Abstract
Electrochemotherapy is a local ablative therapy that increases the cytotoxicity of either bleomycin or cisplatin by applying electric pulses (electroporation) to tumors. It has already been widely used throughout Europe for the treatment of various types of human and veterinary cutaneous tumors, with an objective response rate ranging from 70%-90%, depending on the tumor histotype. Recently, electrochemotherapy was introduced for the treatment of primary liver tumors, such as hepatocellular carcinoma (HCC). The complete response rate was 85% per treated lesion, with a durable response. Therefore, electrochemotherapy could become a treatment of choice for HCC, especially after achieving a transition from an open surgery approach to a percutaneous approach that uses dedicated electrodes. Electrochemotherapy elicits a local immune response and can be considered an in situ vaccination. HCC, among others, is a potentially immunogenic tumor; thus, electrochemotherapy could boost adjuvant immunotherapy to achieve a better and longer-lasting antitumor response. Therefore, therapeutic strategies that combine electrochemotherapy with immune checkpoint inhibitors or adjuvant treatment with cytokines are indicated for HCC. Immunogene therapy using electroporation as a delivery system for plasmid DNA coding for interleukin-12 is a highly promising approach. This electroporation approach has shown efficacy in preclinical settings and veterinary oncology and is awaiting translation for the treatment of liver tumors, i.e., HCC.
Keywords: Electroporation, Electrochemotherapy, Gene therapy, Gene electrotransfer, Interleukin-12, Immune checkpoint inhibitors, Hepatocellular carcinoma
Core Tip: Electrochemotherapy was found to be feasible, safe and highly effective for the treatment of hepatocellular carcinoma (HCC). A local immune response is induced through the destruction of tumor cells; therefore, the electrochemotherapy approach can be considered an in situ vaccination. Electrochemotherapy combined with immune checkpoint inhibitors had an interactive effect on melanoma tumors and HCC. Furthermore, electrochemotherapy can be combined with immunostimulation with cytokines. Electrochemotherapy involving the gene electrotransfer of a plasmid DNA coding for interleukin-12 (IL-12) has already been shown to have clinical value. The combination of electrochemotherapy and immunogene therapy with IL-12 via electroporation might be a feasible new treatment strategy for HCC that is also potentially applicable to other liver tumors.
INTRODUCTION
Liver tumors represent a group of tumors that arise in liver tissue. Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed tumor, the fourth leading cause of cancer-related death worldwide and is responsible for over 850000 deaths annually. Outcomes are poor overall, with an estimated 5-year survival rate of approximately 20%[1].
The most common type of primary liver tumor is HCC, which represents approximately 90% of all primary liver tumors, followed by intrahepatic cholangiocarcinoma. The incidence of liver tumors varies from Europe to Asia, mostly because of regional differences in the prevalence of risk factors. This difference is most clearly seen in HCC. HCC generally occurs in the presence of liver cirrhosis or liver disease. The incidence of HCC in eastern Asia is 3.5-fold higher than the incidence in Europe, mainly because of the difference in the incidence of hepatitis B/C in Asia and Europe[2].
In addition to hepatitis B/C infection, one of the most common risk factors for HCC is alcohol abuse. Other risk factors include dietary aflatoxin exposure, smoking, nonalcoholic fatty liver disease associated with obesity, and diabetes, which is increasingly emerging as a key contributor to the incidence of HCC in the United States and other western countries[2,3].
Therapy options are individualized and based on the stage of disease, liver function, and performance status of the patient.
Therapy options can be divided into three categories as follows: (1) Curative options for early-stage tumors are surgery, liver transplantation and ablation, e.g., microwave ablation (MWA) or radiofrequency ablation (RFA); MWA is more convenient for the treatment of larger lesions, especially those in close proximity to blood vessels[4]; (2) Locoregional therapy, such as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE), for intermediate-stage tumor; TACE is the standard of care for patients without curative treatment options with liver-only disease and without macrovascular invasion or for patients listed for liver transplantation as “bridging” to transplantation; and (3) Systemic therapy for advanced tumors (atezolizumab and bevacizumab, sorafenib, levatinib, regorafenib, cabozantinib, and ramucirumab). Surgical and locoregional therapies are not covered in this review, as they have been reviewed extensively elsewhere[1,2,5-9].
LOCAL THERAPIES FOR LIVER TUMORS
Local therapies are particularly appropriate for the treatment of liver tumors, mostly due to the feasibility of the percutaneous approach. Thermal ablative therapies have been the most rapidly adopted local therapy approaches. Tumors up to 3 cm in diameter can be successfully ablated with either RFA or MWA. Tumor control is achieved with complete responses (CRs) ranging from 40%-80%[10]. However, the local and locoregional recurrence rates in the liver following thermal ablative therapies are significant due to the localized nature of their efficacy. A local recurrence rate of up to 20% has been reported during follow-up after RFA. Similar results have been seen in patients treated with MWA, considering that patients treated with MWA had larger and more lesions or lesions in the vicinity of blood vessels[11]. Another thermal ablative technique is cryoablation, which is based on repetitive cycles of freezing (argon gas) and thawing (helium gas) of tumors, causing the formation of intracellular ice crystals that lead to cell death. The efficacy of cryoablation is similar to that reported for RFA[12].
