Abstract
With advances in allogeneic hematopoietic stem cell transplant (allo-HCT), disease relapse has replaced transplant-related mortality as the primary cause of treatment failure for patients with acute myeloid leukemia (AML). The efficacy of allo-HCT in AML is a consequence of a graft-versus-leukemia (GVL) effect that is mediated by T lymphocytes, and unique mechanisms of immune evasion underlying post-allo-HCT AML relapses have recently been characterized. Relapsed AML following allo-HCT presents a particularly vexing clinical challenge because transplant-related toxicities, such as graft-versus-host (GVHD) and infections, increase the risk of treatment-related morbidity and mortality. In general, the prognosis of relapsed AML following allo-HCT is poor with most patients failing to achieve a subsequent remission and 2-year survival consistently <15%. The two factors that have been found to predict a better prognosis are a longer duration of post-transplant remission prior to relapse and a lower disease burden at the time of relapse. When considered in combination with a patient’s age; co-morbidities; and performance status, these factors can help to inform the appropriate therapy for the treatment of post-transplant relapse. This review discusses the options for the treatment of post-transplant AML relapse with a focus on the options to achieve a subsequent remission and consolidation with cellular immunotherapy, such as a second transplant or donor lymphocyte infusion (DLI). While intensive reinduction therapy and less intensive approaches with hypomethylating agents have long represented the two primary options for the initial treatment of post-transplant relapse, molecularly targeted therapies and immunotherapy are emerging as potential alternative options to achieve remission. Herein, we highlight response and survival outcomes achieved specifically in the post-transplant setting using each of these approaches and discuss how some therapies may overcome the immunologic mechanisms that have been implicated in post-transplant relapse. As long-term survival in post-transplant relapse necessarily involves consolidation with cellular immunotherapy, we present data on the efficacy and toxicity of both DLI and second allo-HCT including when such therapies are integrated with reinduction. Finally, we provide our general approach to the treatment of post-transplant relapse, integrating both novel therapies and our improved understanding of the mechanisms underlying post-transplant relapse.
Keywords: acute myeloid leukemia, allogeneic hematopietic stem cell transplantation, immunotherapy, targeted therapy, donor lymphocyte infusion (DLI)
Introduction
Allogeneic hematopoietic stem cell transplant (allo-HCT) represents the most effective consolidation strategy to prevent disease relapse for the majority of adult acute myeloid leukemia (AML) patients who achieve remission. Patients with intermediate or poor-risk cytogenetics comprise more than 90% of all newly diagnosed AML patients (1), and myeloablative (MAC) allo-HCT in first complete remission (CR1) leads to improved overall survival (OS) and leukemia-free survival (LFS) when compared to non-transplant approaches in these risk groups (2). Furthermore, a randomized trial in AML and myelodysplastic syndrome (MDS) has demonstrated improved overall survival with MAC allo-HCT compared to reduced-intensity conditioning (RIC) when an HLA-matched donor is used (3). However, the risk of transplant-related mortality (TRM) following MAC makes this approach unsuitable for older patients. Two separate European Group for Blood and Marrow Transplantation (EBMT) analyses demonstrate no benefit to MAC compared to RIC approaches for AML patients aged 40-60 regardless of donor type or patients ≥50 when an unrelated donor is used, as the increased incidence of relapse following RIC is offset by the increased incidence of TRM following MAC, yielding comparable LFS and OS (4, 5). As a consequence of improvements in supportive care that have reduced TRM following MAC and the increased use of RIC, relapse has replaced TRM as the primary cause of treatment failure following allo-HCT for AML and occurs after >30% of transplants (6–8). With more than 3,500 allogeneic transplants performed for AML in the United States alone each year, post-transplant relapse is a common problem (9). The prognosis for patients who relapse following transplant is dismal, as the vast majority do not achieve a subsequent remission, and 2-year OS is consistently <15%. (7, 10). An understanding of the clinical factors influencing survival following post-transplant relapse can help to set expectations and influence the choice of subsequent therapy, which may include intensive induction therapy, low-intensity therapy, targeted therapy, additional cellular immunotherapy, and novel immunotherapy agents. The latter two approaches are of particular interest because the efficacy of allo-HCT in AML is primarily driven by a graft-versus-leukemia (GVL) effect, and there is increasing recognition of unique immunologic mechanisms underlying post-allo-HCT relapse.
Among AML patients who relapse following allo-HCT, the single factor that most consistently predicts subsequent survival is the post-transplant remission duration. In retrospective analyses, the Center for International Bone Marrow Transplant Registry (CIBMTR) demonstrated a 3-year OS of just 4% among AML patients relapsing within 6 months of allo-HCT (10), while the EBMT demonstrated a 2-year OS of 7.5% in patients relapsing within 5 months of RIC allo-HCT for AML (7). Unfortunately, such early relapses are common, as the median time to post-transplant relapse is 7 months, and 43% occur within 6 months (10). The dismal outcomes for early relapse patients suggest that curative outcomes are extremely unlikely, and a palliative approach focused on minimizing therapy toxicity and prioritizing quality of life is often warranted. In contrast, the ability to achieve durable long-term survival following post-transplant relapse increases with the duration of the initial post-transplant remission with the small percentage of patients who relapse 2-3 or 3+ years after transplant having 3-year OS of 26% and 38%, respectively (7, 10). Thus curative outcomes can reasonably be pursued in fit patients with late post-transplant relapses, and an aggressive approach is often merited. Other factors that have been associated with improved survival following post-transplant relapse include: a lower disease burden as assessed by the percentage of bone marrow blasts at relapse, the use of RIC with prior transplant, the absence of acute GVHD prior to or at the time of relapse, the absence of adverse cytogenetics, and age ≤40 years (7, 10). For patients who relapse from 6-24 months post-transplant, these additional factors in combination with the patient’s performance status, co-morbidities, and underlying leukemia biology may help to guide the appropriate approach to subsequent treatment. While the initial goal of therapy in all patients who undergo allo-HCT for AML is cure, a post-transplant relapse should prompt a re-evaluation of this goal with the aforementioned risk factors predicting the likelihood of subsequent durable survival, which can help to inform the choice of a palliative or aggressive approach to subsequent therapy.
There is compelling evidence to support a GVL effect in AML that is mediated by T lymphocytes and correlates with the development of GVHD. The occurrence of both acute and chronic GVHD following transplant has been shown to lead to a reduced risk of post-transplant relapse (11, 12). This suggests correlated post-transplant alloimmune effects that prevent relapse (GVL) and cause side effects (GVHD). Notably, patients who relapse in spite of developing GVHD have poorer survival (7, 10), which is likely a consequence of both the morbidity of GVHD and the ineffectiveness of the GVL effect in these patients. In contrast, an effective GVL effect can be utilized in further therapy, as donor lymphocyte infusion (DLI) alone can produce a subsequent remission in 29% of AML patients who relapse post-transplant and such remissions can be durable when DLI is given after achieving remission with chemotherapy (13, 14). These outcomes with DLI further demonstrate that AML is an immune-responsive disease. T cells are the lymphocytes that are specifically responsible for the GVL effect and the efficacy of DLI, as leukemia patients who received a syngeneic or T-cell depleted allogeneic graft had 3-year relapse incidences of 49% and 35%, respectively, compared to <25% in patients who received a T-cell replete allogeneic graft (11). The ability of AML cells to evade detection or destruction by T lymphocytes could underly post-transplant relapses, and a better understanding of these mechanisms may allow for the safe augmentation of the GVL effect without exacerbating GVHD.
In AML patients who have undergone allo-HCT, two immunologic mechanisms underlying relapse have been characterized. Nearly 50% patients relapsing after transplant demonstrate downregulation of HLA Class II on leukemic blasts irrespective of the number of donor-recipient HLA incompatibilities, which is not seen in AML patients who relapse following chemotherapy alone (15, 16). For AML patients who underwent HLA haploidentical transplant, HLA haplotype loss through acquired uniparental disomy of chromosome 6p occurs in up to 1/3rd of relapsed patients, and donor lymphocytes do not respond to these relapsed leukemic blasts in vitro (17, 18). Thus the downregulation of HLA class II molecules or elimination of the non-shared HLA haplotype allows leukemic blasts to evade the GVL effect. A second mechanism that seems to underly AML relapse following allo-HCT is the development of T cell exhaustion. The early detection of severely exhausted bone marrow memory T cells (PD-1+Eomes+T-bet-) predicts relapse, and memory T cells in relapsing patients have increased expression of inhibitory receptors when compared to those who maintain remission at one year (19). These findings suggest that the GVL effect is diminished when allogeneic T cells become exhausted, which can lead to relapse. These two well characterized immunologic mechanisms of relapse (T cell exhaustion and HLA Class II downregulation/haplotype loss) may account for up to 2/3rds of all post-transplant relapses (16), and may have significant implications for the choice of subsequent therapy including immunotherapy.
In the current review, we will outline the current evidence supporting various options for the treatment of relapsed AML following allo-HCT. We will broadly highlight the evidence supporting the use of intensive reinduction with cytotoxic chemotherapy, lower intensity chemotherapy regimens, and targeted therapies including the frequency of achieving remission and long-term survival data. Secondly, we will focus on available immunotherapy strategies for reinduction including DLI, immune checkpoint inhibitors, and other monoclonal antibodies with a focus on how such strategies may overcome known mechanisms of immune evasion and augment the GVL effect. We will additionally discuss the utility of DLI and second allogeneic transplant as consolidation strategies for patients who achieve a second remission. Finally, we will provide our algorithm for how to use these strategies in relapsed AML patients following allo-HCT.
Intensive Reinduction Therapy
There is a paucity of studies describing the optimal intensive reinduction chemotherapy regimen for post-allo-HCT relapse. Many of the studies describing intensive reinduction chemotherapy regimens for AML have either excluded patients with post-transplant relapse entirely (20), provide no data on outcomes specific to patients who underwent a prior allo-HCT (21–23), or include ≤10 patients with post-transplant relapse (24–27). The dearth of data on intensive reinduction for post-allo-HCT relapse likely reflects the difficulty enrolling such patients on clinical trials due to their myriad complications, such as infections and GVHD. As a consequence of this issue, many of the studies describing intensive reinduction for post-allo-HCT relapse are retrospective; include a variety of reinduction regimens; and often include response endpoints that are assessed after chemotherapy and DLI, making the independent contribution of intensive chemotherapy difficult to assess.
The published literature on intensive reinduction chemotherapy for post-allo-HCT relapse of AML demonstrates reasonable response rates but poor long-term survival in the absence of subsequent cellular therapy in the form of either DLI or a 2nd allo-HCT. Select studies are summarized in Table 1 , rely largely on cytarabine-based reinduction regimens and demonstrate remission rates ranging from 13-71% with the largest studies showing ≥40% remission rates when chemotherapy is combined with DLI (7, 28–32). While these studies represent a combination of prospective trials and retrospective reviews, larger series suggest that ≥20% of patients receive only supportive care following post-allo-HCT relapse and 33% receive less intensive therapy (7, 10). Thus while intensive reinduction therapy for post-allo-HCT relapse can yield a reasonable response rate, the patients included in these studies are highly selected and the results should not be extrapolated to all patients with post-transplant relapse. The other notable conclusion is that patients who receive intensive reinduction chemotherapy without subsequent DLI or 2nd allo-HCT have dismal long-term outcomes with overall survival of 0-4.4% (7, 30). These poor outcomes partially reflect a selection bias in that patients who fail to respond to reinduction often will not receive subsequent cellular therapy; however, 13-27% of patients in these cohorts did achieve remission and veritably none had durable survival. These outcomes suggest the importance of having a viable option for subsequent cellular therapy when pursuing intensive reinduction therapy.
