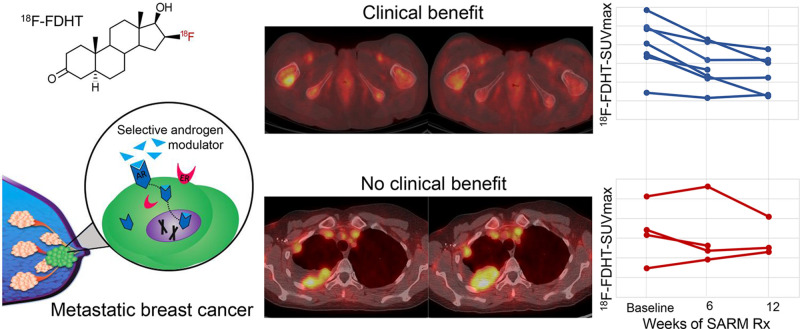
Visual Abstract
Keywords: 18F-FDHT, androgen receptor, AR, PET, breast cancer
Abstract
Most breast cancers express androgen receptors (ARs). This prospective imaging substudy explored imaging of ARs with 18F-fluoro-5α-dihydrotestosterone (18F-FDHT) PET in patients with metastatic breast cancer (MBC) receiving selective AR modulation (SARM) therapy (GTx-024). Methods: Eleven postmenopausal women with estrogen receptor–positive MBC underwent 18F-FDHT PET/CT at baseline and at 6 and 12 wk after starting SARM therapy. Abnormal tumor 18F-FDHT uptake was quantified using SUVmax. AR status was determined from tumor biopsy specimens. 18F-FDHT SUVmax percentage change between scans was calculated. Best overall response was categorized as clinical benefit (nonprogressive disease) or progressive disease using RECIST 1.1. Results: The median baseline 18F-FDHT SUVmax was 4.1 (range, 1.4–5.9) for AR-positive tumors versus 2.3 (range, 1.5–3.2) for AR-negative tumors (P = 0.22). Quantitative AR expression and baseline 18F-FDHT uptake were weakly correlated (Pearson ρ = 0.39, P = 0.30). Seven participants with clinical benefit at 12 wk tended to have larger declines in 18F-FDHT uptake than did those with progressive disease both at 6 wk after starting GTx-024 (median, −26.8% [range, −42.9% to −14.1%], vs. –3.7% [range,−31% to +29%], respectively; P = 0.11) and at 12 wk after starting GTx-024 (median, −35.7% [range, −69.5% to −7.7%], vs. –20.1% [range, −26.6% to +56.5%], respectively; P = 0.17). Conclusion: These hypothesis-generating data suggest that 18F-FDHT PET/CT is worth further study as an imaging biomarker for evaluating the response of MBC to SARM therapy and reiterate the feasibility of including molecular imaging in multidisciplinary therapeutic trials.
The androgen receptor (AR), the most abundantly expressed steroid hormone receptor in breast cancer, is coexpressed in 75%–95% of estrogen receptor (ER)–positive (+) and 10%–35% of triple-negative (ER-negative [−], progesterone receptor−, and human epidermal growth factor receptor 2−) tumors (1). Steroidal androgens, notably dihydrotestosterone and fluoxymesterone, were widely used in the 1970s to treat metastatic breast cancer (MBC), but virilizing side effects, concern for aromatization to estrogen, and the survival benefit found with tamoxifen led to their disfavor (2,3). Recently, AR has reemerged as a therapeutic target in MBC because of elucidation of the complex relationship between the AR axis and breast cancer growth and the development of selective AR modulators (SARMs).
In ER+ breast cancer, AR primarily inhibits tumor proliferation (4,5). GTx-024 is a novel oral nonsteroidal SARM that specifically binds AR-promoting agonist activity. GTx-024 does not bind other steroidal receptors and cannot be aromatized to estrogen (6). GTx-024 slowed tumor growth in preclinical models of ER+ breast cancer and was well tolerated, without virilizing effects (6).
Derived from dihydrotestosterone, 18F-fluoro-5α-dihydrotestosterone (18F-FDHT) was developed for imaging AR with PET (7,8). In prostate cancer, 18F-FDHT PET can quantitate relative levels of AR and be used as a pharmacodynamic imaging biomarker after antiandrogen therapy to provide information about drug targeting, dose optimization, and response (9).
Overmoyer et al. conducted a prospective phase II clinical trial of GTx-024 in postmenopausal women with ER+ MBC (10). As part of this trial, we performed a prospective imaging substudy to determine the feasibility of using 18F-FDHT PET/CT for noninvasive imaging of AR expression in ER+ MBC and to explore the potential of 18F-FDHT PET as an imaging biomarker for evaluating response to SARM therapy.
MATERIALS AND METHODS
Study Design and Participants
The study was a single-site prospective imaging substudy performed as part of a larger open-label, multicenter, international, randomized, parallel-design phase II trial exploring the clinical benefit (CB) of GTx-024 (G200802; GTx, Inc. [NCT02463032]). Participants were randomized 1:1 to receive 9 or 18 mg of GTx-024 orally per day. The trial followed Declaration of Helsinki principles and good clinical practice and was approved by our institutional review board. All participants gave written informed consent.
