Abstract
A 105-day experimental trial was conducted to assess different levels of dietary Aleo vera extract supplementation on water quality parameters, proximate composition, growth performance and haematological parameters of fry Oreochromis niloticus. Four different percentages of dietary leaf extract powder of Aleo vera (ALE) with a basal feed, designated as, i.e., T0 (Control group; without ALE), T1 (1% ALE), T2 (2% ALE), and T3 (3% ALE). Fish fry was reared in concrete tanks (7.0 m, 1.6 m, 1.0: L, W, H; water volume 11.2 m3/tank), with an average initial weight 4.04 ± 0.03 g/ fry, and each treatment was triplicated. Fry was randomly distributed at a stocking rate of 450 individuals/ tanks. The water quality parameters revealed that temperature, pH, salinity, dissolved oxygen (DO) and nitrates were found in a promising range as given by FAO/WHO limits. However, the record values obtained for Electric Conductivity (EC), Total dissolved solids (TDS), and alkalinities were not found in all tanks' suitable range according to FAO/WHO limits. The results revealed a significant impact of different percentages of dietary ALE supplementation on fry's body composition and haematological parameters. Moreover, the final body weight, final body length, average daily weight gain (g), net weight gain (g) and specific growth rate (%) were significantly higher (p < 0.05) in T1 and T2 compared with T0 and T3 treatments. The poorest feed conversion ratio was recorded in the T2 group compared with other treatments. Thus, the current study provides information about the nutritional quality of Nile tilapia culturing in Pakistan.
Keyword: Leaf extract, Proximate composition, Growth performance, Immune response
Abbreviations: ALE, Aloe vera; PER, Protein Efficiency Ratio; ANOVA, Analysis of Variance; IBL, Initial Body Length; FBL, Final Body Length; DE, Digestible Energy; DM, Dry Matter; DP, Digestible Protein; EAA, Essential Amino Acid(s); ADWG, Average Daily Weight Gain; FA, Fatty Acid(s); EFA, Essential Fatty Acid; WG, Weight Gain; FCR, Feed Conversion Ratio; FM, Fish Meal; IBW, Initial Body Weight; FBW, Final Body Weight; K, Condition Factor; PO, Crude Palm Oil; RO, Rapeseed Oil; SGR, Specific Growth Rate; VO, Vegetable Oil(s); VSI, Viscerosomatic Index; HSI, Hepatosomatic Index
1. Introduction
Aquaculture is one of the fastest emerging food-producing sectors globally and is flourishing day by day (Habib et al., 2020). Tilapia is one of the popular aquaculture species known as “aquatic chicken” due to its rapid growth, great adaptability to survive even in harsh environmental conditions, more resistance against a specific disease, high protein composition in meat (El-Sayed, 2006; Abdel-Aziz et al., 2021). Oreochromis niloticus is commonly known as “Nile tilapia”, and is the third most famous cultured species for providing good quality fish protein in the human diet worldwide (FAO, 2012). More recently, tilapia production has been sharing up to 75% of total aquaculture production globally due to its ability to tolerate a wide range of several environmental factors. Therefore, considered a significant cultural species in freshwaters or even in brackish water conditions of hatcheries and fish farms in 100 countries worldwide, including Pakistan (Esselman 2009), FAO (2012) reported that tilapia is the most dominant group of warm water fishes used for aquaculture purposes in various tropical regions globally. Tilapia can tolerate a wide range of temperatures; even can survive for few days in temperature below 10 °C and increases up to 40 °C. Nile tilapia is multiple spawners that is reproduced throughout the year, and gonads mature even during early life stages, which also depend upon the availability of food rich protein and lipid contents. Among 70 aquaculture tilapia species, nine species are widely used for aquaculture purposes, including i.e. Tilapia zillii and Oreochromis niloticus.
Furthermore, most of these species are native to western Africa (FAO, 2012). In sustainable aquaculture practice, the management of fish nutrition is necessary to minimize costs and maximize growth performance is needed to handle fish nutrition for the best possible growth performance and fish health (Hassan et al., 2021a, Hassan et al., 2021b). Today, the world is faces a shortage of fish meal production due to its increased demand in aquaculture and prices. Therefore, many least developing countries are now not using such expensive fish meal as a major source of protein in fish feed and replacing it partially or totally with cheap other animal origin protein sources such as poultry by-products, blood meal and meat, bone meals (FAO, 2012, Hussain et al., 2021). These fish feeds are sometimes deficient, particularly in a few crucial amino acids like isoleucine, lysine and methionine. Tilapia meat can also be used as a major replacing ingredient in the production of fish meal to improve the quality of fish feed in proper ratio to maintain essential amino acid composition in their diet for commercially farmed fish species (Admasu et al., 2017).
The use of herbal immune stimulants in the aquaculture sector, has spread worldwide, specifically to improve the immune system and increase fish tolerance to a range of infectious diseases. Many studies have shown that plant immune stimulants can enhance specific and non-specific immune protective mechanisms and increase or decrease fish losses against various of pathogens. (Yin et al., 2009). Aloe vera is among the essential plants in the Liliaceae family, native to tropical and subtropical regions. (Mandrioli et al., 2011). This plant has a wide range of pharmacological impacts, including skin lesions and wound healing, anti-viral, antibacterial, and other effects. However, the results of immune stimulation on warm-blooded animal development have not been confirmed.
Furthermore, little is known about the effects of immunogenicity and anti-toxicity. No research has been conducted lately to examine the possible effects of Aloe vera extract mixtures in aquaculture. Reports on their combination with other herbs/natural products, on the other hand, support the finding that the benefits of herbal extracts in fish could be enhanced, mainly when applied as mixtures. Case in point, a dietary mix of thyme was reported to have significantly improved growth, overall health, and resistance of Sparidentex hasta fry against Photobacterium damselae (Jahanjoo et al., 2018). Positive synergistic effects of different Aloe vera extracts mixed with other herbal extracts were also reported in O. niloticus and Litopenaeus vannamei (Huang et al., 2018).
