Abstract
Tasks that measure correlates of prosocial decision-making share one common feature: agents can make choices that increase the welfare of a beneficiary. However, prosocial decisions vary widely as a function of other task features. The diverse ways that prosociality is defined and the heterogeneity of prosocial decisions have created challenges for interpreting findings across studies and identifying their neural correlates. To overcome these challenges, we aimed to organize the prosocial decision-making task space of neuroimaging studies. We conducted a systematic search for studies in which participants made decisions to increase the welfare of others during functional magnetic resonance imaging. We identified shared and distinct features of these tasks and employed an unsupervised graph-based approach to assess how various forms of prosocial decision-making are related in terms of their low-level components (e.g. task features like potential cost to the agent or potential for reciprocity). Analyses uncovered three clusters of prosocial decisions, which we labeled as cooperation, equity and altruism. This feature-based representation of the task structure was supported by results of a neuroimaging meta-analysis that each type of prosocial decisions recruited diverging neural systems. Results clarify some of the existing heterogeneity in how prosociality is conceptualized and generate insight for future research and task paradigm development.
Keywords: prosocial decision-making, meta-analysis, cooperation, equity, altruism
Introduction
Prosocial decisions—choices that increase the welfare of others—are universal across cultures (Henrich et al., 2005) and are integral for supporting interpersonal relationships at multiple scales, including between dyads (Rusbult and Van Lange, 2003; Declerck et al., 2013), among groups and social networks (Fehr et al., 2002; Fehr and Fischbacher, 2003; Fehr and Camerer, 2007; de Waal, 2008; Fowler and Christakis, 2010; FeldmanHall, 2017) and within societies (Nowak, 2006). And, while largely conserved across species (de Waal, 2008; Burkart et al., 2014; Hare, 2017), the prevalence and variety of prosociality exhibited by humans is unique (Zaki and Mitchell, 2013; Fehr and Schurtenberger, 2018). Although cognitive and neural processes underlying various forms of prosociality have been studied extensively across disciplines spanning psychology, neuroscience, economics and biology, the heterogeneity of prosocial decisions has led to inconsistencies in how they are operationalized and categorized (Fehr and Schmidt, 1999; Fehr et al., 2002; Rilling et al., 2002; Batson and Powell, 2003; de Waal, 2008; Declerck et al., 2013; Rand and Nowak, 2013; Ruff and Fehr, 2014; Tricomi and Sullivan-Toole, 2015; Marsh, 2016; Parnamets et al., 2020). This can create challenges when interpreting findings across neuroimaging studies or when attempting to understand how different types of prosocial decisions vary in terms of their underlying processes.
Derived from the Latin stem pro and root socius, signifying ‘for a companion’, prosocial decision-making refers to ‘decisions made for the benefit of another’ (Houghton Mifflin Harcourt, 2000). Laboratory tasks that measure correlates of prosocial decision-making share one common feature: allowing deciding participants (or agents) to make choices that increase the welfare of a beneficiary. However, prosocial decisions vary widely as a function of other task features. For example, although choosing to forgo resources (usually money) to alleviate the suffering of a stranger (in a charitable donation task) vs choosing to contribute money to maximize equity among known members in a group (in a public goods game) both share a common prosocial core of increasing the welfare of others, these decisions diverge along multiple other characteristics. In the first example, a prosocial agent sacrifices resources in response to another person’s distress with the understanding that they will not receive anything in return—suggesting a likely role for empathic concern and planning for prosocial action without any anticipation of reward. In the second example, all prosocial agents are relying on the decisions of others and are hoping to increase the total pool of resources for everyone involved. This suggests a role for monitoring the expected actions of others’ decisions and includes the anticipation of self-rewarding outcomes.
In addition to charitable donation tasks and public goods games, other common prosocial paradigms include dictator games, prisoner’s dilemmas and trust games. Such tasks can be implemented with multiple variations, and the vast number of combinations of task features is a major source of heterogeneity. This heterogeneity raises the question of whether common mechanisms underlie all prosocial choices. One possibility is that prosocial decisions in each distinct task are supported by distinct mechanisms. But it is more likely that taxonomic clusters exist within the task space of prosocial decision-making that reflect common underlying neural processes (Cutler and Campbell-Meiklejohn, 2019). One way to identify such clusters would be via a bottom-up approach aimed at characterizing the task structure of prosocial decision-making by analyzing the way specific tasks cluster according to their low-level features. In other words, developing one level of a formal representation (or ontology) of cognitive tasks and their inter-relationships (Turner and Laird, 2012; Poldrack and Yarkoni, 2016). The first goal of this paper was to clarify how different prosocial tasks are inter-related and how their low-level features give rise to broad categories of prosocial decisions. Then, using this information across various studies, we employed an unsupervised graph-based approach to generate a preliminary characterization of the neuroimaging task space comprised of the distinct and shared task features of prosocial decision-making paradigms. Finally, we conducted an functional magnetic resonance imaging (fMRI) meta-analysis to identify patterns of distinct and overlapping neural activation that correspond to the identified clusters of prosocial processes.
Breaking prosocial decision processes down into their relevant task features may allow a better understanding of how prosocial decisions are inter-related, and how they diverge. In general, the features that distinguish these tasks involve those related to the beneficiary (Is the beneficiary a real or imaginary person (or persons) or an organization like a charity? Is their identity apparent to the agent? Is their need or distress known to the agent?), to the interaction (Does the beneficiary also make decisions that will affect ultimate outcome? Will the agent and beneficiary interact only once or more than once?) and to the outcomes of the agent’s decision (What is the magnitude of the benefit to the beneficiary? Will the decision result in rewarding outcomes for the agent? Will it be costly? Will the decision conform to social norms, such as equity? How certain is the outcome?). Multiple combinations of these features likely shape the context, motivations and outcomes of prosocial decisions and thus should recruit diverging neural systems.
Features related to the beneficiary
Various features related to the beneficiary of a prosocial decision are known to influence such decisions. Beneficiaries can include specific people, such as close or familiar others (Sharp et al., 2011; Telzer et al., 2011; Fareri et al., 2015; Hill et al., 2017; Schreuders et al., 2018) or in-group members (Balliet et al., 2014; Telzer et al., 2015; Hackel et al., 2017; Wills et al., 2018) or can be hypothetical or even non-human (e.g. computers) (Delgado et al., 2005; Fareri et al., 2012). Across contexts, agents are typically more willing to help people than computers (Fareri et al., 2015) and are more willing to help people close to them than strangers (Jones and Rachlin, 2006, 2009; Safin et al., 2013; Strombach et al., 2015). Neural activation during decisions that affect real vs imaginary beneficiaries (e.g. computer) is increased in regions important for theory of mind or inferring the mental states of others such as the temporoparietal junction (TPJ) (FeldmanHall et al., 2012).
In tasks that include real beneficiaries who are previously unknown to the agent, the beneficiary may be another participant in study (Weiland et al., 2012) or an anonymous stranger (Bault et al., 2014; Hutcherson et al., 2015; Strombach et al., 2015) who the agent may have briefly met before the task (Shaw et al., 2018; Abe et al., 2019) or seen in a photograph (Genevsky et al., 2013; Park et al., 2017). Receiving any identifying information about a beneficiary generally increases prosociality, in line with the identifiable victim effect (Jenni and Loewenstein, 1997; Kogut and Ritov, 2005; Lee and Feeley, 2016). This effect also results in greater prosociality toward single individuals vs collectives (Kogut and Ritov, 2005; Lee and Feeley, 2016), including charitable organizations, whether predetermined (Greening et al., 2014), of the agent’s choosing (Kuss et al., 2013) or from a list of charities (Hare et al., 2010; Izuma et al., 2010; Tusche et al., 2016). Increases in prosocial decision-making are particularly robust when the need or distress of the beneficiary is salient (Genevsky et al., 2013; FeldmanHall et al., 2015; Kuss et al., 2015; Tusche et al., 2016). Cues that signal need or distress typically elicit empathic concern, which motivates the desire to alleviate it (Preston and de Waal, 2002; de Waal, 2008; Batson, 2011; Marsh, 2016). This form of empathy is supported by activity in neural regions including the anterior insula, anterior cingulate cortex (ACC) and pre-supplementary motor area (pre-SMA) (Lamm et al., 2011; Jauniaux et al., 2019; Fallon et al., 2020; Kogler et al., 2020; Schurz et al., 2020) and empathic neural responding predicts prosocial decision-making both in and out of the laboratory (Tusche et al., 2016; Vekaria et al., 2020).
Features related to the interaction
Aspects of the interaction between agents and beneficiaries (or other agents) in prosocial tasks also influence agents’ decisions, particularly when agents can learn about those with whom they are interacting. In some interactions, only one agent can influence the outcome. For example, in dictator games, agents unilaterally allocate resources between themselves and a beneficiary (Engel, 2011). In others, multiple agents can shape the outcome. For example, in social dilemmas or trust games, agents can choose to cooperate with others in order to increase the total pool of available resources for everyone involved or can defect to obtain better outcomes for self (Balliet et al., 2011). Alternatively, in ultimatum games, agents receive feedback about their decisions from beneficiaries, who can accept or reject the offer (Güth et al., 1982).
Some prosocial decisions involve repeated interactions, which, unlike one-shot interactions, provide opportunities to reciprocate or respond to feedback about prior choices (Thielmann et al., 2020). When repeated interactions are expected, it typically motivates cooperation, with agents motivated to pay short-term cooperation ‘costs’ to increase future reciprocity from a partner (Milinski et al., 2001; Rand and Nowak, 2013) and more willing to cooperate with partners who have cooperated previously (Fehr and Schurtenberger, 2018). This may be related to the ability to update expectations of others’ likely behavior, a type of social learning is supported by the subgenual ACC (Christopoulos and King-Casas, 2014). During these repeated interactions, agents may also act to influence interaction partners to reciprocate, for example, when a cooperative exchange is broken and partners coax others back into cooperation via generosity (Bendor et al., 1921; King-Casas et al., 2008).
Features related to outcomes
Across interaction types, prosocial decisions are also shaped by their anticipated outcomes. In some cases, prosocial decisions may benefit the agent directly. In prisoner’s dilemmas or public goods games, for example, decisions to cooperate increase the probability of future reciprocity. In such tasks, agents also take on the role of beneficiaries (Chaudhuri, 2011; Rand and Nowak, 2013) and, thus, must arbitrate between their own and others’ rewards. These tasks often recruit neural systems that support subjective valuation and reward expectancy, such as the ventromedial prefrontal cortex (PFC) and ventral striatum (Wills et al., 2018, 2020; Parnamets et al., 2020). These tasks also carry an element of uncertainty (Bellucci et al., 2017), with the agent’s outcome often dependent on a beneficiary or trustee’s choices (Mayer et al., 1995). Uncertainty during these decisions may be reflected through activation in the dorsal ACC (Aimone et al., 2014).
Prosocial choices may also yield more abstract rewards, such as conformity to desirable social norms like maximizing equity among multiple parties (via, for example, a 50–50 split of resources) (López-Pérez, 2008; Krupka and Weber, 2013; Fehr and Schurtenberger, 2018). In some cases, agents may choose to act prosocially and forgo resources to avoid deviating from desirable norm, which is known as disadvantageous inequity aversion (Tricomi and Sullivan-Toole, 2015). Equitable interpersonal decisions are thought to engage neural structures involved in a computing subjective value such as the medial PFC and ventral striatum and thus may be motivated through increased intrinsic value placed on the decision (Zaki and Mitchell, 2011), perhaps via their goal of producing increased subjective happiness for agents and beneficiaries (Tabibnia et al., 2007; Tabibnia and Lieberman, 2007). In tasks with repeated interactions, these decisions may also reflect the maintenance of abstract, norm-based rules regarding fairness or reciprocity, in that repeated interactions can enable norms to become established among the interacting parties and maintained in dorsolateral PFC (van den Bos et al., 2009; Guroglu et al., 2014).
In other prosocial decision-making tasks (such as dictator games or charitable giving tasks), agents can forgo resources (including money, time, effort or safety) solely to benefit others. In this case, prosocial choices are made despite certain concrete costs to the agent, often to alleviate the beneficiary’s distress or need. As described above, such decisions are thought to be driven by activation in regions like anterior insula, which represent negative affective states (e.g. pain or distress) of the beneficiary (FeldmanHall et al., 2015; Tusche et al., 2016). Such choices also may yield indirect gains, including increases in mood or well-being (Dunn et al., 2008; Aknin et al., 2012; Curry et al., 2018), possibly related to the vicarious reward of improving the beneficiary’s welfare (Mobbs et al., 2009); such vicarious reward may be supported by activity in ventral striatum and ventromedial PFC.
Given the diversity of extant prosocial decision tasks, two recent meta-analytic studies have been very valuable in describing the neural correlates of prosocial behaviors aggregated across tasks that reflect divergent constellations of the above variables. Bellucci et al. (2020) aggregated across a wide range of tasks in which participants made decisions about others, rated others’ traits or judged others’ behaviors in an effort to find neural activation overlap among prosociality, empathy and mentalizing. They found four regions to be preferentially engaged across the tasks they incorporated: dorsolateral PFC, ventromedial PFC, dorsal posterior cingulate cortex (PCC) and middle cingulate cortex (MCC). Of these regions, they found a conjunction in dorsal PCC activation during tasks involving prosocial behavior and tasks involving mentalizing (understanding another person’s needs and inferring goals across contexts); they also found a conjunction in MCC activation across tasks involving prosocial behavior and tasks involving empathy (resonating with another’s needs) (Bellucci et al., 2020). Activation during prosocial behavior in the dorsolateral PFC and ventromedial PFC did not overlap with activation during mentalizing or empathy tasks. This work identified common neural patterns underlying a range of behaviors related to prosociality, but, by not considering key differences among types of prosocial decisions, it was not able to identify whether they are supported by distinct processes. Cutler and Campbell-Meiklejohn (2019) provided preliminary evidence that distinct neural regions do indeed support different forms of prosocial decision-making, finding diverging patterns of activation for prosocial behaviors that do not provide an opportunity to gain extrinsic rewards (and thus likely are intrinsically motivated) vs those with the probability of gaining an extrinsic reward. For example, extrinsically motivated decisions recruited greater activity in striatal regions relative to intrinsically motivated decisions. In contrast, intrinsically motivated decisions recruited increased activation in ventromedial PFC relative to extrinsically motivated decisions. Activation in ventromedial PFC also differentiated these types along a posterior (intrinsic) to anterior (extrinsic) axis.
