol of phosphate per mol of pRB compared to ∼10 mol of phosphate per mol of hyperphosphorylated pRB (40; S. A. Ezhevsky and S. F. Dowdy, unpublished observations). These observations suggest that hypophosphorylated pRB may be comprised of multiple phospho-isoforms, the summation of which gives a 2D phosphopeptide map that is nearly identical to that of the much more heavily hyperphosphorylated pRB species. Indeed, we detected multiple phospho-isoforms of endogenous hypophosphorylated pRB by 2D-IEF analysis. In addition, consistent with Brown et al. (3), we found that hypophosphorylated pRB contains phosphates throughout the length of the protein, including the N terminus. Taken together, these observations suggest that Cdk sites on pRB are used to both activate pRB by hypophosphorylation and inactivate it by hyperphosphorylation, dependent on the phosphate stoichiometry and perhaps location. Although this paradigm is not a new concept in biology, as many proteins are both activated and inactivated by phosphorylation, including Cdks (42), the pRB, p107, and p130 pocket proteins may be unique in their use of the stoichiometry of phosphorylation at the same sites used for regulation. Likewise, activating hypophosphorylation sites may represent a subset of N-terminal phosphorylation sites on pRB.
Several studies have generated altered pRB forms containing mutations of up to 9 of the 16 total Cdk phosphorylation sites on pRB (6, 20, 24, 28, 32). When overexpressed in cells, these altered pRB forms bind E2Fs and result in an enhanced and, in some instances, irreversible cell cycle arrest. However, where investigated with in vivo [32P]orthophosphate labeling (24), the remaining Cdk sites on these altered pRB proteins were hypophosphorylated. Moreover, due to overexpression of pRB to levels not achieved physiologically, these studies may very well have bypassed the intricate regulatory mechanisms that cells have devised to regulate G1 cell cycle progression. Indeed, physiological induction of pRB above basal levels of expression in G1 has not been observed as a biological mechanism for eliciting a G1 arrest whereas regulation of cyclin-Cdk activity is commonly observed.
The demonstration here that hypophosphorylated pRB is the biologically active form of pRB in vivo and that it is phosphorylated on Cdk sites raised the question as to what the early-G1-pRB-hypophosphorylating kinase is. Either there is an unknown cyclin-Cdk complex that performs this function or a known cyclin-Cdk has been overlooked. Based on our previous observations (16) and those of others (33), we initially focused on cyclin D-Cdk4/6 complexes. Cyclin D-Cdk4/6 activity is constitutive in cycling cells during the entire G1 phase and is also induced when G0 quiescent cells are stimulated with growth factors to enter early G1 (23, 36, 38, 44). However, under both circumstances (cycling and stimulation of G0 cells), cyclin D-Cdk4/6 complexes are fully active in early G1 when pRB is present in its active, hypophosphorylated form bound to E2Fs and repressing E2F-responsive genes, such as the DHFR and cyclin E genes (Fig. 5). Indeed, with T cells, cyclin D-Cdk6 complexes are active for ∼20 h prior to pRB hyperphosphorylation by cyclin E-Cdk2 complexes and for ∼8 h in human diploid fibroblasts (Fig. 1, 3, 5, and 7). In addition, introduction of the Cdk4/6-specific negative regulator p16 into primary cells by protein transduction demonstrated that cyclin D-Cdk4/6 complexes are the early-G1-pRB-hypophosphorylating kinase. Furthermore, these observations are entirely consistent with reports demonstrating the presence of both active cyclin D-Cdk4/6 complexes and active hypophosphorylated pRB (5, 16, 17, 29, 44, 45). Moreover, and importantly, cyclin D-Cdk4 function in Drosophila is involved in growth regulation (accumulation of mass) and not cell cycle progression (7, 37). Lastly, as demonstrated in Fig. 7, even leukemic T cells that contain deregulated cyclin D-Cdk4/6 complexes, due to deletion of the p16INK4a gene, maintain active, hypophosphorylated pRB in early G1 and repress E2F-responsive genes. Taken together, these observations demonstrate that cyclin D-Cdk4/6 complexes are the early-G1-pRB-hypophosphorylating kinase.
