Abstract
Objective:
Subjective Cognitive Decline (SCD) may be an early indicator of risk for Alzheimer’s disease (AD). Findings regarding sex differences in SCD are inconsistent. Studying sex differences in SCD within cognitively unimpaired individuals with autosomal-dominant AD (ADAD), who will develop dementia, may inform sex-related SCD variations in preclinical AD. We examined sex differences in SCD within cognitively unimpaired mutation carriers from the world’s largest ADAD kindred and sex differences in the relationship between SCD and memory performance.
Methods:
We included 310 cognitively unimpaired Presenilin-1 (PSEN1) E280A mutation carriers (51% females) and 1998 non-carrier family members (56% females) in the study. Subjects and their study partners completed SCD questionnaires and the CERAD word list delayed recall test. ANCOVAs were conducted to examine group differences in SCD, sex, and memory performance. In carriers, partial correlations were used to examine associations between SCD and memory performance covarying for education.
Results:
Females in both groups had greater self-reported and study partner-reported SCD than males (all p<0.001). In female mutation carriers, greater self-reported (p=0.02) and study partner-reported SCD (p<0.001) were associated with worse verbal memory. In male mutation carriers, greater self-reported (p=0.03), but not study partner-reported SCD (p=0.11), was associated with worse verbal memory.
Conclusions:
Study partner-reported SCD may be a stronger indicator of memory decline in females versus males in individuals at risk for developing dementia. Future studies with independent samples and preclinical trials should consider sex differences when recruiting based on SCD criteria.
Keywords: Sex-differences, Familial Alzheimer’s disease, Presenilin-1, Episodic Memory, Preclinical Dementia, Memory Disorders
INTRODUCTION:
Increasing evidence has highlighted the urgency to identify individuals at greater risk for developing Alzheimer’s disease (AD), as AD-related pathology (i.e. amyloid-beta and tau tangles) begins to accumulate in the brain many years before dementia onset (Benzinger et al., 2013; Fleisher et al., 2012; Fagan et al., 2015). Greater Subjective Cognitive Decline (SCD), defined as “subjectively reported change in cognitive performance” (Jessen et al., 2014), has been shown to be an early indicator of subtle cognitive decline and to be associated with higher levels of amyloid-beta and tau in the entorhinal cortex in cognitively normal older adults at risk for AD (Buckley et al., 2017; Buckley et al., 2019). As such, measuring SCD may be useful for identifying individuals at increased risk to develop dementia (Buckley et al., 2019). However, while evidence supports that SCD is associated with early disease progression, it is not well understood how SCD may vary with other factors such as biological sex in the preclinical stage of AD.
Sex differences in AD have been understudied despite data showing that there are more women than men diagnosed with the disease (Plassman et al., 2007; Mielke, 2018; Alzheimer’s Association, 2020). While some studies suggest that this may be largely due to women living longer than men on average (Fiest et al., 2016), other studies have reported that there are sex differences in cognitive performance and AD-related pathology burden after controlling for age or survival (Sundermann et al., 2018; Buckley et al., 2018; Buckley et al., 2019; Vila-Castelar et al., 2020). For instance, recent studies showed that cognitively normal older women exhibited a steeper objective cognitive decline and higher levels of entorhinal tau burden compared to men with similar amyloid levels (Buckley et al., 2018; Buckley et al., 2019).
Studies examining sex differences in SCD in AD have reported inconsistent findings. Some studies in cognitively unimpaired individuals found that SCD is more frequently reported in males (Holmen et al., 2013; Paradise et al., 2011), others that SCD is more frequently reported in females (Heser et al., 2019), and others found no difference between males and females (Sundermann et al., 2018). These discrepancies may be related to differences in methods or the age of the cognitively unimpaired participants being studied (Heser et al., 2019). In studies with younger individuals, males tended to report greater SCD than females (Holmen et al., 2013; Paradise et al., 2011) whereas in studies with older individuals, females tended to report greater SCD than males (Heser et al., 2019). Other possible explanations that have been posited for why males may have higher SCD than females in some of these studies include possible selection bias caused by lower participation rates in men compared to women, thus having participating men more likely to report memory concerns than non-participating men, as well as lower education or greater cardiovascular risk factors which may be more present in males than females (Holmen et al., 2013; Paradise et al., 2011).
On the other hand, it has been suggested that females may be more sensitive to subtle pathological changes and more likely to report cognitive problems than males (Heser et al., 2019; Pérès et al., 2011; Sundermann et al., 2018). Sundermann and colleagues showed that both cognitively unimpaired males and females exhibited an association between greater SCD and poorer verbal memory performance. It was only once the groups were further along in the disease progression that females with amnestic mild cognitive impairment (aMCI) showed a stronger association than males with aMCI (Sundermann et al., 2018).
Studies examining SCD in cognitively unimpaired individuals with autosomal dominant AD (ADAD), who have a well-characterized disease progression (Fuller et al., 2019), have provided great insight on the role of SCD in preclinical AD (Gatchel et al., 2020; Norton et al., 2017). Our group previously examined a sample of cognitively unimpaired individuals from the world’s largest kindred with ADAD from Colombia, due to the E280A mutation in the Presenilin-1 (PSEN-1) gene. Carriers within the kindred have a median age of mild cognitive impairment (MCI) onset at 44 years (95% confidence interval [CI] 43–45) and 49 years (95% CI 49–50) for dementia onset. Mutation carriers reported greater self-reported SCD than non-carriers who are from the same families and have the same risk to carry the mutation, but not the study partners (Norton et al., 2017). Further, both self-reported and study partner-reported SCD in carriers were associated with older age (Norton et al., 2017; Gatchel et al., 2020), a proxy for disease progression, such that those who were closer to dementia onset had greater concerns. Study partner-reported SCD began to differ from non-carriers 5.7 years before the age of expected MCI onset and 10.7 years before the age of expected dementia onset (Norton et al., 2017). Within carriers, study partner-reported SCD were related to amyloid-beta and tau in the entorhinal cortex and in the inferior temporal lobe, while self-reported SCD was only related to amyloid burden (Gatchel et al., 2020). However, the role of sex differences in relation to SCD within this preclinical AD cohort remains to be elucidated.
