Abstract
Coronary 18F-sodium fluoride (18F-NaF) PET and CT angiography–based quantitative plaque analysis have shown promise in refining risk stratification in patients with coronary artery disease. We combined both of these novel imaging approaches to develop an optimal machine-learning model for the future risk of myocardial infarction in patients with stable coronary disease. Methods: Patients with known coronary artery disease underwent coronary 18F-NaF PET and CT angiography on a hybrid PET/CT scanner. Machine-learning by extreme gradient boosting was trained using clinical data, CT quantitative plaque analysis, measures and 18F-NaF PET, and it was tested using repeated 10-fold hold-out testing. Results: Among 293 study participants (65 ± 9 y; 84% male), 22 subjects experienced a myocardial infarction over the 53 (40–59) months of follow-up. On univariable receiver-operator-curve analysis, only 18F-NaF coronary uptake emerged as a predictor of myocardial infarction (c-statistic 0.76, 95% CI 0.68–0.83). When incorporated into machine-learning models, clinical characteristics showed limited predictive performance (c-statistic 0.64, 95% CI 0.53–0.76) and were outperformed by a quantitative plaque analysis-based machine-learning model (c-statistic 0.72, 95% CI 0.60–0.84). After inclusion of all available data (clinical, quantitative plaque and 18F-NaF PET), we achieved a substantial improvement (P = 0.008 versus 18F-NaF PET alone) in the model performance (c-statistic 0.85, 95% CI 0.79–0.91). Conclusion: Both 18F-NaF uptake and quantitative plaque analysis measures are additive and strong predictors of outcome in patients with established coronary artery disease. Optimal risk stratification can be achieved by combining clinical data with these approaches in a machine-learning model.
Keywords: myocardial infarction, CT, 18F-NaF PET, quantitative plaque analysis, machine-learning
In everyday clinical practice, prediction of myocardial infarction is challenging and is typically based on cardiovascular risk factors and scores, especially in subjects with suspected coronary artery disease (1). However, in patients with established coronary artery disease, the performance of risk scores is limited, with c-statics ranging from 0.60 to 0.68 (1). Recently, advanced imaging techniques have demonstrated considerable promise in refining risk stratification in patients with established coronary artery disease. We have demonstrated that assessment of disease activity in the coronary arteries with 18F-sodium fluoride (18F-NaF) PET outperforms clinical variables and risk scores for the prediction of myocardial infarction in patients with a high burden of coronary artery disease (2,3). Similarly, in observational studies and a subanalysis of the SCOT-HEART trial, quantitative plaque analysis investigating both plaque type and burden on contrast enhanced CT angiography has emerged as a major predictor of adverse outcomes (4,5). To date, no study has investigated whether these 2 promising methods (which can be obtained during a single imaging session on a hybrid PET/CT scanner) are interchangeable or can provide superior predictive performance when used in combination.
In this study, we used machine-learning to investigate whether the prognostic information provided by quantitative CT plaque analysis and assessments of disease activity by 18F-NaF PET are complementary, and to develop an optimized model to determine the future risk of myocardial infarction in patients with established coronary artery disease (6).
MATERIALS AND METHODS
Study Population
The current study is based on a cohort of patients with established coronary artery disease on guideline-recommended medical treatments, which we assembled for our previous publication regarding the prognostic utility of 18F-NaF PET (2). However, in the current study, we have included longer follow-up and used novel quantitative plaque analysis of coronary CT angiography. Our work is focused specifically on whether machine-learning methods can combine the prognostic information provided by clinical factors, quantitative CT plaque analysis and 18F-NaF PET to improve the prediction of myocardial infarction. All participants underwent hybrid coronary 18F-NaF PET and contrast CT coronary angiography within prospective observational research studies (NCT01749254, NCT02110303, NCT02607748) (3,7,8). All patients had established coronary artery disease and underwent a comprehensive baseline clinical assessment with evaluation of their cardiovascular risk factor profile including calculation of the Secondary Manifestations of ARTerial disease (SMART) risk score (supplemental materials, available at http://jnm.snmjournals.org) (1). Studies were conducted with the approval of the local research ethics committee, in accordance with the Declaration of Helsinki, and with written informed consent from each participant.
CT Angiography and 18F-Sodium Fluoride PET
Acquisition and Reconstruction
Patients underwent 18F-NaF PET on hybrid PET/CT scanners (128-slice Biograph mCT, Siemens Medical Systems; or Discovery 710, GE Healthcare) 60 min after intravenous administration of 18F-NaF (250 MBq). We acquired a noncontrast CT attenuation correction scan followed by a 30-min PET emission scan in list mode, a low-dose noncontrast ECG-gated CT for calculation of the coronary calcium, and a contrast-enhanced ECG-gated coronary CT angiogram, which was obtained in mid-diastole and end-expiration on the same PET/CT system without repositioning the patient. The ECG-gated PET list-mode dataset was reconstructed using harmonized protocols as described previously (supplemental materials) (8–10).
