Abstract
Choledochoscopy, or cholangioscopy, is an endoscopic procedure for direct visualization within the biliary tract for diagnostic or therapeutic purposes. Since its conception in 1879, many variations and improvements are made to ensure relevance in diagnosing and managing a range of intrahepatic and extrahepatic biliary pathologies. This ranges from improved visual impression and optical guided biopsies of indeterminate biliary strictures and clinically indistinguishable pathologies to therapeutic uses in stone fragmentation and other ablative therapies. Furthermore, with the evolving understanding of biliary disorders, there are significant innovative ideas and techniques to fill this void, such as nuanced instances of biliary stenting and retrieving migrated ductal stents. With this in mind, we present a review of the current advancements in choledo-choscopy with new supporting evidence that further delineates the role of choledochoscopy in various diagnostic and therapeutic interventions, complications, limitations and put forth areas for further study.
Keywords: Choledochoscopy, Cholangioscopy, Indeterminate biliary strictures, Difficult bile stones, Primary sclerosing cholangitis, Cholangiocarcinoma
Core Tip: The role of choledochoscopy (for extrahepatic biliary procedures) and cholangioscopy (for intrahepatic biliary procedures) is one and a half centuries old. It is a reliable tool in the visualization of indeterminate strictures and subsequent biopsy for diagnostic purposes. Furthermore, it serves as the “safety net” in therapeutic measures where endoscopic retrograde cholangiopancreatography cannot manage, such as biliary stone fragmentation and retrieving migrated equipment. With the advent of new techniques and adjuncts, its potential has further evolved to improve the procedure's accuracy. We provide a comprehensive update on the current and future potential of choledochoscopy.
INTRODUCTION
Choledochoscopy, or cholangioscopy, refers to an endoscopic procedure for direct visualization within the biliary tract for diagnostic or therapeutic purposes. Attempts to directly visualize the bile duct lumen began as early as 1879. However, it was only with the Wildegans choledochoscope in 1953 that choledochoscopes started having some interventional capabilities. Other milestones in choledochoscopy include developing a flexible choledochoscope by Shore and Lipman in 1965, improved imaging quality with the Hopkins rod lens system in 1975, and cameras attached to the choledochoscopes to televise images for simultaneous viewing in 1985[1].
Regarding currently available choledochoscopes, peroral choledochoscopy was introduced in 1976 using the dual-operator "mother-baby" scope. Subsequently, single-operator choledochoscopes such as the direct peroral choledochoscopes (D-POC) and SpyGlass Direct Visualisation system choledochoscopes (Boston Scientific Corporation, Natick, MA, United States) were introduced[2]. Table 1 enlists technical specifications and details of commonly available choledochoscopes. Spurred by an improved understanding of biliary disorders and innovative technological advances, choledochoscopy remains an evolving field. Choledochoscopy and cholangioscopy are used interchangeably in the literature. However, for this review, choledochoscopy refers to the extrahepatic biliary tree procedure, and cholangioscopy refers to the intrahepatic biliary tree procedure. This review aims to update the technical advances in choledochoscopy, new evidence that further delineates the role of choledochoscopy in various diagnostic and therapeutic interventions, complications, limitations, and put forth areas for further study.
Table 1.
Technical specifications of commonly discussed choledochoscopes
|
Type of choledochoscope
|
Fibreoptic or digital-based imaging systems1
|
Outer diameter (mm)
|
Accessory working channel diameter (mm)
|
Tip deflections |
| Percutaneous | ||||
| CHF-CB30 L/S (Olympus Medical Systems, Tokyo, Japan)[13] | Digital | 2.8 | 1.2 | 2-way (up-down) |
| Peroral – dual-operator | ||||
| Mother-baby[4] | Fibreoptic | “Mother”: 12.6 mm “Baby”: 2.8–3.4 mm | 0.8 – 1.2 | 2-way (up-down) |
| Short-access-mother-baby (Karl Storz, Tuttlingen, Germany)[4] | Fibreoptic | “Mother”: 12.6 mm “Baby”: 3.4 mm | 1.5 | 2-way (up-down) |
| Videocholangioscope (CHF-B290; Olympus Medical Systems, Tokyo, Japan )[6] | Digital | 3.3 | 1.3 | 2-way (up-down) |
| Peroral – Single-Operator | ||||
| SpyGlass Legacy 2007 (Boston Scientific Corporation, Natick, MA, United States)[5] | Fibreoptic | 3.3 | 1.2 | 4-way (up-down, left-right) |
| SpyGlass Direct Visualisation 2015 (Boston Scientific Corporation, Natick, MA, United States)[5] | Digital | 3.6 | 1.2 | 4-way (up-down, left-right) |
| SpyGlass Direct Visualisation II 2018 (Boston Scientific Corporation, Natick, MA, United States) | Digital | Data has not been published yet | ||
| Direct peroral choledochoscopy using variety of ultra-thin endoscopes[5] | Digital | 5.0 – 5.9 | 2.0 | 4-way (up-down, left-right) |
Fibreoptic and digital catheters differ in the modality used to illuminate, acquire and transmit endoscopic images back to the camera. Fibreoptic catheters utlitise multiple individual fibre-optic bundles to reflect light off cable walls and into a camera. Digital catheters use imaging chips to convert reflected light into a digital signal, to produce a higher resolution digital image.
LITERATURE RESEARCH
An electronic search of PubMed was conducted in February 2021 for literature published in English. The following terms were used, and relevant articles were considered: [(choledochoscopy) OR (cholangioscopy)]. The last date of the search was 28th February 2021.
TYPES OF CHOLEDOCHOSCOPY
Choledochoscopy can be performed by peroral, percutaneous transhepatic, percutaneous transenteric via access loop, intra-operative transcystic, or intra-operative transcholedochal access (Figure 1). Table 2 summarizes types of chol-edochoscopy according to access routes, with each route's advantages and limitations. Peroral and percutaneous transhepatic access are the most widely discussed in the literature and are further elaborated on in this section.
Figure 1.
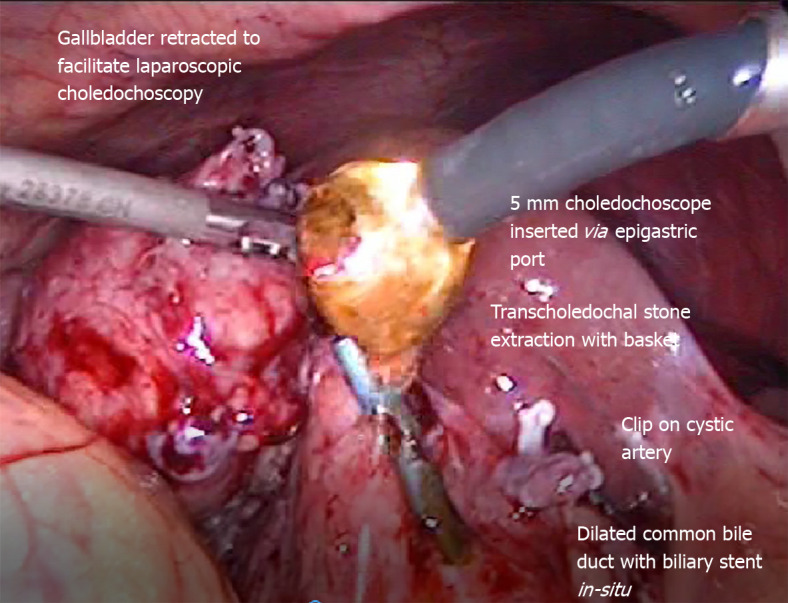
Laparoscopic transcholedochal common bile duct stone extraction by operative choledochoscopy.
Table 2.
Types of choledochoscopy
|
Type of choledochoscopy
|
Advantages
|
Disadvantages
|
| Peroral (endoscopic) | Natural orifice | (1) Technical expertise; (2) Sedation or anesthesia; and (3) Not possible in patients with previous gastric resections or Roux-en-Y gastric bypass |
| Percutaneous transhepatic (interventional radiology) | (1) Shorter scope length; (2) Repeated with ease; and (3) Therapeutic interventions | (1) Need dilated intra-hepatic ducts; and (2) Risk of bleeding, bile leak, tumor seeding, biliary fistula and skin excoriation |
| Percutaneous transenteric via access loop (interventional radiology, surgical) | (1) Shorter scope length; (2) Repeated with ease; (3)Therapeutic interventions; (4) Ductal dilatation not necessary; and (5) In patients with RPC | (1) Previous access loop creation; and (2) Risk of small bowel injury, peritonitis, biliary fistula and skin excoriation |
| Intra-operative transcystic (surgical) | (1) Avoid CBD incision; (2) Therapeutic interventions; (3) Can document CBD clearance; and (4) It can be done laparoscopically | (1) The spiral valve of Heister; (2) Anatomy of the cystic duct; (3) Size of the cystic duct; (4) Need thin scopes (3 mm); (5) Technical expertise; and (6) Risks of bleeding, bile leak |
| Intra-operative transcholedochal (surgical) | Most direct access | (1) Need dilated extra-hepatic biliary system; (2) Risk of bleeding, bile leak; (3) Can put an internal stent; and (4) Can put T tube |
RPC: Recurrent pyogenic cholangitis; CBD: Common bile duct.
Peroral choledochoscopes (POC) are further categorized into dual-operator or single-operator systems. Dual-operator systems require two endoscopists to operate "mother-baby" scopes, where a choledochoscope is inserted through the instrumentation channel of a duodenoscope. This includes original fibreoptic scopes and newer videocholangioscopes with Narrow Band Imaging (NBI) capacity. The original fibreoptic scopes were necessary for peroral choledochoscopy but have limited use currently due to its disadvantages: requires two endoscopists, low image quality with fibreoptic imaging, suboptimal working or irrigation channels, poor maneuverability with two-way tip deflection, and scope fragility[3,4]. In contrast, interest in video-cholangioscopes (CHF-B260, latest version: CHF-B290; Olympus Medical Systems, Tokyo, Japan) remains despite the need for two endoscopists. Advantages include using NBI for improved image quality, the stability of baby scope positioning in bile ducts, and a small outer diameter for use in intrahepatic bile ducts[5,6]. However, its role, especially considering the latest CHF-B290 model, is still being defined and is not currently available for clinical use.
To minimize drawbacks associated with the dual-operator technique, single-operator systems such as the SpyGlass Direct Visualisation peroral choledochoscopy system and D-POC using ultra-thin endoscopes were developed. Currently, three versions of SpyGlass are available – the first-generation SpyGlass Legacy 2007 (Fibre-optic) (FSOC), second-generation SpyGlass Digital System delivery, and access catheter 2015 (Digital) (DSOC) and third-generation SpyGlass Digital System II delivery and access catheter 2018 (Digital). Advantages of FSOC include a four-way deflectable tip for better maneuverability and a dedicated irrigation channel for continuous irrigation. It is limited by the inferior image quality and field-of-view (70˚), poor durability of the reusable fibreoptic probe, small therapeutic channel, and cumbersome setup[7]. Thus, DSOC improved on FSOC by having digital images with 400% greater resolution and 60% wider field-of-view (110˚), improved accessory channel, and easy "plug and play" set up[8]. The third-generation SpyGlass Direct Visualisation II delivery and access catheter 2018 (Digital) is touted to have 250% better resolution than DSOC and adjusted lighting to reduce flare. However, clinical data on its efficacy is not yet available[9].
D-POC utilizes a variety of ultra-slim endoscopes designed initially for pediatric and transnasal use. Key advantages are the variety of endoscopes already available, four-way deflectable tip, and the ability to use NBI for improved image quality. Disadvantages include relatively large outer diameters (5.0-5.9 mm), which may complicate scope insertion and advancement in smaller bile ducts, requiring prior large sphincterotomy to accommodate scope diameters gastric and duodenal looping[5].
Novel multi-bending choledochoscopes are developed to improve the ease of bile duct cannulation. This avoids accessory devices as two bending sections allow more acute angulation and control the choledochoscope while preventing choledochoscope dislodgement. Three prototype models exist. For the first two prototypes, freehand insertion had a 0% technical success rate in a study by Itoi et al[10] involving seven patients. Compared to the second prototype, the third prototype has more excellent distal tip angulation (200˚ vs 160˚) and a smaller outer diameter distal end (4.9 mm vs 5.2 mm) to improve the scope's pushability to minimize loop formation. This translated into improved technical success rates and shorter procedure time with reduced radiation exposure than conventional choledochoscopes and previous generations of multi-bending choledochoscopes. In a randomized controlled trial by Lee et al[11] involving 92 patients, while efficacy in diagnostic and therapeutic interventions was equivalent, multi bending choledochoscope had high technical success rates of freehand biliary insertion (89.1% vs 30.4%, P < 0.001) and shorter mean procedure time with reduced radiation exposure (3.2 ± 1.8 vs 6.0 ± 3.0 min, P = 0.004) than conventional D-POC.
