Summary
Background
Population characteristics can be used to infer vulnerability of communities to COVID-19, or to the likelihood of high levels of vaccine hesitancy. Communities harder hit by the virus, or at risk of being so, stand to benefit from greater resource allocation than their population size alone would suggest. This study reports a simple but efficacious method of ranking small areas of England by relative characteristics that are linked with COVID-19 vulnerability and vaccine hesitancy.
Methods
Publicly available data on a range of characteristics previously linked with either poor COVID-19 outcomes or vaccine hesitancy were collated for all Middle Super Output Areas of England (MSOA, n=6790, excluding Isles of Scilly), scaled and combined into two numeric indices. Multivariable linear regression was used to build a parsimonious model of vulnerability (static socio-ecological vulnerability index, SEVI) in 60% of MSOAs, and retained variables were used to construct two simple indices. Assuming a monotonic relationship between indices and outcomes, Spearman correlation coefficients were calculated between the SEVI and cumulative COVID-19 case rates at MSOA level in the remaining 40% of MSOAs over periods both during and out with national lockdowns. Similarly, a novel vaccine hesitancy index (VHI) was constructed using population characteristics aligned with factors identified by an Office for National Statistics (ONS) survey analysis. The relationship between the VHI and vaccine coverage in people aged 12+years (as of 2021-06-24) was determined using Spearman correlation. The indices were split into quintiles, and MSOAs within the highest vulnerability and vaccine hesitancy quintiles were mapped.
Findings
The SEVI showed a moderate to strong relationship with case rates in the validation dataset across the whole study period, and for every intervening period studied except early in the pandemic when testing was highly selective. The SEVI was more strongly correlated with case rates than any of its domains (rs 0·59 95% CI 0.57-0.62) and outperformed an existing MSOA-level vulnerability index. The VHI was significantly negatively correlated with COVID-19 vaccine coverage in the validation data at the time of writing (rs -0·43 95% CI -0·46 to -0·41). London had the largest number and proportion of MSOAs in quintile 5 (most vulnerable/hesitant) of SEVI and VHI concurrently.
Interpretation
The indices presented offer an efficacious way of identifying geographical disparities in COVID-19 risk, thus helping focus resources according to need.
Funding
Funder: Integrated Covid Hub North East
Award number
n/a
Grant recipient
Fiona Matthews
Research in context.
Evidence before this study
A five-variable index of COVID-19 vulnerability at MSOA level in England was published by Daras et al (2021) and shown to correlate with mortality rates. Indices from other parts of the world have been shown to correlate with case rates and mortality. No indices of vaccine hesitancy have yet been published.
Added value of this study
We present a comprehensive vulnerability index that is composed of four domains so that case rate disparities can be tentatively attributed to differences in underlying characteristics. Our index is computationally simple, uses public data, and correlates more strongly with case rates than others. Our vaccine hesitancy index is the first such to be published, and its intersection with vulnerability allows for more nuance in understanding health inequalities posed by COVID-19.
Implications of all the available evidence
We can now identify areas of England that require extra resources and focus to minimise case rates thus allowing for reductions in NHS pressures, deaths and cases of ‘long COVID’ through intelligent resource allocation.
Alt-text: Unlabelled box
Introduction
The COVID-19 pandemic has had disproportionate effects across age bands, ethnic groups, deprivation levels and geographies in the UK.1, 2, 3, 4 Population characteristics such as overcrowding and deprivation have led to increased risk of infection for those people unable to (or who otherwise do not) adhere to social distancing guidelines or to fully self-isolate when necessary, and prevalent pre-existing long term health conditions have rendered some more at risk of adverse clinical outcomes once infected.4, 5, 6, 7 The COVID-19 pandemic is not over, and future viral variants and novel pandemics are likely, therefore understanding who is at most risk and how to safeguard the vulnerable, benefits all of society.
Despite the introduction of numerous successful vaccinations against COVID-19 and their widespread dissemination, as of summer 2021 large unvaccinated populations exist worldwide, and vaccine hesitancy limits uptake.8,9 Novel viral variants against which current vaccines are less effective may emerge in future. The Office for National Statistics (ONS) published a report on a study of four waves of an online survey that included a question on vaccine hesitancy.9 Their UK-wide logistic regression analysis revealed a number of individual-level characteristics significantly associated with individual level vaccine hesitancy, including being a parent of young children, female sex, and lower education. These are similar to those characteristics found to be associated with vaccine hesitancy in other works from the UK and further afield.10,11
Avoiding unchecked viral spread is important for reasons beyond the obvious need to safeguard life. COVID-19 infection comes with at least two additional major risks; the risk of inducing ‘Long COVID’ in a substantial proportion of patients,12 and the risk of allowing yet more virulent or transmissible variants to emerge which may evade the current vaccines.
The success of local public health responses relies upon managing finite resources and tailoring/targeting messaging to be most impactful in influencing healthy behaviours. To this end, identification of vulnerable populations is of vital importance so that interventions can mitigate the worst effects of the disease. We define vulnerability here as an increased likelihood of suffering relatively high case rates.
We have created a Socio-Ecological COVID-19 Vulnerability Index (SEVI) to pinpoint small areas of England that are more vulnerable to potentially high COVID-19 case numbers, and a second index that ranks small areas by their potential for vaccine hesitancy, using the results of the published ONS report. The intersection of these indices offers a novel way to target finite resources around communications, testing and vaccination efforts to support local public health teams, and is being used in the North East of England to tackle COVID-19 in a targeted, evidence-based way.
Population vulnerability to a respiratory pandemic is related to the ease of viral transmission and size of the susceptible population, the level of socioeconomic disadvantage of communities (which contributes to chronic stress-related health implications and disadvantage), the physical vulnerability of the those infected, and the ability of the health services to cope with demand. We used these ‘themes’ to construct four domain indices, noting that some indicators could relate to multiple themes.
Role of the Funding Source
The funders took no part in the study design, collection, analysis or interpretation of the data used in this study, nor in the writing of the report or the decision to submit for publication.
Methods
Data sources
Geographical level indicators that have been found to be associated with COVID-19 cases or disease severity/mortality were identified through published literature searches (see Table 1 for reference lists associated with individual indicators). Four thematic domains of vulnerability were informally identified; socioeconomic (deprivation and inequality), ecological (environmental factors that are linked with viral spread and population susceptibility to infectious disease), and health service provision/cost and epidemiological (comorbidities and unhealthy behaviours). Publicly available data on factors associated with these domains were obtained from a variety of sources (see Table 1). Where possible, Middle Super Output Area (MSOA, mutually exclusive small level census geographies that contain on average around 8000 people, n=6791) level data were obtained, as these were the smallest census geographies at which COVID-19 case data were available.13 Where data were only available at Lower Super Output Area (LSOA, smaller census geographies wholly within MSOA boundaries) level, if numerators and denominators were provided, the MSOA values were calculated, otherwise the mean of the associated LSOAs was calculated. For data available only at local authority (LA) level (larger geographic areas), all MSOAs within the LA were assumed to share the same value for the indicator. For data obtained at NHS Clinical Commissioning Group (CCG) level, indicators for MSOAs that are contained wholly within the footprint of the CCG were assumed to be the same as that of the CCG. MSOAs spanning more than one CCG were assigned the mean of the indicator for the CCGs in which it lay (n=91, 1·3%). The Isles of Scilly were excluded due to data quality issues, leaving 6790 MSOAs for analysis.
Table 1.
