Abstract
Background
Beclomethasone dipropionate (BDP) and budesonide (BUD) are used widely in the treatment of chronic asthma. The two drugs have different in vitro pharmacokinetic characteristics. It is unclear whether this translates into clinically significant differences in efficacy or safety when treating children and adults with chronic asthma.
Objectives
To assess clinical outcomes in studies which have compared inhaled BDP and BUD in the treatment of chronic asthma.
Search methods
We searched the Cochrane Airways Group Trial Register (1999) and reference lists of articles. We contacted trialists and pharmaceutical companies for additional studies and searched abstracts of major respiratory society meetings (1997‐1999).
Selection criteria
Prospective, randomised trials comparing BDP to BUD in the treatment of chronic asthma. Two reviewers independently assessed articles for inclusion and methodological quality.
Data collection and analysis
One reviewer extracted data; authors were contacted to clarify missing information. Quantitative analyses where undertaken using Review Manager 4.0.3 with MetaView 3.1.
Main results
24 studies met the criteria for inclusion (1174 participants). Methodological quality was variable. A meta‐analysis of crossover studies did not demonstrate a significant difference between BDP and BUD for FEV1, morning PEF, evening PEF, asthma symptoms or rescue beta2 agonist use, over a dose range of 400 to 1000 mcg/d. The majority of crossover trials had significant design flaws related to a lack of washout and/or failure to exclude carryover effects so the results must be viewed with caution. A single crossover study with adequate washout showed that BUD 400 mcg/d delivered via Turbohaler dry powder inhaler (DPI) may be more effective than BDP 400 mcg/d delivered via Rotahaler DPI in reducing histamine bronchial hyper‐responsiveness: Weighted Mean Difference (WMD) 0.43 log10 PC20 FEV1 (95% Confidence Intervals (CI) 0.05, 0.81 log10 PC20 FEV1). A meta‐analysis of two parallel group, dose down‐titration studies (231 patients) showed that less BUD delivered via a Turbohaler DPI was required to maintain control in adults asthmatics compared to BDP delivered via metered dose inhaler with or without a spacer: WMD 444 mcg/d (95% CI 332, 556 mcg/d).
Authors' conclusions
There is limited high quality randomised controlled trial data comparing the relative efficacy of BDP and BUD. Current guidelines (BTS 1997; GINA 1995; NHLBI 1997) assume BDP and BUD to have equal efficacy, such that for each defined level of asthma severity, the recommended doses BDP and BUD are the same. Although there is some data to suggest that BUD via Turbohaler is more effective than BDP via either Rotahaler or MDI (with and without spacer), these comparisons are confounded by use of different delivery devices, and are not sufficient to warrant a change in guideline recommendations.
Plain language summary
The effects of beclomethasone or budesonide for chronic asthma
Beclomethasone and budesonide are commonly used to treat people with asthma in the long‐term. Despite the large amount of research which has been conducted with these two steroids, very little can be concluded as to how effective they are, because the quality of the research to date has not been strong enough. The available research does not therefore provide a clear answer as to whether beclomethasone or budesonide are better for treating asthma.
Background
Beclomethasone dipropionate (BDP) was the first inhaled corticosteroid (ICS) marketed for use in the treatment of chronic asthma in 1972. Budesonide had been available since the early 1980's. Extensive clinical experience has been gained with these compounds in the treatment of chronic asthma, both are regarded as having a highly favourable therapeutic index when compared to oral corticosteroids, i.e. offering large advantages in terms of their efficacy at the cost of few side‐effects.
In vitro studies assessing pharmacodynamic properties have shown differences between BDP and BUD. Receptor affinity is a measure of the strength with which an active molecule binds to the glucocorticoid receptor. This is 1.5 fold higher for 17‐beclomethasone monopropionate (17‐BMP), the bronchially active metabolite of BDP, compared to BUD (Boobis 1998). However when assessing drugs in terms of their relative in vitro potency, i.e. the concentration of drug required producing a standard effect, BUD appears more potent than BDP. This has been shown in early experimental studies for the MacKenzie skin‐blanching test (Johansson 1982), and more recently in terms of several in vitro assays of anti‐inflammatory action including eosinophil survival, basophil histamine release and expression of vascular cell adhesion molecule‐1 (Stellato 1999). It is noteworthy that in these in vitro assays, the maximum effect of BDP and BUD (i.e. their intrinsic activity) was similar, and at very high concentrations (on the 'plateau' of the dose response curve) the quantitative effects of the two drugs were closely matched.
Do differences in the in vitro pharmacodynamic actions of BDP and BUD translate into differences in clinical efficacy? This may depend on dose. At low inhaled dose, more likely to be on the 'steep' portion of the dose response curve for each drug, differences in potency might lead to differences in clinical efficacy. Conversely, at higher inhaled dose (approaching or on the 'plateau' of the dose response curve for each drug) differences in clinical efficacy may not be apparent. A retrospective analysis of prescription records for BDP and BUD in primary care in New Zealand has recently been published (Pethica 1998). The investigators concluded that BDP was more 'potent' than BUD because the mean daily‐prescribed dose of BDP over a one‐year period was 635 mcg/d compared to a mean daily BUD dose 979 mcg/d. This study has a number of limitations however including the fact that drug exposure could not validated and other known and unknown biases may have been operating in this non‐randomised, retrospective cross‐sectional study.
The purpose of this review is to assess the relative clinical efficacy and safety of BDP and BUD delivered at equal nominal dose in the treatment of chronic asthma by systematically reviewing the prospective randomised trials that have compared these inhaled corticosteroids.
Objectives
To assess clinical outcomes in studies which have compared inhaled beclomethasone (BDP) and inhaled budesonide (BUD) in the treatment of chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised studies. Double, single and non‐blinded studies were considered. Studies could be of either parallel group or crossover design.
Types of participants
Studies of both children and adults were included, but patients under two years of age were excluded. To be eligible, participants had to have a diagnosis of chronic asthma. Studies conducted in both primary and secondary care settings were considered.
Types of interventions
BDP versus BUD delivered by oral inhalation. Each drug had to be delivered at the same nominal daily dose. Nominal dose was calculated as the valve dose multiplied by the number of actuations per day. Each drug could be delivered by different device including pressurised metered dose aerosol inhaler (MDI), MDI+spacer or dry powder device (DPI). Studies using nebuliser devices were excluded.
Types of outcome measures
All outcomes were considered. Those identified as important a priori were as follows:
Efficacy related 1. Clinic measured FEV1 and PEF, diary card morning and evening PEF, diurnal variability in PEF 2. Symptoms 3. Rescue beta2 agonist use 4. Histamine and methacholine bronchial hyper‐responsiveness (BHR) 5. Asthma exacerbations: hospital admission, emergency room attendance, unscheduled primary care visits, days off school or work 6. Health‐related quality of life (HRQOL) Safety related 1. Hypothalamo‐pituitary‐adrenal (HPA) axis function reflected in serum and urinary cortisol measures and clinical adrenal insufficiency 2. Oropharyngeal side effects (hoarseness, sore throat, oral Candidiasis)
Search methods for identification of studies
Electronic searches
Stage 1: a search was carried out of the Cochrane Airways Group Trials Register (1999). The following search terms were applied:
steroid* OR glucocorticoid* OR corticosteroid* OR beclomethasone OR budesonide OR fluticasone OR triamcinolone OR flunisolide OR Becotide OR Becloforte OR Pulmicort OR Flixotide
Abstracts from this search were imported into a bibliographic database termed the Inhaled Steroid Register. This was hand‐searched by two reviewers (NPA and JB). Duplicate publications were removed.
Stage 2: two further registers were created by undertaking searches of the Inhaled Steroid Register using the following terms:
beclomethasone OR Becotide OR Becloforte: the Beclomethasone Register
budesonide OR Pulmicort : the Budesonide Register
Each register was hand‐searched for RCTs meeting the inclusion criteria. Citations were excluded based on abstract if it was clear that the study was a) not concerned with the treatment of asthma in humans b) not an RCT c) did not include a treatment arm with inhaled corticosteroid (ICS). Where uncertainty existed, the publication was retrieved in full text form.
Searching other resources
Reference lists of all included trails and relevant narrative reviews were searched for additional RCTs. Authors of identified trials, Glaxo Wellcome (UK) and Astra Zeneca (Sweden) were contacted and were asked about any further published or unpublished trials that may have been conducted. There were no language restrictions. The British Journal of Clinical Research and the European Journal of Clinical Research, which are not electronically indexed on MEDLINE or EMBASE, were hand searched for relevant studies. The proceedings of the British Thoracic Society (1997/1998), European Respiratory Society (1997/1998) and the American Thoracic Society (1997‐1999) were searched for relevant trials.
Data collection and analysis
Selection of studies
The decision to exclude studies prior to full paper retrieval was made by one reviewer (NPA). Papers retrieved in full text form were assessed independently by two reviewers (NPA and JB); disagreement as to which papers to include was resolved by consensus.
Data extraction and management
One reviewer (NPA) extracted data for each outcome from the published results of included trials. In the case of continuous outcomes such as FEV1 measures:
1. Where outcomes were evaluated at a number of time points only data from the last evaluable time point was used. 2. Data were extracted from graphical plots when presented in this form; attempt was made to verify such data by contacting authors. 3. If an intention‐to‐treat analysis was not used by the investigators, and it was not explicit in the presentation of results how many subjects (N) were in each group at the time of last evaluation of that outcome, the appropriate N value for each intervention group was calculated by subtracting the number of patients who withdrew in each intervention group from those randomised to each intervention group.
Authors were written to (by mail, fax and/or electronic mail) to clarify details of randomisation and/or request missing outcome data. Attempt was made to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital World Wide Web (WWW) sites for up‐to‐date contact details.
Assessment of risk of bias in included studies
Two reviewers (NPA and JB) who were blinded to the author's names, institution and funding sources independently assessed each study for methodological quality. The trials were scored using the Cochrane approach:
Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
The methodological quality of included studies was also assessed using a 5 point scoring instrument (Jadad 1996):
a) Was the study described as randomised? (yes=1 no=0) b) Was the study described as double blind? (yes=1 no=0) c) Was there a description of withdrawals and dropouts? (yes=1 no=0) d) Was the method of randomisation well described and appropriate? (yes=1 no=0) e) Was the method of double blinding well described and appropriate? (yes=1 no=0) f) deduct 1 point if method of randomisation or blinding inappropriate
Inter‐rater agreement was measured using the kappa statistic. Disagreement was resolved by consensus.
Assessment of heterogeneity
Heterogeneity of effect size across studies pooled was calculated using a chi square test, with P< 0.05 used as the cut‐off level for significance. Sensitivity analyses were performed on the basis of methodological quality. Results were re‐analysed using studies of only the highest quality scores (Jadad 3 to 5). Subgroup analyses based upon patient age, delivery device and study duration were planned.
Data synthesis
A weighted treatment effect across trials was calculated using the Cochrane statistical package RevMan 4.0.3 with MetaView 3.1. For continuous outcomes, a weighted mean difference (WMD) or standardised mean difference (SMD) was calculated as appropriate. For dichotomous outcomes a relative risk (RR) was calculated. Pooled treatments effects are expressed with their 95% confidence intervals (95% CI). A fixed effect model was used throughout. A number of a priori conditions were established regarding the comparisons made:
1. Studies were distinguished as those in which patients were a) not treated with regular oral corticosteroid (OCS), b) dependent upon regular oral steroid treatment prior to study. Trials involving OCS‐dependent patients in which the efficacy of inhaled corticosteroid (ICS) is being assessed may have an 'OCS down‐titration' design using reduction in the use of oral steroid as an outcome measure, whilst maintaining a given level of asthma control. However, studies in which patients were not treated with regular OCS are more likely to have a design aimed at detecting improvements in asthma control. It is inappropriate to combine trials with these different designs and objectives.
2. The results of parallel and crossover trials were not pooled.
3. It was anticipated that measures of bronchial hyper‐responsiveness (PD20 FEV1, PC20 FEV1) would often be reported as geometric means. Presentation of results in this way indicates that data has been logarithmically transformed prior to analysis by investigators to take account of a skewed distribution. Data for such outcomes was only pooled across studies where the mean and standard deviation of logged values (from which geometric means are derived) could be calculated.
Results
Description of studies
Results of the search
Stage 1 electronic search: 6494 citations retrieved, 2162 original citations
Stage 2 electronic search:
BDP register BUD register
Electronic citations 1149 1036
Citations excluded from abstract description: Not RCT 379 331 Not chronic asthma in humans 190 195 Not concerned with ICS treatment 177 129 Clearly not concerned with BDP v BUD 113 40
Papers retrieved in full text 290 341
Excluded after full paper retrieval: Not RCT 94 46 Not involving BDP v BUD comparison at 176 275 equal nominal daily dose
Included studies 20 (same studies identified from both registers)
One study (Greefhorst 1992) was identified after searching the bibliographies of included studies, three studies (Dal Negro 1997, Hamalainen 1998, Micheletto 1997) were identified by searching the proceedings of respiratory society meetings.
Included studies
24 publications were identified met the inclusion criteria for the review representing 24 studies. Publication dates ranged from 1982 to 1998. Details of the trials are in the table of Characteristics of Included Studies. Four studies were identified that were published in abstract form only (Dal Negro 1997, Greefhorst 1992, Hamalainen 1998, Micheletto 1997). No outcome data for these studies is available. The studies have been included as further information may be available at a future date when the review is updated. Two further publications (Hernandez 1995, Lee 1995) require translation and are awaiting assessment. We would also like to draw the reader's attention to the six further citations (Baxter‐Jones 2000, Brand 2001, Dal Negro 1999b, Jager 2000, Kraszko 1999, Reichel 2001) listed under Studies awaiting assessment. Due to unforeseen delays in the publication of this review, a number of potentially relevant trials have been identified after an up dated search of the electronic sources from April 1999 ‐ August 2001. These studies will be fully evaluated and included as appropriate in a future update at the earliest possible opportunity.
Study Populations
The majority of studies were conducted in Europe (Belgium, Denmark, Finland, France, Germany, Ireland, Italy, Sweden, The Netherlands, UK). One study (Springer 1987) was conducted in Israel. One study (Baran 1987) was conducted in a residential centre for asthmatic children; in one study (Greefhorst 1992) the setting was unclear. All other studies were conducted in a hospital outpatient clinic setting. Six studies were in children, 18 in adults.
Diagnosis of asthma
In 17 studies (71%) there was no clear indication of the criteria upon which a diagnosis of asthma was made and appears to have been at the discretion of the investigators. In one study (Boe 1989) diagnosis was based upon ATS 1962 criteria. In three studies (Bisgaard 1988, Ebden 1986, Petrie 1990) diagnosis was supported by demonstrable reversibility of clinic PEF or FEV1 following inhaled beta2 agonist; in three studies (Brambilla 1994, Svendsen 1992, Tjwa 1995) diagnosis was supported by demonstration of a 15% or greater reversibility in FEV1 following inhaled beta2 agonist or variability in FEV1 in combination with bronchial hyper‐responsiveness (BHR) to methacholine or histamine.
Treatment with oral corticosteroids
In three early studies (Rafferty 1985, Stiksa 1982a, Stiksa 1982b) requirement for oral corticosteroid (OCS) for asthma control in the form of prednisolone was an inclusion criterion. In one study (Stiksa 1985) a proportion of enrolled patients were OCS‐treated, although this was not a specific inclusion criterion. In 20 studies regular OCS treatment was either a specific exclusion criterion or it was clear from baseline demographic characteristics that patients were not receiving regular OCS prior to the study.
Asthma severity and asthma control
Asthma severity may be defined in terms of the amount of medication required to achieve a certain level of asthma control, or in terms of the frequency of symptoms experienced and/or the degree to which FEV1 (% predicted) is depressed before commencing regular prophylactic therapy. Asthma control is best considered the degree to which patients symptoms are uncontrolled and/or the degree to which their FEV (% predicted) is depressed, irrespective of the amount/type of prophylactic medication prescribed. The characteristics of the included studies in terms of these factors have been listed in Table 1, Table 2 and Table 3. Baseline FEV1 (% predicted) and symptom frequency were reported infrequently, so, in the majority of studies, it was not possible to determine the level of asthma control at randomisation.
1. Trial recruiting non‐OCS, non‐ICS treated asthmatics: baseline asthma control.
| Study ID | B.L. FEV1 (% pred) | B.L. symp freq |
| Bisgaard 1988 | not stated | not stated |
| Keelan 1984 | not stated | symptoms 'insufficiently controlled' by bronchodilators and/or anti‐allergy therapy |
| Nicolaizik 1994 | not stated | symptoms 'inadequately controlled' |
| B.L: baseline |
2. Trials recruiting non‐OCS, but ICS‐treated asthmatics: baseline asthma control.
| Study ID | B.L. ICS dose | B.L. FEV1 (% pred) | B.L. symp freq |
| Baran 1987 | not stated | not stated | not stated |
| Bjorkander 1982 | not stated | >70 | not stated |
| Boe 1989 | ICS 400‐800 mcg/d | not stated | not stated |
| Brambilla 1994 | BDP 1000‐2000 mcgd | not stated | recurrent acute exacerbations of dyspoea and wheezing |
| Dal Negro 1997 | BDP 1000 mcg/d | not stated | not stated |
| Ebden 1986 | not stated | not stated | not clearly stated but in opinion of investigator patients were poorly controlled |
| Field 1982 | not stated | not stated | not stated |
| Greefhorst 1992 | not stated | not stated | not stated |
| Hamalainen 1998 | BDP or BUD 800‐1000 mcg/d | not stated | not stated |
| Michleletto 1997 | BDP 1000 mcg/d | not stated | not stated |
| Pedersen 1988 | BDP or BUD 800‐1200 mcg/d | not stated | not stated |
| Petrie 1990 | BDP or BUD 800‐1600 mcg/d | mean 72%, range 34‐114) | not stated |
| Selroos 1994 | BDP 1000 mcg/d | not stated but mean PEFR 75‐77% predicted | not stated |
| Springer 1987 | BDP 400 mcg/d | not stated | not stated |
| Svendsen 1993 | ICS 300‐500 mcg/d | <70 | stated by authors that asthma was 'poorly controlled' |
| Tjwa 1995 | ICS 150‐800 mcg/d | 40‐85 | not stated |
| Willey 1982 | BDP 400 mcg/d or greater | not stated | symptom frequency not stated, but at least 2 courses or oral steroids in last 12 months for exacerbations |
| B.L: baseline |
3. Trials recruiting OCS‐treated asthmatics: baseline asthma control.
| Study ID | B.L. FEV1 (% pred) | B.L. symp freq |
| Rafferty 1985 | not stated | not stated |
| Stiksa 1985 | not stated | not stated |
| Stiksa 1982a | not stated | not stated |
| Stiksa 1982b | not stated | not stated |
| B.L: baseline |
Study design
Five studies (Bisgaard 1988, Brambilla 1994, Dal Negro 1997, Micheletto 1997, Selroos 1994) were parallel group studies. Nineteen studies (79%) were of crossover design.
Only two crossover design studies (Bjorkander 1982a, Tjwa 1995) incorporated a washout (one week or four weeks respectively) between treatment periods. No other crossover studies had a washout period between active treatments. In only two of these (Pedersen 1988, Petrie 1990) was it stated if carryover/sequence effects were tested for and excluded. In all the remaining crossover studies without washout, no comment was made regarding this possible confounding factor.
The length of treatment period varied. Twelve studies (50%) had treatment periods of between two and four weeks, 10 studies (42%) had treatment periods of between six and 12 weeks. The longest study (Selroos 1994) had an effective treatment period of two years. The only study enrolling OCS‐dependent patients (Rafferty 1985) had a complex and unique trial design with treatment periods of variable length and is discussed separately below.
As an inclusion criterion for the review, all studies had to assess equal nominal daily doses of BDP and BUD. Ten studies (42%) assessed 400 mcg/d, seven studies (29%) assessed 800 mcg/d. Two studies (Ebden 1986, Svendsen 1992) assessed higher daily doses i.e. BDP 1500 mcg/d v BUD 1600 mcg/d. Only one study (Baran 1987) assessed 200 mcg/d. Two parallel group studies (Brambilla 1994, Selroos 1994) used a 'dose down‐ titration' design in which ICS dose reduction was attempted at regular intervals during the randomised treatment phase to the minimal effective dose. Conversely, one study (Bisgaard 1988) used a 'dose‐escalation' design whereby patients were randomised to receive BDP or BUD 200 mcg/d, 400 mcg/d then 800 mcg/d each for 4 weeks in succession. Only one study of crossover design (Boe 1989) incorporated more than two treatment periods: BDP 400 mcg/d, BUD 400 mcg/d, BDP 1000 mcg/d and BUD 800 mcg/d.
Eight studies (33%) used a MDI to deliver both BDP and BUD. Different types of delivery device were used to deliver each ICS in 16 studies (66%).
Outcomes
A wide range of efficacy and safety outcomes was assessed. A number of reported outcomes have not been considered. These include plasma ACTH and growth hormone levels (Nicolaizik 1994), and 24 hour urinary tetrahydrocortisone glucosiduronate and tetrahydrocortisol glucosiduronate levels (Springer 1987).
Risk of bias in included studies
Methodological quality of included studies was variable. Only 10 studies (42%) were double blind. 19 studies (79%) provided adequate descriptions of numbers of patients withdrawn and the reasons for withdrawal. As assessed by the Jadad scoring method 15 studies (63%) achieved a score of 3 or 4; no studies achieved a maximum score of 5. In only four studies (17%) was allocation concealment clearly employed. In all other studies allocation concealment was unclear.
