This cross-sectional study examines the association of socioeconomic, geographic, and behavioral factors with the quality of diabetes management for adults.
Key Points
Question
What is the association between area deprivation index, rurality, and diabetes care quality?
Findings
In this cross-sectional study of 31 934 adult patients with diabetes receiving care at primary care practices across 3 US states, census block group–level area deprivation index score and zip code–level rurality were significantly associated with diabetes care quality.
Meaning
Findings from this study showed that adult patients with diabetes who lived in more deprived and rural areas were significantly less likely to attain high-quality diabetes care compared with those in less deprived and urban areas, calling for geographically targeted efforts to improve care quality and health outcomes for disadvantaged populations.
Abstract
Importance
Diabetes management operates under a complex interrelationship between behavioral, social, and economic factors that affect a patient's ability to self-manage and access care.
Objective
To examine the association between 2 complementary area-based metrics, area deprivation index (ADI) score and rurality, and optimal diabetes care.
Design, Setting, and Participants
This cross-sectional study analyzed the electronic health records of patients who were receiving care at any of the 75 Mayo Clinic or Mayo Clinic Health System primary care practices in Minnesota, Iowa, and Wisconsin in 2019. Participants were adults with diabetes aged 18 to 75 years. All data were abstracted and analyzed between June 1 and November 30, 2020.
Main Outcomes and Measures
The primary outcome was the attainment of all 5 components of the D5 metric of optimal diabetes care: glycemic control (hemoglobin A1c <8.0%), blood pressure (BP) control (systolic BP <140 mm Hg and diastolic BP <90 mm Hg), lipid control (use of statin therapy according to recommended guidelines), aspirin use (for patients with ischemic vascular disease), and no tobacco use. The proportion of patients receiving optimal diabetes care was calculated as a function of block group–level ADI score (a composite measure of 17 US Census indicators) and zip code–level rurality (calculated using Rural-Urban Commuting Area codes). Odds of achieving the D5 metric and its components were assessed using logistic regression that was adjusted for demographic characteristics, coronary artery disease history, and primary care team specialty.
Results
Among the 31 934 patients included in the study (mean [SD] age, 59 [11.7] years; 17 645 men [55.3%]), 13 138 (41.1%) achieved the D5 metric of optimal diabetes care. Overall, 4090 patients (12.8%) resided in the least deprived quintile (quintile 1) of block groups and 1614 (5.1%) lived in the most deprived quintile (quintile 5), while 9193 patients (28.8%) lived in rural areas and 2299 (7.2%) in highly rural areas. The odds of meeting the D5 metric were lower for individuals residing in quintile 5 vs quintile 1 block groups (odds ratio [OR], 0.72; 95% CI, 0.67-0.78). Patients residing in rural (OR, 0.84; 95% CI, 0.73-0.97) and highly rural (OR, 0.81; 95% CI, 0.72-0.91) zip codes were also less likely to attain the D5 metric compared with those in urban areas.
Conclusions and Relevance
This cross-sectional study found that patients living in more deprived and rural areas were significantly less likely to attain high-quality diabetes care compared with those living in less deprived and urban areas. The results call for geographically targeted population health management efforts by health systems, public health agencies, and payers.
Introduction
In the US, 34.1 million people, or 13% of the population, are living with diabetes.1 Diabetes and its complications are associated with poor health,2 shortened life expectancy,3,4 and impaired quality of life.5,6 In 2017 alone, $237 billion was spent in the US on direct medical costs related to this disease.2 The burden of diabetes and its complications is disproportionately larger for racial and ethnic minority groups, low-income individuals, and rural residents.7,8,9 Factors in high rates of diabetes complications in these underserved populations include suboptimal control of hyperglycemia and other cardiovascular disease risk factors,10 including hypertension, dyslipidemia, and tobacco smoking.8,11 To improve the quality of diabetes care, health systems and payers rely on publicly reported metrics to evaluate care delivery, identify opportunities for improvement, and support pay-for-performance reimbursement. However, achieving equity in diabetes care also requires these metrics to be leveraged to identify populations and regions with lagging health outcomes. When such data are available in real time, quality metrics can generate actionable information for clinicians, health systems, public health agencies, and payers on subgroups of patients in need of additional support and targeted interventions.
Diabetes management is challenging because of the complex interactions among multiple behavioral, social, and economic factors in a patient's ability to self-manage and access necessary care. Epidemiologic studies on the prevalence of diabetes have found substantial disparities across each of the 5 constructs that are most commonly represented in the social determinants of health framework12: economic stability,13,14 educational level,15 neighborhood and built environment,16 health and health care,17,18 and social and community context.8,19 However, most studies examining disparities in diabetes management and outcomes have focused on select socioeconomic conditions without capturing the full complexity of a patient’s situation, such as poverty and social or environmental context.8
One measure that captures area-level social determinants of health is the area deprivation index (ADI). The ADI score is a composite indicator of area-based socioeconomic disadvantages in the 4 domains outside of the strictly defined health care setting: income, housing, employment, and education. Previous studies have revealed disparities in cancer screening,20 opioid use,21 and drug-related mortality21 as a function of the ADI score. However, evidence is scarce on the association between area-level deprivation (as captured by a multifaceted indicator of social determinants of health, such as the ADI) and diabetes care quality. Even less contemporary evidence is available about the potential differences in diabetes care quality between rural and urban areas as well as about the potential intersection between rurality and deprivation.
To address these critical knowledge gaps and demonstrate how electronic health record (EHR) data can be the basis of real-time evaluations of care quality and equity in routine clinical practice, we examined the associations between 2 complementary area-based metrics, ADI score and rurality, and optimal diabetes care as defined by a quality measure that is frequently used in population health management. Specifically, in this study, we focused on the D5 composite quality metric of optimal diabetes care, a measure that was developed and is tracked by Minnesota Community Measures and is used by health care organizations and health care practices in Minnesota. Minnesota Community Measures defined optimal diabetes care as having the following outcome: a hemoglobin A1c (HbA1c) level that is less than 8.0%; a blood pressure (BP) reading that is less than 140/90 mm Hg; statin use that is appropriate for the patient’s age, low-density lipoprotein cholesterol (LDL-C) level, and history of cardiovascular disease; aspirin use that is appropriate in the setting of ischemic vascular disease; and abstinence from tobacco use.22 Public reporting of the D5 metric performance is mandatory for all health care practices in Minnesota and is used to guide performance-based reimbursement. Understanding the association between these components of optimal diabetes care and the area-based metrics of ADI score and rurality can help inform the multidisciplinary and multifaceted efforts to improve both the quality and equity of diabetes care.
