Abstract
Background
In the intrinsic system of controlling airway calibre, the cholinergic (muscarinic) sympathetic nervous system has an important role. Anticholinergic, anti muscarinic bronchodilators such as ipratropium bromide are frequently used in the management of childhood airway disease. In asthma, ipratropium is a less potent bronchodilator than beta‐2 adrenergic agents but it is known to be a useful adjunct to other therapies, particularly in status asthmaticus. What remains unclear is the role of anticholinergic drugs in the maintenance treatment of chronic asthma.
Objectives
To determine the effectiveness of anticholinergic drugs in chronic asthma in children over the age of 2 years.
Search methods
The Cochrane Airways Group Specialised Register and reference lists of articles were searched. The most recent search was carried out in Febuary 2010.
Selection criteria
Randomised controlled trials in which anticholinergic drugs were given for chronic asthma in children over 2 years of age were included. Studies including comparison of: anticholinergics with placebo, and anticholinergics with any other drug were included.
Data collection and analysis
Eligibility for inclusion and quality of trials were assessed independently by two reviewers.
Main results
Eight studies met the inclusion criteria. Three papers compared the effects of anticholinergic drugs with placebo, and a meta‐analysis of these results demonstrated no statistically significant benefit of the use of anticholinergic drugs over placebo in any of the outcome measures used. The results of one of these trials could not be included in the meta‐analysis but the authors did report significantly lower symptom scores with inhaled anticholinergics compared with placebo. However, there was no significant difference between ipratropium bromide and placebo in the percentage of symptom‐free nights or days. Two trials studied the effects of anticholinergics on bronchial hyper responsiveness to histamine, by measuring the provocation dose of histamine needed to cause a fall of 20 % in FEV1 (PD 20). One study (comparing anticholinergics with placebo) reported a statistically significant increase in PD 20 but this was not found in another study (comparing anticholinergics with a beta‐2 agonist). Both trials also examined the effect of anticholinergic drugs on diurnal variation in peak expiratory flow rate (PEFR) and reported no significant effect. Two studies compared the addition of an anticholinergic drug to a beta‐2 agonist with the beta‐2 agonist alone. Both trials failed to show any significant benefit from the long term use of combined anticholinergics with beta‐2 agonists compared with beta‐2 agonists alone. One trial compared the effects of oral and inhaled anticholinergic drugs with placebo. No statistically significant differences were found in any of the outcome measures except for a higher FEV1 / VC ratio and RV / TLC ratio with oral anticholinergic therapy when compared with placebo.
Authors' conclusions
The present review summarises the best evidence available to date. Although there were some small beneficial findings in favour of anticholinergic therapy, there is insufficient data to support the use of anticholinergic drugs in the maintenance treatment of chronic asthma in children.
Plain language summary
Anticholinergic drugs versus either placebo or other medications for children over two years of age with asthma
Anticholinergic drugs are widely used in the management of both acute and chronic asthma in children. Their effect is achieved through relief of narrowing of the airways that occurs in asthma. Current guidelines for the management of chronic asthma advise that anticholinergic drugs may be used if children are already on high dose inhaled steroids. This study undertook a comprehensive search of the literature unrestricted by country of publication or language. Unfortunately small numbers of relevant trials were found and these were of variable quality. This review found that although anticholinergic drugs are well tolerated, in children over two years of age, there is not enough data to be sure if they are better than placebo in terms of effects on lung function or symptoms.
Background
The cholinergic (muscarinic) sympathetic nervous system plays an important role in the intrinsic system of controlling airway calibre. Anticholinergic, anti muscarinic bronchodilators such as ipratropium bromide are frequently used in the management of childhood airway disease. In asthma, ipratropium is a less potent bronchodilator than beta‐2 adrenergic agents but it is known to be a useful adjunct to other therapies, particularly in status asthmaticus. What remains to be proved is the role of anticholinergic drugs in chronic asthma.
The medicinal benefits of atropine and other naturally occurring anticholinergic alkaloids have been known for many centuries. Atropine, an alkaloid extract of belladonna, is extracted from the Datura stramonium plant. These agents were first used in western medicine in the early 1800s after British colonists learned of their uses in India. By the middle of the 19 th century, belladonna agents were widely used and were the sole bronchodilating agents available. In the 1920s, epinephrine was discovered, followed by the methylxanthines, replacing belladonna alkaloids as bronchodilating agents (Gross 1988). Part of this declining use was due to the side effects of atropine. However, the development of synthetic analogues of atropine, which retained good anticholinergic activity with minimal side effects, resulted in a resurgence of interest. The most studied, and now most widely used of these agents is Ipratropium Bromide (Atrovent, formerly known as Sch 1000) (Goodman 1996).
Ipratropium bromide (IB) is an anticholinergic bronchodilator. It acts at muscarinic receptors, competitively inhibiting the effects of acetylcholine. Acetylcholine acts by causing smooth muscle constriction, which provides broncho motor tone. By antagonising the effects of acetylcholine, cholinergic broncho motor tone is inhibited and the vagal reflexes that mediate bronchoconstriction are blocked.
Since the introduction of inhaled anticholinergic agents, their clinical uses have been explored in many diseases. It is now becoming apparent that these drugs are regarded as first‐line broncho dilatory agents in the treatment of chronic obstructive pulmonary disease (COPD). There is no equivalent in childhood disease. COPD generally occurs after years of smoking and is characterised by chronic mucus secretion, airway infection and variable airway hyper responsiveness (Rubin 1996; Chapman 1996).
In children, anticholinergic agents are used in the management of several diseases, including broncho‐pulmonary dysplasia (BPD), bronchiolitis, viral induced wheeze and asthma.
Many definitions of asthma exist and the term itself is controversial (Keeley 1999). Asthma may be defined as "reversible airways obstruction associated with bronchial hyper reactivity, allergic inflammation of the airways and a response to treatment with bronchodilators with regular prophylactic inhaled anti‐inflammatory agents" (Silverman 1997). More simply, it may be defined as "episodic cough and / or wheeze in a clinical setting, where asthma is likely and other rarer conditions have been excluded" (Doull 1997).
The initial management of acute severe asthma in the paediatric setting involves rapid relief of bronchospasm with inhaled or nebulised bronchodilators. Children who do not fully respond require corticosteroids in addition. Beta‐2 agonists provide a rapid bronchodilating effect and are the most widely used bronchodilators in acute severe asthma (Rubin 1996). However, many studies have examined the effects of adding anticholinergic agents to beta‐2 agonists (Qureshi 1997; Brophy 1998; Craven 2001). Although anticholinergics have a slower onset of action and a weaker bronchodilating effect than beta‐2 agonists, they work through different mechanisms and specifically reduce cholinergic broncho motor tone. A systematic review by the Cochrane Airways Group(Plotnick 2000) reviewed the evidence for a beneficial effect of treating children during acute severe asthma with an anticholinergic agent or both beta‐2 agonist and anticholinergic drugs. They concluded that "a single dose of an anticholinergic agent is not effective in the treatment of mild and moderate exacerbations and is insufficient for acute exacerbations. Adding multiple doses of anticholinergics to beta‐2 agonists appears safe, improves lung function and would avoid admission in one of eleven such treated patients".
Ipratropium bromide has been used for many years in the treatment of asthma. Use of anticholinergic drugs declined with the development of beta‐2 agonists, but interest renewed with the realisation that parasympathetic pathways are important in bronchospasm in some asthmatics. The bronchodilatation produced by ipratropium is slower and less intense than with beta‐2 agonists, but effects can last for up to 6 hours. There appears to be variability in the response of asthmatics to ipratropium. This may be related to the amount of parasympathetic tone and to the degree to which reflex activation of cholinergic pathways participate in generating symptoms in individual patients. Therefore, the effects of ipratropium should be monitored on an individual basis.
