Abstract
Ethnopharmacological relevance
Since ancient times, herbal medicines have been applied in the treatment of cancer. Tea, derivative from the dried leaves of Camellia sinensis (L.) Kuntze plant is the most popular beverage globally after water and is available in various forms. Green tea has been expansively investigated for its beneficial properties of cancer prevention and therapy. The goal of the research: The current study was conducted to evaluate the hepaprotective character of methanolic green tea extract and its mechanism of action contrary to thioacetamide (TAA)-produced liver fibrosis of Sprague Dawley rats.
Materials and Methods
Thirty rodents were equally placed in 5 clusters including normal control, TAA group as a positive control, silymarin as standard drug control, and treatment groups consisting of high dose and a low dose Camellia sinensis. Rats in experimental clusters by mouth fed with C. sinensis at 250 mg/kg or 500 mg/kg daily for 2 months. After 60 days, all rats were sacrificed. Blood specimens were gathered for liver biochemical examination. Livers of all groups were dissected out and subjected to histopathological examination through the Hematoxylin and Eosin stain, Masson trichrome, and immunohistochemistry stains (PCNA). Liver tissue homogenate was also analyzed for antioxidant activity parameters.
Results
Gross morphological examination showed a regular liver architecture in C. sinensis fed collections compared to the TAA sets. Histology of rat’s liver fed with C. sinensis showed an important decrease in the liver index with hepatic cells propagation, mild cellular injury, and immunostaining showed significant down-expression of proliferating cell nuclear antigen (PCNA). TAA produced liver fibrosis through a significant increase in serum alanine transferase, aspartate aminotransferase, alkaline phosphatase, and bilirubin. Total protein and albumin also decreased in the TAA group. Moreover, the reduction of antioxidant enzyme activity including superoxide dismutase and catalase as well as the increase in malondialdehyde was detected in the TAA control group. Meanwhile, an abnormal level of liver biochemical parameters was restored closer to the normal levels in serum of the C. sinensis-fed clusters. In addition, C. sinensis fed assemblies showed elevated antioxidative enzymes activity with a reduction in malondialdehyde level comparable to the levels in silymarin-treated rats.
Conclusions
Green tea potentially inhibited the progression of liver cirrhosis, down -regulation of PCNA proliferation, prevented oxidation of hepatocytes, recovered SOD and CAT enzymes, condensed MDA and reduced cellular inflammation.
Keyword: Liver cirrhosis, Thioacetamide, Green Tea, Hepatoprotective, Silymarin, Histopathology, Immunohistochemistry
1. Introduction
Liver is principal interior and metabolically active structure in human being is that play energetic role in digestion, secretion, blood detoxification, and storage of numerous nutrients and minerals. Due to these fundamental roles of liver in human body, hepatic disorders remain to be amongst the major world public health problem (Abood et al., 2020, Kaur et al., 2020, Chen et al., 2021). Therefore, discovery of most effective and less toxic treatment for liver diseases have been increasingly gaining attention. Various natural products have been extensively evaluated for their hepatoprotective role against liver injury caused by different substances (Salama et al., 2018, Adewale et al., 2019, Zhang et al., 2020, El-Baz et al., 2021).
Thioacetamide (TAA) is an organosulfur compound that is known as hepatotoxic agent to study the liver injury, hepatic fibrosis, liver cancer (Yang et al., 2019, Bashandy et al., 2020, Jantararussamee et al., 2021). Numerous trainings signified potential influence of therapeutic herbs defensive compared to TAA-induced toxicity through in vivo models (Salama et al., 2018, Mousa et al., 2019, Abood et al., 2020, Elnfarawy et al., 2021).
Green tea is amongst the most popular beverage that has been found to promote human health. Although the study of potential health benefits of green tea has been started over the past 30 years, green tea consumption has a history back to over 5000 years. In traditional Indian and Chinese medicine, green tea was prescribed by practitioners for diuresis, mild diarrhea, improving heart health, regulating body temperature and blood sugar (Lin et al., 2019, Dwivedi et al., 2020). Furthermore, green tea historically was used for its beneficial effect on digestive system including liver problems as traditional healers recommended its consumption to sustenance role and remedy illnesses of the liver (Farkhondeh et al., 2020, Wang et al., 2021).
Different kinds of teas result from C. sinensis (L.) Kuntze leaves. It is the degree of fermentation that results in the different categorization of tea. C. sinensis made green leaf phenolic mixtures oxidation not occurs (Bharti and Singh, 2020). In black tea preparation, most of these compounds are oxidized, whereas in case of oolong tea partially oxidized (Lorenzo and Munekata, 2016). Green tea has a considerable higher amount catechins of epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epigallocatechin (EGC), and epicatechin (EC), than the oolong and black tea (Kalathingal et al., 2020, Musial et al., 2020). Earlier studies have revealed that antioxidant potential of green tea is due to catechins of EGCG and EGC (Huang et al., 2020, Farkhondeh et al., 2020). Besides, high total phenolic content with free radical hunting action, antibacterial action and ferric reducing power makes this plant valuable (Cardoso et al., 2020). Green tea catechins have also been contributed to its anti-viral property against influenza virus (Guinobert et al., 2018). In addition, green tea catechins of EGCG and EGC inhibited the liver cancer in human and have wound curing potential in rats (Hajiaghaalipour et al., 2013, Almatroodi et al., 2020, Zhao et al., 2021).
Silymarin was applied as a known hepatoprotective compound. It has been widely used as a reference drug in the screening of hepatoprotective potential of new drugs. Silymarin has shown anti-oxidant, anti-lipid peroxidative, anti-fibrotic, anti-inflammatory, immunomodulating and hepatocytes redeveloping properties (Tighe et al., 2020, Khalil et al., 2021). Silymarin defend rat’s liver compared to TAA-produced hepatotoxicity in experimental animals (Abood et al., 2020, da Silva et al., 2021). Thus far, there is no data available on hepatoprotective activity of green tea. The current training was supposed to assess the conceivable hepatoprotective possessions of C. sinensis in TAA-induced liver fibrosis in rodents.