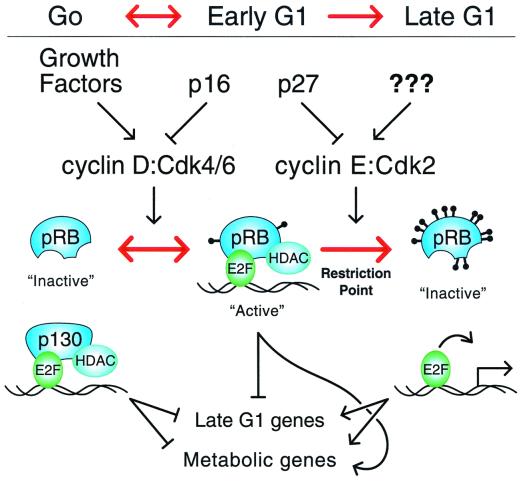
As previously proposed by Sherr (56) and recently supported by genetic experiments with Drosophila (7, 37), these observations are entirely consistent with a role for cyclin D-Cdk4/6 complexes in driving cells out of G0 quiescence into early G1 by replacing G0 p130-E2F complexes with early-G1 hypophosphorylated pRB-E2F complexes and increasing cellular metabolism. However, as demonstrated here and by others (33, 60, 63), under physiological conditions, cyclin D-Cdk4/6 complexes alone are not sufficient to drive cells across the restriction point into late G1. Cyclin E-Cdk2 activity is required to both hyperphosphorylate pRB and drive cells across the restriction point (16, 19, 23, 33, 44, 56). Thus, cyclin D-Cdk4/6 complexes serve as a sensor for external growth factors to help drive cells to the reversible transition from G0 to early G1 while loss of growth factors results in inactivation of cyclin D-Cdk4/6 activity, loss of hypophosphorylated pRB-E2F complexes, subsequent gradual transition back into G0 quiescence, and preferential assembly of p130-E2F4 complexes as hypophosphorylated pRB is degraded and/or gradually dephosphorylated by an as yet unidentified phosphatase. In contrast, activation of cyclin E-Cdk2 complexes results in transition across the irreversible late-G1 restriction point, in part, by hyperphosphorylation of pRB (Fig. 8).
FIG. 8.
Model of G1 cell cycle progression. G0 cells maintain E2F complexed with p130. Growth factor stimulation activates cyclin D-Cdk4/6 complexes driving cells to passage through the reversible transition from G0 into early G1. Cyclin D-Cdk4/6 complexes hypophosphorylate pRB and thereby increase pRB's avidity for E2Fs. Maintenance of cyclin D-Cdk4/6 activity by continuous growth factor stimulation is required during early G1. Loss of growth factor signaling or increases in p16 drive cells back across this reversible transition into G0. Activation of cyclin E-Cdk2 in late G1 hyperphosphorylates pRB, causing the release of E2Fs to activate transcription and drive cells across the irreversible restriction point into late G1.
How does deregulation of cyclin D-Cdk4/6 complexes contribute to oncogenesis?
Due to the strong selection for deregulation of cyclin D-Cdk4/6 complexes in oncogenesis (51, 56), phosphorylation of pRB by cyclin D-Cdk4/6 complexes was previously assumed to be inactivating. Indeed, early studies showed that overexpression of p16INK4a in RB−/− human tumor cells and RB−/− MEFs failed to arrest these cells, supporting a linear model of p16-cyclin D-pRB. Surprisingly, however, are recent observations by Bruce et al. demonstrating that p16 also fails to arrest p130 or p107 nullizygous MEFs that remain wild type for pRB (4). These observations suggest that p16-mediated cell cycle arrest requires p130 or p107, as well as pRB, and are entirely consistent with our observations demonstrating a role for p130-E2Fs in regulating the G0 quiescent state. Moreover, increased levels of p16INK4a are generally detected only in cells that have exited the cell cycle into G0 quiescence and are undergoing senescence (1, 14). These observations further support a role for p16INK4a in driving cells permanently out of early G1 into G0 and for cyclin D-Cdk4/6 complexes to avoid G0 exit by maintaining hypophosphorylated pRB-E2F complexes.