Details of all indicator variables used in the construction of Static Socio-Ecological Vulnerability Index (SEVI), Vaccine Hesitancy Index (VHI), or previously published COVID-19 Vulnerability Index7 (VI). For source URLs see footnote. Reference lists are not exhaustive. Variables in the symbol column correspond to Equation 1.
| INDEX | DOMAIN | INDICATOR | SOURCE | LEVEL | SYMBOL | NOTES | REFERENCES |
|---|---|---|---|---|---|---|---|
| SEVI | 1 | Jobseekers Allowance Rate | NOMIS1 | MSOA | v1 | 6,11,12 | |
| 1 | Rank of Index of Multiple Deprivation (IMD) | Gov.uk2 | LSOA | v2 | Averaged over LSOAs within MSOA | 6,12, 13, 14, 15 | |
| 1 | Long term unemployment, per 100 population of working age† | PHE Fingertips6 | MSOA | v3 | 6,11,12,16 | ||
| 2 | Proportion population over 65 | ONS4 | MSOA | v4 | 17,18 | ||
| 2 | Ethnicity (% non-white population) * | NOMIS1 | MSOA | v5 | 5,19,20 | ||
| 2 | Household overcrowding* | Gov.uk2 | LSOA | v6 | Averaged over LSOAs within each MSOA | 2,6,21 | |
| 3 | Number of care home beds*† | CQC | CCG | v7 | CCG value, or mean of CCG values where MSOA straddles more than one. | 18 | |
| 3 | Number of doctors per 1000 people | LGInform8 | CCG | v8 | CCG value, or mean of CCG values where MSOA straddles more than one. | 18,22,23 | |
| 3 | Average weekly cost of nursing care, £† | LGInform8 | CCG | v9 | CCG value, or mean of CCG values where MSOA straddles more than one. | 3,15,21,24 | |
| 4 | All cause emergency hospital admission rate | PHE Fingertips6 | MSOA | v10 | 12,25 | ||
| 4 | Small Area Mental Health Index (SAMHI) | PLDR7 | LSOA | v11 | Averaged over LSOAs within each MSOA | 13,26 | |
| 4 | Asthma, QOF prevalence | PLDR7 | LSOA | v12 | Averaged over LSOAs within each MSOA | 2,5,12,27 | |
| 4 | Chronic Obstructive Pulmonary Disease, QOF prevalence | PLDR7 | LSOA | v13 | Averaged over LSOAs within each MSOA | 18,28 | |
| 4 | Obesity, % of GP patients > 16 with body mass index > 30 | PLDR7 | LSOA | v14 | Averaged over LSOAs within each MSOA | 5,6,18,22 | |
| 4 | Dementia†, QOF prevalence | PHE Fingertips6 | CCG | v15 | CCG value, or mean of CCG values where MSOA straddles more than one. | 1,5 | |
| 4 | Diabetes†, QOF prevalence | PHE Fingertips6 | CCG | v16 | CCG value, or mean of CCG values where MSOA straddles more than one. | 2,3,5,6,28,29 | |
| 4 | HIV, QOF prevalence | PHE Fingertips6 | LA | v17 | Same value as LA | 13,16,17,29,30 | |
| 4 | Hepatitis, QOF prevalence† | PHE Fingertips6 | LA | v18 | Same value as LA | 16 | |
| VHI | Population under 50 | ONS4 | MSOA | 9 | |||
| Proportion of Black/African/Caribbean ethnic population | NOMIS1 | MSOA | 9 | ||||
| Children under 5 | ONS4 | MSOA | 9 | ||||
| Population with less than degree level qualification | NOMIS1 | MSOA | 9 | ||||
| Renting housing (social or private as proportion of total population) | NOMIS1 | MSOA | 9 | ||||
| VI | Income Domain Indicator* | Gov.UK2 | LSOA | Averaged over LSOAs within each MSOA | 7 | ||
| Long term illness, % of GP patients*,† | PHE Fingertips6 | MSOA | 7 |
1 https://www.nomisweb.co.uk/; 2 https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019; 3 https://www.officeforstudents.org.uk/data-and-analysis/young-participation-by-area/about-tundra/; 4 https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates; 5 https://data.gov.uk/dataset/9c0e093d-d267-4eb8-90d8-54475ab4d1ff/rural-urban-classification-2011-of-middle-layer-super-output-areas-in-england-and-wales; 6 https://fingertips.phe.org.uk/; 7 https://pldr.org/; 8 https://lginform.local.gov.uk/
CCG Clinical Commissioning Group; CQC Care Quality Commission; GP general practitioner; LA local authority; LSOA Lower Super Output Area; MSOA Middle Super Output Area; NHS National Health Service; ONS Office for National Statistics; PHE Public Health England; PLDR Place-Based Longitudinal Data Resource; QOF Quality Outcomes Framework; SEVI Static Ecological Vulnerability Index; VI Vulnerability Index previously published.
Variables used to reconstruct the previously published index by Daras et al.(2021)7
Indicators with greater than 5% missing data. Indices were constructed using means of non-missing variables, so that all MSOA were assigned an index value irrespective of data completeness.
Weekly counts of COVID-19 cases per MSOA were obtained from GOV.UK and converted to rates per 100,000 people using Office for National Statistics mid-2019 population estimates. Case rates were aggregated in three ways; firstly case rates were summed per MSOA across the whole of the available data (2020-03-05 to 2021-06-17), secondly, the dates when national lockdowns were implemented and first began easing were used to sum case rates within each time ‘segment’ as follows:
-
•
Segment 1: Before first lockdown
□ Up to 25/03/2020
-
•
Segment 2: First national lockdown
□ 26/03/2020 to 01/06/2020
-
•
Segment 3: Between first and second national lockdown periods
□ 02/06/2020 to 04/11/2020
-
•
Segment 4: Second national lockdown
□ 05/11/2020 to 02/12/2020
-
•
Segment 5: Between second and third national lockdown periods
□ 03/12/2020 to 05/01/2021
-
•
Segment 6: Third national lockdown
□ 06/01/2021 to 08/03/2021
-
•
Segment 7: Following third national lockdown
□ From 09/03/2021 to 17/06/2021
Lastly, case rates were summed across the lockdown time segments (segments 2,4,6), and separately across the non-lockdown time segments (segments 1,3,5 and 7).
Index construction
We followed a similar methodology to that of Macharia et al. (2021). The choice of indicators to include in the index was decided upon by assessing multivariable statistical significance, but the index itself was composed of all retained variables, combined unweighted, in a simple model. Firstly, all indicators were scaled to a mean of 0 and standard deviation of 1 and oriented so that high numbers implied increased vulnerability. Sixty percent of MSOAs (n=4075) were randomly selected to make up a model development dataset, with the remaining 40% (n=2715) assigned to the validation dataset. In the development dataset, indicators were tested for univariable association with cumulative case rates, ordered by Bayesian Information Criterion (BIC) and considered sequentially for inclusion in a multivariable linear regression model with total cumulative case rate as the dependent variable, using a forward manual stepwise model building process based on likelihood ratio tests and prior knowledge. As our goal was to identify a model that was associated with high case rates at the geographical level, and not to predict COVID-19 per se, nor to examine causal links, only indicators with statistical validity were retained. Discarded indicators are listed in Table S1.