The British Thoracic Society guidelines (BTS 1997; BTS 1993; BTS 1997a) recommended that the management of asthma should be a stepwise approach. Ipratropium was recommended for regular maintenance bronchodilator therapy in schoolchildren who are already on high dose inhaled steroids (Step 4). It was not included in the management of asthma in children under the age of 5 years in these guidelines. The British Thoracic Society guidelines have been recently revised (BTS 2003) and no longer recommend the use of anticholinergic treatment in chronic asthma in children.
Objectives
To determine the efficacy of anticholinergic drugs in chronic asthma in children over the age of 2 years.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled clinical trials in which anticholinergic drugs were given for chronic asthma in children over 2 years of age were included.
Types of participants
Children aged 2 years (24 months) or over with chronic asthma in all settings (emergency department, inpatient or outpatient, general practice and the home). Asthma must have been physician diagnosed or have been diagnosed using internationally established criteria. Treatment in both the inpatient and outpatient setting was included.
Studies involving children under the age of 2 years were excluded since different pathophysiologies may occur in babies and young infants. The effectiveness of anticholinergic agents in children less than 2 years has recently been reviewed elsewhere (Everard 2005).
Studies in which the effects of anticholinergic drugs on exercise‐induced asthma (EIA) were examined were also excluded.
Types of interventions
Inhaled anticholinergic drugs, delivered by any means; nebulised or by metered dose inhaler (MDI); with or without a holding chamber +/‐ a mask. All doses and dosing regimes were included. As this review was examining the effectiveness of anticholinergic therapy on chronic asthma, patients were required to have received the study drug for the duration of at least one week.
Studies including comparison of: anticholinergics with placebo, and anticholinergics with any other drug were included.
Types of outcome measures
Primary outcome measures: days without wheeze or asthma
Secondary outcome measures: changes in lung function changes in symptomatology indicators of exacerbations (for example, increased use of bronchodilators, use of oral steroids, hospital admission or physician attendance). patient and / or parent assessment physician's assessment number of withdrawals from study
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials which is derived from systematic searching of electronic databases including CENTRAL, MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). Additional searches of CENTRAL were also carried out. The general structure of the orginal electronic search strategy was (anticholinergics OR synonyms) AND (children OR synonyms).
All records in the Specialised Register coded as 'asthma' were searched with the following terms:
Anticholinergic* or cholinergic* or antagonist* or muscarinic* or "ipratropium bromide" or atropin* or atrovent* or oxitropium or Sch1000
The following search terms were used in CENTRAL:
(Anticholinergic$.tw. OR cholinergic antagonist$.tw. OR muscarinic antagonist$.tw. OR ipratropium bromide.tw. OR ipratropium.tw. OR atropine.tx. OR atrovent.tw. OR oxitropium bromide.tx. OR sch1000.tx OR EXP ANTICHOLINERGIC$/ OR EXP CHOLINERGIC ANTAGONIST/ OR EXP MUSCARINIC ANTAGONIST/ OR EXP IPRATROPIUM BROMIDE/ OR EXP IPRATROPIUM/ OR EXP ATROPINE/ OR EXP ATROVENT/ OR EXP OXITROPIUM BROMIDE/ OR EXP SCH1000/)
Searching other resources
Handsearching of medical journals through the Cochrane Collaboration.
The reference lists in primary sources were searched.
Personal communication with authors and experts in the field was made in order to trace other unpublished sources.
The pharmaceutical company, Boehringer Ingelheim, was contacted to obtain additional trials and information.
Searches were current as of February 2010.
Data collection and analysis
Selection of studies
The abstract list obtained from the Airways Group search strategy was reviewed by two researchers to identify all potential studies for inclusion. If the study was thought to potentially meet the inclusion criteria, or if further details were needed to assess the study, then the full text of the publication was obtained for further information. Studies were next assessed independently as included or excluded by two reviewers (NM and AB). Disagreement between the two reviewers was resolved by consensus. A list of excluded studies was also made by the two reviewers (NM and AB) with reasons for their exclusion documented (see Table of Excluded Studies). The following questions were asked of each study:
1) Was the study an RCT? 2) Was the study carried out in adults or children? Where the study population included both adults and children, the mean age of the population was noted, and authors contacted for the children only data. If no additional data were obtained, and the mean age was between two and eighteen years of age, then the study was included. 3) Was the study in acute or chronic asthma? 4) Was the research in exercise‐induced asthma? 5) Was the research a single‐dose study?
Data extraction and management
Data for trials were independently extracted by both reviewers (NM and AB). Data were entered into the Cochrane Collaboration software program Review Manager, version 5, by the two reviewers (NM and AB).
Assessment of risk of bias in included studies
Studies to be included underwent quality assessment performed independently by both reviewers (NM and AB), using two methods. The first involved using the Cochrane approach to assessment of allocation of concealment. Trials were scored using the following principles: Grade A: Adequate concealment Grade B: Unclear concealment Grade C: Clearly inadequate concealment
Secondly, each study was also assessed using a 1 to 5 scale described by Jadad 1995 and summarised as follows:
Was the study described as randomised? (1=yes; 0=no) Was the study described as double blind? (1=yes; 0=no) Was there a description of withdrawals and dropouts? (1=yes; 0=no) Was the method of randomisation clearly described and appropriate? (1=yes; 0=no) Was the method of double blinding well described and appropriate? (1=yes; 0=no)
One point was deducted if the methods for randomisation or blinding were inappropriate. Any disagreement between the two reviewers regarding the quality scoring was resolved by consensus.
Data synthesis
Following the integration of generic inverse variance (GIV) in RevMan 5, we have opted to re‐analyse the trial data from crossover studies with this method which analyses studies of that design appropriately. Included trials were combined where possible using Review Manager. For dichotomous variables, individual and pooled statistics were calculated as Odds Ratios with 95% Confidence Intervals. For continuous outcomes, individual and pooled statistics was calculated as Weighted Mean Differences or Standard Mean Differences, as indicated, with 95% Confidence Intervals. A fixed effects model was used to pool the data.
Heterogeneity of effect size across pooled studies was calculated, with p < 0.05 used as the cut‐off level for significance. The results from parallel studies were not pooled with those from cross‐over studies.
It was anticipated that measures of bronchial hyper‐responsiveness (BHR) such as the provocation dose of a challenge substance (e.g. histamine) required to produce a 20% fall in FEV1 (PD 20) would often be reported as geometric means. Presentation of results in this way indicate that the data has been logarithmically transformed prior to analysis by investigators to take account of skewed distribution. Data from such outcomes could only therefore be pooled across studies if the mean and standard deviation of logged values (from which the geometric means are derived) could be calculated.
Subgroup analysis and investigation of heterogeneity
If we had found sufficient data, we could have performed subgroup analysis on
Females versus males. Age (e.g. comparing children aged 2 ‐5 years versus 5 ‐ 18 years). Co‐interventions with corticosteroids versus none. Different doses of anticholinergics ‐ low dose (up to and including 200 mcg/day) versus high dose (over 200 mcg/day). Duration of anticholinergic administration ‐ Less than 6 weeks versus greater than 6 weeks administration.
