Abstract
Umami and sweet sensations provide animals with important dietary information for detecting and consuming nutrients, whereas bitter sensation helps animals avoid potentially toxic or harmful substances. Enormous progress has been made toward animal sweet/umami taste receptor (Tas1r) and bitter taste receptor (Tas2r). However, information about amphibians is mainly scarce. This study attempted to delineate the repertoire of Tas1r/Tas2r genes by searching for currently available genome sequences in 14 amphibian species. This study identified 16 Tas1r1, 9 Tas1r2, and 9 Tas1r3 genes to be intact and another 17 Tas1r genes to be pseudogenes or absent in the 14 amphibians. According to the functional prediction of Tas1r genes, two species have lost sweet sensation and seven species have lost both umami and sweet sensations. Anurans possessed a large number of intact Tas2rs, ranging from 39 to 178. In contrast, caecilians possessed a contractive bitter taste repertoire, ranging from 4 to 19. Phylogenetic and reconciling analysis revealed that the repertoire of amphibian Tas1rs and Tas2rs was shaped by massive gene duplications and losses. No correlation was found between feeding preferences and the evolution of Tas1rs in amphibians. However, the expansion of Tas2rs may help amphibians adapt to both aquatic and terrestrial habitats. Bitter detection may have played an important role in the evolutionary adaptation of vertebrates in the transition from water to land.
Keywords: amphibian, anurans, bitter taste receptor, caecilians, umami/sweet taste receptor
Amphibians have the largest number of Tas2rs across vertebrates. The repertoire of amphibian Tas1rs and Tas2rs was shaped by massive gene duplications and losses. Bitter detection played an important role in the evolutionary adaptation of vertebrates in the transition from water to land.
1. INTRODUCTION
Taste (or gustation) is one of the primary mechanisms that animals use to assess the nutritional quality of foods. Vertebrates can perceive five fundamental taste modalities: bitter, umami, sweet, salty, and sour (Kinnamon & Cummings, 1992; Lindemann, 1996, 2000; Stewart et al., 1997; Yarmolinsky et al., 2009). Each taste sense is thought to have evolved to face a challenge or play a specific role in species evolution (Marco & Davide, 2017; Peng et al., 2020). For instance, sweet and umami tastes are associated with the intake of nutrients such as carbohydrates and protein. Conversely, bitter detection prevents animals from ingesting potentially poisonous foods (Herness & Gilbertson, 1999). Sweet, umami and bitter substances are detected by type II taste receptor cells (TRCs) that function through G protein‐coupled receptors (GPCRs; Adler et al., 2000).
Sweet and umami taste are activated by taste 1 receptors (Tas1r), including Tas1r1, Tas1r2 and Tas1r3 (Bufe & Meyerhof, 2006; Li et al., 2002; Temussi, 2006). They are encoded by Tas1r1, Tas1r2, and Tas1r3 genes, respectively. Each Tas1r contains a GPCR‐like domain and a large N‐terminal extracellular domain (Chen et al., 2009). Tas1r3 is co‐expressed with either Tas1r1 or Tas1r2, which form into heterodimers: a Tas1r1‐Tas1r3 heterodimer functions as an umami taste receptor, whereas a Tas1r2‐Tas1r3 heterodimer senses sweet compound (Li et al., 2002; Nelson et al., 2001). Although most vertebrates have three complete Tas1r genes, the repertoires of Tas1rs in other species may differ in both sequence and numbers. Three main processes have shaped the different numbers of Tas1rs in different species: gene loss, pseudogenization, and duplication (Liu et al., 2014). For example, Tas1r1 is a pseudogene in the giant panda (Ailuropoda melanoleuca; Zhao, Yang, et al., 2010) and six pinniped species (Sato & Wolsan, 2012). Tas1r1 is absent, unamplified, or pseudogenized in 31 species of bats examined (Zhao et al., 2012). Tas1r2 is lost in the genome of the zebra finch (Taeniopygia guttata) and chicken (Gallus gallus) and pseudogenized in some carnivore species (Jiang et al., 2012; Li et al., 2005, 2009; Zhao, Zhou, et al., 2010). In reptiles, Tas1r genes possibly have been lost or pseudogenized in snakes; in testudines and crocodilians, Tas1r genes are either intact or partial (Feng & Liang, 2018). These mutations are thought to result in the disfunction of Tas1r and then affect sweet/umami recognition in animals. Nevertheless, a lineage‐specific increase in the number of Tas1rs has been described in fish. In 15 fish species examined, the number of Tas1r2 genes differs widely, ranging from 1 to 4 (Dong et al., 2018). To date, several studies have investigated the ecological factors that drive the evolution history of Tas1rs in vertebrates. The evolution of Tas1r genes is sometimes explained by feeding ecology (Zhao, Zhou, et al., 2010), sometimes inconsistent with dietary differences (Feng & Liang, 2018; Feng & Zhao, 2013; Zhao et al., 2012; Zhao & Zhang, 2012).
Bitter substances are recognized by taste 2 receptors encoded by the Tas2r gene family (Adler et al., 2000). Tas2r genes possess a GPCR‐like domain and a short extracellular N‐terminus. They are ~900 bp and lack introns. Several studies have indicated that the repertoire of Tas2rs showed a very dynamic evolution among species. For instance, the number of intact (functional) Tas2rs is subject to intense variation: 0 in cetaceans and penguins (Feng et al., 2014; Zhu et al., 2014), 25 in humans (Go et al., 2005; Shi et al., 2003), 3–50 in lizards (Zhong et al., 2017, 2019), 1–5 in teleost (Dong et al., 2018), and 80 in the coelacanth (Latimeria chalumnae; Syed & Korsching, 2014). Not surprisingly, there are varying numbers of pseudogenes among species. Pseudogenization has occurred in almost all Tas2rs in cetaceans (Feng et al., 2014; Zhu et al., 2014).
Numerous studies have attempted to explain the evolutionary and ecological significance of Tas2rs. It is generally accepted that the taste receptor gene family has undergone a complex evolutionary process. They are susceptible to gene duplication, gene deletion, pseudogenization, positive selection, and other factors, resulting in the expansion or contraction of specific gene families among different evolution branches (Dong et al., 2009; Go, 2006; Hayakawa et al., 2014; Li & Zhang, 2014; Shi et al., 2003; Wang & Zhao, 2015). This complexity is assumed to reflect the evolutionary needs of the respective species. For instance, the relationship between the Tas2r numbers of vertebrates and their corresponding dietary habits has been addressed. Actually, some studies have uncovered the probable correlation between diets and Tas2r numbers: herbivores and insectivores who encounter bitter substances more frequently possess more Tas2rs than carnivores (Hu & Shi, 2013; Jiang et al., 2012; Liu et al., 2016; Wang & Zhao, 2015; Zhong et al., 2017). These findings have suggested that the dietary toxin content is one of the primary selective forces for the differences in Tas2rs repertoires among species.
Other than dietary habits, foraging patterns may also affect the repertoires of Tas2r genes. Previous studies have indicated that cetaceans (0–1; Zhu et al., 2014; Feng et al., 2014), snakes (1–2; Zhong et al., 2017), and penguins (0; Zhao et al., 2015) possess a dramatic contraction in the number of functional Tas2r genes. Their behavior of swallowing food whole without mastication reduces the contact of the TRCs with bitter stimuli, resulting in less contact with poisonous foods.
So far, studies on the function and adaptive evolution of Tas1r/Tas2r genes have been carried out extensively in many species. However, data are quite limited in Tas1r/Tas2r families of amphibians, except for the western clawed frog (Behrens et al., 2014; Go, 2006; Shi & Zhang, 2006). Amphibian is the transition lineage from aquatic lifestyle to a terrestrial one in the history of vertebrate evolution and plays an important role in animal evolution. To gain extensive, systematic, and efficient evolution research on Tas1r/Tas2r, it was considered worthwhile to investigate more data and adaptive evolution of amphibian species. Therefore, this study aimed to examine the repertoires of Tas1r/Tas2r genes in amphibians and predict their functionality using available genome assemblies. This study recovered the phylogenetic relationship and determined the duplication and loss events of Tas2rs to understand their birth‐and‐death process in this evolutionary group. Furthermore, the selective pressure of Tas1rs in amphibians was estimated.
2. MATERIALS AND METHODS
2.1. Taxonomic sampling of genome data
Class Amphibia is generally classified into three orders: Anura (anurans), Caudata (urodeles), and Gymnophiona (caecilians). This study focused on currently available genomes of 14 species from the National Center for Biotechnology Information (NCBI) databases. They are Leishan spiny toad (Leptobrachium leishanense), Mexican spadefoot toad (Spea multiplicata), African bullfrog (Pyxicephalus adspersus), American bullfrog (Lithobates catesbeianus), common frog (Rana temporaria), Tibetan Plateau frog (Nanorana parkeri), Eastern banjo frog (Limnodynastes dumerilii), strawberry poison frog (Oophaga pumilio), Asiatic toad (Bufo gargarizans), western clawed frog (Xenopus tropicalis), and African clawed frog (Xenopus laevis) in Anura and two‐lined caecilian (Rhinatrema bivittatum), Gaboon caecilian (Geotrypetes seraphini), and tiny cayenne caecilian (Microcaecilia unicolor) in Gymnophiona. The detailed information of the genome assemblies is provided in Appendix 1. The N50c values of the genomes ranged from 2.9 kb to 20.7 Mb, implying high‐quality assemblies.