The primary aim of the current study was to examine the relationship between SCD and sex in a large sample of cognitively unimpaired PSEN1 E280A mutation carriers. We specifically sought to examine if male and female mutation carriers differed in self-reported and study partner-reported SCD, and if there was a different association between SCD and verbal memory performance in male and female carriers. We hypothesized that: 1) Between PSEN-1 E280A carriers and non-carriers, there would be no sex difference in self-reported or study partner-reported SCD; 2) Within mutation carriers, males and females would not differ in self-reported SCD or study partner-reported SCD; and 3) female carriers would have a stronger association between self- and study partner-reported SCD and verbal memory performance than males.
METHODS:
Participants.
A total of 310 PSEN1 E280A mutation carriers (51% females) and 1998 non-carrier family members (56% females) from the Colombian kindred participated in this study. Participants were cognitively unimpaired, as defined by a Functional Assessment Staging Test (FAST; Sclan & Reisberg, 1992) score of 2 or lower and a Global Deterioration Scale (Reisberg et al., 1982) score of 2 or lower. For both the FAST and the Global Detarioration Scale, a score of 2 denotes subjective memory complaints but no objective impairments and a score of 1 denotes no objective or subjective difficulties. In addition, in order to be classified as cognitively unimpaired, participants had to have a Mini-Mental State Examination (MMSE) score of at least 26/30 (Folstein, 1983) and a score greater than 1.5 standard deviations below the mean on the Spanish-version of the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery (CERAD) word list delayed recall test (Aguirre-Acevedo et al., 2007). Exclusion criteria included current major neurologic or psychiatric disorders. The cognitive tests were administered by trained staff that were blind to the participants’ genetic status. Participants were also blind to their genetic status. This research was completed in accordance with the Helsinki Decleration and was approved by the Ethical Research Committee of the University of Antioquia in Colombia and Masschusetts General Hospital in Boston, MA. Participants provided signed consent forms before any procedures were administered.
Subjective cognitive decline and cognitive measures.
Clinical assessments were administered by trained bilingual clinical staff at the University of Antioquia in Colombia. Self-reported and study partner-reported SCD was assessed using the Memory Complaint Scale in Spanish, a 15-item questionnaire that uses a Likert scale from 0 (no complaints) to 3 (maximal complaints), for a total score that ranges from 0 to 45 (Ardila et al., 2000; Acosta-Baena et al., 2011; Vannini et al., 2020). This scale conforms to the recommendations made by Rabin and colleagues (Rabin et al., 2015) which includes being appropriate for the demographic characteristics of this sample, focusing on a single cognitive construct, and combining specific items versus general items (Norton et al., 2017). The Spanish-version of the CERAD word list delayed recall test was administered to all of the participants as a measure of verbal memory.
Statistical analysis.
We compared age, education, MMSE score, and CERAD word list delayed recall performance between carriers and non-carriers and between males and females using independent two-tailed t tests, and the ratio of males and females using a chi-squared test. We conducted partial correlations controlling for education to test the association between SCD, age and verbal memory performance in male and female carriers. We then conducted partial correlations controlling for education and depression, as measured by the Geriatric Depression Scale, as there was an association between scores on this test and SCD. We conducted two-way ANCOVAs, covarying for education, to assess group*sex interactions when comparing SCD after confirming that all assumptions were satisfied. The two-way ANCOVA was then repeated covarying for education and depression. Effect sizes were measured using Hedges’ g (small effect=0.2, medium effect=0.5, large effect=0.8) and partial eta squared (η2p; small effect=0.01, medium effect=0.06, large effect=0.14). Analyses were conducted using IBM SPSS Statistics Version 24.
RESULTS:
Group demographic and neuropsychological characteristics.
Participant characteristics are presented in Table 1. Cognitively unimpaired mutation carriers were younger [t(455.08)=7.37, p<0.001, Hedges’ g=0.40] and had less years of education [t(2306)=2.73, p=0.006, Hedges’ g=0.17] than non-carriers (Table 1). Carriers performed worse than non-carriers on the MMSE [t(376.03)=3.27, p=0.001, Hedges’ g=0.23] as well as the CERAD word list delayed recall test [t(2306)=2.25, p=0.03, Hedges’ g=0.14].
Table 1.
Demographic and cognitive data for cognitively unimpaired PSEN1 carriers and non-carriers. Group differences for continuous variables were tested using independent t-test and chi-square for categorical variables. MMSE = Mini-Mental State Examination; SCD = Subjective Cognitive Decline.
| Non-carriers | Carriers | |||||
|---|---|---|---|---|---|---|
| n = 1998 Mean (SD) | n = 310 Mean (SD) | t | df | p-value | Hedges’ g | |
| Age (years) | 31.51 (10.38) | 27.46 (8.76) | 7.37 | 455.08 | <0.001 | 0.40 |
| Education (years) | 9.36 (4.23) | 8.66 (4.18) | 2.73 | 2306 | 0.006 | 0.17 |
| Sex, females n, χ2 | 1113 (56%) | 157 (51%) | – | 1 | 0.10 | – |
| MMSE | 29.20 (1.04) | 28.95 (1.25) | 3.27 | 376.03 | 0.001 | 0.23 |
| CERAD Word List Delayed Recall | 6.58 (1.61) | 6.35 (1.71) | 2.25 | 2306 | 0.03 | 0.14 |
| Self-Reported SCD | 12.58 (8.16) | 11.75 (7.76) | 1.68 | 2306 | 0.09 | 0.10 |
| Study Partner-Reported SCD | 8.01 (7.24) | 8.77 (7.21) | −1.72 | 2306 | 0.09 | 0.11 |
SCD in mutation non-carriers and carriers.