Table 1.
Studies including Intensive Chemotherapy for Post-Allo-HCT Relapse.
| Authors | Regimens | Subsequent DLI/2nd Transplant | Relapse within 6 months of Prior Transplant | Median Age | N | %CR | ORR | OS |
|---|---|---|---|---|---|---|---|---|
| Responses Assessed after Chemotherapy Alone | ||||||||
| Koren-Michowitz et al. | Ara-C + GO | 25%/13% | 81% | 53 (31-63) | 16 | 31% | 60% | 25% at 1 year |
| Devillier et al. | HiDAC +/- GO +/- Anthracycline | 8%/25% | 42% | 42 | 24 | 71% | 33% at 1 year | |
| Schmid et al. | Ara-C + Anthracycline +/-Other, HiDAC +/-Other, Anthracycline + Other | 0%/0% | >50%# | 56 (18-76)# | 47 | 27% | 4.4% at 2 years | |
| Sauer et al. | HiDAC +/- Anthracycline OR ICE | 0% | >50%$ | 52 (17-73)$ | 16 | 13% | 34.4% at 1 year | |
| Responses Assessed after Chemotherapy and DLI | ||||||||
| Motabi et al. | FLAG, FLAG-Ida, FLAG-IM, CLAG, CLAM, MEC, 7+3 | 56%/7% | 58% | 52 (18-70) | 73 | 40% | 51% | 32% at 1 year |
| Levine et al.* | 7+3 (Dauno 30) or Other | 100%/3% | 55% | 42 (2-59) | 65 | 42% | 19% at 2 years | |
| Sauer et al. | HiDAC +/- Anthracycline OR ICE | 100% | >50%$ | 52 (17-73)$ | 31 | 48% | 29% at 1 year | |
#Median age and time to relapse reflect the full cohort of 776 patients with post-transplant relapse. $Median age and time to relapse reflect the full cohort of 108 patients with post-tramsplant relapse. *Study includes 4 patients with CML and 11 with MDS.
While intensive reinduction chemotherapy for post-allo-HCT relapse of AML can produce a reasonable response rate, the optimal regimen is undefined, and there is a significant risk of complications including non-relapse mortality (NRM) that may increase with additional agents. In prospective randomized trials in relapsed/refractory AML, the addition of a second agent to high-dose cytarabine leads to an increased response rate without yielding a statistically significant improvement in OS (21, 23, 33). While the increased response rate with multi-agent chemotherapy may be beneficial in the post-transplant setting, as it may facilitate more patients receiving subsequent cellular therapy, there is also a risk of increased toxicity, so the use of multi-agent therapy for reinduction should remain an individualized decision in the absence of a clear survival benefit vis-à-vis high-dose cytarabine in the post-transplant setting. Among patients receiving intensive reinduction post-allo-HCT, the early mortality rate is 8-13% (29, 31, 32), but the addition of gemtuzumab to multi-agent chemotherapy led to 15% of patients dying from veno-occlusive disease (VOD) (22). This latter findings highlights the potential risk of additional agents in intensive reinduction for post-allo-HCT relapse. Ultimately, intensive reinduction chemotherapy has a clear role for the treatment of AML relapse following allo-HCT. High-dose cytarabine alone can yield reasonable and durable responses when combined with subsequent cellular therapy, and the use of multi-agent chemotherapy has the potential to increase both response rates and toxicity without a demonstrated survival benefit.
Lower Intensity Therapy
Hypomethylating agents (HMAs), specifically azacitidine and decitabine, serve as the backbone of lower intensity approaches to reinduction therapy for post-transplant relapse based on their known, modest efficacy in AML, and pre-clinical data suggesting that they can potentially enhance the GVL effect without exacerbating GVHD. In older patients (age ≥65 years) with newly diagnosed AML, azacitidine yields remission in 24.8% of patients and improves OS compared to conventional care regimens (CCR) when censoring for subsequent therapy (34). In addition to its known efficacy, azacitidine is safe and leads to dramatically reduced hospitalizations compared to CCR, which makes it an ideal agent to use in the post-transplant setting. Furthermore, HMAs have been shown to upregulate the expression of tumor cell antigens, HLA class I antigens, and costimulatory molecules on tumor cells (35–38). These changes enhance the ability of allogeneic T cells to both recognize and react to leukemia cells when present, thereby augmenting the GVL effect. HMAs can also increase FOXP3 expression and expand regulatory T cell populations, which animal models suggest may reduce GVHD by suppressing early expansion of alloreactive T cells without inhibiting T cell activation (39–42). Ideally, this means that the GVL effect is preserved in spite of the potential to reduce GVHD. Given the modest clinical efficacy of HMAs in the non-transplant setting and pre-clinical evidence suggesting their particular utility with an alloreactive immune system, numerous studies exploring HMAs in combination with cellular therapy and other agents for the treatment of post-transplant relapse of AML have been completed and select studies are listed in Table 2 .
Table 2.
Studies using lower-intensity, HMA-based regimens for Post-Allo-HCT Relapse.
| Authors | Treatment | AML/MDS | DLI/2nd Transplant | Median time to relapse post-allo-HCT (mos) | Median Age | N | %CR/CRi | ORR | OS |
|---|---|---|---|---|---|---|---|---|---|
| HMA +/- DLI | |||||||||
| Rautenberg et al.# | AZA | 60%/40% | 70%/11% | 4.9 (1-214) | 54 (19-71) | 151 | 41% | 46% | 38% at 2 years |
| Schroeder et al. | DAC | 81%/19% | 61%/25% | 12.3 (1-87) | 56 (21-72) | 36 | 17% | 25% | 11% at 2 years |
| Lubbert et al. | AZA (3 days) | 92%/8% | 65%/27% | 8.3 (2-47) | 62 (28-75) | 26 | 16% | 66% | 16% at 2 years |
| Tessoulin et al. | AZA | 61%/39% | 39%/3% | 3.7 (1.7-37.6) | 57 (17-69) | 31 | 14% | 35% | Median 5.1 mos |
| Craddock et al. | AZA | 64%/36% | 38%/19% | 8 (1-71) | NR | 181 | 15% | 25% | 12.4% at 2 years |
| HMA + Lenalidomide | |||||||||
| Craddock et al. | AZA/LEN | 83%/17% | 10%/7% | 10 (1-39) | 54 (18-73) | 29 | 21% | 24% | Median 27 mos in responders, 10 mos in non-responders |
| HMA + Venetoclax | |||||||||
| Schuler et al. | HMA/VEN | 81%/19% | 34%/6% | 5.7 (1.1-67.8) | 54 (31-72) | 32 | 31% | 44% | Median 3.7 mos |
| Joshi et al.& | HMA/VEN | 66%/34% | 0%/3% | 9 (2-37) | 58 (20-72) | 29 | 28% | 38% | Median 2.6 mos |
#39% of patients in this study were treated for molecular relapse vs. 61% with hematologic relapse. &Three patients received VEN without HMA
Hypomethylating Agents With DLI
While most large studies describing treatment with HMAs for post-transplant relapse include response assessment following subsequent DLI, it is clear that HMAs can be effective as single agents with particular efficacy as an early intervention for molecular relapse. In a series of ten patients with AML and MDS who relapsed a median of 16 months after transplant (range 0-132 months), Bolaños-Meade et al. demonstrated a CR rate of 60% with azacitidine without unexpected or severe toxicities and survival >1 year in 5 patients of whom only 2 received subsequent DLI (43). While the frequency of responses is notable, there are a number of important caveats: most responses were seen in patients with a remission duration >1 year post-transplant, responses were more common in patients with MDS, and the authors did not rigorously define relapse such that some patients may have only had decreasing donor chimerism at the time of treatment. The RELAZA study demonstrated that the initiation of azacitidine for decreasing post-transplant donor chimerism in AML/MDS led to stabilization or improvement in chimerism in 80% of patients with a few patients sustaining >80% donor chimerism following the cessation of therapy without DLI (44). In this study declining donor chimerism served as a proxy for molecular relapse, and the findings suggest that azacitidine may be more effective when used to treat a low disease burden. This has inspired numerous studies of prophylactic post-transplant azacitidine (45–50), although a randomized trial versus best supportive care recently failed to demonstrate a survival benefit (51). In contrast, a randomized trial of prophylactic low-dose decitabine in combination with recombinant human granulocyte colony-stimulating factor (rhG-CSF) given post-transplant led to a reduction in AML relapses compared to no intervention (52). Thus the evidence does not support prophylactic post-transplant maintenance with HMA alone, but HMAs can serve as an effective treatment for relapses that occur late after transplant or when the disease burden is low and may have a role in combination with other agents as maintenance therapy.
In contrast to the response rates seen with azacitidine for late post-transplant and molecular relapses, response rates to HMAs for morphologic relapse (blasts >5%) are much more modest in spite of the frequent addition of DLI. Four studies of HMAs for morphologic post-transplant relapse in which the majority of patients had relapsed within a year of transplant and received DLI demonstrate remission rates of 14-17% with 2-year OS of 11-16% (53–56). These studies utilized both azacitidine and decitabine including varied dosing schedules of both HMAs without appreciable differences in outcomes, although the retrospective studies suggest that most clinicians choose the conventional dosing schedules for AML/MDS for post-transplant treatment (azacitidine 75 mg/m2 daily on days 1-7 or decitabine 20 mg/m2 daily on days 1-5). A large German series of patients treated with azacitidine for post-transplant relapse demonstrated that duration of post-transplant remission (>=6 months vs. <6 months) and the type of relapse (molecular vs. morphologic) could be used to stratify patients into three groups with markedly different rates of remission (71% for late molecular relapse vs. 29% for early morphologic relapse) and OS (2-year OS: 64% for late molecular relapse vs. 27% for early morphologic relapse) (57). As the majority of remissions in this study occurred after DLI, it is very difficult to draw firm conclusions about the role azacitidine plays in achieving these outcomes. However, similar to HMA monotherapy, the evidence suggests that HMAs in combination with DLI can be an effective treatment for relapses that occur late after transplant or when the disease burden is low, but the optimal strategy in terms of both HMA dosing and the timing of DLI remains undefined.
Hypomethylating Agents With Venetoclax
While pre-clinical evidence supports a role for HMAs in enhancing the GVL effect, the potential impact of BCL-2 inhibition on the GVL effect is unclear, but the remarkable efficacy of the BCL-2 inhibitor venetoclax in combination with HMAs for newly diagnosed AML in the elderly suggests that this could be a viable strategy for the treatment of post-transplant relapse. In a murine transplant model, pre-transplant NK cell depletion through BCL-2 inhibition/knock-out leads to improved engraftment, and also improves survival without worsening GVHD in mice inoculated with an MLL-rearranged AML that is insensitive to BCL-2 inhibition (58). However, if post-transplant BCL-2 inhibition robustly depletes alloreactive NK cells, then this could have significant implications in haploidentical transplant, where KIR mismatching of NK cells leads to significant reductions in relapse rates in AML. (59) Future studies of venetoclax in the pre- and post-transplant setting will need to carefully assess its impact on engraftment and relapse, especially following HLA-mismatched transplantation, as it may attenuate the GVL effect that seems to be mediated by NK cells in these transplants. The efficacy of venetoclax in the treatment of newly diagnosed AML in the elderly was demonstrated in a randomized phase III trial in which azacitidine in combination with venetoclax improved both response rates (66.4% vs. 28.3%, p<0.001) and survival (median OS 14.7 vs. 9.6 months, p<0.001) compared to azacitidine alone. While the incidence of febrile neutropenia was significantly higher in the combination therapy group (30% vs. 10%), this did not translate into an increased incidence of early mortality (i.e. within 30 days of treatment initiation) (60). Given the increased risk of infectious complications in the post-transplant setting, one concern is that any disease-control advantage conferred by the addition of venetoclax to HMAs could be offset by increased infectious complications.