2. Materials and Methods
2.1. Ethics for Animal use
The trial protocol accepted by the Morals Commission for Animal Investigation, College of Science, Cihan University-Erbil (Ethic No. 123, 28/10/2020 MAA (R)). During trial, principles of taking attention of animals given through US Nationwide Conservatory of Knowledge and summarized in “director for upkeep and usage of test site” were practical.
2.2. C. sinensis
C. sinensis was bought from marketplace. About 100 g leaves of green tea were macerated in 600 ml methanol in an Erlenmeyer flask for 72 h at 25 °C. The flush extract (Filtrate) was separated by using filter paper (Whatman No.1). The methanol from flush extract was allowed to evaporate under low pressure by using a rotary evaporator. The proportion harvest of resulting concentrated extract 11.6% (w/w). This concentrated further liquefied in 10% Tween 20 and fed by oral gavage once daily in dose of 250 and 500 mg/kg (Kadir et al., 2011).
2.3. Thioacetamide (TAA)
TAA (Sigma-Aldrich, Switzerland) stock solution (0.03% w/v) was prepared by liquefying 30 mg solid crystals in 100 ml sterile distilled water. The resulting solution in concentration of 200 mg/kg (w/w) of TAA to rat was intraperitoneally injected three times per week for two months. At this concentration, TAA brings morphological and biochemical variations in rats comparable to human liver cirrhosis (Abdulaziz Bardi et al., 2013).
2.4. Silymarin
The standard hepatoprotective drug, silymarin, with 80% purity acquired from Universal Test center, USA. The rats were given 50 mg/kg (w/w) of silymarin softened in Tween 20 (10% w/v) (Wong et al., 2012).
2.5. Experimental design
Thirty adult’s healthy rodents (165–195 g, 5–7 weeks old,) were obtained from Animal House Unit (College of Science, Cihan University-Erbil). Before experimentation, rats were kept separately in cages with wide cable bases to avoid coprophagy. Rats were fed with usual pellet food and tap water for 5 days to adapt typical laboratory circumstances and preserve normal circadian rhythm (Alshawsh et al., 2011).
In order to induce liver cirrhosis, rodents arbitrarily separated to 5 clusters with six rats individually. Cluster 1, as ordinary group, obtain d orally 10% Tween 20 every day and intraperitoneal (I.P) inoculated with sterilized distilled water 3 times weekly for 2 months. Collections 2–5 were I. P. inserted with 200 mg/kg TAA, three times per week for 8 weeks to induce liver cirrhosis. However, Group 2 orally received 10 % Tween 20 (5 mg/kg) for 60 days and Group 3 orally takes silymarin (50 mg/kg) as hepatoprotective medicine for 2 months. Collections 4 and 5 orally administered 250 mg/kg and 500 mg/kg of green tea extract for 2 months. Body masses for whole rats weekly documented. At the end of experimental period (2 months), all the rats were starved for 12 h, sacrificed with anesthetized via intramuscular inoculation of 50 mg/kg ketamine and xylazine 5 mg/kg (Amin et al., 2012, Kadir et al., 2013).
For microscopic analysis, livers were separated and rinsed in cold phosphate buffer saline (pH 7.2). Livers were fixed and analyzed for any gross pathological aberrations by taking images. The rat livers of all groups were subjected to histopathological investigation and liver tissue homogenate for antioxidant activities. Blood serum was assessed for biochemical markers of liver in Laboratory of Rizgary Hospital (Salama et al., 2013).
2.6. Liver function test
Liver function tests provide information about liver injury through measurement of level of ALT, ALP, AST, bilirubin, albumin, and total protein in the blood serum. Moreover, SOD, CAT and MDA were assessed in tissue homogenate. For this purpose, liver specimens were washed instantly by cold 10% (w/v) PBS, pH 7.2. The samples were standardized by Teflon Homogenizer (Polytron, Heidolph RZR 1, Germany). After removing tissue fragments by centrifuge at 4500 rpm for 10 min 4 °C, supernatants were assessed for antioxidant activity using assay kits (Cayman Chemical Company, USA) for SOD and CAT. The MDA is indicator of cellular oxidative stress and assessed by thiobarbituric acid as the lipid peroxidation marker (Alkiyumi et al., 2012).
2.7. Histopathology and immunostaining
For histopathological analysis, liver tissue was dipped in 10% phosphate buffered formalin followed by using automated tissue processor machine and then implanted in paraffin to get liver tissue blocks. Eventually, cuts of 5 μm thickness were made for biopsies of the liver. All sections were stained using hematoxylin and eosin stain (Amin et al., 2012), Masson Trichrome staining (Salama et al., 2012), and immunostaining (Bardi et al., 2014, Kadir et al., 2014) for histopathological valuation were observed under microscope.
2.8. Immunohistochemistry
Poly-L-lysine-treated glass slides used for PCNA staining method as previously described in detail by Bardi et al. (2014).
2.9. Statistical analysis
Data analysis performed one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test (SPSS22; USA). Total standards stated as mean ± S.E.M. The p < 0.05 was deliberated numerical important.
3. Results
3.1. Effect of green tea extract in liver injuries
In order to determine influence of green tea on liver injuries, gross microscopic analysis of livers was performed. As revealed in Fig. 1, results disclose the livers of normal control group rats had normal architecture with regular texture besides even superficial. In contrast, TAA-treated cluster had coarse, uneven granular surface with micronodules indicating liver cirrhosis. However, the livers of silymarin- and green tea extract-fed rats were found to have nearly smooth surfaces without micronodules. In addition, although the reduction in body weight was observed due to the treatment of TAA (Table 1), in the presence of silymarin and green tea extracts (250 and 500 mg/kg), the rats gained weight comparable to that of normal control group. Concomitantly, liver to body mass proportion exhibited conforming substantial change among the silymarin also green tea extracts (250 and 500 mg/kg) – fed collections comparison to TAA set (Table 1).
