Abstract
Novel therapeutic strategies are needed in the fight against pancreatic cancer. We have previously documented the chemopreventive effect of MDC-22 in preclinical models of pancreatic cancer. In the present work, we examined the therapeutic effects of MDC-22 in patient-derived tumor xenografts (PDTXs) and in LSL-KrasG12D/+, LSL-Trp53R172H/+, Pdx1-Cre (KPC) genetically engineered mice, two complementary and clinically relevant animal models of pancreatic cancer. In addition, we evaluated whether MDC-22 could synergize with current chemotherapeutic drugs used in the clinic. MDC-22 reduced the growth of various human pancreatic cancer cell lines in a concentration-dependent manner. In vivo, MDC-22 strongly reduced patient-derived pancreatic tumor xenograft growth by 50%, and extended survival of LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx1-Cre (KPC) mice by over a month (5.3 months versus 7.0 months). In both models, MDC-22 inhibited EGFR activation and its downstream signals, including ERK and FAK phosphorylation. In human pancreatic cancer cell lines, MDC-22 enhanced the growth inhibitory effect of irinotecan, and to a lesser degree those of gemcitabine and nab-paclitaxel. Normal human pancreatic epithelial cells were more resistant to the cytotoxic effects of, both, MDC-22 alone or in combination with irinotecan, indicating selectivity. Furthermore, MDC-22 enhanced irinotecan's effect on cell migration, in part, by inhibiting EGFR/FAK signaling. Collectively, our results indicate that MDC-22 is an effective anticancer drug in preclinical models of pancreatic cancer, and suggest that MDC-22 plus irinotecan as drug combination strategy for pancreatic cancer treatment, which warrants further evaluation.
Keywords: Pancreatic cancer, KPC, Phospho-Aspirin, EGFR, irinotecan, FAK, MDC-22
Introduction
Pancreatic cancer (PC) is a leading cause of cancer-related deaths worldwide, with a current overall 5-year survival rate of approximately 10% [1]. Several reasons explain this dismal prognosis. For example, PC is usually diagnosed at advanced stages, and lacks sensitive and specific tumor markers to help detect it at an early-stage. In addition, pancreatic tumors metastasize early, making patients ineligible for surgery. Finally, pancreatic tumors are resistant to existing treatments, being surrounded by a complex and dense tumor microenvironment, which limits the effectiveness of chemotherapy and radiotherapy [2].
Indeed, the current chemotherapeutic options offer limited help. For instance, the chemotherapeutic drug gemcitabine, given alone or in combination with nab-paclitaxel, is minimally effective in PC treatment, improving patients' survival by only a few months 3, 4, 5. Irinotecan is a cytotoxic drug largely used in the treatment of solid tumors. It is often used for the treatment of metastatic PC in combination with fluorouracil, leucovorin, and oxaliplatin (FOLFIRINOX), and leads to longer overall survival than gemcitabine therapy [6]. Unfortunately, irinotecan-based chemotherapy has a large spectrum of side effects, and therefore it is only suitable for patients with tolerance. Therefore, it is critical to develop safe and effective agents for the management of PC.
Epidermal growth factor receptor (EGFR) has a relevant physiological function regulating epithelial tissue development. Aberrant activation of EGFR plays a key role in PC through the activation of downstream cascades that promotes cell survival and proliferation [7,8]. One of the most important EGFR-downstream protein is the focal adhesion kinase (FAK). FAK is a non-receptor cytoplasmic tyrosine kinase that regulates several cell functions such as proliferation, migration and survival. It has been reported that elevated FAK expression enhances tumor malignancy driving the fibrotic and immunosuppressive microenvironment that protects tumors from immune surveillance and drives resistance to immunotherapy 9, 10, 11, 12. Previous studies have shown that pharmacological targeting of FAK in PC models results in decreased stromal density and thus increases the responsiveness of the tumor to chemotherapy, while simultaneously suppressing tumor progression [10]. Thus, the EGFR and FAK are attractive targets for PC treatment.
We have previously identified MDC-22 (Fig. 1A) as a potent EGFR inhibitor [13,14], and documented that MDC-22 possesses strong chemopreventive properties in PC [14]. This novel agent has been synthesized based on a general approach where a specific chemical modification of known drugs enhances their desired anticancer properties, mainly their safety and efficacy [15,16]. In the present study, we examined the therapeutic efficacy of MDC-22 in two clinically relevant models of PC. These included patient-derived tumor xenografts (PDTXs); and the LSL-KrasG12D/+, LSL-Trp53R172H/+, Pdx1-Cre (KPC) genetically engineered mice [17], which develop pancreatic tumors, whose pathophysiological and molecular features resemble those of human pancreatic ductal adenocarcinoma [18]. Our data show that MDC-22 reduces the growth of PDTXs and extends survival in KPC mice, in part, by inhibiting EGFR activation, ERK phosphorylation and FAK signaling. Furthermore, MDC-22 enhances the anticancer effect of irinotecan in vitro and reduces PC cell migration.
Fig. 1.
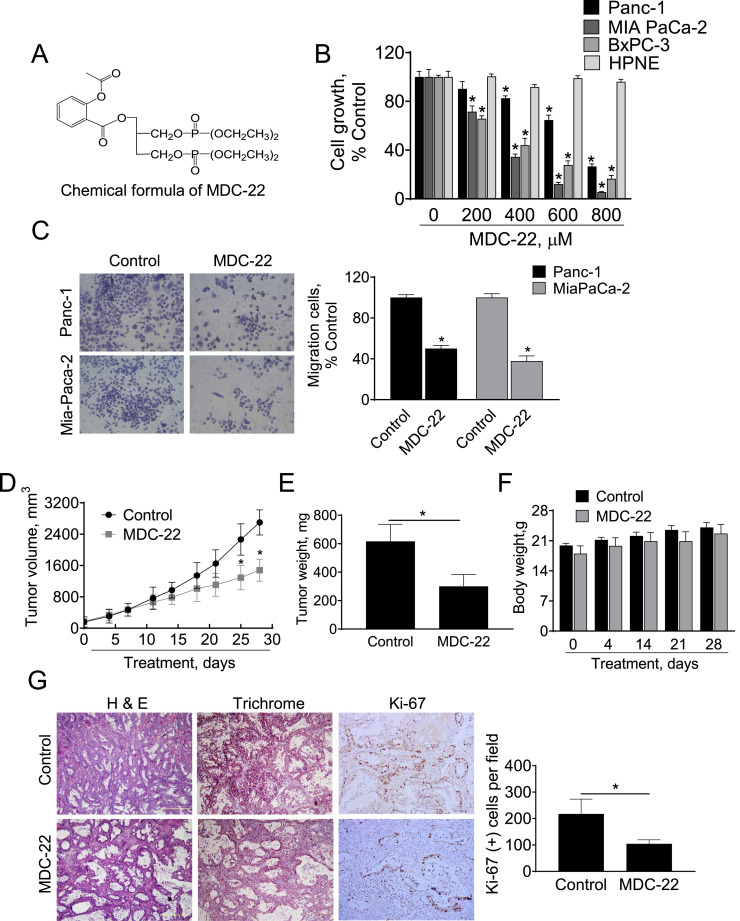
MDC-22 reduces the growth of human pancreatic cancer cells and patient-derived pancreatic tumor xenografts. A: Chemical structure of MDC-22. B: Cell growth was determined in Panc-1, Mia PaCa-2, BxPC-3 and HPNE cells treated with escalating concentrations of MDC-22 (0-800 µM), for 48 h. Results are expressed as % control; *p < 0.01. C: Transwell migration assay were performed in human Panc-1 and MIA PaCa-2 PC cells treated with MDC-22 0.25x IC50 as described under Materials and Methods. Results are expressed as % control. *p < 0.01. D: Patient-derived tumor volume growth over time for vehicle control and MDC-22-treated mice. *Significantly different compared to control group (p < 0.05 vs. control). E: Tumor weight at euthanasia. *Significant compared to control group; p < 0.05. F: Body weight progression between the groups. G: H&E staining, trichrome staining and immunostaining for Ki-67 performed on patient-derived tumor sections. Photographs were taken at x10 magnification. Representative images are shown. Results were expressed as percent of Ki-67+ cells ± SEM per 20x field; *Significant compared to control group; p < 0.05.