Effects of interventions
A significant amount of data for reported outcomes could not be included in the meta‐analysis. This included all data for the four studies published in abstract form only, and a number of outcomes reported in crossover studies published as full journal papers. These data are listed in Table 4. In most cases a narrative description of these results has not been made. This is because the study was either a) low methodological quality (Jadad score 2 or less) and/or b) it was not stated whether carryover effects had been excluded in the case of crossover studies (see Discussion). However, an exception has been made in the case of Pedersen 1990. This was the only fair quality crossover study (Jadad score 3) in which significant carryover effects had been excluded by the investigators. The results are reported in narrative form below.
4. Outcome data not included in meta‐analysis.
| Study ID | Missing data |
| Bisgaard 1988 | Morning PEFR Evening PEFR Daily beta3 agonist use No numerical data presented for above outcomes |
| Boe 1989 | FEV1 FVC Morning PEFR Evening PEFR Daily asthma symptom score No numerical data for above outcomes |
| Dal Negro 1997 | FEV1 Unclear if error bars plotted represent standard deviation values or standard error FVC FEF25‐75 FEF 50 Rescue use beta2 agonist Daily wheeze score Outcomes reported as medians with ranges |
| Ebden 1986 | Rescue use beta2 agonist Daily wheeze score Outcomes reported as medians with ranges |
| Greefhorst 1992 | Morning PEFR Evening PEFR No SD values for above outcomes Asthma symptom score Rescue beta2 agonist use No numerical data available |
| Hamalainen 1998 | Morning PEFR Morning plasma cortisol No standard deviation values available for above outcomes |
| Keelan 1984 | FEV1 FVC Morning PEFR Evening PEFR Asthma symptom score Beta2 agonist use Serum cortisol (time not specified) No numerical data presented for above outcomes |
| Micheletto 1997 | FEV1 Serum ECP Unclear if error bars plotted represent standard deviation values or standard error for above outcomes FVC Clinic PEFR FEF 25‐75 Morning PEFR Evening PEFR Daily beta2 agonist use Morning serum cortisol No numerical data available for above outcomes |
| Nicolaizik 1994 | Serum cortisol Serum ACTH 24 hour urinary free cortisol Above outcomes expressed using medians and analyses using non‐parametric tests Histamine BHR (PC20 FEV1) No SD values for log transformed data available |
| Petrie 1990 | FEV1 Morning PEFR Evening PEFR Daily beta2 agonist use (puffs/day) No numerical data presented for above outcomes Questionnaire concerning delivery device: ease of use, carrying convenience, overall preference No SD values presented for above outcomes |
| Svendsen 1993 | FEV1 FVC Morning PEFR Evening PEFR Clinic PEFR Histamine BHR (PC20 FEV1) Sleep disturbance, wheeze and activity restriction score Rescue beta2 agonist use (puffs/day) Plasma cortisol (time not stated) Plasma cortisol 30 min post 250 mcg iv tetracosactrin No SD values available for any of the above outcomes |
ASTHMA NOT TREATED WITH ORAL STEROIDS
CROSSOVER STUDIES
Spirometry
FEV1 (litres) was reported in six studies (227 subjects). FEV1 (% predicted) was reported in two studies (74 subjects). No significant difference between BDP and BUD was apparent. Studies that reported FEV1 using different measurement scales were pooled in order to increase the power of the analysis (eight studies, 301 subjects). The pooled treatment effect was expressed as a standardised mean difference. No significant difference between BDP and BUD was apparent: SMD ‐0.15, 95% CI ‐0.38 to 0.07. A similar result was found for FVC (litres), FVC (% predicted), and FVC (litres, % predicted combined), with no significant difference between BDP and BUD.
Peak expiratory flow rate (PEF)
Six studies (220 subjects) reported diary card recorded morning PEF (L/min). One study (Field 1982, 54 subjects) reported diary card recorded PEF (% predicted). No significant difference between BDP and BUD was demonstrated, either when outcomes were considered according to the measure used, or when studies were pooled with the treatment effect expressed as a standardised mean difference: SMD ‐0.04 (95% CI ‐0.28 to 0.20).
One study (Springer 1987, 20 subjects) reported clinic measured PEF (% predicted). No significant difference between treatments was demonstrated. Five studies (111 subjects) reported evening PEF. No significant difference between BDP and BUD was apparent, WMD ‐5 L/min (95% CI ‐32 to 21 L/min).
Symptoms
A variety of symptom scores were reported. These included daily, daytime, night‐time, morning and evening cough, breathlessness and wheeze scores. No significant difference between treatments was apparent for any of these symptom measures. Those studies reporting daytime breathlessness, morning breathlessness and daily symptom scores were pooled (six studies, 256 subjects). No significant difference between BDP and BUD was apparent: SMD 0.06 (95% CI ‐0.18 to 0.31). Studies reporting night‐time breathlessness and evening breathlessness scores were pooled (three studies, 134 subjects). Again, no significant difference between treatments was apparent: SMD ‐0.09 (95% CI ‐0.43 to 0.25).
Rescue beta‐2 agonist use
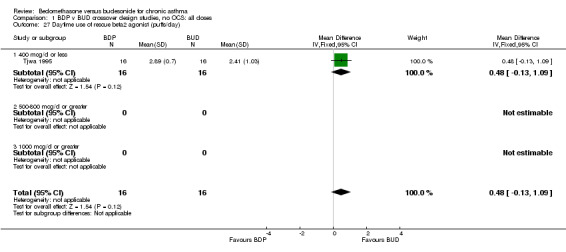
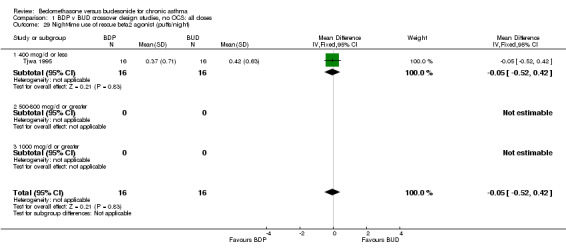
Numerical data for rescue beta2 agonist use was only reported in two studies. Tjwa 1995 assessed daytime and night‐time use; Willey 1982 assessed morning and evening use. No significant differences between treatments were apparent.
Bronchial hyper‐responsiveness (BHR)
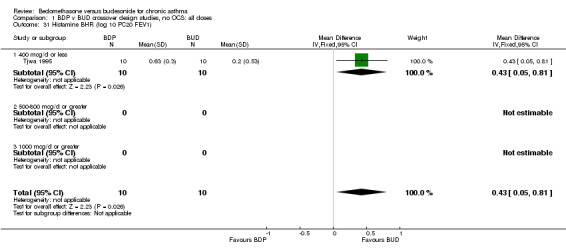
Only one study with numerical data (Tjwa 1995) assessed histamine BHR. This study in adult patients was of fair methodological quality (Jadad score 3), and assessed the effects of BDP and BUD at a nominal daily dose of 400 mcg, with BUD administered via Turbohaler DPI and BDP via a Rotahaler DPI. A significantly lower histamine BHR (expressed as log 10 PC20 FEV1) was apparent after BUD treatment for eight weeks compared to BDP, WMD 0.43 log 10 PC20FEV1 (95% CI 0.05 to 0.81 log10 PC20 FEV1). This translates into a 1.4 fold greater doubling concentration of histamine required to produce a 20% fall in FEV1 compared to BDP (95% CI 0.4 to 2.4 doubling concentrations, investigator's analysis).
Hypothalamo‐pituitary adrenal axis (HPA) function
Three studies (118 subjects) reported morning plasma cortisol. Two studies (76 subjects) reported plasma cortisol following a short cosyntropin test. No significant differences between BDP and BUD treatment groups were evident. In a single crossover study (Pedersen 1988), conducted in children and of fair methodological quality (Jadad score 3) 24 hour urinary free cortisol excretion was assessed. In this study subjects treated with BDP 800‐1200 mcg/d had significantly lower 24 hour urinary cortisol levels compared to BUD 800‐1200 mcg/d: BDP 7.6 nmol cortisol/mmol creatinine/day v BUD 10.2 nmol cortisol/mmol creatinine/day P<0.01.
Local oral side effects
The incidence of local oral side effects was reported in a number of crossover studies (Baran 1987, Boe 1989, Ebden 1986, Petrie 1990, Svendsen 1992). However, interpretation of the results is extremely difficult. In each study, the incidence of side effects was reported by treatment (BDP or BUD), rather than by individual treatment period. It was not stated if, in the case of a patient experiencing an adverse event during the first period of the trial when receiving one ICS, this had resolved by the point of crossover. Because none of the studies incorporated washout periods, this was especially unclear. In summary, the data regarding local oral side effects from the crossover studies comparing BDP to BUD are uninterpretable.
Sensitivity analyses
No heterogeneity in effect size was present when studies were pooled for any outcome. Sensitivity analyses were undertaken by re‐analysing outcomes and only including studies with a Jadad score of 3 or greater. This eliminated two studies (Bjorkander 1982a, Nicolaizik 1994). This did not result in a significant change in the size or direction of the treatment effect for any reported outcome.
Subgroup analyses
Subgroup analyses based upon nominal daily dose, age, study duration, delivery device and asthma control did not identify any groups who appeared to preferentially benefit.
PARALLEL GROUP STUDIES: DOSE‐DOWN TITRATION DESIGN
Two parallel group studies adopted a dose down‐titration design. Both were non‐blinded open studies of variable methodological quality. One study (Brambilla 1994) had a Jadad score of 2, the other (Selroos 1994) had a Jadad score of 3. Both were undertaken in adult asthmatics treated with BDP 1000 mcg/d or greater. In one study (Brambilla 1994) patients entered a 4‐week run‐in phase during which time they were maintained on the same daily dose of BDP that they received before entering the study. In Selroos 1994 study patients entered a two‐year run‐in period during which time the minimal effective dose of BDP was established in order to maintain the 'best possible level' of symptom control, such that patients were free of symptoms. Patients were randomised to either 12 weeks of treatment (Brambilla 1994) or two years of treatment (Selroos 1994). During this time the daily dose of ICS was down‐titrated following pre‐defined symptom‐based criteria whilst maintaining symptom control. The primary outcome measure in both studies was the end of study daily ICS dose. A significantly lower daily dose of BUD was required to maintain symptom control: WMD 444 mcg/d (95% CI 332 to 556 mcg/d). As would be anticipated given a trial design aimed at maintaining optimal control there were no significant differences in FEV1, PEF, rescue beta2 agonist use or withdrawals due to asthma exacerbation. There were no significant differences between treatments with regard to the incidence of local oral side effects (hoarseness/sore throat or Candidiasis).
PARALLEL GROUP STUDIES: DOSE ESCALATION DESIGN
A single parallel group study of three months duration in children (Bisgaard 1988), of high methodological quality (Jadad score 4) had a dose escalation design. Subjects were randomised to treatment with BDP or BUD. Patients in each randomised arm received 200 mcg/d, 400 mcg/d then 800 mcg/d for four week periods in succession with no washout between treatments. Patients were therefore randomised according to ICS, but not by daily dose of ICS. Outcomes reported included 24‐hour urinary free cortisol excretion and plasma cortisol 30 min post 250 mcg i.v. tetracosactrin. No statistically significant difference between BDP and BUD were apparent for either outcome.
ASTHMATICS TREATED WITH ORAL STEROIDS
CROSSOVER STUDIES: OCS‐SPARING STUDY DESIGN
One crossover study (Rafferty 1985) assessed the relative efficacy of BDP and BUD at a nominal daily dose of 800 mcg/d in adult patients treated with and dependent upon oral prednisolone at dose of 5mg/d or greater. This study was of fair quality (Jadad score 3). The use of a crossover design to assess OCS sparing efficacy is unique to this study. During each treatment period the daily dose of prednisolone was down titrated until asthma symptoms had deteriorated to 'an unacceptable level', although no a priori defined scheme was used to do this, with the decision to down‐titrate dose appearing to have been at the discretion of the investigator. The treatment period for each patient concluded at the point of loss of symptom control. Following this, patients entered a 'washout' period during which time their pre‐study dose of oral prednisolone was administered for four weeks before entering the second period of the study on the second ICS. Mean reduction in daily dose of oral prednisolone in BDP treated patients was 2.65 mg/d compared to 1.80 mg/d in BUD treated patients, with difference in reduction between groups of 0.85 mg/d in favour of BDP being statistically significant.
CROSSOVER STUDIES: NON OCS‐SPARING STUDY DESIGN
Three studies enrolled OCS treated patients, but did not assess OCS‐sparing effects as part of the study design. In two studies (Stiksa 1982a, Stiksa 1982b) requirement for OCS therapy was an inclusion criterion. In one study (Stiksa 1985) OCS treatment was not an inclusion requirement, but 10 out of 27 subjects were being treated with oral prednisolone at the time of enrolment. All studies were of fair methodological quality (Jadad score 3). The outcomes for these studies were pooled (3 studies, 144 subjects). No significant difference between BDP and BUD was apparent for morning PEF, evening PEF, daytime or night‐time breathlessness scores, sleep disturbance scores or rescue beta2 agonist use.
Discussion
This systematic review has assessed the randomised controlled trials that compared BDP and BUD at equal nominal daily dose, with the objective of assessing their relative efficacy and safety. 24 trials with 1174 children and adults were included.
RELATIVE EFFICACY
The trials designed to assess the relative efficacy of inhaled BDP and BUD in non oral steroid treated asthmatic patients have been mainly of two period crossover design. Children and adults are represented, with daily doses of ICS ranging from 400 mcg/d or less to 1000 mcg/d or greater. Treatments were compared over a period of up to five weeks. A meta‐analysis of the available data from these trials showed no significant difference between BDP and BUD when considering FEV1, morning PEF, or symptoms. Rescue beta2 agonist use was reported infrequently and the two trials that did report it used different scoring systems. Individually, they did not demonstrate a statistically significant difference between treatment groups.
The results of this analysis require cautious interpretation for the following reasons. Particular strengths of a crossover design relate to the fact that subjects recruited to the trial effectively act as their own controls. This results in a) efficiency in design as only half as many subjects are required in order to achieve the same degree of statistical power as an equivalent parallel design study and b) the effect of inter‐individual variation in response to treatment is reduced, which may otherwise mask a true treatment effect. Unfortunately this design has some serious limitations when assessing the effects of relatively long acting interventions such as inhaled corticosteroids. Although most of the trials in this group were of fair methodological quality according to recognised grading systems, including that developed by Jadad, only two out of 19 trials had a washout of any length between treatment periods. Inhaled corticosteroids almost certainly exert an action that persists over days if not weeks after they have been discontinued. When the performance of long acting agents are assessed in trials of crossover design, there is also a concern that the effects of treatment administered in the first period of the trial may persist into, and modify the effects of any active agent administered in the second period of the trial. This so called 'carryover' or 'sequence' effect will influence the results of the study and lead to biased judgement concerning the relative efficacy and safety of the agents being assessed. In situations where the control intervention is inactive or a placebo, this effect will tend to produce an under estimate of the efficacy of the active treatment. In trials when two active treatments (such as BDP and BUD) are studied the influence of carryover effects are unpredictable. It is possible that a less effective drug could falsely appear to be the more effective one, if for example the carryover effect was strong and confined to the truly more effective drug. There are well‐established statistical methods for assessing the presence or otherwise of carryover effects and excluding them. However a statement as to whether such tests were undertaken was rarely reported.
These considerations somewhat undermine the results of the individual trials and necessitate additional caution in drawing conclusions from any meta‐analyses. When considering the crossover trials, bronchial hyper‐responsiveness to histamine was the only outcome for which a significant difference between BDP and BUD was reported. This was from a single study (Tjwa 1995) in which BUD 400 mcg/d was delivered via the Turbohaler DPI and BDP 400 mcg/d was delivered via the Rotahaler DPI. This study incorporated a four‐week washout period and the investigators excluded carryover effects, so this study is not subject to the criticisms as discussed above. Caution still needs to taken in interpreting these results because neither physicians nor patients were blinded to treatment allocation. The study was sponsored by Astra, the manufacturers of the Pulmicort Turbohaler, so the possibility of bias in favour of BUD cannot be excluded. It also possible that the observed difference in treatment effect was due to the delivery characteristics of the two dry powder delivery devices, rather than differences in the efficacy of BDP and BUD.
Only two parallel group design studies (Brambilla 1994, Selroos 1994) assessed the relative efficacy of BDP and BUD in non oral steroid treated subjects and had usable data. These studies had fundamentally different objectives to the studies of crossover design. They used a dose down‐titration design to compare the doses of BDP and BUD needed to maintain a good level of asthma control. A significantly lower dose of BUD was required to maintain control compared to BDP: WMD 444 mcg/d (95% CI 332 to 556 mcg/d). No heterogeneity in effect size was apparent between these studies, which differed greatly in duration (12 weeks and two years). It is important to appreciate that the drugs were administered using different delivery devices. In both studies, BUD was delivered by the Turbohaler DPI and BDP was delivered either by an MDI (Brambilla 1994) or an MDI+Volumatic spacer (Selroos 1994). Unfortunately there were no studies of this type that compared drugs delivered by the same type of delivery device. As with the study by Tjwa 1995, a weakness common to both of these trials lies in the fact neither physicians nor patients were blinded to treatment allocation. Because both studies were sponsored Astra who manufacture the Pulmicort Turbohaler the possibility of systematic bias in favour of BUD is a possibility that cannot be excluded.
The relative oral steroid sparing efficacy of BDP and BUD was only assessed in one study (Rafferty 1985). In an approach unique in the inhaled corticosteroid literature, the relative oral prednisolone sparing effect of two interventions was assessed in a trial of crossover design. This study has two significant flaws. Firstly, the minimal effective dose of prednisolone was not established for each patient either at the beginning of the study, or during the 'washout' phase between treatment periods when each patient received 4‐6 weeks of treatment with prednisolone. Enrolling subjects who are over treated with oral prednisolone could lead to an overestimate of the absolute prednisolone sparing efficacy of both BDP and BUD and lead to distortion in assessment of their relative effects. The second problem concerns the system used to taper the prednisolone dose. Dose reduction was attempted at regular intervals throughout each treatment period, until the point at which asthma symptoms deteriorated to 'an unacceptable level'. However no a priori definition for unacceptable symptoms was used. This could have led to a large degree of variation in the thresholds used by individual investigators in determining whether or not to continue dose reduction or to stop further dose reduction when assessing patients at clinic visits. The study was double blind and patients were randomised to each treatment sequence so these variations should have affected all patients equally. However in a study of small size (40 subjects) this factor may have had an unpredictable effect on the overall assessment of relative efficacy of the two inhaled steroids.
RELATIVE SAFETY
24 hour urinary free cortisol and plasma cortisol levels following short tetracosactrin test were assessed in the single high quality parallel group dose, escalation design study (Bisgaard 1988) undertaken in children. No significant difference between BDP and BUD delivered by identical delivery device (MDI) were apparent.
No significant difference in post tetracosactrin plasma cortisol levels or basal morning plasma cortisol levels were apparent when BDP and BUD were compared in trials of crossover design. Because of an absence of washout periods and elimination of carryover effects in the trials reporting these outcomes, the same caution in interpretation needs to be taken for the reasons outlined above. Only one crossover study (Pedersen 1988) reported a sensitive measure of HPA function in the form of 24 urinary cortisol excretion corrected for creatinine. It should be noted that although a range of daily doses of ICS were assessed in this study (800‐1200 mcg/d) individual children were randomised to receive identical daily nominal doses of BDP or BUD in each period of the crossover study, via identical delivery devices (MDI). Carryover effects were tested for and excluded in this study, despite the fact that a washout was not used. This study appears to be free of the limitations concerning the majority of crossover design studies. Although these differences are statistically significant, their clinical significance is uncertain. The concern regarding ICS use and HPA function, especially in children, is the long‐term risk of adrenal insufficiency/crisis at times of stress. The predictive value of differences in results obtained in any of the available sensitive measures when comparing inhaled steroids and long term risk of adrenal insufficiency is unknown.
STUDY POWER
The primary purpose of these studies was never clearly defined ‐ i.e. whether their objective was to determine therapeutic superiority or equivalence. This is important since the process of hypothesis testing is different between the two types of study, most noticeably in the use of upper and lower limits of equivalence in equivalence studies. For statistical reasons, equivalence studies usually have to be larger than superiority studies. None of these points were discussed in the trials included here, and all were too small to have been adequate for a test of equivalence.
Authors' conclusions
Implications for practice.
Current asthma guidelines (BTS 1997, GINA 1995, NHLBI 1997) assume beclomethasone and budesonide are equally effective, such that for each defined level of asthma severity, the recommended doses are the same. However, there is little reliable evidence from randomised controlled studies to support this conclusion, and none to refute it. There are some data which suggest that budesonide via a Turbohaler is more effective than BDP delivered by either a Rotahaler or a MDI with or without spacer, but this difference may be due to the delivery device rather than the drug and is not sufficient to warrant any change in guideline recommendations.
Implications for research.
There is a place for a large, parallel group randomised controlled trial to test the equivalence of BDP and BUD. Ideally this would include treatment arms assessing low dose ICS (400 mcg/d or less) and high dose ICS (1000 mcg/d or greater) in asthmatic children and adults. Currently available dry powder delivery devices differ for BDP and BUD. To assess relative drug efficacy without the confounding influence of differences in inhaler performance the newer non‐CFC propellant metered dose inhalers could be used as a standardised delivery device if both drugs become available in this form. An intervention period of 6 months or longer would allow assessment of important outcomes such as asthma exacerbation rates, hospital admissions, days lost from work/school and heath status which have not been reported in any studies to date.
