Abstract
Background
The Food and Drug Administration (FDA) has recently banned flavours from pod-style electronic cigarettes (e-cigarettes), except for menthol and tobacco. JUUL customers have quickly discovered that flavoured disposable e-cigarettes from other manufacturers, such as Puff, are readily available. Our goal was to compare flavour chemicals, synthetic coolants and pulegone in mint-flavoured/menthol-flavoured e-cigarettes from JUUL and Puff, evaluate the cytotoxicity of the coolants and perform a cancer risk assessment for pulegone, which is present in both JUUL pods and disposable Puff products.
Methods
Identification and quantification of chemicals were performed using gas chromatography/mass spectrometry. Cytotoxicity of the coolants was evaluated with BEAS-2B cells using the MTT 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide assay. The cancer risk of pulegone was calculated using the margin of exposure (MOE).
Results
Menthol was the dominant flavour chemical (>1 mg/mL) in all products from both manufacturers. Minor flavour chemicals (<1 mg/mL) differed in the JUUL and Puff fluids and may produce flavour accents. The concentrations of WS-3 and WS-23 were higher in Puff than in JUUL. WS-23 was cytotoxic in the MTT assay at concentrations 90 times lower than concentrations in Puff fluids. The risk of cancer (MOE<10 000) was greater for mint than for menthol products and greater for Puff than for JUUL.
Conclusions
Switching from flavoured JUUL to Puff e-cigarettes may expose users to increased harm due to the higher levels of WS-23 and pulegone in Puff products. Cancer risk may be reduced in e-cigarettes by using pure menthol rather than mint oils to produce minty-flavoured e-cigarette products.
Keywords: carcinogens, electronic nicotine delivery devices, toxicology, global health
Introduction
JUUL was the first popular pod-style e-cigarette with a large share of its sales going to middle and high school students.1–5 JUUL initially marketed eight flavours of pods, including Cool Mint and Classic Menthol, which were later replaced by Mint and Menthol, respectively.6 The rapid spike in JUUL popularity concerned parents, public health officials and regulatory agencies, leading JUUL in 2019 to remove all flavours from their product line in the USA, except for Classic Tobacco, Virginia Tobacco, and Menthol. Puff products, which appear similar to JUUL, did not fall under the Food and Drug Administration’s (FDA) limitations on flavours, and many JUUL users switched to Puff, which rapidly became a dominant e-cigarette brand.7–9 In spite of their popularity, we know little about the relative safety of Puff and JUUL products.
This study compares three classes of chemicals in Puff and JUUL e-cigarette fluids. These include flavour chemicals, in particular menthol, two synthetic coolants and pulegone, a potential carcinogen that has been reported in mint-flavoured e-cigarettes.10 11 Because the use of menthol is permitted by the Family Smoking Prevention and Tobacco Control Act of 2009,12 it is one of the most widely used flavour chemicals in tobacco products,13 sometimes appearing in e-cigarettes that are not explicitly labelled ‘mint’ or ‘menthol’.14 The cooling properties and pleasant minty flavour of menthol may make smoking initiation easier among novice users.15 16 Although generally regarded as safe (GRAS) for ingestion by the Flavour and Extract Manufacturers Association (FEMA),17 menthol is often used in e-cigarette products at high concentrations,14 which are cytotoxic in vitro.14 18 19
The synthetic coolants WS-3 (N-ethyl-p-menthane-3-carboxamide, CAS # 39711-79-0) and WS-23 (2-isopropyl-N,2,3-trimethylbutyramide, CAS # 51115-67-4) are popular cooling agents and were initially developed by Wilkinson Sword Ltd. in the 1970s.20 These coolants are considered safe for ingestion by FEMA and are used extensively in consumer products, including breath fresheners, confectionaries and cosmetics.21–23 WS-3 and WS-23 activate the TRPM8 and TRPA1 receptors, creating a cool relaxing sensation24 while imparting little or no flavour to products that are ingested. WS-23 has been reported in JUUL pods purchased in the European Union25 but was not found in JUUL pods purchased in the USA.6 Bloggers have discussed the addition of coolants to e-cigarette fluids, suggesting they are more widely used than generally recognised.26–28 However, apart from one report on JUUL,25 very little is known about the identities and concentrations of coolants used in e-cigarrette fluids, and the range of concentrations of these coolants in JUUL and Puff e-cigarettes have not previously been compared.
Mint oil, which is often used in e-cigarettes to create mint flavour, can contain pulegone,29 30 a known carcinogen.31 32 In several recent studies, a margin of exposure (MOE) analysis found pulegone to be sufficiently high in some e-cigarettes to present a cancer risk,10 11 which motivated us to examine pulegone in JUUL and Puff products.
This study compares menthol, WS-3 and WS-23, and pulegone in menthol-flavoured and minty-flavoured products made by JUUL and Puff to gain insight into their relative safety. Specifically, we have compared the following: (1) the concentrations of the flavour chemicals, (2) the concentrations and cytotoxicity of WS-3 and WS-23, and (3) the MOEs, which predict cancer risk.
Methods
Sample acquisition
In 2018 and 2019, JUUL Cool Mint, Classic Menthol and their replacements, Mint and Menthol, were purchased online (www.juul.com) and from local stores in Riverside, California and Portland, Oregon. Of the four minty-flavoured/menthol-flavoured pods produced by JUUL, only Menthol is currently available. JUUL Cool Mint, Classic Menthol, Mint and Menthol pods were analysed to compare chemical composition in all minty/menthol JUUL pods. All pods were stored in the dark and analysed close to the time of purchase.
Two types of disposable Puff devices were purchased: the 1.3 mL Puff Bar Menthol, labelled to deliver 300 puffs/device, and the 3.2 mL Puff Plus Cool Mint, labelled to deliver 800 puffs/device. Puff devices were purchased at vape shops in Los Angeles, California, and Riverside, California, in 2020. All devices were stored in the dark and analysed close to the time of purchase.
