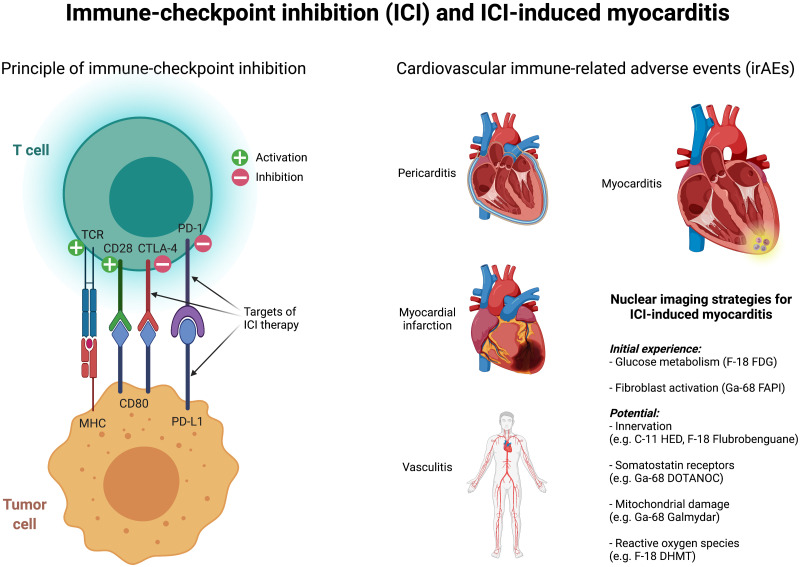
Visual Abstract
Immune checkpoint inhibitors (ICIs) are a novel therapy that directs the immune system to recognize cancer cells and promotes their elimination. Briefly, T cells are activated by antigen-presenting cells or tumor cells primarily via the interactions of the major histocompatibility complex with T-cell receptors and of CD80 (antigen-presenting cells) with CD28 (T cells). Cytotoxic T-lymphocyte–associated protein 4 is an inactivating coreceptor on T cells that has a higher affinity for CD80 and leads to inactivation of T cells. Another inactivating receptor on T cells is programmed death 1 (PD1), which interacts with programmed death ligand 1, which in turn is induced by inflammatory signals and is thought to preserve collateral damage in healthy tissue. Monoclonal antibodies that block cytotoxic T-lymphocyte–associated protein 4 (e.g., ipilimumab), PD1 (e.g., nivolumab and pembrolizumab), or programmed death ligand 1 (atezolizumab) result in T-cell reactivation, which in turn increases the antitumor response but also causes immune-related adverse events (irAEs).
ICI therapy is based on knowledge of these processes, is undoubtedly one of the most significant and promising developments in cancer therapy in recent years, and is increasingly being used for various tumors (1). As impressive as the results may seem for certain tumor types (e.g., the administration of ipilimumab achieves a long-term remission in about 25% of patients with an advanced stage of malignant melanoma, which was considered a terminal disease before immunotherapy (2)), the so-called ICI-associated side effects must not be underestimated (3). Generally speaking, irAEs occur frequently during therapy with ICIs, depending on the affected organ. For example, irAEs occur in up to 60% of patients treated with ipilimumab, with 10%–30% of the cases being considered serious (grade 3–4 according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events) (4).
The most common irAEs include diarrhea and colitis, and the earliest-occurring adverse events include skin rashes. Less common irAEs include hepatitis and endocrinopathies (such as thyroiditis) (4). Cardiovascular irAEs are substantially less common but may be associated with substantially higher fatality rates (3,5). In one study, an evaluation of VigiBase (the World Health Organization’s global database of individual case safety reports from over 130 countries) identified more than 16 million adverse events, of which approximately 31,000 were cardiovascular in origin (6). In this work, it was observed that myocarditis, pericardial disease, and vasculitis increased with ICI therapy and that these cardiovascular irAEs were severe in most cases (>80%) and were partly associated with high mortality (50%, 21%, and 6% in cases of myocarditis, pericardial disease, and vasculitis, respectively). This finding clearly demonstrates that cardiovascular irAEs under ICI therapy need to be detected early in order to adjust the therapy regimen if necessary and monitor patients closely.
