Abstract
Introduction
Local steroid administration during anterior cervical spine surgery has been shown to improve postoperative dysphagia. However, concerns over potential complications remain. This study aims to evaluate the effect of local steroid administration on bone regeneration and spine fusion in a preclinical model, as well as the impact on osteogenic differentiation of human bone marrow‐derived mesenchymal stem cells (hBM‐MSCs) in a 3D culture system.
Materials and methods
Forty‐five rats underwent bilateral L4‐L5 posterolateral lumbar fusion (PLF) utilizing local delivery of low‐dose recombinant human bone morphogenetic protein‐2 (rhBMP‐2; 0.5 μg/implant). Rats were divided into three groups: no steroid (control), low dose (0.5 mg/kg), and high dose (2.5 mg/kg) of triamcinolone. Bone growth and fusion were assessed using radiography, blinded manual palpation, and micro‐CT analysis and were visualized by histology. The impact of triamcinolone exposure on osteogenic differentiation of hBM‐MSCs was evaluated by gene expression analysis, alkaline phosphatase activity assay, and alizarin red staining.
Results
No significant differences in fusion scores or rates were seen in the low‐ or high‐dose steroid treatment groups relative to untreated controls. Quantification of new bone formation via micro‐CT imaging revealed no significant between‐group differences in the volume of newly regenerated bone. Triamcinolone also had no negative impact on pro‐osteogenic gene transcript levels, and ALP activity was enhanced in the presence of triamcinolone. Mineral deposition appeared comparable in cultures grown with and without triamcinolone.
Conclusions
Local steroid application does not seem to inhibit rhBMP‐2‐mediated spine fusion in rats, though our study may not be adequately powered to detect differences in fusion as measured by manual palpation or bone volume as measured by micro‐CT. These findings suggest that local triamcinolone may not increase pseudarthrosis in spine fusion procedures. Further large animal and clinical studies to verify its safety and efficacy are warranted.
Keywords: local steroid, osteogenesis, pseudarthrosis, spine fusion
This study aimed to evaluate the effect of local steroid administration on bone regeneration and spine fusion in an established rodent posterolateral spinal fusion model, in vivo, and on the osteogenic differentiation of human mesenchymal stem cells in a 3D in vitro model. Local steroid administration did not inhibit fusion rates at a clinically relevant dose, nor did it have any adverse effect at a weight‐percentage equivalent dose 5‐fold higher than what is used in humans. In a 3D culture model, we did not find detrimental effects of triamcinolone on the osteogenic differentiation of hBM‐MSCs.
1. INTRODUCTION
There is significant interest in the efficacy and safety of steroids to reduce the incidence of postoperative complications attributable to soft‐tissue edema in patients undergoing anterior cervical procedures. 1 , 2 , 3 , 4 These studies have evaluated the effects of pre‐, 1 intra‐, 2 , 3 , 5 , 6 , 7 , 8 and postoperative 7 , 9 , 10 steroid use through various routes, including intravenous, 4 , 7 intramuscular, 11 and local 2 , 3 , 6 , 8 administration. Outcome variables included steroid‐related complications, dysphagia/odynophagia, prevertebral soft tissue swelling (PSTS), airway compromise, and length of hospital stay. 1 , 2 , 9 , 12 Prospective randomized controlled trials (RCT) involving perioperative intravenous steroid administration 7 , 9 , 10 in the setting of anterior cervical discectomy and fusion (ACDF) have demonstrated reduction in the severity of edematous pharyngolaryngeal lesions 9 and the incidence of postoperative dysphagia. 7 , 10 RCTs investigating the effects of local steroid administration 2 , 3 , 5 , 6 , 8 have also reported reduced incidence and severity of postoperative dysphagia, and better patient‐reported outcomes (PROs) with respect to postoperative dysphagia. 8 However, whether this practice affects arthrodesis and bone growth remains unknown, since most of these studies utilize short‐term follow‐up. As a result, local steroid administration has not been widely adopted. 2 , 7
There remains a paucity of literature regarding the potential but unsubstantiated inhibitory effect of corticosteroids on bone healing and graft incorporation. At 6‐month follow‐up, Jeyamohan et al 7 demonstrated significantly lower fusion rates in cervical patients receiving intravenous steroid intraoperatively but no difference at later time points. Inhibition of graft incorporation and consequent pseudarthrosis were reported after postoperative intramuscular dexamethasone administration in a rabbit posterolateral lumbar fusion (PLF) model. 11 To date though, there have been no animal studies reporting the effect of local steroid application on bone regenerative capacity in spine fusion.
Clinical studies have failed to provide a definitive determination regarding the effects of perioperative steroid on fusion rates in anterior cervical spine surgery. 7 , 9 , 10 Concern regarding steroid‐induced pseudarthrosis is supported by in vitro studies that suggest steroid exposure inhibits osteogenesis. 13 , 14 Therefore, this study sought to quantify the impact of local steroid application on new bone formation and spinal fusion rates in a rat PLF model, and to clarify the impact on osteogenic differentiation of human bone marrow‐derived mesenchymal stem cells (hBM‐MSCs) in a 3D culture system that mimics the in vivo setting.