Major eligibility criteria for both the parent therapeutic trial and the imaging substudy were postmenopausal women with ER+, human epidermal growth factor receptor 2− metastatic or locally recurrent advanced breast cancer; radiologic or clinical disease recurrence or progression within 30 d of randomization onto the therapeutic trial; at least 1 prior hormonal treatment but no more than 1 course of chemotherapy for metastatic disease; available biopsy or archival tumor tissue; bone-only nonmeasurable or measurable disease by RECIST 1.1; adequate organ function; and an Eastern Cooperative Oncology Group performance status of no more than 1. Participants received only the study drug (GTx-024) and no other hormonal treatment for breast cancer while on study.
18F-FDHT PET/CT Scan Acquisition and Image Interpretation
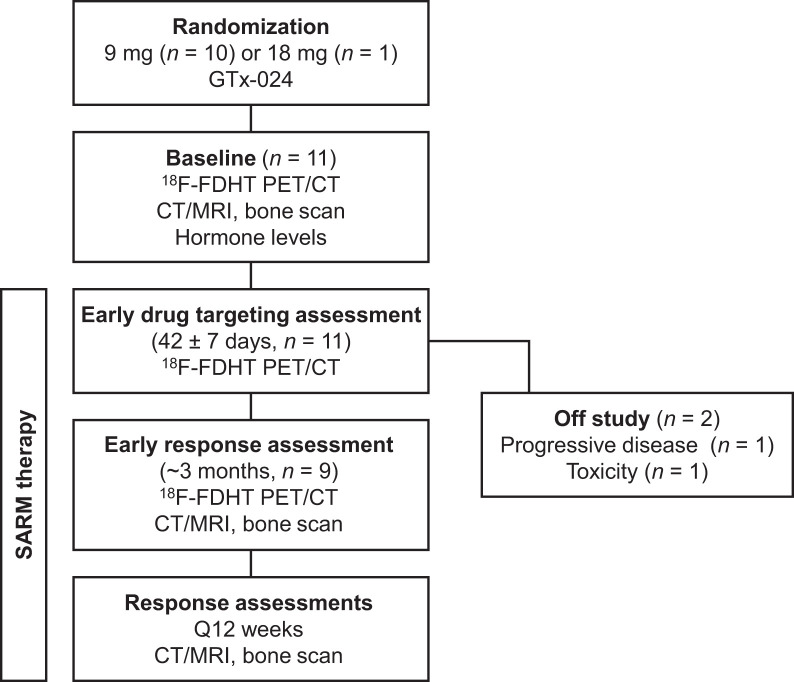
18F-FDHT PET/CT scans were obtained at baseline and at 6 and 12 wk after starting GTx-024 (Fig. 1). The Brigham and Women’s Nuclear Medicine/Biomedical Imaging Research Core manufactured the 18F-FDHT under investigational-new-drug application 122,852 per previously published methods (11,12). The final formulated 18F-FDHT passed all quality control tests required for clinical use and had radiochemical purity of more than 99% and specific activity of more than 18.5 GBq/μmol for all batches.
FIGURE 1.
Study schema.
No specific patient preparation was given for the 18F-FDHT PET/CT scans. Forty-five minutes after intravenous 18F-FDHT administration (333 MBq; 9 mCi), PET scans were obtained in 3-dimensional mode from skull vertex to mid thighs using 3–5 min per bed position (Discovery ST or Discovery MI; GE Healthcare). Images were reconstructed using iterative methods. Unenhanced low-dose CT imaging (3.75- to 5-mm axial slice thickness) was performed over the same range without a breath-hold, for anatomic correlation and attenuation correction. For 7 participants, all 18F-FDHT PET/CT scans were obtained on the same scanner. A scanner upgrade during the study required that the 12-wk scan be obtained on a different scanner for 4 participants.
Lesions on the 18F-FDHT PET/CT scan were determined by comparison to the diagnostic contrast-enhanced chest, abdomen, and pelvis CT scans used to determine eligibility for the parent therapeutic trial. 18F-FDHT uptake was quantitated in measurable lesions larger than 1 cm in the greatest dimension and in nonmeasurable bone lesions, which were allowed on this trial. The following semiquantitative parameters of 18F-FDHT uptake in tumor were recorded at all imaging time points: SUVmax corrected for body weight and for lean body mass, SUVpeak (average SUV in 1-cm3 volume of interest at the tumor’s hottest part) corrected for body weight and for lean body mass, and SUVmean in a 70% isocontour around SUVmax.
Response Assessments
Objective disease response was determined according to RECIST 1.1 in the parent therapeutic trial using contrast-enhanced CT or MRI and bone scans per standard institutional protocols at baseline, week 12 after starting GTx-024, and every 12 wk until progressive disease (PD) or study drug discontinuation. CB was defined as complete or partial response or stable disease per RECIST 1.1. No CB was defined as PD. Best overall response was defined as best tumor response achieved from treatment start until treatment end. Because 18F-FDG PET/CT is not considered the standard of care for assessing the response of MBC to treatment, per National Comprehensive Cancer Network guidelines (13), and was not available at all international sites participating in the parent therapeutic trial, 18F-FDG PET/CT was not included for baseline and disease response assessments.