Identification and quantification of chemicals using gas chromatography/mass spectrometry (GC/MS)
E-cigarette fluids were extracted from the pods and devices, and 50 µL was dissolved in 0.95 mL of isopropyl alcohol (Fisher Scientific, Fair Lawn, New Jersey, USA). Chemical analysis was performed with an Agilent 5975C GC/MS system (Santa Clara, California) using internal standard-based calibration procedures and methods previously described in detail.6 33 The method analyses 180 flavour chemicals plus nicotine.
Culturing of BEAS-2B cells
Human bronchial epithelial cells (BEAS-2B) from American Type Culture Collection (ATCC), Manassas, Virginia, USA were cultured in a growth medium made with 500 mL of Airway Epithelial Cell Basal Medium supplemented with 1.25 mL HLL supplement containing human serum albumin (500 µg/mL), linoleic acid (0.6 µM) and lecithin (0.6 µg/mL), 15 mL of L-glutamine (6 mM), 2 mL of extract P (0.4%) and 5.0 mL Airway Epithelial Cell Supplement containing epinephrine (1.0 µM), transferrin (5 µg/mL), T3 (10 nM), hydrocortisone (0.1 µg/mL), rh EGF (5 ng/mL) and rh Insulin (5 µg/mL) from ATCC. Nunc T-25 tissue culture flasks (Thermo Scientific, Waltham, Massachusetts, USA) were coated overnight with a coating medium made with basal medium (69.3%) (ATCC), collagen (29.7%) (Sigma-Aldrich, St Louis, Missouri, USA), bovine serum albumin (0.99%) (Sigma-Aldrich) and fibronectin (0.01%) (Sigma-Aldrich) before culturing and passaging cells. At 85%–90% confluency, cells were harvested using Dulbecco’s phosphate-buffered saline (DPBS) without calcium or magnesium (Lonza, Walkersville, Maryland, USA) for washing and incubated with a trypsin solution containing trypsin–EDTA (0.25% trypsin/0.53 mM EDTA; ATCC) and 0.5% poly-vinyl-pyrrolidone (Sigma-Aldrich) for 3 min at 37°C to allow detachment. Cells were cultured in T-25 flasks at 75 000 cells/flask, and the medium was replaced every other day. Cells were then plated at 10 000 cells/well in precoated 96-well tissue culture plates (Thermo Scientific) and allowed to attach overnight before a 24-hour treatment.
MTT 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide cytotoxicity of WS-3 and WS-23
The effects of WS-3 and WS-23 on mitochondrial reductases were evaluated in concentration–response experiments. BEAS-2B cells were seeded, allowed to attach overnight and treated with 0.5–5.0 mg of each coolant/mL of culture medium for 24 hours at 37°C. After treatment, 20 µL of MTT reagent (Sigma-Aldrich) dissolved in 5 mg/mL of DPBS (Fisher Scientific, Chino, CA) were added to wells and incubated for 2 hours at 37°C. Solutions were removed from wells, and 100 µL of dimethyl sulfoxide (DMSO) (Fisher Scientific) were added to each well and gently mixed on a shaker to solubilise formazan crystals. Absorbance readings of control and treated wells were taken against a DMSO blank at 570 nm using an Epoch microplate reader (Biotek, Winooski, VT). The MTT assay quantifies the conversion of a yellow tetrazolium salt (MTT) to purple formazan. For each coolant tested, three independent experiments on different passages of the same culture were performed.
MOE calculations for pulegone
To assess the cancer risk associated with pulegone in pod/device fluids, the MOE was calculated using the no-observed adverse effect level (NOAEL) of pulegone and the estimated exposure dose (EED) from pods/devices. Regulatory agencies, including the FDA, use the MOE to assess the cancer risk of food additives.31 Chemicals with MOE values below 10 000 require strategies to limit exposure. The risk associated with pulegone concentration in JUUL and Puff e-cigarettes was evaluated using a daily EED of 1–3 mL,34–37 a NOAEL of 13.39 mg/kg and an adult body weight of 60 kg.31 32
Data analysis and statistics
For GC/MS data, and the means and standard deviation for at least three pods/devices were plotted using Prism software (GraphPad, San Diego, California, USA). For the MTT assay, treatment groups were expressed as percentages of the untreated control. IC50 values were computed using the log inhibitor versus normalised response–variable slope in GraphPad Prism, and IC70 values were evaluated visually. Statistical significance in the MTT assay was determined in GraphPad using a one-way analysis of variance on the raw data. When means were significant (p<0.05), treated groups were compared with the untreated control using Dunnett’s post hoc test.
