Abstract
This work aims to enhance the flavor of functional cucumber juice using herbal extracts of peppermint, basil, lavender, and lemongrass ethanolic extracts and extend its lifetime by controlling the chemical and microbial fluctuations. Cucumber juices were processed as; non-supplemented (J-Con), J-PME, J-BE, J-LE, and J-LEE supplemented with peppermint, basil, lavender, and lemongrass ethanolic extracts, respectively. Peppermint extract was significantly scavenged 88% of DPPH radicals and inhibited the growth of tested gram-positive, gram-negative bacteria and fungi followed by the lemongrass extract. The antioxidant activity of cucumber juices increased due to polyphenols and aroma compounds in the added extracts. However, the antioxidant content was decreased after two months of storage at 4 °C, due to the decrease in polyphenols. The flavor compounds were determined using GC mass, wherein hydrocarbons, acids, alcohols, and carbonyl compounds were the main aroma contents in cucumber juices, and their contents decreased with storage time. Peppermint and lemongrass extracts were significantly (p ≤ 0.05) increased the whiteness of J-PME, and J-LEE, respectively. The highest score of flavor and taste was observed in J-PME that scored 8.3 based on panelists' reports followed by J-LEE. The PME was significantly maintained 91% of the odor and color of J-PME as compared to other juices.
Keywords: Volatile compounds, Flavor, Herbal extract, Cucumber, Antioxidants, Antimicrobial, Quality
1. Introduction
The gourd family member, cucumber (Cucumis sativus L.) is of Indian origin with extensive production every year (Mukherjee et al., 2013). The overproduction of the cucumber crop every year makes it spoiled, and the production of cucumber juice finished this problem (Saad et al., 2021a). Another problem represented in prolonging the cucumber juice shelf life and grantee its safety. The use of chemical preservatives such as organic acids resolved this problem (Mishra et al., 2011, Del Nobile et al., 2012). The chemical additives are effective in preventing microbial contamination and chemical changes, which cause food spoilage, but they are very expensive and cause health disorders (Davidson et al., 2012). Natural preservatives like peptides, salt, sugar, lemon, ginger, e.t.c., are cost-effective, nutritious, willingly available, and do not cause any health risks (Davidson et al., 2012, El-Saadony et al., 2020). The extraction method and the used devices were determined the quantity and quality of volatile compounds in herbal extracts. Basil (Ocimum basilicum L.) extract was enriched with terpenes and oxygenated compounds. Linalool, methyl cinnamate, eucalyptol, α-bergamotene, and eugenol were the main volatile compounds in basil detected by GC–MS (Ahmed et al., 2019). It exhibits considerable antioxidant, antimicrobial, and anticancer activity, therefore it can be used in food preservation for months and even years (Park et al., 2016, Salehi et al., 2018). Peppermint (Mentha piperita L.) is a Lamiaceae member. Park et al., 2016, Beigi et al., 2018 and Salehi et al. (2018) found that menthol, menthone, menthofuran, 1,8-cineole, and menthyl acetate were the main flavor compounds in peppermint extract. Fresh or dried Mentha piperita L is implemented in several foods as a flavoring and preservative agent. Lemongrass (Cymbopogon citratus) is an aromatic plant cultivated for essential oils (EO) production; the main flavor compounds in lemongrass extract are citral and geraniol. Because of aroma compounds, it can be employed in industrial sectors (Muala et al., 2021), it exhibits antimicrobial and immunological activity (Alagawany et al., 2021a). On the other hand, lavender (Lavandula) extract is rich in volatile compounds i.e., linalool, linalyl acetate, lavandulyl acetate, which belong to terpenes. The detected compounds act as a flavor enhancers in foods, reveal antimicrobial, and antioxidant activities; therefore, used in food preservation, besides thier anticancer activity, and cure illness (Ouedrhiri et al., 2017, Guo and Wang, 2020). Blending cucumber juice with herbal extracts leads to increasing antioxidant, antimicrobial, and nutritional value and improving sensory properties, as they increase the taste and flavor of the juice (El-Saadony et al., 2020, Saad et al., 2021a).
This work aimed to investigate the effect of peppermint, basil, lavender, and lemongrass ethanolic extracts on flavoring and store stability of cucumber juice at 4 °C for the period of 0, 15, 30, 45, and 60 days). In addition, estimating the antiradical and antimicrobial activities of these extracts. Finally, identifying the volatile compounds: hydrocarbons, acids, alcohols, and carbonyl compounds by GC-mass additionally, evaluating the quality properties of juice during the storage period.
2. Materials and methods
Peppermint, basil, lavender, lemongrass, and cucumber were acquired from a market in Zagazig City, Egypt. All chemicals in this study were in analytical grade and purchased from Sigma (USA). The bacterial media; plate count agar (PCA), and Müller Hinton Agar (MHA) from (Oxoid, UK). The activated microbial isolates of Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, Escherichia coli, Klebsiella pneumonia, and Salmonella typhi. In addition, Candida gelbeta, Candida tropicalis, Candida albicans, Aspergillus niger, Aspergillus fumigatus, and Aspergillus flavus were obtained from the Microbiology Department, Faculty of Agriculture, Zagazig University, Egypt.
2.1. The preparation of herbal extracts
Peppermint, basil, lavender, and lemongrass herbs were dried in DE 66812464 vacuum oven, Germany (45 °C) and were grounded to a fine powder in Moulinex AR110010 mill, France. Powdered material of each herb was extracted for 3 h with ethanol 50% (1:3, w/v) using a magnetic stirrer at room temperature then was filtrated by filtration paper (Whatman No.1) (Saad et al., 2020a, Saad et al., 2021a). The obtained extracts were stored at 4 °C for further analyses.
2.2. The preparation of herbal enriched cucumber juice
The cucumber juices, control, and enriched juices were processed following (El-Saadony et al., 2020; Saad et al., 2021a) with some modifications. The juice was pasteurized at 95 °C for 3 min in TOMY Sx-300 autoclave (Japan) and then directly cooled. The processed juices were filled into 350 mL sterilized bottles and were stored at 4 °C for two months, further analyses were carried out at intervals of storage period (0, 15, 30, 45, and 60 days).
