Abstract
Biodiesel is considered as a potential alternative energy source, but problem exists with the quantity and quality of feedstock used for it. To improve the feedstock quality of biodiesel, a field experiment was conducted under natural conditions. Cultivar Thori of kasumbha was used in the experiment. Commercialized biofertilizers were applied at the rate of 20 kg per acre and chemical fertilizer (diammonium phosphate) was applied as half dose (15 kg/ha). Results indicated that number of leaf plant−1, leaf area, number of seeds capitulum−1 was significantly increased by biofertilizer treatment alone (BF) and combine treatment of biofertilizer and chemical fertilizer (BFCF). Agronomic traits such as plant height, no. of branches of a plant, no. of capitulum/plant was improved significantly by BF treatment over the control. Maximum 1000 seed weight (41%) and seed yield (23%) were recorded in half dose of chemical fertilizers treatment (CFH). Seed oil content and seed phenolics were significantly improved by BF and CF treatments while maximum biodiesel yield was recorded by BF treatment. Maximum oleic acid was recorded by BF treatment while other fatty acids being maximum in control except linoleic acid in BFCF treatment. Results for specific gravity were non-significant while acid value and free fatty acid contents were substantially reduced by BF treatment as compared to other treatments. Maximum value of iodine number was recorded in BFCF treatment while tocopherol contents were improved by BF treatment. It is inferred that biofertilizer treatment alone perform better as compared to other treatments and 50% chemical fertilizer can be replaced using biofertilizer which is a good approach for sustainable environmental-friendly agriculture.
Keyword: Green energy, Biofuel, Biodiesel, Kasumbha, Biofertilizers, Fatty acid, NMR
1. Introduction
Energy is the one of the fundamental aspects for the viable commodity and responsible for the socio-economic development of a country (Khan et al., 2019). Energy produced from fossil fuel will run out soon due to its uncontrollable consumption. Similarly, change of climate and global warming due to environmental pollution and greenhouse gases have drastic effects on the productivity of the crops (Takase et al., 2021). Therefore, it is very important and need of the day to search the environmental-friendly renewable and sustainable energy resources in order to reduce the emission of greenhouse gases and to improve the economy of the country. It is indispensable to increase the supply of energy by diversification of the energy sources and utilizing the biomass from those sources which have no competition with the conventional food resources (Mizik and Gyarmati, 2021).
Biodiesel is an alternative energy source that is renewable, sustainable and environmental-friendly. It is one of the promising alternative fuels which has almost similar properties to that of petro-diesel (Singh et al., 2021).
This study was designed to enhance the plant growth, yield, quality, quantity and antioxidant properties of oil produced from high oil yielding plant kasumbha with the objective to biodiesel production. Kasumbha is a drought resistant crop and can also be grown in drought prone areas and is considered as a good source of oil with total oil contents of 25–35% (Nosheen et al., 2018). In order to improve the growth and yield of crops, chemical fertilizers are used, although chemical/synthetic fertilizers are essential source of nutrients for development and growth of the plant, yet the excessive use of chemical fertilizers is linked with soil, water and environmental pollution (Nosheen et al., 2021). In order to cope with these detrimental effects, there is a growing interest to find out alternative ways that are sustainable, cost effective and environmental-friendly. One such alternative is the use of biofertilizers to reduce the quantity of chemical/synthetic fertilizers (Nosheen et al., 2009). The biofertilizers are relatively sustainable and environmental-friendly that also circulate the nutrients and reduce the excessive use of chemical/synthetic fertilizers to noticeable extent (Fasusi et al., 2021). The positive role of biofertilizers on oilseed crops has been often stated. Nosheen et al., 2011, Nosheen et al., 2013 reported the application of PGPR not only improved the yield but also the nutritional value of canola oil to a considerable extent. The current research was emphasized on improving the oil contents and oil quality of a crop with the objective for biodiesel production.
2. Materials and methods
A field experiment was conducted with three replications under natural conditions having a plot size of 2 × 2 m2. The design used for the experiment was randomized complete block design (RCBD) and about 45 cm distance was created between the rows. Kasumbha cv. Thori seeds were obtained from National Agricultural Research Centre (NARC). Sterilization of seeds was carried out with 95% (v/v) ethanol for 3 min and washed with autoclaved distilled water successively for 3–4 times before sowing. The commercialized biofertilizers containing living organisms e.g phosphate solubilizing bacteria were mixed with the soil as 20 kg ha−1 to study their effect on plant growth and yield. Chemical fertilizer used was diammonium phosphate and was applied as half dose (DAP as 15 kg ha−1). At the time of sowing, entire amount of DAP fertilizer was mixed with the soil and irrigations were applied as recommended.
Treatments were C (control treatment was without biofertilizer and DAP application), BF (Biofertilizers), CFH (treatment with half dose of Diamonium Phosphate 15 kg/ha) and BFCF (combined application of biofertilizer and half dose of DAP).
2.1. Sampling and measurements of agronomic traits
Agronomic traits such as leaf area, plant height, no. of branches and sub-branches plant−1, number of capitulum plant−1 were recorded. Plants were harvested on maturity when the capitulum turned brown in color and further work on oil contents, oil extraction, oil analysis, biodiesel production and oil quality analysis was carried out.
Leaf area was measured following the method of Ahmed and Morsy (1999) and following formula was used for the calculation.
2.2. Oil extraction
Kasumbha seed oil was extracted according to the AOCS Ag 1–65 method (AOCS, 1993) using Soxhlet apparatus in which hexane was used as a solvent and extraction was carried out at 60 °C for 6 h . The seeds were dried in an oven at 50 °C prior to extraction of oil by Soxhlet apparatus. The 30 g of the ground seeds was placed in the thimble which was loaded in the main chamber of Soxhlet extractor. The Soxhlet extractor with ground seeds was placed onto a solvent containing flask. The solvent used for oil extraction was n-hexane and the chamber containing desired compound with solvent was filled slowly. Chamber was automatically emptied by the siphon side arm when the Soxhlet chamber was almost full. This cycle was repeated multiple times till about 6 h to complete for each sample. After extraction, hexane was removed using rotary evaporator.