Feedback
Data entry error
Summary
In table 1.2 the numbers extracted from Willey 1982 do not seem to match the original paper. I believe the numbers should be BDP 2.00 (0.80) and BUD 2.10 (0.80).
Reply
Response from Cochrane Airways editorial base: Thank you for bringing this error to our attention. The data has been corrected in analyses 1.2.1, 1.3.1, 2.2.2, 2.3.2, 3.2.1, 3.3.1, 4.2.2 and 4.3.1 and the relevant text in the results section updated to reflect this change. The impact on the results of the analyses over all is negligible.
Contributors
Stephanie Weinreich
Academic Medical Center, Amsterdam, The Netherlands
What's new
| Date | Event | Description |
|---|---|---|
| 8 December 2014 | Feedback has been incorporated | Feedback added and typos corrected. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 21 July 2008 | Amended | Converted to new review format. |
| 14 November 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Anna Bara for running the initial electronic search and Steve Milan for assistance with statistical methods. We also like to acknowledge the authors who kindly responded to requests for additional information regarding their studies even in cases where information was not available including Dr M Boorsma, Dr C Brambilla, Dr P Ebden, Professor S Godfrey, Dr S Pedersen, Dr S Selroos, Dr G Stiksa, Dr UG Svendsen, Dr MKT Tjwa, Professor JO Warner and Dr RF Willey.
Data and analyses
Comparison 1. BDP v BUD crossover design studies, no OCS: all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐11.98, 1.89] |
| 1.1 400 mcg/d or less | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐11.98, 1.89] |
| 1.2 500‐800 mcg/d | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 6 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.43, ‐0.03] |
| 2.1 400 mcg/d or less | 5 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.50, ‐0.04] |
| 2.2 500‐800 mcg/d | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 1000 mcg/d or greater | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.51, 0.29] |
| 3 FEV1 (% predicted, litre measures combined) | 8 | 301 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.38, 0.07] |
| 3.1 400 mcg/d or less | 7 | 248 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 3.2 500‐800 mcg/d | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 1000 mcg/d or greater | 1 | 53 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.68, 0.40] |
| 4 FVC (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.1 400 mcg/d or less | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 FVC (litres) | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.29, 0.17] |
| 5.1 400 mcg/d or less | 4 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.32, 0.22] |
| 5.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 1000 mcg/d or greater | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.54, 0.38] |
| 6 FVC (% predicted, litre measures combined) | 6 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.29, 0.20] |
| 6.1 400 mcg/d or less | 5 | 204 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.31, 0.24] |
| 6.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 1000 mcg/d or greater | 1 | 53 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.63, 0.45] |
| 7 Clinic PEFR (% predicted) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.1 400 mcg/d or less | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Morning PEFR (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.1 400 mcg/d or less | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Morning PEFR (L/min) | 6 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐2.99 [‐28.43, 22.45] |
| 9.1 400 mcg/d or less | 5 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐3.02 [‐32.36, 26.32] |
| 9.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 1000 mcg/d or greater | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐52.00, 48.20] |
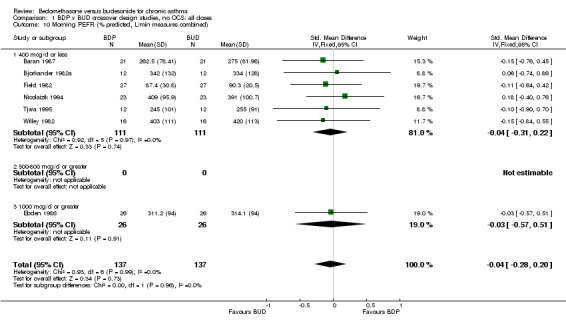
| 10 Morning PEFR (% predicted, L/min measures combined) | 7 | 274 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| 10.1 400 mcg/d or less | 6 | 222 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.31, 0.22] |
| 10.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 1000 mcg/d or greater | 1 | 52 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.57, 0.51] |
| 11 Evening PEFR (L/min) | 5 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐5.47 [‐31.50, 20.56] |
| 11.1 400 mcg/d or less | 4 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐7.12 [‐38.59, 24.35] |
| 11.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 1000 mcg/d or greater | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐48.21, 44.41] |
| 12 Daytime breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.20, 0.72] |
| 12.1 400 mcg/d or less | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.20, 0.72] |
| 12.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Morning breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 13.1 400 mcg/d or less | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 13.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Daytime wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 14.1 400 mcg/d or less | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 14.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Morning wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 15.1 400 mcg/d or less | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 15.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Daytime cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 16.1 400 mcg/d or less | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 16.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
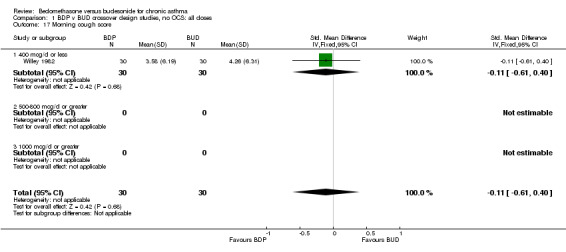
| 17 Morning cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 17.1 400 mcg/d or less | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 17.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Daily asthma symptom score | 5 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.22, 0.39] |
| 18.1 400 mcg/d or less | 5 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.22, 0.39] |
| 18.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined) | 7 | 256 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.18, 0.31] |
| 19.1 400 mcg/d or less | 7 | 256 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.18, 0.31] |
| 19.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
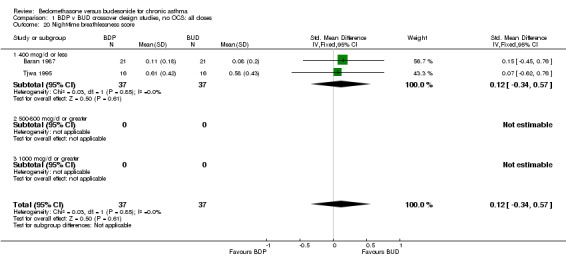
| 20 Night‐time breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.34, 0.57] |
| 20.1 400 mcg/d or less | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.34, 0.57] |
| 20.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
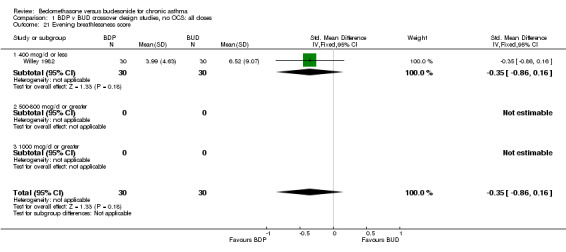
| 21 Evening breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 21.1 400 mcg/d or less | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 21.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
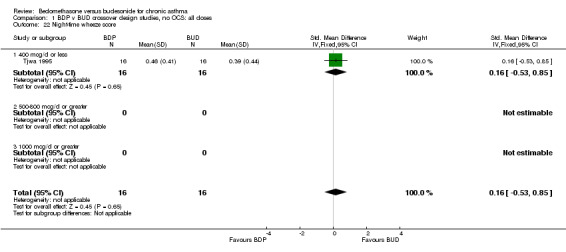
| 22 Night‐time wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 22.1 400 mcg/d or less | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 22.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Evening wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 23.1 400 mcg/d or less | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 23.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Night‐time cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 24.1 400 mcg/d or less | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 24.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Evening cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 25.1 400 mcg/d or less | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 25.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined) | 3 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.43, 0.25] |
| 26.1 400 mcg/d or less | 3 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.43, 0.25] |
| 26.2 500‐800 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 1000 mcg/d or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Daytime use of rescue beta2 agonist (puffs/day) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 27.1 400 mcg/d or less | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 27.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Morning use of rescue beta2 agonist (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 28.1 400 mcg/d or less | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 28.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Night‐time use of rescue beta2 agonist (puffs/night) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 29.1 400 mcg/d or less | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 29.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
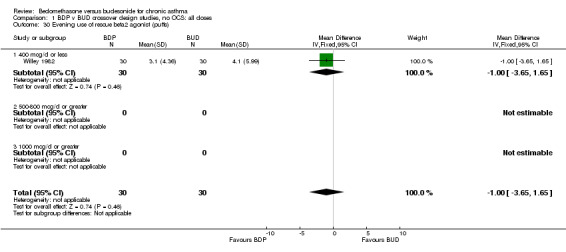
| 30 Evening use of rescue beta2 agonist (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 30.1 400 mcg/d or less | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 30.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Histamine BHR (log 10 PC20 FEV1) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 31.1 400 mcg/d or less | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 31.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 1000 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Morning plasma cortisol (nmol/L) | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | 24.32 [‐15.78, 64.41] |
| 32.1 400 mcg/d or less | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | 22.29 [‐37.29, 81.86] |
| 32.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 1000 mcg/d or greater | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 26.0 [‐28.21, 80.21] |
| 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 26.84 [‐24.79, 78.48] |
| 33.1 400 mcg/d or less | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐254.22, 254.22] |
| 33.2 500‐800 mcg/d or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 1000 mcg/d or greater | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 28.00 [‐24.74, 80.74] |
1.1. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 1 FEV1 (% predicted).
1.2. Analysis.
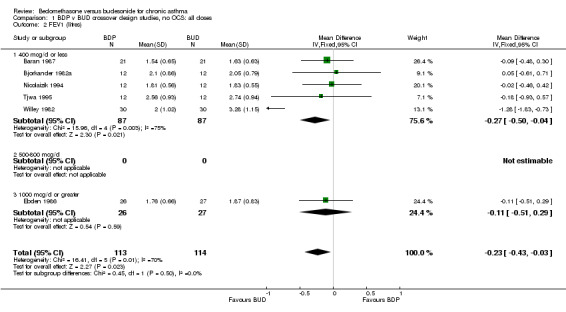
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 2 FEV1 (litres).
1.3. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 3 FEV1 (% predicted, litre measures combined).
1.4. Analysis.
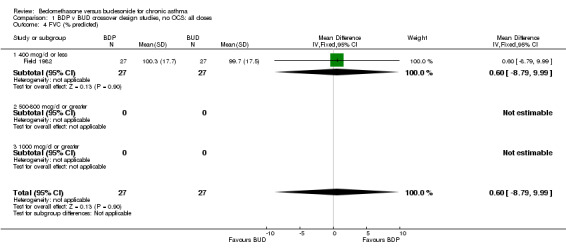
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 4 FVC (% predicted).
1.5. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 5 FVC (litres).
1.6. Analysis.
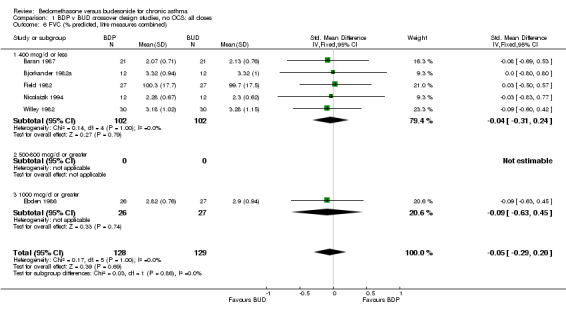
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 6 FVC (% predicted, litre measures combined).
1.7. Analysis.
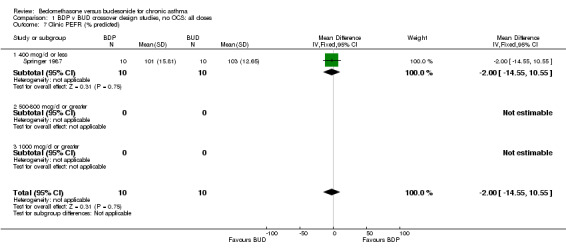
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 7 Clinic PEFR (% predicted).
1.8. Analysis.
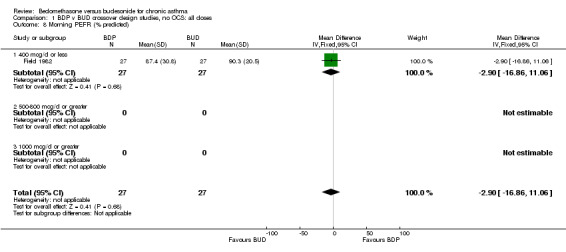
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 8 Morning PEFR (% predicted).
1.9. Analysis.
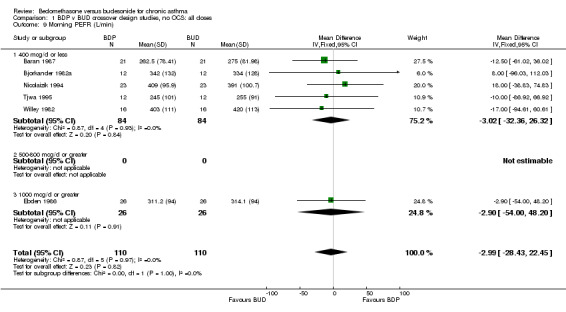
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 9 Morning PEFR (L/min).
1.10. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 10 Morning PEFR (% predicted, L/min measures combined).
1.11. Analysis.
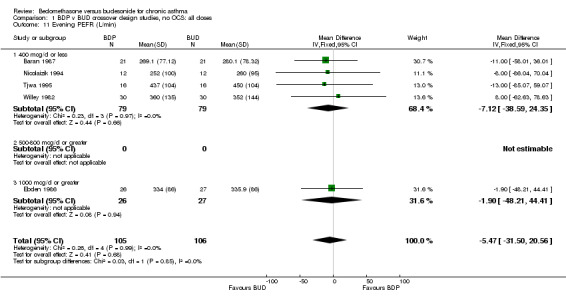
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 11 Evening PEFR (L/min).
1.12. Analysis.
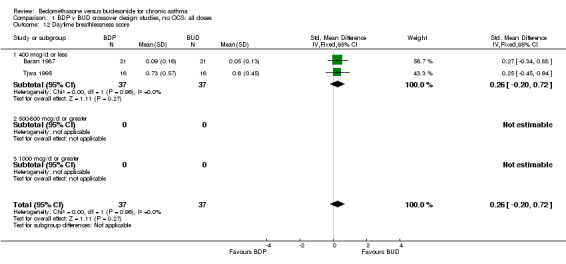
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 12 Daytime breathlessness score.
1.13. Analysis.
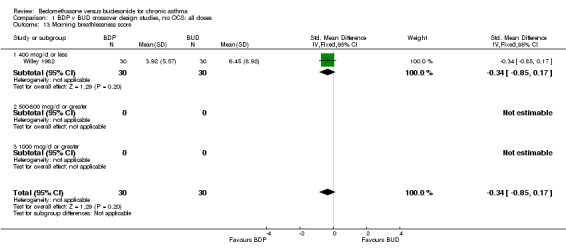
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 13 Morning breathlessness score.
1.14. Analysis.
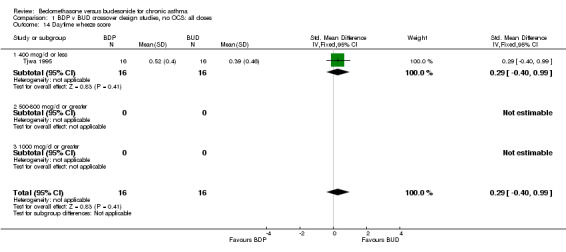
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 14 Daytime wheeze score.
1.15. Analysis.
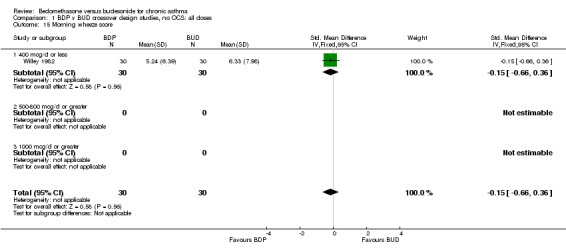
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 15 Morning wheeze score.
1.16. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 16 Daytime cough score.
1.17. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 17 Morning cough score.
1.18. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 18 Daily asthma symptom score.
1.19. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined).
1.20. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 20 Night‐time breathlessness score.
1.21. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 21 Evening breathlessness score.
1.22. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 22 Night‐time wheeze score.
1.23. Analysis.
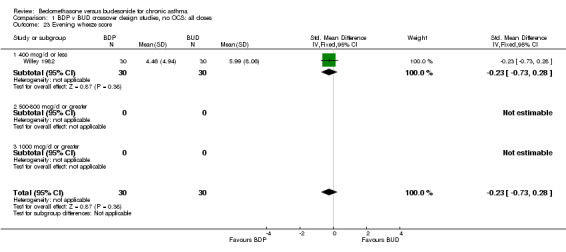
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 23 Evening wheeze score.
1.24. Analysis.
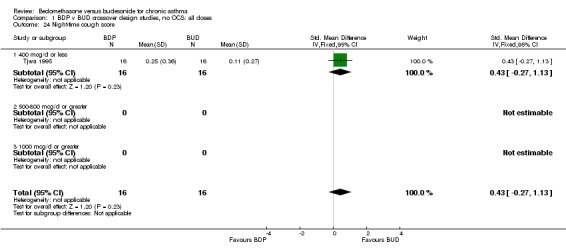
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 24 Night‐time cough score.
1.25. Analysis.
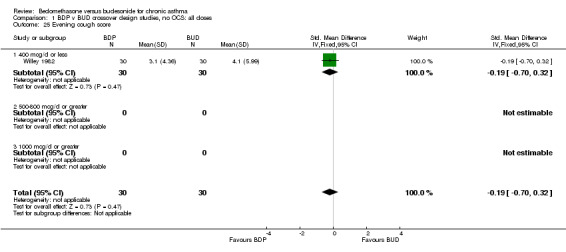
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 25 Evening cough score.
1.26. Analysis.
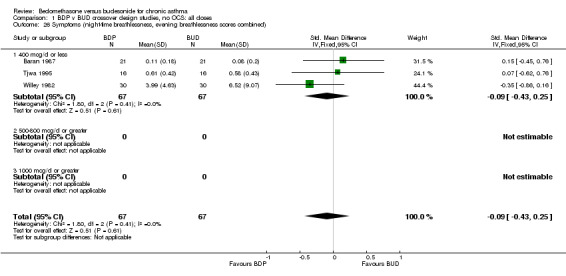
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined).
1.27. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 27 Daytime use of rescue beta2 agonist (puffs/day).
1.28. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 28 Morning use of rescue beta2 agonist (puffs).
1.29. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 29 Night‐time use of rescue beta2 agonist (puffs/night).
1.30. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 30 Evening use of rescue beta2 agonist (puffs).
1.31. Analysis.
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 31 Histamine BHR (log 10 PC20 FEV1).
1.32. Analysis.
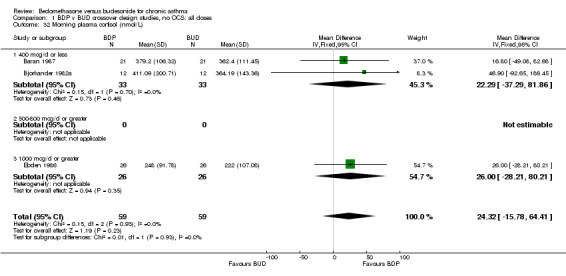
Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 32 Morning plasma cortisol (nmol/L).
1.33. Analysis.

Comparison 1 BDP v BUD crossover design studies, no OCS: all doses, Outcome 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L).
Comparison 2. BDP v BUD crossover design studies, no OCS: all study age groups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐11.98, 1.89] |
| 1.1 Children | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐11.98, 1.89] |
| 1.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 6 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.27, 0.11] |
| 2.1 Children | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 2.2 Adults | 4 | 161 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.34, 0.16] |
| 3 FEV1 (% predicted and litre measures combined) | 8 | 301 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.38, 0.07] |
| 3.1 Children | 4 | 140 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.53, 0.14] |
| 3.2 Adults | 4 | 161 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.42, 0.20] |
| 4 FVC (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.1 Children | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 FVC (litres) | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.29, 0.17] |
| 5.1 Children | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.38, 0.29] |
| 5.2 Adults | 3 | 137 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.39, 0.25] |
| 6 FVC (% predicted and litre measures combined) | 6 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.29, 0.20] |
| 6.1 Children | 3 | 120 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.38, 0.34] |
| 6.2 Adults | 3 | 137 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.41, 0.26] |
| 7 Clinic PEFR (% predicted) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.1 Children | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Morning PEFR (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.1 Children | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.2 Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Morning PEFR (L/min) | 6 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐2.99 [‐28.43, 22.45] |
| 9.1 Children | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐36.54, 37.26] |
| 9.2 Adults | 4 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐6.03 [‐41.15, 29.10] |
| 10 Morning PEFR (% predicted, L/min measures combined) | 7 | 274 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| 10.1 Children | 3 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.36, 0.30] |
| 10.2 Adults | 4 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.40, 0.29] |
| 11 Evening PEFR (L/min) | 5 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐5.47 [‐31.50, 20.56] |
| 11.1 Children | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐10.20 [‐50.47, 30.07] |
| 11.2 Adults | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐36.19, 32.04] |
| 12 Daytime breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.20, 0.72] |
| 12.1 Children | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.34, 0.88] |
| 12.2 Adults | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.45, 0.94] |
| 13 Morning breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 13.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Adults | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 14 Daytime wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 14.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 15 Morning wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 15.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Adults | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 16 Daytime cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 16.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Adults | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 17 Morning cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 17.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 18 Daily asthma symptom score | 5 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.22, 0.39] |
| 18.1 Children | 4 | 140 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.24, 0.42] |
| 18.2 Adults | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.78, 0.82] |
| 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined) | 7 | 256 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.18, 0.31] |
| 19.1 Children | 4 | 140 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.13, 0.53] |
| 19.2 Adults | 3 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.47, 0.27] |
| 20 Night‐time breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.34, 0.57] |
| 20.1 Children | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.45, 0.76] |
| 20.2 Adults | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.62, 0.76] |
| 21 Evening breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 21.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Adults | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 22 Night‐time wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 22.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Adults | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 23 Evening wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 23.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Adults | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 24 Night‐time cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 24.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Adults | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 25 Evening cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 25.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Adults | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined) | 3 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.43, 0.25] |
| 26.1 Children | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.45, 0.76] |
| 26.2 Adults | 2 | 92 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.61, 0.21] |
| 27 Daytime use of rescue beta2 agonists (puffs/day) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 27.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 28 Morning use of rescue beta2 agonists (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 28.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Adults | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 29 Night‐time use of rescue beta2 agonists (puffs/night) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 29.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Adults | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 30 Evening use of rescue beta2 agonists (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 30.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Adults | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 31 Histamine BHR (log 10 PC20 FEV1) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 31.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Adults | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 32 Morning plasma cortisol (nmol/L) | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | 24.32 [‐15.78, 64.41] |
| 32.1 Children | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 16.80 [‐49.08, 82.68] |
| 32.2 Adults | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 28.74 [‐21.79, 79.27] |
| 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 26.84 [‐24.79, 78.48] |
| 33.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Adults | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 26.84 [‐24.79, 78.48] |
2.1. Analysis.
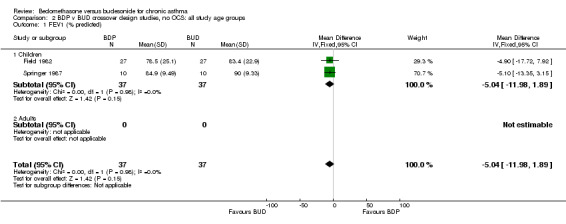
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 1 FEV1 (% predicted).