Non-carriers and carriers did not differ in self-reported SCD [t(2306)=1.68, p=0.09, Hedges’ g=0.10] or study partner-reported SCD [t(2306)=−1.72, p=0.09, Hedges’ g=0.11]. In both non-carriers and carriers, greater age was associated with greater self-reported SCD (r=0.09, p<0.001; r=0.13, p=0.02, respectively) and study partner-reported SCD (r=0.10, p<0.001; r=0.30, p<0.001, respectively).
Male and female characteristics within groups.
Females non-carriers were older [t(1933.69)=−2.14, p=0.03, Hedges’ g=0.10], had higher levels of education [t(1823.18)=−5.11, p<0.001, Hedges’ g=0.23], and greater self-reported [t(1978.06)=−5.99, p<0.001, Hedges’ g=0.27] and study partner-reported SCD [t(1996)=−3.42, p=0.001, Hedges’ g=0.15] than males (Table 2). There were no differences between MMSE [t(1996)=0.19, p=0.85, Hedges’ g=0.01] or CERAD word list delayed recall performance [t(1996)=−1.73, p=0.08, Hedges’ g=0.07] between male and female noncarriers. Female non-carriers showed an association between greater age and greater self-reported (r=0.11, p<0.001) as well as study partner-reported SCD (r=0.12, p<0.001), but not male non-carriers (r=0.03, p=0.33; r=0.06, p=0.10, respectively). In carriers, females had higher levels of education [t(298.63)=−2.39, p=0.02, Hedges’ g=0.27; Table 2] and self-reported SCD [t(298.11)=−2.56, p=0.01, Hedges’ g=0.29] than males, but there were no differences between males and females in age [t(308)=0.29, p=0.77, Hedges’ g=0.03], MMSE performance [t(308)=−0.87, p=0.39, Hedges’ g=0.10], CERAD word list delayed recall performance [t(308)=−1.11, p=0.27, Hedges’ g=0.13], or study partner-reported SCD [t(308)=−1.30, p=0.19, Hedges’ g=0.15]. Carrier males and females exhibited a positive correlation between age and study partner-reported SCD (r=0.27, p=0.001; r=0.31, p<0.001, respectively), but not self-reported SCD (r=0.10, p=0.21; r=0.13, p=0.10, respectively).
Table 2.
Demographic and cognitive data comparing cognitively unimpaired male and female PSEN1 non-carriers and male and female carriers. Group differences for continuous variables were tested using independent t-test and chi-square for categorical variables. MMSE = Mini-Mental State Examination; CERAD Word List = Consortium to Establish a Registry for Alzheimer’s Disease word list delayed recall test; SCD = Subjective Cognitive Decline.
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Non-carriers n=1998 | n = 88 Mean (SD) |
n = 1113 Mean (SD) |
t | df | p-value | Hedges’ g | |
| Age (years) | 30.95 (10.08) | 31.95 (10.60) | −2.14 | 1933.69 | 0.03 | 0.10 | |
| Education (years) | 8.82 (4.38) | 9.80 (4.05) | −5.11 | 1823.18 | <0.001 | 0.23 | |
| MMSE | 29.20 (1.03) | 29.19 (1.02) | 0.19 | 1996 | 0.85 | 0.01 | |
| CERAD Word List | 6.51 (1.62) | 6.63 (1.61) | −1.73 | 1996 | 0.08 | 0.07 | |
| Self-Reported SCD | 11.38 (7.47) | 13.53 (8.55) | −5.99 | 1978.06 | <0.001 | 0.27 | |
| Study Partner-Reported SCD | 7.39 (7.08) | 8.50 (7.32) | −3.42 | 1996 | 0.001 | 0.15 | |
| Carriers n = 310 | n = 15 | n = 157 | |||||
| Age (years) | 27.61 (8.91) | 27.32 (8.64) | 0.29 | 308 | 0.77 | 0.03 | |
| Education (years) | 8.09 (4.45) | 9.22 (3.82) | −2.39 | 298.63 | 0.02 | 0.27 | |
| MMSE | 28.89 (1.28) | 29.01 (1.23) | −0.87 | 308 | 0.39 | 0.10 | |
| CERAD Word List | 6.24 (1.63) | 6.46 (1.79) | −1.11 | 308 | 0.27 | 0.13 | |
| Self-Reported SCD | 10.62 (6.83) | 12.85 (8.44) | −2.56 | 298.11 | 0.01 | 0.29 | |
| Study Partner-Reported SCD | 8.23 (7.04) | 9.29 (7.34) | −1.30 | 308 | 0.19 | 0.15 |
Group and sex differences in SCD.
