Abstract
Disorders of consciousness (DoCs) pose a significant clinical and ethical challenge because they allow for complex forms of conscious experience in patients where intentional behaviour and communication are highly limited or non-existent. There is a pressing need for brain-based assessments that can precisely and accurately characterize the conscious state of individual DoC patients. There has been an ongoing research effort to develop neural measures of consciousness. However, these measures are challenging to validate not only due to our lack of ground truth about consciousness in many DoC patients but also because there is an open ontological question about consciousness. There is a growing, well-supported view that consciousness is a multidimensional phenomenon that cannot be fully described in terms of the theoretical construct of hierarchical, easily ordered conscious levels. The multidimensional view of consciousness challenges the utility of levels-based neural measures in the context of DoC assessment. To examine how these measures may map onto consciousness as a multidimensional phenomenon, this article will investigate a range of studies where they have been applied in states other than DoC and where more is known about conscious experience. This comparative evidence suggests that measures of conscious level are more sensitive to some dimensions of consciousness than others and cannot be assumed to provide a straightforward hierarchical characterization of conscious states. Elevated levels of brain complexity, for example, are associated with conscious states characterized by a high degree of sensory richness and minimal attentional constraints, but are suboptimal for goal-directed behaviour and external responsiveness. Overall, this comparative analysis indicates that there are currently limitations to the use of these measures as tools to evaluate consciousness as a multidimensional phenomenon and that the relationship between these neural signatures and phenomenology requires closer scrutiny.
Keywords: disorders of consciousness, vegetative state, minimally conscious state, brain injury, multidimensional
Introduction
Disorders of consciousness (DoCs) are a devastating range of clinical conditions caused by severe damage to the brain that results in profound neurological impairment and often leaves individuals with limited or non-existent abilities for communication or intentional motor behaviour. It is well established that sophisticated forms of conscious experience, and potentially a rich mental life, can persist in some patients with DoC even in the absence of intentional behavioural responses (Owen et al. 2006; Monti et al. 2010; Naci et al. 2014). However, we currently lack reliable objective markers of consciousness and have a very limited understanding of what first-person experience is like in these conditions. There is a pressing clinical and ethical need to accurately detect and characterize consciousness in these patients, as this information is central to judging the well-being, best interests and moral status of individuals with DoC, and is likely to facilitate more precise and well-informed medical decisions about prognosis, possible therapeutic interventions, day-to-day care needs and in some cases whether life-sustaining treatment should be withdrawn. In this article, I will consider the emerging, well-supported view that consciousness is multidimensional and examine the implications of this approach for the clinical use of some proposed neurophysiological assessments of consciousness in DoC.
Establishing the neural basis of consciousness remains an ongoing scientific challenge, although significant progress has been made in recent decades (Koch et al. 2016; Michel et al. 2019). In healthy individuals and most other clinical populations, subjective reports and behaviour can provide insight into conscious experience. In DoCs, understanding consciousness from a scientific, third-person perspective is highly challenging as subjective reports are either impossible to obtain or highly limited. Given also that behavioural data have limitations in assessing the presence of consciousness in DoC, there has been an ongoing effort to develop accurate measures of consciousness based on neural data. Neural measures of consciousness that can be applied in a task-free setting are particularly appealing as they could, in theory, indicate whether consciousness is present or absent regardless of an individual’s cognitive and motor functioning.
The theoretical idea that conscious states are properties that can be described in hierarchically ordered levels is central to many efforts to develop neural measures of consciousness (Bayne et al. 2016). The majority of this article will be devoted to discussing unidimensional measures of signal complexity applied to resting-state brain data in a task-free setting. Patients in a minimally conscious state (MCS) are thought to have a higher level of consciousness, as potentially indexed by properties like resting-state brain complexity, than those in a vegetative state (VS), but lower than that of healthy, awake individuals without DoC.
However, the soundness of conscious levels as a theoretical construct has recently been challenged. It has been proposed that consciousness cannot always be described in terms of a single dimension of analysis and is in fact a multidimensional phenomenon with many components that can vary independently, such as various elements of attentional control, sensory processing, executive function and semantic comprehension (Bayne et al. 2016; Fazekas and Overgaard 2016; Bayne and Carter 2018; Birch et al. 2020). There is also growing recognition of the need for greater diagnostic precision in clinical practice for DoC (Provencio et al. 2020; Kondziella et al. 2021). Due to the highly heterogeneous pathophysiology amongst DoC patients and the fluctuating, dynamic nature of conscious experience, the traditional emphasis on diagnosing a patient according to a conscious–unconscious binary or unidimensional conscious level is not sufficient in capturing the clinically significant forms of variance to consciousness that can occur in DoC.
In this article, I will examine the implications of the multidimensional view of consciousness for several proposed neural measures of consciousness, particularly those designed as indexes of conscious level, in the context of their use in DoC diagnosis. In attempting to evaluate neural measures against any framework, we encounter an epistemic challenge in that we lack a ground truth about both the presence and the subjective quality of consciousness in many DoC patients (Peterson 2016). We cannot confirm with certainty that consciousness is absent based on the lack of overt or covert behaviours, which depend on specific mental capacities that may be lost even when consciousness itself persists. It is also very challenging to understand the subjective quality of experience in these patients, where communication is impossible or highly limited. Furthermore, I contend that within a multidimensional approach to consciousness, DoC diagnosis will shift to involve a greater emphasis on the subjective quality as well as the presence or absence of conscious experience.
As we lack a ground truth about consciousness in many DoC patients with which to validate these measures, I propose to explore a range of indirect evidence from studies where similar neural properties have been investigated in other conscious states where more evidence is available about the subjective quality of consciousness. This includes states occurring temporarily within healthy, neurotypical participants (e.g. sleep, anaesthesia and psychedelics) and conscious states resulting from longer-term neuropathology and other factors (e.g. psychiatric, neurodevelopmental and neurodegenerative disorders and normal ageing). I will use this evidence to examine the relationship between these unidimensional neural properties and conscious experience when conceived of as a multidimensional phenomenon.
This comparative, translational approach, as well as allowing us to evaluate the behaviour of unidimensional neural measures in states where much more is known about the subjective quality of consciousness, also allows us to advance our understanding of these measures despite lacking a ground truth with which to directly validate them. Although studying evidence of the behaviour of these measures in other conscious states is limited in that it cannot provide direct evidence about their relationship to consciousness in DoC, this analysis will highlight uncertainties and knowledge gaps about these neural measures that may raise important practical and ethical questions surrounding their use in DoC assessment.
Consciousness as a multidimensional phenomenon
Consciousness is often thought to be made up of two interrelated dimensions: arousal and awareness (Laureys 2005). Arousal, or wakefulness, is often thought of as the extent to which an agent is responsive to the external environment and is physiologically regulated by the reticular activating system in the brainstem and basal forebrain. Awareness, sometimes referred to as the content of conscious experience, is governed by cortical mechanisms and interactions. It is possible for awareness to become dissociated from arousal, producing states like rapid eye movement (REM) dreaming and ketamine anaesthesia, where an individual has a sensorily rich conscious experience despite lacking wakefulness and responsiveness to the external environment.
Although the distinction between arousal and awareness is helpful in the context of DoC, where sophisticated forms of conscious experience may be present in individuals with limited behavioural responsiveness, it seems that there is more than one dimension along which the contents of consciousness can vary. It has recently been proposed that consciousness can be more accurately characterized by a multidimensional model that accounts for a wider range of factors that may structurally modulate the subjective quality of conscious experience (Bayne et al. 2016). Even if some conscious states, and some different DoC patients and diagnostic categories, can be intuitively ordered according to conscious level, the multidimensional model suggests that we may be missing clinically and ethically relevant forms of variance to consciousness in these patients if we primarily focus on evaluating consciousness along a singular or a small number of dimensions.
There is not yet an accepted taxonomy of the dimensions that are optimal for describing human consciousness. It is likely that we do not currently know enough about consciousness to establish such a taxonomy, which may also depend on which conscious state is being characterized. Bayne et al. (2016) have suggested that content-related and functional dimensions may be two broad families of dimensions that describe consciousness (Table 1). Conscious states may differ from each other in terms of which kinds of contents are present in conscious awareness—e.g. the extent to which there is gating of sensory stimuli, and awareness of higher-order objects and semantic representation versus merely lower-level perceptual features. Conscious states may also differ in terms of how and the extent to which different contents of consciousness are available to the organism for different functional purposes. For example, they may vary depending on which and whether certain contents are encoded in working and short-term memory and available for reasoning and other executive and cognitive functions and whether the organism has attentional agency and meta-awareness. As Bayne et al. (2016) point out, we can conceive of consciousness coming apart along a range of these dimensions in conscious patients with DoC, and due to the heterogeneity of these conditions, conscious experience may vary considerably between individuals with DoC. However, if this is the case, it is unclear which dimensions of consciousness an increase or decrease in our proposed unidimensional neural measures will reflect, and deepening our understanding of this relationship may have important implications for the use of these measures in diagnostic assessments for consciousness in DoC.
Table 1.
Possible dimensions of consciousness. One possible taxonomy of a multidimensional model of conscious experience, divided into two families of dimensions (content-related and functional; Bayne et al. 2016)
| Possible dimensions of consciousness | |
|---|---|
| Content-related dimensions | Sensory richness |
| High-order object representation | |
| Semantic comprehension | |
| Functional dimensions | Executive functioning |
| Memory consolidation | |
| Intentional agency | |
| Reasoning | |
| Attentional control | |
| Vigilance | |
| Meta-awareness | |
How is consciousness assessed in DoC patients?
Standard forms of DoC assessment typically focus on establishing whether a patient retains the capacity for consciousness. Patients thought to be unconscious based on clinical evaluation are diagnosed as being in a VS (Jennett and Plum 1972), while those found to retain the capacity for consciousness are diagnosed as being in a MCS (Giacino et al. 2002). The dominant method for evaluating the presence of consciousness is bedside behavioural testing using instruments such as the Coma Recovery Scale-Revised (CRS-R; Giacino et al. 2004). These instruments consider overt behaviours including reproducible responses to command, visual fixation and functional object use. VS patients may display periods of eye opening and wakefulness, as well as reflexive responses to stimuli, but no signs of intentional behaviours (Jennett and Plum 1972). MCS patients intermittently display these intentional behaviours inconsistently but clearly discernibly (Giacino et al. 2002).