Consistent with our in vivo observations, Harbour et al. have recently demonstrated by in vitro and overexpression experiments that phosphorylation of pRB by cyclin D-Cdk4 complexes does not result in the release of E2Fs from pRB (21). That study also showed that phosphorylation of pRB by overexpressed cyclin D-Cdk4 complexes releases HDAC from pRB, suggestive of a role for deregulated cyclin D-Cdk4/6 in tumors. In direct contradiction to that study, Brown et al. showed that cyclin D-Cdk4 complexes could not dissociate pRB-E2Fs or relieve transcriptional repression of E2F-responsive genes by pRB but that cyclin E-Cdk2 complexes efficiently relieved the repression (3). Moreover, the in vivo pRB-HDAC association was first reported from Jurkat T cells that have deregulated cyclin D-Cdk6 kinase activity due to a homozygous deletion of the p16INK4a gene (2, 34, 47). Furthermore, as shown in Fig. 7, endogenous E2F-responsive genes, such as those for DHFR and cyclin E, continue to be repressed during early G1 in these p16-minus Jurkat tumor cells.
We now know that nonphysiologically overexpressed cyclin D-Cdk4/6 complexes can perform at least three distinct functions. (i) These complexes can phosphorylate pRB, p130, and p107. (ii) The LXCXE domain on D-type cyclins can compete for binding to pRB with cellular LXCXE binding proteins, including HDAC (22). (iii) Overexpressed cyclin D-Cdk4/6 complexes can inappropriately activate cyclin E-Cdk2 complexes by sequestration of p21 and p27 (57). However, our observations do not exclude the possibility that deregulated cyclin D-Cdk4/6 activity in tumors may generate hypophosphorylated pRB isoforms with reduced avidity for other cellular transcription factors. Indeed, we note that some hypophosphorylated pRB isoforms present in normal cells do not bind E2F4 (Fig. 2).
Conclusions.
In summary, our observations point to a role for p16 and cyclin D-Cdk4/6 in regulating the reversible transition from G0 to early G1 and not the late G1 restriction point or S-phase entry. We conclude that deregulation of cyclin D-Cdk4/6 complexes in human malignancy prevents cells from exiting early G1 into G0 and that additional mutational events are required to inappropriately activate cyclin E-Cdk2 complexes to drive cells across the irreversible restriction point into late G1 and S phase. In addition, activation of cyclin D-Cdk4/6 complexes leads directly or indirectly to activation of metabolic genes in early G1 (Fig. 8). While this may superficially appear to be a rather unimpressive event to select for during nascent oncogenesis compared with inactivation of a tumor suppressor protein, in fact, upregulation of metabolism is a critical determinant for cancer cells to maintain their cellular mass and sustained deregulated cellular division. Moreover, and importantly, this is a subtle phenotype that likely does not induce apoptosis in a primary cell harboring a newly mutated p16 or cyclin D gene.
ACKNOWLEDGMENTS
We thank J. Lees and K. Moberg (Massachusetts Institute of Technology) for anti-E2F4 antibodies.
S.A.E. was supported by an NCI training grant, and A.H. was supported by an NIH Medical Scientist Training Program grant. This work was supported by the Howard Hughes Medical Institute.