2. MATERIAL AND METHODS
2.1. Study design
This study was conducted in accordance with IACUC‐approved protocol #IS00009754, and with Animal Welfare Assurance from the Office of Laboratory Animal Welfare. Forty‐five female Sprague‐Dawley rats (80% power, α = .05), aged 12 to 16 weeks, underwent bilateral PLF at L4‐L5 with implantation of low‐dose recombinant human BMP‐2 (rhBMP‐2; 0.5 μg per side) and high‐ or low‐dose triamcinolone delivered on an absorbable collagen sponge (ACS). An additional five rats underwent sham surgery and received no implant. Three different treatment combinations were tested: (a) rhBMP‐2/ACS (no steroid, n = 15), rhBMP‐2/ACS‐LD (low‐dose steroid, 0.5 mg/kg, n = 15), and rhBMP‐2/ACS‐HD (high dose steroid, 2.5 mg/kg, n = 15). The low‐dose was calculated as the weight‐percentage equivalent of triamcinolone used in humans, 8 and the high‐dose was 5‐fold greater. Figure 1 summarizes the experimental design and workflow. Each ACS (Integra LifeSciences, Plainsboro, New Jersey) was cut to 15 × 5 × 5 mm prior to saturation with rhBMP‐2, and the respective weight‐percentage equivalent dose of triamcinolone for 15 minutes prior to implantation.
FIGURE 1.
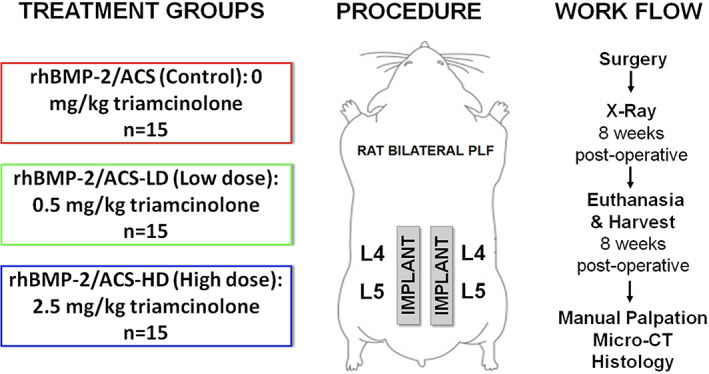
Schematic showing the treatment groups and overall work flow of the study
2.2. Surgical technique
Rats were maintained under anesthesia with an isoflurane inhalational delivery system. The animals were monitored by an assistant for temperature regulation and any cardiac or respiratory anomalies throughout the surgery. The PLF procedure was performed as previously described, 15 , 16 , 17 with a midline posterior skin incision over the lumbar spinous processes and bilateral fascial incisions 3 to 5 mm from the midline. The L4 and L5 transverse processes were exposed bilaterally followed by decortication of the transverse processes with a burr drill. Graft materials were implanted bilaterally to bridge the L4‐L5 transverse processes. Fascial incisions were closed with absorbable sutures and skin incisions with wound clips. Buprenorphine and meloxicam were administered subcutaneously for pain control for 3 days. Rats were housed separately and allowed to eat, drink, and bear weight ad libitum. There were no complications evident in the postoperative period among the cohorts. Notably, we did not encounter behavioral or neurologic changes throughout the study period in our postoperative animals, and there were no infections.
2.3. Determination of fusion
Radiographs were obtained using an APR‐VET Console (Sedecal USA, Inc.) at 4 and 8 weeks postoperatively. After euthanasia at 8 weeks postoperative, lumbar spines were harvested and manually palpated for evidence of fusion by three independent, blinded observers using a previously established scoring system, 15 , 16 , 17 , 18 , 19 whereby 0 = no bridging bone, 1 = fusion unilaterally with bridging bone, and 2 = fusion bilaterally with bridging bone (Table 1). Individual fusion scores for each specimen were averaged, and spines with an average score ≥1.0 were considered successfully fused. 15 , 16 , 17 , 18 , 19
TABLE 1.
Fusion scoring
| Fusion score | 0 | 1 | 2 |
|---|---|---|---|
| Findings based on palpation | No bridging bone | Unilateral bridging bone | Bilateral bridging bone |
Note: Palpation performed by three blinded, independent observers, and scores were averaged. Spines with greater than or equal to 1 were considered fused.
2.4. Quantitative micro‐CT imaging
Specimens were imaged using micro‐computed tomography (micro‐CT) (nanoScan PET/CT, Mediso, Hungary) (n = 6). Native bone volume was also quantified for five animals that underwent sham surgery. Data were acquired with a 2.17× magnification, <60 μm focal spot, 1 × 1 binning, with 720 projection views over a full circle, using 70 kVp/72 μA, and with a 300 ms exposure time. The projection data were reconstructed with a voxel size of 34 μm. The images were then filtered, visualized, segmented, and analyzed using Amira 6.7 image analysis software (ThermoFisher Scientific, Waltham, MA). Total bone volume was determined by including only voxels in the region of interest above a threshold of 500 Houndsfield units (HU). This threshold was chosen by determining the value that best reproduced the newly formed bone structure in several specimens. The total bone volume (TV) for each specimen was determined by segmentation and quantification of the native L4‐L5 processes and the fusion bed. The new bone volume (BV) for each specimen was then calculated by subtracting the mean TV of the sham surgery specimens from the TV of each specimen in the treatment groups.