Percutaneous transhepatic choledochoscopy (PTCS) is reserved for cases when peroral choledochoscopy is unsuitable, such as in complicated anatomy. This percutaneous approach permits shorter endoscopes with better maneuverability to reach areas that are less accessible perorally[12]. A variety of endoscopes can be used, such as those used for other indications (e.g., nephroscope, ureteroscope, bronchoscope) and those specifically designed for choledochoscopy (e.g., CHF-CB30 L/S; Olympus Medical Systems, Tokyo, Japan)[13]. However, it remains second-line to peroral choledochoscopy due to the invasive and time-consuming need to create and mature a large-diameter percutaneous tract several days before choledochoscopy and complications such as bile leak and bleeding metastatic spread to the peritoneum or sinus tract[14].
CHOLEDOCHOSCOPE ADJUNCTS AND ACCESSORIES
This section will discuss the advancements in accessories that facilitate chole-dochoscope advancement, optimize view, improve image quality and efficacy in specific interventions.
Choledochoscope advancement
Devices are developed to guide the advancement of D-POC into bile ducts. An example is how in a study by Yang et al[15] involving 79 patients, the use of D-POC enabled high rates of scope insertion (72.0%). Another device to increase chol-edochoscope stability is a hybrid balloon catheter anchoring device using a 0.021-inch guidewire attached to a balloon catheter's distal end. In a single-center retrospective study by Li et al[16] involving 55 patients, this device-guided D-POC achieved significantly higher technical success rates compared to the conventional wire-guided method (92.7% vs 47.1%, P < 0.05). Another anchoring technique is advancing D-POC over a reusable guide probe of the Kautz device (MTW, Wesel, Germany), designed initially for non-transendoscopic placement of biliary stents. This method increases probe stiffness to prevent choledochoscope looping and had an 85% technical success rate[17].
Optimize view by medications
Ways to optimize view across various modes of choledochoscopy have been described. In D-POC, intraductal simethicone reduces the surface tension of gas bubbles and improves mucosal visualization by anti-foaming action. This is particularly useful in the presence of pneumobilia following a sphincterotomy for choledochoscope access[18].
Optimize view by structural modification
In percutaneous choledochoscopy, Demmert et al[19] devised a novel choledochoscopy expander using microwires to create a flexible whisk-like shape to distend the gallbladder lumen before visualization by choledochoscopy mechanically. A case report showed its use improved gallbladder visualization with reduced infolding of gallbladder lumen and minimal mucosal injury. Other accessories include a transparent cap to the choledochoscope in gallbladder-preserving surgery. According to Jian et al[20] in a retrospective study of 50 patients, the addition of a transparent cap for patients undergoing laparoscopic choledochoscopy significantly reduced gallbladder exploration time (12.04 ± 6.01 min vs 27.96 ± 12.24 min). Reasons put forth include eliminating blind spots as the transparent cap promoted distance between the lens and mucosa, allowing complete visualization. Other benefits include protection of the scope. Sometimes direct visualization by choledochoscopy is not possible due to complete ductal obstruction. In such instances, microcatheters made of the 3-French outer sheath of a basket catheter (MicroCatch; MTW Endoskopie, Düsseldorf, Germany) and 3-French endoscopic nasobiliary drainage tube (Daimon-PTCD set, Hanaco Medical, Saitama, Japan) can aid injection of contrast medium to facilitate guidewire manipulation[21].
Image-enhanced function systems
To improve direct visualization capabilities, choledochoscopy can harness various preexisting image-enhanced function systems, such as NBI, probe-based confocal laser endomicroscopy, i-Scan, chromocholangioscopy, and autofluorescence imaging. NBI utilizes filtered light to improve visualization of ductal mucosa and vessels compared to conventional white-light imaging. It is compatible with videocholangioscopes and D-POC[5]. NBI can improve visual differentiation of benign from malignant strictures[22]. However, improved visualization via NBI may not translate into improved rates of malignancy detection. Dysplasia detection rate did not increase even when 48% more suspicious lesions were biopsied when using NBI in patients with primary sclerosing cholangitis (PSC)[23]. i-Scan, a computed virtual chromoendoscopy system, may also improve visualization of ductal mucosa and vasculature compared to conventional white-light imaging. While diagnostic accuracy using i-Scan was not significantly better, surface structure, surface microvascular architecture, and margins were significantly better visualized[24]. Probe-based confocal laser endomicroscopy captures microscopic images of living tissue for real-time histological tissue assessment under direct visualization. Compatibility with DSOC was demonstrated in a study by Tanisaka et al[25] involving 30 patients with indeterminate biliary strictures (IBS). While probe-based confocal laser endomicroscopy during DSOC had lower sensitivity compared to DSOC alone (94.1% vs 100%), higher specificity (92.3% vs 76.9%) and accuracy [93.3% (95%CI: 78.7%-98.8%) vs 90% (95%CI: 74.4%-96.5%)] was reported. Chromocholangioscopy can show differences between inflamed, ischaemic, and dysplastic biliary lesions based on different gross surface staining patterns using methylene blue injections during choledochoscopy[26]. However, data on the efficacy of chromocholangioscopy in IBS are limited. Lastly, autofluorescence imaging, which compares colors of lesions when blue excitation light and green and red field cameras, are utilized to distinguish between normal and neoplastic mucosa. Itoi et al[27] evaluated autofluorescence imaging as an adjunctive imaging technique during PTCS. Amongst 65 biliary tract lesions, PTCS with autofluorescence imaging had higher specificity (87.5% vs 52.5%) and accuracy (87.7% vs 70.8%) than PTCS alone, though sensitivity decreased (88% vs 100%).
Nevertheless, most image-enhanced function systems have not yet been validated for clinical use in choledochoscopy. Further studies need to evaluate different choledochoscopes with these current imaging systems and if better biliary visualization indeed translates into improved diagnostic and therapeutic accuracy.
Tissue diagnosis
For the acquisition of larger tissue samples, the SpyBite Max biopsy forceps acquire twice the amount of tissue than the SpyBite biopsy forceps[9]. This is particularly promising given how the diagnostic accuracy of biopsy samples of IBS obtained via the legacy SpyBite biopsy forceps has been hampered by inadequate tissue samples[28].
Stone retrieval and fragmentation
For stone retrieval, a variety of equipment is available for the retrieval of stones. Commonly, stone retrieval baskets are the foremost choice, as there are many variable shapes and sizes that can suit most situations. These include Dormia baskets, SpyGlass Retrieval Basket (SpyBasket), and SpyGlass Retrieval Snare (SpySnare)[29]. However, the baskets require expansion and retraction to securely surround the stones, which may be difficult due to limited space[13]. In those cases, open-ended graspers such as alligator forceps are an option.
When the stone is too large to fit into a retrieval basket or difficult to remove after securing the forceps, fragmentation of the stones is possible[30]. Lithotripsy, either electrohydraulic lithotripsy (EHL), extracorporeal shockwave lithotripsy (ESWL), or laser lithotripsy (LL), can aid fragmentation. Traditionally, mechanical lithotripsy is less commonly used due to its limitations in breaking large pigment stones and challenging maneuverability[31]. In addition, EHL has a higher risk of duct damage due to relative imprecision. Furthermore, the probe's caliber may be too large to enter more miniature endoscopes if needed[13]. LL probes are small caliber and allow accurate and precise fragmentation. Commonly, pulse and non-pulsed lasers are available depending on the penetration depth required. However, LL is notably more expensive than EHL.
Migrated hardware retrieval
Choledochoscopic visualization of the hepatobiliary ducts is also valuable for retrieving migrated hardware such as stents using SpyBasket and SpySnare[32], broken baskets[33,34], and migrated coils[35]. However, such instances have yet to be reported on a larger scale and currently lack power. With the garnering of more reported cases, it would then be possible to truly delineate the potential of chol-edochoscopy in therapeutic interventions and other instances.
Stricture ablation
Choledochoscopy can perform therapeutic interventions like ablation of cholangiocarcinoma (CCA) via photodynamic therapy or radiofrequency ablation. Chole-dochoscopy can confirm successful radiofrequency ablation administration and immediate post-procedure complications. Novel choledochoscopy-guided balloon-radiofrequency ablation techniques demonstrated in animal models also show potential for clinical use[36]. Case reports by Chandrasekar et al[37] and Brunaldi et al[38] describe the use of digital cholangioscopy to evaluate photodynamic therapy.
Scope handling techniques
The use of different techniques when handling the choledochoscope has also been proposed in lithotripsy. For example, Zhang et al[39] proposed the J maneuver when performing choledochoscopy in a freehand technique, described as retroflection of the upper endoscope while in the second part of the duodenum, simultaneous rotation and retraction of the endoscope towards the papilla. Zhang et al[39] claimed that this maneuver would eliminate the need for surgical bile duct exploration.
CLINICAL APPLICATIONS
Choledochoscopy can be used for diagnostic and therapeutic indications (Table 3), with main indications in diagnosing IBS and lithotripsy. This section will discuss the efficacy of choledochoscopy compared to conventional methods and recent advances in various diagnostic and therapeutic indications.
Table 3.
Diagnostic and therapeutic indications for choledochoscopy
|
Diagnostic indications
|
Therapeutic indications
|
| Visual impression and visually-guided biopsies of: (1) Indeterminate biliary strictures (IBS); (2) Dominant strictures in primary sclerosing cholangitis (PSC); and (3) IgG4-related sclerosing cholangitis (IgG4-SC) | Stone fragmentation: (1) Electrohydraulic lithotripsy (EHL); and (2) Laser lithotripsy (LL) |
| Precise preoperative mapping of the extent of tumor involvement in CCA | Ablative therapies in cholangiocarcinoma (CCA): (1) Radiofrequency ablation; (2) Photodynamic therapy; (3) Nd:YAG laser ablation; and (4) Argon plasma coagulation |
| Choledochal cysts | Cystic duct stent placement |
| Intraductal papillary neoplasms of the bile duct | Guidewire passage through strictures, surgically altered anatomy |
| Cholangioadenoma | Resection of ductal masses |
| Biliary papillomatosis | Retrieval of migrated ductal stents |
| Eosinophilic cholangitis | Gallbladder stenting and drainage |
| Biliary varices | |
| Right Hepatic Artery Syndrome | |
| Congenital pancreaticobiliary maljunction | |
| Post-liver transplant ductal ischemia | |
| Tissue sampling and visual evaluation for infections: (1) Cytomegalovirus; and (2) HIV | |
| Evaluation of intrahepatic biliary tracts during minimally invasive surgery |
HIV: Human immunodeficiency virus.
IBS
IBS is defined as biliary strictures with aetiologies that cannot be established after standard diagnostic investigations such as laboratory tests, imaging (such as computed tomography or magnetic resonance cholangiopancreatography), or procedures (such as endoscopic retrograde cholangiopancreatography (ERCP)-guided tissue biopsy)[40]. This section will discuss the role of choledochoscopy in diagnosing IBS, specifically when along with the diagnostic algorithm it should be done, optimal choledochoscope choice, the two main ways choledochoscopy can be used, and factors affecting its diagnostic accuracy.
The imperative in biliary strictures is to exclude malignancies, where ERCP with brush cytology is the initial modality of choice. However, despite its high specificity with brush cytology (> 95%), sensitivity remains low. In a review of 16 studies involving 1556 patients, Burnett et al[41] reported that ERCP brush cytology had a sensitivity of 41.6% ± 3.2% (99%CI) and a negative predictive value (NPV) of 58.0% ± 3.2% (99%CI). Thus, adjunctive diagnostic modalities such as choledochoscopy are required. Per the 2018 Asia-Pacific ERCP Club consensus guidelines, chole-dochoscopy-guided biopsies are recommended to improve diagnostic accuracy in situations where conventional ERCP-based brush cytology and forceps biopsy are inconclusive despite clinical suspicion[42].
Choledochoscopy is a valuable diagnostic modality as it can affect the aggressiveness of management. In a multicentre study by Prat et al[43] involving 61 IBS patients, choledochoscopy prevented unnecessary surgical resection in 33 out of 57 patients with initially-suspected carcinoma, and significantly improved management adequacy rates (P < 0.001) than before choledochoscopy despite a moderate overall diagnostic sensitivity (52%-63.6%). Hence given differences in morbidity in surgical compared to conservative management, there is value in choledochoscopy for patients with unclear diagnoses.