S. A. Ezhevsky and A. Ho contributed equally to this work.
REFERENCES
- 1.Alcorta D A, Xiong Y, Phelps D, Hannon G, Beach D, Barrett J C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 3.Brown V D, Phillips R A, Gallie B L. Cumulative effect of phosphorylation of pRB on regulation of E2F activity. Mol Cell Biol. 1999;19:3246–3256. doi: 10.1128/mcb.19.5.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce J L, Hurford R K, Classon M, Koh J, Dyson N. Requirements for cell cycle arrest by p16INK4a. Mol Cell. 2000;6:737–742. doi: 10.1016/s1097-2765(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 5.Brugarolas J, Moberg K, Boyd S D, Taya Y, Jacks T, Lees J A. Inhibition of cyclin-dependent kinase-2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after gamma-irradiation. Proc Natl Acad Sci USA. 1999;96:1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datar S A, Jacobs H W, de La Cruz A F, Lehner C F, Edgar B A. The Drosophila cyclin D-cdk4 complex promotes cellular growth. EMBO J. 2000;19:4543–4554. doi: 10.1093/emboj/19.17.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeCaprio J A, Furukawa T, Ajchenbaum F, Griffin J D, Livingston D M. The retinoblastoma-susceptibility gene product becomes phosphorylated in multiple stages during cell cycle entry and progression. Proc Natl Acad Sci USA. 1992;89:1795–1798. doi: 10.1073/pnas.89.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich C, Wallenfang K, Oesch F, Wieser R. Differences in the mechanisms of growth control in contact-inhibited and serum-deprived human fibroblasts. Oncogene. 1997;15:2743–2747. doi: 10.1038/sj.onc.1201439. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 11.Dowdy S F, Van Dyk L, Schreiber G S. Cell cycle synchronization by elutriation. In: Adolph K W, editor. Human genome methods. Boca Raton, Fla: CRC Press; 1997. pp. 121–132. [Google Scholar]
- 12.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 13.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 14.Erickson S, Sangfelt O, Heyman M, Castro J, Einhorn S, Grander D. Involvement of the Ink4 proteins p16 and p15 in T-lymphocyte senescence. Oncogene. 1998;17:595–602. doi: 10.1038/sj.onc.1201965. [DOI] [PubMed] [Google Scholar]
- 15.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 16.Ezhevsky S A, Nagahara H, Vocero-Akbani A M, Gius D R, Wei M C, Dowdy S F. Hypophosphorylation of the retinoblastoma protein by cyclin D:Cdk4/6 complexes results in active pRB. Proc Natl Acad Sci USA. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 18.Firpo E J, Koff A, Solomon M J, Roberts J M. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1994;14:4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gius D R, Ezhevsky S A, Becker-Hapak M, Nagahara H, Wei M C, Dowdy S F. Transduced p16INK4a peptides inhibit hypophosphorylation of the retinoblastoma protein and cell cycle progression prior to activation of Cdk2 complexes in late G1. Cancer Res. 1999;59:2577–2580. [PubMed] [Google Scholar]
- 20.Hamel P A, Cohen B L, Sorce L M, Gallie B L, Phillips R A. Hyperphosphorylation of the retinoblastoma gene product is determined by domains outside the simian virus 40 large-T-antigen-binding regions. Mol Cell Biol. 1990;10:6586–6595. doi: 10.1128/mcb.10.12.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbour J W, Luo R X, Dei Santi A, Postigo A A, Dean D C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 22.Harbour J W, Dean D C. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–E67. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, Chou H S, Zhu L. Requirement of cyclin E-Cdk2 inhibition in p16INK4a-mediated growth suppression. Mol Cell Biol. 1998;18:5284–5290. doi: 10.1128/mcb.18.9.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudsen E S, Wang J Y. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Mol Cell Biol. 1997;17:5771–5783. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh J, Enders G H, Dynlacht B D, Harlow E. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995;375:506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- 26.Lee W H, Shew J Y, Hong F D, Sery T W, Donoso L A, Young L J, Bookstein R, Lee E Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987;329:642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- 27.Lees J A, Buchkovich K J, Marshak D R, Anderson C W, Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991;10:4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leng X, Connell-Crowley L, Goodrich D, Harper J W. S-phase entry upon ectopic expression of G1 cyclin-dependent kinases in the absence of retinoblastoma protein phosphorylation. Curr Biol. 1997;7:709–712. doi: 10.1016/s0960-9822(06)00301-0. [DOI] [PubMed] [Google Scholar]
- 29.Lissy N A, Van Dyk L F, Becker-Hapak M, Vocero-Akbani A, Mendler J H, Dowdy S F. TCR-antigen induced cell death (AID) occurs from a late G1 phase cell cycle check point. Immunity. 1998;8:57–65. doi: 10.1016/s1074-7613(00)80458-6. [DOI] [PubMed] [Google Scholar]