Coronary Microcalcification Activity (CMA) Quantification
Image analysis was performed in FusionQuant (Cedars-Sinai Medical Center) (11). We used a recently described measure of coronary 18F-NaF uptake, CMA, that quantifies PET activity across the entire coronary vasculature (12). CMA is a highly reproducible and robust measure of disease activity predicting both disease progression and myocardial infarction (2,13). We calculated the per-vessel and per-patient CMA (Fig. 1), maximum coronary SUV, and target-to-background ratio (TBR) as described previously (supplemental materials) (3,12).
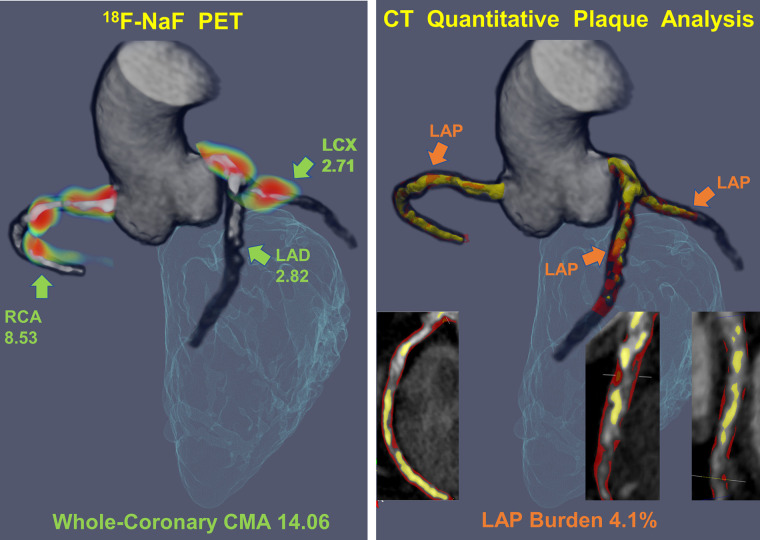
FIGURE 1.
Measuring disease activity across the coronary vasculature with 18F-NaF CMA and the low-attenuation plaque burden with quantitative plaque analysis. Three-dimensional (3D) rendering of coronary CT angiography coregistered with PET for evaluation of 18F-NaF uptake (blue and red; left panel). The CMA is a summary measure of 18F-NaF activity across the entire coronary vasculature as it includes all counts originating from the coronary artery 3D rendering of CT angiography–based quantitative plaque analysis with orange low-attenuation plaque (LAP) and yellow calcified plaque. The low-attenuation plaque burden was defined as the LAP volume × 100%/vessel volume. LAD = left anterior descending; LCX = left circumflex; RCA = right coronary artery.
CT
The coronary artery calcium score was measured in Agatston units (AU) using clinical software (NetraMD, ScImage) on noncontrast CT scans. The presence, extent, and severity of coronary artery disease were evaluated on contrast-enhanced CT angiography by defining the segment involvement score, DUKE coronary artery disease index, and the number of vessels with >50% luminal stenosis (14). Multivessel coronary artery disease was defined as at least 2 major epicardial vessels with any combination of either >50% stenosis, or previous revascularization.
Quantitative Plaque Analysis of CT Angiography
We performed quantitative plaque analysis of all coronary segments with a lumen diameter greater than 2 mm using semiautomated software (AutoPlaque, version 2.0, Cedars-Sinai Medical Center) (4,5). Proximal and distal limits of lesions were manually marked by an experienced reader after examination of coronary CT angiography images in multiplanar format. Subsequent plaque quantification was fully automated using adaptive scan-specific thresholds. Total, calcified, noncalcified as well as low attenuation plaque volumes were calculated. The plaque burden was calculated according to the following equation (plaque volume × 100%/vessel volume). The contrast density difference was the maximal difference in contrast density (mean Hounsfield unit/cross-sectional area) in the plaque and the reference proximal vessel cross section.
Machine Learning
Machine learning was used to derive a joint score for myocardial infarction by incorporating the key clinical variables, quantitative CT variables, and 18F-NaF PET findings.
Model Building
XGBoost is a recent implementation of a gradient boosting algorithm, which iteratively trains a set of weak learners (simple decision trees) using a given set of patient data, to build a combined strong classifier to identify an outcome (15). For every patient, the XGBoost algorithm computes an individualized probability of outcome, considering all input variables.
We applied XGBoost for prediction of myocardial infarction by building 3 models. First, a clinical model with baseline clinical characteristics: age, sex, comorbidities, medication, biomarkers, past medical history, and coronary calcium score (model 1). The second model was derived from quantitative plaque analysis variables (including low attenuation plaque burden and the contrast density difference). A final model incorporated clinical, CT and 18F-NaF PET data in combination. All variables used in the machine-learning modeling are presented in Supplemental Table 1.