There are some nonthermal ablative therapies available in addition to these thermal ablative therapies. Electroporation-based treatment is one of them. Irreversible electroporation is a relatively well-established treatment approach, and the use of electrochemotherapy is on the rise. Irreversible electroporation is a well-accepted therapy for liver tumors, including HCC[13]. This percutaneously performed approach has been practiced in renowned centers, and their reports have demonstrated the feasibility and safety of the approach. Irreversible electroporation is based on the delivery of a long train of up to 100 electric pulses of 1000 V per cm of the distance between the electrodes to destabilize the cell membrane and induce necrotic cell death. Some reports have also indicated the induction of immunogenic cell death following irreversible electroporation[14]. The drawback of irreversible electroporation is that it takes a considerable amount of time to deliver all the electric pulses, and the repetitive delivery of electric pulses increases the temperature of the area around the electrodes; therefore, irreversible electroporation cannot be considered a completely nonthermal technique. Electrochemotherapy is a nonthermal therapy since only 8 electric pulses are delivered between the electrodes to permeabilize the cell membrane, which leads to reversible electroporation[15]. The train of 8 pulses induces permeable structures in the cell membrane, which immediately start to reseal after the pulses are delivered. The application of electric pulses does not affect cell viability per se. The cytotoxic effect is exerted by the drug, which is delivered into the cells due to the permeabilization of the cell membrane. The cytotoxic effect of bleomycin or cisplatin on tumor cells is slowly exerted by the induction of apoptotic, mitotic, and immunogenic cell death[16]. Therefore, the advantage of electrochemotherapy is the slow reaction and the exertion of a cytotoxic effect from the drug only, which avoids the clinical problem of massive necrosis[14]. The drug dosage needed to exert the cytotoxic action is very small due to the increased intracellular delivery of the drugs by electroporation; therefore, there are no severe systemic side effects even when the drug is delivered systemically. Electrochemotherapy can prevent tumor bleeding through the disruption of small tumor vessels; furthermore, electrochemotherapy can promote hemostasis in bleeding tumors[15].
ELECTROCHEMOTHERAPY FOR THE TREATMENT OF HCC
The first study of electrochemotherapy for the treatment of liver tumors was a preliminary study on colorectal liver metastases that indicated the safety and feasibility of the approach[17]. The approach was described from a technical point of view during open surgery. The standard operating procedures for the electrochemotherapy of cutaneous tumors were followed but adapted for the specifics of the liver tumors, especially for tumors larger than 3 cm in diameter[18]. These protocols were then followed in the subsequent application of electrochemotherapy for the treatment of colorectal liver metastases and HCC. The pilot and subsequent phase II study of the treatment of colorectal liver metastases with electrochemotherapy demonstrated a significant benefit of electrochemotherapy as a treatment for patients for whom electrochemotherapy was the only remaining treatment option. A 75% CR rate of metastases was achieved. Effective treatment provided long-term local tumor control as well as a long, progression-free survival rate. The success of electrochemotherapy enabled patients to receive successive treatments and consequently a prolonged life expectancy[19].
Electrochemotherapy was also performed on HCC tumors in patients for whom other curative treatment options had been exhausted. We observed slow resolution of the treated tumors, those associated with cirrhotic livers, and in situations when tumors were adjacent to or embraced major liver vessels or bile ducts. We took advantage of the nonthermal action of electrochemotherapy and demonstrated the feasibility of the approach in patients with difficult-to-treat situations[20,21]. It was demonstrated in a separate study on pig livers that electrochemotherapy does not affect the function and architecture of larger tumor vessels[22]. Furthermore, in that study, no specific pathological effects of electrochemotherapy on healthy liver parenchyma, vessels, or bile ducts were observed, which provided a good starting point for the use of electrochemotherapy in the treatment of HCC, especially in cases where tumors are in contact with larger hollow structures of the liver.
The results obtained for the treated tumors described above demonstrated that electrochemotherapy has similar effectiveness to other ablative therapies. We achieved CR in 84.4% of treated lesions in the phase II trial with a median follow-up time of 50 mo. Thus, the effectiveness of electrochemotherapy is comparable to the effectiveness of MWA, which achieves disease-free survival in 67.2% of patients at 36 mo and 49.1% at 60 mo[11]. Early reports for percutaneous irreversible electroporation show an efficacy of 72%-100% across different studies[13,23].
The main limitation of electrochemotherapy for liver tumors in previous studies was that the procedure was performed intraoperatively during open surgery. This was necessary to maximally control the execution of the treatment and explore the limits of the treatment. Based on the experience gained and the results obtained, we can now claim that electrochemotherapy could produce equally beneficial treatment effects for HCC tumors as other ablative therapies and could be used for the treatment of other liver tumors and metastases. The limitation of not being a percutaneous technique has been recently overcome[21,24].