2.2. Analysis.
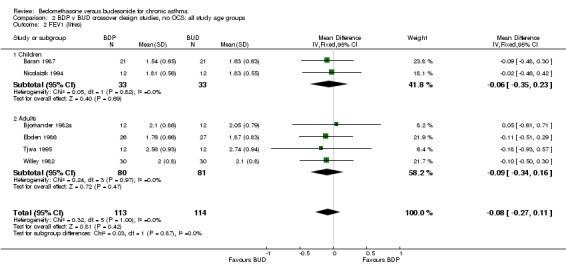
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 2 FEV1 (litres).
2.3. Analysis.
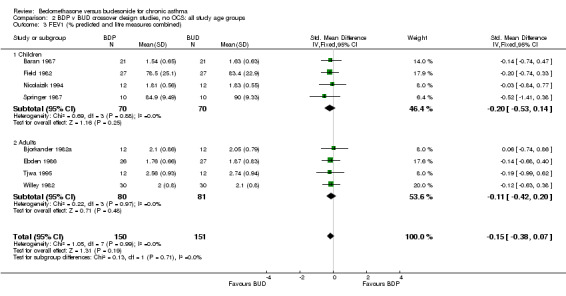
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 3 FEV1 (% predicted and litre measures combined).
2.4. Analysis.
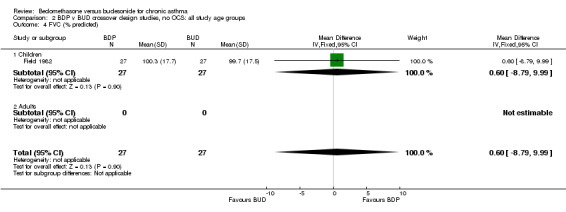
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 4 FVC (% predicted).
2.5. Analysis.
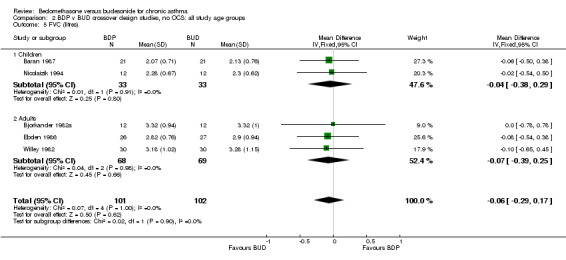
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 5 FVC (litres).
2.6. Analysis.
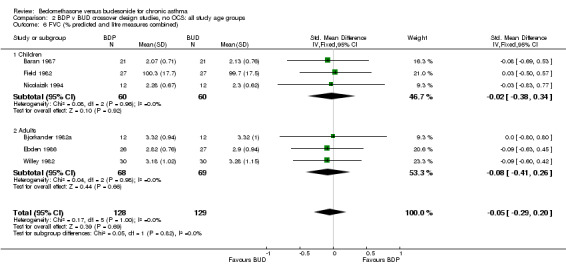
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 6 FVC (% predicted and litre measures combined).
2.7. Analysis.
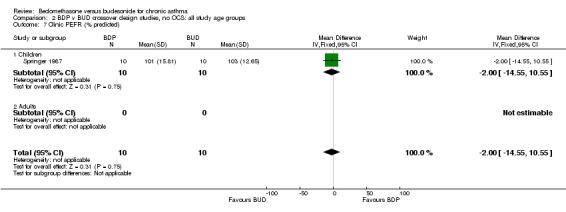
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 7 Clinic PEFR (% predicted).
2.8. Analysis.
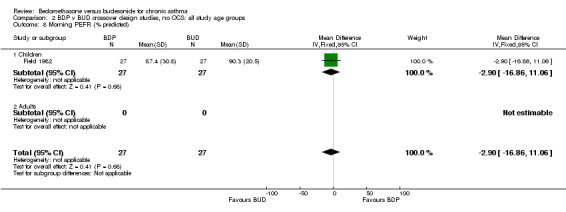
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 8 Morning PEFR (% predicted).
2.9. Analysis.

Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 9 Morning PEFR (L/min).
2.10. Analysis.
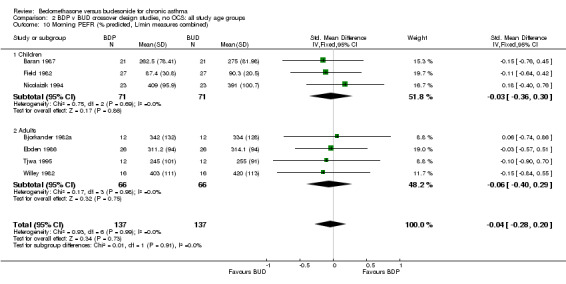
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 10 Morning PEFR (% predicted, L/min measures combined).
2.11. Analysis.
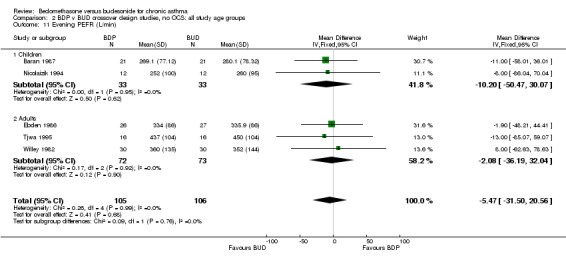
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 11 Evening PEFR (L/min).
2.12. Analysis.
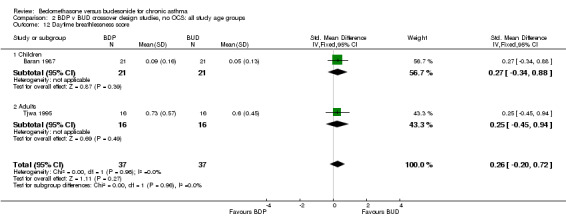
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 12 Daytime breathlessness score.
2.13. Analysis.
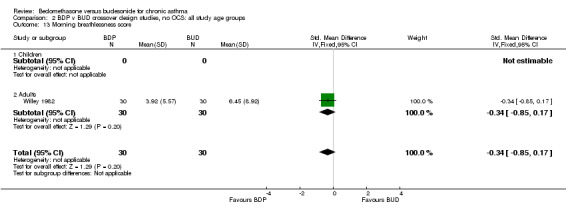
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 13 Morning breathlessness score.
2.14. Analysis.
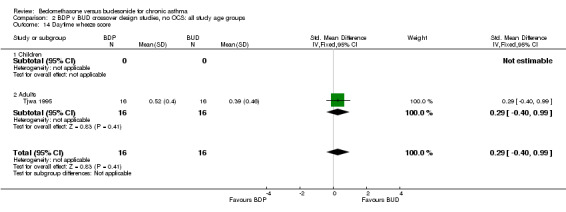
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 14 Daytime wheeze score.
2.15. Analysis.
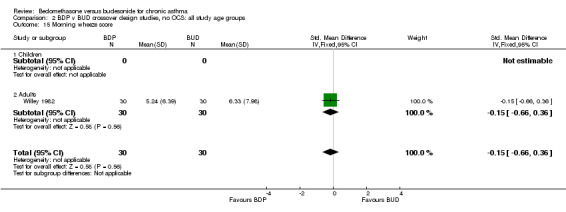
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 15 Morning wheeze score.
2.16. Analysis.
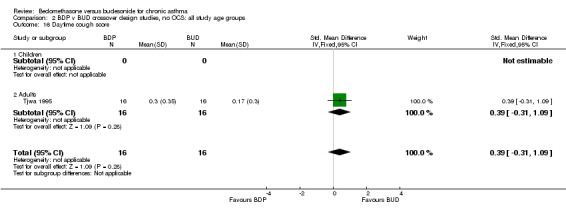
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 16 Daytime cough score.
2.17. Analysis.
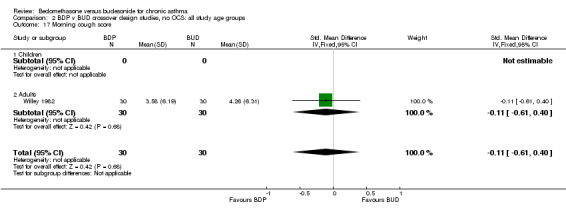
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 17 Morning cough score.
2.18. Analysis.
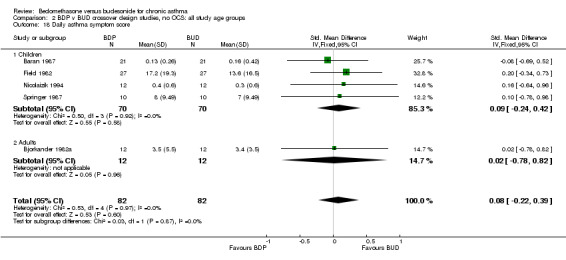
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 18 Daily asthma symptom score.
2.19. Analysis.
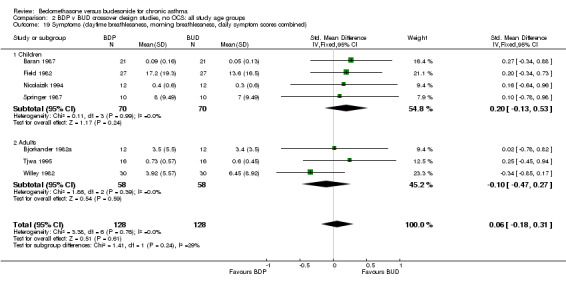
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined).
2.20. Analysis.
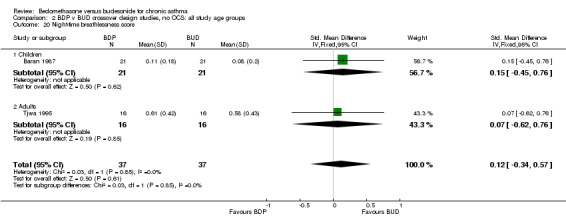
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 20 Night‐time breathlessness score.
2.21. Analysis.
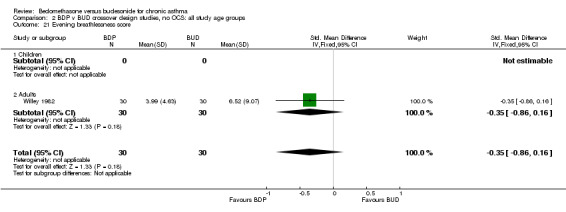
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 21 Evening breathlessness score.
2.22. Analysis.
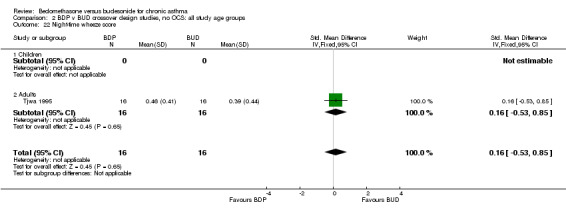
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 22 Night‐time wheeze score.
2.23. Analysis.
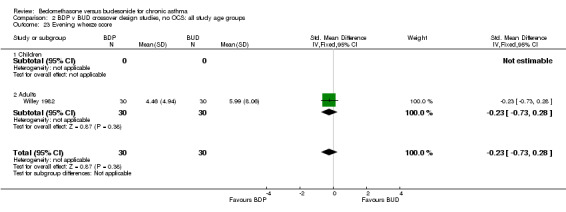
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 23 Evening wheeze score.
2.24. Analysis.
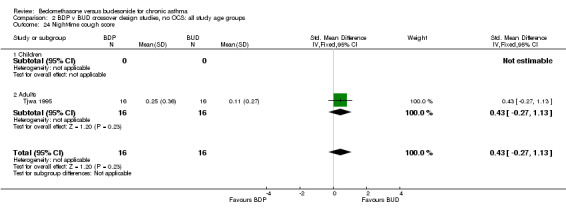
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 24 Night‐time cough score.
2.25. Analysis.
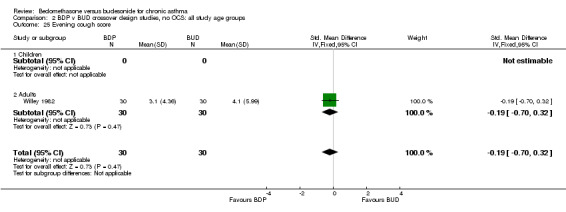
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 25 Evening cough score.
2.26. Analysis.
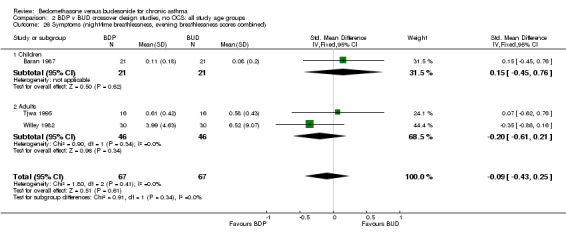
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined).
2.27. Analysis.
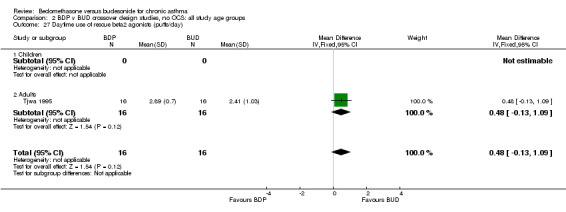
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 27 Daytime use of rescue beta2 agonists (puffs/day).
2.28. Analysis.
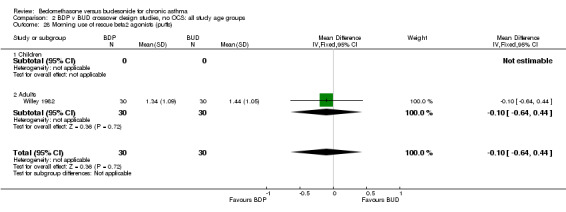
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 28 Morning use of rescue beta2 agonists (puffs).
2.29. Analysis.
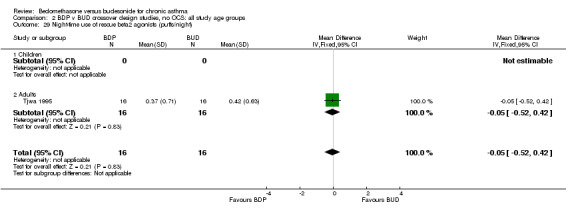
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 29 Night‐time use of rescue beta2 agonists (puffs/night).
2.30. Analysis.
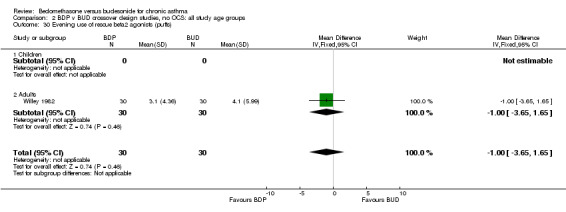
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 30 Evening use of rescue beta2 agonists (puffs).
2.31. Analysis.
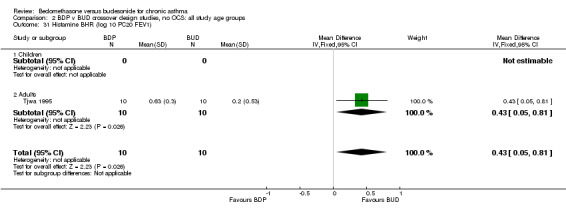
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 31 Histamine BHR (log 10 PC20 FEV1).
2.32. Analysis.

Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 32 Morning plasma cortisol (nmol/L).
2.33. Analysis.
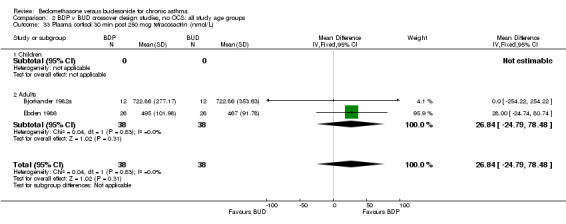
Comparison 2 BDP v BUD crossover design studies, no OCS: all study age groups, Outcome 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L).
Comparison 3. BDP v BUD crossover design studies, no OCS: all study durations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐11.98, 1.89] |
| 1.1 1‐4 weeks | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐11.98, 1.89] |
| 1.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 6 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.27, 0.11] |
| 2.1 1‐4 weeks | 4 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.28, 0.16] |
| 2.2 1‐5 months | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.48, 0.23] |
| 2.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 FEV1 (% predicted, litre measures combined) | 8 | 301 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.38, 0.07] |
| 3.1 1‐4 weeks | 6 | 224 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.41, 0.11] |
| 3.2 1‐5 months | 2 | 77 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.60, 0.29] |
| 3.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 FVC (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.1 1‐4 weeks | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 FVC (litres) | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.29, 0.17] |
| 5.1 1‐4 weeks | 4 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.32, 0.22] |
| 5.2 1‐5 months | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.54, 0.38] |
| 5.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 FVC (% predicted, litre measures combined) | 6 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.29, 0.20] |
| 6.1 1‐4 weeks | 5 | 204 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.31, 0.24] |
| 6.2 1‐5 months | 1 | 53 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.63, 0.45] |
| 6.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Clinic PEFR (% predicted) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.1 1‐4 weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Morning PEFR (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.1 1‐4 weeks | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Morning PEFR (L/min) | 6 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐2.99 [‐28.43, 22.45] |
| 9.1 1‐4 weeks | 4 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐33.57, 29.90] |
| 9.2 1‐5 months | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐5.07 [‐47.64, 37.49] |
| 9.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Morning PEFR (% predicted, L/min measures combined) | 7 | 274 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| 10.1 1‐4 weeks | 5 | 198 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.32, 0.24] |
| 10.2 1‐5 months | 2 | 76 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.50, 0.40] |
| 10.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Evening PEFR (L/min) | 5 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐5.47 [‐31.50, 20.56] |
| 11.1 1‐4 weeks | 3 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐5.74 [‐40.72, 29.25] |
| 11.2 1‐5 months | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐5.14 [‐44.11, 33.82] |
| 11.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Daytime breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.20, 0.72] |
| 12.1 1‐4 weeks | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.34, 0.88] |
| 12.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.45, 0.94] |
| 12.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Morning breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 13.1 1‐4 weeks | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 13.2 1‐5 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Daytime wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 14.1 1‐4 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 14.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Morning wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 15.1 1‐4 weeks | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 15.2 1‐5 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Daytime cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 16.1 1‐4 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 16.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Morning cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 17.1 1‐4 weeks | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 17.2 1‐5 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Daily asthma symptom score | 5 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.22, 0.39] |
| 18.1 1‐4 weeks | 5 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.22, 0.39] |
| 18.2 1‐5 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined) | 7 | 256 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.18, 0.31] |
| 19.1 1‐4 weeks | 6 | 224 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.23, 0.30] |
| 19.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.45, 0.94] |
| 19.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Night‐time breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.34, 0.57] |
| 20.1 1‐4 weeks | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.45, 0.76] |
| 20.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.62, 0.76] |
| 20.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Evening breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 21.1 1‐4 weeks | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 21.2 1‐5 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Night‐time wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 22.1 1‐4 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 22.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Evening wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 23.1 1‐4 weeks | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 23.2 1‐5 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Night‐time cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 24.1 1‐4 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 24.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Evening cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 25.1 1‐4 weeks | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 25.2 1‐5 months | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined) | 3 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.43, 0.25] |
| 26.1 1‐4 weeks | 2 | 102 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.53, 0.25] |
| 26.2 1‐5 months | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.62, 0.76] |
| 26.3 6 months or longer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Daytime use of rescue beta2 agonists (puffs/day) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 27.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 1‐5 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 27.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Morning use of rescue beta2 agonists (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 28.1 1‐4 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 28.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Night‐time use of rescue beta2 agonists (puffs/night) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 29.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 1‐5 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 29.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Evening use of rescue beta2 agonists (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 30.1 1‐4 weeks | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 30.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Histamine BHR (log 10 PC20 FEV1) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 31.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 1‐5 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 31.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Morning plasma cortisol (nmol/L) | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | 24.32 [‐15.78, 64.41] |
| 32.1 1‐4 weeks | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | 22.29 [‐37.29, 81.86] |
| 32.2 1‐5 months | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 26.0 [‐28.21, 80.21] |
| 32.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 26.84 [‐24.79, 78.48] |
| 33.1 1‐4 weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐254.22, 254.22] |
| 33.2 1‐5 months | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 28.00 [‐24.74, 80.74] |
| 33.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
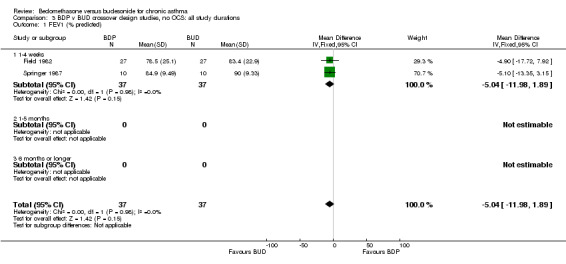
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 1 FEV1 (% predicted).