Pathology and Laboratory Correlates
Tumor tissue from biopsy or archival tissue was reviewed for AR status using standard immunohistochemical techniques with a monoclonal antibody specific for human AR by a central laboratory (QualTek). AR was reported qualitatively as positive (i.e., >1% positive nuclei) or negative and quantitatively as percentage of positive nuclei. The local laboratory evaluated serum for estradiol, testosterone, and sex-hormone–binding globulin (SHBG) levels at baseline and for serum prostate-specific antigen (PSA) levels at baseline and at 6 and 12 wk after therapy.
Statistical Analyses
The primary endpoint of the parent therapeutic trial was CB at 24 wk after starting GTx-024. Therefore, participants in the imaging substudy were followed until the 24-wk assessment. The lesion with the highest 18F-FDHT uptake at baseline was correlated with AR status. Percentage change in 18F-FDHT uptake using the single hottest lesion and the sum of all measured lesions was calculated between baseline (S0), week 6 (S1), and week 12 (S2) scans: We also explored the correlation between the percentage change in the 18F-FDHT uptake of the single hottest lesions at baseline and follow-up and the best overall response (PERCIST-like criteria) (14).
Descriptive statistics summarized baseline and percentage change in 18F-FDHT uptake. The Mann–Whitney U test compared baseline and percentage change in 18F-FDHT uptake between the best-overall-response groups. Pearson testing was used for continuous data to correlate 18F-FDHT uptake versus AR status. At each time point, to account for nonindependence among multiple lesions per patient, repeated-measures correlation was used to assess the common intrapatient association of the various paired quantitative PET parameters to each other (15). All P values are 2-sided, and all CIs are at the 95% level, with statistical significance defined as a P value of no more than 0.05.
RESULTS
Participants and Lesions
Eleven women (median age, 59 y; range, 47–73 y) were enrolled in the 18F-FDHT PET/CT substudy (Table 1) and were scanned between March 2017 and February 2018. Ten were randomized to receive 9 mg of GTx-024 and one to receive 18 mg. Nine women completed baseline, 6-wk, and 12-wk 18F-FDHT PET/CT scans. Two were taken off the study before the 12-wk scan: one at week 6 because of toxicity and one at week 7 for PD (Fig. 1). The best overall response was CB for 7 participants and no CB for 4 participants.
TABLE 1.
Participant and Breast Cancer Tumor Characteristics (n = 11)
| Characteristic | Data |
|---|---|
| Age (y) | |
| Median | 59 |
| Range | 49–73 |
| Histology (n) | |
| Invasive ductal carcinoma | 9 |
| Invasive lobular carcinoma | 2 |
| Receptor status (n) | |
| ER+/PR+/HER2− | 6 |
| ER+/PR−/HER2− | 5 |
| Metastases at diagnosis (n) | |
| Yes | 4 |
| No | 7 |
| Disease-free interval* (y) | |
| Metastases at diagnosis (n = 4) | Not applicable |
| No metastases at diagnosis (n = 7) | 7 (range, 3–19) |
| Median lines of treatment before enrollment (n) | |
| Adjuvant chemotherapy | |
| Metastases at diagnosis (n = 4) | Not applicable |
| No metastases at diagnosis (n = 7) | 1 (0–1) |
| Adjuvant endocrine therapy | |
| Metastases at diagnosis (n = 4) | Not applicable |
| No metastases at diagnosis (n = 7) | 1 (0–2) |
| Chemotherapy for metastatic disease | |
| Metastases at diagnosis (n = 4) | 1 (0–1)† |
| No metastases at diagnosis (n = 7) | 0 (0–1) |
| Endocrine therapy for metastatic disease | |
| Metastases at diagnosis (n = 4) | 2 (1–4) |
| No metastases at diagnosis (n = 7) | 2 (1–6) |
| CDK4/6 inhibitor | |
| Metastases at diagnosis (n = 4) | 2 |
| No metastases at diagnosis (n = 7) | 6 |
| mTOR inhibitor | |
| Metastases at diagnosis (n = 4) | 1 |
| No metastases at diagnosis (n = 7) | 2 |
| Dual PI3 kinase and mTOR inhibitor | |
| Metastases at diagnosis (n = 4) | 0 |
| No metastases at diagnosis (n = 7) | 1 |
| Radiation therapy to metastatic disease | |
| Metastases at diagnosis (n = 4) | 1 (bone) |
| No metastases at diagnosis (n = 7) | 2 (bone) |
| Median metastatic sites at enrollment (n) | 2 (range, 1–4) |
| Location of metastatic sites at enrollment (n) | |
| Bone | 8 (bone only, 5) |
| Viscera (liver, vaginal cuff) | 4 |
| Pleura | 5 |
| Serosa/peritoneum | 2 |
| Lymph node | 2 |
*Time from start of adjuvant therapy to first diagnosis of recurrence or metastatic disease.
†n = 1 with high-dose chemotherapy and stem cell transplantation.
PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; CDK = cyclin-dependent kinase; mTOR = mammalian target of rapamycin; PI3 = phosphoinositide 3.
Table 2 shows all participants’ results regarding AR status, 18F-FDHT uptake at all time points, and outcomes. 18F-FDHT uptake was measured in 40 lesions (median, 4 per participant; range, 1–8). Although all lesions were larger than 1 cm in 1 dimension, for 13 tumors a 2-dimensional region of interest was used for SUVmax and SUVmean corrected for body weight and for lean body mass because either a 1-cm3 sphere could not be placed within the tumor’s anatomic boundaries or because low uptake prevented determination of SUVpeak corrected for body weight and for lean body mass. At all imaging time points, high correlations were observed between all PET parameters measured (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). Therefore, the primary analyses are presented with SUVmax.