Methods
This cross-sectional analysis of EHR data included adults aged 18 years or older who received primary care at any of the 75 Mayo Clinic and Mayo Clinic Health System primary care practices across 54 communities (towns or cities) in Minnesota, Iowa, and Wisconsin. All data were abstracted and analyzed between June 1 and November 30, 2020. The study was approved by the Mayo Clinic Institutional Review Board, which waived the requirement for informed consent because the study was deemed to pose minimal risk. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.23
Study Population
The primary analysis was conducted among patients aged 18 to 75 years who had an established diagnosis of diabetes as of December 31, 2019, and who were empaneled to a Mayo Clinic or Mayo Clinic Health System primary care practice in Minnesota, Iowa, and Wisconsin. Patients with a diabetes diagnosis during a clinical encounter were identified using International Statistical Classification of Diseases, Tenth Revision, Clinical Modification codes. The age subgroup was selected according to the eligibility criteria for the quality measure reported to Minnesota Community Measures.22
Each patient’s primary address was linked to a block group using the Census Geocoder, a program for obtaining latitude and longitude points for each address.24 The coordinates were spatially joined to a TIGER/Line block group Shapefile in ArcMap 10.7 (Esri). We excluded patients without a valid zip code and patients whose address could not be geocoded with a match score greater than 60, a measure that represents how reliably the patient address matched a candidate in the reference data. Patients were assigned a Rural-Urban Commuting Area (RUCA) code based on the zip code of their residence.25,26
Outcomes
The primary outcome was the attainment of all 5 components of the D5 metric of optimal diabetes care,22 as recorded in the EHR in 2019. The EHR was used to ascertain the performance on the D5 metric not only in this research but also in clinical practice for reporting to Minnesota Community Measures. Achievement of the D5 metric components means showing glycemic control (HbA1c <8.0%), BP control (systolic BP <140 mm Hg and diastolic BP <90 mm Hg), lipid control (use of statin therapy according to recommended guidelines), aspirin use (for patients with ischemic vascular disease), and confirmed (self-reported) no tobacco use.27
Guideline-recommended statin use is dependent on age, LDL-C level within the past 5 years, and history of cardiovascular disease. Specifically, the following criteria need to be present for the statin indicator to be successfully met: 18 to 20 years of age regardless of LDL-C level (ie, they are not required to be treated with a statin but meet this metric regardless); 21 to 39 years of age with either an LDL-C level that is less than 190 mg/dL or treatment with a statin; 40 to 75 years of age with either an LDL-C level that is less than 70 mg/dL or treatment with a statin; and any age with a history of vascular disease and either an LDL-C level that is less than 40 mg/dL or treatment with a statin (to convert LDL-C level to millimoles per liter, multiply by 0.0259). Patients with a documented contraindication (eg, pregnancy), intolerance, or allergy to statin therapy automatically meet the statin measure without the requirement for a documented statin prescription or particular LDL-C level. An active prescription for daily aspirin use is required only for individuals with established ischemic vascular disease and no documented allergy, intolerance, or contraindication; all patients without ischemic vascular disease or patients with allergy, intolerance, or contraindication automatically meet this measure even without an aspirin prescription.
The secondary outcomes were meeting each subcriterion of the D5 metric. The exception was aspirin use because nearly all (99.3%) patients in the study population met this metric.
At Mayo Clinic Health System, performance on the D5 metric is calculated and internally shared at the regional, clinic, care team, and individual clinician levels. Regions and clinics function semiautonomously with regional approaches to care quality, including for diabetes. Each practice within the Mayo Clinic Health System has autonomy to implement local quality improvement initiatives, with no standard approach across the health system.
Area Deprivation Index
We examined achieving the D5 metric (primary outcome) and individually meeting the 4 nonaspirin subcriteria of the D5 metric (secondary outcomes) as a function of the block group ADI score. Block group–level information that was necessary for ADI score derivation was obtained from the 5-year American Community Survey, an annual survey conducted by the US Census Bureau that provides population-level estimates that are representative of the noninstitutionalized US population.28 In-depth survey methods are found on the US Census Bureau website.28,29
We used 17 block-group indicators, representing income, employment, housing, and education, to compute ADI scores for all US Census block groups.30,31,32,33 All block groups were ranked by ADI scores. Each block group was assigned to a quintile of ADI scores from the least deprived 20% of block groups (quintile 1) to the most deprived 20% of block groups (quintile 5). Weights that were assigned to each variable in the ADI are presented in the eTable in the Supplement. A geographic hot spot map of block group ADI scores in Minnesota, Iowa, and Wisconsin (n = 11 230) was created and has been previously described.20
Independent Variables
Rurality was ascertained from patient zip codes to identify corresponding RUCA codes. Based on published definitions, the RUCA codes classified areas as urban (1.0, 1.1, 2.0, 2.1, 3.0, 4.1, 5.1, 7.1, 8.1, 10.1), rural (4.0, 4.2, 5.0, 5.2, 6.0, 6.1, 7.0, 7.2, 7.3, 7.4, 8.0, 8.2, 8.3, 8.4, 9.0, 9.1, 9.2), or highly rural (10.0, 10.2, 10.3, 10.4, 10.5, 10.6).34 Zip code–level RUCA codes were used as individual risk factors to make patient-level inferences, given that block group–level RUCA codes were not available through the US Department of Agriculture.25,26 Detailed information regarding RUCA codes can be found on the US Department of Agriculture website.26,34
Patient demographic characteristics (sex, race and ethnicity, and age), history of coronary artery disease, and primary care team specialty (internal medicine, family medicine, or other) were ascertained from the EHR. Race and ethnicity were classified as White or racial and ethnic minority group. This group comprised African, African American, American Indian/Alaskan Native, Asian (including subcategories that were based on country of origin such as Cambodian, Chinese, Filipino, Indian, Japanese, Korean, Laotian, Pakistani, Taiwanese, Thai, and Vietnamese), Black, Caribbean Black, Native Hawaii/Pacific Islander, and Samoan; those who did not provide race and ethnicity, responded with “other,” or identified 2 or more affiliations were also included. These categories were combined because of the small number of patients representing each category, which would preclude analyses. Race and ethnicity were self-reported by the patient during registration and documented in the EHR.