3.2. Analysis.
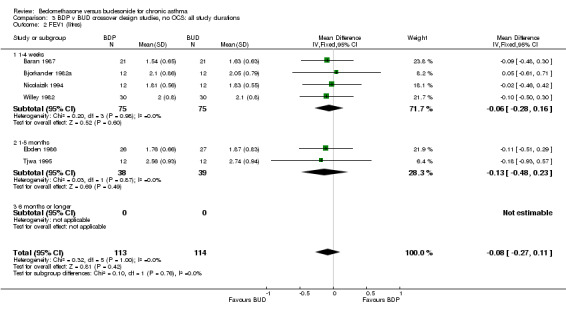
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 2 FEV1 (litres).
3.3. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 3 FEV1 (% predicted, litre measures combined).
3.4. Analysis.
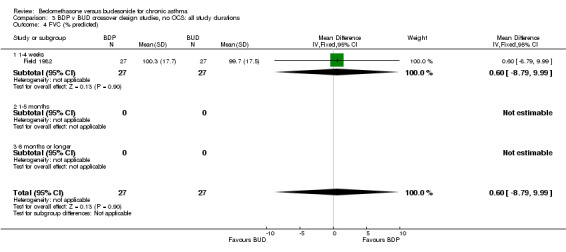
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 4 FVC (% predicted).
3.5. Analysis.
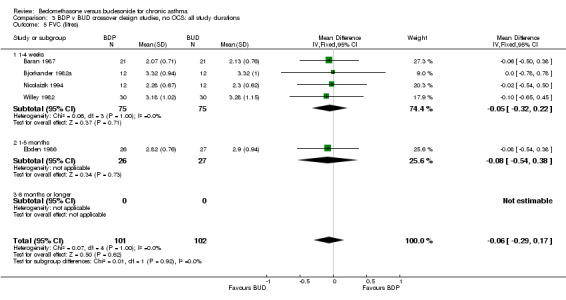
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 5 FVC (litres).
3.6. Analysis.
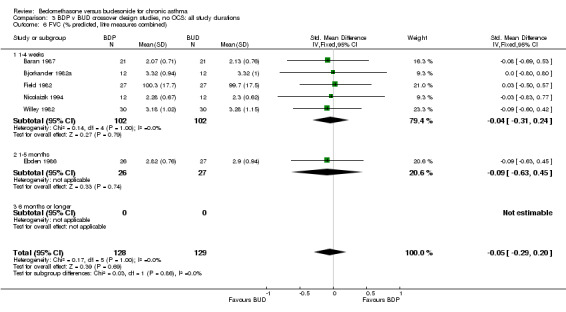
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 6 FVC (% predicted, litre measures combined).
3.7. Analysis.
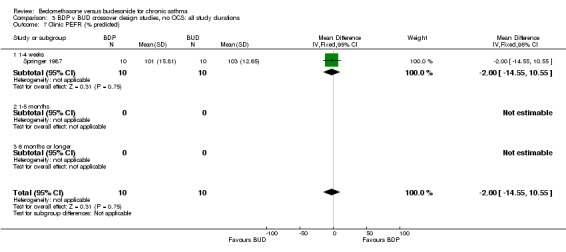
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 7 Clinic PEFR (% predicted).
3.8. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 8 Morning PEFR (% predicted).
3.9. Analysis.
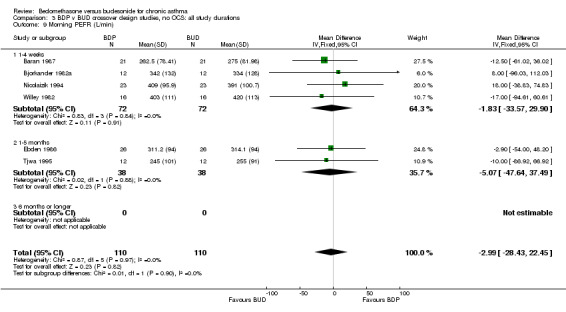
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 9 Morning PEFR (L/min).
3.10. Analysis.
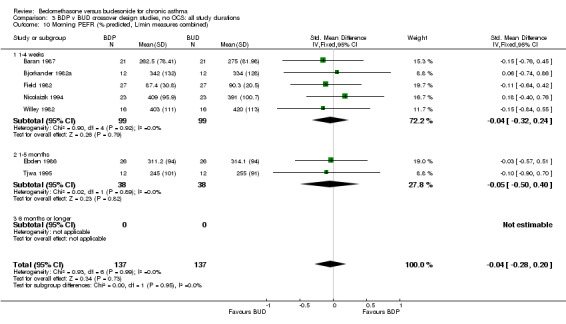
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 10 Morning PEFR (% predicted, L/min measures combined).
3.11. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 11 Evening PEFR (L/min).
3.12. Analysis.
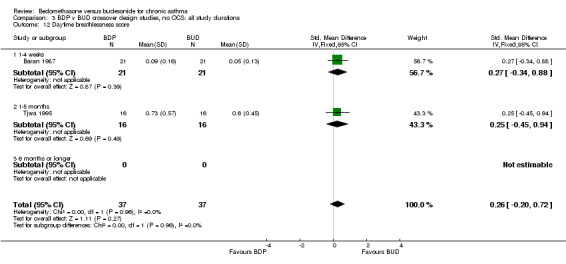
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 12 Daytime breathlessness score.
3.13. Analysis.
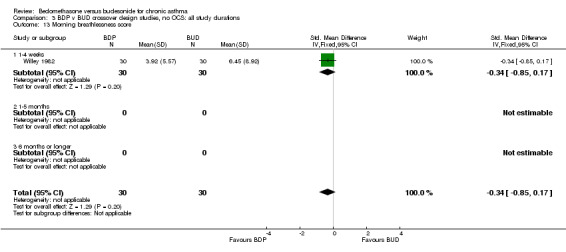
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 13 Morning breathlessness score.
3.14. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 14 Daytime wheeze score.
3.15. Analysis.
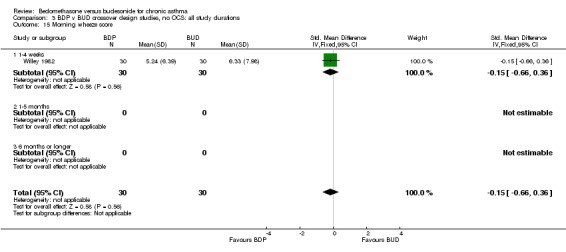
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 15 Morning wheeze score.
3.16. Analysis.
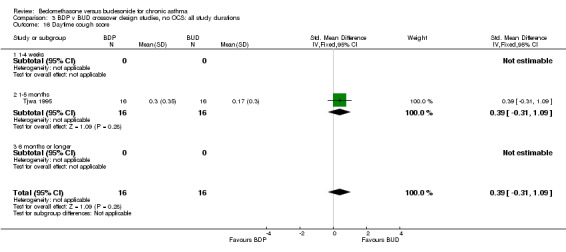
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 16 Daytime cough score.
3.17. Analysis.
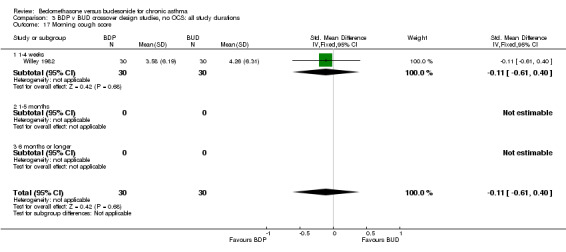
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 17 Morning cough score.
3.18. Analysis.
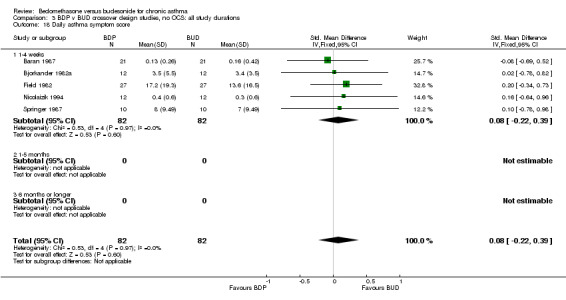
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 18 Daily asthma symptom score.
3.19. Analysis.
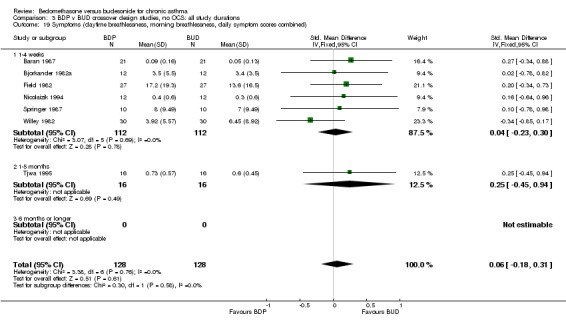
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined).
3.20. Analysis.
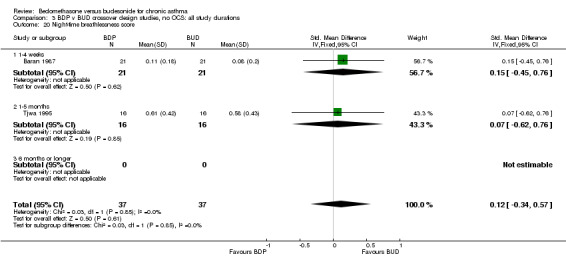
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 20 Night‐time breathlessness score.
3.21. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 21 Evening breathlessness score.
3.22. Analysis.
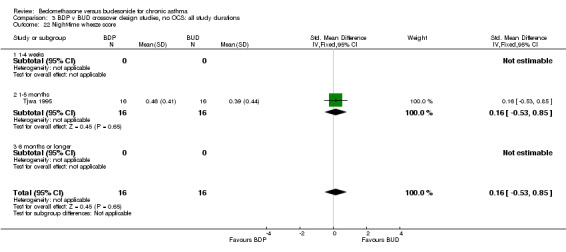
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 22 Night‐time wheeze score.
3.23. Analysis.
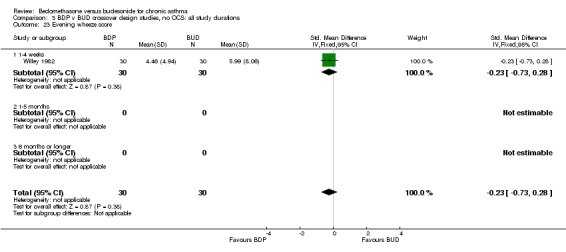
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 23 Evening wheeze score.
3.24. Analysis.
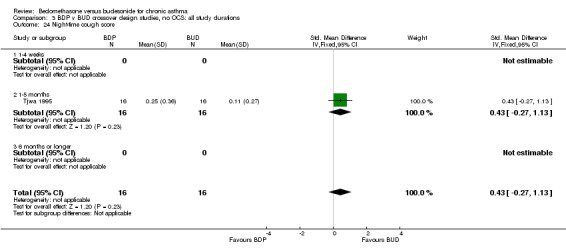
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 24 Night‐time cough score.
3.25. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 25 Evening cough score.
3.26. Analysis.
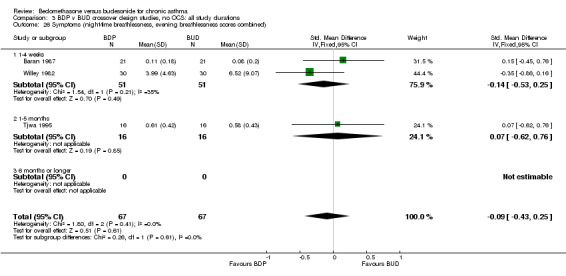
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined).
3.27. Analysis.
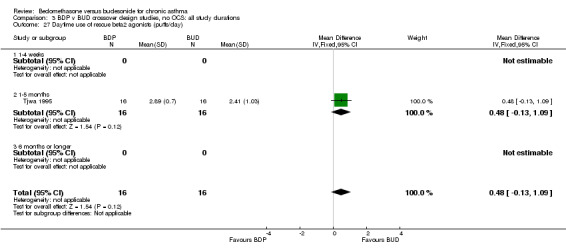
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 27 Daytime use of rescue beta2 agonists (puffs/day).
3.28. Analysis.
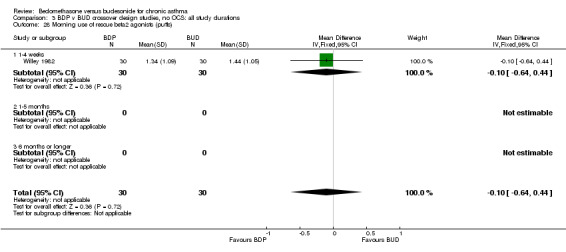
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 28 Morning use of rescue beta2 agonists (puffs).
3.29. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 29 Night‐time use of rescue beta2 agonists (puffs/night).
3.30. Analysis.
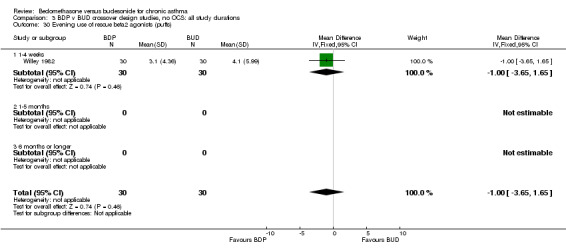
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 30 Evening use of rescue beta2 agonists (puffs).
3.31. Analysis.
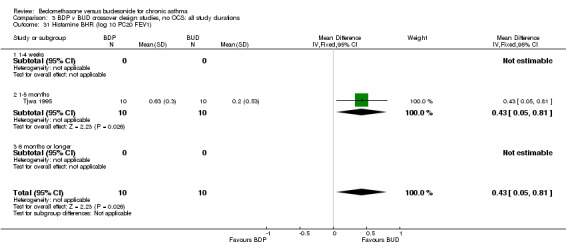
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 31 Histamine BHR (log 10 PC20 FEV1).
3.32. Analysis.
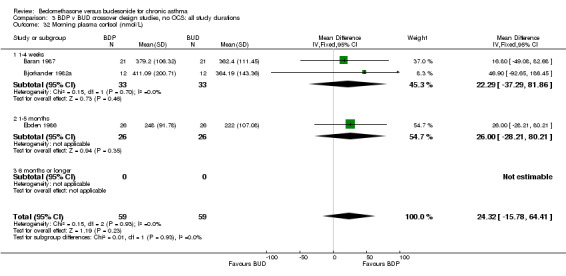
Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 32 Morning plasma cortisol (nmol/L).
3.33. Analysis.

Comparison 3 BDP v BUD crossover design studies, no OCS: all study durations, Outcome 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L).
Comparison 4. BDP v BUD crossover design studies, no OCS: all delivery devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.04 [‐11.98, 1.89] |
| 1.1 MDI v MDI | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐13.35, 3.15] |
| 1.2 MDI v MDI+spacer | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐17.72, 7.92] |
| 1.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (litres) | 6 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.27, 0.11] |
| 2.1 MDI v MDI | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.37, 0.37] |
| 2.2 MDI v MDI+spacer | 3 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 2.3 DPI v DPI | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.93, 0.57] |
| 3 FEV1 (% predicted, litre measures combined) | 7 | 248 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 3.1 MDI v MDI | 3 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.62, 0.34] |
| 3.2 MDI v MDI+spacer | 3 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.47, 0.16] |
| 3.3 DPI v DPI | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.99, 0.62] |
| 4 FVC (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.1 MDI v MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 MDI v MDI+spacer | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐8.79, 9.99] |
| 4.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 FVC (litres) | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.29, 0.17] |
| 5.1 MDI v MDI | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.44, 0.42] |
| 5.2 MDI v MDI+spacer | 3 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.35, 0.20] |
| 5.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 FVC (% predicted, litre measures combined) | 6 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.29, 0.20] |
| 6.1 MDI v MDI | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.58, 0.55] |
| 6.2 MDI v MDI+spacer | 4 | 209 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.33, 0.21] |
| 6.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Clinic PEFR (% predicted) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.1 MDI v MDI | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐14.55, 10.55] |
| 7.2 MDI v MDI+spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Morning PEFR (% predicted) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.1 MDI v MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 MDI v MDI+spacer | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐16.86, 11.06] |
| 8.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Morning PEFR (L/min) | 6 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐2.99 [‐28.43, 22.45] |
| 9.1 MDI v MDI | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 15.70 [‐34.17, 65.58] |
| 9.2 MDI v MDI+spacer | 3 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐9.49 [‐41.54, 22.55] |
| 9.3 DPI v DPI | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐86.92, 66.92] |
| 10 Morning PEFR (% predicted, L/min measures combined) | 7 | 274 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| 10.1 MDI v MDI | 2 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.33, 0.61] |
| 10.2 MDI v MDI+spacer | 4 | 180 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.40, 0.19] |
| 10.3 DPI v DPI | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.90, 0.70] |
| 11 Evening PEFR (L/min) | 5 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐5.47 [‐31.50, 20.56] |
| 11.1 MDI v MDI | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐86.04, 70.04] |
| 11.2 MDI v MDI+spacer | 3 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐3.81 [‐33.70, 26.09] |
| 11.3 DPI v DPI | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐13.00 [‐85.07, 59.07] |
| 12 Daytime breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.20, 0.72] |
| 12.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 MDI v MDI+spacer | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.34, 0.88] |
| 12.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.45, 0.94] |
| 13 Morning breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 13.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 MDI v MDI+spacer | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.85, 0.17] |
| 13.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Daytime wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 14.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 MDI v MDI+spacer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.99] |
| 15 Morning wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 15.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 MDI v MDI+spacer | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 15.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Daytime cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 16.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 MDI v MDI+spacer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 17 Morning cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 17.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 MDI v MDI+spacer | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 17.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Daily asthma symptom score | 5 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.22, 0.39] |
| 18.1 MDI v MDI | 3 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.38, 0.57] |
| 18.2 MDI v MDI+spacer | 2 | 96 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.33, 0.47] |
| 18.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined) | 7 | 256 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.18, 0.31] |
| 19.1 MDI v MDI | 3 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.38, 0.57] |
| 19.2 MDI v MDI+spacer | 3 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.30, 0.33] |
| 19.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.45, 0.94] |
| 20 Night‐time breathlessness score | 2 | 74 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.34, 0.57] |
| 20.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 MDI v MDI+spacer | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.45, 0.76] |
| 20.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.62, 0.76] |
| 21 Evening breathlessness score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 21.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 MDI v MDI+spacer | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.86, 0.16] |
| 21.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Night‐time wheeze score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 22.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 MDI v MDI+spacer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.53, 0.85] |
| 23 Evening wheeze score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 23.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 MDI v MDI+spacer | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.28] |
| 23.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Night‐time cough score | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 24.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 MDI v MDI+spacer | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.27, 1.13] |
| 25 Evening cough score | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 25.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 MDI v MDI+spacer | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.70, 0.32] |
| 25.3 DPI v DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined) | 3 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.43, 0.25] |
| 26.1 MDI v MDI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 MDI v MDI+spacer | 2 | 102 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.53, 0.25] |
| 26.3 DPI v DPI | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.62, 0.76] |
| 27 Daytime use of rescue beta2 agonists (puffs/day) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 27.1 MDI v MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 MDI v MDI+spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 DPI v DPI | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.13, 1.09] |
| 28 Morning use of rescue beta2 agonists (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 28.1 MDI v MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 MDI v MDI+spacer | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 28.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Night‐time use of rescue beta2 agonists (puffs/night) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 29.1 MDI v MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 MDI v MDI+spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 DPI v DPI | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 30 Evening use of rescue beta2 agonists (puffs) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 30.1 MDI v MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 MDI v MDI+spacer | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.65, 1.65] |
| 30.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Histamine BHR (log 10 PC20 FEV1) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 31.1 MDI v MDI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 MDI v MDI+spacer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 DPI v DPI | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 32 Morning plasma cortisol (nmol/L) | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | 24.32 [‐15.78, 64.41] |
| 32.1 MDI v MDI | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 46.90 [‐92.65, 186.45] |
| 32.2 MDI v MDI+spacer | 2 | 94 | Mean Difference (IV, Fixed, 95% CI) | 22.29 [‐19.57, 64.14] |
| 32.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 26.84 [‐24.79, 78.48] |
| 33.1 MDI v MDI | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐254.22, 254.22] |
| 33.2 MDI v MDI+spacer | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 28.00 [‐24.74, 80.74] |
| 33.3 DPI v DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 1 FEV1 (% predicted).