Assessment of clinical heterogeneity The influence of trial characteristics that may have influenced the observed treatment effect were examined. If sufficient numbers of trials and the necessary data had been available, clinical heterogeneity would have be investigated according to:
Treatment setting Asthma severity
Sensitivity analysis
Sensitivity analyses provide an approach for testing how robust the results of a review are relative to key decisions and assumptions that have been made in the process of conducting the review.
Two factors would have been investigated if sufficient numbers of included studies had been found.
Publication bias ‐ the existence of publication bias would have been examined using a funnel plot. Trial quality ‐ the effects of overall trial quality on the pooled result would have been examined using both the Cochrane approach and that of Jadad 1995.
Results
Description of studies
Eight studies met the inclusion criteria (194 participants). For details of each included study, see 'Characteristics of included studies'. An updated search conducted in February 2010 identified 12 references, none of which met the inclusion criteria. For details of previous searches see Table 1.
1. Search history.
| Years (from/to) | Detail |
| All years to January 2002 | References identified: 438 titles and abstracts Possibly relevant: 107 studies for inclusion. Full text versions of these papers were obtained & assessed independently by NM and AB. Where a query arose about a study's fulfilment of the inclusion criteria, the authors were contacted for further confirmation. If the trial had presented both child and adult data together, authors were asked if they could provide the data for children separately. No further information was provided by study authors. N included: Eight. All study authors contacted to clarify information. N excluded: 99 papers (reasons for exclusion detailed in 'Characteristics of Excluded Studies' . Nine out of the 107 studies were published in a foreign language (one in French, one in Polish, three in Spanish, two in Italian, one in German, and one in Dutch). No study was excluded on the basis of language of publication. Translations have been obtained for all studies. Two non‐English language papers met the inclusion criteria for this review and were included (Vazquez 1984; Zimmermann 1984). |
| January 2002 to February 2005 | References identified: 32 titles and abstracts. Possibly relevant: Six (Dutt 1990; Krasnowska 1994; Krasnowska 2004; Li 2003; Miraglia 1983; Pauwels 1995). N included: 0 N excluded: six |
| February 2005 to February 2006 | References identified: 26 Possibly relevant: 0 |
| February 2006 to February 2007 | References identified: 20 Possibly relevant: 0 |
| February 2007 to February 2008 | References identified: 14 Possibly relevant: 0 |
| February 2008 to February 2009 | References identified: 10 Possibly relevant:0 |
The patients included in these studies were between the ages of 4 and 18 years. A total of 194 patients were included with 97 males and 46 females reported. One study (Vazquez 1984 ‐ 49 patients) did not report the numbers of males and females. All participants were studied in the home and outpatient setting.
The studies used a variety of delivery systems (metered dose inhalers with holding chambers and masks, nebulisers and tablets), doses and dosing regimes. Ipratropium bromide, atropine, beta‐2 agonists, disodium cromoglycate and placebo were utilised. With one exception, (Hutchison 1980) ipratropium bromide was the anticholinergic agent.
Asthma severity varied between studies. All patients had chronic asthma, either physician diagnosed or diagnosed according to ATS definitions. In two studies, asthma was described as moderate to severe asthma requiring daily medication (Bratteby 1986; Mann 1982). Freeman 1989 included patients with moderate asthma with the majority on daily medication. Sly 1987 did not specifically state the asthma severity of the trial patients but all were on daily maintenance treatment. Hutchison 1980 included patients with severe chronic asthma which was inadequately controlled despite maximal daily medication. Zimmermann 1984 selected children with mild, moderate and severe asthma. Raes 1989 included those with mild chronic symptomatic asthma and Vazquez 1984 studied those with asymptomatic asthma.
Maintenance treatment included regular inhaled sympathomimetic drugs, disodium cromoglycate, inhaled steroids, oral theophyllines and oral steroids.
Study periods varied from 2 weeks to 4 months. In studies using a cross‐over design, a washout period was only described in one study (Sly 1987 ‐ washout period 4 weeks). In another cross‐over study (Bratteby 1986), data were analysed only from days 5‐14 (out of a trial of 2 x 2 weeks) to provide a wash‐out period.
Symptom diaries were recorded in all eight studies. A variety of symptoms were recorded on a daily basis including nocturnal wheeze and cough, daytime wheeze and cough, exercise tolerance, days of school missed and the need for additional medications (e.g. the need for additional courses of oral steroids). In seven studies, daily PEFRs were also recorded; before and after treatment twice daily. Several of the studies attempted to examine the effects of anticholinergic drugs on the diurnal variation in airway calibre seen in children with asthma.
None of the studies reported the validity of their symptom diaries. In addition, at certain time points in each study, each patient underwent lung function tests. These were performed in the hospital setting.
Risk of bias in included studies
All studies were described as randomised and double‐blind. Five out of eight studies were cross‐over studies (Bratteby 1986; Freeman 1989; Hutchison 1980; Mann 1982; Sly 1987). The three remaining studies were parallel studies (Raes 1989; Vazquez 1984; Zimmermann 1984).
Jadad score 4 Allocation concealment B ‐ Unclear This study was described as a randomised, double‐blind, cross‐over study. The method of blinding was described in the text but the method of randomisation was not stated. The study was in two parts with the second part of the study being relevant to this review. The first part of this trial was a single dose study over two days and was not included in the review. No withdrawals or dropouts were reported.
Jadad score 2 Allocation concealment B ‐ Unclear This study was described as a randomised, double‐blind, cross‐over study. The methods of randomisation and blinding were not stated. The study was in two parts. The second part of the study was relevant to this review. The first part of this trial studied hospitalised children with an acute asthma attack, in the last two days of their admission and this was not included in the review. Withdrawals or dropouts were not described.
Jadad score 3 Allocation concealment B ‐ Unclear This study was described as a randomised, double‐blind, cross‐over study. The method of randomisation was not stated. The method of blinding was described in the text. No withdrawals or dropouts were reported.
Jadad score 3 Allocation concealment A ‐ Adequate This study was described as a randomised, double‐blind, cross‐over study. The method of randomisation was not stated. The method of allocation concealment was described in the text. Two patients (out of 20) were withdrawn from the study for failing to complete adequate records.
Jadad score 3 Allocation concealment B ‐ Unclear This study was described as a randomised, double‐blind, parallel study. The method of randomisation and blinding was described in the text. Three patients (out of 20) were withdrawn or dropped out of the study.
Jadad score 3 Allocation concealment B ‐ Unclear This study was described as a randomised, double‐blind, cross‐over study. The method of randomisation was not stated. The method of blinding was described in the text. No withdrawals or dropouts were reported.
Jadad score 2 Allocation concealment B ‐ Unclear This study was described as a randomised, double‐blind, parallel study. The method of randomisation and blinding was not stated. The study was in two parts. The second part of the study was relevant to this review. The first part of this trial was a single dose study over two days. Data from 32 out of 49 patients were obtained for the second part of the study. We are unable to report the reasons for this due to poor result reporting in the paper.
Jadad score 2 Allocation concealment B ‐ Unclear This study was described as a randomised, double‐blind, parallel study. The method of randomisation and blinding was not stated. No withdrawals or dropouts were reported.
Effects of interventions
Five comparison groups were identified within the studies:
1) Inhaled anticholinergics versus placebo 2) Inhaled anticholinergics versus beta‐2 agonist 3) Inhaled anticholinergics + beta‐2 agonist versus beta‐2 agonist 4) Inhaled anticholinergics versus beta‐2 agonist +inhaled anticholinergics 5) Inhaled anticholinergics versus oral anticholinergics
Ipratropium bromide was the most common anticholinergic drug used but in one trial atropine (inhaled and oral) was used. These results have been analysed separately in two subgroups as it is well recognised that ipratropium bromide is associated with fewer side effects than atropine.