Statistical Analysis
We calculated overall frequencies (percentages) and means (SDs) for baseline patient characteristics. We used multivariable logistic regression to examine the associations between ADI score, rurality, and the outcomes (ie, primary outcome of attaining the D5 metric and secondary outcomes of meeting the nonaspirin subcriteria of the D5 metric). Independent variables in the models included ADI scores by quintile, rural status, age, race and ethnicity, sex, history of coronary artery disease, and care team specialty. We used Huber-White robust SEs clustered at the practice level to adjust SEs for variation in care delivery, resources, and recommendations across the primary care clinics. Analyses were conducted to test for an interaction between ADI score and rurality, and no interaction was found.
A 2-sided P = .05 was used as the threshold of statistical significance. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc), and Stata, version 15.1 (StataCorp LLC).
Results
The study cohort comprised 31 934 patients (17 645 men [55.3%] and 14 289 women [44.8%]) who were eligible for the D5 metric assessment. These patients had a mean (SD) age of 59 (11.7) years and were predominantly White individuals (n = 29 180 [91.4%]). Overall, 1614 patients (5.1%) lived in the most deprived quintile (quintile 5) and 4090 (12.8%) lived in the least deprived quintile (quintile 1); 9193 patients (28.8%) lived in rural areas and 2299 (7.2%) lived in highly rural areas (Table 1).
Table 1. Patient Characteristics at the Start of the Measurement Year.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Patients who attained the D5 metric (n = 13 138) | Patients who did not attain the D5 metric (n = 18 796) | All patients (n = 31 934) | |
| Age, y | |||
| Mean (SD) | 57.8 (12.0) | 61.8 (10.9) | 59 (11.7) |
| 18-44 | 1070 (8.1) | 2671 (14.2) | 3741 (11.7) |
| 45-64 | 5505 (41.9) | 9700 (51.6) | 15 205 (47.6) |
| 65-75 | 6536 (49.9) | 6425 (34.2) | 12 988 (40.7) |
| Sex | |||
| Female | 6119 (46.6) | 8170 (43.5) | 14 289 (44.8) |
| Male | 7019 (53.4) | 10 626 (56.5) | 17 645 (55.3) |
| Race and ethnicitya | |||
| Racial and ethnic minority groupb | 948 (7.2) | 1806 (9.6) | 2754 (8.6) |
| White | 12 190 (92.8) | 16 990 (90.4) | 29 180 (91.4) |
| Coronary artery disease | |||
| Present or previous diagnosis | 2096 (16.0) | 2544 (13.5) | 4640 (14.5) |
| No history | 11 042 (84.1) | 16 252 (86.5) | 27 294 (85.5) |
| ADI score quintile | |||
| 1 (least deprived) | 1793 (13.6) | 2297 (12.2) | 4090 (12.8) |
| 2 | 4669 (35.5) | 6109 (32.5) | 10 778 (33.6) |
| 3 | 3971 (30.2) | 5720 (30.4) | 9691 (30.4) |
| 4 | 2163 (16.5) | 3598 (19.1) | 5761 (18.0) |
| 5 | 542 (4.1) | 1072 (5.7) | 1614 (5.1) |
| Rurality | |||
| Urban | 8771 (66.8) | 11 671 (62.1) | 20 442 (64.0) |
| Rural | 3492 (26.5) | 5701 (30.3) | 9193 (28.8) |
| Highly rural | 875 (6.7) | 1424 (7.6) | 2299 (7.2) |
| Practice specialty | |||
| Internal medicine | 3311 (25.2) | 4521 (24.1) | 7832 (24.5) |
| Family medicine | 9413 (71.6) | 13 779 (73.3) | 23 192 (72.6) |
| Otherc | 414 (3.2) | 496 (2.6) | 910 (2.9) |
| D5 metric components | |||
| Glycemic control | 13 138 (100.0) | 7833 (41.7) | 20 971 (65.7) |
| Blood pressure control | 13 138 (100.0) | 11 188 (60.0) | 24 326 (76.2) |
| Lipid control | 13 138 (100.0) | 14 581 (77.6) | 27 719 (86.8) |
| No tobacco use | 13 138 (100.0) | 12 820 (68.2) | 25 958 (81.3) |
| Aspirin use | 13 138 (100.0) | 18 566 (98.8) | 31 704 (99.3) |
Abbreviation: ADI, area deprivation index.
Race and ethnicity were self-reported by the patient and documented in the electronic health record.
This group comprised African, African American, American Indian/Alaskan Native, Asian (including subcategories that were based on country of origin such as Cambodian, Chinese, Filipino, Indian, Japanese, Korean, Laotian, Pakistani, Taiwanese, Thai, and Vietnamese), Black, Caribbean Black, Native Hawaii/Pacific Islander, and Samoan. Those who did not provide race and ethnicity, responded with “other,” or identified 2 or more affiliations were also included. These categories were combined because of the small number of patients representing each category, which would preclude analyses.
Other included mixed team, nursing home, pediatric resident, pediatrics, and women’s health.
Overall, 13 138 of 31 934 patients (41.1%) achieved the composite D5 metric of optimal diabetes care. Patients who attained the D5 metric compared with those who did not (n = 18 796) often were older (aged 65-75 years: 6536 [49.9%] vs 6425 [34.2%]), women (6119 [46.6%] vs 8170 [43.5%]), White individuals (12 190 [92.8%] vs 16 990 [90.4%]), and those who resided in less deprived and less rural areas. Patients who met the D5 metric had a slightly higher mean (SD) number of clinician visits for primary care, endocrinology, and diabetes education compared with those who did not meet the D5 metric (5.00 [5.68] vs 4.32 [6.00]).
Association of ADI Score With the D5 Metric
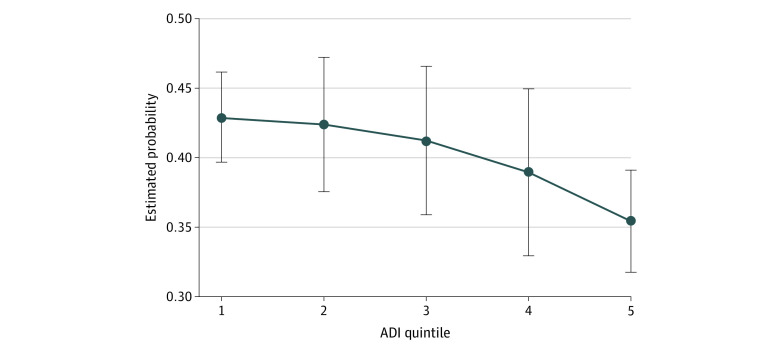
Block group–level ADI score was associated with achieving the composite D5 metric and the individual glycemic control and no tobacco use components. As shown in the Figure, the adjusted probability of attaining the D5 metric decreased incrementally across ADI score quintiles (Table 2). The odds of meeting the D5 metric goals were approximately 28% lower for individuals who were living in quintile 5 vs those living in quintile 1 (odds ratio [OR], 0.72; 95% CI, 0.67-0.78).