Model Testing
Given the limited number of cases, we refrained from performing data-specific hypertuning and applied fixed XGBoost parameters established in our previous studies (15). Furthermore, to avoid biased results and limit overfitting, we tested all of our models using repeated 10-fold cross-testing, which separates training and testing data (16). The dataset was randomly split into 10-folds with similar myocardial infarction rates in each fold (stratified 10-folds). Ten models were created each from 90% of the data, and each tested in held-out test sample (10% of the data). These 10 held-out samples containing nonoverlapping test results were subsequently concatenated to evaluate the average performance of XGBoost in unseen data.
Feature Importance
To elucidate the influence of each of the variables included in the machine-learning model, we provided machine-learning feature importance scores. Importance is the relative amount that each attribute improves the XGBoost performance measure. The variable importance was determined directly from the XGBoost model separately in each fold and returned from the XGBoost model for each variable. The variable importance represents the relative improvement in the log loss objective function of the XGBoost (17).
Clinical Follow-up
The primary endpoint of the study was fatal or nonfatal myocardial infarction. Outcome information was obtained in June 2020 from the local and national health-care record systems that integrates primary and secondary health-care records. Categorization of these outcomes was performed blinded to the coronary CT angiography and PET data.
Statistical Analysis
We assessed the distribution of data with the Shapiro–Wilk test. Continuous parametric variables were expressed as mean ± SD, and nonparametric data were presented as median (interquartile interval). Fisher exact test or χ2 test was used for analysis of categoric variables. The performance of machine-learning models and single clinical characteristics in predicting myocardial infarction was assessed using receiver operator characteristic (ROC) analysis, and the area under the curve (c-statistic) values were compared with the DeLong test (18). Statistical analysis was performed with SPSS, version 24 (IBM SPSS Statistics for Windows, version 24.0, IBM Corp.) and R studio and R software, version 4.01 (R Foundation for Statistical Computing). A 2-sided P < 0.05 was considered statistically significant.
RESULTS
All 293 study participants (65 ± 9 y; 84% male) had established coronary artery disease and were on guideline-recommended medical treatments (Table 1). Two-hundred thirty-seven (81%) patients had a history of revascularization, 191 (65%) had multivessel obstructive coronary artery disease, and the median coronary calcium score was 334 (76 to 804) AU. Over the 53 (40–59) months of follow-up, 22 subjects experienced a fatal (n = 3) or nonfatal (n = 19) myocardial infarction.
TABLE 1.
Baseline Clinical Characteristics
| Category | Variable | Mean ± SD/median [Q1-Q3]/n (%) |
|---|---|---|
| Baseline clinical characteristics | Age | 65 ± 9 |
| Men | 245 (84%) | |
| Body-mass index (kg/m2), | 29 ± 5 | |
| Systolic blood pressure (mm Hg) | 141 ± 20 | |
| Diastolic blood pressure (mm Hg) | 79 ± 11 | |
| Cardiovascular history | History of acute coronary syndrome | 161 (55.1%) |
| History of percutaneous coronary intervention | 182 (62.3%) | |
| History of coronary artery bypass graft surgery | 48 (16.4%) | |
| History of angina | 136 (46.6%) | |
| Recent acute coronary syndrome | 61 (21%) | |
| Cerebrovascular accident or transient ischemic attack | 9 (3.1%) | |
| Comorbidities/risk factors | Hypertension | 174 (59.6%) |
| Hyperlipidemia | 257 (88%) | |
| Diabetes mellitus | 61 (20.8%) | |
| Current smoking | 58 (19.9%) | |
| Ex-smoker | 137 (46.9%) | |
| Atrial fibrillation | 10 (3.4%) | |
| Peripheral vascular disease | 16 (5.5%) | |
| Medications* | Aspirin | 268 (91.8%) |
| Dual antiplatelet therapy | 62 (21.2%) | |
| Statin | 262 (89.7%) | |
| β-Blocker | 196 (67.1%) | |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 197 (67.4%) | |
| Insulin | 4 (1.4%) | |
| Oral diabetic medications | 48 (16.4%) | |
| Calcium blockers | 63 (21.6%) | |
| Diuretics | 38 (16.0%) | |
| Biomarkers | Total cholesterol (mg/dL) | 159 [139–182] |
| LDL cholesterol (mg/dL) | 73 [46–93] | |
| HDL cholesterol (mg/dL) | 46 [39–66] | |
| Triglycerides (mg/dL) | 133 [97–204] | |
| Creatinine (mg/dL) | 0.9 [0.8–1.0] | |
| Risk scores | SMART | 18 [13–26] |
| CT – qualitative & noncontrast | - Single vessel disease | 87 (29.8%) |
| - Two vessel disease | 110 (37.7%) | |
| - Three vessel disease | 81 (27.6%) | |
| - Left main stem involvement | 18 (6.1%) | |
| Coronary stent | 218 (73.4%) | |
| Segment involvement score | 5 [3–7] | |
| Segment involvement score > 5 | 145 (73.5%) | |
| Coronary calcium score | 334 [76–804] | |
| Coronary calcium score category | ||
| 0–99 | 84 (28.7%) | |
| 100–399 | 76 (25.9%) | |
| 400–999 | 74 (25.3%) | |
| >1,000 | 59 (20.1%) | |
| CT – quantitative | Total plaque volume, mm3 | 1174 [716, 1772] |
| Noncalcified plaque volume, mm3 | 1099 [647, 1574] | |
| Calcified plaque volume, mm3 | 77 [23, 180] | |
| Low-attenuation plaque volume, mm3 | 88 [44, 167] | |
| Total plaque burden, % | 55 [49, 63] | |
| Noncalcified plaque burden, % | 51 [45, 57] | |
| Calcified plaque burden, % | 3.5 [1.4, 7.9] | |
| Low-attenuation plaque burden, % | 4.4 [2.6, 7.0] | |
| Area stenosis, % | 58 [47, 75] | |
| Contrast density difference, % | 29 [24, 37] | |
| Ischemia score | 31 [21, 47] | |
| 18F-NaF PET | CMA | 0.66 [0–2.84] |
| TBRmax | 1.22 [1.1–1.42] | |
| SUVmax | 1.44 [1.19, 1.71] | |
| Outcome | Myocardial infarction | 22 (7.5%) |
Recent acute coronary syndrome was defined as an event within less than 14 days before PET imaging.