However, the distinction between extrinsic and intrinsic motivation was determined in advance, rather than being driven by objective features of the data. This is also only one of many possible distinctions among forms of prosocial behavior. An alternative means of investigating neural substrates of various prosocial decision tasks could instead take a more bottom-up approach that identifies distinct clusters of tasks that emerge from statistical variation in their objective features or outcomes. For example, a recent behavioral study analyzed the behavioral outcomes of different economic prosocial tasks (such as the percentage of prosocial decisions during each task, the ratio of other-regarding to self-regarding decisions in each, average monetary donations or summary scores of self-reported measures). Using factor analysis, they determined that the prosocial tasks clustered into four factors that the authors termed: altruistically motivated prosocial behavior, norm-motivated prosocial behavior, strategically motivated prosocial behavior and self-reported prosocial behavior (Böckler et al., 2016).
We sought to use a similar bottom-up approach to meta-analytically investigate the neural correlates of prosocial decision-making during fMRI. We focused on objective features that distinguish the tasks themselves, which included features related to outcomes of decisions, to the beneficiaries of the decision and to the interaction between agents and beneficiaries. We first compiled data from 43 unique fMRI studies of prosocial decision-making (including 25 maps and 18 coordinate tables across 1423 participants). We then dummy-coded task features related to the beneficiary, interaction and outcome of each decision and employed a data-driven, graph-based approach to identify clusters of studies based on their overlapping vs distinct task features. As described in detail below, this approach indicated that the prosocial decision-making task-space comprises three clusters, which we labeled as cooperation, equity and altruism. We next used a meta-analytic approach that combined group-level statistical parametric images with reported peak-coordinates to identify divergent neural activation patterns across these clusters of studies. In so doing, the present study resolves some discrepancies in how prosocial decisions are conceptualized, expands understanding regarding how prosocial decisions are related and distinct and generates insight for future research.
Method
Literature search and study selection
A literature search using PubMed identified research published prior to June 2019 using keywords either (‘fMRI’ or ‘neur*’) and one of the following: (‘prosocial’, ‘altruis*’, ‘trust game’, ‘fairness’, ‘reciproc*’, ‘cooperat*’, ‘charitable’, ‘public goods’, ‘dictator’, ‘ultimatum’, ‘prisoner*’).1 The search returned 201 articles. We removed 124 articles that did not meet key criteria, such as non-neuroimaging studies, neuroimaging studies that did not use fMRI and literature reviews or meta-analyses. Independently, we identified 123 potential articles from the reference lists of the remaining 77 articles. After removing all duplicate titles from the combined lists, 146 articles remained. We then selected only articles that reported novel whole-brain fMRI data (i.e. data only published once) that were collected while participants made decisions that benefitted another individual (prosocial decisions). We also limited our search to include only data from studies that were able to examine differences in activation during prosocial decisions relative to decisions that benefited the agent alone (selfish decisions). In some cases, this was a contrast between prosocial choices and selfish choices within a task condition or parametric modulation of the amount given. For other studies, the contrast was between decisions during a prosocial condition and a self-only condition. We did not include contrasts involving alternate control conditions (e.g. rest and visuomotor controls), even when these were available, due to significant variation in brain activation (Cutler and Campbell-Meiklejohn, 2019). Upon review of the remaining 146 articles, 69 were identified that met our inclusion criteria.
We sent emails to the corresponding authors of all included studies to request unthresholded, group-level t-statistic map(s) from the study that best fit our criteria. For studies that included pharmacological manipulations or clinical populations, we requested data from only the control group. If maps were not available, we requested coordinates for contrasts of interests or extracted them from manuscripts. If a coordinate table reported Z-scores or Talaraich coordinates, peak values were transformed to t-statistics and Montreal Neurological Institute (MNI) coordinates, respectively. If the contrast of interest was reported in both directions (e.g. cooperate > defect and defect > cooperate), the selfish contrast peaks were assigned as negative t-values. Ultimately, we obtained the necessary data from 43 unique fMRI studies, including 25 maps and 18 coordinate tables that included data from 1423 subjects (Table 1).
Table 1.
Descriptions of studies included in meta-analysis
| Study | N | Proportion female | Map or peak | Task | T | FWHM | program | Sig | Contrast selected | Cluster label |
|---|---|---|---|---|---|---|---|---|---|---|
| Abe et al. (2019) | 19 | 9/19 | map | Joint force-production task | 3T | 8 mm3 | Statistical parametric mapping (SPM)12 | Pcorr < 0.001 | Joint performance vs single performance | C |
| Aimone et al. (2014) | 28 | 15/30 total | peak | Trust game, one-shot, binary (investor) | 3T | 8 mm3 | SPM8 | Puncorr <0.0001 | Share vs keep | C |
| Bault et al. (2014) | 25 | 12/29 total | map | Public goods game | 3T | 5 mm3 | FSL | Pcorr < 0.05 (FWE) | PM monetary choice | C |
| Chen et al. (2016) | 93 | 50/104 total | map | Prisoner’s dilemma, iterative | 3T | 5 mm3 | FSL | Pcorr <0.05 (FWE) | Cooperation vs defect (placebo only) | C |
| Decety et al. (2004) | 12 | 6/12 | peak | Cooperative pattern game | 3T | 4.5 × 4.5 × 6 mm | SPM2 | Pcorr < 0.05 | Cooperation vs competition | C |
| Delgado et al. (2005) | 12 | 6/14 total | peak | Trust game, binary, iterative (investor) | 3T | 4 mm3 | Brain Voyager | Puncorr < 0.001 | Share vs keep | C |
| Fareri et al. (2015) | 26 | 14/26 | map | Trust game, binary, iterative (investor) | 3T | 4 mm3 | Brain Voyager | N/A | Share vs keep (across all partners) | C |
| FeldmanHall et al. (2012) | 14 | 8/14 | peak | Your pain, my gain task | 3T | 8 mm3 | SPM | Pcorr < 0.05 | PM monetary choices, no covariates | A |
| FeldmanHall et al. (2015) | 17 | 11/17 | peak | Your pain, my gain task | 3T | 8 mm3 | SPM5 | Pcorr < 0.05 (FWE) | PM monetary choice | A |
| Fermin et al. (2016) | 33 | 18/33 | map | Prisoner’s dilemma, one shot | 3T | 8 mm3 | SPM8 | Puncorr <0.005 | Cooperation vs defect | C |
| Fouragnan et al. (2013) | 18 | 0/18 | peak | Trust game (with priors manipulation) | 4T | 8 mm3 | SPM8 | Pcorr < 0.005 | Share vs keep | C |
| Garbarini et al. (2014) | 16 | 8/16 | map | Trust game, one-shot (responder) | 1.5T | 4 mm3 | Brain Voyager | Pcorr < 0.05 | Reciprocate vs defect | E |
| Genevsky et al. (2013) | 11 | 6/11 | peak | Charitable donation task with identifiable victim | 3T | 4 mm3 | AFNI | Puncorr < 0.005 | Donation vs no donation | A |
| Greening et al. (2014) | 18 | 9/18 | peak | Charity task | 3T | 4 mm3 | AFNI | Pcorr < 0.05 | Charity vvs self | A |
| Guroglu et al. (2014) | 22 | 17/28 total | peak | Modified dictator game (SMI game) | 3T | 8 mm3 | SPM8 | Puncorr < 0.005 | Equal split vs unequal | E |
| Hare et al. (2010) | 22 | 22/22 | map | Charitable donation task | 3T | 8 mm3 | SPM5 | Puncorr < 0.0005 | PM monetary choice | A |
| Hutcherson et al. (2015) | 51 | 0 /51 | map | Modified dictator game, with probable outcomes | 3T | 8 mm3 | SPM8 | Puncorr < 0.05 | Prosocial choice vs self | A |
| Izuma et al. (2010) | 23 | 12/23 | map | Charitable donation task in presence or absence of observer | 3T | 6 mm3 | SPM5 | Puncorr < 0.001 | Donation vs no donation (no observers) | A |
| Koban et al. (2014) | 17 | 10/22 total | map | Modified dictator game, sharing/keeping during conflict | 3T | 8 mm3 | SPM8 | Puncorr < 0.001 | Equal split vs self during human interpersonal conflict | E |
| Kuss et al. (2013) | 33 | 14/33 | map | Charitable donation task, with probable outcomes | 3T | 6 mm3 | SPM8 | Puncorr < 0.001 | Costly donation vs self | A |
| Lee et al. (2018) | 16 | 0/16 | map | Tetris-like game, with self, helping and harming conditions | 3T | 8 mm3 | SPM5 | Pcorr < 0.05 | Help vs self | A |
| Lelieveld et al. (2013) | 26 | 17/26 | map | Dictator game with emotional manipulation | 3T | 6 mm3 | SPM5 | Puncorr < 0.001 | Equal split vs unequal split | E |
| Morishima et al. (2012) | 27 | 17/30 total | map | Dictator game | 3T | 8 mm3 | SPM8 | Pcorr < 0.05 (FWE) | Donation vs self | A |
| Park et al. (2017) | 159 | 100/166 total | map | Dictator game with faces | 3T | 4 mm3 | AFNI | Puncorr < 0.005 | Giving vs not giving (offer only) | A |
| Ramsøy et al. (2015) | 30 | 14/30 | peak | Prisoner’s dilemma, with belief prompts about partner’s decision | 3T | 8 mm3 | SPM8 | Puncorr < 0.001 | Cooperation vs defect | C |
| Schneider-Hassloff et al. (2015) | 164 | 78/164 | map | Prisoner’s dilemma with measures of attachment style | 3T | 8 mm3 | SPM8 | Puncorr < 0.001 | Cooperation vs defect | C |
| Schreuders et al. (2018) | 22 | 12/27 total | map | Modified dictator game (SMI game) | 3T | 8 mm3 | SPM8 | Pcorr < 0.05 | Equal split vs self (across interaction partners) | E |
| Schreuders et al. (2019) | 39 | 29/50 total | map | Modified dictator game (SMI game) | 3T | 8 mm3 | SPM8 | Pcorr < 0.001 | Equal split vs self (across interaction partners) | E |
| Sharp et al. (2011) | 20 | 0/20 | map | Trust game (investor) | 3T | 4 mm3 | AFNI | Puncorr < 0.001 | Share vs keep (controls) | C |
| Shaw et al. (2018) | 38 | 0/38 | peak | Ultimatum game (proposer) | 3T | 5 mm3 | FSL (preproc) SPM12 (GLM) |
Pcorr < 0.001 (FWE) | PM monetary choice (proposers only) | C |
| Smith-Collins et al. (2013) | 24 | 24/24 | peak | Trust game, iterative (investor) | 1.5T | 8 mm3 | SPM8 | Puncorr < 0.001 | Cooperation vs defect | C |
| Stanley et al. (2012) | 40 | 22/40 | map | Trust game, one-shot (investor) | 3T | 6 mm3 | SPM8 | Pcorr < 0.05 (FWE) | PM monetary choices (only human partners) | C |
| Strombach et al. (2015) | 27 | 13/27 | map | Modified dictator game with social distance manipulation | 3T | 8 mm3 | SPM8 | Pcorr < 0.005 | Equal split vs self | E |
| Telzer et al. (2011) | 25 | 13/25 | map | Family assistance task | 3T | 8 mm3 | SPM5 | Pcorr < 0.05 | Costly donation vs self | A |
| Telzer et al. (2015) | 29 | 13/29 | map | Modified dictator game with group membership manipulation | 3T | 8 mm3 | SPM8 | Pcorr < 0.05 | Donation vs self | A |
| Tusche et al. (2016) | 23 | 15/33 total | map | Charitable donation task | 3T | 8 mm3 | SPM8 | Pcorr < 0.05 (FWE) | High donation vs low donation | A |
| van den Bos et al. (2009) | 18 | 9/18 | peak | Trust game, one-shot (responder) | 3T | 6 mm3 | SPM2 | Puncorr < 0.001 | Reciprocate vs defect | C |
| Watanabe et al. (2014) | 48 | N/A | peak | Pay-it-forward + reputation-based indirect reciprocity game | 3T | 8 mm3 | SPM8 | Pcorr < 0.05 (FWE) | Cooperation vs defect (pay-it-forward and reputation-based conditions) | C |
| Weiland et al. (2012) | 14 | 8/14 | peak | Dictator game | 3T | 8 mm3 | Brain Voyager | Puncorr < 0.005 | Fair split (6:6, 7:5) vs unfair (8:4, 9:3, 10:2, 11:1) | E |
| Will et al. (2016) | 43 | 17/43 | map | Dictator game after cyberball | 3T | 8 mm3 | SPM8 | Puncorr < 0.001 | Equitable choice vs inequitable | E |
| Wills et al. (2018) | 42 | 32/47 | peak | Public goods game | 3T | 6 mm3 | SPM12 | Pcorr < 0.05 | Give vs keep | C |
| Wittmann et al. (2016) | 24 | 9/24 | peak | Cooperation task | 3T | 5 mm3 | FSL | Pcorr < 0.05 (FWE) | Cooperation vs competition | C |
| Zaki and Mitchell (2011) | 15 | 6/15 | peak | Modified dictator game | 3T | 6 mm3 | SPM | Pcorr < 0.005 | Equitable choice vs inequitable | E |
Note. Some studies only reported gender as a proportion of the total sample, but not the final analytic sample. Researcher labels: C = Cooperative; E = Equitable; A = Altruistic.
Abbreviation: SMI, self-maximizing inequity.