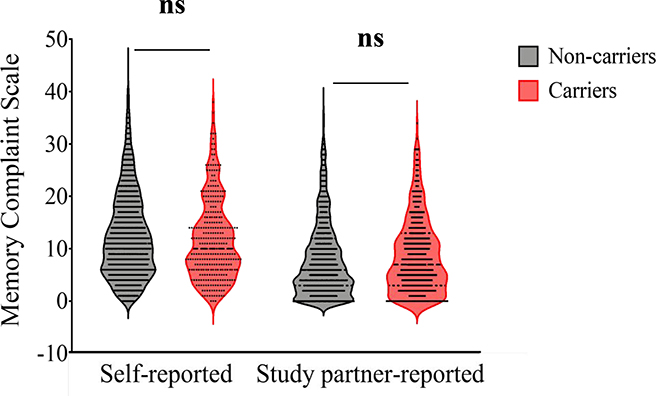
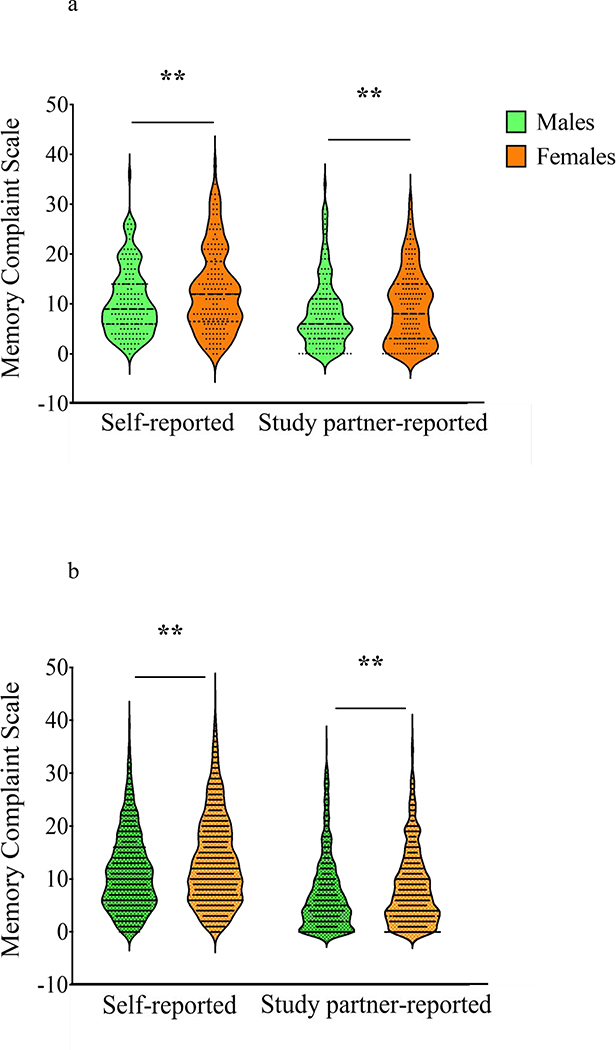
Carriers and non-carriers did not differ in self-reported SCD [F(1, 2303)=3.32, p=0.07, η2p =0.001] or study partner-reported SCD [F(1, 2303)=1.64, p=0.20, η2p =0.001; Fig. 1]. Regarding sex differences, female participants self-reported more SCD compared to males [sex main effect, F(1, 2303)=25.31, p<0.001, η2p =0.011] and had study partners that reported greater SCD compared to male participants [F(1, 2303)=12.48, p<0.001, η2p =0.005; Fig. 2a and Fig. 2b], regardless of carrier status. There was no group*sex interaction between carriers and non-carriers for self-reported SCD [F(1, 2303)=0.02, p=0.90, η2p <0.001] or study partner-reported SCD [F(1, 2303)<0.001, p=0.99, η2p <0.001]. The results of the two-way ANCOVA are summarized in Table 3.
Fig. 1.
Violin plot displaying distribution of self-reported and study partner-reported SCD in mutation carriers and non-carriers. There were no differences between mutation carriers and non-carriers in self-reported SCD (2-way ANCOVA covarying for education, [F(1, 2303)=3.32, p=0.07, η2p=0.00]) and study partner-reported SCD [F(1, 2303)=1.64, p=0.20, η2p=0.001]. There was also no group*sex interaction for self-reported SCD [F(1, 2303)=0.02, p=0.90, η2p <0.001] or study partner-reported SCD [F(1, 2303)<0.001, p=0.99, η2p <0.001]. ns = not significant
Fig. 2a & 2b.
Violin plots displaying distribution of self-reported and study partner-reported SCD. Figure 2a displays female versus male mutation carriers. Figure 2b shows female versus male mutation non-carriers. Females had greater self-reported SCD ( [F(1, 2303)=25.31, p<0.001, η2p =0.011]) and greater study partner-reported SCD [F(1, 2303)=12.48, p<0.001, η2p =0.005] compared to males regardless of carrier status. ** p < .005
Table 3.
ANCOVA summary data covarying for education and comparing cognitively unimpaired male and female PSEN1 non-carriers and male and female carriers. SCD = Subjective Cognitive Decline.
| Dependent Variable | df | F | p-value | η2p | |
|---|---|---|---|---|---|
| Carrier Status (Carriers vs. Non-Carriers) | Self-Reported SCD | 1 | 3.32 | 0.07† | 0.001 |
| Study Partner-Reported SCD | 1 | 1.64 | 0.20 | 0.001 | |
| Sex (Males vs Females) | Self-Reported SCD | 1 | 25.31 | <0.001† | 0.011 |
| Study Partner-Reported SCD | 1 | 12.48 | <0.001† | 0.005 | |
| Carrier Status*Sex | Self-Reported SCD | 1 | 0.02 | 0.90 | <0.001 |
| Study Partner-Reported SCD | 1 | <0.001 | 0.99 | <0.001 | |
| Error | Self-Reported SCD | 2303 | |||
| Study Partner-Reported SCD | 2303 |
p < 0.05 (Covarying for depression & education)
These analyses were repeated covarying for education and depression. Non-carriers displayed greater self-reported SCD than carriers [F(1, 2299)=7.63, p=0.006, η2p =0.003], but there was still no difference between carriers and non-carriers in study partner-reported SCD[F(1, 2299)=1.08, p=0.30, η2p<0.001]. Consistent with our previous findings, females had greater self-reported SCD [F(1, 2299)=7.05, p=0.008, η2p =0.003] and study partner-reported SCD [F(1, 2299)=5.23, p=0.02, η2p =0.002] than males regardless of carrier status. There was no group*sex interaction between carriers and non-carriers for self-reported SCD [F(1, 2299)=0.16, p=0.69, η2p <0.001] or study partner-reported SCD [F(1, 2299)=0.06, p=0.81, η2p <0.001].