2.2. Gene annotation
As Tas2r genes contain no introns, and most have a similar gene length of ~900 bp, gene annotation was performed by sequence alignment with TBlastN (Altschul et al., 1990), which was implanted in TBtools (Chen et al., 2020). First, previously known Tas2r protein sequences were retrieved from the GenBank or literature and used as initial queries (25 from human, 35 from mouse, 3 from chicken, and 80 from coelacanth). Second, the queries were used to blast against a genome assembly by TBlastN (Altschul et al., 1990), with an e‐value of 1 × 10−10. BLAST hits of <100 bp were discarded, and the overlapping hits were merged. The remaining records were prolonged for 500 bp in both 5′ and 3′ directions, which were regarded as the genomic locations of the homologous genes. Third, genomic nucleotide sequences were extracted as candidate Tas2r genes. The outputs were divided into three categories: intact genes, partial genes, and pseudogenes according to a previous study (Li & Zhang, 2014). Intact genes refer to sequences with >270 amino acids with both start and stop codons. Partial genes refer to sequences that lack either a start or a stop codon. They may be complete genes, but their open reading frames (ORFs) are truncated due to incomplete genome sequencing or assembling. The homology of partial genes in their corresponding genome was analyzed through alignment. If multiple fragment sequences of the same species can be aligned with overlapping regions, they are considered to be from different gene loci. If there are no overlapping regions during alignment, they are considered to be from the same gene site, which may be caused by sequence spacing due to sequencing or assembly. Sequences with premature stop codons and/or ORF‐disrupting mutations were regarded as pseudogenes.
As Tas1r genes contain introns, a more complex bioinformatic pipeline was employed. First, previously known Tas1r1, Tas1r2, and Tas1r3 protein sequences were used as queries to identify the genomic locations of homologous genes in a genome. Second, genomic DNA sequences were extracted and used to perform pairwise alignments with query protein sequences by Genewise (Madeira et al., 2019), which provided the exon/intron structures and frameshifting errors. When receiving negative BLAST results, synteny analysis was performed to examine Tas1r genes with closely related species as the reference. If neighboring genes flanking Tas1r genes could be found, Tas1r genes were regarded as absent.
The obtained protein sequences of each Tas1r/Tas2r were verified by the TMHMM method (Krogh et al., 2001) for the presence of seven transmembrane domains. A Tas1r/Tas2r sequence was regarded as a pseudogene if it did not have seven transmembrane domains. In addition, annotated sequences were examined by reciprocal SmartBLAST (https://blast.ncbi.nlm.nih.gov/blast/smartblast/) as well as phylogenetic analyses to ensure that the best hits are known Tas1r/Tas2r genes. The gene nomenclature was named with a four‐letter prefix corresponding to the species names as well as a numerical suffix consecutively. For example, the Tas2r1 gene of L. leishanense is referred to as Lele_Tas2r1.
2.3. Phylogeny of taste receptor genes in amphibians
To explore the evolutionary relationship among Tas1r/Tas2r genes in amphibians, a phylogenetic analysis of intact genes was performed. Partial genes or pseudogenes were not included in the phylogenetic analysis due to a large number of gap sites after alignments. Multiple sequence alignments of amino acid sequences were performed by MAFFT with the L‐INS‐I strategy (Katoh et al., 2002). GBLOCKS (Castresana, 2000) was then used to optimize the quality of alignment results. The selected conserved region was used in the following analysis. The phylogenetic relationship of the genes was inferred by the maximum likelihood (ML; Dempster, 1977) method. ML phylogenies were inferred using IQ‐TREE (Lam‐Tung et al., 2015) under the model selected by ModelFinder (Kalyaanamoorthy et al., 2017). The branch support analysis was evaluated with 1000 ultrafast bootstraps (Minh et al., 2013). The tree was rooted with vertebrate V1R/V2R vomeronasal receptor. The procedures processed in ML phylogenies were all implemented in PhyloSuite (Zhang et al., 2020). The visualization of ML tree was performed using iTOL (Letunic & Bork, 1988).
2.4. Reconstruction of Tas2r repertoire evolution
Large‐scale gene births and deaths are the major forces of functional genetic innovation. To infer the history of births (duplication) and deaths (deletion) of Tas2r genes across the amphibian phylogeny, a reconciliation analysis was performed with NOTUNG 2.6 (Chen et al., 2000). This method estimates the history of gene duplication and deletion times by comparing gene tree with species tree. The species tree was estimated from the TimeTree database, which provided the generally accepted phylogenetic tree (Hedges et al., 2006). All birth‐and‐death events of Tas2rs were placed in each branch of the species tree to show the evolutionary trajectories of Tas2r repertoires in Amphibia.
2.5. Adaptive evolution analysis of Tas1r genes
The selective pressure of Tas1r genes was tested by two steps. First, the number of nonsynonymous substitutions per nonsynonymous site (dN) and the number of synonymous substitutions per synonymous site (dS) were used to compute overall ω (dN/dS) values. ω = 1, ω < 1, and ω > 1 represent neutral, purifying, and positive selection, respectively. The mean ω for each Tas1r was calculated by the CodeML method (Yang, 2007) with EasyCodeML (Gao et al., 2019). The generally accepted phylogenetic tree was inferred from TimeTree (Hedges et al., 2006). Moreover, positive selection could act on individual amino acid residue. Therefore, in the second step, codon‐based analyses with CodeML and FUBAR (Murrell et al., 2013) were performed to detect potential positive selection sites. In CodeML, the models between M7 (purifying selection) with M8 (positive selection) with EasyCodeML were compared. A likelihood ratio test was used to estimate whether there was a significant difference between the models. FUBAR analysis on the Datamonkey server (http://classic.datamonkey.org/; Pond et al., 2005) was used to find evidence of episodic positive/diversifying selection with a posterior probability of 0.9.
3. RESULTS
3.1. Tas1r and Tas2r repertoires
This study annotated 16 Tas1r1, 9 Tas1r2, and 9 Tas1r3 intact sequences that appear to be functional genes (Table 1). One truncated Tas1r1 and Tas1r2 in common frog and one truncated Tas1r3 in Tibetan Plateau frog were also identified as pseudogenes (see Appendix 2 for the genomic location). We failed to identify the Tas1r1 gene from genome assemblies of the western clawed frog and the African clawed frog. Thus, synteny analysis was performed to examine whether Tas1r is lost or not. Tas1r1 is flanked by NOL9 and ZBTB48. This linearity is conserved across human, mouse, and two‐lined caecilian. The presence of NOL9 and ZBTB48 next to Tas1r1 was confirmed, providing evidence of whole Tas1r1 deletion in the two taxa (Appendix 3). Genes that flank Tas1r2 in mice and most species surveyed (MIB2, GOLIM4) were located on the same contig in American bullfrog, Tibetan Plateau frog, African clawed frog, western clawed frog, and Gaboon caecilian. In the genome of the two‐lined caecilian, Tas1r3 is flanked by DVL1 and CPTP. DVL1 and CPTP were adjacent to each other on the same contig in Leishan spiny toad, African bullfrog, American bullfrog, strawberry poison frog, African clawed frog, and western clawed frog (Appendix 3). Thus, it was speculated that perhaps the Tas1r2/Tas1r3 gene is lost in the respective species. Hence, the absence could lead to the inactivation of both umami and sweet taste functions in Leishan spiny toad, African bullfrog, American bullfrog, strawberry poison frog, Asiatic toad, western clawed frog, and African clawed frog and the loss of sweet taste function in Tibetan Plateau frog and tiny cayenne caecilian (Table 1). In addition, multiple copies of Tas1r1 genes in five amphibian genomes were found. Nevertheless, duplication of Tas1r2 only occurred in the common frog, and duplication of Tas1r3 only occurred in common frog, Tibetan Plateau frog, and tiny cayenne caecilian.
TABLE 1.
