Abstract
Background
Nasopharyngeal carcinoma (NPC) is a rare malignant tumor developing from epithelial linings of nasopharynx, and 10–50 out of 100,000 NPC cases were recorded globally particularly in the Asian countries.
Methodology
The cytotoxicity of geraniin against the NPC C666-1 cells were analyzed using MTT assay. The influences of geraniin on the C666-1 cell viability with the presence of ROS and apoptosis inhibitors were also studied. The expressions of PI3K, Akt, mTOR, and autophagic markers LC3, ATG7, P62/SQSTM1 expressions in the C666-1 cells were studied by western blotting analysis. The ROS production was assayed using DCFH-DA staining. The immunofluorescence assay was performed to detect the NF-κB and β-catenin expressions in the C666-1 cells.
Results
The cell viability of C666-1 cells were appreciably prevented by the geraniin. The geraniin treatment also inhibited the C666-1 cell growth with the presence of apoptotic inhibitor Z-VAD-FMK. The geraniin-treatment effectively improved the ROS production and inhibited the NF-κB and β-catenin expressions in the C666-1 cells. Geraniin appreciably modulated the PI3K/Akt/mTOR signaling axis and improved the autophagy-mediated cell death via improving the autophagic markers LC3 and ATG7 expressions in the C666-1 cells.
Conclusion
In conclusion, our results proved that geraniin inhibits C666-1 cell growth and initiated autophagy-mediated cell death via modulating PI3K/Akt/mTOR cascade and improving LC3 and ATG7 expressions in the C666-1. Geraniin and it could be a hopeful and efficient candidate to treat the human NPC in the future.
Keywords: Nasopharyngeal cancer, Geraniin, Autophagy, C666-1 cells, PI3K/Akt pathway
1. Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor arising from the upper mucosal epithelium of the nasopharynx. NPC is regarded as a rare carcinoma worldwide, however it is prevalent in the countries of south east Asia, especially in China (Chen et al., 2019a, Chen et al., 2019b). The initiation of NPC is tightly allied with the infection from Epstein-Barr virus (EBV), whereas there are several risk factors also reported in the NPC development like drinking, smoking, and other environmental factors. The cases of NPC is varied from 10 to 50 out of 100,000 globally (Casanova et al., 2016).
Autophagy is a highly controlled catabolic event participated in the recycling and degradation cellular constituents upon manifold cellular stresses (Denton and Kumar, 2019). As the well-maintained mechanism, autophagy participates in the development of double membrane vesicles called autophagosomes that swallow cellular organelles and components for provide to the lysosome in order to degradation (Limpert et al., 2018). The several preceding researches in the last years has recommended that the clinical and biological impacts of autophagy on the tumor development are connected with the types of tumor and genetic histories (Mulcahy and Thorburn, 2020). Autophagy demonstrates the tumor-preventive actions through the several processes that the tumor cells experience programmed cell death (Chen et al., 2019a, Chen et al., 2019b). Autophagy is essential for the development, differentiation, survival, and homeostasis of cells. The dysregulation in autophagy participates in the variety of ailments, including cancer (Levine and Kroemer, 2008).
The molecular mechanisms of autophagy are not completely discovered, but it is believed that several autophagy-related genes (ATGs) and mediatory proteins are participated in the autophagy (Feng et al., 2014). The ATG7 regulates the expansion and assembly of autophagosomal membranes that makes it vital for autophagy (Xiong, 2015). The p62 protein is a well known biomarker of autophagy, along with the microtubule-associated protein-1 light chain-3 (LC-3). This protein actively participates as a crucial receptor that recognizes and binds to ubiquitinated proteins, transporting them through membrane-bound LC-3 to the phagophore for the degradation. While, p62 itself is degraded via autolysosomes subsequent to the initiation of autophagy. Consequently, the accumulation of p62 was reported as common biomarker of decreased autophagic flux (Yoshii and Mizushima, 2017). LC3 is a protein contributing to the autophagy via conjugating with phosphatidylethanolamine to develop the LC3-II that is a biomarker of autophagosome. The stimulation of autophagy is sensitized via inactivating the systematic target of mTOR that is the vital cellular energy sensor kinase (Lopez-Perez et al., 2020).
PI3K/AKT/mTOR signaling axis is well established pathway, which reported to escalate the cancer cell multiplication, survival and metastasis in several cancers. PI3K/AKT/mTOR axis also impacts in the several imperative cellular events like nutrient intake, cell growth, anabolic reactions, and metastasis motility (Wee and Wang, 2017). In 90% of head and neck cancers, the PI3K/AKT/mTOR signaling is up-regulated. The up-regulated PI3K/AKT/mTOR expression could improve the tumor cell resistance towards radiotherapy and chemotherapeutic drugs (Freudlsperger et al., 2015). The preceding researches has mentioned that the PI3K/AKT cascades is a vital pathway in the signal transduction of several growth factors, and is tightly connected to the cell physiological activities and initiation and progression of numerous cancers. In recent times, the PI3K/AKT signaling axis has received much interests among researchers in order to block the tumor growth (Zhao et al., 2016).
Clinically, the 70–80% of NPC patients has the increased risks of cervical lymph node metastasis and/or distant metastasis during diagnosis that further complicates the successful treatment and survival of NPC patients (Fan et al., 2018). Currently, the application of chemo/radiotherapy and targeted therapy was employed as a major therapeutic options for the NPC. Regrettably, it is unavoidable that the normal nasopharynx epithelium and tissues could be damaged in post radiotherapy (Sidaway, 2019, Zhang et al., 2019). Hence, the new therapeutic approaches with lesser toxicity to normal tissues were needed immediately for the successful management of NPC. Geraniin is a ellagitannin (hydrolysable tannin) naturally present in several edible fruits and nuts like strawberries, pomegranate, blackberries, walnuts, and almonds with many medicinal values (Ito, 2011). Conventionally, the geraniin-rich plant extracts exhibited several biological activities like antidiarrhetic (Okabe et al., 2001), anti-rheumatic arthritis (Li et al., 2008) and curative action against enteritis and dysentery (Yang et al., 2010). Also, geraniin was reported to demonstrate to several pharmacological activities like anti-hypertensive (Moreno et al., 2018), hepatoprotective (Xiao et al., 2015), anti-hyperglycaemic (Chung et al., 2014), anti-cancer (Wang et al., 2017, Yeh et al., 2019), and antiviral (Ahmad et al., 2017, Choi et al., 2019) properties. Nonetheless, the therapeutic actions of geraniin against the NPC was not systematically studied yet. Consequently, here we planned to scrutinize the in vitro anticancer role of geraniin against the NPC C666-1 cells via inhibiting the PI3K/Akt/mTOR pathway and NF-κB, and β-catenin expressions.