Fig. 1.
Influence of C. sinensis extract on gross morphology in TAA-induced liver toxicity. Differences in macroscopic liver appearances were observed. Silymarin besides green tea C. sinensis-fed showed nearly smooth surface similar to normal group. Groups: G1, normal control; G2, thioacetamide control; G3, Silymarin; G4, Low dosage (250 mg/kg); G5, high dosage (500 mg/kg).
Table 1.
Influence of green tea extract on body heaviness, liver mass and liver index in TAA produced hepatotoxicity in rodents.
| Groups | Body mass (gm) |
Liver mass (gm) |
Liver Index (%) (LW/BW %) |
|---|---|---|---|
| Normal Control | 210.83 ± 2.08# | 6.68 ± 0.02# | 3.17 ± 0.04# |
| TAA Control | 163.66 ± 2.46* | 9.17 ± 0.01* | 5.60 ± 0.09* |
| Silymarin + TAA | 228.33 ± 21.73# | 6.21 ± 0.01*# | 2.83 ± 0.24# |
| LD + TAA | 186 ± 1.93 | 6.39 ± 0.01*# | 3.42 ± 0.04# |
| HD + TAA | 191 ± 1.92 | 6.28 ± 0.01*# | 3.28 ± 0.04# |
The data were represented as mean (n = 6 rats/group) ± S.E.M. Means with dissimilar superscripts are meaningfully diverse. *P < 0.05 against ordinary control assembly and # P < 0.05 against TAA control group. TAA stances for thioacetamide. LD and HD stand for low dose (250 mg/kg) and high dose (500 mg/kg), respectively.
3.2. Liver biochemical markers
In the present study, biochemical parameters of liver function were measured as demonstrated in Table 2. The TAA group showed notably elevated ALP, ALT, AST and Bilirubin levels lengthways with noticeable reduction in albumin and total protein comparison to control collection. While the levels of liver function biomarker restored closer to the normal levels in serum of silymarin also C. sinensis-fed groups.
Table 2.
The effects of green tea on biochemical limits in TAA-induced hepatotoxicity in rodent.
| Groups |
ALP IU/L |
ALT IU/L |
AST IU/L |
T.Bilirubin (uM) |
Protein g/L |
Albuming/L |
|---|---|---|---|---|---|---|
| Normal Control | 79 ± 1.53# | 62 ± 1.15# | 163 ± 1.65# | 2.6 ± 0.09# | 71 ± 1.73# | 13.6 ± 0.17# |
| TAA Control | 257 ± 3.05* | 232 ± 2.27* | 347 ± 1.71* | 9.58 ± 0.13* | 53 ± 0.85* | 7.65 ± 0.08* |
| Silymarin + TAA | 126 ± 1.93*# | 70.83 ± 1.64*# | 178 ± 1.81# | 5.82 ± 0.01*# | 67 ± 1.41# | 10.87 ± 0.03*# |
| LD + TAA | 186 ± 0.96*# | 83 ± 1.53*# | 226 ± 1.18*# | 7.04 ± 0.15*# | 61 ± 1.41*# | 10 ± 0.04*# |
| HD + TAA | 140 ± 1.18*# | 76 ± 1.53*# | 176.33 ± 14.87# | 6.25 ± 0.07*# | 65 ± 1.15*# | 10.57 ± 0.02*# |
The data were represented as mean (n = 6 rats/group) ± S.E.M. Means with diverse superiors are suggestively dissimilar. * P < 0.05 against normal control group and # P < 0.05 against TAA control collection. TAA stands for thioacetamide. LD and HD stand for low dose (250 mg/kg) and high dose (500 mg/kg), respectively.
3.3. SOD, CAT and MDA content of liver homogenate
Influence of C. sinensis on SOD, CAT and MDA activity are presented in Table 3. The results demonstrated that the antioxidant activities of SOD besides CAT were knowingly condensed in liver tissue homogenate of the hepatotoxic control group as compared with the green tea extract-fed rats (Table 3). Green tea extract significantly restored the SOD and CAT activity by protecting the tissues from the hepatotoxic effect of TAA. In addition, significantly increased amount of MDA as indicator of lipid peroxidation was detected in TAA control group. However, administrations of C. sinensis caused substantial reduction of MDA level (Table 3).
Table 3.
The effect of C. sinensis on the SOD, CAT and MDA contents of liver homogenate in TAA-induced fibrosis in rodents.
| Groups |
SOD (U/ml) |
CAT (nmol/min/ml) |
MDA (µM) |
|---|---|---|---|
| Normal Control | 0.35 ± 0.006# | 3.40 ± 0.024# | 10.02 ± 0.13# |
| TAA Control | 0.166 ± 0.003* | 1.52 ± 0.024* | 32.40 ± 0.22* |
| Silymarin + TAA | 0.36 ± 0.006# | 3.32 ± 0.019# | 11.05 ± 0.048*# |
| LD + TAA | 0.27 ± 0.007*# | 2.75 ± 0.032*# | 14.94 ± 0.089*# |
| HD + TAA | 0.35 ± 0.009# | 3.18 ± 0.054*# | 12.03 ± 0.06*# |
The data were represented as mean (n = 6 rats/group) ± S.E.M. Means with unlike superiors are pointedly dissimilar. * P < 0.05 contrasted with usual control assemblage and # P < 0.05 against TAA control collection. TAA stands for thioacetamide. LD and HD stand for low dosage (250 mg/kg) and high amount (500 mg/kg), respectively. SOD, CAT and MDA stand for superoxide dismutase, catalase and malondialdehyde, respectively.