2.3. Chemical analysis
2.3.1. Total phenolic compounds (TPC)
The TPC was estimated according to Folin-Ciocalteu method (Škerget et al., 2005) with some modifications. 50 µL of sodium carbonate 7.5% and diluted Folin-Ciocalteu was added to 50 µL of herbal extracts in microtiter plate. The plate was incubated in microtiter plate reader (BioTek Elx808, USA) and the absorbance was read after 30 min at 750 nm as mg GAE/mL using a standard curve of Gallic acid liner equation.
| y = 0.005x + 0.1455, R2 = 0.9957; y is the absorbance, and x is the TPC concentration (mg GAE/mL). |
2.3.2. Antioxidant activity
The radical scavenging activity of herbal extracts (800 µg/mL) was deduced from the ability to convert the purple color of DPPḢ to yellow as compared to TBHQ as synthetic antioxidant according to Hatano et al., (1988) with some modifications. An aliquot (50 µL) of herbal extracts (800 µg/mL) was mixed with 100 µL of ethanolic DPPH in microtiter plate and incubated in microtiter plate reader (BioTek Elx808, USA), the absorbance was recorded after 30 min at 517 nm as compared to control. DPPḢ scavenging activity (%) was calculated in the following equation:
2.3.3. Flavor compounds profile by GC-Mass
The flavor components in cucumber juices, control, and enriched were isolated following (Saad et al., 2020a). 10 mL of each juice was extracted with diethyl ether (1:10, w: v) three times (15 min each). The solvent was separated using BUCHI R-114 rotary evaporator, Germany. The solvent-free extracts were diluted with 1 mL of anhydrous ethyl alcohol: hexane (1:1; v, v) and centrifuged (Sigma 3-30KHS, UK) at 4000g for 10 min; the supernatant was collected, dried over sodium sulfate, and filtered. One µL of the filtrate was injected into GC/MS. The flavor compounds analysis was carried out on a mass spectrometer Agilent 5975C, carrier gas helium, column HP-5 ms (30 m × 250 μm × 0.25 μm), and temperature: 35 °C/3 min, 5 °C/min to 250 °C for 3 min, total 49 min, carrier gas helium 1 mL/min constant speed; split ratio 30:1. The identification of chemical compounds was made by comparison to their relative retention time and library data. The identified components were arranged in order to compounds groups.
2.4. Microbial analysis
2.4.1. Antibacterial
The antibacterial activity of peppermint, basil, lavender, and lemongrass was tested against six pathogenic bacterial isolates. The bacterial isolates were cultured into fresh broth media and brought into a log phase of growth (1x108 CFU/mL) by incubating at 37° C for 24 h before being used. The antibacterial activity was estimated using disc diffusion assay (Alagawany et al., 2021b, El-Saadony et al., 2021a, Abou-Kassem et al., 2021). Petri plates were poured with sterilized MHA and left undisturbed for 24 h. 100 µL of each bacterial culture was spread over MHA plates. Paper discs (6 mm) were saturated with herbal extract concentrations (200, 400, and 800 µg/mL) and then were put on both sides of the plates.Discs were saturated with sterilized distilled water were control. The plates were incubated at 37 °C for 24 h. The inhibition zones around the discs were observed. The minimum inhibitory concentration (MIC) was the lowest concentration of herbal extracts, which inhibited the bacterial growth. The herbal extracts' concentrations were mixed with bacterial inoculum in Muller Hinton broth (MHB) and incubated at 37 °C for 24 h, and the turbidity measured at 660 nm (El-Saadony et al., 2021b, El-Saadony et al., 2021c). The minimum bactericidal concentration (MBC) was the lowest concentration of herbal extracts, which killed the bacteria and was estimated by spreading an aliquot of MIC tubes at MHA plates and the bacterial growth was monitored (El-Saadony et al., 2021d, Abd El-Hack et al., 2021a).
2.4.2. Antifungal
The antifungal activity of herbal extracts against tested fungi was estimated according to Saad et al., 2021b, El-Saadony et al., 2021e. The spore suspension of fungal inoculum (1 × 103 CFU/mL) was prepared by taking a small portion of fungal mycelium, inoculated in potato dextrose broth (PDB), and incubated at 28 °C for five days. The fungal inoculum was spread over the potato dextrose agar (PDA) plates' surface. Paper discs (6 mm) were saturated with different herbal extracts' concentrations (200, 400, and 800 µg/ml) and placed on the PDA plates surface. The PDA plates were incubated at 28 °C for five days. The inhibition zones diameters (mm) produced by varying concentrations of the herbal extract against the tested fungi was measured after incubation. The MIC and minimum fungicidal concentration (MFC) were determined following El-Saadony et al. (2019), and El-Saadony et al. (2021f).
2.4.3. Bacterial count in cucumber juices
The bacterial load in cucumber juices: control, and enriched was performed during storage period (0, 15, 30, 45, and 60 days) at 4 °C. Ten mL of herbal enriched cucumber juices was stirred with 90 mL of sterilized saline peptone water for 10 min at room temperature to obtain 10−1 dilution. Serial dilutions from the previous dilution were prepared up to 10−6 (APHA., 1992, Ashour et al., 2020, Reda et al., 2021a). 1 mL of each dilution was aseptically added to the specific agar plate. The PCA was used for the enumeration of total viable count in the samples after incubation for a day at 37 °C, also, PCA was used to calculate psychrophilic bacterial count after seven days incubation at 10 °C. Colonies counts were converted logarithms (CFU/mL) (Lee, 2009, Reda et al., 2020, Sheiha et al., 2020, Reda et al., 2021b).
2.5. Color parameters and sensory evaluation
The color parameters (L*, a* and b*) of cucumber juices were measured by Hunter Lab spectrophotometer (Color Flex EZ's 45°/0°, USA) according to Hunter (1975).
The cucumber juices; control and enriched with herbal extracts were served to 12 semi-trained panelists in Food science department, Fac. Agric., Zagazig Univ., Egypt. The sensorial traits (color, flavor, odor, overall acceptability) were measured using 9-point hedonic scale as described by Amerine et al. (1965) to award a score (from 1 = dislike extremely to 9 = like extremely). The panelists were used plain water to change mouth feel between sessions (Joshi et al., 2017).
2.6. Statistical analysis
One-way ANOVA test was used to analyze the triplicate data means at p ≤ 0.05. LSD test was used to compare the significant differences between means. The analysis was carried out using SPSS program (v 2019).
3. Results
3.1. Phenolic content and scavenging activity of herbal extracts
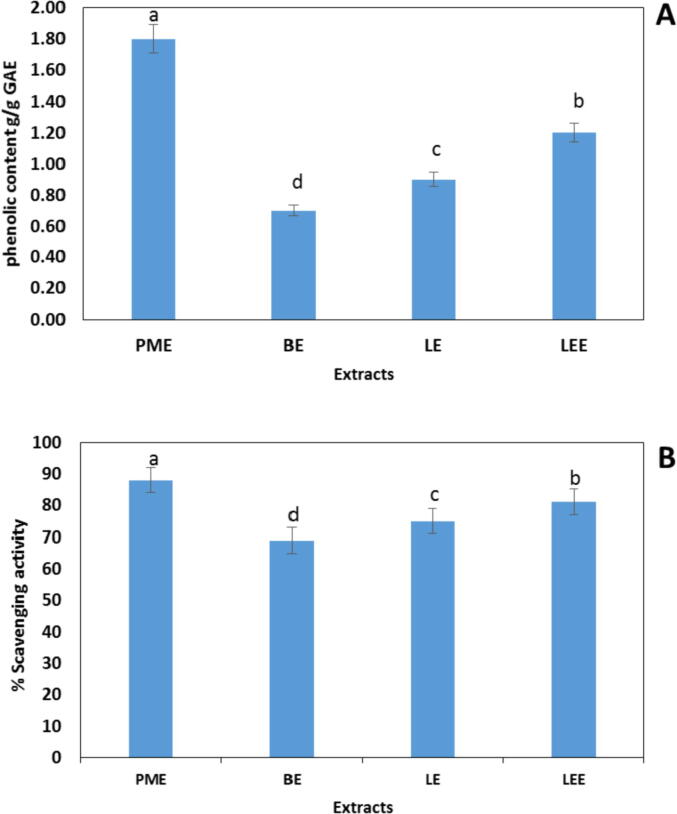
Fig. 1A showed the phenolic contents of peppermint extract (PME), basil extract (BE), lavender extract (LE), and lemongrass extract (LEE). The PME had the highest phenolic content i.e., 1.8 g/g GAE with a relative increase of 33–61% about other extracts. The antioxidant activity of extracts depends on the phenolic content, where PME significantly scavenged 88% of DPPḢ followed by LEE, which scavenged 81% of DPPH radicals (Fig. 1B).