2. Materials and methods
2.1. Chemicals
Geraniin (≥99%) was procured from the SelleckChem, Houston, USA. The 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and other reagents were attained from Sigma-Aldrich, USA. The assay kits were procured from Biocompare, USA, and the antibodies for target markers were purchased from Thermofisher, USA.
2.2. NPC C666-1 cell collection and maintenance
The human NPC C666-1 cells were collected from the American Type Culture Collection (ATCC), Manassas, Virginia, USA. Then procured C666-2 cells were grown on a DMEM supplemented 10% of FBS at 37 °C in a humidified chamber with CO2 (5%) aeration. Followed by the 80% of confluence, cells were separated by trypsinization and utilized for the additional researches.
2.3. Cytotoxicity assay
The influence of geraniin on the viability of C666-1 cells along with the apoptotic inhibitor (Z-VAD-FMK) and ROS inhibitor N-acetyl-l-cysteine (NAC) were scrutinized by MTT cytotoxic assay. To investigate the cytotoxicity of geraniin, C666-1 cells were cultured on the 96-wellplate enriched with DMEM 5 × 103 cells per well population and conserved at 37 °C for 24 h. Subsequently, the C666-1 cells from each plate were supplemented with the varied dosages of geraniin (0–10 μM/mL), 20 µM of Z-VAD-FMK, and 3 mM NAC, respectively, and maintained at same incubatory conditions for 24 h. afterwards, the MTT (20 μl) with DMEM (100 µl) was mixed to all the wells and additionally preserved for 4 h at same incubation conditions. The DMSO at 100 μl concentration was mixed to all wells to liquefy the formed formazan crystals. Finally, the impacts of geraniin on the C666-1 cells were examined by measuring absorbance at 570 nm.
3. Quantification of intracellular reactive oxygen species (ROS) production
The influence of geraniin on the accumulation of ROS in the C666-1 cells were investigated by the DCFH-DA staining. To measure this, C666-1 cells were cultured on the 24-wellplate at 1 × 106 cells per well population maintained at 37 °C for 24 h. Later, the C666-1 cells were administered with the 10 μM/mL of geraniin and preserved for 24 h at same incubation conditions. The ROS production in the geraniin supplemented C666-1 sells were scrutinized in 0, 15, 30, 60, and 90 min interval by staining the C666-1 cells with 10 μl of DCFH-DA stain. The ROS accumulation in the geraniin supplemented C666-1 cells were visualized under fluorescent microscope and ROS accumulation status was quantified.
3.1. Western blotting analysis
For the separation of total proteins from the geraniin treated C666-1 cells, cells were gathered and lysed by the addition of RIPA lysis buffer (Thermofisher, USA). Then the supernatant was gathered followed by the centrifugation at 12,000g at 4 °C for 15 min. The purity and concentration of isolated proteins from the geraniin supplemented C666-1 cells were scrutinized with the aid of BCA Protein Assay Kit (Thermofisher, USA), as per the protocols suggested by the manufacturer. Afterwards, the protein samples at 40μg per lane was standardized, subjected to SDS-PAGE separation, relocated to PVDF membrane (Millipore, USA). Subsequent to blocking in 5 % non-fat milk at 37 °C for 1.5 h, the membranes were then conserved with primary antibodies for 12 h at 4 °C. After the incubation with anti-mouse IgG HRP-Conjugated secondary antibodies (Thermofisher, USA), the protein expressions were pictured with the help of ECL-enhanced chemiluminescent reagent (Thermofisher, USA). The levels of proteins were standardized by β-actin. The primary antibodies utilized in this research for the PI3K, pPI3K, Akt, p-Akt, mTOR, p-mTOR, LC3 I/II, ATG7, and P62/SQSTM1 were procured from the Thermofisher, USA.
3.2. Immunofluorescence analysis
For the immunofluorescence analysis was carried out to visualize the NF-κB and β-catenin expressions in the geraniin supplemented C666-2 cells. For this, the geraniin supplemented C666-1 cells were collected and fixed in formaldehyde (4%) for 15 min, and stained with fluorescent stain DAPI for 5 min at 37 °C. Afterwards, microphotographs were taken using the confocal microscope. Followed by the geraniin supplementation, C666-1 cells were gathered and utilized for the immunofluorescence analysis. Shortly, C666-1 cells were fixed in formaldehyde (4%) for 30 min and permeabilized with 0.5% Triton X-100 for 20 min at 37 °C. Followed by the blocking with 5% BSA for 1 h at 37 °C, the cells were conserved with primary antibodies (1:50) against NF-κB and β-catenin for overnight at 4 °C. After that, cells were cleansed with PBS, and exposed to FITC-conjugated secondary antibodies (1:100 at 37 °C for 45 min. Lastly, cells were stained with DAPI for 30 min to stain the cell nuclei. The microphotographs were taken using confocal microscope. The taken images were examined using Image Pro Plus 6.0 software.
3.3. Statistical analysis
Data are given as mean ± SD of triplicates, which is examined statistically using SPSS software. The variations between sample groups were analyzed by the one-way ANOVA and Tukey’s post doc assay and p < 0.05 was considered as significant.
4. Results
4.1. Effect of geraniin on the C666-1 cell viability
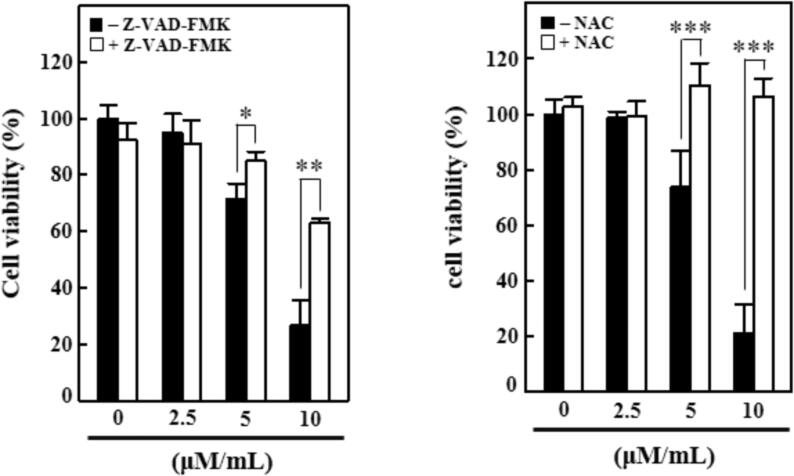
The cytotoxic effects of geraniin against the NPC C666-1 cells were scrutinized in the presence and absence of ROS and apoptosis inhibitors. Fig. 1 demonstrates that the control cells did not showed reduction in the viability. Conversely, the geraniin supplementation at various doses (2.5, 5, and 10 µM/mL) displayed the drastic diminution in the viability of C666-1 cells, when compared with control. The cytotoxic effects of geraniin against the C666-1 cells were also investigated with the presence of apoptosis inhibitor Z-VAD-FMK (Fig. 1). Interestingly, the geraniin treatment also inhibited the C666-1 cell growth with the presence of Z-VAD-FMK, which proves the cytotoxic potential of geraniin against NPC.