SMART = Secondary Manifestations of ARTerial disease risk score; SUVmax = maximum SUV; TBRmax = maximum target to background ratio.
The high burden of atherosclerosis was reflected in the quantitative plaque analysis derived from coronary CT angiography. The median total plaque volume was 1,174 (716 to 1,772) mm3 and consisted largely of noncalcified plaque (1,099 [647 to 1,574] mm3) with a substantial volume of low-attenuation plaque (88 [44 to 167] mm3). Over half of the study population (166 [56%]) had a low-attenuation plaque burden exceeding 4%. On PET, 109 (37.2%) patients presented with a high 18F-NaF CMA (>1.56; Fig. 2).
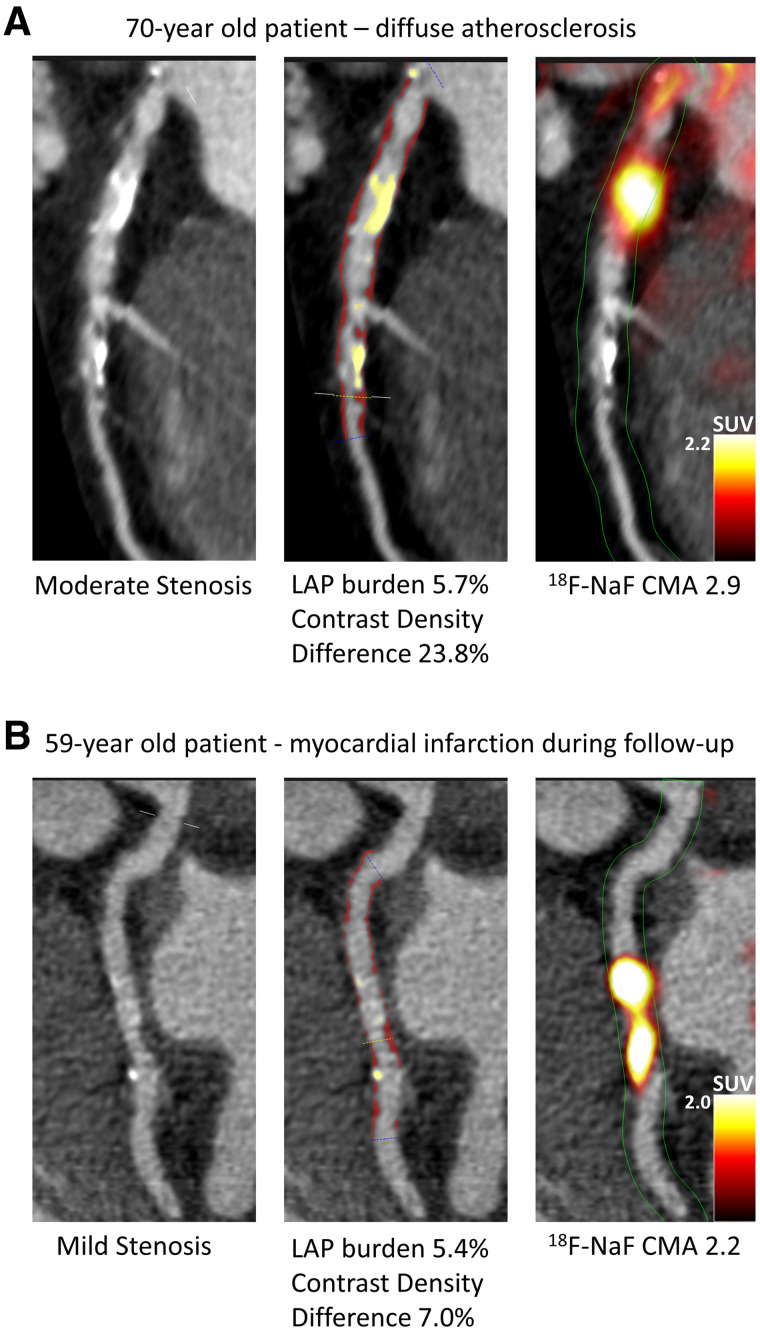
FIGURE 2.
