Abstract
Setting nutritional standards for larval zebrafish (Danio rerio) that maximize growth, survival, and reproductive success is challenging. We evaluated the effects of different feeding regimens on larval zebrafish by comparing Gemma Micro 75 pelleted diet and live-type L rotifers (Brachionus plicatilis) in 3 feeding regimens starting at 9 days postfertilization (dpf): bolus feeding of live diet (BL), continuous feeding of live diet (CL), and pelleted diet (PD). Animals in the PD and CL groups were longer than the BL group at 4–5 weeks postfertilization. The PD group was also greater in body depth than both live diet groups. There was no significant difference in weight between the groups. There were also no significant differences in fecundity or sex ratios indicating that all feeding methods successfully promote growth of a useful breeding stock of fish. In addition, we quantified the equipment, consumable, and labor costs associated with these methods, and found that the PD regimen was superior to both live diet regimens. These data suggest that providing a high nutrient-density pelleted diet to larval and juvenile zebrafish is an effective means to increase early growth and to decrease cost and labor associated with nursery care.
Keywords: zebrafish, nursery, feeding
Introduction
The zebrafish (Danio rerio) has become an important model species for studying many human diseases and metabolic disorders.1 The increased use of this model has led many laboratories to invest in equipment and labor to breed and maintain their fish colonies.
Zebrafish larvae are able to absorb nutrients from the yolk sac until 5–7 days postfertilization (dpf).2 Overlapping absorption of the yolk sac with exogenous feeding beginning around 5 dpf is advantageous for zebrafish rearing and is a common practice among zebrafish facilities. Traditional live feed used for zebrafish larvae include Paramecium, saltwater or fresh water rotifers (Brachionus plicatilis or Brachionus calyciflorus), and Artemia sp.2–7 Live feeds are commonly used due to their small size, high digestibility, and encouragement of prey-capture behavior.2,4,6,7
Intensive feeding schedules and live feeding protocols for rearing larval and juvenile zebrafish present challenges in terms of equipment and labor costs.8 These require daily monitoring, culturing, and counting to maintain the feed stock, the protocols are labor-intensive, and the quality of the feed stock can be variable.6 Rotifer species such as B. plicatilis may need to be provided to the zebrafish multiple times per day since rotifers may not reproduce or survive for long periods when placed in the relatively hypotonic zebrafish environment. Due to their complexity, feeding protocols for larval and juvenile zebrafish may vary greatly between facilities. Standardization of these protocols may improve fish welfare as well as reproducibility of research.
The goal of this study was to evaluate if feeding larval and juvenile zebrafish via a continuous supply of live rotifers or a high nutrient density pelleted feed would improve growth, survival, and reproductive success compared to standard twice-daily bolus feeds of live rotifers. We compared Gemma Micro 75 pelleted diet (Skretting, UT) and live-type L rotifers (B. plicatilis) fed in three different feeding regimens after 9 dpf: bolus feeding of live diet (BL), continuous feeding of live diet (CL), and the pelleted diet (PD). Growth measurements, age at transition to adult feed, age at sexual maturity, sex ratios, and fecundity were measured for each feeding group. In addition, we quantified the equipment, consumable, and labor costs associated with these methods, compared to providing intermittent boluses of live feed.
Materials and Methods
Ethical statement
All procedures were reviewed and approved by the Ohio State University's IACUC before study initiation. The study was performed under IACUC protocol 2018A00000012-R1. The program is AAALAC accredited and compliant with all applicable federal regulations.
Zebrafish husbandry
Broodstock casper and GESTALT zebrafish were kind gifts of L. Zon and A Schier.9,10 Adult zebrafish were maintained in 3.5 L polycarbonate tanks at a stocking density of 6–7 fish per liter, on a Tecniplast ZebTEC active blue recirculating system (West Chester, PA). Fish were maintained under a 14-h light and 10-h dark cycle at 28°C. Water quality was monitored at weekly intervals using API freshwater fish products (Chalfront, PA, Table 1). Excessive debris (algae, food) was siphoned from the bottom of each tank using a pipet as needed. Routine visual health examinations of all fish were conducted daily.