No data could be entered for our primary outcome measure ‐ "days without wheeze or asthma" as none of the studies specifically used this measure, although in some studies it was incorporated into symptom diaries.
1) INHALED ANTICHOLINERGICS versus PLACEBO
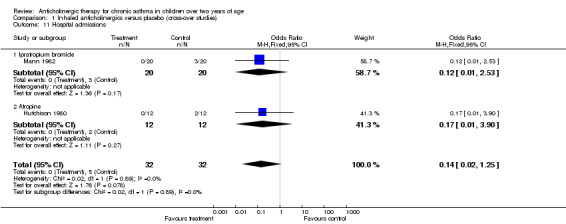
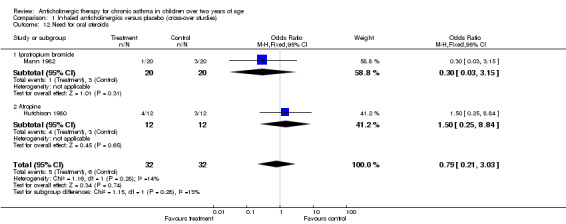
Three studies could be included within this comparison group. All three studies were cross‐over studies. Mann 1982 and Sly 1987 both used IB as the anticholinergic agent. Combining the results of the studies by Mann 1982 and Sly 1987 produced a total of 49 patients. Hutchison 1980 used inhaled atropine as the anticholinergic agent and 12 patients were included in their study. All three studies could be combined in a meta‐analysis only for PEFR (am) and PEFR (pm). Two studies could be combined for the outcomes of hospitalisation rates and the need for additional oral steroids. The other results presented are from a single study only.
Data from these three studies produced the following results (see Comparisons and Data):
No statistically significant effect of inhaled anticholinergic therapy compared to placebo was observed for the following outcome measures:
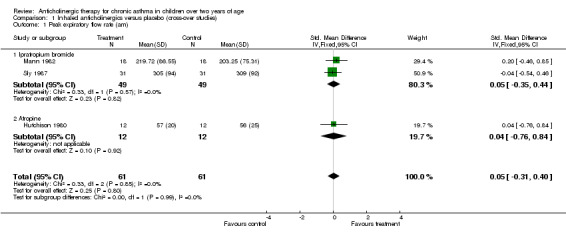
Peak expiratory flow rate (am) ‐ SMD (95%CI) = 0.05 (‐0.31, 0.40)
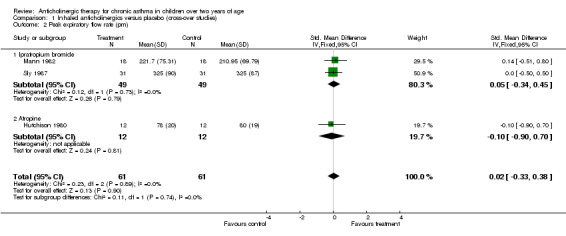
Peak expiratory flow rate (pm) ‐ SMD (95% CI) = 0.02 (‐0.33, 0.38)
Hospital admissions ‐ Odds Ratio (95 % CI) = 0.14 ( 0.02, 1.25)
Need for additional oral steroids ‐ Odds Ratio (95% CI) = 0.79 (0.21, 3.03)
Symptom scores from Mann 1982 could not be included in the meta‐analysis because data were not available for entry into Review Manager. In this study, daily records were kept of day and night cough and wheeze, scoring zero for no symptoms, one for occasional wheeze and/or cough, two for troublesome wheeze and/or cough, and three for very ill with severe wheeze and /or cough.
The authors reported that symptom scores were significantly lower with IB compared to placebo for both night symptom scores (IB 47.8 % compared with placebo 42.9% with score 1; 15.1% IB compared to 21.8% placebo with score 2; 1% IB compared with 3.1% placebo with score 3) and day symptom scores (51.4% IB compared to 48.8% placebo at score 1; 10.2% IB compared with 16.6% placebo at score 2 and 1.4% IB compared with 3.9% placebo at score 3). No standard deviations were given with these results but a p value of < 0.005 was obtained.
However, there was no significant difference between IB and placebo in the percentage of symptom‐free nights or days.
Sly 1987 examined the effect of IB on diurnal variation in airway calibre and also on bronchodilator responsiveness. Sly 1987 postulated that the nocturnal exacerbation of asthma might be due to an exaggeration in the diurnal variation in airway calibre and an increase in airway sensitivity. He postulated that by treating this, a reduction in the effects of nocturnal asthma might be obtained. To study this, PEFRs were recorded twice daily. In addition, the diurnal variation in bronchodilator responsiveness was assessed by examining the increase in PEFR after salbutamol.
Sly 1987 found that 74% of his subjects recorded significant diurnal variation in PEFR during the baseline period of this study. Treatment with IB did not have an effect on this diurnal variation. 21 out of the 31 patients inhaled three times daily salbutamol. The study initially proposed that all trial patients should take regular salbutamol. However, the reasons for 10 patients not taking salbutamol were not given. An increase in morning PEFRs was observed in 12 out of 21 patients (i.e. 57%) but this increase was not affected by treatment with IB compared to placebo.
The study period in this trial was four periods each lasting four weeks (a baseline period and two treatment periods separated by a washout period). At the end of each study period histamine challenges were performed. Subjects did not use salbutamol or treatment aerosols for between 8 and 12 hours before the challenge test. The provocation dose of histamine needed to cause a fall of 20% in FEV1 (PD 20) was recorded. It was found that treatment with IB was associated with significantly higher mean PD 20 values (geometric mean 0.78 versus 0.49 mg/ml, P<0.01).
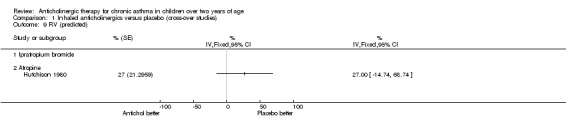
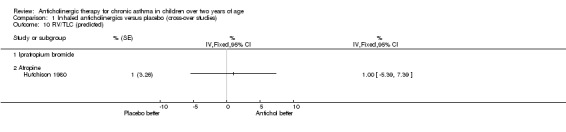
The trial by Hutchison 1980 was the only trial within this comparison group where the effects of anticholinergic drugs were compared to placebo by using formal lung function testing. Vital capacity (VC), forced expiratory volume in one second (FEV1), FEV1/VC, and average forced expiratory flow from 25% to 75% of the vital capacity (FEF 25‐75%) were calculated. In addition, residual volume (RV) and total lung capacity (TLC) were calculated. Results were expressed as percentages of predicted values for FEV1, FEV1/VC, FEF 25‐75% and RV. As mentioned above, inhaled atropine did not have an effect on lung function testing. However, the use of oral atropine resulted in significantly higher results (p<0.05) for FEV1/VC and a significant reduction (p<0.05) in RV/VC (see below). However, there were no differences between oral atropine, inhaled atropine or placebo for the number of days of school missed, night time waking, the need for additional oral steroids, or morning and evening PEFRs. (see also Comparison of Inhaled anticholinergics versus oral anticholinergics).
