Abstract
Being able to predict the mechanical properties of vertebrae in patients with osteoporosis and other relevant pathologies is essential to prevent fractures and to develop the most favorable fracture treatments. Furthermore, a reliable prediction is important for developing more patient‐ and pathology‐specific biomaterials. A plethora of studies correlating bone density to mechanical properties has been reported; however, the results are variable, due to a variety of factors, including anatomical site and methodological differences. The aim of this study was to provide a comprehensive literature review on density and mechanical properties of human vertebral trabecular bone as well as relationships found between these properties. A literature search was performed to include studies, which investigated mechanical properties and bone density of trabecular bone. Only studies on vertebral trabecular bone tissue, reporting bone density or mechanical properties, were kept.
A large variation in reported vertebral trabecular bone densities, mechanical properties, and relationships between the two was found, as exemplified by values varying between 0.09 and 0.35 g/cm3 for the wet apparent density and from 0.1 to 976 MPa for the elastic modulus. The differences were found to reflect variations in experimental and analytical processes that had been used, including testing protocol and specimen geometry. The variability in the data decreased in studies where bone tissue testing occurred in a standardized manner (eg, the reported differences in average elastic modulus decreased from 400% to 10%). It is important to take this variability into account when analyzing the predictions found in the literature, for example, to calculate fracture risk, and it is recommended to use the models suggested in the present review to reduce data variability.
Keywords: bone density, mechanical properties, trabecular bone, vertebrae

1. INTRODUCTION
Osteoporosis is a systemic skeletal disease that involves a reduction in the quantity and quality of bone, resulting in low mineral density and increased bone fragility. It affects more than 200 million people worldwide, 1 , 2 , 3 and the lifetime risk for an osteoporotic fracture, spontaneous or due to, for example, a fall, is estimated to be approximately 40%. 3 , 4 , 5 With the worldwide increase in the active, aging population, the number of fractures is expected to grow significantly. Women are affected by osteoporosis to a greater extent than men, and in the spine, the incidence of fractures due to osteoporosis is 1.6 to 3 times higher in women. 3 , 6 Although less common than osteoporosis, other diseases may also give rise to an increased fracture risk in the spine. However, the effect of other pathologies has not been widely studied except for multiple myeloma. Multiple myeloma is a chronic malignant cell disorder, which occurs in 2.5 to 7.2 people per 100 000 within Western countries. 7 , 8 Apart from other paraneoplastic phenomena such as anemia, renal dysfunction, or hypercalcemia, multiple myeloma is primarily associated with both widespread osteolytic bone destruction and a generalized bone loss. 9 , 10 The presence of lesions is related to significant disease‐related morbidity, 11 and in fact, 50% to 70% of patients diagnosed with multiple myeloma will experience a spinal fracture. 12 Metastases may also give rise to a change in vertebral morphology, with subsequent fractures and/or neurological deficit as a result. 13 Approximately 70% of cancer patients present metastases at the time of death, and the most common site of bone lesions is the spine. 13 , 14
A disease like osteoporosis or metastatic infiltration may not become evident until fracture occurs. 3 , 11 Many studies have, therefore, focused on trying to estimate fracture risk, by determining bone quality through non‐invasive techniques, so that patients can be treated to possibly prevent painful fractures. Clinical fracture prediction tools are currently based on empirical models. The most common clinical method of estimating fracture risk is to measure the bone mineral density (BMD) of the patient's bone and compare it to the mean BMD of a reference group. 3 In several studies, 15 , 16 , 17 , 18 , 19 the BMD, together with the geometry of the vertebra, has been found to correlate with the bone strength. The areal BMD (aBMD), that is, the bone mineral content divided by the projected bone area (g/cm2), is usually determined by dual‐energy X‐ray absorptiometry (DXA), whereas the mean volumetric BMD (vBMD), that is, the bone mineral content per volume (generally as equivalent content of hydroxyapatite per volume, in gHA/cm3), can be determined using quantitative computed tomography (QCT). Areal BMD measurements are faster and give a lower dose of radiation to the patient; however, volumetric BMD measurements give 3D distribution information, which can be combined with finite element analysis (FEA) to enable 3D simulation in which element's properties are given according to respective BMD. 20 FEA has also been widely used to study the mechanical behavior of human vertebral bone 21 , 22 , 23 , 24 , 25 , 26 , 27 based on clinical computed tomography images (CT/FEA). Studies have indicated that nonlinear CT/FEA is a better method to predict failure than lumbar spine aBMD and vBMD for vertebral fractures. 23 , 28 , 29 Numerical modeling could also be used to understand the biomechanics of different pathologies or to develop and improve treatments for the spine. 30 Relating bone density to mechanical properties is important for the correct representation of bone tissue in computational models. 31 , 32 , 33 In the literature, numerous studies correlating bone density to mechanical properties can be found, with a large variation in their results, and most of them have been summarized in a comprehensive review. 32 However, the review gives no recommendation for which density‐mechanical property relationships to use. Furthermore, considering only site‐specific relationships should decrease the data scattering, since it has been found that the results are strongly anatomical site‐dependent. 34 , 35
In addition, the large variation in density‐mechanical property relationships is problematic for the development of biomaterials: in vertebroplasty, for example, the development of a bone cement that matches the modulus of non‐pathological trabecular bone could be of interest, 36 as the large difference in moduli between acrylic bone cement and the surrounding bone has been suggested to increase the risk of fractures in vertebrae adjacent to treated ones. 37 , 38 However, a target value is difficult to define due to the large variation in reported properties. One reason for this variation could be that studies have not divided vertebrae into different pathologies, which may affect the bone quality in different ways, but only related the apparent density or vBMD with, for instance, the strength. 19 , 39 , 40 , 41 , 42 However, different studies have confirmed that osteoporotic vertebrae or vertebrae infiltrated with lytic metastasis have notably reduced mechanical properties in terms of both local material properties, such as compressive elastic modulus and compressive yield strength of the trabecular bone 43 , 44 as well as structural properties such as vertebral body stiffness. 45 On the basis of these results, the prediction of mechanical properties of pathological tissue would benefit from pathology‐specific relationships.
Reliable data of the mechanical properties of vertebral trabecular bone and how they correlate to density are, hence, required to achieve CT‐based FE methods to calculate fracture risk of vertebrae with higher precision, as well as to develop biomaterials with mechanical properties optimized for the loading scenarios in the vertebrae.
The aim of this review was to provide a comprehensive literature review on density and mechanical properties of human vertebral trabecular bone tissue as well as the relationships found between these properties with, where possible, to facilitate fracture prediction and the development of vertebral biomaterials. Furthermore, the relationships from the literature were evaluated in order to propose the use of more specific models, for use in, for example, numerical models.
2. METHODS
A literature search was carried out on the database PubMed to find relevant publications. The following keywords were used for the literature search: human AND (spine OR vertebra*) AND (trabecular OR cancellous OR spongy) AND (density OR BMD) AND (mechanical OR compress* OR tens* OR shear OR torsion* OR bending OR flex*) AND (strength OR stress OR modulus). The literature search resulted in 438 publications that were manually filtered to include only studies where a mechanical property or a density had been investigated. Furthermore, only studies on trabecular bone tissue were investigated, in total 27 publications. Therefore, studies on whole vertebrae, for example, Dall'Ara et al, 22 McBroom et al, 46 Oravec et al, 47 and Fields et al 48 were excluded. All studies in this review were performed at the thoracic‐lumbar region. The reference lists of the included publications were also scanned and crosschecked to search for any other studies that encompassed the above stated criteria.
For clarity, it should be noted that all densities and mechanical properties reviewed in this study were measured on macroscopic samples, that is, local (micro‐level) trabecular properties (as evaluated through, for example, nano‐ or micro‐indentation) were not included in the scope of this study.
3. RESULTS
3.1. Bone density
Traditional density measurements (eg, weighing and/or the Archimedes method) as well as QCT measurements have been performed in order to estimate parameters such as wet, dry, and ash apparent density (ie, wet, dry, and ash weight of bone tissue per unit volume, respectively) and vBMD for vertebral trabecular bone. Table 1 summarizes the studies found for these parameters.
TABLE 1.