Figure. Estimated Probability of Attaining the D5 Metric by Area Deprivation Index (ADI) Score Quintile.
Error bars represent 95% CIs. Estimated probabilities were adjusted for the covariates shown in Table 2.
Table 2. Association Between Area Deprivation Index Score, Rurality, and Quality of Diabetes Carea.
| OR (95% CI) | |||||
|---|---|---|---|---|---|
| All D5 metric components | Glycemic control | Blood pressure control | Lipid control | No tobacco use | |
| ADI score quintile | |||||
| 1 (least deprived) | 1 [Referent] | [Referent] | [Referent] | [Referent] | [Referent] |
| 2 | 0.98 (0.91-1.05) | 0.99 (0.86-1.13) | 1.12 (1.02-1.22)b | 0.94 (0.86-1.02) | 0.70 (0.60-0.80)b |
| 3 | 0.93 (0.84-1.04) | 0.95 (0.83-1.09) | 1.19 (1.03-1.38)b | 0.99 (0.87-1.14) | 0.54 (0.48-0.61)b |
| 4 | 0.84 (0.74-0.97)b | 0.85 (0.74-0.98)b | 1.17 (0.99-1.39) | 0.88 (0.78-1.00)b | 0.46 (0.39-0.55)b |
| 5 | 0.72 (0.67-0.78)b | 0.78 (0.74-0.84)b | 1.00 (0.82-1.21) | 1.12 (1.03-1.22)b | 0.38 (0.31-0.48)b |
| Rurality | |||||
| Urban | [Referent] | [Referent] | [Referent] | [Referent] | [Referent] |
| Rural | 0.84 (0.73-0.97)b | 0.87 (0.75-1.00) | 0.82 (0.67-1.01) | 0.88 (0.81-0.95)b | 0.95 (0.88-1.03) |
| Highly rural | 0.81 (0.72-0.91)b | 0.90 (0.84-0.98)b | 0.93 (0.75-1.16) | 0.83 (0.77-0.89)b | 0.92 (0.78-1.08) |
| Age, y | |||||
| 18-44 | [Referent] | [Referent] | [Referent] | [Referent] | [Referent] |
| 45-64 | 1.41 (1.27-1.57)b | 1.65 (1.48-1.84)b | 0.95 (0.90-1.01) | 0.94 (0.81-1.08) | 1.49 (1.39-1.59)b |
| 65-75 | 2.49 (2.25-2.76)b | 3.00 (2.51-3.60)b | 1.03 (0.93-1.15) | 1.74 (1.51-2.00)b | 3.33 (3.20-3.48)b |
| Sex | |||||
| Female | [Referent] | [Referent] | [Referent] | [Referent] | [Referent] |
| Male | 0.87 (0.83-0.92)b | 0.87 (0.84-0.90)b | 0.84 (0.80-0.89)b | 1.10 (0.98-1.24) | 0.69 (0.63-0.75)b |
| Race and ethnicityc | |||||
| Racial and ethnic minority groupd | 0.85 (0.71-1.02) | 0.72 (0.61-0.84)b | 0.83 (0.72-0.95)b | 0.76 (0.67-0.85)b | 1.18 (1.02-1.36)b |
| White | [Referent] | [Referent] | [Referent] | [Referent] | [Referent] |
| Coronary artery disease | |||||
| No history | [Referent] | [Referent] | [Referent] | [Referent] | [Referent] |
| Present or previous diagnosis | 1.04 (0.98-1.09) | 1.01 (0.90-1.14) | 1.23 (1.18-1.27)b | 3.52 (3.02-4.10)b | 0.76 (0.74-0.78)b |
| Practice specialty | |||||
| Internal medicine | [Referent] | [Referent] | [Referent] | [Referent] | [Referent] |
| Family medicine | 1.04 (0.94-1.16) | 1.08 (0.96-1.21) | 1.17 (0.87-1.58) | 0.89 (0.73-1.09) | 0.79 (0.73-0.85)b |
| Othere | 1.16 (0.83-1.62) | 1.12 (0.93-1.34) | 1.24 (0.97-1.59) | 1.08 (0.66-1.76) | 0.96 (0.74-1.26) |
Abbreviations: ADI, area deprivation index; OR, odds ratio.
Multivariable logistic regression analysis examined the association between ADI score, rurality, and achieving the D5 metric components of optimal diabetes care (primary outcome) and meeting the subcriteria of the D5 metric (secondary outcomes) after adjusting for the patient-level demographic and clinical factors in this table.
P < .05.
Race and ethnicity were self-reported by the patient and documented in the electronic health record.
This group comprised African, African American, American Indian/Alaskan Native, Asian (including subcategories that were based on country of origin such as Cambodian, Chinese, Filipino, Indian, Japanese, Korean, Laotian, Pakistani, Taiwanese, Thai, and Vietnamese), Black, Caribbean Black, Native Hawaii/Pacific Islander, and Samoan. Those who did not provide race and ethnicity, responded with “other,” or identified 2 or more affiliations were also included. These categories were combined because of the small number of patients representing each category, which would preclude analyses.
Other included mixed team, nursing home, pediatric resident, pediatrics, and women’s health.
Within the D5 metric, the most variability was observed for the glycemic control and no tobacco use components. The odds of achieving an HbA1c level that was less than 8.0% were 22% lower for individuals living in quintile 5 vs quintile 1 (OR, 0.78; 95% CI, 0.74-0.84). The odds of meeting the no tobacco use metric decreased progressively as deprivation increased, with patients residing in quintile 5 being 62% less likely to achieve no tobacco use compared with patients living in quintile 1 (OR, 0.38; 95% CI, 0.31-0.48). The odds of meeting the BP control component were highest in patients residing in quintile 2 (OR, 1.12; 95% CI, 1.02-1.22) and quintile 3 (OR, 1.19; 95% CI, 1.03-1.38) compared with those residing in quintile 1 block groups. For lipid control, patients in quintile 5 block groups were significantly more likely to meet the component compared with those in quintile 1 block groups (OR, 1.12; 95% CI, 1.03-1.22).