Summary of Tas1r gene family and functional prediction of umami/sweet taste in amphibian
| Name | Species | Order | Tas1r1 | Tas1r2 | Tas1r3 | Umami (Tas1r1‐Tas1r3) | Sweet (Tas1r2‐Tas1r3) |
|---|---|---|---|---|---|---|---|
| Leishan spiny toad | Leptobrachium leishanense | Anura | 1 | 1 | 0 | × | × |
| Mexican spadefoot toad | Spea multiplicata | Anura | 2 | 1 | 1 | √ | √ |
| African bullfrog | Pyxicephalus adspersus | Anura | 2 | 1 | 0 | × | × |
| American bullfrog | Lithobates catesbeianus | Anura | 2 | 0 | 0 | × | × |
| Common frog | Rana temporaria | Anura | 2(1PS) | 2(1PS) | 2 | √ | √ |
| Tibetan Plateau frog | Nanorana parkeri | Anura | 2 | 0 | 2 (1PS) | √ | × |
| Eastern banjo frog | Limnodynastes dumerilii | Anura | 1 | 1 | 1 | √ | √ |
| Strawberry poison frog | Oophaga pumilio | Anura | 1 | 1 | 0 | × | × |
| Asiatic toad | Bufo gargarizans | Anura | 1 | 1 | 0 | × | × |
| African clawed frog | Xenopus laevis | Anura | 0 | 0 | 0 | × | × |
| Western clawed frog | Xenopus tropicalis | Anura | 0 | 0 | 0 | × | × |
| Two‐lined caecilian | Rhinatrema bivittatum | Gymnophiona | 1 | 1 | 1 | √ | √ |
| Gaboon caecilian | Geotrypetes seraphini | Gymnophiona | 1 | 0 | 1 | √ | × |
| Tiny cayenne caecilian | Microcaecilia unicolor | Gymnophiona | 1 | 1 | 2 | √ | √ |
√, putative function; ×, putative disfunction.
Abbreviation: PS, Pseudogene.
This study annotated 1400 Tas2r genes in amphibian genome assemblies, including 1156 intact, 30 partial, and 214 pseudo Tas2rs (Figure 1). The genomic locations of each Tas2r gene are uploaded in Zenodo (https://doi.org/10.5281/zenodo.5642517). Each species in Gymnophiona possessed a medium‐sized Tas2r repertoire, ranging from 4 to 19. In sharp contrast, the Tas2rs number in order Anura was from 44 to 178. The American bullfrog presented the largest number of Tas2r genes (n = 178) not only in amphibians but also for any species investigated so far. The nucleotide length of intact genes was 816–1272 bp, with an average of 942 bp. The number of partial genes was from 0 to 14. The number and the percentage of pseudogenes ranged from 1 to 47 and from 5.0% to 33.3%, respectively. Overall, it showed a considerable variance in the number of intact genes between the two orders in amphibians. The Tas2r gene repertoire size in Anura species was much larger than in not only Gymnophiona but also other vertebrate groups.
FIGURE 1.
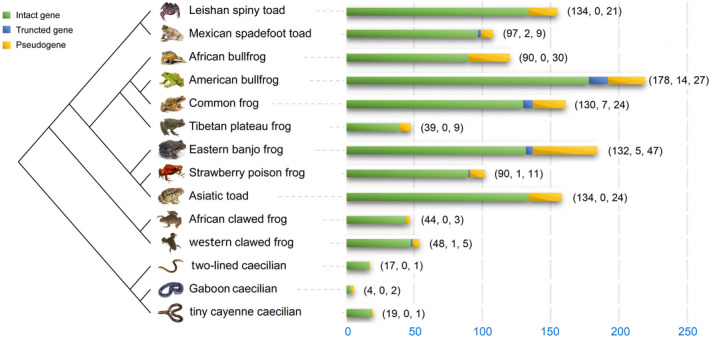
Numbers of intact, partial, and pseudo Tas2r genes in 14 amphibian species. The numbers in brackets denote intact genes, partial genes, and pseudogenes, respectively
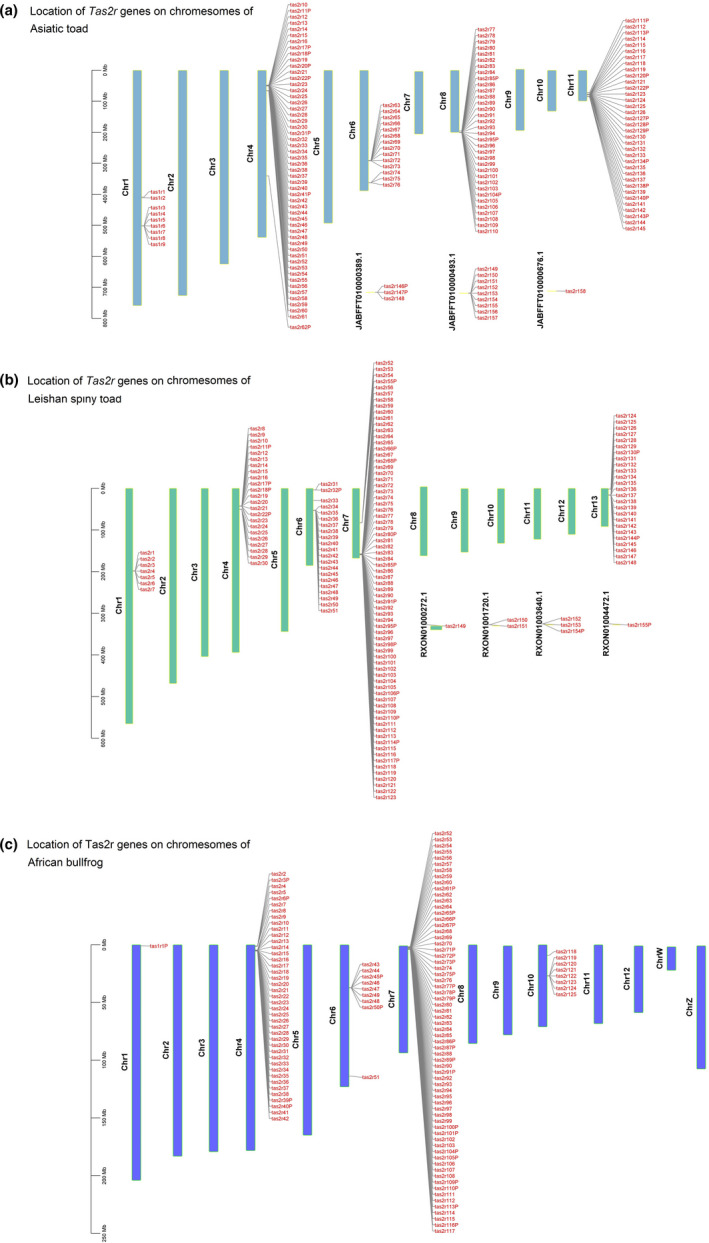
To detect whether tandem duplication happens in amphibians, the genomic location of Tas2r genes was examined. Indeed, some Tas2rs were organized into clusters on specific chromosomes or scaffolds (Appendix 4). For example, Tas2r genes were mainly located on chromosomes 4, 6, 7, and 13 in Leishan spiny toad, chromosomes 4 and 7 in African bullfrog, and chromosomes 4, 8, and 11 in the Asiatic toad. Interestingly, Tas1rs also occurred in neighboring intergenic regions. For instance, multiple copies of the Tas1r1 gene of Mexican spadefoot toad, African bullfrog, common frog, and Tibetan Plateau frog were located in the same chromosome or scaffold of each species. Similar results were also found in Tas1r3 (Appendix 2). These results suggested that tandem duplications of Tas1rs/Tas2rs could be one cause of the expansion of the two gene families.
3.2. Phylogeny of Tas1r and Tas2r genes
To delineate the evolutionary history and relationships among amphibian Tas1r/Tas2r genes, phylogenetic analysis by the ML method based on all intact genes was performed. Figure 2 shows the phylogenetic relationship of intact Tas1r genes. Most branches in the tree showed high bootstrap support, indicating the reliability of phylogenetic relationships among Tas1rs. Clusters of Tas1r1, Tas1r2, and Tas1r3 could separate from each other. Each gene cluster formed into two clades, including anurans and caecilians.
FIGURE 2.