The oil extracted by Soxhlet apparatus was dried and its weight was determined. Then seed oil contents were calculated by the following equation
2.3. Transesterification reaction
Kasumbha oil was preheated over hot plate in order to remove moisture at a temperature of 120 °C for about 1 h before transesterification process. Then kasumbha oil was converted to biodiesel using NaOH as a catalyst (w/w) at 1: 6 M ratio of oil/methanol. The temperature was retained at 65 °C during the reaction and continuous stirring was carried out for an hour. Then separating funnel was used for the cooling and separation of mixture. Upper layer containing biodiesel was separated and washed and the residual water was removed by filtering with anhydrous sodium sulphate (Na2SO3). Methanol present in the biodiesel was removed by rotary evaporator at 45 °C for 30 min. A yellowish transparent liquid thus obtained was a pure biodiesel. Calculation for the biodiesel yield was done through the formula used by Rashid and Anwar (2008).
2.4. Phenolic contents
Phenolic contents of kasumbha seeds were estimated according to the method of Sadasivam and Manickam (1992) which is called as Folin-Ciocalteu method. The sample was lyophilized and dissolved (0.1 g) in deionized water (1 mL). Then it was mixed with 2.8 mL of deionized water and 2 mL of Na2CO3 (2%) and 0.1 mL of Folin-Ciocalteau reagent (50% v/v) was added to the mixture, incubation was carried out for 30 min at room temperature. The absorbance was measured on spectrophotometer at wavelength of 750 nm using deionized water as a blank. Gallic acid was used to plot a standard curve. The data were expressed as mg gallic acid equivalents (GAE)/100 g lyophilized powder.
2.5. Fatty acid analysis
For fatty acid analysis, the samples were analysed by NIRS (Nuclear Infrared Spectroscopy) at NIFA (Nuclear Institute of Food and Agriculture) Oilseed Quality Laboratory, Peshawar.
2.6. Acid value and free fatty acid
Cox and Pearson (1962) method was used for the determination of acid value. A solution of ethanol:diethylether was prepared in the ratio of 1:1 (v/v) and oil (0.2 g) was mixed with 2.5 mL of above solution and then titration was carried out with 0.1 N NaOH. Indicator used for this analysis was phenolphthalein and then calculations for acid values were made.
Acid value was multiplied with 0.503 factor for the measurement of kasumbha oil free fatty acid contents.
2.7. Specific gravity
The AOAC (1970) method was used for the determination of specific gravity of kasumbha oil. The weight of empty specific gravity bottle was measured (A) and filled with 5 mL of oil sample and weighed again (B). Then specific gravity bottle was filled with 5 mL water and weighed again (C). The specific gravity of sample was calculated by the following formula:
2.8. Iodine value
The AOAC (1984) standard method was followed for the iodine value analysis. Oil (0.2 g) was mixed with carbon tetrachloride solution (15 mL) and then 25 mL Wij’s reagent (0.1 mol/L iodine monochloride acetic acid solution) was added in the flask. This mixture was allowed to stand for 2 h at 25 °C in the dark and then addition of potassium iodide was carried out. Then titration was done with 0.2 N sodium thiosulphate and starch was used as an indicator. A blank was prepared by the same procedure and then iodine value was calculated.
2.9. 1H NMR analysis
For 1H NMR analysis of the kasumbha oil biodiesel, Avan CE 300 MHz spectrometer was used with deuterated chloroform (CDCl3) as a solvent and internal standard used was tetramethylsilane (TMS).
2.10. Statistical analysis
Data of the experiment was analyzed through Statistix software and comparison between the treatments was made through Least Significant Difference (LSD) test of Steel and Torrie (1980) at P < 0.05.
3. Results
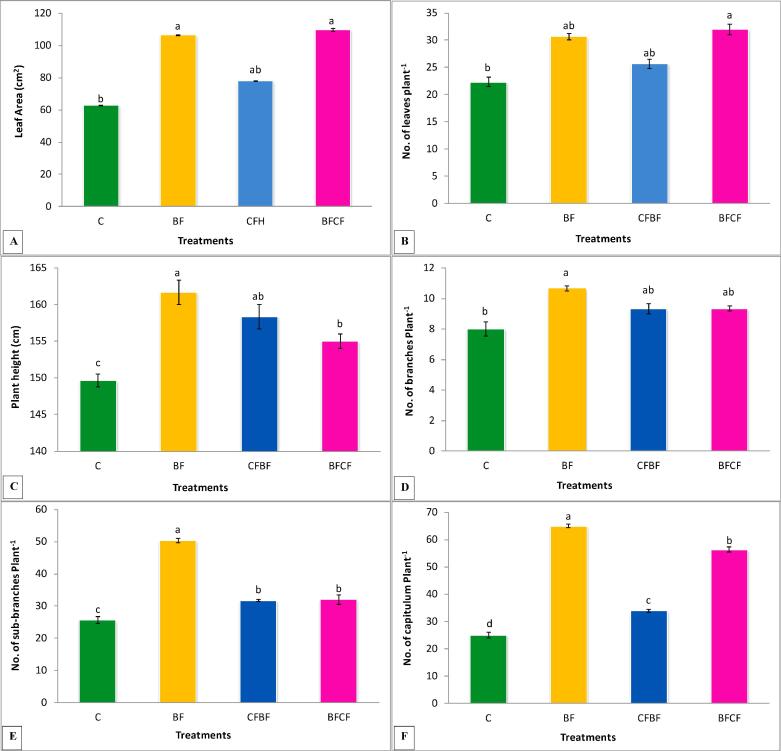
The results indicated that leaf area was significantly increased (74%) by biofertilizer treatment alone (BF) and combine treatment of biofertilizer and chemical fertilizer (BFCF) (69%) as compared to the control (Fig. 1A). The chemical fertilizer treatment alone (CFH) showed 24% nonsignificant increase in leaf area over control (C). The treatment BFCF (combined) showed 3% and 29% increase over BF and CFH treatments respectively while treatment BF showed 26% increase in leaf area over CF treatment.
Fig. 1.