Materials and methods
Reagents
MDC-22 was a gift from Medicon Pharmaceuticals Inc. (Stony Brook, NY). Gemcitabine (> 99%) was purchased from BIOTANG (Waltham, MA). 5-Fluorouracil (≥ 99%) was purchased from Alfa Aesar (Haverhill, MA). Irinotecan hydrochloride was purchased from Sigma-Millipore (Saint Louis, MO). In all cell culture media, the final DMSO concentration was adjusted to 1% (v/v). All general solvents and reagents were HPLC grade or the highest grade commercially available.
Cell culture
MIA PaCa-2 (Cat# CRL-1420), Panc-1 (Cat# CRL-1469) and BxPC-3 (Cat# CRL-1687) human PC cell lines were purchased from American Type Culture Collection (ATCC) and grown according to the supplier's recommendations. Each culture medium was supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin. The human pancreatic normal epithelial cell line, hTERT-HPNE, was cultured in 75% (v/v) DMEM without glucose (Gibco 11966), 25% (v/v) M3:Base medium (InCell, M300F), 5% (v/v) FBS, 5.5 mM D-glucose, 750 ng/mL puromycin, and 10 ng/mL human recombinant epidermal growth factor (EGF). We have not authenticated these cell lines, however we routinely test for mycoplasma contamination in every cell line every three months. These cells were grown as monolayers in the specific medium and under conditions suggested by ATCC. All the cell lines were characterized by cell morphology and growth rate and passaged in our laboratory less than 6 months after being received.
Cell viability assay
Following treatment with increasing concentrations of MDC-22 alone or in combination with gemcitabine, nab-paclitaxel, 5-FU, or irinotecan, the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye (MTT) Millipore-Sigma (Saint Louis, MO) was determined as previously described [19,20].
Transwell migration assay
For the migration assay, MIA PaCa-2 and Panc-1 cells (5.0 × 105cells/well) were seeded in serum-free DMEM in the top chambers of 24-well plates (Corning, New York, NY), as previously described [21,22]. The bottom chambers of the plates were filled with DMEM containing 10% FBS. The cells were incubated for 24 h at 37˚C. Afterwards, MDC-22 alone or in combination with irinotecan were added to the top chambers for an additional incubation of 24 h. At that time, cells in the top chambers were removed, and the cells, which migrated to the bottom chambers were fixed in 4% paraformaldehyde, were stained with 0.5% crystal violet for 1 h at room temperature. The stained cells were counted in 3 independent fields under a microscope. All experiments were performed in triplicate.
Western blot analysis in cells
Following treatment with MDC-22 alone or in combination with irinotecan, cells were collected, total cell fractions obtained and western blots performed as previously described [23,24]. Membranes were probed overnight with the following antibodies from Cell Signaling Technologies (Danvers, MA): p-FAK (Cat #3283), FAK (Cat #3285), p-ERK (Cat #4376), ERK (Cat #9102), cyclinD1 (Cat #2978), p-EGFRTyr1068 (Cat #3777) and EGFR (Cat #2085), using a 1:1,000 dilution of antibody in 5% (w/v) fat-free milk. β-actin (Cat #A1978) from Millipore-Sigma, Saint Louis, MO, was used as a loading control. The membranes were allowed to incubate in secondary antibody, HRP conjugated (1:5,000 dilution), for 60 min. Conjugates were visualized by chemiluminescence.
Animal Studies. Animal studies were approved by the Institutional Animal Care and Use Committee at Stony Brook University.
Patient-derived tumor xenografts (PDTXs)
Human de-identified PC tumor samples were obtained from the Cooperative Human Tissue Network (CHTN), which assigned a patient code and a sample code prior to their release to researchers. Upon delivery, PC tumor tissues were immediately transplanted into female NOD/SCID mice (5-6 weeks old) for PDTX modeling, as previously described [21]. Tumors were collected when they reached approximately 10 mm in diameter, and cut into small pieces (approximately 2 × 2 × 2 mm). Single pieces were subcutaneously implanted into the right and left flanks of additional female NOD/SCID mice. Once the tumor reached ∼200 mm3, mice (n=7/group) were divided randomly into two groups; one receiving PBS (vehicle control), and the other MDC-22 (100 mg/kg) suspended in PBS [14], given intraperitoneally five times per week for 28 days. Body weight and tumor size were measured twice a week [19]. At the end of the study, the animals were euthanized, and tumor weights were measured after their careful resection. Tumor tissue was collected for analysis.
KPC survival
To determine the anticancer capacity of MDC-22, six-week-old male and female KPC mice were randomized and dosed by oral gavage with MDC-22 100 mg/kg in corn oil [14], 5x/week (n=12; 6 males and 6 females) or with corn oil (n=18; 9 males and 9 females) from the time of enrollment until endpoint criteria were met. All animals were weighed 2x/week. Endpoint criteria included the development of hemorrhagic abdominal ascites, severe cachexia, weight loss exceeding 20% of the initial weight, or extreme weakness or inactivity. Mice were observed daily for signs of significant weight loss / cachexia; hemorrhagic ascites; and for other clinical failure including loss of thermoregulation, inactivity, and presence of malignant ascites. When an animal reached its endpoint, it was euthanized by CO2 asphyxiation and pancreas and tumor were carefully resected and stored for further analysis.
Evaluation of the acute toxicity of MDC-22 in hamsters
Five-week old female Syrian golden hamsters (n=2 per group) were treated with increasing doses of MDC-22 for 8 days. On the initial two days (day 0 and 1), hamsters were gavaged with MDC-22 at a dose of 100 mg/kg once daily for 2 days. On the next two days (days 2 and 3), the dose was increased to 200 mg/kg gavaged once daily for 2 days. On the following next two days (days 4 and 5), MDC-22’s dose was increased to 300 mg/kg. Finally, on days 6 and 7, the dose was increased to 400 mg/kg for two additional days. MDC-22 was administered once daily by oral gavage. At the conclusion of the intervention (on day 8), hamsters were euthanized and serum collected for biochemical assay determinations.