3.4. Histopathologic Examination: Hematoxylin and eosin stain
Histopathologic examination revealed regular and normal cellular architecture with separated normal cords of hepatocytes, sinusoid, and central vein in liver tissues of ordinary control assembly (Fig. 2). Whereas liver tissues of TAA control group showed inflammation, congestion, vacuolation, fatty alteration, sinusoid dilatation, propagation of bile ducts and necrosis. The liver lobes were disconnected through fibrous septa spreading from the central vein to portal triad and altered liver construction. Furthermore, the liver sections of green tea extract-treated rats showed mild inflammation and necrosis of hepatocytes and vacuolation comparable to silymarin treated group (Fig. 2).
Fig. 2.
Histopathological analysis of liver sections using H&E stain (20X). G1: normal control set, G2: thioacetamide control group, G3: silymarin group, G4: Low dosage (250 mg/kg), G5: high dose (500 mg/kg). Slices revealed from demonstrative examples (n = 6 rats/assembly).
3.5. Histopathological Examination: Masson trichrome stain
Masson trichrome stain of liver sections accomplished to evaluate the amount of fibrosis. Hepatotoxic cluster (TAA-treated group) disclosed propagation of bile duct with thick fibrous connective tissue septa as well as improved statement of collagen fibers everywhere overfilled central vein, designated extensive fibrosis (Fig. 3). However, rats fed with green tea demonstrated less and mild fibrous septa and regenerating hepatocytes analogous to that of the silymarin cluster (Fig. 3). These explanations established the hepatoprotective action of methanolic extract of green tea.
Fig. 3.
Histopathological analysis of liver sections using Masson Trichrome staining (20x).G1: normal control group, G2: thioacetamide control group, G3: Silymarin group, G4: Low dose (250 mg/kg), G5: high dose (500 mg/kg). Slices exposed from illustrative trials (n = 6 rats/cluster).
3.6. Immunohistochemistry staining of hepatic tissue segments
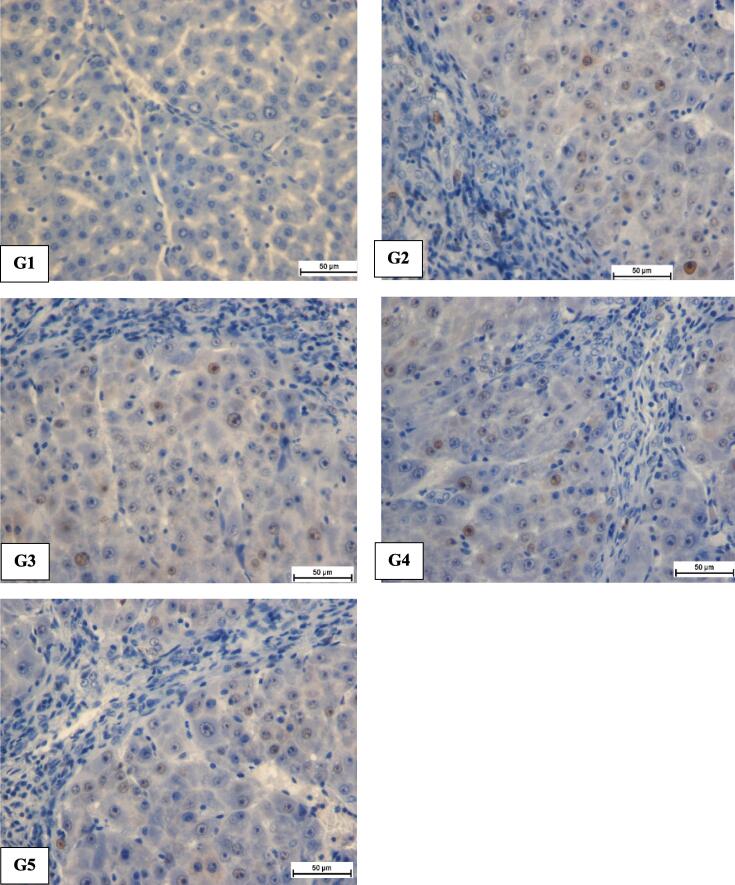
TAA-induced liver injury and significance green tea studied via immunostaining of PCNA appearance in the hepatic parenchymal tissue by means of exact antibodies. Typical cluster obtainable down-expression of PCNA staining, representative no cell rebirth occurring (Fig. 4). In contrast, hepatotoxic control collection had outstanding PCNA expression signifying up-regulation of PCNA proteins with advanced level of hepatic cells cirrhosis. Also, hepatotoxic control cluster raised mitotic figure index meaningfully, suggesting proliferation to renewal widespread hepatic damages formed through TAA (Table 2).
Fig. 4.
Influence of Camellia sinensis on PCNA in histology of liver slices. (A) Standard controls show normal liver construction and no PCNA staining. (B) TAA controls cluster have numerous PCNA-positive hepatic nucleus (C) TAA + silymarin assemblage have few PCNA-positive cells. (D) TAA + 250 mg/kg Camellia sinensis collection have reasonable PCNA-positive nuclei. (E) TAA + 500 mg/kg Camellia sinensis collection has minor PCNA appearance cells (Magnification 20X).
Rat fed with green tea had reduced hepatocytes restitution in comparison to hepatotoxic set, as designated via PCNA countenance besides substantial decrease of mitotic index. These outcomes were quite analogous to that of silymarin fed assembly.