Identifying task features across studies
We first reviewed the details of the methodologies of all available tasks and identified 13 distinct task features that varied among existing prosocial decision-making tasks across neuroimaging studies (Figure 1). These task features can be broken down into those that vary as a function of the beneficiary, the interaction and the outcome. Although in theory features related to the agent can also vary, all participants included in the present study were healthy control adults and we did not identify consistent features related to these participants—for example, consistent individual difference measures—in the available literature. Four independent raters dummy coded (‘present’ or ‘absent’) the 13 features during the prosocial decision phase for the available contrast in each study with high initial agreement among coders (ICC = 0.82, CI95% = [0.79, 0.84]). Discrepancies in the initial coding were then resolved through a consensus agreement across each of the four coders.
Fig. 1.
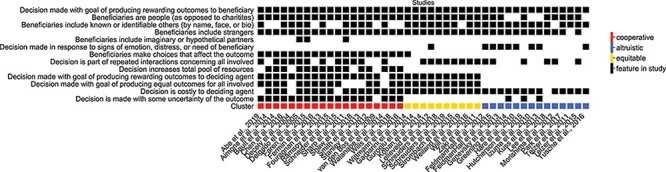
Bipartite feature x study incidence matrix.
Note. Studies were labeled with task features to construct a bipartite graph where edges (black boxes in incidence matrix) exist between a feature node and study node if the study contrast contained the task feature. The final row in this matrix represents each of three clusters, which were labeled by the researchers as cooperative (red), equitable (yellow) and altruistic (blue) decisions based on the task features shared within each cluster.
Identifying clusters within the task structure of prosocial decision-making
To assess differential clusters of prosocial decisions based on their task features, we next applied an unsupervised, graph-based approach. Importantly, we selected an approach that allowed us to identify potential clusters of studies among tasks that all shared a common feature of ‘producing a rewarding outcome to the beneficiary’ (a crucial criterion for inclusion in our meta-analysis). Thus, we sought to construct a fully connected, weighted graph with studies as nodes and the degree of overlapping task features as weighted edges. To accomplish this, we first used the identified features to construct a bipartite graph. This graph contained two sets of nodes: nodes representing the 13 different task features and nodes representing the 43 different prosocial decision study contrasts. In this graph, an edge exists between a feature node and a study node if the study contrast contained the task feature (Figure 1 depicts this graph in matrix form). Next, we projected this bipartite graph onto a weighted network of studies, where edge weights between studies represented the Dice similarity coefficient (Dice, 1945) or the degree of overlapping task features relative to the total possible task features (Figure 2). We then ran the Louvain community detection algorithm (Blondel et al., 2008)—a common unsupervised clustering algorithm used across the biological, psychological and social sciences that detects clustering of nodes in a fully-connected, weighted graph (Matthews et al., 2016; Ito et al., 2017; Gonzalez-Castillo et al., 2019; Miele et al., 2019; Paquola et al., 2019; Allegra et al., 2020; Tian et al., 2020; Wang et al., 2020). This approach assigned study nodes to clusters in two steps. First, the algorithm finds small clusters of studies by optimizing local modularity. Second, it aggregates studies of the same cluster in a hierarchical fashion and builds a new network whose nodes are these clusters. These steps were repeated iteratively until the global modularity was maximized (i.e. global modularity is achieved when the connections between studies within the same clusters are strongest and connections between nodes in different clusters are weakest). The resulting hierarchy of studies across clusters in the study network is depicted in Supplementary Figure S1. To assess the stability of the identified three-cluster solution, we implemented a Jackknife sensitivity analysis, which exhaustively left one study out prior to generating the study network and applying Louvain community detection. We then computed (i) the percentage of iterations that yielded the same number of clusters (three clusters) in the study network and (ii) the proportion of studies that switched to a different cluster.
Fig. 2.
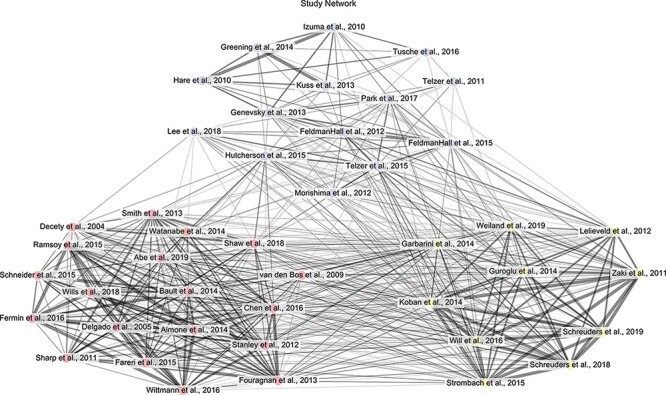
Graph depiction of study network generated from overlapping task features.
Note. The bipartite graph was projected onto a weighted study network, where wider edge weights between studies represented a larger Dice similarity coefficient (or greater similarity according to task features). A community detection algorithm revealed three clusters of studies, which were then labeled by the researchers as cooperative (red), equitable (yellow) and altruistic (blue) decisions based on the task features shared within each cluster. For visualization purposes, the depicted graph was thresholded to only display edges with a Dice similarity coefficient greater than 0.70 (note that the actual graph was fully-connected).
Neuroimaging preprocessing and meta-analyses
We next conducted meta-analyses combining reported peak information (coordinates and t-statistics) with original statistical parametric maps using the anisotropic effect size signed differential mapping software (AES-SDM, version 5.141; Radua et al., 2014). We selected this analytical technique rather than alternatives, such as coordinate-based activation likelihood (Turkeltaub et al., 2002; Eickhoff et al., 2009), because this approach enabled the utilization of precise, continuous estimates of effect sizes, assessment of between-study heterogeneity and identification of potential publication bias (Radua et al., 2012). Using AES-SDM, within-study, voxel-level maps of effect sizes (Hedge’s g) and their variances were re-created for each study. When only reported coordinates and statistics were available for a study, we calculated the effect size at each peak and estimated effect sizes in neighboring voxels based on the Euclidean distance between voxels and the peak using a 20 mm full-width at half maximum (FWHM) Gaussian function (Radua et al., 2012, 2014). This method of estimation is similar to the estimation of activation likelihood used in peak-probability meta-analytic methods, but the use of effect sizes in the calculation increases the accuracy of estimation of the true signal (Radua et al., 2012). When the t-statistics of the peak coordinates were unknown (one study: Delgado et al., 2005), we imputed the effect size with the extent threshold reported in the study.
We used three random-effects models to compute a meta-analytic activation for each prosocial category identified using the Louvain community detection algorithm. Each individual study was weighted by the inverse sum of its variance plus the between-study variance as obtained by the DerSimonian–Laird estimator of heterogeneity (DerSimonian and Laird, 1986). Within this random-effects framework, studies with larger sample sizes or lower variability contribute more and effects are assumed to randomly vary between study samples. To assess statistical significance, we implemented a modified permutation test that empirically estimated a null distribution for each meta-analytic brain map. We thus tested the hypothesis that each map’s true effect sizes were not the result of a random spatial association among studies within a prosocial category. We applied a threshold of P < 0.005 as recommended by Radua et al. (2012) to optimally balance specificity and sensitivity while yielding results approximately equivalent to P < 0.05 corrected for multiple comparisons. Reported z-scores are specified as SDM-Z, as they do not follow a standard normal distribution. We also conducted three pairwise comparisons of activation maps across each of the three prosocial categories, which followed the same procedures (Supplementary Table S1).
The effect size maps were imported into Analysis of Functional NeuroImages (AFNI) (Cox, 1996), and a conjunction analysis was conducted to examine the overlap of consistently activated regions across altruism, cooperation and equity. Conjunction was determined using 3dcalc by overlaying the thresholded meta-analytic maps for each category to determine activation overlay.
Results
Clustering tasks into categories of prosocial decision-making
The Louvain clustering algorithm revealed three clusters of prosocial decision-making tasks (Figure 2). Upon inspection, we labeled these clusters based on the task features (or lack of features) shared among decisions within each cluster. Decisions in the first cluster (red; N = 19; 8 maps, 11 coordinate tables) involved multiple agents acting prosocially to maximize resources. These decisions were characterized by features like outcomes depended on the decisions of others and decisions in conditions of uncertainty. Tasks included prisoner’s dilemmas, public goods games, ultimatum games played by proposers, trust games and non-economic cooperative tasks. We labeled this cluster as cooperative decisions. A second cluster of decisions (yellow; N = 10; 7 maps, three coordinate tables) were those in which unilateral decisions to apportion resources equally were possible. These decisions were characterized by features like adherence to social norms such as producing equitable outcomes for the agent and beneficiary and unilateral decisions made by a single agent (and that thus resulted in no uncertainty). Tasks included dictator games in which 50–50 splits were possible. We labeled this cluster as equitable decisions. Finally, a third cluster of decisions (N = 14; 10 maps, four coordinate tables) was those in which agents made unilateral decisions to forgo resources for others. These decisions were characterized by losses without receiving anything in return and decisions that were made in response to the need or distress of the beneficiaries. Tasks included charitable donation tasks, dictator games in which 50–50 splits were not possible, your pain, my gain tasks and assistance tasks. We labeled this cluster as altruistic decisions. We will refer to each cluster using these terms, with the recognition that alternate descriptions for each cluster could also be appropriate. For example, the cluster of tasks we labeled as ‘cooperative’ could alternately be labeled as ‘strategic’ (Böckler et al., 2016). Crucially, sensitivity analyses (iteratively leaving one study out prior to generating the network and applying community detection) revealed that 100% of iterations yielded a three-cluster solution. The stability of these clusters was also high with 97.7% of nodes in the study network remaining in the same cluster; only one study (van den Bos et al., 2009) out of the forty-three studies ever switched from the cluster labeled as ‘cooperative’ to the cluster labeled as ‘equity’.
Meta-analyses
Neural correlates of cooperative decisions.
In the cooperative decision cluster, prosocial decisions (in contrast to selfish decisions) were associated with increased activation in right inferior frontal gyrus, bilateral subgenual ACC, left ventral striatum (including caudate nucleus), bilateral insula, bilateral MCC, left supramarginal gyrus extending to superior temporal gyrus (STG), left lateral postcentral gyrus, bilateral ventral tegmental area (VTA), left thalamus, left precuneus, right cerebellum lobule VIII and bilateral occipital cortex. We did not find significantly increased activation in any region during selfish vs cooperative decision-making (Figure 3, Table 2).
Fig. 3.
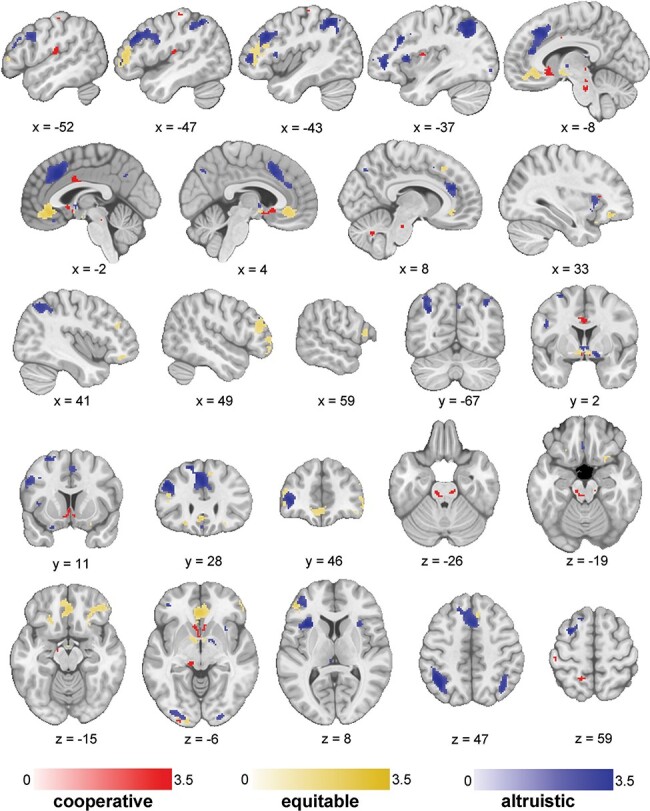
Thresholded results from meta-analyses.
Note. Results from each of three mixed-effects models displaying the main effects of decision-making category (cooperative: red; equitable: yellow; altruistic: blue). SDM-Z maps are corrected using a threshold of P < 0.005 and k > 10.
Table 2.