Several studies have been revealed that various endogenous and exogenous factors, including water pH, salinity, temperature, total dissolved solids, dissolved oxygen concentration and fish feeding composition and its frequency, can affect the proximate composition of fish found in any ecosystem. Furthermore, several genetic and life-related factors, including size, age, gender, and physiology are the endogenous factors governing the fish body composition (El-Sayed et al., 2006). Some factors include dietary protein sources, culturing conditions, and water quality, which is also responsible for changing fish body composition, e.g., small-sized fry stages required more protein in their diets than adult stages. Moreover, the protein composition in the fish diet is an essential factor affecting fish growth and protein composition (Ahmad et al., 2004, Abdel-Tawwab and Ahmad, 2009). The proper development and production of some commercially important tilapia species need 25 to 56% dietary protein in its diet (FAO, 2012). Being an omnivorous fish, Nile tilapia can also use less expensive plant origin sources as major feed ingredients in their diet (Chowdhury, 2011). Therefore, the present study was based on analyzing the impact of diets containing different percentages of Aleo vera leaf extract powder (ALE) on water quality parameters growth and protein composition of Nile tilapia O. niloticus cultured in a close aquaculture system
2. Materials and methods
2.1. Experimental Design
This study was conducted in National Agricultural Research Centre (NARC) located in Islamabad, Pakistan. It consisted of four treatments in three replicates and were designated as the following; T0 (control group: a basal feed without ALE), T1 (1% of ALE in fish feed), T2 (a diet supplemented with 2% ALE), and T3 (a diet supplemented with 3% ALE) fry were cultured (7.0 m, 1.6 m, 1.0 m) each; length, width, depth) and volume 11.2 m3 with an average initial weight of 4.04 ± 0.03 g and were randomly distributed at a stocking rate of 450 individuals/ tanks for total 105 days. Each fish tank was dried, cleared, and add cattle dung 50% of the total organic fertilizers, Urea 1.25 kg, poultry manure 45.0 kg, and di-ammonium phosphate (DAP) 2.5 kg and left the tanks for 15 days before experimental study and culture of Nile tilapia by following the methods of Haraz et al. (2018).
2.2. Fish feed formulation
Fish feed was prepared to contain 30 % crude protein (CP) from Oryza Organics fish fed twice a day/week at 8:30–9:00 AM and 4:30–5:00 PM. to fry stages at the ratio of 3% of total body-weight of fish. A daily meal was readjusted at every fortnight sampling according to the wet-weight gain by following the methods of Admasu et al. (2017).
2.3. Aloe vera feed
Dried leaves of Aloe vera in 80% methanol at room temperature were extracted and filtered. Then this filtrate was dehydrated in a rotary for getting a dark-greenish residue, which was later used as feed addictive as 1%, 2% and 3% along with the major ingredient percentages recorded in Table 1, Table 2 to enhance the immune system of fish and improve its health condition by following the methods of Gabriel et al. (2015), as given below in Table 1, Table 2.
Table 1.
Fish feed composition with different doses of dried Aloe vera leaf extract used for four experimental trials.
| Ingredient used/100 g feed | Control group diet % |
Experimental group diets % |
||
|---|---|---|---|---|
| T0(0%) | T1 (1%) | T2 (2%) | T3 (3%) | |
| Fish meal (g) | 39.01 | 39.01 | 39.01 | 39.01 |
| Corn gluten (30 % CP) | 40.54 | 40.54 | 40.54 | 40.54 |
| Cotton seed meal (g) | 1.72 | 1.72 | 1.72 | 1.72 |
| Rice polish (g) | 7.17 | 7.17 | 7.17 | 7.17 |
| Wheat bran(g) | 5.0 | 4.0 | 3.0 | 2.0 |
| Sun flower oil (mL) | 3.56 | 3.56 | 3.56 | 3.56 |
| Vitamins-mineral premix (quantity in g/2.5 kg) | 3.0 | 3.0 | 3.0 | 3.0 |
| Dried leaf extract of Aloe vera (g) | 0.0 | 1.0 | 2.0 | 3.0 |
Table 2.
Tilapia Fish feed ingredients and composition used during 105 days experimental trials.
| Ingredients | Ingredients of CP % | CP %Contribution |
|---|---|---|
| Wheat bran | 14 | 3.36 |
| Rice polish | 12 | 2.88 |
| Cotton seed meal | 40.95 | 1.64 |
| Corn gluten | 28.50 | 2.85 |
| Fish meal | 56.72 | 10 |
| Sun flower oil | _ | 0.00 |
| Mineral premix | _ | 0.00 |
| Soya bean meal | 46.60 | 9.32 |
| Total | 30.05 |
2.4. Water quality parameters
Water temperature (C), salinity, dissolved oxygen (ppm) and pH were recorded every day, and they were measured by (Celsius glass thermometer), and Handheld refractometer, mobile digital DO-meter (Model: HI9146), and digital pH mete), while alkalinity (mg/l) and nitrate (mg/l) were determined with the chemical methods according to (APHA, 1995).
2.5. Analysis of the proximate composition of fish
At the end of this experimental study, about ten specimens of fingerling fishes from each fish pond were capture for the chemical analysis of their carcass composition.
2.6. Growth and morphological indices
Sampling was performed weekly. At the time of each sample, the fish growth parameters were calculated (Makori et al., 2017, Hassan et al., 2020, Hassan et al., 2021c). Indices for the evaluation of growth performance have been determined as follows:
Net weight gain (NWG) = Average final body weight (AFW) (g) - Average initial body weight (AIW) (g)
Average daily weight gain = {(Final body weight-Initial body weight)/Days}
Specific growth rate (SGR) = {(Final body Weight-Initial body weight)/Days} × 100
Feed Conversion Ratio (FCR) = Feed intake ((g)/Wet weight gain (g)
Feed Conversion Efficiency (FCE) = (Weight gain/Feed intake) × 100
Hepatosomatic index HSI, % = (liver weight/body weight) × 100
Viscerosomatic index,VSI, % = (weight of viscera and associated fat tissue/body weight) × 100
2.7. Hematological parameters
The total red blood cells (RBC) were calculated using an improved method of Neopor emission. Blood was diluted with 5% EDTA with a ratio of 1: 250. Red blood cells were measured by the hemocytometer chamber and then calculated to x106 mm3. For total white blood cells, blood was diluted with 1: 100 Dacciefluid and measured using a hemocytometer and then calculated on x103 mm-3. Both measurements were run under a 100 X microscope (Olympus).