Table 1.
Water Quality Measurements
| Group | N | Ammonia (ppm) | Nitrite (ppm) | pH | Conductivity (μS) | Temp (C) |
|---|---|---|---|---|---|---|
| BL | 30 | 0.20 ± 0.03 | 0.18 ± 0.17 | 6.9 ± 0.05 | 1039 ± 44 | 27.8 ± 0.3 |
| CL | 30 | 1.3 ± 0.82 | 0 ± 0 | 6.8 ± 0.05 | 1262 ± 47 | 27.7 ± 0.2 |
| PD | 30 | 2.5 ± 0.55 | 0.35 ± 0.17 | 7.0 ± 0.04 | 922 ± 21 | 27.9 ± 0.3 |
Data are presented as mean ± SEM.
Embryo rearing
Fish were set up one female to one male per breeding cage in individual Tecniplast 1.7 L static breeding tanks (West Chester, PA) containing a grated insert and a transparent polycarbonate insert on the evening before spawning. The grated insert was used to prevent consumption of eggs by fish before collection and the transparent polycarbonate insert was used to separate fish and prevent breeding until the morning of spawning.
On the morning of spawning, the divider was removed, and fish were transferred to a new, clean 1.7 L tank containing fresh system water and an artificial plant. Fish were allowed to breed uninterrupted for at least 5 h after the lights were on, before embryos were collected. Both male and female fish were placed back in their original home tanks so that embryos could be collected. The grated insert was removed and water from each tank was drained using a mesh tea strainer, which allowed retention of the embryos only. A squirt bottle containing fresh system water was used to rinse the embryos from the strainer into a Petri dish (Greiner Bio-One Polystyrene) containing system water.
At 1 dpf, nonviable embryos were identified using a Leica S9 series stereo microscope (Wetzlar, Germany), removed from the embryo cultures, and discarded in a 10% bleach solution. Viable embryos were transferred to dishes containing embryo medium (E3).11 No more than 50 embryos were placed per dish. Each dish was incubated at 28.5°C and examined daily for swim bladder inflation and embryo viability. When more than 50% of each dish showed inflation of the swim bladder and were swimming upright, first-feeding larvae were transferred into static 3.5 L tanks containing a rotifer (B. plicatilis)/algae (Rotigrow, Reed Mariculture) polyculture solution.6 Larvae remained in the polyculture mixture until 9 dpf, after which each 3.5 L tank of 21–24 fish was randomly assigned to the experimental feeding groups and placed on flow. Water flow into the tanks was also increased to a slow drip.
Culture of rotifers and determination of rotifer densities
Saltwater rotifers (B. plicatilis; Reed Mariculture, Campbell, CA) were cultured in two 5-gallon vessels in 15 g/L Instant Ocean Aquarium salts with continuous air bubbling (Blacksburg, VA). Rotifers were counted and examined daily (before feeding) for rotifer density and viability using a 1 mL counting slide and a Laxco LMZ-Z200 stereo microscope (Mill Creek, WA). After stirring the water, 10 mL of rotifer solution was collected from each culture vessel. Of the 10 mL sample, 1 mL was collected using a pipette and placed on a 1 mL counting slide. Viability assessments included the visualization of egg production, which indicated that rotifers were actively reproducing, green coloration of the rotifers, which indicated that they were actively consuming their algae food source, and rotifer activity (swimming and moving about the slide). Following viability assessments, iodine was used to fix the rotifers for counting.
