Abstract
In recent years green nanotechnology gained significant importance to synthesize nanoparticles due to their cost effectiveness and biosafety. In the current study, silver nanoparticles were synthesized by using extract of Spirogyra hyalina as a capping and reducing agent. The synthesized nanoparticles were characterized by UV–Visible spectroscopy, Fourier transform infrared spectroscopy, Scanning electron microscopy, energy dispersive X-ray spectroscopy, and X-ray diffractive analysis. Silver nanoparticles give a characteristic Surface Plasmon Resonance peak of 451 nm at 2.21 a.u (arbitrary unit). SEM micrograph revealed the spherical morphology and average grain size of 52.7 nm. Furthermore, antibacterial, antifungal, insecticidal, antioxidant and membrane damage activities were determined. The maximum antibacterial and antifungal activity was observed for Pseudomonas aeruginosa (18 ± 1.2 mm) and Fusarium solani (14.3 ± 0.6 mm), respectively. In membrane damage assay, Pseudomonas aeruginosa absorbed A260 wavelength and gave maximum peak values of 0.286, 0.434 and 0.629 at 25, 35 and 45 µg/mL of silver nanoparticles. The membrane damage assay confirmed that nanoparticles are involved in bacterial cell membrane damage. At 500 ppm silver nanoparticles showed 30% mortality against Tribolium castaneum (a common grain pest). The silver nanoparticles also showed potent antioxidant activity and successfully scavenged the DPPH free radicals upto 53.43 ± 0.17, 43.26 ± 0.97, 31.39 ± 0.33, 24.62 ± 0.85, and 14.13 ± 0.12% at a concentration of 400, 200, 100, 50, and 25 µg/mL of nanoparticles, respectively. It is concluded that silver nanoparticles can easily be synthesized by using green algae Spirogyra hyalina as a capping and reducing agent. Silver nanoparticles showed potent biomedical activities and thus can be used for therapeutic applications invitro and invivo.
Keywords: Spirogyra, Silver, Nanoparticles, Biosynthesis, Bio catalytic efficacy
1. Introduction
Doors are opened to exciting knowledge in the field of Nanomedicine in the recent years by nanotechnology (Desireddy et al., 2013; Al-Radadi and Adam, 2020). Nanoparticles are particles having size range from 1 to 100 nm, and have gained much importance in the drug delivery and biomedical engineering. Nanoparticles are of different types such as semiconductor, carbon, metallic and non-metallic nature but the metallic nanoparticles are more important and have played a vital role in the field of cosmetics, medicine, and electronics (Mulfinger et al., 2007, Veeraraghavan et al., 2021, Al-Radadi, 2019; Al-Radadi and Al-Youbi, 2018). Different chemical and physical methods have been utilized for the synthesis of nanoparticles but the chemical and physical methods are always discredited due to their costly and unsafe nature. Sol–gel, Solvothermal, Chemical reduction, Laser ablation and Inert gas condensation are some methods to be avoided due to their toxic and non-environment friendly nature (Iravani et al., 2014; Al-Radadi, 2019). In this regard the synthesis of nanoparticles via green approach is preferred over chemical and physical methods due to it's stability, high yield, cost effectiveness, less toxicity and eco-friendly nature. The chemicals used in the chemical synthesis of nanopartciles are easily adsorbed on the surface of nanoparticles, disturb their charge distribution and thus limit their therapeutic applications. Nanoparticles having strong positive charge can stay for longer times in the lumen of blood vessels and deliver the drug to the right region (Faisal et al., 2021). Synthesis of metallic NPs via green route is more eco-friendly as compared to chemical synthesis (Al-Radadi, 2018; Al-Radadi, 2021a, Al-Radadi, 2021b, Al-Radadi, 2021c). The biological approach of Ag-NPs allow the use of natural sources like plants, bacteria, algae, fungi, and actinomycetes in a safer way (Khodashenas & Ghorbani, 2019). The antibacterial potential of silver nanoparticles has been known from long ago and it is revealed by the use of silver in traditional medicine (Rai et al., 2009). The biomolecules present in the extract of Spirogyra hyalina act as a capping and reducing agent during the synthesis of silver nanoparticles. The intrinsic cytotoxic effectiveness of Ag-NPs contribute to their antibacterial, antifungal, antioxidant, insecticidal and anticancer activities . The current study aims to biosynthesize Spirogyra halina mediated silver nanoparticles and evaluate their antibacterial, antifungal, antioxidant, insecticidal, and effect of nanoparticles on the integrity of cell membrane.
2. Methods and materials
2.1. Collection of material and extract preparation
Spirogyra hyalina is collected from a pond and was identified by the experts in Department of Botany, Abdul Wali Khan University Mardan, Pakistan. After confirmation, the material was air dried under shade for 7 days and then subjected to grinding to obtain a fine powder form. 50gm of algal powder were mixed with 100 ml of deionized water and boiled at 60 ℃ for 15 min (Merin et al., 2010). Just after boiling the extract was filtered thrice by whatmann filter paper and followed by centrifugation at 12,000 rpm to remove any residues. The pellets were discarded and supernatant was collected into separate tubes and stored at 7 ℃ for further use in the experiment.
2.2. Preparation of the reagent and bio-inspired synthesis of silver-NPs
A well-established protocol reported by (Faisal et al., 2020) with minor modification was used for the synthesis of silver nanoparticles. Briefly, 1 mM solution of silver nitrate AgNO3 (Sigma Aldrich) was prepared, and 50 ml of the AgNO3 solution was mixed with 50 ml (1:1) of algal extract at room temperature to synthesize silver nanoparticles. The flask was covered with aluminum foil to protect the reduced silver ions from oxidation by light as silver ions are very sensitive to light. The mixed reagent were exposed to heat (60 ℃) on magnetic stirrer for 45 min. The solution was further left for 24 h in dark for proper reduction. The preliminary indication of the formation of silver nanoparticles is color transformation from light brown to dark brown. After proper reduction reaction the solution was subjected to centrifugation at 12,000 rpm for 20 min to obtain pure nanoparticles. The supernatant was discarded and pellets were subjected to threefold washing with distilled water followed by drying in over at 80 ℃. . The dried nanoparticles were grinded and stored at 7℃ for characterization.
2.3. Characterization of biosynthesized Ag-NPs
Characterization of Ag-NPs was done through UV–Visible spectroscopy, Scanning electron microscopy (SEM), Energy dispersive analysis of X-rays (EDAX), Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) in Centralized Resource Laboratory, University of Peshawar, Pakistan.
