Abstract
In veterinary medicine plant based medicine is achieving a huge importance worldwide. This research was subjected to rectify the hydrophilic Moringa Oleifera alcoholic leaves extract could improve the immune system in vaccinated and non-vaccinated broiler Hubbard chickens experimentally exposed to Newcastle disease (ND) virus. Seventy five chicks with age one day old were splitted randomly into five groups equally in distribution with fifteen chick in each group. Group I was untreated unvaccinated (control negative group) while group IV was infected group with NDV (control positive group). The experimental Groups II and V were given daily oral treatment of hydrophilic alcoholic leaves extract of M. oleifera at 200 mg/kg body weight until day 21 of age while groups III and V were ND vaccinated with La Sota strain of ND vaccines. The four groups (II, III, IV, V) were infected with ND virus velogenic strain (VNDV) on day 21. Following to infection, Monitoring of birds were done daily for clinical signs, postmortem examination, morbidity and mortality. Cellular, humeral immune response and phagocytic activity were evaluated and the data were statistically analyzed using (SPSS). Total and differential cell numbers as well as Haemagglutination inhibition (HI) titre increased in the extract treated and vaccinated group which give total protection against NDV much more than treated and unvaccinated group. As a result it could be recommended to use M. Olifera extract from the first day of rearing in Hubbard chicken with ND vaccination program as a prophylactic treatment in protection of birds against ND infection.
Keywords: Broilers, Moringa Oleifera, Immunity, Phagocytic activity, Velogenic Newcastle disease
1. Introduction
The poultry industry is one of the world's most fundamental agricultural sectors. Poultry provides humans with an excellent source of animal protein in the form of eggs and meat. Poultry meat accounts for 20% of total daily fish and animal protein consumption per capita (Alders et al., 2018). Poultry production showed ecumenical increase from backyard farm to advanced poultry farm including commercial meat and egg production. Diseases result from viral agents in poultry ranches cause a tremendous economic losses. Newcastle disease is a highly contagious and infectious viral disease of domestic, aviary, caged, and wild birds that causes severe morbidity, mortality, economic losses, and risks to the global chicken industry (Rehman et al., 2020). Newcastle disease virus is mainly controlled by vaccination but some challenges particularly in rural areas with pauper ranches as high vaccines costs, unavailability of cold chain systems used theses vaccines in addition to small flock sizes with multi-aged birds may effect on the success of vaccination control system (El-Masry et al., 2019) Vaccination failures and outbreaks of the Newcastle disease have also been happened as a result of neutralization of the vaccine virus at the time of vaccination by maternal antibodies as well as the presence of antiemetic variants in the field that are kept in continuous circulation (Garba et al., 2013, Hegazy et al., 2021). Ameliorating the immunogenicity of the vaccines by the enforcement of natural herbal plants, for example, are complementing ways that may be a pretty way to conquer such infectious diseases. Some studies reported the enhancement in the B and T cell performance and antibody response after co-administration of medicinal plants extracts with vaccine (Hu et al., 2003, Sun et al., 2007). The use of curative plants is progressively earning vale as products of natural sources. They offer medicinal value for a variety of ailments and are considered one of the most essential components of indigenous medical systems that have survived in developing countries (Abd-Rabou et al., 2017, Abdel Rahman et al., 2019). One of these plants is Moringa Oleifera lam belongs to family Moringaceae. It is now widely distributed throughout subtropical and tropical areas in the world. It is Native to India and contains various active substances as phytochemicals, phenolics, vitamins, amino acids macronutrients, and micronutrients which have antioxidant, antiviral activity, anti-inflammatory, antimicrobial and use to expel intestinal worms also has an immunostimulatory effects (Brilhante et al., 2017, Desoky et al., 2019). Therefore the hypothesis of this study is to detect the antiviral and protective effect of Moringa Oleifera leaf extract in broiler Hubbard chickens against velogenic strain of Newcastle virus and study the effect of this leaf meal on the action of Newcastle virus vaccine.
2. Material and methods
2.1. Birds and handling
A total of seventy-five one day old commercial chickens obtained from El-Bana Company were applied and put in separate units until the beginning of the experimental study. The chickens were divided in five experimental groups in Negative Pressure Isolators, under bio-safety conditions in experimental units in Faculty of Veterinary Medicine, Zagazig University. Birds were fed on commercial starter, pelleted ration and kept under the managemental conditions as lightening was provided 23hr light and 1 h dark with cyclic temperatures (minimum 24°C, maximum 32 °C). Feed and water were provided ad libitum. All experimental chickens were used according to the Committee of Animal Welfare and Research Ethics (protocol #ZU-IACU/2/F/9/2018) in Zagazig University, Egypt.
2.2. NDV propagation and titration
NDV were utilized in the present study was velogenic NDV class II, genotype VIId (A/chicken /Egypt /AB3/2018) under an accession number of MK968881 was isolated in department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Prior to use in experimental infection, virus propagation and titeration were carried in embryonated chicken eggs according to (Aldous and Alexander, 2001, Sheble and Reda, 1976), as well as, virus titers were calculated according to (Reed and Muench, 1938). The inoculation dose was 106(ELD50) of 0.1 mL of infectious allantoic fluid. Live vaccines used in this our study including LaSota (LS) (BAL-ND (B1 type LaSota strain live vaccine), G.O.V.s Batch no:L 141/1 NDV strain.