Immunohistochemical staining
Human PC xenograft tissue and KPC pancreas/tumor samples were Immunohistochemical stained for Ki-67 (Cat # sc-15402) from Santa Cruz Biotechnology (Santa Cruz, CA), cyclinD1 (Cat # 2978), p-EGFR (Cat #3777) and p-ERK1/2 (Cat #4376), from Cell Signaling Technology (Danvers, MA), as previously described [25,26]. Scoring: At least 5 fields per sample (at magnification x200) were scored. We calculated the percentage of positive cells (brown staining) by dividing the number of labeled cells by the number of cells in each field and multiplying by 100.
Western blot analysis in tissues
Human PC xenograft tissue was homogenate as previously described [27,28] and western blots were performed as described above. Membranes were probed overnight with the antibodies mentioned above.
Statistical analysis
The data, obtained from at least three independent experiments, were expressed as the mean ± SEM. Statistical evaluation was performed by t-test or one-factor analysis of variance (ANOVA) followed by the Tukey test for multiple comparisons. Graph plotting and statistical analysis by the log-rank (Mantel–Cox) test were performed by GraphPad Prism (version 7.0.5; San Diego, CA, USA). P < 0.05 was regarded as statistically significant.
Results
MDC-22 selectively reduces the growth and migration of human pancreatic cancer cells
We first assessed the selectivity of MDC-22 in vitro by comparing the growth inhibitory effect of MDC-22 in human PC cells to that of human pancreatic normal epithelial cells (HPNE). For this purpose, we treated multiple human PC cells or HPNE cells with or without increasing concentrations of MDC-22 (200–800 μM) for 48 h. MDC-22 reduced human PC cell growth in a concentration-dependent manner and more potently in human PC cells compared to HPNE cells (Fig. 1B). For instance, at 48 h, MDC-22 600 µM reduced Panc-1, MIA PaCa-2 and BxPC-3 cell growth by 35%, 88% and 73% (p < 0.05), respectively. In contrast, MDC-22 at all concentrations tested (up to 800 µM) for 48 h had no significantly effect on the HPNE cell growth, reducing cell growth by < 8% (Fig. 1B), being unable to obtain an Inhibitory Concentration-50 at 48 h (48h-IC50) in HPNE cells with the concentrations of MDC-22 tested. In contrast, the 48h-IC50 for MDC-22 in Panc-1, MIA PaCa-2 and BxPC-3 cells were 525 ± 22 µM, 247 ± 16 µM and 300 ± 17 µM, respectively.
Furthermore, we examined the capacity of MDC-22 to regulate cell migration, using transwell inserts in PC cells. Compared to vehicle-treated controls, MDC-22 at a concentration of 0.25xIC50 reduced cell migration by 50% and 62% in Panc-1 and MIA PaCa-2 cells, respectively (p < 0.01 for both; Fig. 1C).
MDC-22 reduces the growth of patient-derived pancreatic tumor xenografts
We previously documented a chemopreventive effect of MDC-22 in preclinical models of PC [14]. We now evaluated the chemotherapeutic effect of MDC-22 in vivo, in a PDTX model. A human pancreatic tumor obtained from a surgical resection was implanted in a NOD/SCID mouse and then expanded to multiple mice. Once the expanded tumors reached a size of 200 mm3, mice were divided randomly into two groups receiving PBS (vehicle control), or MDC-22 (100 mg/kg) suspended in PBS given by intraperitoneal injections to mice five times per week during 4 weeks. At sacrifice, MDC-22 treatment reduced the rate of growth over baseline by 50.1% (p < 0.01; Fig. 1D). At the end of the treatment tumor weight were 51.2% lower in MDC-22 group compared to control group (Fig. 1E). This reduction in tumor growth was accompanied with a 51.9% inhibition on tumor proliferation, as determined by measuring the expression of the proliferating marker Ki-67 by immunohistochemistry (Fig. 1G). Treatment with MDC-22 showed no apparent adverse effects in mice during the whole treatment period, as can be evidenced by body weight. Throughout the study, the body weight of mice receiving MDC-22 was comparable to that of the control group. For instance, at the start of the treatment, the body weight (mean ± SEM) of control and MDC-22-treated mice was 19.9±0.5 g and 18.0±1.8 g, respectively. At the end of the 28 day-experimental period, the mean body weight in both groups of mice was as follows: Control = 24.1 ± 1.1 g; and MDC-22 = 22.7 ± 2.1 g (Fig. 1F).
MDC-22 inhibits EGFR, FAK and ERK phosphorylation in PC cells and in PDTXs
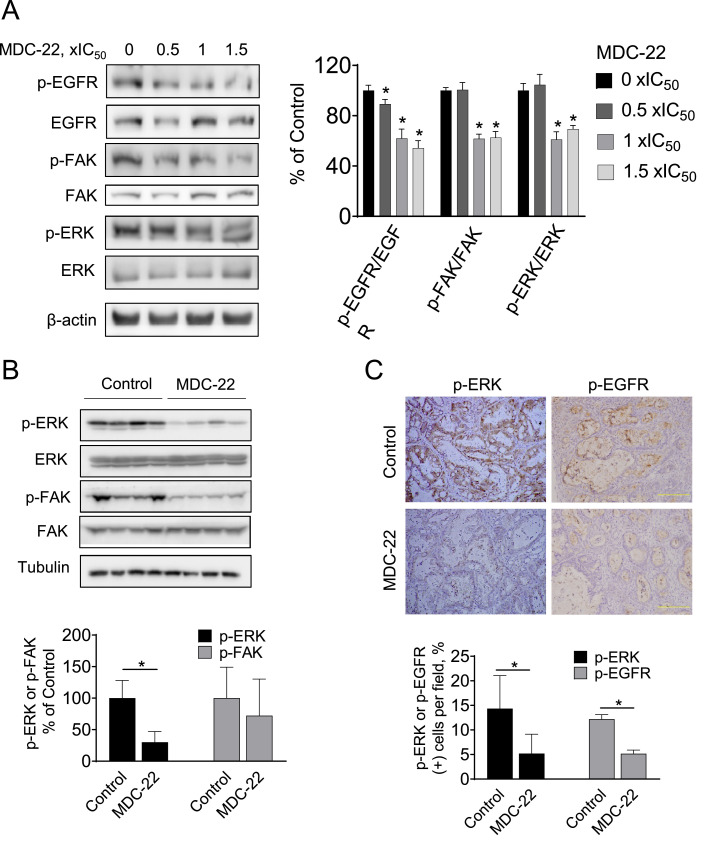
To investigate the mechanisms by which MDC-22 reduces tumor growth, we assessed the activation status of EGFR, and the downstream proteins FAK and ERK, all known to play a key role in pancreatic cancer growth and migration [9,14,29]. In vitro, PC cells were treated with escalating concentrations of MDC-22 for 48 h. MDC-22 at 1x and 1.5x IC50 significantly reduced phosphorylation of EGFR, FAK and ERK, compared to vehicle-treated control cells (Fig. 2A).
Fig. 2.