In practice the rates of serious and fatal infectious complications have proven much higher in post-transplant and relapsed/refractory than newly diagnosed AML patients treated with HMAs and venetoclax, but the regimen consistently induces remissions post-transplant, even in the absence of DLI. When used in the post-transplant setting, the incidence of fatal infectious complications in patients treated with HMAs and venetoclax is 16%, while the incidence of invasive fungal infections with such regimens more broadly in the relapsed/refractory setting is 19% (61, 62). Thus the use of appropriate, broad antimicrobial prophylaxis is critical in patients treated with HMAs and venetoclax for post-allo-HCT relapse. Venetoclax-containing regimens induce remissions in 28-31% of relapsed AML after allo-HCT without appreciable differences in response among patients who receive DLI, but significantly increased response rates when such regimens are used as the first post-transplant salvage treatment. In these studies, OS was significantly improved in responding patients and those treated for molecular relapse (61, 63). As the combination of HMAs and venetoclax can induce remissions post-transplant, it may serve as a useful bridge to subsequent cellular therapy. However, long-term survival data with this approach is lacking, and the significant risk of fatal infectious complications may nullify the response benefit of combination of therapy in the post-transplant setting. Thus HMAs and venetoclax may be an appropriate lower-intensity therapy for some post-transplant patients, especially in combination with appropriate antimicrobial prophylaxis, but further studies are needed to define its role vis-à-vis the more conventional approach combining HMAs with DLI.
Hypomethylating Agents With Lenalidomide
Lenalidomide represents another agent with known antileukemic activity and immunomodulatory properties with a recent study demonstrating its efficacy in treating post-transplant relapse in combination with azacitidine. AML patients in remission have been shown to have increased levels of TNFR2+ CD4+ T cells and regulatory T cells, leading to reduced IL-2 and IFN-gamma production, which can be reversed by treatment with azacitidine and lenalidomide (64). This could prove particularly relevant in post-transplant relapse patients with downregulation of HLA Class II, as IFN-gamma can rapidly reverse this phenotype in vitro (15, 16). However, prior studies of lenalidomide monotherapy in the post-transplant setting have been hampered by the development of GVHD with 43-60% of patients discontinuing for this reason (65, 66). Fortunately, when used in combination with azacitidine for the treatment of post-transplant AML relapse, the incidence of GVHD with lenalidomide was just 10% and 21% of patients achieved a subsequent remission without DLI. Interestingly, there was no evidence that this therapy reversed the exhausted T cell phenotype seen at relapse or led to enhanced cytokine production by T cells in responding patients (67). Further, the low rates of GVHD following lenalidomide treatment seen in this study could be a consequence of the universal use of T cell-depleting GVHD prophylaxis with alemtuzumab or anti-thymocyte globulin in the study population (67). This raises the possibility that an enhanced GVL effect could be seen with azacitidine and lenalidomide among patients who receive T cell-replete grafts, but it also raises the concern for more significant GVHD exacerbations following T cell-replete allo-HCT. Thus additional studies are needed to better define the generalizability of this approach to the more common T cell-replete setting.
Targeted Therapy
The dramatic expansion of gene sequencing technology over the last two decades has facilitated a better understanding of the important role that somatic gene mutations and gene fusions play in the prognosis of AML and led to the development of molecularly targeted therapies. The most common gene mutations in de novo AML include NPM1 (27%), FLT3 (28%), DNMT3A (26%), and IDH1/IDH2 (20%) with roughly 7% being IDH1 and 13% being IDH2 (68, 69). While somatic mutations in the tumor suppressor gene TP53 (8%) and 11q23 rearrangements (4%) are more rare in de novo AML, these alterations tend to be enriched in secondary AML (sAML), which is defined as leukemia that has progressed from an antecedent hematologic disorder or is related to prior therapy with cytotoxic agents or radiation (68, 70). As a consequence of the poor prognosis of sAML, transplant is indicated in appropriate patients, but they have a poor post-transplant prognosis due to high rates of relapse and NRM (71, 72). Approved targeted therapies that have been tested in the post-allo-HCT relapse setting include midostaurin and gilteritinib for FLT3+ AML, (73, 74) enasidenib for IDH2+ AML (75), and ivosidenib for IDH1+ AML (76). Additional therapies that have shown efficacy in patients with post-allo-HCT relapse include sorafenib for FLT3+ AML (77, 78), eprenetapopt for TP53-mutated AML (79), and menin inhibitors for NPM1-mutated and MLL-rearranged AML (80, 81). Studies for which specific data on responses to targeted therapy in the post-allo-HCT setting are available are listed in Table 3 . Interestingly, some targeted therapies yield better responses in the post-allo-HCT setting than in patients without prior transplant, suggesting that alloimmune effects may potentiate their efficacy.
Table 3.
Studies using Targeted Therapy for Post-Allo-HCT Relapse.
| Authors | Agent | Median Age | N& | ORR post-transplant | ORR non-transplant | ORR all | OS |
|---|---|---|---|---|---|---|---|
| FLT3-targeted Therapies | |||||||
| Perl | Gilteritinib | 48 | 36% (CR) | 18% | HR 0.48 (0.27-0.84) vs. chemo for all patients with prior transplant | ||
| Metzelder | Sorafenib | 45 (14-70) | 29 | 48% | 30% | 38% | |
| Bazarbachi | Sorafenib | 48 (19-69) | 34 | 39% (CR) | 2-year OS 38% vs. 9% for controls-->HR 0.44, p=0.001 | ||
| IDH1-targeted Therapy | |||||||
| DiNardo | Ivosidenib | 36 | 42%^ | ||||
| IDH2-targeted Therapy | |||||||
| Stein | Enasidenib | 29 | 35% | 40% | 39% | ||
&Refers to the number of post-allo-HCT patients included in the study. ^The authors note no difference in response rate based on pre-treatment characteristics of which prior allo-HCT was included.
FLT3 Inhibitors
While not an approved therapy for FLT3-mutated AML, the pankinase inhibitor sorafenib is a potent FLT3 inhibitor and the first targeted therapy to prove effective for post-allo-HCT relapse of AML (82). A study of FLT3-ITD AML patients who had relapsed a median of just 2.8 months post-transplant demonstrated a 39% remission rate in those treated with sorafenib with dramatically improved survival compared to a control group that did not receive sorafenib salvage, and a 2-year OS of 38% among those treated with sorafenib of whom 33% and 13% received subsequent DLI or a second allo-HCT, respectively (77). While the poor results of the control group are difficult to interpret given the potential biases that may have led them not to receive sorafenib in this retrospective study, it is clear that sorafenib can lead to durable responses for post-allo-HCT relapse including those with early relapse. In a separate study of relapsed/refractory FLT3-ITD AML, sorafenib led to high response rates with resistance developing later in patients with a history of allo-HCT, and the only durable responses (i.e. >12 months) seen in post-transplant patients (78). Notably, sorafenib has been shown to increase IL-15 production in mutant FLT3-ITD leukemia cells, increase IFN-γ production, and lead to metabolic reprograming of leukemia reactive T cells among relapsed patients post-allo-HCT who respond to treatment (83). The latter finding may be particularly relevant when there is underlying HLA Class II downregulation, as IFN-γ can reverse this process (15). Sorafenib has also been combined with azacitidine in a prospective trial for the treatment of relapsed/refractory FLT3-ITD+ AML with a response rate of 46%, but only seven patients in this study had received a prior allo-HCT (84) which precludes any conclusions about the specific efficacy of this combination in the post-transplant setting. Thus while prospective trial data supporting the use of sorafenib for post-allo-HCT relapse of FLT3-ITD+ AML is lacking, there is retrospective clinical data to support its use as monotherapy.
In contrast to sorafenib, the FLT3 inhibitors midostaurin and gilteritinib have both been investigated in prospective trials for relapsed/refractory FLT3-mutated AML that enrolled post-allo-HCT relapse patients. Midostaurin, which is approved for use in patients with newly diagnosed FLT3-mutated AML based on the RATIFY trial (85), in combination with azacitidine was investigated in a phase I/II trial in newly diagnosed, unfit and relapsed/refractory AML that enrolled primarily patients with FLT3 mutations (74%), and demonstrated a dramatically reduced response duration (median 6 weeks) in patients who had received a prior allo-HCT compared to those who had not (74). In spite of the heterogeneity of the patient population in this study, including patients who had received no prior treatment and those without FLT3 mutations, the results suggest that the combination of midostaurin and azacitidine is not effective for the treatment of post-allo-HCT relapse of FLT3-mutated AML. In contrast, the phase III ADMIRAL trial, which randomized relapsed/refractory FLT3-mutated AML patients to gilteritinib or chemotherapy salvage, demonstrated a dramatically improved response rate among gilteritinib-treated patients who had previously undergone allo-HCT (36% vs. 18% for non-transplant patients) and improved survival among post-transplant patients randomized to gilteritinib versus chemotherapy (73). The improved responses among patients with a prior allo-HCT suggests that alloimmune effects may also potentiate the activity of gilteritinib. The compelling response and survival data from the ADMIRAL trial makes FLT3-mutated AML the rare scenario in which the optimal initial therapy for post-transplant relapse is data driven. However, additional data are needed to better understand the necessity of subsequent cellular therapy to facilitate durable survival, and whether there is a role for combining gilteritinib with either intensive therapy, such as high-dose cytarabine, or hypomethylating agents.
IDH1/2 Inhibitors
After FLT3 mutations, IDH1 and IDH2 mutations represent the next two most common types of gene mutations in AML for which there are approved, targeted therapies, and there is evidence to support their use for the treatment of post-allo-HCT relapse. In a trial in relapsed/refractory IDH1-mutated AML that treated 36 post-transplant patients at the FDA-approved dose, ivosidenib produced an overall response rate (CR, CRi, CRp, or bone marrow CR) of 41.6% without any differences in response noted based on most baseline clinical characteristics, which included prior allo-HCT (76). Further data from this trial may help to elucidate the durability of responses with ivosidenib in the post-allo-HCT setting, although the overall response rate compares favorably with most studies of intensive and lower-intensity salvage therapies following allo-HCT. In a similar trial of enasidenib for IDH2-mutated, relapsed/refractory AML that enrolled 29 patients following allo-HCT, the overall response rate (CR, CRi, CRp, or bone marrow CR) among post-transplant patients was 34.5% compared to 39.5% among patients without a history of transplant (86). As with ivosidenib, additional studies are needed to better understand the durability of responses to enasidenib in the post-transplant setting including the need for further cellular therapy in responding patients. One appealing aspect of ivosidenib and enasidenib use in the post-transplant setting is that their toxicity profile generally compares favorably with intensive chemotherapy, except for the more frequent occurrence of differentiation syndrome (75, 76). Thus targeted therapy for IDH1 and IDH2-mutated AML that has relapsed following allo-HCT represents an effective and potentially less toxic alternative to conventional chemotherapy to achieve subsequent remission.