Fig. 1.
(A) Phenolic contents of peppermint extract (PME), basil extract (BE), lavender extract (LE), and lemongrass extract (LEE); (B) DPPḢ Scavenging activity of PME, BE, LE, and LEE after 30 min.
3.2. Antimicrobial activity of herbal extracts
Table 1 showed that PME, BE, LE, and LEE have considerable antimicrobial activity against tested bacteria and fungi assessed by inhibition zones diameters (mm). The inhibition zones diameters (IZDs) significantly increased with increasing concentrations. The IZDs of PME against tested bacteria ranged from 13 to 28 mm, BE in the range of 8–21 mm, LE from 9 to 23 mm, and LEE in the 11–24 mm range. Staphylococcus aureus and Klebsiella pneumonia were the most vulnerable gram positive and negative bacteria to tested extracts, i.e., 21–28 mm, and 17–22 mm, respectively; however, Listeria monocytogenes and Salmonella typhi were the most resistant. On the other hands, the fungi affected by herbal extracts in the IZDs ranges of (12–30 mm), (10–23 mm), (11–25 mm), and (14–30 mm), respectively. The PME and LEE have similar antifungal activity against tested fungi. Candida gelbeta and Aspergillus niger were the most resistant fungi; however, Candida albicans and Aspergillus fumigatus were the most sensitive fungi to herbal extracts concentrations. Table 2 showed the least concentrations, which inhibited the tested bacteria and fungi. The PME (60–150 µg/mL) was inhibited the pathogenic bacteria and fungi, while LEE (50–140 µg/mL) made the same action; however, BE, and LE inhibited the microorganism at higher levels. The lowest concentration of PME that killed tested bacteria and fungi was in the range of 110–270 µg/mL followed by LEE with 140–250 µg/mL.
Table 1.
Antimicrobial activity of peppermint extract (PME), basil extract (BE), lavender extract (LE), and lemongrass extract (LEE) against tested bacteria and fungi (mean ± SD).
| Microorganisms | PME (µg/mL) |
BE (µg/mL) |
LE (µg/mL) |
LEE (µg/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | 200 | 400 | 800 | 200 | 400 | 800 | 200 | 400 | 800 | 200 | 400 | 800 |
| Bacillus cereus | 17 ± 0.2b | 22 ± 0.1b | 26 ± 0.2b | 11 ± 0.1ab | 15 ± 0.3b | 19 ± 0.3b | 12 ± 0.1b | 16 ± 0.6b | 21 ± 0.6b | 14 ± 0.1b | 16 ± 0.7b | 21 ± 0.2b |
| Staphylococcus aureus | 19 ± 0.3a | 25 ± 0.2a | 28 ± 0.1a | 12 ± 0.2a | 17 ± 0.4a | 21 ± 0.1a | 14 ± 0.4a | 18 ± 0.4a | 23 ± 0.2a | 16 ± 0.3a | 19 ± 0.2a | 24 ± 0.2a |
| Listeria monocytogenes | 16 ± 0.4bc | 21 ± 0.3bc | 24 ± 0.4c | 10 ± 0.5b | 14 ± 0.1bc | 18 ± 0.2bc | 11 ± 0.3bc | 15 ± 0.1bc | 19 ± 0.1c | 13 ± 0.4bc | 15 ± 0.3bc | 20 ± 0.1c |
| Escherichia coli | 14 ± 0.1 cd | 17 ± 0.4d | 20 ± 0.8e | 9 ± 0.2c | 12 ± 0.8 cd | 16 ± 0.4 cd | 9 ± 0.5 cd | 13 ± 0.2 cd | 16 ± 0.3de | 11 ± 0.5 cd | 13 ± 0.5 cd | 18 ± 0.4d |
| Klebsiella pneumonia | 15 ± 0.5c | 20 ± 0.8c | 22 ± 0.9d | 10 ± 0.9bc | 13 ± 0.4c | 17 ± 0.3c | 10 ± 0.2c | 14 ± 0.3c | 17 ± 0.4d | 12 ± 0.6c | 14 ± 0.1c | 19 ± 0.5 cd |
| Salmonella typhi | 13 ± 0.8d | 16 ± 0.7e | 19 ± 0.1f | 8 ± 0.5c | 11 ± 0.3d | 14 ± 0.6d | 9 ± 0.4d | 12 ± 0.4d | 15 ± 0.3e | 10 ± 0.1d | 12 ± 0.4d | 17 ± 0.7e |
| Fungi | ||||||||||||
| Candida gelbeta | 14 ± 0.7d | 18 ± 0.5d | 21 ± 0.5d | 11 ± 0.2c | 13 ± 0.4d | 18 ± 0.3e | 12 ± 0.2c | 15 ± 0.5d | 17 ± 0.1d | 16 ± 0.3 cd | 20 ± 0.2 cd | 25 ± 0.3 cd |
| Candida tropicalis | 16 ± 0.5bc | 20 ± 0.1c | 23 ± 0.2 cd | 12 ± 0.1bc | 16 ± 0.8bc | 20 ± 0.5 cd | 14 ± 0.3bc | 16 ± 0.1 cd | 19 ± 0.2 cd | 18 ± 0.2bc | 21 ± 0.3c | 24 ± 0.2d |
| Candida albicans | 17 ± 0.4b | 23 ± 0.5b | 26 ± 0.4b | 15 ± 0.4a | 18 ± 0.4ab | 22 ± 0.1ab | 16 ± 0.4ab | 19 ± 0.2b | 22 ± 0.4b | 19 ± 0.5b | 23 ± 0.1b | 26 ± 0.4c |
| Aspergillus niger | 12 ± 0.1e | 15 ± 0.8e | 19 ± 0.2e | 10 ± 0.1d | 14 ± 0.3c | 19 ± 0.2d | 11 ± 0.2d | 14 ± 0.4e | 16 ± 0.6e | 14 ± 0.8d | 18 ± 0.4d | 21 ± 0.1e |
| Aspergillus fumigatus | 19 ± 0.2a | 25 ± 0.1a | 30 ± 0.4a | 15 ± 0.2a | 19 ± 0.5a | 23 ± 0.4a | 17 ± 0.5a | 21 ± 0.6a | 25 ± 0.2a | 21 ± 0.2a | 25 ± 0.2a | 30 ± 0.3a |
| Aspergillus flavus | 15 ± 0.4c | 19 ± 0.4 cd | 24 ± 0.3c | 13 ± 0.6b | 17 ± 0.2b | 21 ± 0.7b | 15 ± 0.6b | 17 ± 0.9c | 20 ± 0.3c | 17 ± 0.3c | 22 ± 0.5bc | 28 ± 0.4b |
Means with different lowercase letters in the same column indicate significant difference (p ≤ 0.05)
Table 2.