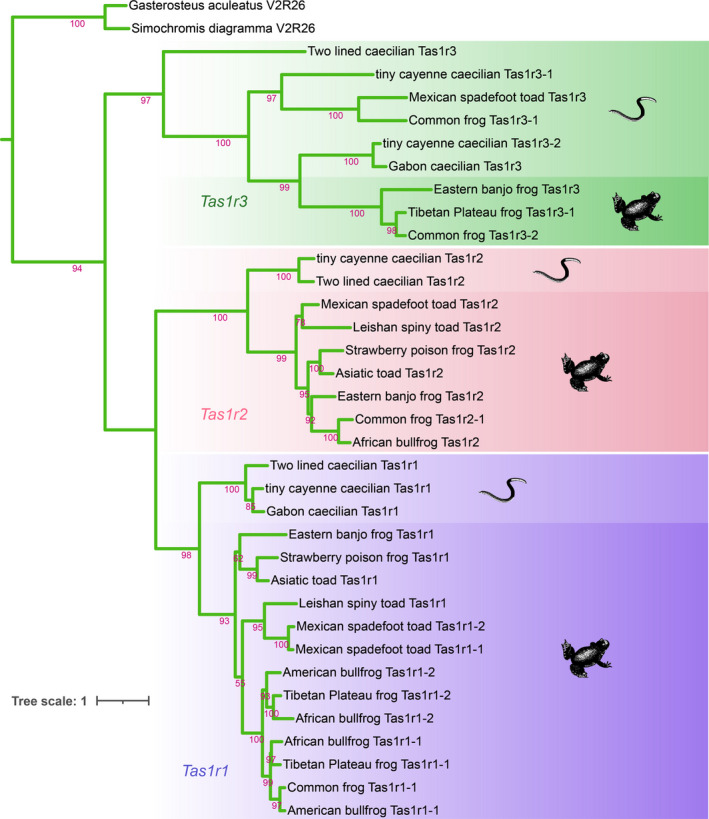
Evolutionary relationships of intact Tas1r genes in amphibians. The phylogenetic tree was constructed using the ML method. Phylogeny was rooted with the vomeronasal 2 receptor 26 gene (V2R26) of two fish species Gasterosteus aculeatus (NCBI accession no. XM_040193080.1) and Simochromis diagramma (NCBI accession no. XM_040009575.1). This is because V2R genes are relatively close to Tas1r genes among GPCRs. The numbers at the branches indicate the percentage of posterior probability values
Based on the phylogenetic tree, intact Tas2r genes were categorized into five large and eight small clades (Figure 3). Some lineages showed a cluster of Tas2r genes from the same species (marked with one color), suggesting that these lineages are enriched with species‐specific gene duplications. In contrast, other lineages showed genes from distantly related species.
FIGURE 3.
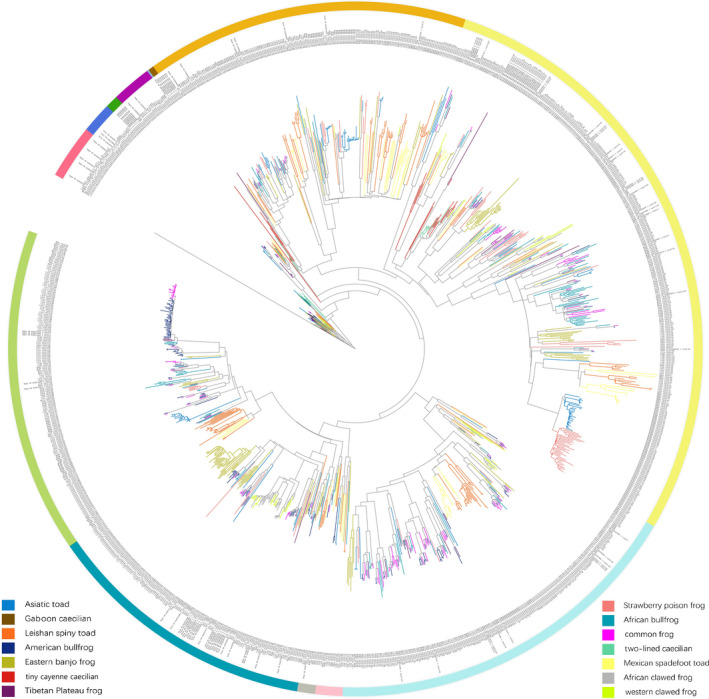
Evolutionary relationships of 1156 intact Tas2r genes in amphibians. The phylogenetic tree was constructed using the ML method. The vomeronasal 1 receptor 3 gene (V1R3; NCBI accession no. AB670529) of East African cichlids (Lithochromis xanthopteryx) was used to root the tree because V1R genes are relatively close to Tas2r genes among GPCRs. Genes from different species are indicated by different colors of branches
3.3. Lineage‐specific gene births and deaths of Tas2rs
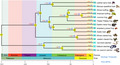
The phylogeny of Tas2r genes implies that extensive gene expansions may have occurred in the Anura lineage or the contractions took place in the Gymnophiona lineage. To infer the evolutionary changes of Tas2r numbers in amphibians, a reconciliation analysis was performed by comparing the gene tree and species tree. Results showed that total duplications and losses were 851 and 555, respectively (Figure 4). Overall, frequent and dramatic gene birth and death events occurred in almost each branch. Conservatively, results indicated that the common ancestor of amphibians had at least 18 intact Tas2rs. After a gene gain (n = 33) and loss (n = 8) in the ancestral lineage of Anura, the Tas2rs number of the common ancestor of Anura increased to 43. Moreover, further reductions (−7, −5) were observed in the branch of Gymnophiona. Because the increase occurred in Anura (43 intact Tas2rs) compared to Gymnophiona (15 intact Tas2rs), data suggested that the reduction of Tas2rs may have occurred before the divergence between Anura and Gymnophiona. As shown in the evolutionary trajectory tree, extensive species‐specific gene duplications may be responsible for the considerably larger Tas2r repertoires in some anurans, for instance, 92 gains in Leishan spiny toad and 81 gains in the Asiatic toad (Figure 4).
FIGURE 4.
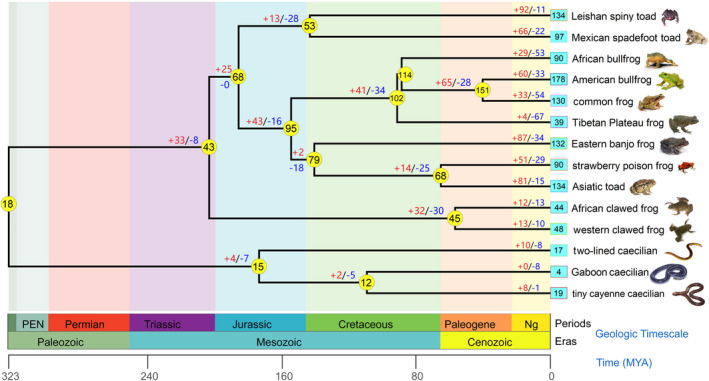
Evolutionary trajectories of amphibian Tas2r gene repertoires. The numbers in circles and boxes denote the number of intact Tas2rs. The numbers on branches denote gene increases (+; caused by gene duplication) and decreases (−; caused by gene deletion). For example, Leishan spiny toad gained 92 Tas2rs and lost 11 Tas2rs after branching off from its common ancestor with the Mexican spadefoot toad. The phylogenetic relationships and divergence times of these species were referred to TimeTree (Hedges et al., 2006)
3.4. Purifying selection in the Tas1r gene family
To understand the selective pressure of the Tas1r gene family, the ratio (dN/dS) of nonsynonymous mutation rate (dN) to synonymous mutation rate (dS) was calculated. All Tas1r genes were under strong purifying selection (0.169–0.210; Table 2). This result indicated that Tas1rs were evolving‐constrained, and the function was conserved in the species, which remained as the relevant Tas1r genes. A small number of positively selected sites were found in each gene interspecies.
TABLE 2.
Selection Analysis of Amphibian Tas1rs
| Gene | Number of sequences a | aa Length | dN/dS | Positively selected sites b | Negatively selected sites b |
|---|---|---|---|---|---|
| Tas1r1 | 16 | 557 | 0.169 | 3 | 428 |
| Tas1r2 | 9 | 450 | 0.187 | 7 | 302 |
| Tas1r3 | 9 | 620 | 0.210 | 6 | 369 |
Number of sequences for each Tas1r gene.
Positively/negatively selected sites were detected with CodeML (M7/M8) and FUBAR methods (posterior probability = 0.9).
4. DISCUSSION
Studies on umami, sweet, and bitter taste receptors have made enormous progress in recent years. Despite the special evolutionary status of amphibians, their taste receptor families have been rarely described except for the western clawed frog (Behrens et al., 2014; Go, 2006; Shi & Zhang, 2006). In this study, the repertoire of Tas1r and Tas2r genes from a wide collection of amphibian species was presented for the first time. Unlike the conservation of only one copy of Tas1r1/Tas1r2/Tas1r3 in numerous vertebrates (Shi & Zhang, 2006), two copies of the Tas1r gene in several amphibian species were found. Similarly, duplication events of the Tas1r gene (n = 1–4) were also reported in several teleost (Dong et al., 2018). The result may support the fact that amphibians diverge from the ancestral fish‐tetrapod stock during the evolution of animals from strictly aquatic forms to terrestrial types. This study confirmed previous findings and showed that all three Tas1rs were absent in the western clawed frog (Shi & Zhang, 2006). Note that the annotations of the Tas1r gene in the genome database are sometimes incorrect. For example, V2R genes of the African clawed frog and the western clawed frog were annotated as Tas1r1 in the NCBI probably due to the sequential similarity of the two GPCR families. To ensure our prediction accuracy, annotated genes were verified in the genome database with reciprocal BLAST, synteny analysis, and phylogenetic analyses.