Although behavioural assessments can sometimes provide strong evidence for the presence of consciousness, they have been shown to misdiagnose up to 40% of conscious patients as unconscious (Schnakers et al. 2009; Kondziella et al. 2016). The motor behaviours measured by the CRS-R can be absent even when consciousness is present, a phenomenon labelled cognitive motor dissociation (CMD; Schiff 2015). It has been shown that some patients initially diagnosed as VS and thought to be completely unconscious are capable of displaying intentional mental behaviours that are detectable with functional magentic resonance imaging (fMRI), such as wilfully modulating their mental activity in response to command (Owen et al. 2006; Coleman et al. 2009b; Monti et al. 2010; Naci and Owen 2013). These active paradigms requiring volitional agency allow for the detection of consciousness in a further subset of DoC patients with CMD and often for a range of other information about consciousness and mental functioning to be inferred in the patient. However, as with active behavioural tests, the fMRI mental imagery test and other neural task-based assessment paradigms for DoC do not provide evidence for the absence of consciousness and risk missing conscious DoC patients.
To address the limitations of active paradigms, there have been efforts to develop task-free neural measures of consciousness. Some proposed measures evaluate properties in resting-state neural data thought to be related to conscious ‘level’, in theory irrespective of the presence of any particular cognitive capacities (Casali et al. 2013). Other measures aim to elicit event-related potentials (ERPs) in response to certain sensory stimuli that may indicate conscious processing; some of these do not require the individual to intentionally attend to the stimulus (Bekinschtein et al. 2009).
As we have no ground truth about consciousness in unresponsive DoC patients with which to directly validate these measures, I propose to examine evidence from studies where these measures were applied in other conscious states, which will allow for an initial, comparative approach to understanding their relationship with conscious experience in a multidimensional sense. I will discuss this evidence and use it to explore the utility of these neural measures in clinically evaluating individuals with DoC when consciousness is conceived of as a multidimensional property.
The remainder of this article will examine evidence for the relationship between conscious experience and the following three electroencephalogram (EEG)-based neural measures, drawn from a range of studies outside of DoC science: measures of resting-state neural complexity (Wu et al. 2011), the perturbational complexity index (PCI), a transcranial magnetic stimulation (TMS)–EEG-based complexity measure (Casali et al. 2013) and the local global paradigm (LGP; Bekinschtein et al. 2009), an ERP-based EEG measure. Many EEG-based measures in particular tend to be informed by the idea of conscious level. As fMRI has higher spatial resolution than EEG, fMRI-based measures tend to be applied less frequently than EEG as measures of global conscious level, although would perhaps lend themselves to differentiating more precise dimensions of conscious experience that are associated with particular spatial regions in the brain.
There are some key limitations to the approach I am taking. Firstly, for resting-state complexity measures, it relies largely on qualitative comparison between studies (in terms of how these measures vary relative to a baseline level), as a direct quantitative comparison is often not possible due to methodological differences between studies. Furthermore, this discussion is largely restricted to results that are comparable in terms of unimodal levels and does not consider the varied spatial patterns these measures may display across the brain. These spatial patterns, as has been noted elsewhere, may be an important target for further investigation of the relationship between brain activity and phenomenological dimensions of consciousness (Boly et al. 2017; Andrillon et al. 2021), but are beyond the scope of this article.
Resting-state neural complexity and dimensions of consciousness
Resting-state complexity in healthy adults
Measures of signal complexity have been widely applied to human brain activity in a range of conscious states and clinical conditions (Table 2) and to data from a range of neuroimaging modalities including EEG, fMRI and magnetoencephalography (MEG). Complexity has no precise definition and a range of methods are thought to capture dimensions of the complexity of a signal, including Lempel–Ziv complexity (Lempel and Ziv 1976) permutation entropy (Liang et al. 2015), multiscale entropy (Li et al. 2010) and fractal dimension (Varley et al. 2020b). While there are differences between methods for quantifying complexity, I will consider them together for the purpose of this article. There is some evidence suggesting that different complexity measures are convergent for neural data (Varley et al. 2020c). Overall, resting-state complexity is typically found to be lower during states where consciousness is thought to be largely absent, including non-rapid eye movement (NREM) sleep and anaesthesia, and is lower on an average for patients behaviourally diagnosed as VS than those with an MCS diagnosis (Gosseries et al. 2011; Wu et al. 2011; Sitt et al. 2014; Thul et al. 2016; Engemann et al. 2018; Varley et al. 2020b).
Table 2.
Resting-state complexity in healthy individuals and clinical populations. Neural complexity in a range of conscious states related to both long- and short-term brain changes. For states in the left and right columns, studies typically draw comparisons against healthy wakeful controls without further discrimination between different standard wake states, although complexity can in fact vary for these as well (middle column). Due to methodological differences between studies, it is not yet possible to draw quantitative comparisons between the states and conditions within each column
| Resting-state complexity | ||||
|---|---|---|---|---|
| Lower complexity ← | ← Normal waking → complexity |
→ Higher complexity | ||
| Vegetative state | Minimally conscious state | Task focus | Mind wandering | Psychedelics |
| NREM sleep | REM sleep | Meditation | Viewing movie | Ketamine |
| Anaesthesia | Viewing visual noise | Depressiona | ||
| Traumatic brain injury (non-DoC) | Hearing speech = hearing random noise | Schizophreniaa | ||
| Dementia ADHD Autism Anorexia nervosa |
Peak at age 60 years | |||
Mixed results have been obtained for schizophrenia and depression, but in the majority of studies, resting-state complexity has been found to be higher in patients than in healthy controls.
There have been several theoretical links drawn between neural complexity and consciousness. Based on the arousal-awareness model, it has been suggested that complexity corresponds more to the awareness dimension and could be thought to index some element of conscious contents, whereas other unidimensional measures like spectral power may be more closely linked to arousal (Schartner et al. 2017b; Scott and Carhart-Harris 2019). One further suggestion is that measures of resting-state neural complexity, which may often indicate the diversity and variability of possible neuronal configurations in a given brain state, may be linked to the possible repertoire of conscious contents (Tagliazucchi et al. 2014; Schartner et al. 2017b; Schwartzman et al. 2019). Kolmogorov theory also posits that the complexity and compressibility of brain data are related to conscious level and the extent to which conscious experience is structured and that states with higher conscious levels are characterized by the brain’s ability to form more complex, less compressible models of the world (Ruffini 2017). These suggestions are speculative but seem to posit a link between neural complexity and dimensions of consciousness related to sensory richness, the presence of high-level perceptual content and the variability of mental content.
In this section, I will examine the characteristics of a range of states within healthy individuals where complexity has been found to be either higher or lower than the restful waking state often used in a baseline in such studies (summarized in Table 2), including the extent to which certain key dimensions of consciousness such as sensory richness, attentional control, arousal, executive function, memory and meta-awareness may be affected.
Sleep
Human sleep can be differentiated into two physiologically distinct components: REM sleep and NREM sleep (Loomis et al. 1935). REM sleep is associated with low muscle tone, wake-like EEG activity and REMs (Matarazzo et al. 2011). NREM sleep, which is commonly divided into three substages, is characterized by the deactivation of many brain areas relative to wake and REM, synchronized EEG activity and slow waves and higher muscle tone than REM sleep.
REM sleep has been associated with levels of resting-state complexity that are similar to the early sleep onset period but higher than other stages of NREM sleep (Pereda et al. 1998; Schartner et al. 2017a). Conscious experience is reported for the majority of REM awakenings (Nielsen 2000; Siclari et al. 2013). REM dreams tend to be immersive and bizarre with a complex narrative and perceptual structure that is mainly instantiated in audio-visual modalities and often involves emotions and movement sensations (Carr and Solomonova 2019). REM dreaming is also reported to be characterized by a relatively high level of sensory richness, especially high levels of visual detail (Fazekas et al. 2019). REM dreaming has been described as a form of spontaneous thought that involves the weakening of a range of dimensions that can serve to constrain the contents and flow of conscious experience over time (Christoff et al. 2016; Girn et al. 2020). This includes a reduction in various dimensions of consciousness that modulate attention and the flow of thought, including ‘deliberate’ constraints on thought (those under cognitive control, such as executive function, volition and typically meta-awareness), as well as ‘automatic’ constraints on thought (those operating outside of cognitive control, such as mechanisms of affective and sensory salience; Christoff et al. 2016).
NREM sleep, especially deep slow-wave sleep, is associated with substantially lower levels of resting-state complexity compared to both wake and REM sleep (Pereda et al. 1998; Andrillon et al. 2016; Schartner et al. 2017a). In studies of brain complexity, NREM is sometimes used as a proxy for unconsciousness or highly diminished conscious level (for discussion and critique of this view, see Windt et al. 2016). Although NREM sleep involves a significant decrease in cortical excitability, it is now widely accepted that conscious experience can occur in any stage of NREM sleep, although waking reports indicate that it occurs less frequently in NREM sleep than in REM sleep (Nielsen 2000; Noreika et al. 2009; Siclari et al. 2013; Windt 2020). There is also evidence that a broad range of cognitive processes are conserved in NREM sleep, including the processing of auditory, visual or somatosensory stimuli and the preparation of motor responses (Andrillon and Kouider 2020). Reports upon awakening indicate that NREM sleep experiences, when compared to REM dreams, have lower sensory richness and are more likely to be described as feeling like thoughts rather than perceptual experiences (Nielsen 2000; Noreika et al. 2009; Siclari et al. 2013). They tend to be more fragmented and mundane in content and have a shorter subjective duration (Carr and Solomonova 2019). When awoken during NREM sleep, it is also common to report no experience or to report having had a conscious experience without being able to remember any specific content (white dream), which could be a result of reduced or absent perceptual imagery and thought contents (Windt 2015; Windt et al. 2016; Fazekas et al. 2019) and suggests that memory loss may be a confounding factor for lower recall rates during NREM sleep. As complexity has been studied over extended periods of NREM sleep without awakening subjects to collect subjective reports, it is possible that intermittently occurring periods of consciousness are associated with higher complexity than non-conscious periods of NREM sleep.