The percutaneous application of electrochemotherapy was enabled by the development of a new pulse generator Cliniporator®VITAE (IGEA SpA, Carpi, Italy), which can generate sufficient power to treat deep-seated tumors. Additionally, long needle electrodes are available, which are similar to those used for irreversible electroporation[15]. The first attempt to treat HCC with percutaneous electrochemotherapy was performed in Ljubljana and demonstrated the feasibility, safety and efficacy of the percutaneous approach to electrochemotherapy for the treatment of HCC (Figure 1)[21]. We are currently gaining new experience in the percutaneous approach, and the process of transition from intraoperative to percutaneous electrochemotherapy is underway. Additionally, other authors have reported the feasibility of percutaneous electrochemotherapy for the treatment of HCC portal vein tumor thrombus at the hepatic hilum in six patients[24].
Figure 1.
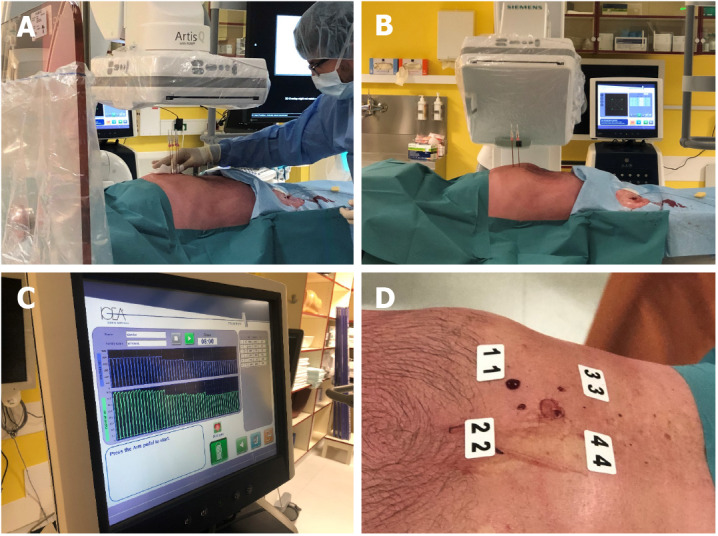
Electrochemotherapy using long needle electrodes in the percutaneous procedure. A: Positioning of the electrodes; B: Verification of the electrode positioning with computerized tomography; C: Treatment of the tumor, electric pulse delivery; D: Posttreatment.
Another percutaneous electrochemotherapy application was performed for the treatment of cholangiocarcinoma in the hepatic hilum[25]. The treatment proved to be safe and effective in five patients and improved the prognosis and quality of life of patients with unresectable perihilar cholangiocarcinoma.
The design and production of new multineedle electrodes for percutaneous use will enable easier and reliable placement of electrodes, avoiding the tedious and laborious placement of single needle electrodes. Currently, needle electrodes need to be placed in the right position with the prerequisite of being in a parallel position to obtain adequate electric field distribution. The treatment plan needs to be prepared for the placement of the electrodes to cover the whole tumor with an electric field sufficient to permeabilize the whole tumor mass. Electrodes are placed at the edge of the tumor or in normal tissue to ensure appropriate safety margins[26]. Minimally invasive endoscopic and laparoscopic electrodes were recently developed as an alternative to this procedure of placing single needles and are now available for clinical use. The shaft of the electrode is inserted in the abdomen, and then the electrode array is inserted into the tumor, extending in an umbrella-like fashion[21]. Endoscopic electrodes have also been developed and are available on the market. The electrode is mounted on the endoscope. The electrodes are parallel plates in a chamber in which the tumor tissue is pulled for injection with bleomycin followed by electroporation. The results of the pilot study using these electrodes have already been published[27]. Seven patients with colorectal tumors who were deemed ineligible for or had declined standard treatment were included. They were treated with bleomycin either intratumorally or intravenously, and the electric pulses were delivered through the endoscopic electrode device. Safety and efficacy were assessed clinically and by scans immediately after treatment, and adverse events were reported. This first-in-human study showed that electrochemotherapy for colorectal tumors using an endoscopic electrode device can induce a local tumor response and is safe for fragile elderly patients with comorbidities.
The intraoperative approach might still be an option in surgical situations in which an unexpected, difficult-to-surgically treat situation occurs; in such a situation, electrochemotherapy can represent a viable treatment option.