2.4. Collection of bacterial and fungal strains
A total of four previously identified bacterial isolates were collected from the Department of Microbiology, Abdul Wali Khan University Mardan. These included Pseudomonas aeruginosa, Bacillus cereus, Staphylococcus aureus, and Klebsiella pneumoniae. Fungal isolates included Fusarium solani, Rhizoctonita solani, and Fusarium proliferatum. The fungal isolates were collected from Department of Botany, Abdul Wali Khan University Mardan, Pakistan.
2.5. Bactericidal activity
To evaluate the bactericidal activity of Ag-NPs 1 mg of Silver nanoparticles was dissolved in 1 ml of DMSO. McFarland standard (104–106 CFU/mL) were spread on the surface of Muller-Hinton Agar plates with the help of sterile cotton swabs, and uniform bacterial lawns were prepared (Ghazala & Shameel., 2005). 6 mm wells were bored by sterilized metallic well borer and each well was filled with DMSO dissolved nanoparticles (100 µL into each well). The plates were left for 10 min in biosafety cabinet for proper diffusion and followed by incubation at 37 ℃ for 24 h. Ciprofloxacin was used as a positive, while DMSO was used as a negative control. After incubation the inhibition zones were recorded with the help of Vernier caliper. The activity was repeated thrice and average was taken as final result.
2.6. Membrane damage assay
The mechanism of nanoparticles to kill bacteria and their effect on bacterial cell membrane integrity was analyzed using UV–Visible spectrophotometer (Shimadzu UV-160A) and the A260 value was measured accurately. A260 is a specific absorbance wavelength that is absorbed by the intracellular materials when get released on the interaction of nanoparticles with bacterial cell membrane (Kora & Arunachalam., 2011). Bacterial inoculum was cultured in broth and after incubation the inoculum was centrifuged at 10,000 rpm for 10 min. After centrifugation the inoculum were washed and suspended in 0.01 mol L−1 PBS solution. Varying concentration of nanoparticles were added to 1.5 ml of bacterial inoculum followed by incubation and the absorbance was recorded at 260 nm (A260).
2.7. Antifungal activity
Agar well diffusion method was used to evaluate the antifungal potential of the biosynthesized Silver nanoparticles (Holder & Boyce, 1994). Fungal isolates were spread on potato dextrose agar (PDA) plates and uniform lawns were prepared by spread plate technique. Wells of 5 mm were bored in each plate by sterile well borer and different concentrations of nanoparticles were poured into each well and the plates were incubated for 72 h at 28 ℃. Amphotericin B was used as a positive, while DMSO as a negative control. The activity was repeated three times and mean inhibition zones were recorded with the help of Vernier caliper.
2.8. Insecticidal activity
Test compounds were exposed to insects Tribolium castaneum, which is a common grain pest by direct contact toxicity method (Ghazala & Shameel., 2005). 10 adult Tribolium castaneum, insects were transferred to petri plates and different concentration of nanoparticles were sprayed on it. Afterwards a check batch of negative control was treated with solvent for determination of solvent effect. As a positive control another batch was exposed to insecticide Mortein Coopex (Reckilt Benckiser Pak. Ltd.). All the batches were incubated for 24 h without food and mortality counts were carried out after exposure.
2.9. Antioxidant assay
DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radicals were exposed to test sample of varying concentrations in order to evaluate the antioxidant potential of silver nanoparticles (Bhakya et al., 2016). DPPH solution was prepared and 180 μL were poured into specified wells of 96-well titer plate, afterwards 20 μL of different concentration (25–400 μg/mL) of nanoparticles were added to each well and incubated for 1 h at 37C. DMSO was used as a negative, while Ascorbic acid was used as a -positive control. After incubation, the samples were exposed to absorbance at 517 nm under microplate reader and absorbance was recorded for each well. The antioxidant potential was calculated by following formula.
Above formula was used for free radicle scavenging activity (FRSA).
3. Results
3.1. Synthesis of silver nanoparticles and algae extract preparation
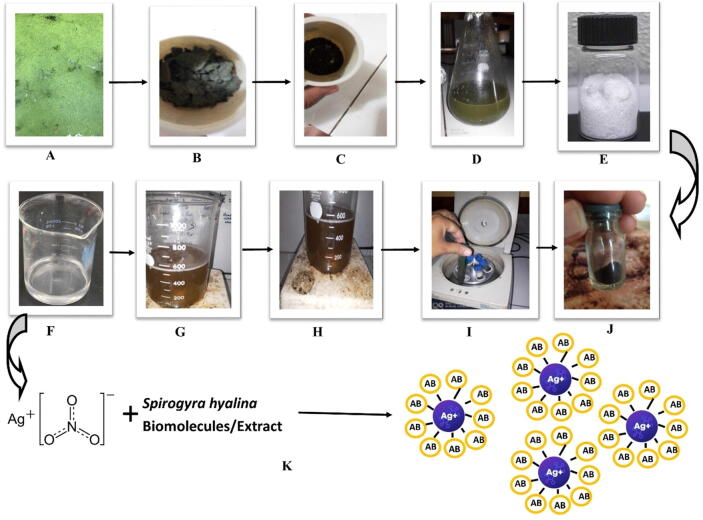
Spirogyra hyalina is a common green alga which was collected from a local pond, and was identified by the experts in Department of Botany, Abdul Wali Khan University Mardan, Pakistan. After confirmation the material was washed thrice and air dried under shade for 7 days and then subjected to grinding to obtain a fine powder. 50 gm of algal powder was mixed with 100 ml of deionized water and boiled at 60 ℃ for 15 min. Just after boiling the extract was filtered thrice by nylon cloth and whatmann filter paper, and followed by centrifugation at 12,000 rpm to remove any residues. The pellets were discarded and supernatant was collected into separate tubes and stored at 7 ℃ for further use in the experiment. 1 mM solution of silver nitrate AgNO3 was prepared, and 50 ml of the AgNO3 solution was mixed with 50 ml (1:1) of algal extract at room temperature to synthesize silver nanoparticles. The flask was covered with aluminum foil to protect the reduced silver ions from oxidation by light as silver ions are very sensitive to light. The mixed reagents were exposed to heat (60 ℃) on magnetic stirrer for 45 min. The solution was further left for 24 h in dark for proper reduction. The preliminary indication of the formation of silver nanoparticles is color transformation from light brown to dark brown as shown in Fig. 1. After proper reduction reaction, the solution was subjected to centrifugation at 12,000 rpm for 20 min to obtain pure nanoparticles. The supernatant was discarded and pellets were subjected to threefold washing with distilled water followed by drying in over at 80 ℃. The dried nanoparticles were grinded and stored at 7 ℃ for characterization.