Stricture location determines if choledochoscopy should be done at all and, if done, when along with the diagnostic algorithm after ERCP-based sampling[42]. Firstly, strictures can be intrinsic (e.g., cholangiocarcinoma, periampullary bile duct cancer) or extrinsic to bile duct (e.g., pancreatic cancer, gallbladder cancer, metastatic disease)[44]. Peroral choledochoscopy is more helpful in evaluating intrinsic than extrinsic strictures. The sensitivity for diagnosing malignancy in intrinsic strictures was higher than extrinsic strictures in both FSOC visual impression and FSOC-guided biopsy[44]. Secondly, strictures are either proximal or distal strictures. Martinez et al[45] recommend that peroral choledochoscopy can be used immediately after the first inconclusive ERCP-based sampling for proximal biliary strictures. On the contrary, for distal biliary strictures, peroral choledochoscopy is recommended only if both ERCP-based sampling and endoscopic ultrasound-guided fine-needle aspiration are negative.
Choledochoscopy should be used in both ways for the diagnosis of IBS – visual impression and choledochoscopy-guided biopsies. Direct visualization by choledochoscopy permits the identification of mucosal features suspicious for malignancy and targeted biopsies. In a recent meta-analysis by Wen et al[40] involving 356 patients across 11 studies, the visual impression was more sensitive than choledochoscopy-guided biopsy across DSOC, FSOC, and D-POC (95% vs 74%, 84.5% vs 60.1%, 83%-92% vs 43%-89.5%). However, specificity was higher in choledochoscopy-guided biopsy than visual impression across DSOC, FSOC and D-POC (98% vs 92%, 98% vs 82.6%, 97% vs 84%-92%)[40]. Furthermore, the lack of a standardized visual classification system necessitates that biopsy results confirm visual findings. Thus, it is insufficient to use either visual impression or biopsy findings alone.
Various choledochoscopes have been studied in the diagnosis of IBS. However, an ideal choledochoscope has not yet been established for IBS diagnosis in clinical practice. POC are more frequently used in IBS. However, PTCS can also be used when POC instability prevents adequate bile duct visualization[46]. When comparing POC without the use of image-enhanced function systems, DSOC has an excellent diagnostic yield in both visual impression and choledochoscopy-guided biopsies[40,47,48]. In a study by Mizrahi et al[47] involving 324 patients, DSOC had a significantly higher diagnostic yield of visual impression for malignancy than FSOC (78% vs 37%, P = 0.004). However, studies comparing the efficacy of different choledochoscopes when image-enhanced function systems are used are lacking. For instance, NBI, which is compatible only with videocholangioscopes and D-POC, may significantly improve the efficacy of these two choledochoscopes compared to others.
Several factors confound the diagnostic accuracy of choledochoscopy in IBS. This section will explore these confounders in visual impression and choledochoscopy-guided biopsies and advances made to mitigate them.
For both visual impression and biopsies, the diagnostic accuracy of chole-dochoscopy may decrease with increasing hyperbilirubinemia levels[49] and in specific patient populations such as patients with PSC[50]. This highlights the importance of patient optimization pre-procedure and identification of other confounding patient factors. Other factors include inadequate experience amongst endoscopists (< 25 cases performed)[49].
A major drawback of visual impression using choledochoscopy is the lack of a standardized visual classification system[40], especially because diagnostic accuracy is experience and operator-dependent. Several studies have proposed novel classification systems. However, there is a lack of comparative studies to standardize one classification system. Tumor vessels, which are dilated and tortuous vessels, are markers of malignancy that provide moderate diagnostic accuracy when coupled with biopsy[51]. Other malignant characteristics include nodular mucosa, neovascularization, friability, and papillary characteristics[52]. More recently, in 2018, a new classification system by Robles-Medranda et al[53] classified lesions based on morphological and vascular characteristics (i.e., polypoid, ulcerated, honeycomb, etc.). This had a high sensitivity (96.3%) and specificity (92.3%) amongst 106 patients. However, there was a discrepancy in an inter-observer agreement between experts and non-experts (κ > 80% and 64.7%-81.9% respectively). Better inter- and intra-observer agreement between both expert and non-expert operators (κ > 80%; P < 0.001) was seen in the use of neovasculature morphology, defined as irregular or ‘spider’ vascularity as proposed by Robles-Medranda et al[53] in 2020. This had a sensitivity of 94%, a specificity of 63%, a positive predictive value (PPV) of 75%, NPV of 90% amongst the 95 patients studied[54]. In 2020, Sethi et al[55] proposed the Monaco Classification, which combined eight observable criteria (presence of stricture, lesion, mucosal features, papillary projections, ulcerations, abnormal vasculature, scarring, pronounced pit pattern). A fair diagnostic accuracy (70%) and inter-observer agreement (κ = 0.31, SE = 0.02) was reported, with ulceration (OR = 10.3, P = 0.01) and papillary projections (OR = 7.2, P = 0.02) being most associated with malignancy.
Two main issues limit the use of choledochoscopy-guided biopsies in IBS – challenges in analyzing small biopsy samples obtained during choledochoscopy and lack of consensus on the optimum number of sample sizes required.
Firstly, choledochoscopy-guided tissue samples are often too small for accurate offsite histopathological examination and thus decrease sensitivity. Adequate tissue acquisition is primarily limited by the technical ability of choledochoscopy forceps jaw[28]. Other factors include age less than 65 years old (OR = 0.170, 95%CI: 0.044–0.649, P = 0.010) and previous biliary stenting before POC (OR = 0.199, 95%CI: 0.053–0.756, P = 0.017)[56]. Thus, one approach improves the choledochoscopy forceps jaw's technical ability to acquire large tissue samples per bite, such as in the SpyBite Max biopsy forceps[57]. Alternatively, specimen processing techniques that can process smaller tissue samples have been proposed as adjuncts to conventional histopathological examination. One method is rapid onsite evaluation of touch imprint cytology (ROSE-TIC) during choledochoscopy-guided biopsies. Touch imprint cytology is useful as an adjunct in cases where clinical suspicion for malignancy is high, but offsite sampling is negative or indeterminate[58]. In a study by Varadarajulu et al[59] involving 31 FSOC- and DSOC-guided biopsy procedures, ROSE-TIC provided an additional opportunity for onsite specimen processing and demonstrated sensitivity (100%), specificity (88.9%), PPV (86.7%), NPV (100%), and diagnostic accuracy (93.5%). However, the use of ROSE-TIC in the context of choledochoscopy has yet to be validated in large-size trials. Another method already used for processing smaller specimens is cell block cytology. A study by Baars et al[60] involving 240 SpyBite specimens from the upper gastrointestinal tract in 10 patients found that cellblock cytology results in fewer crush artifacts and requires a significantly smaller specimen to achieve equivalent diagnostic accuracy (1.49 mm vs 2.02 mm, P < 0.001) compared to standard histopathology. However, as this comparative analysis was performed using gastrointestinal samples, a pilot study involving six IBS patients was performed. All 20 SpyBite samples were successfully processed by cell block cytology[60].
Secondly, the optimum number of biopsies to be taken during choledochoscopy remains unestablished. This may depend on specimen processing techniques (onsite vs offsite) and stricture location (intrinsic vs extrinsic). In a randomized control trial using DSOC by Bang et al[58] involving 62 patients, three biopsies were recommended for offsite specimen processing and one biopsy for onsite specimen processing to achieve equivalent diagnostic accuracy (90%). Additional biopsies for offsite specimen processing did not improve diagnostic accuracy. However, other retrospective studies by Onoyama et al[28] and Varadarajulu et al[59] recommend minimally four biopsies when using offsite and onsite[60] processing techniques, respectively. Furthermore, Varadarajulu et al[59] observed that extrinsic strictures required more biopsies than intrinsic strictures for onsite processing techniques.
PSC
Diagnosis of current studies on choledochoscopy in PSC has focused on identifying CCA in PSC strictures and subtyping PSC through visual impression and choledochoscopy-guided biopsies. While the accuracy of visual impression and choledochoscopy-guided biopsies have been well-studied in IBS, the same conclusions cannot simply be applied to PSC. Underlying ductal inflammation and scarring may mimic CCA visually and complicate the passage of choledochoscopes through bile ducts to evaluate strictures[61]. However, large-scale studies specifically on PSC patients are limited.
The ability to accurately exclude CCA in PSC is critical as PSC patients have an increased CCA risk[61]. Various investigations such as imaging and serological tumor markers such as carbohydrate antigen 19-9 are possible but lack sufficient sensitivity and specificity when used alone[62]. Tissue diagnosis is thus crucial in this workup. A meta-analysis by Njei et al[61] across 21 studies found that single-operator choledochoscopy-guided biopsies are the most accurate in diagnosing CCA in PSC patients as compared to brush cytology, fluorescence in situ hybridization, and probe-based confocal laser endomicroscopy, with a sensitivity of 65% (95%CI: 35%-87%) and specificity of 97% (95%CI: 87%-99%). A study by Majeed et al[63] involving 225 PSC patients found that the use of DSOC in addition to second brush cytology improved sensitivity than second brush cytology alone (100% vs 82%) in detecting CCA in PSC. However, another retrospective study by Kaura et al[64] involving 36 PSC patients found that the addition of SpyGlass choledochoscopy-guided biopsy to fluorescence in situ hybridization did not significantly increase sensitivity compared to brush cytology alone. Hence, there remains uncertainty on whether choledochoscopy with other diagnostic investigations can improve CCA detection in PSC.
Furthermore, choledochoscopy findings on visual inspection can subtype PSC into early or late stages of the disease. Sandha et al[65] proposed the novel Edmonton Classification, which categorizes PSC's visual impression features on FSOC and DSOC into three phenotypes – “inflammatory type”, “fibrostenotic type”, and “nodular or mass-forming type”. Fujisawa et al[66] further correlated these findings with time course – “'inflammatory type” correlated to active phase and early-stage PSC, “fibrostenotic type” with chronic phase and late-stage PSC, and “nodular or mass-forming type” in either phase. Stratification into the disease stages is vital in informing each patient's disease and guiding targeted treatment[65].
In the management of PSC, the role of POC has also been considered, specifically when managing patients with dominant strictures. A dominant stricture is defined as a stricture of ≤ 1.5 mm in the common bile duct or ≤ 1 mm in the hepatic duct within 2 cm of the intrahepatic confluence. In a prospective study by Awadallah et al[67] involving 55 patients with PSC, POC was able to help with the diagnosis of PSC-associated biliary strictures and discovered the presence of choledocholithiasis, which was missed in 30.0% of similar patients undergoing cholangiography, improving therapeutic yield. In bacterial cholangitis superimposed, temporary drainage and flushing measures to keep the biliary ducts patent can be performed. This includes the use of naso-biliary tubes for drainage, biliary lavage for decanting and flushing[68], as well as percutaneous transhepatic cholangioplasty for relief of jaundice[69].
IgG4-sclerosing cholangitis
Choledochoscopy is primarily used to visually differentiate IgG4-related sclerosing cholangitis (IgG4-SC) from PSC and CCA. Accurate differentiation is essential as the prognosis and management of the three conditions differ[66]. A study by Itoi et al[70] using peroral videocholangioscopes on 33 patients found a significant discrepancy in the incidence of visual findings such as the presence of dilated and tortuous vessels, scarring, and pseudodiverticula between patients with IgG4-SC and PSC (P = 0.015, P = 0.001, P = 0.0007 respectively). There is a significant discrepancy in the incidence of partially enlarged vessels and dilated vessels between IgG4-SC patients and distal CCA (P = 0.004) and hilar CCA (P = 0.015)[70]. Another study by Ishii et al[71] using peroral videocholangioscopes on 17 IgG4-SC and 53 CCA patients reported that the use of vessel morphology seen on choledochoscopy could distinguish IgG4-SC patients from CCA patients with sensitivity (96%), specificity (89%), interobserver agreement (κ = 0.719), and the intraobserver agreement (κ = 0.768 and 0.754).
CCA
Choledochoscopy may be helpful in the precise preoperative mapping of CCA before surgical resection. This section will discuss the utility of choledochoscopy regarding its rate of adequate tissue acquisition, diagnostic accuracy in mapping the lateral extent of tumor involvement, ability to impact management, therapeutic interventions, and caveats to its use in CCA.