Sweet and umami tastes help animals to recognize dietary information for nutritious carbohydrates and proteins, respectively, and thus are pivotal for the survival of animals. However, given the similarity of feeding preferences in amphibians and their distinct phylogenetic positions, this study failed to discover a correlation between feeding ecology and Tas1r evolution. Most of the dietary preference of anurans are similar to each other, but their Tas1r genes can be intact, pseudogenized, or absent, suggesting that no correlation exists between Tas1r functionality and feeding ecology. It seemed that loss of umami/sweet tastes could occur in any species. This study found that Tas1r1 genes of the African clawed frog are absent from its genome assembly. Surprisingly, robust glossopharyngeal nerve responses have been recorded in amphibians when various amino acids are applied to taste organs on the tongue (Feder & Burggren, 1992; Gordon & Caprio, 1985; McPheeters & Roper, 1985; Yoshii et al., 1982). The African clawed frog has been reported to have high gustatory sensitivity to amino acid, such as arginine (0.1–1.0 μM; Yoshii et al., 1982). The above contradiction between the absence of Tas1r1 genes and amino acid sensitivity could be explained by the following evidence. Although Tas1r1+3 functions as the main umami receptor in mammals, non‐Tas1r1 genes responsible for detecting amino acids likely exist. For example, an odorant receptor, preferentially tuned to recognize basic amino acids, was identified in goldfish (Speca et al., 1999). The odorant receptor shares sequence similarities with calcium sensing, metabotropic glutamate, and V2R vomeronasal receptors (Speca et al., 1999). To the authors’ knowledge, the western clawed frog has the largest V2R repertoire in 14 species investigated (Urszula Brykczynska et al., 2013; Chen et al., 2019; Shi & Zhang, 2007). It was supposed that a mass of V2Rs may be involved in detecting amino acids. Analysis of gustatory nerve responses in metabotropic glutamate receptor 4 (mGluR4) knockout mice provided functional evidence for the involvement of mGluR4 in umami taste responses (Yasumatsu et al., 2014). As for sweet taste, research on gustatory transduction in taste cells demonstrated that the ability to detect sweet substances is present in frogs (Kusano & Sato, 1958; Toshihide et al., 1995). The cAMP or cGMP cascade may be involved in the transduction of sweet stimuli in bullfrog TRCs (Kolesnikov & Margolskee, 1995). It would be interesting to examine whether there are other transduction mechanisms involved in the umami/sweet sensation of amphibians.
This study reported the largest Tas2rs family for any species so far. Meanwhile, the number of Tas2rs genes (especially intact genes) varies greatly among different amphibian species. Although the African clawed frog, derived from the diploid species western clawed frog, has undergone a whole‐genome duplication (WGD) event to be a tetraploid species, it possesses a moderate size of Tas1r/Tas2r repertoire compared to other amphibians. This finding suggests that WGD may not have played a major role in the evolution of the amphibian Tas1r/Tas2r repertoire.
A large repertoire of Tas2rs in amphibians likely reflects their adaptation to variable lifestyles and environments. Most frogs and toads inhabit both aquatic and terrestrial habitats, which necessarily contain a larger variety of toxic substances. Accordingly, their ecological needs should encompass vastly different requirements for their taste system. For instance, aquatic factors, such as pH were proven to have influenced the divergence of taste receptor genes (Caprio et al., 2014; Lin et al., 2004). A great number of Tas2rs could also fulfill their dietary needs. Although some larger amphibian species eat vertebrates, most frogs feed on worms, insects, and other small arthropods that contain more potentially toxic substances.
Aside from environmental selections, amphibians’ dietary preferences may change. On the one hand, the diet of some anurans tends to vary ontogenetically. For instance, the metamorphic transition from aquatic larvae to terrestrial adults imposes dietary shifts. Larval anurans are almost exclusively microphagous herbivores or detritivores (Altig et al., 2007; Duellman & Trueb, 1986; Montaa et al., 2019; Wassersug & Heyer, 1988). After metamorphosis, most anurans become insectivores (Duellman & Trueb, 1986). Plant‐eating frogs are scant in the literature and include only Bufo marinus, Bufo regularis, Rana esculenta, and Rana hexadactyla (Da Silva & De Britto‐Pereira, 2006). Interestingly, the hylid frog Xenohyla truncata is unique among frogs with its frugivorous feeding biology (Da Silva et al., 1989; Da Silva & De Britto‐Pereira, 2006). Among these diets, plant materials are rich in bitter substances (Glendinning, 1994; Wang et al., 2004), and insects can secrete defensive poisonous chemicals (always tastes bitter) to deter predators (Howse, 1975). Hence, bitter tasting compounds should have strongly affected the diversity of the Tas2rs repertoire in herbivorous and insectivorous amphibians. On the other hand, diet tends to vary seasonally along with prey availability (Donnelly, 1991). As anurans are primarily visual, opportunistic predators (sit‐and‐wait foraging), their selection of prey is limited by the gape of the predator. As a result, the biological importance of bitter taste likely resides in the ability to detect and reject unpalatable, potentially dangerous prey once it is captured, rather than detecting prey (Barlow, 1998). Only after visually selected prey have reached the mouth, does the taste system function as a toxin detector such that unpalatable and potentially poisonous food is spat out. Hence, we speculate that the lifestyle, ecological, and dietary complexity of amphibians elevate their evolutionary pressure for a wide variety of Tas2r genes. The large number of Tas2rs is consistent with anatomical evidence showing more taste buds and a larger number of taste receptors in amphibians than other vertebrates (Kinnamon & Cummings, 1992; Kinnamon & Margolskee, 1996; Lindemann, 1996). Terrestrial and aquatic anurans have enlarged, specialized organized taste disks that often are found atop large epithelial papillae (Barlow, 1998; Reutter & Witt, 1993), perhaps contributing to specific adaptations for tasting in air and water. Moreover, large numbers of Tas2rs may be answered by the exquisite sensitivity and tuning properties in amphibians. Behrens et al. detected the tuning breadth of six Tas2rs of the western clawed frog with 46 bitter compounds. Their results showed that three Tas2rs recognize numerous agonists, whereas the other three Tas2rs are narrowly tuned. That said, a large Tas2r repertoire of the western clawed frog may allow the development of specialized receptors, possibly for toxins with species‐specific relevance (Behrens et al., 2014).
Caecilians are highly adapted for a burrowing existence. They primarily dwell in highly organic, friable surface layers of the soil, where they maintain tunnel systems. As far as is known, all caecilians are carnivores. Free‐ranging diet includes earthworms, platyhelminths, arthropods, frog eggs, tadpoles, and anoline lizards (Bogert, 1970; Daniel, 1998; Wake, 1994). Because animal tissues contain fewer toxic chemicals, it implies reduced importance of bitter taste in caecilians compared with anurans.
In general, this study characterized Tas1r/Tas2r genes and investigated their evolution. It will not only provide abundant raw data but also further recover the evolution dynamics of Tas1r/Tas2r genes. In particular, studying these genes in the large‐scale evolutionary unit can reflect the evolutionary process more comprehensively and systematically. It will also help provide accurate data support for research on function and feeding behavior.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Huaming Zhong: Conceptualization (lead); Data curation (lead); Methodology (lead). Jie Huang: Data curation (equal); Methodology (equal). Shuai Shang: Data curation (equal); Methodology (equal). Baodong Yuan: Funding acquisition (lead); Project administration (lead).
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation of Henan Province (grant number: 182300410071) and Doctoral Research Foundation of Shangqiu Normal University (grant number: 7001‐700218).
APPENDIX 1.
Whole‐genome assemblies analyzed in the present study
| Name | Species | Family | Prefix a | Assembly | N50c b | Genome coverage (×) |
|---|---|---|---|---|---|---|
| Leishan spiny toad | Leptobrachium leishanense | Megophryidae | Lele | GCA_009667805.1 | 1,946,319 | 80.3 |
| Mexican spadefoot toad | Spea multiplicata | Pelobatidae | Spmu | GCA_009364415.1 | 30,692 | 21 |
| African bullfrog | Pyxicephalus adspersus | Pyxicephalidae | Pyad | GCA_004786255.1 | 30,445 | 189 |
| American bullfrog | Lithobates catesbeianus | Ranidae | Lica | GCA_002284835.2 | 5,415 | 66 |
| Common frog | Rana temporaria | Rate | aRanTem1.1 | 2,889 | 68 | |
| Tibetan Plateau frog | Nanorana parkeri | Dicroglossidae | Napa | GCA_000935625.1 | 32,798 | 83 |
| Eastern banjo frog | Limnodynastes dumerilii | Limnodynastidae | Lidu | GCA_011038615.1 | 10,550 | 156 |
| Strawberry poison frog | Oophaga pumilio | Dendrobatidae | Oopu | GCA_009801035.1 | 5,836 | 136 |
| Asiatic toad | Bufo gargarizans | Bufonidae | Buga | GCA_014858855.1 | 1,738,317 | 103 |
| African clawed frog | Xenopus laevis | Pipidae | Xela | GCA_001663975.1 | 19,713 | 30 |
| Western clawed frog | Xenopus tropicalis | Xetr | GCA_000004195.4 | 14,634,335 | 111.5 | |
| Two‐lined caecilian | Rhinatrema bivittatum | Rhinatrematidae | Rhbi | GCA_901001135.1 | 3,216,284 | 43 |
| Gaboon caecilian | Geotrypetes seraphini | Dermophiidae | Gese | GCA_902459495.1 | 20,656,571 | 67 |
| Tiny cayenne caecilian | Microcaecilia unicolor | Siphonopidae | Miun | GCA_901765105.1 | 3,661,507 | 53 |
The prefix is used to discriminate the name of orthologs in species. For example, Leptobrachium leishanense Tas2r1 is referred to as LeleTas2r1.