Density properties of vertebral trabecular bone
| Parameter | Average ± SD (range) | Number of subjects, age | Further division | Reference |
|---|---|---|---|---|
| Volumetric density (g/cm3) | ||||
| Apparent density (wet) a | ||||
| Female | N/A (≈0.11‐0.15) | 2F, 78 and 82y | No lesions | 43 |
| N/A (≈0.17‐0.22) | 2F, 78 and 82y | Osteoblastic lesions | 43 | |
| N/A (≈0.09‐0.13) | 2F, 78 and 82y | Osteolytic lesions | 43 | |
| Mixed sex | 0.18 ± N/A (0.11‐0.35) | 9F, 16M, 20‐90y | N/A | 39 |
| 0.17 ± 0.04 (0.11‐0.27) | 6F, 5M, 32‐65y | Compression b | 41 | |
| 0.19 ± 0.04 (0.11‐0.27) | 6F, 5M, 32‐65y | Tension b | 41 | |
| 0.18 ± 0.05 (N/A) | 9F, 16M, 20‐90y | Compression b | 59 | |
| 0.19 ± 0.04 (N/A) | 9F, 16M, 20‐90y | Tension b | 59 | |
| 0.14 ± 0.06 (0.09‐0.28) | 6F, 9M, 46‐91y | N/A | 58 | |
| N/A (≈0.10‐0.35) | 14, N/A | N/A | 57 | |
| Apparent density (dry) c | ||||
| Male | 0.15 ± 0.056 (0.048‐0.297) | 5M, 70‐84y | N/A | 39 |
| Mixed sex | 0.22 ± 0.05 (0.15‐0.36) | 10F, 12M, 47‐95y | N/A | 72 |
| Apparent density (ash) d | ||||
| Male | 0.091 ± 0.035 (0.028‐0.182) | 5M, 70‐84y | N/A | 39 |
| 0.126 ± 0.035 (0.08‐0.217) | US, 4M, <60 | Healthy inferior‐superior direction | 53 | |
| 0.116 ± 0.028(0.08‐0.187) | US, 4M, <60 | Healthy mediolateral direction | 53 | |
| Mixed sex | 0.133 ± 0.006 (0.07‐0.24) | 27F, 15M, 15‐87y | 55 | |
| vBMD—apparent density (CT) | ||||
| Male | N/A (≈0.04‐0.2) | 5M, 53‐80y | N/A | 19 |
| Mixed sex | 0.124 ± 0.011 (≈0.05‐0.330) | 4F, 3M, 23‐67y | N/A | 42 |
| N/A (≈0.02‐0.21) | 13F, 19M, 20‐91y | N/A | 40 | |
| N/A (≈0.1‐0.39) e | 21F, 22M, 23‐93y | Healthy | 44 | |
| N/A (≈0.06‐0.19) e | 21F, 22M, 23‐93y | Osteoporotic | 44 | |
| N/A (≈0.03‐0.55) e | 7F, 8M, 36‐83y | Metastatic | 44 | |
Evaluated, after removing nonmineralized tissue in a wet state, as the wet weight divided by the apparent volume.
Specimens for compressive and tensile testing, respectively.
Evaluated, after removing nomineralized tissue and drying (ie, in furnace at 100°C for 1 h 39 or room temperature 24 h 72 ), as the dry weight divided by the apparent volume.
Evaluated, after removing nomineralized tissue and ashing (ie, in furnace at 650°C for 18 h, 39 700°C for 24 h, 53 or at 580°C for 24 h 55 ), as the ash weight divided by the apparent volume.
Mixture of femoral and vertebral specimens.
3.2. Mechanical properties
Generally, the mechanical compressive properties are determined by cutting out trabecular bone specimens from a whole bone and loading them in a materials testing machine. In the literature, the use of different test setups can be found. 32 Most mechanical testing on vertebral trabecular bone has been performed using simple compression tests on cylindrical or cubic cores. However, a few studies have also been performed in tension, torsion, and shear. A summary of the reported mechanical properties can be found in Tables 2 and 3.
TABLE 2.
Compressive mechanical properties of vertebral trabecular bone. If not otherwise specified, the properties were measured in the inferior‐superior direction
| Parameter | Average ± SD (range) | Number of subjects, age | Further division | Test geometry | Reference |
|---|---|---|---|---|---|
| Compressive strength (MPa) | |||||
| Ultimate strength | |||||
| Male | N/A (≈0.04‐4) | 5M, 53‐80y | N/A | Cylinder | 19 |
| N/A (≈0.05‐5) | 5M, 70‐84y | N/A | Cube | 39 | |
| Mixed sex | 2.23 ± 0.95 (0.70‐4.33) | 6F, 5M, 32‐65y | N/A | Cylinder | 41, a |
| 0.91 ± 0.63 (0.05‐2.8) | 27F, 21M, 54‐95y | N/A | Cylinder | 64 | |
| 3.3 ± 2.4 (0.4‐10.6) | 16F, 12M, 23‐91y | N/A | Cylinder | 73 | |
| 1.3 ± 0.2 (≈0.1‐3) | 4F, 3M, 23‐67y | N/A | Cube | 42 | |
| 1.28 ± 1.06 (0.038‐2.92) | 7, 23‐67y | N/A | Cube | 54 | |
| 1.6 ± 0.9 (0.6‐3.9) | 10F, 12M, 47‐95y | N/A | Cube | 72 | |
| Yield strength | |||||
| Male | 0.86 ± 0.32 (0.4‐1.56) | 4M, <60y | N/A | Cylinder | 53 |
| 0.37 ± 0.16 (0.21‐0.67) | 4M, <60y | ML direction b | Cylinder | 53 | |
| Mixed sex | 1.92 ± 0.84 (0.56‐3.71) | 6F, 5M, 32‐65y | N/A | Cylinder | 41, a |
| 10.0 ± 2.2 (≈2‐14) | 21F, 22M, 23‐93y | Healthy | Cylinder | 44, c , d | |
| 4.0 ± 2.2 (≈0.1‐7) | 21F, 22M, 23‐93y | Osteoporotic | Cylinder | 44, c , d | |
| 4.0 ± 1.0 (≈0.1‐24) | 7F, 8M, 36‐83y | Metastatic | Cylinder | 44, c , d | |
| 2.05 ± 0.94 (N/A) | 14, N/A | Strain range 0%‐0.10% | Cylinder | 57, a | |
| 2.11 ± 0.97 (N/A) | 14, N/A | Strain range 0.02%‐0.24% | Cylinder | 57, a | |
| 2.02 ± 0.92 (N/A) | 9F, 16M, 20‐90y | N/A | Cylinder | 59, a | |
| N/A (≈0.2‐5.5) | 13F, 19M, 20‐91y | N/A | Cylinder | 40, a | |
| Compressive modulus (MPa) | |||||
| Male | 189.7 ± 67.5 (93.5‐365) | 4M, <60y | IS direction b | Cylinder | 53 |
| 59.9 ± 31.7 (27.2‐143.5) | 4M, <60y | ML direction b | Cylinder | 53 | |
| 99.0 ± 38.5 (58‐154.2) | 5M, 63‐80y | IS direction b | Cube | 74 | |
| 28.1 ± 16.3 (11.9‐48.8) | 5M, 63‐80y | AP direction b | Cube | 74 | |
| 14.3 ± 5.1 (7.2‐19.1) | 5M, 63‐80y | ML direction b | Cube | 74 | |
| N/A (20‐300) | 5M, 53‐80y | N/A | Cylinder | 19 | |
| N/A (≈1‐70) | 5M, 70‐84y | N/A | Cube | 39 | |
| Mixed sex | 319 ± 189 (≈30‐870) | 13F, 19M, 20‐91y | N/A | Cylinder | 40, a |
| 291 ± 113 (90‐536) | 6F, 5M, 32‐65y | N/A | Cylinder | 41, a | |
| 356.2 ± 89.7 (≈120‐480) | 21F, 22M, 23‐93y | Healthy | Cylinder | 44, c | |
| 189.9 ± 95.4 (≈20‐270) | 21F, 22M, 23‐93y | Osteoporotic | Cylinder | 44, c | |
| 201.5 ± 59.7 (≈40‐640) | 7F, 8M, 36‐83y | Metastatic | Cylinder | 44, c | |
| 336 ± 145 (N/A) | 14, N/A | Strain range 0%‐0.10% | Cylinder | 57, a | |
| 322 ± 134 (N/A) | 14, N/A | Strain range 0.02%‐0.24% | Cylinder | 57, a | |
| 165 ± 110 (32‐355) | 6F, 9M, 46‐91y | Endcaps | Cylinder | 58, a | |
| 121 ± 97 (4‐261) | 6F, 9M, 46‐91y | Platen | Cylinder | 58 | |
| 344 ± 148 (N/A) | 9F, 16M, 20‐90y | N/A | Cylinder | 59, a | |
| 75 ± 32 (10‐139) | 27F, 21M, 54‐95y | N/A | Cylinder | 64 | |
| 430 ± 130 (≈200‐600) | 3F, 7M, 37‐84y | N/A | Cylinder | 65 | |
| 317 ± 227 (51.1‐976) | 16F, 12M, 23‐91y | N/A | Cylinder | 73 | |
| 83 ± 16 (≈1‐200) | 4F, 3M, 23‐67y |
N/A Destructive testing |
Cube | 42 | |
| 63 ± 10 (N/A) | 4F, 3M, 23‐67y | N/A | Cube | 42 | |
|
29 ± 6 (N/A) 25 ± 5 (N/A) |
4F, 3M, 23‐67y | AP direction b | Cube | 42 | |
| 63 ± 10 (N/A) | 4F, 3M, 23‐67y | ML direction b | Cube | 42 | |
|
77 ± 43 (N/A) 47 ± 28 (N/A) |
2F, 78 and 82y | No lesions | Cube | 43 | |