Fig. 1.
Effect of geraniin on the C666-1 cell viability. The C666-1 cells treated with 0, 2.5, 5, and 10 µM/mL exhibited the marked reduction in the cell viability. The geraniin treatment also suppressed the C666-1 cell viability with the presence of apoptosis inhibitor Z-VAD-FMK but it did not affected the C666-1 viability with the presence of ROS inhibitor NAC. Each bar illustrates the mean ± SD of triplicates, which is examined by one-way ANOVA consequently Tukey post doc assay. ‘*’ p < 0.05 ‘**’ & ‘***’ p < 0.01 were indicates the statistical significance.
To further confirm the cytotoxic nature of geraniin, the viability assay was continued with the presence of ROS inhibitor NAC. The findings revealed that the geraniin alone at various doses (2.5, 5, and 10 µM/mL) effectively decreased the C666-1 cell growth. On the other hand, the geraniin with the presence of NAC did not affected the C666-1 cell viability (Fig. 1). Hence, the present finding confirmed that the geraniin treatment effective against the suppression of C666-1 growth via promoting the apoptosis.
4.2. Effect of geraniin on the ROS production in the C666-1 cells
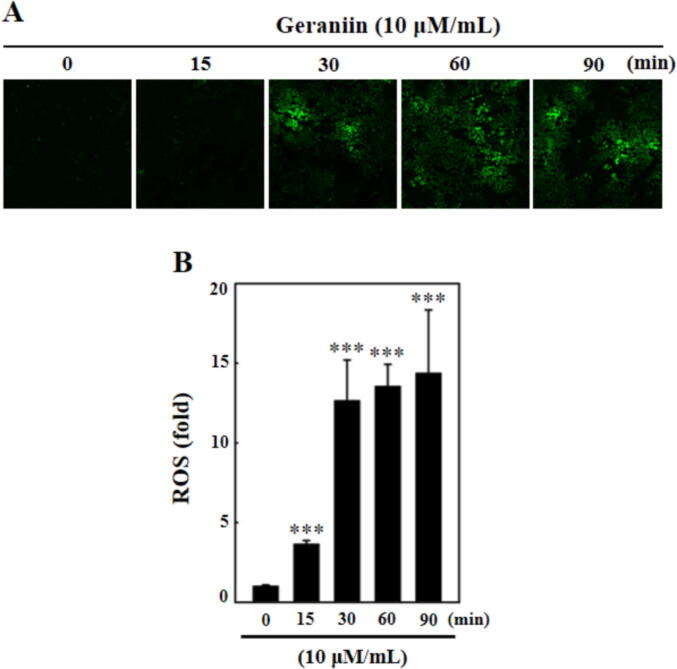
The intracellular ROS accumulation in the geraniin supplemented C666-1 cells were detected using DCFH-DA staining and the outcomes were represented in Fig. 2. The geraniin at 10 µM/mL dose supplemented C666-1 cells were displayed increased production of ROS, which is detected in different time period (0–90 min). The increase in geraniin exposure time also increase the ROS production in the C666-1 cells. These finding proves that the geraniin could speed up the ROS accumulation in the NPC C666-1 cells (Fig. 2).
Fig. 2.
Effect of geraniin on the ROS production in the C666-1 cells. The ROS production in the geraniin-supplemented C666-1 cells were investigated by DCFH-DA staining. The images exhibits that the geraniin gradually improved the ROS production in the C666-1 cells at different time period (0, 15, 30, 60, 90 min). Each bar illustrates the mean ± SD of triplicates, which is examined by one-way ANOVA consequently Tukey post doc assay. ‘***’ p < 0.01 were indicates the statistical significance.
4.3. Effects of geraniin on the PI3K/AKT pathway in the C666-1 cells
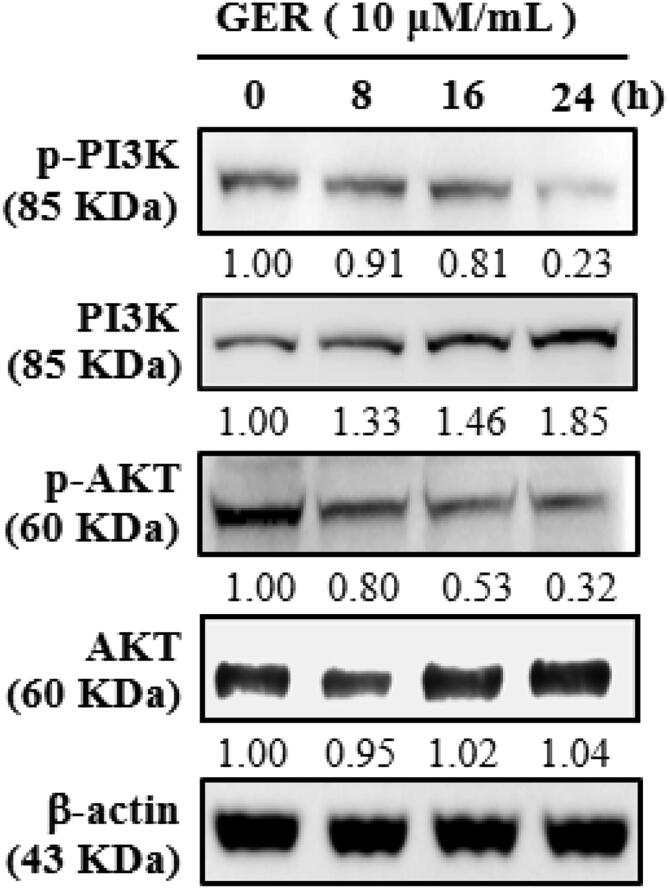
The inhibitory role of geraniin on the expression of PI3K and AKT in the C666-1 cells were studied by western blotting and the findings were displayed in Fig. 3. The geraniin at 10 µM/mL dose slightly improved the PI3K and AKT expression, however, the phosphorylation of both PI3K and AKT was appreciably prevented by the geraniin treatment. The expression of these markers were normalized to the β-actin (Fig. 3). These finding evidenced that the geraniin treatment appreciably blocked the PI3K and AKT signaling in the C666-1 cells.