TABLE 2.
AR Tumor Status, 18F-FDHT PET/CT, and Clinical Outcomes
| Participant no. | Lesions (n) | AR status | Archival tissue location | 18F-FDHT SUVmax of hottest lesion at baseline | Change in 18F-FDHT SUVmax from baseline to… | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|
| Week 6 | Week 12 | Best overall response | Week of best overall response | Week 24 response | |||||
| 1* | 3 | Positive | Primary | 4.1 | −43% | −70% | NonCR/nonPD | 12 | CB |
| 2 | 2 | Positive | Metastasis | 3.5 | −37% | −36% | NonCR/nonPD | 12 | CB |
| 3 | 2 | Positive | Metastasis | 1.4 | −20% | −8% | NonCR/nonPD | 12 | No CB |
| 4 | 1 | Not assessed | 3.3 | −20% | Off study† | NonCR/nonPD | 6 | No CB | |
| 5 | 8 | Positive | Metastasis | 4.8 | −14% | −22% | PR | 12 | No CB |
| 6 | 4 | Positive | Metastasis | 4.9 | −36% | −35% | SD | 12 | No CB |
| 7 | 5 | Positive | Primary | 5.9 | −27% | −48% | SD | 12 | No CB |
| 8 | 1 | Negative | Metastasis | 1.5 | +30% | +56% | PD | 12 | No CB |
| 9 | 5 | Not assessed | 5.1 | +10% | −20% | PD | 12 | No CB | |
| 10 | 4 | Negative | Metastasis | 3.2 | −17% | Off study† | PD | 7 | No CB |
| 11 | 5 | Positive | Metastasis | 3.4 | −31% | −27% | PD | 12 | No CB |
*Received 18 mg of GTx-024; all others received 9 mg.
†Baseline and week 6 scan only; patient 4 off study week 6 because of toxicity, patient 10 off study week 7 because of progression.
NonCR/nonPD = incomplete response but no PD for participants with nonmeasurable disease by RECIST 1.1; CR = complete response; PR = partial response; SD = stable disease.
AR Status Versus Baseline 18F-FDHT Uptake
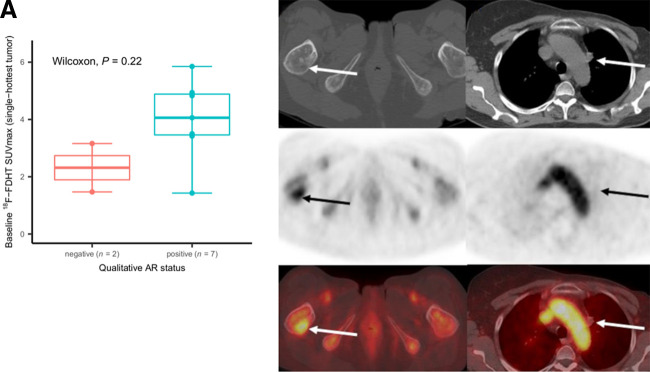
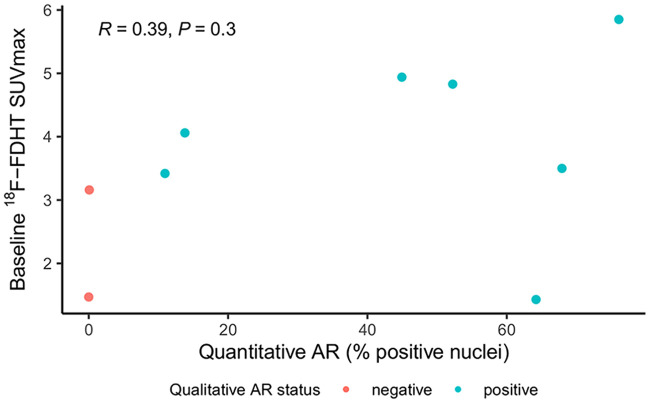
AR status was assessed in 9 of 11 women; 2 from the primary tumor and 7 from metastases. Seven tumors were AR+ and 2 were AR− (both from metastatic disease). Two women had inadequate archival tissue available to determine AR status. Median baseline 18F-FDHT SUVmax was 4.1 (range, 1.4–5.9) for AR+ tumors and 2.3 (range, 1.5–3.2) for AR− tumors (P = 0.22, Fig. 2). A weak, not significant, correlation was found for baseline 18F-FDHT SUVmax versus quantitative AR expression level (Pearson ρ = 0.39, P = 0.30; Fig. 3). SUVmean had similar results (Supplemental Fig. 2).
FIGURE 2.
Baseline 18F-FDHT uptake and qualitative AR status. (A) For 9 participants with archival tissue, median baseline 18F-FDHT SUVmax was 4.1 (range, 1.4–5.9) for 7 participants with AR+ tumors and 2.3 (range, 1.5–3.2) for 2 with AR− tumors (P = 0.22). Individual dots on box plot represent individual-participant data. (B, top row: axial CT; middle row: axial PET; bottom row: axial fused PET/CT) Participant 6, with AR+ tumor and 18F-FDHT uptake in right femur metastasis (arrows, SUVmax of 4.9). (C, top row: axial CT; middle row: axial PET; bottom row: axial fused PET/CT) Participant 8, with AR− tumor and no 18F-FDHT uptake in prevascular lymph node (arrows, SUVmax of 1.5).