4. Discussion
Liver is metabolic structure of the body with high susceptibility to the toxic effects of thioacetamide. Thioacetamid (TAA) has been chemical compound frequently used to induce liver injury in experimental animals, the chronic effects of this chemical lead to liver cirrhosis (Su et al., 2019, Abood et al., 2020, Gratte et al., 2021). In existing training, TAA was practiced to induce the liver toxicity to appraise the hepatoprotective influence of methanolic extract of green tea. Our outcomes confirmed that TAA decreases body weight and increases liver mass, suggesting chronic toxicity. The increased liver mass is attributed to the buildup of fat and disintegration of hepatic tissues. In addition, TAA encourages oxidative stress and lipid peroxidation, a marker of liver fibrosis. However, when rats were orally fed with methanolic extract of green tea for 2 months, liver weight/body weight ratio was increased and found to comparable to that silymarin-treated group. This result corroborated with earlier findings representing the effect of plant extracts with hepatoprotective activity on the liver weight/body weight ratio (Salama et al., 2013, Abood et al., 2020, Elnfarawy et al., 2021). Reduction in body weights seen in green tea-treated group may be attributed to hypolipidemic effect of green tea extract (Deng et al., 2021). Furthermore, the assessment of liver damage caused by TAA was based on measurements of plasma levels of liver markers enzymes. Elevated quantity of ALP, ALT, AST and bilirubin in blood serum are markers of liver injury and several researchers correlated these markers in the TAA-induced hepatotoxicity experiments (Mi et al., 2019, Gowifel et al., 2020, Tsai et al., 2021). In present investigation, significant elevation in serum liver biochemical markers was seen in TAA control group. However, green tea extract-treated groups exhibited hepatoprotective activity by restoring the altered serum level of liver biochemical markers compared to the effects of silymarin (50 mg/kg). Moreover, TAA control group revealed lowered total protein and albumin, whereas green tea extract restored the total protein and albumin to nearly normal range. Our results suggest that green tea extract may prevent cellular leak and damage of useful honesty of the liver cell casing. In liver tissues homogenate of TAA-treated rats, the activities of antioxidant enzymes, SOD and CAT were meaningfully condensed compared with the normal control collection. In damaged liver, activity of these enzymes declines due to loss of tertiary architecture of these proteins due to the action of free radicals (El Awdan et al., 2019, Osman et al., 2021). Conversely, the activities of SOD and CAT reversed back to normal range in liver homogenate of green tea extract-treated cluster. The elevated amount of MDA signifies oxidative pressure due to the lipid peroxidation (El-Baz et al., 2019, Zhan et al., 2019). Administration of green tea extract reduced MDA level as found in silymarin-treated rats.
Macroscopically, green tea extract displayed protective effect compared to TAA-induced liver fibrosis. TAA provokes liver fibrosis in rodents. In a normal control cluster, the liver has even and plane superficial, whereas TAA groups, the liver seemed irregular and nodular through the development of micronodules. Besides, histopathology study of liver of TAA group shows ample mechanical injury, development of rough pseudo lobules through condensed fibrotic septum, propagation of bile canals in the existence of centrilobular and inflammatory cells. Administration of green tea extract and silymarin reconstitute liver from cirrhosis and lead to extraordinary decrease in liver fibrosis. In addition, Masson trichrome stain shows extent liver fibrosis with the examination of collagen statement in liver tissues. In the normal healthy liver, deposition of collagen was found to be negligible. In contrast, the livers of TAA-treated rats illustrated severe fibrosis around bile ducts. The hepatoprotective agent, silymarin, and green tea extract compact collagen admission and the cellular architecture was resembled to a normal control group. These observations were also noted by additional revisions (Kaur et al., 2019, Abood et al., 2020, Elnfarawy et al., 2021).
The results of the current study showed that normal liver cluster or silymarin treated collections displayed down-expression of PCNA, signifying the absence of cell renewal. Over-regulation of PCNA expression hepatic cells noticed in a hepatotoxic cluster, illustrating wide-ranging production, conceivable exertion to rebuilding tissue damage (Keshk et al., 2019, Abood et al., 2020, Abd Abd Eldaim et al., 2021, El-Maadawy et al., 2019). Otherwise, rodents fed with silymarin or green tea expressively condensed cell propagation by means of a reduction in PCNA stain. In the scientific literatures, enormous number of medicinal plants with hepatoprotective potential has been pronounced by several investigators (Bardi et al., 2014, Salama et al., 2018, Kaur et al., 2020, Abood et al., 2020, Elnfarawy et al., 2021).
In conclusion, our study demonstrated that green tea extract exhibited significantly hepatoprotective effect against TAA-induced hepatic damage in rodents as confirmed through gross morphology, histopathology, and biochemistry parameter analysis. Green tea expressively raised the serum concentration of SOD and CAT along with a significant decrease in level of hepatic MDA and down regulation of PCNA stains hepatic sections. The defensive effect of green tea extract against TAA-produced hepatotoxicity might be contributed to its bulk to avoid hepatocyte oxidation and hepatic cells proliferation, reduce oxidative stress, antioxidant, and free radical scavenging effects. The results of this study provide valid evidence of promising hepatoprotective effect of green tea, worthy of further investigations.
Author contributions
All authors contributed to the study conception and design. S.H.S and M.A.A: conducting the experiments, data collection and analysis, manuscript preparation. S.W.B and N.F.S: analysis and interpretation of the data, reviewed drafts of the paper. Ibrahim Abdel Aziz Ibrahim I.A.A.I.: contributed reagents and materials, interpretation of the data, reviewed drafts of the paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to the staffs of the Department of Biology. This study was financially supported by the Cihan University-Erbil through Research Grant (Erbil, 2020).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Suhayla Hamad Shareef, Email: suhayla.shareef@cihanuniversity.edu.iq, suhayla.shareef@su.edu.krd.
Abdullah R. Alzahrani, Email: aralzahrani@uqu.edu.sa.
Morteta H. Al-Medhtiy, Email: d.mortetah.mohamed@uokufa.edu.iq.
Mahmood Ameen Abdulla, Email: a.mahmood.ameen@cihanuniversity.edu.iq.