MDC-22 inhibits EGFR, FAK and ERK phosphorylation in PC cells and in PDTXs A: Immunoblots of EGFR, p-EGFR, FAK, p-FAK, ERK and p-ERK in total cell protein extracts from PC cells treated with escalating concentrations of MDC-22 from 0x to 1.5x IC50 for 48 h, B: Immunoblots of FAK, p-FAK, ERK and p-ERK from total tumor homogenates collected from PDTX tumor samples. Loading control: β-actin. Each lane represents a different tumor sample. Bands were quantified and results are expressed as the ratio of phospho/total and normalized by the protein levels of the loading control. Values are mean ± SEM. *p < 0.002 vs. control. C: Immunohistochemistry p-EGFR and p-ERK were performed on KPC tumor sections and photographs were taken at 20x magnification Representative images are shown. Results were expressed as percent of p-EGFR+ or p-ERK+ cells ± SD per x20 field. *Significant compared to control group; p < 0.01.
To confirm these results, we assessed FAK and ERK activation by immunoblotting in PDTXs homogenates. MDC-22 reduced FAK and ERK activation in PDTXs homogenates compared to vehicle-treated controls (Fig. 2B). Similar results were obtained by immunohistochemistry of tumor sections prepared from PDTXs from control and MDC-22-treated mice, in which MDC-22 significantly reduced p-ERK and p-EGFR levels by 54% and 64%, respectively (Fig. 2C; p < 0.05 for both).
MDC-22 extends survival in KPC mice
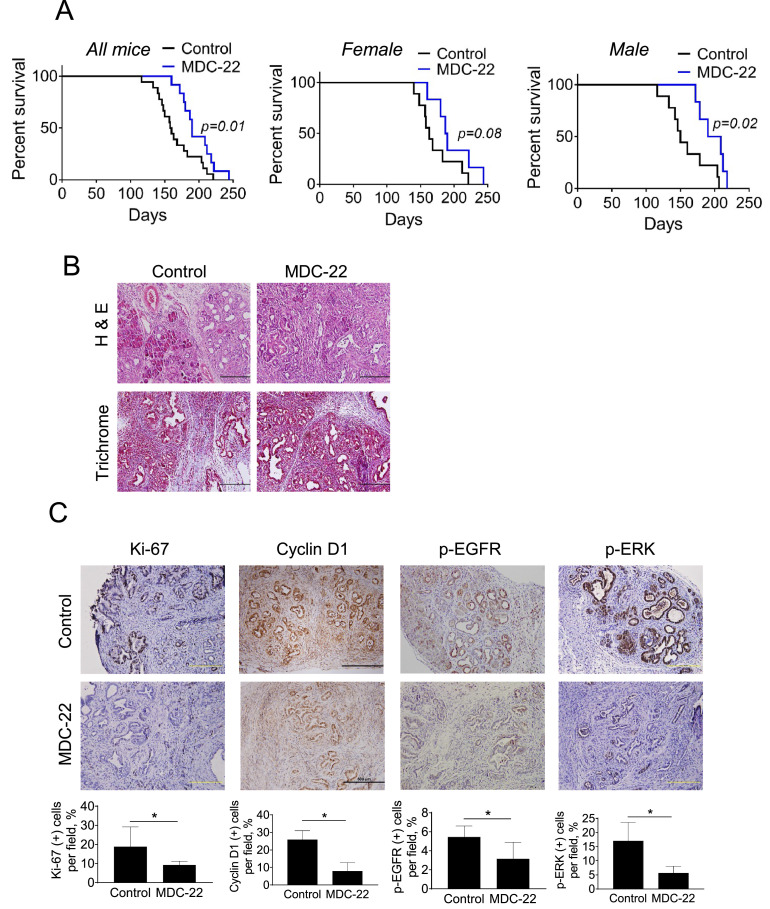
To rule-out an animal model-dependent effect, we next assessed the anticancer efficacy of MDC-22 using a KPC mouse model. Six weeks-old male and female KPC mice were randomized in vehicle-treated controls and MDC-22-treated mice, which were treated with MDC-22 100 mg/kg by oral gavage, 5x/week until mice reached their end-point. As shown in Fig. 3A, MDC-22-treated KPC mice exhibited a significant 19.5% increase in overall median survival compared to vehicle-treated mice (median survival was 159 days for vehicle-treated control versus 190 days for MDC-22 treated mice, log-rank p=0.01). Interestingly, the effect of MDC-22 was significantly more pronounced in male KPC mice (median survival was 150 days for vehicle-treated control versus 199 days for MDC-22 treated mice, log-rank p=0.02), than in female KPC mice, which did not reach statistical significance (median survival was 163 days for vehicle-treated control versus 188 days for MDC-22 treated mice, log-rank p=0.08; Fig. 3A). Consistent with the effect observed in PDTXs, MDC-22 reduced Ki-67 levels in tumor samples isolated from KPC mice by 51% (Fig. 3C).
Fig. 3.
MDC-22 enhances survival of KPC mice. Six weeks-old male and female KPC mice were randomized in vehicle-treated controls and MDC-22-treated mice until endpoint was met. A: Kaplan-Meier survival curve of vehicle control (black) or MDC-22-treated KPC mice (blue). KPC survival curves for all mice (left), only female mice (center) or only male mice (right) are shown. The effect of MDC-22 extending median survival was more pronounce in male KPC mice than in female KPC mice. B: H&E staining and trichrome staining performed on tumor sections. Photographs were taken at x10 magnification. Representative images are shown. C: Immunohistochemistry staining for Ki-67, cyclin D1, p-EGFR and p-ERK were performed in tumor sections. Photographs were taken at 20x magnification. Representative images are shown. Quantification is displayed below the images. Results were expressed as percent of Ki-67+, cyclin D1+, p-EGFR+ and p-ERK+ cells ± SEM per 20x field; *Significant compared to control group; p < 0.05 (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
We next evaluated whether MDC-22 could modulate key molecular targets in KPC mice. For this purpose, we assessed the levels of EGFR phosphorylation, ERK phosphorylation and cyclin D1 by immunohistochemistry in KPC tumor sections from control and MDC-22-treated mice. MDC-22-treated mice reduced cyclin D1, p-EGFR and p-ERK expression levels by 73.7%, 35.9% and 66.7%, respectively, compared to control mice (Fig. 3C).
