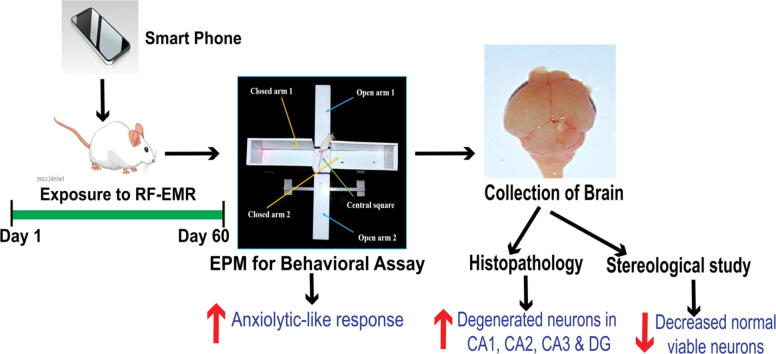
Graphical abstract
Keyword: Electromagnetic fields, Mobile phone, Mice, Behavior, Hippocampus, Pyramidal neuron
Abstract
Electromagnetic field exposure to the nervous system can cause neurological changes. The effects of extremely low-frequency electromagnetic fields, such as second-generation and third-generation radiation, have been studied in most studies. The current study aimed to explore fourth-generation cellular phone radiation on hippocampal morphology and behavior in mice. Swiss albino male mice (n = 30) were randomly categorized into 3 groups; control, 40 min, and 60 min exposure to 2400 MHz radiofrequency electromagnetic radiation (RF-EMR) daily for 60 days. The control mice were housed in the same environments but were not exposed to anything. Anxiety-like behaviors were tested using the elevated plus-maze. For histological and stereological examination, the brain was dissected from the cranial cavity. On Cresyl violet stained brain slices, the number of pyramidal neurons in the cornu ammonis of the hippocampus were counted. In exposed mice compared to control mice, a significant increase in anxiety-like behavior has been observed. Histological observations have shown many black and dark blue cytoplasmic cells with shrunken morphology degenerative alterations in the neuronal hippocampus in the radiation exposed mice. In the RF-EMR mouse hippocampus, stereological analyses revealed a significant decrease in pyramidal and granule neurons compared to controls. Our findings suggest that 2400-MHz RF-EMR cell phone radiation affects the structural integrity of the hippocampus, which would lead to behavioral changes such as anxiety. However, it alerts us to the possible long-term detrimental effects of exposure to RF-EMR.
1. Introduction
Mobile phones emit radiofrequency electromagnetic radiation (RF-EMR) surrounding them, which has negative health consequences. RF-EMR exposure can contribute to neurological and physiological alterations and behavioral abnormalities in adolescents and young adults. The radiation emitted from the mobile phone not only damages the brain and creates cognitive and behavioral deterioration (Cassel et al., 2004). Numerous experimental investigations on the impact of electromagnetic fields (EMF) on the brain and nervous system have been conducted (Bas et al., 2009a, Chen et al., 2014, Odaci et al., 2008). During the conversation with mobile phones, people usually bring one’s cellphone near to their heads. As a result, the brain is more exposed to radiation than any other body part (Irmak et al., 2002). The frequency range of RF-EMR of mobile phones using the Global System for Mobile communication (GSM) system is 300 MHz (MHz) to 300 GHz (GHz) (Dubreuil et al., 2002). Smartphones operate on the 900–2600 MHz frequency all around the world. Male mice were exposed to 2400 MHz frequency RF-EMR for a long time in our study.
Previous findings have shown that oxidative stress responds to exposure to RF-EMR (Consales et al., 2012, Yüksel et al., 2016, Yurekli et al., 2006). Oxidants could interrupt cellular membrane enzyme activity, impair cellular activities, and perhaps lead to cell death (Achudume et al., 2012, Hasan et al., 2021b, Karim et al., 2020, Kobir et al., 2020). Most noticeable behavioral and cognitive disorders of the central nervous system identified in both people and animals regarding long-term smartphone radiation exposure (Babadi-Akashe et al., 2014, Söderqvist et al., 2015, Zhao et al., 2012). Exposure to EMF has indeed been documented to have a variety of neurological consequences, such as decreased number of Cornu Ammonis (CA) pyramidal and Purkinje cells (Aslan et al., 2017, Baş et al., 2013;Odaci et al., 2008, Şahin et al., 2015), increased dark cell cytoplasm (Salford et al., 2003), behavioral changes (Ikinci et al., 2013) and concentration disturbances (Durusoy et al., 2017). Many research focused on behavioral problems caused by RF-EMR exposure, including learning and memory loss in rats (Lai et al., 1994, Wang and Lai, 2000). They reported that low-frequency RF-EMR affects spatial memory and places learning tasks in rodents through water mazes or radial arm mazes. Such findings suggest that people have no conscious awareness of EMF consequences due to the extensive overuse of mobile phones. EMF exposure has many reversible or permanent effects on nerve tissue. No long-term studies on the consequences of 2400 MHz fourth-generation (4G) cell phone radiation exposure on the nervous system have been done.
Therefore, this study has been conducted to explored and evaluate the impact of a 2400 MHz 4G RF-EMR from a cell phone on the brain of mice models, with particular attention to hippocampus morphology.
2. Materials and methods
2.1. Procedures involving animals and ethics
The study took place in the Anatomy and Histology Department, Faculty of Veterinary Science, Bangladesh. This study was approved by the Bangladesh Agricultural University Animal Ethics Committee (AWEEC/BAU/2019–46). The International Center for Diarrheal Disease Research (ICDDRB), Mohakhali, Dhaka, supplied healthy Swiss male albino mice (6 weeks old). At the time of collection, the average physical weight of the mice was 29 ± 5 g. The mice were housed in plastic cages 52 cm × 36 cm × 25 cm in a regulated temperature (24 ± 2 °C) setting with 12-hour light and 12 h dark cycle, 40 percent humidity, and regular feed and ad libitum drinking water.
2.2. Groups of experiment
Thirty male mice were used in this study. The mice were categorized into three groups (Groups I, II, and III) after 1-week acclimatization; each group contained ten mice (n = 10). The body weights were documented before radiation exposure begins. Group I was considered to control fed mice standard diet and fresh drinking water ad libitum without radiation exposure. Group II: Mice were exposed to 2400 MHz 4G modulated RF-EMR for 40 min/day for two months. Group III: Mice were exposed to 2400 MHz 4G modulated RF-EMR for 60 min/day for two months, simultaneously. Each group was kept in a rectangular plastic cage enclosed in a compartmentalized wire mesh.