Case examples of quantitative plaque analysis on coronary CT angiography and 18F-NaF PET in patients with established coronary artery disease. Hybrid CT angiography and 18F-NaF PET of coronary arteries. (A) A 70-y-old male, who presented with diffused largely noncalcified disease (middle panel in red) in the LAD and demonstrated increased 18F-NaF uptake in the LAD on PET. (B) A 59-y-old male with mild LCX atherosclerosis, who presented with a high noncalcified plaque burden (middle panel in red) on CT angiography, significant 18F-NaF uptake and experienced a lateral non–ST-segment elevation myocardial infarction during follow-up. LAD = left anterior descending; LCX = left circumflex; LAP = low attenuation plaque.
On receiver operator curve analysis, 18F-NaF CMA (c-statistic 0.76, 95% CI 0.68 to 0.83; P < 0.001), maximum 18F-NaF TBR (c-statistic 0.72, 95% CI 0.63 to 0.82; P < 0.001) and maximum 18F-NaF SUV (c-statistic 0.70, 95% CI 0.59 to 0.81; P = 0.002) were the only statistically significant predictors of myocardial infarction. In contrast, baseline clinical characteristics, luminal stenosis severity, qualitative or quantitative CT-derived variables were not significant predictors of myocardial infarction on their own (Table 2). However, when incorporated into machine-learning models, the aforementioned variables emerged as predictors of adverse events. Although a model based on clinical characteristics only showed limited predictive performance with a c-statistic of 0.64 (95% CI 0.53–0.76), the quantitative plaque analysis-based machine-learning model outperformed the former with a c-statistic of 0.72 (95% CI 0.60–0.84, P = 0.02), which was comparable to 18F-NaF CMA alone (P = 0.47). Inclusion of clinical data improved the 18F-NaF CMA and quantitative plaque analysis–based models only slightly (0.77 [95% CI 0.69–0.84] and 0.74 [95% CI 0.64–0.83], respectively). Importantly, after inclusion of all available data (clinical, quantitative plaque and 18F-NaF PET), we achieved an increase in model performance with a c-statistic of 0.85 (95% CI 0.79–0.91, P < 0.001), which was higher than the quantitative CT plaque model (P = 0.008) and the 18F-NaF CMA (P = 0.01; Figs. 3 and 4) as well as the clinical characteristics model (P < 0.001).
TABLE 2.
Prediction of Myocardial Infarction in Patients with Advanced Coronary Artery Disease
| Category | Variable | Area under the curve (95% CIs) | P value |
|---|---|---|---|
| Baseline clinical characteristics | Age | 0.51 (0.35–0.67) | 0.81 |
| Sex | 0.51 (0.38–0.64) | 0.84 | |
| Body-mass index | 0.58 (0.46–0.70) | 0.23 | |
| Systolic blood pressure | 0.52 (0.37–0.67) | 0.74 | |
| Past medical history | Myocardial infarction | 0.45 (0.33–0.58) | 0.48 |
| Recent acute coronary syndrome | 0.57 (0.43–0.71) | 0.33 | |
| Percutaneous coronary intervention | 0.53 (0.40–0.67) | 0.66 | |
| Coronary artery bypass graft | 0.52 (0.39–0.65) | 0.80 | |
| Cerebrovascular accident | 0.53 (0.40–0.67) | 0.60 | |
| Comorbidities | Hypertension | 0.47 (0.35–0.59) | 0.57 |
| Hyperlipidemia | 0.48 (0.35–0.60) | 0.61 | |
| Diabetes | 0.51 (0.37–0.65) | 0.29 | |
| Smoking | 0.46 (0.32–0.60) | 0.59 | |
| Peripheral vascular disease | 0.52 (0.39–0.66) | 0.80 | |
| Biomarkers | Total cholesterol (mmol/L) | 0.53 (0.38–0.68) | 0.68 |
| LDL cholesterol (mmol/L) | 0.59 (0.43–0.75) | 0.18 | |
| HDL cholesterol (mmol/L) | 0.53 (0.38–0.67) | 0.71 | |
| Triglycerides (mmol/L) | 0.57 (0.44–0.69) | 0.33 | |
| Creatinine (μmol/L) | 0.54 (0.40–0.68) | 0.54 | |
| Risk scores | SMART | 0.57 (0.43–0.70) | 0.35 |
| CT – qualitative & noncontrast | Multivessel disease | 0.55 (0.42–0.68) | 0.48 |
| Segment involvement score | 0.56 (0.41–0.71) | 0.40 | |
| Coronary calcium score | 0.51 (0.37–0.66) | 0.87 | |
| Modified Duke index | 0.61 (0.48–0.74) | 0.11 | |
| CT – quantitative | Total plaque volume | 0.53 (0.39–0.67) | 0.65 |
| Noncalcified plaque volume | 0.54 (0.40–0.68) | 0.53 | |
| Calcified plaque volume | 0.46 (0.33–0.58) | 0.48 | |
| Low-attenuation plaque volume | 0.57 (0.41–0.72) | 0.30 | |
| Total plaque burden | 0.45 (0.33–0.57) | 0.42 | |
| Noncalcified plaque burden | 0.47 (0.35–0.59) | 0.67 | |
| Calcified plaque burden | 0.41 (0.29–0.54) | 0.16 | |
| Low-attenuation plaque burden | 0.61 (0.48–0.75) | 0.071 | |
| Area stenosis | 0.48 (0.35–0.62) | 0.79 | |
| Contrast density difference | 0.56 (0.40–0.71) | 0.33 | |
| Ischemia score | 0.52 (0.38–0.65) | 0.77 | |
| 18F-NaF PET | CMA total | 0.76 (0.68–0.83) | < 0.001 |
| TBRmax | 0.72 (0.63–0.82) | < 0.001 | |
| SUVmax | 0.70 (0.59–0.81) | 0.002 |
Receiver operator curve modeling for prediction of myocardial infarction.