Effects of Biofertilizer and chemical fertilizers on (A) leaf area (cm2), (B) no. of leaves/plant−1, (C) plant height, (D) no. of branches plant−1, (E) no. of sub-branches plant−1, (F) no. of capitulum plants-1of kasumbha. The mean values with different letters are significantly different from each other at P < 0.05 while values which are sharing letter are non-significant to each other. Symbols used for treatments: C: Control, BF: Biofertilizer, CFH: Half dose of chemical fertilizer, CFBF: Combine application of biofertilizer and half dose of chemical fertilizers.
Maximum number of leaf plant−1 (43%) was recorded by BFCF treatment which showed significant increase over the control (Fig. 1B). Treatment BF showed 37% and CFH showed 14% increase over control. The treatment BFCF showed 19% increase in number of leaf plant−1 as compared to CFH.
All the treatments significantly improved the plant height over the control, however, maximum significant increase of 8% was recorded by BF treatment (Fig. 1C). Similarly, half dose of chemical fertilizers and combined application of biofertilizer and chemical fertilizer showed significant increase (6% and 4%) in the plant height over the control respectively.
Results in Fig. 1D indicated that maximum significant increase (33%) in number of branches plant−1 was recorded by BF treatment over that of the control (C). Treatment CFH and BFCF (combined application of bio-chemical-fertilizers) showed non-significant (16%) increase in the no. of branches as compared to that of the control.
Number of sub-branches plant−1 was significantly increased by all the treatments (Fig. 1E), however; maximum significant increase (96%) was recorded by biofertilizer treatment (BF) when compared with the control. The CFH and BFCF treatments resulted in significant increase of 23% and 25% in the number of sub-branches plant−1 over the control. The percentage increase shown by BF treatment was 37% over the CF treatment and 36% over the BFCF treatment respectively.
Fig. 1F showed that all the treatments significantly enhanced no. of capitulum plant−1, however, maximum significant increase (160%) was recorded by BF as compared to the control. Similarly, CFH and BFCF treatment both significantly increased (36 and 65%) the no of capitulum plant−1 over the control. The % increase shown by BF treatment was 61% over CF and 13.33% over BFCF treatments respectively.
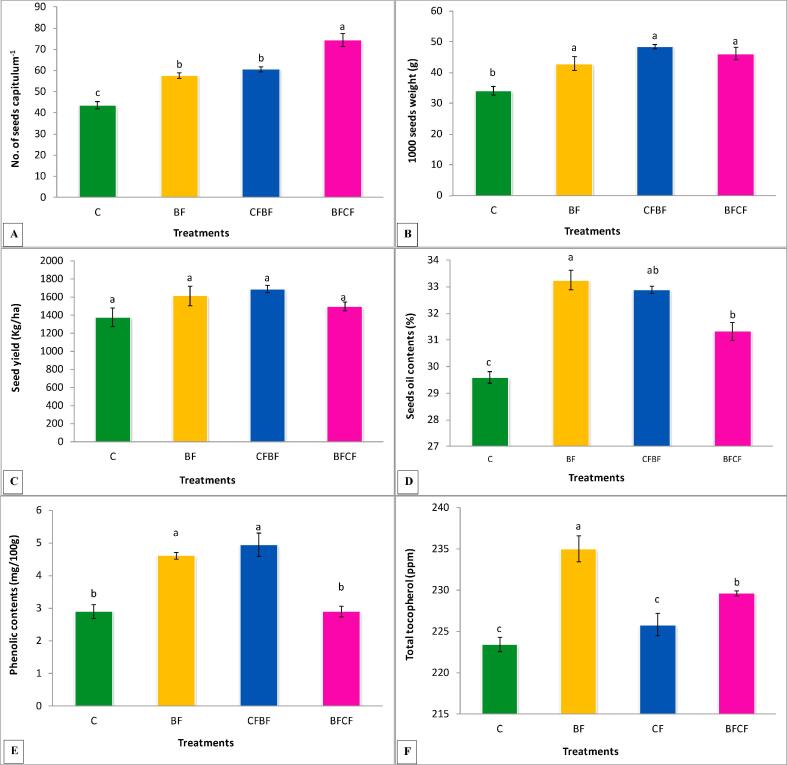
All the treatments significantly enhanced the no. of seeds capitulum−1 over the control as shown in Fig. 2A, however, maximum significant increase (70%) was recorded by BFCF treatment. The biofertilizer and half dose of chemical fertilizer treatments significantly increased (32 and 39%) the number of seeds capitulum−1 over the control. The percent increase by BFCF treatment over BF and CFH was 28 and 22 % respectively.
Fig. 2.
Effects of Biofertilizer and chemical fertilizers on (A) no. of seeds capitulum−1, (B) 1000 Seed weight, (C) seed yield, (D) seed oil content, (E) seed phenolics and (F) total tocopherol of kasumbha. The mean values with different letters are significantly different from each other at P < 0.05 while values which are sharing letter are non-significant to each other. Symbols used for treatments: C: Control, BF: Biofertilizer, CFH: Half dose of chemical fertilizer, CFBF: Combine application of biofertilizer and half dose of chemical fertilizers.
All the treatments significantly improved the 1000 seed weight when compared with the control (Fig. 2B). The maximum 1000 seed weight (41%) was recorded by CFH treatment followed by combine treatment of BFCF (35.4%) as compared to the control. treatment BF also significantly increased (25%) the 1000 seed weight over that of the control.
Results indicated that all the treatment showed increase in seed yield, however, the effect of the treatments was non-significant when compared with that of the control as shown in Fig. 2C. Maximum seed yield (23%) was recorded by the half dose of chemical fertilizers treatment followed by biofertilizer treatment (17%) when compared with that of the control. The combined application of biofertilizer and chemical fertilizer showed 9% non-significant increase over that of the control.
All the treatments significantly enhanced the oil contents of the seeds over the control (Fig. 2D). The treatment BF showed maximum significant increase (12%) in seed oil content followed by CF treatment (11%) compared to the control. The BFCF treatment showed 6% increase in seed oil content over control.
Seed phenolics were significantly improved by BF and CF treatments, however, BFCF showed non-significant effect as compared to the control (Fig. 2E). The maximum (70%) phenolics were recorded by CF treatment while BF showed 59% increase over the control. The treatment CFH showed 41% and 7% increase in seed phenolics as compared to the BFCF and BF treatments respectively.