References
- Abd Eldaim M.A., Tousson E., Soliman M.M., El Sayed I.E.T., Aleem A.A.H.A., Elsharkawy H.N. Grape seed extract ameliorated Ehrlich solid tumor-induced hepatic tissue and DNA damage with reduction of PCNA and P53 protein expression in mice. Environ. Sci. Pollut. Res. 2021:1–13. doi: 10.1007/s11356-021-13904-8. [DOI] [PubMed] [Google Scholar]
- Abdulaziz Bardi D., Halabi M.F., Abdullah N.A., Rouhollahi E., Hajrezaie M., Abdulla M.A. In vivo evaluation of ethanolic extract of Zingiber officinale rhizomes for its protective effect against liver cirrhosis. BioMed. Res. Int. 2013:2013. doi: 10.1155/2013/918460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abood W.N., Bradosty S.W., Shaikh F.K., Salehen N.A., Farghadani R., Agha N.F.S., Al-Medhtiy M.H., Kamil T.D.A., Agha A.S., Abdulla M.A. Garcinia mangostana peel extracts exhibit hepatoprotective activity against thioacetamide-induced liver cirrhosis in rats. J. Funct. Foods. 2020;74 [Google Scholar]
- Adewale O.O., Samuel E.S., Manubolu M., Pathakoti K. Curcumin protects sodium nitrite-induced hepatotoxicity in Wistar rats. Toxicol. Rep. 2019;6:1006–1011. doi: 10.1016/j.toxrep.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkiyumi S.S., Abdullah M.A., Alrashdi A.S., Salama S.M., Abdelwahab S.I., Hadi A.H.A. Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules. 2012;17:6146–6155. doi: 10.3390/molecules17056146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroodi S.A., Almatroudi A., Khan A.A., Alhumaydhi F.A., Alsahli M.A., Rahmani A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules. 2020;25:3146. doi: 10.3390/molecules25143146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshawsh M.A., Abdulla M.A., Ismail S., Amin Z.A. Hepatoprotective effects of Orthosiphon stamineus extract on thioacetamide-induced liver cirrhosis in rats. Evidence-based Complementary Alternative Med. 2011 doi: 10.1155/2011/103039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin, Z.A., Bilgen, M., Alshawsh, M.A., Ali, H.M., Hadi, A.H.A., Abdulla, M.A., 2012. Protective role of Phyllanthus niruri extract against thioacetamide-induced liver cirrhosis in rat model. Evidence-based complementary and alternative medicine. [DOI] [PMC free article] [PubMed]
- Bardi D.A., Halabi M.F., Hassandarvish P., Rouhollahi E., Paydar M., Moghadamtousi S.Z., Al-Wajeeh N.S., Ablat A., Abdullah N.A., Abdulla M.A. Andrographis paniculata leaf extract prevents thioacetamide-induced liver cirrhosis in rats. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0109424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashandy S.A., el Awdan S.A., Mohamed S.M., Omara E.A.A. Allium porrum and Bauhinia variegata mitigate acute liver failure and nephrotoxicity induced by Thioacetamide in male rats. Indian J. Clin. Biochem. 2020;35:147–157. doi: 10.1007/s12291-018-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti R., Singh B. Green tea (Camellia assamica) extract as an antioxidant additive to enhance the oxidation stability of biodiesel synthesized from waste cooking oil. Fuel. 2020;262:116658. doi: 10.1016/j.fuel.2019.116658. [DOI] [Google Scholar]
- Cardoso R., Neto R., Dos Santos D., Almeida C.T., Nascimento C.T., Pressete C.G., Azevedo L., Martino H.S.D., Cameron L.C., Ferreira M.S.L. Barros FAR Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020;128 doi: 10.1016/j.foodres.2019.108782. [DOI] [PubMed] [Google Scholar]
- Chen X., Ding C., Liu W., Liu X., Zhao Y., Zheng Y., Dong L., Khatoon S., Hao M., Peng X. Abscisic acid ameliorates oxidative stress, inflammation, and apoptosis in thioacetamide-induced hepatic fibrosis by regulating the NF-кB signaling pathway in mice. Eur. J. Pharmacol. 2021;891 doi: 10.1016/j.ejphar.2020.173652. [DOI] [PubMed] [Google Scholar]
- da Silva B.S., Paulino A.M.B., Taffarel M., Borba I.G., Telles L.O., Lima V.V., Aguiar D.H., Dias M.C., Nascimento A.F., Sinhorin V.D.G. High sucrose diet attenuates oxidative stress, inflammation and liver injury in thioacetamide-induced liver cirrhosis. Life Sci. 2021;267 doi: 10.1016/j.lfs.2020.118944. [DOI] [PubMed] [Google Scholar]
- Deng X., Hou Y., Zhou H., Li Y., Xue Z., Xue X., Huang G., Huang K., He X., Xu W. Hypolipidemic, anti-inflammatory, and anti-atherosclerotic effects of tea before and after microbial fermentation. Food Sci. Nutrit. 2021;9:1160–1170. doi: 10.1002/fsn3.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi D.K., Jena G., Kumar V. Dimethyl fumarate protects thioacetamide-induced liver damage in rats: Studies on Nrf2, NLRP3, and NF-κB. J. Biochem. Mol. Toxicol. 2020;34 doi: 10.1002/jbt.22476. [DOI] [PubMed] [Google Scholar]
- El-baz F.K., Salama A., Ali S.I., Elgohary R. Haematococcus pluvialis Carotenoids Enrich Fractions Ameliorate Liver Fibrosis Induced by Thioacetamide in Rats: Modulation of Metalloproteinase and Its Inhibitor. BioMed Res. Int. 2021 doi: 10.1155/2021/6631415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-baz F.K., Salama A., Salama R.A. Therapeutic effect of Dunaliella salina microalgae on thioacetamide-(TAA-) induced hepatic liver fibrosis in rats: role of TGF-β and MMP9. BioMed Res. Int. 2019 [Google Scholar]