MIC, MBC, and MFC levels of peppermint extract (PME), basil extract (BE), lavender extract (LE), and lemongrass extract (LEE).
| Microorganisms | PME (µg/mL) |
BE (µg/mL) |
LE (µg/mL) |
LEE (µg/mL) |
||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Bacillus cereus | 70e | 140e | 110e | 200e | 100e | 190e | 80e | 150e |
| Staphylococcus aureus | 60f | 110f | 100f | 190f | 90f | 160f | 70f | 140f |
| Listeria monocytogenes | 90d | 150d | 130d | 260d | 120d | 240d | 100d | 200d |
| Escherichia coli | 110b | 200b | 140b | 250b | 130b | 240b | 120b | 210b |
| Klebsiella pneumonia | 100c | 180c | 130c | 250c | 120c | 220c | 110c | 200c |
| Salmonella typhi | 120a | 210a | 160a | 300a | 150a | 280a | 140a | 250a |
| Fungi | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| Candida gelbeta | 120b | 230b | 140b | 250b | 130b | 250b | 110b | 200b |
| Candida tropicalis | 95d | 160d | 120d | 220d | 110d | 200d | 85d | 150d |
| Candida albicans | 80e | 140e | 110e | 200e | 90e | 160e | 65e | 100e |
| Aspergillus niger | 150a | 270a | 170a | 300a | 160a | 310a | 130a | 240a |
| Aspergillus fumigatus | 65f | 110f | 90f | 150f | 75f | 120f | 50f | 90f |
| Aspergillus flavus | 110c | 200c | 125c | 230c | 120c | 230c | 100c | 180c |
Means with different lowercase letters in the same column indicate significant difference (p ≤ 0.05).
3.3. Flavor compounds profile in cucumber juices control and enriched with herbal extracts
Table 3 showed the flavor compounds profile of herbal-enriched juices. Hydrocarbons, acids, alcohols, aldehydes, and ketones were the main compounds in J-PME. Docosane, 11decyl, Docosane, 7 decyl, and Pentatriacontane were the abundant hydrocarbons in J-PME juices with 25.1, 20.1, and 16.5%, respectively with a relative increase of 5–20% about control and J-BE, J-LE, and J-LEE; however, the other hydrocarbons in low contents. Acetic and nonanoic acids were the prominent acids in juices. Nonanoic acid was higher in J-LE and J-PME with 12.5 and 11.5%, respectively with a relative increase of 40–50% compared to control. Acetic acid, hexanoic acid, pentanoic acid, and octanoic acid were not detected in J-Con; however, the addition of herbal extracts enriched the cucumber juice with these acids. Alcohols, aldehydes, and ketones were the most volatile compounds that flavored the juices. 3, 7, 11, 15 Tetramethyl 2 hexadecen1ol was higher in J-BE, while citral, linalool, menthol, and α-terpinol were higher in J-PME with 48.7, 28.7, 31.6, and 38.2%, respectively as compared to other juices. The contents of citronellol and geraniol were higher in J-LEE than other juices; however, J-BE witnessed higher contents of Eugenol and 1,8 cineole. The odorant compounds in cucumber: Hexanal, (E)-2-hexenal, and (E)-6-nonenal maintained by the addition of peppermint extract; therefore, J-PME had the highest contents of these compounds compared to other juices. In addition, Linalyl acetate, and dihydroactinidiolide were abundant carbonyl compounds in J-PME. However, Linalyl acetate, D-Limonene, and bergamotene were the highest aldehydes and ketones in J-BE. Lavender-enriched juice witnessed higher contents of lavandulyl acetate, linalyl acetate, and linalyl oxide.
Table 3.
Flavor compounds profile in peppermint, basil, lavender, and lemongrass extract enriched juices stored at 4 °C for 45 days.
| Compounds | Relative concentration (%) |
||||
|---|---|---|---|---|---|
| J-Con | J-PME | J-BE | J-LE | J-LEE | |
| Hydrocarbons | |||||
| Tricosane(CAS) | 8.07c | 10.2a | 8.9bc | 7.5c | 9.1b |
| Docosane, 11decyl (CAS) | 22.7b | 25.1a | 21.8bc | 20.5c | 23.2b |
| Docosane, 7 decyl (CAS) | 19.17b | 20.1a | 19.5ab | 18.2c | 19b |
| Pentatriacontane (CAS) | 14.69bc | 16.5a | 15.1b | 13.7c | 15.2ab |
| Neophytadiene | 7.76b | 8.7a | 8.2a | 7.2b | 7.6b |
| Docosane (CAS) | 8.84b | 9.5a | 8.5b | 8.1c | 9ab |
| Dotriacontane (CAS) | 6.41b | 7.2a | 6.5b | 6.3b | 6.9ab |
| 5,5 Diethylheptadecane | 5.42b | 6.2a | 5.7b | 5.1b | 5.3ab |
| Octadecane, 1[2(hexadecyloxy) ethoxy](CAS) | 2.86d | 13.9a | 12.6b | 11.8c | 13ab |
| Supraene | 1.71d | 11.6a | 9.2b | 7.7c | 10.2ab |
| 2-Pentylfuran | 7.06c | 10.5bc | 10.9bc | 11.2b | 12.8a |
| Acids | |||||
| Acetic acid | – | 8.9a | 2.3c | 5.6b | 0.5d |
| Hexanoic acid | – | 1.6a | 0.5c | 1b | 1.3b |
| Pentanoic acid | – | 1.2a | 0.8b | 1.3a | 1.1a |
| Octanoic acid | – | 1.1a | 0.9a | 0.8b | 1a |
| Nonanoic acid | 6.17c | 11.5b | 6.5c | 12.5a | 0.9d |
| Alcohols | |||||
| 3,7,11,15 Tetramethyl 2 hexadecen1ol | 3.11c | 9.2a | 8.9ab | 8.7ab | 7.7b |
| Citral | 17.7c | 48.7a | 1.3e | 5.1d | 36.6b |
| Citronellol | 0.32c | 0.8b | – | – | 2.9a |
| Linalool | 7.27d | 28.7a | 16.7b | 13.5c | 5.4d |
| Geraniol | 1.53c | 1.9c | – | 2.3b | 16.85a |
| Eugenol | 0.94b | 1.2b | 11.3a | 0.5c | 0.9b |
| 1,8 cineole | 0.34d | 0.9c | 16.4a | 2.9b | 0.5d |
| Menthol | 0.57c | 31.6a | tr | 0.3c | 2.2b |
| α-Terpinol | tr | 38.2a | 1.5b | 2.1b | – |
| Carbonyl compounds | |||||
| Hexanal | 1.5ab | 2.2a | tr | 0.3c | 0.9b |
| (E)-2-hexenal | 1.3b | 2.1a | tr | 0.2c | 0.8c |
| (E)-6-nonenal | 2.1b | 3.2a | tr | 0.7c | 1.1c |
| Lavandulyl acetate | – | – | – | 12.5a | – |
| Linalyl acetate | – | 21.2b | 11.2d | 48.7a | 18.9c |
| Linalyl oxide | – | 0.8b | 0.1c | 1.9a | 0.5b |
| D-Limonene | – | 8.1c | 12.5a | 1.5d | 10.2b |
| Bergamotene | – | – | 14.9a | – | – |
| Dihydroactinidiolide | – | 28.1a | – | – | – |
Means with different lowercase letters in the same raw indicate significant difference (p ≤ 0.05).