Anaesthesia
Anaesthesia is another state associated with low levels of neural complexity (Ferenets et al. 2006, 2007; Li et al. 2010; Liang et al. 2015; Schartner et al. 2015; Varley et al. 2020c; Zhang et al. 2001). Like deep NREM sleep, general anaesthesia is often used as a proxy for unconsciousness in studies attempting to validate neural measures of consciousness. Yet consciousness may in fact occur in up to 31% of patients even at surgical levels of anaesthetic dosage (Linassi et al. 2018), a phenomenon called anaesthesia awareness (AA). Detailed subjective reports about AA are typically delayed and affected by the amnesic effects of hypnotic drugs. The subjective quality of consciousness in AA seems to vary based on anaesthetic agent and dosage (Noreika et al. 2011). Like NREM sleep, it is typically perceptually decoupled from external stimuli, fragmented, simple, and mundane in content (Noreika et al. 2011; Radek et al. 2018; Sleigh et al. 2018). However, there is some evidence that aspects of executive functioning (Mashour et al. 2021) and meta-awareness (Sleigh et al. 2018) are more resistant to anaesthesia than they are to natural sleep (Goupil and Bekinschtein 2012). Some of these cognitive or executive functions may persist to a greater extent at low levels of cortical brain complexity during AA. However, we know little about which dimensions of consciousness are most prominent in AA because both impaired memory and false recall may impact subjective reports (Pryor and Root 2013) and, although the presence and subjective quality of conscious experience may fluctuate significantly during anaesthesia, we are largely reliant on delayed reports.
Attentional shifts
There has also been some investigation of neural complexity during different wake states with varying attentional constraints. A ubiquitous form of attentional shift in the general population is that from task-focused or externally engaged attention to task-unrelated thought or internally directed attention, often termed ‘mind wandering’ (Seli et al. 2016). Mind wandering is estimated to comprise 30–50% of waking consciousness (Smallwood and Schooler 2015), and the periods of restful wakefulness where mind wandering tends to occur more frequently have been associated with higher complexity than task-focused states in several studies (Aftanas and Golocheikine 2002; Li et al. 2008; Escrichs et al. 2019; Iglesias-Parro et al. 2020). Like dreaming, it has been described as a form of spontaneous thought that is subject to weakened mechanisms of attentional and executive control, perhaps allowing for more frequent transitions between a wider and more associative range of conscious contents (Christoff et al. 2016; Girn et al. 2020). Experience sampling studies have shown that mind wandering is most often described as being thought-like and stimulus independent, or having content that is unrelated to the immediate environment (Laurens et al. 2017). A smaller proportion of mind wandering experiences were described as perceptual experiences, involving a range of different sensory modalities (Laurens et al. 2017).
Other forms of waking consciousness, like focused attentional states, have stronger deliberate and/or automatic constraints and often involve high executive functioning, attentional control and meta-awareness (Windt 2020). Complexity has been found to be lower during tasks demanding a strong attentional focus, such as arithmetic tasks (Li et al. 2008) and meditation [Sahaja Yoga (Aftanas and Golocheikine 2002) and anapanasati (Escrichs et al. 2019)], compared to during periods of restful wakefulness. Reports are rarely taken during resting periods in these task-based studies, so the precise quality of conscious experience captured at rest is unclear and may fluctuate (Callard and Margulies 2014). Yet, due to limited demands on attention, these rest periods are likely to involve a higher frequency of mind wandering.
Psychedelics and ketamine
During psychedelic experiences, resting-state complexity increases relative to normal wakefulness (Schartner et al. 2017b; Timmermann et al. 2019; Varley et al. 2020a). Although psychedelic experiences can vary qualitatively based on dosage and drug type (Fortier-Davy and Millière 2020), conscious experience under psychoactive doses of lysergic acid diethylamide and psilocybin is significantly altered compared to normal wake and is commonly reported to involve increased sensory (especially visual) vividness, increased richness of experience and of autobiographical memory, hyper-associative thinking and changes in sense of self and meaning attribution (Bayne and Carter 2018; Girn et al. 2020). Several complexity measures have also been found to increase for ketamine-induced states compared to normal waking consciousness (Schartner et al. 2017b; Farnes et al. 2019). Ketamine is a drug used as a dissociative anaesthetic that produces a disconnected but highly vivid hallucinatory state with many similarities to a psychedelic state, although they are not phenomenologically or physiologically identical (Schartner et al. 2017b). Similarities between psychedelic and ketamine states and REM dreaming have long been recognized, but greater meta-awareness is present in psychedelic states (Girn et al. 2020).
Psychedelics also seem to impair many functional and cognitive dimensions of consciousness (Bayne and Carter 2018), although not all dimensions (Fortier-Davy and Raphaël 2020). Although declarative and working memory seem to remain unimpaired (Silverstein and Klee 1958), psychedelics lead to impairments on tasks involving mental manipulation or attentional control and are commonly reported to reduce capacity for concentration (Jackson et al. 1952), sustained attentional focus (Quednow et al. 2012) and speech production (Jackson et al. 1952). Psychedelic states are also reported to lead to greater feelings of insight and creativity, which may be the result of increased capacity for more remote and wide-ranging associations. Overall, the capacity for abstract and evaluative thought, including the ability to identify which thoughts are truly insightful, appear to be diminished (Bayne and Carter 2018).
There has also been some exploration of the relationship between complexity measures and the subjectively rated intensity of phenomenological dimensions during psychedelic experiences. (Schartner et al. (2017a) conducted a questionnaire after the peak effects of the drug had subsided, which revealed that aggregate self-reported scores for the subjective intensity of the psychedelic experience across all phenomenological dimensions (including ego dissolution, muddled thinking, seeing geometric patterns, unusual imagery, distorted time and space perception, vivid imagination, a sense of inner peace and spiritual experience) were correlated with a range of complexity measures applied to MEG data collected during the peak of the psychedelic experience. This correlation was strongest for ketamine-induced states, where the dimensions the most highly correlated with high brain complexity were ego dissolution and a vivid imagination. Although these results rely on the use of subjective scales about conscious experience, for which individual participants may have very different yardsticks, they suggest that the subjective intensity of many of the phenomenological dimensions associated with psychedelic experiences may have a quantitative relationship with neural complexity (Schartner et al. 2017b).
Stimulus information content
The relationship between brain complexity and the meaningfulness of a perceived stimulus has also been studied in fMRI (Boly et al. 2015). When healthy participants were shown a movie, complexity was higher than when they were shown a scrambled version of a movie or TV noise. In the TV noise and scrambled movie conditions, each frame essentially carried the same informational content in terms of its high-level meaning to the participants, so the overall informational content was low. The condition involving a coherent short movie, on the other hand, was experienced as containing a greater amount of meaningful, high-level content. This was despite the fact that in all three conditions (movie, TV noise and scrambled movie), each stimulus was equally as differentiated in terms of low-level perceptual content. A similar study conducted in the auditory domain in EEG data, using normal speech at two different speeds and backwards speech, did not find a difference between these conditions (Bola et al. 2018), although the difference in meaningful information content between these two conditions, which was assumed to be higher for the coherent speech than for the incomprehensible backwards speech, may not be sufficient to differentiate them in neural data.
Dimensions of consciousness and neural complexity in healthy adults
Measures of resting-state neural complexity are probably sensitive to multiple dimensions of consciousness to varying degrees and are likely to display different spatial patterns during different conscious states. However, this initial analysis, which primarily involves considering how complexity behaves at the whole-brain level and reflects its proposed use as a unidimensional measure of conscious ‘level’, can nonetheless highlight potential hypotheses about their relationship with specific features of consciousness.
Multiple states that result in relatively high levels of resting-state complexity involve reduced constraints on mental activity (both deliberate and automatic). These constraints can be thought to be related to a range of possible dimensions governing attention that operate both inside and outside of an agent’s cognitive control. This includes REM sleep relative to other sleep states, as well as psychedelics and ketamine relative to the normal range of wake states (Girn et al. 2020). States where greater attentional resources are engaged or deployed, such as certain kinds of meditation [Sahaja yoga (Aftanas and Golocheikine 2002) and anapanasati (Escrichs et al. 2019)] and arithmetic tasks (Li et al. 2008), involve reduced complexity. It is possible that the weakening of constraints on the flow and content of thoughts allows a richer, more diverse, dynamic and associative repertoire of conscious contents to emerge, as is reported to various extents during REM dreams, mind wandering, and psychedelic and ketamine states. The weakening of attentional and cognitive constraints and increase in sensory richness and variability of contents in these states also seems to be accompanied by a range of functional dimensions being diminished to various degrees, such that less attentional, executive and cognitive control is available to the agent. Furthermore, these high-complexity states, despite rich contents and increased cortical activation, do not seem to be optimal for vigilance and responsiveness to one’s surroundings (Mittner et al. 2016) and for engaging in a range of goal-directed behaviours that may require greater executive and cognitive functioning.
Resting-state complexity also seems to be comparatively higher in states with greater sensory richness, such as psychedelics and ketamine states, and may even have a quantitative relationship with perceived perceptual intensity and vividness (Schartner et al. 2017b). Sensory gating, the process by which irrelevant or redundant stimuli are normally filtered from conscious awareness, has been shown to be reduced in psychedelic states (Vollenweider and Geyer 2001; Vollenweider et al. 2007), perhaps resulting in greater sensory richness due to more stimuli being concurrently perceived. Relative to other sleep states, increases in resting-state complexity also occur during REM sleep, a state characterized by rich and integrated sensory experiences but high levels of sensory gating (McCormick and Bal 1994). Based on this evidence, a working hypothesis may be that measures of resting-state complexity are sensitive to sensorily rich and variable content repertoires regardless of whether this is caused by external inputs or endogenous activations. It may be the case, as has been theorized (Scott and Carhart-Harris 2019), that complexity measures are less sensitive to dimensions of consciousness that affect vigilance to external stimuli and more sensitive to those that modulate the contents of awareness. This feature is helpful in the context of DoCs where perceptually decoupled experience could arise, involving rich conscious experience despite reduced responsiveness in active paradigms (Bayne et al. 2020; Martial et al. 2020).
It is currently more challenging to understand which dimensions of consciousness are present in low complexity states like AA and NREM sleep. The quality and dynamics of consciousness in these states could have important parallels with DoC, where complexity is also lower than in healthy waking controls. There is evidence that a range of qualitatively different states may be possible at low levels of complexity. For example, conscious dimensions like executive function and meta-awareness may in fact be amongst the least impaired during AA. However, a challenging confound for both sleep and anaesthesia experiences, due to our reliance on delayed reports, is the possibility of either impaired or false recall of the occurrence of conscious experience or its subjective quality (Pryor and Root 2013).