FIGURE 3.
Baseline 18F-FDHT uptake and quantitative AR status. Weak, but not statistically significant, correlation was observed between quantitative AR expression levels and baseline 18F-FDHT uptake (Pearson ρ = 0.39, P = 0.30).
Baseline 18F-FDHT Uptake and Change in 18F-FDHT Uptake Versus Best Overall Response
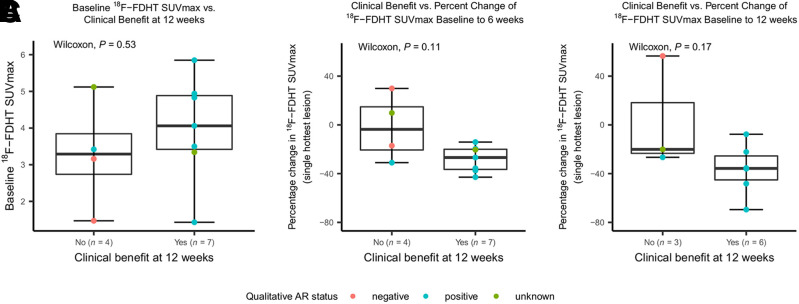
The baseline 18F-FDHT SUVmax of the hottest lesion per participant was similar between the 7 participants with CB at 12 wk after therapy (median, 4.1; range, 1.4–5.9) and the 4 with PD (median, 3.3; range, 1.5–5.1; P = 0.53; Fig. 4A). Results were similar for the hottest-lesion SUVmean and for the summed SUVmax and SUVmean of all lesions (Supplemental Fig. 3).
FIGURE 4.
CB at 12 weeks after starting therapy vs. baseline and change in 18F-FDHT uptake. (A) For 7 participants with CB, median baseline 18F-FDHT SUVmax was 4.1 (range, 1.4–5.9), compared with 3.3 (range, 1.5–5.1) for 4 participants with disease progression (P = 0.53). Individual dots on scatterplot represent individual-participant data. (B and C) Participants with CB at 12 wk tended to have larger declines in 18F-FDHT uptake at 6 wk after starting GTx-024 (median decline, 26.8%; range, −42.9% to −14.1%) than did those with disease progression (median decline, 3.7%; range, −31% to +29%; P = 0.11) (B) and tended to have larger declines in 18F-FDHT uptake at 12 wk after starting GTx-024 (median decline, 35.7%; range, −69.5% to −7.7%) than did those with disease progression (median decline, 20.1%; range, −26.6% to +56.5%; P = 0.17) (C).
Participants with CB at 12 wk tended to have larger declines in 18F-FDHT uptake at 6 wk (median decline, 26.8%; range, −42.9% to −14.1%) after starting GTx-024 than did those with PD (median decline, 3.7%; range, −31% to +29%; P = 0.11). A similar trend was observed at the 12-wk 18F-FDHT PET/CT scan, with a median decline of 35.7% (range, −69.5% to −7.7%) for those with CB compared with a median decline of 20.1% (−26.6% to +56.5%, P = 0.17) for those with PD (Figs. 4B–4C and 5). Similar trends were observed for hottest-lesion 18F-FDHT SUVmean at 6 and 12 wk after starting GTx-024 and for summed SUVmax at 6 wk (Supplemental Figs. 4 and 5). Six-week summed 18F-FDHT SUVmean declines were larger for those with than without CB (P = 0.04, Supplemental Fig. 6). Percentage decrease in 18F-FDHT SUVmax between the single hottest lesions at baseline and follow-up (i.e., PERCIST-like criteria) was significantly larger for those with CB (percentage decline, 21.4%; range, −42.9% to −14.1%) than for those without CB (percentage increase, 7.6%; range, −17.1% to +29.9%; P = 0.01) at week 6 but not at week 12 (P > 0.5, Supplemental Fig. 7).
FIGURE 5.