N50c is the contig length where 50% of the assembled genome lies in blocks of at least N50C.
APPENDIX 2.
The GenBank accession numbers or genomic locations of the Tas1r genes in this study
| Species | Tas1r1 | Tas1r2 | Tas1r3 |
|---|---|---|---|
|
Leishan spiny toad (Leptobrachium leishanense) |
CM019073.1: 49019172–49026858 | CM019073.1: 22996556–23009832 | No BLAST results |
|
Mexican spadefoot toad (Spea multiplicate) |
Tas1r1‐1: VKOC01000007.1: 19288589–19294065 Tas1r1‐2: VKOC01000007.1: 19294512–19300129 |
VKOC01000007.1: 14768290 14774206 | VKOC01000007.1: 14776162–14790438 |
|
African bullfrog (Pyxicephalus adspersus) |
Tas1r1‐1: CM016426.1: 53245503–53258080 Tas1r1‐2: CM016426.1: 53263053–53272308 |
CM016426.1: 4957042–44966384 | No BLAST results |
|
American bullfrog (Lithobates catesbeianus) |
Tas1r1‐1: KV949322.1: 15569–39104 Tas1r1‐2: KV954354.1: 16846–37639 |
No BLAST results | No BLAST results |
|
Common frog (Rana temporaria) |
Tas1r1‐1: Chr10: XM_040326700.1 Tas1r1‐2_PS: Chr10: XM_040326949.1 |
Tas1r2‐1: Chr10: XM_040326656.1 Tas1r2_PS: Chr10: XM_040326657.1 |
Tas1r3‐1: Chr10: XM_040325817.1 Tas1r3‐2 Chr10: XM_040325818.1 |
|
Tibetan Plateau frog (Nanorana parkeri) |
Tas1r1‐1: NW_017306273.1: XM_018576608.1 Tas1r1‐2: |
No BLAST results |
Tas1r3‐1: NW_017307970.1: XM_018571215.1 Tas1r3‐2_PS: |
|
Eastern banjo frog (Limnodynastes dumerilii) |
WWET01001477.1: 12319–39339 | WWET01002568.1: 114560–128901 | WWET01002568.1: 130002–161269 |
|
Strawberry poison frog (Oophaga pumilio) |
VIAB01020550.1: 24836–46751 | VIAB01072821.1: 390–4617+VIAB01059777.1: 1–5576 | No BLAST results |
|
Asiatic toad (Bufo gargarizans) |
CM026466.1: 628630042–628648059 | CM026466.1: 599424218–599444854 | No BLAST results |
|
African clawed frog (Xenopus laevis) |
No BLAST results | No BLAST results | No BLAST results |
|
Western clawed frog (Xenopus tropicalis) |
No BLAST results | No BLAST results | No BLAST results |
|
Two‐lined caecilian (Rhinatrema bivittatum) |
Chr15: XM_029578055.1 | Chr15: XM_029579313.1 | Chr15: XM_029578066.1 |
|
Gaboon caecilian (Geotrypetes seraphini) |
Chr15: XM_033921681.1 | No BLAST results | Chr15: XM_033921380.1 |
| Tiny cayenne caecilian (Microcaecilia unicolor) | Chr13: XM_030222159.1 | Chr13: XM_030185735.1 |
Tas1r3‐1: Chr13: XM_030222148.1 Tas1r3‐2: Chr13: XM_030222149.1 |
APPENDIX 3.
Scaffold and gene location of the flanking genes. The genome of two‐lined caecilian was used as a reference, in which the neighboring genes of Tas1r1 are Nol9 and Zbtb48, Tas1r2 are MIB2 and Golim4, and in Tas1r3, they are Dvl1 and Cptp
| Species | Gene | Scaffold | Flanking gene/position subject | Flanking gene/position subject |
|---|---|---|---|---|
| Tas1r1 | Nol9 (702aa) | Zbtb48 (688aa) | ||
| African clawed frog | NC_054383.1 | 103990021–104013200 | 104132287–104149606 | |
| Western clawed frog | NC_030683.2 | 99469716–99486727 | 99583487–99595828 | |
| Tas1r2 | Mib2 (972aa) | Golim4 (681aa) | ||
| Tibetan Plateau frog | – | NW_017307970.1 | 35880–89028 | 272720–316421 |
| African clawed frog | – | NC_054384.1 | 81727385–81795251 | 81649520–81701317 |
| Western clawed frog | – | NC_030683.2 | 92207281–92280709 | 92329059–92379641 |
| Gaboon caecilian | NC_047098.1 | 20095591–20181939 | 20224665–20270222 | |
| Tas1r3 | Dvl1 (695aa) | Cptp (216aa) | ||
| Leishan spiny toad | – | KN616455.1 | 94046–107939 | 4890–56539 |
| African bullfrog | PZQJ01000011.1 | 45518180 −45584538 | 45466313–45503990 | |
| Strawberry poison frog | – | LVCR01025734.1 | 92–4420 | 10784–12869 |
| African clawed frog | – | KN628019.1 | 106433–139608 | 97714–99564 |
| Western clawed frog | – | LD637301.1 | 281024–324221 | 330574–332913 |
APPENDIX 4.
Tas2rs were organized into clusters on specific chromosomes or scaffolds
Zhong, H. , Huang, J. , Shang, S. , & Yuan, B. (2021). Evolutionary insights into umami, sweet, and bitter taste receptors in amphibians. Ecology and Evolution, 11, 18011–18025. 10.1002/ece3.8398
DATA AVAILABILITY STATEMENT
The genomic locations or GenBank accessions of Tas2r genes are available at Zenodo (https://doi.org/10.5281/zenodo.5642517).
REFERENCES
- Adler, E. , Hoon, M. A. , Mueller, K. L. , Chandrashekar, J. , & Zuker, C. S. (2000). A novel family of mammalian taste receptors. Cell, 100, 693–702. [DOI] [PubMed] [Google Scholar]
- Altig, R. , Whiles, M. R. , & Taylor, C. L. (2007). What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshwater Biology, 52, 386–395. 10.1111/j.1365-2427.2006.01694.x [DOI] [Google Scholar]
- Altschul, S. F. , Gish, W. , Miller, W. , Myers, E. W. , & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Barlow, L. (1998). The biology of amphibian taste. In Heatwole H. & Dawley E. (Eds.), Amphibian biology (pp. 743–782). Surrey Beatty & Sons Pty Ltd. [Google Scholar]
- Behrens, M. , Korsching, S. I. , & Meyerhof, W. (2014). Tuning properties of avian and frog bitter taste receptors dynamically fit gene repertoire sizes. Molecular Biology and Evolution, 31, 3216–3227. [DOI] [PubMed] [Google Scholar]
- Bogert, C. M. (1970). The caecilians of the world. A taxonomic review. The Quarterly Review of Biology, 45, 96–97. [Google Scholar]
- Brykczynska, U. , Tzika, A. C. , Rodriguez, I. , & Milinkovitch, M. C. (2013). Contrasted evolution of the vomeronasal receptor repertoires in mammals and squamate reptiles. Genome Biology and Evolution, 5, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufe, B. , & Meyerhof, W. (2006). Chapter 18. Taste receptors and their variants. In Brigelius‐Flohé R. & Joost H.‐G. (Eds.), Nutritional genomics: Impact on health and disease (pp. 386–411). Wiley‐VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- Caprio, J. , Shimohara, M. , Marui, T. , Harada, S. , & Kiyohara, S. (2014). Marine teleost locates live prey through pH sensing. Science, 344, 1154. [DOI] [PubMed] [Google Scholar]
- Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17, 540–552. [DOI] [PubMed] [Google Scholar]
- Chen, C. J. , Chen, H. , Zhang, Y. , Thomas, H. R. , Frank, M. H. , He, Y. H. , & Xia, R. (2020). TBtools: An integrative toolkit developed for interactive analyses of big biological data. Molecular Plant, 13, 1194–1202. [DOI] [PubMed] [Google Scholar]
- Chen, J. M. , Prendinic, E. , Wu, Y. H. , Zhang, B. L. , Suwannapoome, C. , Chen, H. M. , Qiong, J. J. , Lemmonf, E. M. , Lemmong, A. R. , Stuarth, B. L. , Raxworthy, C. J. , Murphy, R. W. , Yuan, Z.‐Y. , & Che, J. (2019). An integrative phylogenomic approach illuminates the evolutionary history of Old World tree frogs (Anura: Rhacophoridae). Molecular Phylogenetics and Evolution, 145, 106724. [DOI] [PubMed] [Google Scholar]
- Chen, K. , Durand, D. , & Farach‐Colton, M. (2000). NOTUNG: A program for dating gene duplications and optimizing gene family trees. Journal of Computational Biology, 7, 429–447. [DOI] [PubMed] [Google Scholar]
- Chen, Q. Y. , Alarcon, S. , Tharp, A. , Ahmed, O. M. , Estrella, N. L. , Greene, T. A. , Rucker, J. , & Breslin, P. A. S. (2009). Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. The American Journal of Clinical Nutrition, 90, 770S–779S. 10.3945/ajcn.2009.27462N [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva, H. R. , de Britto‐Pereira, M. C. , & Caramaschi, U. (1989). Frugivory and seed dispersal by Hyla truncata, a neotropical treefrog. Copeia, 1989, 781–783. [Google Scholar]
- da Silva, H. R. , & De Britto‐Pereira, M. C. (2006). How much fruit do fruit‐eating frogs eat? An investigation on the diet of Xenohyla truncata (Lissamphibia: Anura: Hylidae). Journal of Zoology, 270, 692–698. 10.1111/j.1469-7998.2006.00192.x [DOI] [Google Scholar]
- Daniel, H. (1998). Caecilians in the wild and in captivity. Observations from 20 years of amateur research. (Amphibia: Gymnophiona.). Herpetozoa, 11, 37–46. [Google Scholar]