- El-Maadawy W.H., Seif El-Din S., Ezzat S.M., Hammam O., Safar M., Saleh S., El-Lakkany N. Rutin Ameliorates Hepatic Fibrosis via Targeting Hepatic Stellate Cells’ Activation, Proliferation and Apoptosis. J. Herbs, Spices Medicinal Plants. 2021;27:322–341. [Google Scholar]
- El Awdan S.A., Abdel Rahman R.F., Ibrahim H.M., Hegazy R.R., El Marasy S.A., Badawi M., Arbid M.S. Regression of fibrosis by cilostazol in a rat model of thioacetamide-induced liver fibrosis: Up regulation of hepatic cAMP, and modulation of inflammatory, oxidative stress and apoptotic biomarkers. Plos one. 2019;14 doi: 10.1371/journal.pone.0216301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnfarawy A.A., Nashy A.E., Abozaid A.M., Komber I.F., Elweshahy R.H., Abdelrahman R.S. Vinpocetine attenuates thioacetamide-induced liver fibrosis in rats. Hum. Exp. Toxicol. 2021;40:355–368. doi: 10.1177/0960327120947453. [DOI] [PubMed] [Google Scholar]
- Farkhondeh T., Pourbagher-Shahri A.M., Ashrafizadeh M., Folgado S.L., Rajabpour-Sanati A., Khazdair M.R., Samarghandian S. Green tea catechins inhibit microglial activation which prevents the development of neurological disorders. Neural Regener. Res. 2020;15:1792. doi: 10.4103/1673-5374.280300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowifel A.M., Khalil M.G., Nada S.A., Kenawy S.A., Ahmed K.A., Salama M.M., Safar M.M. Combination of pomegranate extract and curcumin ameliorates thioacetamide-induced liver fibrosis in rats: impact on TGF-β/Smad3 and NF-κB signaling pathways. Toxicol. Mech. Methods. 2020;30:620–633. doi: 10.1080/15376516.2020.1801926. [DOI] [PubMed] [Google Scholar]
- Gratte F.D., Pasic S., Bakar N.D.B.A., Gogoi-Tiwari J., Liu X., Carlessi R., Kisseleva T., Brenner D.A., Ramm G.A., Olynyk J.K. Previous liver regeneration induces fibro-protective mechanisms during thioacetamide-induced chronic liver injury. Int. J. Biochem. Cell Biol. 2021;134 doi: 10.1016/j.biocel.2021.105933. [DOI] [PubMed] [Google Scholar]
- Guinobert I., Bardot V., Berthomier L., Ripoche I., Faivre C., Haddioui L., Belkhelfa H. Activité virucide in vitro d’un extrait de cyprès sur des virus humains et bovins. Phytothérapie. 2018;16:281–289. [Google Scholar]
- Hajiaghaalipour F., Kanthimathi M., Abdulla M.A., Sanusi J. The effect of Camellia sinensis on wound healing potential in an animal model. Evidence-Based Complementary and Alternative Medicine. 2013 doi: 10.1155/2013/386734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-T., Cheng T.-L., Lin S.-Y., Ho C.-J., Chyu J.Y., Yang R.-S., Chen C.-H., Shen C.-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants. 2020;9:1136. doi: 10.3390/antiox9111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantararussamee C., Rodniem S., Taweechotipatr M., Showpittapornchai U., Pradidarcheep W. Hepatoprotective effect of probiotic lactic acid bacteria on thioacetamide-induced liver fibrosis in rats. Probiotics Antimicrob. Proteins. 2021;13:40–50. doi: 10.1007/s12602-020-09663-6. [DOI] [PubMed] [Google Scholar]
- Kadir F.A., Kassim N.M., Abdulla M.A., Kamalidehghan B., Ahmadipour F., Yehye W.A. PASS-predicted hepatoprotective activity of Caesalpinia sappan in thioacetamide-induced liver fibrosis in rats. Sci World J. 2014:2014. doi: 10.1155/2014/301879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kadir F.A., Kassim N.M., Abdulla M.A., Yehye W.A. Hepatoprotective role of ethanolic extract of Vitex negundo in thioacetamide-induced liver fibrosis in male rats. Evidence-Based Complementary and Alternative Medicine. 2013 doi: 10.1155/2013/739850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadir F.A., Othman F., Abdulla M.A., Hussan F., Hassandarvish P. Effect of Tinospora crispa on thioacetamide-induced liver cirrhosis in rats. Indian J. Pharmacol. 2011;43:64. doi: 10.4103/0253-7613.75673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalathingal M.S.H., Basak S., Mitra J. Artificial neural network modeling and genetic algorithm optimization of process parameters in fluidized bed drying of green tea leaves. J. Food Process Eng. 2020;43 [Google Scholar]
- Kaur G., Shivanandappa T.B., Kumar M., Kushwah A.S. Fumaric acid protect the cadmium-induced hepatotoxicity in rats: owing to its antioxidant, anti-inflammatory action and aid in recast the liver function. Naunyn-Schmiedeberg's Arch. Pharmacol. 2020;393:1911–1920. doi: 10.1007/s00210-020-01900-7. [DOI] [PubMed] [Google Scholar]
- Kaur S., Sharma D., Singh A.P., Kaur S. Amelioration of hepatic function, oxidative stress, and histopathologic damages by Cassia fistula L. fraction in thioacetamide-induced liver toxicity. Environ. Sci. Pollut. Res. 2019;26:29930–29945. doi: 10.1007/s11356-019-06158-y. [DOI] [PubMed] [Google Scholar]
- Keshk W.A., Soliman N.A., Ali D.A., Elseady W.S. Mechanistic evaluation of AMPK/SIRT1/FXR signaling axis, inflammation, and redox status in thioacetamide-induced liver cirrhosis: The role of Cichorium intybus linn (chicory)-supplemented diet. J. Food Biochem. 2019;43 doi: 10.1111/jfbc.12938. [DOI] [PubMed] [Google Scholar]
- Khalil H.M., Eliwa H.A., El-Shiekh R.A., Al-Mokaddem A.K., Hassan M., Tawfek A.M., El-Maadawy W.H. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J. Ethnopharmacol. 2021;277 doi: 10.1016/j.jep.2021.114141. [DOI] [PubMed] [Google Scholar]