Association of Rurality With the D5 Metric
Patients residing in rural zip codes were 16% less likely to attain the D5 metric compared with those living in urban zip codes (OR, 0.84; 95% CI, 0.73-0.97) (Table 2). Patients residing in highly rural zip codes were 19% less likely to achieve the composite D5 metric (OR, 0.81; 95% CI, 0.72-0.91) and were 10% less likely to achieve glycemic control (OR, 0.90; 95% CI, 0.84-0.98) than those living in urban areas. Patients from both rural (OR, 0.88; 95% CI, 0.81-0.95) and highly rural (OR, 0.83; 95% CI, 0.77-0.89) zip codes were less likely to achieve lipid control.
Blood pressure control and no tobacco use were not associated with rurality. We also tested for an interaction between ADI score and rurality, which was not present; hence, the interaction terms were not included in the final model.
Patient-Level Factors Associated With Diabetes Care Quality
Patients from racial and ethnic minority groups with diabetes had lower odds of meeting the glycemic, lipid, and BP control components of the D5 metric compared with White patients, although no significant association was found between race and ethnicity and the D5 metric (Table 2). Older patients were more likely to attain the D5 metric (age 45-64 years: OR, 1.42 [95% CI, 1.27-1.57]; age 65-75 years: OR, 2.49 [95% CI, 2.25-2.76] vs age 18-44 years) as well as the glycemic control (age 45-64 years: OR, 1.65 [95% CI, 1.48-1.84]; age 65-75 years: OR, 3.00 [95% CI, 2.51-3.60] vs age 18-44 years), lipid control (age 65-75 years: OR, 1.74 [95% CI, 1.51-2.00] vs age 18-44 years), and no tobacco use (age 45-64 years: OR, 1.49 [95% CI, 1.39-1.59]; age 65-75 years: OR, 3.33 [95% CI, 3.20-3.48] vs age 18-44 years) components. Patients with coronary artery disease were more likely to achieve BP control (OR, 1.23; 95% CI, 1.18-1.27) and lipid control (OR, 3.52; 95% CI, 3.02-4.10) but less likely to achieve no tobacco use (OR, 0.76; 95% CI, 0.74-0.78). Men had lower odds of attaining the complete and individual D5 metric components, with the exception of lipid control, which showed no significant association with sex.
Discussion
Population health strategies to improve the quality and equity of diabetes management and health outcomes require real-time tracking of performance and identification of populations in need of focused interventions. Geographic areas with gaps in care quality may benefit from targeted allocation of resources to address the factors associated with suboptimal care delivery and health outcomes. To identify areas of suboptimal diabetes care, we used clinical data from 75 primary care practices across 3 states that were linked to publicly available geographic information from the US Census Bureau. We found that adult patients with diabetes who lived in more deprived and in rural areas were significantly less likely to achieve high-quality diabetes care, as measured by the D5 metric, compared with patients who lived in less deprived and urban areas. These findings not only underscore the implications of area-level social determinants of health for diabetes care quality but also signal the need for geographically targeted population health management efforts by health systems, public health agencies, and payers.
People living in socioeconomically deprived areas face multiple obstacles to optimal diabetes care. Rates of type 2 diabetes are substantially higher in neighborhoods that are characterized by lower income, lower educational attainment, single-parent households, and crowded housing.27,35 Individuals living in areas of greater deprivation often have fewer financial resources, lower health literacy, greater comorbidity burden, and higher food insecurity.8 In addition, deprived neighborhoods are associated with an increased rate of obesity and decreased physical activity.36 These spatial social determinants of health are associated with the risk of developing diabetes, barriers to optimal self-management, and greater risk of diabetes-related complications. Clinics, health systems, payers, and public health agencies can, therefore, improve diabetes management and health outcomes in neighborhoods by addressing some of the structural factors that stymie optimal care.8 For example, health systems can increase the availability of social services in clinics; educate health care staff on screening for social determinants of health; bring medical education and outreach to trusted community centers; support food banks with nutritious food options37 and integrated diabetes self-management education or support; and partner with municipalities and public health agencies to build community centers,38 parks,38 and supportive housing.39 Payers can reduce patient cost-sharing responsibilities for diabetes-related care (eg, medications, durable medical equipment and supplies, and appointments),40,41 reimburse social services and support programs (such as community health worker and community paramedic programs),42 and lower insurance premiums for patients who adhere to treatment recommendations and improve their health.
Rural areas have a 17% higher prevalence of diabetes than urban areas.43,44 Yet few studies have examined the quality of diabetes care in rural communities, mostly because available data are scarce and sample sizes are small.45 The present study involved a large primary care population across 75 clinics in 3 Midwestern states, allowing the examination of both urban and rural settings. We found that patients in rural areas were significantly less likely to receive high-quality diabetes care, as measured by the D5 metric, reinforcing the gaps in access to care and care quality in rural communities.46 The role of rurality in diabetes care quality was independent of the ADI score, signifying the additional constraints on optimal diabetes care posed by rurality. Prior research has found that rural residents had higher rates of being uninsured or underinsured and had fewer resources, both medical and nonmedical, to optimally care for their diabetes. This lack of resources included fewer primary care and specialist clinicians as well as less access to diabetes education, exercise facilities, sidewalks for walking, and grocery stores with affordable produce.8,43,46 Patients who lived in rural areas were often unable to order glucose test strips47,48 and may forgo routine screening appointments.49 All these factors may contribute to the worse quality of diabetes care observed in the current study among patients living in rural and highly rural areas.
These geographic disparities that affect rural populations present major challenges for patients with diabetes and point to the need for geographically tailored interventions that take into consideration the specific resources available in rural sites. Implementing telemedicine capabilities or mobile telehealth units for diabetes care in difficult-to-reach and underserved areas may address access-related issues. However, telemedicine would not be a viable option for patients without broadband internet, which is frequently a barrier in highly rural areas. Community health workers,8 community paramedics,50,51 and endocrinology experts in rural primary care practices (eg, Endo ECHO model)52,53 can help address some of the gaps in rural and remote areas.