2.5. Bone histomorphometry
After micro‐CT imaging, representative spines from each group underwent histological evaluation for visualization of the fusion bed. Samples were demineralized as described elsewhere and embedded in paraffin. 20 Seven‐micrometer thin sections were cut with a RM2255 microtome (Leica), deparaffinized, and stained with Gill hematoxylin and eosin (Sigma‐Aldrich) according to the manufacturer's recommendations. Stained sections were mounted with Cytoseal XYL mounting medium (Thermo Scientific), imaged on a TissueGnostic histological microscope (Zeiss), and visualized using TissueFAXS software.
2.6. 3D in vitro osteogenic differentiation of hBM‐MSCs
hBM‐MSCs were purchased from the ATCC and cultured as described previously. 21 ACS scaffolds were cut to 5 × 5 × 5 mm (one‐third the size of the in vivo implants). Prior to seeding, scaffolds were saturated with rhBMP‐2 and triamcinolone at the equivalent concentrations (per unit volume) used in the in vivo study. Scaffolds were seeded with 3.5 × 105 cells and cultured in standard and osteogenic media (OsteoLife, LifeLine). Samples were collected at increasing time points and processed for gene expression (n = 4) using an RNeasy kit (Qiagen). Taqman primer sequences used are shown in Table S1, Supporting Information. At the same experimental time points, samples were also processed for analysis of alkaline phosphatase (ALP) activity (SensoLyte pNPP Alkaline Phosphatase Colorimetric Assay Kit [AnaSpec]) according to the manufacturer's instructions, normalizing to total protein content. For assessment of mineral deposition, at 3 weeks, scaffolds (n = 3) were fixed with 4% paraformaldehyde, processed and embedded into paraffin blocks, and sliced to 20 μm thickness. The sections were stained with Alizarin Red S (10 mg/mL, Sigma‐Aldrich) for 5 minutes. An unseeded collagen scaffold was used to determine possible background staining. Sections were dehydrated through an ethanol ramp and then cleared in xylene before mounting the coverslips. Additional sections were also stained with Gill hematoxylin as previously described. Two fields of view at both low and high magnification were randomly selected and captured for each specimen.
2.7. Statistical analysis
A one‐way analysis of variance (ANOVA) followed by post hoc Tukey honest significant difference (HSD) test was used to compare the average fusion scores and new bone volume quantity between treatment groups. A Fisher exact test was used to compare fusion rates between groups. For all in vitro assays, a two‐way ANOVA followed by a multiple comparison post hoc test was used. Values are reported as mean ± SD. For all analyses, the cutoff for statistical significance was P < .05.
3. RESULTS
Radiographs showed bridging bone formation or lack thereof at L4‐L5 among all groups (Figure 2). Eight weeks after surgery, blinded manual palpation of the harvested spines showed no significant differences in mean fusion scores in the low‐ or high‐dose steroid groups (mean scores of 0.62 ± 0.59 and 1.1 ± 0.81, respectively) compared to the control group (mean score of 0.58 ± 0.58). Similarly, fusion rates in low‐ and high‐dose steroid groups (57% and 33%, respectively) were not significantly different compared to the control group (38%) (Figure 3). Quantification of newly regenerated bone via micro‐CT imaging demonstrated mean new bone volumes of 29.7 mm3 (±27.2 mm3), 41.7 mm3 (±27.6 mm3), and 25.5 mm3 (±21.7 mm3) in the control, low‐dose, and high‐dose steroid treatment groups, respectively (Figure 4C), which were not significantly different from one another (control vs low‐dose steroid; control vs high‐dose steroid, P > .05) (Figure 4D). 3D reconstructions of micro‐CT images were rendered for representative spines in each treatment group (Figure 4A). Histological visualization of the fusion bed was assessed using hematoxylin and eosin staining of tissue sections (Figure 4B). For comparison, an untreated (unfused) control is shown in Figure S1.
FIGURE 2.
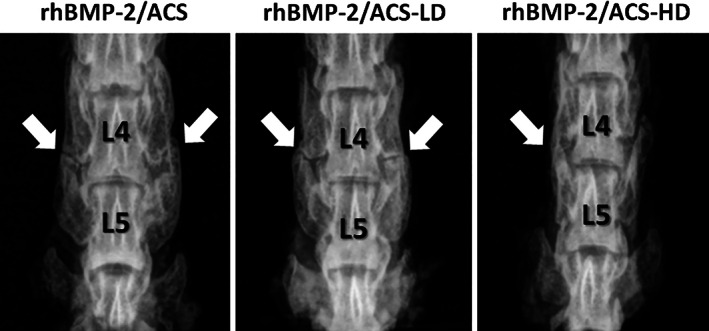
Representative radiographs of fused rat spines from each of the three treatment groups obtained 8 weeks after surgery depicting bridging bone formation between the L‐4 and L‐5 transverse processes. White arrows indicate areas with potential bridging bone
FIGURE 3.
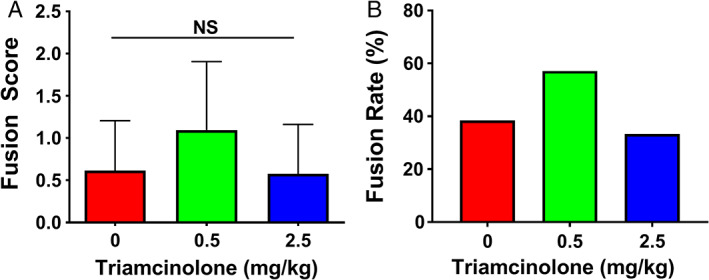
(A) Fusion scores from blinded manual palpation at 8 weeks postoperative. (B) Fusion rates for treatment group are based on manual palpation scores, wherein scores greater than or equal to 1 were considered to be successfully fused spines. Values are reported as mean ± SD (n = 15, per group)
FIGURE 4.