3.4. Fluctuation in polyphenols content and antioxidant activity in cucumber juices during storage
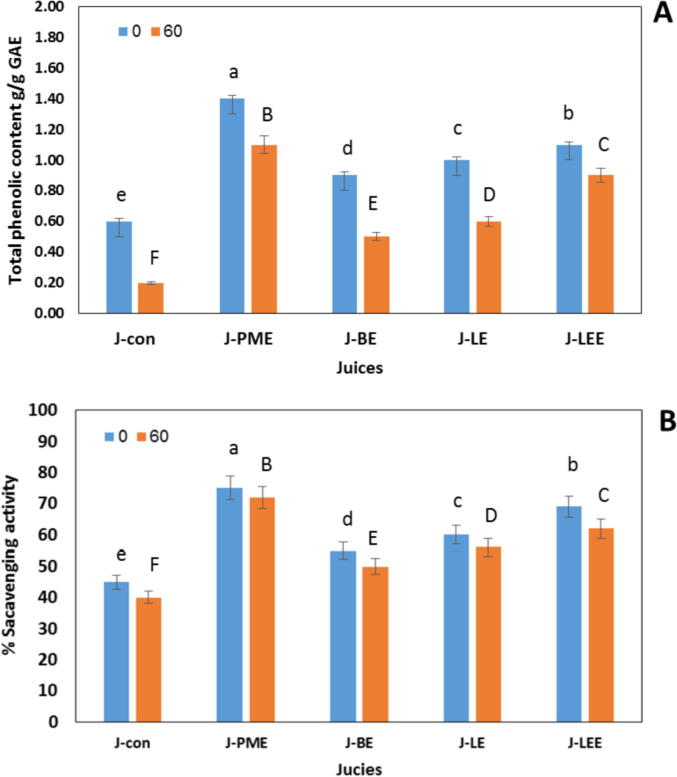
Fig. 2 showed that total phenolic content significantly decreased with the storage period of 2 months. The phenolic compounds in enriched-cucumber juice with lemongrass and peppermint extract were decreased with 20, and 18%, respectively after two monthes. The decrease in J-Con reached 60% followed by J-BE. The same route was observed in Fig. 2B, where the scavenging activity was decreased because of the decline in total phenolic contents. The scavenging activity significantly decreased with 8–11% in J-BE, J-LEE, and control juice at the end of storage.
Fig. 2.
Fluctuations in total phenolic content (A) and antioxidant activity (B) during cold storage for 2 months, means with different lowercase letters indicate significant differences between phenolic and antioxidant contents and uppercase indicate significant differences between phenolic and antioxidant contents during storage.
3.5. Bacterial counts in cucumber juices
The bacterial counts in herbal enriched juices were presented in Table 4. The total viable and psychrophilic bacterial counts were increased in time dependent manner; however, the bacterial count decreased with the herbal supplementation. Peppermint extract was most effective extract in reducing the bacterial count with 40% at the end of storage period as compared to control. After 2 months storage, the bacterial count was log 5 CFU/mL and that was detected in control juice after 15 days storage at 4 °C.
Table 4.
Total viable count and psychrophilic bacteria of herbal enriched juices stored for 2 months at 4 °C (mean ± SD).
| Storage (month) |
0 |
15 |
30 |
45 |
60 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | TVC | PC | TVC | PC | TVC | PC | TVC | PC | TVC | PC |
| J-con | 4.0 ± 0.2a | 1.2 ± 0.5 | 4.8 ± 0.1 | 2.8 ± 0.1 | 5.7 ± 0.1 | 3.4 ± 0.2 | 6.9 ± 0.5 | 4.1 ± 0.1 | 7.5 ± 0.2 | 4.9 ± 0.2 |
| J-PME | 3.1 ± 0.1c | 0.6 ± 0.3 | 3.3 ± 0.2 | 1.5 ± 0.09 | 3.8 ± 0.2 | 2.2 ± 0.1 | 4.2 ± 0.2 | 2.7 ± 0.2 | 5.0 ± 0.1 | 3.0 ± 0.5 |
| J-BE | 3.9 ± 0.3a | 1.1 ± 0.7 | 4.2 ± 0.5 | 2.4 ± 0.1 | 4.9 ± 0.6 | 2.9 ± 0.2 | 5.6 ± 0.7 | 3.5 ± 0.3 | 6.3 ± 0.3 | 3.9 ± 0.1 |
| J-LE | 3.8 ± 0.2b | 1.0 ± 0.5 | 4.1 ± 0.4 | 2.1 ± 0.1 | 4.7 ± 0.3 | 2.7 ± 0.1 | 5.3 ± 0.3 | 3.2 ± 0.1 | 6.0 ± 0.4 | 3.7 ± 0.1 |
| J-LEE | 3.5 ± 0.5b | 0.9 ± 0.3 | 3.7 ± 0.2 | 1.9 ± 0.0 | 4.0 ± 0.4 | 2.5 ± 0.1 | 4.8 ± 0.2 | 3.0 ± 0.0 | 5.7 ± 0.7 | 3.5 ± 0.5 |
TVC; total viable count; PC; psychrophilic count. The data means are presented mean ± SD; means with different lowercase letters in the same column indicate significant difference between treatments.
3.6. Color parameters and sensorial traits
Table 5 showed the fluctuations in color parameters and sensorial traits of tested juices. Generally, the color and sensorial traits declined with the storage time. A lower decrease was observed in J-LEE and J-PME. The supplementation of cucumber juice with LEE and PME significantly increased the L* value from 21.95 to 27.4 and 26.5, respectively. The yellowness of juice increased in J-PME and J-BE. Regarding the sensorial traits, J-PME had the best color, odor, and taste properties, where scored 8.3 based on panelists' report followed by J-LEE. The PME was significantly maintained 91% of juice's color and odor compared to the other juices.
Table 5.