2.3. Preparation of hydro-alcoholic extract of Moringa Oleifera
From the middle aged green trees in agricultural orchards of Faculty of agriculture, Zagazig University the dried leaves of Moringa Oleifera were harvested and grounded into fine coarse powder by hand and mortem. The leaves powder (200 g) was soaked in ethanol 95% (1L): water (1L) 1:1 at 33c for 24 h. The mixture was subjected to filtration using a Watman grade 1 filter paper then the solvent was evaporated and the extract was lyophilized. The extracts were stored in a dark bottle at −4 °C until used. The crude extract was suspended in distilled water (Elrys et al., 2019, Prabsattroo et al., 2012) and by using standard methods according to (Trease and Evans, 1972) Phytochemical analyses of the extracts were performed by Elangovan (2014).
2.4. Experimental infection and sampling
At one day of age, the birds should divided into five equal groups randomly (15 chicks/group). From the other groups, group I (control negative) and group IV (control positive) were isolated Groups II&V were treated The treatment was with the Moringa Oleifera leaves extract 200 mg/kg body weight orally used in drinking water daily for three weeks while groups III&V were vaccinated. At 21 day of age all the groups were challenged intraocular with dose of 0.2 of 106 of vNDV. After infection, clinical signs were monitored daily. For viral shedding evaluation, oropharengeal and cloacal swabs were collected at 3rd 5th 7th and 9th day post infection, placed in sterile phosphate buffer saline and stored at −70 °C until used. Blood samples were collected for hematology at 14, 21 and 28 days of ages and at 21 day of age before inoculation, 3dpi and 5dpi for phagocytic activity. Blood centrifugation was used to separate the serum at 14, 21 day post vaccination and at 14 day post challenge were collected and stored at −20 °C until used for immune response evaluation by haemagglutination test. The birds were vaccinated with LaSota (BAL-ND) (B1 type LaSota strain live vaccine) at 15 day of age eye drop route.
2.5. Serology
The antigen titre for running the HI test was determined by standard HA technique using La Sota ND vaccine as antigen according to (Alexander and Senne, 2003). The reciprocal of the highest dilution of the La Sota ND antigen causing 100% agglutination of an equal volume of standardized RBCs was taken as the HA titre of the antigen. The HI titres were determined, also by the method of (Beard, 1989). The HI titers were reciprocal of the highest dilutions of the sera at which 100% RBC HI occurred. The geometric mean titre (GMT) was calculated according to (Villegas, 1998).
2.6. Hematology
Cellular immune response was assesed by detecting the total leukocytic counts of white blood cells (W BC) which obtained according to standard methods using improved Neubeur heamocytometer and differential leukocytic count which done in stained thin blood smears Coles (1986).
2.7. Phagocytic activity
Blood was collected from three birds weekly before challenge and week after challenge in a test tube containing EDTA anticoagulant then send to Reference Laboratory for Veterinary Quality Control on Poultry Production (RLQP), Zagazig to detect the Phagocytic index of Macrophage cells before and after challenge.
2.8. Virus shedding
RNA was extracted from cloacal and oropharyngeal swabs that collected at 3rd, 5th, 7th and 9th PI according to the manufacturer’s protocol using the QIamp Viral RNA Mini Kit (Qiagen, USA), Quantitative real-time RT-PCR (qRT-PCR) was done by (Wise et al., 2004), Briefly, a one-step qRT-PCR using sequence-specific probes for gene expression analysis was done according to the instructions of the manufacturer (QIAGEN, The Netherlands) and using the ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA). Primers and probes targeting M gene of Newcastle virus indicated in (Table 1). For viral quantification with viral RNA extracted from the titrated challenge virus a standard curve was established.
Table 1.
The primers used for detection of M gene of Newcatle diseae virus.
| Virus | Gene | Primer/probe sequence 5′-3′ | Amplified Segment (bp) | Ref |
|---|---|---|---|---|
| ND | M | M + 4100 AGTGATGTGCTCGGACCTTC-3′ | 121 | Wise et al., 2004 |
| M−4220 CCTGAGGAGAGGCATTTGCTA-3′ | ||||
| M + 4169 [FAM]TTCTCTAGCAGTGGGACAGCCTGC[TAMRA]-3′ |
2.9. Statistical analysis
SPSS software (v.16) and one-way analysis of variance (ANOVA) was used for data analysis, the significance differences between the mean values were calculated using Tukey’s HSD multiple comparison tests. The alpha level for determination of significance was 0.05. Means in the same column followed by different letters were different significantly and the highest value was represented by the letter (a).
3. Results
3.1. Phytochemical analysis
By qualitative phytochemical tests the extract was analyzed into alkaloid, glycoside, saponin, flavonoid, fats, steroid, reducing sugar and oil. The degree of concentrations of glycoside, saponin, reducing sugars and steroid were high in the the methanolic extract of M. oleifera while terpenes and tannins were not appeared as shown in (Table 2).
Table 2.
Results of phytochemical analyses of Moringa oleifera.
| Compounds | Presence in methanol extract |
|---|---|
| Alkaloid | + |
| Saponin | ++ |
| Tannins | – |
| Glycoside | ++ |
| Flavonoid | + |
| Steroid | +++ |
| Terpenes | – |
| Fats and Oil | + |
| Reducing Sugar | ++ |
+ present in low concentration, ++ present with moderate concentration, +++ present in high concentration and – mean abscent.