HDL = high-density lipoprotein; LDL = low-density lipoprotein; SMART = Secondary Manifestations of ARTerial disease risk score; SUVmax = maximum SUV; TBRmax = maximum target-to-background ratio.
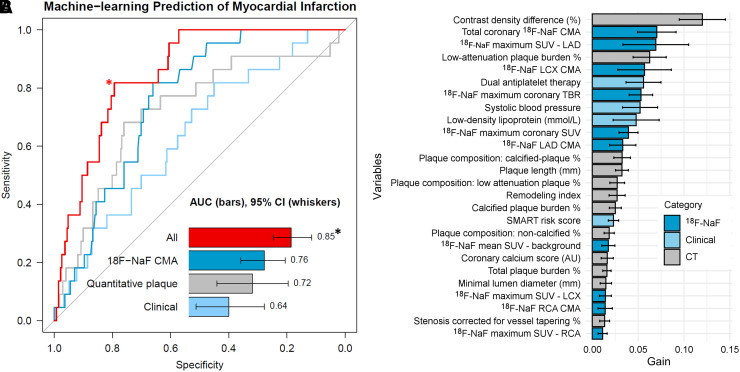
FIGURE 3.
Prediction of myocardial infarction by machine-learning. (A) Receiver operator curves for the risk of myocardial infarction: 18F-NaF CMA alone (dark blue), machine-learning models based on clinical data (light blue), quantitative plaque analysis (gray), clinical + quantitative plaque analysis + 18F-NaF PET (red). The model based on both PET and quantitative CT–based plaque analysis data outperformed the clinical data and both unimodality models (P < 0.01 for all). (B) Feature importance for the machine-learning model based on all variables. Solid bars and error bars represent the mean gain and SD derived from the distribution of the importance within 10-folds of the cross testing, for each variable. *indicates a P < 0.01 for a difference compared with 18F-NAF CMA, quantitative plaque, Clinical and CT (DeLong test). #error bars indicate 95% CIs. TBR = target-to-background ratio.
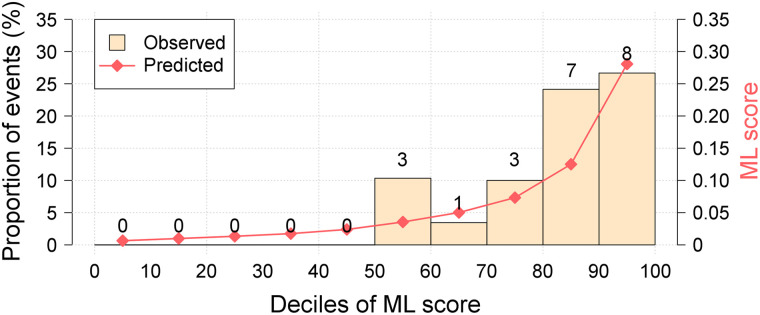
FIGURE 4.
Calibration plot for clinical + quantitative plaque analysis + 18F-NaF PET machine-learning XGBoost model. Calibration plot shows the relationship between the observed and predicted proportion of events, grouped by decile of risk. Our model showed very good calibration with the observed risk of myocardial infarction during follow-up.
DISCUSSION
We have built a machine-learning model for risk stratification in patients with established coronary artery disease. In our cohort of patients with advanced coronary atherosclerosis, we showed that risk prediction does not depend on cardiovascular risk scores, stenosis severity or CT calcium scoring. Rather the risk of myocardial infarction is primarily governed by the analysis of plaque type and plaque burden provided by coronary CT angiography and assessments of disease activity by 18F-NaF PET. Importantly, our machine-learning approach has overcome the challenges posed by colinearity of these variables and, for the first time, has demonstrated that this information is complementary and additive with the combination of both providing the most robust outcome prediction. If confirmed in further studies this comprehensive approach holds major promise in refining risk stratification of patients with established coronary artery disease, a population for which such prediction is currently challenging. Importantly, such stratification in these patients can be achieved objectively with quantitative variables obtained on a single hybrid PET/CT acquisition.