Fig. 1.
This schematic diagram describes the whole process of silver nanoparticles synthesis. A) Collection of Spirogyra hyalina from a local pond. B) Shade Dried Spirogyra hyalina. C) Powder form of Spirogyra hyalina. D) Extract of Spirogyra hyalina. E) Silver nitrate. F) 1 mM solution of Silver nitrate. G) Mixing of Silver nitrate and Spirogyra hyalina extract. H) Reaction of Algae extract and Silver nitrate, kept on magnetic stirrer. H) Centrifugation after reaction. J) Pure Silver nanoparticles. K) Reaction mechanism of silver nitrate and algae extract for silver nanoparticles synthesis.
3.2. UV-analysis
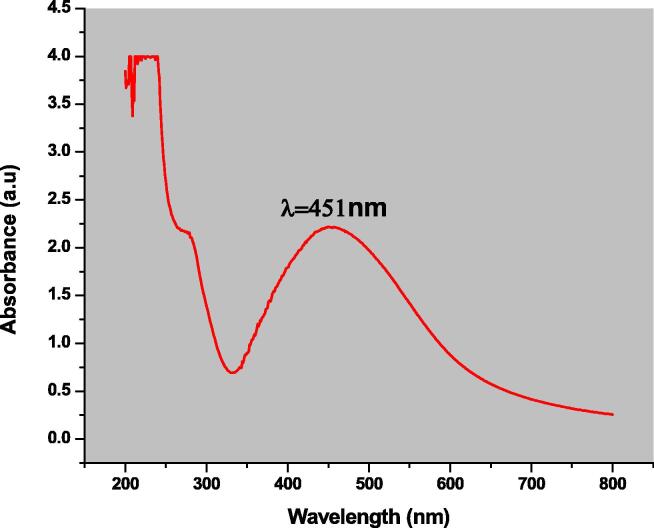
Spirogyra hyalina mediated silver nanoparticles were synthesized and their formation was confirmed by performing UV–visible spectroscopy in the standard wavelength range of 200–700 nm. A well-established protocol was utilized for this purpose. The initial color transformation (from light brown to dark brown) of the reaction mixture is the indication of reduction of Ag+1 ions to Ag0 by the bioactive molecules present in the aqueous extract of Spirogyra hyalina. The reaction mixture was left for 40 min at room temperature after color transformation for proper reduction, and then subjected to absorbance of characteristic wavelength under Spectrophotometer (Shimadzu UV-1800). Fig. 2 analysis revealed the silver nanoparticles absorbed a specific portion of characteristic wavelength and gave a Surface Plasmon Resonance peak at 451 nm at 2.21 a.u. some other absorption peaks were also observed which may be due the presence of the bioactive molecules that took part in the reduction and capping of silver nanoparticles. The characteristic absorbance peak confirmed that silver nanoparticles is successfully synthesized.
Fig. 2.
UV–visible spectra of silver nanoparticles.
3.3. EDX analysis
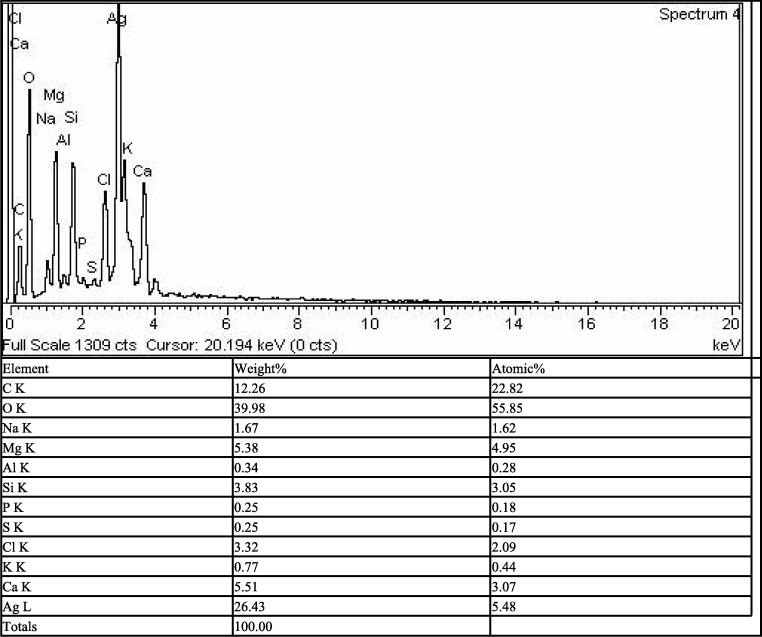
To know about the elemental composition of the biosynthesized silver nanoparticles the Energy dispersive X-ray analysis was carried out using EDX with SEM (JSM5910) INCA200/Oxford instruments, U.K) in the voltage range of 0-20KeV. A strong EDX spectra was observed at 2.5KeV for silver which confirmed the synthesis of silver nanoparticles as shown in Fig. 3. Some additional peaks were also observed for chlorine, carbon, oxygen, phosphorus, Sulphur, potassium, calcium, sodium and magnesium. The additional peaks were due to the bioactive molecules of Spirogyra hyalina that surrounded the Ag ions in the nanoparticles synthesis. However, the additional peaks had no effect on actual nanostructure of silver nanoparticles.
Fig. 3.
Energy dispersive X-ray analysis of silver nanoparticles.
3.4. XRD analysis
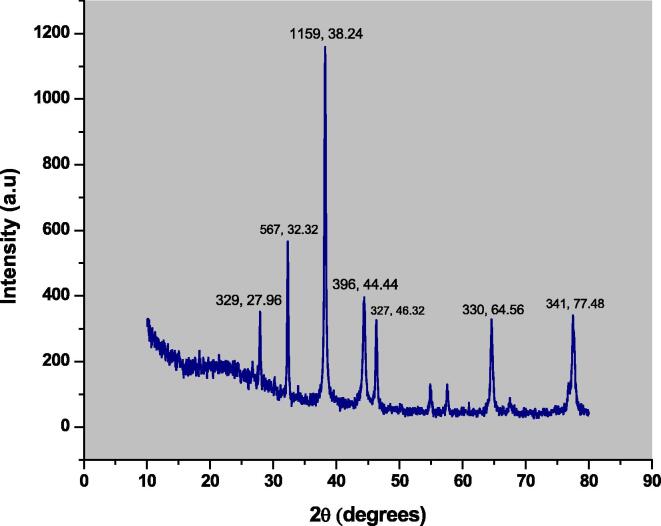
To know about the phase identification and nano-crystalline structure of the biosynthesized silver nanoparticles, the X-ray diffractive analysis was carried out using (JDX-3532, JEOL, Japan) at a voltage and current of 20–40 kV and 2.5–30 mA respectively, while the X-Rays were CuKa (Wavelength = 1.5418 Å) and 2 Theta-Range were kept 0 to 100°. The major observed XRD peaks are 329, 567, 1159, 396, 327, 330, and 341 at 2 Theta or diffraction angle of 27.96, 32.32, 38.24, 44.44, 46.32, 64.56, and 77.48°respectively as shown in Fig. 4. The distinct XRD reflection planes confirms the fcc crystal morphology as confirmed by JCPDS Card No. 36–1451. The average crystal size of 48.81 nm according to Scherer’s equation as stated below.