3.2. Clinical signs and postmortem lesions
On 2 day post-infection (P.I), slight conjunctivitis, depression, ruffled feathers, watery diarrhea, anorexia, closed eyes with watery secretion drilled from the mouth were showed in addition to respiratory signs in form of dyspnea, sneezing and nasal discharge. These signs became clearer with time so that at 3dpi were obviously observed. Neurological signs were detected at 6 day post infection in infected group. On day 3PI, group II had a morbidity rate of 35%, group III had a rate of 16%, group V had a rate of 0%, and group IV had a rate of 100%. Morbidity was 36% in group II, 7% in group III, and 0% in group V on day 6 PI. No mortalities were recorded in all groups except in infected group with Newcastle the mortality was 100% within 7 day post infection. The Post mortem examination showed slight congestion in thigh and breast muscle with slight petecheal hemorrhage in trachea in group II, III, IV and V were 20%, 40%, 60% and 0% respectively and there was hemorrhage between oesophagus and proventriculus in group II, III and V were 0% except group IV was 50%.The cecal tonsils were also enlarged and hemorrhagic with percentage 20%, 10% 65% and 0% in group II, III, IV and V receptively.
3.3. Serology
All pre-vaccination sera collected before virus inoculation were negative for ND antibodies in the Haemagglutination test. In Experimental group II, treated and non-vaccinated. The mean HI titers was 5.0 ± 0.6 log2 at 14 day post challenge while in Experimental group III, vaccinated and challenged group the The mean HI titers at 21 days post vaccination was 6.4 ± 0.16 log2 higher than the mean HI titers 3.4 ± 0.11 log2 at 14 days post vaccination but at 14 day post challenge the HI titre was 7.4 ± 0.17log2. In Experimental group V Mean HI titers were 6.5 ± 0.16 log2 & 7.6 ± 0.11 log2 of chickens immunized with vaccines in combination with Moringa Oleifera at 14 and 21 day post vaccination respectively and 8.0 ± 0.11 log2 post challenge. The data were analyzed by HI test (Table 3).
Table 3.
The HI antibody titers in vaccinated chickens with Newcastle vaccine before and after challenge and vaccination.
| Groups | 14 day post vaccination | 21 day post vaccination | 14 day post challenge |
|---|---|---|---|
| III | 3.4 ± 0.11 | 6.4 ± 0.16 | 7.4 ± 0.17 |
| V | 6.5 ± 0.16 | 7.6 ± 0.11 | 8.0 ± 0.11 |
3.4. Hematological parameters
Leukogram was detailed in Table 4. There is a significant decrease in TLC, lymphocyte, neutrophil, basophil and eosinophil in Group IV when compared with group I at 1st and 2nd week. In group V there is a significant increase in TLC, lymphocyte, neutrophil, basophil and eosinophil when compared with group IV at 1st and 2nd week. While there was no significant difference between group (II) and group (III) in the same parameters.
Table 4.
Effect of oral administration of Moringa hydro alcoholic extract (200 mg/kg b.wt) once daily for 21 successive days on total leukocytic count, lymphocyte, neutrophil, basophile and eosinophil.
|
TIME |
Groups |
I |
II |
III |
IV |
V |
|
|---|---|---|---|---|---|---|---|
| parameters | Control negative | Plant + Virus | Vaccine + Virus | Control positive | Vaccine + Virus + Plant | ||
| At zero time | TLC(103/μl) | 79.2 ± 0.14 d | 80.04 ± 0.02 c | 82.6 ± 0.11 a | 81.4 ± 0.11 b | 82.4 ± 0.23 a | |
| lymphocyte | 70.5 ± 0.14 d | 72.3 ± 0.05 b | 73.1 ± 0.05 a | 72.1 ± 0.08 b | 71.1 ± 0.08 c | ||
| Neutrophil | 2.1 ± 0.28 c | 2.8 ± 0.20 a, b | 3.2 ± 0.14 a | 2.5 ± .18 b c | 3.1 ± 0.05 a b | ||
| basophil | 5.1 ± 0.05 a | 5.3 ± 0.05 a | 5.2 ± 0.05 a | 5.1 ± 0.29 a | 5.2 ± 0.05 a | ||
| eosinophil | 2.1 ± 0.05 b | 2.3 ± 0.05 b | 2.6 ± 0.05 a | 2.2 ± 0.05 b | 2.2 ± 0.12 b | ||
| 1st week | TLC (103/μl) | 79.30 ± 0.05 a | 60.4 ± 0.14 c | 61.4 ± 0.05 c | 40.2 ± 0.12 d | 75.5 ± 1.2 b | |
| lymphocyte | 72.2 ± 0.11 a | 60.8 ± 0.03 c | 61.2 ± 0.14 c | 4 5.1 ± 0.08 d | 70.2 ± 0.60 b | ||
| Neutrophil | 2.2 ± 0.05 a | 1.3 ± 0.08 c | 1.3 ± 0.08 c | 0.6 ± 0.04 d | 1.9 ± 0.05 b | ||
| basophil | 5.2 ± 0.15 a | 3.2 ± 0.12 b | 3.1 ± 0.08 b | 1.9 ± 0.05 c | 5.00 ± 0.05 a | ||
| eosinophil | 2.5 ± 0.21 a | 1.3 ± 0.08 b | 1.4 ± 0.05 b | 0.6 ± 0.02 c | 2.2 ± 0.15 a | ||
| 2nd week | TLC (103/μl) | 80.4 ± 0.21 a | 66.06 ± 0.29 c | 66.6 ± 0.43 c | 38.86 ± 0.47 d | 78.6 ± 0.35 b | |
| lymphocyte | 72.4 ± 0.2 a | 63.5 ± 0.14 c | 63.8 ± 0.05 c | 43.1 ± 0.05 d | 70.9 ± 0.12 b | ||
| Neutrophil | 2.3 ± 0.12 a | 1.4 ± 0.05 c | 1.3 ± 0.06 c | 0.4 ± 0.1 d | 1.9 ± 0.05 b | ||
| basophil | 5.2 ± 0.14 a | 4.6 ± 0.08 b | 4.6 ± 0.06 b | 1.7 ± 0.05 c | 5.1 ± 0.05 a | ||
| eosinophil | 2.2 ± 0.2 a | 1.7 ± 0.08 b,c | 1.6 ± 0.05 c | 0.5 ± 0.02 d | 2.00 ± 0.05 a,b |
3.5. Phagocytic activity
The result of phagocytic activity is indicated by increasing in the mean number of Saccharomyces cerevisiae per macrophage cells as shown in group II due to pretreatment of plant in virus infected group also the same result in group III showing effect of vaccine in increase phagocytic capacity of Macrophage against NDV. In group V showed the highest phagocytic ability of macrophage cells related to synergistic effect of vaccine and plant against NDV. The phagocytic index (PhI) was calculated by the percentage of phagocytes involved in phagocytosisthe mean number of adhered/ingested Saccharomyces cerevisiae per phagocytozing cell Table 5, Table 6; Fig. 1.