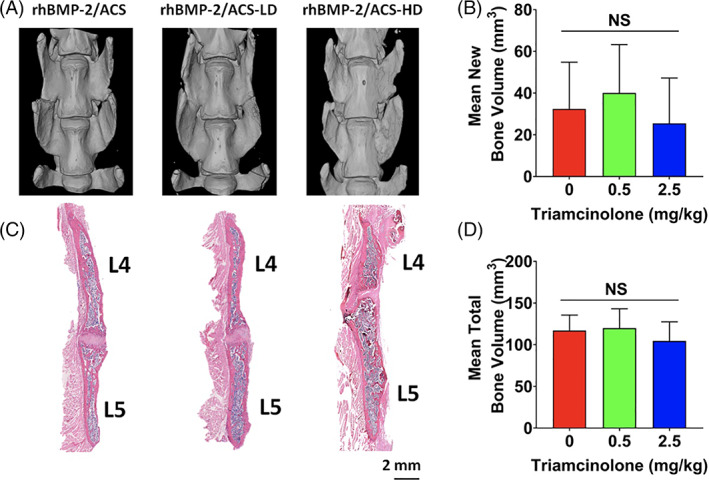
Representative micro‐CT 3D rendering of specimens per each experimental group (A) and hematoxylin and eosin staining of representative spines (B) for each treatment group: rhBMP‐2/ACS, rhBMP‐2/ACS‐LD and rhBMP‐2/ACS‐HD (LD = low‐dose triamcinolone, 0.5 mg/kg; HD = high‐dose triamcinolone, 2.5 mg/kg). New bone volume (C) and total bone volume (D) as quantified by micro‐CT scan for each treatment group. Values are reported as mean ± SD (n = 6, per each group). The threshold for statistical significance was P < .05 (NS = not significant)
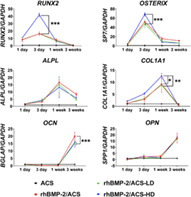
hBM‐MSCs cultured on rhBMP‐2/ACS yielded significantly increased expression of pro‐osteogenic transcripts relative to untreated control cultures (Figure 5). Among rhBMP‐2/ACS‐treated groups, statistically significant differences were found in the expression of RUNX2, OSTERIX, COL1A1, and OPN (P < .05). A complete statistical analysis report is depicted in Table S2. In the case of all but OCN, treatment with steroid increased the expression of the pro‐osteogenic transcript at one or more of the time points tested. OCN expression levels were slightly, yet significantly, lower after triamcinolone treatment relative to controls. ALP activity was significantly enhanced after 3 days, 1 week, and 3 weeks (P < .05) in the presence of triamcinolone relative to controls (Figure 6A). Mineral deposition among treatment groups appeared comparable over the 3‐week experiment (Figure 6B). High‐resolution images of the 3D cultures are presented in Figure S2. Images of the hematoxylin and eosin stained samples show the presence of cells in all treatment groups, including the ACS (no steroid) negative control (Figure S3). Figure S4 shows a representative control scaffold (ie, without cells) stained with alizarin red, confirming the absence of background staining.
FIGURE 5.
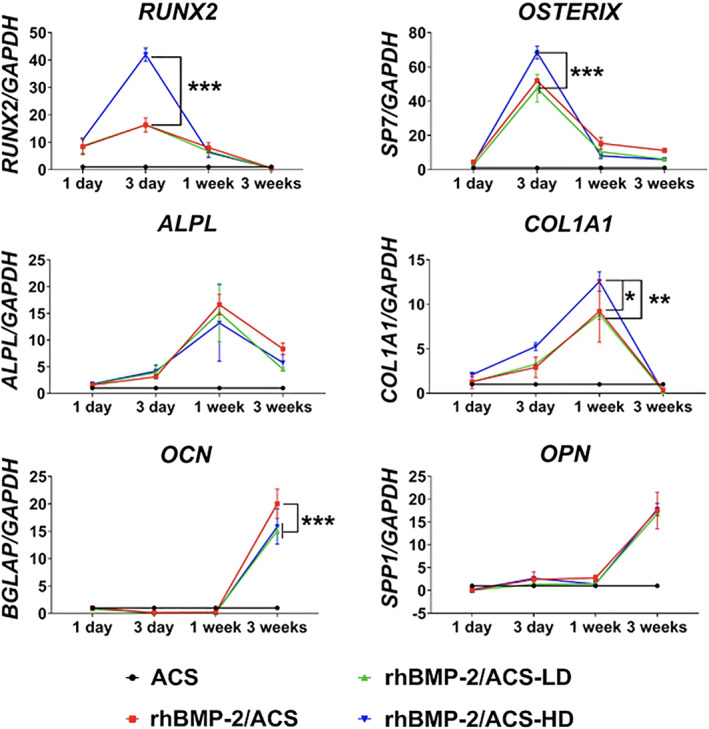
Expression of osteogenesis‐associated marker genes in hBM‐MSCs cultured on ACS (control), rhBMP‐2/ACS, rhBMP‐2/ACS‐LD, and rhBMP‐2/ACS‐HD (LD = low‐dose triamcinolone, 0.5 mg/kg; HD = high‐dose triamcinolone, 2.5 mg/kg). Values are reported as mean ± SD (n = 4, per group). *P < .05, **P < .01, ***P < .001
FIGURE 6.