Retained variables were arranged into the four domains of vulnerability (1: socioeconomic, 2: ecological, 3: healthcare provision/cost, 4: epidemiological), and the arithmetic mean per domain (for all non-missing indicators) was calculated using the following formula:
| (1) |
where v1 – v18 are scaled variables grouped into each of the four domains. The indicator associated with the scaled variables, v1-v19, are provided in Table 1. The SEVI was then calculated as the mean of all 4 (non-missing) unweighted domains and split into quintiles where quintile 5 indicated the highest vulnerability. Domains and/or indicators were not weighted due to insufficient evidence in published literature to directly compare the influence of these indicators on outcomes when combined into a single index.
To compare the performance of our SEVI with a previously published index, a second vulnerability index was constructed using the five indicators detailed in Table 1 and combined following the published methodology.7
Data were missing for some MSOAs for the following variables: doctors per 1000 people n=323 (4.8%), HIV n=323(4.8%), Hepatitis n=1690 (24.9%), weekly cost of nursing care n=2791 (41.1%) and adult social care 232 (4.8%), long term unemployment n=2700 (39.8%), and number of care home beds n=148 (2.2%). Missingness was assumed to be non-informative, and indices were constructed using only non-missing data, thus all 6790 MSOAs in England were assigned index values.
Construction of Vaccine Hesitancy Index (VHI)
A separate set of indicators conforming as closely as possible to those cited by the ONS vaccine hesitancy survey9 as associated with individual-level vaccine hesitancy in the under 50s were obtained, ordered, scaled and summed similarly to the SEVI above to create a Hesitancy Index (VHI) which was also split into quintiles and mapped (Table 1). This VHI model was not subjected to a formal model building procedure, all variables were included.
Validation
Spearman correlation coefficients were calculated between the SEVI and each domain of vulnerability in the validation dataset, and case rates for the whole pandemic and each time segment. Correlations were also examined for the previously published MSOA-level vulnerability index for England, for comparison.
Latest (24/06/2021) two-dose COVID-19 vaccination coverage in people aged 12+ years was obtained from NHS England and correlated with the VHI.
Patient and Public Involvement
Patients and the public were not involved in this research.
Results
Static Socio-Ecological Vulnerability Index (SEVI)
Among the 6790 MSOAs, the SEVI ranged from -0.94 (East Riding of Yorkshire 011, Pocklington) to 2.01 (City of London 001). The SEVI, total cumulative case rates and all domains were significantly positively correlated with each other in the validation dataset (Table 2, Figure 1). Although each vulnerability domain correlated with the total cumulative case rate, the SEVI was the most strongly correlated (rs 0·59, 95% CI 0.57-0.62).
Table 2.
Spearman correlation coefficients (95% CI) between domains of vulnerability, Static Socio-Ecological Vulnerability index (SEVI) and cumulative case rates per 100,000 people between 2020-03-05 and 2021-06-17 in a random sample of 2716 MSOAs in England. All p-values <0·0001.
| Domain 1 | Domain 2 | Domain 3 | Domain 4 | SEVI | |
|---|---|---|---|---|---|
| Domain 1: Socioeconomic | |||||
| Domain 2: Ecological | 0·33 (0.29-0.36) | ||||
| Domain 3: Healthcare provision/cost | 0·09 (0.05-0.13) | 0·16 (0.14-0.20) | |||
| Domain 4: Epidemiological | 0·49 (0.47-0.52) | 0·06 (0.03-0.10) | 0·04 (0.00-0.07) | ||
| SEVI | 0·49 (0.46-0.51) | 0·51 (0.48-0.54) | 0·64 (0.62-0.67) | 0·64 (0.62-0.67) | |
| Cumulative COVID-19 case rate | 0·57 (0.54-0.59) | 0·48 (0.45-0.51) | 0·23 (0.20-0.27) | 0·43 (0.40-0.46) | 0·59 (0.57-0.62) |
Figure 1.
Scatter plots of each separate domain of vulnerability with the overall SEVI and cumulative COVID-19 case rate per MSOA among a validation dataset of 2715 MSOAs in England (2020-03-05 to 2021-06-17). Histograms are shown on the diagonal plots. Domain 1: socioeconomic, domain 2: ecological, domain 3: healthcare provision/cost, domain 4: epidemiological.
The published vulnerability index of Daras et al. (2021) was not optimized for case rates but for mortality. When an analogous index was constructed from the data presented here (see Table 1), it correlated with cumulative case rates (rs 0·36, 95% CI 0.32-0.39) but was outperformed by our more comprehensive index. The SEVI and the Daras et al. (2021) indices were correlated with each other (rs 0·58, 95% CI 0.55-0.61).
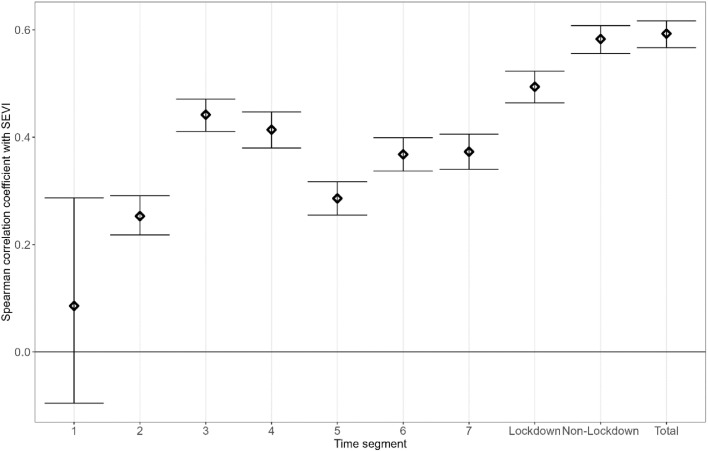
Figure 3 shows the correlations between the SEVI and the cumulative case rate across a number of different ‘segments’ of time in the validation dataset. The SEVI was not correlated with the cumulative case rates from the start of the study period to the start of the first national lockdown (2020-03-26 to 2020-06-01) but was positively correlated with case rates at all other times. The strongest correlation was with cumulative case rate across the whole period. The SEVI was correlated more strongly with cumulative case rates outside of lockdown periods than within lockdowns.
Figure 3.
Spearman correlation coefficients between cumulative COVID-19 case rates per 100,000 per MSOA over different segments of time. Segment 1: before 2020-03-26, segment 2: first national lockdown 2020-03-26 to 2020-06-01, segment 3: 2020-06-02 to 2020-11-04, segment 4: second national lockdown 2020-11-05 to 2020-12-02, segment 5: 2020-12-03 to 2021-01-05, segment 6: third national lockdown 2021-01-06 to 2021-03-08, segment 7: after 2021-02-08, ‘total’ is from 2020-02-05 to 2021-06-17.
Vaccine Hesitancy Index
The VHI ranged from the lowest hesitancy of -1·50 in Westminster 018 (Strand, St James & Mayfair) to highest hesitancy of 2·93 in Milton Keynes 017 (Broughton, Middleton & Kents Hill).