| 2F, 78 and 82y | Osteolytic lesions | Cube | 43 | ||
| 45 ± 18 (N/A) | 2F, 78 and 82y | Osteoblastic lesions | Cube | 43 | |
| 58.5 ± 54 (7‐180) | 7, 23‐67y | N/A | Cube | 54 | |
| 32.7 ± 35 (1‐137) | 7, 23‐67y | AP direction b | Cube | 54 | |
| 33 ± 33 (1‐102) | 7, 23‐67y | ML direction b | Cube | 54 | |
|
67 ± 7 (≈9‐175) 20 ± 3 (≈5‐67) |
27F, 15M, 15‐87y | N/A | Cube | 55 | |
| 33 ± 33 (1‐102) | 27F, 15M, 15‐87y | AP/ML direction b | Cube | 55 | |
| 62.2 ± 57.8 (≈0.1‐225) | 7, 23‐67y | N/A | Cube | 56 | |
| 23.5 ± 22.8 (N/A) | 7, 23‐67y | AP direction b | Cube | 56 | |
| 22.6 ± 21.6 (N/A) | 7, 23‐67y | ML direction b | Cube | 56 | |
| 134 ± 81 (15‐294) | 10F, 12M, 47‐95y | N/A | Cube | 72 | |
| Compressive failure strain (%) | |||||
| Ultimate strain | 1.45 ± 0.33 (0.96‐2.30) | 6F, 5M, 32‐65y | N/A | Cylinder | 41, a |
| 2.9 ± 0.2 (N/A) | 4F, 3M, 23‐67y | N/A | Cube | 42 | |
| Yield strain | 0.78 ± 0.06 (≈0.65‐0.87) | 13F, 19M, 20‐91y | N/A | Cylinder | 40, a |
| 0.84 ± 0.06 (0.75‐0.95) | 6F, 5M, 32‐65y | N/A | Cylinder | 41, a | |
| 0.68 ± 0.11 (0.46‐0.93) | US, 4M, <60y | IS direction b | Cylinder | 53 | |
| 0.88 ± 0.16 (0.65‐1.2) | US, 4M, <60y | ML direction b | Cylinder | 53 | |
| 0.80 ± 0.06 (N/A) | 14, N/A | Strain range 0%‐0.10% | Cylinder | 57 | |
| 0.85 ± 0.06 (N/A) | 14, N/A | Strain range 0.02%‐0.24% | Cylinder | 57 | |
| 0.77 ± 0.06 (N/A) | 9F, 16M, 20‐90y | N/A | Cylinder | 59, a | |
| 0.69 ± 0.03 (N/A) | 5F, 8M, 48‐87y | On‐axis | Cylinder | 60, a | |
| 0.74 ± 0.07 (N/A) | 5F, 8M, 48‐87y | 45° off‐axis | Cylinder | 60, a | |
Studies fulfilling the recommendations in the literature for trabecular testing (ie, endcaps with extensometer, cylindrical geometry with a diameter of at least 7.5 mm, and a height‐diameter ratio of at least 2:1).
Specimens tested in the IS (inferior‐superior) direction, ML (mediolateral) direction, or AP (anteroposterior) direction.
Mixture of femoral and vertebral specimens.
Average values 10 times as large were reported in the reference but due to the range found in the graphs, this was assumed to be a mistake.
TABLE 3.
Mechanical (except compressive) properties of vertebral trabecular bone. All properties were measured in the inferior‐superior (caudal‐cranial) direction
| Parameter | Average ± SD (range) | Number of subjects, age | Further division | Test geometry | Reference |
|---|---|---|---|---|---|
| Tensile strength (MPa) | |||||
| Ultimate strength | |||||
| 2.23 ± 0.76 (1.33‐3.53) | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| Yield strength | |||||
| 1.75 ± 0.65 (0.77‐2.75) | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| 1.76 ± 0.65 (N/A) | 14, N/A | Strain range 0%‐0.10% | Cylinder | 57 | |
| 1.82 ± 0.68 (N/A) | 14, N/A | Strain range 0.02%‐0.24% | Cylinder | 57 | |
| 1.72 ± 0.64 (N/A) | 9F, 16M, 20‐90y | Cylinder | 59 | ||
| Tensile modulus (MPa) | |||||
| 301 ± 100 (139‐472) | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| 338 ± 128 (N/A) | 14, N/A | Strain range 0%‐0.10% | Cylinder | 57 | |
| 319 ± 119 (N/A) | 14, N/A | Strain range 0.02%‐0.24% | Cylinder | 57 | |
| 349 ± 133 (N/A) | 9F, 16M, 20‐90y | N/A | Cylinder | 59 | |
| 450 ± 150 (≈190‐620) | 3F, 7M, 37‐84y | N/A | Cylinder | 65 | |
| Tensile strain (%) | |||||
| Ultimate strain | |||||
| 1.59 ± 0.33 (1.09‐2.51) | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| Yield strain | |||||
| 0.78 ± 0.04 (0.71‐0.88) | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| 0.72 ± 0.05 (N/A) | 14, N/A | Strain range 0%‐0.10% | Cylinder | 57 | |
| 0.78 ± 0.05 (N/A) | 14, N/A | Strain range 0.02%‐0.24% | Cylinder | 57 | |
| 0.70 ± 0.05 (N/A) | 9F, 16M, 20‐90y | N/A | Cylinder | 65 | |
| Shear strength (MPa) | |||||
| 3.1 ± 1.6 (1.4‐7.8) | 10F, 12M, 47‐95y | N/A | Cylinder | 72 | |
| 1.56 ± 0.39 (N/A) | US, 9M, 47‐98y | N/A | Cylinder disk | 75 | |
| 0.68 ± 0.29 (N/A) | US, 6F, 47‐98y | N/A | Cylinder disk | 75 | |
| Torsional modulus (MPa) | |||||
| 88 ± 31 (≈40‐120) | 3F, 7M, 37‐84y | N/A | Cylinder | 65 | |
| Creep modulus (MPa) | |||||
| Loading modulus | |||||
| 251 ± 126 | USA, 3F, 3M, 63‐85y | N/A | Cylinder | 76 | |
| Unloading modulus | |||||
| 274 ± 132 | USA, 3F, 3M, 63‐85y | N/A | Cylinder | 76 | |
3.3. Relationship between density and mechanical properties
Several equations have been proposed to relate bone density to mechanical properties. These are summarized in Table 4. As can be seen in Table 4, in several cases, the same mechanical property was correlated with different types of density (eg, vBMD, apparent wet, dry, and ash density). To make a comparison possible among the correlations, all densities were transformed to ash density based on transformation equations from the literature, 49 , 50 see Table 5. However, in the case of compressive yield strength, all relationships reported were with apparent wet density, and hence, they were not transformed. For the other mechanical properties, apparent ash density was chosen to avoid more than one transformation. Keyak et al 49 studied the relationships between apparent ash density and both apparent wet density and vBMD (using a dipotassium phosphate phantom). The relationship between apparent density and vBMD is dependent on the type of phantom used to calibrate. Schileo et al 50 also related apparent ash density to vBMD, but used a hydroxyapatite phantom. Both types of phantoms have been used in the studies included in this review. Some authors presented both a linear and a power equation for the same analysis or reported relationships between a mechanical property and several densities; in those cases, the equation with the higher coefficient of determination (R 2) was used. It has been found, both theoretically 51 and experimentally, 52 that the testing direction with respect to the trabecular main direction has a significant effect on the mechanical properties of trabecular bone. Samples tested along the inferior‐superior direction have been reported to have mechanical properties more than twice those of samples tested in the mediolateral direction. 42 , 53 , 54 However, very few studies 42 , 53 , 54 , 55 , 56 investigate the mediolateral direction, and only two reported a relationship between density and mechanical properties. 42 , 53 Therefore, only the regressions for trabecular tissue tested along the inferior‐superior direction were included in the analysis.