Table 5.
The phagocytic index of the birds experimentally treated with Moringa Oleifera and Newcastle vaccine.
| Groups |
Phagocytic index |
||||
|---|---|---|---|---|---|
| Control negative Neither infected nor treated | Treated group with M. Olifera and infected | Vaccinated and infected group | Control positive infected not vaccinated | Vaccinated treated and infected | |
| At zero time | 0.45 ± 0.03a,b | 0.44 ± .05b | 0.45 ± .05b,a | 0.46 ± .05a | 0.46 ± 0.03a,b |
| 3rd day | 0.46 ± 0.03e | 0.64 ± .03b | 0.61 ± .03c | 0.51 ± .03d | 0.82 ± 0.05a |
| 5th day | 0.44 ± 0.01e | 0.61 ± .01b | 0.58 ± .03c | 0.49 ± 0.03d | 0.79 ± 0.05a |
Table 6.
The phagocytic percentage of the birds experimentally treated with Moringa Oleifera and Newcastle vaccine.
| Groups |
Phagocytic % |
||||
|---|---|---|---|---|---|
| Control negative Neither infected nor treated | Treated group with M. Olifera and infected | Vaccinated and infected group | Control positive infected not vaccinated | Vaccinated treated and infected | |
| At zero time | 41 %±.3b | 41%±.3b | 41%±.2b | 40%±.3b | 42%±0.3a |
| 3rd day | 41%±0.3 e | 46%± .2b | 44%±.3c | 43%±0.1 d | 64%±.3a |
| 5th day | 40%±0.3 e | 44%±.1b | 42%±.3c | 41%±0.3 d | 57%±.3a |
The phagocytic percentage = Number of PMN that have engulfed bacteria /Number of PMN counted.
Fig. 1.
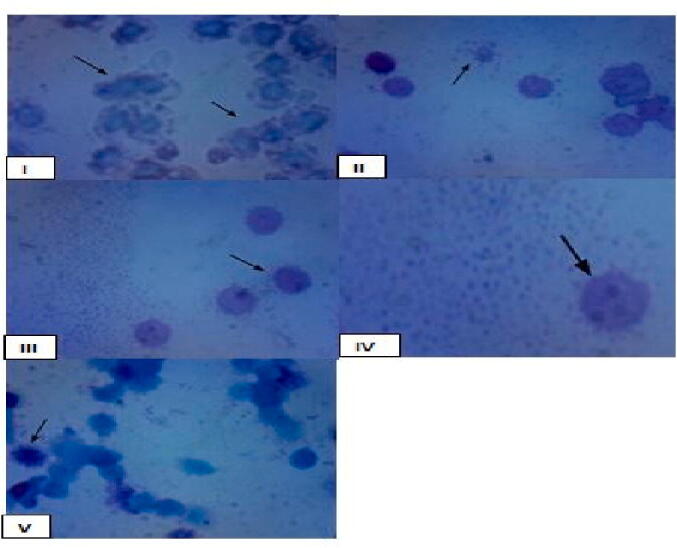
Microscopical examination of phagocytic index of Macrophage cells three days after experiments. Group (1): showing normal phagocytic ability of Macrophage cells. (control −ve), Group II: showing increase in the mean number of Saccharomyces cerevisiae per Macrophage cells due to pretreatment of plant in virus infected group. Group III: showing effect of virus and vaccine in increase phagocytic capacity of Macrophage. Group IV: showing effect of virus presence in the mean number of Saccharomyces cerevisiae per Macrophage cells. (control +ve) and Group V: showing the highest phagocytic ability of macrophage cells related to synergistic effect of virus, vaccine and plant.
3.6. Analysis of viral shedding
Cloacal and Oropharyngeal swabs were taken to reveal virus shedding at 3rd, 5th, 7th and 9th days post challenge. The result of virus shedding revealed that oropharengeal swabs at 3rd day post infection showed the highest value of mean log10EID50 in infected groups of NDV more than in cloacal swabs. Since the mean threshold cycle (Ct) values were observed ranged between (29.3–34.62), all groups shed virus at a comparable level. The mean Ct in Group V were 29.8 ± 1.57, 32.85 ± 0.18, 33.92 ± 1.02, 34.62 ± 1.49 whereas the viral load continued to decrease at 5th, 7th day post challenge. However, NDV RNA levels were dropped at 9 th day post challenge as in Table 7.