Changes in color parameters (L, a, b) and sensorial traits of herbal enriched juices stored for 2 months at 4 °C.
| Color parameters |
J-con |
J-PME |
J-BE |
J-LE |
J-LEE |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Storage period (day) | 0 | 60 | 0 | 60 | 0 | 60 | 0 | 60 | 0 | 60 |
| L* | 21.95 ± 0.2 | 20.8 ± 0.2 | 26.5 ± 0.2 | 25.9 ± 0.2 | 25.9 ± 0.3 | 24.8 ± 0.2 | 23.3 ± 0.2 | 22.8 ± 0.7 | 27.4 ± 0.2 | 26.8 ± 0.2 |
| a* | −3.3 ± 0.3 | −3.9 ± 0.1 | −2.7 ± 0.3 | −2.8 ± 0.0 | −2.8 ± 0.2 | −3.0 ± 0.1 | −3.2 ± 0.1 | −3.9 ± 0.2 | −2.5 ± 0.1 | −2.9 ± 0.3 |
| b* | 9.8 ± 0.1 | 8.95 ± 0.3 | 10.2 ± 0.6 | 9.9 ± 0.3 | 10.5 ± 0.6 | 9.8 ± 0.3 | 8.8 ± 0.3 | 8.0 ± 0.3 | 9.5 ± 0.3 | 9.0 ± 0.1 |
| Sensorial traits | 0 | 60 | 0 | 60 | 0 | 60 | 0 | 60 | 0 | 60 |
| Color | 8.1 ± 0.3a | 7.5 ± 0.2a | 8.5 ± 0.4a | 8.2 ± 0.2 | 7.7 ± 0.3 | 7.0 ± 0.5 | 7.9 ± 0.3 | 7.3 ± 0.2 | 8.4 ± 0.2 | 8.0 ± 0.1 |
| Odor | 7.5 ± 0.4b | 7.2 ± 0.3a | 8.2 ± 0.3b | 8.0 ± 0.3 | 8.0 ± 0.8 | 7.5 ± 0.3 | 8.1 ± 0.5 | 7.8 ± 0.3 | 8.1 ± 0.1 | 7.6 ± 0.3 |
| Taste | 8.0 ± 0.2a | 7.0 ± 0.2b | 8.3 ± 0.5a | 8.1 ± 0.5 | 7.8 ± 0.8 | 7.1 ± 0.8 | 8.0 ± 0.2 | 7.5 ± 0.3 | 8.4 ± 0.3 | 8.1 ± 0.4 |
| Overall acceptability | 7.9 ± 0.1ab | 7.2 ± 0.3a | 8.3 ± 0.6a | 8.1 ± 0.2 | 7.8 ± 0.7 | 7.2 ± 0.2 | 8.0 ± 0.3 | 7.6 ± 0.1 | 8.2 ± 0.6 | 7.9 ± 0.6 |
Mean in the same column with different letters are significantly different (p ≤ 0.05).
4. Discussion
Flavor and color are the most important sensory quality, which contributes the juice acceptance and attracts the consumer (Lasekan and Yap, 2018). Nowadays, non-thermal technologies have increased with the increasing demand of consumers to take fresh or minimally processed food products (Lau et al., 2013). These types of food products contain bioactive ingredients that are destroyed during thermal treatments, and minimal loss of sensory attributes and nutritional quality (Bhat et al., 2011, Zafra-Rojas et al., 2013). The change in color parameter may be due to the Millard reaction between sugars and amino acids (Pandhare et al., 2018).
Among the healthy compounds are polyphenols especially, aroma compounds. Polyphenols discriminated with the highly volatile and quickly oxidized rate; however, they play a crucial role in determining the color and flavor of a product (Winata and Lorenz, 1996). Saad et al. (2020a) identified a broad spectrum of volatile compounds, i.e., hydrocarbons, alcohols, phenol derivatives, esters, aldehydes, ketones, sulfur compounds, fatty acids, and silicone compounds in cucumber juice supplemented with cinnamon clove, mint, and ginger extracts as a flavor enhancer. On the other hand, Elwakeel and Hussein (2021) detected thirty volatile compounds in the cactus pear and guava juice mix, the main compounds were alcohols represented in hexanol and linalool with 12.35% and 7.23%, respectively. Flavor features are important indicators to achieve consumer acceptance (Onetto et al., 2020). The specific aroma compounds depends on plant species. The major aroma compounds contain carbonyl compounds, amino acids, lipids-derived compounds, phenolics, and sesquiterpenes (Tucker, 1993). Flavor formation in fruits and vegetables is due to several mechanisms of autoxidation reactions and various enzymes like lipoxygenase (Yilmaz et al., 2002). In addition, a great number of volatile compounds can be formed during maturation processing steps such as cutting, thermal treatments especially, in fruits like apples, plums, and pears (Arena et al., 2001, Housseiny et al., 2013). In previous study, Mohamed et al. (2014) found that octanal, nonanal, hexnal and (E)-2-hexenal were belong to characteristic volatile compounds in prickly pear juice or its blend also in cucumber juice. Processing of cucumber results in flavor compounds including esters, terpenoids, lactones, and sulfur compounds (Schwab et al., 2008). Packaging materials, temperature, storage time, and microbial load alter juice flavor (Toldrá et al., 2020). The C9 compounds, especially (E,Z)-2.6-nonadienal, were responsible for the flavor of watermelon juice pasteurized at ultra-high temperatures (Wang et al., 2018). The low odor threshold values of aldehydes compared to alcohols explain the important role of these compounds in characteristic flavor of cactus pear even in low concentrations.
Herbal-enriched cucumber juices figured by enhanced antioxidant activity, prevented lipids oxidation, enriched organoleptic characters, and extended shelf life. The presence of phenolics and flavonoids in herbal extracts enriched the antioxidant and antimicrobial activity of juices. According to Pensec et al. (2016), there was a relation between scavenging activity and phenolic compounds content, the tested herbal extracts consists of a high quantity of phenolic compounds. Natural additives are rich in antioxidants content i.e., phenolic compounds with antioxidant and antimicrobial activity, they can used to preserve milk (El-Saadony et al., 2021c), yoghurt (El-Saadony et al., 2021d). Buffalo meat (El-Saadony et al., 2021b, Saad et al., 2020b), wheat-based noodles (Saad et al., 2021c), cucumber juice (El-Saadony et al., 2020, Saad et al., 2021a) enhancing the bread quality (Saad et al., 2015, Saad et al., 2016). Samaranayaka and Li-Chan (2011) reported that the use of antioxidants in nutrition improves health and foodstuff quality.
Saad et al. (2021a) found that IDZ of clove and mint extract (800 µg/mL) were 38, and 35 mm against tested bacteria, also they found that the tested bacteria were inhibited in the range of 100–190 µg/mL and killed at 180–370 µg/mL of herbal extracts. Also, found that clove extract reduced the fungal progress as compared to mint, cinnamon, and ginger extracts. Phytogenic compounds (polyphenols) are of plant origin. The mechanism of antibacterial action of herbal extracts because of phenolic compounds content comes through interacting with many sites at the cell membrane (Sikkema et al., 1995, El-Saadony et al., 2021g, Abd El-Hack et al., 2020; Abd El-Hack et al., 2021b; El-Tarabily et al., 2021). Additionally, phenolic hydrophobicity play a precious role in disturbing the bacterial cell wall and membrane, so, the added substances involved in cell contents (Cushnie and Lamb, 2011). The antifungal mechanism of herbal extracts belongs to the high phenolic compound contents. The permeability and rigidity of cell walls were altered by binding phenolic compounds hydrogen to enzymes to brief the mechanism of phenolic compounds (Bouarab-Chibane et al., 2019, Daglia, 2012, Duggirala et al., 2014, Upadhyay et al., 2014). Another mechanism related to the number and structure of phenolic compounds functional groups, like OH, COO, alkyl, and aldehyde groups (quantitive structure–activity relationship (QSAR) might induce the antimicrobial activity.