Results from the meta-analyses
| Cooperative > selfish decisions (increased activity) | |||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann Area | SDM-Z | P | Voxels | MNI-x | MNI-y | MNI-z |
| Inferior frontal gyrus, triangular part extending to insula (R) | 48 | 3.824 | 0.000581 | 11 | 34 | 24 | 10 |
| Ventral striatum extending to caudate and subgenual cingulate (L) | 4.682 | 0.000021 | 89 | −6 | 16 | −2 | |
| Subgenual anterior cingulate cortex extending to olfactory cortex (R) | 25 | 3.650 | 0.001053 | 28 | 4 | 12 | −10 |
| MCC extending to paracingulate gyri (L, R) | 24 | 3.853 | 0.000523 | 45 | −2 | 4 | 32 |
| Insula (L) | 48 | 3.497 | 0.001740 | 14 | −38 | −4 | 12 |
| Hippocampus (L) | 3.408 | 0.002305 | 11 | −16 | −12 | −12 | |
| VTA (L) | 4.102 | 0.000212 | 51 | −10 | −24 | −28 | |
| VTA (R) | 30 | 3.814 | 0.000600 | 30 | 12 | −24 | −22 |
| Postcentral gyrus, lateral part (L) | 3 | 3.861 | 0.000509 | 14 | −50 | −24 | 60 |
| Supramarginal gyrus extending to STG (L) | 48 | 3.888 | 0.000464 | 95 | −60 | −26 | 22 |
| Thalamus (L) | 50 | 3.931 | 0.000400 | 42 | −14 | −28 | −6 |
| White matter (middle cerebellar peduncles) | 3.614 | 0.001188 | 13 | 14 | −30 | −34 | |
| Precuneus (L) | 3.744 | 0.000764 | 14 | −14 | −50 | 58 | |
| Cerebellum lobule VIII (R) | 3.568 | 0.001378 | 15 | 8 | −62 | −34 | |
| Middle occipital gyrus (L) | 19 | 4.292 | 0.000103 | 49 | −22 | −88 | 18 |
| Middle occipital gyrus (L, R) | 18 | 3.736 | 0.000785 | 11 | −28 | −98 | −6 |
| Equitable > selfish decisions (increased activity) | |||||||
| Inferior frontal gyrus, triangular part (L) | 45 | 2.366 | 0.000113 | 305 | −48 | 42 | 8 |
| Middle frontal gyrus (R) | 45 | 2.093 | 0.000468 | 205 | 48 | 40 | 20 |
| Middle frontal gyrus extending to superior orbital gyrus (R) | 10, 11 | 2.391 | 0.000098 | 234 | 30 | 38 | −12 |
| Anterior cingulate cortex extending to paracingulate gyri (L, R) | 11 | 2.731 | 0.000014 | 488 | −2 | 32 | −10 |
| Inferior frontal gyrus, orbital part (L) | 11 | 2.302 | 0.000158 | 24 | −24 | 26 | −16 |
| Superior frontal gyrus, medial part (R) | 8 | 1.957 | 0.000904 | 18 | 8 | 26 | 48 |
| Inferior frontal gyrus, opercular part (R) | 44 | 1.996 | 0.000748 | 36 | 60 | 16 | 14 |
| Striatum (L) | 1.994 | 0.000752 | 68 | −6 | −6 | −8 | |
| Middle occipital gyrus (L) | 18 | 2.390 | 0.000099 | 113 | −18 | −98 | −2 |
| Equitable < selfish decisions (decreased activity) | |||||||
| Middle frontal gyrus (L) | 46 | −2.534 | 0.000820 | 125 | −22 | 42 | 26 |
| Rolandic operculum (L) | −2.341 | 0.001943 | 36 | −52 | 0 | 16 | |
| Inferior temporal gyrus (L) | 20 | −2.548 | 0.000771 | 15 | −48 | −2 | −32 |
| Precentral gyrus (R) | 6 | −2.533 | 0.000827 | 32 | 48 | −4 | 40 |
| SMA (L) | 6 | −2.831 | 0.000197 | 252 | −6 | −4 | 68 |
| Precentral gyrus (L) | 6 | −2.546 | 0.000778 | 27 | −50 | −8 | 52 |
| Precentral gyrus (L) | 6 | −2.518 | 0.000883 | 17 | −38 | −12 | 40 |
| Caudate nucleus (R) | −3.431 | 0.000007 | 91 | 18 | −16 | 24 | |
| Precentral gyrus (L) | 6 | −2.355 | 0.001822 | 34 | −30 | −18 | 60 |
| Inferior temporal gyrus (L) | 20 | −2.449 | 0.001206 | 12 | −44 | −18 | −24 |
| Thalamus extending to caudate (L) | −3.007 | 0.000079 | 288 | −14 | −20 | 20 | |
| Posterior insula (R) | −3.078 | 0.000054 | 27 | 32 | −28 | 18 | |
| Supramarginal gyrus (R) | 48 | −2.651 | 0.000474 | 70 | 54 | −36 | 28 |
| PCC, ventral portion (R) | 30 | −2.347 | 0.001894 | 10 | 14 | −38 | 6 |
| Posterior STS (R) | 39 | −3.021 | 0.000073 | 65 | 48 | −40 | −2 |
| Supramarginal gyrus (L) | 48 | −2.581 | 0.000663 | 84 | −50 | −40 | 28 |
| Inferior temporal gyrus (L) | 20 | −3.117 | 0.000043 | 105 | −42 | −44 | −10 |
| Posterior STS (R) | 39 | −2.428 | 0.001329 | 11 | 44 | −48 | 12 |
| Postcentral gyrus (L) | 5 | −2.937 | 0.000113 | 182 | −20 | −52 | 64 |
| Posterior STS (L) | 39 | −2.592 | 0.000627 | 27 | −40 | −60 | 12 |
| Extrastriate cortex extending to parahippocampal gyrus (R) | 36 | −3.239 | 0.000021 | 211 | 30 | −62 | 6 |
| Primary visual cortex (L) | 17 | −2.488 | 0.00101 | 57 | −24 | −68 | 8 |
| Altruistic > selfish decisions (increased activity) | |||||||
| Middle orbital gyrus (L) | 3.397 | 0.000130 | 200 | −38 | 44 | 0 | |
| Middle frontal gyrus (R) | 45 | 2.827 | 0.001091 | 31 | 46 | 40 | 28 |
| Anterior cingulate cortex extending to paracingulate gyri and pre-SMA (L, R) | 32 | 3.871 | 0.000021 | 1278 | 10 | 36 | 22 |
| Gyrus rectus, ventromedial (L, R) | 11 | 2.564 | 0.002750 | 10 | −2 | 30 | −18 |
| Middle frontal gyrus (L) | 44 | 3.761 | 0.000031 | 568 | −40 | 24 | 32 |
| Anterior insula (L) | 48 | 3.961 | 0.000014 | 203 | −34 | 20 | 8 |
| Anterior insula (R) | 48 | 2.923 | 0.000773 | 64 | 30 | 18 | 6 |
| Inferior frontal gyrus, orbital part (L) | 38 | 2.982 | 0.000624 | 12 | −24 | 12 | −24 |
| Ventral striatum (R) | 3.150 | 0.000331 | 50 | 14 | 2 | −8 | |
| Thalamus (L) | 2.814 | 0.001146 | 40 | −6 | −6 | 0 | |
| Thalamus (L) | 2.803 | 0.001191 | 23 | −14 | −30 | 16 | |
| Inferior parietal (excluding supramarginal and angular) gyri (L) | 40 | 3.917 | 0.000017 | 705 | −40 | −54 | 50 |
| Inferior parietal (excluding supramarginal and angular) gyri (R) | 40 | 3.105 | 0.000394 | 219 | 42 | −56 | 52 |
| Precuneus (L) | 7 | 2.830 | 0.001082 | 13 | −4 | −60 | 38 |
| Precuneus (R) | 7 | 2.960 | 0.000673 | 26 | 6 | −70 | 44 |
| Middle occipital gyrus (L) | 18 | 2.761 | 0.001381 | 194 | −22 | −96 | 0 |
| Inferior occipital gyrus (R) | 18 | 2.594 | 0.002483 | 65 | 24 | −96 | −6 |
| Altruistic < selfish decisions (decreased activity) | |||||||
| Superior frontal gyrus, dorsolateral (R) | 9 | −2.602 | 0.002379 | 13 | 18 | 50 | 30 |
| Inferior frontal gyrus, pars triangularis part (R) | 48 | −3.033 | 0.000539 | 104 | 48 | 34 | 6 |
| Rolandic operculum (L) | 6, 48 | −3.372 | 0.000147 | 38 | −56 | 0 | 8 |
| Posterior insula (L) | 84 | −2.627 | 0.002198 | 15 | −42 | 0 | 8 |
| Anterior STS (R) | −3.524 | 0.000080 | 69 | 48 | −2 | −14 | |
| Putamen (lenticular nucleus) (R) | 48 | −2.541 | 0.002913 | 10 | 30 | −8 | 2 |
| Posterior insula (R) | 48 | −3.902 | 0.000016 | 780 | 42 | −10 | 2 |
| Superior frontal gyrus, dorsolateral (R) | 6 | −3.119 | 0.000394 | 13 | 18 | −10 | 72 |
| Posterior insula (L) | 48 | −4.006 | 0.000010 | 70 | −38 | −14 | 6 |
| Inferior temporal gyrus (L) | 20 | −3.238 | 0.000252 | 12 | −42 | −14 | −28 |
| MTG (R) | 21 | −4.377 | 0.000002 | 232 | 58 | −24 | −8 |
| Inferior temporal gyrus extending to parahippocampal gyrus (L) | 20 | −3.175 | 0.000317 | 32 | −42 | −26 | −18 |
| Postcentral gyrus, medial (R) | 3 | −2.787 | 0.001283 | 15 | 32 | −34 | 50 |
| Postcentral gyrus, medial (L) | 3 | −3.018 | 0.000567 | 18 | −36 | −38 | 56 |
| Fusiform gyrus (L) | 37 | −3.181 | 0.000311 | 88 | −22 | −44 | −18 |
| MTG (R) | −3.156 | 0.000342 | 195 | 48 | −48 | 0 | |
| Superior parietal gyrus (R) | 5 | −3.479 | 0.000096 | 195 | 14 | −50 | 70 |
| MTG (L) | 37 | −4.052 | 0.000008 | 764 | −44 | −68 | 6 |
| MTG (R) | 37 | −3.774 | 0.000029 | 136 | 42 | −70 | 14 |
| Cerebellum, crus I extending to crus II (L) | −2.648 | 0.002050 | 11 | −22 | −82 | −30 | |
Neural correlates of equitable decisions.
In the equitable decision cluster, prosocial decisions (in contrast to selfish decisions) were associated with increased activation in bilateral orbital frontal cortex (OFC), bilateral ventrolateral PFC, bilateral dorsolateral PFC, bilateral medial PFC including rostral ACC, bilateral ventral striatum and caudate and left occipital cortex. Activation was increased during selfish relative to equitable decisions in left dorsolateral PFC, medial portion of left precentral gyrus, lateral portion of bilateral precentral gyrus, left thalamus, bilateral supramarginal gyrus, left inferior temporal gyrus, bilateral posterior superior temporal sulcus (STS) and bilateral occipital cortex (Figure 3, Table 2).
Neural correlates of altruistic decisions.
In the altruistic decision cluster, prosocial decisions (in contrast to selfish decisions) were associated with increased activation in several regions including left ventromedial PFC, bilateral ACC and paracingulate gyrus, bilateral pre-SMA, bilateral anterior insula, right ventrolateral PFC, left bilateral dorsolateral PFC, thalamus, right ventral striatum, right precuneus and bilateral interior parietal gyrus.
We also found increased activation during selfish decision-making, in contrast to altruistic decision-making, in several regions including right dorsolateral PFC, right ventrolateral PFC right putamen, bilateral posterior insula, bilateral precentral gyrus, right middle temporal gyrus (MTG), STG, right STS left parahippocampal gyrus, right superior parietal gyrus, bilateral middle occipital gyrus and left cerebellum crus I and II (Figure 3, Table 2).
Pairwise meta-analytic map comparisons
Pairwise comparisons confirmed that the meta-analytic maps derived from each cluster of studies were distinct from one another. When comparing activation for cooperative relative to equitable decisions, we found increased activation in regions that included left ventrolateral PFC, left SMA, right caudate, left hippocampus, bilateral thalamus, left VTA, left supramarginal gyrus and left superior parietal gyrus (Supplementary Figure S2A). We did not find increased activity in any region for equitable relative to cooperative decisions. When comparing activation for equitable relative to altruistic decisions, however, we found increased activation for equitable relative to altruistic decisions in regions that included right ventrolateral PFC, bilateral posterior insula and right STG (Supplementary Figure S2B).
When comparing activation for altruistic relative to equitable decisions, we found increased activation in regions that included dorsolateral PFC, bilateral pre-SMA, left anterior insula, bilateral caudate, left thalamus and left hippocampus (Supplementary Figure S2C). Finally, when comparing activation for altruistic relative to cooperative decisions, we found increased activation in regions that included left dorsolateral PFC, left SMA, left MTG and left angular gyrus (Supplementary Figure S2D). We did not find increased activity in any region for cooperative relative to altruistic decisions. Supplementary Table S1 for results.
Conjunction across meta-analytic maps
Conjunction analyses identified overlapping regions of activation for altruism ∩ equity and cooperation ∩ equity. Overlapping activation for altruism ∩ equity was observed in bilateral dorsolateral PFC (BA9/46; left: [−44, 34, 20], k = 19; right: [46, 40, 24], k = 24), left ventrolateral PFC (BA10; [−42, 44, −4], k = 32) and left visual cortex (BA18, [−20, −96, −8], k = 34). Overlapping activity for equity ∩ cooperation was observed in bilateral ventral striatum (left: [−2, 4, −8]; right: [4, 4, −8]; k = 4). We did not find overlapping activation for cooperation ∩ altruism nor did we find overlapping activation across all three clusters of studies (cooperation ∩ equity ∩ altruism).
Discussion
Using data from 43 unique fMRI studies that included 25 statistical maps and 18 coordinate tables across 1423 subjects, we identified 13 features that distinguish prosocial decisions tasks. We used these features to generate a feature-based representation of prosocial decision tasks that classified prosocial decisions into three sub-clusters that we subsequently labeled as cooperation, equity and altruism. The feature-based structure we generated identified conceptually and motivationally coherent categories of prosocial decisions. This conclusion is supported by the results of our fMRI meta-analysis, which found evidence suggesting that each category of decision recruits diverging neural systems. The first cluster of decisions (which we labeled as cooperative) primarily recruited regions such as dorsal and ventral striatum, VTA and subgenual ACC. A second cluster of decisions (which we labeled as equitable) recruited neural regions such as ventral striatum, dorsolateral PFC and ventromedial PFC. A third cluster of decisions (which we labeled as altruistic) recruited neural regions such as ventral striatum, dorsolateral PFC, ventromedial PFC, pre-SMA, dorsal ACC and anterior insula.
Our approach demonstrates that the dozens of tasks that have been used to assess the neural correlates of prosocial decisions generally cluster together according to specific shared features. These tasks are adaptations of those used in studies of prosocial decision-making outside the scanner and more broadly. We identified key features that distinguish prosocial decisions, including features related to the identity of the beneficiary, the nature of the interaction between agents and beneficiaries and various outcomes associated with the decision. Then, using an unsupervised graph-based approach, we identified three clusters of tasks that tend to share common core features. For example, cooperative decisions included outcomes that depended on the decisions of others (e.g. ‘beneficiaries make choices that affect the outcome’) and decisions in conditions of uncertainty. Features shared by equitable decisions included adherence to social norms such as producing equal outcomes and unilateral decisions made by a single agent and that thus resulted in no uncertainty. Features shared by altruistic decisions included outcomes that did not produce any benefit to the deciding agent and unilateral decisions that were made in response to the need or distress of the beneficiaries. Of note, the prosocial decision-making tasks included in this meta-analysis were described by the original authors using at least 10 different terms that did not consistently correspond to the features of the tasks being used—including cooperation, collaboration, reciprocity, trust, equity, fairness, prosocial behavior, interpersonal behavior, charitable behavior and altruism—reinforcing the value of a clearer and more consistent prosocial decision-making task space.
