Abstract
Lower back pain (LBP) occurs in 80% of adults in their lifetime; resulting in LBP being one of the biggest causes of disability worldwide. Chronic LBP has been linked to the degeneration of the intervertebral disc (IVD). The current treatments for chronic back pain only provide alleviation of symptoms through pain relief, tissue removal, or spinal fusion; none of which target regenerating the degenerate IVD. As nucleus pulposus (NP) degeneration is thought to represent a key initiation site of IVD degeneration, cell therapy that specifically targets the restoration of the NP has been reviewed here. A literature search to quantitatively assess all cell types used in NP regeneration was undertaken. With key cell sources: NP cells; annulus fibrosus cells; notochordal cells; chondrocytes; bone marrow mesenchymal stromal cells; adipose‐derived stromal cells; and induced pluripotent stem cells extensively analyzed for their regenerative potential of the NP. This review highlights: accessibility; expansion capability in vitro; cell survival in an IVD environment; regenerative potential; and safety for these key potential cell sources. In conclusion, while several potential cell sources have been proposed, iPSC may provide the most promising regenerative potential.
Keywords: biologic therapies, regenerative medicine, stem cell, tissue engineering
Cell sources and lineages proposed for regeneration of the intervertebral disc. This review highlights: accessibility; expansion capability in vitro; cell survival in an IVD environment; regenerative potential; and safety for these key potential cell sources. In conclusion, while several potential cell sources have been proposed, induced pluripotent stem cells may provide the most promising regenerative potential.
1. INTRODUCTION
1.1. Lower back pain and treatment
Lower back pain (LBP) is the biggest cause of disability worldwide, around 80% of adults will suffer from LBP in their lifetime. 1 , 2 Most people experience mild pain and recover quickly; however, it is common for episodes of LBP to relapse contributing to a lifelong disability and a large societal burden. 1 , 3 The current mainstay of treatments to combat LBP are split into pharmacological (opioids, non‐steroidal anti‐inflammatory drugs, antidepressants) and non‐pharmacological (physical therapy, exercise, massage and manipulation, and alternative therapies (acupuncture, magnet therapy, and reiki). 4 , 5 , 6 , 7 , 8 Although these treatments are potentially helpful in acute LBP, there are limited therapies that are efficient in the management of chronic LBP. 9 , 10 , 11 , 12 For example, treatments available to relieve chronic LBP and recurrent onset back pain include an invasive operation to remove the IVD and potentially fuse adjacent vertebrae, which can lead to the alteration of the normal physiological and biomechanical function of the spine. 13 In some cases spinal fusion can increase the risk of adjacent segment disease, where the spinal segment directly below or above the site of the fused IVD experiences increased biomechanical demands, which can lead to accelerated IVD degeneration. 14 , 15 The use of painkillers for the treatment of chronic LBP has also had repercussions, with 24% of chronic LBP cases reported to result in aberrant medication‐taking behaviors. 16 Such behaviors are contributing to the opioid epidemic in the United States 17 and the development of an opioid crisis in the United Kingdom and other countries. 18 In the United States there were 70 237 opioid overdose deaths in 2017, which constituted 67.8% of overall drug related overdoses. 19 Therefore, there has been an emphasis to reassess the management of chronic LBP. 20 Approximately 40% of all chronic LBP cases are associated with IVD degeneration. 21 , 22 , 23 However, none of the treatments stated above address the underlying cause of IVD degeneration and target the restoration of the damaged IVD. 24
1.2. Intervertebral disc, degeneration, and discogenic back pain
The IVD is a complex structure, where the biomechanical functioning of the IVD relies on a balance between the three main tissues that compose it. The central aspect of the IVD contains a glycosaminoglycan‐rich gel‐like tissue called the nucleus pulposus (NP), which is enclosed by the annulus fibrosus (AF). The third distinct region are the cartilaginous endplates (CEP), which are composed of cartilage and situated superiorly and inferiorly to the IVD, and act to separate and anchor the IVD to the vertebrae via the bony end plates, the CEP also act as a gateway for nutrient transport into the IVD. 25 , 26 The different compositions of the three areas of the IVD allow the support of spinal compressive loads, the NP osmotically exerts the swelling pressure while the AF constrains the NP, preventing it from protruding transversely and the CEP constrains the NP from bulging into the adjacent vertebrae; thus creating tensile stresses and absorbing the considerable hydrostatic pressure. 26 , 27 A healthy IVD requires maintenance of this homeostatic environment, a simple shift in the matrix properties, cells, or molecular signals in these three areas of the IVD has implications to the distribution of the mechanical load and begins the cascade of IVD degeneration. The disruption of this homeostatic balance is multifactorial, and may start at the cellular level caused by, for example, nutrient deprivation due to ossification of the endplates, or could initiate via a structural defect that can cause subsequent cellular changes. 28
The regulation of cellular turnover is vital to tissue homeostasis. In IVD degeneration the number of functional cells are decreased, through apoptosis and cellular senescence, 29 , 30 , 31 , 32 with an accompanied phenotypic shift toward catabolism; which leads to altered NP matrix maintenance and an increase in catabolic responses by the IVD cells themselves. 33 , 34 , 35 , 36 NP matrix composition is regulated through cellular matrix synthesis and matrix degradation via the activity of matrix metalloproteinases (MMPs) and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), enzyme families, which can be inhibited by tissue inhibitors of metalloproteinases (TIMPS). 36 Healthy NP tissue contains a ratio of 27:1 glycosaminoglycan‐to‐hydroxyproline (collagen) ratio, and aggrecan and its associated glycosaminoglycans (GAGs) preserves the high water content of the NP. 37 During degeneration, NP matrix synthesis and degradation becomes dysregulated leading to the loss of proteoglycans and dehydration. The condensed NP and increase in intradiscal pressure results in reduced capacity of mechanical loading of the IVD leading to the creation of microfissures throughout the IVD. 38 Once the outer AF is ruptured or the CEP has fractured, inflammatory cells can migrate into the IVD which propagates an inflammatory cascade. This together with the catabolic factors produced by the native IVD cells, contributes to discomfort and the stimulation of pain sourced from the IVD, also known as discogenic pain. 38 , 39 There is no definitive mechanism to explain the link between IVD and the discogenic pain patients experience. However, the combination of structure disruption with the production of angiogenic and neurotrophic factors by NP and AF cells, stimulates angiogenesis and promotes neural ingrowth to the largely avascular and aneural IVD. 39 , 40 The IVD cells also produce pain sensitizing factors such as substance P, which can sensitize in‐growing nerves and local nerve roots to pain. 40 , 41 Thus, it is important to deduce a regenerative approach that will restore the balance of cells, extracellular matrix and the biomechanics of the IVD and interrupt the viscous cycle of degeneration. 42 , 43
1.3. Potential approaches to regenerate the nucleus pulposus
There has been a focus for a tissue engineering approach to restore the appropriate cell and matrix content of the NP, which would benefit the function of the IVD as a whole and thus resolve discogenic back pain. 43 , 44 , 45 These studies have ranged from biomaterial‐based to cellular approaches or a combination thereof. Some studies have investigated implanting biomaterials to stimulate the resident NP cells, 46 , 47 , 48 while others have used biomaterials to act as a combination of mechanical support and as a cell carrier system. 49 , 50 , 51 Cell therapy has been proposed to restore the NP cell population, as the loss of viable cells and a shift to a catabolic phenotype is one of the characteristics of IVD pathogenesis. 52 , 53 , 54
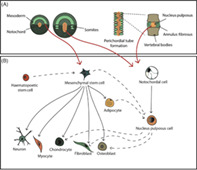
An important consideration in the choice of cell source for therapeutic use is the origin of cells of the IVD. Developmentally, the IVD's unique structure is formed from cells of at least two embryonic origins: the notochord, which develops into the NP and the sclerotome which makes up all other connective tissue of the spine including the AF and vertebrae. 44 , 55 The notochord and sclerotome are derived from one of the three primary embryonic germ layers: the mesoderm (Figure 1A). 56 , 57 Therefore, a cell‐based therapy approach could be performed using cells that have the capacity to develop into, and from, the mesoderm‐derived lineages (Figure 1B). Such as differentiated cells: notochordal (NC) cells, 58 NP cells, 59 , 60 AF cells, 61 and chondrocytes 62 , 63 ; adult stromal cells from bone marrow or adipose 64 , 65 , 66 ; embryonic stem cells, 67 , 68 or more recently the use of induced pluripotent stem cells (iPSC) have also been explored. 69 , 70 , 71 However, this straightforward cell therapy strategy is made much more difficult by the fact that the microenvironment of the IVD is very harsh. The IVD environment has an acidic pH, due to high concentrations of lactate, 29 low concentrations of glucose and oxygen, 72 and fluctuating osmolarity 73 , 74 , 75 ; the degenerate IVD also contains increased expression of catabolic cytokines, 36 , 76 that further disrupt this harsh environment. Therefore, the cells will also need to be able to adapt and survive in the IVD niche.
FIGURE 1.
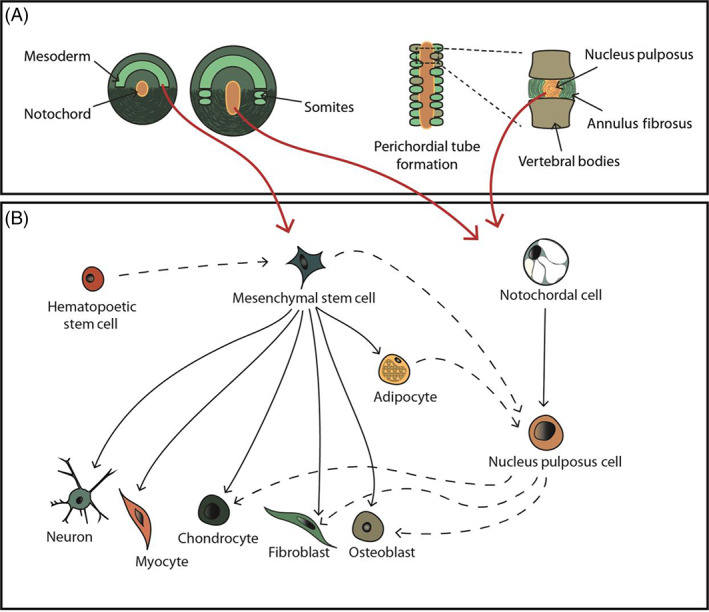
Cell sources and linages involved in cellular therapy in regenerating the intervertebral disc. (A) Schematic illustration depicting key stages of intervertebral disc development, highlighting the mesodermal origin of the notochord and sclerotome that evolve into the nucleus pulposus, annulus fibrosus and vertebral bodies. Red arrows show the potential cell sources. (B) An illustration of the mesenchymal stem cell and notochordal cell differentiation lineages (black arrows). Under appropriate culture conditions transdifferentiation can be induced to develop different cell types (black dash arrows), which can interlink different cell lines
There have been a wide range of potential cell sources explored for the repair and regeneration of the IVD which will be explored within this systematic literature review. Specifically, this systematic literature review will discuss the cell sources investigated for the regeneration of the NP, from terminally differentiated cell sources, to the use of adult stem cells and recent studies investigating iPSC.
2. METHODS
PubMed was used as the principal database with a primary search of “([disc degeneration] OR [intervertebral] NOT [retinal]) AND ([embryonic stem cells] OR [progenitor cells] OR [fibroblasts] OR [stem cells] OR [induced pluripotent stem cells] OR [adult stem cells] OR [mesenchymal] OR [adipose] OR [hematopoietic] OR [synovial] OR [disc stem cells] OR [disc cells] OR [nucleus pulposus] OR [chondrocytes] OR [notochordal] OR [notochord]) AND ([cell therapy] OR [regeneration] OR [therapy] OR [treatment]).” Clinical trials, in vivo and in vitro studies were all included, and the search was limited to the English language and published prior to 31 December 2020. These keywords were chosen to explore the different types of cell sources to be used as potential regenerative therapies for the IVD. This search generated a total of 3566 publications. The title and abstracts were initially screened based on their relevance to cellular therapies for the regeneration of the NP region only. A total of 355 articles were identified.
In order to review the regenerative potential of each cell, the studies identified from the literature search was used systematically to first identify their native phenotype. Followed by the ability of the cell type to be handled in an in vitro setting, such as, cell expansion capability, and maintenance of phenotype. Next, the survival of the cell type within the IVD environment was evaluated. Finally, regenerative effects, if any, of the selected cell type was reported predominately in vivo systems. From these analyses each cell source was ranked based on the cells accessibility, expansion capability in vitro, survival in IVD, ECM production, and potential adverse effects on a scale of 0 to 3. Using the sum of each feature, the cell source was given an overall rating on a scale of 0 to 15 to enable a semiquantitative comparison of potential for IVD regeneration.
3. TRENDS IN CELLULAR THERAPY RESEARCH FOR INTERVERTEBRAL DISC REGENERATION
There has been a gradual increase in the number of studies investigating potential cellular therapy to regenerate the NP, with the first studies reported in 1994. Over the last 16 years, a 50% increase has been seen (Figure 2). Initial studies focused on the use of NP cells, either alone or augmented with growth factor stimulation or anti‐catabolic therapies (Figure 3). From 2003 onwards, initial studies were reported investigating the use of adult stem cells and alternative terminally differentiated cells (Figure 3C). From then on bone marrow stem cells (BMSC) have been the predominant cell choice, with 40% of the literature reporting studies using BMSC, followed by NP cells and then ADSC (Figure 3B). From 2013 onwards, cells that have pluripotent and multipotent capability were first reported, with the use of human umbilical cord stem cells (hUCSC) and iPSC (Figure 3). Most of the studies have utilized in vitro cell culture (42%) and in vivo small and large animal models (39%) (Figure 4A,B), as organ and tissue culture models were only introduced in 2008 (Figure 4C). One of the earliest in vivo studies was conducted in a dog IVD degeneration model in 1994, after that smaller animal models were favored; in 2009, studies progressed to a higher incidence of large animal models, with the first clinical human trial being implemented in 2006, 12 years after the first study on cell therapy. The first human clinical trial was reported using hematopoietic stem cells 77 ; 9‐fold more clinical trials have been conducted on humans than in dogs (Figure 4C). Greater than 50% of the in vivo studies utilized small animal models, with rabbit being the dominant species (Figure 5B); due to the ease of accessibility and cost implications. 78 Less than half of studies utilized large animal models, with dog being the most utilized large animal model and no studies used cow models (Figure 5A). Similarities between human and animal IVD have been previously reviewed by Alini et al. (2008) and will not be part of the discussion of this review. However, it is worth noting that a significant limitation when analyzing animal models for regenerative approaches is that many of the species utilized retain their immature NCs and thus have an increased regenerative capability which can skew results. Thus, model systems which lose NCs postnatally, such as sheep, goats and chondrodystrophic dogs, would be more appropriate for investigations into regenerative approaches. 79
FIGURE 2.
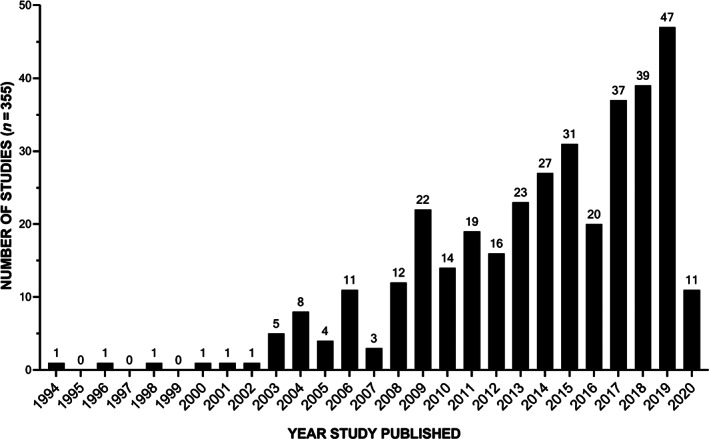
Publication intensity for cellular therapy for NP regeneration. The number of studies investigating potential cellular therapy to regenerate the NP extracted from the literature review search expanding over 26 years (from January 1994 to December 2020)
FIGURE 3.
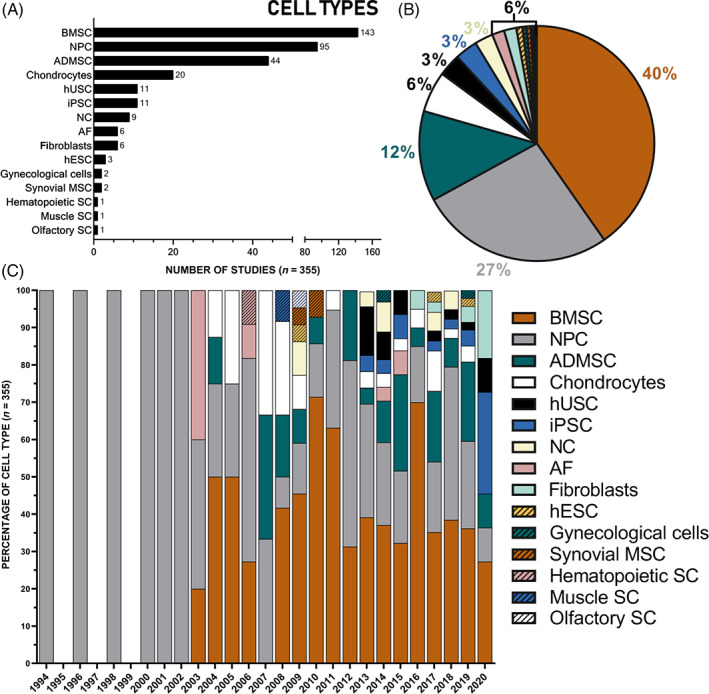
Cell sources proposed for NP regeneration: Studies investigating potential cellular therapy to regenerate the NP expanding over 26 years (from January 1994 to December 2020) were classified according to cell source proposed: the number (A), percentage (B) of cell type and the percentage of cell type relative to the year (C) which used the following cell types: Adipose derived mesenchymal stem cells (ADMSC), annulus fibrosus (AF) cells, bone marrow derived mesenchymal stem cells (BMSC), chondrocytes (subgroups include: endplate chondrocytes, hyaline chondrocytes, articular chondrocytes, nasal chondrocytes, and auricular chondrocytes), fibroblast cells, gynecological cells (subgroups include: menstrual blood derived stem cells, and human amniotic cells), hematopoietic stem cells, human embryonic stem cells (hESC), human umbilical cord stem cells (hUSC; including placenta derived mesenchymal stem cells), induced pluripotent stem cells (iPSC), muscle derived stem cells, notochordal cells (NC), nucleus pulposus cells (NPC), olfactory stem cells, and synovial derived mesenchymal stem cells. (B) From the literature extracted: 40% used BMSC; 26% used NPC; 12% ADMSC; 6% used chondrocytes; 3% used iPSC; 3% used hUSC; 3% used NC cells; 2% used AF cells; 2% used fibroblasts; <1% used hESC; <1% used synovial MSC; <1% used gynecological cells; <1% used olfactory SC; <1% used muscle SC; <1% hematopoietic SC
FIGURE 4.