The haemoglobin (Hb, g dL-1) concentrations were determined by the cyanomethaemoglobin method (Klontz, 1994). All the values of red blood cell indices, the mean values of cell haemoglobin (MCH pg), cell haemoglobin concentration (MCHC %), and cell haemoglobin volume (MCV fl) were calculated according to Wintrobe formulae (Anderson and Klontz, 1965). The percentage composition of leukocytes was determined based on their identification characters listed (Ivanova, 1983)
2.8. Statistical analysis
The experimental results were analyzed by one-way analysis of variance (ANOVA) using SPSS version 26 .The differences among the treatments were determined by Duncan Waller at a significant level (p < 0.05).
3. Results
3.1. Water quality parameter
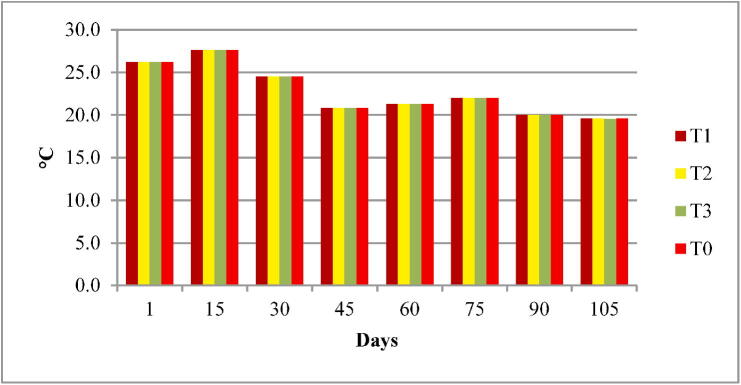
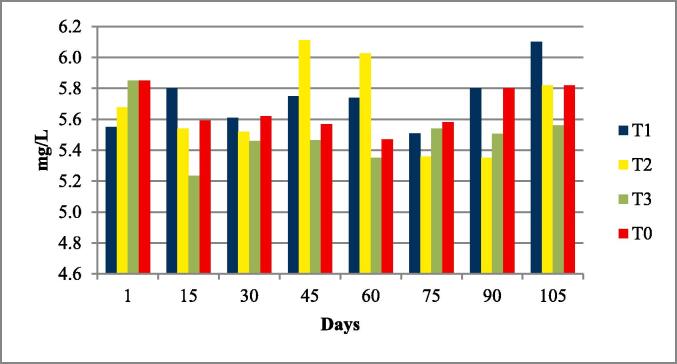
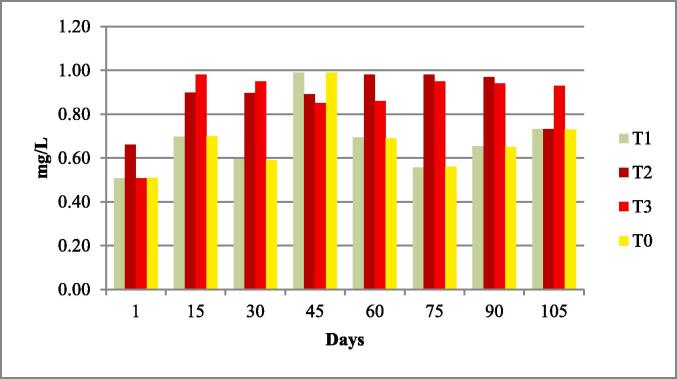
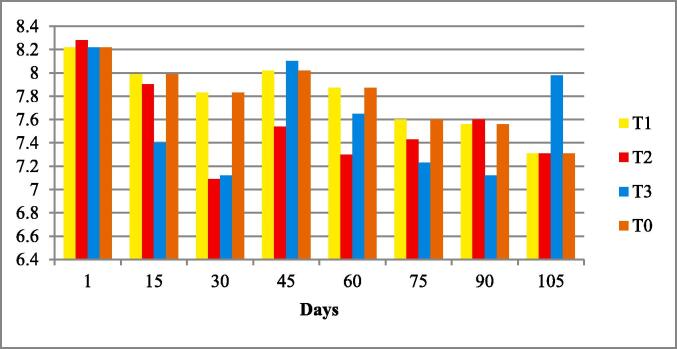
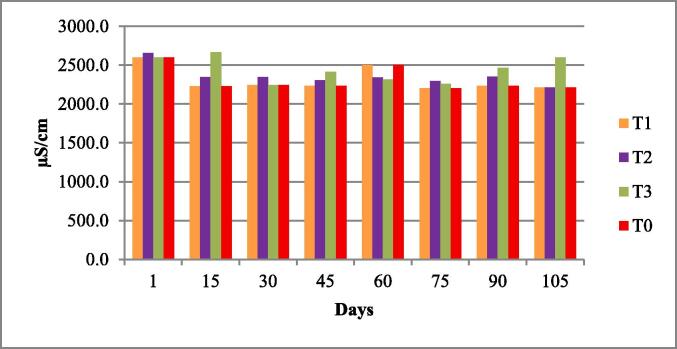
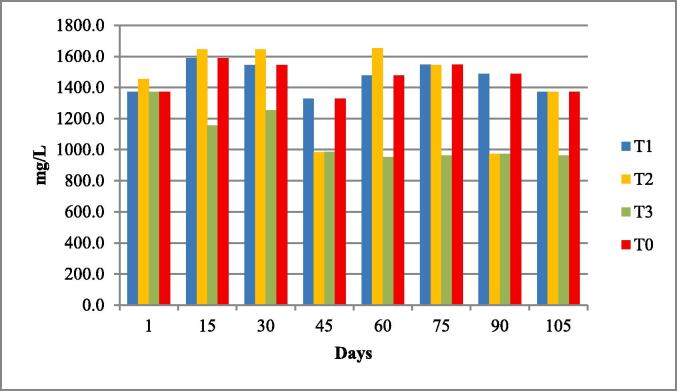
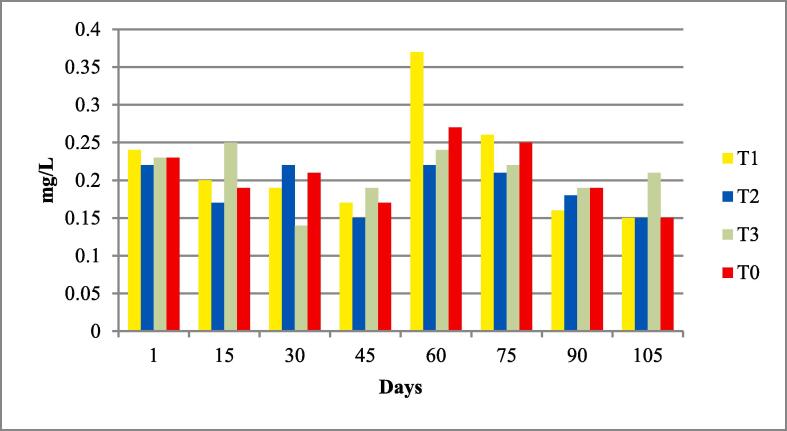
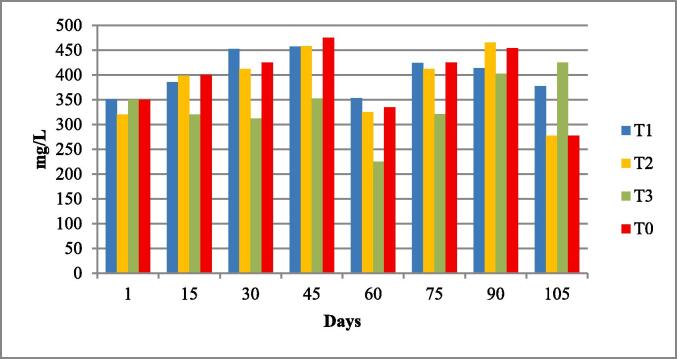
The water quality parameters were analyzed for all experimental tanks. Means of water temperature, dissolved oxygen (DO), salinity, pH and nitrates were in range of standard value as given by FAO/WHO; however, the electrical conductivity (EC), total dissolved solids (TDS). According to the FAO/WHO, the total alkalinity was not lied in standard values according to the FAO/WHO as shown in Table 3 and Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8.