Supporting the identified task structure, the clusters yielded by our approach map closely onto the results of a previous behavioral characterization of prosocial paradigms that applied factor analysis to the behavioral outcomes of these paradigms (Böckler et al., 2016). These outcomes included the percentage of prosocial decisions during tasks, the ratio of other-regarding vs self-regarding decisions, average monetary donations or summary scores of self-reported measures. Using a similar bottom-up, but otherwise completely distinct approach across 329 participants who completed multiple tasks-assessing prosocial behavior, the authors identified clusters of prosocial tasks that correspond to those we identified: altruistically motivated prosocial behavior (corresponding to the cluster labeled as altruistic decisions), norm-motivated prosocial behavior (corresponding to the cluster labeled as equitable decisions) and strategically motivated prosocial behavior (corresponding to the cluster labeled as cooperative decisions). They also identified self-reported prosocial behavior (a category not included in our meta-analysis) as comprising a fourth distinct cluster.
Our findings also extend this work by showing that tasks cluster similarly even when completed by different participants across tasks. This suggests task features are crucial in determining the category of a prosocial decision and has implications for comparing results across different tasks, such as in previous meta-analyses. In addition, our findings suggest that even within a type of task, specific features may determine the category of prosocial decision at hand. For example, dictator games that offered an option to split available resources equally (50–50) clustered with equitable decisions, whereas dictator games with the option to make other prosocial splits clustered with altruistic decisions. The existence of strong norms related to equity may explain why 50–50 is the most common non-selfish split across dictator games when this choice is available (Engel, 2011). Future analyses of dictator game tasks, particularly those conducted using fMRI, could benefit from considering that there may be something unique about the decision to split resources equally (50%) rather than it simply existing as an option on a parametric continuum between 49 and 51%.
Our approach also yielded several important observations about the neural substrates of prosocial decisions. Notably, all three clusters of prosocial decisions recruited the striatum, but each category of decisions elicited activation in different regions within the striatum. We found that cooperation recruited the left caudate and bilateral ventral striatum, equity recruited right caudate and bilateral ventral striatum and altruism recruited right ventral striatum. Previous work suggests these differences may be explained by how tasks in the three clusters vary in value and uncertainty. Activity in the striatum has been consistently found to encode action value during learning and decision-making (Daw and Doya, 2006; Guitart-Masip et al., 2014). In some tasks (primarily cooperative and equitable decisions), prosocial decisions increased the agent’s own welfare. In other tasks (primarily altruistic and equitable decisions), prosocial decisions meant forgoing resources. Importantly, in economic games involving simultaneous decisions by multiple agents, cooperative decisions are usually made with some uncertainty about the ultimate outcome. It is possible that the striatal activity observed during cooperative decisions also reflects the uncertainty of decisions, which is recruited during decision-making under risky or uncertain conditions (Krain et al., 2006; Lopez-Paniagua and Seger, 2013; Farrar et al., 2018). The only overlap of striatal activation we identified occurred in a small volume of four voxels during both cooperative and equitable decisions. This conjunction may reflect the fact that cooperative and equitable decisions benefit both agents and beneficiaries, which may be recapitulated in ventral striatal activity.
This suggests the possibility of an additive effect of striatal activity—an interpretation consistent with observations of a parametric effect of striatal activation and reward magnitude for self (Miller et al., 2014). Ventral striatum is also preferentially engaged in response to rewarding social stimuli relative to rewarding nonsocial stimuli, for instance, when participants cooperate with a human partner relative to a computer partner despite identical monetary gains (Rilling et al., 2002, 2004). This also might explain why we did not observe any consistent regions that were more active during selfish decisions than cooperative decisions. Cooperative decisions, which yield outcomes benefiting both the agent and other beneficiaries, are also potentially more rewarding than decisions that only yield self-rewarding outcomes. Cooperative decisions also uniquely recruited activity in bilateral VTA, which projects dopamine to the ventral striatum in response to positive prediction errors and reward cues (D’Ardenne et al., 2008), and activity in which likely reflects the anticipation of both the self- and social-rewards gained from cooperating with others.
Striatal activation to anticipatory reward cues occurs within a larger subjective valuation system, which consistently involves activity in the medial PFC during reward-based decision-making (Bartra et al., 2013) and prosocial decision-making (Cutler and Campbell-Meiklejohn, 2019; Bellucci et al., 2020). As has been previously found (Cutler and Campbell-Meiklejohn, 2019), we observed activation in more anterior portions of the ventromedial PFC (including the rostral ACC) during equitable decisions that produce self-enhancing, norm-based outcomes and activation in more posterior portions during altruistic decisions. These results are consistent with a hypothesized spatial gradient of activation along the medial PFC during prosocial decision-making (Sul et al., 2015), which may integrate information about self and others to encode an overall value during a prosocial decision (Hutcherson et al., 2015).
Activation in both ventral striatum and medial PFC did not overlap between altruistic decisions and cooperative decisions. In contrast to altruistic decisions, which recruited the lingual gyrus portion of left ventromedial PFC, we found activation in bilateral subgenual ACC during tasks that require agents to cooperate with others to achieve a common goal. This suggests that altruistic and cooperative decision represent distinct processes, despite frequent conflation these two categories of decisions in the literature, for example, when altruistic behavior (choosing to benefit others without any self-gain) is labeled as ‘cooperation’ (Declerck et al., 2013; Balliet et al., 2014; Gintis, 2014; Peysakhovich et al., 2014; Yang et al., 2019). Because the subgenual ACC supports prosocial learning computations (Christopoulos and King-Casas, 2014; Lockwood et al., 2016) as well as preferences for socially rewarding outcomes (Smith et al., 2010), it may also play a role in updating expectations of others’ actions or the value of others’ outcomes during iterative cooperative decision-making.
In addition to a subjective-valuation sub-system, altruistic decisions seemed to recruit two other distinct sub-systems underlying goal-directed behavior and empathy. The goal-directed sub-system included regions typically implicated in controlling action and directing goal-directed behaviors, including the lateral PFC (Hoshi and Tanji, 2004; Kaller et al., 2011; Morris et al., 2014). Importantly, activation for equitable and altruistic decision-making overlapped in dorsolateral PFC, involved in modulating subjective value representations (Carlson and Crockett, 2018; Tusche and Hutcherson, 2018) and in making norm-related decisions (Knoch et al., 2006; Baumgartner et al., 2011). Thus, it may play an important role in guiding prosocial action in accordance with abstract, social rules during social decision-making tasks (Bellucci et al., 2020).
The second sub-system comprised regions commonly involved in representing and empathizing with the distress of others, and included the dorsal ACC, pre-SMA and anterior insula (Decety and Lamm, 2006; Lamm et al., 2011; Ashar et al., 2017). Activation in these regions emerged only during altruistic decisions, consistent with the theory that affective resonance with others’ distress give rise to empathic concern and altruistic motivation, which is a primary motivator of prosocial behavior in the absence of cooperative or equity-maintaining goals (Batson, 2009, 2011; Decety et al., 2016; Brethel-Haurwitz et al., 2018; O’Connell et al., 2019). This finding was likely driven by eight out of the fourteen identified altruistic studies including stimuli that depicted or implied the need or distress of beneficiaries. We also found activation in the precuneus—a key node of the mentalizing system (Koster-Hale et al., 2017)—during altruistic decisions. This region has also been found to be active in response to observing emotional suffering (Immordino-Yang et al., 2009; Masten et al., 2011; Meyer et al., 2013). Although some hypothesize the right TPJ—another core region of the mentalizing system—to be recruited during prosocial decision-making (Chakroff and Young, 2014; Parnamets et al., 2020), we did not find differential activation in this region across prosocial relative to selfish decisions. It is possible that we did not observe differences in mean activation because both selfish and prosocial decisions require the maintenance of others’ beliefs and intentions, whereas studies finding TPJ activation usually contrast decisions pertaining to other people with hypothetical decisions pertaining to imaginary people (or computers) (FeldmanHall et al., 2012) (for further evidence regarding the role of TPJ during domain-general social vs self-specific decision-making, also see: Lockwood et al., 2018, 2020).
Limitations and future directions
These results should be considered in light of some limitations. We could not obtain complete data from a number of potentially relevant studies. At least 69 studies would have been eligible for the analysis if we had been able to retrieve the necessary data. In addition, while the 13 features we identified captured the distinctions across the tasks included in our analysis, they may not be representative of all features that could potentially overlap across tasks. With access to more data, future work could test how clustering algorithms such as the one we employed can generalize to unseen tasks with different combinations of features or even generate new tasks with unique sets of task features. Another limitation of our approach is that we used a binary coding for our features (1 = present and 0 = absent), which did not make any prior assumptions about the features (e.g. no assumption that features were parametrically weighted). Therefore, we were unable to test how strongly each feature contributed to the three-cluster solution (since they were all weighted equally).
We identified how tasks were inter-related using a bottom-up feature-based approach (assuming equally important features), and how these inter-relationships give rise to overarching prosocial categories. This is in contrast to the more top-down approaches based on expert-models that have been used to map cognitive constructs like creativity (Kenett et al., 2020), cognitive control (Lenartowicz et al., 2010) or theory of mind (Schurz et al., 2020) onto tasks. Future work could combine these approaches to generate a finer grained task space for prosocial decision-making including all possible levels of its cognitive ontology: the categories identified in the present study, finer detailed sub-categories, task paradigms and their features weighted according to their relative importance and contrast estimates. In so doing, we could go further in solving discrepancies within the prosocial decision-making literature, such as delineating more specific categories of prosocial decision-making within the identified task-space, which may only reflect the top level of a prosocial decision-making hierarchy. Our data show evidence of such a prosocial decision hierarchy across clusters of studies (Supplementary Figure S1). However, future work, with an adequate number of studies on prosocial decisions, will be needed to demonstrate that such a hierarchy can be recapitulated using meta-analytic brain maps (see Schurz et al., 2020) (especially with increasing availability of other studies that prompt prosocial decisions). Other categories of decisions likely also exist within this hierarchy. For example, active decisions to forgive (Fourie et al., 2020), norm-enforcing decisions (i.e. social influence on agreements or valuation) (Chang and Sanfey, 2013; Wu et al., 2016; Zinchenko and Arsalidou, 2018; Yang et al., 2019) and third-party altruistic punishment decisions for norm violations (Fehr et al., 2004; Buckholtz et al., 2008; Jordan et al., 2016; David et al., 2017) were not considered in this study because we sought to only examine decisions that directly benefited another person but may reflect more specific prosocial decisions under the umbrella of the identified categories.
As with many neuroimaging tasks, prosocial decision-making tasks adapted for neuroimaging are tightly controlled and often designed to minimize variability and maximize the statistical power of detecting effects. However, they are not designed with high ecological validity and may not map onto the contexts of real-world prosocial decisions such as holding a door open, splitting a meal, volunteering or donating blood or an organ. Instead, they are primarily monetary in nature, repetitive and may increase behaviors related to social desirability in the laboratory (Richman et al., 1999). Recent work has focused on making neuroimaging paradigms more ‘naturalistic,’ such as viewing or listening to narratives or interacting in real time with another person in the laboratory (Hasson and Frith, 2016; Redcay and Moraczewski, 2019; Redcay and Schilbach, 2019; Wheatley et al., 2019) or in the real world (Dikker et al., 2021). Other work has concentrated on characterizing the behavioral and neural features of individuals who engage in extreme forms of real-world prosociality (Marsh et al., 2014; Brethel-Haurwitz et al., 2018; O’Connell et al., 2019; Vekaria et al., 2020). Understanding the neurobiology underlying more ecologically valid altruistic decisions will be crucial for understanding the broader picture of prosocial decision-making.
Related to this, we were not able to consider how individual differences in phenotypic traits may contribute to the neural activity patterns observed across prosocial decision-making tasks due to the limited number of studies that collect or report consistent data on participant characteristics, yet this remains an open question. Finally, we only considered univariate maps that contrasted prosocial vs selfish decisions (or assessed a parametric increase). Due to the high variability of contrasts across studies, this allowed us to generate consistent neuroimaging contrasts that only indexed activation during the decision phase across studies. This approach is distinct from other meta-analytic work compiling coordinate-based maps across any contrasts in prosocial tasks (Yang et al., 2019; Bellucci et al., 2020), which run the risk of creating dependence across experiment maps that negatively impacts the validity of meta-analytic results (Müller et al., 2018). Ideally, future work would incorporate neural activation maps derived from computational modeling of behavior (Tognoli et al., 2017; Charpentier and O’Doherty, 2018; Lockwood et al., 2020; Lockwood and Klein-Flügge, 2020; Suzuki and O’Doherty, 2020), which holds promise for understanding individual differences in social learning and decision-making (Patzelt et al., 2018). For example, computational modeling of decisions can identify latent subjective states (e.g. mood, anxiety), beliefs about others (e.g. trust, morality) or other subjective biases about agents that are not directly observable from behavior. Thus, mapping these latent parameters onto the task features that give rise to them and their neural representations will be a necessary next step in characterizing a cognitive ontology of prosociality.
Conclusion
Despite limitations, the present study provides a framework for understanding how prosocial decisions are inter-related and distinct and can be applied to a variety of experimental task paradigms. Using a bottom-up approach, we identified a feature-based representation of the task-space underlying prosocial decisions. Results revealed that three clusters of prosocial decisions identified this way—cooperative, equitable and altruistic decisions—recruit neural systems that diverge in ways that shed light on the key motivations and mechanisms that support each category of prosocial decision compared to selfish decisions. These findings clarify some of the existing heterogeneity in how prosociality is conceptualized and generate insight for future research in task paradigm development and the improvement of formal cognitive ontologies.