Results indicated that tocopherol contents were improved by all the treatments (Fig. 2F). Maximum increase (5%) in tocopherol contents were recorded in BF treatment over the control. Treatments CF and BFCF also showed significant increase in tocopherol contents over that of the control.
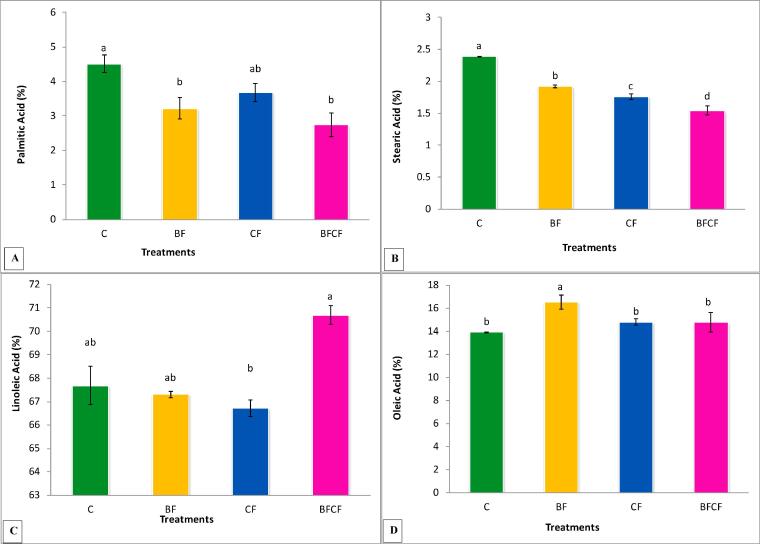
Variations in fatty acid profile by different treatments were observed (Fig. 3A-D). The results indicated that maximum palmitic acid contents were found in control treatment while other treatments showed reduction in palmitic acid over the nontreated control (Fig. 3A). Maximum reduction was observed in BFCF treatment.
Fig. 3.
Effects of Biofertilizer and chemical fertilizers on (A) Palmitic acid (%), (B) Stearic Acid (%), (C) Linoleic acid (%), (D) Oleic acid (%) of kasumbha. The mean values with different letters are significantly different from each other at P < 0.05 while values which are sharing letter are non-significant to each other. Symbols used for treatments: C: Control, BF: Biofertilizer, CFH: Half dose of chemical fertilizer, CFBF: Combine application of biofertilizer and half dose of chemical fertilizers.
Similarly, reduction in stearic acid fatty acid was also recorded by all treatments over that of the control as shown in Fig. 3B. The treatment BF showed 19% reduction over control while maximum reduction (35%) was recorded in BFCF treatment. Chemical fertilizer treatment also showed considerable reduction in stearic acid contents over control.
The treatment BFCF showed maximum increase of 4% in linoleic acid over the control (Fig. 3C). The other two treatments BF and CF showed non-significant decline in linoleic acid when compared with the control.
The results in Fig. 3D indicated that oleic acid contents were significantly enhanced (16%) by BF treatment when compared with the control. The magnitude of increase in oleic acid contents recorded by treatments CF and BFCF was non-significant compared to the control.
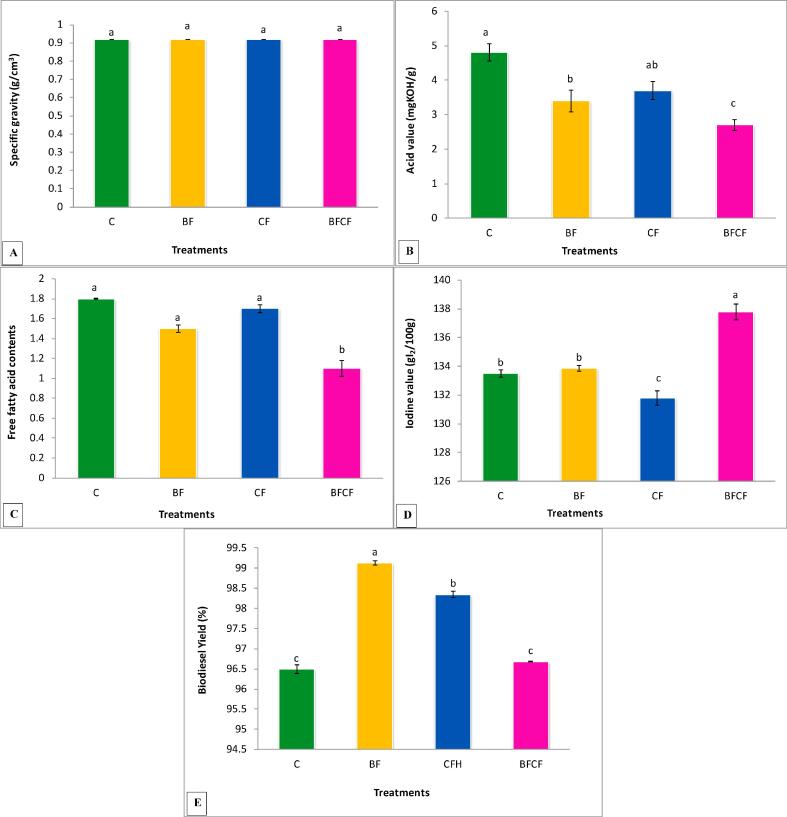
Results in Fig. 4A showed that there was no effect of treatments on specific gravity of oil. All the treatments were non-significant as compared to the control and among each other as well.
Fig. 4.
Effects of Biofertilizer and chemical fertilizers on (A) Specific gravity (g/cm3), (B) Acid value (mgKOH/g), (C) Free Fatty acid contents, (D) Iodine value (gI2/100g) (E) Biodiesel yield of kasumbha. The mean values with different letters are significantly different from each other at P < 0.05 while values which are sharing letter are non-significant to each other. Symbols used for treatments: C: Control, BF: Biofertilizer, CFH: Half dose of chemical fertilizer, CFBF: Combine application of biofertilizer and half dose of chemical fertilizers.
All the treatments reduced the acid value of kasumbha when it was compared with the control (Fig. 4B). The BF treatment showed 29% significant reduction in acid value while CF treatment showed non-significant reduction over control. However, BFCF treatment showed maximum reduction (43%) in acid value.