CURRENT DEVELOPMENTS IN ABLATIVE THERAPIES COMBINED WITH IMMUNOTHERAPY
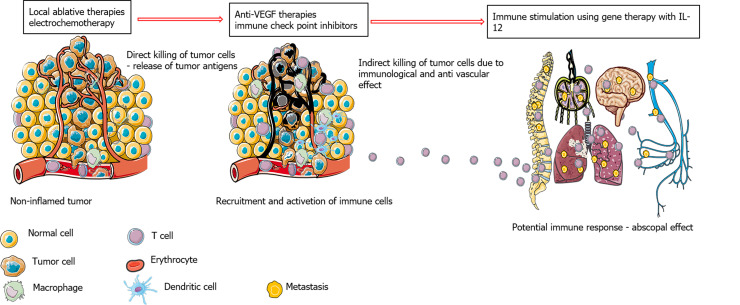
The current paradigm is that local and locoregional ablative therapies can elicit a local immune response that can be boosted by immunotherapeutic approaches. This approach is currently being explored, predominantly with a combination of radiotherapy and immune checkpoint inhibitors; however, other ablative techniques are already in clinical trials in combination with immune therapies. These clinical trials explored which tumors would benefit the most and the optimal timing, sequence, dose of immune therapy, and the number of fractions and dose per fraction for radiotherapy. Radiotherapy can stimulate a proinflammatory environment by killing tumor cells and stimulating the infiltration of immune cells, thus turning immunologically cold tumors into immunologically hot tumors. Radiation damage resulting in micronuclei in cells stimulates cytosolic nucleic acid sensor pathways, such as cyclic GMP-AMP synthase, which is a stimulator of interferon genes. Additionally, irradiation modulates neoantigen expression, which impacts immune surveillance and sets the stage for combined treatment with immune checkpoint inhibitors[28,29]. However, as stated, questions arise regarding the appropriate doses and fractionation of tumor irradiation to elicit an adequate immunogenic response. Clinical studies indicate that stereotactic body radiation therapy is more powerful in enhancing antitumor immunity and works better with immune checkpoint inhibitors than fractionated conventional radiotherapy. This effect was observed when this combination was tested in non-small cell lung cancer, melanoma, head and neck cancer, HCC, pancreatic cancer, and genital tumors[30].
Several clinical studies have been initiated on the treatment of HCC with the combination of locoregional and local ablative therapies with immune checkpoint inhibitors based on promising results from studies testing immune checkpoint inhibitors in advanced HCC. It is known from retrospective studies of other tumor types that the clinical efficacy of immune checkpoint inhibitors correlates with tumor burden; therefore, it is better to treat smaller tumors with this approach. Another reason for combining electrochemotherapy with immune checkpoint inhibitors is that although immune therapies are also combined with surgical approaches, the immunological effects that are observed after local and locoregional therapies favor such combinations. Current clinical studies are evaluating immune checkpoint inhibitors as an adjuvant therapy with RFA in neoadjuvant settings and are investigating whether the combination with immune checkpoint inhibitors in tumors larger than 3 cm can be performed with curative intent (NCT03847428 and NCT03630640). Furthermore, the role of anti-vascular endothelial growth factor (VEGF) therapies in combination with immune checkpoint inhibitors and local ablative therapies should be determined in the future. It has been shown that anti-VEGF therapies overcome intrinsic resistance to immune checkpoint inhibitors (Figure 2). Additionally, an increase in VEGF after RFA was observed in patients with HCC; thus, inhibiting VEGF can enhance the effect of immune therapy in combination with the local ablative therapies required to achieve a complete response of HCC[9].
Figure 2.
Potential benefits of combining local ablative therapies with immune checkpoint inhibitors and anti-vascular therapies. IL-12: Interleukin-12.
CAN ELECTROCHEMOTHERAPY FOR IN SITU VACCINATION IN COMBINATION WITH IMMUNE CHECKPOINT INHIBITORS BE EXPLOITED?
Similar findings to those outlined above were found with electroporation-based treatments. The results of these reports showed that both irreversible electroporation and electrochemotherapy could induce immunogenic cell death[14]. A recent study on electrochemotherapy in mice compared the response of different tumors to electrochemotherapy and correlated it with the immune status of those tumors. The response of tumors correlated with the immune status; specifically, more immunogenic tumors responded significantly better than less immunogenic tumors. Furthermore, the response varied according to the drug used for electrochemotherapy. The study indicated that intratumoral cisplatin electrochemotherapy seems to be very effective for immunogenic tumors. All these data indicate that electrochemotherapy elicits immunogenic cell death in situ by releasing ATP and high-mobility group box and calreticulin translocation, which is dependent on tumor immunogenicity and the drug used for electrochemotherapy[14,16,31].
The results following electrochemotherapy performed for patients with melanoma during therapy with immune checkpoint inhibitors against either cytotoxic T-lymphocyte antigen or programmed cell death ligand 1 were published in a retrospective study[32]. The local response rate was higher than the reported local response rate for electrochemotherapy only. Ipilimumab combined with electrochemotherapy was feasible, tolerable, and showed a high systemic response rate. The second report to date is a case report, where a symptomatic melanoma lesion that was refractory to nivolumab was successfully treated with electrochemotherapy and achieved a 4-year durable response[33].