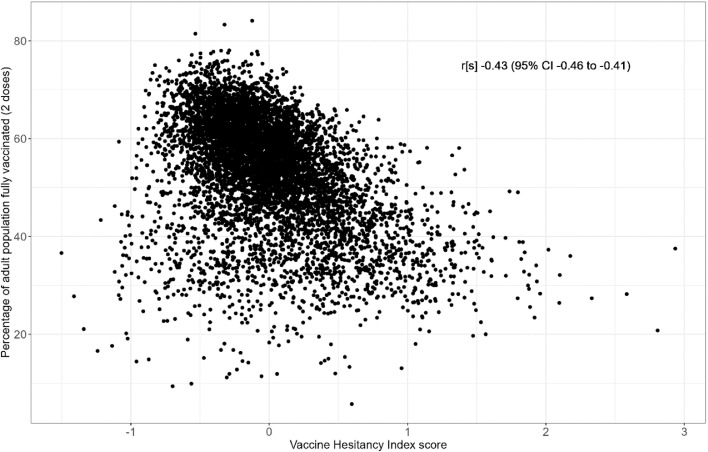
The correlation of VHI with the total cumulative two dose COVID-19 vaccination coverage across England was -0·43 (95% CI -0.46 to -0.41, Figure 2), suggesting that higher hesitancy index score was associated with lower vaccination coverage, at the time of writing.
Figure 2.
Relationship between the Vaccine Hesitancy Index and adult vaccination coverage (as of 2021-06-24) in 6790 MSOAs in England.
SEVI and VHI were weakly correlated with each other (rs 0·23, 95% CI 0.21-0.26), as expected given overlap in their included variable sets (age, ethnicity, education etc).
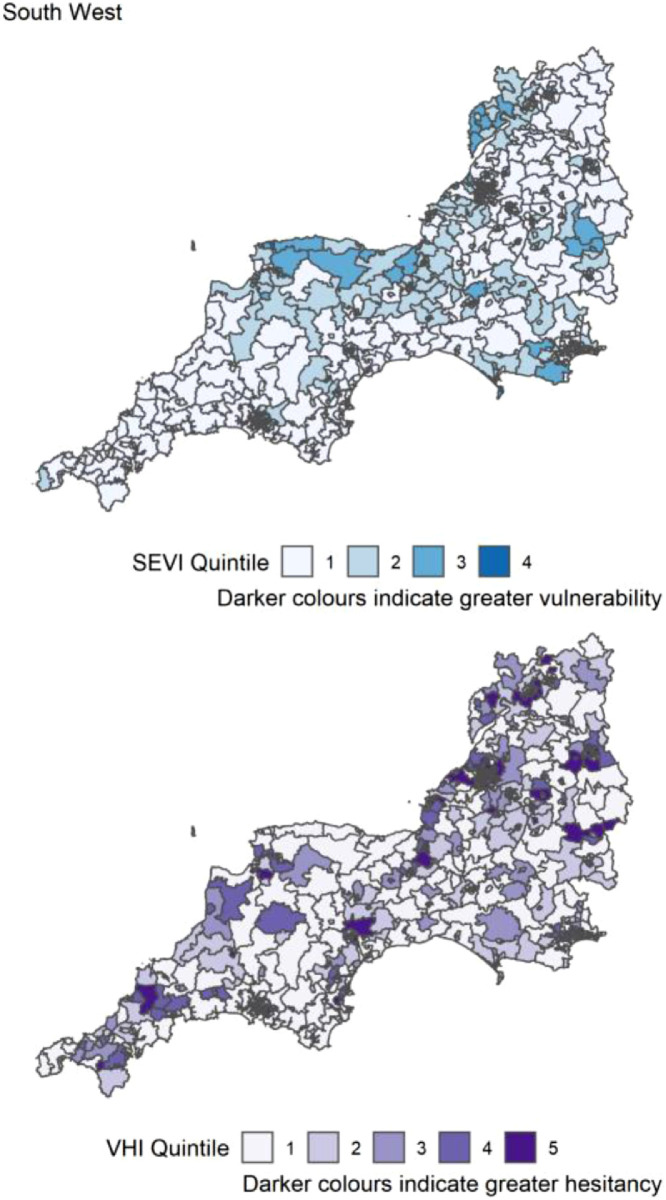
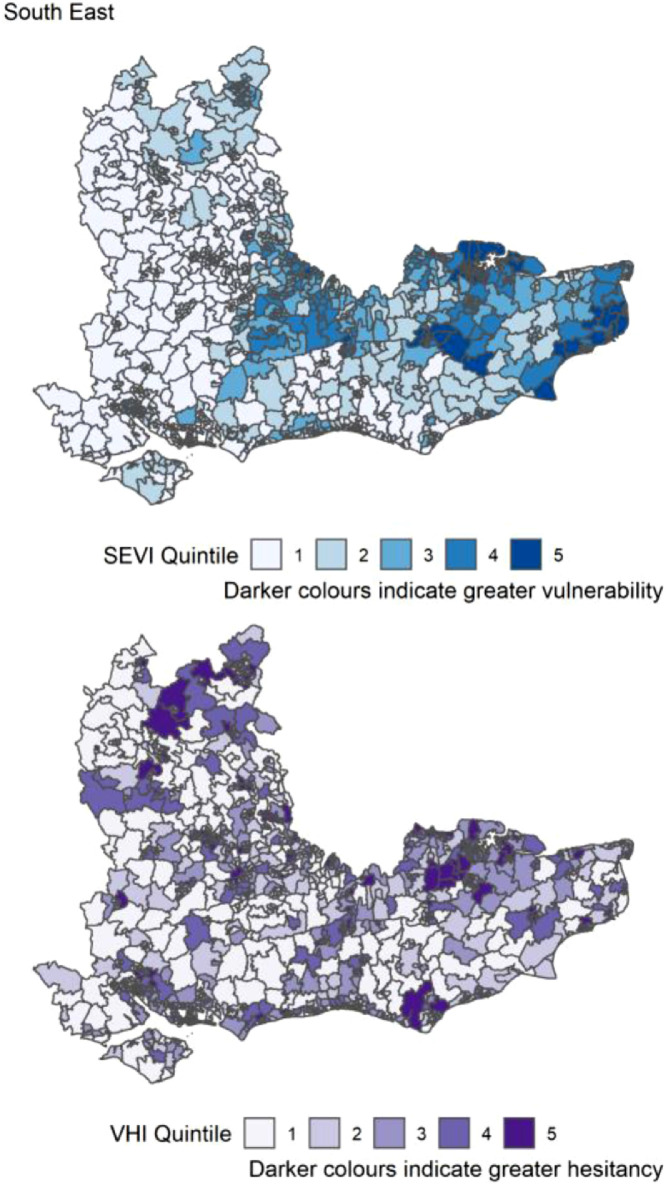
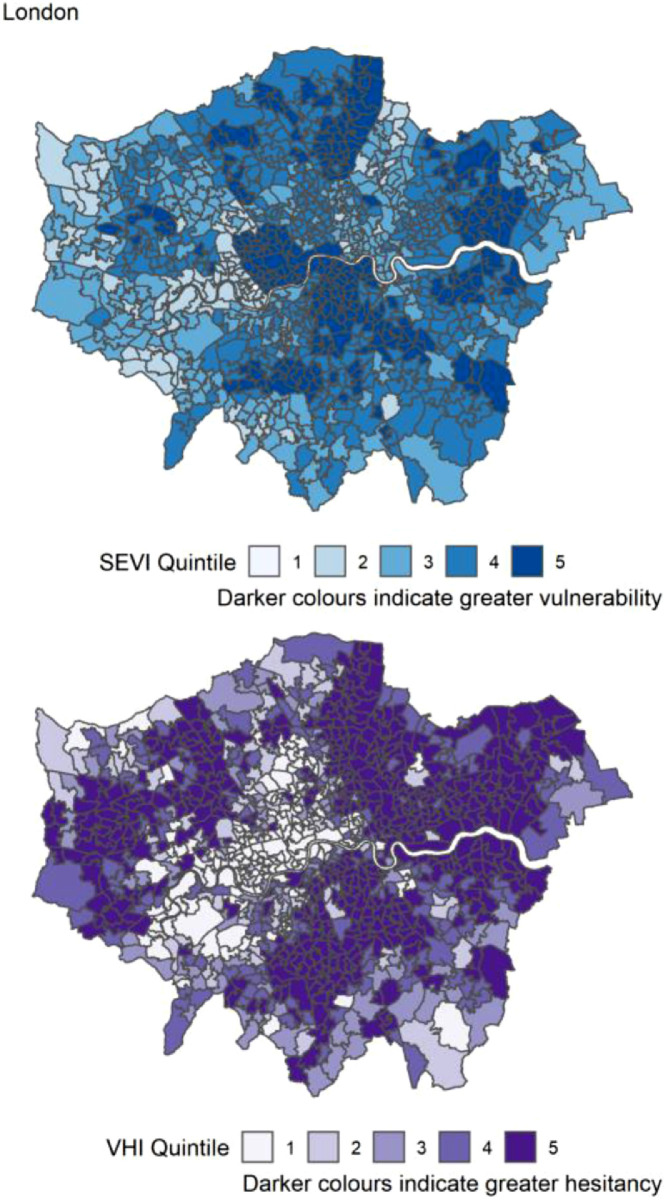
Table 3 reports the count of MSOAs per region of England in the top 20% (highest index score) for the SEVI, VHI and both. The highest number of MSOAs in quintile 5 of the SEVI (most vulnerable) were in the London (Table 3). London had most of the highest scoring MSOAs for vaccine hesitancy (n=480, 49%) and for both indices concurrently (n=158, 16%). There were no MSOAs in the South West region that were in quintiles 5 for both SEVI and VHI. The distribution of both SEVI and VHI across each region of England is shown in Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12. The distribution of vulnerability scores for each domain per region are shown in Figures S1-S9.