Results
Concentrations of flavour chemicals in JUUL and Puff e-cigarettes
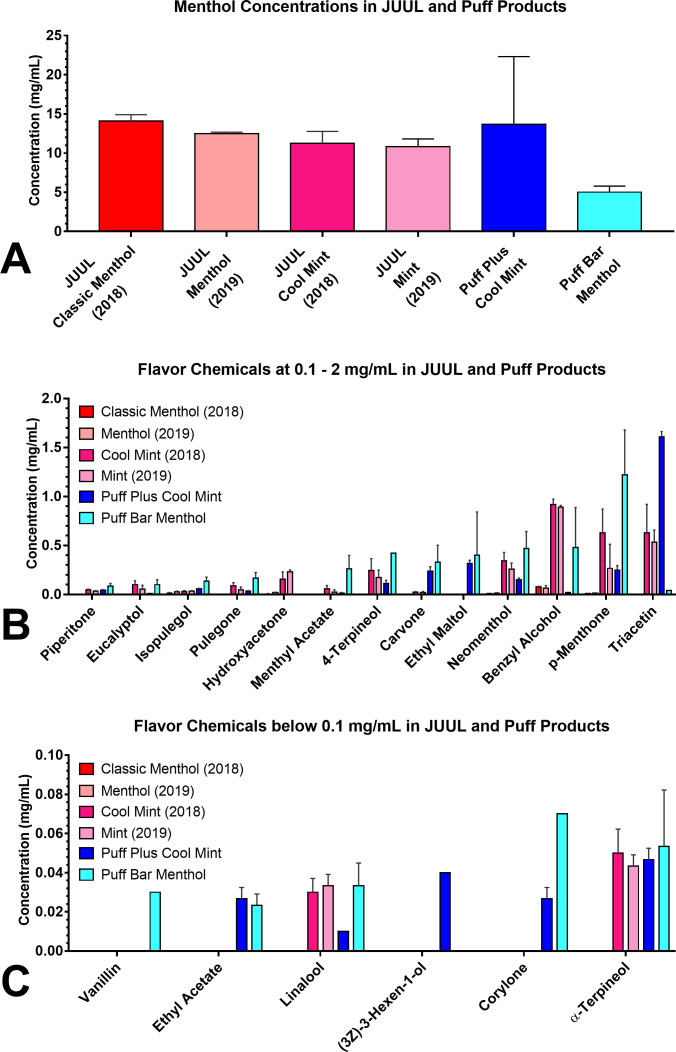
Menthol was the dominant flavour chemical in the JUUL and Puff samples (concentration range 5–14 mg/mL) (figure 1A). Menthol concentrations were similar in all products, except Puff Bar Menthol, in which the concentration was lower. Other flavour chemicals were generally <1 mg/mL (figure 1B, C), except for triacetin and p-menthone, which were >1 mg/mL in Puff Plus Cool Mint and Puff Bar Menthol, respectively (figure 1B). In JUUL fluids, minor flavour chemicals (<1 mg/mL) were generally present in the two mint flavours from JUUL but absent or lower in concentration in the menthol flavours. Puff products had more minor flavour chemicals than JUUL (figure 1B, C). In Puff, minor flavour chemicals were generally higher in the Menthol devices (figure 1B, C). Estimated concentrations of flavour chemicals identified at levels below the Limit of Quantification (20 µg/mL for 50 µL samples) are shown in online supplemental table S1.
Figure 1.
Flavour chemicals in JUUL and Puff Mint and Menthol e-cigarette fluids. (A) Menthol was the dominant flavour chemical in all six products. (B) Chemicals present at concentrations ranging from 0.1 to 2.0 mg/mL. (C) Chemicals present at concentrations lower than 0.1 mg/mL. Data are means±SD of at least three samples for each group.
tobaccocontrol-2021-056582supp001.pdf (68.8KB, pdf)
WS-3 and WS-23 concentrations in JUUL and Puff
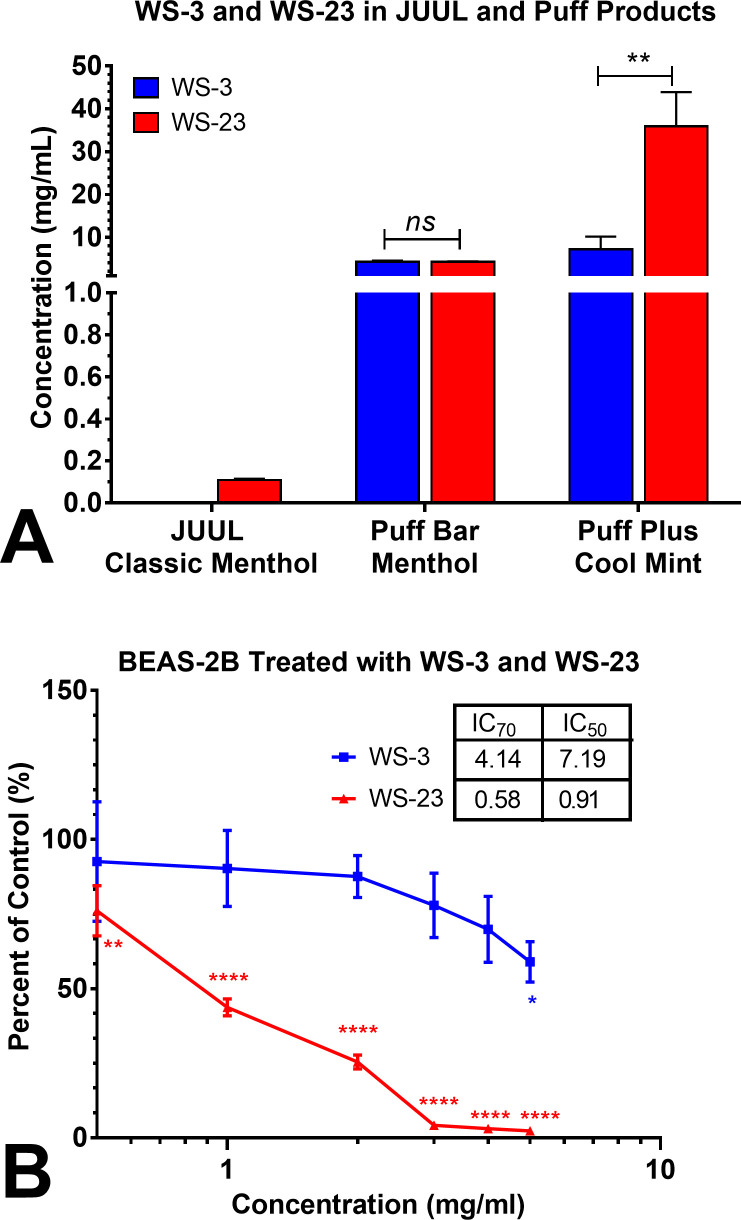
While WS-3 was absent in all JUUL pods, WS-23 was present in the JUUL Menthol pods at an average concentration of 0.1 mg/mL (figure 2A). Both coolants were in Puff fluids at much higher concentrations. WS-23 in Puff Plus Cool Mint averaged 36 mg/mL with one device having 45 mg/mL of WS-23. In the other Puff products, the average concentrations of WS-3 and WS-23 were similar and ranged between 4.3 and 7.2 mg/mL.