TABLE 4.
Relationship between mechanical properties and density for vertebral trabecular bone
| Parameter | Material model: (ρ[g/cm3]) | R 2 | Number of subjects, age | Further division | Test geometry | Reference |
|---|---|---|---|---|---|---|
| Compressive strength (MPa) | ||||||
| Ultimate strength | ||||||
| σ u = 3.84 × 10−5 × vBMD2.12 | .50 | 5M, 53‐80y | N/A | Cylinder | 19, a , b | |
| σ u = −1.46 + 21.9 × ρ wet or σ u = 33.2 × ρ wet 1.53 | .71 or .68 | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| σ u = −0.971 + 16.9 × ρ dry or σ u = 97.9 × ρ dry 2.3 | .74 or .79 | 5M, 70‐84y | N/A | Cube | 39 | |
| σ u = −0.953 + 27.5 × ρ ash or σ u = 284 × ρ ash 2.27 | .74 or .78 | 5M, 70‐84y | N/A | Cube | 39 | |
| σ u = −0.9 + 0.019 × vBMD | .91 | 4F, 3M, 23‐67y | N/A | Cube | 42, b , c | |
| σ u = −1.89 + 0.021 × ρ ash or σ u = 78.2 × ρ ash 1.8 | .89 or .91 | 27F, 15M, 15‐87y | N/A | Cube | 55 | |
| Yield strength | ||||||
| σ y = −0.75 + 24.9 × vBMD or σ y = 37.4 × vBMD1.39 | .91 or .95 | 13F, 19M, 20‐91y | N/A | Cylinder | 40, c | |
| σ y = −0.84 + 14.4 × ρ wet or σ y = 23.2 × ρ wet 1.60 | .84 or .88 | 13F, 19M, 20‐91y | N/A | Cylinder | 40 | |
| σ y = −1.4 + 19.6 × ρ wet or σ y = 32.6 × ρ wet 1.60 | .73 or .70 | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| σ y = −1.52 + 16.0 × ρ wet | .81 | 21F, 22M, 23‐93y | Healthy + osteoporotic | Cylinder | 44, d | |
| σ y = −1.67 + 15.5 × ρ wet | .76 | 7F, 8M, 36‐83y | Metastatic | Cylinder | 44, d | |
| σ y = 6.9 × vBMD − 0.13 | .58 | 4M, <60y | IS direction e | Cylinder | 53 | |
| σ y = 18.81 × vBMD1.83 | .70 | 4M, <60y | ML direction e | Cylinder | 53 | |
| σ y = 37.1 × ρ wet 1.74 | .80 | 9F, 16M, 20‐90y | N/A | Cylinder | 59 | |
| Compressive modulus (MPa) | ||||||
| E = 0.00148 × vBMD2.26 | .31 | 5M, 53‐80y | N/A | Cylinder | 19, a , b | |
| E = 4730 × ρ wet 1.56 | .73 | 9F, 16M, 20‐90y | N/A | Cylinder | 34 | |
| E = −34.7 + 3230 × vBMD or E = 2980 × vBMD1.05 | .91 or .90 | 13F, 19M, 20‐91y | N/A | Cylinder | 40, c | |
| E = −97.1 + 2130 × ρ wet or E = 2580 × ρ wet 1.34 | .88 or .93 | 13F, 19M, 20‐91y | N/A | Cylinder | 40 | |
| E = 2100 × ρ wet or E = 2350 × ρ wet 1.20 | .61 or .60 | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| E = 498 × ρ wet + 8.9 | .77 | 21F, 22M, 23‐93y | Healthy + osteoporotic | Cylinder | 44, d | |
| E = 433 × ρ wet + 20.8 | .87 | 7F, 8M, 36‐83y | Metastatic | Cylinder | 44, d | |
| E = 1493.8 × vBMD | .59 | US, 4M, <60y | IS direction e | Cylinder | 53 | |
| E = 3349.1 × vBMD1.94 | .79 | US, 4M, <60y | ML direction e | Cylinder | 53 | |
| E = 1540 × ρ wet − 58 | .64 | 6F, 9M, 46‐91y | Endcaps | Cylinder | 58 | |
| E = 935 × ρ wet − 15 | .31 | 6F, 9M, 46‐91y | Platen | Cylinder | 58 | |
| E = 203 × ρ wet − 7.47 or E = 7570 × ρ dry 1.94 | .54 or .70 | 5M, 70‐84y | N/A | Cube | 39 | |
| E = 334 × ρ wet − 7.61 or E = 1890 × ρ ash 1.92 | .55 or .70 | 5M, 70‐84y | N/A | Cube | 39 | |
| E = −53 + 1.1 × vBMD | .82 | 4F, 3M, 23‐67y | Destructive testing (IS direction e ) | Cube | 42, c | |
| E = −49 + 1.0 × vBMD | .73 | 4F, 3M, 23‐67y | IS direction e , E measured at ε = 0.4% | Cube | 42, c | |
| E = −21 + 0.41 × vBMD | .53 | 4F, 3M, 23‐67y | AP direction e , E measured at ε = 0.4% | Cube | 42, c | |
| E = −1 + 0.23 × vBMD | .33 | 4F, 3M, 23‐67y | ML direction e , E measured at ε = 0.4% | Cube | 42, c | |
| Compressive failure strain (%) | ||||||
| Ultimate strain | ||||||
| NS f vs ρ wet | N/A | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| Yield strain | ||||||
| ε = 0.73 + 0.45 × vBMD | .58 | 13F, 19M, 20‐91y | N/A | Cylinder | 40, c | |
| ε = 0.66 + 1.09 × ρ wet or ε = 1.24 × ρ wet 0.21 | .49 or .48 | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| ε = 1.16‐2.414 × vBMD | .17 | US, 4M, <60y | ML direction e | Cylinder | 53 | |
| Tensile strength (MPa) | ||||||
| Ultimate strength | ||||||
| σ u = 13.2 × ρ wet or σ u = 13.3 × ρ wet 1.07 | .47 or .47 | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| Yield strength | ||||||
| σ y = 10.1 × ρ wet or σ y = 10 × ρ wet 1.04 | .51 or .51 | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| σ y = 21.7 × ρ wet 1.52 | .53 | 9F, 16M, 20‐90y | N/A | Cylinder | 59 | |
| Tensile strain (%) | ||||||
| Ultimate strain | ||||||
| NS f | N/A | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| Yield strain | ||||||
| NS f | N/A | 6F, 5M, 32‐65y | N/A | Cylinder | 41 | |
| Compressive fatigue strength | ||||||
| N f = 4.57 × 10−18 × (σ/E 0)−8.54 (at 1.4‐2.9 Hz) | N/A | 11, 37‐101y | N/A | 77 | ||
| σ = 74.3 × ρ 1.76m2.97Nf −0.069 | N/A | 29, 29‐86y | N/A | Cylinder | 78, g | |
A dipotassium phosphate phantom was used to determine vBMD.
vBMD was measured in mg/cm3.
A hydroxyapatite phantom was used to determine vBMD.
Mixture of femoral and vertebral specimens. In the reference, the measured apparent density was called vBMD. However, it was defined as the product of wet tissue density times the average bone volume fraction; hence according to the present study, it is not vBMD but wet apparent density.
Specimens tested in the IS (inferior‐superior) direction, ML (mediolateral) direction, or AP (anteroposterior) direction.
NS is short for not significant.
ρ is the volume fraction and m = 0.00069.
TABLE 5.
Transformation equations between densities, together with coefficient of determination (R2) and when reported, SE of the estimate (SEestimate)
vBMD was measured in mg.
Equations established based on trabecular bone from proximal human tibiae.
Equations established based on trabecular bone from human and bovine femora.
The relationships between density and compressive ultimate strength, yield strength, and elastic modulus, respectively, are presented in Figures 1, 2, 3 (after the above described transformation). For the remaining mechanical properties (tensile strength, compressive and tensile yield strain, and ultimate strain), only one or a maximum of two relationships were reported, and they were, therefore, not reproduced graphically. All relationships were only plotted within the range of densities reported in the respective study. For the studies that included their raw data, 19 , 40 , 41 , 55 the raw data together with the reported relationships were also replicated in Figures 1, 2, 3. In one study, 40 the reported relationships were found not to be the best fit for the reported raw data; in that case, both the originally reported relationship and the locally reproduced relationship based on least squares best‐fit were illustrated.