Evaluation of the acute toxicity of MDC-22 in hamsters
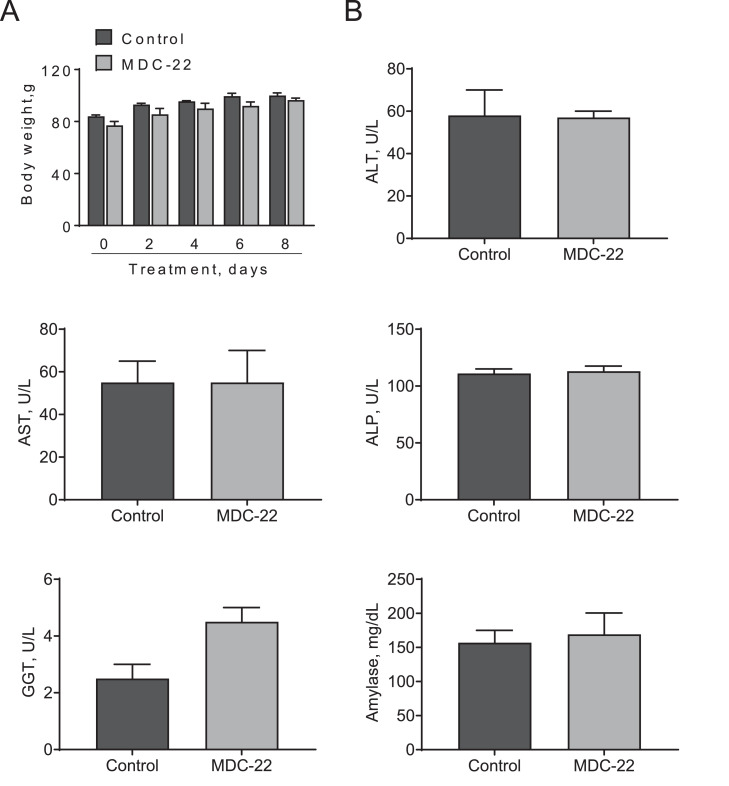
We previously documented that MDC-22 is safe in mice and rats [13,14,30]. We now expanded these studies and evaluated the acute toxicity of MDC-22 in hamsters, which represents an appropriate model for carcinogenic studies [31], and complements the safety findings in mice and rats. Five-week old Syrian golden hamsters were treated with increasing doses of MDC-22 for 8 days. Even at a dose of 400 mg/kg, MDC-22 was well tolerated, with the hamsters showing no weight loss or other signs of toxicity during treatment (Fig. 4A). Indeed, the body weights of vehicle- and MDC-22-treated animals increased during the treatment days and were comparable throughout the study. Moreover, no adverse effects of MDC-22 on liver and pancreas function were noted, with no changes in the levels of multiple liver enzymes (alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and Gamma-glutamyl transferase) and amylase between the groups (Fig. 4B).
Fig. 4.
MDC-22 displays no acute toxicity in hamsters. Syrian female hamsters were treated with escalating doses of MDC-22, as described in Materials and Methods. A: Hamster's body weight progression during treatment days for control and MDC-22 treated groups. Results are presented as the mean ± SD. B: Serum levels of liver enzymes and amylase for control and MDC-22-treated hamsters at the end of the treatment period. Results are presented as the mean ± SD. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, Gamma-glutamyl transferase.
MDC-22 enhances the growth inhibitory effect of chemotherapeutic drugs in vitro
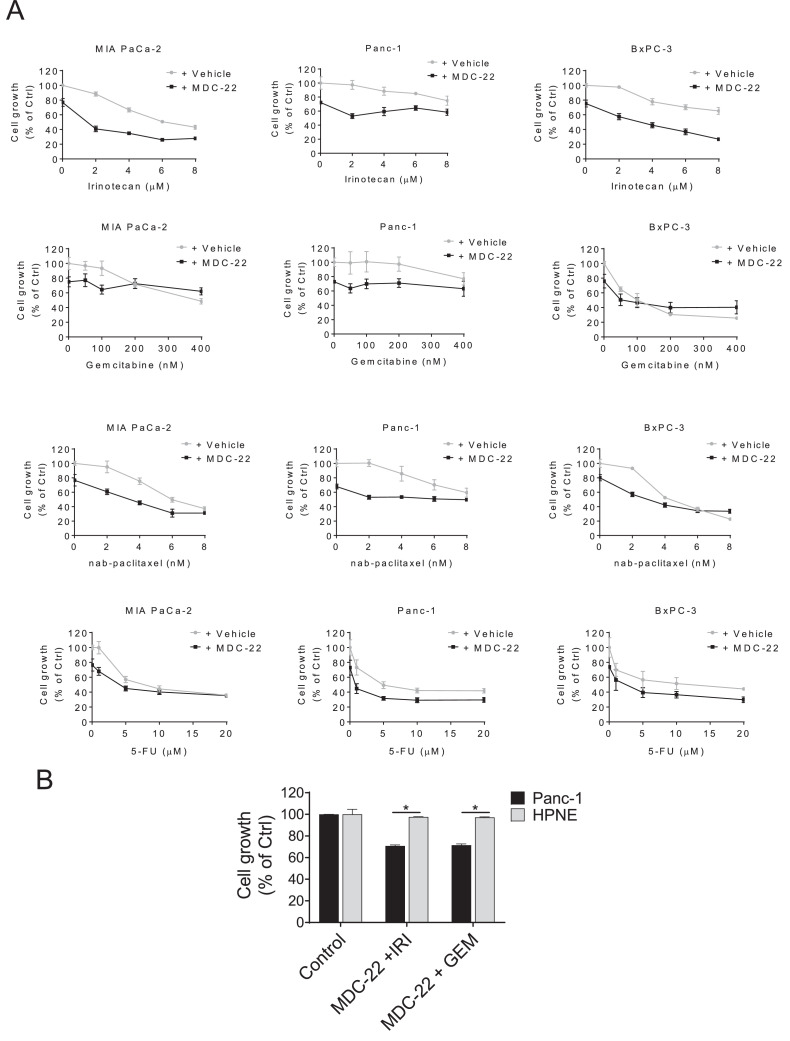
Given that drug combination for the treatment of PC is a common practice, we next evaluated the efficacy of MDC-22 in combination with various chemotherapeutic agents in a panel of three human PC cells lines. MIA PaCa-2, Panc-1 and BxPC-3 cells were treated for 48 h with MDC-22 0.25xIC50 alone or in combination with escalating concentrations of irinotecan (0 to 8 µM), gemcitabine (0 to 400 nM), nab-paclitaxel (0 to 8 nM), or 5-FU (0 to 20 µM). The concentrations were chosen based on growth inhibitory effects by these chemotherapeutic drugs [21]. Even though the cell growth inhibitory additive outcomes of the drug combinations were variable in the multiple cell lines, MDC-22 showed a strong combination effect, enhancing the anticancer effect of irinotecan, gemcitabine and nab-paclitaxel, and to a lesser effect that of 5-FU (Fig. 5A). Among the multiple drug combinations, MDC-22 plus irinotecan was the best drug combination to reduce cancer cell growth. For instance, treatment with MDC-22 0.25xIC50 for 48h reduced cell growth by 24%, 27% and 25% in MIA PaCa-2, Panc-1 and BxPC-3 cells, respectively. Treatment with irinotecan 2 µM for 48 had minimum effect on cell growth reducing it by 12%, 3% and 3% in MIA PaCa-2, Panc-1 and BxPC-3 cells, respectively. Interestingly, combination of MDC-22 0.25xIC50 and irinotecan 2 µM reduced cell growth by 60, 48 and 43% (p<0.01 for all three) in MIA-PaCa-2, Panc-1 and BxPC-3 cells, respectively (Fig. 5A), highlighting a strong additive effect between MDC-22 and irinotecan in all three cell lines.
Fig. 5.
MDC-22 enhances the growth inhibitory effect of chemotherapeutic drugs in vitro
A: Cell growth was determined in MIA PaCa-2, Panc-1 and BxPC-3 cells treated for 48h with DMSo (Vehicle) or MDC-22 0.25xIC50 alone or in combination with gemcitabine, nab-paclitaxel, 5-FU or irinotecan in escalating concentrations, 0 to 400 nM, 0 to 8 nM, 0 to 20 µM and 0 to 8 µM, respectively. Results are expressed as % control. B: Cell growth was determined in Panc-1 and HPNE cells with MDC-22 400 µM alone or in combination with irinotecan 2 µM (IRI) or gemcitabine 50 nM (GEM) for 48 h. Results are expressed as % control; *p < 0.01.