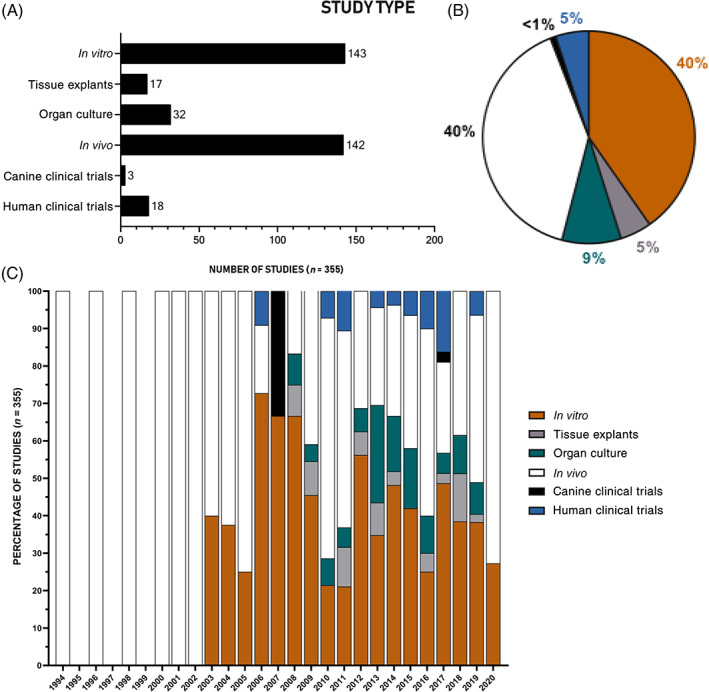
Study type utilized to investigate cellular regeneration of the NP. Studies investigating potential cellular therapy to regenerate the NP expanding over 26 years (from January 1994 to December 2020) were classified according to the type of study performed; the number (A), percentage (B) of study type and the percentage of study type relative to the year (C) from the literature search that used the following model systems: in vitro (including 2D and 3D culture), tissue explants, organ culture, in vivo (subcutaneous or injected into a healthy or degenerate intervertebral disc), canine clinical trials and human clinical trials
FIGURE 5.
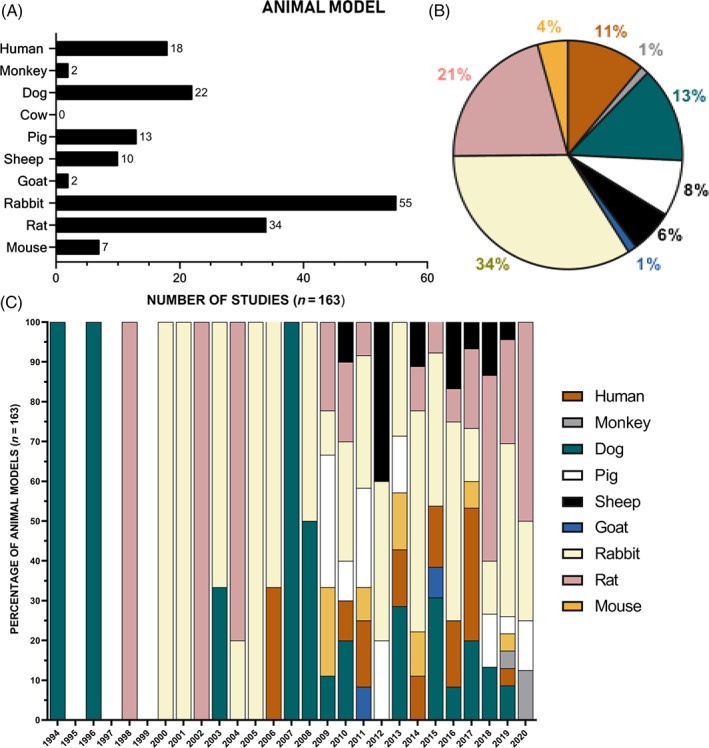
Animal model utilized for investigation of cell therapies for NP regeneration. In vivo studies (including human clinical trials, canine clinical trials, and in vivo study types) investigating potential cellular therapy to regenerate the NP expanding over 26 years (from January 1994 to December 2020) were classified according to the animal model utilized; the number (A), percentage of animal model used (B) and the percentage of animal model used relative to the year (C) from the literature search that used the following living animal model system: human, monkey, dog, cow, pig, sheep, goat, rabbit, rat, and mouse
4. PROPOSED CELL SOURCES FOR NUCLEUS PULPOSUS REGENERATION
This review discusses the key cell types investigated for regenerative approaches and discusses their potential applicability for lower back pain. With a focus on native phenotype, accessibility, survival, expansion capability, and tolerance of the IVD environment. Leading to recommendations for potential cell sources for tissue regeneration approaches and their limitations. Cell types will be discussed in order of terminal differentiation, namely differentiated cells (NP, AF, NC, and chondrocytes); followed by adult stromal cells (BMSC, ADMSC, and UCSC); and finally, iPSC. Other cell types were not further analyzed as only a limited number of studies utilized them. These included: fibroblast cells, 80 , 81 , 82 , 83 gynecological cells (menstrual blood derived stem cells and human amniotic cells), 84 , 85 hematopoietic stem cells, 77 hESC, 86 , 87 , 88 muscle derived stem cells, 89 olfactory stem cells, 90 and synovial derived mesenchymal stem cells. 91 , 92 Cellular therapies have been proposed either as cells alone or together with instructive biomaterials or growth factors to support regenerative properties. This review aims to focus on the choice of cell source for regenerative approaches and thus will not discuss the combinations with biomaterial and growth factors which have been reviewed elsewhere. 68 , 93 , 94 , 95 , 96
5. NUCLEUS PULPOSUS CELLS
5.1. Native phenotype
NP cells within the mature human IVD are rounded and situated within a lacunae,* the NP cell produces abundant proteoglycans and collagen type II, with phenotypic makers of Forkhead Box F1 (FOXF1), paired box 1 (PAX1), Keratin 19 (KRT19) among others. 44 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 NP tissue also contains a population of NP progenitor cells 98 , 101 , 102 , 103 which have been suggested to be NP‐derived stem cells although their full characterization as stem cells has not been completed. NP progenitors in vitro are presented as elongated spindle shape cells, 104 which are positive for Tie2 and express stem cell genes (eg, Sox2, Oct3/4, Nanog, CD133, Nestin, and neural cell adhesion molecule). 8 , 103 , 105 These NP progenitors were positive for CD73, CD90, and CD105. 70 NP cells show potential for osteogenic, adipogenic and, in comparison to AF and CEP cells, maintain the greatest chondrogenic potential. 106 , 107
5.2. Expansion capability and maintenance of phenotype
Human NP (hNP) cells are versatile, they can be harvested from cadavers, surgical samples 99 and can be cryopreserved without altering cell integrity. 108 However, the numbers of cells retrieved are relatively low and in vitro expansion would be required to yield enough cells for further therapy. Furthermore, hNP cells extracted from degenerative IVDs undergo cellular senescence at an accelerated rate; and express a decreased replicative potential when compared with IVD cells extracted from non‐degenerate IVDs.30 † Rapid de‐differentiation and phenotypic alterations of NP cells happen within the first passage of monolayer expansion or serial passaging in NP. 107 , 109 Whereas continuous expansion 110 and other three‐dimensional (3D) culture systems such as NP pellet, 111 alginates 33 , 109 or spheroid culture system 103 leads to the maintenance and restores NP phenotype. Co‐culture of hNP cells with other cell types can result in a positive effect on cell viability and proliferation, for example, doubling proliferation was seen when co‐cultured with autologous hBMSC for 3 days. 112 Unfortunately, many studies utilizing hNP cells for regenerative studies do not report the passage number where cells were utilized, however the majority of studies that state this, limit passage number to a maximum of three passages 62 , 98 , 110 , 112 , 113 , 114 , 115 , 116 , 117 , 118 and thus limit induction of cellular senescence and retain re‐differentiation capacity. 33 , 119 , 120
5.3. Cell survival in the intervertebral disc environment
Naturally, NP cells are adapted to survive within the harsh environment of the IVD, 121 , 122 , ‡ however when these cells are removed from the IVD and cultured within monolayer in non‐physiological conditions they may lose their adaptations to this environment. In vitro culture is often utilized when testing cells in IVD conditions such as pH, osmolarity, and oxygen concentrations are easier to manipulate. 123 , 124 , 125 In altered pH conditions that resemble mild to severe degenerative IVD, NP cell viability and proliferation was sustained in low pH 124 ; rabbit NP (rbNP) cells cultured in pH 7.4 displayed an increase in apoptosis and decrease in cell proliferation compared with pH 6.5, indicating that NP cells prefer mild acidic conditions. When directly comparing rat NP (rtNP) cells to rtADMSC, it has been demonstrated that NP cells were less sensitive to acidic conditions and produced lower catabolic metabolism. 124 It has previously been reported that matrix metabolic activity is also enhanced when cultured under acidic pH in bovine NP (bNP) cells. 126 This is an indication that bNP favors physiological conditions in comparison to hNP; high glucose also increased extracellular matrix gene expression in bNP cells compared with hNP cells. 127 A report of rtNP progenitors cultured in IVD‐like high osmolarity using NaCl (400 mOsm/L) showed increased proliferation than isolated rtNP cells, whether under standard (280 mOsm) or high osmolarity conditions (400 mOsm/L). 123 However, hNP cells cultured in high osmolarity increased proteoglycan production. 128 Low oxygen was not detrimental to matrix synthesis for bNP and even promotes the ideal NP phenotype, through an increase in aggrecan, and collagen type 2 markers. 129 At low oxygen concentration hypoxia‐inducible factor‐1 (HIF‐1α) was mostly localized to NP cells, more so than other cells. 130 , 131 HIF‐1α is a crucial physiological regulator in anaerobic metabolism and the constitutive expression of HIF‐1α by a NP cell indicates their ability to survive and adapt to hypoxic conditions within the IVD. 132
IVD conditions of altered pH, osmolarity and oxygen have a strong influence on metabolic rates, matrix production and cell survival. 133 In monolayer, NP and NP progenitor cells have the ability to adapt and survive in IVD culture conditions, favoring altered pH, osmolarity, and oxygen to IVD physiological standards, which results in proteoglycan production. Given these findings NP cells seeded into a IVD organ, have the potential to survive, proliferate and produce regenerative extracellular matrix. However, the culture of isolated cells prevents all cell‐matrix interactions and signaling and thus, NP cells could act differently when cultured in in vivo systems. 133
NP cells transplantation into in vivo models have also been investigated and studies have shown that the transplanted NP cell remain viable for a number of weeks and months; allogeneic expanded rtNP cells remained viable for 4 weeks in a rat model 134 ; in a canine model, cryopreserved autologous cNP cells were observed at 12 weeks 115 and 12 months of allogeneic expanded cNP cells 62 ; xenogeneic transplantation of a hNP established cell line were sustained in a rabbit model for 8 and 24 weeks, 98 , 135 12 weeks in a monkey model 100 and 12 weeks in a canine model. 116 Interestingly, transplanted NP cells were mainly localized in the injected zone, with observation of cells migrating into the inner AF in canine and rat treated models. Suggesting that injected NP cells have the ability to migrate and integrate with native cells. 115 , 136 Once migrated to the inner AF, the cell takes upon the morphology of the native cell, such as an AF spindle‐like shape. Whereas, the rtNP cells which stayed within the NP maintained their rounded shape. 134 Establishing that NP cells can remain viable and proliferate in most conditions and differing animal host.
5.4. Regenerative effect of nucleus pulposus cells
hNP cells produced aggrecan and type II collagen and low levels of type I collagens, for up to 24 weeks when compared with the degenerate control in a number of in vivo systems. 62 , 98 , 118 , § However, it has been observed that regenerative effects may differ depending upon the cell sources administered. Rosenzweig et al. (2017) observed a difference proteoglycan expression of bNP and hNP cells; bNP cells resulted in higher expression of collagen type II and aggrecan. 110 Whereas, in expanded hNP cells protein expression was not preserved and less collagen type II and aggrecan were observed. 110 These differences may have been due to the source of hNP cells, which were from herniated degenerate IVDs, 110 which have been shown to have higher levels of cellular senescence and increased inflammatory factors than non‐degenerate discs. 30 There are reports that herniated cells have limited regenerative potential as they show signs of de‐differentiation, degeneration, and decreased aggrecan and collagen type II production. 30 , 114 , ¶
hNP cells implanted into in vivo models, displayed partial regeneration of IVD height, 116 specifically, the results showed initial IVD narrowing due to degeneration prior to implantation, followed by regenerated IVD height of 14.8%, after 3 weeks analyzed using magnetic resonance imaging (MRI), 115 18.91% and 9.7% after 4 weeks using X‐ray, 135 , 137 and 7% to 15% after 8 weeks using X‐ray, 118 in comparison to the degenerate control. Chen et al. (2016) reported that hNP and hNPSC injected into rabbit degenerate induced IVDs, and after 12 weeks there appeared to be no significant difference in the degenerate IVD control and was actually significantly lower than in the normal “healthy” IVD. 98 An MRI analysis using relative signal intensity index suggested that the NPSC group restored IVD height greater than the NP cell and degenerate control, however, in most studies the IVD height was still less than that of a healthy IVD. 98 , 115 , 116 , 118 , 135 Despite improvement to IVD height seen in most studies and GAG production, NP cells used to regenerate the IVD were able to halt further degeneration, but not significantly improve the degenerate condition in the context of Pfirrmann classification; which was reported at Grade 2 to 3, 112 , 115 whereas the degenerate IVDs displays progressive degeneration to Grade 3 to 5. 115
5.5. Concluding remarks
NP cells are able to demonstrate long term survival in vivo and display their ability to adapt to the IVD microenvironment, including differences to osmolarity, oxygen, and pH conditions, however NP progenitor cells also have this ability and further displayed regenerative effectiveness. Recently, NP progenitor cells have been successfully expanded from NP cell populations following spheroid culture system. 103 It was reported that animal NP cells differed in responsiveness and bNP cells were less representative of hNP cells. NP cells demonstrated their ability to produce GAG for regenerating a degenerate IVD, with slight restoration. However, the main issue with the use of native NP cells in a regenerative approach is the sourcing of these cells as harvesting from a normal IVD would induce IVD degeneration, but if cells are harvested from an already degenerate or herniated IVD, these cells would show increased catabolism and thus reduced regenerative capacity, although sourcing from cadavers could be a possibility. NP progenitor cells are currently in clinical trials (DiscGenics), 118 while NP cell‐based clinical trials 138 have been completed and the results of these studies will be interesting to follow.
6. ANNULUS FIBROSUS CELLS
6.1. Native phenotype
AF cells in the inner AF are rounded, chondrocyte‐like cells. Progressing to the outer AF, cells are more elongated in morphology, similar to fibroblasts. 139 The outer AF is mechanically strong matrix composed of a higher ratio of collagen type I to type II, resulting from expression of COL5A1, a gene that regulates collagen type I assembly. 59 , 140 , 141
6.2. Expansion capability and maintenance of phenotype
The expansion capability for AF cells is very similar in some ways to NP cells. The same method of cell isolation** and expansion is often used 107 , 142 which extracts, per grams of tissue, roughly the same low density yield of cells as NP. 143 , 144 , †† AF cells also share a similar phenotype: positive for CD44, CD73, CD90, CD105, CD151, and CD166 and negative for CD34, CD45, CD146, and similar transcriptome profiling to NP cells. 145 However, van den Akker et al. (2020) published a set of novel membrane‐associate markers for NP and AF cells, thus distinguishing the few markers specific for AF, for example, secreted frizzled related protein 2 (SFRP2) and COL1A1. 146 Similar to NP cells, AF cells show osteogenic and chondrogenic potential, but with a greater number of highly expressed stemness genes. 107 This review focuses on the cell sources for NP regeneration, and while AF cells have been more commonly studied as potential regenerative strategies for AF tissues and have been reviewed elsewhere, 147 , 148 , 149 some studies have also investigated their use for NP regeneration which will be explored further here.
6.3. Cell survival in the intervertebral disc environment
As AF cell are native to the IVD environment, it is no surprise that when transplanted into in vivo rabbit IVDs, >90% of allogeneic AF cells were viable 12 weeks post‐transplantation. 141 , ‡‡
6.3.1. Regenerative effect of annulus fibrosus cells
The majority of studies utilizing AF cells in this literature search were in vitro methods, with two papers studying AF cells in rabbit IVD degeneration models. 141 , 150 AF cells in vitro have been shown to produce elastin 24 , 141 , 151 and predominantly collagen type I, where collagen type II remained undetected. 49 , 142 , 152 Even in different biomaterial culture conditions, AF cells seen to favor the synthesis of collagen type I, a characteristic of native fibrocartilaginous AF tissue. 49 , 142 , 152 , §§ The two in vivo studies highlighted that collagen type II and aggrecan were upregulated. 153 The structure of the inner AF was significantly preserved, 153 suggesting the AF cells are drawn toward the AF region rather than staying in the NP and strong safranin‐O staining was observed in the AF cell‐transplanted NP tissue, which is very histologically similar to hyaline‐like cartilage and normal AF. 141
6.4. Concluding remarks
The use of AF cells for NP tissue regeneration would not be a preferred cell choice due to the fact that they predominately produce an unflavored collagen type I and cartilage‐type matrix, which did not resemble the native extracellular matrix of the NP.¶¶ Thus, in the IVD regeneration field, AF cells in are mainly utilized for AF repair. 151 , 152 , 154 , 155 , 156 However, where AF cells are targeted for AF repair, the use of cell therapies for such an approach would need to also consider the risk of implanted cells leaking from the disc following AF damage and rupture. There are a couple of reviews that have discussed large animal and clinical trials studies that target the “sealing” of AF to prevent NP herniation which is outside the scope of the current review. 144 , 157
7. NOTOCHORDAL CELLS
7.1. Native phenotype
Notochordal (NC) cells are distinct through their relatively large size (>20 μm) and presence of vacuoles. 158 , 159 , *** In many studies this classic physaliphorous phenotype was used to distinguish between other NP cell types. 160
7.2. Expansion capability and maintenance of phenotype
In standard monolayer culture, the NC vacuoles cells were lost with expansion of porcine NC (pNC) cells after 28 days 161 , 162 or within 1 to 3 passages 160 ; canine NC (cNC) cells observed a loss in NC characterization after 6 days in monolayer culture. 163 It was reported that NC cells acquired a NP‐like morphology (small, round cells) and became indistinguishable from NP cells. 160 , 161 There are additional reports of a decrease in NC cell marker expression such as Brachyury, 160 Keratin (KRT) 8, and KRT19. 161 Gantenbein et al. (2014) reported that when pNC cells are cultured in monolayer, they are outcompeted by smaller (<8 μm) NP cells, despite the culture starting as 80% NC cell population. Kim et al. (2009) highlighted that NC cells grown in monolayer culture had a significantly slower growth rate of 135 hours population doubling time compared with NP cells which showed a growth rate of 23 hours population doubling time. This is in conjunction with the observation that single isolated NC cells morphologically differentiated into three distinct cell types: NC vacuolated cells, giant cells, and small NP cells. 74 , 158 As a result, alternative methods such as co‐culturing NC with other cells and culturing in 3D in vitro culture models have been investigated. When cNC cells are co‐cultured with cMSC, an increase in proteoglycan production and maintenance of NC phenotype was observed. 164 pNC cells were shown to have high cell viability for up to 42 days in alginate bead culture, 165 , 166 with one study reporting up to 80% cell viability at 10 days in rbNC. 158 Gantenbein et al. (2014) reported that a higher fraction of around 50% of pNC cells were recoverable after 34 days of culture when compared with the identical cell population grown in monolayer. Providing evidence that keeping NC cells in 3D cultures, which would resemble their in vivo cluster form, rather than completely isolating them in monolayer, could preserve the NC cell phenotype better during in vitro culture. 74 , 160 , 167 , †††
7.3. Cell survival in intervertebral disc environment
NC cells are sensitive to culture conditions. Osmolarity has been shown to affect NC cell phenotype, Spillekom et al. (2014) demonstrated cNCs cultured in αMEM at 400 mOsm/L contained more vacuolated cells and showed significantly higher brachyury expression compared with high glucose DMEM/F12 and αMEM both at 300 mOsm/L. Guehring et al. (2009) established that NC cells are also highly metabolically active; consuming more oxygen and glucose and producing more lactate compared with NP cells. Thus, NC cells exhibit a strong nutrient dependency, 160 , 166 which resulted in some studies altering the culture condition when culturing NC cells: such as adding 10% fetal calf serum supplement. 160 In addition, co‐culturing NC with other cells (eg, NP cells) at a lower ratio of 30:70 166 reduces nutrient depletion, preventing NC cell death. 166 Further, NC cells were more sensitive to nutrient deprivation than other IVD cells and were found to not survive under conditions which NP cells were still viable. Interestingly, the porosity of the cartilage endplate is correlated with the nutrient supply and presence of NC cells. 168 , 169 , 170 , 171 Despite NC cell sensitivity in nutrient deprivation, culture preference is with low glucose media αMEM at 400 mOsm/L. 74 Finally, oxygen content as Gantenbein et al. (2014) observed, also plays a role in NC cell marker expression. Brachyury and CD24 was only expressed in the 2% oxygen conditions and downregulated in the 20% oxygen, indicating that NC cells were functional only in physioxia conditions. 107 , 160 Despite these findings, to date ideal culture conditions have not been established for NC cells.