Fig. 4.
X-ray diffractive peaks of silver nanoparticles.
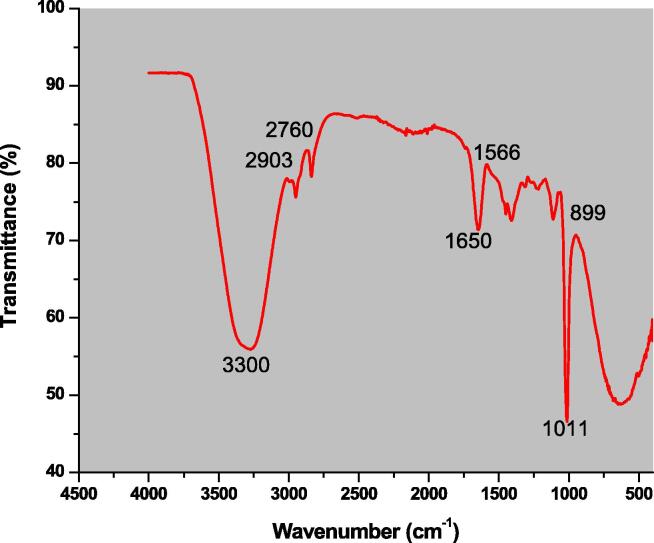
3.5. FTIR analysis
Functional groups are atoms or group of atoms that give a characteristic property to a compound. The Fourier transform infrared spectroscopy was performed in order to know what type of functional groups took part in the capping and reduction of Ag+1 ions to Ag0. Spectrum 3™ FT-IR Spectrometer were used in FTIR analysis of the biosynthesized silver nanoparticles in the spectral range of 400 to 4500 cm−1. As shown in Fig. 5 major peaks were observed at 3300, 2903, 2760, 1650, 1566, 100, and 899 cm−1. These obtained peaks correspond to stretching of N-H bond in amines and O-H in alcohols, stretching in aromatic CH3 groups, C-H bond stretching in alkene and alkane, C = C bond stretching in alkene, N-O bond stretching in nitro compound, O-H bond stretching in carboxylic acid, and C = C bond bending in alkenes respectively. The different functional groups associated with silver nanoparticles are first, the Spirogyra hyalina extract biomolecules that have been later become the part of silver nanoparticles after the reaction and took part in the capping and reduction of silver ions.
Fig. 5.
Fourier transform infrared spectroscopy of silver nanoparticles.
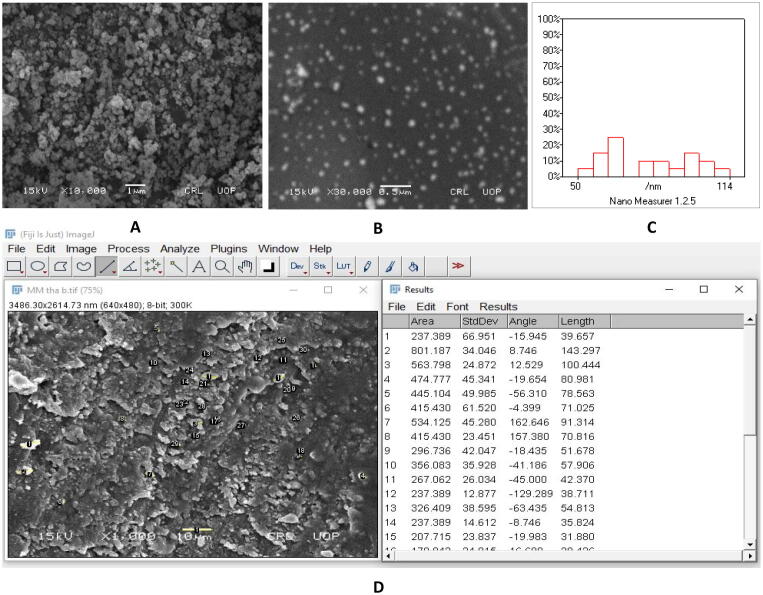
3.6. SEM analysis
The silver nanoparticles were further characterized by scanning electron microscopy to know about the physical dimension and size of the nanoparticles. SEM (JSM5910) was used in this analysis. As shown in Fig. 6, the white patches in the SEM micrograph indicate the agglomeration of the particles, however, some particles are uniform while some are polydispersed having average size of 52.7 nm (confirmed by ImageJ and Nano Measurer analysis). The particle agglomeration is the product of the biomolecules that quickly reduce silver to silver nanoparticles during the synthesis phase. The scale of the electro-microscopic silver nanoparticles showed it to be a successful therapeutic candidate. The particle size, however, also depends on the salt and pH levels of the reaction. In comparison with low pH, high pH results in greater concentration and greater scale of nanoparticles.
Fig. 6.
Scanning electron micrograph of silver nanoparticles. A) SEM micrograph at 10,000x. B) SEM micrograph at 30,000x. C) Nano measurer analysis. D) ImageJ analysis of Ag-NPs.