2) INHALED ANTICHOLINERGICS versus BETA‐2 AGONIST
Raes 1989 compared the effects of IB with the beta‐2 agonist, fenoterol, on bronchial hyper responsiveness (BHR) to histamine. Their study group was in children with mild stable asthma. Daily diary cards were kept and lung function was assessed formally at monthly intervals through the study. The authors reported no statistically significant differences between the treatment groups in terms of:
‐ symptom scores of cough, wheeze, number of asthma attacks ‐ PEFR diurnal variation ‐ FEV1 ‐ PD 20 values
In the IB group, three of the 12 patients were withdrawn. The exact timing of this withdrawal is not documented by the authors. Two of these patients had an increase in asthma symptoms in the weeks prior to entering the study and the third patient's symptoms worsened during the study (in all patients in the trial, medications apart from trial drugs had been stopped one week before the start of the study).
3) INHALED ANTICHOLINERGICS PLUS BETA‐2 AGONISTS versus BETA‐2 AGONISTS
Two trials compared the effects of inhaled anticholinergic drugs combined with a beta‐2 agonist with a beta‐2 agonist alone (Bratteby 1986; Freeman 1989). Unfortunately data could not be combined in a meta‐analysis due to data presentation in the Freeman 1989 study. Both trials were cross‐over studies.
Bratteby 1986 studied 13 children in a two‐part study, the second part being relevant to this review. The authors reported that there was no statistically significant difference in symptom scores (recording daytime breathlessness, cough, physical activity, tremor, palpitations and sleep) between the two comparison groups except for a slightly higher score for disturbed sleep during the IB period (p < 0.05). The number of additional doses of salbutamol used were not significantly different between the two groups. The authors also reported that there was no statistically significant difference in the changes in PEFRs or in FEV1 between the two comparison groups.
Freeman 1989 studied 10 children in a two‐part study, the second part being relevant to this review. Once again, the authors reported no statistically significant differences in symptom scores (recording nocturnal wheeze and cough, daytime wheeze, daytime activity and sputum production) between the two study groups. In addition, there were no statistically significant differences in PEFRs, which were recorded twice daily. The authors did not examine the effect of trial drugs on diurnal variation. At three time points in the study, lung function tests were performed and results compared to those found at the baseline point in the study. No statistically different changes in lung function (as measured by FEV1, FVC, FEF 25‐75% and heart rate) were found between the two study groups.
In summary, both trials failed to show any significant benefit from the long term use of combined anticholinergics with beta‐2 agonists compared with beta‐2 agonists alone.
4) INHALED ANTICHOLINERGICS PLUS BETA‐2 AGONISTS versus INHALED ANTICHOLINERGICS
The trial by Zimmermann 1984 involved 39 children over a two week study period in a parallel trial. Children were either given fenoterol plus IB or IB alone. However, the dose of IB in the IB alone arm of the study was 5 times that of the dose in the combination arm, making data comparison difficult.
Results were reported by using means but no standard deviations were given. Data could therefore not be entered into the RevMan statistical software package. No tests for significance were calculated by the authors and the baseline data of the two groups were different. Therefore the results from this study will not be reported further. Despite diary cards for symptom scores being recorded by the patients, the results of these were not given in the text.
5) INHALED ANTICHOLINERGICS versus ORAL ANTICHOLINERGICS
The trial by Hutchison 1980 has already been discussed in the comparison above, "Inhaled anticholinergics versus placebo". This trial compared the effects of both inhaled and oral anticholinergics with placebo on chronic asthma in 12 children. The authors reported no statistically significant differences between any of the three study groups in terms of the number of days missed from school, night time waking, the subjective assessment of adequate asthma control or the need for additional doses of oral steroids. In addition, no significant difference in morning and evening PEFRs between the three groups was noted. The effects on diurnal variation were not examined in the trial. The authors reported a significantly higher FEV1 / VC ratio (p< 0.05) while subjects were taking oral atropine. In addition, the residual volume expressed as a percentage of total lung capacity (RV / TLC) was significantly reduced with oral atropine. Inhaled atropine was not found to be significantly different to placebo.
The trial by Vazquez 1984, has not been included in the results section above. The trial was a two‐part parallel study, the second part of which was relevant to this review. The first part examined and compared the effects of disodium cromoglycate, salbutamol and IB on exercise‐induced bronchospasm in 49 patients. 32 patients were then given the trial medications for one month and then reassessed. Outcome measures included symptom diaries, PEFRs and formal lung function (including FEV1) testing at the end of the trial. The study was published in Spanish but a translation was obtained. Unfortunately, the results section of the paper concentrated on reporting the results from the initial phase of the study. No results were given for symptom diaries, PEFRS or FEV1. In addition, the authors did not explain why only 32 of the original 49 patients continued into the second phase of the trial. The study authors have been contacted to obtain more information and their response is awaited.
Heterogeneity
Data were merged from the trials by Hutchison 1980; Mann 1982 and Sly 1987 so these were examined for heterogeneity for treatment setting and asthma severity. In all three studies, the trial settings were similar in that all patients were assessed in both the home and in the outpatient clinic. The three studies were then examined for heterogeneity in asthma severity. In Hutchinson, patients were included with severe chronic asthma. Patients were all said to be inadequately controlled despite maximal daily medication, including regular inhaled sympathomimetics, oral theophylline and inhaled and oral steroids. Mann enrolled patients with chronic asthma who were all on regular daily medication, although none were on oral steroids. Sly did not specifically state the asthma severity of their trial group but all children were on salbutamol, oral theophyllines and inhaled steroids, suggesting at least moderate asthma. The PEFR data analysed in the meta‐analysis from these three studies showed no evidence of heterogeneity (chi square = 0.23, p = 0.89), and was therefore not explored further.
The existence of publication bias would have been assessed by using a funnel plot. However, as only three studies could be merged these data were considered insufficient to examine for this. However, in future updates of this review, if more data can be merged then the presence of publication bias will be sought.
Mann was graded A by the Cochrane assessment of allocation of concealment, whereas the other seven trials scored B. Where data from three trials were compared (Mann 1982; Hutchison 1980; Sly 1987), similar results were found. So, although a formal sensitivity analysis could not be performed due to the low numbers, it was encouraging that similar data were found when trials of different allocation concealment were compared.
Secondly, each study was also assessed using a 1 to 5 scale described by Jadad 1995 as discussed above. One trial obtained a Jadad score of 4 (Bratteby 1986); four trials obtained a score of 3 (Mann 1982; Sly 1987; Hutchison 1980) and three studies a score of 2 (Freeman 1989; Vazquez 1984; Zimmermann 1984). However, despite the variation in trial quality, once again when data were compared between studies, findings were similar, suggesting that trial quality did not affect the findings of the review.
ADVERSE EVENTS AND EFFECTS
Very few adverse events or side effects were reported in any of the trials. Mann reported no apparent side effects but one child disliked the taste of IB. Sly did not discuss or monitor side effects. Hutchison 1980 reported no side effects for either inhaled or oral atropine. They did report however, that if they increased the dose of oral atropine, a small increase in their selected dose caused dry mouth and mydriasis. Bratteby 1986 reported mild tremor and palpitations in both their treatment groups (i.e. IB plus beta‐2 agonists and beta‐2 agonists alone). Zimmermann 1984 reported cough occurring in seven children in both treatment groups. Raes 1989; Freeman 1989; Vazquez 1984 reported no side effects during their study period.
Discussion
Anticholinergic agents are widely used in the treatment of acute asthma and in infants with airways obstruction with associated wheeze. A systematic review by the Cochrane Collaboration (Plotnick 2000) concluded that using multiple doses of anticholinergic in addition to beta‐2 agonists was beneficial in the management of acute asthma in children. It is also well recognised that a single dose of anticholinergic has bronchodilating effects although the effect may be slightly less and slower in onset of action than with beta‐2 agonists. However, the effectiveness of anticholinergics as part of the regular maintenance treatment in chronic asthma remains unclear, resulting in the need for this review.