Figure 2.
Synthetic coolant concentrations in e-cigarette fluids and their toxicities. (A) WS-3 and WS-23 were higher in Puff fluids than in JUUL pods. (B) Cytotoxicity of WS-3 and WS-23 in the MTT assay. Data are the means±SD of at least three independent biological experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. MTT, 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide.
Cytotoxicity of WS-3 and WS-23
The cytotoxicity of WS-3 and WS-23 was evaluated using the MTT assay in conjunction with ISO protocol #10 993–5, which measures mitochondrial reductase activity (figure 2B).38 BEAS-2B cells were tested using concentrations of coolant that were lower than those found in the e-cigarettes. While concentrations of WS-3 below 5 mg/mL produced little to no response in the MTT assay, BEAS-2B cells were adversely affected by all concentrations of WS-23 that were tested (IC70=0.59).
Hazard analysis of pulegone in JUUL and Puff e-cigarettes
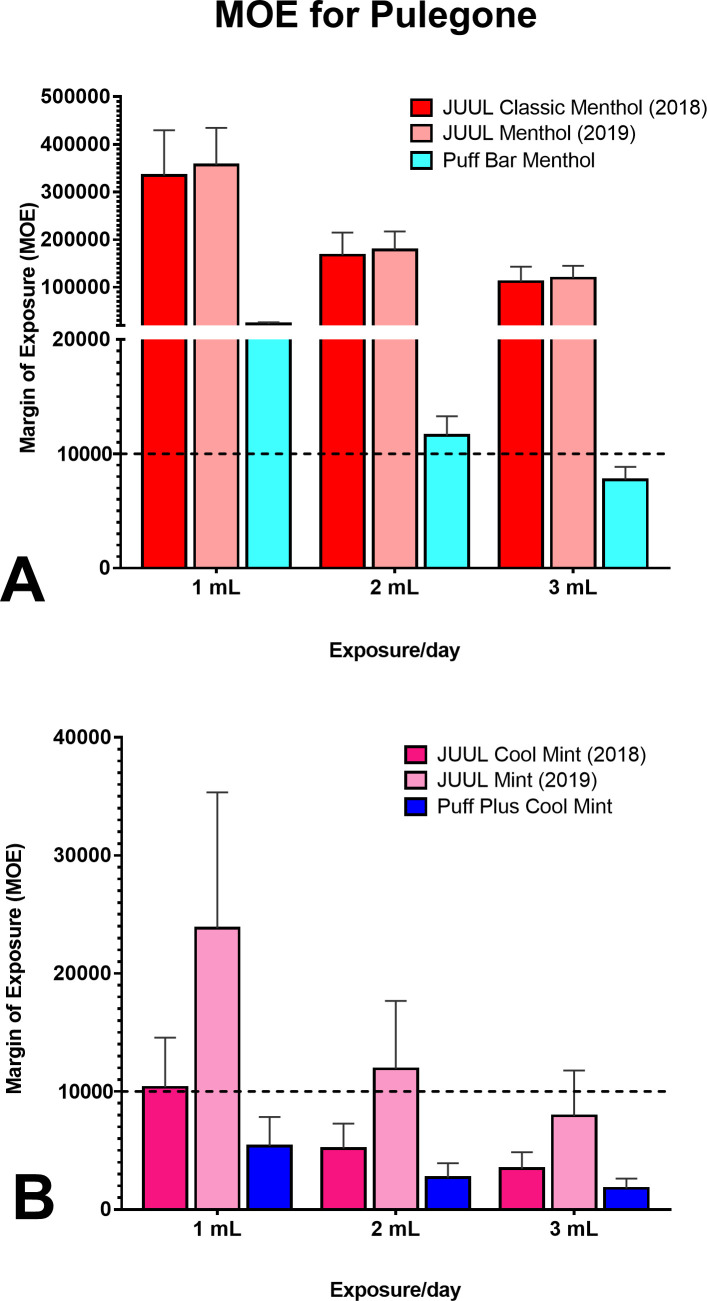
The concentrations of pulegone in JUUL pods and disposable Puff devices ranged from 0.002 to 0.2 mg/mL and were higher in the mint labelled products (figure 1). For menthol products from both manufacturers, only the 3 mL/day exposure scenario for Puff Bar Menthol generated an MOE of <10 000, which was below the safety threshold (figure 3A). In contrast, for all mint-flavoured samples, most scenarios produced an MOE of <10 000 (figure 3B). For all scenarios for both mint-flavoured and menthol-flavoured products, the MOEs for Puff were consistently lower than those for JUUL, suggesting a greater risk with Puff.
Figure 3.
MOE for pulegone in JUUL and Puff products. (A) MOE for ‘menthol’ labelled JUUL and Puff e-cigarette fluids. (B) MOE for ‘mint’ labelled JUUL and Puff e-cigarette fluids. MOEs below the threshold of 10 000 indicate a high carcinogenic potential and concern for human health. MOE, margin of exposure.