Supplementary Material
Acknowledgements
We would like to thank Peter E. Turkeltaub, Katherine O’Connell, Kathryn Berluti, Adam E. Green and Joscelin Rocha-Hidalgo for helpful suggestions, discussions and feedback regarding this project. We are also grateful to Daniel Campbell-Meiklejohn for support in collecting data, Lin Gan, Alexa King and Kathleen Neill for their help identifying the task features, and all of the researchers who contributed their time and data for our meta-analysis.
Footnotes
After conducting this initial search, we conducted an additional PubMed search using the criteria [(“fMRI” OR “neur*”) AND “equity”] and did not identify any studies published prior to 2019 that would have been eligible for the study and were not already included.
Contributor Information
Shawn A Rhoads, Department of Psychology, Georgetown University, Washington, DC 200057, USA.
Jo Cutler, Centre for Human Brain Health, University of Birmingham, Birmingham, B15 2TT, UK.
Abigail A Marsh, Department of Psychology, Georgetown University, Washington, DC 200057, USA.
Funding
This work was supported by the National Science Foundation Graduate Research Fellowship Program Award to S.A.R.
Conflicts of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
Data availability
All thresholded and unthresholded meta-analytic activation maps are openly available on the Open Science Framework at https://osf.io/3vu9w/.
Supplementary data
Supplementary data are available at SCAN online.
References
- Abe M.O., Koike T., Okazaki S., et al. (2019). Neural correlates of online cooperation during joint force production. NeuroImage, 191, 150–61. doi: 10.1016/j.neuroimage.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Aimone J.A., Houser D., Weber B. (2014). Neural signatures of betrayal aversion: an fMRI study of trust. Proceedings of the Royal Society B: Biological Sciences, 281(1782), 1–6. doi: 10.1098/rspb.2013.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aknin L.B., Dunn E.W., Norton M.I. (2012). Happiness runs in a circular motion: evidence for a positive feedback loop between prosocial spending and happiness. Journal of Happiness Studies, 13(2), 347–55. doi: 10.1007/s10902-011-9267-5 [DOI] [Google Scholar]
- Allegra M., Seyed-Allaei S., Schuck N.W., Amati D., Laio A., Reverberi C. (2020). Brain network dynamics during spontaneous strategy shifts and incremental task optimization. NeuroImage, 217, 116854. doi: 10.1016/j.neuroimage.2020.116854 [DOI] [PubMed] [Google Scholar]
- Ashar Y.K., Andrews-Hanna J.R., Dimidjian S., Wager T.D. (2017). Empathic care and distress: predictive brain markers and dissociable brain systems. Neuron, 94(6), 1263–73. e4. doi: 10.1016/j.neuron.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balliet D., Mulder L.B., Van Lange P.A.M. (2011). Reward, punishment, and cooperation: a meta-analysis. Psychological Bulletin, 37(4), 594–615. doi: 10.1037/a0023489 [DOI] [PubMed] [Google Scholar]
- Balliet D., Wu J., De Dreu C.K.W. (2014). Ingroup favoritism in cooperation: a meta-analysis. Psychological Bulletin, 140(6), 1556–81. doi: 10.1037/a0037737 [DOI] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–27. doi: 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson C.D. (2009). These things called empathy: eight related but distinct phenomena. In: Decety, J., Ickes, W., editors. The Social Neuroscience of Empathy, Cambridge, MA: MIT Press, 4–14. doi: 10.7551/mitpress/9780262012973.003.0002 [DOI] [Google Scholar]
- Batson C.D. (2011). [Google Scholar]
- Batson C.D., Powell A.A. (2003). Altruism and prosocial behavior. In: Weiner, I.B., editor. Handbook of Psychology. New York, NY: Wiley. doi: 10.1002/0471264385.wei0519 [DOI] [Google Scholar]
- Bault N., Pelloux B., Fahrenfort J.J., Ridderinkhof K.R., Van Winden F. (2014). Neural dynamics of social tie formation in economic decision-making. Social Cognitive and Affective Neuroscience, 10(6), 877–84. doi: 10.1093/scan/nsu138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T., Knoch D., Hotz P., Eisenegger C., Fehr E. (2011). Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nature Neuroscience, 14(11), 1468–74. doi: 10.1038/nn.2933 [DOI] [PubMed] [Google Scholar]
- Bellucci G., Chernyak S.V., Goodyear K., Eickhoff S.B., Krueger F. (2017). Neural signatures of trust in reciprocity: a coordinate-based meta-analysis. Human Brain Mapping, 38(3), 1233–48. doi: 10.1002/hbm.23451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci G., Camilleri J.A., Eickhoff S.B., Krueger F. (2020). Neural signatures of prosocial behaviors. Neuroscience and Biobehavioral Reviews, 118, 186–95. doi: 10.1016/j.neubiorev.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor J., Kramer R.M., Stout S. (1921). When in doubt: cooperation in a noisy prisoner’s dilemma. Journal of Conflict Resolution, 35(4), 691–719. [Google Scholar]
- Blondel V., Guillaume J., Lambiotte R., Lefebvre E. (2008). Fast unfolding of community hierarchies in large networks. Journal of Statistical Mechanics: Theory and Experiment, 2008(10), 1–12. doi: 10.1088/1742-5468/2008/10/P10008 [DOI] [Google Scholar]
- Böckler A., Tusche A., Singer T. (2016). The structure of human prosociality: differentiating altruistically motivated, norm motivated, strategically motivated, and self-reported prosocial behavior. Social Psychological and Personality Science, 7(6), 530–41. doi: 10.1177/1948550616639650 [DOI] [Google Scholar]
- Brethel-Haurwitz K.M., Cardinale E.M., Vekaria K.M., et al. (2018). Extraordinary altruists exhibit enhanced self–other overlap in neural responses to distress. Psychological Science, 29(10), 1–11. doi: 10.1177/0956797618779590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz J.W., Asplund C.L., Dux P.E., et al. (2008). The neural correlates of third-party punishment. Neuron, 60(5), 930–40. doi: 10.1016/j.neuron.2008.10.016 [DOI] [PubMed] [Google Scholar]
- Burkart J.M., Allon O., Amici F., et al. (2014). The evolutionary origin of human hyper-cooperation. Nature Communications, 5(1), 1–9. doi: 10.1038/ncomms5747 [DOI] [PubMed] [Google Scholar]
- Carlson R.W., Crockett M.J. (2018). The lateral prefrontal cortex and moral goal pursuit. Current Opinion in Psychology, 24, 77–82. doi: 10.1016/j.copsyc.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Chakroff A., Young L. (2014). The prosocial brain: perceiving others in need and acting on it. In: Padilla-Walker, L.M., Carlo, G., editors. Prosocial Development: A Multidimensional Approach, Oxford, UK: Oxford University Press, 90–111. [Google Scholar]
- Chang L.J., Sanfey A.G. (2013). Great expectations: neural computations underlying the use of social norms in decision-making. Social Cognitive and Affective Neuroscience, 8(3), 277–84. doi: 10.1093/scan/nsr094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier C.J., O’Doherty J.P. (2018). The application of computational models to social neuroscience: promises and pitfalls. Social Neuroscience, 13(6), 637–47. doi: 10.1080/17470919.2018.1518834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A. (2011). Sustaining cooperation in laboratory public goods experiments: a selective survey of the literature. Experimental Economics, 14(1), 47–83. doi: 10.1007/s10683-010-9257-1 [DOI] [Google Scholar]
- Chen X., Hackett P.D., DeMarco A.C., et al. (2016). Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging and Behavior, 10(2), 581–93. doi: 10.1007/s11682-015-9411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos G.I., King-Casas B. (2014). With you or against you: social orientation dependent learning signals guide actions made for others. NeuroImage, 104, 326–35. doi: 10.1016/j.neuroimage.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Curry O.S., Rowland L.A., Van Lissa C.J., Zlotowitz S., McAlaney J., Whitehouse H. (2018). Happy to help? A systematic review and meta-analysis of the effects of performing acts of kindness on the well-being of the actor. Journal of Experimental Social Psychology, 76, 320–9. doi: 10.1016/j.jesp.2018.02.014 [DOI] [Google Scholar]
- Cutler J., Campbell-Meiklejohn D. (2019). A comparative fMRI meta-analysis of altruistic and strategic decisions to give. NeuroImage, 184, 227–41. doi: 10.1016/j.neuroimage.2018.09.009 [DOI] [PubMed] [Google Scholar]
- D’Ardenne K., McClure S.M., Nystrom L.E., et al. (2008). BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science, 319(5867), 1264–7. doi: 10.1126/science.1150605 [DOI] [PubMed] [Google Scholar]
- David B., Hu Y., Krüger F., Weber B. (2017). Other-regarding attention focus modulates third-party altruistic choice: an fMRI study. Scientific Reports, 7, 1–12. doi: 10.1038/srep43024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N.D., Doya K. (2006). The computational neurobiology of learning and reward. Current Opinion in Neurobiology, 16(2), 199–204. doi: 10.1016/j.conb.2006.03.006 [DOI] [PubMed] [Google Scholar]
- de Waal F.B.M. (2008). Putting the altruism back into altruism: the evolution of empathy. Annual Review of Psychology, 59, 279–300. doi: 10.1146/annurev.psych.59.103006.093625 [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P.L., Sommerville J.A., Chaminade T., Meltzoff A.N. (2004). The neural bases of cooperation and competition: an fMRI investigation. NeuroImage, 23(2), 744–51. doi: 10.1016/j.neuroimage.2004.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Bartal I.B.A., Uzefovsky F., Knafo-Noam A. (2016). Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1686), 20150077. doi: 10.1098/rstb.2015.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Lamm C. (2006). Human empathy through the lens of social neuroscience. The Scientific World Journal, 6, 1146–63. doi: 10.1100/tsw.2006.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck C.H., Boone C., Emonds G. (2013). When do people cooperate? The neuroeconomics of prosocial decision making. Brain and Cognition, 81(1), 95–117. doi: 10.1016/j.bandc.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Frank R.H., Phelps E.A. (2005). Perceptions of moral character modulate the neural systems of reward during the trust game. Nature Neuroscience, 8(11), 1611–8. doi: 10.1038/nn1575 [DOI] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Dice L.R. (1945). Measures of the amount of ecologic association between species. Ecology, 26(3), 297–302. doi: 10.2307/1932409 [DOI] [Google Scholar]
- Dikker S., Michalareas G., Oostrik M., et al. (2021). Crowdsourcing neuroscience: inter-brain coupling during face-to-face interactions outside the laboratory. NeuroImage, 227, 1–49. [DOI] [PubMed] [Google Scholar]
- Dunn E.W., Aknin L.B., Norton M.I. (2008). Spending money on others promotes happiness. Science, 319(5870), 1687–8. doi: 10.1126/science.1150952 [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–26. doi: 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel C. (2011). Dictator games: a meta study. Experimental Economics, 14(4), 583–610. doi: 10.1007/s10683-011-9283-7 [DOI] [Google Scholar]
- Fallon N., Roberts C., Stancak A. (2020). Shared and distinct functional networks for empathy and pain processing: a systematic review and meta-analysis of fMRI studies. Social Cognitive and Affective Neuroscience, 15(7), 709–23. doi: 10.1093/scan/nsaa090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Chang L.J., Delgado M.R. (2012). Effects of direct social experience on trust decisions and neural reward circuitry. Frontiers in Neuroscience, 6, 1–17. doi: 10.3389/fnins.2012.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Chang L.J., Delgado M.R. (2015). Computational substrates of social value in interpersonal collaboration. Journal of Neuroscience, 35(21), 8170–80. doi: 10.1523/jneurosci.4775-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar D.C., Mian A.Z., Budson A.E., Moss M.B., Killiany R.J. (2018). Functional brain networks involved in decision-making under certain and uncertain conditions. Neuroradiology, 60(1), 61–9. doi: 10.1007/s00234-017-1949-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E., Fischbacher U., Gächter S. (2002). Strong reciprocity, human cooperation, and the enforcement of social norms. Human Nature, 13(1), 1–25. doi: 10.1007/s12110-002-1012-7 [DOI] [PubMed] [Google Scholar]
- Fehr E., Rockenbach B., Borst A., Schultz W. (2004). Human altruism: economic, neural, and evolutionary perspectives. Current Opinion in Neurobiology, 14(6), 784–90. doi: 10.1016/j.conb.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Fehr E., Camerer C.F. (2007). Social neuroeconomics: the neural circuitry of social preferences. Trends in Cognitive Sciences, 11(10), 419–27. doi: 10.1016/j.tics.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Fehr E., Fischbacher U. (2003). The nature of human altruism. Nature, 425(6960), 785–91. doi: 10.1038/nature02043 [DOI] [PubMed] [Google Scholar]
- Fehr E., Schmidt K.M. (1999). A theory of fairness, competition, and cooperation. The Quarterly Journal of Economics, 114(3), 817–68. [Google Scholar]
- Fehr E., Schurtenberger I. (2018). Normative foundations of human cooperation. Nature Human Behaviour, 2(7), 458–68. doi: 10.1038/s41562-018-0385-5 [DOI] [PubMed] [Google Scholar]
- FeldmanHall O., Dalgleish T., Thompson R., Evans D., Schweizer S., Mobbs D. (2012). Differential neural circuitry and self-interest in real vs hypothetical moral decisions. Social Cognitive and Affective Neuroscience, 7(7), 743–51. doi: 10.1093/scan/nss069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FeldmanHall O., Dalgleish T., Evans D., Mobbs D. (2015). Empathic concern drives costly altruism. NeuroImage, 105, 347–56. doi: 10.1016/j.neuroimage.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FeldmanHall O. (2017). How does social network position influence prosocial behavior? Trends in Cognitive Sciences, 21(8), 572–3. doi: 10.1016/j.tics.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Fermin A.S.R., Sakagami M., Kiyonari T., Li Y., Matsumoto Y., Yamagishi T. (2016). Representation of economic preferences in the structure and function of the amygdala and prefrontal cortex. Scientific Reports, 6, 1–11. doi: 10.1038/srep20982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouragnan E., Chierchia G., Greiner S., Neveu R., Avesani P., Coricelli G. (2013). Reputational priors magnify striatal responses to violations of trust. Journal of Neuroscience, 33(8), 3602–11. doi: 10.1523/jneurosci.3086-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie M.M., Hortensius R., Decety J. (2020). Parsing the components of forgiveness: psychological and neural mechanisms. Neuroscience and Biobehavioral Reviews, 112, 437–51. doi: 10.1016/j.neubiorev.2020.02.020 [DOI] [PubMed] [Google Scholar]