Table 3.
Shown the average values and ranged of water quality parameters measured during 105 days trial for the four experimental ponds of this study.
| Water Parameters | Mean ± S.D | Range | WHO/FAO limits | References |
|---|---|---|---|---|
| Temperature (°C) | 22.78 ± 0.05 | 22.7–22.8 | 20 to 36 °C | (FAO, 2012) |
| Dissolved Oxygen (DO) mg/L | 5.66 ± 2.83 | 5.66–5.7 | 3 to 5 mg/L | (El-Sayed, 2006) |
| Salinity (g/L) | 0.79 ± 0.11 | 0.68–0.9 | 0.5 to 2.5 g/L | (Yanbo et al., 2006) |
| pH | 7.70 ± 0.12 | 7.6–7.8 | 5.5 to 9.5 | (Rebouças et al., 2016) |
| Electric Conductivity (EC) µS/cm | 2354.5 ± 65.03 | 2307.6–2445.5 | 100 to 2000 μS/cm | (Stone et al., 2013). |
| Total Dissolve Solids (TDS) mg/L |
1353.8 ± 186.37 | 1077.1–1464.8 | <400 ppm | (Boyd et al., 2016) |
| Nitrates mg/L |
0.20 ± 0.01 | 0.19–0.21 | 0.2 to 219 mg/l | (Boyd et al., 2007) |
| Alkalinity mg/L |
379.0 ± 28.07 | 338.4–401.6 | 20 mg/l | (Boyd et al., 2016). |
Fig. 1.
Water temperature (T) of four experimental fish tanks.
Fig. 2.
Dissolved oxygen of four experimental fish tanks.
Fig. 3.
Salinity of four experimental fish tanks.
Fig. 4.
Water pH of four experimental fish tanks.
Fig. 5.
Electric conductivity of four experimental fish tanks.
Fig. 6.
Total dissolved solids of four experimental fish tanks.
Fig. 7.
Nitrates of four experimental fish tanks.
Fig. 8.
Total alkalinity of four experimental fish tanks.
3.2. Proximate composition
Table 4 presented the chemical composition of fish carcasses at the end of the study. The crude protein was high and ranged between 60 and 70%, while crude lipid content ranged 2.5–2.7%, ash content was 2.5–2.8, and moisture was much high range from 93.0 to 95.0% in the body composition of Oreochromis niloticus, as given below.
Table 4.
Proximate composition of Oreochromis niloticus whole fish (carcass) of four experimental fish ponds from (initial and final exp.)15 th July to 15 th September 2020
| Ingredients (%) | Initial | C(T0) | T1 | T2 | T3 | Permissible range (WHO) | References |
|---|---|---|---|---|---|---|---|
| Moisture | 95.78 ± 0.24 | 93.5ab ± 0.21 | 94.0ab ± 0.20 | 95.0a ± 0.5 | 93.0b ± 0.56 | 78.9 ± 0.5 | (FAO,2012) |
| Ash | 2.50 ± 0.11 | 2.7 ± 0.20 | 2.8 ± 0.1 | 2.5 ± 0.22 | 2.7 ± 0.57 | 2.6 ± 0.2 | (Boyd et al., 2016) |
| Crude Lipid | 2.10 ± 0.16 | 2.6 ± 0.1 | 2.7 ± 0.10 | 2.7 ± 0.25 | 2.5 ± 0.40 | 5.0 ± 12.0 | (El-Sayed, 2004) |
| Crude Protein | 60.14 ± 2.16 | 65.0ab ± 4.01 | 67.0ab ± 0.5 | 70.0a ± 0.30 | 60.0b ± 1.05 | 30.0 ± 40.0 | (El-Sayed, 2006) |
Values are the mean ± SD of groups in the same row with different superscripts are significantly different (p < 0.05).
3.3. Growth performance and feed utilization parameters
Results obtained in Table 5 indicated that the growth performance of Nile tilapia significantly varied with a difference in dietary ALE supplementation. The highest growth was recorded in T1, while the FBW (82.6 ± 0.64 g), FBL (18.10 ± 0.10 cm) ADWG (0.74 ± 0.01 g), SGR (3.03 ± 0.06), NWG (78.70 ± 0.80 g) followed by dietary ALE (T3) and (T0) while these parameters were were the least with fish fed a diet containing 3% ALE (T3) where:- ADWG, NWG, SGR, 0.67 ± 0.01 (g), 70.7 ± 0.62 (g), 2.96 ± 0.50 %d-1 respectively. There was a significant difference among treatments at level (p < 0.05) in FCR. The best FCR was recorded in (T1) and (T2) compared to other treatments. No significant changes were found among T0, T3 and T3 in HSI and VSI. They were significantly higher than T1.
Table 5.