Sex differences in the association between verbal memory and SCD.
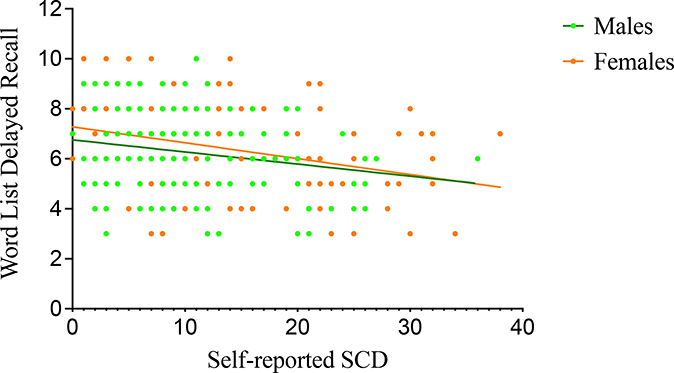
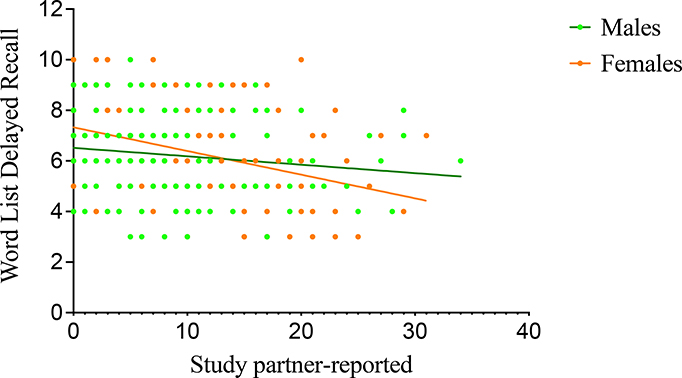
Within non-carriers, males and females displayed an association with worse CERAD word list delayed recall performance and greater self-reported (r=−0.11, p=0.001; r=−0.10, p=0.001, respectively) as well as study partner-reported SCD (r=−0.13, p<0.001; r=−0.18, p<0.001, respectively). In female carriers, worse CERAD word list delayed recall performance was associated with greater self-reported (r=−0.19, p=0.02; Fig. 3) and study partner-reported SCD (r=−0.28, p<0.001; Fig. 4). Male carriers displayed an association between worse CERAD word list delayed recall performance and greater self-reported (r=−0.17, p=0.03; Fig. 3) but not study partner-reported SCD (r=−0.13, p=0.11; Fig. 4).
Figure 3.
Association between word list delayed recall and self-reported SCD in mutation carriers. Greater self-reported SCD was associated with worse memory performance in both male (CERAD word list mean=6.24, SD=1.63; self-reported SCD mean=10.62, SD=6.84, r=−0.17, p=0.03) and female mutation carriers (CERAD word list mean=6.46, SD=1.79; self-reported SCD mean=12.85, SD=8.44; r=−0.19, p=0.02).
Figure 4.
Association between word list delayed recall and study partner-reported SCD in mutation carriers. In female carriers, greater study partner-reported SCD was associated with worse memory performance after covarying for education (CERAD word list mean=6.46, SD=1.79; study partner-reported SCD mean=9.29, SD=7.34; r=−0.28, p<0.001). Male carriers did not display an association between study partner-reported SCD and worse memory performance after covarying for education (CERAD word list mean=6.24, SD=1.63; study partner-reported SCD mean=8.23, SD=7.04; r=−0.13, p=0.11).
When controlling for depression and education, both male and female non-carriers exhibited associations between worse CERAD word list delayed recall performance and greater self-reported (r=−0.10, p=0.002; r=−0.09, p=0.004, respectively) as well as study partner-reported SCD (r=−0.13, p<0.001; r=−0.17, p<0.001, respectively). In mutation carriers, females had a negative association between CERAD word list performance and self-reported (r=−0.16, p=0.05) as well as study partner-reported SCD in females (r=−0.26, p=0.001). In male carriers, there was a negative association between CERAD word list delayed recall performance and self-reported SCD (r=−0.17, p=0.04), but not study partner-reported SCD (r=−0.13, p=0.12)
DISCUSSION:
Previous studies examining sex differences in SCD in individuals at risk for sporadic AD have yielded mixed results, likely due to various confounding factors such as age and cardiovascular risk factors (Heser et al., 2019; Holmen et al., 2013). In this study, we leveraged our access to a large sample of cognitively unimpaired individuals from the largest single-mutation ADAD cohort to test whether there were sex differences in SCD in mutation carriers and noncarriers during the preclinical stage of the disease. Our findings showed that PSEN-1 mutation carriers did not differ from non-carriers in self-reported or study partner-reported SCD when accounting for education. However, when accounting for education and depression symptoms, non-carriers self-reported more SCD than carriers. This finding suggests that the self-reported SCD within carriers, but not in non-carriers, may be driven by symptoms of depression, which has been suggested to be an early clinical marker of AD (Gatchel et al., 2019). Sex did not play a role in this observed outcome between carriers and non-carriers in study partner-reported SCD or self-reported SCD. These findings do not support previous studies reporting sex differences in SCD in the preclinical stage of AD. Perhaps sex differences within SCD may not manifest until later in the AD trajectory. Sundermann and colleagues, for instance, found that the sex differences in SCD within their overall sample were driven by their aMCI group. Once their sample was stratified by group, there were no sex differences in the mean of self-reported or study partner-reported SCD in their normal control group (Sundermann et al., 2018).