- Dempster, A. P. (1977). Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society, 39, 1–38. [Google Scholar]
- Dong, C. J. , Chen, L. , Feng, J. Y. , Xu, J. , Mahboob, S. , Alghanim, K. , Li, X. J. , & Xu, P. (2018). Genome wide identification of taste receptor genes in common carp (Cyprinus carpio) and phylogenetic analysis in teleost. Gene, 678, 65–72. [DOI] [PubMed] [Google Scholar]
- Dong, D. , Jones, G. , & Zhang, S. Y. (2009). Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evolutionary Biology, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly, M. A. (1991). Feeding patterns of the strawberry poison frog, Dendrobates pumilio (Anura: Dendrobatidae). Copeia, 1991, 723–730. [Google Scholar]
- Duellman, W. E. , & Trueb, L. (1986). Integumentary, sensory, and visceral systems. In Duellman W. E. & Trueb L. (Eds.), Biology of amphibians (pp. 367–414). McGaw‐Hill. [Google Scholar]
- Feder, M. E. , & Burggren, W. W. (1992). Environmental physiology of the amphibians. BioScience, 43, 492–493. [Google Scholar]
- Feng, P. , & Liang, S. (2018). Molecular evolution of umami/sweet taste receptor genes in reptiles. PeerJ, 6, e5570. 10.7717/peerj.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, P. , & Zhao, H. (2013). Complex evolutionary history of the vertebrate sweet/umami taste receptor genes. Chinese Science Bulletin, 58, 108–114. [Google Scholar]
- Feng, P. , Zheng, J. S. , Rossiter, S. J. , Wang, D. , & Zhao, H. B. (2014). Massive losses of taste receptor genes in toothed and baleen whales. Genome Biology and Evolution, 6, 1254–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, F. L. , Chen, C. J. , Arab, D. A. , Du, Z. G. , He, Y. H. , & Ho, S. Y. W. (2019). EasyCodeML: A visual tool for analysis of selection using CodeML. Ecology and Evolution, 9, 3891–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning, J. I. (1994). Is the bitter rejection response always adaptive? Physiology & Behavior, 56, 1217–1227. [DOI] [PubMed] [Google Scholar]
- Go, Y. (2006). Lineage‐specific expansions and contractions of the bitter taste receptor gene repertoire in vertebrates. Molecular Biology and Evolution, 23, 964–972. [DOI] [PubMed] [Google Scholar]
- Go, Y. , Satta, Y. , Takenaka, O. , & Takahata, N. (2005). Lineage‐specific loss of function of bitter taste receptor genes in humans and nonhuman primates. Genetics, 170, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, K. D. , & Caprio, J. (1985). Taste responses to amino acids in the southern leopard frog, Rana sphenocephala . Comparative Biochemistry and Physiology Part A Physiology, 81, 525–530. [DOI] [PubMed] [Google Scholar]
- Hayakawa, T. , Suzuki‐Hashido, N. , Matsui, A. , & Go, Y. (2014). Frequent expansions of the bitter taste receptor gene repertoire during evolution of mammals in the Euarchontoglires clade. Molecular Biology and Evolution, 31, 2018–2031. [DOI] [PubMed] [Google Scholar]
- Hedges, S. B. , Dudley, J. , & Kumar, S. (2006). TimeTree: A public knowledge‐base of divergence times among organisms. Bioinformatics, 22, 2971–2972. [DOI] [PubMed] [Google Scholar]
- Herness, M. S. , & Gilbertson, T. A. (1999). Cellular mechanisms of taste transduction. Annual Review of Physiology, 61, 873–900. [DOI] [PubMed] [Google Scholar]
- Howse, P. E. (1975). Chemical defences of ants, termites and other insects: some outstanding questions. International Union for the Study of Social Insects; (French Section). [Google Scholar]
- Hu, L. L. , & Shi, P. (2013). Smallest bitter taste receptor (T2Rs) gene repertoire in carnivores. Zoological Research, 34, 249–255. [PubMed] [Google Scholar]
- Jiang, P. H. , Josue, J. , Li, X. , Glaser, D. , Li, W. H. , Brand, J. G. , Margolskee, R. F. , Reed, D. R. , & Beauchamp, G. K. (2012). Major taste loss in carnivorous mammals. Proceedings of the National Academy of Sciences of the United States of America, 109, 4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy, S. , Minh, B. Q. , Wong, T. K. F. , von Haeseler, A. , & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , Misawa, K. , Kuma, K. , & Miyata, T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon, S. C. , & Cummings, T. A. (1992). Chemosensory transduction mechanisms in taste. Annual Review of Physiology, 54, 715–731. [DOI] [PubMed] [Google Scholar]
- Kinnamon, S. C. , & Margolskee, R. F. (1996). Mechanisms of taste transduction. Current Opinion in Neurobiology, 9, 1–9. [DOI] [PubMed] [Google Scholar]
- Kolesnikov, S. S. , & Margolskee, R. F. (1995). A cyclicnucleotidesuppressible conductance activated by transducin in taste cells. Nature, 376, 85. [DOI] [PubMed] [Google Scholar]
- Krogh, A. , Larsson, B. , von Heijne, G. , & Sonnhammer, E. L. (2001). Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. Journal of Molecular Cell Biology, 305, 567–580. [DOI] [PubMed] [Google Scholar]
- Kusano, K. , & Sato, M. (1958). The influence of anions on the activity of gustatory receptors. The Japanese Journal of Physiology, 8, 254–274. [DOI] [PubMed] [Google Scholar]
- Lam‐Tung, N. , Schmidt, H. A. , Arndt, V. H. , & Quang, M. B. (2015). IQ‐TREE: A fast and effective stochastic algorithm for estimating maximum‐likelihood phylogenies. Molecular Biology and Evolution, 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic, I. , & Bork, P. (1988). Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. FEBS Letters, 232, 78–82. [DOI] [PubMed] [Google Scholar]
- Li, D. Y. , & Zhang, J. Z. (2014). Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Molecular Biology and Evolution, 31, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Glaser, D. , Li, W. , Johnson, W. E. , O'Brien, S. J. , Beauchamp, G. K. , & Brand, J. G. (2009). Analyses of sweet receptor gene (Tas1r2) and preference for sweet stimuli in species of Carnivora. Journal of Heredity, 100(Suppl. 1), S90–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Li, W. H. , Wang, H. , Cao, J. , Kenji, M. , Huang, L. Q. , Bachmanov, A. A. , Reed, D. R. , Legrand‐Defretin, V. , Beauchamp, G. K. , & Brand, J. G. (2005). Pseudogenization of a sweet‐receptor gene accounts for cats’ indifference toward sugar. PLoS Genetics, 1, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. D. , Staszewski, L. , Xu, H. , Durick, K. , Zoller, M. , & Adler, E. (2002). Human receptors for sweet and umami taste. Proceedings of the National Academy of Sciences of the United States of America, 99, 4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W. H. , Burks, C. A. , Hansen, D. R. , Kinnamon, S. C. , & Gilbertson, T. A. (2004). Taste receptor cells express pH‐sensitive leak K+ channels. Journal of Neurophysiology, 92, 2909–2919. [DOI] [PubMed] [Google Scholar]
- Lindemann, B. (1996). Chemoreception: Tasting the sweet and the bitter. Current Biology, 6, 1234–1237. [DOI] [PubMed] [Google Scholar]
- Lindemann, B. (2000). A taste for umami. Nature Neuroscience, 3, 99–100. [DOI] [PubMed] [Google Scholar]
- Liu, G. J. , Walter, L. , Tang, S. N. , Tan, X. X. , Shi, F. L. , Pan, H. J. , Roos, C. , Liu, Z. J. , & Li, M. (2014). Differentiated adaptive evolution, episodic relaxation of selective constraints, and pseudogenization of umami and sweet taste genes TAS1Rs in catarrhine primates. Frontiers in Zoology, 11, 79. 10.1186/s12983-014-0079-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. J. , Liu, G. J. , Hailer, F. , Orozco‐Terwengel, P. , Tan, X. X. , Tian, J. D. , Yan, Z. Z. , Zhang, B. W. , & Li, M. (2016). Dietary specialization drives multiple independent losses and gains in the bitter taste gene repertoire of Laurasiatherian Mammals. Frontiers in Zoology, 13, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, F. , Park, Y. M. , Lee, J. , Buso, N. , Gur, T. , Madhusoodanan, N. , Basutkar, P. , Tivey, A. R. N. , Potter, S. C. , Finn, R. D. , & Lopez, R. (2019). The EMBL‐EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Research, 47, W636–W641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco, A. , & Davide, R. (2017). A matter of taste: Lineage‐specific loss of function of taste receptor genes in vertebrates. Frontiers in Molecular Biosciences, 4, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters, M. , & Roper, S. D. (1985). Amiloride does not block taste transduction in the mudpuppy, Necturus maculosus . Chemical Senses, 10, 341–352. [Google Scholar]