Effects of Aloe vera on growth, feed utilization and survival indices of Oreochromis niloticus
| Parameters |
Treatments |
|||
|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |
| Initial body weight IBW (g) | 4.0 ± 0.01a | 4.0 ± 0.03a | 4.0 ± 0.01a | 4.0 ± 0.02a |
| Final body weight FBW (g) | 77.0 ± 0.21a | 82.6 ± 0.64b | 78.0 ± 0.66c | 74.7 ± 0.63b |
| Specific growth rate (%d-1) | 2.98 ± 0.04a | 3.03 ± 0.06b | 2.99 ± 0.50c | 2.96 ± 0.50b |
| Feed conversion ratio | 0.68 ± 0.12a | 0.69 ± 0.32b | 0.66 ± 0.01a | 0.72 ± 0.32b |
| Feed conversion efficiency | 7.059 ± 0.02a | 7.058 ± 0.02a | 7.062 ± 0.012a | 7.054 ± 0.01a |
| Average daily weight gain (g/day) | 0.69 ± 0.00a | 0.74 ± 0.01b | 0.70 ± 0.00a | 0.67 ± 0.01b |
| Net weight gain (g) | 73.0 ± 0.20a | 78.70 ± 0.80b | 74.01 ± 0.65c | 70.7 ± 0.62b |
| Hepatosomatic index | 3.10 ± 0.1a | 3.21 ± 0.2a | 3.02 ± 0.1a | 3.41 ± 0.2a |
| Viscerosomatic index | 4.20 ± 0.3a | 4.70 ± 0.2b | 3.80 ± 0.2c | 4.90 ± 0.2b |
| Survival % | 89.76 ± 0.02a | 97.82 ± 0.01b | 92.88 ± 0.03b | 90.72 ± 0.03c |
3.4. Haematological analysis
Blood samples of fish were collected from each treatment with the help of syringes in vacationers in which EDTA (anticoagulant) and the following parameters were calculated for each treatment: TLC (total leukocyte count), Neutrophil, lymphocytes, monocytes, eosinophil, platelets, total RBC, and haemoglobin. While in total, proteins, albumins and globulins were also calculated. Haemoglobin concentration was recorded maximum in T2 with 10.85/µL of blood among all treatments, while minimum in T0 with 7.65/µL. The values recorded regarding to the TLC and RBC were maximum in T2 with 76000, and 2.28/µL neutrophils concentration were analyzed maximum in T1 with 8%, while minimum in T0 with 6%. Lymphocytes in T2 were recorded maximum with 90.5% and minimum in T3 with 89.5%. Platelets count for all the treatments in decreasing manner as T1 > T2 > T0 > T3. The highest value of total proteins and globulins were recorded in T2 with 7.6 and 2.7, while the lowest values were 6.4 and 2.3 g/L in T1, respectively. Albumins were analyzed and recorded maximum during liver function test in T0 with 4.8, while minimum in T1 with 4.14 g/L as shown in Table 6.
Table 6.
Haematological analysis of Oreochromis niloticus in four experimental fish ponds during the study period
| Blood Parameters |
Treatments |
|||
|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |
| Hemoglobin/µL | 7.65 ± 0.04a | 7.7 ± 0.07b | 10.85 ± 0.06b | 7.8 ± 0.07b |
| TLC/µL | 48,900 ± 8.44a | 54800 ± 8.54b | 76000 ± 9.62c | 56000 ± 9.64b |
| Neutrophils % | 6 ± 0.99a | 8 ± 0.98b | 7.5 ± 1.55b | 6.5 ± 0.89a |
| Lymphocytes% | 90 ± 1.16a | 89 ± 0.88b | 90.5 ± 0.81a | 89.5 ± 0.92b |
| Monocytes % | 1.5 ± 0.66a | 1.5 ± 0.64a | 1.5 ± 0.88b | 1.5 ± 0.86b |
| Eosinophil’s% | 2.5 ± 0.66a | 2.5 ± 0.60a | 2.5 ± 0.88b | 2.0 ± 0.99b |
| Platelets count/µL | 135,500 ± 10.043a | 154500 ± 8.043a | 149500 ± 9.043a | 44500 ± 6.043a |
| Total RBC/µL | 1.42 ± 0.05a | 1.32 ± 0.04 a | 2.28 ± 0.06 a | 1.44 ± 0.05 a |
| HCT (%) | 23.05 ± 0.20a | 21.05 ± 0.30a | 31.02 ± 0.20a | 21.5 ± 0.40a |
| MCV | 163.1 ± 2.52a | 161.9 ± 0.54a | 158.65 ± 0.66b | 147.1 ± 0.52b |
| MCH | 53.95 ± 0.56a | 54.6 ± 0.56a | 53.5 ± 0.56a | 56.35 ± 0.56a |
| MCHC | 28.65 ± 0.26a | 35.22 ± 0.24a | 36.3 ± 0.16a | 37.25 ± 0.18a |
| WBC count/µL | 5.9 ± 0.8a | 7.6 ± 0.94b | 8.9 ± 1.52c | 7.9 ± 1.53c |
| albumin(g/L) | 4.8 ± 0.22a | 4.15 ± 0.24a | 4.3 ± 0.23a | 4.35 ± 0.24a |
| Globulin(g/L) | 2.3 ± 0.99a | 2.55 ± 0.99a | 2.7 ± 0.99a | 2.9 ± 0.99a |
| Total protein(g/L) | 6.4 ± 0.86a | 7.2 ± 0.88a | 7.6 ± 0.96b | 7.4 ± 0.99b |
4. Discussion
Aloe vera has been utilized as a popular folk remedy for as long as civilization has existed. In aquaculture, it has recently been reported that it has the potential to act as an alternative growth booster, anti-stressor, immunological stimulant, appetizer, and digestive stimulant (Heidarieh et al., 2013). The present study revealed that dietary extract of Aloe vera in T1 and T3 could improve the feed utilization growth performance and health condition of Nile tilapia. It was reported that the level of Aloe vera leaf extract/kg feeds of less than 2% seemed to be more suitable for growth and immunity response against certain diseases of this species (Mahdavi et al., 2013, Gabriel et al., 2015).