Many studies investigating complexity and consciousness have significant methodological limitations in that they do not take reports from subjects while measuring their brain activity. Instead, they rely on assumptions about the general characteristics or the conscious level of the states in question to judge the efficacy of a particular neural measure, even in states that are very heterogeneous or where little is known about consciousness. Because of this limitation, much of the present discussion has involved considering evidence that stems from independent lines of research investigating resting-state complexity and phenomenology. Detailed subjective reports that go beyond probing the conscious–unconscious binary in participants, by using either free reports or more targeted questionnaires, will need to be collected alongside neural data to evaluate the relationship between resting-state complexity and consciousness on a more fine-grained level, as has been done for some studies conducted in healthy, awake volunteers (and even during sleep). This level of precision will be particularly important in low complexity states like anaesthesia and NREM sleep where the presence of consciousness may be more intermittent, and low levels of complexity could be the result of the relative infrequency of higher-complexity conscious periods within the data.
Do changes in resting-state complexity always reflect changes in consciousness?
The neurophysiological processes underlying changes in neural signal complexity remain unclear. Although proposed as potential indexes of consciousness, this family of measures may be sensitive to both conscious and non-conscious forms of neurophysiological processing, both of which occur simultaneously in the brain during conscious experience. Resting-state brain complexity also appears to be impacted by demographic factors and clinical conditions. An individual’s baseline level of complexity during task-free wakefulness can vary based on their age, sex and certain medications and due to a range of long-term conditions impacting the brain, which in addition to acquired brain injury include psychiatric, neurodevelopmental and neurodegenerative disorders. It is not clear to which extent (if at all) these factors are directly associated with fundamental differences in conscious experience itself, but further exploration of this question will be important for evaluating the kind of reliable diagnostic information about consciousness these measures could provide for individuals with DoC.
Effect of age and sex on complexity
There is evidence that age has a significant effect on resting-state brain complexity. Complexity has been found to increase until mid-adulthood, peaking between ages 41 and 60 years, before gradually starting to decline (Anokhin et al. 1996; Yang et al. 2013a; Shumbayawonda et al. 2018), which suggests that even healthy ageing has a substantial fingerprint on individual resting-state complexity. This change in background electrophysiology across the lifespan has been suggested to reflect underlying processes of brain maturation, synaptic pruning, changes in axonal volumes and hormonal changes (Shumbayawonda et al. 2018).
Biological sex has also been found to have some effect on resting-state complexity levels. The effects have been mixed depending on the modality and complexity measure used: EEG fractal dimension was found to be higher on average in women (Ahmadlou et al. 2012), whereas permutation Lempel–Ziv complexity of MEG activity was found to be slightly higher in men (although whole-brain values for men and women overlapped considerably; Shumbayawonda et al. 2018). The existence of a sex fingerprint in resting-state complexity may be attributable to a range of factors (such as white matter and grey matter changes between the sexes, the effects of hormones and other unknown or environmental factors; Shumbayawonda et al. 2018) and may merit further investigation as a potential confounding factor for complexity measures as signatures of consciousness in DoC.
Complexity in neurological and psychiatric conditions
Although some factors impacting baseline complexity levels during wakefulness, such as those related to sex and healthy ageing, may not be directly associated with changes to conscious experience, some neurological and psychiatric conditions are associated with a decrease in baseline levels of resting-state complexity that may reflect significant and direct changes to the individual’s conscious experience in addition to the underlying changes occurring on a neurobiological level as a result of the disorder. For example, the majority of studies have found decreased neural complexity in those with dementia caused by Alzheimer’s disease (AD; Jeong 2004; Dauwels et al. 2010; Yang et al. 2013b; Liu et al. 2016), although increases have also been reported in some studies investigating preclinical AD (Gaubert et al. 2019). AD is associated with a range of pathological changes in the brain, including amyloid plaque and neurofibrillary tangles, engendering changes in neural connectivity (Braak and Braak 1991; Raskin et al. 2015). It also involves a range of progressive impairments to memory, visuospatial function, language, attention, problem-solving, meta-awareness, mood and personality (Salloway and Correia 2009). There is also some evidence that a loss of neural complexity is associated with the extent of cognitive impairment in AD, with complexity being inversely correlated with scores in neuropsychological tests (Yagyu et al. 1997). However, measures of resting-state complexity are normally studied as a signature that is informative relative to a reference point (e.g. healthy controls) rather than as an absolute measure that can facilitate cross-disorder comparisons. Therefore, even if a linear relationship with some aspects of phenomenology exists for this population, we cannot assume that it will translate to other populations, including DoC, where we may encounter different absolute ranges of these complexity values for which the same linear relationships may not apply.
Furthermore, even in dementia, impairments present in a heterogeneous way and are often difficult to evaluate where communication is impaired, so we cannot assume uniform impacts on every dimension of consciousness. For example, high levels of meta-awareness are sometimes reported, if apparently intermittently, even at late stages of disease progression (O’shaughnessy et al. 2020). The abnormal whole-brain complexity values observed in a range of other neurological and psychiatric disorders also suggests that the relationship between complexity, neuropathology and cognitive impairment is complex and many-to-one. Both increases (Li et al. 2008; Ahmadlou et al. 2012; Méndez et al. 2012) and decreases (Nandrino et al. 1994) in complexity have been observed for depression, which has been discussed as involving fundamental shifts in conscious experience (Whiteley 2021) and schizophrenia [increase: Li et al. 2008; Takahashi et al. 2010; Fernández et al. 2011; Ibáñez-Molina et al. 2018; Xiang et al. 2019; decrease: Lee et al. 2001; Yang et al. 2015). This suggests that some forms of psychopathology may be associated with abnormally high complexity. There are several possible reasons for the inconsistent results obtained for these conditions, including the use of different complexity measures and methodological differences between studies. Furthermore, schizophrenia can have a range of both positive symptoms (hallucinations, paranoia and delusions) and negative symptoms (cognitive impairment, flattened affect and disorganized thinking) that can present very differently between individuals and in different phases of the disorder. Other conditions that could be thought to involve abnormalities on the level of conscious experience like autism (Lai et al. 2010; Catarino et al. 2011) and attention deficit hyperactivity disorder (ADHD; Sokunbi et al. 2013) have been more consistently found to be associated with abnormally low levels of complexity. The translatability of results observed in different studies remains unclear, but it is possible that quantitatively equivalent decreases in complexity can be associated with a range of qualitatively different neurological and psychiatric impairments affecting consciousness and cognition and that they sometimes do not reflect changes in consciousness at all. As such it is likely that we will face challenges in drawing inferences about specific dimensions of consciousness, or general inferences about the extent of cognitive impairment, based on whole-brain complexity measures alone.
While some clinical conditions that affect baseline complexity, like dementia, clearly also have profound impacts on consciousness, it is possible that resting-state complexity levels are also affected in some conditions where a fundamental shift in conscious experience does not appear to be amongst the core symptoms. For example, individuals with anorexia nervosa (AN) have lower resting-state complexity than healthy controls (Tóth et al. 2004; Collantoni et al. 2020), but changes to conscious experience do not seem to be directly implicated in the psychopathology of AN. Lowered complexity may be due rather to the fact that affected individuals have neurobiological damage caused by starvation and prolonged malnutrition (Collantoni et al. 2020). A range of neurobiological changes unrelated to the neural mechanisms of consciousness may also impact values of brain complexity in other disorders affecting the brain, including DoC, where severe brain injury is present. The impact of such neurobiological factors may pose challenges for operationalizing resting-state complexity as a reliable measure of consciousness and in determining the extent to which inferences about their relationship with phenomenology can be translated across clinical populations.
Perturbational complexity index
The following section will review a range of evidence for the PCI (Casali et al. 2013; Table 3), another proposed task-free, unidimensional neural measure of consciousness. PCI is a more recently developed measure that involves locally applying TMS to the brain and then calculating the complexity of the resulting EEG signal. PCI was inspired by theoretical approaches in consciousness science postulating that the emergence of consciousness requires an optimal balance of integration and differentiation in functional neuronal circuitry (Tononi and Edelman 1998; Tononi 2004; Seth et al. 2006). PCI is a method designed to capture these two properties in a single unidimensional index: the extent to which the TMS pulse propagates in the brain is a function of the level of integration of widespread neuronal populations and the complexity of the resulting EEG activity reflects the extent to which this neural response is differentiated (Casali et al. 2013). As such it has been suggested that PCI may be a more sensitive quantitative measure of the fundamental brain activity linked to consciousness than resting-state complexity (Casarotto et al. 2016). PCI has been less widely applied than measures of resting-state complexity (TMS equipment is more costly and less widely available than EEG in clinical settings), so less evidence is available. Yet this brief discussion of the available PCI results from a range of states will serve as a useful comparison point, particularly for highlighting how different passive neural measures of consciousness may correlate with different features of consciousness and reflect different underlying neural mechanisms.
Table 3.
PCI values for a range of conscious states (Casali et al. 2013; Sarasso et al. 2015; Casarotto et al. 2016). PCI value of 0.31 has been proposed as an empirically supported threshold for distinguishing between conscious and unconscious individuals with DoC
| State | PCI |
|---|---|
| Healthy awake volunteers | 0.44–0.67 |
| Minimally conscious state | 0.37–0.52 |
| Vegetative state | 0.19–0.31 |
| Deep anaesthesia (propofol, xenon and midazolam) | 0.08–0.31 |
| REM sleep | 0.35–0.56 |
| NREM sleep | 0.12–0.31 |
| Ketamine | 0.35–0.55 |
PCI has been designed as a measure that is quantitatively comparable across individuals and conscious states. A PCI value of 0.31 has been proposed as an approximate threshold for distinguishing conscious and unconscious individuals, based on empirical data collected in both DoC and sleeping and anaesthetized healthy volunteers. In healthy, awake volunteers, PCI values have been found to range from 0.44 to 0.67 (Casali et al. 2013).
During NREM sleep, a deeper state of sleep where conscious experience is thought to be less frequent and vivid, PCI values range from 0.12 to 0.31, whereas in REM sleep, where vivid conscious experience occurs more frequently, PCI values were within the range of healthy awake individuals (0.35–0.56). Across several studies examining anaesthetized individuals who were deeply sedated and had lost responsiveness, PCI values were also significantly reduced (between 0.008 and 0.31; Casali et al. 2013; Sarasso et al. 2015). However, during ketamine anaesthesia, which unlike other anaesthetic agents like xenon and propofol does not aim to eliminate consciousness but rather produces a disconnected but vivid conscious state, PCI values were comparable to those obtained for healthy, awake volunteers (0.35–0.55). This is a key point of contrast so far between PCI and resting-state measures of EEG complexity, where ketamine has been found to lead to significantly higher values than during normal waking consciousness.