Decrease in free-fatty-acid of oil was recorded by all treatments as shown in Fig. 4C; however, maximum reduction of 38% was resulted in BFCF treated plants oil over the value of control. Other treatments showed non-significant reduction compared to the control.
Iodine value of the kasumbha oil was increased by 3% by BFCF treatment while the iodine value of BF treatment was almost at par to that of the control. Treatment CF showed significant reduction in iodine value as compared to the control (Fig. 4D).
The maximum biodiesel yield was recorded in BF treatment that showed 3% significant increase over the control (Fig. 4E). Chemical fertilizer treatment showed 2% significant increase over the control while BFCF treatment showed non-significant effect on biodiesel yield as compared to the control.
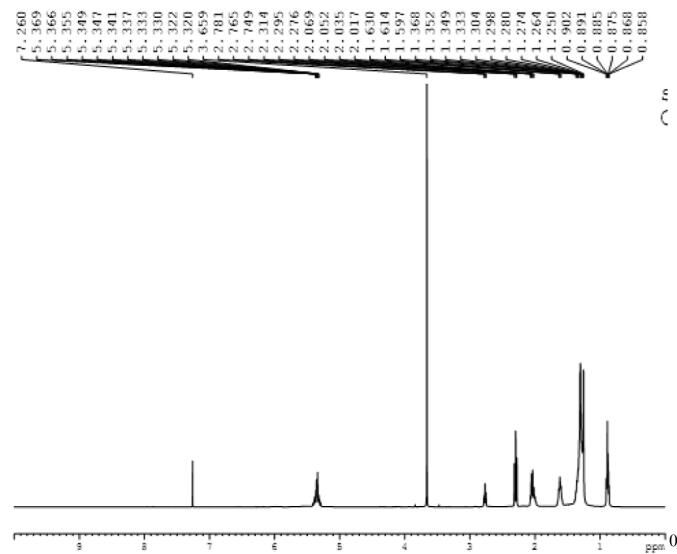
Fig. 5 showed the 1HNMR spectra for the yield of biodiesel. The appearance of signals at 3.659 ppm of methoxy protons indicated the conversion of kasumbha oil into biodiesel. The BF treatment showed 99% conversion while CFH showed 98% conversion of kasumbha oil into biodiesel.
Fig. 5.
1H NMR Spectra for biodiesel yield.
4. Discussion
The application of biofertilizer and chemical fertilizers improved the growth and yield of the kasumbha. The stimulatory effect of biofertilizer and combine application of biofertilizer and chemical fertilizer on leaf area and number of leaves/plant might be due to production of phytohormones by the bacteria which improve the growth attributes of the plant. Karakurt and Aslantas (2010) reported the similar results who indicated that growth parameters like leaf area and contents of pigment fractions in plant seedlings were increased by bacterial inoculation. The application of biofertilizer enhanced the number of branches plant−1, plant height, number of sub-branches plant−1, and number of capitulum plant−1 as compared to the control. Increased in growth of plants inoculated with phosphate solubilizer might be due to the increased phosphate accumulation and resulted in better growth of roots, which increased the uptake of water and nutrients to the plants (Yasmin et al., 2021). The treatments BFCF and CFH resulted in increase in no. of seeds capitulum−1, 1000 seed weight and seed yield over the control and treatment BF also have significant effect on the yield attributes and these findings such as increased in height of plants, spike/spikelets number, number of tillers, straw and grain yield and 1000 grain weight were reported by Akhtar et al. (2009) using PGPR inoculated seeds along with recommended dose of chemical fertilizers and compost in wheat. Similarly, it has been reported in a previous study that plant growth, nutrient contents (phosphorus and nitrogen) and yield were improved by combined application of PGPR and chemical fertilizers to the plant (Adesemoye et al., 2009). Increase in the weight of the seeds might be due to the fact that biofertilizer improved the dry matter accumulation in the seed weight whereas, the higher seed yield in CFH treatment might be due to the increase supply of nutrients which improved leaf area, and number of branches plant−1 and these results were according to previous studies who described that the usage of phosphorus and nitrogen fertilizers plus Azospirillum and Azotobacter in combined application in barley resulted in maximum 1000 grain weight and grain yield when compared with that of the control (Mirshekari et al., 2012).
In the present study, improvement in seed oil contents by BF and BFCF are in line with the results of Mirzaei et al. (2010) who stated that significantly enhanced in the seed yield and oil contents of safflower was recorded by the inoculation of Azospirillum and Azotobacter in addition of half dose of chemical fertilizers. Nosheen and Bano (2014) reported the similar results that PGPR inoculation along with urea and DAP application increased 1000-seed weight, height of the plants, and oil content over the uninoculated control in safflower. The increase in oil content might be due to the increase in endogenous level of phytohormones as it was reported previously by Sharma et al. (2008) that phytohormones played a critical role in seed maturation and accumulation of seed oil contents in Brassica. Yasmin and Bano (2011) reported that phosphate solubilizing bacteria not only improved plants growth of chickpea but also enhanced the level of abscisic acid and to our understanding, this phytohormone (ABA) might be involved in the increase of oil contents of an oilseed crop. Another reason behind the increased oil contents might be the improving the function of enzymes which are involved in the oil production by the application of PGPR. It has been reported that hydroxymethylglutaryl-CoA (Hmgr-CoA) and phytoene desaturase (crtl) genes isolated from bacteria (E. uredovora) were manipulated in sunflower resulted in enhanced oil contents and quality of the plant (Dağüstü et al., 2008).
In the present investigation maximum improvement in seed phenolics and tocopherol was recorded by BF and CF treatments. These results confirm the findings of Raj et al. (2005) who stated that PGPR improved the phenolic contents. Seed phenolics and tocopherol are the natural antioxidants that improve the oil quality by increasing the oxidation stability and enhancing the storage qualities of kasumbha oil. Siger et al. (2008) demonstrated that oxidation stability of polyunsaturated fatty acid was increased by the seed phenolics. Enhancement in the yield of kasumbha biodiesel by BF treatment might be due to its noticeable effect on acid value and free fatty acids of kasumbha. A similar improvement in yield of biodiesel was reported by Nosheen et al. (2009) in soybean inoculated with commercialized biofertilizers (Biozote and Biopower) in comparison with the control. Presence of free fatty acids in the biodiesel results in soap formation and required higher quantities of basic catalyst in order to neutralize them. The soap formation resulted in an increase in viscosity and hence formation of gels that affects reaction and also the separation of glycerol takes place. Low acid value vegetable oils neutralize the effect of free fatty acids (Freedman et al., 1984) and also the other factors can affect the biodiesel yield such as fatty acid silhouette and other properties of oil.