FIGURE 1.

Regressions found in the literature between compressive ultimate strength and ash apparent density for vertebral trabecular bone. Models in black represent studies following current literature recommendations for mechanical testing of bone tissue, while models in gray did not. The three calculated densities, Kopperdahl et al, 41 Lang et al, 19 and Augat et al, 42 were transformed from wet apparent density (Equation (1)), vBMD (Equation (2)), and vBMD (Equation (3)), respectively. To the right, the raw data for Lang et al 19 and Mosekilde et al 55 are reproduced. The remaining studies did not include raw data, 41 or it was not possible to distinguish vertebral data from data for other anatomical locations 39 , 42
FIGURE 2.
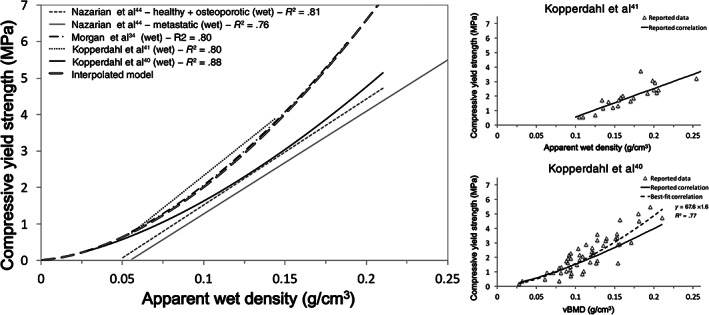
Regressions found in the literature between compressive yield strength and apparent wet density for vertebral trabecular bone, divided by pathology where reported. Models in black represent studies following current literature recommendations for mechanical testing of bone tissue, while models in gray did not. The proposed model is also included. To the right, the raw data for Kopperdahl et al 41 and Kopperdahl et al 40 are reproduced. In the latter, the reported relationship was found not to be the best fit for the reported raw data. Both the reported relationship and the best‐fit relationship were illustrated. In the remaining studies, 44 , 57 it was not possible to distinguish vertebral data from data for other anatomical locations
FIGURE 3.

Regressions found in the literature between compressive elastic modulus and ash apparent density for vertebral trabecular bone, divided by pathology where reported. Models in black represent studies following current literature recommendations for mechanical testing of bone tissue, while models in gray did not. The proposed model is also included. Densities from Keaveny et al, 58 Kopperdahl et al, 41 Kopperdahl et al, 40 Morgan et al, 34 and Nazarian et al 44 were transformed from wet apparent density (Equation (1)), and Lang et al 19 and Augat et al 42 were transformed from vBMD (Equation (2) and Equation (3), respectively). To the right, the raw data for Kopperdahl et al, 41 Kopperdahl et al, 40 and Lang et al 19 are reproduced. The remaining studies did not include raw data, 34 , 42 , 58 or it was not possible to distinguish vertebral data from data for other anatomical locations 39 , 44
4. DISCUSSION
The aim of the present review was to collect and compare the available data in the literature on both mechanical properties and density of vertebral trabecular bone tissue, as well as to evaluate the relationships between these properties. Furthermore, the review aimed to recommend the use of more specific models for numerical modeling, to improve fracture prediction models and facilitate the development and improvement of biomaterials for the spine.
A large range of density values was found, 58 for example, reported apparent wet density values ranged between 57 0.09 and 0.35 g/cm3. It has been found that the density and trabecular structure not only vary between anatomical sites, 34 , 35 , 59 , 60 , 61 but also between different regions in the same anatomical site. 62 , 63 Furthermore, underlying pathologies could have an effect on both density and bone structure. Several authors did not specify whether the vertebrae had been examined for diseases or other factors affecting the bone mineralization prior to testing. Lang et al, 19 for example, did not specify whether the vertebrae had been examined. Nevertheless, they reported that two specimens from one donor had extremely low bone mineral density. Furthermore, different protocols were used to measure the density of the bone tissue, which might have influenced the results. In the case of wet and dry density, the cleaning procedure used is of importance to obtain a correct density. 50 Moreover, there is still a lack of a precise protocol for assessing bone density using CT; factors, such as geometrical calibration, range of density of the calibration phantom used, resolution, scanning parameters, beam hardening correction, position of the vertebra with respect to the scanner, and so forth, all influence the obtained density.
The reported mechanical properties also showed a large range of values, for example, reported average compressive elastic modulus values varied between 75 ± 32 MPa 64 and 430 ± 130 MPa 65 in healthy bone tested in the superior‐inferior direction. Furthermore, comparing the reported regression equations between density and mechanical properties, a large discrepancy was found in all cases (ie, for compressive elastic modulus, compressive yield and ultimate strength). For instance, for the linear relations (y = A × x + B) for elastic modulus, only including relationships with apparent wet density, a variation in A between 203 and 2100 and of B between7 and 58 was reported. This gives rise to a difference in elastic modulus of an order of magnitude, which would have a large impact on, for example, numerical modeling or development of patient‐specific biomaterials.
The differences in elastic modulus could be, partially, explained by the different testing techniques used in the included studies, that is, platen, 19 , 39 , 42 , 43 endcaps with an extensometer, 34 , 40 , 41 , 57 , 58 , 59 , 65 and step‐wise loading. 44 It has been demonstrated that not measuring strain directly on the tissue and using the platen technique causes a systematic underestimation in elastic modulus in trabecular bone. 58 In fact, all studies using the platen techniques 19 , 39 , 42 showed a lower elastic modulus (see Figure 3). Another factor that influences the mechanical properties is the cross‐sectional area of the studied trabecular bone. Since trabecular bone has a heterogeneous microstructure, a certain area is needed in order to assume it to be a continuum. Linde et al 66 reported that the elastic modulus is directly proportional to specimen cross section (up to a diameter of 7.5 mm or a side length of 6.5 mm) and aspect ratio. The studies with a smaller diameter (4 mm 19 and 5.4 mm 44 ) had, in fact, lower elastic moduli than the studies with larger specimen sizes (>8 mm 34 , 40 , 41 , 57 , 58 , 59 , 60 ). Bevill et al 35 found that correction for side‐effects (ie, interruption of the trabecular network along the sides of the machined specimen) improved the prediction of mechanical properties. However, none of the other studies corrected for side effects.
Furthermore, bone tissue is a viscoelastic material and is slightly dependent on strain rate, if the strain rate is increased by an order of magnitude, the measured bone strength will increase by approximately 15%. 67 Many of the tests were performed at the same strain rate, that is, 0.005 second−1. 34 , 40 , 41 , 42 , 57 , 58 , 59 , 64 , 65 However, some studies used a higher 32 , 43 or lower 19 strain rate, which might have influenced the measured mechanical properties. Strain rates in vivo range between 0.002 second−1 (walking) and 1 second−1 (impact). 67 Drying is one of the factors that affect the mechanical properties of bone tissue. It causes an increase in elastic modulus and strength and a decrease in toughness. 68 , 69 However, many of the studies did not specify to which degree the bone tissue was humid at the time of mechanical testing, and it is, hence, difficult to draw any conclusions whether this caused a variability in the data. Furthermore, different pre‐conditioning was applied (ranging from no pre‐conditioning to 10 cycles between 0% and 0.8% strain). It is not clear to what extent pre‐conditioning affects the mechanical properties; however, it is possible that it had an effect on the collected data.