Table 3.
Count of MSOAs per region that scored in the highest 20% of areas for COVID-19 vulnerability (SEVI), vaccine hesitancy (VHI) or both. Quintile 5 is the most vulnerable/extreme quintile.
| Region | Number of MSOAs in region | Number (% in region) of MSOAs in quintile 5 of SEVI | Number (%) of MSOAs in quintile 5 of VHI | Number (and %) of MSOAs in quintile 5 of both SEVI and VHI |
|---|---|---|---|---|
| North East | 340 | 186 (54.7) | 22 (6.5) | 7 (2.1) |
| North West | 924 | 256 (27.7) | 128 (13.9) | 56 (6.1) |
| East Midlands | 573 | 112 (19.5) | 108 (18.8) | 35 (6.1) |
| West Midlands | 735 | 227 (30.9) | 159 (21.6) | 78 (10.6) |
| East of England | 736 | 48 (6.5) | 168 (22.8) | 20 (2.7) |
| Yorkshire and the Humber | 692 | 189 (27.3) | 91 (13.2) | 46 (6.7) |
| London | 983 | 262 (26.7) | 480 (48.8) | 158 (16.1) |
| South East | 1108 | 78 (7.0) | 132 (11.9) | 10 (1.0) |
| South West | 699 | 0 (0) | 70 (10.0) | 0 (0) |
VHI Vaccine hesitancy index; MSOA Middle Super Output Area; SEVI Static Socio-Ecological Vulnerability Index.
Figure 4.
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across the South West of England. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 5.
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across the South East of England. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 6.
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across London. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 7.
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across Yorkshire and the Humber region. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 8.
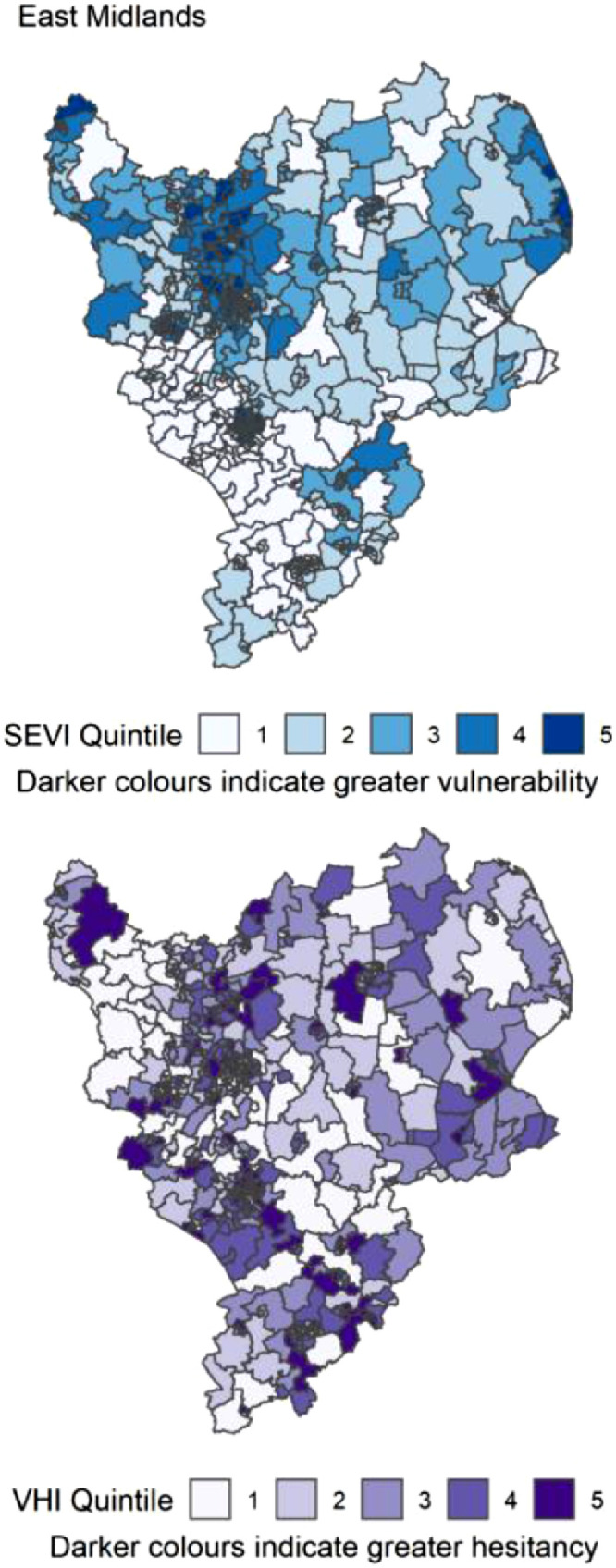
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across the East Midlands of England. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 9.