Concentrations of flavour chemicals in edible consumer products
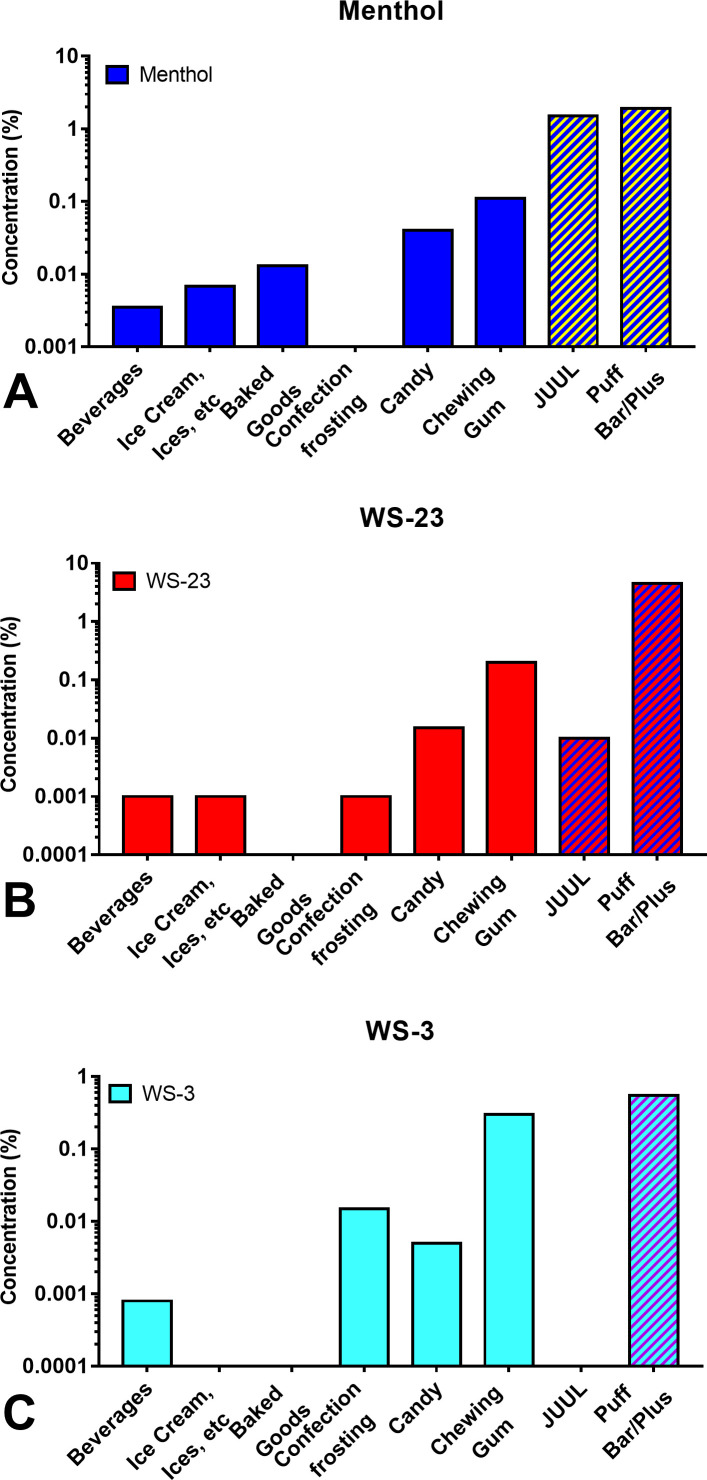
Synthetic coolants and menthol in edible consumer goods were compared with concentrations in JUUL and Puff e-cigarette fluids (figure 4). Concentrations of menthol in JUUL and Puff were similar, yet 14-543 times higher than in other consumer products (figure 4A). WS-23 in Puff was 450 times higher than concentrations in JUUL pods, and 23–4500 times higher than the concentration in edible consumer products (figure 4B). WS-3, which was absent in JUUL pods, was 2–688 times higher in Puff when compared with edible products (figure 4C).
Figure 4.
Concentrations of menthol and synthetic coolants in JUUL and Puff e-cigarette fluids and edible consumer products. (A) Menthol, (B) WS-23 and (C) WS-3.
Discussion
Four main observations come from our comparison of three classes of chemicals in JUUL and Puff e-cigarettes. First, in both brands, menthol was the dominant flavour chemical in mint-flavoured and menthol-flavoured fluids, which likely have similar, although not identical, minty flavours. Second, while low concentrations of WS-23 were present in JUUL Classic Menthol, both WS-3 and WS-23 were present at much higher concentrations in Puff products with the concentration of WS-23 exceeding that of menthol in Puff Plus Cool Mint. Third, WS-23 was cytotoxic in the MTT assay at concentrations well below those found in Puff devices. Fourth, pulegone concentrations in mint products from JUUL and Puff were high enough to present a cancer risk based on MOE evaluations. While the FDA flavour ban has reduced sales of JUUL to minors, young users appear to have rapidly adopted other brands, such as Puff,22 which has high concentrations of WS-23 and concerning levels of pulegone. Ironically, the flavour ban may have caused youth to migrate to a potentially more harmful e-cigarette.
Since the dominant flavour chemical in mint and menthol-flavoured JUUL and Puff products was menthol, banning the sale and distribution of mint-flavoured pods may not adequately address the widespread use of this popular flavour. While current federal regulations limit the distribution and sale of flavoured cartridge-based pod products, such as JUUL, they do not solve the problem that menthol-flavoured e-cigarettes are apparently similar, although not identical to mint. Consequently, a minty flavour is still sold by JUUL as Menthol and is also available as mint in disposable devices from other manufacturers, such as Puff. Although our study deals only with JUUL and Puff, any e-cigarette manufacturer can produce menthol-flavoured pods or cartridges that may be an acceptable substitute for mint.