- 30.Lissy N A, Davis P K, Irwin M, Kaelin W G, Dowdy S F. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature. 2000;407:642–645. doi: 10.1038/35036608. [DOI] [PubMed] [Google Scholar]
- 31.Lukas J, Parry D, Aagaard L, Mann D J, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 32.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain J P, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 35.Mahony D, Parry D A, Lees E. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene. 1998;16:603–611. doi: 10.1038/sj.onc.1201570. [DOI] [PubMed] [Google Scholar]
- 36.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer C A, Jacobs H W, Datar S A, Du W, Edgar B A, Lehner C F. Drosophila cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J. 2000;19:4533–4542. doi: 10.1093/emboj/19.17.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittnacht S. Control of pRB phosphorylation. Curr Opin Genet Dev. 1998;8:21–27. doi: 10.1016/s0959-437x(98)80057-9. [DOI] [PubMed] [Google Scholar]
- 40.Mittnacht S, Lees J A, Desai D, Harlow E, Morgan D O, Weinberg R A. Distinct sub-populations of the retinoblastoma protein show a distinct pattern of phosphorylation. EMBO J. 1994;13:118–127. doi: 10.1002/j.1460-2075.1994.tb06241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moberg K, Starz M A, Lees J A. E2F4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan D O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 43.Nagahara H, Vocero-Akbani A M, Snyder E L, Ho A, Latham D G, Lissy N A, Becker-Hapak M, Ezhevsky S A, Dowdy S F. Transduction of full-length TAT fusion proteins into mammalian cells: p27Kip1 mediates cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 44.Nagahara H, Ezhevsky S A, Vocero-Akbani A M, Kaldis P, Solomon M J, Dowdy S F. TGF-β targeted inactivation of cyclin E:Cdk2 complexes by inhibition of Cdk2 activating kinase activity. Proc Natl Acad Sci USA. 1999;96:14961–14966. doi: 10.1073/pnas.96.26.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nath N, Bian H, Reed E F, Chellappan S P. HLA class I-mediated induction of cell proliferation involves cyclin E-mediated inactivation of RB function and induction of E2F activity. J Immunol. 1999;162:5351–5358. [PubMed] [Google Scholar]
- 46.Nevins J R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 47.Quesnel B, Preudhomme C, Lepelley P, Hetuin D, Vanrumbeke M, Bauters F, Velu T, Fenaux P. Transfer of p16inka/CDKN2 gene in leukaemic cell lines inhibits cell proliferation. Br J Haematol. 1996;95:291–298. doi: 10.1046/j.1365-2141.1996.d01-1913.x. [DOI] [PubMed] [Google Scholar]
- 48.Rabilloud T, Adessi C, Giraudel A, Lunardi J. Improvement of the solubilization of proteins in two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 1997;18:307–316. doi: 10.1002/elps.1150180303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruas M, Peters G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys Acta. 1998;1378:115–177. doi: 10.1016/s0304-419x(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 52.Saris C J, van Eenbergen J, Jenks B G, Bloemers H P. Hydroxylamine cleavage of proteins in polyacrylamide gels. Anal Biochem. 1983;132:54–67. doi: 10.1016/0003-2697(83)90425-6. [DOI] [PubMed] [Google Scholar]
- 53.Schwarze S R, Ho A, Vocero-Akbani A, Dowdy S F. In vivo protein transduction: delivery of biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 54.Schwarze S R, Dowdy S F. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol. 2000;21:45–48. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 55.Schwarze S R, Hruska K A, Dowdy S F. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- 56.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 57.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 58.Smith E J, Leone G, DeGregori J, Jakoi L, Nevins J R. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol Cell Biol. 1996;16:6965–6976. doi: 10.1128/mcb.16.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsutsui T, Hesabi B, Moons D S, Pandolfi P P, Hansel K S, Koff A, Kiyokawa H. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol. 1999;19:7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 61.Vocero-Akbani A, Vander Heyden N, Lissy N A, Ratner L, Dowdy S F. Killing HIV infected cells by direct transduction of an HIV protease-activated caspase-3 protein. Nat Med. 1999;5:29–33. doi: 10.1038/4710. [DOI] [PubMed] [Google Scholar]
- 62.Vocero-Akbani A, Lissy N A, Nagahara H, Snyder E L, Ho A, Latham D G, Becker-Hapa M, Gius D R, Ezhevsky S A, Dowdy S F. Transduction of full length TAT fusion proteins directly into mammalian cells: analysis of TCR-activation induced cell death (AID) Methods Enzymol. 2000;322:508–521. doi: 10.1016/s0076-6879(00)22046-6. [DOI] [PubMed] [Google Scholar]
- 63.Wagner M, Greten F R, Weber C K, Koschnick S, Mattfeldt T, Deppert W, Kern H, Adler G, Schmid R M. A murine tumor progression model for pancreatic cancer recapitulating the genetic alterations of the human disease. Genes Dev. 2001;15:286–293. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]