- Fowler J.H., Christakis N.A. (2010). Cooperative behavior cascades in human social networks. Proceedings of the National Academy of Sciences, 107(12), 5334–8. doi: 10.1073/pnas.0913149107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarini F., Boero R., D’Agata F., et al. (2014). Neural correlates of gender differences in reputation building. PLoS One, 9(9), e106285. doi: 10.1371/journal.pone.0106285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevsky A., Vastfjall D., Slovic P., Knutson B. (2013). Neural underpinnings of the identifiable victim effect: affect shifts preferences for giving. Journal of Neuroscience, 33(43), 17188–96. doi: 10.1523/JNEUROSCI.2348-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintis H. (2014). The Bounds of Reason: Game Theory and the Unification of the Behavioral Sciences. Princeton, NJ: Princeton University Press. doi: 10.1080/10848770.2013.838043 [DOI] [Google Scholar]
- Gonzalez-Castillo J., Caballero-Gaudes C., Topolski N., Handwerker D., Pereira F., Bandettini P. (2019). Imaging the spontaneous flow of thought: distinct periods of cognition contribute to dynamic functional connectivity during rest. NeuroImage, 202(116129), 1–14. doi: 10.1101/527804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening S., Norton L., Virani K., Ty A., Mitchell D., Finger E. (2014). Individual differences in the anterior insula are associated with the likelihood of financially helping versus harming others. Cognitive, Affective and Behavioral Neuroscience, 14(1), 266–77. doi: 10.3758/s13415-013-0213-3 [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M., Duzel E., Dolan R., Dayan P. (2014). Action versus valence in decision making. Trends in Cognitive Sciences, 18(4), 194–202. doi: 10.1016/j.tics.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroglu B., Will G.J., Crone E.A. (2014). Neural correlates of advantageous and disadvantageous inequity in sharing decisions. PLoS One, 9(9), 12–4. doi: 10.1371/journal.pone.0107996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth W., Schmittberger R., Schwarze B. (1982). An experimental analysis of ultimatum bargaining. Journal of Economic Behavior and Organization, 3(4), 367–88. doi: 10.1016/0167-2681(82)90011-7 [DOI] [Google Scholar]
- Hackel L.M., Zaki J., Van Bavel J.J. (2017). Social identity shapes social valuation: evidence from prosocial behavior and vicarious reward. Social Cognitive and Affective Neuroscience, 12(8), 1219–28. doi: 10.1093/scan/nsx045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B. (2017). Survival of the friendliest: homo sapiens evolved via selection for prosociality. Annual Review of Psychology, 68(1), 155–86. doi: 10.1146/annurev-psych-010416-044201 [DOI] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Knoepfle D.T., O’Doherty J.P., Rangel A. (2010). Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. Journal of Neuroscience, 30(2), 583–90. doi: 10.1523/JNEUROSCI.4089-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Frith C.D. (2016). Mirroring and beyond: coupled dynamics as a generalized framework for modelling social interactions. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1693), 20150366. doi: 10.1098/rstb.2015.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J., Boyd R., Bowles S., et al. (2005). “Economic man” in cross-cultural perspective: ethnography and experiments from 15 small-scale societies. Behavioral and Brain Sciences, 28(6), 795815. [DOI] [PubMed] [Google Scholar]
- Hill P.F., Yi R., Spreng R.N., Diana R.A. (2017). Neural congruence between intertemporal and interpersonal self-control: evidence from delay and social discounting. NeuroImage, 162, 186–98. doi: 10.1016/j.neuroimage.2017.08.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E., Tanji J. (2004). Area-selective neuronal activity in the dorsolateral prefrontal cortex for information retrieval and action planning. Journal of Neurophysiology, 91(6), 2707–22. doi: 10.1152/jn.00904.2003 [DOI] [PubMed] [Google Scholar]
- Houghton Mifflin Harcourt . (2000). The American Heritage Dictionary of the English.
- Hutcherson C.A., Bushong B., Rangel A. (2015). A neurocomputational model of altruistic choice and its implications. Neuron, 87(2), 451–63. doi: 10.1016/j.neuron.2015.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang M.H., McColl A., Damasio H., Damasio A. (2009). Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences, 106(19), 8021–6. doi: 10.1073/pnas.0810363106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Kulkarni K.R., Schultz D.H., et al. (2017). Cognitive task information is transferred between brain regions via resting-state network topology. Nature Communications, 8(1), 1–13. doi: 10.1038/s41467-017-01000-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2010). Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience, 22(4), 621–31. doi: 10.1162/jocn.2009.21228 [DOI] [PubMed] [Google Scholar]
- Jauniaux J., Khatibi A., Rainville P., Jackson P.L. (2019). A meta-analysis of neuroimaging studies on pain empathy: investigating the role of visual information and observers’ perspective. Social Cognitive and Affective Neuroscience, 14(8), 789–813. doi: 10.1093/scan/nsz055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni K., Loewenstein G. (1997). Explaining the “identifiable victim effect”. Journal of Risk and Uncertainty, 14(3), 235–57. doi: 10.1023/A:1007740225484 [DOI] [Google Scholar]
- Jones B.A., Rachlin H. (2006). Social discounting. Psychological Science, 17(4), 283–6. doi: 10.1111/j.1467-9280.2006.01699.x [DOI] [PubMed] [Google Scholar]
- Jones B.A., Rachlin H. (2009). Delay, probability, and social discounting in a public goods game. Journal of the Experimental Analysis of Behavior, 91(1), 61–73. doi: 10.1901/jeab.2009.91-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J.J., Hoffman M., Bloom P., Rand D.G. (2016). Third-party punishment as a costly signal of trustworthiness. Nature, 530(7591), 473–6. doi: 10.1038/nature16981 [DOI] [PubMed] [Google Scholar]
- Kaller C.P., Rahm B., Spreer J., Weiller C., Unterrainer J.M. (2011). Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cerebral Cortex, 21(2), 307–17. doi: 10.1093/cercor/bhaq096 [DOI] [PubMed] [Google Scholar]
- Kenett Y.N., Kraemer D.J.M., Alfred K.L., Colaizzi G.A., Cortes R.A., Green A.E. (2020). Developing a neurally informed ontology of creativity measurement. NeuroImage, 221, 117166. doi: 10.1016/j.neuroimage.2020.117166 [DOI] [PubMed] [Google Scholar]
- King-Casas B., Sharp C., Lomax-Bream L., Lohrenz T., Fonagy P., Read Montague P. (2008). The rupture and repair of cooperation in borderline personality disorder. Science, 321(5890), 806–10. doi: 10.1126/science.1156902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D., Pascual-Leone A., Meyer K., Treyer V., Fehr E. (2006). Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science, 314(5800), 829–32. doi: 10.1126/science.1129156 [DOI] [PubMed] [Google Scholar]
- Koban L., Pichon S., Vuilleumier P. (2014). Responses of medial and ventrolateral prefrontal cortex to interpersonal conflict for resources. Social Cognitive and Affective Neuroscience, 9(5), 561–9. doi: 10.1093/scan/nst020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L., Müller V.I., Werminghausen E., Eickhoff S.B., Derntl B. (2020). Do I feel or do I know? Neuroimaging meta-analyses on the multiple facets of empathy. Cortex, 129, 341–55. doi: 10.1016/j.cortex.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut T., Ritov I. (2005). The “identified victim” effect: an identified group, or just a single individual? Journal of Behavioral Decision Making, 18(3), 157–67. doi: 10.1002/bdm.492 [DOI] [Google Scholar]
- Koster-Hale J., Richardson H., Velez N., Asaba M., Young L., Saxe R. (2017). Mentalizing regions represent distributed, continuous, and abstract dimensions of others’ beliefs. NeuroImage, 161, 9–18. doi: 10.1016/j.neuroimage.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain A.L., Wilson A.M., Arbuckle R., Castellanos F.X., Milhama M.P. (2006). Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. NeuroImage, 32(1), 477–84. doi: 10.1016/j.neuroimage.2006.02.047 [DOI] [PubMed] [Google Scholar]
- Krupka E.L., Weber R.A. (2013). Identifying social norms using coordination games: why does dictator game sharing vary? Journal of the European Economic Association, 11(3), 495–524. doi: 10.1111/jeea.12006 [DOI] [Google Scholar]
- Kuss K., Falk A., Trautner P., Elger C.E., Weber B., Fliessbach K. (2013). A reward prediction error for charitable donations reveals outcome orientation of donators. Social Cognitive and Affective Neuroscience, 8(2), 216–23. doi: 10.1093/scan/nsr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss K., Falk A., Trautner P., Montag C., Weber B., Fliessbach K. (2015). Neuronal correlates of social decision making are influenced by social value orientation-an fMRI study. Frontiers in Behavioral Neuroscience, 9(40), 1–8. doi: 10.3389/fnbeh.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–502. doi: 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Lee M., Ahn H.S., Kwon S.K., Kim S. (2018). Cooperative and competitive contextual effects on social cognitive and empathic neural responses. Frontiers in Human Neuroscience, 12, 1–17. doi: 10.3389/fnhum.2018.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Feeley T.H. (2016). The identifiable victim effect: a meta-analytic review. Social Influence, 11(3), 199–215. doi: 10.1080/15534510.2016.1216891 [DOI] [Google Scholar]
- Lelieveld G.J., van Dijk E., Güroǧlu B., et al. (2013). Behavioral and neural reactions to emotions of others in the distribution of resources. Social Neuroscience, 8(1), 52–62. doi: 10.1080/17470919.2012.735621 [DOI] [PubMed] [Google Scholar]
- Lenartowicz A., Kalar D.J., Congdon E., Poldrack R.A. (2010). Towards an ontology of cognitive control. Topics in Cognitive Science, 2(4), 678–92. doi: 10.1111/j.1756-8765.2010.01100.x [DOI] [PubMed] [Google Scholar]
- Lockwood P.L., Apps M.A.J., Valton V., Viding E., Roiser J.P. (2016). Neurocomputational mechanisms of prosocial learning and links to empathy. Proceedings of the National Academy of Sciences, 113(35), 9763–8. doi: 10.1073/pnas.1603198113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood P.L., Wittmann M.K., Apps M.A.J., et al. (2018). Neural mechanisms for learning self and other ownership. Nature Communications, 9(1), 4747. doi: 10.1038/s41467-018-07231-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood P.L., Apps M.A.J., Chang S.W.C. (2020). Is there a “Social” Brain? Implementations and algorithms. Trends in Cognitive Sciences, 24(10), 802–13. doi: 10.1016/j.tics.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood P.L., Klein-Flügge M. (2020). Computational modelling of social cognition and behaviour—a reinforcement learning primer. Social Cognitive and Affective Neuroscience, 1–11. doi: 10.1093/scan/nsaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Paniagua D., Seger C.A. (2013). Coding of level of ambiguity within neural systems mediating choice. Frontiers in Neuroscience, 7, 1–19. doi: 10.3389/fnins.2013.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pérez R. (2008). Aversion to norm-breaking: a model. Games and Economic Behavior, 64(1), 237–67. doi: 10.1016/j.geb.2007.10.009 [DOI] [Google Scholar]
- Marsh A.A., Stoycos S.A., Brethel-Haurwitz K.M., Robinson P., VanMeter J.W., Cardinale E.M. (2014). Neural and cognitive characteristics of extraordinary altruists. Proceedings of the National Academy of Sciences, 111(42), 15036–41. doi: 10.1073/pnas.1408440111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A. (2016). Neural, cognitive, and evolutionary foundations of human altruism. Wiley Interdisciplinary Reviews Cognitive Science, 7(1), 59–71. doi: 10.1002/wcs.1377 [DOI] [PubMed] [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. (2011). An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. NeuroImage, 55(1), 381–8. doi: 10.1016/j.neuroimage.2010.11.060 [DOI] [PubMed] [Google Scholar]
- Matthews L.J., Passmore S., Richard P.M., Gray R.D., Atkinson Q.D. (2016). Shared cultural history as a predictor of political and economic changes among nation states. PLoSOne, 11(4), e0152979. doi: 10.1371/journal.pone.0152979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R.C., Davis J.H., Schoorman F.D. (1995). An integrative model of organizational trust. The Academy of Management Review, 20(3), 709–34. doi: 10.2307/258792 [DOI] [Google Scholar]
- Meyer M.L., Masten C.L., Ma Y., et al. (2013). Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience, 8(4), 446–54. doi: 10.1093/scan/nss019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele V., Matias C., Robin S., Dray S. (2019). Nine quick tips for analyzing network data. PLOS Computational Biology, 15(12), e1007434. doi: 10.1371/journal.pcbi.1007434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinski M., Semmann D., Bakker T.C.M., Krambeck H.J. (2001). Cooperation through indirect reciprocity: image scoring or standing strategy? Proceedings of the Royal Society B: Biological Sciences, 268(1484), 2495–501. doi: 10.1098/rspb.2001.1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.M., Shankar M.U., Knutson B., McClure S.M. (2014). Dissociating motivation from reward in human striatal activity. Journal of Cognitive Neuroscience, 26(5), 1075–84. doi: 10.1162/jocn [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Meyer M., et al. (2009). A key role for similarity in vicarious reward. Science, 324(5929), 900. doi: 10.1126/science.1170539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y., Schunk D., Bruhin A., Ruff C.C., Fehr E. (2012). Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron, 75(1), 73–9. doi: 10.1016/j.neuron.2012.05.021 [DOI] [PubMed] [Google Scholar]