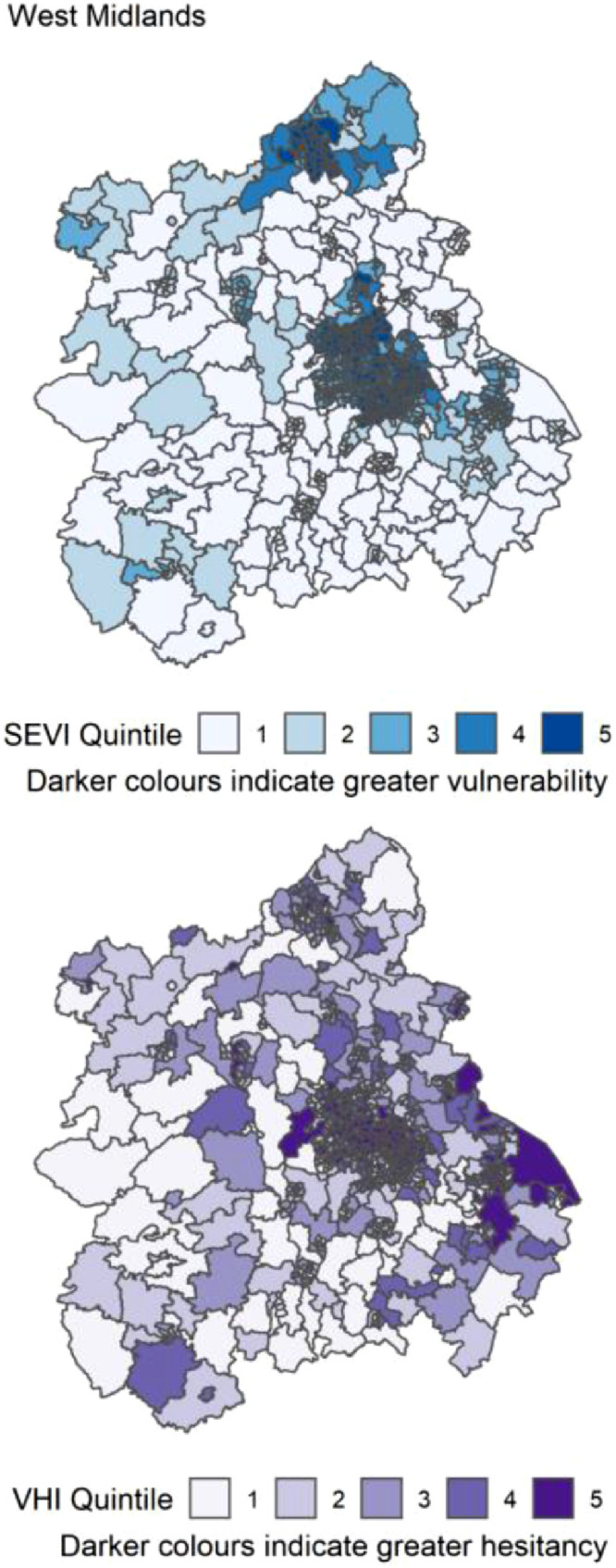
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across the West Midlands of England. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 10.

Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across the East of England. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 11.
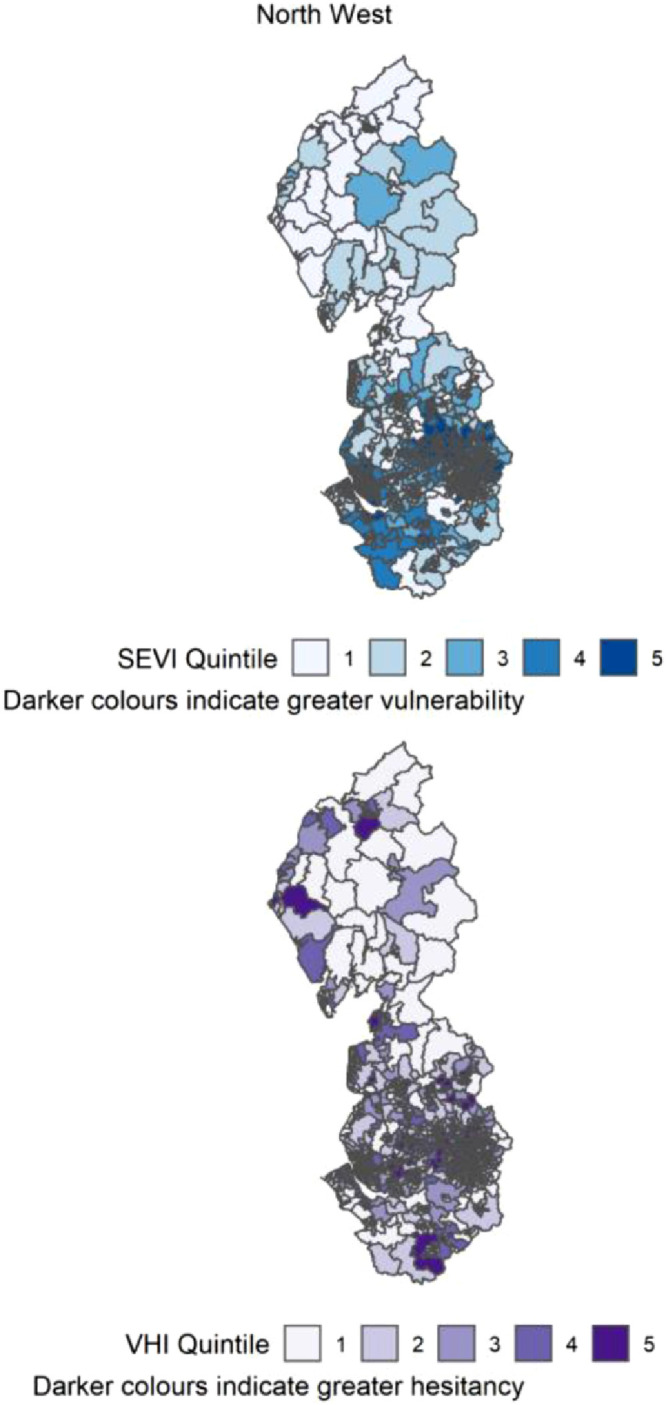
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across the North West of England. Quintile 5 indicates 20% of highest scoring MSOAs.
Figure 12.
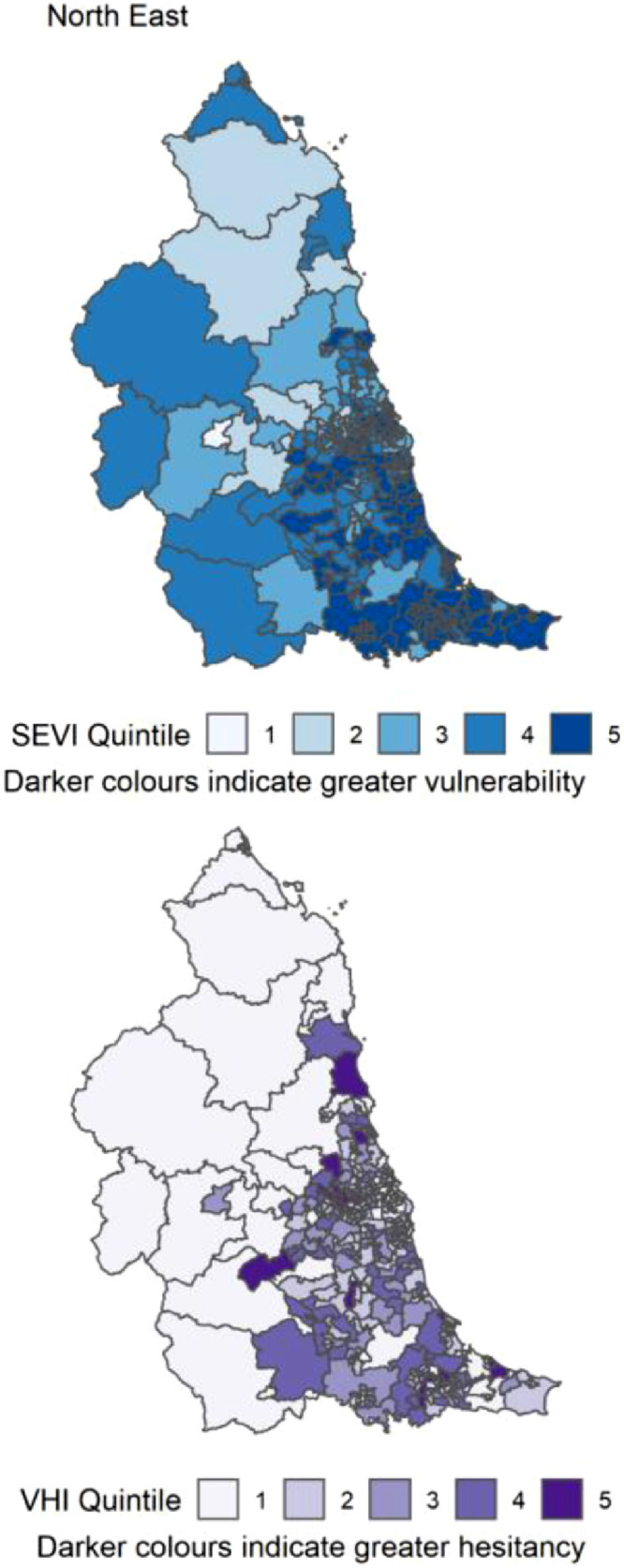
Distribution of quintiles of the static socio-ecological vulnerability index (SEVI) and vaccine hesitancy index (VHI) across the North East of England. Quintile 5 indicates 20% of highest scoring MSOAs.