IVDs are subject to other stimuli which could impede NC cell's ability to regenerate the IVD, such as mechanical loading exerted in vivo and its effect on the NC cell metabolism and biosynthesis. Purmessur et al. (2013) used an ex vivo model of pNC cell‐rich NP tissue loaded into a hydrostatic pressure chamber and subjected the tissue to a daily load of 0.5 to 2 MPa at 0.1 Hz for 2 hours. 165 Despite the reported increase in proteoglycan accumulation observed in the daily loading control, the histological images show the cell population transitions from ~75% of large NC cells being observed in the control to ~25% in the daily pressurization control. Which together with the lack of evidence of apoptosis, supports potential differentiation into small NP cells under load. The study concluded that NC cells were able to withstand the hydrostatic loading, with the daily loading regime causing little effect on cell viability in comparison to the controls. Nonetheless, the results showed that the large vacuolated morphology of NC cells decreased under load, suggesting alteration in cell phenotype.‡‡‡
7.4. Regenerative effect of notochordal cells
Due to the loss of NC cell phenotype in culture, efforts have been made to improve regenerative effects by co‐culturing NP cells with other cells or 3D culture. Most studies investigated NC cells with a co‐culture of NP cells. A significant increase in the GAG/DNA ratio at 7 days, irrelevant of oxygen concentration was observed with the co‐culture of pNC cells and bNP, 160 which after 14 days GAG/DNA ratio was only significantly increased in specifically the 2% oxygen culture conditions. However, other studies observed only slight increase in GAG or insignificant change in extracellular matrix content when cNC and cNP were co‐cultured 164 ; and pNC and bNP cells were co‐cultured. 162 , 166 In respect of specific extracellular gene expression: Arkesteijn et al. (2017) demonstrated the inhibitory potential of NC cells on collagen type I expression, as an increased expression in collagen type I was significantly increased in the degenerative control and not in the pNC cells control. 166 Aggrecan was upregulated in the co‐culture pNC cells and bNP at 14 days 160 and cNC cells cultured in αMEM 400 mOsm/L conditions at 28 days. 74 In Arkesteijn et al. (2017) and Potier and Ito (2014) there was no significant increase in collagen type II reported in in vitro NC controls, however both studies also documented the loss of NC cell morphology, 161 , 166 whereas Spillekom et al. (2014) observed collagen type II in cNC cultured at 28 days in higher osmolarity and lower glucose conditions. 74 A major limitation with the co‐culture of NC and NP cells, is that, as previously discovered, both cells proliferate at different rates and thus NCs could be overcrowded by the proliferation of NP cells, 74 , 162 and NC demanding dependency of culture conditions. NC cells show constant DNA content throughout culture and are able to display an increase in proteoglycan production through a high GAG/DNA ratio, thus demonstrating an efficient phenotype for producing extracellular matrix within the limited nutrient environment of the IVD. Furthermore, Cappello et al. (2006) reported cNC cells were capable of producing proteoglycans at a 1.5‐fold greater rate of synthesis than cNP cells; thus, indicating that the extracellular matrix produced by NC cells is assembled in a distinct manner different to NP cells, as proteoglycans secreted from NC cells migrate and aggregate quicker compared with NP cell synthesized proteoglycans§§§. 172 The next question would be: is the extracellular matrix produced by NC cells more favorable to regenerating the degenerative IVD in vivo?
There have been limited use of NC cells in vivo models, however the studies that have used NC cells for regeneration of degenerative IVDs have shown promising results. Liu et al. (2018) used rtNC cells in a rat model in which degeneration was induced via puncture, where NC cells restored the loss of proteoglycans and maintained NP/AF boundary. 173 In the rtNC‐treated IVDs, collagen type II was significantly increased when compared with the degenerative control. As a result, IVD height was increased compared with degenerative controls. 173
7.5. Concluding remarks
NC cells are highly viable in the conditions related to the IVD and have been shown to upregulate collagen type II and down‐regulate collagen type I, however limited studies have been able to preserve their large vacuolated morphology and most suggest that they differentiate into small NP‐like cells when they are cultured in the harsher degenerative IVD conditions. 74 , 160 , 162 , 165 There are inconclusive results for the ideal culture conditions of NC cells. The general consensus is that due to their in vivo cluster form, 3D alginate bead culture is preferred; however, from all the studies extracted from the literature review, no studies have investigated NC cells in biomaterials, other than alginate culture, 43 , 161 to date. The lack of progression with NC cells in studies could be explained by species‐specific difference,¶¶¶ but also, and most likely, by variances in isolation protocols and/or in culture conditions. As stated above, a valuable characteristic of NC cells is their anti‐angiogenic effects; the natural IVD conditions and mechanical stimuli promote vascular growth, which if unchecked may lead to a worsened state of IVD degeneration and contribute to the symptoms of pain. 165 , 174 The anti‐angiogenic molecules produced by NC cells have the potential to prevent such unfavorable consequences. However, a key limitation of utilizing NCs is obtaining a suitable cell source as human IVDs do not retain NCs beyond adolescence; xenografts would need to be deployed which also have limitations. Furthermore, the difficulties seen in NC cell expansion/culture, but the advantageous characteristics has led to alternative approaches of developing hNC cells through iPSC 60 , 70 , 71 , 175 (see Section 13) or non‐cell based therapy with the introduction of NC‐conditioned media and the potential use of NC matrix or growth factors derived from NC cells. 43 , 58
8. CHONDROCYTES
Native characteristics, expansion capability and maintenance of phenotype in an intervertebral disc environment.
Chondrocytes harvested from different sources**** are able to adapt their phenotype and differentiate into a spherical shape with well‐formed lacunae irrelevant of their in‐situ culture system, for example, nasal chondrocytes in in vitro 3D pellet culture, 63 alginate beads 176 or in hydrogel, 177 and auricular chondrocytes in vivo rabbit IVD. 178 Numerous reports evaluated the following cell surface proteins as potential markers of chondrogenesis, namely CD29 in combination with CD49, CD146, and CD166, 179 with Sox9 being the strongest indicator of chondrocytic lineage. 180 , 181
As chondrocytes also exhibit favorable properties for cartilage repair, there are systematic reviews that have collated information on the culture and expansion capability of chondrocytes; highlighting that chondrogenic phenotype can be maintained using low glucose and hypoxic conditions. 182 Chondrocytes retain good expansion capability with Fellows et al. (2017) reporting human chondrocytes retained good viability after passage 9.††††, 182 , 183 Gay et al. (2019) conducted an insightful study comparing articular and nasal chondrocytes, with the chondrocytes harvested from the same animal model. The study investigated how the cell types respond to different environments that are associated with the IVD, such as altered oxygen and glucose concentrations, within an in vitro pellet culture model. In this study nasal chondrocytes were the cell type that displayed an increase in DNA content in each condition, indicating that nasal chondrocytes can survive, adapt, and proliferate favorably when directly compared with articular chondrocytes. 63 , 176 In vivo chondrocytes demonstrated the potential for long‐term survival of transplanted cells; transplanted autologous auricular chondrocytes were shown to survive for at least 6 months in a rabbit model, 178 and porcine articular cartilage remained viable at 12 months post injection into a porcine model. 184 , ‡‡‡‡ Throughout the studies using chondrocytes for cell therapy for IVD regeneration, the cells were shown to be tolerant to the IVD environment, most probably due to its similarities to the condition of cartilage the chondrocytes are derived, and remain viable post‐transplantation in small animal models. 63
8.1. Regenerative effect of chondrocytes
With the confirmation of sustained cell viability, the majority of studies also investigated potential for restoration of the IVD through analyzing matrix production. In normal conditions of 21% oxygen in a monolayer, articular chondrocytes were able to up‐regulate aggrecan, type I and type II collagen mRNA, when compared with AF cells in the same conditions. 185 In conditions related to the IVD (hypoxic and low glucose), nasal chondrocytes and articular chondrocytes were both capable of producing GAGs and collagen type I and type II. 185 Furthermore, in the IVD conditions nasal chondrocytes produced a ratio of low collagen to high GAG content, whereas articular cells produced a less favorable high collagen content. 176 However, when acidic and inflammatory cytokines were introduced to represent a degenerative IVD environment, neither articular chondrocytes nor nasal chondrocytes deposited GAGs. 63
In vivo transplantation of auricular and articular chondrocytes within the degenerative NP resulted in the production of proteoglycans for up to 12 months in a porcine model 184 and tissue formation which resembled hyaline‐like cartilage was apparent in a rabbit model. 178 Despite the chondrocytes' ability to express extracellular matrix components, it was duly noted that the values were not in the same range of magnitude as native tissue, with native healthy NP tissue having a unique biochemical composition with a GAG to collagen ratio of 27:1. 37 , §§§§ Studies also assumed that the filling of the degenerate IVD with matrix produced from the chondrocytes, were deemed “restored” and did not review how the composition, for example of type I and type II collagens (which are usually understood to be an unfavorable structure of scar tissue in knee joints), would fare in restoring the biomechanical properties of the IVD. Therefore, it would be logical to favor a cell source which can accumulate a similar, if not equal, amount and type of extracellular matrix as a healthy NP. 63
8.2. Concluding remarks
Several different sources of chondrocytes have been utilized in studies where regenerating the IVD is proposed, including articular chondrocytes, nasal chondrocytes, endplate chondrocytes and auricular chondrocytes. Signifying chondrocytes are readily available from multiple sources and could be autologous, although some sources are more accessible than others. The ultimate issue with using chondrocytes as cell therapy, refers to the fact that they maintain their chondrocyte phenotype in the IVD. This is not the characteristic we want to observe in the NP, as chondrocytes produce cartilage and extracellular matrix that is macroscopically more solid in comparison to the gelatinous healthy NP, 178 and does not contain the same composition as native NP. 141 Despite these limitations, studies investigating chondrocytes for IVD regeneration, identify a cell type that is reliable and stable, easy to expand in vitro and remains viable under the harsh conditions of the IVD. Throughout these studies there was no evidence of necrotic change, unfavorable bone growth, transplanted cell migration, nor were there any active signs of tissue vascularization. 62 , 178 , 186 , ¶¶¶¶ In fact, Acosta et al. (2011) demonstrated that chondrocyte treated IVDs produced high levels of the anti‐angiogenic/neurogenic factor, chondromodulin‐I, which lasted for at least 12 months post injection. 184 Chondrocytes are a viable option for regeneration, if the correct IVD phenotype can be reproduced by transplanted cells. Notably, there was a phase I clinical trial initiated employing juvenile articular chondrocytes delivered in fibrin carrier (“NuQu,” NCT01771471) which reported preliminary results in 15 patients in 2013, 187 but the final outcome of the follow up phase II study initiated for 44 patients has not yet been reported. Most probably, the availability of other more promising cell source alternatives that are able to exhibit these NP like phenotypes, has resulted in chondrocytes being a less lucrative cell source for NP regeneration.
9. ADULT STROMAL CELL SOURCES
Adult stromal cells are favorable for their self‐renewal properties and have a much greater interest than embryonic stem cells (ESCs) because of several disadvantages of ESCs. hESC show notable tumorigenic properties, through an increase of telomerase activity, leading to high proliferation and potential formation of teratomas. Additionally, using embryonic cells is surrounded by several ethical issues and there are limited use of embryonic cell lines, which have gained approval from national legislations, 188 therefore it is a less preferable option for future IVD regeneration approaches. 189 In contrast, adult stromal cells can be isolated from various tissues including umbilical cord, bone marrow, and adipose tissue and thus show greater accessibility for use. 190
10. BONE MARROW STROMAL CELLS
10.1. Native characteristics, expansion capability, and maintenance of phenotype
BMSC display similar fibroblastic morphologies, with an apparent marginally extending cytoplasm in monolayer culture, indicating plastic adhesion ability. 139 , 191 , 192 , 193 BMSC express cell surface markers, including CD29, CD44, CD73, CD90, CD105, and CD166 and are negative for typical markers of hematopoietic stem cells, CD34, leukocytes, CD45, and endothelial cells such as CD14. 177 , 193 , 194 , 195 BMSC can maintain cell markers in 90% of cells after passage 4/5 177 , 196 ; however, this cell type favors high‐glucose condition (4.5 g/L) independent of oxygen concentration. 63 BMSC are easily manipulated, if exposed to the appropriate stimuli in vitro, they can differentiate into osteogenic, chondrogenic and adipogenic lineages.177, 192, 193, 195, *****
10.2. Cell survival in the intervertebral disc environment
In vitro, altered hypoxic conditions resulted in an increase in cell viability in rBMSC 197 , 198 and reports of no change in metabolic activity hBMSC. 63 , 199 , 200 An observation that pro‐inflammatory cytokine stimulation does not change the hBMSC metabolic and cell activity. 199 In contrast, when BMSC were cultured in IVD‐like pH (6.8) or osmolarity of 485 mOsm, cell proliferation and expression of matrix proteins were strongly decreased in rBMSC 98 and in hBMSC. 63 Suggesting that acidic condition and high osmolarity were critical factors that reduced biosynthesis and proliferation of BMSC in vitro. In a in vivo animal model of IVD degeneration hBMSC have been reported to remain viable and survive in porcine IVDs for 6 months, 196 rabbits for 3 months, 201 rats for 6 weeks, 202 and bovine for 3 weeks. 195 Reports from in vitro and ex vivo analysis show that when BMSC are subject to IVD‐conditions they differentiate toward NP‐cell like phenotype, 196 , 202 with elevated SOX‐9 gene expression after 7 days 203 and 14 days in vitro, 63 , 191 , 204 and after 1 month 202 and 3 to 6 months in vivo. 196 , ††††† Recently more efforts have been made to differentiate BMSC into NP cells in vitro, utilizing the IVD environment, such as using NP conditioned medium and hypoxia 205 or co‐cultured with NC‐rich NP tissue. 193
10.3. Regenerative effect of bone marrow stem cells
Human aspirated BMSC is frequently used to inject into models of IVD degeneration in rabbits, 201 rats, 202 porcine, 196 and bovine, 195 , 206 , 207 and are one of a few cell types that has been established in human clinical trials. 65 , 196 , 208 , 209 , 210 , 211 , 212 The introduction of hBMSC in animal models resulted in increased collagen type II and aggrecan expression, 195 , 196 , 207 collagen type I 196 , 207 was also observed in small areas of NP, increased compared with degenerative controls but less than healthy IVD. Interestingly, proteoglycans were expressed throughout the NP region in hBMSC treated bovine IVDs, even in areas void of cells. 195 Analysis using transmission electron microscopy displayed degenerative IVDs treated with hBMSCs preserved some lamellar organization, as well as a denser matrix in both AF and NP, resembling the healthy bovine IVD control group. 207 These results could be due to the observation that after the injection of hBMSC studies have shown that the expression of SOX9 was detected in the injected cells, indicating that when hBMSC are subject to the IVD environment they tend to differentiate toward IVD‐like cells.‡‡‡‡‡, 196 , 200 , 202 However, BMSC have been shown to have potentially adverse characteristics of migrating away from the injected site. Henriksson et al.(2009) reported areas of tissue with limited injected hBMSC cells and many studies have observed that BMSC migrate away from the injection site; BMSC have been found distributed throughout the NP region, 195 in the border zone between NP and AF, 196 and cells seeded onto the CEP migrated into the NP. 207 , 213 , §§§§§ Animal studies have demonstrated hBMSC capability to differentiate into an NP‐like phenotype, display matrix producing properties and with no adverse bone mineralization being detected in large animal models, 196 therefore BMSC have transitioned to clinical trials on human patients which has been reviewed recently. 214
Patients used for BMSC clinical trials were selected based on lower back pain diagnosed with IVD degeneration, 65 , 209 , 211 , 212 , 215 lumbago, 216 or their candidacy for spinal fusion or total discectomy. 212 More specifically, the inclusion symptoms were pain 65 , 209 , 210 , 212 , 216 ; the presence of a posterior IVD bulge or small protrusion 209 , 210 ; or IVD height loss. 65 , 212 Interestingly, two clinical studies requested an intact annulus fibrosus ring capable of holding cells. 65 , 211 Only one study injected allogeneic hBMSC, 65 while the rest used patient derived autologous hBMSC. The allogeneic therapy posed no safety concerns and was concluded to be a valid and a more convenient alternative to autologous BMSC‐treated patients. 65 Quality control tests of karyotyping the BMSC were monitored and displayed that the injected BMSC characteristics remained stable over time, 211 with no adverse effects being reported in either allogeneic 65 or autologous BMSC injection, also demonstrating safety. 210 The methods that were used to analyze the regenerative effects of BMSC treatment differed between each clinical trial. In one study, 40% of patients improved one modified Pfirrmann grade, with no radiographic worsening. 212 MRI analysis was used to assess the visual bulging of the treated IVD. Reports of the reduction of the IVD posterior bulge by an average of 23% after 6‐month post‐treatment, 209 and another study observed four out of five patients posterior protrusion reduced by 20%, 43%, 40%, and 48%, with one patient displaying mild progression and a 25% increase in size of their posterior protrusion, after 4 to 6 year post‐treatment. 210 Water content was also analyzed through MRI; BMSC treatment resulted in an observed increase in water content in the IVD in patients after 12 months post‐treatment 65 , 211 and visualized after 2‐year post‐treatment. 216 Orozco et al. (2011) reported that despite the increase in water content, IVD height was not recovered through treatment. 123 Throughout clinical trails the assessment of pain and disability was the method of analyzing regeneration. However, the studies had differing methods of scoring and analyzing pain and disability, with some patients self‐reported overall quality of life improvements despite reports of continued IVD degeneration. 210 Four clinical trials utilized the Oswestry Disability Index (ODI) to assess disability and visual analogue scale (VAS) to evaluate pain; disability was reduced 3 months post‐treatment, which was maintained till 12 months, 65 12 months post‐treatment, 208 and a slight reduction in disability was observed. 65 Lumbar pain was acutely reduced 3 months post‐treatment, followed by minimal difference to 12 months, 65 or patients that reported >25% reduction of posterior bulge also reported lower pain score at 6 months post‐treatment. 209 Sciatic pain improved at 6 to 12 months post‐treatment 211 and 4‐year post‐treatment lumbar and radicular pain improved. 208 However, the placebo effect has not been considered in clinical trials to date, which should be investigated in randomized controlled trials.