Fig. 3.
Effects of geraniin on the PI3K/Akt signalling pathway in the C666-1 cells. The protein expressions of PI3K and Akt in the C666-1 cells, which is administered with the 2.5, 5, 7.5, 10 µM/mL of geraniin were examined using western blotting assay. Assay was done in triplicate and the outcomes were noted. The geraniin treatment appreciably modulated the PI3K/Akt pathway.
4.4. Effect of geraniin on the NF-κB expression in the C666-1 cells
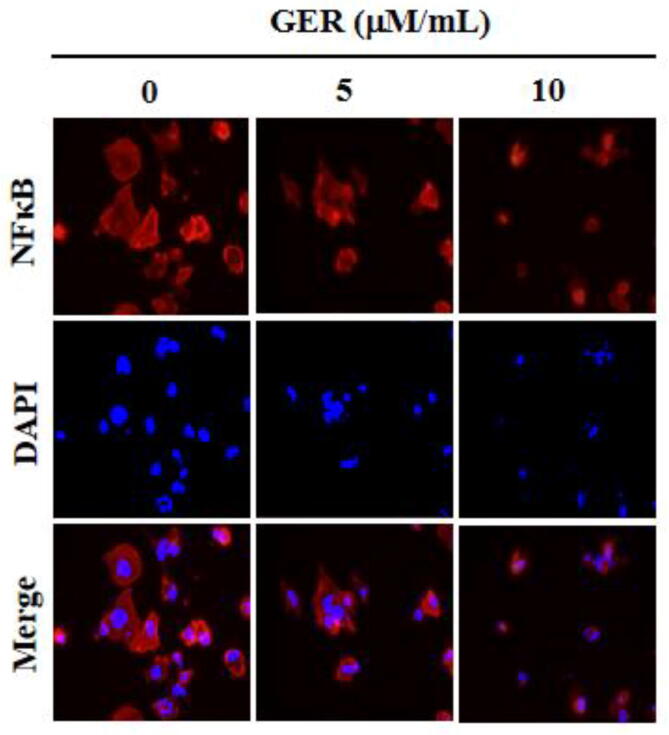
The inhibitory role of geraniin on the NF-κB expression in the C666-1 cells were investigated using immunofluorescence assay and the finding was illustrated in the Fig. 4. As depicted in the Fig. 4, the elevated expression of NF-κB expression was noted in the control C666-1 cells. Conversely, the NF-κB expression were remarkably decreased in the C666-1 cells, which is supplemented with the 5 and 10 µM/mL of geraniin. This finding evidenced that the geraniin was appreciably blocked the NF-κB expression in the NPC C666-1 cells.
Fig. 4.
Effect of geraniin on the NF-κB expression in the C666-1 cells. The NF-κB expression in the 5 and 10 µM/mL of geraniin treated C666-1 cells were investigated using immunofluorescence assay. Assay was done in triplicate and the outcomes were noted. The remarkable reduction in the NF-κB expression was noted in the geraniin supplemented C666-1 cells.
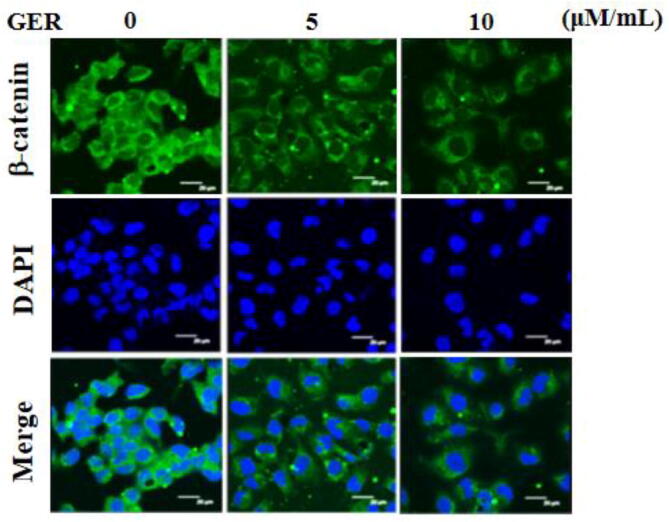
4.5. Effect of geraniin on the β-catenin expression in the C666-1 cells
The impacts of geraniin on the expression of β-catenin in the NPC C666-1 cells were examined by immunofluorescence analysis and the finding was illustrated in Fig. 5. The untreated C666-1 cells were displayed the improved expression of β-catenin. In contrast, the diminished expression of β-catenin was witnessed in the C666-1 cells, which is administered with the 5 and 10 µM/mL of geraniin. Hence, it was clear that the geraniin could block the expression of β-catenin in the C666-1 cells (Fig. 5).
Fig. 5.
Effect of geraniin on the β-catenin expression in the C666-1 cells. The 5 and 10 µM/mL of geraniin administered C666-1 cells were subjected to detect the β-catenin expression by immunofluorescence assay. Assay was done in triplicate and the outcomes were noted. The decreased β-catenin expression was witnessed in the geraniin supplemented C666-1 cells.