18F-NaF PET provides an assessment of vascular injury and disease activity across a wide spectrum of cardiovascular conditions including aortic stenosis, mitral annular calcification, abdominal aortic aneurysm, erectile dysfunction, bioprosthetic valve degeneration and coronary artery disease (2,19–22). Indeed, baseline 18F-NaF PET is consistently associated with future disease progression and adverse events in each of these conditions. On the other hand, quantitative assessment of atherosclerotic plaque on contrast-enhanced CT angiography allows us to measure the burden of different types of plaque across the coronary arteries (4). We recently demonstrated that the low-attenuation plaque burden provides powerful prediction of myocardial infarction, outperforming cardiovascular risk scores, Agatston coronary artery calcium scoring, or the presence and severity of obstructive coronary artery disease (5). Whether these 2 exciting developments can be used in combination to further advance risk prediction was previously unknown.
Using the information from these approaches and by leveraging machine learning, we were able to build an integrated model for prediction of events in patients with established coronary artery disease, a group of patients in whom risk prediction is currently challenging. The XGBoost algorithm has been successfully implemented for risk prediction in a wide range of clinical scenarios (15,23). It enables the incorporation of numerous predictors into the model even when these variables are correlated—a major limitation with conventional regression analyses. Although we have previously shown that 18F-NaF uptake is associated with quantitative plaque analysis indices, our current analysis highlights the complementary prognostic information that PET and quantitative CT plaque assessments provide together (24,25). Indeed, our machine-learning model incorporating the information from these 2 modalities alongside clinical factors outperformed the individual components analyzed separately with a high c-statistic of 0.85. Importantly, our study also underscores that in patients with advanced coronary artery disease, markers of disease activity, plaque type and plaque burden provide risk prediction superior to clinical risk scores and conventional coronary calcium CT analyses.
According to societal guidelines, patients with clinically manifest atherosclerotic arterial disease are considered to be at very high risk of a recurrent cardiovascular events and cardiovascular mortality. However, in everyday clinical practice, it is apparent that there is a wide distribution of actual risk for recurrent vascular events in patients with clinically established arterial disease. Although the population of subjects with manifested coronary artery disease is rapidly growing, accurate risk prediction in this important population remains challenging. The guideline-recommended SMART risk score was shown to have only a moderate c-statistic (0.64–0.68), and there is a paucity of data regarding the role imaging could play in this cohort (1). In our study we have targeted this important high-risk population. We have demonstrated that quantitative plaque analysis measures and the coronary microcalcification activity considerably improve stratification of patients’ risk (c-statistic 0.85). In a conservative 10-fold cross testing machine-learning model, we showed that CT and PET data need to be used together for optimal stratification.
Limitations
With the limited number of patients and events, our findings require confirmation in future studies. Machine-learning models can perform better when trained within bigger datasets, and therefore further studies are needed to confirm our findings and allow further testing to refine and to calibrate the machine-learning models. External validation of our findings in other cohorts is needed. Although this is currently challenging given that 18F-NaF PET is an emerging technique, this will be possible in the future using outcome data from the Prediction of Recurrent Events With 18F-Fluoride (PREFFIR) study, which is prospectively investigating the ability of 18F-NaF coronary PET and CT angiography to predict recurrent events in patients with multivessel disease and recent myocardial infarction. Since most of the study participants had multivessel disease, future studies should characterize the utility of 18F-NaF PET in patients with single vessel disease.
CONCLUSION
Both 18F-NaF uptake and quantitative plaque analysis measures from contrast CT are strong predictors of outcome in patients with established coronary artery disease. Optimal risk stratification can be achieved by combining these imaging assessments of plaque type, burden, and activity with clinical variables in a machine-learning model.
DISCLOSURE
This research was supported in part by grants R01HL135557 and R01HL133616 from the National Heart, Lung, and Blood Institute/National Institute of Health (NHLBI/NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. David E. Newby (CH/09/002, RE/18/5/34216, RG/16/10/32375), Marc R. Dweck (FS/14/78/31020), Mohammed N. Meah (FS/19/46/34445), and Michelle C. Williams (FS/11/014, CH/09/002, FS/ICRF/20/26002) are supported by the British Heart Foundation. Philip D. Adamson is supported by Heart Foundation of New Zealand Senior Fellowship (1844). Evangelos Tzolos was supported by a grant from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. David E. Newby is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA) and Marc R. Dweck of the Sir Jules Thorn Award for Biomedical Research Award (2015). Edwin J.R. van Beek is supported by SINAPSE (Scottish Imaging Network – A Platform of Scientific Excellence). Nikhil V. Joshi is supported by the Medical Research Council through MRC Clinical Academic Research Partnership grant (MR/T005459/1). No other potential conflict of interest relevant to this article was reported.
KEY POINTS.
QUESTION: Does combining information provided by CT plaque analysis and assessments of disease activity by 18F-NaF PET with machine-learning enhance risk stratification in established coronary artery disease?