This point of difference may indicate that PCI, as theorized, reflects different underlying neural mechanisms to resting-state complexity, and so may have a different relationship with the mechanisms underlying consciousness. A possible interpretation is that very ‘high entropy’ states like those induced by ketamine and psychedelic drugs involve high neural complexity in terms of maximal randomness or unpredictability, but not necessarily high levels of causal integration. Because PCI is also sensitive to cortical integration, ketamine states, which like psychedelics involve significant impairments to cognitive and executive functioning, do not cause PCI to increase relative to normal wakefulness. Although the relationship between PCI and conscious states is many-to-one, high PCI values may have a stronger association with states characterized by normal levels of cognitive and executive functioning compared to resting-state complexity.
For DoC, PCI has been found to have very high sensitivity and specificity for discriminating behaviourally diagnosed MCS and VS patients at the individual level (over 94% accuracy; Casali et al. 2013; Casarotto et al. 2016), much higher than reported for measures of resting-state complexity. However, the validity of these results remains unclear because we cannot assume that the VS patients in this study were unconscious just because they were behaviourally unresponsive. VS patients had PCI values ranging from 0.19 to 0.31, within the range of healthy subjects during deep anaesthesia and NREM sleep (Casali et al. 2013). MCS patients had PCI values ranging from 0.32 to 0.49, above the values observed in NREM sleep and deep sedation in anaesthesia. PCI values very close to the 0.31 threshold were reported for some patients in both groups.
There has not been any explicit discussion so far about the extent to which factors such as age, sex, medication use, brain damage and other neuropsychiatric conditions may affect PCI, although such factors will be important targets for investigation if PCI is to be operationalized as a reliable, disorder-agnostic tool in the context of DoC assessment.
Local global paradigm
ERPs are another potentially useful way of detecting consciousness in the brain (Kotchoubey 2005). They represent a different but important approach to consciousness assessment that focuses on detecting specific brain functions that may require consciousness. For example, the conscious detection of an oddball tone in a sequence of auditory stimuli is known to be associated with several EEG events. Characteristic neural responses to tone changes that are local in time, mismatch negativity (MMN; Ulanovsky et al. 2003) and P3a (Squires et al. 1975), are thought to be automatic responses associated with preattentive and preconscious processes. The later P3b ERP component (Squires et al. 1975) has been associated with the conscious processes involved in the detection of a global pattern violation occurring over several seconds, although whether this response is in fact a signature of conscious processes in the brain remains a point of contention (Koch et al. 2016; Silverstein et al. 2015; Naccache et al. 2016).
Here, I will discuss the data available for the LGP, a method closely related to the oddball paradigm that separates analysis based on the sensory events expected to produce either a local or a global effect response rather than looking for specific MMN, P3a and P3b ERP components, which can be difficult to disentangle. This paradigm can be applied both passively and actively, either by presenting stimuli with no instruction or by asking participants to pay attention to the sounds. Eliciting a neural response to global pattern violations seems to require conscious attention, although experiments can be designed such that attention can be exogenously attracted, e.g. by using highly engaging audiovisual stimuli, as was done for 3-month-old infants (Kouider et al. 2013).
There have been several studies applying the LGP to DoC patients (Bekinschtein et al. 2009; Faugeras et al. 2011; King et al. 2013). Bekinschtein et al. (2009) found that the global effect was absent in all four VS patients and in one of four MCS patients. Faugeras et al. (2011) found the global response to be present in 2 of the 22 VS patients studied. King et al. (2013) found that 14% of VS patients displayed a significant global effect, compared to 31% of MCS patients and 52% of healthy controls. The global effect has been found to be absent in both NREM and REM sleep (Strauss et al. 2015) and in anaesthesia (Nourski et al. 2018; Table 4). It is typically present in awake, healthy participants where attention is being paid to the stimulus. In macaques, the global effect has also been found to be absent after ketamine anaesthesia (Uhrig et al. 2016). Ketamine and REM sleep both involve vivid conscious experiences that are highly disconnected from the external environment. These findings emphasize that the global effect can be absent where consciousness is present and that we cannot rule out that this is the case in DoC patients where no global effect is detected.
Table 4.
Results of the LGP in a range of conscious states. The LGP, a variant of the auditory oddball paradigm, in a range of conscious states. The presence of the global effect has been thought to indicate the presence of consciousness, whereas the local effect is associated with preconscious, preattentive processing of auditory stimuli
| State | Finding |
|---|---|
| Minimally conscious state | Local effect sometimes present, global effect sometimes present (less frequent than local effect; Bekinschtein et al. 2009) |
| Vegetative state | Local effect sometimes present (less frequent than in MCS), global effect uncommon (less frequent than in MCS; Bekinschtein et al. 2009) |
| General anaesthesia | Local effect present in auditory regions only, global effect absent (Nourski et al. 2018) |
| REM sleep | Local effect present but distorted, global effect absent (Strauss et al. 2015) |
| NREM sleep | Local effect present but distorted, global effect absent (Strauss et al. 2015) |
| Attention on task | Local effect present, global effect amplified (Bekinschtein et al. 2009) |
| Attention off task | Local effect present, global effect diminished (Bekinschtein et al. 2009) |
| Infant (3 months) | Local effect present, global effect present but delayed (Kouider et al. 2013) |
Implications of the multidimensional approach for neural measures of consciousness in DoC
Neural measures of conscious level and the multidimensional framework
An appraisal of neural measures of conscious ‘level’ within a multidimensional framework of consciousness suggests that these measures may be more sensitive to some specific dimensions of consciousness than others. This may mean that such neural measures do not straightforwardly measure conscious ‘level’ or provide a generalizable or hierarchical characterization of the conscious state or extent of residual mental functioning in an individual with a DoC.
There are clear limitations to this comparative and translational approach, one of which is that indirect evidence about neural measures cannot provide a direct ground truth with which to validate these measures. Furthermore, it is unclear to what extent insights based on studies conducted in healthy participants and clinical populations outside of DoC can generalize to the relationship between these measures of consciousness in individuals with DoC, who have severe acquired brain damage. Despite these limitations, the qualitative observations that can be drawn from the comparative evidence considered in this article may provide insight into how the three measures considered (resting-state complexity, PCI and LGP) could fit into a multidimensional framework for clinically assessing consciousness in DoC and highlight potential limitations to their use in this context.
A multidimensional appraisal indicates some ways in which these measures, all of which were proposed as measures that can provide generalizable evidence about the presence of consciousness or conscious ‘level’, can come apart. Figure 1 offers a rough sketch indicating the possible relationship between each of these measures and four of the conscious dimensions discussed in this article. These dimensions have been selected to highlight informative instances of dissociation that we may observe between the three measures, rather than to be broadly representative of the dimensions that it may be important to evaluate in individuals with DoC.
Figure 1.
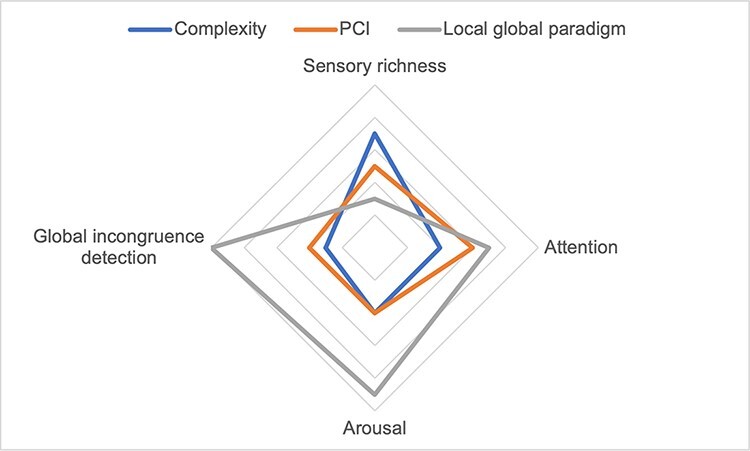
Possible sensitivity of three proposed neural measures of consciousness [resting-state complexity measures (complexity), PCI and the global effect in the LGP] to four dimensions of consciousness (or families of dimensions)
The mechanisms underlying the presence of the global effect in the LGP are relatively well understood, so they can provide clear evidence for some dimensions of neurocognitive processing, such as global incongruence detection (although whether this capacity necessitates consciousness remains a matter of debate). The presence of the global effect also depends on the individual being awake and responsive to the external environment (high arousal) and is more likely to be present when the individual is attending to the stimulus. However, the global effect is absent for sensorily rich internally generated experiences (e.g. REM dreams and ketamine).
As highlighted in Fig. 1, unidimensional measures of resting-state brain complexity seem to have a more general, less precise relationship with the neural mechanisms underlying conscious experience. Evidence suggests that complexity is especially sensitive to states with high levels of sensory richness and a dynamic and variable range of conscious content resulting from minimal attentional constraints (including, to varying extents, psychedelics, ketamine, REM dreaming and mind wandering). Unlike the global effect in the LGP, it appears to be sensitive to sensory richness irrespective of whether the individual displays high levels of external responsiveness or arousal. Because complexity is lower in states with greater constraints on attention, it may also be inversely correlated with many dimensions of cognitive and executive functioning. However, complexity has a more general relationship with the mechanisms underlying a range of conscious dimensions, so it is unlikely to allow for a more fine-grained dissection of precise conscious and neurocognitive functions such as global incongruence detection.
Like measures of resting-state complexity, PCI was also designed as a unidimensional measure of conscious ‘level’, so like measures of resting-state complexity, it seems to be associated with a more general range of neural mechanisms and conscious dimensions. However, evidence indicates that PCI may have a more balanced sensitivity to states with high levels of executive and attentional control and those with high levels of sensory richness but minimal attentional constraints. In this sense, PCI values may not have an inverse relationship with attentional control, as may be the case for resting-state complexity measures. PCI values in the same quantitative range can correspond to—e.g. normal wakefulness, REM dreaming and ketamine—states displaying varying levels and forms of attentional control. Further studies examining PCI in a wider range of conscious states and patient groups may help to clarify the ways in which it may dissociate from measures of resting-state complexity, perhaps as a result of having a closer causal relationship with the neural mechanisms of consciousness in the brain.
Limitations of neural measures of conscious level in DoC diagnosis
Scrutinizing unidimensional neural measures of conscious level in terms of their relationship with a wider range of phenomenological dimensions highlights several potential limitations to their use in the diagnosis of consciousness in individuals with DoC. Firstly, comparative evidence suggests that measures of neural complexity tend to have a higher sensitivity to the presence of certain qualitative forms of conscious experience than others, rather than corresponding to an increase in conscious ‘level’ per se. However, as their relationship with more specific conscious states is broad and appears to be many-to-one, they are unlikely to provide a reliable basis for drawing inferences about the presence of any particular conscious dimension or neurocognitive mechanism (in the way that is possible using ERPs like the LGP).