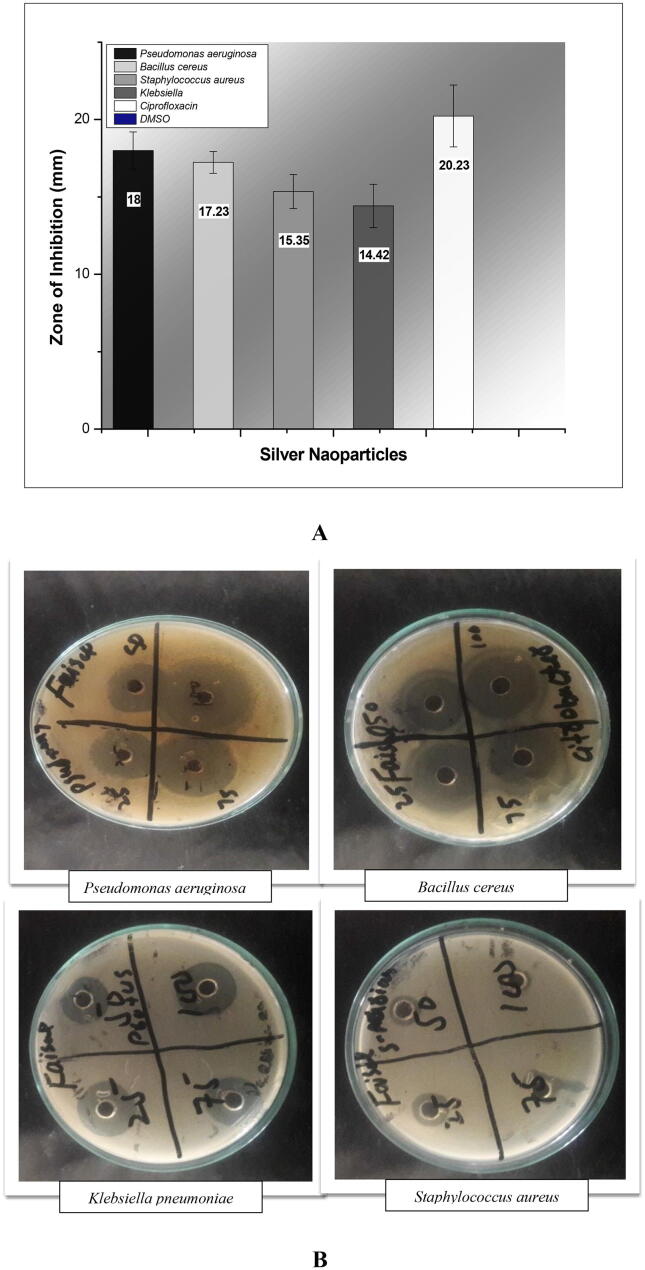
3.7. Bactericidal activity
1 mg of silver nanoparticles were dissolved in 1 ml DMSO. 100 µl of nanoparticles were taken and poured in to each well according to Agar well diffusion Assay. The plates were left for 15 min to ensure proper diffusion of nanoparticles into wells and then incubated for 24 h at 37 ℃. After incubation, the zones of inhibition were measured in X, Y, Z-axis manner and their average was taken as mean inhibition zone, the assay was repeated three times. The largest zones of inhibition were observed for Pseudomonas aeruginosa (18 ± 1.2 mm), followed by Bacillus cereus (17.23 ± 0.7 mm), Staphylococcus aureus (15.35 ± 1.1 mm), and Klebsiella pneumoniae (14.42 ± 1.4 mm) respectively as shown in the figure. The negative control DMSO showed no antibacterial activity against the test organisms, while Ciprofloxacin showed an average antibacterial activity of 20.23 ± 2.0 as shown in Fig. 7.
Fig. 7.
A & B) Antibacterial assay of silver nanoparticles.
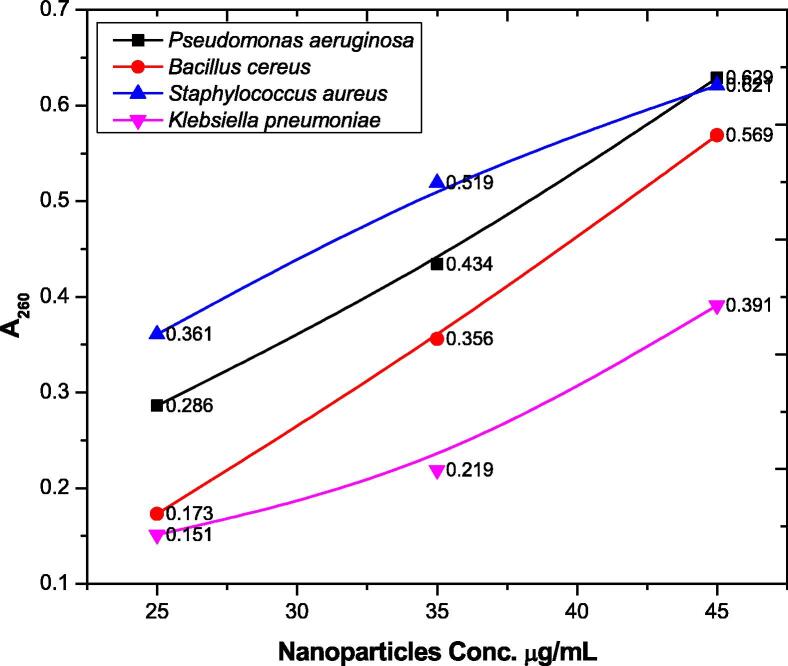
3.8. Effect of silver nanoparticles on the integrity of cell membrane
Release of intracellular materials upon interaction of nanoparticles with bacterial cell membrane and detecting the release of intracellular materials by absorbance at 260 nm on spectrophotometer was determined. Different concentrations of silver nanoparticles were applied against Pseudomonas aeruginosa, Bacillus cereus, Staphylococcus aureus, and Klebsiella pneumoniae. Nanoparticles treated culture suspension of Pseudomonas aeruginosa showed an increase in the A260 value with the increase in nanoparticles concentration. The A260 values of Pseudomonas aeruginosa are 0.286, 0.434 and 0.629, for Bacillus cereus the values are 0.173, 0.356 and 0.569, for Staphylococcus aureus, the values are 0.361, 0.519 and 0.621, for Klebsiella pneumoniae the values are 0.151, 0.219 and 0.391 at 25, 35 and 45 µg/ml of silver nanoparticles and at 1 h of exposure respectively as shown in Fig. 8.
Fig. 8.
Membrane damage assay of silver nanoparticles.