Choledochoscopy allows good access laterally along the bile duct to reach lateral margins of CCA. For example, in a study by Ogawa et al[72] involving 118 target sites along the extrahepatic bile duct, DSOC-guided mapping biopsies could reach 100% of target sites compared to fluoroscopy-guided mapping biopsy (78%).
Diagnostic accuracy of the preoperative mapping of CCA using choledochoscopy requires further validation, owing to the small sample sizes studied[73]. In a study by Pereira et al[74] involving 43 patients, the accuracy of DSOC-guided visual impression and DSOC-guided biopsy was 95% and 81% respectively in the diagnosis of CCA. To further increase diagnostic accuracy in identifying the superficial spread of CCA based on visual impression, Fukasawa et al[75] proposed the novel Form-Vessel Classification (F-V scores), stratifying the form of biliary surface and vessel structure seen on peroral choledochoscopy into four and three grades, respectively. Amongst the 30 biopsy samples from 11 patients, higher F-V scores corresponded with a higher histological malignancy rate and frequency of mutant alleles[75].
Furthermore, choledochoscopy has been shown to alter management. Tyberg et al[76] reported that DSOC-guided mapping biopsy altered the surgical plan in 32 out 105 patients, where six patients required less extensive surgery, 12 had more extensive disease precluding surgery, and 14 were found to have the benign disease.
Caveats to the use of choledochoscopy in the preoperative mapping of CCA include suboptimal rates of successful biopsies attributable to inadequate sample size[72] and limited ability to visualize proximal tumor margin and submucosal tumor extension in all patients[77].
The use of choledochoscopy to perform therapeutic interventions in CCA has also been explored. As mentioned in the section on adjuncts to choledochoscopes above, the use of radiofrequency ablation, photodynamic therapy, and modalities like Nd-YAG laser ablation or Argon plasma coagulation in treating hemobilia have been explored in recent years[78]. However, further studies should be reported to broaden the currently lacking literature as therapies like photodynamic therapy are currently rarely used due to their complex logistical requirements and unclear role in managing biliary pathologies such as malignant biliary strictures[12].
Extrahepatic stones
The primary use of the choledochoscopy resides as an option in managing large or complicated extrahepatic stones in the biliary tree after endoscopic measures have been considered or found unsuitable. Endoscopic treatment via ERCP with standard sphincterotomy or endoscopic papillary large balloon dilatation (EPLBD) is currently recognized as the first-line treatment for extrahepatic bile duct stones, using a combination of basket or balloon catheterization for the exploration and then extraction[79].
Choledochoscopy can be considered for the removal of difficult extrahepatic bile stones. POC-guided clearance is was highly effective in clearing difficult bile stones defined as large stones ≥ 15 mm in diameter and with a prior attempt at stone clearance or impacted multiple stones[80]. Any stones in the hepatic duct or above a stricture were also considered difficult. Choledochoscopy has also been touted to have surpassed the previous second-line therapy of mechanical lithotripsy. In a study involving 32 patients with huge common bile duct stones, defined as stones not cleared by endoscopic sphincterotomy and EPLBD or not amenable to EPLBD, Angsuwatcharakon et al[81] claimed a higher success rate in choledochoscopy-guided laser lithotripsy over mechanical lithotripsy in the first session (63.0% vs 100%, P < 0.01) and lower radiation exposure (20989 vs 40745 mGycm2).
Additionally, the use of EHL and LL assisted by POC also has excellent duct clearance rates. Both EHL and LL had higher ductal clearance rates when compared to ESWL in dealing with retained biliary stones[82]. However, complications and length of hospital say were similar between the two. In a meta-analysis of 49 studies, Korrapati et al[83] noted the accuracy of POC to be 89.0% (95%CI: 84%-93%) for the visualization of the pathology and a clearance rate of 88.0% (95%CI: 85%-91%).
The safety and reduced radiation exposure make choledochoscopy an excellent alternative to conventional management of extrahepatic biliary stones. In a study by Franzini et al[4] involving 100 patients, the use of choledochoscopy-guided EHL was non-inferior to ERCP with EPLBD in the removal of complex biliary stones (defined as > 15 mm, > 10 stones, the disproportion of ≥ 2 mm between stone and distal common bile duct or biliary stricture with a stone upstream)[84]. However, some still consider POC to be relatively complicated and time-consuming despite its safety and benefits compared to the conventional and more straightforward mechanical lithotripsy technique[85]. In a study by Buxbaum et al[86] consisting of 60 patients comparing POC-assisted lithotripsy and conventional therapy (defined as mechanical lithotripsy), the duration for lithotripsy procedure was significantly longer (120.7 vs 81.2 min, P = 0.0008). In contrast, Angsuwatcharakon et al[81] claimed that there was no significantly different procedure time (66 vs 83 min, P = 0.23) between POC-assisted lithotripsy and mechanical lithotripsy in stone management after the failure of EPLBD. While more trials with higher power should be performed to establish the significance of this disparity in procedural time, the efficacy and non-inferior complications rate of POC-assisted lithotripsy against manual lithotripsy in the management of large bile duct stones has been established. Therefore, it can be used as a standard of care after failing endoscopic treatment with ERCP and sphincterotomy.
The efficacy of different types of POC in stone removal is also a consideration. In a retrospective study involving 32 patients who failed conventional ERCP for stone removal, Murabayashi et al[87] noted that both DSOC and videocholangioscope (CHF-B260) achieved a 100% complete stone removal with similar adverse event rates. However, DSOC was noted to have significantly shorter procedural time (67 ± 30 minutes vs 107 ± 64 min), and a lesser number of endoscopic sessions were needed (1.35 ± 0.49 vs 2.00 ± 0.85)[87].
Alternative therapeutic options like ESWL, where direct contact with the stone is unnecessary, are valuable when patients cannot undergo endoscopic therapy[88]. However, the risk of recurrence was notably higher when compared to POC. A prospective study of 58 patients by Aljebreen et al[89] compared ESWL and SpyGlass-guided EHL. Bile duct stone clearance rate was 100% in the SpyGlass-guided EHL group and 64.4% in the ESWL group. Historically, the role of chemical dissolution (such as methyl) of stones had been entertained by perfusing the common bile duct with solvents. However, the success rate remains low (66%-74%), with high complication rates (67%), including haemorrhage, duodenal ulceration, acute pancreatitis, and anaphylaxis[90].
Intrahepatic stones
The use of cholangioscopy for hepatolithiasis is limited due to relatively smaller hepatic ducts and strictures within the intrahepatic lumens[12]. Consequently, the literature is scarce, with few large patient studies. In a case series involving 190 patients, Cheng et al[91] reported a high intrahepatic stone clearance rate via POC (88.4%). However, a higher recurrence rate is reported with such an approach. In a retrospective study by Huang et al[92] of 245 patients undergoing PTCS to treat hepatolithiasis, recurrence rates was 63.2% overall, depending on the type of hepatolithiasis. Cholangioscopy via a percutaneous transenteric approach via access loop is another alternative for hepatolithiasis extraction. Access loops are preemptively created during hepaticojejunostomy for ease of future biliary interventions. This is particularly relevant for patients with intrahepatic strictures, predisposed to recurrent hepatolithiasis and cholangitis requiring repeated biliary intervention[93]. In cases with altered surgical anatomy, the use of cholangioscopy is valuable, allowing access to pathology sites without a choledochotomy, hence sparing the patient from a T-tube insertion. This helps lower complication rates and operative duration, and the length of hospital stay[94].
Other indications
In terms of diagnostic indications, choledochoscopy has also been used in diseases with a higher probability of malignant transformation, such as in the detection of dysplasia[95] and intraoperative determination of resection planes[96] in choledochal cysts, or diagnosis of malignant lesions such as intraductal papillary neoplasms of the bile duct[97]. In addition, recent reports demonstrate a role in the diagnosis of benign biliary pathologies such as cholangioadenoma[98], biliary papillomatosis[99], eosinophilic cholangitis[100], choledochal varices[101], right hepatic artery syndrome[102], congenital pancreaticobiliary maljunction[103], post-transplant ductal ischemia[104], infections such as cytomegalovirus and human immunodeficiency virus-associated cholangiopathy[105,106] and intraoperative evaluation for intrahepatic biliary duct injury during surgery[107].
For therapeutic interventions, choledochoscopy is useful in visualization and subsequent guidewire placement in the context of surgically altered anatomy. One example is PTCS in severe biliary-enteric strictures that have failed conventional fluoroscopic techniques[108]. Other examples include DSOC-guided direct visualization of late fibrotic strictures of anastomotic regions after deceased donor transplantation. This enabled guidewire placement, followed by subsequent dilation and stent placement[109,110]. Other surgically altered anatomy to which choledochoscopy is used successfully includes strictures in hepaticojejunostomy, afferent loop syndrome[111], and other complex biliary strictures that previously failed conventional guidewire placement[112]. Treatment of haemobilia has also been reported[78].
Choledochoscopy-assisted endoscopic transpapillary gallbladder stenting (ETGS) and subsequent drainage in acute cholecystitis is a potential use that has been recently explored. ETGS is an alternative for acute cholecystitis patients with significant co-morbidity who are at prohibitive risk for cholecystectomy or even percutaneous cholecystostomy[113]. However, ETGS is commonly limited by poor cystic duct cannulation rates. In a retrospective study by Cao et al[114] of 226 patients with acute cholecystitis requiring ETGS, the use of single-operator choledochoscope guidance increased the overall technical success of cannulation rates to 75%-86.4%.
COMPLICATIONS
Complications arising from choledochoscopy can be divided into procedure-related complications (including preparatory and intra-procedure complications) as well as technical complications of choledochoscopy. We will discuss a possible preventive measure that can be taken.
Procedure-related
For percutaneous choledochoscopy, complications occur during preparatory procedures such as percutaneous transhepatic biliary drainage and tract dilation than during choledochoscopy itself[115]. Regarding mild complications, a study by Wang et al[116] on 826 patients reported bleeding (1.9%), T-tube dislodgement (0.8%), infection (0.7%), basket incarceration (0.6%), and bile leaks (0.4%). Additionally, post-operative choledochoscopy could result in damage to T-tube systems, preventing extraction of retained stones, and causing bleeding and intestinal fistulas[117]. Severe complications include severe haemobilia, haemoperitoneum, sinus tract rupture, and ductal injury[115].
Peroral choledochoscopy is generally regarded as a low-risk procedure. Complications such as cholangitis, pancreatitis, haemobilia, bile leak, air embolization, bile duct perforation have been reported[44]. A meta-analysis by Korrapati et al[83] involving 2193 patients across 49 studies who underwent peroral choledochoscopy reported an overall adverse event rate of 7% (95%CI: 6%–9%), where complications primarily included cholangitis, followed by pancreatitis and perforation. However, Lenze et al[118], reported a 16.4% adverse event rate (pancreatitis, cholangitis, or significant bleeding) amongst 67 patients who underwent DSOC. While all complications in this study were successfully treated conservatively, it reinforces that choledochoscopy should only be used in patients failing conventional procedures.
Technical-related
Rates of adverse events arising from choledochoscopy have been compared against conventional procedures used in biliary disorders. A large retrospective study by Sethi et al[119] compared the adverse event rates occurring in 3475 ERCP procedures and 402 ERCP with additional choledochoscopy. It was found that the additional choledochoscopy contributed to a significantly higher rate of cholangitis than when the only ERCP was done (1.0% vs 0.2%; OR = 4.98; 95%CI: 1.06-19.67), which is postulated to be secondary to intermittent intraductal irrigation during chole-dochoscopy[119]. A caveat when comparing adverse events rates across procedures is the selection bias in patients undergoing choledochoscopy. They are likely to have failed conventional methods like ERCP, possibly due to underlying complicated anatomy or lesions, which in itself may predispose to complications[83].
Prevention of complications
Risks of complications can be mitigated. A retrospective multicentre study by Ang et al[120] analyzing 250 DSOC procedures found that prophylactic pre-procedural antibiotics significantly decreased the rate of cholangitis in patients who received antibiotics (n = 102) than those who did not (n = 148) (1% vs 12.8% respectively, P < 0.001).