Another practical limitation to these unidimensional measures of consciousness is that baseline values for an individual may be impacted by demographic and disorder-specific factors. Resting-state complexity varies based on age and possibly also sex, as well as in a range of brain-based disorders including DoC. Furthermore, some of the factors impacting measures of complexity in these cases may not be directly linked to changes in conscious experience (e.g. in AN). This further complicates the process of drawing inferences about consciousness in DoC based on these measures, as many of these factors may need to be accounted for in diagnosis, and the specific forms of brain damage present in DoC may have their own fingerprint for some measures of resting-state complexity.
The extent to which these factors like age, sex, and neurological and psychiatric conditions impact PCI has not been studied, but as a unidimensional measure, it may face similar limitations to resting-state complexity in the context of DoC assessment. However, unlike measures of resting-state complexity, PCI has the advantage of being designed as an absolute index of consciousness, such that PCI values are intended to be comparable between individuals and between different clinical populations (Casarotto et al. 2016). As such, it may eventually be easier to quantify the factors that impact this measure and to operationalize how they should be accounted for in DoC assessment. The fact that PCI is theorized to be mechanistically linked to conscious processing, rather than just detecting side effects of this processing, may also mean that it will be easier to eventually establish clearer associations between PCI and specific dimensions of consciousness, possibly independently of other neural differences between individuals and populations.
Overall, the limitations of unidimensional neural measures in providing information about consciousness within a multidimensional framework raise potential ethical issues for the use of these measures in DoC assessment. Our lack of knowledge about how these measures relate to consciousness both in general and in a translational, DoC-specific context could lead to either an overestimation or underestimation of an individual’s general level of neurocognitive impairment or otherwise to a mischaracterization of the qualitative nature of their conscious experience and mental functioning in ways that may impact diagnosis, prognosis and treatment.
By contrast, the precise constraints and limitations of ERP-based paradigms like the LGP as tools for evaluating consciousness are better understood. We know that the absence of a signature like the global effect does not necessarily equal the absence of the underlying neural capacity or mechanism, so with this understanding its risks within DoC assessment are minimal. However, there is still an important open question about whether the global effect necessitates consciousness. Furthermore, one possible advantage of measures of conscious ‘level’ like resting-state complexity and PCI may be their potential for detecting signatures of perceptually decoupled conscious experiences—something that may be more challenging to achieve with ERP-based paradigms.
How should we evaluate consciousness as a multidimensional phenomenon in DoC assessment?
With increasing awareness of the fact that conscious experience, both in general and in DoC, is dynamic, heterogeneous and multidimensional, there has been wider recognition of the need for greater diagnostic precision in DoC assessment and endophenotypes (Provencio et al. 2020; Kondziella et al. 2021). There is also wider acknowledgement of the benefits of multimodal and multitechnological (Demertzi et al. 2017) approaches to diagnosis in DoC, where multiple neurophysiological and behavioural assessments and modalities are combined to improve diagnostic reliability (Giacino et al. 2018; Kondziella et al. 2020). Several multimodal paradigms have been proposed and implemented (Coleman et al. 2009a; Gibson et al. 2014; Sergent et al. 2016; Hermann et al. 2020; Møller et al. 2020). Such paradigms may be used to increase the reliability of a binary diagnostic judgement about the presence of consciousness in a patient, but could also be an important tool for improving the richness and precision of our diagnostic approach to consciousness in DoC.
Within a multimodal diagnostic framework, each neural assessment could be seen to provide different information about consciousness and neurocognitive functioning in a patient, such that combining differently designed paradigms with underlying links to different conscious dimensions may eventually allow for richer insight into the subjective quality of consciousness in individuals with DoC. ERPs such as the LGP fit into this framework in quite a clear way. This ERP and others allow for clear inferences about specific neural mechanisms that may shed light on an individual’s capacity for specific forms of perceptual and cognitive functioning (incongruence detection, semantic comprehension, as well as more basic forms of sensory processing) without necessarily being generalizable to all dimensions of consciousness or neurocognitive functioning. Assessment paradigms for DoC that incorporate a range of ERPs have been proposed (Møller et al. 2020). Although it is challenging to establish with certainty whether any ERP is a true signature of consciousness (Kotchoubey 2005), the functional neural circuitry highlighted by these measures may nonetheless provide information that can inform prognosis (Sergent et al. 2016) and care, treatment and rehabilitation decisions for these patients.
Other forms of neural assessment, such as active, task-based fMRI paradigms, may also be informative within a multidimensional assessment paradigm. Active paradigms have clear limitations, but a patient’s ability to participate in such a paradigm by, e.g. generating mental imagery in response to command, can provide strong positive evidence not only for consciousness, but for a range of specific dimensions of consciousness (depending on the exact task, these include semantic comprehension, reasoning, long-term memory, memory consolidation and sustained attention to internal imagery; Naci et al. 2017). Passive fMRI paradigms that assess neural activity in a task-free setting, such as that which occurs in response natural speech (Sokoliuk et al. 2021) or an engaging short movie (Naci et al. 2014), also have the potential to be highly informative with regard to specific dimensions of conscious experience such as speech comprehension; recognition of familiar voices, objects and faces; theory of mind; executive function and experiencing affective states (Naci et al. 2017). However, in the absence of volitional mental activity, it remains unclear to what extent evidence about conscious experience itself can be drawn based on the neural responses observed in these passive paradigms.
The diagnostic value of levels-based neural measures of consciousness is less clear within a multimodal, multidimensional framework for DoC assessment. Resting-state complexity and PCI cannot be used to draw general inferences about an individual’s level of consciousness or neurocognitive functioning as they are more sensitive to certain dimensions and forms of conscious experience than others. However, these unidimensional measures seem to have only a relatively general association with a range of conscious mechanisms and dimensions that does not allow for fine-grained distinctions or inferences about any single property and that therefore leads to considerable diagnostic ambiguity within a multidimensional framework. The limitations of neural measures of conscious ‘level’ within a multidimensional understanding of consciousness means that their use in DoC diagnosis will need to be carefully scrutinized. However, investigation of the spatial patterns of properties like neural complexity in the brain using more spatially precise modalities like fMRI or high-density EEG may eventually allow for more precise relationships with phenomenology to be inferred.
Conclusion
There is a pressing clinical and ethical need for reliable, precise methods for characterizing consciousness in individuals with DoC, who are a highly heterogeneous and challenging clinical group. There is also a growing view that consciousness is multidimensional, even though some proposed neural measures of consciousness are centred on the idea that consciousness is a property that can be described in terms of hierarchical ‘levels.’ Here, I have examined a range of comparative evidence about the relationship between these measures, such as those indexing resting-state neural complexity and the TMS–EEG-based PCI, and conscious experience, suggesting that elevated levels of these measures correlate with an increase in some dimensions of consciousness and not others. Furthermore, these measures may be affected by a range of disorder-specific and demographic factors that are not directly reflective of fundamental changes to consciousness. Analysing such neural measures within a multidimensional framework for consciousness has emphasized the need for closer scrutiny of their relationship to both phenomenology and neurobiological impairment before they can ethically be used to support inferences about conscious experience in individuals with DoC. This analysis also suggests that, based on our current level of knowledge, neural measures with clearer links to more specific elements of phenomenology and/or neurocognitive functioning, such as ERP-based measures like the LGP, may currently be the most informative assessments within a diagnostic paradigm that adequately accounts for the multidimensionality, variability and possible heterogeneity that may characterize conscious experience amongst those with DoC.
Acknowledgements
I thank Lionel Naccache, Athena Demertzi and two anonymous reviewers for their insightful comments on this manuscript. I also thank Jennifer Windt and Thomas Andrillon for their valuable feedback and contributions at every stage of the preparation of this manuscript.
Funding
This research was supported by an Australian Government Research Training Program (RTP) Scholarship.
Conflict of interest statement
None declared.
References
- Aftanas LI, Golocheikine SA. Non-linear dynamic complexity of the human EEG during meditation. Neurosci Lett 2002;330:143–46. [DOI] [PubMed] [Google Scholar]
- Ahmadlou M, Adeli H, Adeli A. Fractality analysis of frontal brain in major depressive disorder. Int J Psychophysiol 2012;85:206–11. [DOI] [PubMed] [Google Scholar]
- Andrillon T, Burns A, Mackay T et al. Predicting lapses of attention with sleep-like slow waves. Nat Commun 2021;12:3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrillon T, Kouider S. The vigilant sleeper: neural mechanisms of sensory (de)coupling during sleep. Curr Opin Physiol 2020;15:47–59. [Google Scholar]
- Andrillon T, Poulsen AT, Hansen LK et al. Neural markers of responsiveness to the environment in human sleep. J Neurosci 2016;36:6583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Birbaumer N, Lutzenberger W et al. Age increases brain complexity. Electroencephalogr Clin Neurophysiol 1996;99:63–8. [DOI] [PubMed] [Google Scholar]
- Bayne T, Carter O. Dimensions of consciousness and the psychedelic state. Neurosci Conscious 2018;2018:niy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne T, Hohwy J, Owen AM. Are there levels of consciousness? Trends Cogn Sci 2016;20:405–13. [DOI] [PubMed] [Google Scholar]
- Bayne T, Seth AK, Massimini M. Are there islands of awareness? Trends Neurosci 2020;43:6–16. [DOI] [PubMed] [Google Scholar]
- Bekinschtein TA, Dehaene S, Rohaut B et al. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci U S A 2009;106:1672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J, Schnell AK, Clayton NS. Dimensions of animal consciousness. Trends Cogn Sci 2020;24:789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bola M, Orłowski P, Baranowska K et al. Informativeness of auditory stimuli does not affect EEG signal diversity. Front Psychol 2018;9:1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Massimini M, Naotsugu Tsuchiya BR et al. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J Neurosci 2017;37:9603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Sasai S, Gosseries O et al. Stimulus set meaningfulness and neurophysiological differentiation: a functional magnetic resonance imaging study. PLoS One 2015;10:e0125337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59. [DOI] [PubMed] [Google Scholar]
- Callard F, Margulies DS. What we talk about when we talk about the default mode network. Front Hum Neurosci 2014;8:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M, Solomonova E. Dream recall and content in different stages of sleep and time-of-night effect. 2019, 167–72.