Algorithms for early detection of ICI therapy–induced myocarditis have already been proposed and include recording cardiovascular symptoms, performing electrocardiography, and determining troponin (7,8). However, additional procedures such as cardiac MRI may be required to establish the diagnosis of myocarditis. Recently, the potential of nuclear imaging to detect, in particular, the cardiac complications of ICI therapy has been recognized. We have already shown in previous work that 18F-FDG PET/MRI is well suited for high-sensitivity detection of myocarditis and for assessment of the course of disease (9). There are preliminary reports demonstrating the utility of 18F-FDG PET in ICI-induced myocarditis. A recent case report demonstrated that 18F-FDG PET/CT can detect ICI-induced myocarditis, which was also manifested by mild troponin elevation, despite unremarkable electrocardiography, echocardiography, and cardiac MRI results (10). Furthermore, 18F-FDG PET was also shown to be suitable for assessing the course of disease in this case.
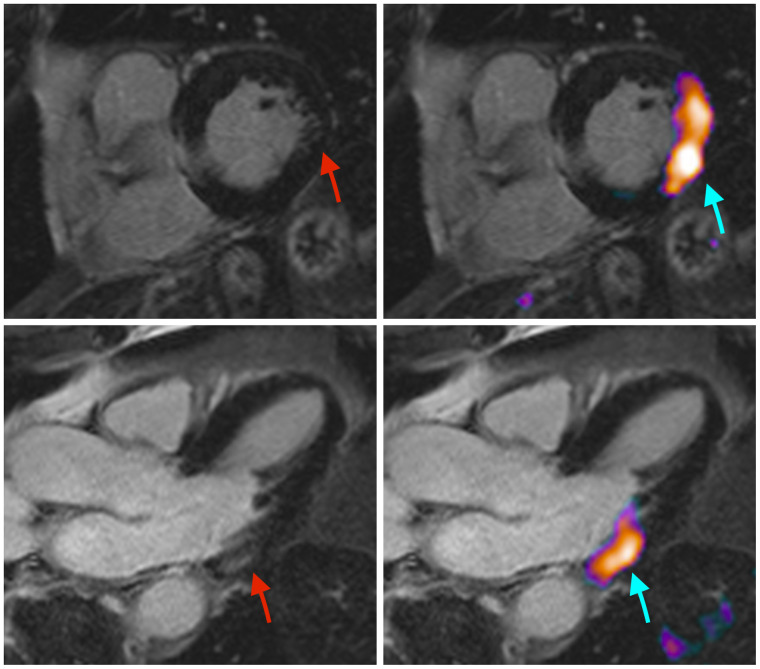
In a recent landmark paper, we demonstrated that PD1 and its ligand, which are the targets of ICI therapy, are strongly expressed on cardiac endothelial cells (11). In a melanoma mouse model, we demonstrated that anti-PD1 therapy causes myocardial infiltration of activated CD4- and CD8-positive T lymphocytes, which led to a significant impairment of left ventricular function under dobutamine stress testing. Furthermore, anti-PD1 ICI therapy significantly affected myocardial metabolism, as evidenced by changes in the proteome and lipidome. Analogous to these findings from the mouse model is that patients receiving anti-PD1 therapy with nivolumab for metastatic melanoma showed an ejection fraction reduction on echocardiography both at rest and under dobutamine stress. In some patients recruited for this study, 18F-FDG PET/MRI detected active ICI-induced myocarditis (Fig. 1), also demonstrating the potential of 18F-FDG PET to detect this irAE. Although the case shown in Figure 1 appears promising, the disadvantage of 18F-FDG PET imaging is that 18F-FDG uptake is relatively nonspecific and that successful imaging depends strongly on patient preparation and cooperation to successfully suppress myocardial glucose metabolism. Accordingly, imaging is compromised in certain patient populations with preexisting conditions (e.g., diabetes mellitus) or under certain medications such as glucocorticoids.
FIGURE 1.
In this patient, clinical suspicion of ICI-induced myocarditis was raised because of elevated troponin. MRI (left) reveals faint late gadolinium enhancement in basal lateral wall (red arrows). 18F-FDG PET/MRI (right) shows clear tracer accumulation in this area (blue arrows), which is indicative of active myocarditis.
An alternative and promising target is the fibroblast activation protein (FAP). FAP is strongly overexpressed in the stroma of various tumors (so-called cancer-associated fibroblasts) and is already used as a target for imaging and radioligand therapy (12,13). However, FAP is also known to be strongly overexpressed in benign remodeling processes of tissue healing and thus can be detected after, for example, an acute myocardial infarction, which could reduce the specificity for imaging inflammation in the setting of irAEs (14,15). Initial work has demonstrated the potential of 68Ga-FAPI PET in a small group of patients with suspected ICI-induced myocarditis (16). In this work, patients with elevated troponin, electrocardiography changes, lymphocytic infiltration of the myocardium on biopsy, and wall motion abnormalities on echocardiography showed increased 68Ga-FAPI uptake compared with ICI-treated patients without evidence of myocarditis. Although the results appear promising, studies with larger patient collectives are warranted, and these initial results should be viewed with caution.