- Morris R.W., Dezfouli A., Griffiths K.R., Balleine B.W. (2014). Action-value comparisons in the dorsolateral prefrontal cortex control choice between goal-directed actions. Nature Communications, 5(1), 1–10. doi: 10.1038/ncomms5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V.I., Cieslik E.C., Laird A.R., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 84, 151–61. doi: 10.1016/j.neubiorev.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M.A. (2006). Five rules for the evolution of cooperation. Science, 314(5805), 1560–3. doi: 10.1126/science.1133755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell K., Brethel-Haurwitz K.M., Rhoads S.A., et al. (2019). Increased similarity of neural responses to experienced and empathic distress in costly altruism. Scientific Reports, 9(10774), 1–11. doi: 10.1038/s41598-019-47196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquola C., De Wael R.V., Wagstyl K., et al. (2019). Microstructural and functional gradients are increasingly dissociated in transmodal cortices. PLoS Biology, 17(5), 1–28. doi: 10.1371/journal.pbio.3000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.K., Blevins E., Knutson B., Tsai J.L. (2017). Neurocultural evidence that ideal affect match promotes giving. Social Cognitive and Affective Neuroscience, 12(7), 1083–96. doi: 10.1093/scan/nsx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnamets P., Shuster A., Reinero D.A., Van Bavel J.J. (2020). A value-based framework for understanding cooperation. Psychological Science, 29(3), 227–34. doi: 10.1177/0963721420906200 [DOI] [Google Scholar]
- Patzelt E.H., Hartley C.A., Gershman S.J. (2018). Computational phenotyping: using models to understand individual differences in personality, development, and mental illness. Personality Neuroscience, 1, 1–9. doi: 10.1017/pen.2018.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peysakhovich A., Nowak M.A., Rand D.G. (2014). Humans display a “cooperative phenotype” that is domain general and temporally stable. Nature Communications, 5(1), 1–8. doi: 10.1038/ncomms5939 [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Yarkoni T. (2016). From brain maps to cognitive ontologies: informatics and the search for mental structure. Annual Review of Psychology, 67(1), 587–612. doi: 10.1146/annurev-psych-122414-033729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S.D., de Waal F.B.M. (2002). Empathy: its ultimate and proximate bases. Behavioral and Brain Sciences, 25(1), 1–20. doi: 10.1017/S0140525X02000018 [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips M.L., et al. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27(8), 605–11. doi: 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Radua J., Rubia K., Canales-Rodríguez E.J., Pomarol-Clotet E., Fusar-Poli P., Mataix-Cols D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Frontiers in Psychiatry, 5(13), 1–8. doi: 10.3389/fpsyt.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsøy T.Z., Skov M., Macoveanu J., Siebner H.R., Fosgaard T.R. (2015). Empathy as a neuropsychological heuristic in social decision-making. Social Neuroscience, 10(2), 179–91. doi: 10.1080/17470919.2014.965341 [DOI] [PubMed] [Google Scholar]
- Rand D.G., Nowak M.A. (2013). Human cooperation. Trends in Cognitive Sciences, 17(8), 413–25. doi: 10.1016/j.tics.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Redcay E., Moraczewski D. (2019). Social cognition in context: a naturalistic imaging approach. NeuroImage, 216, 116392. doi: 10.1016/j.neuroimage.2019.116392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Schilbach L. (2019). Using second-person neuroscience to elucidate the mechanisms of social interaction. Nature Reviews: Neuroscience, 20(8), 496–505. doi: 10.1038/s41583-019-0179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman W.L., Kiesler S., Weisband S., Drasgow F. (1999). A meta-analytic study of social desirability distortion in computer-administered questionnaires, traditional questionnaires, and interviews. Journal of Applied Psychology, 84(5), 754. doi: 10.1037//0021-9010.84.5.754 [DOI] [Google Scholar]
- Rilling J.K., Gutman D.A., Zeh T.R., Pagnoni G., Berns G.S., Kilts C.D. (2002). A neural basis for social cooperation. Neuron, 35(2), 395–405. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., Sanfey A.G., Aronson J.A., Nystrom L.E., Cohen J.D. (2004). The neural correlates of theory of mind within interpersonal interactions. NeuroImage, 22(4), 1694–703. doi: 10.1016/j.neuroimage.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Fehr E. (2014). The neurobiology of rewards and values in social decision making. Nature ReviewsNeuroscience, 15(8), 549–62. doi: 10.1038/nrn3776 [DOI] [PubMed] [Google Scholar]
- Rusbult C.E., Van Lange P.A.M. (2003). Interdependence, interaction, and relationships. Annual Review of Psychology, 54(1), 351–75. doi: 10.1146/annurev.psych.54.101601.145059 [DOI] [PubMed] [Google Scholar]
- Safin V., Locey M.L., Rachlin H. (2013). Valuing rewards to others in a prisoner’s dilemma game. Behavioural Processes, 99, 145–9. doi: 10.1016/j.beproc.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Hassloff H., Straube B., Nuscheler B., Wemken G., Kircher T. (2015). Adult attachment style modulates neural responses in a mentalizing task. Neuroscience, 303, 462–73. doi: 10.1016/j.neuroscience.2015.06.062 [DOI] [PubMed] [Google Scholar]
- Schreuders E., Klapwijk E.T., Will G.J., Güroğlu B. (2018). Friend versus foe: neural correlates of prosocial decisions for liked and disliked peers. Cognitive, Affective and Behavioral Neuroscience, 18(1), 127–42. doi: 10.3758/s13415-017-0557-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuders E., Smeekens S., Cillessen A.H.N., Güroğlu B. (2019). Friends and foes: neural correlates of prosocial decisions with peers in adolescence. Neuropsychologia, 129, 153–63. doi: 10.1016/j.neuropsychologia.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Schurz M., Radua J., Tholen M.G., et al. (2020). Toward a hierarchical model of social cognition: a neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychological Bulletin, 2, 999. doi: 10.1037/bul0000303 [DOI] [PubMed] [Google Scholar]
- Sharp C., Burton P.C., Ha C. (2011). “Better the devil you know”: a preliminary study of the differential modulating effects of reputation on reward processing for boys with and without externalizing behavior problems. European Child and Adolescent Psychiatry, 20(11–12), 581–92. doi: 10.1007/s00787-011-0225-x [DOI] [PubMed] [Google Scholar]
- Shaw D.J., Czekóová K., Staněk R., et al. (2018). A dual-fMRI investigation of the iterated Ultimatum Game reveals that reciprocal behaviour is associated with neural alignment. Scientific Reports, 8(1), 1–13. doi: 10.1038/s41598-018-29233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.V., Hayden B.Y., Truong T.K., Song A.W., Platt M.L., Huettel S.A. (2010). Distinct value signals in anterior and posterior ventromedial prefrontal cortex. Journal of Neuroscience, 30(7), 2490–5. doi: 10.1523/JNEUROSCI.3319-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Collins A.P.R., Fiorentini C., Kessler E., Boyd H., Roberts F., Skuse D.H. (2013). Specific neural correlates of successful learning and adaptation during social exchanges. Social Cognitive and Affective Neuroscience, 8(8), 887–97. doi: 10.1093/scan/nss079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D.A., Sokol-Hessner P., Fareri D.S., et al. (2012). Race and reputation: perceived racial group trustworthiness influences the neural correlates of trust decisions. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1589), 744–53. doi: 10.1098/rstb.2011.0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strombach T., Weber B., Hangebrauk Z., et al. (2015). Social discounting involves modulation of neural value signals by temporoparietal junction. Proceedings of the National Academy of Sciences, 112(5), 1619–24. doi: 10.1073/pnas.1414715112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul S., Tobler P.N., Hein G., et al. (2015). Spatial gradient in value representation along the medial prefrontal cortex reflects individual differences in prosociality. Proceedings of the National Academy of Sciences, 112(25), 7851–6. doi: 10.1073/pnas.1423895112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., O’Doherty J.P. (2020). Breaking human social decision making into multiple components and then putting them together again. Cortex, 127, 221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G., Satpute A.B., Lieberman M.D. (2007). The sunny side of fairness: preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry). Psychological Science, 19(4), 339–47. doi: 10.1111/j.1467-9280.2008.02091.x [DOI] [PubMed] [Google Scholar]
- Tabibnia G., Lieberman M.D. (2007). Fairness and cooperation are rewarding: evidence from social cognitive neuroscience. Annals of the New York Academy of Sciences, 1118, 90–101. doi: 10.1196/annals.1412.001 [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. (2011). Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage, 58(1), 242–9. doi: 10.1016/j.neuroimage.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Ichien N., Qu Y. (2015). The ties that bind: group membership shapes the neural correlates of in-group favoritism. NeuroImage, 115, 42–51. doi: 10.1016/j.neuroimage.2015.04.035 [DOI] [PubMed] [Google Scholar]
- Thielmann I., Spadaro G., Balliet D. (2020). Personality and prosocial behavior: a theoretical framework and meta-analysis. Psychological Bulletin, 146(1), 30–90. doi: 10.1037/bul0000217.supp [DOI] [PubMed] [Google Scholar]
- Tian Y., Margulies D.S., Breakspear M., Zalesky A. (2020). Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nature Neuroscience, 23(11), 1421–32. doi: 10.1101/2020.01.13.903542 [DOI] [PubMed] [Google Scholar]
- Tognoli E., Dumas G., Kelso J.A.S. (2017). A roadmap to computational social neuroscience. Cognitive Neurodynamics, 12(1), 135–40. doi: 10.1007/s11571-017-9462-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E., Sullivan-Toole H. (2015). Fairness and inequity aversion. In: Toga, A., editor. Brain Mapping: An Encyclopedic Reference, Vol. 3, New York, NY: Elsevier Inc. doi: 10.1016/B978-0-12-397025-1.00142-1 [DOI] [Google Scholar]
- Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage, 16(3), 765–80. doi: 10.1006/nimg.2002.1131 [DOI] [PubMed] [Google Scholar]
- Turner J.A., Laird A.R. (2012). The cognitive paradigm ontology: design and application. Neuroinformatics, 10(1), 57–66. doi: 10.1007/s12021-011-9126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A., Bockler A., Kanske P., Trautwein F.-M., Singer T. (2016). Decoding the charitable brain: empathy, perspective taking, and attention shifts differentially predict altruistic giving. Journal of Neuroscience, 36(17), 4719–32. doi: 10.1523/JNEUROSCI.3392-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A., Hutcherson C.A. (2018). Cognitive regulation alters social and dietary choice by changing attribute representations in domain-general and domain-specific brain circuits. ELife, 7, e31185. doi: 10.7554/eLife.31185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W., van Dijk E., Westenberg M., Rombouts S.A.R.B., Crone E.A. (2009). What motivates repayment? Neural correlates of reciprocity in the Trust Game. Social Cognitive and Affective Neuroscience, 4(3), 294–304. doi: 10.1093/scan/nsp009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekaria K.M., O’Connell K., Rhoads S.A., et al. (2020). Activation in bed nucleus of the stria terminalis (BNST) corresponds to everyday helping. Cortex, 127, 67–77. doi: 10.1016/j.cortex.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Metoki A., Smith D.V., et al. (2020). Multimodal mapping of the face connectome. Nature Human Behaviour, 4(4), 397–411. doi: 10.1038/s41562-019-0811-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Takezawa M., Nakawake Y., et al. (2014). Two distinct neural mechanisms underlying indirect reciprocity. Proceedings of the National Academy of Sciences, 111(11), 3990–5. doi: 10.1073/pnas.1318570111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland S., Hewig J., Hecht H., Mussel P., Miltner W.H.R. (2012). Neural correlates of fair behavior in interpersonal bargaining. Social Neuroscience, 7(5), 537–51. doi: 10.1080/17470919.2012.674056 [DOI] [PubMed] [Google Scholar]
- Wheatley T., Boncz A., Toni I., Stolk A. (2019). Beyond the isolated brain: the promise and challenge of interacting minds. Neuron, 103(2), 186–8. doi: 10.1016/j.neuron.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will G.J., Crone E.A., Van Lier P.A.C., Güroǧlu B. (2016). Neural correlates of retaliatory and prosocial reactions to social exclusion: associations with chronic peer rejection. Developmental Cognitive Neuroscience, 19, 288–97. doi: 10.1016/j.dcn.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J.A., Hackel L., FeldmanHall O., Pärnamets P., Van Bavel J.J. (2020). The social neuroscience of cooperation. In: Poeppel, D., Gazzaniga, M., Mangun, G.R., editors. The Cognitive Neurosciences, 6th edn, Cambridge, MA: MIT Press. doi: 10.31234/osf.io/8kcd4 [DOI] [Google Scholar]
- Wills J.A., Hackel L., Van Bavel J. (2018). Shifting prosocial intuitions: neurocognitive evidence for a value based account of group-based cooperation. PsyArXiv, 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M.K., Kolling N., Faber N.S., Scholl J., Nelissen N., Rushworth M.F.S. (2016). Self-other mergence in the frontal cortex during cooperation and competition. Neuron, 91(2), 482–93. doi: 10.1016/j.neuron.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Luo Y., Feng C. (2016). Neural signatures of social conformity: a coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 71, 101–11. doi: 10.1016/j.neubiorev.2016.08.038 [DOI] [PubMed] [Google Scholar]
- Yang Z., Zheng Y., Yang G., Li Q., Liu X. (2019). Neural signatures of cooperation enforcement and violation: a coordinate-based meta-analysis. Social Cognitive and Affective Neuroscience, 14(9), 919–31. doi: 10.1093/scan/nsz073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Mitchell J.P. (2011). Equitable decision making is associated with neural markers of intrinsic value. Proceedings of the National Academy of Sciences, 108(49), 19761–6. doi: 10.1073/pnas.1112324108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Mitchell J.P. (2013). Intuitive prosociality. Current Directions in Psychological Science, 22(6), 466–70. doi: 10.1177/0963721413492764 [DOI] [Google Scholar]
- Zinchenko O., Arsalidou M. (2018). Brain responses to social norms: meta-analyses of fMRI studies. Human Brain Mapping, 39(2), 955–70. doi: 10.1002/hbm.23895 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All thresholded and unthresholded meta-analytic activation maps are openly available on the Open Science Framework at https://osf.io/3vu9w/.