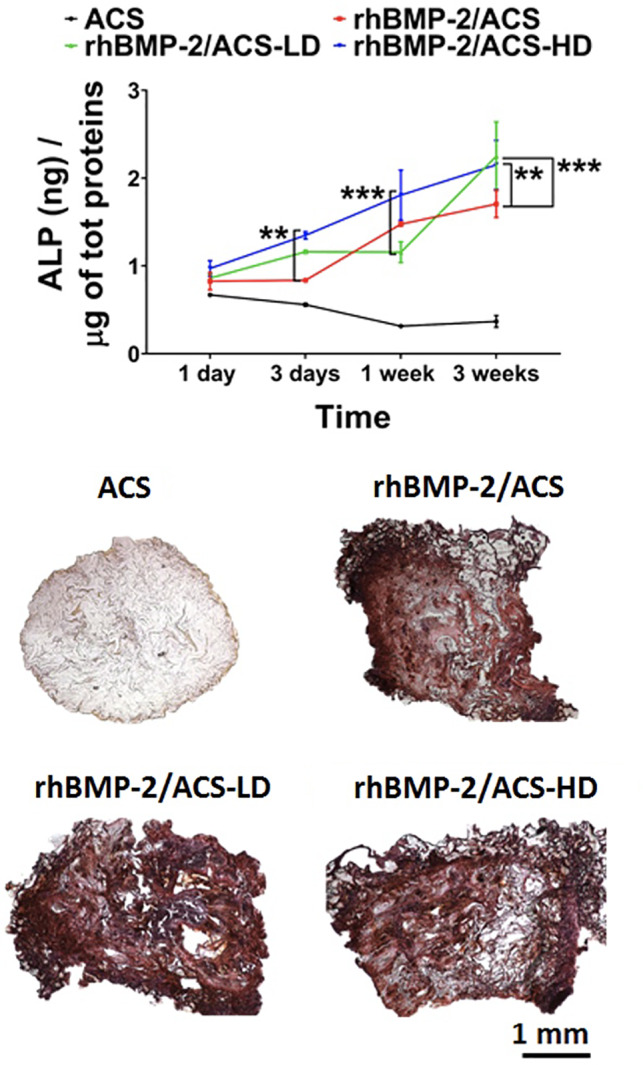
ALP activity (A) and alizarin red staining (B) of hBM‐MSCs cultured on ACS (control), rhBMP‐2/ACS, rhBMP‐2/ACS‐LD and rhBMP‐2/ACS‐HD (LD = low‐dose triamcinolone, 0.5 mg/kg; HD = high‐dose triamcinolone, 2.5 mg/kg). Values are reported as mean ± SD (n = 4, per each group). *P < .05, **P < .01, ***P < .001
4. DISCUSSION
Postoperative dysphonia and dysphagia have been reported in up to 51% 22 and 60% 22 , 23 of patients undergoing anterior cervical spine surgery, respectively, with surgeon underreporting of these complications as high as 80%. 24 Dysphagia results from postoperative soft tissue swelling, 25 and both intravenous 1 , 4 , 7 , 9 , 10 and local 2 , 3 , 5 , 6 steroid application have shown clinical efficacy as treatment modalities.
Corticosteroids are established anti‐inflammatory agents that inhibit the production of inflammatory prostaglandins and cytokines. 5 Few studies have investigated the effects of perioperative steroid administration on fusion rates, and those that exist report mixed results. Lee et al reported complete cervical fusion (based on radiography) at 16 to 32 months postoperative with local steroids (n = 25). 2 To the contrary, Jeyamohan et al completed postoperative CT scans at 6, 12, and 24 month time points and found significantly lower cervical fusion rates in the steroid treatment group at 6‐month follow‐up, but no significant differences in fusion rates 2 years after surgery (n = 56). 7 Therefore, despite clinical trials demonstrating decreased postoperative soft tissue swelling, dysphagia, and dysphonia with steroid use, surgeons have been slow to adapt local steroid application in routine anterior cervical spine procedures.