Results of current study depicted a noteworthy finding that BF treatment showed higher improvement in the oleic acid contents. Oleic acid improves the biodiesel properties i-e oxidation stability, viscosity, cetane number, specific gravity, and cold flow properties and is considered as a vital fatty acid to produce biodiesel of better quality. In the present work, single application of biofertilizers enhanced the oleic acid contents as compared to that of control. Present results are in accordance to that of the Choudhury and Kennedy (2004) that sunflower inoculation with nitrogen fixing bacteria increased the phosphorus level that influenced the seed oil content and ultimately altered the proportion of fatty acids (unsaturated/saturated fatty acids ratio. Similarly, Jha et al. (2007) explained that after the transformation and functional expression Azospirillum brasilense ACP in Brassica juncea enhanced the overall fatty acid profile mainly oleic acid (C18:1) fatty acid. The prime improvement was observed for oleic acid (C18:1) and linoleic acid (C18:2) in the seeds with parallel reduction of C22:1 (erucic acid) which indicated that biofertilizers played a critical role in improving the oleic acid content in the Brassica seed. According to our results, CFH took part in enhancing the composition of fatty acid in term of monounsaturated fatty acid (oleic acid). Nitrogen and phosphate fertilizers play a very key role in altering the composition of fatty acid of oil seed crops during the seed development process which requires N and C skeleton for biosynthesis which is under genetic control (Texier, 1993). It was reported by Gopinath et al. (2009) that cetane number of the biodiesel is significantly influenced by the fatty acid methyl esters. Increase in unsaturation decreases the cetane number and while increase in chain length of fatty acid methyl ester increase the cetane number. Therefore, increase in the degree of unsaturation, decreased the cetane number of unsaturated fatty acids and vice versa for saturated fatty acids. Oxidation stability is an important indicator of fuel quality, and it depends upon degree of unsaturation. Polyunsaturated-fatty-acids are more susceptible to oxidation and leads to bad biodiesel-storage-properties when compared with monounsaturated fatty acids (Atabani et al., 2012). Hence, the application of biofertilizers improved the cetane number and oxidation stability safflower by improving the oil quality which are variables affecting biodiesel yield and quality significantly.
The oil properties play an important role for good quality biodiesel production. An important variable called acid value affect the biodiesel quality during transesterification process and strongly need to be monitored (Babderra et al. 2016). The presence of higher acid contents causes the corrosion of the engine (Atabani et al., 2012, Dodos et al., 2012). According to the present study, all the treatments decreased the acid value of kasumbha oil; however, maximum reduction was brought about by BFCF treatment followed by BF treatment. Our results confirm the findings of Abd El-Gawad et al. (2009) who stated that peroxide and acid value of canola was decreased by Azotobacter chroococcum and Bacillus megaterium inoculation as compared to untreated control. It has been stated by Ullah et al. (2011) that production of phytohormone by PGPR might decrease the acid value as he indicated that the treatment of cytokinin reduced the safflower oil acid value. Bondioli et al. (2002) reported that improved-storage-properties and oxidation stability of biodiesel can be achieved by lowering the acid value of vegetable oil and biodiesel. Presence of large amount of free fatty acids in the oil highly influenced the yield and quality of biodiesel (Babderra et al. 2016) during base catalyzed transesterification reaction which results in the formation of soap and eventually leads to the reduction of biodiesel quality and yield (Demirbas, 2009).
Specific gravity is of paramount importance and basic property of biodiesel as heating value and cetane number which are the most important indicator of performance are correlated with it (Tat and Gerpen, 2000). The results indicated that there is no significant effect of treatments on the specific gravity of kasumbha oil. The increase in specific gravity leads to poor biodiesel quality and creates problem during the operation process. Tat and Gerpen (2000) reported that specific gravity is very important variable that is linked with the storage of fuel and transportation. Increase in specific gravity tends to lower the cetane number (Evangelos and Giakoumis, 2013).
The CF treatment showed maximum reduction in the iodine value in the current study while BFCF treatment resulted in higher iodine value resulting in the formation of deposit, consequently, cause problems of storage stability of the oil and biodiesel. Iodine value has a strong correlation with the degree of unsaturation of fatty acids and strongly affect the biodiesel yield and quality. The oils with monounsaturated fatty acid have lower iodine value as compared to the oil with higher polyunsaturated fatty acid (Ibeto et al., 2012). Iodine value and refractive index are in direct correlation with each other, oxidation stability and quality of oil can be improved by lowering the value of both variables (Eid et al., 2010).
4.1. Conclusion
It is inferred from the present investigation that biofertilizer and chemical fertilizers treatments alone significantly improved the growth and agronomic attributes such as leaf area, plant height, number of branches plant−1, number of sub-branches plant−1, number of capitulum plant−1, yield attributes. The combine treatment BFCF also stimulatory effect on the growth and yield of kasumbha, however its effect was not much pronounced on some traits. The seed oil contents which play an important role in improving the oil quantity were significantly improved by BF treatment. Similarly, maximum biodiesel yield was also recorded in biofertilizer treatment. The seed phenolics and tocopherols which are considered as antioxidant were improved by both biofertilizer treatment. The fatty acid profile in term of increase in oleic acid and the oil quality were significantly improved by biofertilizer treatment. It can be concluded that application of biofertilizer alone impart a very effective role in improving the growth, yield and oil quality of kasumbha followed by chemical fertilizer alone as compared when applied as a combine treatment.
Ethic approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this article.
Code availability
Not Applicable.
Funding
Authors are thankful to the Higher Education for providing the funding (IPFP/HRD/HEC/2014/1672) to accomplish this piece of work.