Components of the D5 metric that were associated with the ADI score were glycemic control and no tobacco use, with residents of the most deprived areas being 22% less likely to achieve an HbA1c level that was less than 8.0% and 62% less likely to not use tobacco compared with residents of the least deprived areas. Both of these metrics were outcome indicators that relied heavily on individual behavior change, medication adherence (for glycemic control), and self-efficacy. This finding contrasts with the lipid control and aspirin use metrics, which were process indicators that were achieved simply by clinicians prescribing the appropriate pharmacotherapy. Although an outcome indicator of lipid control would face the same challenges as the glycemic control, BP control, and no tobacco use components, the process indicator of having an active statin prescription on file (regardless of whether that statin prescription was filled or what LDL-C level was achieved) was easier to meet. Lifestyle therapy (ie, medical nutrition therapy) is a core component of successful glycemic control, and patients in socioeconomically deprived areas are more likely to experience food insecurity, lack of nutritious food choices, and inadequate safe spaces for physical activity. In addition, many glucose-lowering medications are expensive, making it challenging for low-income patients to access and fully adhere to clinically preferred treatment regimens. Previous studies found that access to diabetes self-management education in low-income and rural areas was inadequate,54,55 which was associated with greater probability of forgoing medical care.56,57 All of these structural factors need to be addressed to improve diabetes care quality in socioeconomically deprived areas.
Similarly, although the prevalence of smoking has declined in the US over the past 60 years, tobacco use remains concentrated among low-income and other socioeconomically disadvantaged populations.58 Therefore, focused smoking and other tobacco use–cessation interventions are needed that are tailored to residents of socioeconomically deprived areas. Such efforts include referral pathways for free smoking-cessation programs59; expanded access to smoking-cessation counseling and medication benefits; antismoking media, social media, and community campaigns60,61,62,63; and smoking bans in public housing.64
Blood pressure control is another outcome measure but is generally more amenable to pharmacologic intervention than glycemic control. Still, it is unclear why patients in both the least and the most deprived ADI score quintiles were less likely to achieve the BP control metric than patients in the middle quintiles (which is a finding that is consistent with results of previous work).65 A recent population-based study of patients with diabetes across the US found that patients with both high and low incomes, according to the federal poverty level, were less likely to be treated with antihypertensive medications than patients with an intermediate income level,66 suggesting that those in both extremes of income may be more likely to be undertreated for their hypertension. However, in other studies, no significant association was found between BP control and either income or rurality.67 Thus, the reasons behind better BP control among patients residing in lower-income areas will need to be explored in future research.
Independent of area-level factors (ie, ADI score and rurality), patients from racial and ethnic minority groups were significantly less likely to attain high-quality diabetes care. Our findings build on robust literature that confirmed racial disparities in diabetes-related health outcomes, including higher rates of both acute (ie, severe hypoglycemia68,69,70,71 and hyperglycemia or ketoacidosis68,69) and chronic (ie, kidney failure,72 amputation,72 and cardiovascular disease72) complications among Black patients with diabetes compared with White patients. As a result of historical and contemporary residential segregation, predominantly Black neighborhoods are more likely to be characterized by food deserts, fewer recreational facilities, environmental chemicals and toxins, and lower-quality housing vs predominantly White neighborhoods.8,73 These structural barriers have all been found to be associated with diabetes prevalence and health outcomes. This finding signals a need for policy makers to tighten environmental safety regulations in areas of concentrated poverty, which are most often disadvantaged and multiracial and multiethnic neighborhoods, as well as to improve insurance access and coverage for those who are unable to afford it.
Improving health equity and eliminating health disparities are urgent national priorities.74 We believe this study provides a framework for evaluating diabetes care quality and equity through the lens of geographic disparities, yielding rapidly actionable information for health systems, policy makers, and payers to drive innovation and improvement in underserved areas.
Strengths and Limitations
This study has some strengths. To our knowledge, this study was the first multisite investigation into area-level variation in diabetes care quality that focused on socioeconomic deprivation and rurality across a diverse geographic area in 3 states. The study is strengthened by granular patient-level data that allowed us to contextualize geospatial disparities at the census block-group level with the patient’s clinical context and care.
This study also has some limitations. Because we used survey data when calculating ADI scores, the calculations are susceptible to nonresponse bias. In addition, the findings may not be generalizable to other settings because of the lower representation of racial and ethnic minority groups in the included clinical sites than in the general US population. Nevertheless, the study population was representative of the upper Midwest and rural communities across the country.75 Although we cannot draw causal inferences from this observational study, the findings show how readily available EHR data and tools can be used to track not only the quality but also the equity of chronic disease care. Such data, when available in real time, can inform and support interventions to improve the health of all people with diabetes.
Conclusions
This cross-sectional study found that adult patients with diabetes in areas that were more socioeconomically deprived and rural were significantly less likely to attain the D5 metric of optimal diabetes care compared with patients who lived in less deprived and urban areas. Geographically targeted population health management efforts by health systems, public health agencies, and payers are needed to improve the care quality and health outcomes for disadvantaged populations.
eTable. American Community Survey 5-Year Estimates and Factor Score Coefficients
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020: estimates of diabetes and its burden in the United States. Accessed August 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 2.American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi: 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985-2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867-874. doi: 10.1016/S2213-8587(14)70161-5 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2014: estimates of diabetes and its burden in the United States. Accessed May 23, 2020. https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