2.3. Radiofrequency electromagnetic radiation (RF-EMR) exposure system
In this experiment, two 4G interconnected 2400 MHz mobile phones (Huawei GR5 2017) were used for radiation exposure with a Specific Absorption Rate (SAR) of 2.7 W/kg. During the exposure period, test animals were placed in plastic cages in the EMF exposure room. There are no other electric devices such as a PC, laptop, camera, or any EMF emitting sources in the EMF exposure room. The control room is separate from the EMF exposure room. The elevated plus-maze was situated in another room next to the EMF exposure room for the behavioral study. So, we ensured that there were no other sources of EMF. For uniform radiation exposure during the exposure time, the automatic answer mode mobile phone was positioned in the center of the mouse cage's roof (Kumar et al., 2009, Narayanan et al., 2009a). During the RF-EMR application, the temperature and humidity of the exposure room and the mouse cage were monitored daily with a digital thermometer. When radiation exposure was provided to mice, the intensity of electromagnetic radiation released by cell phones was measured in an interactive active call by the ED-78S Electrosmog frequency meter (CORNET Microsystem Inc., Santa Clara, CA 95,050 USA) with frequencies ranging from 100 kHz to 2.5 GHz and ensured that radiation exposure was evenly distributed on mice. The average power density in this study just before the radiation exposure was 530 µW/cm2 and during the radiation exposure was 738 µW/cm2. Every day, before RF-EMR exposure began, the intensity of RF-EMR of the mobile phone was determined. A call was given from another 4G connected 2400 MHz mobile phone to expose the mice. The mobile phone batteries were charged continuously. The experimental groups were continuously exposed to RF-EMR from mobile phones from 10.00 AM to 10.40 AM and 10.00 AM to 11.00 AM, daily for 60 days. Under standard lab conditions, the mice of the control group were maintained without exposure to electromagnetic radiation. The electric field or power density of radiofrequency electromagnetic radiation where the control group was also measured by the ED-78S Electrosmog frequency meter (CORNET Microsystem Inc., Santa Clara, CA 95,050 USA) and the frequencies ranging from 100 kHz to 2.5 GHz. This exposure system is similar to that used in earlier studies for the applications of 900 to 2400-MHz RF-EMR (Hasan et al., 2021a, Hasan and Islam, 2020, Narayanan et al., 2009b, Saikhedkar et al., 2014, Sokolovic et al., 2008).
2.4. Behavioural study
The elevated plus maze (EPM) is a method of assessing fear and anxiety-like responses in mice based on their natural preference for open, elevated areas (Pellow et al., 1985). In our study, the EPM framework was made from wood consisting of four arms (50 cm in length × 5 cm in wide) attached to the central pillar (5 cm × 5 cm): 40 cm high fences in closed arms but open arms have no wall. The maze is standing with four legs 70 cm above the floor. Mice are allowed to access all arms and walk freely around them. The closed arms, therefore, gave the animals a safe zone. Above the maze, a video camera was installed, and behind a white curtain, a monitor was placed. The testing room consisted of a large laboratory without sound attenuation facilities. After two months of exposure, all mice were tested. At the time of behavior testing, individual mice were weighed daily. Mice were familiar with the examination room half an hour before the behavior examination. Behavioral assessment was performed in a luminous red room. The EPM examination was conducted even though explained previously (Kauppila et al., 1991, Liu et al., 2002). On the central square, the Mice were placed with an open arm and allowed to explore the maze for five minutes; following that, the mouse was brought from the maze to its cage.
Before a new mouse was tested, the EPM apparatus was cleaned up with 20 percent alcohol to eliminate any putrid odor index. The movement and other behavioral activities of each mouse in the EPM were recorded used a video camera which was then analyzed using the automated tracking program Ethovision XT 10.1 version (Fig. 1A). The number of open and closed arms entrances and the length of time spent in the open arms were considered indications of fear and anxiety-like behavior. When the mouse had entirely reached the arm (4 legs in the arm), it counted one entry in a particular arm. The frequency of open and closed arms entrances, as well as the length of time spent on open and closed arms as well as the number of head dipping (mouse lowered its head toward the floor over the sides of the open arm), and rearing (mouse standing on hind legs with front paws against walls of the maze) behaviors were calculated and recorded from the plus-maze. The increase in the number of mice entering and the duration of stay in the open arms was considered an indicator of reduced anxiety (Pellow et al., 1985). The mice were sacrificed after the behavioral test, and the brain tissue was collected and separated from the hippocampus for histopathological study after proper perfusion fixation.
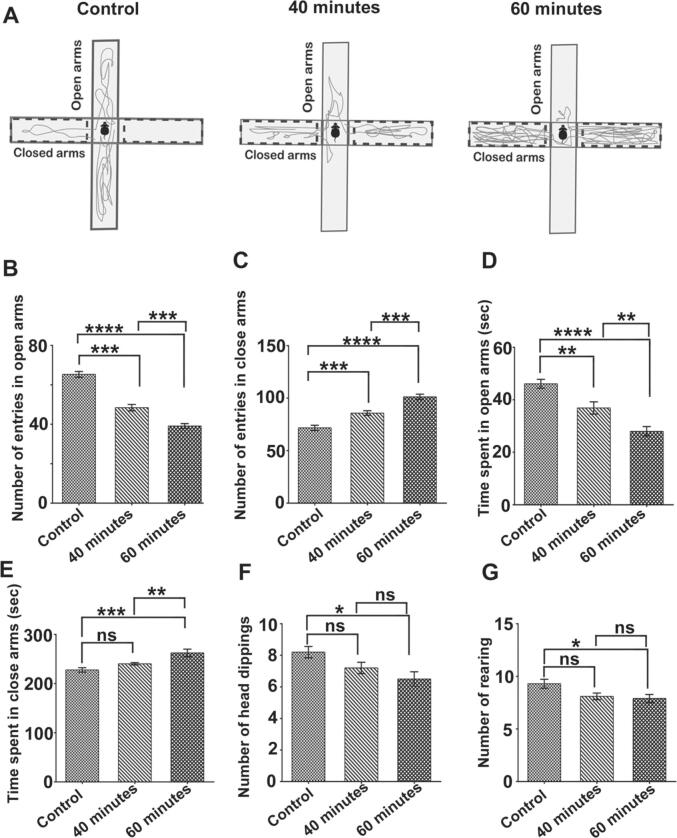
Fig. 1.
(A). The movement track report of the elevated plus maze (EPM). (B-G) Histograms showing the behavioral parameters of the EPM test in the different experimental groups. The 40 and 60 min of exposed mice had significantly decreased in entry (B) and time spent (D) in the open arms relative to the control mice, while entry (C) and time spent (E) in closed arms were significantly increased. Compared to the 40-minute exposed mice, the 60-minute exposed mice had lower entrances (B) and time spent in the open arms (D). In 40 min, there were no significant changes in the number of head dippings (F) or rearing (G). However, a significant difference was observed in 60 min compared with the control mice. Results presented as Mean ± SEM (n = 10 mice in each group). ‘‘ns’’ indicates not significant (p > 0.05). (*) shows a statistically significant difference at the p < 0.05 level; (**) indicates a statistically significant difference at the p < 0.01 level; (***) indicates a statistically significant difference at the p < 0.001 level; (****) shows a statistically significant difference at the p < 0.0001 level.
2.5. Tissue processing for histopathological studies
Each mouse was weighed and placed on an autopsy board after being thoroughly anesthetized.
Then the mice were preserved by perfusion method. Then the brain was removed from the cranial cavity, and it was kept in 10% formalin solution for post-fixation of 48 h. The gross analysis took into consideration factors such as color and weight. For the histological study, the brain's left hemisphere was trimmed into 5 mm2 sizes. Then, the tissues were washed in PBS four times and each for 30 min; dehydration was done using an ascending grade of ethyl alcohol (70%, 80%, 95%, 100%, 100%, and 100%) solutions. The tissues were immersed in 70% ethyl alcohol overnight. The incubation time for 80% and 95% ethyl alcohol was 8 h; 100% ethyl alcohol was 2 h each time. The tissues were cleared in a series of xylene and embedded in paraffin. Then, a Leica rotary microtome was used, 5-µm thick serial slices were cut. Each mouse had twenty-five sections fixed sequentially on slides. Hematoxylin & Eosin (H & E) staining was performed for histopathological observation. Tissue processing and staining procedures have been carried out following (Drury, 1983).