CRediT authorship contribution statement
Asia Nosheen: Conception, Design of the experiment, Supervision, Data curation, Interpretation of data, Formal analysis, Project administration. Syed Babar Hussain: Investigation, Methodology, Writing-original draft, Data collection, Interpretation of data. Humaira Yasmin: Software, Review and editing, Statistical analysis. Rabia Naz: Writing, Review and editing, Statistical analysis. Rumana Keyani: Drafting, Validation, Reviewing, Editing. Saqib Mumtaz: Simulation, Drafting and revising the manuscript. Muhammad Nadeem Hassan: Visualization, Reviewing, Validation, Editing, Data analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are thankful to National Agricultural Research Center for providing the seeds for the experiment. The authors would like to extend their sincere appreciation to the Taif University Researchers Supporting Project number (TURSP-2020/262), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Asia Nosheen, Email: asia.nosheen@comsats.edu.pk.
Humaira Yasmin, Email: humaira.yasmin@comsats.edu.pk.
Rabia Naz, Email: rabia.naz@comsats.edu.pk.
Rumana Keyani, Email: rumana.keyani@comsats.edu.pk.
Saqib Mumtaz, Email: saqib.mumtaz@comsats.edu.pk.
Syed Babar Hussain, Email: babarhussain679@gmail.com.
Muhammad Nadeem Hassan, Email: nadeem_hassan@comsats.edu.pk.
Othman M. Alzahrani, Email: o.alzahrani@tu.edu.sa.
Ahmed Noureldeen, Email: a.noureldeen@tu.edu.sa.
Hadeer Darwish, Email: hadeer@tu.edu.sa.
References
- Abd El-Gawad A.M., Hendawey M.H., Farag H.I.A. Interaction between biofertilization and canola genotypes in relation to some biochemical constituents under Siwa Oasis conditions. Res. J. Agri. Biol. Sci. 2009;5(1):82–96. [Google Scholar]
- Adesemoye A.O., Torbert H.A., Kloepper J.W. Plant Growth-Promoting Rhizobacteria allow reduced application rates of chemical fertilizers. Micro. Eco. 2009;58(4):921–929. doi: 10.1007/s00248-009-9531-y. [DOI] [PubMed] [Google Scholar]
- Ahmed F.F., Morsy M.H. A new method for measuring leaf area in different fruit species. Minia J. Agri Res. Develop. 1999;19:97–105. [Google Scholar]
- Akhtar M.J., Asghar H.N., Shahzad K., Arshad M. Role of plant growth promoting rhizobacteria applied in combination with compost and mineral fertilizers to improve growth and yield of wheat (Triticum aestivum L.) Pak. J. Bot. 2009;41:381–390. [Google Scholar]
- AOAC, 1970. Official method of analysis XI Edn. Association of official Analytical chemists, Washington D. C.
- AOAC. 1984. Official methods of Analysis; 14th ed. Association of Official Analytical chemists; V.A. Arlington.
- AOCS . 4th ed. American Oil Chemist’s Society Press; Champaign, IL, USA: 1993. Official Methods and Recommended Practices of the American Oil Chemist’s Society. [Google Scholar]
- Atabani A.E., Silitonga A.S., Badruddin I.A., Mahlia T.M.I., Masjuki H.H., Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sust. Ener. Rev. 2012;16(4):2070–2093. [Google Scholar]
- Babderra, B., Vázquez, R., Martínez, M., José, A., 2016. Effect of free fatty acids contents on biodiesel quality. Pilot plant studies. Fuel. 174. DOI: 10.1016/j.fuel.2016.01.018.
- Bondioli P., Gasparoli A., Della Bella L., Tagliabue S. Evaluation of biodiesel storage stability using reference methods. Euro. J. Lipid Sci. Technol. 2002;104(12):777–784. [Google Scholar]
- Choudhury A.T.M.A., Kennedy I.R. Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol. Fert. Soil. 2004;39(4):219–227. [Google Scholar]
- Cox, H.E., Pearson, D., 1962. The chemical analysis of foods, chemical publishing Co., Inc. New York, p. 421.
- Dağüstü N., Fraser P., Enfıssi E., Bramley P. Screening for high callus induction and agrobacterium-mediated transformation of sunflower (Helianthus annuus L.) Biotech. Biotechnol. Equip. 2008;22(4):933–937. doi: 10.1080/13102818.2008.10817582. [DOI] [Google Scholar]
- Demirbaş A. Production of biodiesel from algae oils. Energy Sources. 2008;31(2):163–168. [Google Scholar]
- Dodos G.S., Konstantakos T., Longinos S., Zannikos F. Effects of microbiological contamination in the quality of biodiesel fuels. Global NEST J. 2012;14(2):175–182. [Google Scholar]
- Eid R.A., Taha L.S., Ibrahim S.M.M. Physiological properties studies on essential oil of Jasminum grandiflorum L. as affected by some vitamins. Ozean J. Appl. Sci. 2010;3:87–96. [Google Scholar]
- Evangelos, G., Giakoumis, 2013. A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renew. Ener. 50: 858–878.
- Fasusi O.A., Cruz C., Babalola O.O. Agricultural Sustainability: Microbial Biofertilizers in Rhizosphere Management. Agriculture. 2021;11(163) doi: 10.3390/agriculture11020163. [DOI] [Google Scholar]
- Freedman B., Pryde E.H., Mounts T.L. Variables affecting the yields of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984;61(10):1638–1643. [Google Scholar]
- Gopinath A, Puhan S, Nagarajan G. Relating the cetane number of biodiesel fuels to their fatty acid composition: a critical study. J. Auto. Proce. Inst. Mech. 2009;223(4):565–583. doi: 10.1243/09544070JAUTO950. [DOI] [Google Scholar]
- Ibeto Cynthia Nkolika, Okoye Chukwuma Obiajulu Benedict, Ofoefule Akuzuo Uwaoma. Comparative study of the physicochemical characterization of some oils as potential feedstock for biodiesel production. Renew. Ener. 2012;2012:1–5. [Google Scholar]
- Jha Jyoti K., Sinha Saheli, Maiti Mrinal K., Basu Asitava, Mukhopadhyay Ujjal K., Sen Soumitra K. Functional expression of an acyl carrier protein (ACP) from Azospirillum brasilense alters fatty acid profiles in Escherichia coli and Brassica juncea. Plant Physiol. Biochem. 2007;45(6-7):490–500. doi: 10.1016/j.plaphy.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Karakurt H., Aslantas R. Effect of some plant growth promoting rhizobacteria (PGPR) strains on plant growth and leaf nutrient contents of Apple. J. Fruit Ornament. Plant Res. 2010;18(1):101–110. [Google Scholar]
- Khan, S., Raza, M., Nosheen, A., Naz, R., Shah, S.M.U., Hassan, M.N. 2019. Quality comparison of biodiesel produced from waste cooking oil of restaurant and domestic kitchen, Inter. J. Green Ener. DOI: 10.1080/15435075.2019.1700123.