Despite an extensive and thorough literature search in which hundreds of papers examining anticholinergic drugs were found, only eight met the inclusion criteria of this review. Three papers compared the effects of anticholinergic drugs with placebo, and a meta‐analysis of these results demonstrated no statistically significant benefit of the use of anticholinergic drugs over placebo in any of the outcome measures (including symptom scores, PEFRs and formal lung function testing). The results of one trial could not be included in the meta‐analysis but the authors did report significantly lower symptom scores with inhaled anticholinergics compared with placebo. However, there was no significant difference between IB and placebo in the percentage of symptom‐free nights or days.
Two trials studied the effects of anticholinergics on bronchial hyper responsiveness to histamine, by measuring the provocation dose of histamine needed to cause a fall of 20 % in FEV1 (PD 20). One study (comparing anticholinergics with placebo) reported a statistically significant increase in PD 20 but this was not found in another study (comparing anticholinergics with beta‐2 agonist). A meta‐analysis could not be performed to view the overall effect as the comparison groups were different.
Both of these trials also examined the effect of anticholinergic drugs on diurnal variation in PEFR and both reported no effect.
Two studies compared the addition of an anticholinergic drug to a beta‐2 agonist with a beta‐2 agonist alone. Although the results could not be combined in a meta‐analysis, both trials failed to show any significant benefit from the long term use of combined anticholinergics with beta‐2 agonists compared with beta‐2 agonists alone (symptom scores, PEFRs and lung function tests were used as outcome measures).
One trial compared the effects of oral and inhaled anticholinergic drugs with placebo. No statistically significant differences were found in any of the outcome measures except for a higher FEV1 / VC ratio and RV / TLC ratio (i.e. reduced "gas‐trapping") with oral anticholinergic therapy when compared with placebo.
Two non‐English language trials were included in our review. Unfortunately, data reporting in these papers was poor so conclusions are difficult to draw from these trials. In addition, the IB doses in the two arms of the Zimmermann 1984 study were different and therefore, comparison could not be made.
It was noted that three out of the five cross‐over studies did not incorporate a formal wash‐out period. However, we think that this is unlikely to have affected the study results as inhaled anticholinergic drugs are known to have a relatively short duration of action.
The present review summarises the best evidence available to date. Although there were some small beneficial findings in favour of anticholinergic therapy as detailed above, the results presented here do not support the use of anticholinergic drugs in the maintenance treatment of chronic asthma in children. Further work is required to clarify their exact role if any.
The British Thoracic Society guidelines (BTS 1993; BTS 1997; BTS 1997a) recommended the use of IB as regular maintenance therapy in schoolchildren who are already on high dose inhaled steroids (Step 4). They were not recommended for use in the management of chronic asthma in children under the age of 5 years. The new BTS/SIGN guidelines have recently been published (BTS 2003). From this review, it seems that there is little evidence to support their use for the maintenance treatment of chronic asthma in children, and this is reflected in the new guidelines that withdrawn the previous recommendation.
METHODOLOGICAL LIMITATIONS
Systematic bias Bias related to missed trials: a large volume of literature was searched for relevant studies. Despite a comprehensive search strategy it is possible that relevant studies were missed.
Power A significant proportion of outcome data from individual trials could not be included in the meta‐analysis because either standard deviation values for mean effect sizes were not presented in the original citation or no numerical data were presented at all. These missing data will diminish the power of the meta‐analysis.
Generalisability The studies included in this review included children with a spectrum of asthma severity being treated with a range of drug doses delivered by various means (nebuliser, MDI, MDI plus spacer) over a range of treatment periods. These factors should be taken into account when considering the findings of this review.
Authors' conclusions
Implications for practice.
In children with chronic asthma (ranging in severity from mild to severe), there is not enough data to assess the efficacy of adding long term anticholinergic therapy to maintenance treatment.
It does however appear that anticholinergic agents are safe and well tolerated.
Implications for research.
Future trials could improve on several aspects: interventions, choice of outcomes, and stratified reporting of results by asthma severity level. Several of the trials that were included in this review discussed the uncertainty about selecting appropriate doses and delivery methods of anticholinergic drugs. A variety of doses were therefore selected and drugs were delivered by a variety of delivery systems. In some of the trials, it was unclear how the drug had been delivered and if by inhaler +/‐ holding chamber, whether the inhaler technique had been checked.
It is clearly difficult to monitor the effects of the trial drug on a child's quality of life and asthma control over a long period of time when the child is at home. Completing symptom and PEFR diaries is time consuming and results are difficult to validate. Future trials should include more sensitive and reliable endpoints and should perhaps consider the use of researchers in the community environment to monitor outcome measures. When children are assessed in the hospital environment for formal lung function testing, a wider and more consistent choice of outcome measures would help to compare results from a number of studies.
Finally, the small number of trials found during this review meant that results could not be separated meaningfully with regard to asthma severity. This may be important and should be considered if further trials are planned.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2014 | Amended | PLS title amended |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 16 February 2010 | New search has been performed | Literature search re‐run; no new studies found. |
| 18 February 2009 | New search has been performed | Literature search run: no new studies identified. |
| 7 April 2008 | Amended | Converted to new review format. |
| 20 February 2008 | Amended | Search re‐run; no new eligible trials for inclusion in the review |
| 10 September 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank the Airways Group Editorial team, especially Karen Blackhall, Toby Lasserson and Bettina Reuben. In addition, we are most grateful to our translators; Celia Almeida, Molly Gong and Gianni Ferrara, and to Claire Allen for providing consumer advice on the synopsis. Finally, we are very grateful for the ongoing editorial support and advice from Dr Mike McKean.
No source of funding was available for this review.
Data and analyses
Comparison 1. Inhaled anticholinergics versus placebo (cross‐over studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak expiratory flow rate (am) | 3 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.31, 0.40] |
| 1.1 Ipratropium bromide | 2 | 98 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.35, 0.44] |
| 1.2 Atropine | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.76, 0.84] |
| 2 Peak expiratory flow rate (pm) | 3 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.33, 0.38] |
| 2.1 Ipratropium bromide | 2 | 98 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.34, 0.45] |
| 2.2 Atropine | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.90, 0.70] |
| 3 Number of participants with significant diurnal variation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ipratropium bromide | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Atropine | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Amplitude of diurnal variation in PEFRs | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 4.1 Ipratropium bromide | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Atropine | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Symptom score | 1 | Symptoms (Fixed, 95% CI) | Totals not selected | |
| 5.1 Ipratropium bromide | 1 | Symptoms (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Atropine | 0 | Symptoms (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 FEV1 (% predicted) | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 6.1 Ipratropium bromide | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Atropine | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 FEV1/VC (predicted) | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 7.1 Ipratropium bromide | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Atropine | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 FEF 25‐75% (predicted) | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 8.1 Ipratropium bromide | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Atropine | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 RV (predicted) | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 9.1 Ipratropium bromide | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Atropine | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 RV/TLC (predicted) | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 10.1 Ipratropium bromide | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Atropine | 1 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Hospital admissions | 2 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.25] |
| 11.1 Ipratropium bromide | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.53] |
| 11.2 Atropine | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.90] |
| 12 Need for oral steroids | 2 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.21, 3.03] |
| 12.1 Ipratropium bromide | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.15] |
| 12.2 Atropine | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.25, 8.84] |
| 13 Additional doses of beta‐2 agonists | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Ipratropium bromide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Atropine | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Use of additional medication (physician directed) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 Ipratropium bromide | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Atropine | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 1 Peak expiratory flow rate (am).