The two 5-gallon vessels, referred to as maintenance cultures, were maintained at a concentration of 600–1500 rotifers/mL. A separate rotifer culture vessel, referred to as the feed out culture (FCV), was maintained at a concentration of 1500 rotifers/mL and restocked daily with fresh rotifers from the maintenance cultures. Salinity in the FCV was kept at 5 g/L aquarium salts to acclimate the rotifers to the hypotonic zebrafish water. Rotifers from the FCV were used to supply rotifers for the polyculture solution for larvae less than 9 dpf, the continuous live rotifer system, and the rotifer bolus for the feeding of larval and juvenile zebrafish. RG Complete Algae (Reed Mariculture) diluted 1:5 was used as a food source for the rotifers.
Continuous live rotifer system
A continuous feeding system was developed in-house to provide a constant drip of live saltwater rotifers into each fish tank over a 24-h period (Fig. 1A). The continuous system was allowed to run a complete 24 h cycle without interruption before restocking each morning. The feeding system consisted of a rotifer holding tank (13 L cone-bottom bucket), standard adjustable IV lines serving as feed lines (1 feed line/tank), an air stone, and a Masterflex C/L Series single-channel peristaltic pump (Cole-Parmer, Vernon Hills, IL) (Fig. 1A).
FIG. 1.
Development of a continuous live rotifer feeding system. (A) A feeding system was developed in-house to provide a constant drip of live saltwater rotifers into each fish tank over a 24-h period. The feeding system consisted of a rotifer holding tank (13 L cone-bottom bucket, white star), standard adjustable IV lines serving as feed lines (1 feed line/tank), an air stone, and a Masterflex C/L Series single-channel peristaltic pump (red arrow). (B) Zebrafish in the CL group were seen swimming near the source of rotifers for extended periods, demonstrating prey-seeking behavior. CL, continuous feeding of live diet.
Drip rate for each feeding line and pump speed were adjusted to maintain a similar drip rate and deliver a similar number of rotifers per day between all tanks. A mixture of rotifers (1 L at 1500 rotifers/mL), reverse osmosis (RO) water containing 1 g/L aquarium salts, and 2–3 mL RG complete algae was added to the holding tank of the continuous system daily. The holding tank and feed lines were cleaned every 7–10 days to remove the build-up of algae.
Bolus live feed
Rotifers for the BL group were delivered by introducing a dose of rotifers into the fish tank fish over the course of a few seconds. To make the feeding solution, rotifers from the FCV were filtered into a squirt bottle and mixed with RO water containing 5 g instant ocean aquarium salts per liter. RG complete algae (1–1.5 mL) was also added to the squirt bottle until a dark green slurry was formed. Each tank in the BL group received two boluses of rotifers per day on the weekdays and one bolus per day on the weekends. Rotifers were fed in excess amounts relative to what the zebrafish were able to consume based on the buildup of food debris in the bottom of the tanks.
Pelleted diet feed
Each tank in the PD group received 0.1 g of Gemma Micro 75 pelleted diet daily. Pelleted diet was delivered using a handheld granular feed dispenser (Pentair, Cat # ah20) and the standardized amount delivered was determined on an analytical balance. The amount of food provided was determined to be in excess based on the buildup of debris in the bottom of the tanks, similar to the other feeding methods. The amount was not increased based on the size or age of the fish. All fish in the PD group were fed once daily.
Time and labor costs
To determine the time and labor costs associated with each feeding regimen, the aquaculture technician was timed while performing tasks associated with feeding for each group. Tasks measured included time to complete rotifer harvesting (counting and culture maintenance), time to maintain the continuous live rotifer system, and the time to feed the pelleted diet. Equipment and consumable costs were also tabulated and compared between each feeding group.
Growth measurements
Fish were anesthetized using tricaine methanesulfonate (MS-222). Two milliliters of 4 g/L tricaine (dissolved in embryo medium) was added to 100 mL of fish water in a Petri dish (Greiner Bio-One Polystyrene) and mixed by swirling the plate before adding the fish. Anesthetized fish were then placed on grid paper and photographed with a Leica S9 series stereo microscope and attached digital camera. Images were analyzed using Adobe Photoshop to determine fork length and body depth.