3.9. Antifungal activity
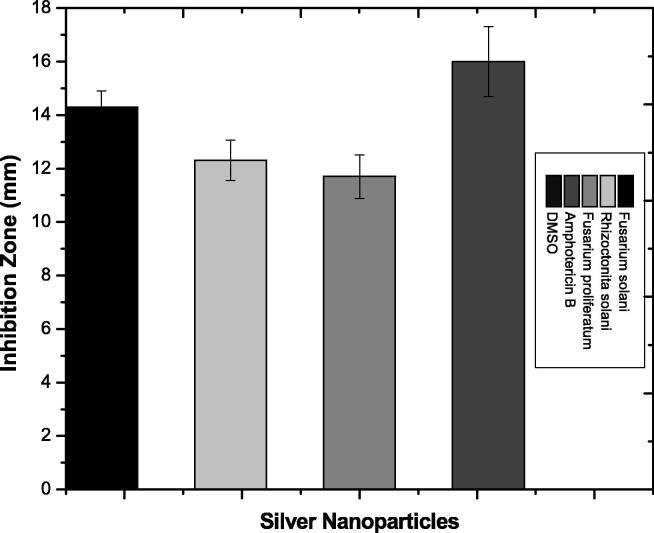
To evaluate the antifungal potential of biosynthesized silver nanoparticles, 1 mg of silver nanoparticles were dissolved in 1 ml of DMSO. 100 µL of nanoparticles were pipetted out and poured into wells that were already made in sterilized PDA plates and inoculated with fungal isolates. Maximum zones of inhibition were observed against Fusarium solani (14.3 ± 0.6 mm), followed by Rhizoctonita solani (12.3 ± 0.76 mm), and Fusarium proliferatum (11.7 ± 0.81 mm) as shown in Fig. 9. Amphotericin B and DMSO were used as a positive and negative control, respectively. The activity was performed three times and their means were taken as actual inhibition zones.
Fig. 9.
Antifungal activity of silver nanoparticles.
3.10. Insecticidal activity
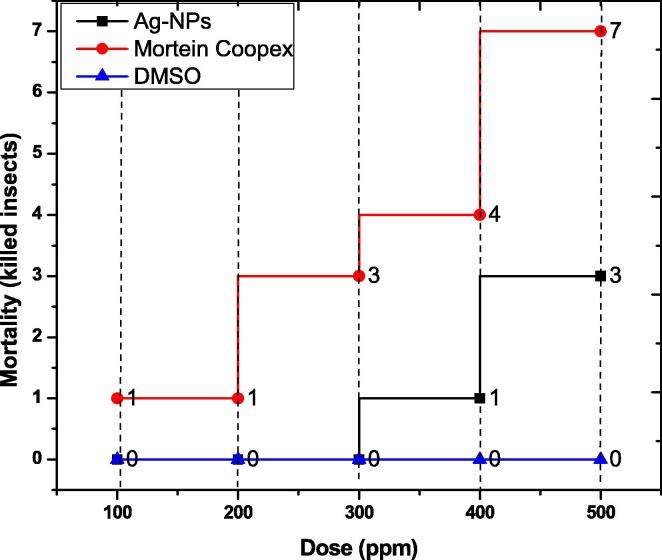
Test compound of dose in ppm (100, 200, 300, 400, and 500) were sprayed on insects Tribolium castaneum, followed by incubation for 24 h without food, and mortality counts were noted after exposure. The activity was performed on 10 insects. Silver nanoparticles showed maximum mortality of 30% (3 insects killed) at a concentration of 500 ppm, followed by 10% (1 insect killed) at 400 ppm as shown in the figure. The silver nanoparticles showed no activity at 100–300 ppm. Positive control Mortein Coopex showed maximum mortality at 500 ppm which is 70% (7 insects killed) as shown in Fig. 10. Negative control DMSO showed no activity and all the insects were found alive after 24 h of exposure.
Fig. 10.
Insecticidal activity of silver nanoparticles.
3.11. Antioxidant assay
DPPH (2,2-diphenyl-1-picrylhydrazyl) free radicals were exposed to test sample of varying concentrations in order to evaluate the antioxidant potential of silver nanoparticles. The silver nanoparticles successfully scavenged the DPPH free radicals upto 53.43 ± 0.17, 43.26 ± 0.97, 31.39 ± 0.33, 24.62 ± 0.85, and 14.13 ± 0.12% at a Conc. of 400, 200, 100, 50, and 25 µg/mL respectively as shown in Table 1. The antioxidant activity of silver nanoparticles were found dose dependent. The activity increased with increase in the concentration of nanoparticles.
Table 1.
Antioxidant potential of silver nanoparticles.
| NPs | Conc (µg/mL) | DPPH (%FRSA) |
|---|---|---|
| Ag-NPs | 400 | 53.43 ± 0.17 |
| 200 | 43.26 ± 0.97 | |
| 100 | 31.39 ± 0.33 | |
| 50 | 24.62 ± 0.85 | |
| 25 | 14.13 ± 0.12 |
4. Discussion
Green technology used for the synthesis of nanoparticles gained significant importance in research community in recent years due to their simple, nontoxic, less time consuming, and cost effective nature, and also due to their feasibility for large scale production . In the current study, Ag-NPs were prepared from the extract of Spirogyra hyalina by utilizing green synthesis approach and then their antibacterial, antifungal, insecticidal, antioxidant and membrane damage activity were determined. In addition to this, different analytical techniques such as UV–visible spectroscopy, FTIR, SEM, EDAX and XRD were used for the characterization of Ag-NPs. From UV–visible spectroscopy, it was observed that sample had absorbed energy at 451 nm which was a characteristic peak value of Ag-NPs, same results were also found by (Bolea et al., 2014). Beside this, absorption peak at 451 nm with no other peak displayed high purity of the nanoparticles. Different researchers observed strong absorption peak of Ag-NPs below 450 nm wavelength and concluded that it was due to red shift of the samples appealed at 500 °C and 700 °C and also stated that in materials transition, when an electron gain some energy, it makes transition from lower energy level to higher energy level (Gurunathan et al., 2009, Kouvaris et al., 2012).
FTIR analysis of Spirogyra hyalina silver nanoparticles showed interrelation of different functional groups and vibrations of functional groups i.e. alkanes, phenol, alcohols, aromatics, alkenes, alkayl-halids and aliphatic amines, which were the stretching vibration peaks of Ag-NPs. Dinesh et al., 2012, also reported similar results. Furthermore, it was determined that the peaks were due to –C = O–, C–O–C and C–O stretching vibrations in carboxylic acid, polysaccharide and amino acid respectively. Similar results were also reported by Umoren et al., 2014 by utilizing red apple.
The SEM micrograph of the synthesized Ag-NPs confirmed the spherical morphology of the Ag-NPs showing particle size in the range of 52.7 nm confirmed by Nano-measurer and ImageJ analysis. Furthermore, the morphology of Ag-NPs synthesized was hexagonal which is in agreement with our results but the size of the nanoparticle in the current study was greater as compared to (Faisal et al., 2020; Al-Radadi., 2018) which might be due to different synthesis conditions like temperature, time of incubation, nature of plant extract and handling applications.