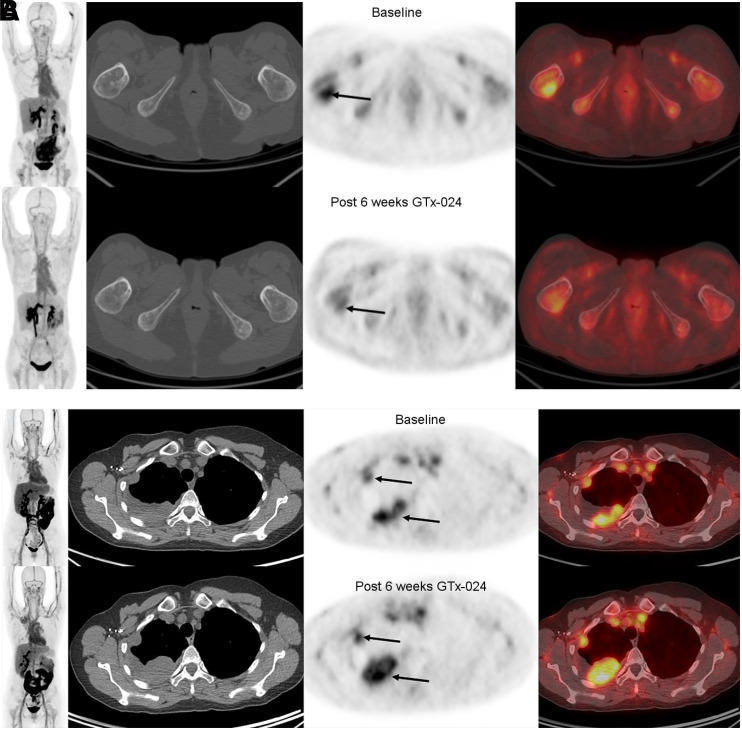
(A, left-most panel: maximum-intensity-projection PET; 2nd panel: axial CT; 3rd panel: axial PET; 4th panel: axial fused PET/CT) 18F-FDHT–avid AR+ tumor at baseline (top row, arrow [SUVmax, 4.9]) and decline in 18F-FDHT uptake 6 wk after starting GTx-024 (bottom row, arrow). Best overall response was stable disease 12 wk after starting therapy. (B, left-most panel: maximum-intensity-projection PET; 2nd panel: axial CT; 3rd panel: axial PET; 4th panel: axial fused PET/CT) 18F-FDHT–avid AR+ tumor at baseline (top row, lower arrow [SUVmax, 5.1]) and no decline in 18F-FDHT uptake and increased tumor size 6 wk after starting GTx-024 (bottom row, arrows). Best overall response was PD 12 wk after starting therapy.
Five of 7 participants with CB at week 12 after starting GTx-024 progressed by week 24. The 2 participants with continued CB at 24 wk had the largest declines in 18F-FDHT uptake at week 6 and were among the top 3 for the largest decline in 18F-FDHT uptake at week 12.
18F-FDHT Uptake Versus Hormone and PSA Levels
No correlations were found between baseline 18F-FDHT uptake, estradiol, and testosterone levels (Supplemental Figure 8). There tended to be higher baseline 18F-FDHT uptake with lower baseline SHBG (Supplemental Fig. 9). No correlations were found at baseline or during treatment when comparing SUVmax or SHBG with AR status and CB (Supplemental Fig. 10). There were no correlations between baseline 18F-FDHT SUVmax and PSA levels. Although the participant with the largest decline in 18F-FDHT SUVmax also had categoric declines in PSA levels at 6 and 12 wk after starting GTx-024 and CB, no correlations between changes in PSA, 18F-FDHT SUVmax, or best overall response were observed (Supplemental Fig. 11).
DISCUSSION
Despite the shift in therapeutic paradigms brought about by targeted therapy in many cancers, tumor heterogeneity, inability to biopsy every lesion, and target conversion within the tumor remain challenges in predicting who will benefit from specific therapeutic agents. Noninvasive, whole-body molecular imaging evaluates the entire tumor burden, providing one potential solution, but remains a globally underutilized tool for optimizing therapeutic strategies in large clinical trials (16,17). Our data supplement prior work by demonstrating the feasibility of using 18F-FDHT PET/CT for evaluating the response to SARM therapy in a large therapeutic clinical trial.
In 13 patients with ER+ MBC who underwent 18F-FDHT PET/CT and metastatic tumor biopsy within 8 wk, Venema et al. found a correlation between 18F-FDHT uptake and AR expression (r2 = 0.47, P = 0.01) using a semiquantitative assessment of more than 10% nuclear staining as positive for AR (18). Although not statistically significant, all but one AR+ tumor in our dataset had a baseline 18F-FDHT SUVmax higher than the findings in AR− tumors, suggesting a trend for higher baseline 18F-FDHT uptake in AR+ tumors. One AR− tumor had baseline 18F-FDHT uptake greater than the optimal SUVmax cutoff of 1.9 suggested for differentiating AR+ from AR− tumor by 18F-FDHT PET/CT (18). The small sample size, tumor heterogeneity, and nonpaired lesions for AR status and 18F-FDHT uptake may drive the lack of significance in our dataset. Our results, as well as those of Venema et al., support further investigation of 18F-FDHT PET/CT as an imaging biomarker of AR expression (18).
AR expression is heterogeneous between primary breast cancer and metastases, with discordance rates of up to 33% (19), and in metastases over the natural disease history (20). Venema et al. reported an AR+ primary tumor with AR− metastatic disease in 2 of 13 patients and substantial intrapatient 18F-FDHT heterogeneity (18), with patients having both 18F-FDHT+ and 18F-FDHT− lesions and 18F-FDHT SUVs ranging from 0.8 to 6.5.
The therapeutic trial inclusion criteria mandated objective evidence of progression within 30 d of randomization, but standard imaging modalities, that is, CT and bone scans, for this purpose often fail to differentiate active disease from disease that previously responded to treatment. This failure likely contributed to tumor heterogeneity in our imaging substudy. 18F-FDG PET/CT may be useful to supplement or replace standard imaging to better identify the metabolically active tumor burden and guide interpretation of 18F-FDHT PET/CT. Such a strategy was used with 18F-FES PET/CT imaging for breast cancer bone metastases (21).