Discussion
We have constructed a vaccine hesitancy index using publicly available data, to identify areas with relatively high proportions of people with characteristics associated with individual level hesitancy. Good vaccine coverage benefits all by protecting against morbidity and mortality in those vaccinated, reducing pressures on health services, and also helps to reduce the circulating viral load in the population so emergence of new viral variants becomes less likely. The combination of a tool for identifying vulnerable communities and those with potentially large vaccine hesitant populations offers a novel, data driven way of allocating resources based on potential need. This tool, initially developed using smaller geographical units that are not publicly available, is already being employed in the local COVID-19 response in the North East via the Integrated COVID Hub North East (@CovidhubNE), to target resources toward the most vulnerable communities, for example by siting pop-up vaccination clinics. We present it here as a simple but effective method for local health protection teams and other stakeholders to use in their acute pandemic management and envisage its usefulness to extend to other infectious diseases in future. For instance, identification of lower than expected vaccine coverage in a particular area could lead public health analysts to assess the potential vaccine hesitancy of the area using the VHI, in order to understand the relative potential benefits of addressing low coverage through rectification of any supply or access issues, or through hesitancy-related community interactions.
The construction of COVID-19 vulnerability indices to highlight inequalities in susceptibility to case and mortality rates has been performed for several countries (including the UK, US, Germany and Kenya) and geographical designations.7,15,16,20,31,32 These indices vary in the number of indicators included, the complexity of construction, the size of geographical areas used and the type of outcome used for validation. As yet, none have published on the effectiveness of these indices in real-time pandemic response. A previous vulnerability index for England was constructed at MSOA level by Daras et al. (2021). Their approach used only five indicators, but in a more complex design than presented here, and validated the index against age-adjusted mortality rates. Our approach employed a more comprehensive list of indicators that are publicly available, combined in a simple way. We chose to include variables such as HIV and hepatitis, which may correlate with urbanity, population density etc as they may add additional useful information to the index without adding to the ‘cost’ of index construction. We argue that local public health responses are designed to prevent COVID-19 cases rather than ensuing mortality, since cases are the source of acute morbidity, mortality, and protracted morbidity from ‘long COVID’, which is likely to exert significant pressure on NHS resources in future. Therefore, we designed our index to detect both vulnerability to increased case numbers and mortality concurrently, with a focus on the former, as this better aligns with the needs of public health interventions. Our index is also more easily implemented by local public health bodies due to its simplicity. We have shown that our SEVI correlates more strongly with cumulative COVID-19 case rates over the period studied than that of Daras et al. (2021) and compares favourably in terms of correlation with cases compared to other indices.32
Each domain of vulnerability was positively correlated with total cumulative case rates, independently of the SEVI, however, combining all together into a single index led to the highest correlation, indicating the benefit of a composite index rather than focusing on single domains. The SEVI correlated well with cumulative case rates across all segments of time excluding the time before the first national lockdown. This is likely because the timing of local epidemics varied due to seeding of the virus across the country at different times. Once the virus was established across England, the SEVI correlated well with case rates.
In summary, the combination of a simple, easily implemented vulnerability index with a simple index of vaccine hesitancy provides a novel, data-driven way for local public health bodies to target resources aimed at ameliorating the pandemic. Future work is needed to understand the usefulness of these indices in ‘real-world’ pandemic scenarios, and its applicability in different areas.
Strengths and Limitations
The strengths of this work include a simple yet effective methodology for ranking small geographies in terms of four domains of vulnerability and their composite. Simplicity in the methodology allows for expedience in a time-critical scenarios and widens the group of people with sufficient analytic skills to use the tool, thus improving its usefulness. Publicly available data were used so that reconstruction of the indices presented can be accomplished without data costs. Combining four domains of vulnerability into a single index allows for some differentiation of the reasons underlying geographical disparities in case rates, although we do not imply causality.
Our unit of study was the MSOA, as it was the lowest level available for analysis, and heterogeneity of vaccination uptake and disease are better elucidated at small geographical scales.13 This geography contains a number of ‘communities’ since the average population size exceeds 8000. Lower-level geographies would likely show more nuance in patterns of behaviour and thus vulnerability, however case numbers at lower geographies are not publicly available, but should be used for more targeted local understanding.
Early in the pandemic in England, case numbers were not a good reflection of underlying disease prevalence, as testing was predominantly in hospital patients rather than the general public, therefore areas with older populations would have systematically recorded greater case rates due to (likely) higher case hospitalisation rates. As testing was rolled out to the public (mid-April 2020) the case rate would have more accurately represented the underlying COVID rate. Due to precarious employment and other factors (more prevalent in deprived areas), some people may have been unwilling to have COVID tests as a positive result would have negative implications for their lives and livelihoods, thus case rates may be underestimates of COVID prevalence. This reduction in case rates may therefore correlate with areas scoring high on our SEVI due to the socioeconomic domain indicators used, potentially artificially reducing the associations reported.
COVID-19 vaccine hesitancy is likely to combine some more static factors like general mistrust of public health bodies, and some more variable factors, such as mistrust of new technologies. Vaccine hesitancy toward different vaccines, and over time, may therefore vary. The extent to which this VHI could be used in other scenarios is unknown, and beyond the scope of this study.
Some data were not available for all MSOAs, therefore assumptions were made about the characteristics and resources of the populations involved, for example, MSOAs that fell within the catchment area of more than one CCG were assigned the mean value of the CCGs for resources. This occurred in a minority of MSOAs therefore it was unlikely to bias results meaningfully.
We chose to represent the ethnic diversity of MSOAs using only two categories, ‘white’ and ‘non-white’. We recognise that COVID-19 susceptibility varies widely within the diversity of the ‘non-white’ group, but we chose to retain this grouping for two reasons. Firstly, our aim was to construct a parsimonious model from indicators in existing literature, and at the time of writing, most studies of COVID-19 and ethnicity lacked ethnicity granularity. Secondly, greater numbers of ethnic categories could lead to very small counts per MSOA per group, which could cause index users issues with model implementation and interpretation. For parsimony, speed, and ubiquity of usefulness, we therefore opted to retain the original two-group ethnicity coding.