During juice storage, chemical and microbial fluctuations occurred where a decrease in the antioxidant activity content in juices was observed. The scavenging activity of cucumber juice supplemented with clove extract decreased from 88% to 83% after six months of storage (Saad et al., 2021a). The scavenging activity in pomegranate squash decreased after 3-month storage because of the gradual loses in phenolic content (Karpagavalli and Amutha, 2015). Other opinions observed an increase in polyphenols content (Martínez-Flores et al., 2015). Herbal extracts increased the phenolic and flavonoid compounds in juice samples. Since many plant extracts are rich in phenolic compounds, this is of particular interest for the development of natural alternatives to synthetic preservatives in the food and cosmetic applications (Bouarab Chibane et al., 2019, Kočevar Glavač and Lunder, 2018). There was a significant decrease (p ≤ 0.05) in phenolic compounds and flavonoids content of cucumber juice from zero days to six months of storage (Abu-Reidah et al., 2012).
The bacterial count; TVC and PC in stored cucumber juices enriched with herbal extracts; mint, clove, ginger, and cinnamon were significantly decreased (p ≤ 0.05) from 1.1 to 0.3 and 0.5, respectively, at zero-day storage; however, the bacterial count was increased with storage period (Saad et al., 2021a). The volatile compounds in added herbal extracts exhibited several biological activities where limonene and menthol were reported as powerful antimicrobial and anticance (Skalicka-Wozniak et al., 2009). The antibacterial mechanism of phenolic compounds depended on the interaction between polyphenols and many sites at the cell membrane; this might be altered the cell membranes' permeability. Besides, the binding polyphenols with cell wall enzymes modified the cell wall rigidity (Sikkema et al., 1995, Cushnie and Lamb, 2011). In addition, the hydrophobicity of phenolic compounds enhances their antimicrobial activity by interacting with the cell membrane (Bouarab-Chibane et al., 2019). This may cause defoliation of the cytoplasmic membrane and coagulation of the cell content that can lead to inhibition in intracellular enzymes' activity.
5. Conclusions
The high content of phenolic and flavor compounds in herbal extracts used as natural preservatives and flavor agents. This work concluded that blending PME and LEE with cucumber juice able to satisfy the consumer demand from color, taste and flavor. The obtained values for mixture indicated that they are a rich source for polyphenols and flavor compounds. The blends showed a remarkable increase in total phenolic content to higher level in J-PME. Therefore, further studies of storage for this blend are in need with analysis of non-volatile as well as volatile compounds during storage. Several studies carried out on the isolation and identification of volatile compounds in cucumber and tested herbal extracts in this respect. To the best knowledge of authors, no studies performed on the volatile compounds of blend cucumber juice with peppermint, lemongrass, lavender and basil extract and use it as flavor enhancer. Preservation method protected cucumber crop from spoilage and increased manufacture of high-quality and valuable juice.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021 doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of dietary herbal blend, feed restriction on growth, carcass characteristics, and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20(1):896–910. [Google Scholar]
- Abu-Reidah I.M., Arráez-Román D., Quirantes-Piné R., Fernández-Arroyo S., Segura-Carretero A., Fernández-Gutiérrez A. HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012;46:108–117. [Google Scholar]
- Ahmed A.F., Attia F.A., Liu Z., Li C., Wei J., Kang W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness. 2019;8:299–305. [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100(5) doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Amerine M., Pangborn R., Roessler E. Academic Press; New York 602: 1965. Principles of sensory evaluation of food. [Google Scholar]
- APHA., 1992. Compendium of methods for the microbiological examination of foods 3rd Ed.
- Arena E., Campisi S., Fallico B., Lanza M., Maccarone E. Aroma value of volatile compounds of prickly pear (Opuntia ficus indica (L.) Mill., Cactaceae). Ital. J. Food Sci. 2001;13(3):311–319. [Google Scholar]
- Ashour E.A., El-Hack M.E.A., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Beigi M., Torki-Harchegani M., Ghasemi Pirbalouti A. Quantity and chemical composition of essential oil of peppermint (Mentha× piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018;21:267–276. [Google Scholar]
- Bhat R., Kamaruddin N.S.B.C., Min-Tze L., Karim A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011;18:1295–1300. doi: 10.1016/j.ultsonch.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Bouarab Chibane L., Degraeve P., Ferhout H., Bouajila J., Oulahal N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019;99:1457–1474. doi: 10.1002/jsfa.9357. [DOI] [PubMed] [Google Scholar]
- Bouarab-Chibane L., Forquet V., Lantéri P., Clément Y., Léonard-Akkari L., Oulahal N., Degraeve P., Bordes C. Antibacterial properties of polyphenols: characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie T.T., Lamb A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Davidson P.M., Taylor T.M., Schmidt S.E. Chemical preservatives and natural antimicrobial compounds. Food microbiol.: Fundamentals front. 2012:765–801. [Google Scholar]
- Del Nobile M.A., Lucera A., Costa C., Conte A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012;3:287. doi: 10.3389/fmicb.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala S., Nankar R.P., Rajendran S., Doble M. Phytochemicals as inhibitors of bacterial cell division protein FtsZ: coumarins are promising candidates. Appl. Biochem. Biotechnol. 2014;174:283–296. doi: 10.1007/s12010-014-1056-2. [DOI] [PubMed] [Google Scholar]
- El-Saadony, M. T., Saad, A. M., Taha, T. F., Najjar, A. A., Zabermawi, N. M., Nader, M. M., ... & Salama, A. (2021e). Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 28(12), 6782–6794. https://doi.org/10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Zabermawi N.M., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: A review. Food Rev. Int. 2021:1–23. [Google Scholar]
- El-Saadony, M., El-Wafai, N., El-Fattah, H., Mahgoub, S., 2019. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 7, 238-249.