Cresyl violet (CV) stain was also used for stereological analysis and quantification of neurons in the hippocampal subregions. The serial sections of the brain were deparaffinized in three changes of xylene for 5 min each. Then the sections were rehydrated in descending grade of ethyl alcohol of 5 concentrations for 5 min in each step. Then the sections were hydrated in deionized water for 5 min and finally stained with 0.1% aqueous solution of Cresyl violet stain for 20 min. Then the sections were differentiated with 70% alcohol for optimum staining and finally dehydrated with two changes of 90% absolute ethanol for 5 min each step. The sections were then air-dried for 10 min and cleared in two changes of xylene for 5 min each, and lastly, coverslipped with DPX.
2.6. Quantification of neurons in the hippocampus
In CV-stained brain sections, the numbers of normal viable neuron cells in different hippocampus areas were quantified. Fig. 3 displays areas that have been confined to the quantitative analysis of neurons in the hippocampus. The number of normal viable neurons in CA1, CA2, CA3 & DG regions of the hippocampus was anticipated for each animal. For Quantitative analysis, the brain sections were obtained from five different hippocampal areas of each group. The high-quality pictures were taken using a Nikon digital camera mounted to an Olympus microscope with 100X lenses (Olympus BX 51 photographic light microscope).
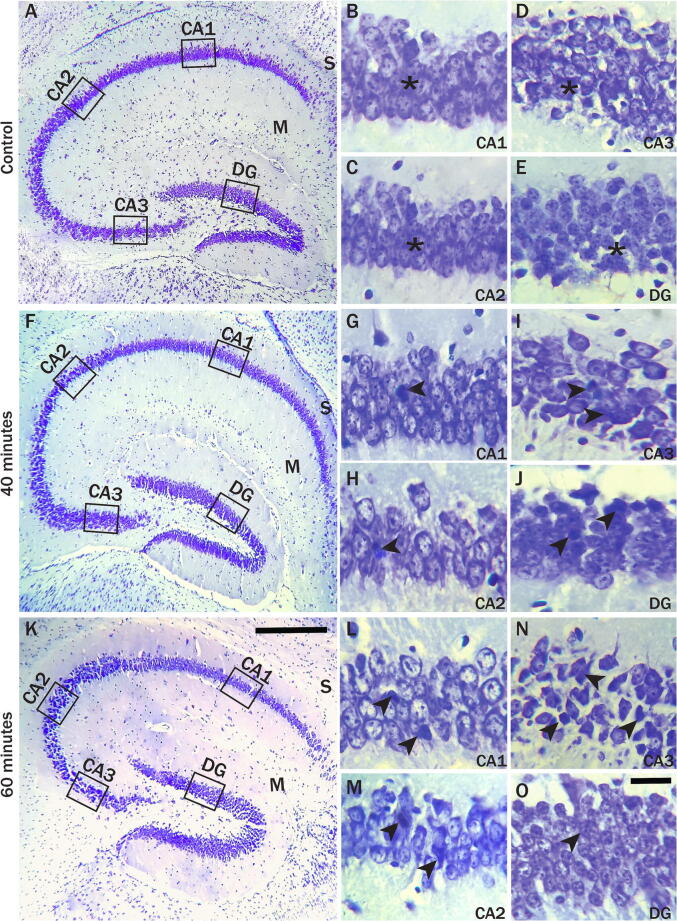
Fig. 3.
Cresyl violet stained sections hippocampus of control (A-E), 40 min (F-J), and 60 min (K-O) exposed mice formed by Cornu Ammonis (CA) as CA1, CA2 & CA3 areas. It continued as a subiculum (S). Around the upper and lower limbs of CA3, the dentate gyrus (DG) is located. In control mice, the star indicates healthy neurons. The nuclei of cells and Nissl granular constituents of the cytoplasm appeared dark violet in all four regions in control mice (B-E). The rest of the cytoplasm appeared lightly stained. In radiation-exposed mice, the arrowheads represent degenerated neurons with dark cytoplasm and shrinkage in the cell body. In 40 min, exposed mouse pyramidal cells in CA1, CA2, CA3 regions (G, H, I) and granule cells (J) in the DG of the showed degenerated with darkly stained nuclei (arrowhead). In CA1, CA2, CA3 regions (L, M, N) and granular layer of the DG (O) of 60 min exposed mice showed the increased number of disorganized degenerated with darkly stained shrunken morphology pyramidal neuron cells (arrowhead) compared to the control and 40 min mice. Scale bar = 100 μm in A, F, K, and 20 μm in B-E, G-J, and L-O. (n = 10 mice in each group).
The normal viable neurons were quantified using the imaging program NIS Elements Br version 4.30 software (Joy et al., 2018, Madhyastha et al., 2002, Massand et al., 2020). CA1, CA2, CA3 cornu ammonis sub-regions have a length of 350 µm, and a 50-μm × 100-μm rectangle for the dentate gyrus (DG) granular layer of the hippocampus was chosen for quantification of viable neurons in each hippocampal image. The cell counts in the CA1, CA, and CA3 regions were represented as the number of cells per unit length of the cell field (cells/350 µm length) and the granule cells in the dentate gyrus were presented as the number of cells per unit area (cells/150 µ2) (Govindaiah et al., 1997, Madhyastha et al., 2002, Wood et al., 1993). Degenerating neurons were characterized as deformed, shrunken, pyknotic, and hyperchromatic neurons, which had an indistinct border between the cytoplasm and nucleus. Neurons with a distinctive structure as well as a spherical nucleus were the normal assessment criteria. To avoid manual bias while counting the cells, slides from different groups of mice were coded. This same method of stereological analysis was also used by (Joy et al., 2018, Madhyastha et al., 2002).
2.7. Analysis of statistics
Graph pad prism software version 7.0 was used to conduct statistical analysis. To identify the differences between experimental control and exposed groups, one-way analysis of variance (ANOVA) followed by Tukeýs multiple comparison test was used. Statistical significance was considered as a P-value ≤ of 0.05.
3. Results
3.1. Effects of RF-EMR exposure on anxiety-like behaviors
In this study, an EPM test was performed to assess the mouse's anxiogenic-like behavior exposed to 2400 MHz RF-EMR for two months. The frequency of entrances and duration spent in EPM's close arms and open arms is a measure of anxiety in mice. All mice in the control group were healthy and active without any unusual behavioral alterations during the entire experimental period. During the experiment, the mice' health conditions and their water and food consumption were checked daily. The Track Report (Fig. 1A) clearly shows that the control mice spent a significant amount of time in the open arms, but the exposed mice spent a greater amount of time in the closed arms. This means that the exposed mice have shown a reduced exploratory behavior and are more anxious than the control. This study indicated a significant reduction in open arm entrances in exposed mice, reflecting anxiety symptoms. In 40 min (P ≤ 0.001), (P ≤ 0.01), and 60 min (P ≤ 0.0001), (P ≤ 0.0001) of exposed mice compared with control mice, the entries (Fig. 1B) and time spent (Fig. 1D) in the open arm was reduced significantly.
In contrast, entry (Fig. 1C) and time spent (Fig. 1E) in closed arms were significantly enhanced in 40 min (P ≤ 0.001), (P ≤ 0.0001), and 60 min (P ≤ 0.0001), (P ≤ 0.001) exposed mice, respectively. The frequency of head dippings (Fig. 1F) and the number of rearing (Fig. 1G) was not significantly different in 40 min. However, a significant difference was found in 60 min (P ≤ 0.05) than in the control mice. Statistical analysis indicated that 60 min of exposed mice showed a marked decline in open arm entry (Fig. 1B) and an increase in closed arm entry (Fig. 1C) compared with the 40 min group. It indicates that the mice exposed for 60 min (P ≤ 0.0001) showed higher fear and anxiety-like behavior.