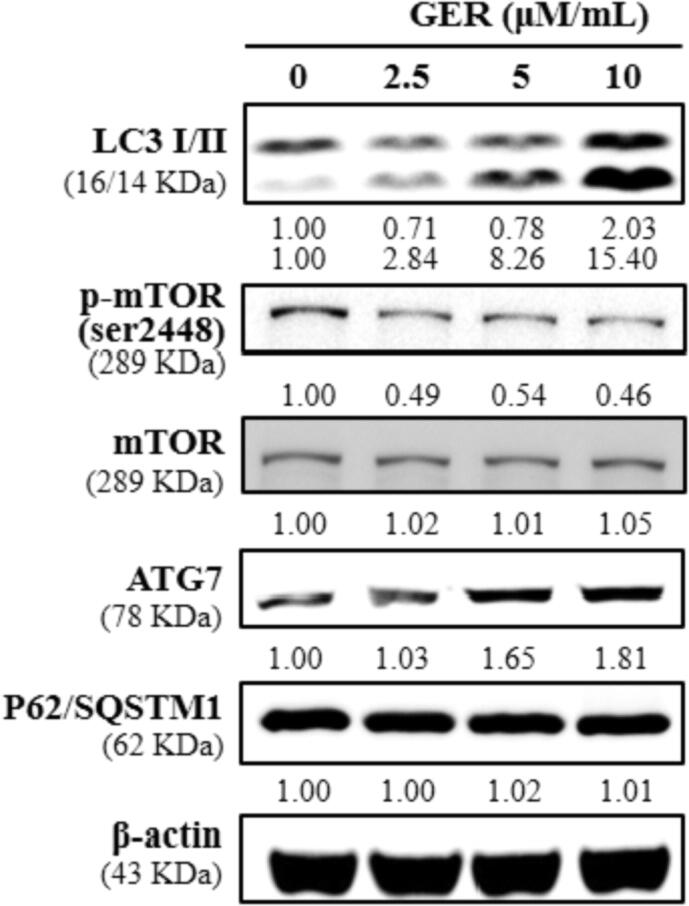
4.6. Effect of geraniin on the expressions of LC3, ATG7, and P62/SQSTM1 in the C666-1 cells
The impacts of geraniin on the expressions of autophagic biomarkers i.e. LC3, ATG7, and P62/SQSTM1 in the C666-1 cells were inspected using western blotting and the finding was displayed in Fig. 6. Our finding clearly showed that the geraniin treatment at 2.5, 5, and 10 µM/mL was appreciably improved the expressions of LC3 I/II and ATG7 in the C666-1 cells. The geraniin treatment slightly improved the mTOR expression and decreased the phosphorylation of mTOR in the C666-1 cells. Additionally, the geraniin treatment did not displayed notable changes on the expressions of P62/SQSTM1 in the C666-1 cells. This findings proposed that the geraniin appreciably improved the autophagic markers in the C666-1 cells (Fig. 6).
Fig. 6.
Effect of geraniin on the expressions of LC3, ATG7, and P62/SQSTM1 in the C666-1 cells. The expressions of autophagy related proteins i.e. LC3, ATG7, and P62/SQSTM1 in the 2.5, 5, 7.5, 10 µM/mL of geraniin treated C666-1 cells were investigated using western blotting analysis. Assay was done in triplicate and the outcomes were noted. The geraniin treatment improved the LC3 and ATG7 expressions and did not affected the P62/SQSTM1 expression in the C666-1 cells.
5. Discussion
NPC is a rare malignant tumor developing from the epithelial cell linings of the nasopharynx (Ferlay et al., 2015). In recent times, autophagy was reported to be dysregulated in the NPC cells and autophagy sensitize the tumor cells to the chemotherapy (Lin et al., 2014).
Autophagy is a vital event that plays a double-edged functions in mediating the tumor development that is connected with various cell types and specific tumor development stages. Many studies has mentioned that the detection of LC3-I/II is regularly operated as an biomarker of autophagy (Tanida et al., 2004). As mentioned earlier, the deficient in autophagy demonstrates the positive impact on the malignant transformation, signifying that autophagy acts as the tumor suppressor. Though, extreme autophagy may result in the cell necrosis in many cancer cells, preserving the regular cellular mechanisms via stimulating autophagy (Lorente et al., 2018). Nonetheless, autophagy has the pro-survival impact, hence exploring the hopeful options to provoke the autophagy was believed to be hopeful target to inhibit the tumor development (Lin et al., 2020). The present research has focused to discover the anticancer role of geraniin against the NPC C666-1 cells via up-regulating autophagic biomarker expressions. Similarly, a preceding study has mentioned that the curcumin provoked autophagy in pancreatic cancer cells that was contributed to the tumor growth suppression (Zhu and Bu, 2017). A clinical research also stated that autophagy is enhanced followed by the cancer therapy (Chung et al., 2020). In our study, we also evidenced that the geraniin sensitized the C666-1 cells to the autophagy via up-regulating the autophagic markers.
The p62/SQSTM1 is an selective autophagy adaptor, demonstrates the crucial functions in the identification of cytoplasmic protein insertion bodies, a event of conformational ailments like neurodegenerative and several chronic disorders. p62 has the innate capacity to aggregate/polymerize and an potential to precisely identify the substrates (Ichimura and Komatsu, 2010). p62 contributes to the direct protein–protein communications with ubiquitinated proteins via its ubiquitin-associated domain and with LC-3 confined to the separation membrane through the LC3-interacting region motif, thereby enabling the ubiquitinated protein sequestrations in a nascent autophagosome (Matsumoto et al., 2011). Our finding demonstrated that the geraniin treatment demonstrated a very slight increase on the p62/SQSTM1 expressions in the C666-1 cells.
The ATG7 regulates many critical processes during the tumorigenesis that can stimulate cell apoptosis and autophagy via controlling several caspase species (Baquero et al., 2015). Several researches demonstrate that ATG7 is contributed to cell apoptosis of numerous tumors, and it is thought to be a novel target in the case of cancer treatment. Zeng et al. (2015) specified that the miR137 impacts apoptosis of glioblastoma cell via regulating ATG7 in U87 cells. In agreement with these reports, here we also witnessed that the geraniin treatment effectively up-regulated the ATG7 expression in the C666-1 cells.
The increasing reports were highlighted that the autophagy performs the critical functions in the NPC development. As agreeing with this statement, Tan et al. (2019) reported that the triggering autophagy via cisplatin is one of the extensively employed chemotherapeutic option for the NPC treatment. Whereas, the drug-resistance and deleterious side effects of cisplatin often experienced in the NPC patients (Hsieh et al., 2019). Consequently, the exploration of new therapeutic agent from natural sources with possessing toxicity to normal tissues were highly demanded in recent times to efficiently treat the NPC via triggering autophagy. Similarly, here we evidenced that the geraniin treatment appreciably improved the autophagy in the C666-1 cells via up-regulating the autophagic markers i.e. LC3 and ATG7 expressions.
The intracellular ROS accumulation has been reported to facilitate the autophagy in several tumor cells (Poillet-Perez et al., 2015). A drastic elevation in the ROS accumulation could provoke the tumor cell cycle arrest and apoptosis. This could be reached with the chemotherapy and antioxidants lessening in tumor cells (Cadenas, 2004). A preceding research has demonstrated that the elevation in the ROS could improve the cisplatin-sensitivity in lung cancer cells. The improved ROS production connected with the cellular autophagy. Various researches has highlighted the participation of ROS in the regulation of autophagy (Ferro et al., 2019). Similarly, we also witnessed that the geraniin appreciably improved the ROS accumulation in the C666-1 cells.