Fork length was measured from the tip of the snout to the middle of the caudal fin as demonstrated in Figure 2A. Body depth is defined as the dorsal-ventral length and was measured at the central part of the fish using the start of the anal fin as a landmark (Fig. 2A). After imaging, excess water was removed by placing the fish on a moist sponge and then fish were weighed on a tared analytical balance (Mettler Toledo). Fish were transferred to a recovery tank containing fresh system water and allowed to completely recover from anesthesia before they were returned to their home tanks.
FIG. 2.
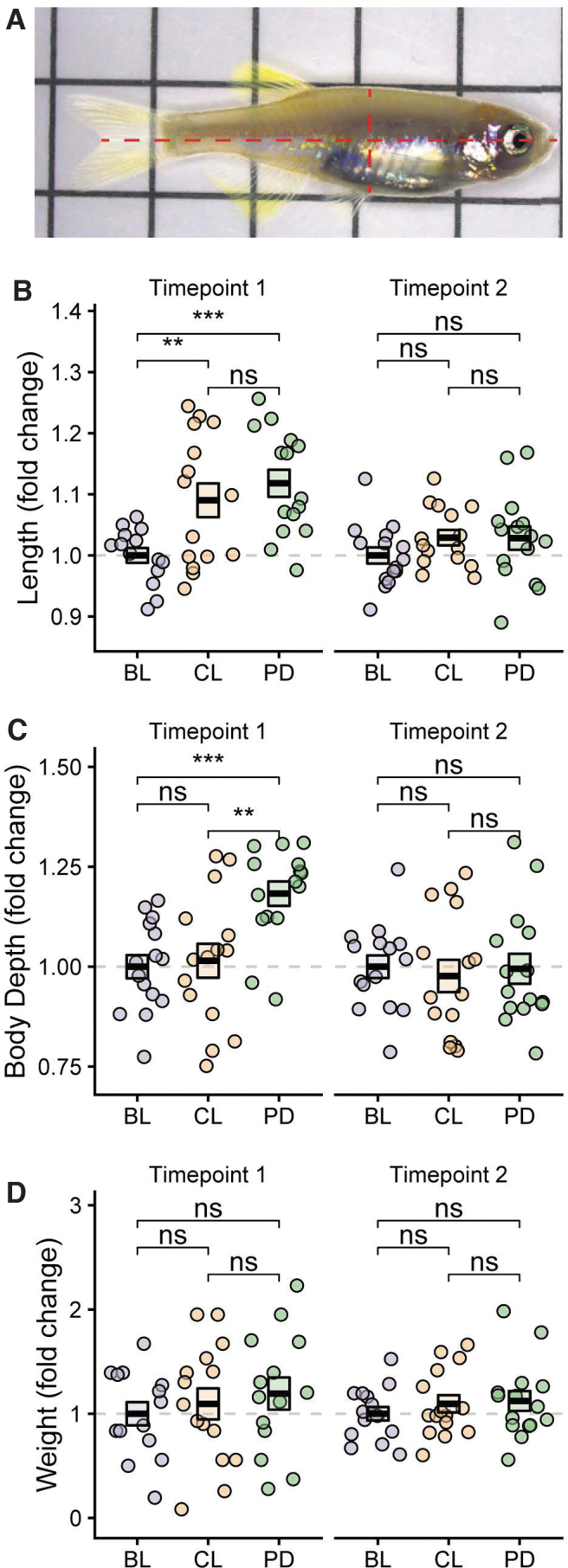
Effect of feeding regimen on fish growth. (A) Representative image used for zebrafish measurements. (B–D) Length (B), body depth (C), and weight (D) were measured at 4–5 weeks (Timepoint 1) and 113–115 days (Timepoint 2) postfertilization. Data are presented as fold change relative to the BL group. Box indicates mean ± SEM; ns: p > 0.05; **: p ≤ 0.01; ***: p ≤ 0.001. The combined results from two independent experiments are shown. BL, feeding of live diet; ns, not significant.