3.2. Histopathological study
3.2.1. Staining with hematoxylin and eosin
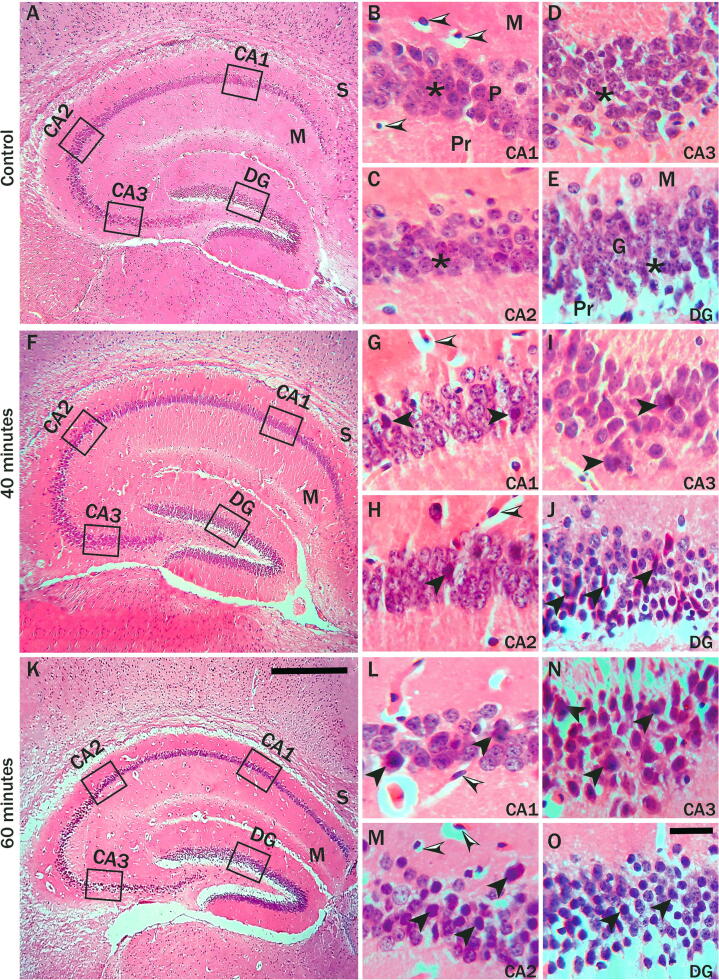
Microscopic observation of Hematoxylin and Eosin (H & E) stained hippocampus of control, 40- and 60-minutes exposed mice groups showing different regions of the hippocampus (Fig. 2). The Cornu Ammonis (CA) of the hippocampus is divided into CA1, CA2, and CA3 areas, with the dentate gyrus (DG) surrounding the upper and lower limbs of CA3. There was a regular form of pyramidal neuron cells in CA1, CA2, & CA3 regions (Fig. 2B, 2C, 2D) and granule cells in the DG (Fig. 2E), as well as there, were no dark cells in control mice. There have been some pathological changes in the hippocampus of mice that have been exposed to radiation compared to control mice. Pyramidal neurons exhibited degenerate neurons with black nuclei in CA1, CA2, CA3 area of 40 min exposed mouse (Fig. 2G, 2H, 2I). There was marked cellular degeneration with many dark granule cells in the granular layer of DG of 40-minute exposed mice (Fig. 2J). The pyramidal cells in the CA1, CA2, & CA3 areas of the hippocampus of 60 min exposed mice showed many dark stained irregular shape pyramidal neuronal cells with small dark nuclei compared to 40 min and control mice. In the DG of 60 min exposed mice, the granule cells showed excess degenerated dark cells (Fig. 2O).
Fig. 2.
Hematoxylin and Eosin-stained sections hippocampus of control (A-E), 40 min (F-J), and 60 min (K-O) exposed mice formed by Cornu Ammonis (CA) as CA1, CA2 & CA3 areas. It continued as a subiculum (S). Around the upper and lower limbs of CA3, the dentate gyrus (DG) is located. The Cornu Ammonis (CA) as CA1, CA2 & CA3 areas formed by three layers: the molecular layer (M), the pyramidal layer (P), and the polymorphic layer (Pr). The pyramidal layer contains pyramidal cells (stars), and both molecular & polymorphic layers contain astrocytes (black-white arrowheads). The dentate gyrus consists of the molecular layer (M), granular layer (G), and polymorphic layer (Pr). The granule cells in the granule layer were arranged in dense columns with large vesicular nuclei in control (E). In control mice (B-D), large vesicular healthy nuclei of pyramidal neuron cells. (G-I) representative microphotographs of CA1, CA2, CA3 region pyramidal layers of 40 min exposed mice showed degenerating neurons with darkly stained nuclei (arrowhead) with normal small dark nuclei astrocytes (black-white arrowheads) were observed. In the CA1, CA2, CA3 region 60 min radiation-exposed mice (L-N), there were more dark stained pyramidal neuron cells (arrowheads) compared to 40 min and control mice with normal small dark nuclei astrocytes (black-white arrowheads). In the DG of 40- and 60-minutes exposed mice (J & O), there was degeneration of granule cells with many dark-stained nuclei (arrowheads). However, the degenerated granule cells were more in 60 min compared to 40 min and control mice. Scale bar = 100 μm in A, F, K, and 20 μm in B-E, G-J, and L-O. (n = 10 mice in each group).
3.2.2. Neuronal assay by cresyl violet staining
Cresyl Violet (CV) staining was performed to quantify the total number of normal pyramidal neurons of CA1, CA2, CA3 hippocampal regions, and granule cells in the granular layer of DG in different groups (Fig. 3). The nucleus and cytoplasm of neuron cells are shown in CV staining (Fig. 3A, 3F, 3 K). The nuclei are dark and appeared dark violet in all four regions in control mice (Fig. 3B, 3C, 3D, 3E). There were many abnormal sizes, and shaped cells among the normal pyramidal neurons in the RF-EMR exposed mice compared to the control. It is clearly shown that radiation exposure causes an irregular arrangement of the neurons, increasing darkly stained degenerated neurons and pyknosis. In CA1, CA2, CA3, and DG regions of 40 min, exposed mice showing darkly stained degenerated nuclei are interspersed between normal nerve cells (Fig. 3G, 3H, 3I, 3 J). Wherein 60 min exposed mice, indicating an increased number of disorganized and degenerated darkly stained shrunken morphology neuron cells compared to the control and 40 min mice (Fig. 3L, 3 M, 3 N, 3O).