TP53-Targeted Therapy
Mutations in the tumor suppressor gene TP53 are frequent in AML patients with adverse cytogenetics and confer an extremely poor prognosis even with allo-HCT with subsequent relapse rates of 60% (87). However, the tumor suppressor function of TP53 can be restored with molecularly targeted therapy. (88) APR-246, now known as eprenetapopt, represents one such therapy, and a patient with relapsed TP53-mutated AML following allo-HCT achieved a complete response following treatment with this agent in combination with sorafenib and hydroxyurea (79). Unfortunately, the combination of therapies that this patient received makes it difficult to isolate the role eprenetapopt played in achieving remission. While two large trials of eprenetapopt in combination with azacitidine for newly diagnosed TP53-mutated myeloid malignancies demonstrated promising results (89, 90), a subsequent randomized phase III trial versus azacitidine monotherapy failed to show a survival benefit (91). Eprenetapopt has also been studied as a prophylactic post-transplant maintenance therapy in combination with azacitidine for patients with TP53-mutated AML and MDS leading a 1-year relapse-free survival of 58% (92). Given the dismal prognosis of TP53-mutated myeloid malignancies following transplant, this result is promising, but a randomized trial versus azacitidine is needed to establish the contribution of eprenetapopt to the durable post-transplant remissions seen in this study. Ultimately, evidence that eprenetapopt is useful as post-transplant prophylaxis is likely to suggest that it may also have a role for the treatment of post-transplant relapse.
Menin Inhibitors
Breakage of the KMT2A gene, commonly known as the MLL gene and located at 11q23, leads to a poor prognosis AML with relapse rates of 35% following allo-HCT in adult patients (93), while AML with an isolated mutation of NPM1 has an incidence of post-allo-HCT relapse of 12-22% when transplant is performed in first remission (94). KMT2A-rearranged and NPM1-mutated AML are linked by highly upregulated HOX expression, and menin inhibitors lead to cellular differentiation of leukemic cells in both types of AML both in vitro and in vivo. (95). This is similar to the cellular differentiation that is seen with IDH inhibitors in IDH-mutated AML. Two ongoing trials of menin inhibitors (SNDX-5613 and KO-539) in relapsed/refractory KMT2A-rearranged and NPM1-mutated acute leukemias including patients with a history of allo-HCT are currently enrolling with preliminary results demonstrating their efficacy in producing remissions (80, 81). At the moment, there is no specific data on the efficacy of these agents in post-allo-HCT patients, but they have demonstrated a favorable safety profile to date that suggests the potential for menin inhibitors to achieve similar success for post-transplant relapse in KMT2A-rearranged and NPM1-mutated AML as FLT3 and IDH inhibitors in their respective subtypes.
Immunotherapy
Given that the GVL effect in AML seems to be primarily mediated by T lymphocytes (11). there is substantial interest in T-cell-directed therapies to combat post-allo-HCT relapse. While post-transplant immunosuppression is critical for the prevention of GVHD, its withdrawal in relapsed patients without evidence of GVHD can unleash alloreactive T cells and even produce remissions in patients with morphologic relapse with low blast counts (<10%) (96). Other T cell-based therapies include DLI, immune checkpoint inhibitors, and dual affinity retargeting antibodies (DARTs), among others. However, stimulating alloreactive T cells post-transplant also dramatically increases the risk of GVHD, which greatly increases the potential morbidity of these therapies. A second allogeneic transplant, which relies on a combination of the GVL effect and conditioning chemotherapy, can also produce prolonged responses, both with and without antecedent chemotherapy to induce remission. One potential intervention that can help to maximize the GVL effect and reduce the post-allo-HCT relapse risk is the use of mobilized peripheral blood stem cells as opposed to a bone marrow graft (97, 98). However, as with other interventions that augment the GVL effect, the use of peripheral blood grafts leads to an increased risk of both acute and chronic GVHD (97, 98). As KIR mismatching has been shown to prevent AML relapse following haploidentical transplantation (59), haploidentical NK cell-based therapy is emerging as another cellular therapy option that may augment the GVL effect. Studies for which there are specific response and outcomes data for the use of immunotherapy for post-allo-HCT relapse of AML are highlighted in Table 4 .
Table 4.
Studies using Immunotherapies for Post-Allo-HCT Relapse.
| Authors | Therapy | DLI/2nd Transplant | Median time to relapse post-allo-HCT (mos) | Median Age | N | %CR/CRi | ORR | OS |
|---|---|---|---|---|---|---|---|---|
| DLI | ||||||||
| Kolb et al. | DLI | 100% | 7.9 (4.3-33.6) | 37 (4-54) | 23 | 22% | Median 248 days in AML/MDS | |
| Schmid et al. | DLI | 100%/8% | 5.5 (0.1-55) | 39 (16-65) | 171 | 35% | 38% | 2-year OS 20% with DLI |
| Kharfan-Dabaja et al. | DLI | 100%/18% | 7.0 (0.7-129) | 49 (19-75) | 281 | 24% | 5-year OS 15% (10-19%) | |
| Immune Checkpoint Inhibitors | ||||||||
| Davids et al. | Ipilimumab | 12 | 33% | 42% | ||||
| Davids et al. | Nivolumab | 10 | 0% | 0% | ||||
| Haploidentical NK Cell Infusion | ||||||||
| Shaffer et al. | NK cell infusion | 3.5 (1-94) | 19 (1.9-55.9) | 8 | 25% | 38% | Median 12.9 months (0.8-65.3 months) | |
| 2nd Allogeneic Transplant | ||||||||
| Kharfan-Dabaja et al. | 2nd Transplant (MUD) | NR/100% | 15 (6-31) | 46 (35-58) | 320 | 2 year OS 31% (26-37%) | ||
| Kharfan-Dabaja et al. | 2nd Transplant (Haplo) | NR/100% | 11 (5-25) | 44(33-53) | 135 | 2-year OS 29% (20-39%) | ||
| Kharfan-Dabaja et al. | 2nd Transplant | 1%/100% | 11.6 (1-152) | 43 (18-67) | 137 | 39% | 5-year OS 19% (12-25%) | |
Withdrawal of Immunosuppression
For AML patients, discontinuation of post-transplant immunosuppression can prevent impending relapse and even induce remission in some cases of morphologic relapse. Leukemia and MDS patients with serially decreasing levels of donor chimerism post-transplant are at significantly increased risk of relapse compared to patients with full donor chimerism and rising levels of donor chimerism (99). This suggests that interventions that reverse declining chimerism may prevent subsequent relapse. In a cohort of 26 patients with hematologic malignancies with incomplete donor chimerism following allo-HCT, 27% achieved full donor chimerism following the early withdrawal of immunosuppression with the majority remaining in durable remission (≥31 months) (100). Among 45 AML and MDS with morphologic relapse post-transplant in the absence of GVHD, the withdrawal of immunosuppression led to complete remission in 6.6%, all of whom had less than 10% blasts in the marrow at relapse (96). While the withdrawal of immunosuppression for post-transplant relapse increases the risk of GVHD, it can yield remission in a limited subset of cases and may also potentiate the GVL effect of subsequent therapy. Thus for relapsed post-transplant patients without GVHD, withdrawal of immunosuppression should generally be the first intervention.
DLI
Following the withdrawal of immunosuppression for relapsed post-allo-HCT patients without GVHD, DLI can induce remissions, but durable responses are seen only in patients who achieve remission before DLI and those with a favorable karyotype. In a cohort of 171 AML patients who received DLI for post-allo-HCT relapse, 35% achieved remission with 43% and 46% of evaluable patients developing acute and chronic GVHD, respectively (14). This highlights the significant morbidity that is caused by DLI, which makes it essential to carefully select patients who are likely to benefit prior to initiating therapy. In this cohort of patients who universally received DLI for post-transplant relapse, three prognostic groups were defined with patients in remission at the time of DLI and/or with a favorable karyotype having a 2-year overall survival of 56% compared to 21% and 9% in female patients who were not in remission at the time of DLI but had a low tumor burden (<35% blasts) at relapse and all remaining patients, respectively (14). While durable responses are achieved even among patients with active disease, this must be weighed against the risk of morbidity from GVHD. Furthermore, the recently characterized immunoediting capabilities of post-transplant AML with downregulation of HLA Class II genes or loss of haplotype raises the possibility that DLI could be ineffective in relapses involving this underlying mechanism of immune evasion (15–17). If studies clearly demonstrate the ineffectiveness of DLI in this setting, then it will be critical to develop standardized assays to screen for this mechanism, as its presence may necessitate alternative therapy to achieve a durable remission. Finally, there is also no data to support the optimal salvage regimen to achieve remission prior to DLI such that the choice and intensity of chemotherapy necessarily depends on the patient’s performance status, co-morbidities, and the presence of targetable gene mutations.
Checkpoint Inhibitors
A second immunologic mechanism that seems to underly post-transplant relapse is the development of T cell exhaustion with the increased expression of immune checkpoints (17). This raises the possibility that immune checkpoint blockade could be a useful treatment approach for post-transplant relapse of AML. In a study including 12 AML patients with post-transplant relapse, blockade of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) with ipilimumab produced a response rate of 42% including complete remission in 4 patients with extramedullary leukemia. A total of 22 post-transplant patients received the maximum tolerated dose of 10 mg/kg of ipilimumab with three patients developing GVHD that precluded further treatment on the study and three patients developing immune-related adverse events (immune thrombocytopenia, colitis, and pneumonitis) of whom two patients were able to resume therapy following treatment with steroids (101). While the side effects of post-transplant ipilimumab are significant, its unique efficacy in patients with extramedullary relapse may justify its use in this scenario. A separate study of PD-1 blockade with nivolumab for post-allo-HCT relapse enrolled 10 patients with AML of whom none had an objective response to treatment, and nivolumab led to dose limiting toxicity in 18% of patients including 2 deaths from GVHD in spite of dose de-escalation (102). The lack of efficacy and significant toxicity seen in this small cohort of AML patients tempers enthusiasm for post-transplant PD-1 blockade, although additional studies are needed to define whether specific toxicity mitigation strategies could make this a tolerable treatment option. In addition to T cell checkpoint inhibitors, a recent addition to the armamentarium is the macrophage checkpoint inhibitor magrolimab, which targets CD47, and has shown promising activity in combination with azacitidine in newly diagnosed AML patients who are unfit for intensive therapy with an objective response rate of 71% in TP53-mutated AML (103). As detailed previously, the presence of a TP53 mutation confers an extremely poor prognosis even among AML patients who undergo allo-HCT, so it is hopeful that magrolimab’s promising activity in the newly diagnosed setting will translate to post-transplant, relapsed patients. Overall, studies of immune checkpoint inhibition for post-allo-HCT relapse have demonstrated significant toxicity with encouraging results restricted to AML patients with extramedullary relapse who were treated with ipilimumab.
DARTs
Flotetuzumab, a CD123 x CD3 DART, represents another promising immunotherapy for the treatment of TP53-mutated AML. In a phase I/II trial, 47% of patients with relapsed/refractory AML and TP53 abnormalities had a complete response to flotetuzumab with responders demonstrating a significantly higher baseline tumor inflammation score than nonresponders. (104) While this study did not enroll patients with post-allo-HCT relapse, a study of flotetuzumab in combination with DLI is currently recruiting (NCT04582864).
CAR T Cells
CAR T cells are approved for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) and represent a promising modality for the treatment of post-transplant, relapsed AML. Among patients with ALL, response rates to CD19-targeted CAR T cells and 12-month overall survival are similar regardless of prior receipt of allo-HCT (105). Importantly, the comparable survival outcomes in adult patients regardless of prior transplant status suggests that prior allo-HCT does not potentiate the risk of mortal CAR T-cell toxicities. For relapsed AML after allo-HCT, CD19 CAR T cells have proven effective in a number of patients with t(8;21), which often aberrantly expresses CD19 (106, 107). While CD19 is rarely expressed in AML cells, CAR T cells targeting CD33; CD38; and CD123 have demonstrated efficacy for the treatment of post-allo-HCT AML relapse (108, 109), while CAR T cells targeting CLL1; CD13; and TIM3 among others are in development for the treatment of relapsed/refractory AML (110, 111). Thus the early evidence points to the relative safety of CAR T cells for the treatment of relapsed ALL after allo-HCT, and early studies treating relapsed AML after allo-HCT with CAR T cells targeting a variety of different antigens have yielded promising results.