Herein, we have evaluated whether steroid treatment detrimentally affects bone augmentation and fusion in a preclinical rat PLF model. Triamcinolone was selected given its use in ACDF as described in previous clinical trials and for its potential to provide anti‐inflammatory effects up to several weeks postoperatively. 8 Our in vivo results demonstrated no significant differences in outcomes among groups. If there exists a critical threshold above which triamcinolone inhibits bone healing in this model, such a weight‐equivalent dose is likely higher than the range that might be considered reasonable for clinical use. However, it is pertinent to keep in mind that rhBMP‐2 delivery was the mode of fusion in this study (as it provides the most reliable bone regenerative response in this model). While an autograft‐ or allograft‐based treatments would have made our findings more broadly applicable to clinical use, these options do not heal as predictably in the rat arthrodesis model. 26 The dose of rhBMP‐2 used in this study was intentionally subtherapeutic (0.5 μg/implant) and was chosen to avoid an oversaturation of the fusion response, thereby increasing the chance of observing a true negative influence of triamcinolone on fusion rate. In fact, while 10 μg rhBMP‐2/ACS (per animal; 5 μg/implant) has elicited 100% fusion rates in prior studies utilizing the rat PLF model, 15 , 17 , 27 , 28 it has been shown that 0.5 μg/implant rhBMP‐2 on ACS yields a substantially lower fusion rate (50%‐66%). 15 , 17
Corticosteroids (glucocorticoids and mineralocorticoids) have anti‐inflammatory and immunosuppressive effects and alter metabolic and endocrine pathways. 5 , 11 , 13 Chronic steroid exposure can have a significant impact on skeletal health, most notably causing osteoporosis. 29 However, there has been some uncertainty as to the actual effect of acute glucocorticoid exposure on osteoblast differentiation. For example, it has been suggested that glucocorticoid effects on osteogenesis are dependent on the stage of cell growth and differentiation, including decreased osteoblast number and impaired osteoblast function. 30 Li et al investigated steroid‐induced osteonecrosis in vitro, demonstrating impairment of BMSC differentiation and a diminishment of blood supply by suppression of vascular endothelial growth factor (VEGF). 13 However, some studies report that glucocorticoids actually induce osteoblastic cell differentiation. 31 Our study demonstrates that when administered at a dose equivalent to that routinely used clinically on ACS, local steroid does not adversely impact fusion in this preclinical model. It is known that drugs are released from collagen sponges very quickly (within a few hours) 32 ; thus, the lack of an inhibitory effect of triamcinolone on bone formation may be related to its burst release and lack of persistence at the site of bone healing. Other modes of delivery may not result in the same outcomes.
In a 3D culture model, we did not find detrimental effects of triamcinolone on the osteogenic differentiation of hBM‐MSCs. In fact, the expression of all the osteogenic marker genes was enhanced or unaffected by the presence of triamcinolone, with the exception of OSTEOCALCIN. This correlates with established evidence suggesting that osteocalcin is a sensitive marker of the corticosteroid‐induced depression of osteoblast activity. 33 , 34 , 35 , 36 However, it should be noted that OPN, another crucial osteoblast marker, was not found downregulated by triamcinolone. Thus, it is reasonable to infer that the differences we found in the expression of OCN are not biologically significant, especially considering there were no differences in the fusion outcomes.
Although these results are promising, the use of a preclinical, in vivo rodent spinal arthrodesis model presents inherent limitations. Such limitations include anatomical differences and higher bone regenerative capacity in rats. 37 Given that there was uncertainty regarding the expected differences in fusion rate, bone volume, and osteogenic gene expression between the different treatment groups, an a priori power analysis was difficult. Post hoc power analysis suggested that the RT‐PCT analyses were adequately powered. However, post hoc analysis demonstrated that the fusion scoring and micro‐CT analyses were likely underpowered. Therefore, the in vivo results should be interpreted with caution. Further investigation with a more robust sample size is warranted.
Notably, this study evaluated local steroid application in the lumbar spine as opposed to the retropharyngeal space, where the steroid is typically applied during anterior cervical fusion surgery. 2 , 3 This decision was based on (a) the lack of a reliable small animal preclinical cervical model, (b) experience with an established rat PLF model, and (c) the technical challenges inherent to accessing the cervical spine in rats. Additionally, while ACDF is certainly one area in which these scientific questions are currently under debate, the results of this study could be relevant to all spine procedures. For future investigation, it would be pertinent to assess the impact of local steroid on autograft‐mediated, rather than rhBMP‐2‐mediated, spinal fusion. Furthermore, local steroid application could also be investigated in the setting of autograft plus bone graft extender‐mediated spine fusion in large animal models.
5. CONCLUSIONS
Local steroid application does not seem to inhibit rhBMP‐2‐mediated spine fusion in rats at doses that are clinically relevant in humans undergoing similar procedures. However, our study may not be adequately powered to detect differences in fusion as measured by manual palpation or bone volumes as measured by micro‐CT. Additionally, local steroid exposure does not adversely impact osteogenic differentiation in hBM‐MSCs grown in 3D rhBMP‐2/ACS scaffolds. If there is a dose‐dependent threshold above which steroid inhibits bone healing, such a dose may be out of the range that would generally be considered for clinical use.
CONFLICT OF INTEREST
Wellington K. Hsu: Royalties: Stryker; Consulting: Stryker, AlloSource, Wright Medical, Medtronic, Mirus; Speaking and/or Teaching Arrangements: AONA; Trips/Travel: Stryker, Medtronic, Micro Medicine; Board of Directors: Lumbar Spine Research Society, American Academy of Orthopedic Surgeons, North American Spine Society, Cervical Spine Research Society; Scientific Advisory Board: Bioventus; Grants: Medtronic. Erin Hsu: Royalties: Stryker (E); Other Office: Orthopedic Research Society (Media Relations and Communications Committee).