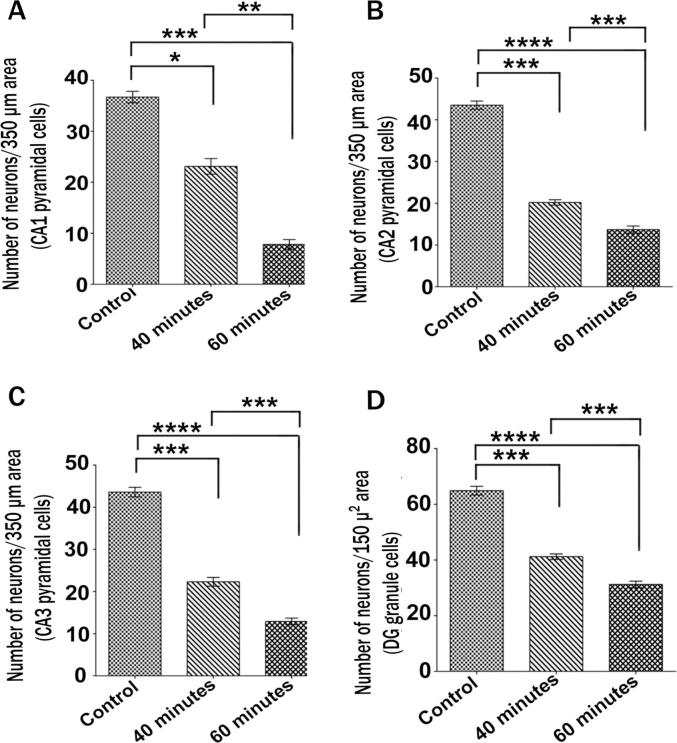
The number of normal neurons in the hippocampus of exposed mice was significantly lower than the control (P ≤ 0.001) (Fig. 4). CA1, CA2 and CA3 regions showed many pyknosis and degenerated neurons in exposed mice on CV staining (Fig. 3). In the CA1, CA2, & CA3 areas of the hippocampus of control mice, the total number of normal viable pyramidal neurons was 36.70 ± 1.13, 43.50 ± 0.98, 43.60 ± 1.14, and granule cell was 64.90 ± 1.57 in the DG region. In 40–60-minutes exposed mice, the total numbers of normal viable neurons were CA1 [23.10 ± 1.52, 7.80 ± 0.95], CA2 [20.20 ± 0.65, 13.70 ± 0.86], CA3 [22.30 ± 1.05, 12.90 ± 0.80] and DG [41.20 ± 0.99, 31.20 ± 1.19] respectively, which were significantly lower than control (Fig. 4). The loss of viable neurons in CA1 (P ≤ 0.01) and CA3 (P ≤ 0.01) areas was higher (Fig. 4A & 4C). The number of dark cells increased while typical pyramidal neuronal cells reduced in the exposure group relative to the control. This study's findings showed that prolonged exposure to radiation causes more damage to the CA1, CA2, and CA3 pyramidal neurons and granule cells of the DG region of the brain hippocampus.
Fig. 4.
The histogram shows the numbers of normal pyramidal neuron cells in different areas of the hippocampus of control, 40- and 60-minutes exposed mice (A-D). For measurement of viable neurons in each hippocampal imaging, a length of 350 µm of CA1, CA2, CA3 and 150 µ2 area of DG layer was selected. Healthy viable neurons were decreased significantly in 60 min compared to 40 min and control mice. Results were presented as Mean ± SEM. (n = 10 mice in each group). (*) indicates a statistically significant difference at the p < 0.05 level; (**) indicates a statistically significant difference at the p < 0.01 level; (***) indicates a statistically significant difference at the p < 0.001 level; (****) indicates a statistically significant difference at the p < 0.0001 level. CA, Cornu Ammonis; DG, Dentate Gyrus.
4. Discussion
The increasing use of cell phones has increased the risk of radiation exposure at ever earlier ages. The deleterious effects on the brain caused by RF-EMR released by smartphones are of great concern. The EMF effect depends on different factors such as frequency, duration of the exposure. The permissible limit for mobile phones is 2 W/kg SAR (This, 2010), but many of them have a 1.4 W/kg SAR value (Agarwal et al., 2011). RF-EMR has many effects on the biological system, especially the brain, liver, kidney, and heart (Hasan et al., 2021a, Odacı et al., 2015, Topal et al., 2015).
The mice in our experiment have been exposed to 2400-MHz RF-EMR because the mobile phones run in Bangladesh at this frequency. In contrast to previous studies, we allowed the mice to move around in their cages freely. The main reason for the mouse's free movement in the cages was to alleviate the anxiety caused by the immobilization inside the cage. From that viewpoint, our study is different from related literature studies. To mimic real-life conditions, we irradiated mice using a commonly used cell phone. We then performed behavioral and histological studies on brain tissue. We performed an Elevated Plus Maze (EPM) test after exposure to 2400 MHz RF-EMR and assessed anxiogenic-like behavior. This study has shown that exposure to mobile phone radiation increases anxiety levels related to neurobiological and cellular alterations. The frequency of entries in the EPM's open and closed arms indicates anxiety (Ehlers and Todd, 2017). The exposed mice showed less exploration and significantly higher avoidance of open arms than the control. The exposed mice also exhibited decreased rearing and head dipping behavior, which indicates reduced exploratory behavior.
In many experiments, the anxiolytic level was confirmed by a reduced entry in the close arm. In our study RF-EMR exposed mice revealed a decreased number of open arm entries because anxiolytic animals avoided open arm entrance and stayed in a nearby or safe area (Cui et al., 2007, Liu et al., 2008, Rodgers and Dalvi, 1997, Wąsik et al., 2019). Exploratory behaviors include open arm activity and more head dipping, showing a greater exploration level (Brown et al., 1999). In this experiment, a significant decline in exploratory behavior was assessed by a decreased time spent in the open arm in all exposed groups relative to the control. This is confirmed by earlier research of several authors who have reported lower exploratory behaviors and decreased anxiety in animals exposed to RF-EMR, vibration, and noise (Abbate et al., 2004, Khirazova et al., 2012). The statistically significant difference that has often been seen between the control groups confirmed the combined effects of RF-EMR and other stressors (Kumar et al., 2009). The open-arm avoidance index is an indicator of anxiety (Trullas and Skolnick, 1993).
Excessive amounts of reactive oxygen species (ROS) produced during electromagnetic radiation exposure were closely connected with apoptosis of neural cells (Kesari et al., 2011). Histopathological considerations have further confirmed the oxidative and behavior effects. The hippocampus of the exposed mouse brains, particularly CA1, CA2, CA3, and DG hippocampal areas, displayed severe neurodegeneration. This change was seen in degenerated neurons with darkly stained nuclei; some neurons are apoptotic than normal cells, stained by CV and H & E. The brains of control mice revealed normal pyramidal neuron cells with spherical shape visible nucleus no degenerated cells. The finding suggested that serious neurodegeneration is influenced by electromagnetic radiation. Our study results revealed that neurodegeneration in the CA and DG regions of the hippocampus due to exposure to 2400 MHz RF-EMR. Many studies have already highlighted the effect of 900 to 2100 MHz RF-EMR on the central nervous system. They found pyknotic neurons in the hippocampus's CA region (Baş et al., 2013), darkly stained granule cells in the DG areas (Odaci et al., 2008), and dark cytoplasm pyramidal neurons in the hippocampus of the experimental model after exposure of 9oo MHz RF-EMR (Şahin et al., 2015). Previous research also shows evidence of such an impact (Salford et al., 2003). They reported that neuronal alteration was represented as dispersed, darkly stained neurons distributed in all regions of the brain. O. Bas et al., 2009 reported many abnormal black and shrunken neurons with pyramidal cell loss in the EMR exposed rats. However, there are some variations between the present study and two previous research (Bas et al., 2009b, Odaci et al., 2008) in terms of the experimental method. In those two studies, the pregnant rats were exposed to 900 MHz RF-EMR. Then the number of neuron cells in the newborn rat’s hippocampus was studied, wherein our study we used 2400 MHz RF-EMR exposure to mice. The damaged neurons were characterized by irregular cellular shapes with an increased nucleus and cytoplasm chromatin content and intense and homogenous stained nuclei (Sugimoto et al., 1990). The exposed mouse neurons had all these features, while none of the control mice exhibited these characteristics. The hippocampal cell damage gives us an indication of behavioral changes in exposed mice. In this study, anxiety-like behavior was significantly affected by long-term exposure to 2400 MHz RF-EMR. The histological observations of our study confirm elevated plus-maze parameters. Future studies should include detailed TUNEL Assay and immunohistochemistry to confirm the hippocampal cell damage.