Except for feed intake (T1), which appeared to have a beneficial range of 1% A. vera/kg feed, this affected WG, SGR, and AGR in a similar method. Fish fed T0, T2, and T3 dietary A. vera had the lowest feed intake of the test treatments, but they utilized their feed more efficiently, resulting in increased organo-somatic indices (VSI and HSI), condition, and growth (Mahdavi et al., 2013, Gabriel et al., 2015). In aquaculture, diet supplements are the major aspects for consideration during fish culture, particularly in intensive or semi-intensive culture systems, for increasing fish production. Even the metabolic rate of fish directly depends on the fish feed intake (Imsland et al., 2006). The present study revealed the significant impact of different percentages of dietary Aleo vera leaf extracts on the body composition and haematological parameters of fry stage found in four groups as, T3 > T0 > T1 > T2, respectively. In this study, the highest FBW, ADWG, NWG, and SGR recorded in T3 compared to other treatments and the best FCR recorded in a similar trend to our finding (Ali et al., 2005).
Abdel-Tawwab et al. (2007). Reported that the variation in the physiological parameters of water and fish body composition was usually interrelated, and it had been noted that most fish body composition is affected by water salinity and several other environmental factors. Gan et al. (2013) reported that low dissolved oxygen (DO) water concentration could affect protein production in grass carp fish. Abdel-Tawwab et al. (2010) had found the effect of dietary level of protein on growth, increase in initial body weight, physiology and feed intake of Nile tilapia O. niloticus. The optimum growth of tilapia fry s ranged from 0.4 to 0.5 g was obtained by consuming 45% crude protein in its diet. At the same time, the juvenile stage (37–43 g) and fingerlings (17–22 g) showed their optimum growth at 35% of crude protein in its diet. Moreover, the optimum growth of Nile tilapia depended on the protein quality in its diet and the dietary sources (NRC, 2011).
In the present study, the range of water temperature, pH, salinity, dissolved oxygen (DO) and nitrates of four experimental trials was found favourable for the growth and reproduction of Nile tilapia. However, the electric conductivity (EC), total dissolved solids (TDS), and alkalinity were inappropriate during this study. Ross (2002) reported that the lower limit of DO concentration for the optimum growth of Nile tilapia was 3.0 mg/L. Even the growth defects reported in the juvenile’s stage of Nile tilapia was only due to the increases in water temperature at 34 °C. Favourable water temperatures of Nile tilapia was lies between 20 and 35 °C, while its high reproduction rate found between 25 °C and 36 °C; however, its feeding activity almost stops at water temperature fall below 16 to 17 °C (Lim and Webster, 2006). The most favourable range of pH required for the optimal growth of Oreochromis niloticus was 6.0 to 9.0 (Stone et al., 2013) observed that the desirable range of electric conductivity (EC) for tilapia was lies between 100 and 2000 μS/cm. For maintaining the normal water pH range or buffering, a minimum value of alkalinity, 20 mg L-1 CaCO3 is suitable for fish culture, as reported by Boyd et al., (2016), had also given the appropriate amount of total dissolved solids (TDS) in fish ponds was 400 ppm. Yanbo et al. (2006) found 0.3 mg/l nitrates in fresh water ponds were found suitable for Nile tilapia growth. Though Nile tilapia are well-known examples of freshwater fishes, some of its strains are euryhaline and can tolerate high salinity or even can grow and reproduce well if salinity reach > 36 ppt; however, the optimal growth and reproduction performance were attained at high at 19 ppt (El-Sayed,2006). Thus, tilapia fish has been suggested as marine origin fishes in terms of evolution (Beveridge and Mc Andrew, 2000).
Nowadays, Nile tilapia is considered a commercially critical cultural species as the other tilapia species because of its wide tolerant range of pH, 6.2–8.3, and even survive in low DO (dissolved oxygen) contents. That is ranged from 4.81 and 6.79 mg/L (Njiru et al., 2006, Yongo and Outa, 2016). So, all concerns regarding the water parameters of fish ponds were directly proportional to their gross production. Thus, the physicochemical conditions of the fish pond have an enormous correlation with fish farming or fish culture (Bryan et al., 2011). A broad range of positive and negative impacting factors are independent of each other and are generally necessary for the optimal growth of fish. It has been reported that fish growths are highly dependent on feed consumption and quality. In addition, there are other biotic (i.e., age and sex, genetic variances) and abiotic factors (i.e., stocking density, water temperature, dissolved amount of oxygen) (Imsland et al., 2007, Bhatnagar and Devi, 2013). Thus, it is essential to keep the DO level up to 5 ppm and salinity as 10 to 25 ppt for most euryhaline species, as suggested by Bhatnagar et al., 2004, Bhatnagar and Singh, 2010 for supporting the fish production.
5. Conclusion
This study concluded that water parameters and dietary ALE supplements had shown a substantial effect on the growth, immune responses, and body composition of Nile tilapia O. niloticus. Thus, our study would also benefit inproviding information to the fish farmers about the culturing of good quality tilapia species and fulfils the protein requirement in human diets.
Data availability statement
All data analyzed during this study are included in this published article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Habib Ul Hassan, Email: habib5447@gmail.com, Habib.ulhassan@yahoo.com.
Ahasan Habib, Email: a.habib@umt.edu.my.
References
- Abdel-Aziz M.F.A., Hassan H.U., Yones A.M., Abdel-Tawwab Y.A., Metwalli A.A.A.T., Hassan, H. U., Yones, A. M., Abdel-Tawwab, Y. A., Metwalli, A. A. A. T., 2021 Assessing the effect of different feeding frequencies combined with stocking density, initial weight, and dietary protein ratio on the growth performance of tilapia, catfish and carp. Sci. Afr. 2021;12,(00806) doi: 10.1016/j.sciaf.2021.e00806. [DOI] [Google Scholar]
- Abdel-Tawwab M., Abdelghany A.E., Ahmad M.H. Effect of diet supplementation on water quality, phytoplankton community structure, and the growth of Nile tilapia, Oreochromis niloticus (L.), Common carp, Cyprinus carpio (L.), and Silver carp, Hypophthalmichthys molitrix (V.), polycultured in fertilized earthen ponds. J. Appl. Aquac. 2007;19(1):1–24. [Google Scholar]
- Abdel-Tawwab M., Ahmad M.H. Effect of dietary protein regime during the growing period on growth performance, feed utilization and whole-body chemical composition of Nile Tilapia, Oreochromis niloticus (L.) Aquac. Res. 2009;40(13):1532–1537. [Google Scholar]
- Abdel-Tawwab M., Ahmad M.H., Khattab Y.A.E., Shalaby A.M.E. Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus(L.) Aquaculture. 2010;298(3-4):267–274. [Google Scholar]
- APHA (American Public Health Association), (1995). Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, DC.