Contributors
CW designed the study with help from FM and DS. CW gathered the data, completed all analyses and drafted the manuscript with input from FM and DS. CW and DS verified the underlying data and accept submission responsibility.
Declaration of interests
DS and CW are part-funded by a Department of Health and Social Care grant to the Newcastle upon Tyne Hospitals NHS Foundation Trust for the Integrated COVID Hub North East for the purposes of local pandemic response, paid via the University of Newcastle (the primary employer of all authors). Grant awarded to FM.
Data sharing statement
All data used are publicly available at no cost. The indices are available at the following DOI: 10.25405/data.ncl.17135066.
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100296.
Appendix. Supplementary materials
References
- 1.Platt L, Warwick R. COVID-19 and Ethnic Inequalities in England and Wales*. Fiscal Studies. 2020;41:259–289. doi: 10.1111/1475-5890.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazroo J, Becares L. Evidence for ethnic inequalities in mortality related to COVID-19 infections: Findings from an ecological analysis of England and Wales. medRxiv. 2020 doi: 10.1101/2020.06.08.20125153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England. Disparities in the risk and outcomes of COVID-19. 2020 ;: 89.
- 4.Bambra C, Riordan R, Ford J, Matthews F. The COVID-19 pandemic and health inequalities. Journal of Epidemiology and Community Health. 2020;74:964–968. doi: 10.1136/jech-2020-214401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niedzwiedz CL, O'Donnell CA, Jani BD, et al. Ethnic and socioeconomic differences in SARS-CoV2 infection in the UK Biobank cohort study. medRxiv. 2020 doi: 10.1186/s12916-020-01640-8. 2020.04.22.20075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daras K, Alexiou A, Rose TC, Buchan I, Taylor-Robinson D, Barr B. How does vulnerability to COVID-19 vary between communities in England? Developing a Small Area Vulnerability Index (SAVI) Journal of Epidemiology and Community Health. 2021 doi: 10.1136/jech-2020-215227. ;: jech-2020-215227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevallier C, Hacquin A-S, Mercier H. COVID-19 Vaccine Hesitancy: Shortening the Last Mile. Trends in Cognitive Sciences Science. 2021;25:331–333. doi: 10.1016/j.tics.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis J, Shine C, Bonang L, Vizard T. Coronavirus and vaccine hesitancy, Great Britain: 17 February to 14 March 2021. 2021;: 1–27.
- 10.Stead M, Jessop C, Angus K, et al. National survey of attitudes towards and intentions to vaccinate against COVID-19: implications for communications. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-055085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McElfish PA, Willis DE, Shah SK, Bryant-Moore K, Rojo MO, Selig JP. Sociodemographic Determinants of COVID-19 Vaccine Hesitancy, Fear of Infection, and Protection Self-Efficacy. Journal of Primary Care and Community Health. 2021;12 doi: 10.1177/21501327211040746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Dowd A. Covid-19: Third of people infected have long term symptoms. BMJ (Clinical research ed) 2021;373:n1626. doi: 10.1136/bmj.n1626. [DOI] [PubMed] [Google Scholar]
- 13.Delamater PL, Kay M, Matthew L. Erratum: Fine-scale spatial clustering of measles nonvaccination that increases outbreak potential is obscured by aggregated reporting data (Proceedings of the National Academy of Sciences of the United States of America (2020) 117 (28506-28514) DOI: 10.1. Proceedings of the National Academy of Sciences of the United States of America. 2021;118 doi: 10.1073/pnas.2111722118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plümper T, Neumayer E. The pandemic predominantly hits poor neighbourhoods? SARS-CoV-2 infections and COVID-19 fatalities in German districts. European journal of public health. 2020;30:1176–1180. doi: 10.1093/eurpub/ckaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder BF, Parks V. Spatial variation in socio-ecological vulnerability to Covid-19 in the contiguous United States. Health and Place. 2020;66 doi: 10.1016/j.healthplace.2020.102471. [DOI] [PubMed] [Google Scholar]
- 16.Nicodemo C, Barzin S, Cavalli N, et al. Measuring geographical disparities in England at the time of COVID-19: results using a composite indicator of population vulnerability. BMJ open. 2020;10 doi: 10.1136/bmjopen-2020-039749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHSA. COVID-19 and the Northern Powerhouse: Tackling inequalities for UK health and productivity. 2020.
- 18.Caul S. Deaths involving COVID-19 by local area and socioeconomic deprivation. Office for National Statistics. 2020 ;: 1–23. [Google Scholar]
- 19.Vahabi N, Salehi M, Duarte JD, Mollalo A, Michailidis G. County-level longitudinal clustering of COVID-19 mortality to incidence ratio in the United States. Scientific Reports. 2021;11:1–22. doi: 10.1038/s41598-021-82384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharya R, Porwal A. A vulnerability index for the management of and response to the COVID-19 epidemic in India: an ecological study. The Lancet Global Health. 2020;8:e1142–e1151. doi: 10.1016/S2214-109X(20)30300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiaghadi A, Rifai HS, Liaw W. Assessing COVID-19 risk, vulnerability and infection prevalence in communities. PLoS ONE. 2020;15:1–21. doi: 10.1371/journal.pone.0241166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clouston SAP, Natale G, Link BG. Socioeconomic inequalities in the spread of coronavirus-19 in the United States: A examination of the emergence of social inequalities. Social Science and Medicine. 2021;268 doi: 10.1016/j.socscimed.2020.113554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards-Belle A, Orzechowska I, Gould DW, et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Medicine. 2020;46:2035–2047. doi: 10.1007/s00134-020-06267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JT, Krieger N. Revealing the unequal burden of COVID-19 by income, race/ethnicity, and household crowding: US county versus zip code analyses. Journal of Public Health Management and Practice. 2021;27:S46–S56. doi: 10.1097/PHH.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 25.Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Social Science and Medicine. 2020;258 doi: 10.1016/j.socscimed.2020.113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Barneveld K, Quinlan M, Kriesler P, et al. The COVID-19 pandemic: Lessons on building more equal and sustainable societies. Economic and Labour Relations Review. 2020 doi: 10.1177/1035304620927107. [DOI] [Google Scholar]
- 27.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. The Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palladino R, Bollon J, Ragazzoni L, Barone-Adesi F. Excess deaths and hospital admissions for COVID-19 due to a late implementation of the lockdown in Italy. International Journal of Environmental Research and Public Health. 2020;17:1–6. doi: 10.3390/ijerph17165644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freese KE, Vega A, Lawrence JJ, Documet PI. Social Vulnerability Is Associated with Risk of COVID-19 Related Mortality in U.S. Counties with Confirmed Cases. Journal of health care for the poor and underserved. 2021;32:245–257. doi: 10.1353/hpu.2021.0022. [DOI] [PubMed] [Google Scholar]
- 30.Laster Pirtle WN. Racial Capitalism: A Fundamental Cause of Novel Coronavirus (COVID-19) Pandemic Inequities in the United States. Health Education and Behavior. 2020 doi: 10.1177/1090198120922942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macharia PM, Joseph NK, Okiro EA. A vulnerability index for COVID-19: spatial analysis at the subnational level in Kenya. BMJ Global Health. 2020;5 doi: 10.1136/bmjgh-2020-003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar A, Chouhan P. COVID-19: District level vulnerability assessment in India. Clinical Epidemiology and Global Health. 2021;9:204–215. doi: 10.1016/j.cegh.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.