The up-regulation of β-catenin expression in cancer cells were tightly allied with the resistance to the radiotherapy (Chen et al., 2007). In this decade, the NPC patients who experienced chemoradiotherapy has the poor life quality, along with serious adverse effects. The dysregulation in the β-catenin expression was tightly connected with the initiation and progression of several cancers (Clevers and Nusse, 2012). Likewise, our finding has proved that the geraniin treatment appreciably decreased the β-catenin expression in the C666-1 cells.
NF-κB is a vital multipotent transcription factor responsible for several cellular inflammatory responses (Hayden and Ghosh, 2012). Researches has unveiled that the stimulation of NF-κB expression is tightly connected with the EBV infection, the development of immortal nasopharyngeal epithelial cells, accumulation of tumor stem cells, and reprogramming of metabolism. Many researches has recognized the vital functions of NF-κB in the survival, multiplication, and cell cycle regulation, adhesion, and drug-resistance of the tumor cells during therapy (Yi et al., 2018). In several tumors, the NF-κB is uncoupled from its regular modes of regulation and demonstrates elevated activity (Basseres and Baldwin, 2006, Biswas and Iglehart, 2006). The NF-κB is reported to enhance the tumorigenesis, and the proteins transcribed by NF-κB may elevate the tumor development. NF-κB may speed up the tumorigenic process via hindering the tumor cell apoptosis (Shin et al., 2016). It was reported that the NF-κB could be a crucial marker in the early detection of tumors (Zheng et al., 2016). Here, we witnessed that the geraniin effectively suppressed the NF-κB in the C666-1 cells.
The PI3K/Akt/mTOR signaling axis has the vital roles in the progression of NPC via facilitating cell proliferation and hindering apoptosis (Lin et al., 2018). It was previously been reported to be connected to cell functions like multiplication, migration, and metastasis. The PI3K/AKT/mTOR signaling could be activated through manifold events, all of that elevate the stimulation of the signaling in cancer cells (Yang et al., 2017, Zhong et al., 2017). A preclinical research exhibited that the AKT/mTOR pathway is normally stimulated in variety of tumors and hindering of this cascade may elevate chemosensitivity, which recommends that the AKT/mTOR axis could be hopeful target for the NPC treatment (Lin et al., 2017). Moreover, a growing evidences has highlighted that the AKT/mTOR cascade controls the autophagy that can be define the survival and necrosis of calls and performs a crucial functions in the tumorigenesis (Wang et al., 2018, Wu et al., 2018). The stimulation of AKT was already reported to hinder autophagy via regulating mTOR signaling (Wu et al., 2020). The dysregulation in the PI3K/Akt/mTOR signaling axis is tightly connected with the malignant conversion and apoptosis of cancer cells, radiotherapy resistance, and metastasis of cancer tissues (Ni et al., 2014). In this work, our findings evidenced that the geraniin appreciably blocked the PI3K/Akt/mTOR signaling in the C666-1 cells, which facilitates to the cell growth inhibition via stimulating apoptosis/autophagy in C666-1 cells.
6. Conclusion
In conclusion, our results evidenced that geraniin could inhibit the NPC C666-1 cell proliferation and trigger autophagy-mediated cell death. The ROS accumulation and cell viability inhibition with the presence of apoptosis inhibitor were highly initiated in geraniin-supplemented C666-1 cells. Moreover, geraniin appreciably modulated the PI3K/Akt/mTOR signaling and improved the LC3 and ATG7 expressions in the C666-1, which is necessary for the autophagy initiation. These findings justified the in vitro anticancer role of geraniin and it could be a talented and efficient candidate for the management of human NPC in the future. Also, more researches are still needed in future particularly for its clinical applications in order to develop the novel chemotherapeutic agent.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was funded by Yunnan first people's Hospital Clinical center open program and Yunnan Natural Science Foundation of China(2021LCZXXFHX07).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad S.A.A., Palanisamy U.D., Tejo B., Chew M.F., Tham H.W., Hassan S.S. Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol. J. 2017;14:229. doi: 10.1186/s12985-017-0895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero P., Karvela M., Mitchell R., Mukhopadhyay A., Pellicano F., Hopcroft L., Holyoake T.L., Helgason G.V. The autophagy related protein ATG7 regulates differentiation and energy metabolism of CML cells. Br. J. Haematol. 2015;6 [Google Scholar]
- Basseres D.S., Baldwin A.S. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- Biswas D.K., Iglehart J.D. Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J. Cell Physiol. 2006;209(3):645–652. doi: 10.1002/jcp.20785. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Mitochondrial free radical production and cell signaling. Mol. Aspects Med. 2004;25(1-2):17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Casanova M., Özyar E., Patte C., Orbach D., Ferrari A., Veyrat-Follet C., Errihani H., Pan J., Zhang L., Shen L., Grzegorzewski K.J., Varan A. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5–fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother. Pharmacol. 2016;77(2):289–298. doi: 10.1007/s00280-015-2933-2. [DOI] [PubMed] [Google Scholar]