- Mirshekari B., Hokmalipour S., Sharifi R.S., Farahvash F., Ebadi-Khazine-Gadim A. Effect of seed biopriming with plant growth promoting rhizobacteria (PGPR) on yield and dry matter accumulation of spring barley (Hordeum vulgare L.) at various levels of nitrogen and phosphorus fertilizers. J. Food Agri. Environ. 2012;10:314–320. [Google Scholar]
- Mirzaei A., Vazan S., Naseri R. Response of yield and yield component of safflower (Carthamus tinctorious L.) to seed inoculation with Azotobacter and Azospirillum and different nitrogen levels under dry land conditions. World Appl. Sci. J. 2010;11:1287–1291. [Google Scholar]
- Mizik T., Gyarmati G. Economic and Sustainability of Biodiesel Production—A Systematic Literature Review. Clean Technol. 2021;3:19–36. doi: 10.3390/cleantechnol3010002. [DOI] [Google Scholar]
- Nosheen, A., Bano, A., Faizanullah, 2009. Comparative study for the effect of biofertilizers and chemical fertilizers on Soybean oil content and its potential for biodiesel production. Pak. J. Sci. Ind. Res. 52 (5):264–269.
- Nosheen A., Bano A. Growth enrichment of Carthamus tinctorius (L.) and reduction in dosage of chemical fertilizer with application of plant growth promoting rhizobacteria. Int. J. Agron. Agri. Res. (IJAAR). 2014;4(6):75–84. [Google Scholar]
- Nosheen A., Naz R., Tahir A.T., Yasmin H., Keyani R., Mitrevski B., et al. Improvement of safflower oil quality for biodiesel production by integrated application of PGPR under reduced amount of NP fertilizers. PLoS ONE. 2018;13(8) doi: 10.1371/journal.pone.0201738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheen S., Ajmal I., Song Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability. 2021;13:1868. doi: 10.3390/su13041868. [DOI] [Google Scholar]
- Nosheen A., Bano A., Ullah F. The role of plant growth promoting rhizobacteria on oil yield and biodiesel production of canola (Brassica napus L.) Energy Sources, Part A: Recovery, Utiliz., Environ. Effects. 2013;35(16):1574–1581. doi: 10.1080/15567036.2010.529561. [DOI] [Google Scholar]
- Nosheen A., Bano A., Ullah F. Nutritive value of canola (Brassica napus L.) as affected by plant growth promoting rhizobacteria. Eur. J. Lipid Sci. Technol. 2011;113:1342–1346. [Google Scholar]
- Raj, N.S., Shetty, H.S., Reddy, M.S., Siddiqui, Z.A., 2005. Plant Growth-promoting Rhizobacteria: Potential green alternative for plant productivity PGPR: Biocontrol and Biofertilization, Siddiqui Z.A. (ed.), PP. 197–216, Dordrecht, The Netherlands.
- Rashid Umer, Anwar Farooq. Production of biodiesel through base-catalyzed transesterification of Safflower oil using an optimized protocol. Energy Fuels. 2008;22(2):1306–1312. [Google Scholar]
- Sadasivam S., Manickam A. Wiley Eastern Ltd.; New Delhi, India: 1992. Biochemical Methods for Agricultural Sciences; pp. 187–188. [Google Scholar]
- Sharma N., Anderson M., Kumar A., Zhang Y., Giblin E.M., Abrams S.R., Zaharia L.I., Taylor D.C., Fobert P.R. Transgenic increases in seed oil content are associated with the differential expression of novel Brassica-specific transcripts. BMC Genomics. 2008;9:619. doi: 10.1186/1471-2164-9-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siger A., Nogala-Kalucka M., Lampart-Szczapa E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipid. 2008;15(2):137–149. [Google Scholar]
- Singh R., Arora A., Singh V. Biodiesel from oil produced in vegetative tissues of biomass–A review. Biores. Technol. 2021;326:124772. doi: 10.1016/j.biortech.2021.124772. [DOI] [PubMed] [Google Scholar]
- Steel R.G.D., Torrie G.H. 2nd ed. McGraw Hill Book Co. Inc.; Singapore: 1980. Principles and procedures of statistics; pp. 172–177. [Google Scholar]
- Takase M., Essandoh P.K., Kipkoech R. New non-edible Allanblackia parviflora seed oil as an alternative feedstock for biodiesel production and characterization of the fuel. Discov. Sustain. 2021;2:8. doi: 10.1007/s43621-021-00019-w. [DOI] [Google Scholar]
- Tat M.E., Gerpen J.H.V. The specific gravity of biodiesel and its blends with diesel fuel. J. Amer. Oil Chem. Soc. 2000;77(2):115–119. [Google Scholar]
- Texier P.H. Le-cotton, cinquieme producteur mondial d huile alimentaire. Cotton Develop. 1993;8:2–3. [Google Scholar]
- Ullah F., Bano A. Effect of plant growth regulators on oil yield and biodiesel production of safflower (Carthamus tinctorius L.) Braz. J. Plant Physiol. 2011;23:27–31. [Google Scholar]
- Yasmin H., Bano A., Wilson N.L., Nosheen A., Naz R., Hassan M.N., et al. Drought-tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiol. Plant. 2021;1–15 doi: 10.1111/ppl.13497. [DOI] [PubMed] [Google Scholar]
- Yasmin H., Bano A. Isolation and characterization of phosphate solubilizing bacteria from rhizosphere soil of weeds of khewra salt range and attock. Pak. J. Bot. 2011;43(3):1663–1668. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article.