A previous review 32 summarized most of the elasticity‐density relationships found in the literature for human bone tissue, that is, not for vertebral bone specifically. Although the studies were normalized by strain rate and type of density and further split by the main factors influencing the mechanical properties (ie, testing technique, specimen geometry, and anatomical site), variation between studies was still found. However, the studies were split by one factor at the time and not by all three of them, due to the limited number of studies. An attempt was made to group the relationships in the present review by the three remaining parameters (testing technique, specimen geometry, and strain rate). However, it was only possible for two properties, that is, compressive elastic modulus and yield strength. If including only the elasticity‐density and compressive yield strength‐density relationships fulfilling the recommendations in the literature for trabecular testing (ie, endcaps with extensometer, 58 cylindrical geometry with a diameter of at least 7.5 mm 66 and a height‐diameter ratio of at least 2:1 70 ), the variation decreased considerably (see Figures 1, 2, 3). All of those relationships were based on tests performed at the same strain rate, so in this case, there was no need to normalize by this parameter. In the case of compressive elastic modulus, it was possible to see that the range of average modulus decreased drastically, from 83‐319 MPa 40 , 42 to 291‐319 MPa 40 , 41 (the study of Nazarian et al 44 was excluded, since it included a mixture of femoral and spinal specimens). At higher densities, the differences are however still large (eg, in Figure 3, elastic modulus was found to vary between 300 MPa 58 and 450 MPa 34 at 0.12 g/cm3), a 50% variation. For yield strength, only three studies 40 , 41 , 57 were performed according to the recommendations, and an average value of 1.92‐2.11 MPa was reported 41 , 57 (as above Nazarian et al 44 was excluded); hence, no comparison was possible in this case. However, it should be noted that all equations, in both cases, have been subjected to a density transformation. Even though the correlations equations used (Equations (1)‐(3)) were reported to have high coefficients of determination (R 2 > .99 for all three) and when reported, the SE of the estimate was low (SEestimate < 0.007 g/cm3), and it might still have influenced the outcome, since the transformation equations were determined based on human tibial trabecular bone 49 and femoral trabecular bone (human and bovine). 50 It should be confirmed that the equations are valid also for human vertebral trabecular bone.
Although there is still some data scattering, the use of relationships only from studies that have tested vertebral trabecular bone specimens in a standardized way 34 , 40 , 41 , 58 , 59 should improve the precision. Therefore, the raw data (when available) and data points based on the reported equations, density range, and sample size were interpolated to achieve new average relationships between apparent wet density and both compressive yield strength and elastic modulus (for compressive ultimate strength only one study 41 fulfilled the criteria). Apparent wet density was chosen as the density to correlate the mechanical properties with, since most included studies had reported these relationships, Kopperdahl et al 40 being the only exception where the raw data included was vBMD. vBMD was transformed to apparent wet density using Equations (2) and (3). In the cases were raw data were not available and both power and linear relationships, the relationship with the higher R2 was chosen. The new models are the best‐fit power regression to the data points from the included studies (raw data or based on reported relationships) and can be found in Table 6. The SE of the estimates was also calculated and was found to be in the range of 0.06‐0.61 and 74‐127 MPa, for compressive yield strength and compressive modulus, respectively. It was noted that in general, the error increased with increasing density. The anisotropy and inhomogeneity of the trabecular tissue also influence the mechanical properties 71 and need to be included for improved predictions. However, the diagnostic tools of today (ie, CT) only provide density and, hence, limit the use of more complex patient‐specific models. Further studies should investigate whether the use of these new relationships improves, for example, the accuracy of numerical models of vertebral biomechanics.
TABLE 6.
Proposed relationships between mechanical properties and apparent wet density for vertebral trabecular bone and calculated SE of the estimate (SEestimate) of the proposed model against the reported raw data or model from the literature
| Parameter | Input data | N° data points | Ref. | Proposed model | SEestimate |
|---|---|---|---|---|---|
| Compressive strength (MPa) | |||||
| Yield strength | |||||
| Raw data | 21 | 41 | σ y = 38.0 × ρ wet 1.77 | 0.57 | |
| σ y = 37.1 × ρ wet 1.74 | 8 a | 34 | 0.06 | ||
| Raw data b | 52 | 40 | 0.61 | ||
| Compressive modulus (MPa) | |||||
| E = 4730 × ρ wet 1.56 | 8 a | 34 | E = 3180 × ρ wet 1.38 | 127 | |
| E = 1540 × ρ wet − 58 | 9 c | 58 | 88 | ||
| Raw data b | 73 | 40 | 96 | ||
| Raw data | 22 | 41 | 74 | ||
22 out 30 samples came from Kopperdahl et al; 41 therefore, eight data points were evenly distributed over the reported density range (0.11‐0.35 g/cm3), and the reported model was used to calculate the corresponding yield stress and compressive modulus.
vBMD was transformed to apparent wet density using Equations (2) and (3).
Nine samples had been used to determine the reported model; hence, nine data points were evenly distributed over the reported density range (0.09‐0.28 g/cm3), and the reported model was used to calculate the corresponding compressive modulus.
One limitation to the proposed models is that the studies they are based on all come from the same group of authors. The testing methods and sample geometries used as inclusion criteria are, however, well established and are being more and more implemented. Nevertheless, the validity of the included studies should be verified by other authors.
It was also of interest to look into differences between healthy bone and pathological bone, that is, osteoporotic and metastatic bone. However, only two of the studies included in the present review investigated pathological (osteoporotic or metastatic) trabecular bone tissue from the spine. 43 , 44 The results of Hipp et al 43 indicated that the models based on bone density for healthy bone can also be used to estimate Young's modulus and compressive strength of bone tissue with lytic changes, but that tissue with blastic changes, associated with osseous metastases, would need the use of adjusted models. Nazarian et al 44 found that the transaxial subregion with the minimum bone volume fraction (BV/TVmin) could estimate variations in both compressive strength and Young's modulus for trabecular bone specimens independently of skeletal site (ie, femur or vertebra) or bone pathology (ie, osteoporosis or metastatic cancer). However, no differentiation was made between lytic and blastic bone in the study of Nazarian et al 44 Furthermore, none of the pathology‐specific studies 43 , 44 fulfilled the current literature recommendation of local deformation measurements (platen displacement was used in the former and a step‐wise deformation measurement in the latter). Further studies are needed to determine whether or not specific material models are needed for pathological tissue, especially for metastatic bone tissue.
5. CONCLUSIONS
New CT‐based FE methods to calculate fracture risk of vertebrae are of high importance, as well as developing biomaterials for the spine with optimal mechanical properties. Both of these need reliable data of the mechanical properties of vertebral trabecular bone and how they correlate with density. This review serves as a guide to the best relationships to implement for those purposes. A number of studies on the vertebral trabecular bone tissue were analyzed. A non‐negligible variation (more than 4‐fold) was found in the reported densities and mechanical properties, as well as the given relationships between apparent density and mechanical properties. Nevertheless, if considering only the studies meeting the inclusion criteria for trabecular bone testing (ie, using the endcap/extensometer technique and sufficiently large cross sections), the variability decreased significantly (difference in elastic modulus of approximately 10%). It is important to understand the effect of the material model when choosing the appropriate one during development of, for example, numerical models or more patient‐specific biomaterials. The authors provide evidence for the use of more specific material models, which would improve the model fidelity and proposes two interpolated models. This was based on the data found in the literature on vertebral trabecular bone meeting the discussed criteria in sample dimensions (a diameter of at least 7.5 mm and a height‐diameter ratio of at least 2:1) and the use of endcaps with extensometer. The correlations between density and elastic modulus and compressive yield strength were given, respectively:
E = 3180 × ρ wet 1.38.
σ y = 38.0 × ρwet 1.77.
However, further studies are needed to confirm the validity of these models.
For future studies on trabecular bone properties, the authors believe that it would be beneficial if researchers would at least:
Specify patient pathology, sex, and age as a minimum.
Follow current literature recommendations regarding the mechanical testing, that is, use endcaps, local deformation measurements (through, for example, extensometers or imaging), and adequate sample dimensions (diameter of ≥7.5 mm, height to diameter ratio of 2:1).
Use and report on a strain rate representative for the envisaged real situation.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this article.
AUTHOR CONTRIBUTIONS
The study conception and design was proposed by Cecilia Persson. The literature search and data analysis were performed by Caroline Öhman Mägi, Ondrej Holub, Dan Wu, Richard M. Hall, and Cecilia Persson. The first draft of the manuscript was written by Caroline Öhman Mägi, and all authors commented on previous versions of the manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ms. Ingrid Ajaxon for assistance with data collection. This work was supported by the European Union (FP7‐PEOPLE‐2010‐268134), VINNOVA (VINNMER project 2010‐02073), KVA (project FOA13H‐141), and the Swedish Research Council (project 621‐2011‐6258).