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.a., El-Ghareeb W.R., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20(1):762–776. [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.-S.-M., Fouda S.-E.-S.-E.-S., El-Tahan A.M. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi. J. Biol. Sci. 2021;28(8):4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69(1) [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28(9):5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwakeel M.A., Hussein A.S. Evaluation of quality attributes, antioxidant activity and volatile compounds of two cactus pear juices blended with guava juice. Egyptian Journal of Chemistry. 2021;64:3–4. [Google Scholar]
- Guo X., Wang P. Aroma Characteristics of Lavender Extract and Essential Oil from Lavandula angustifolia Mill. Molecules. 2020;25:5541. doi: 10.3390/molecules25235541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T., Kagawa H., Yasuhara T., Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988;36:2090–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- Housseiny M.M., Abo-Elmagd H.I., Ibrahim G.E. Preliminary studies on microbial polysaccharides from different Penicillium species as flavour stabiliser in cloudy apple juice. Int. J. Food Sci. Technol. 2013;48:2292–2299. [Google Scholar]
- Joshi V.K., Kumar V., Kumar V. Influence of different sugar sources, nitrogen sources and inocula on the quality characteristics of apple tea wine. J. Inst. Brew. J. Inst. Brew. 2017;123:268–276. [Google Scholar]
- Karpagavalli B., Amutha S. Influence of storage condition on the antioxidant activity of pomegranate squash. Plant Arch. 2015;15:405–410. [Google Scholar]
- Kočevar Glavač N., Lunder M. Preservative efficacy of selected antimicrobials of natural origin in a cosmetic emulsion. Int. J. Cosmet. Sci. 2018;40:276–284. doi: 10.1111/ics.12461. [DOI] [PubMed] [Google Scholar]
- Lasekan O., Yap S.P. Characterization of the aroma compounds in fresh and dried sapodilla (Manikara zapota L.) by the application of aroma extract dilution analysis. CYTA-J. Food. 2018;16:801–806. [Google Scholar]
- Lau T.-C., Chan M.-W., Tan H.-P., Kwek C.-L. Functional food: a growing trend among the health conscious. Asian Soc. Sci. 2013;9:198. [Google Scholar]
- Lee P.S. Quantitation of microorganisms. Practical handbook of microbiology. 2009;3:19–38. [Google Scholar]
- Martínez-Flores H.E., Garnica-Romo M.G., Bermúdez-Aguirre D., Pokhrel P.R., Barbosa-Cánovas G.V. Physico-chemical parameters, bioactive compounds and microbial quality of thermo-sonicated carrot juice during storage. Food Chem. 2015;172:650–656. doi: 10.1016/j.foodchem.2014.09.072. [DOI] [PubMed] [Google Scholar]
- Mishra B.B., Gautam S., Sharma A. Shelf life extension of sugarcane juice using preservatives and gamma radiation processing. J. Food Sci. 2011;76:M573–M578. doi: 10.1111/j.1750-3841.2011.02348.x. [DOI] [PubMed] [Google Scholar]
- Mohamed S.A., Hussein A.M.S., Ibraheim E. Physicochemical, sensorial, antioxidant and volatile of juice from prickly pear with guava or mandarin. Int. J. Food Nutr. Sci. 2014;3:44. [Google Scholar]
- Muala W.C.B., Desobgo Z.S.C., Jong N.E. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P.K., Nema N.K., Maity N., Sarkar B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia. 2013;84:227–236. doi: 10.1016/j.fitote.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Onetto C.A., Borneman A.R., Schmidt S.A. Investigating the effects of Aureobasidium pullulans on grape juice composition and fermentation. Food Microbiol. 2020;90 doi: 10.1016/j.fm.2020.103451. [DOI] [PubMed] [Google Scholar]
- Ouedrhiri W., Mounyr B., Harki E.H., Moja S., Greche H. Synergistic antimicrobial activity of two binary combinations of marjoram, lavender, and wild thyme essential oils. Int. J. Food Prop. 2017;20:3149–3158. [Google Scholar]
- Pandhare G., Satwase A., Jaju R., Awalgaonkar G. Effect of natural preservatives on pineapple juice. J. Pharmacogn. Phytochem. 2018;7:746–750. [Google Scholar]
- Park Y.J., Baskar T.B., Yeo S.K., Arasu M.V., Al-Dhabi N.A., Lim S.S., Park S.U. Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus. 2016;5:1628. doi: 10.1186/s40064-016-3283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensec F., Szakiel A., Paczkowski C., Wozniak A., Grabarczyk M., Bertsch C., Fischer M.J.C., Chong J. Characterization of triterpenoid profiles and triterpene synthase expression in the leaves of eight Vitis vinifera cultivars grown in the Upper Rhine Valley. J. Plant Res. 2016;129(3):499–512. doi: 10.1007/s10265-016-0797-0. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100(8) doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26(15):4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., Elmassry R.A., Hamed A.S., Wahdan K.M., Ramadan M.F. Characterization of composition, antioxidant potential and microbial organisms upon submerged Cicer arietinum fermentation. J. Food Meas. Charact. 2016;10:319–326. [Google Scholar]
- Saad A.M., Elmassry R.A., Wahdan K.M., Ramadan F.M. Chickpea (Cicer arietinum) steep liquor as a leavening agent: effect on dough rheology and sensory properties of bread. Acta Periodica Technol. 2015;46(46):91–102. [Google Scholar]
- Saad A.M., El-Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;56:3255–3268. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional Cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148(7) [Google Scholar]
- Saad A.M., Mohamed A.S., Ramadan M.F. Storage and heat processing affect flavors of cucumber juice enriched with plant extracts. Int. J. Veg. Sci. 2020:277–287. [Google Scholar]
- Saad A.M., Osman A.O.M., Mohamed A.S., Ramadan M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: Characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int. J. Pept. Res. Ther. 2020;26:567–577. [Google Scholar]
- Salehi B., Stojanović-Radić Z., Matejić J., Sharopov F., Antolak H., Kręgiel D., Sen S., Sharifi-Rad M., Acharya K., Sharifi-Rad R. Plants of genus Mentha: From farm to food factory. Plants. 2018;7:70. doi: 10.3390/plants7030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayaka A.G., Li-Chan E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods. 2011;3:229–254. [Google Scholar]
- Schwab W., Davidovich-Rikanati R., Lewinsohn E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008;54:712–732. doi: 10.1111/j.1365-313X.2008.03446.x. [DOI] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., El-Hack A., Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema J., de Bont J.A., Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalicka-Wozniak K., Los R., Glowniak K., Malm A. Volatile compounds in fruits of Peucedanum cervaria (Lap.) L. Chem. Biodivers. 2009;6:1087–1092. doi: 10.1002/cbdv.200800236. [DOI] [PubMed] [Google Scholar]
- Škerget M., Kotnik P., Hadolin M., Hraš A.R., Simonič M., Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- Toldrá F., Gallego M., Reig M., Aristoy M.-C., Mora L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020;321 doi: 10.1016/j.foodchem.2020.126689. [DOI] [PubMed] [Google Scholar]
- Tucker G. In: Biochemistry of Fruit Ripening. Taylor J.E., Tucker G.A., editors. Chapman & Hall; Londres: 1993. Introduction en Seymour GB; pp. 1–52. [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: a minireview of the mechanistic basis. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Guo X., Ma Y., Zhao X., Zhang C. Effect of ultrahigh temperature treatment on qualities of watermelon juice. Food Sci. Nutr. 2018;6:594–601. doi: 10.1002/fsn3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winata A., Lorenz K. Antioxidant potential of 5-n-pentadecylresorcinol. J. Food Process. Preserv. 1996;20:417–429. [Google Scholar]
- Yilmaz E., Baldwin E., Shewfelt R. Enzymatic modification of tomato homogenate and its effect on volatile flavor compounds. J. Food Sci. 2002;67:2122–2125. [Google Scholar]
- Zafra-Rojas Q.Y., Cruz-Cansino N., Ramírez-Moreno E., Delgado-Olivares L., Villanueva-Sánchez J., Alanís-García E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013;20:1283–1288. doi: 10.1016/j.ultsonch.2013.01.021. [DOI] [PubMed] [Google Scholar]