FEMA has designated menthol and synthetic coolants (WS-3 and WS-23) as GRAS for ingestion, and they are widely used in food and cosmetic products.17 As pointed out previously, the concentrations of flavour chemicals in e-cigarettes are often very high.14 39 Menthol and WS-23 concentrations in both brands exceeded those used in most edible consumer products (figure 4).22 23 While acceptable exposure to GRAS chemicals is based on ingestion data, the acceptable exposures when inhaled are generally unknown and are likely to be much lower,40 41 raising concerns about the delivery of coolants in e-cigarettes. Unlike the USA, several countries (Canada and Germany) have avoided potential problems with coolants by banning their use in tobacco products.42 43
The concentrations of menthol in JUUL and Puff are high enough to affect cell health. In numerous studies with various cell types, menthol inhibited proliferation and/or caused cell death.44 45 Menthol concentrations in JUUL and Puff would be cytotoxic in the MTT assay based on prior reports with BEAS-2B cells (IC70=1.38 mg/mL) and A549 cells (IC50=0.98 mg/mL–aerosol data).14 18 Even at concentrations below the MTT NOAEL, menthol, when delivered in a PG aerosol using an e-cigarette, binds to TRPM8 receptors on BEAS-2B cells, allowing calcium influx and downstream activation of oxidative stress and inflammatory responses.46 The reported adverse effects of menthol in humans have generally been derived from studies comparing mentholated versus non-mentholated tobacco cigarettes and have ranged from being an irritant to causing cancer, although the data supporting the latter claim have been ambiguous.44 In 2011, it was concluded by the FDA’s Tobacco Products Scientific Advisory Committee that menthol is not a carcinogen.47 Nevertheless, the inhalation of menthol does have an effect on humans. For example, inhalation of a high dose of menthol by a 13-year-old boy resulted in adverse central nervous system effects.48 Workers in a throat lozenge manufacturing plant reported that menthol was an irritant that affected their eyes, nasal passages, throats and larynxes.49 Ingestion of menthol at high doses has resulted in abdominal discomfort, convulsions, nausea, vertigo, ataxia, drowsiness and coma.49 50 In future studies, it will be important to determine if the high concentrations of menthol inhaled in the context of e-cigarette aerosols produce health effects that have not yet been recognised.
High concentrations of WS-23 and WS-3 appeared in our e-cigarette fluid data for the first time in Puff and are likewise concerning, as they produce cytotoxic effects in the MTT assay at concentrations below those in Puff e-cigarettes. In contrast, the concentration of WS-23 in JUUL Classic Menthol was not high enough to produce an IC70 in the MTT assay. The cytotoxicity that could be ascribed to menthol in the six products we tested would be roughly equivalent. However, the toxicity ascribable to WS-23 would be many times greater in the Puff products than in JUUL, suggesting that the removal of most JUUL flavours inadvertently motivated users to try other products, such as Puff, that may be more harmful.
Pulegone in e-cigarette fluids is a concern because of its known carcinogenicity.31 32 Our data are based on acute exposures and do not directly assess the long-term effects of e-cigarette chemicals on human health. Calculation of the MOE enables a prediction to be made about the possibility of cancer developing with long-term exposure to individual chemicals and is useful to regulatory agencies in prioritising their cancer risk.31 51–53 As MOE values fall below 10 000, the possibility of cancer developing increases. Products labelled “menthol” had concentrations of pulegone that produced MOEs above 10 000, indicating they are not likely to cause cancer in users. However, Puff Bar Menthol was much closer to the 10 000 cut-off than the JUUL products, which ranged from 100 000 to >300 000. In contrast, products labelled “mint’ generally had MOEs below 10 000, and in all cases, MOEs for Puff were lower than those for JUUL. These data are consistent with the interpretation that the mint products were flavoured with mint oil, which usually contains pulegone,29 30 while menthol-flavoured products were likely made from crystalline menthol, which would have higher purity and lower concentrations of pulegone. These data support the idea that using pure menthol rather than mint oil in e-cigarette fluids would reduce the risk of developing cancer, which could provide a basis for the regulation of additives to mint-flavoured/menthol-flavoured products. Since our MOE calculations are based on pulegone ingestion, our values probably underestimate inhalation exposure, which generally produces a stronger effect to toxicants, including carcinogens.40 41
Our data are based on concentrations of chemicals in e-cigarette fluids, which we have previously shown generally predict the cytotoxicity of aerosols.18 The concentrations of flavour chemicals and coolants received by a user will depend on the transfer efficiency of each chemical to the aerosol and its retention by the user. Therefore, the actual doses inhaled during vaping may be lower than the concentrations we report in the e-cigarette fluids. The frequency of vaping will also affect the overall exposure a user receives. These factors will eventually need to be determined to understand the concentrations of flavour chemicals, coolants, and pulegone that users of JUUL and Puff products receive.
In summary, flavour chemicals in JUUL Cool Mint, Mint, Classic Menthol and Menthol, and in Puff Plus Cool Mint and Puff Bar Menthol were similar, but not identical, with menthol being the dominant flavour chemical in all products tested. Synthetic coolants are being added to e-cigarettes, sometimes at high concentrations that exceed those used in other consumer products and produced in vitro cytotoxicity. Regulation of mentholated e-cigarettes is now complicated by the sale of ‘mint-like’ flavours under the name menthol, the lack of regulation of flavour chemicals in disposable e-cigarettes, the presence of cytotoxic concentrations of synthetic coolants in menthol and mint e-cigarettes, and the presence of pulegone in mint-flavoured products at concentrations that may be a cancer risk.
What this paper adds.
We compared the flavour chemicals, coolants (WS-3 and WS-23), and pulegone in mint-flavoured and menthol-flavoured Puff (disposable) and JUUL (pod) e-cigarettes.
Menthol was the dominant flavour chemical in all products, suggesting users may interchange mint and menthol products to achieve a ‘minty’ flavour.
Unlike JUUL, Puff products contained cytotoxic concentrations of the synthetic coolant WS-23 and concentrations of pulegone that present a greater cancer risk based on margin of exposure analysis.
Restriction of JUUL flavours may have inadvertently caused a migration of users to a potentially more harmful product.
The use of pure menthol instead of mint oil in e-cigarette fluids may reduce cancer risk.
Acknowledgments
We thank Dr. Careen Khachatoorian for providing the Puff samples.
Footnotes
Contributors: EEO and PT formed the conception and design of the study. WL, KJMcW and JFP were involved in the gas chromatography–mass spectrometry analysis. EEO performed the cell culture experiment. EEO, WL, KJMcW and PT were involved in the data analysis and interpretation. EEO and PT drafted the manuscript. All authors critically reviewed, edited and approved the final manuscript.