PERTINENT FINDINGS: In a post hoc analysis of data collected for prospective observational studies, on a cohort of 293 patients with established coronary artery disease, we have demonstrated that optimal risk stratification can be achieved by combining clinical data with 18F-NaF PET and quantitative coronary CT angiography plaque analysis in a machine-learning model.
IMPLICATIONS FOR PATIENT CARE: This approach has major potential for the risk stratification of patients with established coronary artery disease.
REFERENCES
- 1. Dorresteijn JA, Visseren FL, Wassink AM, et al.Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99:866–872. [DOI] [PubMed] [Google Scholar]
- 2. Kwiecinski J, Tzolos E, Adamson PD, et al. 18F-sodium fluoride coronary uptake predicts outcome in patients with coronary artery disease. J Am Coll Cardiol. 2020;75:3061–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. [DOI] [PubMed] [Google Scholar]
- 4. Hell MM, Motwani M, Otaki Y, et al.Quantitative global plaque characteristics from coronary computed tomography angiography for the prediction of future cardiac mortality during long-term follow-up. Eur Heart J Cardiovasc Imaging. 2017;18:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams MC, Kwiecinski J, Doris M, et al.Low attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction. Circulation. 2020;18:1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Motwani M, Dey D, Berman DS, et al.Machine-learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J. 2017;38:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moss AJ, Dweck MR, Doris MK, et al.Ticagrelor to reduce myocardial injury in patients with high-risk coronary artery plaque. JACC Cardiovasc Imaging. 2020;13:1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doris MK, Otaki Y, Krishnan SK, et al.Optimization of reconstruction and quantification of motion-corrected coronary PET-CT. J Nucl Cardiol. 2020;27:494–504 10.1007/s12350-018-1317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubeaux M, Joshi N, Dweck MR, et al.Motion correction of 18F-sodium fluoride PET for imaging coronary atherosclerotic plaques. J Nucl Med. 2016;57:54–59. [DOI] [PubMed] [Google Scholar]
- 10. Lassen ML, Kwiecinski J, Dey D, et al.Triple-gated motion and blood pool clearance corrections improve reproducibility of coronary 18F-NaF PET. Eur J Nucl Med Mol Imaging. 2019;46:2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massera D, Doris MK, Cadet S, et al.Analytical quantification of aortic valve 18F-sodium fluoride PET uptake. J Nucl Cardiol. 2020;27:962–972 10.1007/s12350-018-01542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwiecinski J, Cadet S, Daghem M, et al.Whole-vessel coronary 18F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur J Nucl Med Mol Imaging. 2020;47:1736–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tzolos E, Kwiecinski J, Lassen ML, et al.Observer repeatability and interscan reproducibility of 18F-sodium fluoride coronary microcalcification activity. J Nucl Cardiol. 2020; 10.1007/s12350-020-02221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leipsic J, Abbara S, Achenbach S, et al.SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. [DOI] [PubMed] [Google Scholar]
- 15. Commandeur F, Slomka PJ, Goeller M, et al.Machine-learning to predict the long-term risk of myocardial infarction and cardiac death based on clinical risk, coronary calcium, and epicardial adipose tissue: a prospective study. Cardiovasc Res. 2020;116:2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J-H. Estimating classification error rate: repeated cross-validation, repeated hold-out and bootstrap. Comput Stat Data Anal. 2009;53:3735–3745. [Google Scholar]
- 17. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning Data Mining, Inference and Prediction. Springer; 2001:367. [Google Scholar]
- 18. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 19. Dweck MR, Jenkins WSA, Vesey AT, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371–378. [DOI] [PubMed] [Google Scholar]
- 20. Cartlidge TRG, Doris MK, Sellers SL, et al.Detection and prediction of bioprosthetic aortic valve degeneration. J Am Coll Cardiol. 2019;73:1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forsythe RO, Dweck MR, McBride OMB, et al.F-18-sodium fluoride uptake in abdominal aortic aneurysms The SoFIA(3) Study. J Am Coll Cardiol. 2018;71:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiecinski J, Tzolos E, Cartlidge TRG, et al. Native aortic valve disease progression and bioprosthetic valve degeneration in patients with transcatheter aortic valve implantation. Circulation. 2021;144:1396–1408. [DOI] [PMC free article] [PubMed]
- 23. van Rosendael AR, Maliakal G, Kolli KK, et al.Maximization of the usage of coronary CTA derived plaque information using a machine-learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J Cardiovasc Comput Tomogr. 2018;12:204–209. [DOI] [PubMed] [Google Scholar]
- 24. Kwiecinski J, Dey D, Cadet S, et al.Predictors of 18F-sodium fluoride uptake in patients with stable coronary artery disease and adverse plaque features on computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2020;21:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwiecinski J, Dey D, Cadet S, et al.Peri-coronary adipose tissue density is associated with 18F-sodium fluoride coronary uptake in stable patients with high-risk plaques. JACC Cardiovasc Imaging. 2019;12:2000–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]