4.2. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 2 FEV1 (litres).
4.3. Analysis.
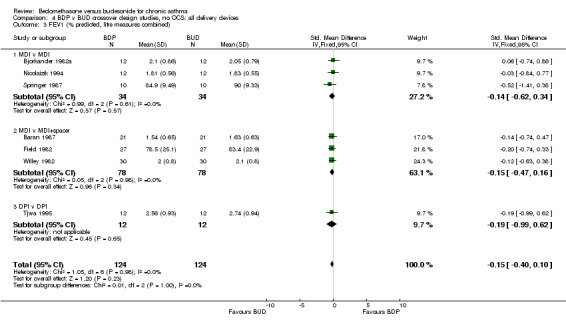
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 3 FEV1 (% predicted, litre measures combined).
4.4. Analysis.
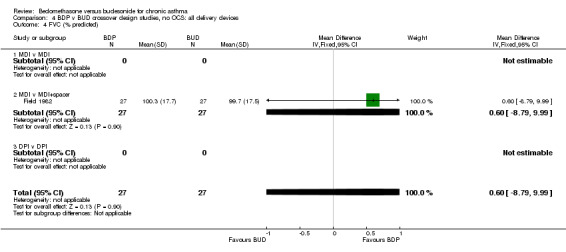
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 4 FVC (% predicted).
4.5. Analysis.
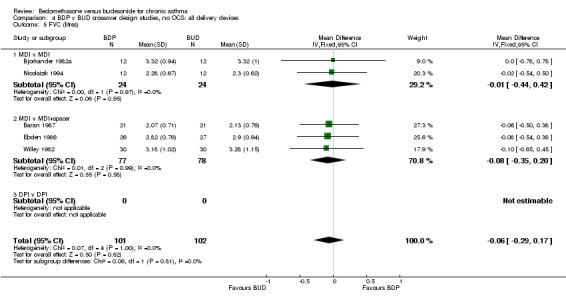
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 5 FVC (litres).
4.6. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 6 FVC (% predicted, litre measures combined).
4.7. Analysis.
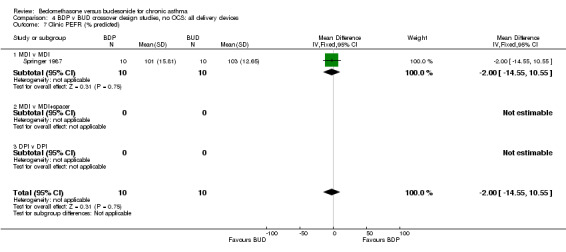
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 7 Clinic PEFR (% predicted).
4.8. Analysis.
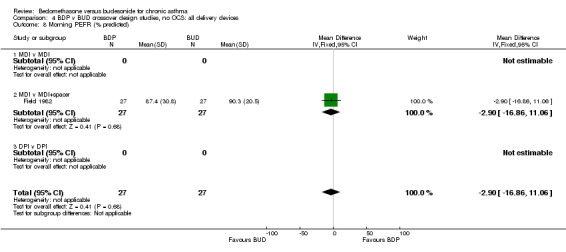
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 8 Morning PEFR (% predicted).
4.9. Analysis.
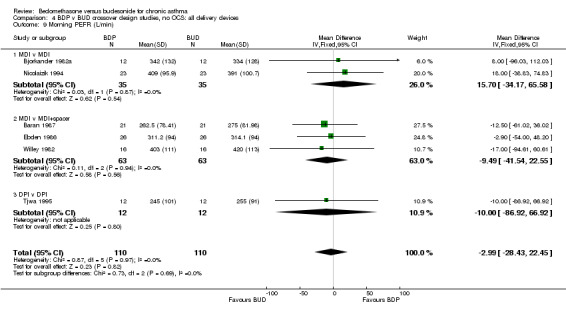
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 9 Morning PEFR (L/min).
4.10. Analysis.
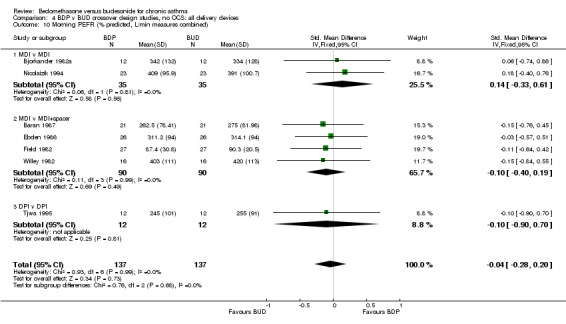
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 10 Morning PEFR (% predicted, L/min measures combined).
4.11. Analysis.
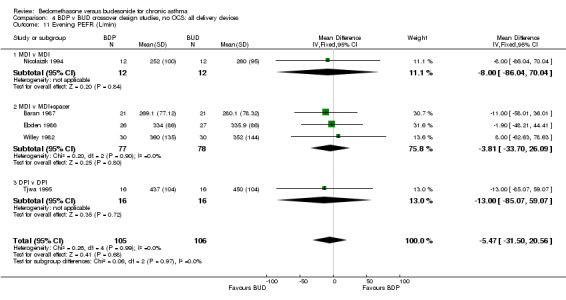
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 11 Evening PEFR (L/min).
4.12. Analysis.
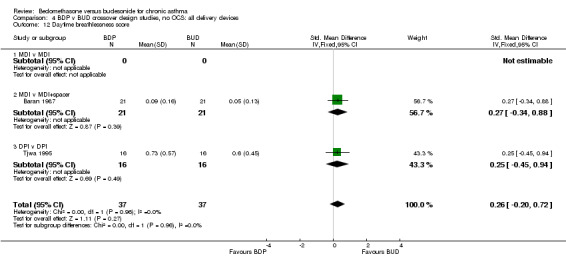
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 12 Daytime breathlessness score.
4.13. Analysis.
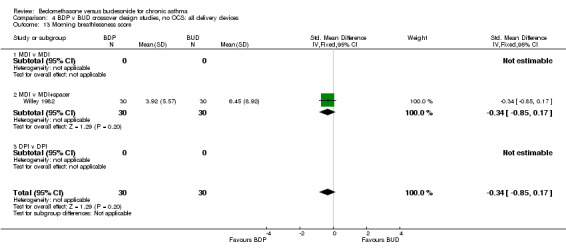
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 13 Morning breathlessness score.
4.14. Analysis.
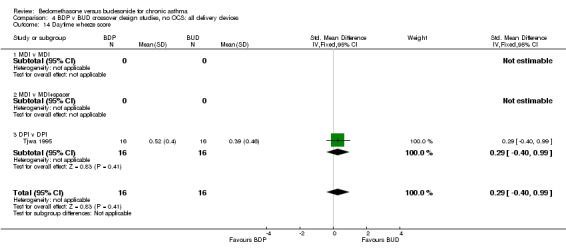
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 14 Daytime wheeze score.
4.15. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 15 Morning wheeze score.
4.16. Analysis.
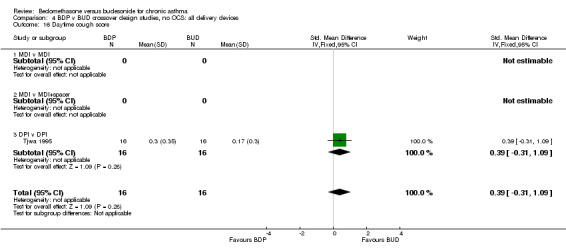
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 16 Daytime cough score.
4.17. Analysis.
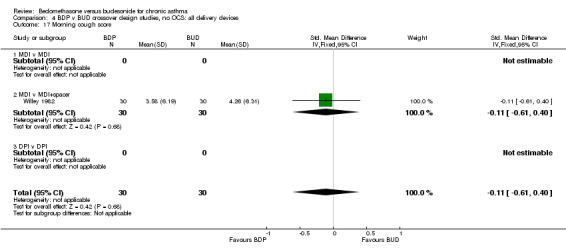
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 17 Morning cough score.
4.18. Analysis.
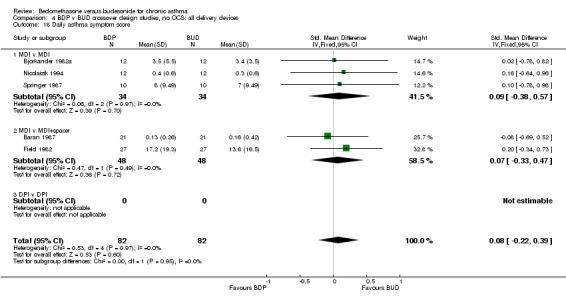
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 18 Daily asthma symptom score.
4.19. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 19 Symptoms (daytime breathlessness, morning breathlessness, daily symptom scores combined).
4.20. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 20 Night‐time breathlessness score.
4.21. Analysis.
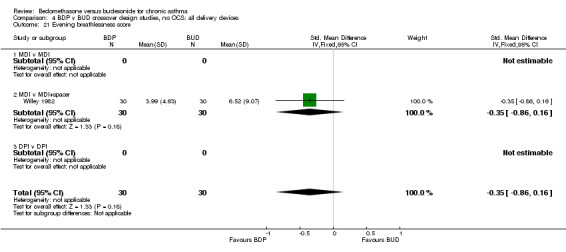
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 21 Evening breathlessness score.
4.22. Analysis.
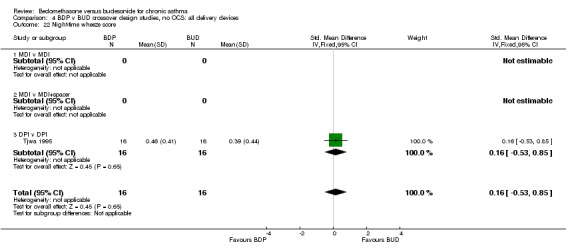
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 22 Night‐time wheeze score.
4.23. Analysis.
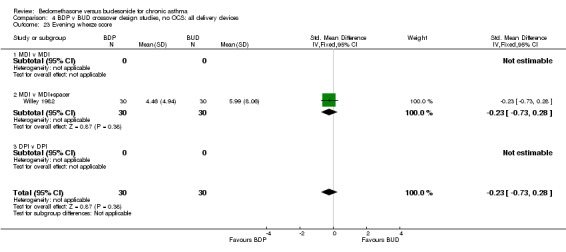
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 23 Evening wheeze score.
4.24. Analysis.
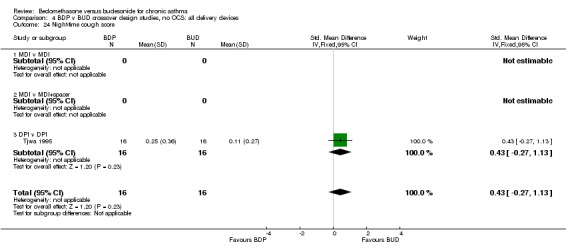
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 24 Night‐time cough score.
4.25. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 25 Evening cough score.
4.26. Analysis.
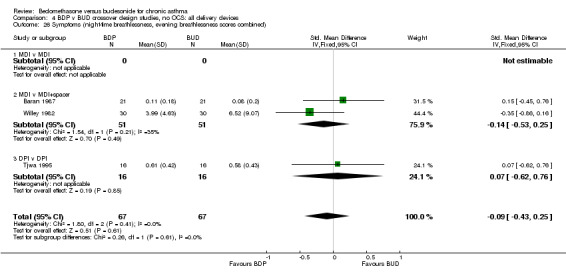
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 26 Symptoms (night‐time breathlessness, evening breathlessness scores combined).
4.27. Analysis.
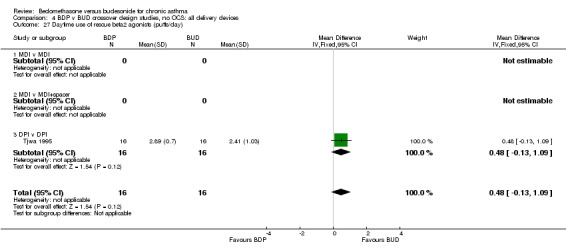
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 27 Daytime use of rescue beta2 agonists (puffs/day).
4.28. Analysis.
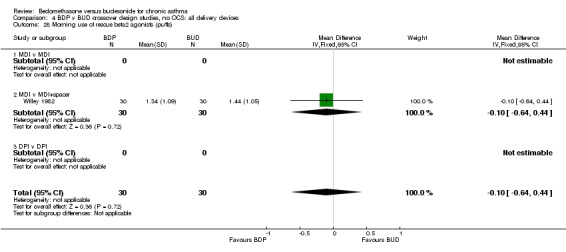
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 28 Morning use of rescue beta2 agonists (puffs).
4.29. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 29 Night‐time use of rescue beta2 agonists (puffs/night).
4.30. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 30 Evening use of rescue beta2 agonists (puffs).
4.31. Analysis.

Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 31 Histamine BHR (log 10 PC20 FEV1).
4.32. Analysis.
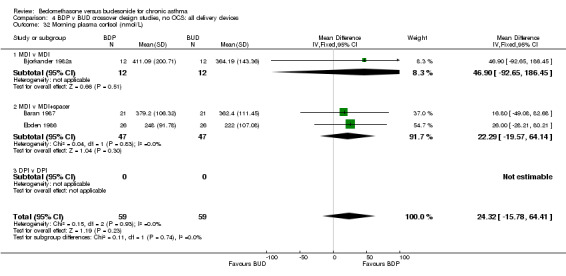
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 32 Morning plasma cortisol (nmol/L).
4.33. Analysis.
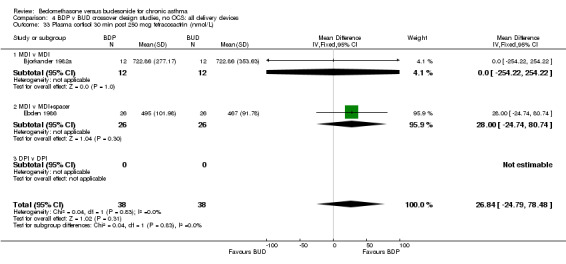
Comparison 4 BDP v BUD crossover design studies, no OCS: all delivery devices, Outcome 33 Plasma cortisol 30 min post 250 mcg tetracosactrin (nmol/L).
Comparison 5. BDP v BUD parallel design dose‐down titration studies, no OCS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily dose inhaled corticosteroid (mcg) | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | 444.22 [332.46, 555.98] |
| 1.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 1‐5 months | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 470.00 [315.00, 625.00] |
| 1.3 6 months or longer | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 416.30 [255.01, 577.59] |
| 2 FEV1 (litres) | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.23, 0.20] |
| 2.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 1‐5 months | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.30, 0.20] |
| 2.3 6 months or longer | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.33, 0.47] |
| 3 Morning PEFR | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐18.0 [‐54.76, 18.76] |
| 3.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 1‐5 months | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐18.0 [‐54.76, 18.76] |
| 3.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Evening PEFR (L/min) | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐49.29, 33.29] |
| 4.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 1‐5 months | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐49.29, 33.29] |
| 4.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Daily PEFR (L/min) | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐58.72, 59.92] |
| 5.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 1‐5 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 6 months or longer | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐58.72, 59.92] |
| 6 Rescue beta2 agonist use (puffs/day) | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.43, 1.43] |
| 6.1 1‐4 weeks | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 1‐5 months | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.43, 1.43] |
| 6.3 6 months or longer | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Withdrawal due to asthma exacerbation (No. of patients) | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.44, 7.10] |
| 7.1 1‐4 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 1‐5 months | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.44, 7.10] |
| 7.3 6 months or longer | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Sore throat/erythematous throat/Candidiasis (No. of patients) | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.92, 2.37] |
| 8.1 1‐4 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 1‐5 months | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.92, 2.37] |
| 8.3 6 months or longer | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Hoarseness (No. of patients) | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.74, 1.76] |
| 9.1 1‐4 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 1‐5 months | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.61, 1.55] |
| 9.3 6 months or longer | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.90 [0.93, 16.27] |
5.1. Analysis.
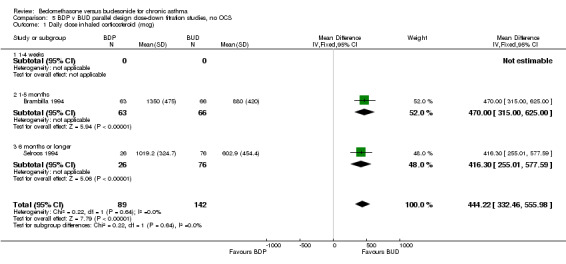
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 1 Daily dose inhaled corticosteroid (mcg).
5.2. Analysis.
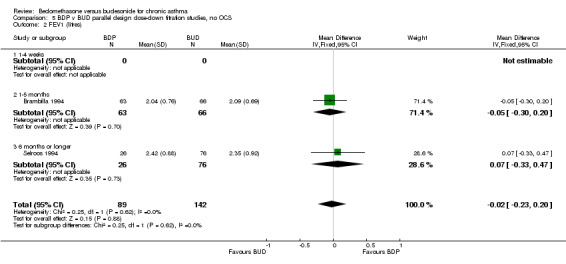
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 2 FEV1 (litres).
5.3. Analysis.
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 3 Morning PEFR.
5.4. Analysis.
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 4 Evening PEFR (L/min).
5.5. Analysis.
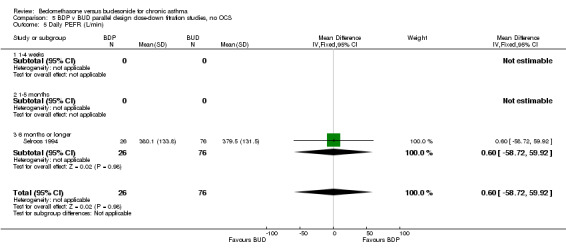
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 5 Daily PEFR (L/min).
5.6. Analysis.
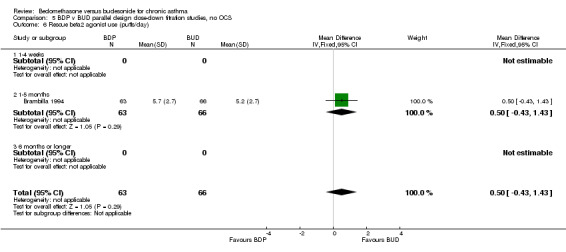
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 6 Rescue beta2 agonist use (puffs/day).
5.7. Analysis.
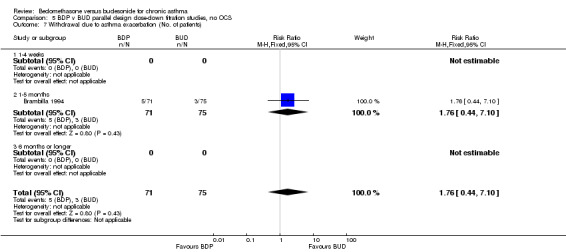
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 7 Withdrawal due to asthma exacerbation (No. of patients).
5.8. Analysis.
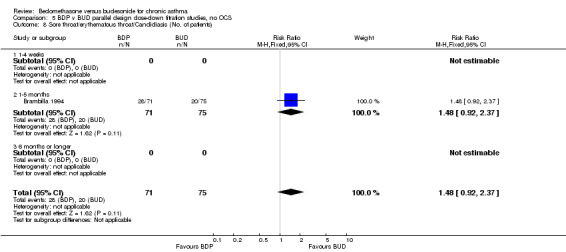
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 8 Sore throat/erythematous throat/Candidiasis (No. of patients).
5.9. Analysis.
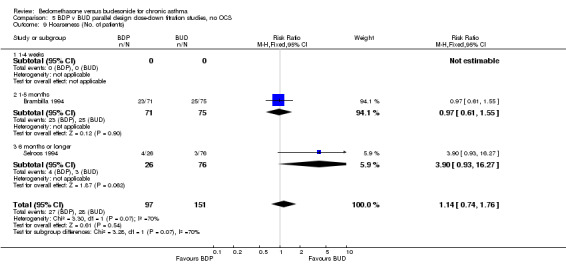
Comparison 5 BDP v BUD parallel design dose‐down titration studies, no OCS, Outcome 9 Hoarseness (No. of patients).
Comparison 6. BDP v BUD crossover design, OCS treated patients, non OCS sparing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Morning PEFR (L/min) | 3 | 144 | Mean Difference (IV, Fixed, 95% CI) | 3.15 [‐30.72, 37.01] |
| 2 Evening PEFR (L/min) | 3 | 144 | Mean Difference (IV, Fixed, 95% CI) | 4.68 [‐29.03, 38.38] |
| 3 Daytime breathlessness score | 3 | 144 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.36, 0.29] |
| 4 Night‐time breathlessness score | 3 | 144 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 5 Sleeping difficulty due to asthma score | 3 | 144 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.25, 0.40] |
| 6 Rescue beta2 agonist use (puffs/d) | 3 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.70, 0.96] |
6.1. Analysis.
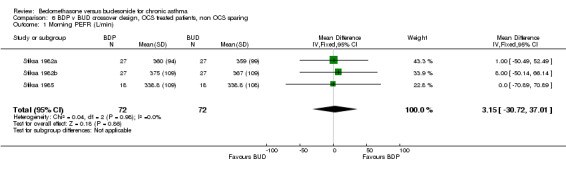
Comparison 6 BDP v BUD crossover design, OCS treated patients, non OCS sparing, Outcome 1 Morning PEFR (L/min).