Table 7.
Showed the shedding of the NDV from oropharengeal and cloacal swabs in experimental infected Hubbard chickens:
| Groups | 3 days post challenge |
5 days post challenge |
7 days post challenge |
9 days post challenge |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log10EID50 |
CT | Log10EID50 |
CT | Log10EID50 |
CT | Log10EID50 |
CT | |||||
| OPa | clb | OPa | clb | OPa | clb | OPa | clb | |||||
| I | 5.2 ± 0.17 | 4.6 ± 0.15 | 29.3 ± 1.57 | 4.0 ± 0.21 | 2.5 ± 0.05 | 31.73 ± 0.31 | 2.0 ± 0.13 | 1.0 ± 0.18 | 33.34 ± 0.98 | – | – | 34.62 ± 2.87 |
| II | 5.7 ± 0.31 | 4.4 ± 0.21 | 28.7 ± 2.12 | 4.3 ± 0.31 | 3.3 ± 0.11 | 30.21 ± 0.31 | 2.5 ± 0.05 | 1.0 ± 0.31 | 32.0 ± 0.98 | – | – | 33.7 ± 0.57 |
| III | 4.3 ± 0.15 | 3.9 ± 0.15 | 31.0 ± 1.64 | 3.2 ± 0.11 | 2.5 ± 0.07 | 32.1 ± 0.18 | 1.5 ± 0.13 | 1.0 ± 0.07 | 33.34 ± 1.47 | – | – | 34.62 ± 1.89 |
| IV | 5.0 ± 0.21 | 4.3 ± 0.21 | 30.7 ± 0.98 | 3.5 ± 0.11 | 2.9 ± 0.05 | 32.0 ± 0.31 | 1.8 ± 0.31 | 1.0 ± 0.13 | 33.18 ± 0.57 | – | – | 34.05 ± 2.87 |
| V | 4.9 ± 0.07 | 4.4 ± 0.21 | 29.8 ± 1.57 | 4.0 ± 0.07 | 3.0 ± 0.11 | 32.85 ± 0.18 | 2.0 ± 0.21 | 1.0 ± 0.13 | 33.92 ± 1.02 | – | – | 34.62 ± 1.49 |
± Means standard error within the same row carrying different superscript were significantly different at P value < 0.05.
4. Discussion
Newcastle disease (ND) is a highly contagious viral disease that affects poultry. Newcastle disease (ND) is an avian disease that affects chickens, turkeys, pigeons, and other birds. It is caused by the Newcastle disease virus, which is a filterable virus (NDV). In unvaccinated birds, morbidity and mortality in susceptible birds can reach 90–100% in the severe stages of the disease. Respiratory, digestive, and neurological manifestations characterized the infection. Despite vaccination and therapy programmers, total average losses in poultry owing to ND are currently estimated to be 40–60% (Bello et al., 2018) but vaccination has some demerits as it take long time to produce protective immunity and also the high cost of Vaccines so (Andleeb et al., 2020, Li et al., 2020) found that using medicinal plant extracts as antiviral medicines is a good way for protection the flocks against NDV. Use of medicinal plant extracts as antiviral medicines against NDV is one of the methods to protect flocks against NDV. So in this research we study the effect of Moringa Oleifera which is considered one of the medicinal plants which has antiviral properties and play a critical role in the body's immune system's defense against the Newcastle disease virus (Chollom et al., 2012) also it has other merits as it is easily handle and available to the bird breeder with low cost and to compare the protective efficacy of Moringa Oleifera extract in chickens with or without NDV vaccination against Newcastle disease virus. Clinical signs in infected groups (depression in appetance, huddle together, greenish diarrhea, respiratory signs and nervous signs) were observed. The same signs recorded by (Alders and Spradbrow, 2001, Okoye et al., 2000).
The gross lesion was as the same reported by Okoye et al. (2000) for vVND. This is because the virulence and the tropism of the virus strain, their immunity and target species (Alexander and Senne, 2003). The lesions were more severe in group IV, followed by groups III and II. The incubation period of experimental Newcastle was ranged between 3 days as reported by Okoye et al. (2000) and from 2 to 16 days as by Hamid et al. (1991) so the morbidity rate was higher in infected and treated unvaccinated group (group II) and in (group IV) infected group the same result recorded by Okoye et al. (2000) who reported the high morbidity of the NDV may reach 100% in unvaccinated birds.
Haemagglutination inhibition test was used to evaluate immunogenicity effect of Moringa Oleifera leave extract with and without NDV vaccines. The mean HI titer at 21 day post vaccination in immunized chickens were more greater than the mean HI titer at 14 day post vaccination in sera of chickens. In the Previous studies mentioned at 21 day post vaccination. Vaccinated chickens could be totally protected from highly pathogenic NDV challenge if antibody titers to the challenge virus were equal to or greater than 4log2, especially 21 days after immunization (Eze et al., 2014). The mean HI titer of immunized chicken and treated with Moringa Oleifera leave extract at 21 day post vaccination was much higher than 14 day post vaccination neither in vaccinated group nor in Moringa Oleifera leave extract group. Two weeks after challenge, vaccinated and treated chickens showed a significant increase in antibody titers. This contradicts the findings of other studies. (Tully et al., 2009), this could be due to a reduction in challenge virus replication in the survivors. The efficacy assessments were based on a challenge study conducted 28 days after vaccination (see Table 7).