In both carriers and non-carriers, greater age was associated with self- and study partner-reported SCD, suggesting that SCD increases as a normal part of aging within the kindred. Alternatively, it may suggest a heightend sensitivity to memory changes particular to this cohort of non-carriers and carriers, as individuals in both groups have at least one parent with the mutation and therefore, the same likelihood of having the mutation themselves. As such, they may be more sensitive to subtle cognitive changes that occur with age, particularly those who are closer to the estimated age of onset of objective memory decline and MCI (i.e. age 44) (Fuller et al., 2019). This sensitivity could be driven by sex in non-carriers given that female non-carriers had an association with greater age and greater self- and study partner-reported SCD, but male non-carriers did not. Conversely, both male and female carriers had positive associations with age and study partner-reported SCD, but not self-reported SCD. Within carriers, it may only be the study partners who exhibit greater sensitivity to memory changes as the carriers age.
Contrary to what we hypothesized, females had greater self-reported and study partner-reported SCD than males in both carriers and non-carriers. The difference in self-reported SCD found between males and females may be explained by a gender bias in endorsing health concerns. Females have been shown to be more likely to report and seek out care for physical and mental health concerns than males (Thompson et al., 2016; Wool and Barsky, 1994). This may reflect a discomfort in males in reporting possible health conditions perhaps due to social or cultural factors, which it is not unique to those with a family history of AD. Mood disorders like depression have been shown to be associated with SCD in both males and females of various ages (Brown, Hill, & Haider, 2020) and may often account for observed differences in SCD between males and females. However, our results remained consistent even when accounting for depression in addition to education.
Although a greater propensity for females to endorse health problems may help explain our findings for self-reported SCD, it may not reasonably explain the higher levels of study partner-reported SCD for female participants compared to male participants. This is because both male and female participants could have had either male or female study partners that reported their concerns (i.e. spouses, children, other family members, or friends). Since the sex of the study partners were unavailable to us in this study, we were unable to examine if study partner sex may have influenced the study partner-reported SCD of the participants (i.e. greater SCD reported by female study partners than male study partners or vice versa).
The study partner’s perception of the severity of cognitive issues may also be influenced by cultural factors and their own understanding of ADAD. Individuals within our cohort may have a hyperawareness of, or even a bias towards, memory decline as they typically know a parent or family member with the disease. Our findings suggest that study partners may have a particular acuity toward the potential cognitive decline of females within our cohort even when accounting for depression and education.
Consistent with prior findings, greater SCD was associated with worse verbal memory performance (Sundermann et al., 2018) in both male and female mutation carriers. Female carriers displayed an association between worse verbal memory performance and greater self-reported, as well as study partner-reported SCD, while male carriers showed an association between worse verbal memory performance and greater self-reported SCD, but not study partner-reported SCD. This finding suggests that study partner-reported SCD may be more sensitive to early cognitive changes in female carriers than in male carriers. These negative associations between verbal memory performance and self-reported as well as study partner-reported SCD were also found in male and female non-carriers suggesting that while SCD may be a good indicator of early memory changes, this may not be particular to mutation carriers. Previous studies have suggested that the correlation between SCD and objective memory performance may be influenced by reported anxiety, depression, and emotional distress (Buckley et al., 2013; Pearman and Storandt, 2004). However, our findings in both carriers and non-carriers remained consistent after controlling for depression and education.
This study has several limitations. The cross-sectional design of this study prevented us from measuring sex differences in changes within SCD and objective verbal memory decline over time. Further, there was a large difference in sample size between carriers and non-carriers and there were more female non-carriers than male non-carriers. Differences in sample size can lead to unequal variance which can decrease statistical power and increase type 1 error rates (Rusticus & Lovato, 2014), but were corrected within our SPSS software automatically using Welch’s Test for Unequal Variances (Welch, 1947). Although we previously examined the relationship between SCD and AD biomarkers (i.e amyloid-beta and tau deposition; Gatchel et al., 2020) as well as the effect of lifestyle factors on the onset and rate of cognitive decline within carriers (Aguirre-Acevedo et al., 2016) in smaller cohorts, in this larger cohort we did not have access to AD biomarker data, neurodegenerative markers, lifestyle factors, or subjective complaints beyond the domain of memory (e.g. executive function, language, etc.). There is still uncertainty regarding the generalizability of our findings in ADAD to late-onset AD even when they are consistent with data from studies in older adults at risk for AD (Pérès et al., 2011; Sundermann et al., 2018; Heser et al., 2019; Buckley et al., 2019). Thus the generalizability of our findings to late onset AD should be interpreted with caution. Future studies should also consider examining sex differences within SCD and AD biomarkers in addition to other cognitive domains in a larger sample. Addtionally, the relationship between SCD, depression, and AD biomarkers should be further studied. It may also be helpful to explore the relation between the sex of the study partner and their reporting of SCD in male and female participants. As previously mentioned, this may provide greater insight on the difference found between males and females in study partner-reported SCD.
In summary, examining sex differences within SCD in PSEN1 E280A carriers gives us the unique opportunity to study individuals in the preclinical stages who do not have comorbidities associated with aging, such as cardiovascular risk or differences in survival bias or mortality between males and females. Our findings support the body of literature suggesting that females may have more SCD than males, and that SCD are strongly associated with objective memory performance, including in the preclinical stage of AD. The results of this study highlight the value of considering sex when assessing SCD in future investigations.
ACKNOWLEDGEMENTS:
The authors thank the PSEN1 Colombian families for contributing their valuable time and effort, without which this study would not have been possible.