Next, we evaluated the selectivity of MDC-22 in combination with irinotecan or gemcitabine on cell growth in PC cells and compared it to that of HPNE cells. For this purpose, HPNE and Panc-1 cells were treated with MDC-22 400 µM either alone or in combination with irinotecan 2 µM or gemcitabine 50 nM for 48 h. While MDC-22 + gemcitabine, or MDC-22 + irinotecan significantly reduced Panc-1 cell growth by 35% and 37%, respectively, MDC-22 + irinotecan or gemcitabine, under the same experimental conditions, was unable to reduce HPNE cell growth (Fig. 5B). These results show that MDC-22 alone and in combination with irinotecan or gemcitabine is selective to PC cells, sparing the normal cells (Fig. 5B).
MDC-22 enhances irinotecan's effect on PC cell migration
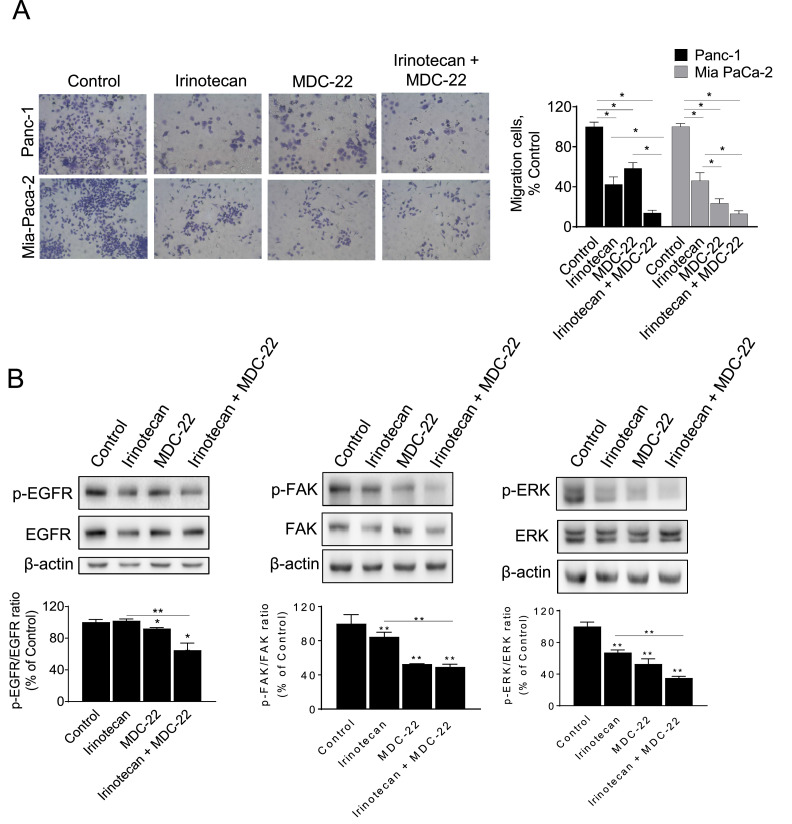
Finally, we evaluated the ability of MDC-22 both alone and in combination with irinotecan to inhibit migration using transwell inserts in PC cells. Treatment with MDC-22 or irinotecan alone resulted in a 41.5% and 57.5% reduction of migratory cells in Panc-1 cells and in a 77.0% and 53.9% in MIA PaCa-2 cells, compared to controls (Fig. 6A). Co-treatment with MDC-22 and irinotecan significantly decreased the number of migratory cells by 86.2% and 87.0% in Panc-1 and MIA PaCa-2 respectively, compared to controls. Of note, this effect was significantly different to the one observed by either drug alone (Fig. 6A).
Fig. 6.
MDC-22 enhances irinotecan's effect on PC cell migration. Human Panc-1 and MIA PaCa-2 PC cells treated with MDC-22 0.25xIC50, Irinotecan 2 µM or both in combination for 48 h and evaluated for their migratory A: Transwell migration assay as described under Materials and Methods. Results are expressed as % control. *p < 0.05. B: Immunoblots of EGFR, p-EGFR, FAK, p-FAK, ERK and p-ERK in total cell protein extracts from Panc-1 cells treated with MDC-22 0.25xIC50, irinotecan 2 µM or both for 24 h. Loading control: β-actin. Bands were quantified and results are expressed as % control; *p < 0.05, **p < 0.01.
Finally, we determined whether MDC-22 ± irinotecan could modulate the activation of EGFR, FAK and ERK in Panc-1 cells. Treatment with MDC-22 0.25xIC50 for 24 h, both alone and in combination with irinotecan 2 µM, significantly reduced the activation of the above mentioned proteins in contrast to vehicle-treated control cells. Cells treated only with irinotecan also shown a reduced activation of these proteins, except for EGFR that remained similar to control values (Fig. 6B).
Discussion
PC is currently one of the deadliest types of cancer, with low improvement in survival rates. During the last few decades, there has been a significant effort to develop effective therapies to fight this disease [32]. We have previously documented that MDC-22 has strong chemopreventive efficacy in preclinical models of PC [14]. Our present data indicate that MDC-22 possess strong chemotherapeutic efficacy in two clinically relevant preclinical models of pancreatic cancer, acting primarily through the inhibition of EGFR pathway.
The majority of the existing chemotherapeutic agents for PC do not offer much benefit [2]. For instance, gemcitabine has been a standard of care for the treatment of PC for over 20 years, but as a single agent gemcitabine is not curative. Currently, newly diagnosed PC patients would likely receive the combination of gemcitabine with Abraxane [33,34], or FOLFIRINOX, which is the use of a combined therapy of leucovorin-modulated 5-FU, irinotecan, and oxaliplatin. Unfortunately, the majority of patients with advanced PC treated with gemcitabine monotherapy or combination will progress after several months, leaving patients with few options. Thus, there has been an extended effort to develop new chemotherapeutic agents as first line and second-line treatment. In this study, we assessed the anticancer effect of MDC-22 in two complementary and clinically relevant animal models that better mimic the human disease. Multiple studies have used PC PDTX models to demonstrate the potential utility of novel single or combination regimens for treating this tumor type [35,36]. Our findings demonstrate that MDC-22 possesses potent chemotherapeutic efficacy in PDTXs, without any sign of toxicity.
To confirm the findings in the PDTX model, we evaluated the efficacy of MDC-22 to enhance survival in the KPC genetically engineered mouse model. Human PDA arises from pancreatic intra-epithelial neoplasias, frequently driven by activating mutations in Kras, followed by loss or mutation of tumor suppressors, such as p53. The KPC model which conditionally express endogenous mutant Kras and p53 alleles in pancreatic cells [17], develop pancreatic tumors as they age, and recapitulate pathophysiological and molecular features that resemble those observed in human pancreatic ductal adenocarcinoma [37]. It is noteworthy that KPC mice succumb from the diseases at around 6 months of age. MDC-22 extended overall median survival compared to vehicle-treated mice, without signs of toxicity, and the effect of MDC-22 was significantly more pronounced in male KPC mice. Overall, these two clinically relevant preclinical models validate our previous findings and indicate that MDC-22 is efficacious for PC treatment.