- Chen H.T., Liu H., Mao M.J., Tan Y., Mo X.Q., Meng X.J., Cao M.T., Zhong C.Y., Liu Y., Shan H., Jiang G.M. Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Mol. Cancer. 2019;18:101. doi: 10.1186/s12943-019-1030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.S., Woodward W.A., Behbod F., Peddibhotla S., Alfaro M.P., Buchholz T.A., Rosen J.M. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J. Cell Sci. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- Chen Y.-P., Chan A.T.C., Le Q.-T., Blanchard P., Sun Y., Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- Choi J.-G., Kim Y.S., Kim J.H., Chung H.-S. Antiviral activity of ethanol extract of Geranii Herba and its components against influenza viruses via neuraminidase inhibition. Sci. Rep. 2019;9:12132. doi: 10.1038/s41598-019-48430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A.P.Y.S., Ton S.H., Gurtu S., Palanisamy U.D. Ellagitannin geraniin supplementation ameliorates metabolic risks in high-fat diet-induced obese Sprague Dawley rats. J. Funct. Foods. 2014;9:173–182. [Google Scholar]
- Chung C., Seo W., Silwal P., Jo E.K. Crosstalks between inflammasome and autophagy in cancer. J. Hematol. Oncol. 2020;13:100. doi: 10.1186/s13045-020-00936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Denton D., Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26(4):605–616. doi: 10.1038/s41418-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Tang Y., Wang J., Xiong F., Guo C., Wang Y., Xiang B., Zhou M., Li X., Wu X.u., Li Y., Li X., Li G., Xiong W., Zeng Z. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J. Cancer. 2018;9(16):2852–2864. doi: 10.7150/jca.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. Int. J. Cancer. 2015;136:e359–e386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Ferro F., Servais S., Besson P., Roger S., Dumas J.F., Brisson L. Autophagy and mitophagy in cancer metabolic remodelling. Semin. Cell Dev. Biol. 2019 doi: 10.1016/j.semcdb.2019.05.029. [DOI] [PubMed] [Google Scholar]
- Freudlsperger C., Horn D., Weißfuß S., Weichert W., Weber K.-J., Saure D., Sharma S., Dyckhoff G., Grabe N., Plinkert P., Hoffmann J., Freier K., Hess J. Phosphorylation of AKT(Ser473) serves as an independent prognostic marker for radiosensitivity in advanced head and neck squamous cell carcinoma. Int. J. Cancer. 2015;136(12):2775–2785. doi: 10.1002/ijc.29328. [DOI] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M.-J., Wang C.-W., Lin J.-T., Chuang Y.-C., Hsi Y.-T., Lo Y.-S., Lin C.-C., Chen M.-K. Celastrol, a plant-derived triterpene, induces cisplatin-resistance nasopharyngeal carcinoma cancer cell apoptosis though ERK1/ 2 and p38 MAPK signaling pathway. Phytomedicine. 2019;58:152805. doi: 10.1016/j.phymed.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Ichimura Y., Komatsu M. Selective degradation of p62 by autophagy. Semin. Immunopathol. 2010;32(4):431–436. doi: 10.1007/s00281-010-0220-1. [DOI] [PubMed] [Google Scholar]
- Ito H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011;77(11):1110–1115. doi: 10.1055/s-0030-1270749. [DOI] [PubMed] [Google Scholar]
- Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Huang H., Zhou W., Feng M., Zhou P. Anti-hepatitis B virus activities of Geranium carolinianum L. Extracts and identification of the active components. Biol. Pharm. Bull. 2008;31(4):743–747. doi: 10.1248/bpb.31.743. [DOI] [PubMed] [Google Scholar]
- Limpert A.S., Lambert L.J., Bakas N.A., Bata N., Brun S.N., Shaw R.J., Cosford N.D.P. Autophagy in cancer: regulation by small molecules. Trends Pharmacol. Sci. 2018;39(12):1021–1032. doi: 10.1016/j.tips.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D.-C., Meng X., Hazawa M., Nagata Y., Varela A.M., Xu L., Sato Y., Liu L.-Z., Ding L.-W., Sharma A., Goh B.C., Lee S.C., Petersson B.F., Yu F.G., Macary P., Oo M.Z., Ha C.S., Yang H., Ogawa S., Loh K.S., Koeffler H.P. The genomic landscape of nasopharyngeal carcinoma. Nat. Genet. 2014;46(8):866–871. doi: 10.1038/ng.3006. [DOI] [PubMed] [Google Scholar]
- Lin Y.-T., Wang H.-C., Chuang H.-C., Hsu Y.-C., Yang M.-Y., Chien C.-Y. Pre-treatment with angiotensin-(1–7) inhibits tumor growth via autophagy by downregulating PI3K/Akt/mTOR signaling in human nasopharyngeal carcinoma xenografts. J. Mol. Med. (Berl.) 2018;96(12):1407–1418. doi: 10.1007/s00109-018-1704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.T., Wang H.C., Hsu Y.C., Cho C.L., Yang M.Y., Chien C.Y. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. Int. J. Mol. Sci. 2017;18:7. doi: 10.3390/ijms18071343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.M., Wang Z.Y., Zhou X.W., Zhang M., Gao D.F., Zhang L., Wang P., Chen Y., Lin Y.X., Zhao B.X., Miao J.Y., Kong F. Discovery of new fluorescent thiazole-pyrazoline derivatives as autophagy inducers by inhibiting mTOR activity in A549 human lung cancer cells. Cell Death Dis. 2020;11:551. doi: 10.1038/s41419-020-02746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Perez O., Badiola J.J., Bolea R., Ferrer I., Llorens F., Martin-Burriel I. An update on autophagy in prion diseases. Front. Bioeng. Biotechnol. 2020;8:975. doi: 10.3389/fbioe.2020.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente J., Velandia C., Leal J.A., Garcia-Mayea Y., Lyakhovich A., Kondoh H., LLeonart ME The interplay between autophagy and tumorigenesis: exploiting autophagy as a means of anticancer therapy. Biol. Rev. Camb. Philos. Soc. 2018;93:152–165. doi: 10.1111/brv.12337. [DOI] [PubMed] [Google Scholar]
- Matsumoto G., Wada K., Okuno M., Kurosawa M., Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol. Cell. 2011;44(2):279–289. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- Moreno E., Gayosso J.A., Montejano José.R., Almaguer G., Vázquez N., Cruz C., Mercado A., Bobadilla N.A., Gamba G., Sierra A., Ramírez V. Geraniin is a diuretic by inhibiting the Na+-K+-2Cl cotransporter NKCC2. Am. J. Physiol. Renal. Physiol. 2018;314(2):F240–F250. doi: 10.1152/ajprenal.00221.2017. [DOI] [PubMed] [Google Scholar]
- Mulcahy L.J.M., Thorburn A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27:843–857. doi: 10.1038/s41418-019-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J., Cozzi P., Hao J., Duan W., Graham P., Kearsley J., Li Y. Cancer stem cells in prostate cancer chemoresistance. Curr. Cancer Drug Targets. 2014;14:225–240. doi: 10.2174/1568009614666140328152459. [DOI] [PubMed] [Google Scholar]