10.4. Concluding remarks
BMSC are easily sourced¶¶¶¶¶ and easy to differentiate in vitro, once in an IVD environment BMSC commonly differentiate toward NP‐cell like phenotype, expressing NP markers CD24, KRT19, SOX‐9, aggrecan, collagen type II, and produce proteoglycans. These characteristics transition into large animal models. However, in vivo BMSC were not able to produce enough matrix to reverse the degenerated IVD, as BMSC which were used to investigate regeneration of enzyme induced degeneration in an animal model with a complete digested NP showed an inability to survive. While in a less harsh degenerative IVD model, injected BMSC were unable to produce enough matrix comparable to a healthy IVD. One major limitation was the ability of BMSC to migrate, as demonstrated when BMSC appeared in the NP despite being seeded onto the CEP; BMSC migrating from the CEP into the vertebrae may give rise to BMSC differentiating into osteophytes, which has been reported of BMSC leakage into adjacent vertebrae in a rabbit model. 217 Despite these potential adverse effects, clinical trials demonstrate the safety and efficacy of autologous and allogeneic BMSC implantation. 65 , 208 , 210 , 211 , 212 MRI displayed BMSC treatment improved moisture content, IVD bulges were reduced, but no significant difference to height of the IVD was observed. An acute decrease of pain and disability was reported post‐treatment with BMSC, followed by modest additional improvements. In one study 23% of patients elected to proceed with surgery within 3 years post‐treatment, as after 1 to 3 years ODI reduced to moderate disability. 65 , 208 All in all, in most cases BMSC treatment was effective at slowing down degeneration but not regenerating the IVD.****** Further useful research may involve NP regeneration in animal models with natural occurring IVD degeneration, which includes all of the degenerative IVD chemical, physical and mechanical microenvironment (as in vitro, BMSC were observed to undergo apoptosis in IVD‐like acidic conditions) 53 , 197 ; to evaluate and improve transplanted BMSC differentiation, regenerative effects and safety. 218
11. ADIPOSE DERIVED MESENCHYMAL STROMAL CELLS
11.1. Native characteristics, expansion capability, and maintenance of phenotype
Isolated hADMSC grow as adherent monolayers with a spindle‐shaped and fibroblast‐like morphology in vitro 219 , 220 , 221 and exhibit high proliferation capability in appropriate culture conditions. 220 , 222 When maintained in standard culture conditions hADMSC expressed mesenchymal stem cell markers CD73, CD90, and CD105, and lacked expression of hematopoietic cell markers CD14, CD34, and CD45, and the immunological cell markers CD19 and HLA‐DR. 224 , 226 Similar to BMSC, these cells have the ability to differentiate into osteogenic, chondrogenic and adipogenic lineages. 220 , 222 , 223 , 224 , ††††††
11.2. Cell survival in intervertebral disc environment
In a two‐dimensional (2D) in vitro culture system, degenerate conditions such as low acidity and high osmolarity impaired the viability and proliferation of rADMSC 124 , 225 and in hADMSC. 226 , 227 Interestingly, despite hyperosmolarity leading to lower cell viability and proliferation, 400 mOsm/L resulted in the highest expression of SOX9, aggrecan, and collagen II in comparison to 300 and 500 mOsm/L. 225 The effect of inflammatory factors resulted in hADMSC producing more pro‐inflammatory cytokines and also enhanced osteogenesis. 228 Low oxygen has also been shown to trigger hADMSC to produce angiogenic and neurotrophic factors which would be detrimental for IVD regeneration. 199 In degenerated IVD models in vivo hADMSC have been observed after 2 weeks, 229 10 weeks, 190 and 12 weeks post‐injection 220 in small animal models and 16 weeks in Porcine animal model. 222 , ‡‡‡‡‡‡
11.3. Regenerative effect of adipose derived stromal cells
hADMSC have been studied in small animal models, using genetically defective biglycan mice, 220 rats and rabbit models subject to needle puncture injury, 190 , 230 , 231 , 232 in one large animal model 222 and in a human clinical trial. 223 hADMSC diffused throughout the IVDs. 220 Degenerative IVDs injected with hADMSC demonstrated an improvement to GAG content with positive staining of collagen type II and aggrecan at 2‐week to 6‐week post‐injection in rat IVDs, 230 at 12‐week post‐injection in mice IVDs, 220 and in rabbits at 18‐week post‐injection. 190 Structural organization of the degenerative IVDs was improved at 4 weeks post‐injury in rabbit models, 232 at 12 weeks post‐injection in a mouse model, 220 and 16 weeks in rat and rabbit models. 190 , 231 Despite the effect of ADMSC demonstrating some regenerative properties throughout all these studies, only partial regeneration of the IVD was demonstrated. Furthermore, the presence of small chondrocytes within the NP was observed in a rabbit model 190 and fibrous connective tissues were observed, which was also present in degenerative controls. 231 hADMSC displayed limited proliferation capability in vivo, as 12 weeks post‐injection hADMSC were negative for the proliferation marker, Ki67, 220 and human nuclear antigen (HNA). HNA was expressed at 2 weeks yet had disappeared by the 4‐week to 6‐week IHC analysis. 230
There was a lack of larger animal studies that used hADMSC, however analysis of the few studies demonstrated that the effects of hADMSC did not change the level of aggrecan, or collagen type II in a porcine model, but did result in a greater expression of Sox 9 in comparison to a degenerative IVD control at 21 days. 222 , §§§§§§ This study also analyzed the effect of human microfragmented adipose tissue (hMFAT) seeded into the porcine model; MFAT contains a mixture of cells including ADSCs and growth factors. 223 MFAT resulted in a homologous distribution of extracellular matrix and cells, additionally, it showed the partial regeneration of the degenerate NP. 223 , 233 Specifically, proteoglycans and collagen type II were easily detected, and GAG content was comparable to normal non‐degenerate controls. 222 MFAT has the same composition as stromal vascular fraction (SVF) of subcutaneous adipose tissue which has been used in human clinical trials 223 ; the difference between the SVF and MFAT is the enzymatic and mechanical techniques used for isolation. 222 In human clinical trials, autologous SVF injection has improved pain, analysis with (VAS) after 6 months post‐treatment in 15 patients with degenerative IVD disease 223 and after 12 months post‐treatment on chronic LBP patients. 221 In three patients who experienced improvement of pain and disability, also simultaneously displayed increased water content on MRI 12 months post‐treatment. 221 In human clinical trials there was no reports of adverse events, including osteophyte formation or incidence of infection. 221 , 223 , ¶¶¶¶¶¶
11.4. Concluding remarks
ADSCs can be obtained by a simple surgical procedure which is routinely used in cosmetic surgery; one clinical trial reported patients were discharged 4 hours from the start of harvesting autologous ADMSCs until after cell transplantation. Harvesting of cells also ascertains large quantities of cells, with additional ease in proliferation in vitro under standard tissue culture condition.******* In vivo, ADMSC results in partial regeneration of the IVD mainly through extracellular matrix production, and there was no report of adverse events. However, injection of ADMSC demonstrated evidence of low cell survival in IVD‐like environments in vitro which translated to poor survival in vivo animal model studies. Moreover, ADMSC are very angiogenic by nature 223 and increased the expression of proinflammatory molecules and promoted inflammation in NPC, 222 , 228 which could contribute further to degeneration.
12. UMBILICAL CORD STEM CELLS
12.1. Native characteristics, expansion capability, and maintenance of phenotype
The umbilical cord Wharton's jelly tissue contains stem cells similar to adult MSC. 234 , 235 In vitro hUCSC displays adherent growth, ability to form cell colonies and exhibits fibroblast‐like morphology. 235 , 236 , 237 , 238 They expressed typical MSC markers CD29, CD44, CD73, CD90, and CD105, but were negative for CD14, CD34, and CD45 235 , 236 , 237 , 238 ; with 98% to 99% of cells positive for these MSC after five passages, with the exception of CD105 which was positive in 75% of cells. 236 hUCSC have self‐renewing capability, leading to high proliferation and have also demonstrated multidifferentiation capacity, 235 , 239 differentiating into cells such as osteogenic, chondrogenic, and adipogenic cell lines, 240 , 241 , 242 , 243 including muscle cells, 244 neural cells, 245 and IVD cells. 235 , †††††††
12.2. Cell survival in the intervertebral disc environment
In vitro cultured within a hypoxic environment irrelevant of culture serum, hUCSC demonstrated NP differentiation, cells displayed a clustering morphology, deposited GAGs, and expressed extracellular matrix proteins. 236 Exposure of NP cells taken from a degenerate IVD to hUCSC conditioned media, has been shown to restore degenerative NP cells to multipotent and self‐renewing NP precursor cells that expressed Tie 2+, OCT4, and Nanog. 243 Furthermore, it has been observed that mechanical IVD stimuli results in hUSC anti‐apoptotic effects; when co‐cultured with hNP cells undergoing compressive stress, the compression‐induced apoptosis of NP cells was suppressed. 246 hUCSC were influenced to differentiate toward NP cells with upregulation of ACAN, COL2A1, FOXF1, and KRT19 at higher levels in vivo than in vitro (normal culture conditions; DMEM, 37°C, 5% CO2. 237 hUCSC survived in rabbit IVD explants for 4 weeks, 247 in vivo rabbit degenerative IVDs 8 weeks post‐injection, 237 and canine degenerative IVDs 24 weeks post‐injection.‡‡‡‡‡‡‡, 235
12.3. Regenerative effect of umbilical cord stem cells
hUCSC have been used in in vivo rabbit models, 237 , 247 , 248 rat models, 238 and larger dog models. 235 In degenerated animal IVD models, treatment with hUCSC demonstrated partial preservation of the NP region and increased structure in rabbit, 237 , 249 rat, 238 and canine models. 235 Aggrecan, type II collagen, and SOX‐9 increased in hUCSC injected groups when compared with the degenerate controls, 235 , 237 , 238 , 249 which also translated into an increase of matrix gene expression. 235 Interestingly collagen type I was downregulated in the hUCSC injected group 235 as well as inflammatory cytokines to levels comparable to healthy controls.§§§§§§§, 238
MRI analysis of the NP displayed increased IVD height and water content in a rabbit degenerate model 8‐weeks post‐injection 237 and in canine models 8‐weeks, 12‐weeks, and 24‐weeks post‐injection. 235 However, there was no reported difference in MRI analysis after 12 weeks post‐injection, 249 and MRI signals remained lower than in healthy control IVDs. 235 One major limitation observed in Beeravolu et al. (2018), was human specific markers were observed in the AF of the injected IVD. Either transplanted cells were not fully placed into the NP during injection or like BMSC they are capable of migrating out of the NP region.¶¶¶¶¶¶¶, 237 The mixed MRI analysis from in vivo testing were also reiterated in a small clinical trial with hUCSC. Two patients that presented with chronic low back pain were treated with hUCSC. 239 After a 2‐year follow up post‐treatment pain and function improved in both patients; the first patient showed an immediate effect of pain relief (VAS), followed by sustained effect up to 24 months, this was also observed in their disability report (ODI), and translated into a greater MRI signal intensity compared with pre‐treatment, indicating higher water content in the NP. In the second patient there was an acute regeneration with an improved pain (VAS) and disability (ODI) score at 6 months post‐treatment, however the scores progressively worsened following 12 and 24 months. Also correlating to no notable increase of MRI signal intensity. 239
12.4. Concluding remarks
hUCSC are harvested from the umbilical cord or placenta, which is non‐invasive, and readily available in blood banks; umbilical cord displays low incidence of graft vs host disease and can be used allogeneically.******** However, social, and ethical issues have been recognized surrounding the practice of blood collection, due to obtaining consent and that blood banking is becoming more commercialized; a case story of a private biotechnology company that enforced obstetricians to halt blood collection to certain banks due to alleged patent infringement, highlights these concern of commercializing. 250 As with mesenchymal stromal cells, hUCSC have the capability of high proliferation rates and differentiating into multiple lineages, including NP cells, especially in the IVD environment. In smaller animal models and larger animal models, hUCSC demonstrated partial regeneration of NP degeneration; aggrecan and collagen type II was deposited with a downregulation in collagen type I and cytokines. However, there are some limitations with the migration ability of hUCSC, which could lead to safety concerns. hUCSC have been tested in human clinical trials with a small cohort, which showed initial improvement to symptoms, although no regeneration was concluded. No adverse events were found in the human patients, 239 however, this study was limited by only using two patients; more clinical studies are needed to identify a reliable outcome for the use of hUCSC for regenerative purposes.
13. INDUCED PLURIPOTENT STEM CELLS
13.1. Native characteristics, expansion capability, and maintenance of phenotype
iPSC can be generated from almost any type of somatic cell by introducing a combination of several reprogramming transcription factors, such as Oct3/4, Sox2, KLf4, and c‐Myc. 251 , 252 , †††††††† Human iPSC for use in NP regeneration has been generated from normal dermal fibroblasts 60 , 71 and NP cells. 69 iPSC have a distinct morphology with a prominent nucleolus and a high nucleus to cytoplasm ratio. Pluripotency is characterized with the expression of OCT4, SOX2, c‐MYC, KLF4, and NANOG. iPSC have an unlimited proliferation capacity and maintain normal karyotype in culture. 69 , 71 iPSC can differentiate into cells of all germ layers. 253 , ‡‡‡‡‡‡‡‡ However, rapid cell growth and high plasticity allow iPSC to form teratomas in vivo, 69 which are used to demonstrate iPSC pluripotency but would not be favorable for treatment approaches. 69 , 71 Thus, for applicability for regeneration it is essential to differentiate the iPSC to the cell type of choice for regeneration and purify by removing non‐differentiated cells or cells from other lineages prior to injection in vivo.
13.2. Differentiation into notochordal‐like cells
To generate induced NC‐like cells (iNC‐LC), iPSC can be subject to differentiation via different methods; the most common method is firstly differentiating iPSC toward primitive streak mesoderm, followed by plasmid transfection with NC transcription factors such as Brachyury, FOXA2 or NOTO to differentiate into an NC phenotype 60 , 70 , 71 , 103 ; Xia et al. (2019) differentiated hiPSC toward mesoblastic cells, followed by differentiation into NP‐LC. 254 Hu et al. (2020) used a lentivirus vector system to transfect GDF5 to differentiate hiPSC 255 ; and hiPSC were successfully differentiated into NC‐like cells under the influence of native devitalized porcine NP matrix powder. 256 iNC‐LC have been shown to express typical notochordal markers of brachyury, KRT8, KRT19, collagen type II, collagen type I, and aggrecan. 254 , 256 Furthermore, iNC‐LC has been demonstrated to survive in IVD retain the expression of KRT18, KRT19, brachyury, 71 collagen type II, and aggrecan 175 phenotype of NC for up to 8 weeks post‐injection in a small 178 and large degenerative animal model.§§§§§§§§, 72
13.3. Regenerative effect of induced pluripotent stem cells
Human iPSC co‐cultured with pNP matrix powder to generate NP‐like tissue in vitro for 28 days, increased expression of Sox9, collagen type II, and aggrecan. 256 When iNC‐LC were translated into animal models, collagen type II, and aggrecan proteins were present following 8 weeks 175 and 16 weeks post‐injection in rat models, 254 leading to increased proteoglycan production and restored NP region.¶¶¶¶¶¶¶¶ In these small animal models, there was an observed IVD height increase at 8 weeks 175 and 24 weeks post‐injection. 254 Interestingly, the injection of iNC‐LC resulted in an increase in intradiscal pH, 12 weeks post‐injection in a porcine model, indicating the cells were able to influence their surroundings to produce a less degenerate environment and potentially play a protective role. 71
13.4. Concluding remarks
iPSC are potentially an abundant cell source, as they can be obtained through reprogramming somatic cells of the patient. iPSC also show proliferation ability and the capacity to differentiate into a chosen cell once appropriate differentiation protocols have been established. Studies using iPSC have differentiated these stem cells into NC‐like cells but not differentiated to fully mature NP cells. 69 Once iNC‐LC are generated, these cells upregulate NP markers in vitro and increase collagen type II and aggrecan expression in vitro and in vivo. Which, in turn has shown a to halt IVD degeneration in animal models. However, iPSC ability to form teratomas in the IVD is a cause for concern, however no teratoma formation was reported in these initial studies, 71 , 175 which may be the result of stable phenotype of iNC‐LC once differentiated into a committed cell lineage.********* In conclusion, the study of iPSC in animal models is novel and no iPSC are used clinically at present, but preliminary results of seeding iNC‐LC into degenerate IVDs in animal models shows promising results, with no safety issues highlighted, or extensive studies, to date.
14. DISCUSSION AND CONCLUSIONS
A key challenge of cell therapy is choosing an appropriate cell source that can not only survive within the natural harsh environment of the IVD, but also is safe to use and produces appropriate extracellular matrix to restore biomechanical and biological function of the IVD. One accommodating factor about the IVD is the avascular nature (although this does change during degeneration); therefore, is considered immunoprivileged and should tolerate autologous or allogeneic cells. 257 , 258 The key characteristics of the ideal cell source are assessed on their ability to proliferate in vitro, in order to obtain sufficient number of cells for preparation as a therapeutic model, survive in the IVD environment, regenerate the NP through the analysis of extracellular matrix production and assure safety and long‐term effectiveness.