- Admasu F., Getahun A., Wakjira M. Supplemental feed formulation for the best growth performance of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) (Pisces: Cichlidae) in pond culture system. J. Chem. Biol. Phys. Sci. 2017;2:599–611. [Google Scholar]
- Ahmad, M. H., Abdel-Tawwab, M., Khattab, Y. A. (2004).Effect of dietary protein levels on growth performance and protein utilization in Nile tilapia (Oreochromis niloticus L.) with different initial body weights. In: The sixth international symposium on tilapia in aquaculture, Manila, Philippine, pp. 249-263.
- Ali M., Iqbal F., Salam A., Iram S., Athar M. Comparative study of body composition of different fish species from brackish water pond. Int. J. Environ Sci. Technol. 2005;2(3):229–232. [Google Scholar]
- Anderson D., Klontz G.W. Basic haematology for the fish culturist. Northwest Fish Culture Conf. 1965;16:38–41. [Google Scholar]
- Rebouças V.T., Lima F.R.D.S., Cavalcante D.D.H. Reassessment of the suitable range of water pH for culture of Nile tilapia Oreochromis niloticus L. in eutrophic water. Acta Sci. - Anim. Sci. 2016;38:361–368. [Google Scholar]
- Beveridge M.C.M., Mc-Andrew B.J. Tilapias: Biology and exploitation. Springer; Dordrecht: 2000. Genetics for the management and improvement of cultured tilapias; pp. 227–266. [Google Scholar]
- Boyd C.E., Tucker C., Mcnevin A., Bostick K., Clay J. Indicators of resource use efficiency and environmental performance in fish and crustacean aquaculture. Rev. Fish. Sci. Aquac. 2007;15(4):327–360. [Google Scholar]
- Bhatnagar A., Devi P. Water quality guidelines for the management of pond fish culture in Kurukshetra. India. Int. J. Environ. Sci. 2013;3(6):1980–2009. [Google Scholar]
- Bhatnagar A., Jana S.N., Garg S.K., Patra B.C., Singh G., Barman U.K. Water quality management in aquaculture. Course Manual of summer school on development of sustainable aquaculture technology in fresh and saline waters, CCS Haryana Agricultural. Hisar (India) 2004;3:203–210. [Google Scholar]
- Bhatnagar A., Singh G. Culture fisheries in village ponds: a multi-location study in Haryana, India. Agr. Biol. J. N.Am. 2010;1(5):961–968. [Google Scholar]
- Boyd Claude E., Tucker Craig S., Somridhivej Benjaporn. Alkalinity and hardness: critical but elusive concepts in aquaculture. J. World Aquac. Soc. 2016;47(1):6–41. [Google Scholar]
- Bryan, R., Soderberg, W., Blanchet, H., Sharpe, W. E. (2011). Management of Fish Ponds in Pennsylvania.1-32.
- Chowdhury, D.K. (2011). Optimal feeding rate for Nile tilapia (Oreochromis niloticus), M.Sc thesis, Department of Animal and Aquacultural Sciences, Norwegian University of Life Sciences, p. 76.
- Esselman, P.C. (2009). Fish Communities and Conservation of Aquatic Landscapes in Northeastern Mesoamerica (Doctoral dissertation). (Natural Resources and Environment) in the University of Michigan. pp, 1-130.
- El-Sayed, A.F.M. (2006). Tilapia culture.CABI. Oceanography Department, Faculty of Science, Alexandria University, Alexandria, Egypt, pp. 1-95.
- El-Sayed A.F.M. In New dimensions in farmed tilapia: proceedings of the Sixth International Symposium on Tilapia Aquaculture. 2004. Protein nutrition of farmed tilapia: searching for unconventional sources; pp. 364–378. [Google Scholar]
- FAO. (2012). The state of world fisheries and aquaculture. Opportunities and challenges. Food and Agriculture Organization of the United Nations, p. 209.
- Gabriel N.N., Qiang J., He J., Ma X.Y., Kpundeh M.D., Xu P. Dietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus in tilapia (GIFT) Fish Shellfish Immunol. 2015;44(2):504–514. doi: 10.1016/j.fsi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Gan L., Liu Y.-J., Tian L.-X., Yue Y.-R., Yang H.-J., Liu F.-J., Chen Y.-J., Liang G.-Y. Effects of dissolved oxygen and dietary lysine levels on growth performance, feed conversion ratio and body composition of grass carp. C tenopharyngodon idella. Aquac. Nutr. 2013;19(6):860–869. [Google Scholar]
- Habib A., Rahman M., Sarker M., Musa N., Hossain M., Shahreza M.A. Breeding performance of riverine Rohu (Labeo rohita) and growth performance of F1 progenies reared in hapas. J.Sustain Sci. Manag. 2020;15(2):24–32. 2672-7226. [Google Scholar]
- Haraz Y.G., El-Hawarry W.N., Shourbela R.M. Culture Performance of Nile tilapia (Oreochromis niloticus) raised in a biofloc based intensive system. Alex. J. Vet. Sci. 2018;58(1):166–172. doi: 10.5455/ajvs.299795. [DOI] [Google Scholar]
- Hussain M., Hassan U.H., Siddique M.A.M., Mahmood K., Abdel-Aziz V., Laghari M.Y., Abro N.A., Gabol K., Nisar Rizwan, Halima S. Effect of varying dietary protein levels on growth performance and survival of milkfish Chanos chanos fingerlings reared in brackish water pond ecosystem. J. Aquat. Res. Egypt. 2021 doi: 10.1016/j.ejar.2021.05.001. [DOI] [Google Scholar]
- Hassan H.U., Ali Q.M., Ahmad N., Masood Z., Hossain M.Y., Gabol K., Khan W., Hussain M., Ali A., Attaullah M., Kamal M. Assessment of growth characteristics, the survival rate and body composition of Asian Sea bass Lates calcarifer (Bloch, 1790) under different feeding rates in closed aquaculture system. Saudi J. Biol. Sci. 2021;28(2):1324–1330. doi: 10.1016/j.sjbs.2020.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H.U., Gabol K., Wattoo J., Chatta A.M., Ali Q.M., Mahmood K., Hussain M., Abro N.A., Attaullah M., Rahman S.U., Rashid A., Rahman M.A., Hossain M.Y. First Pacific White shrimp, Litopenaeus vannamei (Boone, 1931) culture in Pakistan: evaluation of optimum salinity level for the growth performance and survival in the hypo saline and hyper saline condition under pond ecosystem. J. Anim. Plant Sci. 2021;31(5):2021. doi: 10.36899/JAPS.2021.5.0351. [DOI] [Google Scholar]