Safety is a critical consideration for new therapeutic agents. Under the conditions tested, MDC-22 appears to satisfy this requisite of improved safety. The hamster's studies, which complemented our previous MDC-22’s toxicity studies in mice and rats [13,14,30], assessed multiple parameters of acute toxicity. MDC-22, even at 400 mg/kg, a dose 4-times higher than the dose used in the efficacy studies, was well tolerated, showing essentially no signs of liver toxicity. Overall, these additional studies confirm that MDC-22 appears safe and efficacious in the treatment of pancreatic cancer.
It is well accepted that combination drug therapies are usually more effective than monotherapies [32]. Irinotecan is a crucial anticancer drug in treatment regimens for several solid tumors [38]. This natural product-derivative can be used as a monotherapy but it is more frequently combined with other cytotoxic agents, such as in FOLFIRINOX combo (combination of irinotecan, 5-fluorouracil, leucovorin and oxaliplatin) [39]. Irinotecan rapidly kill cancer cells but it also affects non-tumor cells such as blood cells and epithelial cells. Consequently, it is often accompanied by toxicity, primarily neutropenia and diarrhea [39]. For the above mentioned reasons, FOLFIRINOX is reserved for patients that can tolerate the toxicity associated with it, and new drug combination that may lead to less toxicity and better anticancer effects are under active investigation. MDC-22 proved to be a strong combination partner of irinotecan, the both of them strongly inhibiting cell growth in multiple PC cells. This drug combination shows promise and it would be worth exploring in vivo in the near future.
An important feature of MDC-22, both as a single agent and in combination with irinotecan and gemcitabine, is its selectivity. Anticancer agents should preferably kill the tumor and not the normal tissues. MDC-22 exhibits such selectivity. Compared with human pancreatic cancer cell lines, the normal human pancreatic epithelial cells are more resistant to the growth inhibitory effect of MDC22 ± irinotecan. These observations validate our previous findings that MDC-22 is able to target specifically cancer cells and not normal cells, fact of notable importance in order to ensure less toxicity. This selectivity, together with lack of apparent toxicity, is a significant advantage of MDC-22.
EGFR activation plays an important role in the growth, invasion, adhesion, and angiogenesis of numerous cancers, including PC [8,29,40]. A main mechanism involved in the anti-cancer effect of MDC-22 points to EGFR. EGFR overexpression has been correlated with more advanced disease, poor survival and metastasis of PC [41]. MDC-22 inhibits EGFR activation and EGFR-downstream proteins as FAK and ERK, in PDTXs and KPC models. It is important to note that, at this time, we cannot rule out the possibility that MDC-22 exerts an anti-inflammatory effect and that the inhibition of EGFR may be a consequence of such reduced inflammation or other effect [13,14]. Additional studies are warranted to fully characterize the exact mechanism of MDC-22 effect on EGFR inhibition.
The activation of EGFR can regulate migration of PC cells, in part, through the modulation of FAK [42]. FAK is a central hub for multiple signaling pathways, affecting a series of cellular biological behaviors such as proliferation, survival, adhesion, migration, invasion, and metastasis in various tumor cells [43]. Therefore, the above provides evidence for the importance of EGFR and FAK as targets for the development of anti-metastatic drugs. MDC-22 showed strong effects inhibiting PC cell migration. Furthermore, the anticancer and anti-migratory effects were enhanced with MDC-22 and irinotecan combination.
Collectively, the present results indicate that MDC-22 is a safe and effective anticancer agent in preclinical models of PC, acting primarily through the inhibition of EGFR pathway. Moreover, MDC-22 is a promising combination partner of irinotecan, inhibiting PC cell growth and migration. Taken together, our results highlight the potential of MDC-22 as a chemotherapeutic agent for PC.
CRediT authorship contribution statement
Cecilia Rodriguez Lanzi: Conceptualization, Methodology, Visualization, Investigation, Writing – original draft, Writing – review & editing. Ran Wei: Conceptualization, Methodology, Visualization, Investigation, Writing – review & editing. Dingyuan Luo: Methodology, Visualization, Investigation, Writing – review & editing. Gerardo G. Mackenzie: Conceptualization, Visualization, Investigation, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
All authors declare no competing financial interest.
Acknowledgments
Funding: This work was supported, in part, by grants from the University of California, Davis and grants from NIHCA175699, NIHCA181727 to G.G.M. We thank Mr. Joseph LaComb for the assistance with the animal studies.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.Ying J.E., Zhu L.M., Liu B.X. Developments in metastatic pancreatic cancer: is gemcitabine still the standard? World J Gastroenterol WJG. 2012;18:736–745. doi: 10.3748/wjg.v18.i8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnanamony M., Gondi C.S. Chemoresistance in pancreatic cancer: emerging concepts. Oncol Lett. 2017;13:2507–2513. doi: 10.3892/ol.2017.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamisawa T., Wood L.D., Itoi T., Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., Choné L., Francois E., Artru P., Biagi J.J., et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 7.Ardito C.M., Briggs C.D., Crawford H.C. Targeting of extracellular proteases required for the progression of pancreatic cancer. Expert Opin Ther Targets. 2008;12:605–619. doi: 10.1517/14728222.12.5.605. [DOI] [PubMed] [Google Scholar]
- 8.Navas C., Hernandez-Porras I., Schuhmacher A.J., Sibilia M., Guerra C., Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318–330. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecker T.P., Gladson C.L. Focal adhesion kinase in cancer. Front Biosci. 2003;8:s705–s714. doi: 10.2741/1115. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H., Liu X., Knolhoff B.L., Hegde S., Lee K.B., Jiang H., Fields R.C., Pachter J.A., Lim K.H., DeNardo D.G. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut. 2020;69:122–132. doi: 10.1136/gutjnl-2018-317424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H., Hegde S., Knolhoff B.L., Zhu Y., Herndon J.M., Meyer M.A., Nywening T.M., Hawkins W.G., Shapiro I.M., Weaver D.T., et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulzmaier F.J., Jean C., Schlaepfer D.D. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L., Wong C.C., Mackenzie G.G., Sun Y., Cheng K.W., Vrankova K., Alston N., Ouyang N., Rigas B. Phospho-Aspirin (MDC-22) inhibits breast cancer in preclinical animal models: an effect mediated by EGFR inhibition, p53 acetylation and oxidative stress. BMC Cancer. 