Funding: Research reported in this publication was supported by grant R01ES029741-01 from the National Institute of Environmental Health Sciences and the Food and Drug Administration Centre for Tobacco Products, and a Predoctoral Fellowship from the UCR Graduate Division to Esther Omaiye.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are included in the submitted manuscript.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019;28:146–51. 10.1136/tobaccocontrol-2018-054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herzog B, Kanada P. Nielsen tobacco All Channel Biweekly data 11/17/2018: Wells Fargo Equity Research Reports, 2018. Available: https://athra.org.au/wp-content/uploads/2018/12/Wells-Fargo-Nielsen-Tobacco-All-Channel-BiWeekly-Report-Period-Ending-11.17.18.pdf [Accessed 2 Feb 2021].
- 3. Leventhal AM, Miech R, Barrington-Trimis J, et al. Flavors of e-cigarettes used by youths in the United States. JAMA 2019;322:2132–4. 10.1001/jama.2019.17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cullen KA, Gentzke AS, Sawdey MD, et al. E-Cigarette use among youth in the United States, 2019. JAMA 2019;322:2095–103. 10.1001/jama.2019.18387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang TW, Neff LJ, Park-Lee E, et al. E-cigarette Use Among Middle and High School Students - United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:37. 10.15585/mmwr.mm6937e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omaiye EE, McWhirter KJ, Luo W, et al. High-Nicotine electronic cigarette products: toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem Res Toxicol 2019;32:1058–69. 10.1021/acs.chemrestox.8b00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Food US, Administration D. Fda finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint, 2020. Available: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children [Accessed 2 Feb 2021].
- 8. Miech R, Leventhal A, Johnston L, et al. Trends in use and perceptions of nicotine Vaping among US youth from 2017 to 2020. JAMA Pediatr 2021;175:185–90. 10.1001/jamapediatrics.2020.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aubrey A. Parents: Teens Are Still Vaping, Despite Flavor Ban. Here’s What They’re Using, 2020. Available: https://www.npr.org/sections/health-shots/2020/02/17/805972087/teens-are-still-vaping-flavors-thanks-to-new-disposable-vape-pens [Accessed 30 Nov 2020].
- 10. Jabba SV, Jordt S-E. Risk analysis for the carcinogen pulegone in Mint- and Menthol-Flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019;179:1721–3. 10.1001/jamainternmed.2019.3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omaiye EE, Luo W, McWhirter KJ, et al. Electronic cigarette refill fluids sold worldwide: flavor chemical composition, toxicity, and hazard analysis. Chem Res Toxicol 2020;33:2972–87. 10.1021/acs.chemrestox.0c00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Family smoking prevention and tobacco control act H.R.1256, 111Th us Congress 22.
- 13. Giovino G, Sidney S, Gfroerer J, et al. Epidemiology of menthol cigarette use. Nicotine & Tobacco Res. 2004;6:67–81. 10.1080/14622203710001649696 [DOI] [PubMed] [Google Scholar]
- 14. Omaiye EE, McWhirter KJ, Luo W, et al. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep 2019;9:2468. 10.1038/s41598-019-39550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klausner K. Menthol cigarettes and smoking initiation: a tobacco industry perspective. Tob Control 2011;20 Suppl 2:ii12eii19. 10.1136/tc.2010.041954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Villanti AC, Johnson AL, Halenar MJ, et al. Menthol and mint cigarettes and Cigars: initiation and progression in youth, young adults and adults in waves 1–4 of the path study, 2013–2017. Nicotine Tob Res 2020;3. 10.1093/ntr/ntaa224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hallagan J. The safety assessment and regulatory authority to use flavors: focus on e-cigarettes, 2014. Available: https://www.femaflavor.org/node/24344 [Accessed 30 Nov 2020].
- 18. Behar RZ, Luo W, McWhirter KJ, et al. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep 2018;8:8288. 10.1038/s41598-018-25575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fetterman JL, Weisbrod RM, Feng B, et al. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol 2018;38:1607–15. 10.1161/ATVBAHA.118.311156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leffingwell J, Rowsell D. Wilkinson sword cooling compounds: from the beginning to now a chronological review of research into the cooling and therapeutic effects of these types of materials. Available: https://www.researchgate.net/publication/260247091_Wilkinson_Sword_Cooling_Compounds_From_the_Beginning_to_Now [Accessed 1 April 2021].
- 21. Leffingwell JC. Cooling Ingredients and Their Mechanism of Action. In: Paye M, Maibach HI, Barel AO, eds. Handbook of cosmetic science and technology. 3rd ed. New York: Informa Healthcare (Pub.), 2009: 661–75. [Google Scholar]
- 22. Marnett LJ, Cohen SM, Fukushima S. Gras flavoring substances 26: the 26th publication by the expert panel of the flavor and extract manufacturers association provides an update on recent progress in the consideration of flavoring ingredients generally recognized as safe under the food additive Amendment. Food Technol 2013;67:38–56. [Google Scholar]
- 23. Smith RL, Newberne P, et al. , the FEMA Expert Panel . Gras flavoring substances 17. Food Technology 1996;50:72–8. [Google Scholar]
- 24. Behrendt H-J, Germann T, Gillen C, et al. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 2004;141:737–45. 10.1038/sj.bjp.0705652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erythropel HC, Anastas PT, Krishnan-Sarin S, et al. Differences in flavourant levels and synthetic coolant use between USA, EU and Canadian Juul products. Tob Control 2020;30:453–5. 10.1136/tobaccocontrol-2019-055500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. WS-23 expertise. Available: https://www.reddit.com/r/DIY_eJuice/comments/aangb4/ws23_expertise/ [Accessed 1 Apr 2021].
- 27. WS-3 vs. WS-23. Available: https://www.reddit.com/r/DIY_eJuice/comments/9uhdny/ws3_vs_ws23/ [Accessed 1 Apr 2021].