- Hassan H.U., Ali Q.M., Rahman M.A., Kamal M., Tanjin S., Farooq U., Mawa Z., Badshah N., Mahmood K., Hasan M.R., Gabool K., Rima F.A., Islam M.A., Rahman O., Hossain M.Y. Growth pattern, condition and prey-predator status of 9 fish species from the Arabian Sea (Baluchistan and Sindh) Pakistan. Egypt. J. Aquat. Biol. Fish. 2020;24:281–292. doi: 10.21608/ejabf.2020.97439. [DOI] [Google Scholar]
- Hassan H.U., Ali Q.M., Khan W., Masood Z., Abdel-Aziz M.F.A., Shah M.I.A., Gabol K., Wattoo J., Chatta A.M., Kamal K., Zulfiqar T., Hossain M.Y. Effect of feeding frequency as a rearing system on biological performance, survival, body chemical composition and economic efficiency of Asian Seabass Lates calcarifer (Bloch, 1790) reared under controlled environmental conditions. Saudi J. Biol. Sci. 2021;28(12):7360–7366. doi: 10.1016/j.sjbs.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Hui, Pan Luqing, Pan Shanshan, Song Mengsi. Effects of dietary herbal formulae combined by Astragalus polysaccharides, chlorogenic acid and allicin in different combinations and proportions on growth performance, non-specific immunity, antioxidant status, vibriosis resistance and damage indexes of Litopenaeus vannamei. Aquac. Res. 2018;49(2):701–716. [Google Scholar]
- Heidarieh M., Mirvaghefi R.A., Sepahil A., Sheikhzadel N., Shahbazfar A.M., Akbari M. Effects of dietary Aloe vera on growth performance, skin and gastrointestinal morphology in rainbow trout (Oncorhynchus mykiss) Turk. J. Fish Aquat. Sci. 2013;3:367–373. [Google Scholar]
- Imsland A.K., Foss A., Sparboe L.O., Sigurdsson S. The effect of temperature and fish size on growth and feed efficiency ratio of juvenile spotted wolf fish Anarhichas minor. J. Fish Biol. 2006;68(4):1107–1122. [Google Scholar]
- Imsland A.K., Schram E., Roth B., Schelvis-Smit R., Kloet K. Improving growth in juvenile turbot (Scophthalmusmaximus Rafinesque) by rearing fish in switched temperature regimes. Aquac Int. 2007;15(5):403–407. [Google Scholar]
- Jahanjoo V., Yahyavi M., Akrami R., Bahri A.H. Influence of adding garlic (Allium sativum), Ginger (Zingiber officinale), thyme (Thymus vulgaris) and their combination on the growth performance, haematoimmunological parameters and disease resistance to Photobacterium damselae in sobaity sea bream (Sparidentex hasta) Fry. Turkish J. Fish. Aquat. Sci. 2018;18(4):633–645. [Google Scholar]
- Ivanova, N.T.(1983). Atlas of fish blood cells. LPP Mosacow, Russia (in Russian).
- Klontz, G.W. (1994). Fish hematology. In: Techniques in fish immunology. Stolen, J.S., T.C. Flecher, A.F. Rowely, T.C. Zelikoff, S.L. Kaattari and S.A. Smith (Eds.). Vol. 2, SOS Publications, USA., ISBN: 0962550582, 121-132.
- Lim C.E., Webster C.D. In: Tilapia Biology, Culture, and Nutrition. Lim C.E., Webster C.D., editors. Food Products Press; New York: 2006. Feeding Practices; pp. 547–559. [Google Scholar]
- Makori A.J., Abuom P.O., Kapiyo R., Anyona D.N., Dida G.O. Effects of water physico-chemical parameters on tilapia (Oreochromis niloticus) growth in earthen ponds in Teso North Sub-County, Busia County. Fish Aquatic Sci. 2017;20:30. doi: 10.1186/s41240-017-0075-7. [DOI] [Google Scholar]
- Mandrioli R., Mercolini L., Ferranti A., Fanali S., Raggi M.A. Determination of aloe emodin in Aloe vera extracts and commercial formulations by HPLC with tandem UV absorption and fluorescence detection. Food Chem. 2011;126(1):387–393. [Google Scholar]
- Mahdavi M., Hajimoradloo A., Ghorbani R. Effect of Aloe vera extract on growth parameters of common carp (Cyprinus carpio), World. J. Med. Sci. 2013;9:55–60. [Google Scholar]
- NRC Nutrient requirements of fish and shrimp. Washington, DC: National Academy Press. Aquac Int. 2011;20:601–602. [Google Scholar]
- Njiru M., Ojuok J.E., Okeyo-Owuor J.B., Muchiri M., Ntiba M.J., Cowx I.G. Some biological aspects and life history strategies of Nile tilapia Oreochromis niloticus (L.) in Lake Victoria, Kenya. Afr. J. Ecol. 2006;44(1):30–37. [Google Scholar]
- Ross L.L. Environmental physiology and energetic. Fish and Fisheries Series. 2002;25:89–128. http://link.springer.com/chapter/10.1007 [Google Scholar]
- Stone N.M., Shelton J.L., Haggard B.E., Thomforde H.K. Southern Regional Aquaculture Center; Stoneville, Mississippi: 2013. Interpretation of water analysis reports for fish culture. [Google Scholar]
- Yanbo W., Wenju Z., Weifen L., Zirong X. Acute toxicity of nitrite on tilapia (Oreochromis niloticus) at different external chloride concentrations. Fish Physiol. Biochem. 2006;32(1):49–54. doi: 10.1007/s10695-005-5744-2. [DOI] [PubMed] [Google Scholar]
- Yin G., Ardó L., Thompson K.D., Adams A., Jeney Z., Jeney G. Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio and protection against Aeromonas hydrophila. Fish Shellfish Immunol. 2009;26(1):140–145. doi: 10.1016/j.fsi.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Yongo E., Outa N. Growth and population parameters of Nile tilapia, Oreochromisniloticus (L.) in the open waters of Lake Victoria, Kenya. Lakes Reserv. Res. Manag. 2016;21(4):375–379. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this published article.