Funding.
This research was supported by the NIH National Institute of Aging (RO1AG054671 [YTQ]), and Office of the Director (DP5OD019833 [YTQ]), MGH ECOR Clafin Distinguished Scholar Award [YTQ], MGH Physician/Scientist Development Award [YTQ] and the Alzheimer’s Association [YTQ]. Dr. Guzmán-Vélez was supported by the NIA K23AG061276. Dr. Vila-Castelar received funding from the Alzheimer’s Association (2019-AARF-644631). Dr. Pardilla-Delgado was supported by a training grant from the NHLBI (5T32HL007901–22). Dr. Lopera received funding from the National Institutes of Health, Comite para el Desarrollo de la Investigacion (CODI‐UdeA) and COLCIENCIAS.
Footnotes
Conflicts of Interest. The authors have no conflicts of interest to report.
REFERENCES:
- 2020 Alzheimer’s disease facts and figures. (2020). Alzheimer’s & Dementia, 16(3), 391–460. 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, Jaramillo-Elorza MC, Moreno S,…Lopera F (2011). Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. The Lancet. Neurology , 10(3), 213–220. 10.1016/S1474-4422(10)70323-9 [DOI] [PubMed] [Google Scholar]
- Afifi M (2007). Gender differences in mental health. Singapore medical journal, 48(5), 385–391. [PubMed] [Google Scholar]
- Aguirre-Acevedo DC, Gómez RD, Moreno S, Henao-Arboleda E, Motta M, Muñoz C, … Lopera F (2007). Validez y fiabilidad de la batería neuropsicológica CERAD-Col [Validity and reliability of the CERAD-Col neuropsychological battery]. Revista de neurologia, 45(11), 655–660. [PubMed] [Google Scholar]
- Aguirre-Acevedo DC, Lopera F, Hena E, Tirad V, Muño C, Giraldo M…Jaimes F (2016). Cognitive Decline in a Colombian Kindred With Autosomal Dominant Alzheimer Disease: A Retrospective Cohort Study. JAMA Neurology, 73(4), 431–438. 10.1001/jamaneurol.2015.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A, Loper F, Rossell M, Moren S, Madriga L, Arango-Lasprilla JC, …Kosik KS (2000). Neuropsychological profile of a large kindred with familial Alzheimer’s disease caused by the E280A single presenilin-1 mutation. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists, 15(6), 515–528. [PubMed] [Google Scholar]
- Benzinger TLS, Blazey T, Jack CR, Koeppe RA, Su Y, Xiong C, … Morris JC (2013). Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proceedings of the National Academy of Sciences, 110(47), E4502–E4509. 10.1073/pnas.1317918110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Hill NL, & Haider MR (2020). Age and gender disparities in depression and subjective cognitive decline-related outcomes. Aging & mental health, 1–8. Advance online publication. 10.1080/13607863.2020.1861214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, … Amariglio RE (2017). Region-Specific Association of Subjective Cognitive Decline With Tauopathy Independent of Global β-Amyloid Burden. JAMA Neurology, 74(12), 1455. 10.1001/jamaneurol.2017.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, … Sperling RA (2018). Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimer’s & Dementia, 14(9), 1193–1203. 10.1016/j.jalz.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, … Sperling RA (2019). Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured by Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurology, 76(5), 542. 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R, Saling MM, Ames D, Rowe CC, Lautenschlager NT, Macaulay SL, … Australian Imaging Biomarkers and Lifestyle Study of Aging (AIBL) Research Group (2013). Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. International psychogeriatrics, 25(8), 1307–1315. 10.1017/S1041610213000665 [DOI] [PubMed] [Google Scholar]
- Buckley RF, Sikkes S, Villemagne VL, Mormino EC, Rabin JS, Burnham S, … Amariglio RE (2019). Using subjective cognitive decline to identify high global amyloid in community‐based samples: A cross‐cohort study. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 11(1), 670–678. 10.1016/j.dadm.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call JB, & Shafer K (2018). Gendered Manifestations of Depression and Help Seeking Among Men. American Journal of Men’s Health, 12(1), 41–51. 10.1177/1557988315623993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Frank B, Oliffe JL, & Gregory D (2011). Health, Illness, Men and Masculinities (HIMM): A theoretical framework for understanding men and their health. Journal of Men’s Health, 8(1), 7–15. 10.1016/j.jomh.2010.09.227 [DOI] [Google Scholar]
- Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TLS, … Holtzman DM (2014). Longitudinal Change in CSF Biomarkers in Autosomal-Dominant Alzheimer’s Disease. Science Translational Medicine, 6(226), 226ra30–226ra30. 10.1126/scitranslmed.3007901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, … Jetté N (2016). The Prevalence and Incidence of Dementia Due to Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Canadian Journal of Neurological Sciences / Journal Canadien Des Sciences Neurologiques, 43(S1), S51–S82. 10.1017/cjn.2016.36 [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, … Reiman EM (2012). Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: A cross-sectional study. The Lancet Neurology, 11(12), 1057–1065. 10.1016/S1474-4422(12)70227-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, & Helzer JE (1983). The Mini-Mental State Examination. Archives of general psychiatry, 40(7), 812. 10.1001/archpsyc.1983.01790060110016 [DOI] [PubMed] [Google Scholar]