1.2. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 2 Peak expiratory flow rate (pm).
1.3. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 3 Number of participants with significant diurnal variation.
1.4. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 4 Amplitude of diurnal variation in PEFRs.
1.5. Analysis.
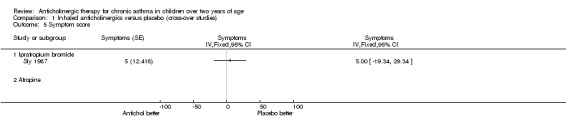
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 5 Symptom score.
1.6. Analysis.
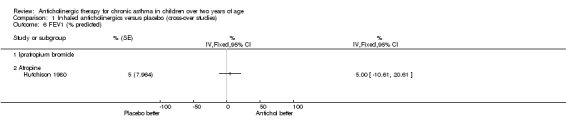
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 6 FEV1 (% predicted).
1.7. Analysis.
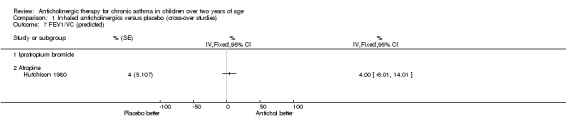
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 7 FEV1/VC (predicted).
1.8. Analysis.
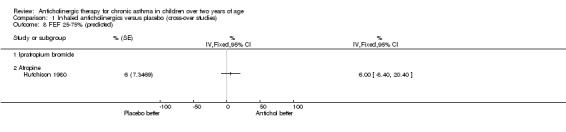
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 8 FEF 25‐75% (predicted).
1.9. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 9 RV (predicted).
1.10. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 10 RV/TLC (predicted).
1.11. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 11 Hospital admissions.
1.12. Analysis.
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 12 Need for oral steroids.
1.13. Analysis.
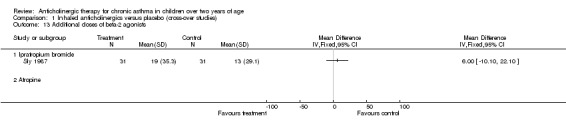
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 13 Additional doses of beta‐2 agonists.
1.14. Analysis.
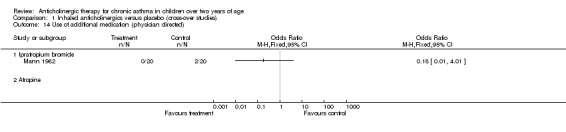
Comparison 1 Inhaled anticholinergics versus placebo (cross‐over studies), Outcome 14 Use of additional medication (physician directed).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bratteby 1986.
| Methods | Design: randomised, double‐blind, cross‐over study. Method of randomisation: not stated. Concealment of randomisation: unclear (B) Blinding: double‐blind, using identical aerosols for trial drug and placebo. Description of withdrawals or dropouts: no. Jadad's score: 4 | |
| Participants | Number enrolled into trial: 13 Number in treatment group: 13 Number in control group: 13 Number of withdrawals or dropouts (treatment/control): 0 Number completing trial (treatment / control): 13. Age (range): 9.3‐16.7 yrs Age (mean): 11.9 yrs Sex: 8 males, 5 females. Asthma diagnosis: Physician diagnosed. Severity of asthma: Chronic bronchial asthma on daily medication. Inclusion criteria: (1) Moderate to severe asthma in a stable state, and (2) Reversibility of bronchial obstruction with >15% improvement in FEV1 after inhaling nebulised salbutamol (0.1 mg/kg of body weight). | |
| Interventions | Setting: Home / outpatient clinic. Types: Ipratropium bromide 40mcg (2 puffs) via metered aerosol QDS ‐ each dose preceded by an inhalation of salbutamol as a metered aerosol (0.1mg per inhalation). Duration: Total 4 weeks (2 X 2 weeks) | |
| Outcomes | Outcomes: Diary card recording:
‐Symptoms (scores graded 0‐3: 0=no symptoms, 1=mild symptoms, 2=moderate symptoms, 3=severe symptoms)
‐Additional medications taken
‐PEFRs 4 times daily
Adverse events: side effects recorded in daily diary, specifically palpitations and tremor. Only results from days 5‐14 used in analysis (to provide wash‐out period). |
|
| Notes | Study in two parts, second part relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Freeman 1989.
| Methods | Design: randomised, double‐blind, cross‐over study. Method of randomisation: not stated. Concealment of randomisation: unclear (B) Blinding: double‐blind Description of withdrawals or dropouts: no. Jadad's score: 2. | |
| Participants | Number enrolled into trial: 10 Number in treatment group: 10 Number in control group: 10. Age (range): 6‐14 yrs Age (mean): 9 yrs Sex: 6 males, 4 females. Asthma diagnosis: Physician diagnosed. Severity of asthma: Moderate asthma, all except one patient on oral theophyllines, 4 pts on sodium cromoglycate (SCG), 3 on inhaled steroids. Inclusion criteria: Chronic asthma. Exclusion criteria: known hypersensitivity to ipratropium bromide +/‐ sympathomimetic drugs or if known cardiac disease / thyrotoxicosis or other major illnesses. Source of participants: hospital outpatient clinic. | |
| Interventions | Setting: Home / outpatient clinic. Types: Ipratropium bromide nebulised (125 ‐ 250mcg according to age) plus nebulised fenoterol (0.2‐0.4mg according to age) vs placebo plus fenoterol., each given three times daily. Duration: Total 8 weeks (2 X 4 weeks). Additional notes: No washout period stated. | |
| Outcomes | Outcome: Diary cards recording nocturnal wheeze and cough, daytime wheeze, daytime activity, sputum production and PEFRs (am and pm) Lung function testes were performed at each of three visits (before starting trial, after the 1st month and after the 2nd month). Adverse events: Daily diary | |
| Notes | Study in two parts, second part relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hutchison 1980.
| Methods | Design: randomised, double‐blind, cross‐over study. Method of randomisation: not stated. Concealment of randomisation: unclear (B). Blinding: double‐blind ‐ placebo tablets or inhalations. Description of withdrawals or dropouts: not stated. Jadad's score: 3 | |
| Participants | Number enrolled into trial: 12 Number in treatment group : 12 Number in control group : 12 Number of withdrawals or dropouts : None stated. Age (range): 7‐17 yrs Sex: 11 males, 1 females. Asthma diagnosis: Physician diagnosed. Severity of asthma: Severe chronic asthma. Inclusion criteria: Inadequate control despite maximum therapy with regular inhaled sympathomimetics, oral theophylline, inhaled and oral steroids. Source of participants: Not stated. | |
| Interventions | Setting: Home / outpatient clinic. Types: (1) Inhaled atropine and oral placebo; (2) Oral atropine and inhaled placebo; (3) Double placebo. Duration: Total 3 months (3 X 4 weeks of each treatment). Additional notes: Medications given TDS, and all regular medication continued. Atropine (inhaled) dose: 20mcg/kg inhalation (i.e. 60mcg/kg/24 hrs) Atropine (oral) dose: 20mcg/kg TDS. No washout period stated. | |
| Outcomes | Outcomes: Weekly diary card recording symptoms, days of school missed, night time waking and amount of medications, particularly use of oral steroids. PEFRs measured daily before morning and evening treatment. Lung function tests performed at end of each study period. VC, FEV1, FEV1/VC and FEF25‐75% were calculated. Adverse events: side effects recorded in daily diary (with particular reference to blurred vision and dry mouth). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mann 1982.