Transition to adult feed
Zebrafish were observed daily for growth and signs of maturity. Visual evaluation of external morphology (muscling, pigment pattern, and fin development) was assessed for each tank of fish.12 Once visual standards for maturity were met by >50% of fish in the tank, fish were immediately switched to Gemma Micro 300, a processed pelleted diet suitable for adult fish.13 Visual standard for maturity included an increase in definition of the muscular portions of the fish, increase in pigment of stripes for wild-type fish and more prominent forking of fins such as the anal fin.
Age at sexual maturity and fecundity
To determine if feeding regimen had effects on chronological age at sexual maturity, fish were started on breeding trials 3 weeks after transition to adult feed. An established breeding line of zebrafish maintained in the facility was used for mating with experimental fish. Five crosses were set up per feed group (BL, CL, and PD). Crosses comprised 3 females and 2 males chosen at random from each feeding group. Each resulting clutch was analyzed for clutch size, embryo viability, and survival to swim bladder inflation. Sexual maturity and breeding success were confirmed at the time point when greater than 50% of all breeding pairs from each replicate tank were able to breed and produce viable embryos. For female casper zebrafish, the visualization of eggs was also used as an adjunct to determine sexual maturity.
Euthanasia of embryos, larval, and adult zebrafish
All euthanasia methods were performed in accordance with the AVMA guidelines on euthanasia.14 Larval and adult zebrafish were euthanized by MS-222 overdose followed by ice water immersion.
Statistical analysis
Statistical analysis was performed in R v4.0. Figures show mean ± standard error of the mean. Pairwise group comparisons were made using the Wilcoxon rank-sum test or Student's t-test depending on the data distribution. p < 0.05 was considered statistically significant.
Results
Continuous live rotifer system
It was necessary to establish a consistent, even rate of delivery of the live rotifers by setting the peristaltic pump rate and valve pressure on each feeding line, and by accurately quantifying the feeding solution delivered. Three separate trials were performed using four off-system sham tanks without zebrafish, to quantify feeding solution delivery. Between 2538 mL and 2913 mL feeding solution was delivered to each tank per day (mean volume of 2712 mL over three trials, Table 2).
Table 2.
Quantification of Feeding Solution Volume Delivered by the Continuous Live Rotifer System
| Trial | Mean volume (mL) | Std. Err. (mL) | Tanks |
|---|---|---|---|
| 1 | 2913 | 623 | 4 |
| 2 | 2538 | 466 | 4 |
| 3 | 2688 | 384 | 4 |
Based on the number of rotifers input into the feeding system, the total number of rotifers delivered in the subsequent feeding trials was calculated to be 375,000 per tank per day. Viable rotifers were identified in each sham tank indicating that rotifers were able to survive migration through the system. Fish in the CL group were seen swimming in a group near the point at where rotifers dripped into the tank for extended periods (Fig. 1B), whereas similar prey-seeking behavior was only transiently observed following bolus feeds.
Effect of feeding regimen on zebrafish growth
The fork length, body depth, and weight of representative fish from each experimental group (BL, CL, and PD) were measured, as demonstrated in Figure 2A, after 4–5 weeks of feeding on the study regimen (38 or 47 dpf; Timepoint 1). At Timepoint 1, representative animals from the CL and PD groups were 9% and 12% longer than the BL group, respectively (p = 0.007, p = 1.3 × 10−4, Fig. 2B). There was no difference in the average length of the PD and CL groups. The body depth of representative animals in the PD group was 18% greater than that of the BL group and 17% greater than that of the CL group at Timepoint 1 (p = 1.7 × 10−4 and p = 0.004, respectively, Fig. 2C). There was no difference in body depth between the BL and CL groups at Timepoint 1. The weight of representative animals was not significantly different between any of the groups at Timepoint 1 (Fig. 2D).