- Fuller JT, Cronin-Golomb A, Gatchel JR, Norton DJ, Guzmán-Vélez E, Jacobs H, … Quiroz YT (2019). Biological and Cognitive Markers of Presenilin1 E280A Autosomal Dominant Alzheimer’s Disease: A Comprehensive Review of the Colombian Kindred. The journal of prevention of Alzheimer’s disease, 6(2), 112–120. 10.14283/jpad.2019.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel JR, Lopera F, Norton DJ, Baena A, Guzman-Velez E, Sanchez JS, … Quiroz YT (2020). Association of subjective cognitive decline with markers of brain pathology in preclinical autosomal dominant Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 91(3), 330–332. 10.1136/jnnp-2019-321205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel JR, Rabin JS, Buckley RF, Locascio JJ, Quiroz YT, Yang H-S…Harvard Aging Brain Study. (2019). Longitudinal Association of Depression Symptoms With Cognition and Cortical Amyloid Among Community-Dwelling Older Adults. JAMA Network Open, 2(8), e198964–e198964. 10.1001/jamanetworkopen.2019.8964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heser K, Kleineidam L, Wiese B, Oey A, Roehr S, Pabst A, … Wagner M (2019). Subjective Cognitive Decline May Be a Stronger Predictor of Incident Dementia in Women than in Men. Journal of Alzheimer’s Disease, 68(4), 1469–1478. 10.3233/JAD-180981 [DOI] [PubMed] [Google Scholar]
- Holmen J, Langballe EM, Midthjell K, Holmen TL, Fikseaunet A, Saltvedt I, & Tambs K (2013). Gender differences in subjective memory impairment in a general population: The HUNT study, Norway. BMC Psychology, 1(1), 19. 10.1186/2050-7283-1-19 [DOI] [Google Scholar]
- Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, … Wagner M (2020). The characterisation of subjective cognitive decline. The Lancet Neurology, 19(3), 271–278. 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM (2018). Sex and Gender Differences in Alzheimer’s Disease Dementia. The Psychiatric times, 35(11), 14–17. [PMC free article] [PubMed] [Google Scholar]
- Niu H, Álvarez-Álvarez I, Guillén-Grima F, & Aguinaga-Ontoso I (2017). Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurología (English Edition), 32(8), 523–532. 10.1016/j.nrleng.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Norton DJ, Amariglio R, Protas H, Chen K, & Aguirre DC (2017). Subjective memory complaints in preclinical autosomal dominant Alzheimer disease. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise MB, Glozier NS, Naismith SL, Davenport TA, & Hickie IB (2011). Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: A cross-sectional study. BMC Psychiatry, 11(1), 108. 10.1186/1471-244X-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearman A, & Storandt M (2004). Predictors of subjective memory in older adults. The journals of gerontology. Series B, Psychological sciences and social sciences, 59(1), P4–P6. 10.1093/geronb/59.1.p4 [DOI] [PubMed] [Google Scholar]
- Pérès K, Helmer C, Amieva H, Matharan F, Carcaillon L, Jacqmin-Gadda H, … Dartigues J-F (2011). Gender Differences in the Prodromal Signs of Dementia: Memory Complaint and IADL-Restriction. A Prospective Population-Based Cohort. Journal of Alzheimer’s Disease, 27(1), 39–47. 10.3233/JAD-2011-110428 [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB (2007). Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology, 29(1–2), 125–132. 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M,…Sikkes SA (2015). Subjective Cognitive Decline in Older Adults: An Overview of Self-Report Measures Used Across 19 International Research Studies. Journal of Alzheimer’s disease : JAD, 48 Suppl 1(0 1), S63–S86. 10.3233/JAD-150154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, & Crook T (1982). The Global Deterioration Scale for assessment of primary degenerative dementia. The American journal of psychiatry, 139(9), 1136–1139. 10.1176/ajp.139.9.1136 [DOI] [PubMed] [Google Scholar]
- Rusticus SA, & Lovato CY (n.d.). Impact of Sample Size and Variability on the Power and Type I Error Rates of Equivalence Tests: A Simulation Study. 10.7275/4S9M-4E81 [DOI]
- Sclan S, & Reisberg B (1992). Functional Assessment Staging (FAST) in Alzheimer’s Disease: Reliability, Validity, and Ordinality. International Psychogeriatrics, 4(3), 55–69. doi: 10.1017/S1041610292001157 [DOI] [PubMed] [Google Scholar]
- Sundermann EE, Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Rubin LH, & Bondi MW (2018). Sex Influences the Accuracy of Subjective Memory Complaint Reporting in Older Adults. Journal of Alzheimer’s Disease, 61(3), 1163–1178. 10.3233/JAD-170425 [DOI] [PubMed] [Google Scholar]
- Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, & Aubrey-Bassler K (2016). The influence of gender and other patient characteristics on health care-seeking behaviour: A QUALICOPC study. BMC Family Practice, 17(1), 38. 10.1186/s12875-016-0440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Castelar C, Guzmán-Vélez E, Pardilla-Delgado E, Buckley RF, Bocanegra Y, Baena A,…Quiroz YT (2020). Examining Sex Differences in Markers of Cognition and Neurodegeneration in Autosomal Dominant Alzheimer’s Disease: Preliminary Findings from the Colombian Alzheimer’s Prevention Initiative Biomarker Study. Journal of Alzheimer’s disease : JAD, 77(4), 1743–1753. 10.3233/JAD-200723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hanseeuw BJ, Gatchel JR, Sikkes SAM, Alzate D, Zuluaga Y…Quiroz YT (2020). Trajectory of Unawareness of Memory Decline in Individuals With Autosomal Dominant Alzheimer Disease. JAMA Network Open, 3(12), e2027472–e2027472. 10.1001/jamanetworkopen.2020.27472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BL (1947). The Generalization of `Student’s’ Problem when Several Different Population Variances are Involved. Biometrika, 34(1/2), 28–35. JSTOR. 10.2307/2332510 [DOI] [PubMed] [Google Scholar]
- Wool CA, & Barsky AJ (1994). Do Women Somatize More Than Men? Psychosomatics, 35(5), 445–452. 10.1016/S0033-3182(94)71738-2 [DOI] [PubMed] [Google Scholar]