The first and most logical cells to regenerate the NP with would be utilizing IVD cells themselves, NP, and AF cells. NP and AF cells can survive in the NP and produce extracellular matrix in vivo. 186 However, AF cells produce extracellular matrix that does not resemble the native extracellular matrix of the NP. Furthermore, the harvesting of NP cells involves a difficult invasive procedure, which results in a limited cell number and inefficient expansion capacity limits the use of NP and AF cells as a cell source potential greatly, although cadaveric sources for NP cells are currently in clinical trials. 118 In addition, NP cells exposed to the degenerated IVD may result in NP cellular senescence and contribute to an inability to produce proteoglycans 30 , 259 ; as this is a key factor in initial stages of IVD degeneration. NP cells display similar phenotypic characteristics to chondrocytes, sharing similar morphology and some gene expression. Chondrocytes are readily available from multiple sources, can be expanded in vitro and remain viable in IVD condition. However, in vivo they retain their chondrocytic phenotype and produce extracellular matrix that involves a slightly different composition compared with NP extracellular matrix, 141 , 178 thus affecting the biomechanical properties of the IVD. Another cell type are IVD derived NC cells; these cells are of notochordal origin and are pre‐existing cells in the NP region of the human IVD prior to adulthood. NC cells are capable of synthesizing a proteoglycan‐rich matrix and play a protective role in a catabolic environment; however, they are hampered by difficulties in handling them and acquisition. In particular the difficulties in maintaining phenotype in monolayer culture in vitro, 74 , 160 , 161 together with difficulties in harvesting sufficient numbers due to difficulties with amplification in vitro and their non‐existence in mature human IVDs. 4 , 158 , 162
Alternatives are to utilize adult stromal cell differentiation into NP‐like cells. BMSC have good differentiation capacity and are easily accessible. Animal models and clinical trials showed promising results for partial regeneration of NP. However, the differentiation fluidity resulted in adverse events such as osteophyte formation 217 and capacity of BMSC to migrate, applies a reason for concern with unwanted bone formation if injected alone. While BMSC are the target of many clinical trials, results to date are limited to short term follow up and thus longer‐term results are awaited. Another source of MSC are ADMSC, harvesting these cells is less invasive and can result with larger cell quantities than BMSC extraction. 229 In vitro they are grown easily under standard conditions. Despite promising in vitro results, and some extracellular matrix production observed in vivo, ADMSC survival rates in IVD conditions in vitro and in vivo are low, triggered by the hypoxic and inflammatory host environment. 220 , 221 BMSC and ADMSC generally proliferate when exposed to inflammatory conditions, especially where there is also oxidative stress; this response is valuable in normal healing conditions and protects from apoptosis. 228 , 260 However, in the context of the degenerative IVD, the increase in cells could result in depleting the already limited nutrient supply of the IVD and thus may not provide regeneration. MSC derived from perinatal tissues have less limitations than using BMSC and ADMSC; hUCSC are more primitive and display a lower risk of rejection, 261 making these cells available for allogeneic transplants. Human UCSC offers good differentiation capabilities and can be subject to differentiation into NP‐like cells in IVD conditions. In smaller and larger animal models, hUCSC demonstrated partial regeneration of degenerate NP tissue, yet their observed migration ability is a limitation, which may lead to safety concerns. In human clinical trials, overall, there was an improvement in self‐assessment of pain and disability and some reports of NP regeneration through MRI analysis. There were no adverse events reported, displaying the safety of hUCSCs in humans. However, this was a small cohort, and more studies are needed.
The final cells evaluated were iPSC, which could be used to generate NP‐like cells. As there are no established precursors of NP cells, differentiating iPSC into iNC‐LC is proposed within the literature to regenerate the IVD in the literature to date, however full characterization of these cells and their function has not been completed. iPSC can be generated from any somatic cell; therefore, they are highly accessible and have an unlimited proliferation ability. As iPSC have been used to differentiate into iNC‐LC, they survive in IVD conditions and have been shown to produce extracellular matrix in vivo, although investigation of the true degenerate environment remains to be evaluated. Furthermore, safety issues with these cells still need to be evaluated, iPSC can form teratomas and the safety of viral transfection, used to induce iPSC differentiation, on the cells' karyotype also needs to be assessed. Based on the evaluation of all cell types (Table 1), iPSC or BMPCs currently provide the highest overall potential for cellular therapy to regenerate the NP, however further investigation is required. If the safety of iPSC can be established, then utilizing iPSC for cell therapy would provide improved accessibility compared with NP, AF, NC, BMSC, and ADMSC; increased proliferation compared with NP, AF, and NC cells; and at least in principle would be on par with the ability of NC and NP cells to survive within the IVD environment and produce the desired extracellular matrix. The next question would be which cell type would possess the greatest regenerative potential in a degenerate IVD. Whereas injecting NP cells could cause the injected cells to contribute to degeneration, limited knowledge is known about the regenerative effects of NC cells.
TABLE 1.
Heat map showing the conclusion of cell type use to regenerate the degenerative nucleus pulposus of the intervertebral disc; nucleus pulpous cells (NP), annulus fibrosis (AF) cells, notochordal (NC) cells, chondrocytes, bone marrow stem cells (BMSC), adipose‐derived stem cells (ADMSC), umbilical cord stem cells (UCSC), and induced pluripotent stem cells (iPSC)
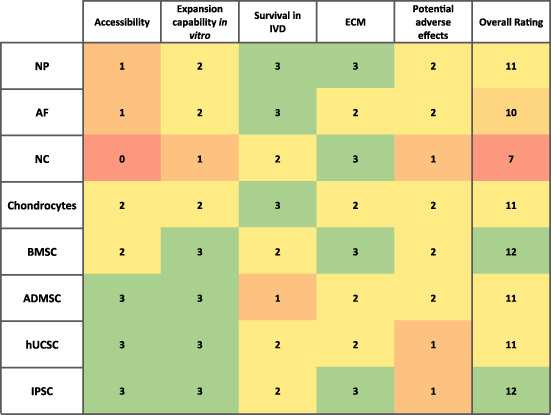
|
Note: These cell type were ranked 1 to 3, 1 being inefficient, and 3 being efficient at accessibility of harvesting the cell type, expansion capability in vitro, the ability of the cell type to survive in a intervertebral disc environment, the ability of the cell to produce extracellular matrix and the potential adverse event that could potentially happen; deeming a safety issue.
This report concentrates on cell sources; however, there are other important considerations that have extensive influence on cell behavior, such as the addition of biomaterials and growth factors. Biomaterials are used as cell carriers, cell anchoring, a guide for extracellular matrix synthesis and mechanical support; reviews have discussed cell‐biomaterial approaches of IVD tissue engineering. 95 , 262 , 263 These materials could be utilized to provide instructive cues and protection to cells and thus must be considered in combination for future therapies. Bowles and Setton (2017) have reviewed bioengineering advances to treat IVD regeneration. Interestingly this biomaterial review concludes too, that the regeneration of IVD must take into consideration biological processes. 263 Growth factors can also be implemented to facilitate correct differentiation, or regenerative properties. 194 , 231 , 255 , 264 For example, GDF5 gene transferred BMSC upregulated aggrecan and SOX9 and KRT19 compared with non‐transfected cells, which was reported to lead to partial recovery of GAGs in bovine degenerated IVD 194 ; also pre‐treated ADSCs with SAG resulted in the improved IVD height, water content, extracellular matrix content, and structure of degenerated IVDs in vivo. 231 Few of the studies cited in this review compared the treatment of cells only and cells with biomaterials, there is evidence that when cells are used in conjunction with a biomaterial, it can result in an enhanced regenerative effect on the IVD. 103 , 238 Combining cells, biomaterial and growth factors is the principle of a tissue engineering review by Tendulkar et al. (2019), whose analysis focuses on the repair of NP utilizing tissue engineering approach of injectable hydrogels, cells, and growth factors. 93
In summary, here we provide a rational discussion of potential cell sources proposed for NP tissue regeneration, based on accessibility, expansion capability in vitro, cell survival in the IVD environment, regenerative effects of cells alone, and potential safety of the cells. We propose that the use of iPSC‐NC‐LC as a cell source could have the potential for regenerating the NP in degenerate IVDs and requires further study to assess their regenerative ability and safety.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following sources of support for this manuscript: European Union's Horizon 2020 research 52 and innovation program iPSpine under the grant agreement #825925 (www.ipspine.eu); MT acknowledges the financial support by the Dutch Arthritis Society (LLP22).
Williams, R. J. , Tryfonidou, M. A. , Snuggs, J. W. , & Le Maitre, C. L. (2021). Cell sources proposed for nucleus pulposus regeneration. JOR Spine, 4(4), e1175. 10.1002/jsp2.1175
Funding information Dutch Arthritis Society, Grant/Award Number: LLP22; European Unions Horizon 2020 Research 52 and Innovation Program iPSpine, Grant/Award Number: 825925
Footnotes
Conclusion; Table 1. Accessibility of NP cells is ranked 1, as these cells are situated in the NP within the IVD. Therefore, difficult to access and unable to use autologously.
Conclusion; Table 1. Expansion capability of NP cells is ranked 2, as these cells have low cell number from harvesting and inability to expand in monolayer culture conditions.
Conclusion; Table 1. Survival capability of NP cells in IVD environment is ranked 3, as these cells are derived from the IVD and are adapted to survive in that physiological environment.
Conclusion; Table 1. NP cell's ability to produce extracellular matrix is ranked 3, as these cells produce aggrecan and collagen type II, which are extracellular matrix physiologically found in the NP region of the disc.
Conclusion; Table 1. The potential adverse effects of using NP cells is ranked 2, as NP cells used from a degenerative disc sources resulted in less regeneration of disc and an increase in catabolic enzymes and inflammatory factors. Subjecting patients through treatments that may result in no significant improvement.
Conclusion; Table 1. Accessibility of AF cells is ranked 1, as these cells are situated in the AF within the IVD. Therefore, difficult to access and unable to use autologously.
Conclusion; Table 1. Expansion capability of AF cells is ranked 2, as these cells have low cell number from harvesting.
Conclusion; Table 1. Survival capability of AF cells in IVD environment is ranked 3, as these cells are derived from the IVD and are adapted to survive in that physiological environment.
Conclusion; Table 1. AF cells ability to produce extracellular matrix is ranked 2, as these cells produce cartilage like matrix, which is not phenotypic of the NP region.
Conclusion; Table 1. The potential adverse effects of using AF cells is ranked 2, as using this cell source would not result in a regeneration of the native matrix production, thus subjecting patients through treatments that results in no significant improvement.
Conclusion; Table 1. Accessibility of NC cells is ranked 0, as these cells are only situated in the NP region of certain species of animals and in humans under the age of 10.
Conclusion; Table 1. Expansion capability of NC cells is ranked 1, as there's limited experience in handling these cells in research and the few research has shown that NC cells are problematic in monolayer and in continuous expansion culture conditions.
Conclusion; Table 1. Survival capability of NC cells in IVD environment is ranked 2, as these cells are derived from the IVD and are adapted to survive in that physiological environment, although they show increased nutrient demand and do not appear in mature human IVDs.
Conclusion; Table 1. NC cells ability to produce extracellular matrix is ranked 3, as these cells produce proteoglycans at a higher fold than NP cells and extracellular matrix at the same physiological GAG/DNA ratio.
Conclusion; Table 1. The potential adverse effects of using NC cells are ranked 1, as NC cells used are xenogenous and could pose a rejection reaction.
Conclusion; Table 1. Accessibility of chondrocyte cells is ranked 2, as these cells can be harvested from multiple sources in the body, however they are invasive procedures and could lead to injury at the site of cell source.
Conclusion; Table 1. Expansion capability of chondrocyte cells is ranked 2, as these cells can be easily expanded in different in vitro culture systems, while still maintaining good viability after several passages. However, they are limited by senescence.
Conclusion; Table 1. Survival capability of chondrocyte cells in IVD environment is ranked 3, as it has be demonstrated that these cells can survival and proliferate in in vitro IVD environment, and survive in the IVD of in vivo animal models.
Conclusion; Table 1. Chondrocyte cell's ability to produce extracellular matrix is ranked 2, as these cells were shown to be able to produce extracellular matrix in vitro and in vivo models, however it did not resembled the phenotypic matrix observed in the NP.
Conclusion; Table 1 . The potential adverse effects of using chondrocyte cells is ranked 2, as using this cell source would not result in a regeneration of the native matrix production, thus subjecting patients through treatments that results in no significant improvement.
Conclusion; Table 1. Expansion capability of BMSCs is ranked 3, as these cells can be easily maintained after multiple passages and are easily manipulated into different cell lineages.
Conclusion; Table 1. Survival capability of BMSCs in IVD environment is ranked 2, as in vitro IVD conditions affected BMSCs biosynthesis and proliferation ability, however these cells were able to survive in vivo animal models as they differentiated towards NP‐like cells.
Conclusion; Table 1. BMSC's ability to produce extracellular matrix is ranked in IVD environment is ranked 3, as in in vivo animal studies BMSCs that differentiate into NP‐like cells have shown their ability to produce NP native matrix, resulting in some disc regeneration.
Conclusion; Table 1. The potential adverse effects of using BMSCs is ranked 2, as in in vivo animal studies BMSCs have shown to migrate away from the injected site, posing a risk of bone formation in unwanted areas of the IVD. Additionally, BMSCs are used in clinical human trails which have shown no overall significant change to LBP patients, as the use of BMSC resulted in slowing down the degeneration of the disc and not regenerating the disc.
Conclusion; Table 1. Accessibility of BMSCs is ranked 2, as these cells are easily sourced from the bone marrow, however it is a painful and invasive procedure.
Conclusion; Table 1. The potential adverse effects of using BMSCs is ranked 2, as in in vivo animal studies BMSCs have shown to migrate away from the injected site, posing a risk of bone formation in unwanted areas of the IVD. Additionally, BMSCs are used in clinical human trails which have shown no overall significant change to LBP patients, as the use of BMSC resulted in slowing down the degeneration of the disc and not regenerating the disc.
Conclusion; Table 1. Expansion capability of ADMSCs is ranked 3, as these cells can be easily maintained in different culture conditions and are easily manipulated into different cell lineages.
Conclusion; Table 1. Survival capability of ADMSCs in IVD environment is ranked 1, as the IVD conditions effected the proliferation and viability of ADMSCs. Additionally, the hypoxic conditions resulted in these cells releasing adverse factors, which would further disc degeneration.
Conclusion; Table 1. ADMSC's ability to produce extracellular matrix is ranked 2, as in in vivo small animal models resulted in improved matrix deposit, however in larger animal models no difference in extracellular matrix production was observed.
Conclusion; Table 1. The potential adverse effects of using ADMSCs is ranked 2, as even though there was no reports of ADMSC differentiation into unwanted bone formation, transplanted ADMSC had low survival rate reducing the potential of regeneration and increasing the risk of ADMSCs excreting detrimental factors, thus subjecting patients through treatments that would result in no significant improvement.
Conclusion; Table 1. Accessibility of ADMSCs is ranked 3, as an abundance of cells are collected in a simple surgical procedure.
Conclusion; Table 1. Expansion capability of hUCSCs is ranked 3, as these cells can be easily maintained in different culture conditions and are easily manipulated into different cell lineages
Conclusion; Table 1. Survival capability of hUCSCs in IVD environment is ranked 2, as these cells were able to survive in vitro IVD conditions and in vivo animal models as they differentiate towards NP‐like cells. However, they have not yet been investigated in a degenerate environment.
Conclusion; Table 1. hUCSCs ability to produce extracellular matrix is ranked 2, as in in vivo animal models these cells were able to produce extracellular matrix native to the NP, which resulted in partial regeneration.
Conclusion; Table 1. The potential adverse effects of using hUCSCs is ranked 1, as some reports of transplanted cells migrating away from treatment site, and human clinical trials have shown limited regeneration with the use of hUCSCs.
Conclusion; Table 1. Accessibility of hUCSCs is ranked 3, as these cells are externally sources and there is an abundance of hUCSCs in blood banks.
Conclusion; Table 1. Accessibility of iPSC is ranked 3, as these cells are generated from almost any somatic cell and then stored in abundance in iPSC banks.
Conclusion; Table 1. Expansion capability of iPSC is ranked 3, as these cells can be easily maintained, expanded in culture conditions, and are theoretically easily manipulated into different cell lineages.
Conclusion; Table 1. Survival capability of iPSC in IVD environment is ranked 2, as these cells were able to survive in vitro IVD conditions and in vivo animal models as they differentiate towards NC‐like cells.
Conclusion; Table 1. iPSC's ability to produce extracellular matrix is ranked 3, as in in vivo animal models these cells were able to produce extracellular matrix native to the NP, which resulted in disc regeneration.
Conclusion; Table 1. The potential adverse effects of using iPSC is ranked 1, as there is potential of iPSC to form teratomas, however there have been no reports in the few initial in vivo studies with these cells.