6.2. Analysis.

Comparison 6 BDP v BUD crossover design, OCS treated patients, non OCS sparing, Outcome 2 Evening PEFR (L/min).
6.3. Analysis.
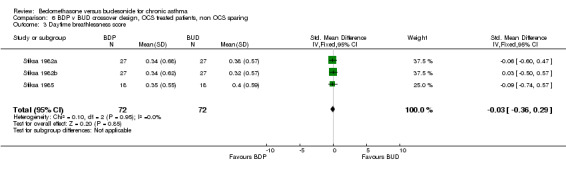
Comparison 6 BDP v BUD crossover design, OCS treated patients, non OCS sparing, Outcome 3 Daytime breathlessness score.
6.4. Analysis.
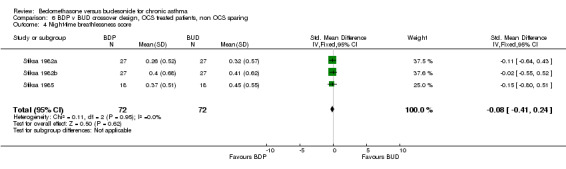
Comparison 6 BDP v BUD crossover design, OCS treated patients, non OCS sparing, Outcome 4 Night‐time breathlessness score.
6.5. Analysis.

Comparison 6 BDP v BUD crossover design, OCS treated patients, non OCS sparing, Outcome 5 Sleeping difficulty due to asthma score.
6.6. Analysis.
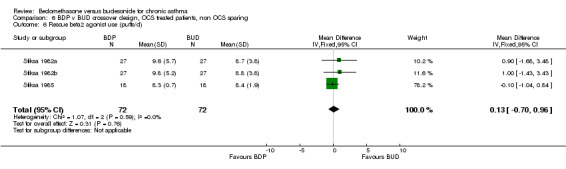
Comparison 6 BDP v BUD crossover design, OCS treated patients, non OCS sparing, Outcome 6 Rescue beta2 agonist use (puffs/d).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baran 1987.
| Methods | Setting: Belgium, residential centre for asthmatic children Length of intervention: 3 weeks Design: crossover, no washout Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: no tabulated demographic data Jadad score: 3 | |
| Participants | 21 children: 15M 6F Age range: 4‐14 Inclusion criteria: Children suffering from chronic asthma and using inhaled corticosteroids to control their disease No other criteria given Exclusion criteria: None stated | |
| Interventions | BDP: 50 mcg 2puffs 2xdaily (200 mcg/d) via MDI BUD: 50 mcg 2puffs 2xdaily (200 mcg/d) via Inhalet spacer |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daytime breathlessness score Night‐time breathlessness score Daily cough score Sleeping difficulties score 8 am plasma cortisol Incidence of oropharyngeal Candidiasis Both active treatment arms demonstrated significant improvements compared to non‐randomised placebo arm for morning PEFR, evening PEFR and symptom scores. | |
| Notes | No reply from author to clarify details of randomisation method Not stated if carryover effects were tested for. Significant improvements in morning PEFR, evening PEFR and symptoms compared to non‐randomised placebo treatment period at end of study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bisgaard 1988.
| Methods | Setting: multicentre Denmark, hospital outpatient clinic Length of intervention: 3 months Design: parallel group, dose escalation Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 41 children Age range: 7‐15 years (of those completing trial) Inclusion criteria: Children with bronchial asthma "airway obstruction reversible by > 20%" All children recruited were using regular beta2 agonists. None of the children recruited had used oral steroids within the previous 6 months, but this was not an explicit exclusion criterion. Exclusion criteria: None stated | |
| Interventions | BDP: step‐wise increased doses (200, 400, 800 mcg daily) via aerosol MDI BUD: step‐wise increased doses (200, 400, 800 mcg daily) via aerosol MDI |
|
| Outcomes | 24 hour urinary free cortisol excretion (corrected for creatinine) Plasma cortisol 30 min post 125 mcg iv tetracosactrin (nmol/L) Morning PEFR (% predicted) Evening PEFR (% predicted) Rescue beta2 agonist use Withdrawal due to asthma exacerbation (No. of patients) | |
| Notes | Dose escalation design: patients were randomised to receive either BDP or BUD. Successive doses (200 mcg/d, 400 mcg/d, 800 mcg/d) were given for 4 week periods in succession, with no washout between each dose. Patients were not randomised to receive each dose. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bjorkander 1982a.
| Methods | Setting: Sweden, hospital outpatient clinic Length of intervention: 2 weeks Design: crossover, 1 week washout Randomised: yes, method not stated Allocation concealment: unclear Masking: not stated Excluded: not stated Withdrawals: none, but 5 patients excluded from analysis due to requirement for oral steroids during washout period Baseline characteristics: no details Jadad score: 2 | |
| Participants | 17 adults randomised, 12 completed the study: 3M 9F Age range: 27‐66 years Inclusion criteria: Stable chronic asthma of moderate severity Demonstrated requirement for treatment with BDP over last year and were steroid dependent i.e. relapsed if steroid medication was withdrawn % predicted FEV1 70 or greater Exclusion criteria: None stated | |
| Interventions | BDP: 100mcg 1 puff 4xdaily (400mcg/day) via MDI BUD: 100mcg 1 puff 4xdaily (400mcg/day) via MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daily use of beta2 agonists (puffs/day) Daily asthma symptom score Morning plasma cortisol Morning plasma cortisol 30 min post 250mg tetracosactrin | |
| Notes | No reply from author to clarify details of randomisation method Published paper reported two studies. The second study was excluded (see Excluded studies). Significantly higher FEV1 and morning PEFR in BDP treated subjects, and higher evening PEFR in BUD treated subjects, compared to placebo washout | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Boe 1989.
| Methods | Setting: multicentre study Sweden, hospital outpatient clinic Length of intervention: 4 weeks Design: crossover, no washout Randomisation: yes, random number table Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no details Jadad score: 4 | |
| Participants | 128 adults: 67M 61F Age range 18‐77 years Inclusion criteria: Asthma as defined by ATS criteria Requirement for inhaled steroids in a dose range of 400‐800 mcg/d Exclusion criteria: Cardiovascular disease or diabetes mellitus | |
| Interventions | Four treatment allocations: 1) BDP 200 micrograms 2xdaily (400 mcg/day) 2) BUD 200 micrograms 2xdaily (400 mcg/day) 3) BDP 500 micrograms 2xdaily (1000 mcg/day) 4) BUD 400 micrograms 2xdaily (800 mcg/day) All via MDI | |
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daily asthma symptom score | |
| Notes | No reply from author to clarify if allocation concealment was employed Not stated if carryover effects were tested for. Significant improvements in morning/evening PEFR, beta2 agonist use, symptoms in BDP and BUD groups compared to non‐randomised placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Brambilla 1994.
| Methods | Setting: multicentre study France, hospital outpatient clinic Length of intervention: 12 weeks Design: parallel group, dose down‐titration Randomisation: yes, method not stated Allocation concealment: unclear Masking: none Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 146 adults Inclusion criteria: Recurrent acute exacerbations of breathlessness and wheezing 15% or greater variability in FEV1 Bronchial responsiveness to methacholine or histamine (PD20 FEV1 2mg or less) Receiving inhaled BDP 1000‐2000 mcg/d for at least 6 months Exclusion criteria: Airways disease other than asthma Smoker > 5 packs cigarettes per year Significant co‐existent disease | |
| Interventions | BDP: 800 to 2000 mcg total daily dose via MDI BUD: 800 to 1600 mcg total daily dose via Turbohaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Number of puffs of inhaled corticosteroid per day Number asthma exacerbations/week Number night awakenings requiring beta2 agonist use/week Daily beta2 agonist use (puffs/day) Withdrawal due to asthma exacerbation Incidence of local oral side effects | |
| Notes | Reply from author but unable to clarify details of randomisation procedure. Dose down titration study design: patients were randomised to receive either BDP or BUD at a dose considered to be equivalent to their pre‐study dose of BDP. BDP 250 mcg/puff via MDI was considered equivalent to BUD 200 mcg/actuation via Turbuhaler DPI. During the study daily dose of ICS was reduced according to pre‐defined criteria based on experience of asthma symptoms in the week prior to assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dal Negro 1997.
| Methods | Setting: multicentre study Italy, hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: not stated Excluded: not stated Withdrawals: stated Baseline characteristics: no demographic data presented Jadad score: 1 | |
| Participants | 79 adult adults Inclusion criteria: Adult patients with stable asthma (not defined) Regular treatment with BDP 1000 mcg/d No further details Exclusion criteria: No details | |
| Interventions | BDP: 200 mcg 4xdaily (800 mcg/d) via Pulvinal DPI BUD: 200 mcg 4xdaily (800 mcg/d) via Turbuhaler DPI |
|
| Outcomes | FEV1 FVC FEF25‐75 MEF50 Morning PEFR Asthma symptom score | |
| Notes | Study in abstract form only. No reply from trial sponsors to clarify details of randomisation procedure, further inclusion/exclusion criteria, patient withdrawals or outcomes data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ebden 1986.
| Methods | Setting: Wales, hospital outpatient clinic Length of intervention: 6 weeks Design: crossover, no washout Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no details Jadad score: 3 | |
| Participants | 28 adults: 20M 8F Age range: 19‐72 years Inclusion criteria Patients with chronic asthma whose "attending physician considered their asthma to be poorly controlled and require further treatment" 15% reversibility in PEFR after inhaled beta2 agonist Good inhaler technique Exclusion criteria: Use of oral steroids within 2 months of the study | |
| Interventions | BDP: 250 mcg 3puffs 2xdaily (1500 mcg/day) via MDI BUD: 200 mcg 4 puffs 2xdaily (1600 mcg/day) via MDI+ collapsible spacer |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daily beta2 agonist (puffs/day) Daytime wheeze score Morning serum cortisol Serum cortisol 30 minutes post 250mcg tetracosactrin | |
| Notes | Reply from author but unable to clarify details of randomisation procedure or if carryover effects were excluded. Majority of patients demonstrated a significant improvement in at least one efficacy outcome compared to baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Field 1982.
| Methods | Setting: UK, paediatric outpatient clinic Length of intervention: 4 weeks Design: crossover, no washout Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no details Jadad score: 3 | |
| Participants | 31 children: 19M 12F Age range: 4‐14 years Inclusion criteria: "fairly severe asthma requiring regular inhaled corticosteroids" Exclusion criteria: not stated | |
| Interventions | BDP: 400 mcg/day in 4 divided doses children remained on delivery device used before trial (either aerosol MDI or Rotahaler DPI) BUD: 200 mcg 2xdaily (400 mcg/day) via aerosol MDI with tube spacer |
|
| Outcomes | FEV1 (% predicted) FVC (% predicted) Morning PEFR (% predicted) Evening PEFR (% predicted) FEF 50 (% predicted) FEF 25 (% predicted) Daily symptom score | |
| Notes | Reply from authors but unable to clarify details of randomisation procedure. Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Greefhorst 1992.
| Methods | Setting: muticentre study, The Netherlands, primary care or hospital outpatient setting unclear Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Masking: none Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic data presented Jadad score: 1 | |
| Participants | 145 adults Inclusion criteria: Adult asthmatic patients already treated with inhaled corticosteroids No further details Exclusion criteria: No details | |
| Interventions | BDP: 200 mcg 2xdaily (400 mcg/d) via Rotahaler DPI BUD: 200 mcg 2xdaily (400 mcg/d) via Turbuhaler DPI |
|
| Outcomes | Morning PEFR Evening PEFR Asthma symptom score Rescue beta2 agonist use | |
| Notes | Study in abstract form only. No reply from author to clarify details of randomisation method used, further inclusion/exclusion criteria, patient withdrawals or data for outcomes. Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hamalainen 1998.
| Methods | Setting: multicentre study Germany, hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Masking: none Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic data presented Jadad score: 1 | |
| Participants | 79 adults: 39M 40F Mean (SD) age: 51 (15) years Inclusion criteria: Adults with asthma currently treated with inhaled BDP 1000 mcg/day via MDI Non smokers No further details Exclusion criteria: No details | |
| Interventions | BDP: 200 mcg 4 actuations/day (800 mcg/d) via Easyhaler DPI BUD: 200 mcg 4 actuations/day (800 mcg/d) via Turbuhaler DPI |
|
| Outcomes | Morning PEFR Morning serum cortisol | |
| Notes | Study in abstract form only. No reply from author to clarify details of randomisation method used, further inclusion/exclusion criteria, patient withdrawals or data for outcomes. Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Keelan 1984.
| Methods | Setting: Ireland, hospital outpatient clinic Length of intervention: 4 weeks Design: crossover, no washout Randomisation: yes, method not stated Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: no tabulated demographic data presented Jadad score: 3 | |
| Participants | 36 adults: 21M 15F Age range: 15‐65 years Inclusion criteria: Adults with asthma insufficiently controlled by bronchodilators and /or anti‐allergic therapy and who required 400 micrograms of BDP per day. Exclusion criteria: Bronchopulmonary disease other than asthma, or other disease Use of oral steroids or ACTH preparation within last month | |
| Interventions | BDP: 50 mcg 2 puffs 4xdaily (400 mcg/day) via MDI and placebo via MDI+spacer BUD: 50 mcg 2 puffs 4xdaily (400 mcg/day) via MDI+ spacer and placebo via MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Asthma symptom score Beta2 agonist use Serum cortisol (time not specified) | |
| Notes | No reply from author to clarify details of randomisation procedure. Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Micheletto 1997.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: unclear Excluded: not stated Withdrawals: not stated Baseline characteristics: no details Jadad score: 1 | |
| Participants | 32 adults Age range: not stated Inclusion criteria: Adult patients with stable asthma receiving inhaled BDP 1000 mcg/day No other details Exclusion criteria: Not stated | |
| Interventions | BDP: 200 mcg 4xdaily (800 mcg/day) via Pulvinal DPI BUD: 200 mcg 4xdaily (800 mcg/day) via Turbuhaler DPI |
|
| Outcomes | FEV1 FVC Clinic PEFR FEF 25‐75 Morning PEFR Evening PEFR Daily beta2 agonist use Serum ECP Morning serum cortisol | |
| Notes | Study in abstract form only. No reply from trial sponsors to clarify details of randomisation procedure, further inclusion/exclusion criteria, patient withdrawals or data for outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nicolaizik 1994.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention: 2 weeks Design: crossover, no washout Randomisation: yes, method not stated Allocation concealment: unclear Masking: none Excluded: not stated Withdrawals: stated Baseline characteristics: no tabulated demographic data presented Jadad score: 2 | |
| Participants | 14 children: 11M 3F Age range: 8 to 14 years Inclusion criteria: Asthma inadequately controlled on inhaled sodium cromoglycate and beta2 agonists and/or optimal doses of slow release oral theophyllines Reliable in medication compliance, diary card recording, use of MDI Exclusion criteria: Any steroid medication within the last month or regular corticosteroid treatment in the last 12 months. Significant bronchopulmonary disease other than asthma | |
| Interventions | BDP: 50 mcg 4 puffs 2xdaily (400 mcg/day) via MDI BUD: 50 mcg 4 puffs 2xdaily (400 mcg/day) via MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daily symptom score Weekly use of beta2 agonists Bronchial responsiveness to histamine PC20 FEV1, PD20 FEV1) Serum cortisol (area under curve for 12 hours, 20min interval samples) Serum growth hormone (area under curve for 12 hours, 20min samples) 12pm serum ACTH 8am serum ACTH 24 hour urinary free cortisol | |
| Notes | Reply from authors but unable to clarify details of randomisation procedure Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pedersen 1988.
| Methods | Setting: Denmark, hospital outpatient clinic Length of intervention: 6 weeks Design: crossover, no washout Randomisation: yes, computer generated algorithm Allocation concealment: yes, using sealed envelopes Masking: none Excluded: not stated Withdrawals: stated Baseline characteristics: no tabulated demographic data presented Jadad score: 3 | |
| Participants | 31 children: 15M 16F Age range: 5‐15 years Inclusion criteria: Children with asthma receiving high dose inhaled corticosteroids (either BDP or BUD) Exclusion criteria: Receipt of oral or parenteral steroids in last 6 months | |
| Interventions | BDP: total daily dose 800‐1200 mcg via either aerosol MDI or MDI+spacer BUD: total daily dose 800‐1200 mcg via either aerosol MDI or MDI+spacer Children continued with the same dose of inhaled steroid throughout the trial, which was their dose at baseline on entering the study |
|
| Outcomes | 24 hour urinary free cortisol | |
| Notes | Author confirmed method of random order generation and use of allocation concealment. Carryover effects were tested for and excluded. Children continued using the delivery device that they had been using prior to study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Petrie 1990.
| Methods | Setting: multicentre study UK, hospital outpatient clinic Length of intervention: 4 weeks Design: crossover, no washout Randomisation: yes, method not stated Allocation concealment: unclear Masking: none Excluded: stated Withdrawals: stated Baseline characteristics: no tabulated demographic data presented Jadad score: 2 | |
| Participants | 40 adults Age range of study completers: 26‐76 years Inclusion criteria: Adults with chronic stable asthma 15% or greater reversibility in PEFR following beta2 agonists or > 20% variability in daily PEFR during 2 week run in period All patients receiving 800‐1600 mcg inhaled BDP or BUD on entry to study. Exclusion criteria: Respiratory tract infection within last 2 weeks | |
| Interventions | BDP: 100 mcg 4puffs 2xdaily ( 800mcg/day) via Diskhaler DPI BUD: 200 mcg 2 puffs 2xdaily (800 mcg/day) via Turbohaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Daily beta2 agonist use | |
| Notes | No reply from author to clarify details of randomisation procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rafferty 1985.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention: variable Design: crossover Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no data regarding baseline spirometry of subjects, wide range for duration of asthma, duration of oral steroid use and inhaled steroid use. Jadad score: 3 | |
| Participants | 40 adults randomised, 26 completed study: 12 M 14F Age range: 23‐72 years Inclusion criteria: "Severe chronic asthma", being treated with at least 5mg oral prednisolone per day and inhaled BDP 400 mcg daily for at least 9 months Exclusion criteria: Any patient whose dose of oral prednisolone had been adjusted in the last 3 months | |
| Interventions | 1. BDP: 200 mcg 1puff 4xdaily (800 mcg/d) via MDI 2. BUD 200 mcg 2 puffs 2xdaily (800 mcg/d) via MDI+Inhalet spacer 3. BUD 200 mcg 4 puffs 1xdaily (800 mcg/d) via MDI+Inhalet spacer |
|
| Outcomes | Reduction in daily prednisolone dose (mg/d) | |
| Notes | No reply from author to clarify details of randomisation procedure or regimen for tapering prednisolone dose. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Selroos 1994.
| Methods | Setting: Finland, hospital outpatient clinic Length of intervention: 2 years Design: parallel group, dose down titration Randomisation: yes, computer randomisation algorithm Allocation concealment: unclear Masking: none Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 102 adults: 38M 74F Inclusion criteria: Not stated explicitly but study included adult asthmatics who required moderate to high doses of inhaled BDP Excluson criteria: None stated | |
| Interventions | BDP: variable dose via aerosol MDI + Volumatic spacer BUD: variable dose via Turbuhaler DPI |
|
| Outcomes | Daily dose inhaled corticosteroid (mg) FEV1 FEV1 (% predicted) Daily PEFR Daily PEFR (% predicted) Incidence of local oral side‐effects | |
| Notes | Author confirmed method of random order generation. Dose down titration study design: two year 'run‐in' period during which time all patients had their daily dose of BDP down‐titrated to the minimal dose effective in controlling symptoms. However there was no pre‐defined scheme based on objective measures of control for doing this. During study dose down titration was attempted if patient was a) completely or almost completely symptom free and b) FEV1 and PEFR were no lower than at previous assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Springer 1987.
| Methods | Setting: Israel, paediatric outpatient clinic Length of intervention: 4 weeks Design: crossover, no washout Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no tabulated demographic data presented Jadad score: 3 | |
| Participants | 10 children Age range: 9‐15 Inclusion criteria: Asthma dependant on treatment with inhaled steroids. Exclusion criteria: Use of oral steroids in the previous month. | |
| Interventions | BDP: 50 mcg 4 puffs 4xdaily (400 mcg/d) BUD: 50 mcg 4 puffs 4xdaily (400 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) PEFR (% predicted) Daily symptom score 24 hour urinary tetrahydrocortisone glucosiduronate 24 hour urinary tetrahydrocortisol glucosiduronate | |
| Notes | Reply from authors but unable to clarify details of randomisation procedure Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Stiksa 1982a.
| Methods | Setting: Sweden, hospital outpatient clinic Length of intervention period: 2 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes Design: crossover, no washout Masking: none Excluded: not stated Withdrawals: stated (none) Baseline characteristics: no baseline demographic data presented Jadad score: 3 | |
| Participants | 27 adults: 19M 8F Age range: 19‐76 years Inclusion criteria: Asthmatics with 'severe steroid‐dependent' asthma Exclusion criteria: Not stated | |
| Interventions | 1. BDP 200 mcg 4xdaily (800 mcg/d) 2. BUD 200 mcg 4xdaily (800 mcg/d) Delivery device: MDI |
|
| Outcomes | Morning PEFR Evening PEFR Daytime breathlessness score Night‐time breathlessness score Sleeping difficulty due to asthma score Rescue beta2 agonist use | |
| Notes | Author confirmed randomisation method and use of allocation concealment. Author also confirmed that all enrolled patients were oral steroid dependent and that there were no withdrawals from the study. Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Stiksa 1982b.