Postnatal exposure to EMF resulted in a significant reduction in CA pyramidal cells in the EMF group, according to (Bas et al., 2009a). Mortazavi et al., 2017 reported that the total number of pyramidal cells were lost in 4-week-old males when exposed to 900 MHz EMF. The number of hippocampus pyramidal neurons (Baş et al., 2013) and granule cells of DG (Odaci et al., 2008) has been decreased following exposure to RF-EMR. This should be mentioned that the electromagnetic exposure process was structured differently in earlier research (Bas et al., 2009b) from that in the current research. However, the RF-EMR exposure process used in this study has been modified. The exposure system used in this study consisted of a 4G connected cell phone that continuously emitted 2400 MHz frequency RF-EMR. The mobile phone was placed just above the mice, allowing the mice to move in the cage freely.
Cell phones with frequencies ranging from 900 to 1800 MHz have been more popular during the last two decades. Such frequencies may have a lot of adverse effects on biological systems. Previous findings have shown that mobile radiation can cause ROS in different tissues (Misa-Agustiño et al., 2015, Ozguner et al., 2005). The excess production of ROS may induce oxidative stress and inflammation, resulting in changes in antioxidant defense mechanisms, as well as oxidative stress. Many studies have shown that electromagnetic radiation can cause oxidative damage to tissues. Increased antioxidant enzyme concentrations such as malondialdehyde, catalase, and superoxide dismutase may help evaluate the correlation between apoptosis and radiation (Dasdag et al., 2008, Kesari et al., 2010). The hippocampus regulates certain cognitive and behavioral functions that include retaining information during the learning process (Altun et al., 2017). There was an abnormal arrangement of neurons and a reduction in normal viable granule and pyramidal neurons in the hippocampus due to radiation exposure. Previous researchers have reported similar results with strong support for these findings (Kakkar and Kaur, 2011, Wang et al., 2017). Our stereological and histopathological findings showed a significant change in the hippocampus of the electromagnetic radiation-exposed mice consistent with the behavioral study findings. The result suggested that anxiety-like behavior was influenced by electromagnetic radiation. The generalization of the effects of mice experiments for human beings is still under question. This study, carried out using the frequency 2400-MHz, commonly used today in many countries for cellular communication, would significantly contribute to the literature about the consequences of long-term cell phone use on living tissue. However, based on our data, we cannot validate the hypothesis that 2400-MHz RF-EMR has the same effect on humans.
5. Conclusion
Our current study suggests that long-term exposure to 4G cell phone radiation has detrimental effects on anxiety-like behavior and changes in the morphological architecture of the hippocampus. The type of neuronal damage discussed here does not have immediate demonstrable results. However, it may lead to decreased brain reserve capacity in the long run, which could be discovered in other late neuronal diseases. However, further detailed experiments using different approaches (such as electron microscopic, autoradiographic, TUNNEL Assay, and immunohistochemistry) are required to determine whether such pathological alterations are reversed or persistent in getting a satisfactory conclusion in this regard. However, our current results might serve as primary data for further comprehensive studies on the effects of 4G cell phone radiation on the brain.
Author Contributions
The planning and research facilities were established by MRI and supervised the entire research. IH carried out the entire laboratory work and actively participated in the histological study and statistical analysis. MRJ and MNI provided and contributed to the cresyl violet staining. IH, MNI and MRI prepared the draft copy of the manuscript. IH, MRJ, MNI and MRI reviewed and edited the whole manuscript and finally approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The scholars extend their appreciation to Bangladesh Agricultural University Research System (BAURES) and University Grants Commission (UGC) of Bangladesh for financing the research.
Data statement
All data generated or analyzed during this study are included in this article.
Funding Sources
Bangladesh Agricultural University Research System (BAURES), Grant number: 2018/558AU-GC and University Grants Commission (UGC) of Bangladesh, Grant number: 37.01.0000.073.03.012.19/3504.
Approval Statement
All experiments were approved by the Bangladesh Agricultural University Animal Ethics Committee (AWEEC/BAU/2019-46)
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Imam Hasan, Email: imamhasan@bau.edu.bd.
Mir Rubayet Jahan, Email: rubayet.lucky@gmail.com.
Md Nabiul Islam, Email: nabiul@yamaguchi-u.ac.jp.
Mohammad Rafiqul Islam, Email: rafiqul.islam@bau.edu.bd.
References
- Abbate C., Micali E., Giorgianni C., Munaò F., Brecciaroli R., Salmaso L., Germanò D. Affective correlates of occupational exposure to whole-body vibration a case-control study. Psychother. Psychosom. 2004;73:375–379. doi: 10.1159/000080391. [DOI] [PubMed] [Google Scholar]
- Achudume A.C., Onibere B., Nwoha P., Alatise O., Aina F. Effects of Radiation Emitted From Base Stations on Bilirubin, Transaminases and Lipid Peroxidation in Exposed Rats. Energy Environ. Res. 2012;2 doi: 10.5539/eer.v2n1p115. [DOI] [Google Scholar]
- Agarwal A., Singh A., Hamada A., Kesari K. Cell phones and male infertility: A review of recent innovations in technology and consequences. Int. Braz J Urol. 2011;37(4):432–454. doi: 10.1590/S1677-55382011000400002. [DOI] [PubMed] [Google Scholar]
- Altun G., Alizadeh M., Altun E., Ozel G. Odd burr lindley distribution with properties and applications. Hacettepe J. Math. Stat. 2017;46:255–276. doi: 10.15672/HJMS.2017.410. [DOI] [Google Scholar]
- Aslan A., İkinci A., Baş O., Sönmez O.F., Kaya H., Odacı E. Long-term exposure to a continuous 900 MHz electromagnetic field disrupts cerebellar morphology in young adult male rats. Biotech. Histochem. 2017;92(5):324–330. doi: 10.1080/10520295.2017.1310295. [DOI] [PubMed] [Google Scholar]
- Babadi-Akashe Z., Zamani B.E., Abedini Y., Akbari H., Hedayati N. The Relationship between Mental Health and Addiction to Mobile Phones among University Students of Shahrekord. Iran. Addict. Heal. 2014;6:93–99. [PMC free article] [PubMed] [Google Scholar]
- Bas O., Odaci E., Kaplan S., Acer N., Ucok K., Colakoglu S. 900 MHz electromagnetic field exposure affects qualitative and quantitative features of hippocampal pyramidal cells in the adult female rat. Brain Res. 2009;1265:178–185. doi: 10.1016/j.brainres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Bas O., Odaci E., Mollaoglu H., Ucok K., Kaplan S. Chronic prenatal exposure to the 900 megahertz electromagnetic field induces pyramidal cell loss in the hippocampus of newborn rats. Toxicol. Ind. Health. 2009;25(6):377–384. doi: 10.1177/0748233709106442. [DOI] [PubMed] [Google Scholar]