AUTHOR CONTRIBUTIONS
Abhishek Kannan: Design, data acquisition, analysis, and interpretation of data; manuscript preparation. Silvia Minardi: Design, data acquisition, analysis, and interpretation of data; manuscript preparation. David J. Ellenbogen: Data acquisition, analysis, and interpretation of data; manuscript preparation. Mitchell J. Hallman: Data acquisition, analysis, and interpretation of data; manuscript preparation. Allison C. Greene: Data acquisition, analysis, and interpretation of data; manuscript preparation. Jonathan T. Yamaguchi: Data acquisition, analysis, and interpretation of data; manuscript preparation. Mark A. Plantz: Data acquisition, analysis, and interpretation of data; manuscript preparation. Soyoen Jeong: Data acquisition, analysis, and interpretation of data. Kennedy C. Sana: Data acquisition, analysis, and interpretation of data. Vivek Shah: Data acquisition, analysis, and interpretation of data. Chawon Yun: Data acquisition, analysis, and interpretation of data; manuscript preparation. Erin L. Hsu: Design, data acquisition, analysis, and interpretation of data; manuscript preparation. Wellington K. Hsu: Design, data acquisition, analysis, and interpretation of data; manuscript preparation.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENT
This study was supported by the Department of Orthopedic Surgery at Feinberg School of Medicine.
Kannan, A. , Minardi, S. , Ellenbogen, D. J. , Hallman, M. J. , Greene, A. C. , Yamaguchi, J. T. , Plantz, M. A. , Jeong, S. , Sana, K. C. , Shah, V. , Yun, C. , Hsu, E. L. , & Hsu, W. K. (2021). The effect of local steroid application on bony fusion in a rat posterolateral spinal arthrodesis model. JOR Spine, 4(4), e1177. 10.1002/jsp2.1177
Abhishek Kannan and Silvia Minardi contributed equally to this study.
Funding information Feinberg School of Medicine
Contributor Information
Erin L. Hsu, Email: erinkhsu@gmail.com.
Wellington K. Hsu, Email: wellington.hsu@nm.org.
REFERENCES
- 1. Emery SE, Akhavan S, Miller P, et al. Steroids and risk factors for airway compromise in multilevel cervical corpectomy patients: a prospective, randomized, double‐blind study. Spine. 2009;34:229‐232. [DOI] [PubMed] [Google Scholar]
- 2. Lee SH, Kim KT, Suk KS, Park KJ, Oh KI. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion: a prospective, randomized study. Spine. 2011;36:2286‐2292. [DOI] [PubMed] [Google Scholar]
- 3. Koreckij TD, Davidson AA, Baker KC, Park DK. Retropharyngeal steroids and dysphagia following multilevel anterior cervical surgery. Spine. 2016;41:E530‐E534. [DOI] [PubMed] [Google Scholar]
- 4. Nam TW, Lee DH, Shin JK, Goh TS, Lee JS. Effect of intravenous dexamethasone on prevertebral soft tissue swelling after anterior cervical discectomy and fusion. Acta Orthop Belg. 2013;79:211‐215. [PubMed] [Google Scholar]
- 5. Cancienne JM, Werner BC, Loeb AE, et al. The effect of local intraoperative steroid administration on the rate of postoperative dysphagia following ACDF: a study of 245,754 patients. Spine. 2016;41:1084‐1088. [DOI] [PubMed] [Google Scholar]
- 6. Edwards CC II, Dean C, Edwards CC, et al. Can dysphagia following anterior cervical fusions with rhBMP‐2 be reduced with local depomedrol application?: a prospective, randomized, placebo‐controlled, double‐blind trial. Spine. 2016;41:555‐562. [DOI] [PubMed] [Google Scholar]
- 7. Jeyamohan SB, Kenning TJ, Petronis KA, Feustel PJ, Drazin D, DiRisio DJ. Effect of steroid use in anterior cervical discectomy and fusion: a randomized controlled trial. J Neurosurg Spine. 2015;23:137‐143. [DOI] [PubMed] [Google Scholar]
- 8. Jenkins TJ, Nair R, Bhatt S, et al. The effect of local versus intravenous corticosteroids on the likelihood of dysphagia and dysphonia following anterior cervical discectomy and fusion: a single‐blinded, prospective, randomized controlled trial. J Bone Joint Surg. 2018;100:1461‐1472. [DOI] [PubMed] [Google Scholar]
- 9. Pedram M, Castagnera L, Carat X, Macouillard G, Vital JM. Pharyngolaryngeal lesions in patients undergoing cervical spine surgery through the anterior approach: contribution of methylprednisolone. Eur Spine J. 2003;12:84‐90. [DOI] [PubMed] [Google Scholar]
- 10. Song KJ, Lee SK, Ko JH, Yoo MJ, Kim DY, Lee KB. The clinical efficacy of short‐term steroid treatment in multilevel anterior cervical arthrodesis. Spine J. 2014;14:2954‐2958. [DOI] [PubMed] [Google Scholar]
- 11. Sawin PD, Dickman CA, Crawford NR, Melton MS, Bichard WD, Sonntag VK. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg. 2001;94:76‐81. [DOI] [PubMed] [Google Scholar]
- 12. Zadegan SA, Jazayeri SB, Abedi A, Bonaki HN, Vaccaro AR, Rahimi‐Movaghar V. Corticosteroid administration to prevent complications of anterior cervical spine fusion: a systematic review. Global Spine J. 2018;8:286‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X, Jin L, Cui Q, Wang GJ, Balian G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int. 2005;16:101‐108. [DOI] [PubMed] [Google Scholar]