Special considerations
Choledochoscopy has demonstrated good safety profiles in diverse patient groups – the elderly, pregnant women, and children. In a multicentre study by Bernica et al[121] across 209 patients, there was no significant difference in adverse events rates even in patients above 75 years old when compared with younger patients (7.30% for patients aged below 65 years, 6.98% for patients aged 65–75 years, and 7.79% for patients aged above 75 years; P < 0.17). Choledochoscopy is a promising alternative procedure for choledocholithiasis in pregnant women who require minimal radiation exposure. Pregnant women with choledocholithiasis have significant radiation exposure when treated conventionally via ERCP. A case report demonstrated the ability to completely reduce radiation exposure during choledocholithiasis identification and removal using DSOC. This combination of DSOC with ERCP was not associated with adverse maternal and fetal outcomes[122]. Case series have also reported successful choledochoscopy with no significant complications in children for indications such as intrahepatic lithotripsy[123], evaluation of biliary strictures, and management before and after liver transplant[124]. While choledochoscopy in children is beyond the scope of this review, it can be extrapolated to be a safe and effective modality used in pediatric biliary pathologies such as Caroli disease, biliary atresia, and monitoring post-Kasai procedure.
In summary, choledochoscopy is generally a low-risk procedure that can be used even in the elderly, pregnant women, and children when indicated. However, given that patients undergoing choledochoscopy have a higher risk of complications than conventional biliary procedures, choledochoscopy should only be used in patients failing conventional procedures.
LIMITATIONS
Overall limitations of choledochoscopy include operator-dependency, cost, and technical limitations in choledochoscopes and accessories.
Firstly, the accuracy of choledochoscopy is highly operator-dependent and may be affected by insufficient endoscopy experience (< 25 cases performed)[49]. Increased choledochoscopy volume could result in a less steep learning curve. This is supported by the concept that repetition allows for accurate anatomical recognition and more straightforward instrumentation guidance[125]. Simulated training models are proposed to improve inter-operator discrepancy. A randomized control trial by Li et al[126] involving 20 resident trainees found that the use of physical three-dimensional printed models for simulated choledochoscopy led to significantly higher accurate anatomical structure identification (P < 0.05) and reduction in time taken to complete simulated choledochoscopy. Other training models include a three-dimensional printed model of a biliary tree integrated with augmented reality by Tang et al[127]. This allows for spatially accurate real-time simulated choledochoscopy. A training model for the freehand double-bending D-POC technique is also reported[128]. The advent of artificial intelligence to aid in customized, individualized learning should also be considered in surgery[129]. Larger studies are needed to validate these training models, determine optimum training time to achieve competency in choledochoscopy and compare if training translates to reduced inter-operator discrepancy in clinical practice.
Another limitation lies in the cost-benefit analysis of choledochoscopy compared to conventional procedures. High capital costs for the initial purchase of processors, scopes, and repair costs are cited as factors against choledochoscopy. For recurring costs for performing a single procedure, Loras et al[130] found that additional choledochoscopy use during ERCP in 2018 can increase procedural costs alone by $3662.71 and $2637.02 for stone extraction and stricture diagnosis, respectively. ERCP with choledochoscopy was the most expensive among advanced endoscopic procedures studied, even though ERCP alone was not more expensive than most other procedures[130]. However, there is an argument for cost-efficacy in choledochoscopy. Choledochoscopy may reduce the need to perform costlier procedures. In a study by Sandha et al[131] across 51 patients with difficult-to-access choledocholithiasis, choledochoscopy-guided lithotripsy circumvented the need for laparoscopic and open surgical bile duct exploration. This decreased costs per procedure by $1619 and $3210 respectively[131]. However, it is essential to consider the potential reusability of the equipment. While it is thought that reusable devices are more cost-effective and environmentally less damaging[132], the use of disposable equipment in other laparoscopic surgeries is noted to be associated with more significant intraoperative problems caused by technical difficulties[133]. Thus, proper handling and technical maintenance of reusable equipment should be emphasized and taught to benefit financially, economically, and technically.
Other limitations include the technical aspects of fiberoptics and accessories. Suboptimal image quality, size of therapeutic channels of current systems, ease of use, and various accessories still limit choledochoscopy use[12]. However, given how new technology could overcome previous models' limitations and develop new accessories quickly, it is promising that current technical limitations can similarly be overcome.
FUTURE DIRECTIONS
Future studies can develop quality indicators to prove the adequacy of chol-edochoscopy, validate technological advances, and identify factors affecting choledochoscopy efficacy and methods to overcome limitations in specific indications such as IBS diagnosis and preferred management of complex bile stone disease.
First, future studies can focus on ways to improve the accuracy of choledochoscopy. Other than hyperbilirubinemia and endoscopists’ experience, patient and procedural factors should be identified[49]. This can guide ways to optimize patients pre-procedure and improve the quality of choledochoscopy. Specifically, studies are still needed to determine the optimal number of biopsies for IBS diagnosis while considering technical improvements in choledochoscopy forceps jaws (e.g., SpyBite Max). Regarding visual impression, many studies have developed novel visual classification systems such as the “tumor vessel sign”[51], characterization of mucosal and vascular features[52-54], and the Monaco Classification[55]. However, these are done using specific choledochoscopes like DSOC. Given how different choledochoscopes have variable imaging quality, studies need to determine if such visual classification systems can be accurately applied even when using choledochoscopes with lower imaging quality. Subsequently, comparative studies are needed to determine a standardized classification system with the highest accuracy and least inter-observer variability.
Secondly, there is a lack of quality indicators to demonstrate the biliary system's complete visualization in real-time during each choledochoscopy. Good advancement of the choledochoscope for complete visualization is often presumed[134]. Zimmer et al[134] proposed the visualization of the “bilio-papillary Z line” as a quality indicator. As it represents the distal-most end of the common bile duct at the bilio-papillary junction, visualization of the “bilio-papillary Z line” is thought to confirm visualization of the entire common bile duct. However, this marker is limited due to occasional difficult access and prolapsing papillary mucosa at this junction[134]. Future studies should evaluate this marker's accuracy and develop other quality indicators easily adaptable in clinical use.
Thirdly, studies can further clarify the role of novel enhanced imaging systems and new video display techniques. Some studies involving NBI and i-Scan reported no increase in diagnostic accuracy rate despite improved duct visualization[23,24]. Future studies need to explore if improved biliary visualization correlates to improved diagnostic or therapeutic efficacy.
To further improve image quality, studies can explore the use of new display techniques during choledochoscopy, which may negate any loss of three-dimensionality and poor spatial orientation associated with choledochoscopy. These include three-dimensional (3D) and two-dimensional-4K ultra-high definition (2D-4K), which has four-fold more pixels than two-dimensional high definition (2D-HD)[135]. While 3D and 2D-4K display techniques have not been studied in choledochoscopy, advantages are reported in laparoscopic surgery. The 3D display enables better laparoscopic performance compared to conventional 2D-HD monitors[136]. However, it is less clear whether 3D or 2D-4K display is better. Some studies demonstrated significantly better laparoscopic performance in 3D display than 2D-4K display, lower operative time, error rates[136], and increased precision in tasks[137]. Other studies found no significant difference in either operative time or error rates[138]. Nevertheless, given that 3D and 2D-4K displays may optimize scope-guided procedures, studies can consider evaluating these new display techniques in choledochoscopy.
Lastly, the role of artificial intelligence in chole-dochoscopy can be explored. Artificial intelligence has shown good accuracy in automating the detection of polyps, neoplasia, and blind spots and documentation of the procedure's technical details when used for colonoscopy and oesophagogastroduodenoscopy[139]. Given how it has shown potential in improving efficiency, particularly in gastrointestinal endoscopy, future studies may consider applying machine learning models to automate certain aspects of choledochoscopy.
CONCLUSION
Choledochoscopy (for extrahepatic biliary procedures) and cholangioscopy (for intrahepatic biliary procedures) is a dynamic instrument, adapting to a myriad of different circumstances. While the two phrases are used interchangeably, a distinction has to be acknowledged. It serves a diagnostic purpose in the evaluation of biliary pathologies and aids in histology sampling. It also serves a therapeutic purpose in stone fragmentation and extraction and manages malignant lesions in the biliary tree. Collectively, the utility of this instrument has advanced tremendously in recent years, potentially overtaking conventional methods of diagnosis and treatment in the near future. Choledochoscopy is complementary to other endoscopic, interventional radiology, and operative techniques for biliary intervention as well. With the increasing ability of artificial intelligence to automate the detection of pathologies and individualise training for endoscopists, a future pioneered by choledochoscopy and cholangioscopy is promising.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 20, 2021
First decision: July 17, 2021
Article in press: November 15, 2021
Specialty type: Surgery
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atstupens K, Hourneaux de Moura EG S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
Contributor Information
Tsinrong Lee, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 308232, Singapore. leet0043@e.ntu.edu.sg.
Thomas Zheng Jie Teng, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 308232, Singapore.
Vishal G Shelat, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 308232, Singapore; Department of General Surgery, Tan Tock Seng Hospital, Singapore 308433, Singapore.
References
- 1.Phillips E, Berci G, Barber K, Williams J. The Role of Choledochoscopy: The Eternal Problem of How to Remove a CBD Stone. Surg Innov. 2015;22:540–545. doi: 10.1177/1553350615594444. [DOI] [PubMed] [Google Scholar]
- 2.Monga A, Ramchandani M, Reddy DN. Per-oral cholangioscopy. J Interv Gastroenterol. 2011;1:70–77. doi: 10.4161/jig.1.2.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raijman I. Choledochoscopy/cholangioscopy. Gastrointest Endosc Clin N Am. 2013;23:237–249. doi: 10.1016/j.giec.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Franzini TA, Moura RN, de Moura EG. Advances in Therapeutic Cholangioscopy. Gastroenterol Res Pract. 2016;2016:5249152. doi: 10.1155/2016/5249152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida Y, Itoi T, Okabe Y. Types of Peroral Cholangioscopy: How to Choose the Most Suitable Type of Cholangioscopy. Curr Treat Options Gastroenterol. 2016;14:210–219. doi: 10.1007/s11938-016-0090-2. [DOI] [PubMed] [Google Scholar]
- 6.Olympus Endoscope Overview. 2020. Available from https://d3a0ilwurc1bhm.cloudfront.net/asset/084438885177/0694ed24d569c1855888028a9fcf522a .
- 7.Derdeyn J, Laleman W. Current role of endoscopic cholangioscopy. Curr Opin Gastroenterol. 2018;34:301–308. doi: 10.1097/MOG.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 8.Yodice M, Choma J, Tadros M. The Expansion of Cholangioscopy: Established and Investigational Uses of SpyGlass in Biliary and Pancreatic Disorders. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boston Scientific. SpyGlass™ DS II Direct Visualization System. 2019. Available from: https://www.bostonscientific.com/content/dam/bostonscientific/endo/portfolio-group/SpyGlass%20DS/SpyGlass-DS-System-ebrochure.pdf .