NK Cell Therapy
The importance of KIR mismatching in preventing AML relapse was initially reported in patients undergoing haploidentical allo-HCT (59), but more recent studies also point to the importance of KIR mismatching following fully matched transplant (112). A small study of eight patients (6 AML and 2 MDS) with post-allo-HCT relapse demonstrated that haploidentical NK cell infusion following lymphodepleting chemotherapy was safe and produced remission in 25% of patients, although the haploidentical NK cells did not persist, leading to relapse within 2 months (113). However, the pre-emptive administration of donor-derived, expanded NK cells after haploidentical transplantation has shown promise as a strategy to decrease early post-transplant relapse (114). While the GVL effect is primarily mediated by donor T lymphocytes, NK cells also play a role, and further studies to improve the persistence of allogeneic NK cells may help to produce more durable outcomes.
Second Transplant
Another form of cellular immunotherapy for AML patients who relapse following allo-HCT is a second allo-HCT. Similar to DLI, a second allogeneic transplant produces the best outcomes when performed in remission and in patients with a longer duration of remission following their initial transplant. In an EBMT registry study including 455 patients with relapsed AML following allo-HCT who received a second transplant from a matched unrelated or haploidentical donor, the two factors that predicted poorer leukemia-free survival (LFS) following second transplant were transplant with active disease versus CR (HR 1.48, p=0.004) and duration of remission >13.2 months following the first transplant (HR 0.53, p<0.0001) without appreciable differences based on donor type (115). Unsurprisingly, the clinical factors that predict outcome following a second allo-HCT are similar to those that predict outcome after DLI. A separate EBMT registry study failed to demonstrate an improvement in outcomes by switching donors for the second allo-HCT for AML (116), but a small study of 40 patients demonstrated improved overall and event-free survival in patients whose second transplant utilized a new mismatched haplotype. While the authors attempted to determine whether acquired uniparental disomy (UPD) of chromosome 6p as a mechanism of genomic HLA loss played a role in relapse for patients who had previously undergone haploidentical transplant, the small patient numbers precluded any definitive conclusions (117). This well characterized mechanism of immune evasion in patients who have undergone haploidentical transplant provides a reasonable rationale for choosing 2nd transplant over DLI as consolidation therapy in certain patients. However, there is a lack of compelling data to guide the choice between these two options as consolidative cellular immunotherapy in most AML patients with post-transplant relapse. A retrospective study of 418 patients from the EBMT attempted to compare outcomes with second transplant versus DLI for AML and demonstrated similar 5-year overall survival (19% for 2nd transplant vs. 15% for DLI), although patients receiving 2nd transplant were significantly younger (median age 43 vs. 49), more likely to have achieved remission prior to cellular therapy (38.7% vs. 18.1%), and had a significantly longer interval from 1st transplant to relapse (median 348 days vs. 211 days). Notably, the incidence of NRM at 2 years was significantly higher among patients who received second transplant (26% vs. 9%) as was the incidence of grade 2-4 aGVHD at day +100 after intervention (37% vs. 20%), but there were no differences in the incidence of cGVHD (118). Based on these results, DLI and 2nd allo-HCT are both reasonable options as consolidation for patients achieving remission, but 2nd transplant clearly carries an increased risk of toxicity.
Discussion
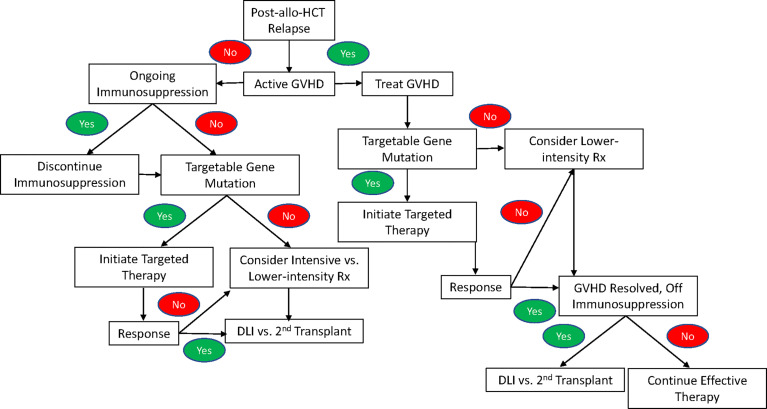
AML relapse following allo-HCT is a common problem, and its management presents additional challenges compared to relapsed/refractory AML in the non-transplant setting due to potential for residual complications from allo-HCT, especially GVHD. While randomized trials are lacking, treatment seems to be most effective when initiated prior to frank morphologic relapse when falling donor chimerism or recurrent leukemia-defining mutations suggestive of measurable residual disease (MRD) are detected. (44). Another area of active investigation is the use of maintenance therapies to prevent post-transplant relapse. This review has primarily focused on options for the management of frank morphologic relapse. As illustrated in Figure 1 , treatment for patients with ongoing or unresolved complications from GVHD at the time of relapse necessarily involves the ongoing management of GVHD, which may limit options for further therapy, particularly due to the potential for intensive therapies to increase toxicity. These patients are also often excluded from clinical trials due to the need for ongoing immunosuppression and their increased risk of complications. Patients with active GVHD may be candidates for targeted therapies against FLT3; IDH1; and IDH2, as there is not currently evidence to suggest that these therapies are likely to exacerbate GVHD. If GVHD can be controlled, then lower-intensity therapies such as azacitidine, decitabine, or azacitidine/venetoclax may be an option for those without targetable mutations or who fail to respond to targeted therapy. Ultimately, any treatment responses achieved with targeted or lower-intensity therapies will not be durable, so subsequent cellular immunotherapy with DLI or a 2nd allogeneic transplant should be considered. However, this must be approached with caution, as a history of grade 2-4 acute GVHD prior to a second transplant or DLI has been shown to lead to significantly increased NRM and poorer survival compared to patients without such a history (118).
Figure 1.
An algorithm for the treatment of relapse of AML after allogeneic hematopoietic stem cell transplant following a standard-of-care approach in the absence of an available clinical trial.
In patients without active or unresolved complications of GVHD who suffer a post-transplant relapse, treatment begins with the withdrawal of immunosuppression. However, this is rarely an effective strategy in isolation, so additional treatment is indicated. Given the lack of a standard-of-care approach in patients with post-allo-HCT relapse, enrollment on a clinical trial is preferred. Figure 1 provides an algorithm for treatment following a standard-of-care approach when a clinical trial is not available. While there are no trials comparing targeted therapy to intensive therapy or lower-intensity treatment specifically in the post-allo-HCT relapse setting, the available data for FLT3, IDH1, and IDH2 inhibitors suggest comparable or improved response rates with decreased toxicity, making targeted therapy the treatment of choice for relapsed AML with a targetable mutation. In patients who lack a targetable mutation or fail to respond to targeted therapy, a number of factors guide the choice between intensive and lower-intensity therapy including patient age, performance status, co-morbidities, and prior lines of therapy. Additionally, poor outcomes with intensive therapy among patients with an early post-transplant relapse (i.e. <6 months) often make it difficult to justify the potential for added toxicity, except in fit, young patients and those with rapidly proliferative disease that cannot be adequately controlled with lower-intensity therapy.
If a response is achieved following intensive; lower-intensity; or targeted therapy, then subsequent cellular immunotherapy with DLI or a 2nd transplant is indicated, as there is not currently any data to suggest that such responses are durable in the absence of cellular immunotherapy. For patients with persistent leukemia despite treatment of post-transplant relapse and reasonable fitness, DLI or a second transplant with active disease still may be an option given a 5-year survival of 11% with either therapy in this scenario (118). However, other factors that influence the likelihood of successful cellular immunotherapy, such as the time from first transplant to relapse, must be carefully considered in such patients due to the significant potential for toxicity.
Ultimately, the recent proliferation of novel agents for the treatment of AML including both targeted therapies and immunotherapies, which may both enhance the GVL effect, is altering the landscape for treatment of post-allo-HCT AML relapse. This review highlights the unmet need for further studies in this area, especially as increases in the frequency of allo-HCT for AML coupled with decreases in NRM due to improvements in supportive care and the use of reduced-intensity conditioning place more patients at risk for post-transplant relapse. As we continue to better understand the immunologic mechanisms underlying the GVL effect, some of these novel therapies may serve to enhance it without exacerbating GVHD. Furthermore, a better understanding of the immunologic mechanisms underlying post-transplant relapse may facilitate the rational selection of treatment strategies to overcome those mechanisms such as 2nd transplant with haplotype switch for patients who have relapsed after haploidentical transplant with leukemic blasts demonstrating loss of the mismatched haplotype. Thus advancements in the laboratory may help to guide our use of the expanding options for the treatment of post-allo-HCT relapse of AML.
Author Contributions
JW and IG contributed equally to the conception of the manuscript. JW drafted the initial manuscript. IG and LL revised the initial draft. JW, LL, and IG agreed upon the final contents of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by NIH grant P30 CA06973.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and Acute Myeloid Leukemia. Blood (2006) 107(9):3481–5. doi: 10.1182/blood-2005-09-3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia in First Complete Remission: Systematic Review and Meta-Analysis of Prospective Clinical Trials. JAMA (2009) 301(22):2349–61. doi: 10.1001/jama.2009.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scott BL, Pasquini MC, Fei M, Fraser R, Wu J, Devine SM, et al. Myeloablative Versus Reduced-Intensity Conditioning for Hematopoietic Cell Transplantation in Acute Myelogenous Leukemia and Myelodysplastic Syndromes-Long-Term Follow-Up of the BMT CTN 0901 Clinical Trial. Transplant Cell Ther (2021) 27(6):483.e1–6. doi: S2666-6367(21)00718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Passweg JR, Labopin M, Cornelissen J, Volin L, Socie G, Huynh A, et al. Conditioning Intensity in Middle-Aged Patients With AML in First CR: No Advantage for Myeloablative Regimens Irrespective of the Risk Group-an Observational Analysis by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant (2015) 50(8):1063–8. doi: 10.1038/bmt.2015.121 [DOI] [PubMed] [Google Scholar]
- 5. Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced Intensity Conditioning Compared With Myeloablative Conditioning Using Unrelated Donor Transplants in Patients With Acute Myeloid Leukemia. J Clin Oncol (2009) 27(27):4570–7. doi: 10.1200/JCO.2008.20.9692 [DOI] [PubMed] [Google Scholar]
- 6. Horowitz M, Schreiber H, Elder A, Heidenreich O, Vormoor J, Toffalori C, et al. Epidemiology and Biology of Relapse After Stem Cell Transplantation. Bone Marrow Transplant (2018) 53(11):1379–89. doi: 10.1038/s41409-018-0171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, Risk Factors, and Outcome of Adults With Relapsed AML After Reduced Intensity Conditioning for Allogeneic Stem Cell Transplantation. Blood (2012) 119(6):1599–606. doi: 10.1182/blood-2011-08-375840 [DOI] [PubMed] [Google Scholar]
- 8. Cornelissen JJ, Versluis J, Passweg JR, van Putten WL, Manz MG, Maertens J, et al. Comparative Therapeutic Value of Post-Remission Approaches in Patients With Acute Myeloid Leukemia Aged 40-60 Years. Leukemia (2015) 29(5):1041–50. doi: 10.1038/leu.2014.332 [DOI] [PubMed] [Google Scholar]
- 9. Center for International Blood and Marrow Transplant Research . Number of HCTs Performed in the United States and Reported to CIBMTR - Cell Source and Donor Category by Donor Type and Year (2019). Available at: https://bloodcell.transplant.hrsa.gov/research/transplant_data/transplant_activity_report/bydiseasecategorycellsource.pdf (Accessed March 7).