The CL and PD groups transitioned to adult feed between 55 and 61 dpf and the BL group transitioned to adult feed between 61 and 69 dpf. A second timepoint was chosen occurring after all groups had transitioned to adult feed and had reached or were near sexual maturity (113–115 dpf, Timepoint 2). There was no significant difference between the groups in any measurement at Timepoint 2 (Fig. 2B–D). These data show that both the continuous live rotifer system and pelleted diet regimens increase early growth of juvenile zebrafish compared to standard feeding with live rotifer boluses.
Age at sexual maturity
Breeding trials were used to determine if feeding regimen had effects on chronological age at sexual maturity. Fish were started on breeding trials 3 weeks after transition to adult feed. Sexual maturity and breeding success were confirmed at the time point when greater than 50% of all breeding pairs from each replicate tank were able to breed and produce viable embryos. The PD and CL groups reached sexual maturity between 92 and 102 dpf. The BL group reached sexual maturity between 92 and 131 dpf. There was no consistent difference in age at sexual maturity between any of the groups. The overall rate of breeding success was 58%, 26%, and 36% in the BL, CL, and PD groups, respectively.
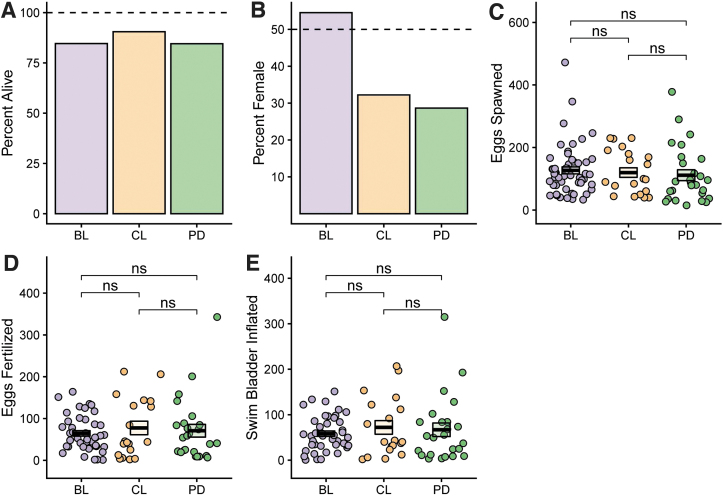
Survival to adulthood, sex ratio, and fecundity
The percent of animals surviving to adulthood was over 80% in all groups (Fig. 3A). The number of male and female animals was determined by visual inspection and quantified. The BL group had 54% female fish while the CL and PD groups had 32% and 28% female, respectively (not statistically different, Fig. 3B).
FIG. 3.
Comparison of survival, sex ratio, and fecundity according to study group. (A) Percentage of fish that survived to adulthood per feeding group. (B) Percentage of fish identified as female per feeding group. (C–E) The number of eggs spawned (C), eggs fertilized (D), and embryos that lived to swim bladder inflation (E) were quantified for all study groups. Boxes show mean and SEM; ns: p > 0.05.
The number of eggs spawned, eggs fertilized, and embryos that lived to swim bladder inflation were quantified for all breeding pairs from each replicate tank. Breeding pairs were set up weekly for each feeding group with each group being bred for a minimum of 3 weeks (Fig. 3C–E). There was no significant difference noted in any of these values between the three feeding groups, indicating that fish in all three feeding groups were capable of successfully reproducing. These data also indicate that the assessment of age at sexual maturity was performed accurately and that fish in all groups were capable of successfully breeding at the time point identified.