- Baş O., Sönmez O.F., Aslan A., Ikinci A., Hanci H., Yildirim M., Kaya H., Akça M., Odaci E. Pyramidal cell loss in the cornu ammonis of 32-day-old female rats following exposure to a 900 megahertz electromagnetic field during prenatal days 13–21. NeuroQuantology. 2013;11:591–599. doi: 10.14704/nq.2013.11.4.701. [DOI] [Google Scholar]
- Brown R.E., Corey S.C., Moore A.K. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2K mice. Behav. Genet. 1999;29:263–271. doi: 10.1023/A:1021694307672. [DOI] [Google Scholar]
- Cassel J.-C., Cosquer B., Galani R., Kuster N. Whole-body exposure to 2.45 GHz electromagnetic fields does not alter radial-maze performance in rats. Behav. Brain Res. 2004;155(1):37–43. doi: 10.1016/j.bbr.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Chen C., Ma Q., Liu C., Deng P., Zhu G., Zhang L., He M., Lu Y., Duan W., Pei L., Li M., Yu Z., Zhou Z. Exposure to 1800 MHz radiofrequency radiation impairs neurite outgrowth of embryonic neural stem cells. Sci. Rep. 2014;4:1–10. doi: 10.1038/srep05103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consales C., Merla C., Marino C., Benassi B. Electromagnetic fields, oxidative stress, and neurodegeneration. Int. J. Cell Biol. 2012;2012:1–16. doi: 10.1155/2012/683897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X.-Y., Zhao X., Chu Q.-P., Chen B.-Q., Zhang Y.-H. Influence of diltiazem on the behavior of zolpidem-treated mice in the elevated-plus maze test. J. Neural Transm. 2007;114(2):155–160. doi: 10.1007/s00702-006-0535-1. [DOI] [PubMed] [Google Scholar]
- Dasdag S., Bilgin H.M., Akdag M.Z., Celik H., Aksen F. Effect of long term mobile phone exposure on oxidative-antioxidative processes and nitric oxide in rats. Biotechnol. Biotechnol. Equip. 2008;22(4):992–997. doi: 10.1080/13102818.2008.10817595. [DOI] [Google Scholar]
- Drury R. Theory and Practice of Histological Techniques. J. Clin. Pathol. 1983;36(5):609. doi: 10.1136/jcp.36.5.609-d. [DOI] [Google Scholar]
- Dubreuil D., Jay T., Edeline J.-M. Does head-only exposure to GSM-900 electromagnetic fields affect the performance of rats in spatial learning tasks? Behav. Brain Res. 2002;129(1-2):203–210. doi: 10.1016/S0166-4328(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Durusoy R., Hassoy H., Özkurt A., Karababa A.O. Mobile phone use, school electromagnetic field levels and related symptoms: a cross-sectional survey among 2150 high school students in Izmir. Environ. Heal. A Glob. Access Sci. Source. 2017;16(1) doi: 10.1186/s12940-017-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M.R., Todd R.M. Acute psychophysiological stress impairs human associative learning. Neurobiol. Learn. Mem. 2017;145:84–93. doi: 10.1016/j.nlm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Govindaiah, Shankaranarayana Rao, B.S., Raju, T.R., Meti, B.L., 1997. Loss of hippocampal CA1 neurons and learning impairment in subicular lesioned rats. Brain Res. 745, 121–126. https://doi.org/10.1016/S0006-8993(96)01135-3. [DOI] [PubMed]
- Hasan I., Amin T., Alam M.R., Islam M.R. Hematobiochemical and histopathological alterations of kidney and testis due to exposure of 4G cell phone radiation in mice. Saudi J. Biol. Sci. 2021;28(5):2933–2942. doi: 10.1016/j.sjbs.2021.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan I., Islam M.R. Biochemical and histopathological effects of mobile phone radiation on the liver of Swiss albino mice. Eur. J. Anat. 2020;24:257–261. [Google Scholar]
- Hasan I., Pervin M., Kobir M.A., Sagor S.H., Karim M.R. Effect of formaldehyde and urea contaminated feed exposure into the liver of young and adult pigeons (Columba livia) Vet. World. 2021;14:769–776. doi: 10.14202/VETWORLD.2021.769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikinci A., Odaci E., Yildirim M., Kaya H., Akça M., Hanci H., Aslan A., Sönmez O.F., Baş O. The effects of prenatal exposure to a 900 megahertz electromagnetic field on hippocampus morphology and learning behavior in rat pups. NeuroQuantology. 2013;11:582–590. doi: 10.14704/nq.2013.11.4.699. [DOI] [Google Scholar]
- Irmak M.K., Fadıllıoğlu E., Güleç M., Erdoğan H., Yağmurca M., Akyol Ö. Effects of electromagnetic radiation from a cellular telephone on the oxidant and antioxidant levels in rabbits. Cell Biochem. Funct. 2002;20(4):279–283. doi: 10.1002/cbf.976. [DOI] [PubMed] [Google Scholar]
- Joy T., Rao M.S., Madhyastha S. N-acetyl cysteine supplement minimize tau expression and neuronal loss in animal model of alzheimer’s disease. Brain Sci. 2018;8:1–15. doi: 10.3390/brainsci8100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar V., Kaur I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011;49(11):2906–2913. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Karim M., Kobir A., Hasan I., Pervin M., Ahmed A. Formaldehyde-contaminated feed induces histopathological changes in the testes of adult pigeons (Columba livia) J. Adv. Biotechnol. Exp. Ther. 2020;3(3):152. doi: 10.5455/jabet.2020.d120. [DOI] [Google Scholar]
- Kauppila T., Tanila H., Carlson S., Taira T. Effects of atipamezole, a novel α2-adrenoceptor antagonist, in open-field, plus-maze, two compartment exploratory, and forced swimming tests in the rat. Eur. J. Pharmacol. 1991;205(2):177–182. doi: 10.1016/0014-2999(91)90817-A. [DOI] [PubMed] [Google Scholar]
- Kesari K.K., Behari J., Kumar S. Mutagenic response of 2.45GHz radiation exposure on rat brain. Int. J. Radiat. Biol. 2010;86:334–343. doi: 10.3109/09553000903564059. [DOI] [PubMed] [Google Scholar]
- Kesari K.K., Kumar S., Behari J. 900-MHz microwave radiation promotes oxidation in rat brain. Electromagn. Biol. Med. 2011;30(4):219–234. doi: 10.3109/15368378.2011.587930. [DOI] [PubMed] [Google Scholar]
- Khirazova E.E., Baizhumanov A.A., Trofimova L.K., Deev L.I., Maslova M.V., Sokolova N.A., Kudryashova N.Y. Effects of GSM-frequency electromagnetic radiation on some physiological and biochemical parameters in rats. Bull. Exp. Biol. Med. 2012;153(6):817–820. doi: 10.1007/s10517-012-1833-2. [DOI] [PubMed] [Google Scholar]
- Kobir M.A., Akther L., Hasan I., Shahid M.A.H., Haque Z., Karim M.R. Effects of Imidacloprid-Contaminated Feed Exposure on Hematological Parameters in Adult Rabbits (Oryctolagus Cuniculus) Res. Agric. Livest. Fish. 2020;7:439–444. doi: 10.3329/ralf.v7i3.51363. [DOI] [Google Scholar]
- Kumar, R.S., Sareesh, N.N., Nayak, S., Mailankot, M., 2009. Hypoactivity of wistar rats exposed to mobile phone on elevated plus maze. Indian J. Physiol. Pharmacol. [PubMed]
- Lai H., Horita A., Guy A.W. Microwave irradiation affects radial-arm maze performance in the rat. Bioelectromagnetics. 1994;15(2):95–104. doi: 10.1002/bem.2250150202. [DOI] [PubMed] [Google Scholar]
- Liu L.i., Ikonen S., Heikkinen T., Heikkilä M., Puoliväli J., van Groen T., Tanila H. Effects of fimbria-fornix lesion and amyloid pathology on spatial learning and memory in transgenic APP+PS1 mice. Behav. Brain Res. 2002;134(1-2):433–445. doi: 10.1016/S0166-4328(02)00058-X. [DOI] [PubMed] [Google Scholar]