Öhman‐Mägi, C. , Holub, O. , Wu, D. , Hall, R. M. , & Persson, C. (2021). Density and mechanical properties of vertebral trabecular bone—A review. JOR Spine, 4(4), e1176. 10.1002/jsp2.1176
Funding information European Union, Grant/Award Number: FP7‐PEOPLE‐2010‐268134; KVA, Grant/Award Number: FOA13H‐141; Swedish Research Council, Grant/Award Number: 621‐2011‐6258; VINNOVA, Grant/Award Number: 2010‐02073
REFERENCES
- 1. Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a world‐wide projection. Osteoporos Int. 1992;2(6):285‐289. [DOI] [PubMed] [Google Scholar]
- 2. Cooper C. Epidemiology of osteoporosis. Osteoporos Int. 1999;9:S2. [DOI] [PubMed] [Google Scholar]
- 3. WHO scientific group on the prevention and management of osteoporosis . Prevention and management of osteoporosis: report of a WHO scientific group. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 4. Melton LJ III. Who has osteoporosis? A conflict between clinical and public health perspectives. J Bone Miner Res. 2000;15(12):2309‐2314. [DOI] [PubMed] [Google Scholar]
- 5. Melton LJ III, Chrischilles EA, Cooper C, Lane AW, Riggs BL. How many women have osteoporosis? J Bone Miner Res. 2005;20(5):886‐892. [DOI] [PubMed] [Google Scholar]
- 6. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726‐1733. [DOI] [PubMed] [Google Scholar]
- 7. Phekoo KJ, Schey SA, Richards MA, et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol. 2004;127(3):299‐304. [DOI] [PubMed] [Google Scholar]
- 8. Kristinsson SY, Landgren O, Dickman PW, Derolf ÅR, Björkholm M. Patterns of survival in multiple myeloma: a population‐baseds of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25(15):1993‐1999. [DOI] [PubMed] [Google Scholar]
- 9. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21‐33. [DOI] [PubMed] [Google Scholar]
- 10. Mahindra A, Hideshima T, Anderson KC. Multiple myeloma: biology of the disease. Blood Rev. 2010;24:S5‐S11. [DOI] [PubMed] [Google Scholar]
- 11. Melton LJ III, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population‐based study. J Bone Miner Res. 2005;20(3):487‐493. [DOI] [PubMed] [Google Scholar]
- 12. Lecouvet FE, Berg BCV, Maldague BE, et al. Vertebral compression fractures in multiple myeloma. Part I. Distribution and appearance at MR imaging. Radiology. 1997;204(1):195‐199. [DOI] [PubMed] [Google Scholar]
- 13. Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine. 1999;24(18):1943. [DOI] [PubMed] [Google Scholar]
- 14. Snyder BD, Cordio MA, Nazarian A, et al. Noninvasive prediction of fracture risk in patients with metastatic cancer to the spine. Clin Cancer Res. 2009;15(24):7676‐7683. [DOI] [PubMed] [Google Scholar]
- 15. Millard J, Augat P, Link T, et al. Power spectral analysis of trabecular bone structure from radiographs: correlation with bone mineral density and biomechanics. Calcif Tissue Int. 1998;63:482‐489. [DOI] [PubMed] [Google Scholar]
- 16. Majumdar S, Lin J, Link T, et al. Fractal analysis of radiographs: assessment of trabecular bone structure and prediction of elastic modulus and strength. Med Phys. 1999;26(7):1330‐1340. [DOI] [PubMed] [Google Scholar]
- 17. Lochmuller E, Miller P, Burklein D, Wehr U, Rambeck W, Eckstein F. In situ femoral DXA related to ash weight, bone size and density and its relationship with mechanical failure loads of the proximal femur. Osteoporos Int. 2000;11:361‐367. [DOI] [PubMed] [Google Scholar]
- 18. Bauer JS, Kohlmann S, Eckstein F, Mueller D, Lochmüller EM, Link TM. Structural analysis of trabecular bone of the proximal femur using multislice computed tomography: a comparison with dual X‐ray absorptiometry for predicting biomechanical strength in vitro. Calcif Tissue Int. 2006;78(2):78‐89. [DOI] [PubMed] [Google Scholar]
- 19. Lang S, Moyle D, Berg E, et al. Correlation of mechanical properties of vertebral trabecular bone with equivalent mineral density as measured by computed tomography. J Bone Joint Surg Am. 1988;70(10):1531‐1538. [PubMed] [Google Scholar]
- 20. Imai K, Ohnishi I, Matsumoto T, Yamamoto S, Nakamura K. Assessment of vertebral fracture risk and therapeutic effects of alendronate in postmenopausal women using a quantitative computed tomography‐based nonlinear finite element method. Osteoporos Int. 2009;20(5):801‐810. [DOI] [PubMed] [Google Scholar]
- 21. Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33(4):744‐750. [DOI] [PubMed] [Google Scholar]
- 22. Dall'Ara E, Pahr D, Varga P, Kainberger F, Zysset P. QCT‐based finite element models predict human vertebral strength in vitro significantly better than simulated DEXA. Osteoporos Int. 2012;23(2):563‐572. [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Sanyal A, Cawthon PM, et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012;27(4):808‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keyak JH, Sigurdsson S, Karlsdottir GS, et al. Effect of finite element model loading condition on fracture risk assessment in men and women: the AGES‐Reykjavik study. Bone. 2013;57(1):18‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29(3):570‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackman TM, DelMonaco AM, Morgan EF. Accuracy of finite element analyses of CT scans in predictions of vertebral failure patterns under axial compression and anterior flexion. J Biomech. 2016;49(2):267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pahr DH, Schwiedrzik J, Dall'Ara E, Zysset PK. Clinical versus pre‐clinical FE models for vertebral body strength predictions. J Mech Behav Biomed Mater. 2014;33:76‐83. [DOI] [PubMed] [Google Scholar]
- 28. Imai K. Vertebral fracture risk and alendronate effects on osteoporosis assessed by a computed tomography‐based nonlinear finite element method. J Bone Miner Metab. 2011;29(6):645‐651. [DOI] [PubMed] [Google Scholar]
- 29. Imai K. Analysis of vertebral bone strength, fracture pattern, and fracture location: a validation study using a computed tomography‐based nonlinear finite element analysis. Aging Dis. 2015;6(3):180‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snyder PJ, Kopperdahl DL, Stephens‐Shields AJ, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177(4):471‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wirtz DC, Schiffers N, Pandorf T, Radermacher K, Weichert D, Forst R. Critical evaluation of known bone material properties to realize anisotropic FE‐simulation of the proximal femur. J Biomech. 2000;33(10):1325‐1330. [DOI] [PubMed] [Google Scholar]
- 32. Helgason B, Perilli E, Schileo E, Taddei F, Brynjólfsson S, Viceconti M. Mathematical relationships between bone density and mechanical properties: a literature review. Clin Biomech. 2008;23(2):135‐146. [DOI] [PubMed] [Google Scholar]
- 33. Austman RL, Milner JS, Holdsworth DW, Dunning CE. The effect of the density–modulus relationship selected to apply material properties in a finite element model of long bone. J Biomech. 2008;41(15):3171‐3176. [DOI] [PubMed] [Google Scholar]
- 34. Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus–density relationships depend on anatomic site. J Biomech. 2003;36(7):897‐904. [DOI] [PubMed] [Google Scholar]
- 35. Bevill G, Easley SK, Keaveny TM. Side‐artifact errors in yield strength and elastic modulus for human trabecular bone and their dependence on bone volume fraction and anatomic site. J Biomech. 2007;40(15):3381‐3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Persson C, Robert E, Carlsson E, et al. The effect of unsaturated fatty acid and triglyceride oil addition on the mechanical and antibacterial properties of acrylic bone cements. J Biomater Appl. 2015;30(3):279‐289. [DOI] [PubMed] [Google Scholar]
- 37. Li Y‐A, Lin C‐L, Chang M‐C, Liu C‐L, Chen T‐H, Lai S‐C. Subsequent vertebral fracture after vertebroplasty: incidence and analysis of risk factors. Spine. 2012;37(3):179‐183. [DOI] [PubMed] [Google Scholar]
- 38. Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. Am J Neuroradiol. 2006;27(1):217‐223. [PMC free article] [PubMed] [Google Scholar]
- 39. Keller TS. Predicting the compressive mechanical behavior of bone. J Biomech. 1994;27(9):1159‐1168. [DOI] [PubMed] [Google Scholar]