- 10.Itoi T, Nageshwar Reddy D, Sofuni A, Ramchandani M, Itokawa F, Gupta R, Kurihara T, Tsuchiya T, Ishii K, Ikeuchi N, Moriyasu F, Moon JH. Clinical evaluation of a prototype multi-bending peroral direct cholangioscope. Dig Endosc. 2014;26:100–107. doi: 10.1111/den.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YN, Moon JH, Lee TH, Choi HJ, Itoi T, Beyna T, Neuhaus H. Prospective randomized trial of a new multibending vs conventional ultra-slim endoscope for peroral cholangioscopy without device or endoscope assistance (with video) Gastrointest Endosc. 2020;91:92–101. doi: 10.1016/j.gie.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Ayoub F, Yang D, Draganov PV. Cholangioscopy in the digital era. Transl Gastroenterol Hepatol. 2018;3:82. doi: 10.21037/tgh.2018.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed S, Schlachter TR, Hong K. Percutaneous Transhepatic Cholangioscopy. Tech Vasc Interv Radiol. 2015;18:201–209. doi: 10.1053/j.tvir.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Mukewar S, Carr-Locke D. Advances in Endoscopic Imaging of the Biliary Tree. Gastrointest Endosc Clin N Am. 2019;29:187–204. doi: 10.1016/j.giec.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Yang JJ, Liu XC, Chen XQ, Zhang QY, Liu TR. Clinical value of DPOC for detecting and removing residual common bile duct stones (video) BMC Gastroenterol. 2019;19:135. doi: 10.1186/s12876-019-1045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Guo SJ, Zhang JC, Wang HY, Li K, Niu SH. A new hybrid anchoring balloon for direct peroral cholangioscopy using an ultraslim upper endoscope. Dig Endosc. 2018;30:364–371. doi: 10.1111/den.12989. [DOI] [PubMed] [Google Scholar]
- 17.Anderloni A, Auriemma F, Fugazza A, Troncone E, Maia L, Maselli R, Carrara S, D'Amico F, Belletrutti PJ, Repici A. Direct peroral cholangioscopy in the management of difficult biliary stones: a new tool to confirm common bile duct clearance. Results of a preliminary study. J Gastrointestin Liver Dis. 2019;28:89–94. doi: 10.15403/jgld.2014.1121.281.bil. [DOI] [PubMed] [Google Scholar]
- 18.Zimmer V. Intraductal simethicone application for optimization of endoscopic view on direct cholangioscopy. Ann Gastroenterol. 2020;33:689. doi: 10.20524/aog.2020.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demmert AC, Nezami N, Singh H. The Cholangioscopy Expander: A Handmade Device to Improve Visualization and Minimize Mucosal Injury during Percutaneous Cholangioscopy. J Vasc Interv Radiol. 2020;31:1956–1958. doi: 10.1016/j.jvir.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Jian W, Song YZ, Xiang QF, Tian HY, Xie ZZ, Yang JB, Zhang YM, Zhang RK, Liu JL. Application of Transparent Cap-assisted Choledochoscopy in Endoscopic Gallbladder-preserving Surgery. Surg Laparosc Endosc Percutan Tech. 2020;30:317–321. doi: 10.1097/SLE.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M, Kato A, Hayashi K, Naitoh I, Miyabe K, Hori Y, Asano G. Novel technique for intraductal cholangioscopy-assisted biliary drainage with over-the-wire microcatheter manipulation. Endoscopy. 2019;51:E398–E399. doi: 10.1055/a-0962-9628. [DOI] [PubMed] [Google Scholar]
- 22.Jang JW, Noh DH, Paik KH, Kim SH, Paik IH, Jung SH. Effectiveness of cholangioscopy using narrow band imaging for hepatobiliary malignancies. Ann Surg Treat Res. 2017;93:125–129. doi: 10.4174/astr.2017.93.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsaitas C, Semertzidou A, Sinakos E. Update on inflammatory bowel disease in patients with primary sclerosing cholangitis. World J Hepatol. 2014;6:178–187. doi: 10.4254/wjh.v6.i4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YN, Moon JH, Choi HJ. Role of Image-Enhanced Endoscopy in Pancreatobiliary Diseases. Clin Endosc. 2018;51:541–546. doi: 10.5946/ce.2018.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanisaka Y, Ryozawa S, Nonaka K, Yasuda M, Fujita A, Ogawa T, Mizuide M, Tashima T, Araki R. Diagnosis of Biliary Strictures Using Probe-Based Confocal Laser Endomicroscopy under the Direct View of Peroral Cholangioscopy: Results of a Prospective Study (with Video) Gastroenterol Res Pract. 2020;2020:6342439. doi: 10.1155/2020/6342439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erim T, Shiroky J, Pleskow DK. Cholangioscopy: the biliary tree never looked so good! Curr Opin Gastroenterol. 2013;29:501–508. doi: 10.1097/MOG.0b013e3283640f4b. [DOI] [PubMed] [Google Scholar]
- 27.Itoi T, Shinohara Y, Takeda K, Nakamura K, Takei K. Improvement of choledochoscopy: Chromoendocholedochoscopy, autofluorescence imaging, or narrow-band imaging. Dig Endosc. 2007;19:S95–S104. [Google Scholar]
- 28.Onoyama T, Hamamoto W, Sakamoto Y, Kawahara S, Yamashita T, Koda H, Kawata S, Takeda Y, Matsumoto K, Isomoto H. Peroral cholangioscopy-guided forceps biopsy versus fluoroscopy-guided forceps biopsy for extrahepatic biliary lesions. JGH Open. 2020;4:1119–1127. doi: 10.1002/jgh3.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S, Shah RJ. Cholangioscopy-guided basket retrieval of impacted stones. VideoGIE. 2020;5:387–388. doi: 10.1016/j.vgie.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponchon T, Genin G, Mitchell R, Henry L, Bory RM, Bodnar D, Valette PJ. Methods, indications, and results of percutaneous choledochoscopy. A series of 161 procedures. Ann Surg. 1996;223:26–36. doi: 10.1097/00000658-199601000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JG, Leung JW. Endoscopic management of difficult common bile duct stones. Gastrointest Endosc Clin N Am. 1996;6:43–55. [PubMed] [Google Scholar]
- 32.Al Lehibi A, Al Mtawa A, Almasoudi T, Al Ghamdi A, Al Otaibi N, Al Balkhi A. Removal of proximally migrated biliary stents by using single-operator cholangioscopy. VideoGIE. 2020;5:213–216. doi: 10.1016/j.vgie.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varshney VK, Sreesanth KS, Gupta M, Garg PK. Laparoscopic retrieval of impacted and broken dormia basket using a novel approach. J Minim Access Surg. 2020;16:415–417. doi: 10.4103/jmas.JMAS_245_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tepox-Padrón A, Romano-Munive F, Ramírez-Polo AI, Téllez-Ávila FI. Three case reports of impacted biliary stone extraction basket. Rev Gastroenterol Mex (Engl Ed) 2020;85:222–224. doi: 10.1016/j.rgmx.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Bent CK, Wright L, Dong PR. "Coildocholithiasis"-Common Bile Duct Obstruction Secondary to Migration of Right Hepatic Artery Pseudoaneurysm Coils. J Vasc Interv Radiol. 2016;27:1741–1743. doi: 10.1016/j.jvir.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Inoue T, Kutsumi H, Ibusuki M, Yoneda M. Feasibility of balloon-based endobiliary radiofrequency ablation under cholangioscopy guidance in a swine model. Sci Rep. 2021;11:14254. doi: 10.1038/s41598-021-93643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandrasekar VT, Faigel D. Diagnosis and treatment of biliary malignancies: biopsy, cytology, cholangioscopy and stenting. Mini-invasive Surg . 2021;5:33. [Google Scholar]
- 38.Brunaldi VO, Brunaldi JE, Vollet-Filho JD, Brunaldi MO, Ardengh JC, Bagnato VS, Dos-Santos JS, Kemp R. Photodynamic therapy of extrahepatic cholangiocarcinoma using digital cholangioscopy. Arq Bras Cir Dig. 2020;33:e1490. doi: 10.1590/0102-672020190001e1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang HC, Dedania B, Thosani N. Management of choledocholithiasis by direct cholangioscopy via freehand intubation using the "J" maneuver. VideoGIE. 2019;4:214–216. doi: 10.1016/j.vgie.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen LJ, Chen JH, Xu HJ, Yu Q, Liu K. Efficacy and Safety of Digital Single-Operator Cholangioscopy in the Diagnosis of Indeterminate Biliary Strictures by Targeted Biopsies: A Systematic Review and Meta-Analysis. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10090666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184:304–311. doi: 10.1016/j.jss.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 42.Sun B, Moon JH, Cai Q, Rerknimitr R, Ma S, Lakhtakia S, Ryozawa S, Kutsumi H, Yasuda I, Shiomi H, Li X, Li W, Zhang X, Itoi T, Wang HP, Qian D, Wong Lau JY, Yang Z, Ji M, Hu B Asia-Pacific ERCP Club. Review article: Asia-Pacific consensus recommendations on endoscopic tissue acquisition for biliary strictures. Aliment Pharmacol Ther. 2018;48:138–151. doi: 10.1111/apt.14811. [DOI] [PubMed] [Google Scholar]
- 43.Prat F, Leblanc S, Foissac F, Ponchon T, Laugier R, Bichard P, Maire F, Coumaros D, Charachon A, Vedrenne B, Boytchev I, Chaussade S, Kaddour N, Laquière A, Gaujoux S. Impact of peroral cholangioscopy on the management of indeterminate biliary conditions: a multicentre prospective trial. Frontline Gastroenterol. 2019;10:236–243. doi: 10.1136/flgastro-2018-100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsa N, Khashab MA. The Role of Peroral Cholangioscopy in Evaluating Indeterminate Biliary Strictures. Clin Endosc. 2019;52:556–564. doi: 10.5946/ce.2019.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez NS, Trindade AJ, Sejpal DV. Determining the Indeterminate Biliary Stricture: Cholangioscopy and Beyond. Curr Gastroenterol Rep. 2020;22:58. doi: 10.1007/s11894-020-00797-9. [DOI] [PubMed] [Google Scholar]
- 46.Mizuno S, Nakai Y, Tanaka M, Ushiku T, Arita J, Hasegawa K, Fukayama M, Koike K. Gastrointestinal: Reappraisal of the usefulness of percutaneous transhepatic cholangioscopy for indeterminate distal biliary strictures. J Gastroenterol Hepatol. 2019;34:961. doi: 10.1111/jgh.14588. [DOI] [PubMed] [Google Scholar]
- 47.Mizrahi M, Khoury T, Wang Y, Cohen J, Sheridan J, Chuttani R, Berzin TM, Sawhney MS, Pleskow DK. "Apple Far from the Tree": comparative effectiveness of fiberoptic single-operator cholangiopancreatoscopy (FSOCP) and digital SOCP (DSOCP) HPB (Oxford) 2018;20:285–288. doi: 10.1016/j.hpb.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Tanisaka Y, Mizuide M, Fujita A, Ogawa T, Suzuki M, Katsuda H, Saito Y, Miyaguchi K, Tashima T, Mashimo Y, Ryozawa S. Diagnostic Process Using Endoscopy for Biliary Strictures: A Narrative Review. J Clin Med. 2021;10 doi: 10.3390/jcm10051048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang S, Stevens T, Kou L, Vargo JJ, Parsi MA. Efficacy of digital single-operator cholangioscopy and factors affecting its accuracy in the evaluation of indeterminate biliary stricture. Gastrointest Endosc. 2020;91:385–393.e1. doi: 10.1016/j.gie.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Fung BM, Fejleh MP, Tejaswi S, Tabibian JH. Cholangioscopy and its Role in Primary Sclerosing Cholangitis. Eur Med J Hepatol. 2020;8:42–53. [PMC free article] [PubMed] [Google Scholar]
- 51.Subhash A, Abadir A, Iskander JM, Tabibian JH. Applications, Limitations, and Expansion of Cholangioscopy in Clinical Practice. Gastroenterol Hepatol (N Y) 2021;17:110–120. [PMC free article] [PubMed] [Google Scholar]
- 52.Sethi A, Shah RJ. Cholangioscopy and pancreatoscopy. Tech Gastrointest Endosc . 2017;19:182–187. [Google Scholar]
- 53.Robles-Medranda C, Valero M, Soria-Alcivar M, Puga-Tejada M, Oleas R, Ospina-Arboleda J, Alvarado-Escobar H, Baquerizo-Burgos J, Robles-Jara C, Pitanga-Lukashok H. Reliability and accuracy of a novel classification system using peroral cholangioscopy for the diagnosis of bile duct lesions. Endoscopy. 2018;50:1059–1070. doi: 10.1055/a-0607-2534. [DOI] [PubMed] [Google Scholar]
- 54.Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S, Niwa H, Mitsui T, Asada Y, Miura S, Ohta T, Nakanuma Y. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–1343. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- 55.Sethi A, Tyberg A, Slivka A, Adler DG, Desai AP, Sejpal DV, Pleskow DK, Bertani H, Gan SI, Shah R, Arnelo U, Tarnasky PR, Banerjee S, Itoi T, Moon JH, Kim DC, Gaidhane M, Raijman I, Peterson BT, Gress FG, Kahaleh M. Digital Single-operator Cholangioscopy (DSOC) Improves Interobserver Agreement (IOA) and Accuracy for Evaluation of Indeterminate Biliary Strictures: The Monaco Classification. J Clin Gastroenterol. 2020 doi: 10.1097/MCG.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 56.Onoyama T, Takeda Y, Kawata S, Kurumi H, Koda H, Yamashita T, Hamamoto W, Sakamoto Y, Matsumoto K, Isomoto H. Adequate tissue acquisition rate of peroral cholangioscopy-guided forceps biopsy. Ann Transl Med. 2020;8:1073. doi: 10.21037/atm-20-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boston Scientific. SpyGlass™ Max Biopsy Forceps. 2020. Available from: https://www.bostonscientific.com/en-US/products/forceps/spysbte-max-biopsy-forceps.html .