Testosterone, dihydrotestosterone, and 18F-FDHT competitively bind AR (7), and SHBG binds sex hormones, including dihydrotestosterone. Categorically low sex hormone levels in this postmenopausal population likely limited our ability to identify any correlations between baseline 18F-FDHT uptake, testosterone, estrogen, or PSA levels. Further, the trend we observed for an inverse relationship between 18F-FDHT uptake and serum SHBG may be explained by the possibility that SHBG binding decreased the 18F-FDHT availability for tumor binding, given the low levels of estrogen and testosterone in our participants. Kramer et al. used a simplified method to correct body-weight–corrected SUV for serum SHBG and found an improved correlation with Patlak Ki derived from dynamic images (22). We did not find any statistically significant correlations at baseline or during treatment between SUVmax/SHBG and AR expression or best overall response. The design of future studies of androgen modulation should take into consideration the hormonal status of the study population.
To our knowledge, this was the first study assessing changes in 18F-FDHT uptake on PET in patients with MBC treated with SARM. Although CB was not associated with baseline 18F-FDHT uptake, those with CB within the first 12 wk of treatment tended to have larger percentage declines in 18F-FDHT uptake after 6 and 12 wk of SARM therapy for most of the semiquantitative parameters we explored. All but 2 participants progressed by 24 wk after starting therapy, but 2 of the 3 participants with the largest 18F-FDHT declines at 6 and 12 wk after starting GTx-024 continued to have CB at 24 wk. Larger studies are needed to determine the percentage decline in SUV that correlates with a clinical disease response.
Boers et al. recently evaluated 18F-FDHT PET/CT for assessing changes in AR availability in 21 patients with AR+ MBC receiving bicalutamide, a pure AR antagonist (23). Like our findings with GTx-024, baseline 18F-FDHT uptake did not predict CB to bicalutamide. Decreases in 18F-FDHT uptake after 4–6 wk of bicalutamide also did not predict CB in the total study population, which included both ER+ and ER− tumors, contrasting with our findings. In a subgroup analysis of 13 patients with ER+ tumor, the authors reported a trend for larger 18F-FDHT uptake declines in 5 patients with CB from bicalutamide than in 8 with PD (n = 8) (23). Our study included only participants with ER+ breast cancer, and our results support the subgroup analysis trend. The different pharmacology between GTx-024 and bicalutamide is also noted and is important to understand when evaluating imaging biomarkers in specific breast cancer subtypes.
Early imaging time points would be most advantageous for limiting use of ineffective therapy and unnecessary toxicities. The PERCIST-like criteria at 6 wk after starting therapy best separated those with from those without CB in our cohort. The optimal parameter still needs to be determined in larger studies.
This study had several limitations, notably the small number of participants enrolled. Prospectively including an imaging study in a larger therapeutic trial, however, ensured standardization of 18F-FDHT PET/CT imaging and response assessments using RECIST 1.1. Lesions were not paired for AR status and 18F-FDHT uptake assessment because the parent therapeutic trial allowed archival tissue specimens. Although metastases represented most archival tissue specimens (n = 7), they still may not have been from the same site or organ. Additional limitations are the use of different PET/CT scanners and randomization of 1 participant to the higher GTx-024 dose level. Finally, not including 18F-FDG PET/CT, in addition to or instead of standard imaging (i.e., CT and bone scans), to identify the metabolically active tumor burden in following for disease response in a new therapeutic trial remains a challenge. We believe that the inclusion of noninvasive whole-body functional imaging combining a metabolic tracer such as 18F-FDG and a specific hormonal targeting agent such as 18F-FDHT should be encouraged in this patient population since it could improve tumor characterization, assess tumor heterogeneity, guide biopsy, and help with decision making and evaluation of therapeutic response while also helping validate the specific investigational radiotracer.
CONCLUSION
Our data suggest that 18F-FDHT PET/CT may be a useful imaging biomarker for evaluating the response of MBC to SARM therapy and other AR-expressing malignancies and reiterate the feasibility of including molecular imaging in multidisciplinary therapeutic trials. Establishing the repeatability and reproducibility of quantitating 18F-FDHT uptake in breast cancer and thresholds for predicting response is a required next step to establish 18F-FDHT PET/CT as a noninvasive molecular imaging biomarker. This step may be challenging, given the underlying tumor heterogeneity seen in hormonally driven breast cancer.
DISCLOSURE
Heather Jacene has received honoraria from Janssen Pharmaceuticals and Bayer Healthcare; research support from Siemens Healthcare, Inc., GTx, Inc., and Blue Earth Diagnostics; consulting fees from Advanced Accelerator Applications; royalties from Cambridge Publishing; and NIH/NCI grant support not related to this work as a coinvestigator (1R01CA235589 - 01A1). Beth Overmoyer has received research support from Genentech, Incyte, GTx, and Eisai. Annick Van den Abbeele has received NCI grant support through a National Comprehensive Cancer Center grant (Dana-Farber/Harvard Cancer Center 2 P30 CA006516-52; principal investigator, Laurie Glimcher) as a co–principal investigator of the Tumor Imaging Metrics Core; is an unpaid board member of the Centre for Probe Development and Commercialization (CPDC), Toronto, Canada; is an unpaid consultant for Fusion Pharmaceuticals and Bristol-Myers Squibb; receives travel expenses from Ipsen, ImaginAb, and CPDC to attend investigators’ or board meetings; and receives royalties from Thieme Publishers. Diane Young and Mayzie Johnston were employees of GTx, Inc. GTx, Inc., provided funding to conduct this research. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank Dr. Myles Brown for his expertise and support throughout all aspects of this study and Dr. Barbara McNeil for critically reviewing this article. We also thank Drs. Alan Packard and Robert Helm for their graphical contributions.