- 40. Kopperdahl DL, Morgan EF, Keaveny TM. Quantitative computed tomography estimates of the mechanical properties of human vertebral trabecular bone. J Orthop Res. 2002;20(4):801‐805. [DOI] [PubMed] [Google Scholar]
- 41. Kopperdahl DL, Keaveny TM. Yield strain behavior of trabecular bone. J Biomech. 1998;31(7):601‐608. [DOI] [PubMed] [Google Scholar]
- 42. Augat P, Link T, Lang TF, Lin JC, Majumdar S, Genant HK. Anisotropy of the elastic modulus of trabecular bone specimens from different anatomical locations. Med Eng Phys. 1998;20(2):124‐131. [DOI] [PubMed] [Google Scholar]
- 43. Hipp JA, Rosenberg AE, Hayes WC. Mechanical properties of trabecular bone within and adjacent to osseous metastases. J Bone Miner Res. 1992;7(10):1165‐1171. [DOI] [PubMed] [Google Scholar]
- 44. Nazarian A, von Stechow D, Zurakowski D, Müller R, Snyder BD. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif Tissue Int. 2008;83(6):368‐379. [DOI] [PubMed] [Google Scholar]
- 45. Windhagen HJ, Hipp JA, Silva MJ, Lipson SJ, Hayes WC. Predicting failure of thoracic vertebrae with simulated and actual metastatic defects. Clin Orthop Relat R. 1997;344:313‐319. [PubMed] [Google Scholar]
- 46. McBroom R, Hayes W, Edwards W, Goldberg R, White A. Prediction of vertebral body compressive fracture using quantitative computed tomography. J Bone Joint Surg Am. 1985;67(8):1206‐1214. [PubMed] [Google Scholar]
- 47. Oravec D, Kim W, Flynn MJ, Yeni YN. The relationship of whole human vertebral body creep to geometric, microstructural, and material properties. J Biomech. 2018;73:92‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fields AJ, Lee GL, Liu XS, Jekir MG, Guo XE, Keaveny TM. Influence of vertical trabeculae on the compressive strength of the human vertebra. J Bone Miner Res. 2011;26(2):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keyak JH, Lee IY, Skinner HB. Correlations between orthogonal mechanical properties and density of trabecular bone: use of different densitometric measures. J Biomed Mater Res. 1994;28(11):1329‐1336. [DOI] [PubMed] [Google Scholar]
- 50. Schileo E, Dall'Ara E, Taddei F, et al. An accurate estimation of bone density improves the accuracy of subject‐specific finite element models. J Biomech. 2008;41(11):2483‐2491. [DOI] [PubMed] [Google Scholar]
- 51. Turner CH, Cowin SC. Errors induced by off‐axis measurement of the elastic propertibes of bone. J Biomech Eng. 1988;110(3):213‐215. [DOI] [PubMed] [Google Scholar]
- 52. Öhman C, Baleani M, Perilli E, et al. Mechanical testing of cancellous bone from the femoral head: experimental errors due to off‐axis measurements. J Biomech. 2007;40(11):2426‐2433. [DOI] [PubMed] [Google Scholar]
- 53. Aiyangar AK, Vivanco J, Au AG, Anderson PA, Smith EL, Ploeg HL. Dependence of anisotropy of human lumbar vertebral trabecular bone on quantitative computed tomography‐based apparent density. J Biomech Eng. 2014;136(9):091003. [DOI] [PubMed] [Google Scholar]
- 54. Hans D, Wu C, Njeh CF, et al. Ultrasound velocity of trabecular cubes reflects mainly bone density and elasticity. Calcif Tissue Int. 1999;64(1):18‐23. [DOI] [PubMed] [Google Scholar]
- 55. Mosekilde L, Mosekilde L, Danielsen CC. Biomechanical competence of vertebral trabecular bone in relation to ash density and age in normal individuals. Bone. 1987;8(2):79‐85. [DOI] [PubMed] [Google Scholar]
- 56. Ouyang X, Majumdar S, Link TM, et al. Morphometric texture analysis of spinal trabecular bone structure assessed using orthogonal radiographic projections. Med Phys. 1998;25(10):2037‐2045. [DOI] [PubMed] [Google Scholar]
- 57. Morgan EF, Yeh OC, Chang WC, Keaveny TM. Nonlinear behavior of trabecular bone at small strains. J Biomech Eng. 2000;123(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 58. Keaveny TM, Pinilla TP, Crawford RP, Kopperdahl DL, Lou A. Systematic and random errors in compression testing of trabecular bone. J Orthop Res. 1997;15(1):101‐110. [DOI] [PubMed] [Google Scholar]
- 59. Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. J Biomech. 2001;34(5):569‐577. [DOI] [PubMed] [Google Scholar]
- 60. Bevill G, Farhamand F, Keaveny TM. Heterogeneity of yield strain in low‐density versus high‐density human trabecular bone. J Biomech. 2009;42(13):2165‐2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Matsuura M, Eckstein F, Lochmüller E‐M, Zysset PK. The role of fabric in the quasi‐static compressive mechanical properties of human trabecular bone from various anatomical locations. Biomech Model Mechanobiol. 2008;7(1):27‐42. [DOI] [PubMed] [Google Scholar]
- 62. Kim D‐G, Hunt CA, Zauel R, Fyhrie DP, Yeni YN. The effect of regional variations of the trabecular bone properties on the compressive strength of human vertebral bodies. Ann Biomed Eng. 2007;35(11):1907‐1913. [DOI] [PubMed] [Google Scholar]
- 63. Chen H, Shoumura S, Emura S, Bunai Y. Regional variations of vertebral trabecular bone microstructure with age and gender. Osteoporos Int. 2008;19(10):1473‐1483. [DOI] [PubMed] [Google Scholar]
- 64. Follet H, Viguet‐Carrin S, Burt‐Pichat B, et al. Effects of preexisting microdamage, collagen cross‐links, degree of mineralization, age, and architecture on compressive mechanical properties of elderly human vertebral trabecular bone. J Orthop Res. 2011;29(4):481‐488. [DOI] [PubMed] [Google Scholar]
- 65. Wolfram U, Wilke H‐J, Zysset PK. Valid μ finite element models of vertebral trabecular bone can be obtained using tissue properties measured with nanoindentation under wet conditions. J Biomech. 2010;43(9):1731‐1737. [DOI] [PubMed] [Google Scholar]
- 66. Linde F, Hvid I, Madsen F. The effect of specimen geometry on the mechanical behaviour of trabecular bone specimens. J Biomech. 1992;25(4):359‐368. [DOI] [PubMed] [Google Scholar]
- 67. Carter D, Hayes W. The compressive behavior of bone as a two‐phase porous structure. J Bone Joint Surg Am. 1977;59(7):954‐962. [PubMed] [Google Scholar]
- 68. Evans FG. Preservation effects. Mechanical properties of bone; Springfield, IL: Thomas; 1973:56‐60. [Google Scholar]
- 69. Dempster WT, Liddicoat RT. Compact bone as a non‐isotropic material. Am J Anat. 1952;91(3):331‐362. [DOI] [PubMed] [Google Scholar]
- 70. Keaveny TM, Borchers RE, Gibson LJ, Hayes WC. Theoretical analysis of the experimental artifact in trabecular bone compressive modulus. J Biomech. 1993;26(4):599‐607. [DOI] [PubMed] [Google Scholar]
- 71. Tassani S, Öhman C, Baleani M, Baruffaldi F, Viceconti M. Anisotropy and inhomogeneity of the trabecular structure can describe the mechanical strength of osteoarthritic cancellous bone. J Biomech. 2010;43(6):1160‐1166. [DOI] [PubMed] [Google Scholar]
- 72. Cendre E, Mitton D, Roux JP, et al. High‐resolution computed tomography for architectural characterization of human lumbar cancellous bone: relationships with histomorphometry and biomechanics. Osteoporos Int. 1999;10(5):353‐360. [DOI] [PubMed] [Google Scholar]
- 73. Hou FJ, Lang SM, Hoshaw SJ, Reimann DA, Fyhrie DP. Human vertebral body apparent and hard tissue stiffness. J Biomech. 1998;31(11):1009‐1015. [DOI] [PubMed] [Google Scholar]
- 74. Ladd AJ, Kinney JH, Haupt DL, Goldstein SA. Finite‐element modeling of trabecular bone: comparison with mechanical testing and determination of tissue modulus. J Orthop Res. 1998;16(5):622. [DOI] [PubMed] [Google Scholar]
- 75. Xavier F, Jauregui JJ, Cornish N, et al. Regional variations in shear strength and density of the human thoracic vertebral endplate and trabecular bone. Int J Spine Surg. 2017;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim DG, Shertok D, Ching Tee B, Yeni YN. Variability of tissue mineral density can determine physiological creep of human vertebral cancellous bone. J Biomech. 2011;44(9):1660‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haddock SM, Yeh OC, Mummaneni PV, Rosenberg WS, Keaveny TM. Similarity in the fatigue behavior of trabecular bone across site and species. J Biomech. 2004;37(2):181‐187. [DOI] [PubMed] [Google Scholar]
- 78. Rapillard L, Charlebois M, Zysset PK. Compressive fatigue behavior of human vertebral trabecular bone. J Biomech. 2006;39(11):2133‐2139. [DOI] [PubMed] [Google Scholar]