- 5.Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120-129. doi: 10.4239/wjd.v8.i4.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478-2483. doi: 10.2337/dc07-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579-E1639. doi: 10.1210/jc.2012-2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258-279. doi: 10.2337/dci20-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):359-361. doi: 10.15585/mmwr.mm6712a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington RA, Califf RM, Balamurugan A, et al. Call to action: rural health: a presidential advisory from the American Heart Association and American Stroke Association. Circulation. 2020;141(10):e615-e644. doi: 10.1161/CIR.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 11.Haw JS, Shah M, Turbow S, Egeolu M, Umpierrez G. Diabetes complications in racial and ethnic minority populations in the USA. Curr Diab Rep. 2021;21(1):2. doi: 10.1007/s11892-020-01369-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asare M, Flannery M, Kamen C. Social determinants of health: a framework for studying cancer health disparities and minority participation in research. Oncol Nurs Forum. 2017;44(1):20-23. doi: 10.1188/17.ONF.20-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):804-818. doi: 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- 14.Brown AF, Ettner SL, Piette J, et al. Socioeconomic position and health among persons with diabetes mellitus: a conceptual framework and review of the literature. Epidemiol Rev. 2004;26:63-77. doi: 10.1093/epirev/mxh002 [DOI] [PubMed] [Google Scholar]
- 15.Borrell LN, Dallo FJ, White K. Education and diabetes in a racially and ethnically diverse population. Am J Public Health. 2006;96(9):1637-1642. doi: 10.2105/AJPH.2005.072884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkowitz SA, Kalkhoran S, Edwards ST, Essien UR, Baggett TP. Unstable housing and diabetes-related emergency department visits and hospitalization: a nationally representative study of safety-net clinic patients. Diabetes Care. 2018;41(5):933-939. doi: 10.2337/dc17-1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005-2016. JAMA Intern Med. 2019;179(10):1376-1385. doi: 10.1001/jamainternmed.2019.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danaei G, Friedman AB, Oza S, Murray CJ, Ezzati M. Diabetes prevalence and diagnosis in US states: analysis of health surveys. Popul Health Metr. 2009;7:16. doi: 10.1186/1478-7954-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flôr CR, Baldoni NR, Aquino JA, et al. What is the association between social capital and diabetes mellitus? a systematic review. Diabetes Metab Syndr. 2018;12(4):601-605. doi: 10.1016/j.dsx.2018.03.021 [DOI] [PubMed] [Google Scholar]
- 20.Kurani SS, McCoy RG, Lampman MA, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open. 2020;3(3):e200618. doi: 10.1001/jamanetworkopen.2020.0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurani S, McCoy RG, Inselman J, et al. Place, poverty and prescriptions: a cross-sectional study using Area Deprivation Index to assess opioid use and drug-poisoning mortality in the USA from 2012 to 2017. BMJ Open. 2020;10(5):e035376. doi: 10.1136/bmjopen-2019-035376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minnesota Community Measures . Measurement resources. Accessed January 6, 2021. https://mncm.org/measurement-resources/
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 24.Goldberg DW, Ballard M, Boyd JH, et al. An evaluation framework for comparing geocoding systems. Int J Health Geogr. 2013;12:50. doi: 10.1186/1476-072X-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson V, Wong E, Lampman M, et al. Comparing patient-centered medical home implementation in urban and rural VHA clinics: results from the patient aligned care team initiative. J Ambul Care Manage. 2018;41(1):47-57. doi: 10.1097/JAC.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 26.US Department of Agriculture . Rural-urban commuting area codes. Accessed July 15, 2019. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- 27.Meyerink BD, Lampman MA, Laabs SB, et al. Relationship of clinician care team composition and diabetes quality outcomes. Popul Health Manag. 2021;24(4):502-508. doi: 10.1089/pop.2020.0229 [DOI] [PubMed] [Google Scholar]
- 28.US Census Bureau . American Community Survey. Accessed July 5, 2019. https://www.census.gov/programs-surveys/acs
- 29.US Census Bureau . Design and methodology report. Accessed July 1, 2019. https://www.census.gov/programs-surveys/acs/methodology/design-and-methodology.html
- 30.Singh GK. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003;93(7):1137-1143. doi: 10.2105/AJPH.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS (Wash DC). 2016;4(3):1238. doi: 10.13063/2327-9214.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RUCA Rural Health Research Center . Four category classification, census division. Accessed April 28, 2021. https://depts.washington.edu/uwruca/ruca-maps.php
- 35.Kolak M, Abraham G, Talen MR. Mapping census tract clusters of type 2 diabetes in a primary care population. Prev Chronic Dis. 2019;16:E59. doi: 10.5888/pcd16.180502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drewnowski A, Buszkiewicz J, Aggarwal A, Rose C, Gupta S, Bradshaw A. Obesity and the built environment: a reappraisal. Obesity (Silver Spring). 2020;28(1):22-30. doi: 10.1002/oby.22672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seligman HK, Lyles C, Marshall MB, et al. A pilot food bank intervention featuring diabetes-appropriate food improved glycemic control among clients in three states. Health Aff (Millwood). 2015;34(11):1956-1963. doi: 10.1377/hlthaff.2015.0641 [DOI] [PubMed] [Google Scholar]
- 38.Bilal U, Auchincloss AH, Diez-Roux AV. Neighborhood environments and diabetes risk and control. Curr Diab Rep. 2018;18(9):62. doi: 10.1007/s11892-018-1032-2 [DOI] [PubMed] [Google Scholar]
- 39.Tsai J, Gelberg L, Rosenheck RA. Changes in physical health after supported housing: results from the Collaborative Initiative to End Chronic Homelessness. J Gen Intern Med. 2019;34(9):1703-1708. doi: 10.1007/s11606-019-05070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown DS, Delavar A. The Affordable Care Act and insurance coverage for persons with diabetes in the United States. J Hosp Manag Health Policy. 2018;2:2. doi: 10.21037/jhmhp.2018.04.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang JX, Bhaumik D, Huang ES, Meltzer DO. Change in insurance status and cost-related medication non-adherence among older U.S. adults with diabetes from 2010 to 2014. J Health Med Econ. 2018;4(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Association of Chronic Disease Directors . Community programs linked to clinical services—community health workers. Reimbursement/advocacy. Accessed April 27, 2021. https://chronicdisease.org/mpage/domain4/extenders/chw_ra/
- 43.Massey CN, Appel SJ, Buchanan KL, Cherrington AL. Improving diabetes care in rural communities: an overview of current initiatives and a call for renewed efforts. Clin Diabetes. 2010;28(1):20-27. doi: 10.2337/diaclin.28.1.20 [DOI] [Google Scholar]
- 44.Keppel KG, Pearcy JN, Klein RJ. Measuring progress in Healthy People 2010. Healthy People 2010 Stat Notes. 2004;(25):1-16. [PubMed] [Google Scholar]