- Okabe S., Suganuma M., Imayoshi Y., Taniguchi S., Yoshida T., Fujiki H. New TNF-areleasing inhibitors, geraniin and corilagin, in leaves of acer nikoense, megusurino-ki. Biol. Pharm. Bull. 2001;24(10):1145–1148. doi: 10.1248/bpb.24.1145. [DOI] [PubMed] [Google Scholar]
- Sidaway P. Induction chemotherapy improves efficacy. Nat. Rev. Clin. Oncol. 2019;16:528. doi: 10.1038/s41571-019-0242-0. [DOI] [PubMed] [Google Scholar]
- Poillet-Perez L., Despouy G., Delage-Mourroux Régis, Boyer-Guittaut M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015;4:184–192. doi: 10.1016/j.redox.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.Y., Kim C.G., Jung Y.J., Lim Y., Lee Y.H. The UPR inducer DPP23 inhibits the metastatic potential of MDA–MB–231 human breast cancer cells by targeting the Akt–IKK–NF-κB–MMP–9 axis. Sci. Rep. 2016;6:34134. doi: 10.1038/srep34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G., Wang X., Tang Y., Cen W., Li Z., Wang G., Jiang J., Wang X. PP-22 promotes autophagy and apoptosis in the nasopharyngeal carcinoma cell line CNE-2 by inducing endoplasmic reticulum stress, downregulating STAT3 signaling, and modulating the MAPK pathway. J. Cell. Physiol. 2019;234(3):2618–2630. doi: 10.1002/jcp.27076. [DOI] [PubMed] [Google Scholar]
- Tanida I., Ueno T., Kominami E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004;36(12):2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li J., Cao N., Li Z., Han J., Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018;11:7777–7786. doi: 10.2147/OTT.S159095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wan D., Zhou R., Zhong W., Lu S., Chai Y. Geraniin inhibits migration and invasion of human osteosarcoma cancer cells through regulation of PI3K/Akt andERK1/2 signaling pathways. Anti Cancer Drugs. 2017;28(9):959–966. doi: 10.1097/CAD.0000000000000535. [DOI] [PubMed] [Google Scholar]
- Wee P., Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 2017;9(5):52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wang H., Teng T., Duan S., Ji A., Li Y. Hydrogen sulfide and autophagy: a double edged sword. Pharmacol. Res. 2018;131:120–127. doi: 10.1016/j.phrs.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang Y., Qin X., Geng H., Zuo D., Zhao Q. PI3K/AKT/mTOR pathway-related long non-coding RNAs: roles and mechanisms in hepatocellular carcinoma. Pharmacol. Res. 2020;160:105195. doi: 10.1016/j.phrs.2020.105195. [DOI] [PubMed] [Google Scholar]
- Xiao F., Zhai Z., Jiang C., Liu X., Li H., Qu X., Ouyang Z., Fan Q., Tang T., Qin A., Gu D. Geraniin suppresses RANKL-induced osteoclastogenesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp. Cell Res. 2015;330(1):91–101. doi: 10.1016/j.yexcr.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Xiong J. Atg7 in development and disease: panacea or Pandora’s Box? Protein Cell. 2015;6(10):722–734. doi: 10.1007/s13238-015-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Qu Y.-J., Cheng Y., Liang T., Zhang M.-N., Zhang D., Dong L.-N., Wang X.-W., Zhang G.-M. Endoplasmic reticulum stress regulates proliferation, migration and invasion of human ovarian cancer SKOV3 cells through PI3K/AKT/mTOR signaling pathway. Cancer Biomark. 2017;19(3):263–269. doi: 10.3233/CBM-160424. [DOI] [PubMed] [Google Scholar]
- Yang Y.-C., Li J., Zu Y.-G., Fu Y.-J., Luo M., Wu N., Liu X.-L. Optimisation of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chem. 2010;122(1):373–380. [Google Scholar]
- Yeh C., Hsieh M., Yang J., Yang S., Chuang Y., Su S., Liang M., Chen M., Lin C. Geraniin inhibits oral cancer cell migration by suppressing matrix metalloproteinase-2 activation through the FAK/Src and ERK pathways. Environ. Toxicol. 2019;34(10):1085–1093. doi: 10.1002/tox.22809. [DOI] [PubMed] [Google Scholar]
- Yi M., Cai J., Li J., Chen S., Zeng Z., Peng Q., Ban Y., Zhou Y., Li X., Xiong W., Li G., Xiang B. Rediscovery of NF-κB signaling in nasopharyngeal carcinoma: How genetic defects of NF-kappaB pathway interplay with EBV in driving oncogenesis? J. Cell Physiol. 2018;233:5537–5549. doi: 10.1002/jcp.26410. [DOI] [PubMed] [Google Scholar]
- Yoshii S.R., Mizushima N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017;18(9):1865. doi: 10.3390/ijms18091865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Huo G., Mo Y., Wang W., Chen H. MIR137 regulates starvation-induced autophagy by targeting ATG7. J. Mol. Neurosci. 2015;56(4):815–821. doi: 10.1007/s12031-015-0514-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen L., Hu G.Q., et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N. Engl. J. Med. 2019;381:1124–1135. doi: 10.1056/NEJMoa1905287. [DOI] [PubMed] [Google Scholar]
- Zhao M., Luo R., Liu Y., Gao L., Fu Z., Fu Q., Luo X., Chen Y., Deng X., Liang Z., Li X., Cheng C., Liu Z., Fang W. miR–3188 regulates nasopharyngeal carcinoma proliferation and chemosensitivity through a FOXO1–modulated positive feedback loop with mTOR–p–PI3K/AKT–c–JUN. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Dai W., Cheung A.K.L., Ko J.M.Y., Kan R., Wong B.W.Y., Leong M.M.L., Deng M., Kwok T.C.T., Chan J.-W., Kwong D.-W., Lee A.-M., Ng W.T., Ngan R.K.C., Yau C.C., Tung S., Lee V.-fun., Lam K.-O., Kwan C.K., Li W.S., Yau S., Chan K.-W., Lung M.L. Whole–exome sequencing identifies multiple loss–of–function mutations of κB pathway regulators in nasopharyngeal carcinoma. Proc. Natl. Acad. Sci. U.S.A. 2016;113(40):11283–11288. doi: 10.1073/pnas.1607606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.T., Yu J., Wang H.J., Shi Y., Zhao T.S., He B.X., Qiao B., Feng Z.W. Effects of endoplasmic reticulum stress on the autophagy, apoptosis, and chemotherapy resistance of human breast cancer cells by regulating the PI3K/AKT/mTOR signaling pathway. Tumour Biol. 2017;39 doi: 10.1177/1010428317697562. 1010428317697562. [DOI] [PubMed] [Google Scholar]
- Zhu Y.P., Bu S.R. Curcumin induces autophagy, apoptosis, and cell cycle arrest in human pancreatic cancer cells. Evid. Complement. Alternat. Med. 2017;2017:5787218. doi: 10.1155/2017/5787218. [DOI] [PMC free article] [PubMed] [Google Scholar]