- 10. Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of Patients With Acute Myeloid Leukemia Relapsing After Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant (2015) 21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-Versus-Leukemia Reactions After Bone Marrow Transplantation. Blood (1990) 75(3):555–62. doi: 10.1182/blood.V75.3.555.555 [DOI] [PubMed] [Google Scholar]
- 12. Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic Effect of Graft-Versus-Host Disease in Human Recipients of Allogeneic-Marrow Grafts. N Engl J Med (1979) 300(19):1068–73. doi: 10.1056/NEJM197905103001902 [DOI] [PubMed] [Google Scholar]
- 13. Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-Versus-Leukemia Effect of Donor Lymphocyte Transfusions in Marrow Grafted Patients. Blood (1995) 86(5):2041–50. doi: 10.1182/blood.V86.5.2041.bloodjournal8652041 [DOI] [PubMed] [Google Scholar]
- 14. Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor Lymphocyte Infusion in the Treatment of First Hematological Relapse After Allogeneic Stem-Cell Transplantation in Adults With Acute Myeloid Leukemia: A Retrospective Risk Factors Analysis and Comparison With Other Strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol (2007) 25(31):4938–45. doi: 10.1200/JCO.2007.11.6053 [DOI] [PubMed] [Google Scholar]
- 15. Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune Escape of Relapsed AML Cells After Allogeneic Transplantation. N Engl J Med (2018) 379(24):2330–41. doi: 10.1056/NEJMoa1808777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, et al. Immune Signature Drives Leukemia Escape and Relapse After Hematopoietic Cell Transplantation. Nat Med (2019) 25(4):603–11. doi: 10.1038/s41591-019-0400-z [DOI] [PubMed] [Google Scholar]
- 17. Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of Mismatched HLA in Leukemia After Stem-Cell Transplantation. N Engl J Med (2009) 361(5):478–88. doi: 10.1056/NEJMoa0811036 [DOI] [PubMed] [Google Scholar]
- 18. McCurdy SR, Iglehart BS, Batista DA, Gocke CD, Ning Y, Knaus HA, et al. Loss of the Mismatched Human Leukocyte Antigen Haplotype in Two Acute Myelogenous Leukemia Relapses After Haploidentical Bone Marrow Transplantation With Post-Transplantation Cyclophosphamide. Leukemia (2016) 30(10):2102–6. doi: 10.1038/leu.2016.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noviello M, Manfredi F, Ruggiero E, Perini T, Oliveira G, Cortesi F, et al. Bone Marrow Central Memory and Memory Stem T-Cell Exhaustion in AML Patients Relapsing After HSCT. Nat Commun (2019) 10(1):1065–1. doi: 10.1038/s41467-019-08871-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Litzow MR, Wang XV, Carroll MP, Karp JE, Ketterling R, Kaufmann SH, et al. A Randomized Phase II Trial of Three Novel Regimens for Relapsed/ Refractory Acute Myeloid Leukemia (AML) Demonstrates Encouraging Results With a Flavopiridol-Based Regimen: Results of Eastern Cooperative Oncology Group (ECOG) Trial E1906. Blood (2014) 124(21):3742. doi: 10.1182/blood.V124.21.3742.3742 [DOI] [Google Scholar]
- 21. Ravandi F, Ritchie EK, Sayar H, Lancet JE, Craig MD, Vey N, et al. Vosaroxin Plus Cytarabine Versus Placebo Plus Cytarabine in Patients With First Relapsed or Refractory Acute Myeloid Leukaemia (VALOR): A Randomised, Controlled, Double-Blind, Multinational, Phase 3 Study. Lancet Oncol (2015) 16(9):1025–36. doi: 10.1016/S1470-2045(15)00201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin MG, Augustin KM, Uy GL, Welch JS, Hladnik L, Goyal S, et al. Salvage Therapy for Acute Myeloid Leukemia With Fludarabine, Cytarabine, and Idarubicin With or Without Gemtuzumab Ozogamicin and With Concurrent or Sequential G-CSF. Am J Hematol (2009) 84(11):733–7. doi: 10.1002/ajh.21545 [DOI] [PubMed] [Google Scholar]
- 23. Karanes C, Kopecky KJ, Head DR, Grever MR, Hynes HE, Kraut EH, et al. A Phase III Comparison of High Dose ARA-C (HIDAC) Versus HIDAC Plus Mitoxantrone in the Treatment of First Relapsed or Refractory Acute Myeloid Leukemia Southwest Oncology Group Study. Leuk Res (1999) 23(9):787–94. doi: 10.1016/S0145-2126(99)00087-9 [DOI] [PubMed] [Google Scholar]
- 24. Pastore D, Specchia G, Carluccio P, Liso A, Mestice A, Rizzi R, et al. FLAG-IDA in the Treatment of Refractory/Relapsed Acute Myeloid Leukemia: Single-Center Experience. Ann Hematol (2003) 82(4):231–5. doi: 10.1007/s00277-003-0624-2 [DOI] [PubMed] [Google Scholar]
- 25. Amadori S, Arcese W, Isacchi G, Meloni G, Petti MC, Monarca B, et al. Mitoxantrone, Etoposide, and Intermediate-Dose Cytarabine: An Effective and Tolerable Regimen for the Treatment of Refractory Acute Myeloid Leukemia. J Clin Oncol (1991) 9(7):1210–4. doi: 10.1200/JCO.1991.9.7.1210 [DOI] [PubMed] [Google Scholar]
- 26. Spadea A, Petti MC, Fazi P, Vegna ML, Arcese W, Avvisati G, et al. Mitoxantrone, Etoposide and Intermediate-Dose Ara-C (MEC): An Effective Regimen for Poor Risk Acute Myeloid Leukemia. Leukemia (1993) 7(4):549–52. [PubMed] [Google Scholar]
- 27. Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Holowiecki J, et al. Cladribine Combined With High Doses of Arabinoside Cytosine, Mitoxantrone, and G-CSF (CLAG-M) Is a Highly Effective Salvage Regimen in Patients With Refractory and Relapsed Acute Myeloid Leukemia of the Poor Risk: A Final Report of the Polish Adult Leukemia Group. Eur J Haematol (2008) 80(2):115–26. doi: 10.1111/j.1600-0609.2007.00988.x [DOI] [PubMed] [Google Scholar]
- 28. Koren-Michowitz M, Maayan H, Apel A, Shem-Tov N, Yerushalmi R, Volchek Y, et al. Salvage Therapy With ARA-C and Gemtuzumab Ozogamicin in AML Patients Relapsing After Stem Cell Transplantation. Ann Hematol (2015) 94(3):375–8. doi: 10.1007/s00277-014-2229-3 [DOI] [PubMed] [Google Scholar]
- 29. Motabi IH, Ghobadi A, Liu J, Schroeder M, Abboud CN, Cashen AF, et al. Chemotherapy Versus Hypomethylating Agents for the Treatment of Relapsed Acute Myeloid Leukemia and Myelodysplastic Syndrome After Allogeneic Stem Cell Transplant. Biol Blood Marrow Transplant (2016) 22(7):1324–9. doi: 10.1016/j.bbmt.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 30. Sauer T, Silling G, Groth C, Rosenow F, Krug U, Gorlich D, et al. Treatment Strategies in Patients With AML or High-Risk Myelodysplastic Syndrome Relapsed After Allo-SCT. Bone Marrow Transplant (2015) 50(4):485–92. doi: 10.1038/bmt.2014.300 [DOI] [PubMed] [Google Scholar]
- 31. Devillier R, Crocchiolo R, Etienne A, Prebet T, Charbonnier A, Furst S, et al. Outcome of Relapse After Allogeneic Stem Cell Transplant in Patients With Acute Myeloid Leukemia. Leuk Lymphoma (2013) 54(6):1228–34. doi: 10.3109/10428194.2012.741230 [DOI] [PubMed] [Google Scholar]
- 32. Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective Trial of Chemotherapy and Donor Leukocyte Infusions for Relapse of Advanced Myeloid Malignancies After Allogeneic Stem-Cell Transplantation. J Clin Oncol (2002) 20(2):405–12. doi: 10.1200/JCO.2002.20.2.405 [DOI] [PubMed] [Google Scholar]
- 33. Vogler WR, McCarley DL, Stagg M, Bartolucci AA, Moore J, Martelo O, et al. A Phase III Trial of High-Dose Cytosine Arabinoside With or Without Etoposide in Relapsed and Refractory Acute Myelogenous Leukemia. A Southeastern Cancer Study Group Trial. Leukemia (1994) 8(11):1847–53. [PubMed] [Google Scholar]
- 34. Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients With Newly Diagnosed AML With >30% Blasts. Blood (2015) 126(3):291–9. doi: 10.1182/blood-2015-01-621664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coral S, Sigalotti L, Gasparollo A, Cattarossi I, Visintin A, Cattelan A, et al. Prolonged Upregulation of the Expression of HLA Class I Antigens and Costimulatory Molecules on Melanoma Cells Treated With 5-Aza-2’-Deoxycytidine (5-AZA-CdR). J Immunother (1999) 22(1):16–24. doi: 10.1097/00002371-199901000-00003 [DOI] [PubMed] [Google Scholar]
- 36. Coral S, Sigalotti L, Altomonte M, Engelsberg A, Colizzi F, Cattarossi I, et al. 5-Aza-2’-Deoxycytidine-Induced Expression of Functional Cancer Testis Antigens in Human Renal Cell Carcinoma: Immunotherapeutic Implications. Clin Cancer Res (2002) 8(8):2690–5. [PubMed] [Google Scholar]
- 37. Gang AO, Frosig TM, Brimnes MK, Lyngaa R, Treppendahl MB, Gronbaek K, et al. 5-Azacytidine Treatment Sensitizes Tumor Cells to T-Cell Mediated Cytotoxicity and Modulates NK Cells in Patients With Myeloid Malignancies. Blood Cancer J (2014) 4:e197. doi: 10.1038/bcj.2014.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, et al. Hypomethylating Agents in Combination With Immune Checkpoint Inhibitors in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Leukemia (2018) 32(5):1094–105. doi: 10.1038/s41375-018-0070-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ehx G, Fransolet G, de Leval L, D'Hondt S, Lucas S, Hannon M, et al. Azacytidine Prevents Experimental Xenogeneic Graft-Versus-Host Disease Without Abrogating Graft-Versus-Leukemia Effects. Oncoimmunol (2017) 6(5):e1314425. doi: 10.1080/2162402X.2017.1314425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, Caballero-Velazquez T, Blanco B, Herrero-Sanchez C, et al. Immunomodulatory Effect of 5-Azacytidine (5-Azac): Potential Role in the Transplantation Setting. Blood (2010) 115(1):107–21. doi: 10.1182/blood-2009-03-210393 [DOI] [PubMed] [Google Scholar]
- 41. Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, et al. In Vivo Administration of Hypomethylating Agents Mitigate Graft-Versus-Host Disease Without Sacrificing Graft-Versus-Leukemia. Blood (2010) 116(1):129–39. doi: 10.1182/blood-2009-12-257253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ Regulatory T Cells Preserve Graft-Versus-Tumor Activity While Inhibiting Graft-Versus-Host Disease After Bone Marrow Transplantation. Nat Med (2003) 9(9):1144–50. doi: 10.1038/nm915 [DOI] [PubMed] [Google Scholar]