- 45.Moscovice I, Rosenblatt R. Quality-of-care challenges for rural health. J Rural Health. 2000;16(2):168-176. doi: 10.1111/j.1748-0361.2000.tb00451.x [DOI] [PubMed] [Google Scholar]
- 46.HealthyPeople.gov . Diabetes. Accessed August 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/diabetes
- 47.Blonde L, Karter AJ. Current evidence regarding the value of self-monitored blood glucose testing. Am J Med. 2005;118(suppl 9A):20S-26S. doi: 10.1016/j.amjmed.2005.07.053 [DOI] [PubMed] [Google Scholar]
- 48.Chin MH, Cook S, Jin L, et al. Barriers to providing diabetes care in community health centers. Diabetes Care. 2001;24(2):268-274. doi: 10.2337/diacare.24.2.268 [DOI] [PubMed] [Google Scholar]
- 49.Gamm LD, Hutchison LL, Dabney BJ, Dorsey AM. Rural Healthy People 2010: A Companion Document to Healthy People 2010. The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center; 2003:1. [Google Scholar]
- 50.Martin AC, O’Meara P. Perspectives from the frontline of two North American community paramedicine programs: an observational, ethnographic study. Rural Remote Health. 2019;19(1):4888. doi: 10.22605/RRH4888 [DOI] [PubMed] [Google Scholar]
- 51.Martin A, O’Meara P, Farmer J. Consumer perspectives of a community paramedicine program in rural Ontario. Aust J Rural Health. 2016;24(4):278-283. doi: 10.1111/ajr.12259 [DOI] [PubMed] [Google Scholar]
- 52.Bouchonville MF, Paul MM, Billings J, Kirk JB, Arora S. Taking telemedicine to the next level in diabetes population management: a review of the Endo ECHO model. Curr Diab Rep. 2016;16(10):96. doi: 10.1007/s11892-016-0784-9 [DOI] [PubMed] [Google Scholar]
- 53.Bouchonville MF, Hager BW, Kirk JB, Qualls CR, Arora S. Endo ECHO improves primary care provider and community health worker self-efficacy in complex diabetes management in medically underserved communities. Endocr Pract. 2018;24(1):40-46. doi: 10.4158/EP-2017-0079 [DOI] [PubMed] [Google Scholar]
- 54.Luo H, Bell RA, Winterbauer NL, et al. Trends and rural-urban differences in participation in diabetes self-management education among adults in North Carolina: 2012-2017. J Public Health Manag Pract. 2020. doi: 10.1097/PHH.0000000000001226 [DOI] [PubMed] [Google Scholar]
- 55.Rutledge SA, Masalovich S, Blacher RJ, Saunders MM. Diabetes self-management education programs in nonmetropolitan counties—United States, 2016. MMWR Surveill Summ. 2017;66(10):1-6. doi: 10.15585/mmwr.ss6610a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Towne SD, Bolin J, Ferdinand A, Nicklett EJ, Smith ML, Ory MG. Assessing diabetes and factors associated with foregoing medical care among persons with diabetes: disparities facing American Indian/Alaska Native, Black, Hispanic, low income, and southern adults in the U.S. (2011-2015). Int J Environ Res Public Health. 2017;14(5):E464. doi: 10.3390/ijerph14050464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lutfiyya MN, McCullough JE, Mitchell L, Dean LS, Lipsky MS. Adequacy of diabetes care for older U.S. rural adults: a cross-sectional population based study using 2009 BRFSS data. BMC Public Health. 2011;11:940. doi: 10.1186/1471-2458-11-940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drope J, Liber AC, Cahn Z, et al. Who’s still smoking? disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin. 2018;68(2):106-115. doi: 10.3322/caac.21444 [DOI] [PubMed] [Google Scholar]
- 59.Kegler MC, Haardörfer R, Berg C, et al. Challenges in enforcing home smoking rules in a low-income population: implications for measurement and intervention design. Nicotine Tob Res. 2016;18(5):976-981. doi: 10.1093/ntr/ntv165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubin R. Successful CDC campaign “Tips From Former Smokers” to be expanded. JAMA. 2015;313(6):558. doi: 10.1001/jama.2014.18588 [DOI] [Google Scholar]
- 61.Fallin A, Neilands TB, Jordan JW, Ling PM. Social branding to decrease lesbian, gay, bisexual, and transgender young adult smoking. Nicotine Tob Res. 2015;17(8):983-989. doi: 10.1093/ntr/ntu265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling PM, Lee YO, Hong J, Neilands TB, Jordan JW, Glantz SA. Social branding to decrease smoking among young adults in bars. Am J Public Health. 2014;104(4):751-760. doi: 10.2105/AJPH.2013.301666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fallin A, Neilands TB, Jordan JW, Hong JS, Ling PM. Wreaking “havoc” on smoking: social branding to reach young adult “partiers” in Oklahoma. Am J Prev Med. 2015;48(1 suppl 1):S78-S85. doi: 10.1016/j.amepre.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy DE, Adams IF, Adamkiewicz G. Delivering on the promise of smoke-free public housing. Am J Public Health. 2017;107(3):380-383. doi: 10.2105/AJPH.2016.303606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doshi T, Smalls BL, Williams JS, Wolfman TE, Egede LE. Socioeconomic status and cardiovascular risk control in adults with diabetes. Am J Med Sci. 2016;352(1):36-44. doi: 10.1016/j.amjms.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med. 2021;384(23):2219-2228. doi: 10.1056/NEJMsa2032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan X, Lee LK, Huynh S, Pawaskar M, Rajpathak S. Sociodemographic disparities in the management of type 2 diabetes in the United States. Curr Med Res Opin. 2020;36(6):967-976. doi: 10.1080/03007995.2020.1756764 [DOI] [PubMed] [Google Scholar]
- 68.McCoy RG, Lipska KJ, Herrin J, Jeffery MM, Krumholz HM, Shah ND. Hospital readmissions among commercially insured and Medicare Advantage beneficiaries with diabetes and the impact of severe hypoglycemic and hyperglycemic events. J Gen Intern Med. 2017;32(10):1097-1105. doi: 10.1007/s11606-017-4095-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCoy RG, Herrin J, Lipska KJ, Shah ND. Recurrent hospitalizations for severe hypoglycemia and hyperglycemia among U.S. adults with diabetes. J Diabetes Complications. 2018;32(7):693-701. doi: 10.1016/j.jdiacomp.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Association of cumulative multimorbidity, glycemic control, and medication use with hypoglycemia-related emergency department visits and hospitalizations among adults with diabetes. JAMA Netw Open. 2020;3(1):e1919099. doi: 10.1001/jamanetworkopen.2019.19099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-Gutierrez R, Herrin J, Lipska KJ, Montori VM, Shah ND, McCoy RG. Racial and ethnic differences in 30-day hospital readmissions among US adults with diabetes. JAMA Netw Open. 2019;2(10):e1913249. doi: 10.1001/jamanetworkopen.2019.13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514-1523. doi: 10.1056/NEJMoa1310799 [DOI] [PubMed] [Google Scholar]
- 73.Gaskin DJ, Thorpe RJ Jr, McGinty EE, et al. Disparities in diabetes: the nexus of race, poverty, and place. Am J Public Health. 2014;104(11):2147-2155. doi: 10.2105/AJPH.2013.301420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.US Department of Health and Human Services , Office of Disease Prevention and Health Promotion. Social determinants of health. Accessed March 15, 2021. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
- 75.Rocca WA, Grossardt BR, Brue SM, et al. Data resource profile: expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol. 2018;47(2):368-368j. doi: 10.1093/ije/dyx268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. American Community Survey 5-Year Estimates and Factor Score Coefficients