REFERENCES
- 1. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356‐2367. doi: 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 2. Traeger AC, Buchbinder R, Elshaug AG, Croft PR, Maher CG. Care for low back pain: can health systems deliver? Bull World Health Organ. 2019;97(6):423‐433. doi: 10.2471/BLT.18.226050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maetzel A, Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract Res: Clin Rheumatol. 2002;16(1):23‐30. doi: 10.1053/berh.2001.0204 [DOI] [PubMed] [Google Scholar]
- 4. Pittler MH, Brown EM, Ernst E. Static magnets for reducing pain: systematic review and meta‐analysis of randomized trials. CMAJ. 2007;177(7):736. doi: 10.1503/CMAJ.061344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu L, Skinner M, McDonough S, Mabire L, Baxter GD. Acupuncture for low back pain: an overview of systematic reviews. Evid‐Based Complement Altern Med: eCAM. 2015;2015:1‐18. doi: 10.1155/2015/328196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514‐530. doi: 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 7. Jahantiqh F, Abdollahimohammad A, Firouzkouhi M, Ebrahiminejad V. Effects of reiki versus physiotherapy on relieving lower back pain and improving activities daily living of patients with intervertebral disc hernia. J Evid‐Based Integr Med. 2018;23:1‐5. doi: 10.1177/2515690X18762745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nice T. Low back pain and sciatica in one over 16s. FEBS Lett. 2018;586:1154‐1159. doi: 10.1016/j.febslet.2012.03.030 [DOI] [Google Scholar]
- 9. Phillips K, Ch'ien APY, Norwood BR, Smith C. Chronic low back pain management in primary care. Nurse Pract. 2003;28(8):26‐31. doi: 10.1097/00006205-200308000-00008 [DOI] [PubMed] [Google Scholar]
- 10. Bogduk N. Management of chronic low back pain. Med J Aust. 2004;180(2):79‐83. doi: 10.5694/j.1326-5377.2004.tb05805.x [DOI] [PubMed] [Google Scholar]
- 11. Steffens D, Maher CG, Pereira LSM, et al. Prevention of low back pain a systematic review and meta‐analysis. JAMA Intern Med. 2016;176:199‐208. doi: 10.1001/jamainternmed.2015.7431 [DOI] [PubMed] [Google Scholar]
- 12. Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391:2368‐2383. doi: 10.1016/S0140-6736(18)30489-6 [DOI] [PubMed] [Google Scholar]
- 13. Berg S, Tullberg T, Branth B, Olerud C, Tropp H. Total disc replacement compared to lumbar fusion: a randomised controlled trial with 2‐year follow‐up. Eur Spine J. 2009;18(10):1512‐1519. doi: 10.1007/s00586-009-1047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6):S190‐S194. doi: 10.1016/J.SPINEE.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 15. Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13(10):1339‐1349. doi: 10.1016/J.SPINEE.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 16. Martell BA, O'Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116‐127. doi: 10.7326/0003-4819-146-2-200701160-00006 [DOI] [PubMed] [Google Scholar]
- 17. Manchikanti L, Helm S 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9‐ES38. [PubMed] [Google Scholar]
- 18. Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: A perspective from different continents. Pain Rep. 2017;2(3):599. doi: 10.1097/PR9.0000000000000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scholl L., Seth P, Karissa M, Wilson N, Baldwin G (2017) Morbidity and Mortality Weekly Report Drug and Opioid‐Involved Overdose Deaths‐United States, 2013–2017 . https://surveillance.cancer.gov/joinpoint/. Accessed April 24, 2020. [DOI] [PMC free article] [PubMed]
- 20. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain‐United States, 2016. JAMA. 2016;315(15):1624‐1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari‐Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25(4):487‐492. doi: 10.1097/00007632-200002150-00016 [DOI] [PubMed] [Google Scholar]
- 22. Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta‐analysis. Am J Neuroradiol. 2015;36(12):2394‐2399. doi: 10.3174/ajnr.A4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng CJ, Chen J. Disc degeneration implies low back pain. Theor Biol Med Model. 2015;12(1):24. doi: 10.1186/s12976-015-0020-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakai D, Andersson GBJ. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11(4):243‐256. doi: 10.1038/nrrheum.2015.13 [DOI] [PubMed] [Google Scholar]
- 25. Bae WC, Statum S, Zhang Z, et al. Morphology of the cartilaginous endplates in human intervertebral disks with ultrashort echo time MR imaging. Radiology. 2013;266(2):564‐574. doi: 10.1148/radiol.12121181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tomaszewski KA, Saganiak K, Gładysz T, Walocha JA. The biology behind the human intervertebral disc and its endplates. Folia Morphol. 2015;74(2):157‐168. doi: 10.5603/FM.2015.0026 [DOI] [PubMed] [Google Scholar]
- 27. Oegema TR. Biochemistry of the intervertebral disc. Clin Sports Med. 1993;12(3):419‐439. [PubMed] [Google Scholar]
- 28. Le Maitre CL, Dahia CL, Giers M, et al. Development of a standardized histopathology scoring system for human intervertebral disc degeneration: an Orthopaedic Research Society spine section initiative. JOR Spine. 2021;4(2):e1167. doi: 10.1002/JSP2.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120‐130. doi: 10.1186/ar629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9(3):R45. doi: 10.1186/ar2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bergknut N, Smolders LA, Grinwis GCM, et al. Intervertebral disc degeneration in the dog. Part 1: anatomy and physiology of the intervertebral disc and characteristics of intervertebral disc degeneration. Vet J. 2013;195:282‐291. doi: 10.1016/j.tvjl.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 32. Jiang L, Zhang X, Zheng X, et al. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: implications for diabetic intervertebral disc degeneration. J Orthop Res. 2013;31(5):692‐702. doi: 10.1002/jor.22289 [DOI] [PubMed] [Google Scholar]
- 33. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732. doi: 10.1186/ar1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FWL. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13(6):R215. doi: 10.1186/ar3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hodgkinson T, Shen B, Diwan A, Hoyland JA, Richardson SM. Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR Spine. 2019;2(1):e1045. doi: 10.1002/jsp2.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(4):652‐655. doi: 10.1042/BST0350652 [DOI] [PubMed] [Google Scholar]
- 37. Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cells Mater. 2004;8:58‐64. doi: 10.22203/eCM.v008a06 [DOI] [PubMed] [Google Scholar]
- 38. Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473(6):1903‐1912. doi: 10.1007/s11999-014-3774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Binch AL, Cole AA, Breakwell LM, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16(5):416. doi: 10.1186/s13075-014-0416-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MIV. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178‐181. doi: 10.1016/S0140-6736(97)02135-1 [DOI] [PubMed] [Google Scholar]
- 41. Ashton K, Walsh DA, Polak JM, Eisenstein SM. Substance P in intervertebral discs: binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 2009;65:635‐639. doi: 10.3109/17453679408994620 [DOI] [PubMed] [Google Scholar]
- 42. Vergroesen PPA, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23:1057‐1070. doi: 10.1016/j.joca.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 43. De Vries S, Van Doeselaar M, Meij B, Tryfonidou M, Ito K. Notochordal cell matrix as a therapeutic agent for intervertebral disc regeneration. Tissue Eng ‐ Part A. 2019;25:830‐841. doi: 10.1089/ten.tea.2018.0026 [DOI] [PubMed] [Google Scholar]
- 44. Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21(1):29‐41. doi: 10.1615/CritRevEukarGeneExpr.v21.i1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vadalà G, Russo F, di Martino A, Denaro V. Intervertebral disc regeneration: From the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med. 2015;9(6):679‐690. doi: 10.1002/term.1719 [DOI] [PubMed] [Google Scholar]
- 46. Endres M, Abbushi A, Thomale UW, et al. Intervertebral disc regeneration after implantation of a cell‐free bioresorbable implant in a rabbit disc degeneration model. Biomaterials. 2010;31(22):5836‐5841. doi: 10.1016/j.biomaterials.2010.03.078 [DOI] [PubMed] [Google Scholar]
- 47. Chan LKY, Leung VYL, Tam V, Lu WW, Sze KY, Cheung KMC. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater. 2013;9(2):5262‐5272. doi: 10.1016/j.actbio.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 48. Lin X, Fang X, Wang Q, et al. Decellularized allogeneic intervertebral disc: natural biomaterials for regenerating disc degeneration. Oncotarget. 2016;7(11):12121‐12136. doi: 10.18632/oncotarget.7735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Helen W, Gough JE. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within PDLLA/bioglass composite foam scaffolds in vitro. Acta Biomater. 2008;4(2):230‐243. doi: 10.1016/j.actbio.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 50. Henriksson HB, Hagman M, Horn M, Lindahl A, Brisby H. Investigation of different cell types and gel carriers for cell‐based intervertebral disc therapy, in vitro and in vivo studies. J Tissue Eng Regen Med. 2012;6(9):738‐747. doi: 10.1002/term.480 [DOI] [PubMed] [Google Scholar]
- 51. Thorpe AA, Boyes VL, Sammon C, Le Maitre CL. Thermally triggered injectable hydrogel, which induces mesenchymal stem cell differentiation to nucleus pulposus cells: potential for regeneration of the intervertebral disc. Acta Biomater. 2016;36:99‐111. doi: 10.1016/j.actbio.2016.03.029 [DOI] [PubMed] [Google Scholar]
- 52. Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: Regulation of proteoglycan synthesis. Exp Cell Res. 1999;246(1):129‐137. doi: 10.1006/excr.1998.4287 [DOI] [PubMed] [Google Scholar]
- 53. Sakai D. Future perspectives of cell‐based therapy for intervertebral disc disease. Eur Spine J. 2008;17:452‐458. doi: 10.1007/s00586-008-0743-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith LJ, Silverman L, Sakai D, et al. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine. 2018;1(4):e1036. doi: 10.1002/jsp2.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alkhatib B, Ban GI, Williams S, Serra R, Development IVD. Nucleus pulposus development and sclerotome specification. Curr Mol Biol Rep. 2018;4(3):132‐141. doi: 10.1007/s40610-018-0100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nieuwkoop PD. Short historical survey of pattern formation in the endo‐mesoderm and the neural anlage in the vertebrates: the role of vertical and planar inductive actions. Cell Mol Life Sci. 1997;53(4):305‐318. doi: 10.1007/pl00000608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31‐41. doi: 10.1242/dmm.006403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bach FC, Tellegen AR, Beukers M, et al. Biologic canine and human intervertebral disc repair by notochordal cell‐derived matrix: from bench towards bedside. Oncotarget. 2018;9(41):26507‐26526. doi: 10.18632/oncotarget.25476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Colombier P, Clouet J, Chariau C, et al. Generation of human nucleus pulposus cells from stem cells: first steps towards intervertebral disc regeneration. Osteoarthr Cartil. 2016a;24:S11‐S12. doi: 10.1016/j.joca.2016.01.051 [DOI] [Google Scholar]
- 60. Colombier P, Halgand B, Chédeville C, et al. NOTO transcription factor directs human induced pluripotent stem cell‐derived mesendoderm progenitors to a notochordal fate. Cells. 2020;9(2):509. doi: 10.3390/cells9020509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gao C, Ning B, Sang C, Zhang Y. Rapamycin prevents the intervertebral disc degeneration via inhibiting differentiation and senescence of annulus fibrosus cells. Aging (Albany, NY). 2018;10(1):131‐143. doi: 10.18632/aging.101364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ganey T, Libera J, Moos V, et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28(23):2609‐2620. doi: 10.1097/01.BRS.0000097891.63063.78 [DOI] [PubMed] [Google Scholar]
- 63. Gay MH, Mehrkens A, Rittmann M, et al. Nose to back: compatibility of nasal chondrocytes with environmental conditions mimicking a degenerated intervertebral disc. Eur Cells Mater. 2019;37:214‐232. doi: 10.22203/eCM.v037a13 [DOI] [PubMed] [Google Scholar]
- 64. Hoogendoorn RJW, Lu ZF, Kroeze RJ, Bank RA, Wuisman PI, Helder MN. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future: tissue engineering review series. J Cell Mol Med. 2008;12:2205‐2216. doi: 10.1111/j.1582-4934.2008.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Noriega DC, Ardura F, Hernández‐Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101(8):1945‐1951. doi: 10.1097/TP.0000000000001484 [DOI] [PubMed] [Google Scholar]
- 66. Omlor GW, Lorenz S, Nerlich AG, Guehring T, Richter W. Disc cell therapy with bone‐marrow‐derived autologous mesenchymal stromal cells in a large porcine disc degeneration model. Eur Spine J. 2018;27(10):2639‐2649. doi: 10.1007/s00586-018-5728-4 [DOI] [PubMed] [Google Scholar]
- 67. Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell‐extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8(1):90. doi: 10.1186/1471-213X-8-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vadalà G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8(5):185‐201. doi: 10.4252/wjsc.v8.i5.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhu Y, Liang Y, Zhu H, et al. The generation and functional characterization of induced pluripotent stem cells from human intervertebral disc nucleus pulposus cells. Oncotarget. 2017;8(26):42700‐42711. doi: 10.18632/oncotarget.17446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tang R, Jing L, Willard VP, et al. Differentiation of human induced pluripotent stem cells into nucleus pulposus‐like cells. Stem Cell Res Ther. 2018;9(1):61. doi: 10.1186/s13287-018-0797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sheyn D, Ben‐David S, Tawackoli W, et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics. 2019;9(25):7506‐7524. doi: 10.7150/thno.34898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huang YC, Leung VYL, Lu WW, Luk KDK. The effects of microenvironment in mesenchymal stem cell‐based regeneration of intervertebral disc. Spine J. 2013;13(3):352‐362. doi: 10.1016/j.spinee.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 73. Urban JPG. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2002;30(6):858‐864. doi: 10.1042/BST0300858 [DOI] [PubMed] [Google Scholar]
- 74. Spillekom S, Smolders LA, Grinwis GCM, et al. Increased osmolarity and cell clustering preserve canine notochordal cell phenotype in culture. Tissue Eng‐Part C. 2014;20(8):652‐662. doi: 10.1089/ten.tec.2013.0479 [DOI] [PubMed] [Google Scholar]
- 75. Li H, Wang J, Li F, Chen G, Chen Q. The influence of hyperosmolarity in the intervertebral disc on the proliferation and chondrogenic differentiation of nucleus pulposus‐derived mesenchymal stem cells. Cells Tissues Organs. 2018;205(3):178‐188. doi: 10.1159/000490760 [DOI] [PubMed] [Google Scholar]
- 76. Phillips KLE, Cullen K, Chiverton N, et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: Interleukin‐1 is a master regulator of catabolic processes. Osteoarthr Cartil. 2015;23(7):1165‐1177. doi: 10.1016/j.joca.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 77. Haufe SMW, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15(1):136‐137. doi: 10.1089/scd.2006.15.136 [DOI] [PubMed] [Google Scholar]
- 78. Thorpe AA, Bach FC, Tryfonidou MA, et al. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine. 2018;1(3):e1027. doi: 10.1002/jsp2.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2‐19. doi: 10.1007/s00586-007-0414-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shi P, Chee A, Liu W, Chou PH, Zhu J, An HS. Therapeutic effects of cell therapy with neonatal human dermal fibroblasts and rabbit dermal fibroblasts on disc degeneration and inflammation. Spine J. 2019;19(1):171‐181. doi: 10.1016/j.spinee.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 81. Chee A, Shi P, Cha T, et al. Cell therapy with human dermal fibroblasts enhances intervertebral disk repair and decreases inflammation in the rabbit model. Global Spine J. 2016;6(8):771‐779. doi: 10.1055/s-0036-1582391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ural IH, Alptekin K, Ketenci A, Solakoglu S, Alpak H, Özyalçın S. Fibroblast transplantation results to the degenerated rabbit lumbar intervertebral discs. Open Orthop J. 2017;11(1):404‐416. doi: 10.2174/1874325001711010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen C, Zhou T, Sun X, et al. Autologous fibroblasts induce fibrosis of the nucleus pulposus to maintain the stability of degenerative intervertebral discs. Bone Res. 2020;8(1):7. doi: 10.1038/s41413-019-0082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Luo TD, Vines JB, Zabarsky ZK, et al. Evaluation of percutaneous intradiscal amniotic suspension allograft in a rabbit model of intervertebral disc degeneration. Spine. 2019;44(6):E329‐E337. doi: 10.1097/BRS.0000000000002851 [DOI] [PubMed] [Google Scholar]
- 85. Hu X, Zhou Y, Zheng X, et al. Differentiation of menstrual blood‐derived stem cells toward nucleus pulposus‐like cells in a coculture system with nucleus pulposus cells. Spine. 2014;39(9):754‐760. doi: 10.1097/BRS.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 86. Diaz‐Hernandez ME, Khan NM, Trochez CM, et al. Derivation of notochordal cells from human embryonic stem cells reveals unique regulatory networks by single cell‐transcriptomics. J Cell Physiol. 2019;235:5241‐5255. doi: 10.1002/jcp.29411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Quintin A, Schizas C, Scaletta C, et al. Isolation and in vitro chondrogenic potential of human foetal spine cells. J Cell Mol Med. 2009;13:2559‐2569. doi: 10.1111/j.1582-4934.2008.00630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hang D, Li F, Che W, et al. One‐stage positron emission tomography and magnetic resonance imaging to assess mesenchymal stem cell survival in a canine model of intervertebral disc degeneration. Stem Cells Dev. 2017;26(18):1334‐1343. doi: 10.1089/scd.2017.0103 [DOI] [PubMed] [Google Scholar]
- 89. Vadalà G, Sobajima S, Lee JY, et al. In vitro interaction between muscle‐derived stem cells and nucleus pulposus cells. Spine J. 2008;8(5):804‐809. doi: 10.1016/j.spinee.2007.07.394 [DOI] [PubMed] [Google Scholar]
- 90. Murrell W, Sanford E, Anderberg L, Cavanagh B, Mackay‐Sim A. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 2009;9(7):585‐594. doi: 10.1016/j.spinee.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 91. Chen S, Emery SE, Pei M. Coculture of synovium‐derived stem cells and nucleus pulposus cells in serum‐free defined medium with supplementation of transforming growth factor‐β1: A potential application of tissue‐specific stem cells in disc regeneration. Spine. 2009;34(12):1272‐1280. doi: 10.1097/BRS.0b013e3181a2b347 [DOI] [PubMed] [Google Scholar]
- 92. Miyamoto T, Muneta T, Tabuchi T, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase‐related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12(6):R206. doi: 10.1186/ar3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tendulkar G, Chen T, Ehnert S, Kaps HP, Nüssler AK. Intervertebral disc nucleus repair: hype or hope? Int J Mol Sci. 2019;20:1‐15. doi: 10.3390/ijms20153622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tong W, Lu Z, Qin L, et al. Cell therapy for the degenerating intervertebral disc. Transl Res. 2017;181:49‐58. doi: 10.1016/j.trsl.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stergar J, Gradisnik L, Velnar T, Maver U. Intervertebral disc tissue engineering: a brief review. Bosn J Basic Med Sci. 2019;19:130‐137. doi: 10.17305/bjbms.2019.3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Harmon MD, Ramos DM, Nithyadevi D, et al. Growing a backbone‐functional biomaterials and structures for intervertebral disc (IVD) repair and regeneration: challenges, innovations, and future directions. Biomater Sci. 2020;8:1216‐1239. doi: 10.1039/c9bm01288e [DOI] [PubMed] [Google Scholar]
- 97. Thorpe AA, Binch ALA, Creemers LB, Sammon C, Le Maitre CL. Nucleus pulposus phenotypic markers to determine stem cell differentiation: fact or fiction? Oncotarget. 2016;7(3):2189‐2200. doi: 10.18632/oncotarget.6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen X, Zhu L, Wu G, Liang Z, Yang L, Du Z. A comparison between nucleus pulposus‐derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model. Int J Surg. 2016;28:77‐82. doi: 10.1016/j.ijsu.2016.02.045 [DOI] [PubMed] [Google Scholar]