- 43. Bolanos-Meade J, Smith BD, Gore SD, McDevitt MA, Luznik L, Fuchs EJ, et al. 5-Azacytidine as Salvage Treatment in Relapsed Myeloid Tumors After Allogeneic Bone Marrow Transplantation. Biol Blood Marrow Transplant (2011) 17(5):754–8. doi: 10.1016/j.bbmt.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for Treatment of Imminent Relapse in MDS or AML Patients After Allogeneic HSCT: Results of the RELAZA Trial. Leukemia (2012) 26(3):381–9. doi: 10.1038/leu.2011.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Webster JA, Yogarajah M, Zahurak M, Symons H, Dezern AE, Gojo I, et al. A Phase II Study of Azacitidine in Combination With Granulocyte-Macrophage Colony-Stimulating Factor as Maintenance Treatment, After Allogeneic Blood or Marrow Transplantation in Patients With Poor-Risk Acute Myeloid Leukemia (AML) or Myelodysplastic Syndrome (MDS). Leuk Lymphoma (2021) 1–11. doi: 10.1080/10428194.2021.1948029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transplant (2016) 22(2):385–90. doi: 10.1016/j.bbmt.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pusic I, Choi J, Fiala MA, Gao F, Holt M, Cashen AF, et al. Maintenance Therapy With Decitabine After Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant (2015) 21(10):1761–9. doi: 10.1016/j.bbmt.2015.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, et al. CC-486 Maintenance After Stem Cell Transplantation in Patients With Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol Blood Marrow Transplant (2018) 24(10):2017–24. doi: 10.1016/j.bbmt.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maples KT, Sabo RT, McCarty JM, Toor AA, Hawks KG. Maintenance Azacitidine After Myeloablative Allogeneic Hematopoietic Cell Transplantation for Myeloid Malignancies. Leuk Lymphoma (2018) 59(12):2836–41. doi: 10.1080/10428194.2018.1443334 [DOI] [PubMed] [Google Scholar]
- 50. Ali N, Tomlinson B, Metheny L, Goldstein SC, Fu P, Cao S, et al. Conditioning Regimen Intensity and Low-Dose Azacitidine Maintenance After Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia. Leuk Lymphoma (2020) 1–11. doi: 10.1080/10428194.2020.1789630 [DOI] [PubMed] [Google Scholar]
- 51. Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A Phase 3 Randomized Study of 5-Azacitidine Maintenance vs Observation After Transplant in High-Risk AML and MDS Patients. Blood Adv (2020) 4(21):5580–8. doi: 10.1182/bloodadvances.2020002544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao L, Zhang Y, Wang S, Kong P, Su Y, Hu J, et al. Effect of rhG-CSF Combined With Decitabine Prophylaxis on Relapse of Patients With High-Risk MRD-Negative AML After HSCT: An Open-Label, Multicenter, Randomized Controlled Trial. J Clin Oncol (2020) 38(36):4249–59. doi: 10.1200/JCO.19.03277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Craddock C, Labopin M, Robin M, Finke J, Chevallier P, Yakoub-Agha I, et al. Clinical Activity of Azacitidine in Patients Who Relapse After Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia. Haematologica (2016) 101(7):879–83. doi: 10.3324/haematol.2015.140996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lubbert M, Bertz H, Wasch R, Marks R, Ruter B, Claus R, et al. Efficacy of a 3-Day, Low-Dose Treatment With 5-Azacytidine Followed by Donor Lymphocyte Infusions in Older Patients With Acute Myeloid Leukemia or Chronic Myelomonocytic Leukemia Relapsed After Allografting. Bone Marrow Transplant (2010) 45(4):627–32. doi: 10.1038/bmt.2009.222 [DOI] [PubMed] [Google Scholar]
- 55. Tessoulin B, Delaunay J, Chevallier P, Loirat M, Ayari S, Peterlin P, et al. Azacitidine Salvage Therapy for Relapse of Myeloid Malignancies Following Allogeneic Hematopoietic SCT. Bone Marrow Transplant (2014) 49(4):567–71. doi: 10.1038/bmt.2013.233 [DOI] [PubMed] [Google Scholar]
- 56. Schroeder T, Rautenberg C, Kruger W, Platzbecker U, Bug G, Steinmann J, et al. Treatment of Relapsed AML and MDS After Allogeneic Stem Cell Transplantation With Decitabine and DLI-A Retrospective Multicenter Analysis on Behalf of the German Cooperative Transplant Study Group. Ann Hematol (2018) 97(2):335–42. doi: 10.1007/s00277-017-3185-5 [DOI] [PubMed] [Google Scholar]
- 57. Rautenberg C, Bergmann A, Germing U, Fischermanns C, Pechtel S, Kaivers J, et al. Prediction of Response and Survival Following Treatment With Azacitidine for Relapse of Acute Myeloid Leukemia and Myelodysplastic Syndromes After Allogeneic Hematopoietic Stem Cell Transplantation. Cancers (Basel) (2020) 12(8). doi: 10.3390/cancers12082255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiao Y, Davis JE, Rautela J, Carrington EM, Ludford-Menting MJ, Goh W, et al. Recipient BCL2 Inhibition and NK Cell Ablation Form Part of a Reduced Intensity Conditioning Regime That Improves Allo-Bone Marrow Transplantation Outcomes. Cell Death Differ (2019) 26(8):1516–30. doi: 10.1038/s41418-018-0228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science (2002) 295(5562):2097–100. doi: 10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- 60. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383(7):617–29. doi: 10.1056/NEJMoa2012971 [DOI] [PubMed] [Google Scholar]
- 61. Schuler E, Wagner-Drouet EM, Ajib S, Bug G, Crysandt M, Dressler S, et al. Treatment of Myeloid Malignancies Relapsing After Allogeneic Hematopoietic Stem Cell Transplantation With Venetoclax and Hypomethylating Agents-a Retrospective Multicenter Analysis on Behalf of the German Cooperative Transplant Study Group. Ann Hematol (2021) 100(4):959–68. doi: 10.1007/s00277-020-04321-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aldoss I, Dadwal S, Zhang J, Tegtmeier B, Mei M, Arslan S, et al. Invasive Fungal Infections in Acute Myeloid Leukemia Treated With Venetoclax and Hypomethylating Agents. Blood Adv (2019) 3(23):4043–9. doi: 10.1182/bloodadvances.2019000930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Joshi M, Cook J, McCullough K, Nanaa A, Gangat N, Foran JM, et al. Salvage Use of Venetoclax-Based Therapy for Relapsed AML Post Allogeneic Hematopoietic Cell Transplantation. Blood Cancer J (2021) 11(3):49–z. doi: 10.1038/s41408-021-00437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Govindaraj C, Madondo M, Kong YY, Tan P, Wei A, Plebanski M. Lenalidomide-Based Maintenance Therapy Reduces TNF Receptor 2 on CD4 T Cells and Enhances Immune Effector Function in Acute Myeloid Leukemia Patients. Am J Hematol (2014) 89(8):795–802. doi: 10.1002/ajh.23746 [DOI] [PubMed] [Google Scholar]
- 65. Kneppers E, van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, et al. Lenalidomide Maintenance After Nonmyeloablative Allogeneic Stem Cell Transplantation in Multiple Myeloma Is Not Feasible: Results of the HOVON 76 Trial. Blood (2011) 118(9):2413–9. doi: 10.1182/blood-2011-04-348292 [DOI] [PubMed] [Google Scholar]
- 66. Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, et al. Lenalidomide Maintenance After Allogeneic HSCT Seems to Trigger Acute Graft-Versus-Host Disease in Patients With High-Risk Myelodysplastic Syndromes or Acute Myeloid Leukemia and Del(5q): Results of the LENAMAINT Trial. Haematologica (2012) 97(9):34. doi: 10.3324/haematol.2012.067629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Craddock C, Slade D, De Santo C, Wheat R, Ferguson P, Hodgkinson A, et al. Combination Lenalidomide and Azacitidine: A Novel Salvage Therapy in Patients Who Relapse After Allogeneic Stem-Cell Transplantation for Acute Myeloid Leukemia. J Clin Oncol (2019) 37(7):580–8. doi: 10.1200/JCO.18.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cancer Genome Atlas Research Network. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N Engl J Med (2013) 368(22):2059–74. doi: 10.1056/NEJMoa1301689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, Clinical Outcome, and Prognostic Significance of IDH Mutations in AML. Am J Hematol (2015) 90(8):732–6. doi: 10.1002/ajh.24072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cheung E, Perissinotti AJ, Bixby DL, Burke PW, Pettit KM, Benitez LL, et al. The Leukemia Strikes Back: A Review of Pathogenesis and Treatment of Secondary AML. Ann Hematol (2019) 98(3):541–59. doi: 10.1007/s00277-019-03606-0 [DOI] [PubMed] [Google Scholar]
- 71. Litzow MR, Tarima S, Perez WS, Bolwell BJ, Cairo MS, Camitta BM, et al. Allogeneic Transplantation for Therapy-Related Myelodysplastic Syndrome and Acute Myeloid Leukemia. Blood (2010) 115(9):1850–7. doi: 10.1182/blood-2009-10-249128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sengsayadeth S, Gatwood KS, Boumendil A, Labopin M, Finke J, Ganser A, et al. Conditioning Intensity in Secondary AML With Prior Myelodysplastic Syndrome/Myeloproliferative Disorders: An EBMT ALWP Study. Blood Adv (2018) 2(16):2127–35. doi: 10.1182/bloodadvances.2018019976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med (2019) 381(18):1728–40. doi: 10.1056/NEJMoa1902688 [DOI] [PubMed] [Google Scholar]
- 74. Strati P, Kantarjian H, Ravandi F, Nazha A, Borthakur G, Daver N, et al. Phase I/II Trial of the Combination of Midostaurin (PKC412) and 5-Azacytidine for Patients With Acute Myeloid Leukemia and Myelodysplastic Syndrome. Am J Hematol (2015) 90(4):276–81. doi: 10.1002/ajh.23924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in Mutant IDH2 Relapsed or Refractory Acute Myeloid Leukemia. Blood (2017) 130(6):722–31. doi: 10.1182/blood-2017-04-779405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions With Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med (2018) 378(25):2386–98. doi: 10.1056/NEJMoa1716984 [DOI] [PubMed] [Google Scholar]
- 77. Bazarbachi A, Labopin M, Battipaglia G, Djabali A, Passweg J, Socie G, et al. Sorafenib Improves Survival of FLT3-Mutated Acute Myeloid Leukemia in Relapse After Allogeneic Stem Cell Transplantation: A Report of the EBMT Acute Leukemia Working Party. Haematologica (2019) 104(9):e398–401. doi: 10.3324/haematol.2018.211615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High Activity of Sorafenib in FLT3-ITD-Positive Acute Myeloid Leukemia Synergizes With Allo-Immune Effects to Induce Sustained Responses. Leukemia (2012) 26(11):2353–9. doi: 10.1038/leu.2012.105 [DOI] [PubMed] [Google Scholar]
- 79. Deneberg S, Cherif H, Lazarevic V, Andersson PO, von Euler M, Juliusson G, et al. An Open-Label Phase I Dose-Finding Study of APR-246 in Hematological Malignancies. Blood Cancer J (2016) 6(7):e447. doi: 10.1038/bcj.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang ES, Altman JK, Pettit K, De Botton S, Walter RP, Fenaux P, et al. Preliminary Data on a Phase 1/2A First in Human Study of the Menin-KMT2A (MLL) Inhibitor KO-539 in Patients With Relapsed or Refractory Acute Myeloid Leukemia. Blood (2020) 136(Supplement 1):7–8. doi: 10.1182/blood-2020-134942 [DOI] [Google Scholar]