The antiviral activity of Moringa Oleifera Plant as it contains alkaloids, flavonoids, saponins and tannins. The best antiviral activity of moringa extract concentration studied by several researchers because The aqueous seed extract of M. oleifera effect versus NDV was inspected according to Chollom et al. (2012) found that virus death was proportional to extract concentration and inversely related to antibody generation against NDV, indicating that M. oleifera seed aqueous extract possesses high antiviral activity against NDV in an ovo experiment. M. olifera extract has nutritional value because it includes a lot of minerals for example calcium ions (Ferreira et al., 2008, Siddhuraju and Becker, 2003). These components could be the source of an increased immunological response. M. oleifera's ability to boost immunological responses is due to the presence of growth factors such as cytokines, which stimulate both adaptive and innate immunity Davis and Kuttan (1998).
The effect of Moringa Oleifera leaf extract on hematological markers was studied. Most broiler chicken groups had leukocytosis, which could be linked to lymphocytosis caused by the vaccine's immunostimulatory impact (Latimer, 2011) a marked response to antigenic stimulation caused by viral infection (Doneley, 2018), and boosting the immune system's reaction Active Moringa Oleifera extract component stimulates immunological response via lymphocyte proliferation (Chollom et al., 2012) in vaccinated, infected, and Moringa Oleifera extract treated broiler chickens, respectively. In the vaccinated broiler group, monocytosis occurred as a result of an effective immune reaction of the body against the vaccine virus. (Šimpraga et al., 2008) and transition of monocytes from bone marrow to the tissues as macrophages for mopping up of necrotic debris in infected broilers group Thomson (1984). In general, leukogram images demonstrated the highest degree of leukocytosis in broilers vaccinated and treated with Moringa Oleifera extract, possibly indicating a synergistic effect of the two to enhance the immune system of birds. White blood cell counts and immunoglobulin levels increased significantly when different doses of Moringa Oleifera were given Adedapo et al. (2005). Similarly, Moringa Oleifera leaf powder supplemented diets improved the immunological response of O. niloticus fry and prevented illness induced by A. hydrophila, according to (Abd El-Gawad et al., 2020).
Virus shedding is one of the parameters that evaluate the protective efficacy of the Newcastle virus after challenge. This study revealed a significant reduce in the virus shedding levels more in vaccinated and treated group than vaccinated group alone or treated group alone. The virus tiers were higher in oropharengeal swabs than cloacal swabs in our experimental study. Tian and his colleague, 2005 registered the shedding of viral tiers from the trachea and observed that they were more than from the cloaca and it may be resulted in the efficiency of the replication for the upper respiratory tract after inoculation the challenge virus (Tian et al., 2005, Webster et al., 2006). The virus shedding was peaked at day 3 post challenge and remained significantly less in consecutive days post challenge while zero virus was detected in chickens on day 9th post challenge by titration in ECE-SPFs as reported by (Lee and Suarez, 2004, Webster et al., 1986). In the opposite side the shedding in the cloacal swabs were low it could be related to substances that make inhibition which present in fecal specimens that decrease PCR amplification and the limitation capacity of most commercial RNA extraction kits to remove these inhibitors from clinical samples Das et al. (2009)
The phagocytic activity of Moringa Oleifera leaf extract on experimentally inoculated Hubbard chickens found that There is no difference in the increase in mean number of saccharomyces cerevisiae per macrophage cells in treated unvaccinated and vaccinated group, while in combined group with vaccines and plant show the highest phagocytic ability of saccharomyces cerevisiae per macrophage cells due to synergistic action to stimulate the immune system of birds. Gupta et al. (2010) reported that moringa extract pretreatment in mice blocked cyclophosphamide bone marrow suppressive effect on phagocytic activity.
5. Conclusion
The protection percentage and serological response in vaccinated chickens with Newcastle virus and treated with Moringa Oleifera leaf extract were improved and gives preferable results than vaccinated chickens only or treated chickens only so Moringa Oleifera leaf extract may be useful in the protection against Newcastle virus when used mixed with the vaccine.