- Lin Y.-Y., Hu C.-T., Sun D.-S., Lien T.-S., Chang H.-H. Thioacetamide-induced liver damage and thrombocytopenia is associated with induction of antiplatelet autoantibody in mice. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-53977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J.M., Munekata P.E.S. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pacific J. Tropical Biomed. 2016;6:709–719. [Google Scholar]
- Mi X.-J., Hou J.-G., Jiang S., Liu Z., Tang S., Liu X.-X., Wang Y.-P., Chen C., Wang Z., Li W. Maltol mitigates thioacetamide-induced liver fibrosis through TGF-β1-mediated activation of PI3K/Akt signaling pathway. J. Agric. Food. Chem. 2019;67:1392–1401. doi: 10.1021/acs.jafc.8b05943. [DOI] [PubMed] [Google Scholar]
- Mousa A.A., El-Gansh H.A.I., Abd Eldaim M.A., Mohamed M.A.E.-G., Morsi A.H., El Sabagh H.S. Protective effect of Moringa oleifera leaves ethanolic extract against thioacetamide-induced hepatotoxicity in rats via modulation of cellular antioxidant, apoptotic and inflammatory markers. Environ. Sci. Pollut. Res. 2019;26:32488–32504. doi: 10.1007/s11356-019-06368-4. [DOI] [PubMed] [Google Scholar]
- Musial C., Kuban-Jankowska A., Gorska-Ponikowska M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020;21:1744. doi: 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A., El-Hadary A., Korish A.A., Alnafea H.M., Alhakbany M.A., Awad A.A., Abdel-Hamid M. Angiotensin-I Converting Enzyme Inhibition and Antioxidant Activity of Papain-Hydrolyzed Camel Whey Protein and Its Hepato-Renal Protective Effects in Thioacetamide-Induced Toxicity. Foods. 2021;10:468. doi: 10.3390/foods10020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama S.M., Abdulla M.A., Alrashdi A.S., Ismail S., Alkiyumi S.S., Golbabapour S. Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC complementary and alternative medicine. 2013;13:1–17. doi: 10.1186/1472-6882-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama, S.M., Bilgen, M., Al Rashdi, A.S., Abdulla, M.A., 2012. Efficacy of Boesenbergia rotunda treatment against thioacetamide-induced liver cirrhosis in a rat model. Evidence-based complementary and alternative medicine. [DOI] [PMC free article] [PubMed]
- Salama S.M., Ibrahim I.A.A., Shahzad N., Al-Ghamdi S., Ayoub N., AlRashdi A.S., Abdulla M.A., Salehen N., Bilgen M. Hepatoprotectivity of Panduratin A against liver damage: In vivo demonstration with a rat model of cirrhosis induced by thioacetamide. Apmis. 2018;126(9):710–721. doi: 10.1111/apm.12878. [DOI] [PubMed] [Google Scholar]
- Su G.-Y., Li Z.-Y., Wang R., Lu Y.-Z., Nan J.-X., Wu Y.-L., Zhao Y.-Q. Signaling pathways involved in p38-ERK and inflammatory factors mediated the anti-fibrosis effect of AD-2 on thioacetamide-induced liver injury in mice. Food Funct. 2019;10(7):3992–4000. doi: 10.1039/c8fo02405g. [DOI] [PubMed] [Google Scholar]
- Tighe S.P., Akhtar D., Iqbal U., Ahmed A. Chronic Liver Disease and Silymarin: A Biochemical and Clinical Review. J. Clin. Translational Hepatol. 2020;8:454. doi: 10.14218/JCTH.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M.-Y., Yang W.-C., Lin C.-F., Wang C.-M., Liu H.-Y., Lin C.-S., Lin J.-W., Lin W.-L., Lin T.-C., Fan P.-S. The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice. Molecules. 2021;26:1937. doi: 10.3390/molecules26071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tian L., Wang Y., Zhao T., Khan A., Wang Y., Cao J., Cheng G. Protective effect of Que Zui tea hot-water and aqueous ethanol extract against acetaminophen-induced liver injury in mice via inhibition of oxidative stress, inflammation, and apoptosis. Food Funct. 2021;12:2468–2480. doi: 10.1039/d0fo02894k. [DOI] [PubMed] [Google Scholar]
- Wong W.-L., Abdulla M.A., Chua K.-H., Kuppusamy U.R., Tan Y.-S., Sabaratnam V. Hepatoprotective effects of Panus giganteus (Berk.) corner against thioacetamide-(TAA-) induced liver injury in rats. Evidence-Based Complementary and Alternative Medicine. 2012 doi: 10.1155/2012/170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.Y., Kim K.S., Lee Y.H., Park J.H., Kim J.-H., Lee S.-Y., Kim Y.-M., Kim I.S., Kacew S., Lee B.M. Dendropanax morbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-β1/Smads pathways. Int. J. Biol. Sci. 2019;15:800. doi: 10.7150/ijbs.30356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan F., Zhao G., Li X., Yang S., Yang W., Zhou S., Zhang F. Inositol-requiring enzyme 1 alpha endoribonuclease specific inhibitor STF-083010 protects the liver from thioacetamide-induced oxidative stress, inflammation and injury by triggering hepatocyte autophagy. Int. Immunopharmacol. 2019;73:261–269. doi: 10.1016/j.intimp.2019.04.051. [DOI] [PubMed] [Google Scholar]
- Zhang M.-Q., Ren X., Zhao Q., Yue S.-J., Fu X.-M., Li X., Chen K.-X., Guo Y.-W., Shao C.-L., Wang C.-Y. Hepatoprotective effects of total phenylethanoid glycosides from Acanthus ilicifolius L. against carbon tetrachloride-induced hepatotoxicity. J. Ethnopharmacol. 2020;256 doi: 10.1016/j.jep.2020.112795. [DOI] [PubMed] [Google Scholar]
- Zhao X., Pei D., Yang Y., Xu K., Yu J., Zhang Y., Zhang Q., He G., Zhang Y., Li A. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv. Funct. Mater. 2021;31:2009442. [Google Scholar]