One question that remains is how the combined treatment affects the local recurrence-free interval and systemic progression-free interval or even influences overall survival. Another question is whether the combined treatment increases long-term survival. The retrospective analysis of the combined electrochemotherapy and pembrolizumab treatment of patients with melanoma demonstrated that all these parameters were increased[34]. This study proved that electrochemotherapy can be considered an in situ vaccination. However, the question arises as to whether this holds true for all tumor types and treatment parameters. Some preclinical data indicate that not all tumors are equally susceptible to electrochemotherapy. Their responses are dependent on some immune response-related parameters in addition to intrinsic sensitivity to chemotherapeutic drugs and vascularization, such as major histocompatibility complex I expression and mutational burden[16]. This is the so-called “immunogenicity” of the tumors. Therefore, the treatment induces immunogenic cell death and the in situ vaccination effect to different degrees in different tumors. This was demonstrated by the adjuvant effects of immunotherapy with the cytokine interleukin-12 (IL-12). The adjuvant effect was more pronounced in less immunogenic tumors, indicating less responsiveness to electrochemotherapy, and less pronounced potentiation was observed in more immunogenic tumors that were more responsive to electrochemotherapy[16].
Therefore, we can expect that not all tumors in the liver will respond equally to adjuvant immunotherapy either with immune checkpoint inhibitors or other immunotherapies. However, a comparison between the responses of colorectal liver metastases and HCC to electrochemotherapy showed that HCC responds better[19,20]. Does this mean that HCC is more immunogenic than colorectal liver metastases and that adjuvant immunotherapy would not contribute significantly? It is well established that HCC is an immunologically hot tumor, and it was demonstrated that HCC is responsive to immune checkpoint inhibitors in clinical trials[35,36]. However, the combination with electrochemotherapy needs to be investigated for all liver cancers. The other aspect is that HCC is better vascularized than colorectal liver metastases; therefore, the disruptive vascular effect of electrochemotherapy is more pronounced and could also account for the overall antitumor effectiveness[19-21].
IS THERE A POSSIBILITY FOR ADJUVANT IMMUNOSTIMULATION?
If ongoing clinical trials on ablative therapies will meet expectations in combination with immune checkpoint inhibitors and other systemic treatments (tyrosine kinase inhibitors and anti-VEGF), then a new line of treatment will be available for cancer patients. The effects will certainly vary between the patients according to the tumor type, the type of ablative technique, and the degree to which the tumors need to be destroyed for the best vaccination effect. These aspects need to be explored, but first, reliable markers are needed for the measurement of immune effects in vivo[36].
Nevertheless, if the current combination of immune checkpoint inhibitors and other drugs does not provide optimal treatment outcomes, we will need to explore additional ways to boost the immune responses of the organism. Adjuvant immunostimulation with cytokines has been explored as one option. Historically, some interleukins, such as IL-2 and IL-12, were evaluated in clinical trials in the form of recombinant proteins. These combinations with radiotherapy have yielded promising results, but the cytokine side effects were overwhelming in some cases. Therefore, this approach was abandoned[37].
There are now new techniques that can provide targeted and controllable expression of the desired molecules. Gene therapy is one such method that is gaining attention, especially with the development of genetically based coronavirus 2019 vaccines. Special attention has been given to naked DNA plasmids that can be delivered to targeted tissues by nonviral delivery techniques, such as electroporation[38]. This technique is called gene electrotransfer and can be used to deliver genes to either healthy tissues or tumors. In healthy tissues, such as muscle or skin, the expression is either systemic or localized. Therefore, if transfection occurs in normal cells, the expression lasts until the cells start to divide, as the plasmid is expressed episomally and is not retained as the cells divide. However, the encoded protein works in a paracrine fashion.
This technique is also gaining recognition because clinical studies in the United States have demonstrated the feasibility, safety, and efficacy of similar gene therapies for cancer treatment using a plasmid coding for IL-12. IL-12 is a potent proinflammatory cytokine with pleiotropic activity[39]. Most importantly, it can engage in multiple effector mechanisms and reverse tumor immunosuppression. Numerous localized delivery strategies are being explored to maximize its effectiveness, among which naked plasmid delivery with electroporation is promising. This approach has already been proven safe and effective for the treatment of cutaneous melanoma, and clinical trials for other tumors are underway[40,41].
Therefore, the immune-gene therapy approach might be the next step in immunotherapy. The approach could be exploited for skin tumors and liver tumors and be used as a monotherapy or in combination with ablative techniques.