| Methods | Setting: Sweden, hospital outpatient clinic Length of intervention period: 2 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes Design: crossover, no washout Masking: none Excluded: not stated Withdrawals: stated (none) Baseline characteristics: no baseline demographic data presented Jadad score: 3 | |
| Participants | 27 adults: 19M 8F Age range: 19‐76 years Inclusion criteria: Asthmatics with 'severe steroid‐dependent' asthma Exclusion criteria: Not stated | |
| Interventions | 1. BDP 400 mcg 2xdaily (800 mcg/d) 2. BUD 400 mcg 2xdaily (800 mcg/d) Delivery device: MDI |
|
| Outcomes | Morning PEFR Evening PEFR Daytime breathlessness score Night‐time breathlessness score Sleeping difficulty due to asthma score Rescue beta2 agonist use | |
| Notes | See Stiksa 1982a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Stiksa 1985.
| Methods | Setting: Sweden, hospital outpatient clinic Length of intervention period: 3 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes Design: crossover, no washout Masking: none Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 20 adults: 11M 9F Mean (SD) age: 56.4 (7) years Inclusion criteria: Asthmatics currently receiving inhaled BDP 200 mcg/d for 8 months or longer Exclusion criteria: Not stated | |
| Interventions | 1. BDP: 200 mcg 1puff 4xdaily (800 mcg/d) via MDI 2. BUD 200 mcg 2 puffs 2xdaily (800 mcg/d) via MDI+Inhalet spacer 3. BUD 200 mcg 4 puffs 1xdaily (800 mcg/d) via MDI+Inhalet spacer |
|
| Outcomes | Morning PEFR Evening PEFR Daytime breathlessness score Night‐time breathlessness score Sleeping difficulty due to asthma score Daily beta2 agonist use | |
| Notes | Author confirmed randomisation method and use of allocation concealment 10 patients were receiving treatment with oral prednisolone at enrolment. Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Svendsen 1992.
| Methods | Setting: Denmark, hospital outpatient clinic Length of intervention: 6 weeks Design: crossover, no washout Randomised: yes, method not stated Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 39 adults Age range: 18 years or older Inclusion criteria: Asthma poorly controlled with inhaled steroids, daily dose 300 ‐ 500 mcg/day FEV1, FVC or PEFR < 70% predicted 15% or greater improvement in FEV1 after inhaled beta2 agonist Bronchial hyper‐responsiveness to histamine (PC20 FEV1 2 mg/ml or less) Exclusion criteria: Regular oral steroids during 3 months prior to the trial Unable to use aerosols correctly Pregnancy or significant co‐existent disease Respiratory infections within last 4 weeks | |
| Interventions | BDP: 250 mcg 3 puffs 2xdaily (1500 mcg/day) via MDI and placebo via MDI+Inhalet spacer BUD: 200 mcg 4 puffs 2xdaily (1600 mcg) via MDI+Inhalet spacer and placebo via MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Clinic PEFR Histamine BHR (PC20 FEV1) Sleep disturbance, wheeze and activity restriction score Rescue beta2 agonist use (puffs/day) Plasma cortisol (time not stated) Plasma cortisol 30 min post 250 mcg iv tetracosactrin | |
| Notes | Reply from author but unable clarify details of randomisation procedure Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tjwa 1995.
| Methods | Setting: The Netherlands, hospital outpatient clinic Length of intervention: 8 weeks Design: crossover, 4 week washout Randomisation: yes, by computerised randomisation algorithm Allocation concealment: unclear Masking: none Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 16 adults: 14M 2F Inclusion criteria: 18 years of age or older Asthma for more than 2 years %predicted FEV1 40‐85 15% or greater improvement in FEV1 after inhaled beta2 agonist Bronchial hyper‐responsiveness to histamine (PC20 FEV1 less than or equal to 1.0mg/ml) Currently receiving inhaled steroid 150‐800 mcg/daily Exclusion criteria: Pregnancy or significant co‐existent disease Asthma exacerbation or respiratory tract infection in previous 2 months | |
| Interventions | BDP: 200 mcg 1 actuation 2xdaily (400 mcg daily) via Rotahaler DPI BUD: 200 mcg 1 actuation 2xdaily (400 mcg daily) via Turbuhaler DPI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Daytime wheeze score Daytime breathlessness score Daytime cough score Night‐time wheeze score Night‐time breathlessness score Night‐time cough score Daytime beta2 agonist use (puffs/day) Night‐time beta2 agonist use (puffs/day) Bronchial responsiveness to histamine (PC20 FEV1) | |
| Notes | Author confirmed method of random order generation and provided original data for all outcomes. Carryover effects were tested for and excluded. Significant improvement in at least one efficacy measure for both BDP and BUD groups compared to placebo washout | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Willey 1982.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention: 4 weeks Design: crossover, no washout Randomisation: yes, computerised randomisation algorithm Allocation concealment: yes Masking: double blind Excluded: not stated Withdrawals: stated Jadad score: 4 | |
| Participants | 30 adults: 8M 12F Age range: 18‐67 years Inclusion criteria: Diagnosis of chronic asthma Current treatment with BDP 400 mcg/day Required at least two courses of oral corticosteroid therapy for exacerbations of asthma within the last 12 months Able to use an aerosol MDI efficiently Exclusion criteria: Oral steroids within the last month | |
| Interventions | BDP: 50 mcg 2 puffs 4xdaily (400 mcg daily) via MDI plus placebo MDI+spacer BUD: 50 mcg 4 puffs 2xdaily (400 mcg daily) via MDI + spacer plus placebo MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Morning wheeze score Evening wheeze score Morning breathlessness score Evening breathlessness score Morning cough score Evening cough score Nightime salbutamol use score Daytime salbutamol use score | |
| Notes | Author confirmed details of randomisation procedure. Not stated if carryover effects were tested for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ACTH: adrenocorticotrophic hormone ATS: American Thoracic Society BDP: beclomethasone dipropionate BUD: budesonide DPI: dry powder inhaler ECP: eosinophil cationic protein FEF25‐75: forced expiratory flow at 25 to 75% of FVC FEF 50: forced expiratory flow rate at 50% of FVC FEV1: forced expired volume in one second FVC: forced vital capacity ICS: inhaled corticosteroid MDI: metered dose inhaler PC20 FEV1: concentration of inhalant required to produce a 20% fall in FEV1 PD20 FEV1: dose of inhalant required to produce a 20% fall in FEV1 PEFR: peak expiratory flow rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Birkebaek 1997 | Study concerned with the effects of BDP and BUD on markers of bone and collagen turnover only |
| Bjorkander 1982b | Unequal nominal daily doses of BDP and BUD compared: BDP 400 mcg/d v BUD 200 mcg/d |
| de Graaff 1996 | Not clear if study randomised. |
| Kiviranta 1993 | Described as randomised in paper publication, however following contact with authors it was apparent that patients were not allocated to intervention groups in a randomised manner |
| Piquet 1996 | Unequal nominal daily doses of BDP and BUD compared: BDP 1500‐2000 mcg/d v BUD 800 mcg/d |
| Rosenhall 1982 | Unequal doses of BDP and BUD compared: BDP 400 mcg/d v BUD 200 or 800 mcg/d |
| Struijs 1997 | Patients enrolled with chronic obstructive airways disease, not asthma |
Contributions of authors
Nick Adams retrieved papers identified by electronic search, handsearched additional sources for relevant studies, assessed trials for methodological quality, contacted authors to clarify details of trial design and/or request missing data, extracted data from included trials and wrote the review.
Janine Bestall retrieved papers identified by search, assessed trials for methodological quality, contacted authors for clarification or trial details and/or request missing data.
Paul Jones provided editorial support .
Sources of support
Internal sources
No sources of support supplied
External sources
NHS Research and Development, UK.
Garfield Weston Foundation, UK.
Declarations of interest
None
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Baran 1987 {published data only}
- Baran D. A comparison of inhaled budesonide and beclomethasone dipropionate in childhood asthma. British Journal of Diseases of the Chest 1987;81(2):170‐5. [DOI] [PubMed] [Google Scholar]
Bisgaard 1988 {published data only}
- Bisgaard H, Damkjaer Nielsen M, Andersen B, Andersen P, Foged N, Fuglsang G, et al. Adrenal function in children with bronchial asthma treated with beclomethasone dipropionate or budesonide. Journal of Allergy & Clinical Immunology 1988;81(6):1088‐95. [DOI] [PubMed] [Google Scholar]
Bjorkander 1982a {published data only}
- Bjorkander J, Formgren H, Johansson SA, Millqvist E. Methodological aspects on clinical trials with inhaled corticosteroids: results of two comparisons between two steroid aerosols in patients with asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:108‐17. [PubMed] [Google Scholar]
Boe 1989 {published data only}
- Boe J, Rosenhall L, Alton M, Carlsson LG, Carlsson U, Hermansson BA, et al. Comparison of dose‐response effects of inhaled beclomethasone dipropionate and budesonide in the management of asthma. Allergy: European Journal of Allergy & Clinical Immunology 1989;44(5):349‐55. [DOI] [PubMed] [Google Scholar]
Brambilla 1994 {published data only}
- Brambilla C, Godard P, Lacronique J, Allaert FA, Duroux P, Blaive B, et al. A 3‐month comparative dose‐reduction study with inhaled beclomethasone dipropionate and budesonide in the management of moderate to severe adult asthma. Drug Investigation 1994;8(1):49‐56. [Google Scholar]
Dal Negro 1997 {published data only}
- Dal Negro R, Micheletto C, Ciani F, Muzzi A, Crimi E, Filippa P, et al. Efficacy and safety of inhaled beclomethasone dipropionate dry powder in the treatment of chronic asthma: A controlled study vs. budesonide. European Respiratory Journal. 1997:351S.
Ebden 1986 {published data only}
- Ebden P, Jenkins A, Houston G, Davies BH. Comparison of two high dose corticosteroid aerosol treatments, beclomethasone dipropionate (1500 micrograms/day) and budesonide (1600 micrograms/day), for chronic asthma. Thorax 1986;41(11):869‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 1982 {published data only}
- Field HV, Jenkinson PM, Frame MH, Warner JO. Asthma treatment with a new corticosteroid aerosol, budesonide, administered twice daily by spacer inhaler. Archives of Disease in Childhood 1982;57(11):864‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greefhorst 1992 {published data only}
- Greefhorst APM. Budesonide and terbutaline delivered via Turbuhaler compared to BDP and salbutamol delivered via Rotahaler. European Respiratory Journal. 1992; Vol. 5, issue Suppl 15:360S.
Hamalainen 1998 {published data only}
- Hamalainen KM, Laurikaninen K, Leinonen M, Jager L. Comparison of two multidose powder inhalers (MDPI) in the treatment of asthma with inhaled corticosteroids. European Respiratory Journal. 1998:61S.
Keelan 1984 {published data only}
- Keelan P, Gray P, Kelly P, Frame M. Comparison of a new corticosteroid aerosol, budesonide, with beclomethasone dipropionate in the treatment of chronic asthma. Irish Medical Journal 1984;77(8):244‐7. [PubMed] [Google Scholar]
Micheletto 1997 {published data only}
- Micheletto C, Mauroner L, Burti E, Turco P, Pomari L, Cantini L, Dal Negro R. Inhaled beclomethasone dipropionate and budesonide dry powder in chronic asthma: Lung function‐serum ECP relationship. European Respiratory Journal. 1997:351S. [PubMed]
Nicolaizik 1994 {published data only}
- Nicolaizik WH, Marchant JL, Preece MA, Warner JO. Endocrine and lung function in asthmatic children on inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 1994;150(3):624‐8. [DOI] [PubMed] [Google Scholar]
Pedersen 1988 {published data only}
- Pedersen S, Fuglsang G. Urine cortisol excretion in children treated with high doses of inhaled corticosteroids: a comparison of budesonide and beclomethasone. European Respiratory Journal 1988;1(5):433‐5. [PubMed] [Google Scholar]
Petrie 1990 {published data only}
- Petrie GR, Choo‐Kang YFJ, Clark RA, Milledge JSSPR, Whitfield RJ, Higgins AJ. An assessment of the acceptability of two breath‐actuated corticosteroid inhalers comparison of Turbohaler(TM) with Diskhaler(TM). Drug Investigation 1990;2(2):129‐31. [Google Scholar]
Rafferty 1985 {published data only}
- Rafferty P, Tucker LG, Frame MH, Fergusson RJ, Biggs BA, Crompton GK. Comparison of budesonide and beclomethasone dipropionate in patients with severe chronic asthma: assessment of relative prednisolone‐sparing effects. British Journal of Diseases of the Chest 1985;79(3):244‐50. [PubMed] [Google Scholar]
Selroos 1994 {published data only}
- Selroos O, Backman R, Forsen KO, Lofroos AB, Niemisto M, Pietinalho A, et al. Clinical efficacy of budesonide Turbuhaler compared with that of beclomethasone dipropionate pMDI with volumatic spacer. A 2‐year randomized study in 102 asthma patients. Allergy: European Journal of Allergy & Clinical Immunology 1994;49(10):833‐6. [DOI] [PubMed] [Google Scholar]
Springer 1987 {published data only}
- Springer C, Avital A, Maayan C, Rosler A, Godfrey S. Comparison of budesonide and beclomethasone dipropionate for treatment of asthma. Archives of Disease in Childhood 1987;62(8):815‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiksa 1982a {published data only}
- Stiksa G, Glennow C, Johannesson N. A open cross‐over trial with budesonide and beclomethasone dipropionate in patients with bronchial asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:266‐7. [Google Scholar]
Stiksa 1982b {published data only}
- Stiksa G, Glennow C, Johannesson N. A open cross‐over trial with budesonide and beclomethasone dipropionate in patients with bronchial asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:266‐7. [Google Scholar]
Stiksa 1985 {published data only}
- Stiksa G, Glennow C. Once daily inhalation of budesonide in the treatment of chronic asthma: a clinical comparison. Annals of Allergy 1985;55(1):49‐51. [PubMed] [Google Scholar]
Svendsen 1992 {published data only}
- Svendsen UG, Frolund L, Heinig JH, Madsen F, Nielsen NH, Weeke B. High‐dose inhaled steroids in the management of asthma. A comparison of the effects of budesonide and beclomethasone dipropionate on pulmonary function, symptoms, bronchial responsiveness and the adrenal function. Allergy: European Journal of Allergy and Clinical Immunology 1992;47(2 Pt 2):174‐80. [DOI] [PubMed] [Google Scholar]
- Svendsen UG, Frolund L, Heinig JH, Madsen F, Nielsen NH, Weeke B. [High dose inhaled steroids in the treatment of bronchial asthma. A comparison of the effects of budesonide and beclomethasone dipropionate on pulmonary function, symptoms, bronchial reactivity and adrenocortical function]. Ugeskrift for Laeger 1993;155(28):2197‐202. [PubMed] [Google Scholar]
Tjwa 1995 {published data only}
- Tjwa MK. Budesonide inhaled via Turbuhaler: a more effective treatment for asthma than beclomethasone dipropionate via Rotahaler. Annals of Allergy, Asthma & Immunology 1995;75(2):107‐11. [PubMed] [Google Scholar]
Willey 1982 {published data only}
- Willey RF, Godden DJ, Carmichael J, Preston P, Frame MH, Crompton GK. Twice daily inhalation of a new corticosteroid, budesonide, in the treatment of chronic asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:138‐42. [PubMed] [Google Scholar]
References to studies excluded from this review
Birkebaek 1997 {published data only}
- Birkebaek NH, Esberg BH, Andersen K, Wolthers OD, Hassager C. [Budesonide and beclomethasone dipropionate inhalation powders. The effect on bone and collagen turnover in children]. Ugeskrift for Laeger 1997;159(17):2559‐62. [PubMed] [Google Scholar]
Bjorkander 1982b {published data only}
- Bjorkander J, Formgren H, Johansson SA, Millqvist E. Methodological aspects on clinical trials with inhaled corticosteroids: results of two comparisons between two steroid aerosols in patients with asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:108‐17. [PubMed] [Google Scholar]
de Graaff 1996 {published data only}
- Graaff CS, Burgh JHAM, Stallaert RALM, Prins J, Lier AA. A double‐blind comparative study of the inhaled corticosteroids budesonide and beclomethasone dipropionate in dry powder inhalers in asthmatic patients. European Respiratory Journal. 1996; Vol. 5, issue Suppl 15:S359‐60.
Kiviranta 1993 {published data only}
- Kiviranta K, Turpeinen M. Effect of eight months of inhaled beclomethasone dipropionate and budesonide on carbohydrate metabolism in adults with asthma. Thorax 1993;48(10):974‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piquet 1996 {published data only}
- Piquet J, Zuck P, Dennewald G, Dugue P, Grivaux M, Brun P, et al. Equally efficacious asthma management with budesonide 800 mug administered by Turbuhaler(TM) or with beclomethasone dipropionate >=1500 mug given through a pressurized metered‐dose inhaler with spacer. Advances in Therapy 1996;13(1):38‐50. [PubMed] [Google Scholar]
Rosenhall 1982 {published data only}
- Rosenhall L, Lundqvist G, Adelroth E, Glennow C. Comparison between inhaled and oral corticosteroids in patients with chronic asthma. European Journal of Respiratory Diseases ‐ Supplement 1982;122:154‐62. [PubMed] [Google Scholar]
Struijs 1997 {published data only}
- Struijs A, Mulder H. The effects of inhaled glucocorticoids on bone mass and biochemical markers of bone homeostasis: a 1‐year study of beclomethasone versus budesonide. Netherlands Journal of Medicine 1997;50(6):233‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baxter‐Jones 2000 {published data only}
- Baxter‐Jones AD, Helms PJ. Early introduction of inhaled steroids in wheezing children presenting in primary care. A pilot study. Clinical & Experimental Allergy 2000;30(11):1618‐26. [DOI] [PubMed] [Google Scholar]
Brand 2001 {published data only}
- Brand PL, Baan‐Slootweg OH, Heynens JW, Vries TW, Versteegh FG, Vreuls RC, et al. Comparison of handling and acceptability of two spacer devices in young children with asthma. Acta Paediatrica 2001;90(2):133‐6. [DOI] [PubMed] [Google Scholar]
Dal Negro 1999b {published data only}
- Dal Negro R, Micheletto C, Tognella S, Mauroner L, Burti E, Turco P, et al. Effect of inhaled beclomethasone dipropionate and budesonide dry powder on pulmonary function and serum eosinophil cationic protein in adult asthmatics. Journal of Investigational Allergology & Clinical Immunology 1999;9(4):241‐7. [PubMed] [Google Scholar]
Hernandez 1995 {published data only}
- Hernandez J, Garcia‐Selles FJ, Negro JM, Pascual A, Sola J, Miralles JC, et al. [Comparative study] of beclomethasone and budesonide, with same posology (400 micrograms every 12 hours), in the control of cortico‐ dependent intrinsic asthma]. Allergologia et Immunopathologia 1995;23(5):193‐201. [PubMed] [Google Scholar]
Jager 2000 {published data only}
- Jager L, Laurikainen K, Leinonen M, Silvasti M. Beclomethasone dipropionate Easyhaler is as effective as budesonide Turbohaler in the control of asthma and is preferred by patients. International Journal of Clinical Practice 2000;54(6):368‐72. [PubMed] [Google Scholar]
Kraszko 1999 {published data only}
- Kraszko P, Vondra D, Malolepszy J, Svensson M, Baly J, Fiserova J, et al. Budesonide via Turbuhaler, 400 mug daily, is as effective as beclomethasone dipropionate via pressurised MDI, 800 mug daily, for control of mild to moderate asthmatic patients. Journal of Clinical Research 1999;2:47‐55. [Google Scholar]
Lee 1995 {published data only}
- Lee YC, Rhee YK. Effect of inhaled steroids on the cortisol concentration by different dosage or delivery method. Tuberculosis & Respiratory Diseases 1995;42(6):888‐99. [Google Scholar]
Reichel 2001 {published data only}
- Reichel W, Dahl R, Ringdal N, Zetterstrom O, Elshout FJ, Laitinen LA. Extrafine beclomethasone dipropionate breath‐actuated inhaler (400 micrograms/day) versus budesonide dry powder inhaler (800 micrograms/day) in asthma. International Journal of Clinical Practice 2001;55(2):100‐6. [PubMed] [Google Scholar]
Additional references
Boobis 1998
- Boobis AR. Comparative physiochemical and pharmacokinetic profiles of inhaled beclomethasone dipropionate and budesonide. Respiratory Medicine 1998;92(Suppl B):2‐6. [DOI] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐20. [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global strategy for asthma management and prevention NHBLI/WHO workshop report. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1995, issue Publication No. 95‐3659.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Johansson 1982
- Johansson SA, Andersson KE, Brattsand R, Gruvstad E, Hedner P. Topical and systemic glucocorticoid potencies of budesonide and beclomethasone dipropionate in man. European Journal of Clinical Pharmacology 1982;22:523‐9. [DOI] [PubMed] [Google Scholar]
NHLBI 1997
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue NIH Publication No. 97‐4051.
Pethica 1998
- Pethica BD, Penrose A, MacKenzie D, Hall J, Beasley R, Tilyard M. Comparison of potency of inhaled beclomethasone and budesonide in New Zealand: retrospective study of computerised general practice records. BMJ 1998;317(7164):986‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stellato 1999
- Stellato C, Atsuta J, Bickel CA, Schleimer RP. An in vitro comparison of commonly used topical glucocorticoid preparations. Journal of Allergy & Clinical Immunology 1999;104(3 Pt 1):623‐9. [DOI] [PubMed] [Google Scholar]