In addition to this, EDAX analysis was accomplished which showed pure Ag-NPs phases and major peak was observed at 2.5 keV in EDAX spectrum representing pure silver in the test sample. The EDAX spectra of Ag-NPs presented from simple precipitation method using silver nitrate was the starting materials for the synthesis of nanoparticles. The results of EDAX spectrum showed that pure Ag-NPs were prepared successfully with intense peaks. However, additional peaks were observed in the spectrum that showed involvement of algae biomolecules during the synthesis of nanoparticles. The same EDAX pattern of Ag-NPs with high purity were recorded by (Jena et al., 2013) in their studies. Abdel-Raouf et al. (2018) also performed similar study to identify the purity of Ag-NPs by utilizing EDAX analysis and observed pure silver and additional peaks in the spectrum that showed purity of the sample.
Moreover, XRD analysis was performed to determine the size and crystallanity of the biosynthesized silver nanoparticles. XRD spectrum displayed the planes orientation and crystalline nature of Ag-NPs. The major observed XRD peaks were 329, 567, 1159, 396, 327, 330, and 341 at 2Theta or diffraction angle of 27.96, 32.32, 38.24, 44.44, 46.32, 64.56, and 77.48 degrees respectively. The distinct XRD reflection planes confirms the fcc crystal morphology and average crystal size of 48.81 nm, this agreed with the International Center of Diffraction Data card (JCPDS-36–1451) and hence confirmed the synthesis of crystalline hexagonal structure which coincide with the results of (Faisalet al., 2020).
In bactericidal activity, the largest zone of inhibition was observed for Pseudomonas aeruginosa (18 ± 1.2), followed by Bacillus cereus (17.23 ± 0.7), Staphylococcus aureus (15.35 ± 1.1), and Klebsiella pneumoniae (14.42 ± 1.4), respectively. Silver nanoparticles have the capability to interact with the cell membrane of bacteria and cause damage to it by releasing their intracellular materials to external medium (Ansari et al, 2014), which was confirmed by the absorbance at 260 nm in the membrane damage assay. Nanoparticles treated culture suspension of Pseudomonas aeruginosa showed an increase in the A260 value with respect to the increase in nanoparticles concentration. The A260 values of Pseudomonas aeruginosa were 0.286, 0.434 and 0.629, for Bacillus cereus the values were 0.173, 0.356 and 0.569, for Staphylococcus aureus the values were 0.361, 0.519 and 0.621, for Klebsiella pneumoniae the values were 0.151, 0.219 and 0.391 at 25, 35 and 45 µg/ml of silver nanoparticles, and at 1 h of exposure, respectively. The bactericidal activity of the silver nanoparticles is used due to their interaction and inhibition of bacterial cell membrane (Slavin et al., 2017). The maximum damage was observed for Pseudomonas aeruginosa at 45 µg/ml of Ag-NPs. The antibacterial, antifungal and other biomedical activities greatly depends on the size and concentration of nanoparticles, small sizes cross the bacterial protective walls easily and penetrate to damage it (Lok et al., 2007). The antifungal potential of the algae mediated nanoparticles were also evaluated and maximum zone of inhibition was observed against Fusarium solani (14.3 ± 0.6 mm), followed by Rhizoctonita solani (12.3 ± 0.76 mm), and Fusarium proliferatum (11.7 ± 0.81 mm). Nanoparticles were sprayed on Tribolium castaneum (a common grain pest) and a dose dependent insecticidal activity was observed. At 500 ppm concentration of nanoparticles, 3 insects were killed by nanoparticles out of 10, and a 30% mortality rate was observed. Our results are contradicting at this stage with the results of (Ghazala and Shameel, 2005) due to different methodologies and insecticidal agents used.
Antioxidants are compounds that help prevent or reduce cell damage caused by free radicals, which are unstable molecules produced by the cell in response to environmental and other stresses. They're also known as “free-radical scavengers”. Antioxidants can come from both natural and synthetic sources. Silver nanoparticles are potent antioxidants (Mittal et al., 2012). The silver nanoparticles successfully scavenged the DPPH free radicals up to 53.43 ± 0.17, 43.26 ± 0.97, 31.39 ± 0.33, 24.62 ± 0.85, and 14.13 ± 0.12% at a Conc. of 400, 200, 100, 50, and 25 µg/mL respectively. DPPH free radicals can be easily scavenged by Ag-NPs and thus proved itself a strong candidate to be used in antioxidant assays.
Our findings thus, endorse the bio-safe nature of the silver nanoparticles and thus gives a clear picture that Spirogyra hyalina mediated silver nanoparticles are stable and can be used for therapeutic applications in-vivo.
5. Conclusion
It is concluded from the study that silver nanoparticles can be easily synthesized by using green algae Spirogyra hyalina as a capping and reducing agent. The biomolecules of Spirogyra hyalina stabilize the silver ions in the reduction process and prevent the oxidation of silver ions from external factors as confirmed by advance spectroscopic characterization via FTIR, UV, XRD, EDX, and SEM. Furthermore, the silver nanoparticles showed potent antibacterial, antifungal, antioxidant and insecticidal activity and thus it can be used as a potent therapeutic agent. However, for in-vivo use, cytotoxicity assay is recommended to be performed to ensure the biosafe nature of silver nanoparticles.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
Department of Microbiology, Abdul Wali Khan University Mardan, Pakistan, Department of Botany, Abdul Wali Khan University Mardan, Pakistan, Department of Biotechnology, Bacha Khan University, Charsadda, Pakistan, and University of Peshawar, Pakistan are deeply acknowledged for providing research facilities.