- Liu T., Wang S., He L., Ye K. Anxiogenic effect of chronic exposure to extremely low frequency magnetic field in adult rats. Neurosci. Lett. 2008;434(1):12–17. doi: 10.1016/j.neulet.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Madhyastha S., Somayaji S.N., Rao M.S., Nalini K., Bairy K.L. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can. J. Physiol. Pharmacol. 2002;80(11):1076–1084. doi: 10.1139/y02-135. [DOI] [PubMed] [Google Scholar]
- Massand A., Rai R., Rai A.R., Joy T., Murlimanju B.V., Marathe A. Effect of Methanolic Leaf Extract of Ficus Religiosa on Neuronal Degeneration: A Pilot Study in Male Albino Wistar Rats. Indian J. Public Heal. Res. Dev. 2020;11:685. [Google Scholar]
- Misa-Agustiño M.J., Leiro-Vidal J.M., Gomez-Amoza J.L., Jorge-Mora M.T., Jorge-Barreiro F.J., Salas-Sánchez A.A., Ares-Pena F.J., López-Martín E. EMF radiation at 2450 MHz triggers changes in the morphology and expression of heat shock proteins and glucocorticoid receptors in rat thymus. Life Sci. 2015;127:1–11. doi: 10.1016/j.lfs.2015.01.027. [DOI] [PubMed] [Google Scholar]
- Mortazavi, S.A.R., Mortazavi, S.M.J., Paknahad, M., 2017. Comments on ‘Neuroprotective effects of melatonin and omega-3 on hippocampal cells prenatally exposed to 900 MHz electromagnetic fields.’ Int. J. Radiat. Biol. https://doi.org/10.1080/09553002.2017.1310403. [DOI] [PubMed]
- Narayanan S.N., Kumar R.S., Potu B.K., Nayak S., Mailankot M. Spatial memory perfomance of wistar rats exposed to mobile phone. Clinics. 2009;64:231–234. doi: 10.1590/s1807-59322009000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S.N., Kumar R.S., Potu B.K., Nayak S., Mailankot M. Spatial memory performance of Wistar rats exposed to mobile phone. Clinics (Sao Paulo). 2009;64:231–234. doi: 10.1590/s1807-59322009000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaci E., Bas O., Kaplan S. Effects of prenatal exposure to a 900 MHz electromagnetic field on the dentate gyrus of rats: A stereological and histopathological study. Brain Res. 2008;1238:224–229. doi: 10.1016/j.brainres.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Odacı E., Ünal D., Mercantepe T., Topal Z., Hancı H., Türedi S., Erol H.s., Mungan S., Kaya H., Çolakoğlu S. Pathological effects of prenatal exposure to a 900 MHz electromagnetic field on the 21-day-old male rat kidney. Biotech. Histochem. 2015;90(2):93–101. doi: 10.3109/10520295.2014.947322. [DOI] [PubMed] [Google Scholar]
- Ozguner F., Altinbas A., Ozaydin M., Dogan A., Vural H., Kisioglu A.N., Cesur G., Yildirim N.G. Mobile phone-induced myocardial oxidative stress: Protection by a novel antioxidant agent caffeic acid phenethyl ester. Toxicol. Ind. Health. 2005;21(7-8):223–230. doi: 10.1191/0748233705th228oa. [DOI] [PubMed] [Google Scholar]
- Pellow S., Chopin P., File S.E., Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Rodgers R.J., Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997;21(6):801–810. doi: 10.1016/S0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Şahin A., Aslan A., Baş O., Ikinci A., Özyilmaz C., Fikret Sönmez O., Çolakoʇlu S., Odaci E. Deleterious impacts of a 900-MHz electromagnetic field on hippocampal pyramidal neurons of 8-week-old Sprague Dawley male rats. Brain Res. 2015;1624:232–238. doi: 10.1016/j.brainres.2015.07.042. [DOI] [PubMed] [Google Scholar]
- Saikhedkar N., Bhatnagar M., Jain A., Sukhwal P., Sharma C., Jaiswal N. Effects of mobile phone radiation (900 MHz radiofrequency) on structure and functions of rat brain. Neurol. Res. 2014;36(12):1072–1079. doi: 10.1179/1743132814Y.0000000392. [DOI] [PubMed] [Google Scholar]
- Salford L.G., Brun A.E., Eberhardt J.L., Malmgren L., Persson B.R.R. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ. Health Perspect. 2003;111(7):881–883. doi: 10.1289/ehp.6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderqvist F., Carlberg M., Hardell L. Biomarkers in volunteers exposed to mobile phone radiation. Toxicol. Lett. 2015;235(2):140–146. doi: 10.1016/j.toxlet.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Sokolovic D., Djindjic B., Nikolic J., Bjelakovic G., Pavlovic D., Kocic G., Krstic D., Cvetkovic T., Pavlovic V. Melatonin reduces oxidative stress induced by chronic exposure of microwave radiation from mobile phones in rat brain. J. Radiat. Res. 2008;49(6):579–586. doi: 10.1269/jrr.07077. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Bennett G.J., Kajander K.C. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42:205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- This N. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz TO 100 kHz) Health Phys. 2010;99:818–836. doi: 10.1097/HP.0b013e3181f06c86. [DOI] [PubMed] [Google Scholar]
- Topal Z., Hanci H., Mercantepe T., Erol H.S., Keleş O.N., Kaya H., Mungan S., Odaci E. The effects of prenatal long-duration exposure to 900-MHz electromagnetic field on the 21-day-old newborn male rat liver. Turkish J. Med. Sci. 2015;45:291–297. doi: 10.3906/sag-1404-168. [DOI] [PubMed] [Google Scholar]
- Trullas R., Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl). 1993;111(3):323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Wang B., Lai H. Acute exposure to pulsed 2450-MHz microwaves affects water-maze performance of rats. Bioelectromagnetics. 2000;21:52–56. doi: 10.1002/(SICI)1521-186X(200001)21:1<52::AID-BEM8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wang K., Lu J.M., Xing Z.H., Zhao Q.R., Hu L.Q., Xue L., Zhang J., Mei Y.A. Effect of 1.8 GHz radiofrequency electromagnetic radiation on novel object associative recognition memory in mice. Sci. Rep. 2017;7 doi: 10.1038/srep44521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wąsik A., Białoń M., Żarnowska M., Antkiewicz-Michaluk L. Comparison of the effects of 1MeTIQ and olanzapine on performance in the elevated plus maze test and monoamine metabolism in the brain after ketamine treatment. Pharmacol. Biochem. Behav. 2019;181:17–27. doi: 10.1016/j.pbb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Wood E.R., Mumby D.G., Pinel J.P.J., Phillips A.G. Impaired Object Recognition Memory in Rats Following Ischemia-Induced Damage to the Hippocampus. Behav. Neurosci. 1993;107:51–62. doi: 10.1037/0735-7044.107.1.51. [DOI] [PubMed] [Google Scholar]
- Yüksel M., Nazıroğlu M., Özkaya M.O. Long-term exposure to electromagnetic radiation from mobile phones and Wi-Fi devices decreases plasma prolactin, progesterone, and estrogen levels but increases uterine oxidative stress in pregnant rats and their offspring. Endocrine. 2016;52(2):352–362. doi: 10.1007/s12020-015-0795-3. [DOI] [PubMed] [Google Scholar]
- Yurekli A.I., Ozkan M., Kalkan T., Saybasili H., Tuncel H., Atukeren P., Gumustas K., Seker S. GSM base station electromagnetic radiation and oxidative stress in rats. Electromagn. Biol. Med. 2006;25(3):177–188. doi: 10.1080/15368370600875042. [DOI] [PubMed] [Google Scholar]
- Zhao L., Peng R.Y., Wang S.M., Wang L.F., Gao Y.B., Dong J., Li X., Su Z.T. Relationship between cognition function and hippocampus structure after long-term microwave exposure. Biomed. Environ. Sci. 2012;25:182–188. doi: 10.3967/0895-3988.2012.02.009. [DOI] [PubMed] [Google Scholar]