Furthermore, there are other potential radiotracers that show high potential but for which data are lacking to date (e.g., imaging of innervation, somatostatin receptors, mitochondrial damage, or reactive oxygen species). In view of the increasing numbers of ICI therapies, the development of new ICIs, and the improved monitoring of these patients, referrals to nuclear medicine institutes will increase, with the question of irAEs and especially of severe ICI-induced myocarditis.
In conclusion, nuclear imaging may be helpful with regard to the detection of cardiotoxicity from ICI therapies. Cardiooncologic monitoring during therapy for early detection of irAEs includes physical examination, electrocardiography and Holter electrocardiography, cardiac biomarkers, and echocardiography. In patients with suspected ICI-induced myocarditis, further workup may include MRI, nuclear medicine imaging, or cardiac catheterization. Nuclear medicine imaging is characterized by a high sensitivity and may help when there are unclear findings on MRI or echocardiography or can be used for follow-up. However, a broader database is still needed before a final recommendation can be made. In addition, given the complexity of cardiovascular irAEs, interdisciplinary teams should determine treatment, and interdisciplinary guidelines need to be elaborated.
DISCLOSURE
Christoph Rischpler reports a research grant from Pfizer; a consultancy for Adacap and Pfizer; and speaker honoraria from Adacap, Alnylam, BTG, GE Healthcare, Pfizer, and Siemens Healthineers. Matthias Totzeck and Tienush Rassaf report personal fees and other fees from Edwards, Novartis, Bristol Myers Squibb, Bayer, Daiichi Sankyo, and AstraZeneca, outside the submitted work. Lale Umutlu reports speaker honoraria from Bayer, speaker honoraria/research grants from Siemens Healthcare, and a research grant from the German Research Foundation (DFG). Ken Herrmann reports personal fees from Bayer, Sofie Biosciences, SIRTEX, Adacap, Curium, Endocyte, BTG, IPSEN, Siemens Healthineers, GE Healthcare, Amgen, Novartis, ymabs, Aktis Oncology, Theragnostics, and Pharma15; nonfinancial support from ABX; grants from BTG; and other fees from Sofie Biosciences, outside the submitted work. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11:3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schadendorf D, Hodi FS, Robert C, et al.Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Totzeck M, Lutgens E, Neilan TG. Are we underestimating the potential for cardiotoxicity related to immune checkpoint inhibitors? Eur Heart J. 2021;42:1632–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martins F, Sofiya L, Sykiotis GP, et al.Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. [DOI] [PubMed] [Google Scholar]
- 5. D’Souza M, Nielsen D, Svane IM, et al.The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. 2021;42:1621–1631. [DOI] [PubMed] [Google Scholar]
- 6. Salem J-E, Manouchehri A, Moey M, et al.Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahmood SS, Fradley MG, Cohen JV, et al.Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rassaf T, Totzeck M, Backs J, et al.Onco-cardiology: consensus paper of the German Cardiac Society, the German Society for Pediatric Cardiology and Congenital Heart Defects and the German Society for Hematology and Medical Oncology. Clin Res Cardiol. 2020;109:1197–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nensa F, Kloth J, Tezgah E, et al.Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol. 2018;25:785–794. [DOI] [PubMed] [Google Scholar]
- 10. Arponen O, Skyttä T. Immune checkpoint inhibitor-induced myocarditis not visible with cardiac magnetic resonance imaging but detected with PET-CT: a case report. Acta Oncol. 2020;59:490–492. [DOI] [PubMed] [Google Scholar]
- 11. Michel L, Helfrich I, Hendgen-Cotta UB, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J. August 14, 2021. [Epub ahead of print]. [DOI] [PubMed]
- 12. Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferdinandus J, Fragoso Costa P, Kessler L, et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced stage solid tumors: a case series of nine patients. J Nucl Med. August 12, 2021. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 14. Siebermair J, Köhler MI, Kupusovic J, et al.Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J Nucl Cardiol. 2021;28:812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Totzeck M, Siebermair J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur Heart J. 2020;41:1060. [DOI] [PubMed] [Google Scholar]
- 16. Finke D, Heckmann MB, Herpel E, et al.Early detection of checkpoint inhibitor-associated myocarditis using 68Ga-FAPI PET/CT. Front Cardiovasc Med. 2021;8:614997. [DOI] [PMC free article] [PubMed] [Google Scholar]