Data availability statement
All the data is available in the manuscript. However if additional or raw data is required then it will be made available upon request.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Raouf N., Alharbi R.M., Al-Enazi N.M., Alkhulaifi M.M., Ibraheem I.B.M. Rapid biosynthesis of silver nanoparticles using the marine red alga Laurencia catarinensis and their characterization. Beni-Suef University J. Basic Appl. Sci. 2018;7(1):150–157. [Google Scholar]
- Al-Radadi N.S. Artichoke (Cynara scolymus L.,) mediated rapid analysis of silver nanoparticles and their utilisation on the cancer cell treatments. Journal of Computational and Theoretical Nanoscience. 2018;15(6–7):1818–1829. doi: 10.1166/jctn.2018.7317. [DOI] [Google Scholar]
- Al-Radadi N.S. Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arabian Journal of Chemistry. 2019;12(3):330–349. doi: 10.1016/j.arabjc.2018.05.008. [DOI] [Google Scholar]
- Al-Radadi, N. S. 2021. Facile one-step green synthesis of gold nanoparticles (AuNp) using Licorice root extract: antimicrobial and anticancer study against HepG2 cell line Arab. J.Chem 14 (2021)1-25. 10.1016/j.arabjc.2020.102956 [DOI]
- Al-Radadi, N. S. 2021. Green Biosynthesis of Flaxseed Gold Nanoparticles (Au-NPs) as Potent Anticancer Agent Against Breast Cancer Cells. J.Saudi.Chem.Society 25(6) (2021)1-22. 10.1016/j.jscs.2021.101243 [DOI]
- Al-Radadi N.S. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: Antimicrobial and anticancer study against HepG2 cell line. Arabian J. Chem. 2021;14(2):102956. doi: 10.1016/j.arabjc.2020.102956. [DOI] [Google Scholar]
- Al-Radadi N.S., Adam S.I.Y. Green biosynthesis of Pt-nanoparticles from Anbara fruits: Toxic and protective effects on CCl4 induced hepatotoxicity in Wister rats. Arabian Journal of Chemistry. 2020;13(2):4386–4403. doi: 10.1016/j.arabjc.2019.08.008. [DOI] [Google Scholar]
- Al-Radadi N.S., Al-Youbi A.N. One-step synthesis of au nano-assemblies and study of their anticancer activities. Journal of Computational and Theoretical Nanoscience. 2018;15(6–7):1861–1870. doi: 10.1166/jctn.2018.7323. [DOI] [Google Scholar]
- Ansari M.A., Khan H.M., Khan A.A., Ahmad M.K., Mahdi A.A., Pal R., Cameotra S.S. Interaction of silver nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Basic Microbiol. 2014;54(9):905–915. doi: 10.1002/jobm.201300457. [DOI] [PubMed] [Google Scholar]
- Bhakya S., Muthukrishnan S., Sukumaran M., Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016;6(5):755–766. [Google Scholar]
- Bolea E., Jiménez-Lamana J., Laborda F., Abad-Álvaro I., Bladé C., Arola L., Castillo J.R. Detection and characterization of silver nanoparticles and dissolved species of silver in culture medium and cells by AsFlFFF-UV-Vis-ICPMS: application to nanotoxicity tests. Analyst. 2014;139(5):914–922. doi: 10.1039/c3an01443f. [DOI] [PubMed] [Google Scholar]
- Desireddy A., Conn B.E., Guo J., Yoon B., Barnett R.N., Monahan B.M., Kirschbaum K., Griffith W.P., Whetten R.L., Landman U., Bigioni T.P. Ultrastable silver nanoparticles. Nature. 2013;501(7467):399–402. doi: 10.1038/nature12523. [DOI] [PubMed] [Google Scholar]
- Dinesh S., Karthikeyan S., Arumugam P. Biosynthesis of silver nanoparticles from Glycyrrhiza glabra root extract. Arch. Appl. Sci. Res. 2012;4(1):178–187. [Google Scholar]
- Faisal, S., Khan, M.A., Jan, H., Shah, S.A., Abdullah, Shah, S., Rizwan, M., Ullah, W., Akbar, M.T., Redaina , 2021 . Edible mushroom (Flammulina velutipes) as biosource for silver nanoparticles: from synthesis to diverse biomedical and environmental applications . Nanotechnology 32(6), 065101. doi: 10.1088/1361-6528/abc2eb. [DOI] [PubMed]
- Faisal, S., Abdullah, Shah, S.A., Shah, S., Akbar, M.T., Jan, F., Haq, I. , Baber, M.E., Aman, K., Zahir, F., Bibi, F., Syed, F., Iqbal, M., Jawad, S.M., Salman, S., 2020 . In vitro biomedical and photo-catalytic application of bio-inspired zingiber officinale mediated silver nanoparticles . J. Biomed. Nanotechnol. 16(4), 492 – 504. [DOI] [PubMed]
- Ghazala B., Shameel M. Phytochemistry and bioactivity of some freshwater green algae from Pakistan. Pharm. Biol. 2005;43(4):358–369. doi: 10.1080/13880200590951838. [DOI] [PubMed] [Google Scholar]
- Gurunathan S., Kalishwaralal K., Vaidyanathan R., Venkataraman D., Pandian S.R.K., Muniyandi J., Hariharan N., Eom S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B: Biointerfaces. 2009;74(1):328–335. doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Holder I.A., Boyce S.T. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns. 1994;20(5):426–429. doi: 10.1016/0305-4179(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharmaceutical Sci. 2014;9(6):385. [PMC free article] [PubMed] [Google Scholar]
- Jena J., Pradhan N., Dash B.P., Sukla L.B., Panda P.K. Biosynthesis and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int. J. Nanomater. Biostruct. 2013;3(1):1–8. [Google Scholar]
- Khodashenas B., Ghorbani H.R. Synthesis of silver nanoparticles with different shapes. Arabian J. Chem. 2019;12(8):1823–1838. [Google Scholar]
- Kora A.J., Arunachalam J. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J. Microbiol. Biotechnol. 2011;27(5):1209–1216. [Google Scholar]
- Kouvaris P., Delimitis A., Zaspalis V., Papadopoulos D., Tsipas S.A., Michailidis N. Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater. Lett. 2012;76:18–20. [Google Scholar]
- Lok C.-N., Ho C.-M., Chen R., He Q.-Y., Yu W.-Y., Sun H., Tam P.-H., Chiu J.-F., Che C.-M. Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007;12(4):527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- Merin D.D., Prakash S., Bhimba B.V. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pacific J. Tropical Med. 2010;3(10):797–799. [Google Scholar]
- Mittal A.K., Kaler A., Banerjee U.C. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. NANO Biomed. Eng. 2012;4(3) [Google Scholar]
- Mulfinger L., Solomon S.D., Bahadory M., Jeyarajasingam A.V., Rutkowsky S.A., Boritz C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007;84(2):322. doi: 10.1021/ed084p322. [DOI] [Google Scholar]
- Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Slavin Y.N., Asnis J., Häfeli U.O., Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology. 2017;15(1):1–20. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umoren S.A., Obot I.B., Gasem Z.M. Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J. Mater. Environ. Sci. 2014;5(3):907–914. [Google Scholar]
- Veeraraghavan V.P., Periadurai N.D., Karunakaran T., Hussain S., Surapaneni K.M., Jiao X. Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929) Saudi J. Biol. Sci. 2021;28(7):3633–3640. doi: 10.1016/j.sjbs.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data is available in the manuscript. However if additional or raw data is required then it will be made available upon request.