- 14. Dalle Carbonare L, Arlot ME, Chavassieux PM, et al. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid‐induced and postmenopausal osteoporosis. J Bone Miner Res. 2001;16:97‐103. [DOI] [PubMed] [Google Scholar]
- 15. Hsu WK, Wang JC, Liu NQ, et al. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J Bone Joint Surg. 2008;90:1043‐1052. [DOI] [PubMed] [Google Scholar]
- 16. Hsu WK, Polavarapu M, Riaz R, et al. Nanocomposite therapy as a more efficacious and less inflammatory alternative to bone morphogenetic protein‐2 in a rodent arthrodesis model. J Orthop Res. 2011;29:1812‐1819. [DOI] [PubMed] [Google Scholar]
- 17. Lee SS, Hsu EL, Mendoza M, et al. Gel scaffolds of BMP‐2‐binding peptide amphiphile nanofibers for spinal arthrodesis. Adv Healthc Mater. 2015;4:131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yee AJM, Bae HW, Friess D, Robbin M, Johnstone B, Yoo JU. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine. 2004;29:1308‐1313. [DOI] [PubMed] [Google Scholar]
- 19. Grauer JN, Patel TC, Erulkar JS, Troiano NW, Panjabi MM, Friedlaender GE. Evaluation of OP‐1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001;26:127‐133. [DOI] [PubMed] [Google Scholar]
- 20. Jakus AE, Rutz AL, Jordan SW, et al. Hyperelastic “bone”: a highly versatile, growth factor–free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med. 2016;8:358ra127. [DOI] [PubMed] [Google Scholar]
- 21. Minardi S, Taraballi F, Wang X, et al. Biomimetic collagen/elastin meshes for ventral hernia repair in a rat model. Acta Biomater. 2017;50:165‐177. [DOI] [PubMed] [Google Scholar]
- 22. Winslow CP, Winslow TJ, Wax MK. Dysphonia and dysphagia following the anterior approach to the cervical spine. Arch Otolaryngol Head Neck Surg. 2001;127:51‐55. [DOI] [PubMed] [Google Scholar]
- 23. Winslow CP, Meyers AD. Otolaryngologic complications of the anterior approach to the cervical spine. Am J Otolaryngol. 1999;20:16‐27. [DOI] [PubMed] [Google Scholar]
- 24. Edwards CC II, Karpitskaya Y, Cha C, et al. Accurate identification of adverse outcomes after cervical spine surgery. J Bone Joint Surg. 2004;86:251‐256. [DOI] [PubMed] [Google Scholar]
- 25. Nagoshi N, Tetreault L, Nakashima H, et al. Risk factors for and clinical outcomes of dysphagia after anterior cervical surgery for degenerative cervical myelopathy: results from the AOSpine International and North America studies. J Bone Joint Surg. 2017;99:1069‐1077. [DOI] [PubMed] [Google Scholar]
- 26. Grauer JN, Bomback DA, Lugo R, Troiano NW, Patel TC, Friedlaender GE. Posterolateral lumbar fusions in athymic rats: characterization of a model. Spine J. 2004;4:281‐286. [DOI] [PubMed] [Google Scholar]
- 27. Wang JC, Kanim LE, Yoo S, et al. Effect of regional gene therapy with bone morphogenetic protein‐2‐producing bone marrow cells on spinal fusion in rats. JBJS. 2003;85:905‐911. [DOI] [PubMed] [Google Scholar]
- 28. Hsu WK, Polavarapu M, Riaz R, et al. Characterizing the host response to rhBMP‐2 in a rat spinal arthrodesis model. Spine. 2013;38:E691‐E698. [DOI] [PubMed] [Google Scholar]
- 29. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid‐induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319‐1328. [DOI] [PubMed] [Google Scholar]
- 30. Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73‐81. [DOI] [PubMed] [Google Scholar]
- 31. Bellows CG, Heersche JN, Aubin JE. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev Biol. 1990;140:132‐138. [DOI] [PubMed] [Google Scholar]
- 32. Radu FA, Bause M, Knabner P, Lee GW, Friess WC. Modeling of drug release from collagen matrices. J Pharm Sci. 2002;91:964‐972. [DOI] [PubMed] [Google Scholar]
- 33. Ekenstam E, Stålenheim G, Hällgren R. The acute effect of high dose corticosteroid treatment on serum osteocalcin. Metabolism. 1988;37:141‐144. [DOI] [PubMed] [Google Scholar]
- 34. Nicholson G, Bryant AE, Macdonald IA, Hall GM. Osteocalcin and the hormonal, inflammatory and metabolic response to major orthopaedic surgery. Anaesthesia. 2002;57:319‐325. [DOI] [PubMed] [Google Scholar]
- 35. Brabnikova Maresova K, Pavelka K, Stepan JJ. Acute effects of glucocorticoids on serum markers of osteoclasts, osteoblasts, and osteocytes. Calcif Tissue Int. 2013;92:354‐361. [DOI] [PubMed] [Google Scholar]
- 36. Frenkel B, White W, Tuckermann J. Glucocorticoid‐induced osteoporosis. In: Wang J‐C, Harris C, eds. Glucocorticoid Signaling: From Molecules to Mice to Man. New York, NY: Springer; 2015:179‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salamon ML, Althausen PL, Gupta MC, Laubach J. The effects of BMP‐7 in a rat posterolateral intertransverse process fusion model. Clin Spine Surg. 2003;16:90‐95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