- 28. Any difference between TFA Koolada and WS-23 in terms of strength? Available: https://www.reddit.com/r/DIY_eJuice/comments/dsg99t/any_difference_between_tfa_koolada_and_ws23_in/ [Accessed 1 Apr 2021].
- 29. Bektašević M, Politeo O, Carev I. Comparative study of chemical composition, cholinesterase inhibition and antioxidant potential of Mentha pulegium L. essential oil. Chem Biodivers 2021;18:e2000935. 10.1002/cbdv.202000935 [DOI] [PubMed] [Google Scholar]
- 30. Grosse Y, Loomis D, Lauby-Secretan B, et al. Carcinogenicity of some drugs and herbal products. Lancet Oncol 2013;14:807–8. 10.1016/s1470-2045(13)70329-2 [DOI] [PubMed] [Google Scholar]
- 31. United States Food and Drug Administration . Food additive regulations; synthetic flavoring agents and adjuvants. Federal Register 2018;83:50490–503 https://www.federalregister.gov/documents/2018/10/09/2018-21807/food-additive-regulations-synthetic-flavoring-agents-and-adjuvants [Google Scholar]
- 32. National Toxicology Program . Toxicology and carcinogenesis studies of pulegone (Cas No. 89−82−7) in f344/n rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Technol. Rep. Ser 2011;563:1–201. [PubMed] [Google Scholar]
- 33. Brown JE, Luo W, Isabelle LM, et al. Candy flavorings in tobacco. N Engl J Med 2014;370:2250–2. 10.1056/NEJMc1403015 [DOI] [PubMed] [Google Scholar]
- 34. Yingst J, Foulds J, Hobkirk AL. Dependence and use characteristics of adult JUUL electronic cigarette users. Subst Use Misuse 2021;56:61–6. 10.1080/10826084.2020.1834582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Is it okay to go through one Juul pod a day? Available: www.quora.com/Is-it-okay-to-go-through-one-Juul-pod-a-day [Accessed 1 Apr 2021].
- 36. How many pods do you go through per day? Available: https://www.reddit.com/r/juul/comments/8w4uen/how_many_pods_do_you_go_through_per_day [Accessed 1 Apr 2021].
- 37. Is it normal to finish a pod in a day? Available: https://www.reddit.com/r/juul/comments/6nj8sq/is_it_normal_to_finish_a_pod_in_a_day/ [Accessed 1 Apr 2021].
- 38. Biological Evaluation of Medical Devices - Part 5: Tests for in Vitro Cytotoxicity; ISO 10993-5:2009(E), iv–34; Geneva, Switzerland, 2009.
- 39. Tierney PA, Karpinski CD, Brown JE, et al. Flavour chemicals in electronic cigarette fluids. Tob Control 2016;25:e10–15. 10.1136/tobaccocontrol-2014-052175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rennen MAJ, Bouwman T, Wilschut A. Oral-to-inhalation route extrapolation in occupational health risk assessment: a critical assessment. Regul Toxicol Pharmacol 2003;39:5–11. [DOI] [PubMed] [Google Scholar]
- 41. Escher SE, Tluczkiewicz I, Batke M, et al. Evaluation of inhalation TTC values with the database RepDose. Regul Toxicol Pharmacol 2010;58:259–74. 10.1016/j.yrtph.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 42. Verordnung über Tabakerzeugnisse und verwandte Erzeugnisse (Tabakerzeugnisverordnung - TabakerzV), Anlage 1 In. Berlin, Germany 2016.
- 43. Order amending the schedule to the tobacco act (menthol) in. Ottawa, on 2017.
- 44. Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tob Induc Dis 2011;9 Suppl 1:S7. 10.1186/1617-9625-9-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stefaniak AB, LeBouf RF, Ranpara AC, et al. Toxicology of flavoring- and cannabis-containing e-liquids used in electronic delivery systems. Pharmacol Ther 2021;224:107838. 10.1016/j.pharmthera.2021.107838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nair V, Tran M, Behar RZ, et al. Menthol in electronic cigarettes: a contributor to respiratory disease? Toxicol Appl Pharmacol 2020;407:115238. 10.1016/j.taap.2020.115238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tobacco Products Scientific Advisory Committee . Menthol cigarettes and public health: review of the scientific evidence and recommendations, 2011. [Google Scholar]
- 48. O'Mullane NM, Joyce P, Kamath SV, et al. Adverse CNS effects of menthol-containing olbas oil. Lancet 1982;1:1121. 10.1016/s0140-6736(82)92297-8 [DOI] [PubMed] [Google Scholar]
- 49. OECD SIDS Program . Menthols 2003.
- 50. Dukes MNG. Camphor and Menthol Volatile Oils. Meyler’s Side Effects Of Drugs: An Encyclopaedia of Adverse Reactions and Interactions; Excerpta Medica. Amsterdam; Princeton; New York, 1980. [Google Scholar]
- 51. Scientific opinion of the panel on food additives, Flavourings, processing AIDS and materials in contact with food on a Request from Commission on flavoring group evaluation 78 (FGE.78). 2009. consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic and aromatic hydrocarbons evaluated by EFSA in FGE.25. Available: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.931 [Accessed 1 Apr 2021].
- 52. Summary and conclusions of the sixty-fourth meeting of the joint FAO/WHO expert Committee on food additives (JECFA), Rome, 8-17 February 2005. Fao.Org, 2005. Available: http://www.fao.org/3/a-at877e.pdf [Accessed 1 Apr 2021].
- 53. Barlow S, Renwick AG, Kleiner J, et al. Risk assessment of substances that are both genotoxic and carcinogenic. Food and Chemical Toxicology 2006;44:1636–50. 10.1016/j.fct.2006.06.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tobaccocontrol-2021-056582supp001.pdf (68.8KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are included in the submitted manuscript.