- Casali AG, Gosseries O, Rosanova M et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013;5:198ra105. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Comanducci A, Rosanova M et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol 2016;80:718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A, Churches O, Baron-Cohen S et al. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin Neurophysiol 2011;122:2375–83. [DOI] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Kieran CR et al. Mind-wandering as spontaneous thought: a dynamic framework. Nat Rev Neurosci 2016;17:718–31. [DOI] [PubMed] [Google Scholar]
- Coleman MR, Bekinschtein T, Monti MM et al. A multimodal approach to the assessment of patients with disorders of consciousness. In: Laureys S, Nicholas DS, Owen AM (eds.), Progress in Brain Research, Vol. 177, Coma Science: Clinical and Ethical Implications. Elsevier, 2009a, 231–48. [DOI] [PubMed] [Google Scholar]
- Coleman MR, Davis MH, Rodd JM et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain 2009b;132:2541–52. [DOI] [PubMed] [Google Scholar]
- Collantoni E, Madan CR, Meneguzzo P et al. Cortical complexity in anorexia nervosa: a fractal dimension analysis. J Clin Med 2020;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Cichocki A. Diagnosis of attention deficit hyperactivity disorder from EEG signals: where are we standing? Curr Alzheimer Res 2010;7:487–505. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Sitt JD, Sarasso S et al. Measuring states of pathological (un)consciousness: research dimensions, clinical applications, and ethics. Neurosci Conscious 2017;2017:nix010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engemann DA, Raimondo F, King J-R et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 2018;141:3179–92. [DOI] [PubMed] [Google Scholar]
- Escrichs A, Sanjuán A, Atasoy S et al. Characterizing the dynamical complexity underlying meditation. Front Syst Neurosci 2019;13. doi: 10.3389/fnsys.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnes N, Juel BE, Nilsen AS et al. Increased signal diversity/complexity of spontaneous EEG, but not evoked EEG responses, in ketamine-induced psychedelic state in humans. BioRxiv 2019:508697. doi: 10.1101/508697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N et al. Probing consciousness with event-related potentials in the vegetative state. Neurology 2011;77:264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas P, Nemeth G, Overgaard M. White dreams are made of colours: what studying contentless dreams can teach about the neural basis of dreaming and conscious experiences. Sleep Med Rev 2019;43:84–91. [DOI] [PubMed] [Google Scholar]
- Fazekas P, Overgaard M. Multidimensional models of degrees and levels of consciousness. Trends Cogn Sci 2016;20:715–6. [DOI] [PubMed] [Google Scholar]
- Ferenets R, Tarmo Lipping A, Jantti AV et al. Comparison of entropy and complexity measures for the assessment of depth of sedation. IEEE Trans Biomed Eng 2006;53:1067–77. [DOI] [PubMed] [Google Scholar]
- Ferenets R, Vanluchene A, Lipping T et al. Behavior of entropy/complexity measures of the electroencephalogram during propofol-induced sedation: dose-dependent effects of remifentanil. Anesthesiology 2007;106:696–706. [DOI] [PubMed] [Google Scholar]
- Fernández A, López-Ibor M-I, Turrero A et al. Lempel-Ziv complexity in schizophrenia: a MEG study. Clin Neurophysiol 2011;122:2227–35. [DOI] [PubMed] [Google Scholar]
- Fortier-Davy M, Raphaël M. The multi-dimensional approach to drug-induced states a commentary on Bayne and Carter’s ‘dimensions of consciousness and the psychedelic state’. Neurosci Conscious 2020;2020:niaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubert S, Raimondo F, Houot M et al. EEG evidence of compensatory mechanisms in preclinical Alzheimer’s disease. Brain 2019;142:2096–112. [DOI] [PubMed] [Google Scholar]
- Giacino J, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–9. [DOI] [PubMed] [Google Scholar]
- Giacino J, Katz DI, Schiff ND et al. Practice guideline update recommendations summary: disorders of consciousness. Neurology 2018;91:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JS, Childs AN, Cranford R et al. The minimally conscious state. Neurology 2002;58:349–53. [DOI] [PubMed] [Google Scholar]
- Gibson RM, Fernández-Espejo D, Gonzalez-Lara LE et al. Multiple tasks and neuroimaging modalities increase the likelihood of detecting covert awareness in patients with disorders of consciousness. Front Hum Neurosci 2014;8:950. doi: 10.3389/fnhum.2014.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girn M, Mills C, Roseman L et al. Updating the dynamic framework of thought: creativity and psychedelics. NeuroImage 2020;213:116726. [DOI] [PubMed] [Google Scholar]
- Gosseries O, Schnakers C, Ledoux D et al. Automated EEG entropy measurements in coma, vegetative state/unresponsive wakefulness syndrome and minimally conscious state. Funct Neurol 2011;26:25–30. [PMC free article] [PubMed] [Google Scholar]
- Goupil L, Bekinschtein T. Cognitive processing during the transition to sleep. Arch Ital Biol 2012;150:140–54. [DOI] [PubMed] [Google Scholar]
- Hermann B, Stender J, Habert M-O et al. Multimodal FDG-PET and EEG assessment improves diagnosis and prognostication of disorders of consciousness. MedRxiv 2020. doi: 10.1101/2020.06.16.20132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Molina AJ, Lozano V, Soriano MF et al. EEG multiscale complexity in schizophrenia during picture naming. Front Physiol 2018;9:1213. doi: 10.3389/fphys.2018.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Parro S, Soriano MF, Prieto M et al. Introspective and neurophysiological measures of mind wandering in schizophrenia. Sci Rep 2020;10:4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DH, Rinkel M, Solomon HC. Mental changes experimentally produced by L. S. D. (d-lysergic acid diethylamide tartrate). Psychiatr Q 1952;26:33–53. [DOI] [PubMed] [Google Scholar]
- Jennett B, Plum F. Persistent vegetative state after brain damage. a syndrome in search of a name. Lancet 1972;1:734–37. [DOI] [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 2004;115:1490–505. [DOI] [PubMed] [Google Scholar]
- King J-RF, Gramfort FA, Schurger A et al. Single-trial decoding of auditory novelty responses facilitates the detection of residual consciousness. NeuroImage 2013;83:726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Massimini M, Boly M et al. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci 2016;17:307–21. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Bender A, Diserens K et al. European academy of neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol 2020;27:741–56. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Friberg CK, Frokjaer VG et al. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016;87:485–92. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Menon DK, Helbok R et al. A precision medicine framework for classifying patients with disorders of consciousness: Advanced Classification of Consciousness Endotypes (ACCESS). Neurocrit Care 2021;35:27–36. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B. Event-related potential measures of consciousness: two equations with three unknowns. In: Laureys S (ed.), Progress in Brain Research, Vol. 150, The Boundaries of Consciousness: Neurobiology and Neuropathology. Elsevier, 2005, 427–44. [DOI] [PubMed] [Google Scholar]
- Kouider S, Stahlhut C, Gelskov SV et al. A neural marker of perceptual consciousness in infants. Science 2013;340:376–80. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Chakrabarti B et al. A shift to randomness of brain oscillations in people with autism. Biol Psychiatry 2010;68:1092–9. [DOI] [PubMed] [Google Scholar]
- Laurens VC, D’Argembeau A, Salmon E et al. Fluctuations of attentional networks and default mode network during the resting state reflect variations in cognitive states: evidence from a novel resting-state experience sampling method. J Cogn Neurosci 2017;29:95–113. [DOI] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci 2005;9:556–9. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Zhu YS, Xu YH et al. Detection of non-linearity in the EEG of schizophrenic patients. Clin Neurophysiol 2001;112:1288–94. [DOI] [PubMed] [Google Scholar]
- Lempel A, Ziv J. On the complexity of finite sequences. IEEE Trans Inf Theory 1976;22:75–81. [Google Scholar]
- Li D, Xiaoli L, Liang Z et al. Multiscale permutation entropy analysis of EEG recordings during sevoflurane anesthesia. J Neural Eng 2010;7:046010. [DOI] [PubMed] [Google Scholar]
- Li Y, Tong S, Liu D et al. Abnormal EEG complexity in patients with schizophrenia and depression. Clin Neurophysiol 2008;119:1232–41. [DOI] [PubMed] [Google Scholar]
- Liang Z, Wang Y, Sun X et al. EEG entropy measures in anesthesia. Front Comput Neurosci 2015:9:16. doi: 10.3389/fncom.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linassi F, Zanatta P, Tellaroli P et al. Isolated forearm technique: a meta-analysis of connected consciousness during different general anaesthesia regimens. Br J Anaesth 2018;121:198–209. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang C, Ji Z et al. Multiple characteristics analysis of Alzheimer’s electroencephalogram by power spectral density and Lempel–Ziv complexity. Cogn Neurodyn 2016;10:121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science 1935;81:597–8. [DOI] [PubMed] [Google Scholar]
- Martial C, Cassol H, Laureys S et al. Near-death experience as a probe to explore (disconnected) consciousness. Trends Cogn Sci 2020;24:173–83. [DOI] [PubMed] [Google Scholar]
- Mashour GA, Palanca BJA, Basner M et al. Recovery of consciousness and cognition after general anesthesia in humans. eLife 2021;10:e59525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarazzo L, Foret A, Mascetti L et al. A systems-level approach to human REM sleep. In: Morrison AR, Mallick BN, McCarley RW et al. (eds.), Rapid Eye Movement Sleep: Regulation and Function. Cambridge: Cambridge University Press, 2011, 71–9. [Google Scholar]
- McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol 1994;4:550–6. [DOI] [PubMed] [Google Scholar]
- Méndez MA, Zuluaga P, Hornero R et al. Complexity analysis of spontaneous brain activity: effects of depression and antidepressant treatment. J Psychopharmacol 2012;26:636–43. [DOI] [PubMed] [Google Scholar]
- Michel M, Beck D, Block N et al. Opportunities and challenges for a maturing science of consciousness. Nat Hum Behav 2019;3:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittner M, Hawkins GE, Boekel W et al. A neural model of mind wandering. Trends Cogn Sci 2016;20:570–8. [DOI] [PubMed] [Google Scholar]
- Møller M, Holm L, Højlund A et al. Applied potential of task-free event-related paradigms for assessing neurocognitive functions in disorders of consciousness. Brain Commun 2020;2:fcaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010;362:579–89. [DOI] [PubMed] [Google Scholar]
- Naccache L, Sébastien Marti JD, Sitt DT et al. Why the P3b is still a plausible correlate of conscious access? A commentary on Silverstein et al., 2015. Cortex 2016;85:126–8. [DOI] [PubMed] [Google Scholar]