- 99. Tang S, Richards J, Khan S, et al. Nonviral transfection with brachyury reprograms human intervertebral disc cells to a pro‐anabolic anti‐catabolic/inflammatory phenotype: a proof of concept study. J Orthop Res. 2019;37(11):2389‐2400. doi: 10.1002/jor.24408 [DOI] [PubMed] [Google Scholar]
- 100. Wang H, Jin CH, Kong J, et al. The research of transgenic human nucleus pulposus cell transplantation in the treatment of lumbar disc degeneration. Kaohsiung J Med Sci. 2019;35(8):486‐492. doi: 10.1002/kjm2.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32(23):2537‐2544. doi: 10.1097/BRS.0b013e318158dea6 [DOI] [PubMed] [Google Scholar]
- 102. Erwin WM. Biologically based therapy for the intervertebral disk: who is the patient? Glob Spine J. 2013;3(3):193‐199. doi: 10.1055/s-0033-1343074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang X, Guerrero J, Croft AS, Albers CE, Häckel S, Gantenbein B. Spheroid‐like cultures for expanding angiopoietin receptor‐1 (aka. Tie2) positive cells from the human intervertebral disc. Int J Mol Sci. 2020;21(24):1‐17. doi: 10.3390/ijms21249423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liang L, Li X, Li D, et al. The characteristics of stem cells in human degenerative intervertebral disc. Medicine (Baltimore). 2017;96(25):e7178. doi: 10.1097/MD.0000000000007178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Erwin WM, Islam D, Eftekarpour E, Inman RD, Karim MZ, Fehlings MG. Intervertebral disc‐derived stem cells. Spine. 2013;38(3):211‐216. doi: 10.1097/BRS.0b013e318266a80d [DOI] [PubMed] [Google Scholar]
- 106. Wu T, Song HX, Dong Y, Li JH. Cell‐based therapies for lumbar discogenic low back pain systematic review and single‐arm meta‐analysis. Spine. 2018;43(1):49‐57. doi: 10.1097/BRS.0000000000001549 [DOI] [PubMed] [Google Scholar]
- 107. De Luca P, Castagnetta M, de Girolamo L, et al. Intervertebral disc and endplate cell characterisation highlights annulus fibrosus cells as the most promising for tissue‐specific disc degeneration therapy. Eur Cells Mater. 2020;39:156‐170. doi: 10.22203/eCM.v039a10 [DOI] [PubMed] [Google Scholar]
- 108. Tanaka M, Sakai D, Hiyama A, et al. Effect of cryopreservation on canine and human activated nucleus pulposus cells: a feasibility study for cell therapy of the intervertebral disc. BioResearch Open Access. 2013;2(4):273‐282. doi: 10.1089/biores.2013.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kluba T, Niemeyer T, Gaissmaier C, Gründer T. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine. 2005;30(24):2743‐2748. doi: 10.1097/01.brs.0000192204.89160.6d [DOI] [PubMed] [Google Scholar]
- 110. Rosenzweig DH, Tremblay Gravel J, Bisson D, Ouellet JA, Weber MH, Haglund L. Comparative analysis in continuous expansion of bovine and human primary nucleus pulposus cells for tissue repair applicatioans. Eur Cell Mater. 2017;33:2240‐2251. doi: 10.22203/eCM.v033a18 [DOI] [PubMed] [Google Scholar]
- 111. Yung Lee J, Hall R, Pelinkovic D, et al. New use of a three‐dimensional pellet culture system for human intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine. 2001;26(21):2316‐2322. doi: 10.1097/00007632-200111010-00005 [DOI] [PubMed] [Google Scholar]
- 112. Mochida J, Sakai D, Nakamura Y, Watanabe T, Yamamoto Y, Kato S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: A three‐year, prospective clinical study of its safety. Eur Cells Mater. 2015;29:202‐212. doi: 10.22203/eCM.v029a15 [DOI] [PubMed] [Google Scholar]
- 113. Sakai D, Mochida J, Iwashina T, et al. Atelocollagen for culture of human nucleus pulposus cells forming nucleus pulposus‐like tissue in vitro: influence on the proliferation and proteoglycan production of HNPSV‐1 cells. Biomaterials. 2006;27(3):346‐353. doi: 10.1016/j.biomaterials.2005.06.040 [DOI] [PubMed] [Google Scholar]
- 114. Hegewald AA, Enz A, Endres M, et al. Engineering of polymer‐based grafts with cells derived from human nucleus pulposus tissue of the lumbar spine. J Tissue Eng Regen Med. 2011;5(4):275‐282. doi: 10.1002/term.312 [DOI] [PubMed] [Google Scholar]
- 115. Nukaga T, Sakai D, Tanaka M, Hiyama A, Nakai T, Mochida J. Transplantation of activated nucleus pulposus cells after cryopreservation: efficacy study in a canine disc degeneration model. Eur Cells Mater. 2016;31:95‐106. doi: 10.22203/eCM.v031a07 [DOI] [PubMed] [Google Scholar]
- 116. Hiraishi S, Schol J, Sakai D, et al. Discogenic cell transplantation directly from a cryopreserved state in an induced intervertebral disc degeneration canine model. JOR Spine. 2018;1(2):e1013. doi: 10.1002/jsp2.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang SZ, Fan WM, Jia J, Ma LY, Bin Yu J, Wang C. Is exclusion of leukocytes from platelet‐rich plasma (PRP) a better choice for early intervertebral disc regeneration? Stem Cell Res Ther. 2018;9(1):1‐11. doi: 10.1186/s13287-018-0937-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Silverman LI, Dulatova G, Tandeski T, et al. In vitro and in vivo evaluation of discogenic cells, an investigational cell therapy for disc degeneration. Spine J. 2020;20(1):138‐149. doi: 10.1016/j.spinee.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 119. Lee J, Lee E, Kim H, Son Y. Comparison of articular cartilage with costal cartilage in initial cell yield, degree of dedifferentiation during expansion and redifferentiation capacity. Biotechnol Appl Biochem. 2007;48(3):149‐158. doi: 10.1042/BA20060233 [DOI] [PubMed] [Google Scholar]
- 120. Kregar Velikonja N, Urban J, Fröhlich M, et al. Cell sources for nucleus pulposus regeneration. Eur Spine J. 2014;23:364‐374. doi: 10.1007/s00586-013-3106-9 [DOI] [PubMed] [Google Scholar]
- 121. Zhang J, Zhang J, Zhang Y, et al. Mesenchymal stem cells‐derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J Cell Mol Med. 2020a;24(20):11742‐11754. doi: 10.1111/jcmm.15784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Baumgartner L, Wuertz‐Kozak K, le Maitre CL, et al. Multiscale regulation of the intervertebral disc: achievements in experimental, in silico, and regenerative research. Int J Mol Sci. 2021;22:1‐42. doi: 10.3390/ijms22020703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tao Y‐Q, Liang CZ, Li H, et al. Potential of co‐culture of nucleus pulposus mesenchymal stem cells and nucleus pulposus cells in hyperosmotic microenvironment for intervertebral disc regeneration. Cell Biol Int. 2013;37(8):826‐834. doi: 10.1002/cbin.10110 [DOI] [PubMed] [Google Scholar]
- 124. Han B, Wang HC, Li H, et al. Nucleus pulposus mesenchymal stem cells in acidic conditions mimicking degenerative intervertebral discs give better performance than adipose tissue‐derived mesenchymal stem cells. Cells Tissues Organs. 2014;199:342‐352. doi: 10.1159/000369452 [DOI] [PubMed] [Google Scholar]
- 125. Suyama K, Sakai D, Hirayama N, et al. Effects of interleukin‐17A in nucleus pulposus cells and its small‐molecule inhibitors for intervertebral disc disease. J Cell Mol Med. 2018;22(11):5539‐5551. doi: 10.1111/jcmm.13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Neidlinger‐Wilke C, Mietsch A, Rinkler C, Wilke H‐J, Ignatius A, Urban J. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J Orthop Res. 2012;30:112‐121. doi: 10.1002/jor.21481 [DOI] [PubMed] [Google Scholar]
- 127. Rinkler C, Heuer F, Pedro MT, Mauer UM, Ignatius A, Neidlinger‐Wilke C. Influence of low glucose supply on the regulation of gene expression by nucleus pulposus cells and their responsiveness to mechanical loading. J Neurosurg Spine. 2010;13(4):535‐542. doi: 10.3171/2010.4.SPINE09713 [DOI] [PubMed] [Google Scholar]
- 128. Krouwels A, Popov‐Celeketic J, Plomp SGM, et al. No effects of hyperosmolar culture medium on tissue regeneration by human degenerated nucleus pulposus cells despite upregulation extracellular matrix genes. Spine. 2018;43(5):307‐315. doi: 10.1097/BRS.0000000000000920 [DOI] [PubMed] [Google Scholar]
- 129. Mwale F, Ciobanu I, Giannitsios D, Roughley P, Steffen T, Antoniou J. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine. 2011;36(2):E131‐E138. doi: 10.1097/BRS.0b013e3181d52b9e [DOI] [PubMed] [Google Scholar]
- 130. Risbud MV, Guttapalli A, Stokes DG, et al. Nucleus pulposus cells express HIF‐1α under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98(1):152‐159. doi: 10.1002/jcb.20765 [DOI] [PubMed] [Google Scholar]
- 131. Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor‐1, glucose transporter‐1 and MMP‐2. Cell Tissue Res. 2002;308(3):401‐407. doi: 10.1007/s00441-002-0563-6 [DOI] [PubMed] [Google Scholar]
- 132. Ziello JE, Jovin IS, Huang Y. Hypoxia‐inducible factor (HIF)‐1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80:51‐60. [PMC free article] [PubMed] [Google Scholar]
- 133. Bibby SRS, Jones DA, Ripley RM, Urban JPG. Metabolism of the intervertebral disc: Effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine. 2005;30(5):487‐496. doi: 10.1097/01.brs.0000154619.38122.47 [DOI] [PubMed] [Google Scholar]
- 134. Wang W, Qiu Y, Huang X, et al. Transplantation of allogeneic nucleus pulposus cells attenuates intervertebral disc degeneration by inhibiting apoptosis and increasing migration. Int J Mol Med. 2018b;41(5):2553‐2564. doi: 10.3892/ijmm.2018.3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Iwashina T, Mochida J, Sakai D, et al. Feasibility of using a human nucleus pulposus cell line as a cell source in cell transplantation therapy for intervertebral disc degeneration. Spine. 2006;31(11):1177‐1186. doi: 10.1097/01.brs.0000217687.36874.c4 [DOI] [PubMed] [Google Scholar]
- 136. Li YY, Diao HJ, Chik TK, et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng‐Part A. 2014a;20:1379‐1391. doi: 10.1089/ten.tea.2013.0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wang X, Meng Q, Qiu C, et al. Potential therapeutic role of co‐Q10 in alleviating intervertebral disc degeneration and suppressing IL‐1β‐mediated inflammatory reaction in NP cells. Int Immunopharmacol. 2018d;64:424‐431. doi: 10.1016/j.intimp.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 138. Tschugg A, Michnacs F, Strowitzki M, Meisel HJ, Thomé C. A prospective multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART Disc plus autologous disc chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disc to avoid secondary disease: Study protocol. Trials. 2016;17(1):108. doi: 10.1186/s13063-016-1239-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: Phenotype and function. J Anat. 2012;221(6):480‐496. doi: 10.1111/j.1469-7580.2012.01521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Poiraudeau S, Monteiro I, Anract P, Blanchard O, Revel M, Corvol MT. Phenotypic characteristics of rabbit intervertebral disc cells comparison with cartilage cells from the same animals. Spine. 1999;24(9):837‐844. doi: 10.1097/00007632-199905010-00002 [DOI] [PubMed] [Google Scholar]
- 141. Sato M, Asazuma T, Ishihara M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue‐engineering method. Spine. 2003;28(6):548‐553. doi: 10.1097/01.brs.0000049909.09102.60 [DOI] [PubMed] [Google Scholar]
- 142. Colombini A, Lopa S, Ceriani C, et al. In vitro characterization and in vivo behavior of human nucleus pulposus and annulus fibrosus cells in clinical‐grade fibrin and collagen‐enriched fibrin gels. Tissue Eng Part A. 2015;21:793‐802. doi: 10.1089/ten.tea.2014.0279 [DOI] [PubMed] [Google Scholar]
- 143. Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan‐based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27(3):388‐396. doi: 10.1016/j.biomaterials.2005.06.037 [DOI] [PubMed] [Google Scholar]
- 144. Torre OM, Mroz V, Bartelstein MK, Huang AH, Iatridis JC. Annulus fibrosus cell phenotypes in homeostasis and injury: implications for regenerative strategies. Ann N Y Acad Sci. 2019;1442(1):61‐78. doi: 10.1111/nyas.13964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Power KA, Grad S, Rutges JPHJ, et al. Identification of cell surface‐specific markers to target human nucleus pulposus cells expression of carbonic anhydrase XII varies with age and degeneration. Arthritis Rheum. 2011;63(12):3876‐3886. doi: 10.1002/art.30607 [DOI] [PubMed] [Google Scholar]
- 146. van den Akker GGH, Eijssen LMT, Richardson SM, et al. A membranome‐centered approach defines novel biomarkers for cellular subtypes in the intervertebral disc. Cartilage. 2020;11(2):203‐220. doi: 10.1177/1947603518764260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bron J, Helder M, Meisel H‐J, Royen B, Smit TH. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301‐313. doi: 10.1007/s00586-008-0856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chu G, Shi C, Wang H, Zhang W, Yang H, Li B. Strategies for annulus fibrosus regeneration: from biological therapies to tissue engineering. Front Bioeng Biotechnol. 2018;1:90. doi: 10.3389/fbioe.2018.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tavakoli J, Diwan AD, Tipper JL. Molecular sciences advanced strategies for the regeneration of lumbar disc annulus fibrosus. Int J Mol Sci. 2020;21:4889. doi: 10.3390/ijms21144889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang Z, Perez‐Terzic CM, Smith J, et al. Efficacy of intervertebral disc regeneration with stem cells: a systematic review and meta‐analysis of animal controlled trials. Gene. 2015b;564(1):1‐8. doi: 10.1016/j.gene.2015.03.022 [DOI] [PubMed] [Google Scholar]
- 151. Bhunia BK, Kaplan DL, Mandal BB. Silk‐based multilayered angle‐ply annulus fibrosus construct to recapitulate form and function of the intervertebral disc. Proc Natl Acad Sci U S A. 2018;115(3):477‐482. doi: 10.1073/pnas.1715912115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Frauchiger DA, Heeb SR, May RD, Wöltje M, Benneker LM, Gantenbein B. Differentiation of MSC and annulus fibrosus cells on genetically engineered silk fleece‐membrane‐composites enriched for GDF‐6 or TGF‐β3. J Orthop Res. 2018;36(5):1324‐1333. doi: 10.1002/jor.23778 [DOI] [PubMed] [Google Scholar]
- 153. Wang YH, Yang B, Li WL, Li JM. Effect of the mixture of bone marrow mesenchymal stromal cells and annulus fibrosus cells in repairing the degenerative discs of rabbits. Genet Mol Res. 2015;14(1):2365‐2373. doi: 10.4238/2015.March.27.22 [DOI] [PubMed] [Google Scholar]
- 154. Nukaga T, Sakai D, Schol J, Sato M, Watanabe M. Annulus fibrosus cell sheets limit disc degeneration in a rat annulus fibrosus injury model. JOR Spine. 2019;2(2):e1050. doi: 10.1002/jsp2.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yang CH, Chiang YF, Chen CH, Wu LC, Liao CJ, Chiang CJ. The effect of annular repair on the failure strength of the porcine lumbar disc after needle puncture and punch injury. Eur Spine J. 2016;25(3):906‐912. doi: 10.1007/s00586-015-4316-0 [DOI] [PubMed] [Google Scholar]
- 156. Gebhard H, Bowles R, Dyke J, et al. Total disc replacement using a tissue‐engineered intervertebral disc in vivo: new animal model and initial results. Evid Based Spine Care J. 2010;1:62‐66. doi: 10.1055/s-0028-1100918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sloan SR, Lintz M, Hussain I, Hartl R, Bonassar LJ. Biologic annulus fibrosus repair: a review of preclinical in vivo investigations. Tissue Eng‐Part B: Rev. 2018;24:179‐190. doi: 10.1089/ten.teb.2017.0351 [DOI] [PubMed] [Google Scholar]
- 158. Kim JH, Deasy BM, Seo HY, et al. Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine. 2009;34(23):2486‐2493. doi: 10.1097/BRS.0b013e3181b26ed1 [DOI] [PubMed] [Google Scholar]
- 159. Ellis K, Bagwell J, Bagnat M. Notochord vacuoles are lysosome‐related organelles that function in axis and spine morphogenesis. J Cell Biol. 2013;200(5):667‐679. doi: 10.1083/jcb.201212095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gantenbein B, Calandriello E, Wuertz‐Kozak K, Benneker LM, Keel MJB, Chan SCW. Activation of intervertebral disc cells by co‐culture with notochordal cells, conditioned medium and hypoxia. BMC Musculoskelet Disord. 2014;15(1):422. doi: 10.1186/1471-2474-15-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Potier E, Ito K. Using notochordal cells of developmental origin to stimulate nucleus pulposus cells and bone marrow stromal cells for intervertebral disc regeneration. Eur Spine J. 2014;23(3):679‐688. doi: 10.1007/s00586-013-3107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Potier E, de Vries S, van Doeselaar M, Ito K. Potential application of notochordal cells for intervertebral disc regeneration: an in vitro assessment. Eur Cells Mater. 2014;28:68‐81. doi: 10.22203/eCM.v028a06 [DOI] [PubMed] [Google Scholar]
- 163. Smolders LA, Meij BP, Onis D, et al. Gene expression profiling of early intervertebral disc degeneration reveals a down‐regulation of canonical Wnt signaling and caveolin‐1 expression: implications for development of regenerative strategies. Arthritis Res Ther. 2013;15(1):R23. doi: 10.1186/ar4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Arkesteijn IT, Smolders LA, Spillekom S, et al. Effect of coculturing canine notochordal, nucleus pulposus and mesenchymal stromal cells for intervertebral disc regeneration. Arthritis Res Ther. 2015;17(1):60. doi: 10.1186/s13075-015-0569-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Purmessur D, Guterl CC, Cho SK, et al. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther. 2013a;15:R122. doi: 10.1186/ar4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Arkesteijn ITM, Potier E, Ito K. The regenerative potential of notochordal cells in a nucleus pulposus explant. Global Spine J. 2017;7(1):14‐20. doi: 10.1055/s-0036-1583174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Humphreys MD, Ward L, Richardson SM, Hoyland JA. An optimized culture system for notochordal cell expansion with retention of phenotype. JOR Spine. 2018;1(3):e1028. doi: 10.1002/jsp2.1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Urban JPG, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: An in vivo study. Biorheology. 1978;15:203‐223. doi: 10.3233/BIR-1978-153-409 [DOI] [PubMed] [Google Scholar]
- 169. Urban MR, Fairbank JCT, Etherington PJ, Loh L, Winlove CP, Urban JPG. Electrochemical measurement of transport into scoliotic intervertebral discs in vivo using nitrous oxide as a tracer. Spine. 2001;26(8):984‐990. doi: 10.1097/00007632-200104150-00028 [DOI] [PubMed] [Google Scholar]
- 170. Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205(5):357‐362. doi: 10.1111/j.0021-8782.2004.00352.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Guehring T, Wilde G, Sumner M, et al. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60(4):1026‐1034. doi: 10.1002/art.24407 [DOI] [PubMed] [Google Scholar]
- 172. Cappello R, Bird JLE, Pfeiffer D, Bayliss MT, Dudhia J. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine. 2006;31(8):873‐882. doi: 10.1097/01.brs.0000209302.00820.fd [DOI] [PubMed] [Google Scholar]
- 173. Liu Z, Zheng Z, Qi J, et al. CD24 identifies nucleus pulposus progenitors/notochordal cells for disc regeneration. J Biol Eng. 2018;12(1):35. doi: 10.1186/s13036-018-0129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kwon WK, Moon HJ, Kwon TH, Park YK, Kim JH. Influence of rabbit notochordal cells on symptomatic intervertebral disc degeneration: anti‐angiogenic capacity on human endothelial cell proliferation under hypoxia. Osteoarthr Cartil. 2017;25(10):1738‐1746. doi: 10.1016/j.joca.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 175. Zhang Y, Zhang Z, Chen P, et al. Directed differentiation of notochord‐like and nucleus pulposus‐like cells using human pluripotent stem cells. Cell Rep. 2020c;30(8):2791‐2806. doi: 10.1016/j.celrep.2020.01.100 [DOI] [PubMed] [Google Scholar]
- 176. Vedicherla S, Buckley CT. In vitro extracellular matrix accumulation of nasal and articular chondrocytes for intervertebral disc repair. Tissue Cell. 2017;49(4):503‐513. doi: 10.1016/j.tice.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 177. Tsaryk R, Silva‐Correia J, Oliveira JM, et al. Biological performance of cell‐encapsulated methacrylated gellan gum‐based hydrogels for nucleus pulpous regeneration. J Tissue Eng Regen Med. 2017;11(3):637‐648. doi: 10.1002/term.1959 [DOI] [PubMed] [Google Scholar]
- 178. Gorenšek M, Jaksimović C, Kregar‐Velikonja N, et al. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett. 2004;9(2):363‐373. [PubMed] [Google Scholar]