COMBINED ELECTROCHEMOTHERAPY AND GENE ELECTROTRANSFER OF PLASMID DNA CODING FOR IL-12
There are two options for the combined electrochemotherapy and gene electrotransfer approach for the treatment of HCC. The first involves combined treatment delivered during the same electroporation session since both drug and gene delivery is based on electroporation. Therefore, the same electroporation session could be exploited to perform both electrochemotherapy and gene electrotransfer. In theory, the two treatments require different electric pulse parameters for optimal/high delivery, but preclinical data indicate that gene electrotransfer can occur with the same electric pulses that are used for electrochemotherapy[42]. Therefore, gene delivery of IL-12 coding plasmids to tumors could be performed during electrochemotherapy. The problem of how to deliver the plasmid into the tumor needs to be resolved. One option could be to adjust the new percutaneous electrodes with a syringe to deliver the plasmid into tumors.
The second approach for combining electrochemotherapy with gene electrotransfer for the treatment of HCC is to perform gene electrotransfer into distant muscle or skin for systemic transgene delivery. For example, localized transfection into the muscle could result in the shedding of IL-12 from the muscle into the bloodstream[43-45]. The shedding of the transgene would be controllable, sustainable and without pharmacological peaks that can produce severe side effects. This approach would provide a more prolonged action of the transgene and could also provide a good treatment effect.
CONCLUSION
Local ablative therapies that destroy tumor cells activate localized immune reactions; thus, these therapies can be considered in situ vaccines. Electrochemotherapy is an ablative therapy that elicits this in situ vaccination effect. Electrochemotherapy has been used for the treatment of HCC tumors in patients where other treatment options have been exhausted. This approach has been proven to be feasible, safe, and highly effective. Its limits were explored in the open surgery approach; however, with the development of new percutaneous electrodes, electrochemotherapy could be performed in a similar percutaneous way to other ablative therapies used for the treatment of liver tumors. Electrochemotherapy combined with immune checkpoint inhibitors has been shown to have an interactive effect as a treatment for melanoma tumors. Similar to the combination of inhibitors with other ablative therapies, the combination of immune checkpoint inhibitors and electrochemotherapy could also be effective for the treatment of HCC. Furthermore, electrochemotherapy could be combined with cytokine immunostimulation methods. The combination of electrochemotherapy with gene electrotransfer of a naked plasmid coding for IL-12 has already proven its value in preclinical work. Therefore, the combination of electrochemotherapy with IL-12 immunogene therapy, which are both delivered via electroporation, could be a new treatment approach for HCC tumors and possibly other liver tumors.
ACKNOWLEDGEMENTS
We gratefully thank American Journal Experts for linguistic editing of the manuscript.
Footnotes
Conflict-of-interest statement: We have no conflict-of-interest to declare. Editing services provided by American Journal Experts was financed through the funding of Slovenian Research Agency - grant P3-0003.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: World Journal of Gastrointestinal Surgery - member of editorial board, 04091635; Slovenian Society for Gastroenterology and Hepatology; International hepatopancreatobiliary association.
Peer-review started: March 26, 2021
First decision: October 16, 2021
Article in press: December 16, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morganti AG S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Blaž Trotovšek, Department of Abdominal Surgery, University Medical Centre Ljubljana, Ljubljana 1000, Slovenia; Medical Faculty Ljubljana, University of Ljubljana, Ljubljana 1000, Slovenia. blaz.trotovsek@kclj.si.
Mihajlo Djokić, Department of Abdominal Surgery, University Medical Centre Ljubljana, Ljubljana 1000, Slovenia; Medical Faculty Ljubljana, University of Ljubljana, Ljubljana 1000, Slovenia.
Maja Čemažar, Department of Experimental Oncology, Institute of Oncology, Ljubljana 1000, Slovenia; Faculty of Health Sciences, University of Primorska, Izola 6310, Slovenia.
Gregor Serša, Department of Experimental Oncology, Institute of Oncology, Ljubljana 1000, Slovenia; Faculty of Health Sciences, University of Ljubljana, Ljubljana 1000, Slovenia.