2014;14:141. doi: 10.1186/1471-2407-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattheolabakis G., Papayannis I., Yang J., Vaeth B.M., Wang R., Bandovic J., Ouyang N., Rigas B., Mackenzie G.G. Phospho-Aspirin (MDC-22) prevents pancreatic carcinogenesis in mice. Cancer Prev Res (Phila) 2016;9:624–634. doi: 10.1158/1940-6207.CAPR-15-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L., Mackenzie G.G., Sun Y., Ouyang N., Xie G., Vrankova K., Komninou D., Rigas B. Chemotherapeutic properties of phospho-nonsteroidal anti-inflammatory drugs, a new class of anticancer compounds. Cancer Res. 2011;71:7617–7627. doi: 10.1158/0008-5472.CAN-11-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackenzie G.G., Sun Y., Huang L., Xie G., Ouyang N., Gupta R.C., Johnson F., Komninou D., Kopelovich L., Rigas B. Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology. 2010;139:1320–1332. doi: 10.1053/j.gastro.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H., Rustgi A.K., Chang S., Tuveson D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Gopinathan A., Morton J.P., Jodrell D.I., Sansom O.J. GEMMs as preclinical models for testing pancreatic cancer therapies. Dis Model Mech. 2015;8:1185–1200. doi: 10.1242/dmm.021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackenzie G.G., Bartels L.E., Xie G., Papayannis I., Alston N., Vrankova K., Ouyang N., Rigas B. A novel Ras inhibitor (MDC-1016) reduces human pancreatic tumor growth in mice. Neoplasia. 2013;15:1184–1195. doi: 10.1593/neo.131368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei R., Hackman R.M., Wang Y., Mackenzie G.G. Targeting glycolysis with epigallocatechin-3-gallate enhances the efficacy of chemotherapeutics in pancreatic cancer cells and xenografts. Cancers (Basel) 2019:11. doi: 10.3390/cancers11101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo D., Digiovanni M.G., Wei R., Lacomb J.F., Williams J.L., Rigas B., Mackenzie G.G. Phospho-valproic acid (MDC-1112) reduces pancreatic cancer growth in patient-derived tumor xenografts and KPC mice: enhanced efficacy when combined with gemcitabine. Carcinogenesis. 2020;41:927–939. doi: 10.1093/carcin/bgz170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei R., Penso N.E.C., Hackman R.M., Wang Y., Mackenzie G.G. Epigallocatechin-3-Gallate (EGCG) suppresses pancreatic cancer cell growth, invasion, and migration partly through the inhibition of AKT pathway and epithelial-mesenchymal transition: enhanced efficacy when combined with gemcitabine. Nutrients. 2019;11 doi: 10.3390/nu11081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenzie G.G., Queisser N., Wolfson M.L., Fraga C.G., Adamo A.M., Oteiza P.I. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin's lymphoma cells. Int J Cancer. 2008;123:56–65. doi: 10.1002/ijc.23477. [DOI] [PubMed] [Google Scholar]
- 24.Huang L., Zhu C., Sun Y., Xie G., Mackenzie G.G., Qiao G., Komninou D., Rigas B. Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine-dependent effect. Carcinogenesis. 2010;31:1982–1990. doi: 10.1093/carcin/bgq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie G.G., Ouyang N., Xie G., Vrankova K., Huang L., Sun Y., Komninou D., Kopelovich L., Rigas B. Phospho-sulindac (OXT-328) combined with difluoromethylornithine prevents colon cancer in mice. Cancer Prev Res (Phila) 2011;4:1052–1060. doi: 10.1158/1940-6207.CAPR-11-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallangada N.A., Vargas J.M., Thomas S., DiGiovanni M.G., Vaeth B.M., Nemesure M.D., Wang R., LaComb J.F., Williams J.L., Golub L.M., et al. A novel tricarbonylmethane agent (CMC2.24) reduces human pancreatic tumor growth in mice by targeting Ras. Mol Carcinog. 2018;57:1130–1143. doi: 10.1002/mc.22830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez Lanzi C., Perdicaro D.J., Gambarte Tudela J., Muscia V., Fontana A.R., Oteiza P.I., Vazquez Prieto M.A. Grape pomace extract supplementation activates FNDC5/irisin in muscle and promotes white adipose browning in rats fed a high-fat diet. Food Funct. 2020;11:1537–1546. doi: 10.1039/c9fo02463h. [DOI] [PubMed] [Google Scholar]
- 28.Mattheolabakis G., Wang R., Rigas B., Mackenzie G.G. Phospho-valproic acid inhibits pancreatic cancer growth in mice: enhanced efficacy by its formulation in poly-(L)-lactic acid-poly(ethylene glycol) nanoparticles. Int J Oncol. 2017;51:1035–1044. doi: 10.3892/ijo.2017.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardito C.M., Gruner B.M., Takeuchi K.K., Lubeseder-Martellato C., Teichmann N., Mazur P.K., Delgiorno K.E., Carpenter E.S., Halbrook C.J., Hall J.C., et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L., Mackenzie G., Ouyang N., Sun Y., Xie G., Johnson F., Komninou D., Rigas B. The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;162:1521–1533. doi: 10.1111/j.1476-5381.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konishi Y., Tsutsumi M., Tsujiuchi T. Mechanistic analysis of pancreatic ductal carcinogenesis in hamsters. Pancreas. 1998;16:300–306. doi: 10.1097/00006676-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Miller A.L., Garcia P.L., Yoon K.J. Developing effective combination therapy for pancreatic cancer: an overview. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein D., El-Maraghi R.H., Hammel P., Heinemann V., Kunzmann V., Sastre J., Scheithauer W., Siena S., Tabernero J., Teixeira L., et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju413. [DOI] [PubMed] [Google Scholar]
- 34.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne A.T., Alferez D.G., Amant F., Annibali D., Arribas J., Biankin A.V., Bruna A., Budinska E., Caldas C., Chang D.K., et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 36.Blasco M.T., Navas C., Martin-Serrano G., Grana-Castro O., Lechuga C.G., Martin-Diaz L., Djurec M., Li J., Morales-Cacho L., Esteban-Burgos L., et al. Complete regression of advanced pancreatic ductal adenocarcinomas upon combined inhibition of EGFR and C-RAF. Cancer Cell. 2019;35(573-587):e576. doi: 10.1016/j.ccell.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive K.P., Politi K. Translational therapeutics in genetically engineered mouse models of cancer. Cold Spring Harb Protoc. 2014;2014 doi: 10.1101/pdb.top069997. [DOI] [PubMed] [Google Scholar]
- 38.de Man F.M., Goey A.K.L., van Schaik R.H.N., Mathijssen R.H.J., Bins S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin Pharmacokinet. 2018;57:1229–1254. doi: 10.1007/s40262-018-0644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailly C. Irinotecan: 25 years of cancer treatment. Pharmacol Res. 2019;148 doi: 10.1016/j.phrs.2019.104398. [DOI] [PubMed] [Google Scholar]
- 40.Stock A.M., Hahn S.A., Troost G., Niggemann B., Zanker K.S., Entschladen F. Induction of pancreatic cancer cell migration by an autocrine epidermal growth factor receptor activation. Exp Cell Res. 2014;326:307–314. doi: 10.1016/j.yexcr.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald T.L., Lertpiriyapong K., Cocco L., Martelli A.M., Libra M., Candido S., Montalto G., Cervello M., Steelman L., Abrams S.L., et al. Roles of EGFR and KRAS and their downstream signaling pathways in pancreatic cancer and pancreatic cancer stem cells. Adv Biol Regul. 2015;59:65–81. doi: 10.1016/j.jbior.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Ricono J.M., Huang M., Barnes L.A., Lau S.K., Weis S.M., Schlaepfer D.D., Hanks S.K., Cheresh D.A. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Z., Jiang G., Blume-Jensen P., Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]