Ethics statement
The protocol was conducted according to the Committee of Animal Welfare and Research Ethics (protocol #ZU-IACU/2/F/9/2018) in Zagazig University, Egypt.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd-Rabou A.A., Abdalla A.M., Ali N.A., Zoheir K.M. Moringa oleifera root induces cancer apoptosis more effectively than leave nanocomposites and its free counterpart. Asian Pacific J. Cancer Prevent.: APJCP. 2017;18:2141. doi: 10.22034/APJCP.2017.18.8.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Gawad E.A., El Asely A.M., Soror E.I., Abbass A.A., Austin B. Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquacult. Int. 2020;28(1):389–402. [Google Scholar]
- Abdel Rahman M., Tolba H., Abdel-Ghany H. Ultrastructure, morphological differentiation and pathological changes of Ascaridia species in pigeons. Adv. Anim. Vet. Sci. 2019;7:66–72. [Google Scholar]
- Adedapo A.A., Abatan M.O., Idowu S.O., Olorunsogo O.O. Toxic effects of chromatographic fractions of Phyllanthus amarus on the serum biochemistry of rats. Phytotherapy Res.: Int. J. Devoted Pharmacol. Toxicol. Eval. Natural Product Derivat. 2005;19(9):812–815. doi: 10.1002/ptr.1721. [DOI] [PubMed] [Google Scholar]
- Alders R., Costa R., Gallardo R.A., Sparks N., Zhou H. Elsevier; 2018. Smallholder poultry: Leveraging for sustainable food and nutrition security, Encyclopedia of Food Security and Sustainability; pp. 340–346. [Google Scholar]
- Alders R., Spradbrow P. Australian Centre for International Agricultural Research (ACIAR) 2001. Controlling Newcastle disease in village chickens: a field manual. [Google Scholar]
- Aldous E.W., Alexander D.J. Detection and differentiation of Newcastle disease virus (avian paramyxovirus type 1) Avian Pathol. 2001;30(2):117–128. doi: 10.1080/03079450120044515. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Senne D. Newcastle disease. Dis. Poultry. 2003;11:64–87. [Google Scholar]
- Andleeb R., Ashraf A., Muzammil S., Naz S., Asad F., Ali T., Rafi R., Al-Ghanim K.A., Al-Misned F., Ahmed Z., Mahboob S. Analysis of bioactive composites and antiviral activity of Iresine herbstii extracts against Newcastle disease virus in ovo. Saudi J. Biol. Sci. 2020;27(1):335–340. doi: 10.1016/j.sjbs.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C. 3rd ed. Kendall/Hunt Publishing Company; Dubuque, Iowa: 1989. Serologic procedures. A laboratory manual for the isolation and identification of avian pathogens; pp. 192–200. [Google Scholar]
- Bello M.B., Yusoff K.M., Ideris A., Hair-Bejo M., Peeters B.P., Jibril A.H., Tambuwal F.M., Omar A.R. Genotype diversity of Newcastle disease virus in Nigeria: Disease control challenges and future outlook. Adv. Virol. 2018;2018 doi: 10.1155/2018/6097291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilhante R.S.N., Sales J.A., Pereira V.S., Castelo D.d.S.C.M., de Aguiar Cordeiro R., de Souza Sampaio C.M., Paiva M.d.A.N., Dos Santos J.B.F., Sidrim J.J.C., Rocha M.F.G. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pacific J. Tropical Med. 2017;10:621–630. doi: 10.1016/j.apjtm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Chollom S., Agada G., Gotep J., Mwankon S., Dus P., Bot Y., Nyango D., Singnap C., Fyaktu E., Okwori A. Investigation of aqueous extract of Moringa oleifera lam seed for antiviral activity against newcastle disease virus in ovo. J. Med. Plants Res. 2012;6:3870–3875. [Google Scholar]
- Coles, E., 1986. Veterinary clinical Pathology 4th ed. WB Saunders company Philadelphia. London, Toronto, Mexico, Riodejenario, Sydney, Tokyo & Hong Kong, pp. 136–170.
- Das A., Spackman E., Pantin-Jackwood M.J., Suarez D.L. Removal of real-time reverse transcription polymerase chain reaction (RT-PCR) inhibitors associated with cloacal swab samples and tissues for improved diagnosis of Avian influenza virus by RT-PCR. J. Vet. Diagn. Invest. 2009;21(6):771–778. doi: 10.1177/104063870902100603. [DOI] [PubMed] [Google Scholar]
- Davis L., Kuttan G. Suppressive effect of cyclophosphamide-induced toxicity by Withania somnifera extract in mice. J. Ethnopharmacol. 1998;62(3):209–214. doi: 10.1016/s0378-8741(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Desoky E.-S., Elrys A.S., Rady M.M. Integrative moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals-contaminated saline soil. Ecotoxicol. Environ. Saf. 2019;169:50–60. doi: 10.1016/j.ecoenv.2018.10.117. [DOI] [PubMed] [Google Scholar]
- Doneley B. CRC Press; 2018. Avian Medicine and Surgery in Practice: Companion and Aviary Birds. [Google Scholar]
- El-Masry S., Nasr-Eldin M., Faiesal A., Othman B. Comparative study of different vaccination routes against Newcastle disease in layer chickens. Arab Universities J. Agric. Sci. 2019;27(1):231–238. [Google Scholar]
- Elangovan M. Dhanarajan. MS Rajalakshmi A, Jayachitra A., Pardhasaradhi Mathi, Narasimharao Bhogireddy.“Analysis of Phytochemicals, Antibacterial and Antioxidant activities of Moringa Oleifera Lam. Leaf extract-an in vitro study”. Int. J. Drug Dev. Res. 2014;6:280–285. [Google Scholar]
- Elrys A.S., Desoky E.-S., Abo El-Maati M.F., Elnahal A.S., Abdo A.I., Raza S., Zhou J. Can secondary metabolites extracted from Moringa seeds suppress ammonia oxidizers to increase nitrogen use efficiency and reduce nitrate contamination in potato tubers? Ecotoxicol. Environ. Saf. 2019;185:109689. doi: 10.1016/j.ecoenv.2019.109689. [DOI] [PubMed] [Google Scholar]