KEY POINTS.
QUESTION: Does 18F-FDHT uptake on PET/CT in MBC correlate with tumor AR status and predict the response to SARM therapy?
PERTINENT FINDINGS: In a prospective imaging substudy of 11 women with MBC receiving SARM therapy, we showed trends toward larger declines in 18F-FDHT uptake after the start of therapy with CB.
IMPLICATIONS FOR PATIENT CARE: With further validation in well-designed clinical trials, 18F-FDHT PET/CT could be a valuable tool to characterize tumors and direct strategies modulating AR signaling in breast cancer and other AR+ malignancies.
REFERENCES
- 1. Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24:924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies C, Pan H, Godwin J, et al.Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tormey DC, Gray R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group. J Natl Cancer Inst. 1996;88:1828–1833. [DOI] [PubMed] [Google Scholar]
- 4. Narayanan R, Dalton JT. Androgen receptor: a complex therapeutic target for breast cancer. Cancers (Basel). 2016;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venema CM, Bense RD, Steenbruggen TG, et al.Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol Ther. 2019;200:135–147. [DOI] [PubMed] [Google Scholar]
- 6. Overmoyer B, Sanz-Altimira P, Partridge AH, et al.Enobosarm for the treatment of metastatic estrogen and androgen receptor positive breast cancer: final results of the primary endpoint and current progression free survival [abstract]. Cancer Res. 2015;75(9 suppl):P1-13-04. [Google Scholar]
- 7. Bonasera TA, O’Neil JP, Xu M, et al.Preclinical evaluation of fluorine-18-labeled androgen receptor ligands in baboons. J Nucl Med. 1996;37:1009–1015. [PubMed] [Google Scholar]
- 8. Liu A, Dence CS, Welch MJ, Katzenellenbogen JA. Fluorine-18-labeled androgens: radiochemical synthesis and tissue distribution studies on six fluorine-substituted androgens, potential imaging agents for prostatic cancer. J Nucl Med. 1992;33:724–734. [PubMed] [Google Scholar]
- 9. Beattie BJ, Smith-Jones PM, Jhanwar YS, et al.Pharmacokinetic assessment of the uptake of 16β-18F-fluoro-5α-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overmoyer B, Rugo HS, Johnston S, et al.First stage of an on-going phase 2, open label, international, randomized, parallel design study investigating efficacy + safety of GTx-024 for advanced ER+/AR+ breast cancer (BC) [abstract]. Ann Oncol. 2017;28(suppl 1):i7–i8. [Google Scholar]
- 11. Ackermann U, Lewis JS, Young K, et al.Fully automated synthesis of [18F]fluoro-dihydrotestosterone ([18F]FDHT) using the FlexLab module. J Labelled Comp Radiopharm. 2016;59:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mori T, Kiyono Y, Asai T, et al.Automated synthesis of 16β-[18F]fluoro-5dihydrotestosterone using a plastic cassette-type FDG synthesizer [abstract]. J Nucl Med. 2010;51(suppl 2):1525. [Google Scholar]
- 13. Gradishar WJ, Anderson BO, Abraham J, et al.Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:452–478. [DOI] [PubMed] [Google Scholar]
- 14. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 (suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ. 1995;310:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Vries EGE, Kist de Ruijter L, Lub-de Hooge MN, Dierckx RA, Elias SG, Oosting SF. Integrating molecular nuclear imaging in clinical research to improve anticancer therapy. Nat Rev Clin Oncol. 2019;16:241–255. [DOI] [PubMed] [Google Scholar]
- 17. Waaijer SJH, Kok IC, Eisses B, et al.Molecular imaging in cancer drug development. J Nucl Med. 2018;59:726–732. [DOI] [PubMed] [Google Scholar]
- 18. Venema CM, Mammatas LH, Schroder CP, et al.Androgen and estrogen receptor imaging in metastatic breast cancer patients as a surrogate for tissue biopsies. J Nucl Med. 2017;58:1906–1912. [DOI] [PubMed] [Google Scholar]
- 19. Bronte G, Bravaccini S, Ravaioli S, et al.Androgen receptor expression in breast cancer: what differences between primary tumor and metastases? Transl Oncol. 2018;11:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cimino-Mathews A, Hicks JL, Illei PB, et al.Androgen receptor expression is usually maintained in initial surgically resected breast cancer metastases but is often lost in end-stage metastases found at autopsy. Hum Pathol. 2012;43:1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurland BF, Peterson LM, Linden HM, Mankoff DA. FDG PET and FES PET predict PFS on endocrine therapy-response. Clin Cancer Res. 2018;24:249–250. [DOI] [PubMed] [Google Scholar]
- 22. Kramer GM, Yaqub M, Vargas HA, et al.Assessment of simplified methods for quantification of 18F-FDHT uptake in patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2019;60:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boers J, Venema CM, de Vries EFJ, et al.Serial [18F]-FDHT-PET to predict bicalutamide efficacy in patients with androgen receptor positive metastatic breast cancer. Eur J Cancer. 2021;144:151–161. [DOI] [PubMed] [Google Scholar]