- 58.Bang JY, Navaneethan U, Hasan M, Sutton B, Hawes R, Varadarajulu S. Optimizing Outcomes of Single-Operator Cholangioscopy-Guided Biopsies Based on a Randomized Trial. Clin Gastroenterol Hepatol. 2020;18:441–448.e1. doi: 10.1016/j.cgh.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 59.Varadarajulu S, Bang JY, Hasan MK, Navaneethan U, Hawes R, Hebert-Magee S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? Gastrointest Endosc. 2016;84:681–687. doi: 10.1016/j.gie.2016.03.1497. [DOI] [PubMed] [Google Scholar]
- 60.Baars JE, Keegan M, Bonnichsen MH, Aepli P, Theyventhiran R, Farrell E, Kench JG, Saxena P, Kaffes AJ. The ideal technique for processing SpyBite tissue specimens: a prospective, single-blinded, pilot-study of histology and cytology techniques. Endosc Int Open. 2019;7:E1241–E1247. doi: 10.1055/a-0950-9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Njei B, McCarty TR, Varadarajulu S, Navaneethan U. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139–1151. doi: 10.1111/apt.13817. [DOI] [PubMed] [Google Scholar]
- 62.Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19-9 - tumor marker: Past, present, and future. World J Gastrointest Surg. 2020;12:468–490. doi: 10.4240/wjgs.v12.i12.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Majeed A, Castedal M, Arnelo U, Söderdahl G, Bergquist A, Said K. Optimizing the detection of biliary dysplasia in primary sclerosing cholangitis before liver transplantation. Scand J Gastroenterol. 2018;53:56–63. doi: 10.1080/00365521.2017.1385840. [DOI] [PubMed] [Google Scholar]
- 64.Kaura K, Sawas T, Bazerbachi F, Storm AC, Martin JA, Gores GJ, Abu Dayyeh BK, Topazian MD, Levy MJ, Petersen BT, Chandrasekhara V. Cholangioscopy Biopsies Improve Detection of Cholangiocarcinoma When Combined with Cytology and FISH, but Not in Patients with PSC. Dig Dis Sci. 2020;65:1471–1478. doi: 10.1007/s10620-019-05866-2. [DOI] [PubMed] [Google Scholar]
- 65.Sandha G, D'Souza P, Halloran B, Montano-Loza AJ. A Cholangioscopy-Based Novel Classification System for the Phenotypic Stratification of Dominant Bile Duct Strictures in Primary Sclerosing Cholangitis-the Edmonton Classification. J Can Assoc Gastroenterol. 2018;1:174–180. doi: 10.1093/jcag/gwy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujisawa T, Ushio M, Takahashi S, Yamagata W, Takasaki Y, Suzuki A, Okawa Y, Ochiai K, Tomishima K, Ishii S, Saito H, Isayama H. Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Awadallah NS, Chen YK, Piraka C, Antillon MR, Shah RJ. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis? Am J Gastroenterol. 2006;101:284–291. doi: 10.1111/j.1572-0241.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 68.Vlăduţ C, Ciocîrlan M, Bilous D, Șandru V, Stan-Ilie M, Panic N, Becheanu G, Jinga M, Costache RS, Costache DO, Diculescu M. An Overview on Primary Sclerosing Cholangitis. J Clin Med. 2020;9 doi: 10.3390/jcm9030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmed O, Mathevosian S, Arslan B. Biliary Interventions: Tools and Techniques of the Trade, Access, Cholangiography, Biopsy, Cholangioscopy, Cholangioplasty, Stenting, Stone Extraction, and Brachytherapy. Semin Intervent Radiol. 2016;33:283–290. doi: 10.1055/s-0036-1592327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Itoi T, Kamisawa T, Igarashi Y, Kawakami H, Yasuda I, Itokawa F, Kishimoto Y, Kuwatani M, Doi S, Hara S, Moriyasu F, Baron TH. The role of peroral video cholangioscopy in patients with IgG4-related sclerosing cholangitis. J Gastroenterol. 2013;48:504–514. doi: 10.1007/s00535-012-0652-6. [DOI] [PubMed] [Google Scholar]
- 71.Ishii Y, Serikawa M, Tsuboi T, Kawamura R, Tsushima K, Nakamura S, Hirano T, Fukiage A, Ikemoto J, Kiyoshita Y, Saeki S, Tamura Y, Chayama K. Usefulness of peroral cholangioscopy in the differential diagnosis of IgG4-related sclerosing cholangitis and extrahepatic cholangiocarcinoma: a single-center retrospective study. BMC Gastroenterol. 2020;20:287. doi: 10.1186/s12876-020-01429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogawa T, Kanno Y, Koshita S, Masu K, Kusunose H, Sakai T, Yonamine K, Miyamoto K, Murabayashi T, Kozakai F, Endo K, Noda Y, Ito K. Cholangioscopy- vs fluoroscopy-guided transpapillary mapping biopsy for preoperative evaluation of extrahepatic cholangiocarcinoma: a prospective randomized crossover study. Surg Endosc. 2020 doi: 10.1007/s00464-020-08141-y. [DOI] [PubMed] [Google Scholar]
- 73.Urban O, Vanek P, Zoundjiekpon V, Falt P. Endoscopic Perspective in Cholangiocarcinoma Diagnostic Process. Gastroenterol Res Pract. 2019;2019:9704870. doi: 10.1155/2019/9704870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pereira P, Santos S, Morais R, Gaspar R, Rodrigues-Pinto E, Vilas-Boas F, Macedo G. Role of Peroral Cholangioscopy for Diagnosis and Staging of Biliary Tumors. Dig Dis. 2020;38:431–440. doi: 10.1159/000504910. [DOI] [PubMed] [Google Scholar]
- 75.Fukasawa Y, Takano S, Fukasawa M, Maekawa S, Kadokura M, Shindo H, Takahashi E, Hirose S, Kawakami S, Hayakawa H, Yamaguchi T, Nakayama Y, Inoue T, Sato T, Enomoto N. Form-Vessel Classification of Cholangioscopy Findings to Diagnose Biliary Tract Carcinoma's Superficial Spread. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21093311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tyberg A, Raijman I, Siddiqui A, Arnelo U, Adler DG, Xu MM, Nassani N, Sejpal DV, Kedia P, Nah Lee Y, Gress FG, Ho S, Gaidhane M, Kahaleh M. Digital Pancreaticocholangioscopy for Mapping of Pancreaticobiliary Neoplasia: Can We Alter the Surgical Resection Margin? J Clin Gastroenterol. 2019;53:71–75. doi: 10.1097/MCG.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 77.Tsuyuguchi T, Fukuda Y, Saisho H. Peroral cholangioscopy for the diagnosis and treatment of biliary diseases. J Hepatobiliary Pancreat Surg. 2006;13:94–99. doi: 10.1007/s00534-005-1064-2. [DOI] [PubMed] [Google Scholar]
- 78.Sum Foong K, Lee A, Kudakachira S, Ramberan H. Hemobilia from Biliary Angiodysplasia Diagnosed with Cholangioscopy. ACG Case Rep J. 2016;3:e132. doi: 10.14309/crj.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karagyozov P, Boeva I, Tishkov I. Role of digital single-operator cholangioscopy in the diagnosis and treatment of biliary disorders. World J Gastrointest Endosc. 2019;11:31–40. doi: 10.4253/wjge.v11.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stefanidis G, Christodoulou C, Manolakopoulos S, Chuttani R. Endoscopic extraction of large common bile duct stones: A review article. World J Gastrointest Endosc. 2012;4:167–179. doi: 10.4253/wjge.v4.i5.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Angsuwatcharakon P, Kulpatcharapong S, Ridtitid W, Boonmee C, Piyachaturawat P, Kongkam P, Pareesri W, Rerknimitr R. Digital cholangioscopy-guided laser vs mechanical lithotripsy for large bile duct stone removal after failed papillary large-balloon dilation: a randomized study. Endoscopy. 2019;51:1066–1073. doi: 10.1055/a-0848-8373. [DOI] [PubMed] [Google Scholar]
- 82.Galetti F, Moura DTH, Ribeiro IB, Funari MP, Coronel M, Sachde AH, Brunaldi VO, Franzini TP, Bernardo WM, Moura EGH. Cholangioscopy-guided lithotripsy vs. conventional therapy for complex bile duct stones: a systematic review and meta-analysis. Arq Bras Cir Dig. 2020;33:e1491. doi: 10.1590/0102-672020190001e1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Korrapati P, Ciolino J, Wani S, Shah J, Watson R, Muthusamy VR, Klapman J, Komanduri S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: a systematic review and meta-analysis. Endosc Int Open. 2016;4:E263–E275. doi: 10.1055/s-0042-100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chin MW, Byrne MF. Update of cholangioscopy and biliary strictures. World J Gastroenterol. 2011;17:3864–3869. doi: 10.3748/wjg.v17.i34.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yasuda I, Itoi T. Recent advances in endoscopic management of difficult bile duct stones. Dig Endosc. 2013;25:376–385. doi: 10.1111/den.12118. [DOI] [PubMed] [Google Scholar]
- 86.Buxbaum J, Sahakian A, Ko C, Jayaram P, Lane C, Yu CY, Kankotia R, Laine L. Randomized trial of cholangioscopy-guided laser lithotripsy versus conventional therapy for large bile duct stones (with videos) Gastrointest Endosc. 2018;87:1050–1060. doi: 10.1016/j.gie.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 87.Murabayashi T, Ogawa T, Koshita S, Kanno Y, Kusunose H, Sakai T, Masu K, Yonamine K, Miyamoto K, Kozakai F, Endo K, Noda Y, Ito K. Peroral Cholangioscopy-guided Electrohydraulic Lithotripsy with a SpyGlass DS Versus a Conventional Digital Cholangioscope for Difficult Bile Duct Stones. Intern Med. 2020;59:1925–1930. doi: 10.2169/internalmedicine.4463-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tandan M, Reddy DN. Extracorporeal shock wave lithotripsy for pancreatic and large common bile duct stones. World J Gastroenterol. 2011;17:4365–4371. doi: 10.3748/wjg.v17.i39.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aljebreen AM, Alharbi OR, Azzam N, Almadi MA. Efficacy of spyglass-guided electrohydraulic lithotripsy in difficult bile duct stones. Saudi J Gastroenterol. 2014;20:366–370. doi: 10.4103/1319-3767.145329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer KR, Hofmann AF. Intraductal mono-octanoin for the direct dissolution of bile duct stones: experience in 343 patients. Gut. 1986;27:196–202. doi: 10.1136/gut.27.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng YF, Lee TY, Sheen-Chen SM, Huang TL, Chen TY. Treatment of complicated hepatolithiasis with intrahepatic biliary stricture by ductal dilatation and stenting: long-term results. World J Surg. 2000;24:712–716. doi: 10.1007/s002689910114. [DOI] [PubMed] [Google Scholar]
- 92.Huang MH, Chen CH, Yang JC, Yang CC, Yeh YH, Chou DA, Mo LR, Yueh SK, Nien CK. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655–2662. doi: 10.1111/j.1572-0241.2003.08770.x. [DOI] [PubMed] [Google Scholar]
- 93.Kwan KEL, Shelat VG, Tan CH. Recurrent pyogenic cholangitis: a review of imaging findings and clinical management. Abdom Radiol (NY) 2017;42:46–56. doi: 10.1007/s00261-016-0953-y. [DOI] [PubMed] [Google Scholar]
- 94.Zhou Y, Zha WZ, Wu XD, Fan RG, Zhang B, Xu YH, Qin CL, Jia J. Biliary exploration via the left hepatic duct orifice vs the common bile duct in left-sided hepatolithiasis patients with a history of biliary tract surgery: A randomized controlled trial. Medicine (Baltimore) 2018;97:e9643. doi: 10.1097/MD.0000000000009643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Angsuwatcharakon P, Kulpatcharapong S, Moon JH, Ramchandani M, Lau J, Isayama H, Seo DW, Maydeo A, Wang HP, Nakai Y, Ratanachu-Ek T, Bapaye A, Hu B, Devereaux B, Ponnudurai R, Khor C, Kongkam P, Pausawasdi N, Ridtitid W, Piyachaturawat P, Khanh PC, Dy F, Rerknimitr R. Consensus guidelines on the role of cholangioscopy to diagnose indeterminate biliary stricture. HPB (Oxford) 2021 doi: 10.1016/j.hpb.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Pandit N, Deo KB, Yadav TN, Gautam S, Dhakal Y, Awale L, Adhikary S. Choledochal Cyst: A Retrospective Study of 30 Cases From Nepal. Cureus. 2020;12:e11414. doi: 10.7759/cureus.11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cocca S, Grande G, Reggiani Bonetti L, Magistri P, Di Sandro S, Di Benedetto F, Conigliaro R, Bertani H. Common bile duct lesions - how cholangioscopy helps rule out intraductal papillary neoplasms of the bile duct: A case report. World J Gastrointest Endosc. 2020;12:555–559. doi: 10.4253/wjge.v12.i12.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oshikiri T, Kashimura N, Katanuma A, Maguchi H, Shinohara T, Shimizu M, Kondo S, Katoh H. Mucin-secreting bile duct adenoma--clinicopathological resemblance to intraductal papillary mucinous tumor of the pancreas. Dig Surg. 2002;19:324–327. doi: 10.1159/000064570. [DOI] [PubMed] [Google Scholar]