- Minh, B. Q. , Nguyen, M. A. T. , & von Haeseler, A. (2013). Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution, 30, 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaa, C. G. , Silva, S. D. G. T. M. , Hagyari, D. , Wager, J. , & Schalk, C. M. (2019). Revisiting “what do tadpoles really eat?” A 10‐year perspective. Freshwater Biology, 64, 2269–2282. [Google Scholar]
- Murrell, B. , Moola, S. , Mabona, A. , Weighill, T. , Sheward, D. , Kosakovsky Pond, S. L. , & Scheffler, K. (2013). FUBAR: A fast, unconstrained Bayesian AppRoximation for inferring selection. Molecular Biology and Evolution, 30, 1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, G. , Hoon, M. A. , Chandrashekar, J. , Zhang, Y. F. , Ryba, N. J. , & Zuker, C. S. (2001). Mammalian sweet taste receptors. Cell, 106, 381–390. [DOI] [PubMed] [Google Scholar]
- Peng, C. J. , Ren, J. L. , Deng, C. , Jiang, D. C. , Wang, J. C. , Qu, J. Y. , Chang, J. , Yan, C. C. , Jiang, K. , Murphy, R. W. , Wu, D.‐D. , & Li, J.‐T. (2020). The genome of Shaw's sea snake (Hydrophis curtus) reveals secondary adaptation to its marine environment. Molecular Biology and Evolution, 37, 1744–1760. [DOI] [PubMed] [Google Scholar]
- Pond, S. L. K. , Frost, S. D. W. , & Muse, S. V. (2005). HyPhy: Hypothesis testing using phylogenies. Bioinformatics, 21, 676–679. [DOI] [PubMed] [Google Scholar]
- Reutter, K. , & Witt, M. (1993). Morphology of the vertebrate taste organs and their nerve supply. In Roper S. D. & Simon S. A. (Eds.), Mechanisms of taste transduction (pp. 29–82). CRC Press. [Google Scholar]
- Sato, J. J. , & Wolsan, M. (2012). Loss or major reduction of umami taste sensation in pinnipeds. Naturwissenschaften, 99, 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, P. , & Zhang, J. Z. (2006). Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Molecular Biology and Evolution, 23, 292–300. [DOI] [PubMed] [Google Scholar]
- Shi, P. , & Zhang, J. Z. (2007). Comparative genomic analysis identifies an evolutionary shift of vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Research, 17, 166–174. 10.1101/gr.6040007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, P. , Zhang, J. Z. , Yang, H. , & Zhang, Y. P. (2003). Adaptive diversification of bitter taste receptor genes in mammalian evolution. Molecular Biology and Evolution, 20, 805–814. [DOI] [PubMed] [Google Scholar]
- Speca, D. J. , Lin, D. M. , Sorensen, P. W. , Isacoff, E. Y. , & Dittman, A. H. (1999). Functional identification of a goldfish odorant receptor. Neuron, 23, 487. [DOI] [PubMed] [Google Scholar]
- Stewart, R. E. , Desimone, J. A. , & Hill, D. L. (1997). New perspectives in a gustatory physiology: Transduction, development, and plasticity. American Journal of Physiology, 272, 1–26. [DOI] [PubMed] [Google Scholar]
- Syed, A. S. , & Korsching, S. I. (2014). Positive Darwinian selection in the singularly large taste receptor gene family of an ‘ancient’ fish, Latimeria chalumnae . BMC Genomics, 15, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temussi, P. (2006). The history of sweet taste: Not exactly a piece of cake. Journal of Molecular Recognition, 19, 188–199. [DOI] [PubMed] [Google Scholar]
- Toshihide, S. , Yukio, O. , & Takenori, M. (1995). Molecular mechanisms of gustatory transductions in frog taste cells. Progress in Neurobiology, 46, 239–287. [PubMed] [Google Scholar]
- Wake, M. (1994). Caecilians in captivity. In Murphy J. B., Adler K. K., & Colllins J. T. (Eds.), Captive management and conservation of amphibians and reptiles (pp. 255–266). Society for the Study of Amphibians and Reptiles. [Google Scholar]
- Wang, K. , & Zhao, H. B. (2015). Birds generally carry a small repertoire of bitter taste receptor genes. Genome Biology and Evolution, 7, 2705–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. X. , Thomas, S. D. , & Zhang, J. Z. (2004). Relaxation of selective constraint and loss of function in the evolution of human bitter taste receptor genes. Human Molecular Genetics, 13, 2671–2678. [DOI] [PubMed] [Google Scholar]
- Wassersug, R. J. , & Heyer, W. R. (1988). A survey of internal oral features of Leptodactyloid Larvae (Amphibia: Anura). Smithsonian Contributions to Zoology, 457, 1–99. [Google Scholar]
- Yang, Z. H. (2007). PAML 4: Phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution, 24, 1586–1591. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky, D. A. , Zuker, C. S. , & Ryba, N. J. P. (2009). Common sense about taste: From mammals to insects. Cell, 139, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu, K. , Manabe, T. , Yoshida, R. , Iwatsuki, K. , & Ninomiya, Y. (2014). Involvement of multiple taste receptors in umami taste: Analysis of gustatory nerve responses in mGluR4 knock‐out mice. Journal of Physiological Sciences, 593, 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii, K. , Yoshii, C. , Ko Ba Take, Y. , & Kurihara, K. (1982). High sensitivity of Xenopus gustatory receptors to amino acids and bitter substances. American Physiological Society Journal, 243, R42–R48. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Gao, F. L. , Jakovlić, I. , Zou, H. , Zhang, J. , Wen, X. L. , & Wang, G. T. (2020). PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20, 348–355. [DOI] [PubMed] [Google Scholar]
- Zhao, H. B. , Li, J. Z. , & Zhang, J. Z. (2015). Molecular evidence for the loss of three basic tastes in penguins. Current Biology, 25, R141–R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Xu, D. , Zhang, S. , & Zhang, J. (2012). Genomic and genetic evidence for the loss of umami taste in bats. Genome Biology and Evolution, 4, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Yang, J. , Xu, H. , & Zhang, J. (2010). Pseudogenization of the umami taste receptor gene Tas1r1 in the giant panda coincided with its dietary switch to bamboo. Molecular Biology and Evolution, 27, 2669–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , & Zhang, J. (2012). Mismatches between feeding ecology and taste receptor evolution: An inconvenient truth. Proceedings of the National Academy of Sciences of the United States of America, 109, E1464; author reply E1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Zhou, Y. , Pinto, C. M. , Charles‐Dominique, P. , Galindo‐González, J. , Zhang, S. , & Zhang, J. Z. (2010). Evolution of the sweet taste receptor gene Tas1r2 in bats. Molecular Biology and Evolution, 27, 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, H. M. , Shang, S. , Wu, X. Y. , Chen, J. , Zhu, W. C. , Yan, J. K. , Li, H. T. , & Zhang, H. H. (2017). Genomic evidence of bitter taste in snakes and phylogenetic analysis of bitter taste receptor genes in reptiles. PeerJ, 5, e3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, H. M. , Shang, S. , Zhang, H. X. , Chen, J. , Wu, X. Y. , & Zhang, H. H. (2019). Characterization and phylogeny of bitter taste receptor genes (Tas2r) in Squamata. Genetica, 147, 131–139. [DOI] [PubMed] [Google Scholar]
- Zhu, K. L. , Zhou, X. M. , Xu, S. X. , Sun, D. , Ren, W. H. , Zhou, K. Y. , & Yang, G. (2014). The loss of taste genes in cetaceans. BMC Ecology and Evolution, 14, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genomic locations or GenBank accessions of Tas2r genes are available at Zenodo (https://doi.org/10.5281/zenodo.5642517).