- 81. Kolinski B. Syndax Announces Positive Interim Data Demonstrating Robust Clinical Activity in Phase 1 Portion of the AUGMENT-101 Trial of SNDX-5613 in Patients With Genetically-Defined Acute Leukemias. Press Release. Waltham, MA: Syndax Pharmaceuticals; (2021). [Google Scholar]
- 82. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res (2004) 64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443 [DOI] [PubMed] [Google Scholar]
- 83. Mathew NR, Baumgartner F, Braun L, O'Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib Promotes Graft-Versus-Leukemia Activity in Mice and Humans Through IL-15 Production in FLT3-ITD-Mutant Leukemia Cells. Nat Med (2018) 24(3):282–91. doi: 10.1038/nm.4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 Study of Azacytidine Plus Sorafenib in Patients With Acute Myeloid Leukemia and FLT-3 Internal Tandem Duplication Mutation. Blood (2013) 121(23):4655–62. doi: 10.1182/blood-2013-01-480228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin Plus Chemotherapy for Acute Myeloid Leukemia With a FLT3 Mutation. N Engl J Med (2017) 377(5):454–64. doi: 10.1056/NEJMoa1614359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular Remission and Response Patterns in Patients With Mutant-IDH2 Acute Myeloid Leukemia Treated With Enasidenib. Blood (2019) 133(7):676–87. doi: 10.1182/blood-2018-08-869008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Middeke JM, Herold S, Rucker-Braun E, Berdel WE, Stelljes M, Kaufmann M, et al. TP53 Mutation in Patients With High-Risk Acute Myeloid Leukaemia Treated With Allogeneic Haematopoietic Stem Cell Transplantation. Br J Haematol (2016) 172(6):914–22. doi: 10.1111/bjh.13912 [DOI] [PubMed] [Google Scholar]
- 88. Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the Tumor Suppressor Function to Mutant P53 by a Low-Molecular-Weight Compound. Nat Med (2002) 8(3):282–8. doi: 10.1038/nm0302-282 [DOI] [PubMed] [Google Scholar]
- 89. Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J Clin Oncol (2021) 39(14):1584–94. doi: 10.1200/JCO.20.02341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cluzeau T, Sebert M, Rahme R, Cuzzubbo S, Lehmann-Che J, Madelaine I, et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone Des Myelodysplasies (GFM). J Clin Oncol (2021) 39(14):1575–83. doi: 10.1200/JCO.20.02342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Korbel G. Aprea Therapeutics Announces Results of Primary Endpoint From Phase 3 Trial of Eprenetapopt in TP53 Mutant Myelodysplastic Syndromes (MDS). Press Release. Boston, MA: Aprea Therapeutics; (2020). [Google Scholar]
- 92. Korbel G. Aprea Therapeutics Announces Positive Results From Phase 2 Trial of Eprenetapopt + Azacitidine for Post-Transplant Maintenance Therapy in TP53 Mutant MDS and AML. Boston, MA: Aprea Therapeutics; (2021). [Google Scholar]
- 93. Pigneux A, Labopin M, Maertens J, Cordonnier C, Volin L, Socie G, et al. Outcome of Allogeneic Hematopoietic Stem-Cell Transplantation for Adult Patients With AML and 11q23/MLL Rearrangement (MLL-R AML). Leukemia (2015) 29(12):2375–81. doi: 10.1038/leu.2015.143 [DOI] [PubMed] [Google Scholar]
- 94. Poire X, Labopin M, Polge E, Blaise D, Chevallier P, Maertens J, et al. Hematopoietic Stem Cell Transplantation for Adult Patients With Isolated NPM1 Mutated Acute Myeloid Leukemia in First Remission. Am J Hematol (2019) 94(2):231–9. doi: 10.1002/ajh.25355 [DOI] [PubMed] [Google Scholar]
- 95. Gundry MC, Goodell MA, Brunetti L. It’s All About MEis: Menin-MLL Inhibition Eradicates NPM1-Mutated and MLL-Rearranged Acute Leukemias in Mice. Cancer Cell (2020) 37(3):267–9. doi: 10.1016/j.ccell.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 96. Oran B, Giralt S, Couriel D, Hosing C, Shpall EJ, de Meis E, et al. Treatment of AML and MDS Relapsing After Reduced-Intensity Conditioning and Allogeneic Hematopoietic Stem Cell Transplantation. Leukemia (2007) 21(12):2540–4. doi: 10.1038/sj.leu.2404828 [DOI] [PubMed] [Google Scholar]
- 97. Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow as a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol (2017) 35(26):3002–9. doi: 10.1200/JCO.2017.72.8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Stem Cell Trialists’ Collaborative Group . Allogeneic Peripheral Blood Stem-Cell Compared With Bone Marrow Transplantation in the Management of Hematologic Malignancies: An Individual Patient Data Meta-Analysis of Nine Randomized Trials. J Clin Oncol (2005) 23(22):5074–87. doi: 10.1200/JCO.2005.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R, et al. Serial and Quantitative Analysis of Mixed Hematopoietic Chimerism by PCR in Patients With Acute Leukemias Allows the Prediction of Relapse After Allogeneic BMT. Bone Marrow Transplant (1998) 21(5):487–95. doi: 10.1038/sj.bmt.1701119 [DOI] [PubMed] [Google Scholar]
- 100. Horn B, Petrovic A, Wahlstrom J, Dvorak CC, Kong D, Hwang J, et al. Chimerism-Based Pre-Emptive Immunotherapy With Fast Withdrawal of Immunosuppression and Donor Lymphocyte Infusions After Allogeneic Stem Cell Transplantation for Pediatric Hematologic Malignancies. Biol Blood Marrow Transplant (2015) 21(4):729–37. doi: 10.1016/j.bbmt.2014.12.029 [DOI] [PubMed] [Google Scholar]
- 101. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients With Relapse After Allogeneic Transplantation. N Engl J Med (2016) 375(2):143–53. doi: 10.1056/NEJMoa1601202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Davids MS, Kim HT, Costello C, Herrera AF, Locke FL, Maegawa RO, et al. A Multicenter Phase 1 Study of Nivolumab for Relapsed Hematologic Malignancies After Allogeneic Transplantation. Blood (2020) 135(24):2182–91. doi: 10.1182/blood.2019004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sallman DA, Asch AS, Al Malki MM, Lee DJ, Donnellan WB, Marcucci G, et al. The First-in-Class Anti-CD47 Antibody Magrolimab (5F9) in Combination With Azacitidine Is Effective in MDS and AML Patients: Ongoing Phase 1b Results. Blood (2019) 134(Supplement_1):569. doi: 10.1182/blood-2019-126271 [DOI] [Google Scholar]
- 104. Vadakekolathu J, Lai C, Reeder S, Church SE, Hood T, Lourdusamy A, et al. TP53 Abnormalities Correlate With Immune Infiltration and Associate With Response to Flotetuzumab Immunotherapy in AML. Blood Adv (2020) 4(20):5011–24. doi: 10.1182/bloodadvances.2020002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults With B-Cell Lymphoblastic Leukemia. N Engl J Med (2018) 378(5):439–48. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Danylesko I, Jacoby E, Yerushalmi R, Shem-Tov N, Besser MJ, Vernitsky H, et al. Remission of Acute Myeloid Leukemia With T(8;21) Following CD19 CAR T-Cells. Leukemia (2020) 34(7):1939–42. doi: 10.1038/s41375-020-0719-y [DOI] [PubMed] [Google Scholar]
- 107. Qu C, Li Z, Kang L, Wang Y, Dai H, Yin J, et al. Successful Treatment of Two Relapsed/Refractory T(8;21) Acute Myeloid Leukemia Patients by CD19-Directed Chimeric Antigen Receptor T Cells. Bone Marrow Transplant (2019) 54(7):1138–40. doi: 10.1038/s41409-018-0423-y [DOI] [PubMed] [Google Scholar]
- 108. Sallman DA, Elmariah H, Sweet K, Talati C, Mishra A, Cox CA, et al. Phase 1/1b Safety Study of Prgn-3006 Ultracar-T in Patients With Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndromes. Blood (2021) 138(Supplement 1):825. doi: 10.1182/blood-2021-152692 [DOI] [Google Scholar]
- 109. Cui Q, Qian C, Xu N, Kang L, Dai H, Cui W, et al. CD38-Directed CAR-T Cell Therapy: A Novel Immunotherapy Strategy for Relapsed Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation. J Hematol Oncol (2021) 14(1):82–4. doi: 10.1186/s13045-021-01092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang H, Wang P, Li Z, He Y, Gan W, Jiang H. Anti-CLL1 Chimeric Antigen Receptor T-Cell Therapy in Children With Relapsed/Refractory Acute Myeloid Leukemia. Clin Cancer Res (2021) 27(13):3549–55. doi: 10.1158/1078-0432.CCR-20-4543 [DOI] [PubMed] [Google Scholar]
- 111. He X, Feng Z, Ma J, Ling S, Cao Y, Gurung B, et al. Bispecific and Split CAR T Cells Targeting CD13 and TIM3 Eradicate Acute Myeloid Leukemia. Blood (2020) 135(10):713–23. doi: 10.1182/blood.2019002779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Boudreau JE, Giglio F, Gooley TA, Stevenson PA, Le Luduec JB, Shaffer BC, et al. KIR3DL1/HLA-B Subtypes Govern Acute Myelogenous Leukemia Relapse After Hematopoietic Cell Transplantation. J Clin Oncol (2017) 35(20):2268–78. doi: 10.1200/JCO.2016.70.7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shaffer BC, Le Luduec JB, Forlenza C, Jakubowski AA, Perales MA, Young JW, et al. Phase II Study of Haploidentical Natural Killer Cell Infusion for Treatment of Relapsed or Persistent Myeloid Malignancies Following Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant (2016) 22(4):705–9. doi: 10.1016/j.bbmt.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ciurea SO, Kongtim P, Soebbing D, Trikha P, Behbehani G, Rondon G, et al. Decrease Post-Transplant Relapse Using Donor-Derived Expanded NK-Cells. Leukemia (2021). doi: 10.1038/s41375-021-01349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kharfan-Dabaja MA, Labopin M, Brissot E, Kroger N, Finke J, Ciceri F, et al. Second Allogeneic Haematopoietic Cell Transplantation Using HLA-Matched Unrelated Versus T-Cell Replete Haploidentical Donor and Survival in Relapsed Acute Myeloid Leukaemia. Br J Haematol (2021) 193(3):592–601. doi: 10.1111/bjh.17426 [DOI] [PubMed] [Google Scholar]
- 116. Shimoni A, Labopin M, Finke J, Ciceri F, Deconinck E, Kroger N, et al. Donor Selection for a Second Allogeneic Stem Cell Transplantation in AML Patients Relapsing After a First Transplant: A Study of the Acute Leukemia Working Party of EBMT. Blood Cancer J (2019) 9(12):88–3. doi: 10.1038/s41408-019-0251-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Imus PH, Blackford AL, Bettinotti M, Iglehart B, Dietrich A, Tucker N, et al. Major Histocompatibility Mismatch and Donor Choice for Second Allogeneic Bone Marrow Transplantation. Biol Blood Marrow Transplant (2017) 23(11):1887–94. doi: 10.1016/j.bbmt.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of Second Allogeneic Hematopoietic Cell Transplant vs Donor Lymphocyte Infusion With Overall Survival in Patients With Acute Myeloid Leukemia Relapse. JAMA Oncol (2018) 4(9):1245–53. doi: 10.1001/jamaoncol.2018.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]