Time and labor costs
The total time to perform tasks related to each feeding group was recorded. Culture and harvesting of rotifers required daily cleaning for both rotifer culture vessels, rotifer counts and viability checks, stocking the FCV, preparing the saltwater for each culture, and preparing the bolus for feeding of the BL group or for placing in the continuous system for the CL group. Total time for rotifer culture and harvest was 35 ± 4 min. Time to maintain and fill the continuous system, which included filling the system and adjusting the flow rates for each tank, in addition to rotifer culture maintenance, required a total of 39 ± 6 min. Cleaning the continuous system occurs every 7–10 days and requires about 6 min to complete when performed. Time to feed the pelleted diet is about 2 s (0.03 min) per tank. Daily consumable costs for the PD group were greater than for either live feeding regimen ($2.77 vs. $1.75 per 4 tanks/day). Time, labor, and consumable costs are summarized in Table 3.
Table 3.
Time, Labor, and Consumable Costs Associated with Feeding Regimens in the Study
| Study group | Total time per day (mins) | Labor cost per day* | Consumable cost per day |
|---|---|---|---|
| CL | 39 ± 6 | $11.05–$12.75 | $1.75 |
| BL | 35 ± 4 | $3.74–$5.44 | $1.75 |
| PD | 0.03 | $0.0085 | $2.77 |
Labor costs were calculated based on an hourly wage of $17. Calculations are based on maintaining four tanks per group.
Discussion
This study was designed to identify an optimal feeding strategy for a small to medium-sized zebrafish nursery with reasonable constraints on equipment and consumable costs and labor. In comparison to the standard approach of intermittent bolus live feeds, providing a continuous supply of live feed or a high nutrient density pelleted feed can increase juvenile zebrafish growth rates while promoting balanced sex ratios and equivalent reproductive success. Moreover, a pelleted diet can be provided with minimal labor input and could be performed by any vivarium technician with minimal training in aquaculture.
The frequency of feeding and amount of feed are important aspects of any larval zebrafish rearing protocol.2,5 Smaller, frequent bolus feeds can be provided by aquatic facilities with a large pool of trained aquaculture technicians who are able maintain the rotifer stocks and feed the nursery animals multiple times per day. Alternatively, robotic feeders can be stocked with live feed, which is administered to nursery tanks automatically. We developed an inexpensive continuous setup that has the advantage of maintaining the rotifers in mildly hypotonic conditions until delivered into the nursery tanks.
Continuous delivery of live rotifers and the pelleted diet were both associated with increased early growth rates of juvenile zebrafish. Animals fed with the continuous system demonstrated consistent prey-seeking behavior in the area where rotifers entered the tanks compared to animals in the bolus group that only demonstrated this behavior at feeding times and generally ignored the food debris on the bottom of the tanks. Animals in the pelleted diet group did not exhibit extended periods of prey-seeking behavior after feeding. All feeding regimens in this study provided nutrients to the nursery tanks in excess of what the juvenile zebrafish could ingest as evidenced by the rapid buildup of uneaten food debris. Our conclusion from these data is therefore that the continuous live food system and the pelleted diet are more efficient means of delivering bioavailable nutrients to the juvenile zebrafish compared to the bolus feeding method.
Despite the early differences in growth rate, the pelleted and continuous live diets did not demonstrate superior fecundity or a younger age at sexual maturity. This may be due, in part, to our study protocol, in which animals graduated from the study-assigned feed to the same adult feeding regimen at ∼2 months postfertilization. Sex determination in zebrafish may be affected by nutrient density relative to fish housing density with high nutrient density favoring female fish development.15 Since our continuous and pelleted diet groups skewed in favor of males, we speculate that these groups may have been relatively underfed following an early growth advantage compared to the bolus group. Regardless, our data show that the continuous and pelleted groups reach sexual maturity at least on par with the bolus feed group.
Labor time and cost are important factors to consider when operating a zebrafish nursery. A feeding regimen that minimizes both while maintaining high feeding standards will be beneficial for zebrafish, aquatic facility managers, and facility users. Daily consumable costs were less for the live rotifer diets compared to the pelleted diet. However, equipment costs to start up and maintain the live rotifer culture vessels are significantly greater than those for a pelleted diet, which are negligible.