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan W, Deng Q, Lin S, Wang Y, Xu G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:264–272. doi: 10.1080/02656736.2018.1562571. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver ; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 7.Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN, Guha C. Radiotherapy for Hepatocellular Carcinoma: New Indications and Directions for Future Study. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020;72:262–276. doi: 10.1016/j.jhep.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293–313. doi: 10.1038/s41575-020-00395-0. [DOI] [PubMed] [Google Scholar]
- 10.Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210–1224. doi: 10.1002/bjs.7669. [DOI] [PubMed] [Google Scholar]
- 11.Sun Q, Shi J, Ren C, Du Z, Shu G, Wang Y. Survival analysis following microwave ablation or surgical resection in patients with hepatocellular carcinoma conforming to the Milan criteria. Oncol Lett. 2020;19:4066–4076. doi: 10.3892/ol.2020.11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song KD. Percutaneous cryoablation for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:509–515. doi: 10.3350/cmh.2016.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugimoto K, Abe M, Yoshimasu Y, Takeuchi H, Kasai Y, Itoi T. Irreversible electroporation of hepatocellular carcinoma: the role of ultrasonography. Ultrasonography. 2020;39:229–237. doi: 10.14366/usg.20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brock RM, Beitel-White N, Davalos RV, Allen IC. Starting a Fire Without Flame: The Induction of Cell Death and Inflammation in Electroporation-Based Tumor Ablation Strategies. Front Oncol. 2020;10:1235. doi: 10.3389/fonc.2020.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sersa G, Ursic K, Cemazar M, Heller R, Bosnjak M, Campana LG. Biological factors of the tumour response to electrochemotherapy: Review of the evidence and a research roadmap. Eur J Surg Oncol. 2021;47:1836–1846. doi: 10.1016/j.ejso.2021.03.229. [DOI] [PubMed] [Google Scholar]
- 16.Ursic K, Kos S, Kamensek U, Cemazar M, Miceska S, Markelc B, Bucek S, Staresinic B, Kloboves Prevodnik V, Heller R, Sersa G. Potentiation of electrochemotherapy effectiveness by immunostimulation with IL-12 gene electrotransfer in mice is dependent on tumor immune status. J Control Release. 2021;332:623–635. doi: 10.1016/j.jconrel.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Edhemovic I, Gadzijev EM, Brecelj E, Miklavcic D, Kos B, Zupanic A, Mali B, Jarm T, Pavliha D, Marcan M, Gasljevic G, Gorjup V, Music M, Vavpotic TP, Cemazar M, Snoj M, Sersa G. Electrochemotherapy: a new technological approach in treatment of metastases in the liver. Technol Cancer Res Treat. 2011;10:475–485. doi: 10.7785/tcrt.2012.500224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mir LM, Gehl J, Sersa G, Collins CG, Garbay J-R, Billard V, Geertsen PF, Rudolf Z, O’sullivan GC, Marty M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator TM by means of invasive or non-invasive electrodes. EJC Sup. 2006 [Google Scholar]
- 19.Edhemovic I, Brecelj E, Gasljevic G, Marolt Music M, Gorjup V, Mali B, Jarm T, Kos B, Pavliha D, Grcar Kuzmanov B, Cemazar M, Snoj M, Miklavcic D, Gadzijev EM, Sersa G. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol. 2014;110:320–327. doi: 10.1002/jso.23625. [DOI] [PubMed] [Google Scholar]
- 20.Djokic M, Cemazar M, Popovic P, Kos B, Dezman R, Bosnjak M, Zakelj MN, Miklavcic D, Potrc S, Stabuc B, Tomazic A, Sersa G, Trotovsek B. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur J Surg Oncol. 2018;44:651–657. doi: 10.1016/j.ejso.2018.01.090. [DOI] [PubMed] [Google Scholar]
- 21.Djokic M, Dezman R, Cemazar M, Stabuc M, Petric M, Smid LM, Jansa R, Plesnik B, Bosnjak M, Tratar UL, Trotovsek B, Kos B, Miklavcic D, Sersa G, Popovic P. Percutaneous image guided electrochemotherapy of hepatocellular carcinoma: technological advancement. Radiol Oncol. 2020;54:347–352. doi: 10.2478/raon-2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zmuc J, Gasljevic G, Sersa G, Edhemovic I, Boc N, Seliskar A, Plavec T, Brloznik M, Milevoj N, Brecelj E, Kos B, Izlakar J, Jarm T, Snoj M, Stukelj M, Miklavcic D, Cemazar M. Large Liver Blood Vessels and Bile Ducts Are Not Damaged by Electrochemotherapy with Bleomycin in Pigs. Sci Rep. 2019;9:3649. doi: 10.1038/s41598-019-40395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman A, Grand D, Charpentier KP. Irreversible electroporation of hepatocellular carcinoma: patient selection and perspectives. J Hepatocell Carcinoma. 2017;4:49–58. doi: 10.2147/JHC.S129063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarantino L, Busto G, Nasto A, Fristachi R, Cacace L, Talamo M, Accardo C, Bortone S, Gallo P, Tarantino P, Nasto RA, Di Minno MN, Ambrosino P. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J Gastroenterol. 2017;23:906–918. doi: 10.3748/wjg.v23.i5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarantino L, Busto G, Nasto A, Nasto RA, Tarantino P, Fristachi R, Cacace L, Bortone S. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A feasibility study. Eur J Surg Oncol. 2018;44:1603–1609. doi: 10.1016/j.ejso.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Miklavcic D, Snoj M, Zupanic A, Kos B, Cemazar M, Kropivnik M, Bracko M, Pecnik T, Gadzijev E, Sersa G. Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed Eng Online. 2010;9:10. doi: 10.1186/1475-925X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falk Hansen H, Bourke M, Stigaard T, Clover J, Buckley M, O'Riordain M, Winter DC, Hjorth Johannesen H, Hansen RH, Heebøll H, Forde P, Jakobsen HL, Larsen O, Rosenberg J, Soden D, Gehl J. Electrochemotherapy for colorectal cancer using endoscopic electroporation: a phase 1 clinical study. Endosc Int Open. 2020;8:E124–E132. doi: 10.1055/a-1027-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, Harrington KJ. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203–217. doi: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- 29.Jesenko T, Bosnjak M, Markelc B, Sersa G, Znidar K, Heller L, Cemazar M. Radiation Induced Upregulation of DNA Sensing Pathways is Cell-Type Dependent and Can Mediate the Off-Target Effects. Cancers (Basel) 2020;12 doi: 10.3390/cancers12113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Shen L. Radiobiology of stereotactic ablative radiotherapy (SABR): perspectives of clinical oncologists. J Cancer. 2020;11:5056–5068. doi: 10.7150/jca.44408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvet CY, Famin D, André FM, Mir LM. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology. 2014;3:e28131. doi: 10.4161/onci.28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heppt MV, Eigentler TK, Kähler KC, Herbst RA, Göppner D, Gambichler T, Ulrich J, Dippel E, Loquai C, Schell B, Schilling B, Schäd SG, Schultz ES, Matheis F, Tietze JK, Berking C. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother. 2016;65:951–959. doi: 10.1007/s00262-016-1856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karaca B, Yayla G, Erdem M, Gürler T. Electrochemotherapy with anti-PD-1 treatment induced durable complete response in heavily pretreated metastatic melanoma patient. Anticancer Drugs. 2018;29:190–196. doi: 10.1097/CAD.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 34.Campana LG, Peric B, Mascherini M, Spina R, Kunte C, Kis E, Rozsa P, Quaglino P, Jones RP, Clover AJP, Curatolo P, Giorgione R, Cemazar M, Terlizzi F, Bosnjak M, Sersa G. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers (Basel) 2021;13 doi: 10.3390/cancers13174289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, Mukherjee SK, Thursz M, Wong CN, Sharma R, Rimassa L. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39:3620–3637. doi: 10.1038/s41388-020-1249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora S, Velichinskii R, Lesh RW, Ali U, Kubiak M, Bansal P, Borghaei H, Edelman MJ, Boumber Y. Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors. Adv Ther. 2019;36:2638–2678. doi: 10.1007/s12325-019-01051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27:58–63. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- 38.Keller H. Malta kENUP F. covidX. 2021 [cited 17 March 2021]. In: covidX [Internet]. Kalkara (Malta) - . Available from: https://www.covidx.eu/covid-evax .
- 39.Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, Gabaldon TA, Zaharoff DA. Localized Interleukin-12 for Cancer Immunotherapy. Front Immunol. 2020;11:575597. doi: 10.3389/fimmu.2020.575597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Algazi A, Bhatia S, Agarwala S, Molina M, Lewis K, Faries M, Fong L, Levine LP, Franco M, Oglesby A, Ballesteros-Merino C, Bifulco CB, Fox BA, Bannavong D, Talia R, Browning E, Le MH, Pierce RH, Gargosky S, Tsai KK, Twitty C, Daud AI. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann Oncol. 2020;31:532–540. doi: 10.1016/j.annonc.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Bhatia S, Longino NV, Miller NJ, Kulikauskas R, Iyer JG, Ibrani D, Blom A, Byrd DR, Parvathaneni U, Twitty CG, Campbell JS, Le MH, Gargosky S, Pierce RH, Heller R, Daud AI, Nghiem P. Intratumoral Delivery of Plasmid IL12 Via Electroporation Leads to Regression of Injected and Noninjected Tumors in Merkel Cell Carcinoma. Clin Cancer Res. 2020;26:598–607. doi: 10.1158/1078-0432.CCR-19-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cemazar M, Golzio M, Sersa G, Hojman P, Kranjc S, Mesojednik S, Rols MP, Teissie J. Control by pulse parameters of DNA electrotransfer into solid tumors in mice. Gene Ther. 2009;16:635–644. doi: 10.1038/gt.2009.10. [DOI] [PubMed] [Google Scholar]
- 43.Chiarella P, Massi E, De Robertis M, Sibilio A, Parrella P, Fazio VM, Signori E. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin Biol Ther. 2008;8:1645–1657. doi: 10.1517/14712598.8.11.1645. [DOI] [PubMed] [Google Scholar]
- 44.Tevz G, Kranjc S, Cemazar M, Kamensek U, Coer A, Krzan M, Vidic S, Pavlin D, Sersa G. Controlled systemic release of interleukin-12 after gene electrotransfer to muscle for cancer gene therapy alone or in combination with ionizing radiation in murine sarcomas. J Gene Med. 2009;11:1125–1137. doi: 10.1002/jgm.1403. [DOI] [PubMed] [Google Scholar]
- 45.Tevz G, Pavlin D, Kamensek U, Kranjc S, Mesojednik S, Coer A, Sersa G, Cemazar M. Gene electrotransfer into murine skeletal muscle: a systematic analysis of parameters for long-term gene expression. Technol Cancer Res Treat. 2008;7:91–101. doi: 10.1177/153303460800700201. [DOI] [PubMed] [Google Scholar]