- Naci L, Cusack R, Anello M et al. A common neural code for similar conscious experiences in different individuals. Proc Natl Acad Sci U S A 2014;111:14277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naci L, Graham M, Owen AM et al. Covert narrative capacity: mental life in patients thought to lack consciousness. Ann Clin Transl Neurol 2017;4:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naci L, Owen AM. Making every word count for nonresponsive patients. JAMA Neurol 2013;70:1235–41. [DOI] [PubMed] [Google Scholar]
- Nandrino J-L, Pezard L, Martinerie J et al. Decrease of complexity in EEG as a symptom of depression. Neuroreport 1994;5:528–30. [DOI] [PubMed] [Google Scholar]
- Nielsen TA. A review of mentation in REM and NREM sleep:‘covert’ REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci 2000;23:851–66. [DOI] [PubMed] [Google Scholar]
- Noreika V, Jylhänkangas L, Móró L et al. Consciousness lost and found: subjective experiences in an unresponsive state. Brain Cogn 2011;77:327–34. [DOI] [PubMed] [Google Scholar]
- Noreika V, Valli K, Lahtela H et al. Early-night serial awakenings as a new paradigm for studies on NREM dreaming. Int J Psychophysiol 2009;74:14–8. [DOI] [PubMed] [Google Scholar]
- Nourski KV, Steinschneider M, Rhone AE et al. Auditory predictive coding across awareness states under anesthesia: an intracranial electrophysiology study. J Neurosci 2018;38:8441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’shaughnessy N, Bhome R, Gallagher P et al. Awareness in severe Alzheimer’s disease: a systematic review. Aging Ment Health 2020;25:602–12. doi: 10.1080/13607863.2020.1711859. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M et al. Detecting awareness in the vegetative state. Science 2006;313:1402. [DOI] [PubMed] [Google Scholar]
- Pereda E, Gamundi A, Rial R et al. Non-linear behaviour of human EEG: fractal exponent versus correlation dimension in awake and sleep stages. Neurosci Lett 1998;250:91–4. [DOI] [PubMed] [Google Scholar]
- Peterson A. Consilience, clinical validation, and global disorders of consciousness. Neurosci Conscious 2016;2016:niw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio J J, Hemphill J C, Claassen J et al. The curing coma campaign: framing initial scientific challenges—proceedings of the first curing coma campaign scientific advisory council meeting. Neurocrit Care 2020;33:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor KO, Root JC. III. Intraoperative awareness: a pound of prevention, an ounce of cure? Br J Anaesth 2013;111:529–31. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA et al. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology 2012;37:630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek L, Kallionpää RE, Karvonen M et al. Dreaming and awareness during dexmedetomidine- and propofol-induced unresponsiveness. Br J Anaesth 2018;121:260–9. [DOI] [PubMed] [Google Scholar]
- Raskin J, Cummings J, Hardy J et al. Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr Alzheimer Res 2015;12:712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini G. An algorithmic information theory of consciousness. Neurosci Conscious 2017;2017:nix019. doi: 10.1093/nc/nix019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Correia S. Alzheimer disease: time to improve its diagnosis and treatment. Cleve Clin J Med 2009;76:49–58. [DOI] [PubMed] [Google Scholar]
- Sarasso S, Boly M, Napolitani M et al. Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr Biol 2015;25:3099–105. [DOI] [PubMed] [Google Scholar]
- Schartner M, Pigorini A, Gibbs SA et al. Global and local complexity of intracranial EEG decreases during NREM sleep. Neurosci Conscious 2017a;2017:4. doi: 10.1093/nc/niw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner M, Carhart-Harris RL, Barrett AB et al. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 2017b;7:46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner M, Seth A, Noirhomme Q et al. Complexity of multi-dimensional spontaneous EEG decreases during propofol induced general anaesthesia. PLoS One 2015;10:e0133532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015;72:1413–5. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman DJ, Schartner M, Ador BB et al. Increased spontaneous EEG signal diversity during stroboscopically-induced altered states of consciousness. BioRxiv 2019:511766. doi: 10.1101/511766. [DOI] [Google Scholar]
- Scott G, Carhart-Harris RL. Psychedelics as a treatment for disorders of consciousness. Neurosci Conscious 2019;2019:niz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P, Risko EF, Smilek D et al. Mind-wandering with and without intention. Trends Cogn Sci 2016;20:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C, Faugeras F, Rohaut B et al. Multidimensional cognitive evaluation of patients with disorders of consciousness using EEG: a proof of concept study. NeuroImage 2016;13:455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, Izhikevich E, Reeke GN et al. Theories and measures of consciousness: an extended framework. Proc Natl Acad Sci U S A 2006;103:10799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumbayawonda E, Tosun PD, Fernández A et al. Complexity changes in brain activity in healthy ageing: a permutation Lempel-Ziv complexity study of magnetoencephalograms. Entropy 2018;20:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siclari F, LaRocque J, Postle B et al. Assessing sleep consciousness within subjects using a serial awakening paradigm. Front Psychol 2013;4:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AB, Klee GD. Effects of lysergic acid diethylamide (LSD-25) on intellectual functions. AMA Arch Neurol Psychiatry 1958;80:477–80. [DOI] [PubMed] [Google Scholar]
- Silverstein BH, Snodgrass M, Shevrin H et al. P3b, consciousness, and complex unconscious processing. Cortex 2015;73:216–27. [DOI] [PubMed] [Google Scholar]
- Sitt JD, King J-R, Karoui IE et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014;137:2258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J, Warnaby C, Tracey I. General anaesthesia as fragmentation of selfhood: insights from electroencephalography and neuroimaging. Br J Anaesth 2018;121:233–40. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The science of mind wandering: empirically navigating the stream of consciousness. Ann Rev Psychol 2015;66:487–518. [DOI] [PubMed] [Google Scholar]
- Sokoliuk R, Degano G, Banellis L et al. Covert speech comprehension predicts recovery from acute unresponsive states. Ann Neurol 2021;89:646–56. [DOI] [PubMed] [Google Scholar]
- Sokunbi MO, Fung W, Sawlani V et al. Resting state FMRI entropy probes complexity of brain activity in adults with ADHD. Psychiatry Res 2013;214:341–8. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol 1975;38:387–401. [DOI] [PubMed] [Google Scholar]
- Strauss M, Sitt JD, King J-R et al. Disruption of hierarchical predictive coding during sleep. Proc Natl Acad Sci U S A 2015;112:E1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart‐Harris R, Leech R et al. Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp 2014;35:5442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Cho RY, Mizuno T et al. Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. NeuroImage 2010;51:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul A, Lechinger J, Donis J et al. EEG entropy measures indicate decrease of cortical information processing in disorders of consciousness. Clin Neurophysiol 2016;127:1419–27. [DOI] [PubMed] [Google Scholar]
- Timmermann C, Roseman L, Schartner M et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep 2019;9:16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC Neurosci 2004;5:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Consciousness and complexity. Science 1998;282:1846–51. [DOI] [PubMed] [Google Scholar]
- Tóth E, Kondákor I, Túry F et al. Nonlinear and linear EEG complexity changes caused by gustatory stimuli in anorexia nervosa. Int J Psychophysiol 2004;51:253–60. [DOI] [PubMed] [Google Scholar]
- Uhrig L, Janssen D, Dehaene S et al. Cerebral responses to local and global auditory novelty under general anesthesia. NeuroImage 2016;141:326–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci 2003;6:391–8. [DOI] [PubMed] [Google Scholar]
- Varley TF, Carhart-Harris R, Roseman L et al. Serotonergic psychedelics LSD & psilocybin increase the fractal dimension of cortical brain activity in spatial and temporal domains. NeuroImage 2020a;220:117049. [DOI] [PubMed] [Google Scholar]
- Varley TF, Craig M, Adapa R et al. Fractal dimension of cortical functional connectivity networks & severity of disorders of consciousness. PLoS One 2020b;15:e0223812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley TF, Luppi AI, Pappas I et al. Consciousness & brain functional complexity in propofol anaesthesia. Sci Rep 2020c;10:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B et al. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 2007;32:1876–87. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull 2001;56:495–507. [DOI] [PubMed] [Google Scholar]
- Whiteley CMK. Depression as a disorder of consciousness. Br J Philos Sci 2021:716838. doi: 10.1086/716838. [DOI] [Google Scholar]
- Windt JM. Just in Time—Dreamless Sleep Experience as Pure Subjective Temporality. Open MIND. Frankfurt am Main: MIND Group, 2015. [Google Scholar]
- Windt JM. Consciousness in sleep: how findings from sleep and dream research challenge our understanding of sleep, waking, and consciousness. Philos Compass 2020;15:e12661. [Google Scholar]
- Windt JM, Nielsen T, Thompson E. Does consciousness disappear in dreamless sleep? Trends Cogn Sci 2016;20:871–82. [DOI] [PubMed] [Google Scholar]
- Wu D-Y, Cai G, Yuan Y et al. Application of nonlinear dynamics analysis in assessing unconsciousness: a preliminary study. Clin Neurophysiol 2011;122:490–8. [DOI] [PubMed] [Google Scholar]
- Xiang J, Tian C, Niu Y et al. Abnormal entropy modulation of the EEG signal in patients with schizophrenia during the auditory paired-stimulus paradigm. Front Neuroinform 2019;13:4. doi: 10.3389/fninf.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagyu T, Wackermann J, Shigeta M et al. Global dimensional complexity of multichannel EEG in mild Alzheimer’s disease and age-matched cohorts. Dement Geriatr Cogn Disord 1997;8:343–7. [DOI] [PubMed] [Google Scholar]
- Yang AC, Hong C-J, Liou Y-J et al. Decreased resting-state brain activity complexity in schizophrenia characterized by both increased regularity and randomness. Hum Brain Mapp 2015;36:2174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AC, Huang C-C, Yeh H-L et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis. Neurobiol Aging 2013a;34:428–38. [DOI] [PubMed] [Google Scholar]
- Yang AC, Wang S-J, Lai K-L et al. Cognitive and neuropsychiatric correlates of EEG dynamic complexity in patients with Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2013b;47:52–61. [DOI] [PubMed] [Google Scholar]
- Zhang X-S, Roy RJ, Jensen EW. EEG complexity as a measure of depth of anesthesia for patients. IEEE Trans Biomed Eng 2001;48:1424–33. [DOI] [PubMed] [Google Scholar]