In this study, all first-feeding larvae were fed in rotifer polycultures, meaning that our results do not argue in favor of eliminating rotifer production from the facility entirely. However, switching to pelleted diet following the polyculture phase would still substantially reduce the overall time and labor cost of operating a zebrafish nursery while preserving some potential benefit of live food for the youngest fish. In addition, this strategy allows rotifer maintenance to be skipped for up to 48 h on days when first-feeding larvae are not added to the system. This reduces weekend/holiday workload and eliminates the need for specialized training for vivarium staff feeding the animals on these days.
Our study has shown that a pelleted zebrafish diet and continuous delivery of live rotifers are superior in promoting early growth rates while helping to produce adult broodstock that perform equivalently compared to those raised on traditional live bolus feeds. This, along with the clear advantages in labor costs makes pelleted diets an attractive option for zebrafish nursery care.
Acknowledgment
We acknowledge Drs. Judy Hickman-Davis and Heather Shive for critical input on the article.
Authors' Contributions
T.A.C. and B.W.B. designed the study, performed experiments, analyzed data, and wrote the article. S.C., E.T., and J.S. performed experiments and analyzed data.
Disclosure Statement
The authors declare no competing interests.
Funding Information
This work was supported by NIDDK K08DK111920 and the Pelotonia Foundation.
References
- 1. Cagan RL, Zon LI, White RM. Modeling cancer with flies and fish. Dev Cell 2019;49:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence C, James A, Mobley S. Successful replacement of Artemia salina nauplii with marine rotifers (Brachionus plicatilis) in the diet of preadult zebrafish (Danio rerio). Zebrafish 2015;12:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aoyama Y, Moriya N, Tanaka S, Taniguchi T, Hosokawa H, Maegawa S. A novel method for rearing zebrafish by using freshwater rotifers (Brachionus calyciflorus). Zebrafish 2015;12:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avdesh A, Chen M, Martin-Iverson MT, et al. : Regular care and maintenance of a zebrafish (Danio rerio) laboratory: an introduction. J Vis Exp 2012;69:e4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hensley MR, Leung YF. A convenient dry feed for raising zebrafish larvae. Zebrafish 2010;7:219–231. [DOI] [PubMed] [Google Scholar]
- 6. Lawrence C, Best J, Cockington J, et al. The complete and updated “Rotifer Polyculture Method” for rearing first feeding zebrafish. J Vis Exp 2016;107:e53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson C. Aspects of larval rearing. ILAR J 2012;53:169–178. [DOI] [PubMed] [Google Scholar]
- 8. Carvalho AP, Araujo L, Santos MM. Rearing zebrafish (Danio rerio) larvae without live food: evaluation of a commercial, a practical and a purified starter diet on larval performance. Aquaculture Res 2006;37:1107–1111. [Google Scholar]
- 9. McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, Shendure J. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 2016;353:aaf7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White RM, Zon LI. Melanocytes in development, regeneration, and cancer. Cell Stem Cell 2008;3:242–252. [DOI] [PubMed] [Google Scholar]
- 11. Westerfield M: The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). University of Oregon Press, Eugene, 2000. [Google Scholar]
- 12. Singleman C, Holtzman NG. Growth and maturation in the zebrafish, Danio rerio: a staging tool for teaching and research. Zebrafish 2014;11:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawrence C, Best J, James A, Maloney K. The effects of feeding frequency on growth and reproduction in zebrafish (Danio rerio). Aquaculture 2012;368:103–108. [Google Scholar]
- 14. AVMA Guidelines for the Euthanasia of Animals. 2020. Edition. American Veterinary Medical Association, Shaumburg, IL, 2020. [Google Scholar]
- 15. Nagabhushana A, Mishra RK. Finding clues to the riddle of sex determination in zebrafish. J Biosci 2016;41:145–155. [DOI] [PubMed] [Google Scholar]