- Eze C.P., Shoyinka V.S., Okoye J.O.A., Ezema W.S., Ogbonna I.O., Eze D.C., Okwor E.C., Ikejiofor O.K. Comparison of the serum proteins and immune responses of velogenic Newcastle disease virus infected chickens and ducks. Open J. Veterinary Med. 2014;2014 [Google Scholar]
- Ferreira P.M.P., Farias D.F., Oliveira J.T.d.A., Carvalho A.d.F.U. Moringa oleifera: bioactive compounds and nutritional potential. Revista de Nutrição. 2008;21(4):431–437. [Google Scholar]
- Garba S., Mera U., Garba H., Musa U., Jimoh A., Raji A. Effect of garlic and neem leaf aqueous extracts on immune response of broilers to live Newcastle disease vaccine. Sci. J. Veterinary Adv. 2013;2:16–20. [Google Scholar]
- Gupta A., Gautam M.K., Singh R.K., Kumar M.V., Rao C.V., Goel R., Anupurba S. Immunomodulatory effect of Moringa oleifera Lam. extract on cyclophosphamide induced toxicity in mice. indian J. of Expermintal Biology. 2010;48(11):1157–1160. [PubMed] [Google Scholar]
- Hamid H., Campbell R.S.F., Parede L. Studies of the pathology of velogenic Newcastle disease: Virus infection in non-immune and immune birds. Avian Pathol. 1991;20(4):561–575. doi: 10.1080/03079459108418796. [DOI] [PubMed] [Google Scholar]
- Hegazy A.M.E., Yehia N., Hassan A.F.I., El-Saadony M.T., Aboelenin S.M., Soliman M.M., Tolba H.M.N. The potency of newly development H5N8 and H9N2 avian influenza vaccines against the isolated strains in laying hens from Egypt during 2019. Saudi J. Biolog. Sci. 2021;28(9):5310–5316. doi: 10.1016/j.sjbs.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Concha C., Lin F., Persson Waller K. Adjuvant effect of ginseng extracts on the immune responses to immunisation against Staphylococcus aureus in dairy cattle. Vet. Immunol. Immunopathol. 2003;91(1):29–37. doi: 10.1016/s0165-2427(02)00264-7. [DOI] [PubMed] [Google Scholar]
- Latimer K.S. John Wiley & Sons; 2011. Duncan and Prasse’s Veterinary Laboratory Medicine: Clinical Pathology; pp. 55–57. [Google Scholar]
- Lee C.-W., Suarez D.L. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J. Virol. Methods. 2004;119(2):151–158. doi: 10.1016/j.jviromet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Li J., Yu Y., Feng C., Wang H. Mechanical characterization of Pinus massoniana cell walls infected by blue-stain fungi using in situ nanoindentation. J. For. Res. 2020;31(2):661–665. [Google Scholar]
- Okoye J., Agu A., Chineme C., Echeonwu G. Pathological characterization in chickens of a velogenic Newcastle disease virus isolated from guinea fowl. Revue D Elevage Et De Medicine Veterinaire des Pays Tropicaux. 2000;53:325–330. [Google Scholar]
- Prabsattroo T., Wattanathorn J., Iamsa-ard S., Muchimapura S., Thukhammee W. Moringa oleifera leaves extract attenuates male sexual dysfunction. Am. J. Neurosci. 2012;3:17–24. [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Rehman Z.U., Ren S., Yang B., Yang X., Butt S.L., Afzal A., Malik M.I., Sun Y., Yu S., Meng C., Ding C. Newcastle disease virus induces testicular damage and disrupts steroidogenesis in specific pathogen free roosters. Vet. Res. 2020;51:1–11. doi: 10.1186/s13567-020-00801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheble, A., Reda, I., 1976. Isolation and characterization of a local velogenic viscerotropic strain of Newcastle disease virus MV Sc. Thesis, Faculty of Veterinary Medicine, Cairo University.
- Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food. Chem. 2003;51(8):2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Šimpraga M., Lukač Novak I., Mazija H., Štoković I., Vojta A. Hematological and biochemical parameters of ostriches after vaccination against Newcastle disease. Period. Biol. 2008;110:91–94. [Google Scholar]
- Sun J., Hu S., Song X. Adjuvant effects of protopanaxadiol and protopanaxatriol saponins from ginseng roots on the immune responses to ovalbumin in mice. Vaccine. 2007;25(6):1114–1120. doi: 10.1016/j.vaccine.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Thomson R.G. WB Saunders; 1984. General veterinary pathology; pp. 15–19. [Google Scholar]
- Tian G., Zhang S., Li Y., Bu Z., Liu P., Zhou J., Li C., Shi J., Yu K., Chen H. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology. 2005;341(1):153–162. doi: 10.1016/j.virol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Trease G., Evans W. Univ. Press; Aberdeen, Great Britain: 1972. Pharmacognosy. [Google Scholar]
- Tully T.N., Dorrestein G.M., Jones A.K. Elsevier/Saunders; 2009. Handbook of Avian Medicine. [Google Scholar]
- Villegas, P., 1998. Titration of biological suspensions. A laboratory manual for the isolation and identification of avian pathogens, 248–254.
- Webster R., Kawaoka Y., Bean W. Vaccination as a strategy to reduce the emergence of amantadine-and rimantadine-resistant strains of A/Chick/Pennsylvania/83 (H5N2) influenza virus. J. Antimicrobial Chemotherapy. 1986;18:157–164. doi: 10.1093/jac/18.supplement_b.157. [DOI] [PubMed] [Google Scholar]
- Webster R.G., Guan Y., Peiris M., Chen H. H5N1 influenza continues to circulate and change. Microbe-Am. Soc. For Microbiol. 2006;1(12):559–565. [Google Scholar]
- Wise M.G., Suarez D.L., Seal B.S., Pedersen J.C., Senne D.A., King D.J., Kapczynski D.R., Spackman E. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 2004;42:329–338. doi: 10.1128/JCM.42.1.329-338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