- 99.Yoon JH. Biliary papillomatosis: correlation of radiologic findings with percutaneous transhepatic cholangioscopy. J Gastrointestin Liver Dis. 2013;22:427–433. [PubMed] [Google Scholar]
- 100.Hirata H, Kuwatani M, Mitsuhashi T. A case with eosinophilic cholangitis which presented the unique cholangioscopic findings. J Hepatobiliary Pancreat Sci. 2021;28:e24–e25. doi: 10.1002/jhbp.862. [DOI] [PubMed] [Google Scholar]
- 101.Senra Lorenzana F, Navaratne L, Martínez Isla A. Choledochal varices: An uncommon cholangioscopic finding. Cir Esp (Engl Ed) 2020;98:361. doi: 10.1016/j.ciresp.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 102.Takeda Y, Onoyama T, Sakamoto Y, Kawahara S, Hamamoto W, Koda H, Yamashita T, Matsumoto K, Isomoto H. A Case of Right Hepatic Artery Syndrome Diagnosed by Using SpyGlassDSTM System. Yonago Acta Med. 2020;63:372–375. doi: 10.33160/yam.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y, Sun W, Zhang F, Huang J, Fan Z. Pancreaticobiliary maljuction combining with pancreas divisum: Report of four cases. Exp Ther Med. 2014;7:8–10. doi: 10.3892/etm.2013.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stadmeyer P, Dalvie P, Hubers J, Gopal D. Percutaneous Transhepatic Single-Operator Cholangioscopy-Guided Intraductal Stone Therapy in a Liver Transplant Patient With Ischemic Cholangiopathy. ACG Case Rep J. 2021;8:e00595. doi: 10.14309/crj.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prasad GA, Abraham SC, Baron TH, Topazian MD. Hemobilia caused by cytomegalovirus cholangiopathy. Am J Gastroenterol. 2005;100:2592–2595. doi: 10.1111/j.1572-0241.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 106.Molina CJR, Eid TI, Arce V, Quigley G, Bowie T, Chuang KY. A Case of CMV Cholangiopathy in an HIV Patient on Antiretroviral Therapy: 1327. Am J Gastroenterol . 2018;113:S761–S762. [Google Scholar]
- 107.Figueira ERR, Franzini T, Costa TN, Madruga-Neto AC, Guedes HG, Romano VC, Ceconello I, de Moura EGH. Laparoscopic SpyGlass cholangioscopy evaluation during bilioenteric anastomosis for hepatolithiasis, a case report. Int J Surg Case Rep. 2021;78:140–144. doi: 10.1016/j.ijscr.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trivedi PS, Pshak TJ. Cholangioscopy-Assisted Crossing of Severe Biliary-Enteric Anastomotic Stricture Following Failure of Standard Fluoroscopic Techniques. Cardiovasc Intervent Radiol. 2021;44:992–995. doi: 10.1007/s00270-020-02743-8. [DOI] [PubMed] [Google Scholar]
- 109.Chokpapone YM, Murray AR, Mehta AP, Puri VC, Mejia A, Mantry P. Morphological Characteristics of Biliary Strictures after Liver Transplantation Visualized Using SpyGlass™ Cholangioscopy. Case Reports Hepatol. 2020;2020:8850000. doi: 10.1155/2020/8850000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Franzini T, Sagae VMT, Guedes HG, Sakai P, Waisberg DR, Andraus W, D'Albuquerque LAC, Sethi A, de Moura EGH. Cholangioscopy-guided steroid injection for refractory post liver transplant anastomotic strictures: a rescue case series. Ther Adv Gastrointest Endosc. 2019;12:2631774519867786. doi: 10.1177/2631774519867786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim SH, Jeong S, Lee DH, Yoo SS, Lee KY. Percutaneous Cholangioscopic Lithotripsy for Afferent Loop Syndrome Caused by Enterolith Development after Roux-en-Y Hepaticojejunostomy: A Case Report. Clin Endosc. 2013;46:679–682. doi: 10.5946/ce.2013.46.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hao J, Huang X. The Status and Development of Oral Choledochoscopy Diagnosis and Treatment of Biliary Tract Diseases. Int J Gen Med. 2021;14:4269–4277. doi: 10.2147/IJGM.S317484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yeo CS, Tay VW, Low JK, Woon WW, Punamiya SJ, Shelat VG. Outcomes of percutaneous cholecystostomy and predictors of eventual cholecystectomy. J Hepatobiliary Pancreat Sci. 2016;23:65–73. doi: 10.1002/jhbp.304. [DOI] [PubMed] [Google Scholar]
- 114.Cao J, Ding X, Wu H, Shen Y, Zheng R, Peng C, Wang L, Zou X. Classification of the cystic duct patterns and endoscopic transpapillary cannulation of the gallbladder to prevent post-ERCP cholecystitis. BMC Gastroenterol. 2019;19:139. doi: 10.1186/s12876-019-1053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee JH, Kim HW, Kang DH, Choi CW, Park SB, Kim SH, Jeon UB. Usefulness of percutaneous transhepatic cholangioscopic lithotomy for removal of difficult common bile duct stones. Clin Endosc. 2013;46:65–70. doi: 10.5946/ce.2013.46.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang H, Ou Y, Ou J, Jian Z. Complication Analysis with Percutaneous Postoperative Choledochoscopy in 826 Patients: A Single-Center Study. J Laparoendosc Adv Surg Tech A. 2019;29:995–999. doi: 10.1089/lap.2019.0110. [DOI] [PubMed] [Google Scholar]
- 117.Kong J, Wu SD, Xian GZ, Yang S. Complications analysis with postoperative choledochoscopy for residual bile duct stones. World J Surg. 2010;34:574–580. doi: 10.1007/s00268-009-0352-4. [DOI] [PubMed] [Google Scholar]
- 118.Lenze F, Bokemeyer A, Gross D, Nowacki T, Bettenworth D, Ullerich H. Safety, diagnostic accuracy and therapeutic efficacy of digital single-operator cholangioscopy. United European Gastroenterol J. 2018;6:902–909. doi: 10.1177/2050640618764943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, Khan AH, Shah RJ. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc. 2011;73:251–256. doi: 10.1016/j.gie.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 120.Ang TL, Kwek ABE. Safety and efficacy of SpyGlass cholangiopancreatoscopy in routine clinical practice in a regional Singapore hospital. Singapore Med J. 2019;60:538–544. doi: 10.11622/smedj.2018158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bernica J, Elhanafi S, Kalakota N, Jia Y, Dodoo C, Dwivedi A, Sealock RJ, Patel K, Raijman I, Zuckerman MJ, Othman MO. Cholangioscopy Is Safe and Feasible in Elderly Patients. Clin Gastroenterol Hepatol. 2018;16:1293–1299.e2. doi: 10.1016/j.cgh.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 122.Uradomo L, Pandolfe F, Aragon G, Borum ML. SpyGlass cholangioscopy for management of choledocholithiasis during pregnancy. Hepatobiliary Pancreat Dis Int. 2011;10:107. doi: 10.1016/s1499-3872(11)60017-9. [DOI] [PubMed] [Google Scholar]
- 123.Agrawal V, Acharya H, Saxena A, Sharma D. Per-operative modified rigid cholangioscopy for removal of intrahepatic stones associated with choledochal cyst in children. J Minim Access Surg. 2019;15:210–213. doi: 10.4103/jmas.JMAS_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weigand K, Küchle M, Zuber-Jerger I, Müller M, Kandulski A. Diagnostic Accuracy and Therapeutic Efficacy of Digital Single-Operator Cholangioscopy for Biliary Lesions and Stenosis. Digestion. 2021;102:776–782. doi: 10.1159/000513713. [DOI] [PubMed] [Google Scholar]
- 125.Kirk RM. Surgical skills and lessons from other vocations: a personal view. Ann R Coll Surg Engl. 2006;88:95–98. doi: 10.1308/003588406X95156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li A, Tang R, Rong Z, Zeng J, Xiang C, Yu L, Zhao W, Dong J. The Use of Three-Dimensional Printing Model in the Training of Choledochoscopy Techniques. World J Surg. 2018;42:4033–4038. doi: 10.1007/s00268-018-4731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang R, Ma L, Li A, Yu L, Rong Z, Zhang X, Xiang C, Liao H, Dong J. Choledochoscopic Examination of a 3-Dimensional Printing Model Using Augmented Reality Techniques: A Preliminary Proof of Concept Study. Surg Innov. 2018;25:492–498. doi: 10.1177/1553350618781622. [DOI] [PubMed] [Google Scholar]
- 128.Naito ST, Itoi T, Yamamoto K, Tsuchiya T, Tsuji S, Tanaka R, Honjo M, Mukai S, Matsunami Y, Asai Y, Nagakawa Y, Ikeuchi N, Sofuni A. Novel ex vivo training model for freehand insertion using a double-bending peroral direct cholangioscope. J Gastroenterol Hepatol. 2018;33:543–547. doi: 10.1111/jgh.13864. [DOI] [PubMed] [Google Scholar]
- 129.Zhou XY, Guo Y, Shen M, Yang GZ. Application of artificial intelligence in surgery. Front Med. 2020;14:417–430. doi: 10.1007/s11684-020-0770-0. [DOI] [PubMed] [Google Scholar]
- 130.Loras C, Mayor V, Fernández-Bañares F, Esteve M. Study of the standard direct costs of various techniques of advanced endoscopy. Comparison with surgical alternatives. Dig Liver Dis. 2018;50:689–697. doi: 10.1016/j.dld.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 131.Sandha J, van Zanten SV, Sandha G. The Safety and Efficacy of Single-Operator Cholangioscopy in the Treatment of Difficult Common Bile Duct Stones after Failed Conventional ERCP. J Can Assoc Gastroenterol. 2018;1:181–190. doi: 10.1093/jcag/gwy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Manatakis DK, Georgopoulos N. Reducing the Cost of Laparoscopy: Reusable vs Disposable Laparoscopic Instruments. Minim Invasive Surg. 2014;2014:408171. doi: 10.1155/2014/408171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Paolucci V, Schaeff B, Gutt CN, Encke A. Disposable vs reusable instruments in laparoscopic cholecystectomy. A prospective, randomised study. Endosc Surg Allied Technol. 1995;3:147–150. [PubMed] [Google Scholar]
- 134.Zimmer V. The "Bilio-Papillary Z Line": Proposal for a Novel Quality Indicator of Direct Cholangioscopy. Clin Endosc. 2018;51:498–499. doi: 10.5946/ce.2018.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kanaji S, Watanabe R, Mascagni P, Trauzettel F, Urade T, Longo F, Guerriero L, Perretta S, Dallemagne B, Kakeji Y, Marescaux J. Three-dimensional imaging improved the laparoscopic performance of inexperienced operators: a prospective trial. Surg Endosc. 2020;34:5083–5091. doi: 10.1007/s00464-019-07308-6. [DOI] [PubMed] [Google Scholar]
- 136.Tan YP, Lim C, Junnarkar SP, Huey CWT, Shelat VG. 3D Laparoscopic common bile duct exploration with primary repair by absorbable barbed suture is safe and feasible. J Clin Transl Res. 2021;7:473–478. [PMC free article] [PubMed] [Google Scholar]
- 137.Rana AM, Rana AA, Hewett PJ. Comparison of three-dimensional and 4K imaging systems in novice surgeons: a cross-over study. ANZ J Surg. 2020;90:1009–1013. doi: 10.1111/ans.15653. [DOI] [PubMed] [Google Scholar]
- 138.Dunstan M, Smith R, Schwab K, Scala A, Gatenby P, Whyte M, Rockall T, Jourdan I. Is 3D faster and safer than 4K laparoscopic cholecystectomy? Surg Endosc. 2020;34:1729–1735. doi: 10.1007/s00464-019-06958-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abadir AP, Ali MF, Karnes W, Samarasena JB. Artificial Intelligence in Gastrointestinal Endoscopy. Clin Endosc. 2020;53:132–141. doi: 10.5946/ce.2020.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