- 179. Vinod E, Boopalan P, Sathishkumar S. Reserve or resident progenitors in cartilage? Comparative analysis of chondrocytes versus chondroprogenitors and their role in cartilage repair. Cartilage. 2018;9(2):171‐182. doi: 10.1177/1947603517736108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Janssen J, Batschkus S, Schimmel S, Bode C, Schminke B, Miosge N. The influence of TGF‐β3, EGF, and BGN on SOX9 and RUNX2 expression in human chondrogenic progenitor cells. J Histochem Cytochem. 2019;67(2):117‐127. doi: 10.1369/0022155418811645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Koelling S, Kruegel J, Irmer M, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4(4):324‐335. doi: 10.1016/J.STEM.2009.01.015 [DOI] [PubMed] [Google Scholar]
- 182. Vinod E, Parameswaran R, Ramasamy B, Kachroo U. Pondering the potential of hyaline cartilage–derived chondroprogenitors for tissue regeneration: a systematic review. Cartilage. 2020;1‐19. doi: 10.1177/1947603520951631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Fellows CR, Williams R, Davies IR, et al. Characterisation of a divergent progenitor cell sub‐populations in human osteoarthritic cartilage: the role of telomere erosion and replicative senescence. Sci Rep. 2017;7:41421. doi: 10.1038/SREP41421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Acosta FL, Metz L, Adkisson HD, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011;17:3045‐3055. doi: 10.1089/ten.tea.2011.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kuh SU, Zhu Y, Li J, et al. A comparison of three cell types as potential candidates for intervertebral disc therapy: annulus fibrosus cells, chondrocytes, and bone marrow derived cells. Joint Bone Spine. 2009;76(1):70‐74. doi: 10.1016/j.jbspin.2008.02.021 [DOI] [PubMed] [Google Scholar]
- 186. Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell‐based therapeutics: disc chondrocyte transplantation. A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5‐21. doi: 10.1016/j.bioeng.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 187. Coric D, Pettine K, Sumich A, Boltes M. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine. 2013;18(1):85‐95. doi: 10.3171/2012.10.SPINE12512 [DOI] [PubMed] [Google Scholar]
- 188. Löser P, Schirm J, Guhr A, Wobus AM, Kurtz A. Human embryonic stem cell lines and their use in international research. Stem Cells. 2010;28(2):240. doi: 10.1002/STEM.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Oehme D, Goldschlager T, Rosenfeld JV, Ghosh P, Jenkin G. The role of stem cell therapies in degenerative lumbar spine disease: a review. Neurosurg Rev. 2015;38(3):429‐445. doi: 10.1007/s10143-015-0621-7 [DOI] [PubMed] [Google Scholar]
- 190. Chun HJ, Kim YS, Kim BK, et al. Transplantation of human adipose‐derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg. 2012;78(364):371. doi: 10.1016/j.wneu.2011.12.084 [DOI] [PubMed] [Google Scholar]
- 191. Shim EK, Lee JS, Kim DE, et al. Autogenous mesenchymal stem cells from the vertebral body enhance intervertebral disc regeneration via paracrine interaction: An in vitro pilot study. Cell Transplant. 2016;25(10):1819‐1832. doi: 10.3727/096368916X691420 [DOI] [PubMed] [Google Scholar]
- 192. Yang SH, Yang KC, Chen CW, Huang TC, Sun YH, Hu MH. Comparison of transforming growth factor‐beta1 and lovastatin on differentiating mesenchymal stem cells toward nucleus pulposus‐like phenotype: An in vitro cell culture study. Asian Spine J. 2019;13(5):705‐712. doi: 10.31616/asj.2018.0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Li D, Zeng Q, Jiang Z, et al. Induction of notochordal differentiation of bone marrow mesenchymal‐derived stem cells via the stimulation of notochordal cell‐rich nucleus pulposus tissue. Mol Med Rep. 2021;23(2):162. doi: 10.3892/mmr.2020.11801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bucher C, Gazdhar A, Benneker LM, Geiser T, Gantenbein‐Ritter B. Nonviral gene delivery of growth and differentiation factor 5 to human mesenchymal stem cells injected into a 3d bovine intervertebral disc organ culture system. Stem Cells Int. 2013;2013:326828. doi: 10.1155/2013/326828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Mwale F, Wang HT, Roughley P, Antoniou J, Haglund L. Link N and mesenchymal stem cells can induce regeneration of the early degenerate intervertebral disc. Tissue Eng ‐ Part A. 2014;20:2942‐2949. doi: 10.1089/ten.tea.2013.0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Henriksson HB, Svanvik T, Jonsson M, et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine. 2009;34(2):141‐148. doi: 10.1097/BRS.0b013e31818f8c20 [DOI] [PubMed] [Google Scholar]
- 197. Wuertz K, Godburn K, Neidlinger‐Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine. 2008;33(17):1843‐1849. doi: 10.1097/BRS.0b013e31817b8f53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Wang W, Wang Y, Deng G, et al. Transplantation of hypoxic‐preconditioned bone mesenchymal stem cells retards intervertebral disc degeneration via enhancing implanted cell survival and migration in rats. Stem Cells Int. 2018c;2018:1‐13. doi: 10.1155/2018/7564159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Binch ALA, Richardson SM, Hoyland JA, Barry FP. Combinatorial conditioning of adipose derived‐mesenchymal stem cells enhances their neurovascular potential: Implications for intervertebral disc degeneration. JOR Spine. 2019;2(4). doi: 10.1002/jsp2.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Peroglio M, Eglin D, Benneker LM, Alini M, Grad S. Thermoreversible hyaluronan‐based hydrogel supports in vitro and ex vivo disc‐like differentiation of human mesenchymal stem cells. Spine J. 2013;13(11):1627‐1639. doi: 10.1016/j.spinee.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 201. Papadimitriou N, Li S, Henriksson HB. Iron sucrose‐labeled human mesenchymal stem cells: in vitro multilineage capability and in vivo traceability in a lapine xenotransplantation model. Stem Cells Dev. 2015;24(20):2403‐2412. doi: 10.1089/SCD.2015.0140 [DOI] [PubMed] [Google Scholar]
- 202. Wei A, Tao H, Chung SA, Brisby H, Ma DD, Diwan AD. The fate of transplanted xenogeneic bone marrow‐derived stem cells in rat intervertebral discs. J Orthop Res. 2009;27(3):374‐379. doi: 10.1002/jor.20567 [DOI] [PubMed] [Google Scholar]
- 203. Le Maitre CL, Frain J, Millward‐Sadler J, Fotheringham AP, Freemont AJ, Hoyland JA. Altered integrin mechanotransduction in human nucleus pulposus cells derived from degenerated discs. Arthritis Rheum. 2009;60(2):460‐469. doi: 10.1002/art.24248 [DOI] [PubMed] [Google Scholar]
- 204. Kumar D, Gerges I, Tamplenizza M, Lenardi C, Forsyth NR, Liu Y. Three‐dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: A potential injectable cellular product for nucleus pulposus regeneration. Acta Biomater. 2014;10(8):3463‐3474. doi: 10.1016/j.actbio.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 205. Sinkemani A, Wang F, Xie Z, Chen L, Zhang C, Wu X. Nucleus pulposus cell conditioned medium promotes mesenchymal stem cell differentiation into nucleus pulposus‐like cells under hypoxic conditions. Stem Cells Int. 2020;2020:8882549. doi: 10.1155/2020/8882549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Chan SCW, Bürki A, Bonél HM, Benneker LM, Gantenbein‐Ritter B. Papain‐induced in vitro disc degeneration model for the study of injectable nucleus pulposus therapy. Spine J. 2013;13(3):273‐283. doi: 10.1016/j.spinee.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 207. Pereira CL, Teixeira GQ, Ribeiro‐Machado C, et al. Mesenchymal stem/stromal cells seeded on cartilaginous endplates promote intervertebral disc regeneration through extracellular matrix remodeling. Sci Rep. 2016;6:33836. doi: 10.1038/srep33836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Blanco JF, Villarón EM, Pescador D, et al. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: results of a prospective phase I/II clinical trial with long‐term follow‐up. Stem Cell Res Ther. 2019;10(1):63. doi: 10.1186/s13287-019-1166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Centeno C, Markle J, Dodson E, et al. Treatment of lumbar degenerative disc disease‐associated radicular pain with culture‐expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med. 2017;15(1):197. doi: 10.1186/s12967-017-1300-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra‐discal injection of autologous, hypoxic cultured bone marrow‐derived mesenchymal stem cells in five patients with chronic lower back pain: A long‐term safety and feasibility study. J Transl Med. 2016;14(1):253. doi: 10.1186/s12967-016-1015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García‐Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92(7):822‐828. doi: 10.1097/TP.0b013e3182298a15 [DOI] [PubMed] [Google Scholar]
- 212. Pettine KA, Suzuki RK, Sand TT, Murphy MB. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three‐year follow‐up. Int Orthop. 2017;41(10):2097‐2103. doi: 10.1007/s00264-017-3560-9 [DOI] [PubMed] [Google Scholar]
- 213. Wangler S, Peroglio M, Menzel U, et al. Mesenchymal stem cell homing into intervertebral discs enhances the Tie2‐positive progenitor cell population, prevents cell death, and induces a proliferative response. Spine. 2019;44(23):1613‐1622. doi: 10.1097/BRS.0000000000003150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Binch ALA, Fitzgerald JC, Growney EA, Barry F. Cell‐based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17(3):158‐175. doi: 10.1038/s41584-020-00568-w [DOI] [PubMed] [Google Scholar]
- 215. Elabd C, Ichim TE, Miller K, et al. Comparing atmospheric and hypoxic cultured mesenchymal stem cell transcriptome: implication for stem cell therapies targeting intervertebral discs. J Transl Med. 2018;16(1):222. doi: 10.1186/s12967-018-1601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine. 2010;35(11):E475‐E480. doi: 10.1097/BRS.0b013e3181cd2cf4 [DOI] [PubMed] [Google Scholar]
- 217. Vadalà G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6(5):348‐355. doi: 10.1002/term.433 [DOI] [PubMed] [Google Scholar]
- 218. Urits I, Capuco A, Sharma M, et al. Stem cell therapies for treatment of discogenic low back pain: a comprehensive review. Curr Pain Headache Rep. 2019;23(9):65. doi: 10.1007/s11916-019-0804-y [DOI] [PubMed] [Google Scholar]
- 219. Gimble JM, Guilak F. Adipose‐derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362‐369. doi: 10.1080/14653240310003026 [DOI] [PubMed] [Google Scholar]
- 220. Marfia G, Campanella R, Navone SE, et al. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: A preliminary study on biglycan‐deficient murine model of chronic disc degeneration. Arthritis Res Ther. 2014;16(1):457. doi: 10.1186/s13075-014-0457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kumar H, Ha DH, Lee EJ, et al. Safety and tolerability of intradiscal implantation of combined autologous adipose‐derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1‐year follow‐up of a phase i study. Stem Cell Res Ther. 2017;8(1):262. doi: 10.1186/s13287-017-0710-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Zhou X, Zhang F, Wang D, et al. Micro fragmented adipose tissue promotes the matrix synthesis function of nucleus pulposus cells and regenerates degenerated intervertebral disc in a pig model. Cell Transplant. 2020;29. doi: 10.1177/0963689720905798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Comella K, Silbert R, Parlo M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med. 2017;15(1):12. doi: 10.1186/s12967-016-1109-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Wang F, Nan LP, Zhou SF, et al. Injectable hydrogel combined with nucleus pulposus‐derived mesenchymal stem cells for the treatment of degenerative intervertebral disc in rats. Stem Cells Int. 2019a;2019:1‐17. doi: 10.1155/2019/8496025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Zhang Y, Wang Y, Zhou X, et al. Osmolarity controls the differentiation of adipose‐derived stem cells into nucleus pulposus cells via histone demethylase KDM4B. Mol Cell Biochem. 2020;472:157‐171. doi: 10.1007/s11010-020-03794-8 [DOI] [PubMed] [Google Scholar]
- 226. Li H, Liang C, Tao Y, et al. Acidic pH conditions mimicking degenerative intervertebral discs impair the survival and biological behavior of human adipose‐derived mesenchymal stem cells. Exp Biol Med. 2012;237(7):845‐852. doi: 10.1258/ebm.2012.012009 [DOI] [PubMed] [Google Scholar]
- 227. Liang C, Li H, Tao Y, et al. Responses of human adipose‐derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med. 2012;10(1):49. doi: 10.1186/1479-5876-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Borem R, Madeline A, Bowman M, Gill S, Tokish J, Mercuri J. Differential effector response of amnion‐ and adipose‐derived mesenchymal stem cells to inflammation; implications for intradiscal therapy. J Orthop Res. 2019;37(11):2445‐2456. doi: 10.1002/jor.24412 [DOI] [PubMed] [Google Scholar]
- 229. Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose‐tissue‐derived stromal cells. Acta Neurochir (Wien). 2010;152(10):1771‐1777. doi: 10.1007/s00701-010-0698-2 [DOI] [PubMed] [Google Scholar]
- 230. Jeong JH, Jin ES, Min JK, et al. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology. 2009;59(1):55‐64. doi: 10.1007/s10616-009-9192-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Hua J, Shen N, Wang J, et al. Small molecule‐based strategy promotes nucleus pulposus specific differentiation of adipose‐derived mesenchymal stem cells. Mol Cells. 2019;42(9):661‐671. doi: 10.14348/molcells.2019.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Ma C, Wang R, Zhao D, et al. Efficacy of platelet‐rich plasma containing xenogenic adipose tissue‐derived stromal cells on restoring intervertebral disc degeneration: a preclinical study in a rabbit model. Pain Res Manag. 2019;2019:6372356. doi: 10.1155/2019/6372356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Colombier P, Clouet J, Boyer C, et al. TGF‐b1 and GDF5 act synergistically to drive the differentiation of human adipose stromal cells toward nucleus pulposus‐like cells. Stem Cells. 2016b;34:653‐667. doi: 10.1002/stem.2249 [DOI] [PubMed] [Google Scholar]
- 234. Breymann C, Schmidt D, Hoerstrup SP. Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev. 2006;2(2):87‐92. doi: 10.1007/s12015-006-0014-y [DOI] [PubMed] [Google Scholar]
- 235. Zhang Y, Tao H, Gu T, et al. The effects of human Wharton's jelly cell transplantation on the intervertebral disc in a canine disc degeneration model. Stem Cell Res Ther. 2015;6(1):154. doi: 10.1186/s13287-015-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Chon BH, Lee EJ, Jing L, Setton LA, Chen J. Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin‐rich pseudo‐three‐dimensional culture system. Stem Cell Res Ther. 2013;4(5):120. doi: 10.1186/scrt331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Beeravolu N, Brougham J, Khan I, McKee C, Perez‐Cruet M, Chaudhry GR. Human umbilical cord derivatives regenerate intervertebral disc. J Tissue Eng Regen Med. 2018;12(1):e579‐e591. doi: 10.1002/term.2330 [DOI] [PubMed] [Google Scholar]
- 238. Choi UY, Joshi HP, Payne S, et al. An injectable hyaluronan–methylcellulose (HAMC) hydrogel combined with Wharton's jelly‐derived mesenchymal stromal cells (WJ‐MSCs) promotes degenerative disc repair. Int J Mol Sci. 2020;21(19):1‐20. doi: 10.3390/ijms21197391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Pang X, Yang H, Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician. 2014;17(4):E525‐E530. doi: 10.36076/PPJ.2014/17/E525 [DOI] [PubMed] [Google Scholar]
- 240. Wang H‐S, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330‐1337. doi: 10.1634/stemcells.2004-0013 [DOI] [PubMed] [Google Scholar]
- 241. Mueller AA, Forraz N, Gueven S, et al. Osteoblastic differentiation of wharton jelly biopsy specimens and their mesenchymal stromal cells after serum‐free culture. Plast Reconstr Surg. 2014;134(1):59e‐69e. doi: 10.1097/PRS.0000000000000305 [DOI] [PubMed] [Google Scholar]
- 242. Choi KS, Harfe BD. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci U S A. 2011;108(23):9484‐9489. doi: 10.1073/pnas.1007566108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Zeng X, Lin J, Wu H, et al. Effect of conditioned medium from human umbilical cord‐derived mesenchymal stromal cells on rejuvenation of nucleus pulposus derived stem/progenitor cells from degenerated intervertebral disc. Int J Stem Cells. 2020;13(2):257. doi: 10.15283/IJSC20027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Zucconi E, Vieira NM, Bueno CR Jr, et al. Preclinical studies with umbilical cord mesenchymal stromal cells in different animal models for muscular dystrophy. J Biomed Biotechnol. 2011;2011:715251. doi: 10.1155/2011/715251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Leite C, Silva NT, Mendes S, et al. Differentiation of human umbilical cord matrix mesenchymal stem cells into neural‐like progenitor cells and maturation into an oligodendroglial‐like lineage. PLoS One. 2014;9(10). doi: 10.1371/journal.pone.0111059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Zhao Y, Qin Y, Yang JS, et al. Wharton's jelly‐derived mesenchymalstem cells suppress apoptosis of nucleuspulposus cells in intervertebral discdegeneration via Wnt pathway. Eur Rev Med Pharmacol Sci. 2020;24:9807‐9814. [DOI] [PubMed] [Google Scholar]
- 247. Anderson DG, Markova D, An HS, et al. Human umbilical cord blood‐derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013;92(5):420‐429. doi: 10.1097/PHM.0b013e31825f148a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Perez‐Cruet M, Beeravolu N, McKee C, et al. Potential of human nucleus pulposus‐like cells derived from umbilical cord to treat degenerative disc disease. Clin Neurosurg. 2019;84(1):272‐283. doi: 10.1093/neuros/nyy012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Leckie SK, Sowa GA, Bechara BP, et al. Injection of human umbilical tissue‐derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013;13(3):263‐272. doi: 10.1016/j.spinee.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kurtzberg J, Lyerly AD, Sugarman J. Untying the Gordian knot: policies, practices, and ethical issues related to banking of umbilical cord blood. J Clin Invest. 2005;115(10):2592‐2597. doi: 10.1172/JCI26690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663‐676. doi: 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 252. Okita K, Yamanaka S. Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci. 2011;366(1575):2198‐2207. doi: 10.1098/rstb.2011.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. doi: 10.1186/s13287-019-1165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Xia K, Zhu J, Hua J, et al. Intradiscal injection of induced pluripotent stem cell‐derived nucleus pulposus‐like cell‐seeded polymeric microspheres promotes rat disc regeneration. Stem Cells Int. 2019;2019:6806540. doi: 10.1155/2019/6806540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Hu A, Xing R, Jiang L, et al. Thermosensitive hydrogels loaded with human‐induced pluripotent stem cells overexpressing growth differentiation factor‐5 ameliorate intervertebral disc degeneration in rats. J Biomed Mater Res Part B Appl Biomater. 2020;108(5):2005‐2016. doi: 10.1002/jbm.b.34541 [DOI] [PubMed] [Google Scholar]
- 256. Liu Y, Fu S, Rahaman MN, Mao JJ, Bal BS. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res Part A. 2015;103(3):1053‐1059. doi: 10.1002/jbm.a.35243 [DOI] [PubMed] [Google Scholar]
- 257. Sun Z, Zhang M, Zhao XH, et al. Immune cascades in human intervertebral disc: the pros and cons. Int J Clin Exp Pathol. 2013;6(6):1009‐1014. [PMC free article] [PubMed] [Google Scholar]
- 258. Sun Z, Liu B, Luo ZJ. The immune privilege of the intervertebral disc: implications for intervertebral disc degeneration treatment. Int J Med Sci. 2020;17(5):685‐692. doi: 10.7150/ijms.42238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J. 2006;15:312‐316. doi: 10.1007/s00586-006-0126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383‐396. doi: 10.1038/nri3209 [DOI] [PubMed] [Google Scholar]
- 261. Arufe MC, de la Fuente A, Fuentes I, de Toro FJ, Blanco FJ. Umbilical cord as a mesenchymal stem cell source for treating joint pathologies. World J Orthop. 2011;2(6):43‐50. doi: 10.5312/wjo.v2.i6.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54‐67. doi: 10.1016/j.biomaterials.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Huang YC, Hu Y, Li Z, Luk KDK. Biomaterials for intervertebral disc regeneration: current status and looming challenges. J Tissue Eng Regen Med. 2018;12(11):2188‐2202. doi: 10.1002/TERM.2750 [DOI] [PubMed] [Google Scholar]
- 264. Clarke LE, McConnell JC, Sherratt MJ, Derby B, Richardson SM, Hoyland JA. Growth differentiation factor 6 and transforming growth factor‐beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16(2):R67. doi: 10.1186/ar4505 [DOI] [PMC free article] [PubMed] [Google Scholar]