| Methods | Design: randomised, double‐blind, cross‐over study. Method of randomisation: not stated. Concealment of randomisation: adequate (A) ‐ inhalers coded by manufacturers, code broken after trial completion. Blinding: double‐blind. Description of withdrawals or dropouts: yes. Jadad's score: 3 | |
| Participants | Number enrolled into trial: 20 Number in treatment group: 20 Number in control group: 20 Number of withdrawals or dropouts (treatment/control): 2 (failed to keep adequate records). Number completing trial (treatment / control): 18. Age (range): 6‐14 yrs Age (mean): 9.2 yrs Sex: 9 males, 11 females. Asthma diagnosis: Physician diagnosed. Severity of asthma: Chronic bronchial asthma on daily medication ‐ 8 pts on regular SCG or inhaled steroids. None on oral steroids. Inclusion criteria: Pts selected from those attending paediatric asthma clinic for > 6 months. Source of participants: Outpatient clinic. | |
| Interventions | Setting: Home / outpatient clinic. Types: Ipratropium bromide 40mcg (2 puffs inhaled via metered dose inhaler) TDS for 4 weeks vs placebo inhalers TDS for 4 weeks. Duration: Total 8 weeks (2 X 4 weeks). Additional notes: No washout period stated | |
| Outcomes | Outcomes: Diary card recording: ‐Symptoms of day and night cough and wheeze (scoring 0 for no symptoms, 1 for occasional wheeze and/or cough, 2 for troublesome wheeze and/or cough, and 3 for very ill with severe wheeze and/or cough). ‐PEFRs measured am and pm before treatment. ‐Need for additional bronchodilators ‐Need for any other drugs e.g. steroids. Adverse events: side effects recorded in daily diary. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Raes 1989.
| Methods | Design: randomised, double‐blind, parallel study. Method of randomisation: allocation to group by study sponsor. Concealment of randomisation: unclear (B). Blinding: double‐blind ‐ pharmaceutical company provided each patient with coded boxes containing drug.. Description of withdrawals or dropouts: yes. Jadad's score: 3 | |
| Participants | Number enrolled into trial: 20 Number in treatment group (ipratropium bromide): 12 Number in control group (salbutamol): 8 Number of withdrawals or dropouts (treatment group): 3. Number completing trial (treatment / control): 17. Age (range): 7‐15 yrs Sex: 14 males, 6 females. Asthma diagnosis: Physician diagnosed. Severity of asthma: Mild chronic symptomatic asthma Inclusion criteria: (1) PD20 of <150mcg, (2) FEV1 and FVC > 80% of predicted, (3) FEV1/FVC >70% of predicted, (4) <20% increase in baseline FEV1 after 0.4mg of inhaled fenoterol. Source of participants: Outpatient clinic. Additional notes: Study performed between February and July (European study). | |
| Interventions | Setting: Home / outpatient clinic. Types: Ipratropium bromide 40mcg (2 puffs inhaled via metered dose inhaler) TDS vs fenoterol 0.2mg via inhaler TDS. Duration: Total 4 months. | |
| Outcomes | Outcomes: Diary card recording: ‐Symptoms of cough, wheeze and frequency of asthma attacks (score severity between 0 to 3 for each item). ‐PEFRs measured am and pm before treatment. ‐Need for additional bronchodilators ‐Need for any other drugs e.g. steroids. At clinic (monthly assessments): PD20 and FEV1. Adverse events: side effects recorded in daily diary. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sly 1987.
| Methods | Design: randomised, double‐blind, cross‐over study. Method of randomisation: not stated. Concealment of randomisation: unclear (B). Blinding: double‐blind ‐ identically labelled aerosols labelled A or B. Description of withdrawals or dropouts: no. Jadad's score: 3 | |
| Participants | Number enrolled into trial: 31 Number in treatment group : 31 Number in control group : 31 Number of withdrawals or dropouts : None stated. Age (range): 8‐18 yrs Age (mean): 12.0 yrs Sex: 21 males, 10 females. Asthma diagnosis: Physician diagnosed. Severity of asthma: Not stated. All children on salbutamol (via nebuliser or inhaler), theophylline (oral) and inhaled steroids as maintenance therapy. Inclusion criteria: Nil stated. Source of participants: Not stated. | |
| Interventions | Setting: Home / outpatient clinic. Types: Ipratropium bromide 40mcg (2 puffs inhaled via metered dose inhaler) TDS for 4 weeks vs placebo inhalers TDS for 4 weeks. At end of each group each patient had histamine challenge. Duration: Total 8 weeks (2 X 4 weeks). Additional notes: Washout period of 4 weeks between trial drugs. | |
| Outcomes | Outcomes: Daily diary card recording: ‐Symptoms of amount of wheeze during the night; amount of cough during the night; amount of wheeze during the day and limitation of activity during the day (score between 0 to 3 for each of the above). Symptom scores were added daily to give a daily total. ‐PEFRs measured on waking, at 1600 hrs and at bedtime. ‐Medications taken. Histamine challenges performed at completion of each study period. Adverse events: side effects recorded in daily diary. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Vazquez 1984.
| Methods | Design: randomised, double‐blind parallel study. Method of randomisation: not stated. Concealment of randomisation: unclear (B). Blinding: double‐blind. Description of withdrawals or dropouts: not stated. Jadad's score: 2. | |
| Participants | Number enrolled into trial: 49 Number in treatment groups: Group A (salbutamol)=13 Group B (disodium cromoglycate)=12 Group C (ipratropium bromide)=12 Group D (placebo)=12 Number of withdrawals or dropouts : 17 but reason unclear. Age (mean): 9.4 yrs (SD=2.05) Sex: not stated. Asthma diagnosis: Diagnosed according to ATS definition. Severity of asthma: Asymptomatic asthma. Inclusion criteria: All showed exercise‐induced asthma, all except 2 had atopic asthma. Source of participants: Allergy or respiratory consultations. | |
| Interventions | Types: Group A = salbutamol 200mcg; Group B = disodium cromoglycate 20mg; Group C = ipratropium bromide 40 mcg; Group D = placebo. All medications were by inhaler at a dose of two puffs administered three times a day. Duration: 1 month. | |
| Outcomes | Outcomes: Diary records, spirometry (FEV1, PEFR), exercise test. Spirometry measured pre‐exercise and immediately post‐exercise, followed by at 5, 10, 15 and 20 minutes post exercise. | |
| Notes | Study in two parts, second part relevant to this review. Data from 32 patients only were obtained for the second part of the study (17 did not return). We are unable to report the reasons for this due to limited translation of the paper. In addition, we suspect that there was a typing error in the paper. In the second part of the study, doses were quoted in 'mg' where we suspect 'mcg' was meant instead. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zimmermann 1984.
| Methods | Design: randomised, double‐blind parallel study. Method of randomisation: not stated. Concealment of randomisation: unclear (B). Blinding: double‐blind. Description of withdrawals or dropouts: not stated. Jadad's score: 2. | |
| Participants | Number enrolled into trial: 39 Group A (ipratropium bromide + fenoterol) = 20. Group B (ipratropium bromide)=19. Number of withdrawals or dropouts : None stated. Age (mean): 4 to 14 years and 9 months. Sex: 30 males, 9 females. Asthma diagnosis: Physician diagnosed. Severity of asthma: Six had severe asthma, 27 had moderate asthma, and six had mild asthma. Inclusion criteria: Asthma for greater than one year. Source of participants: Paediatric outpatient clinic. | |
| Interventions | Types: Group A = 0.1mg fenoterol + 0.04mg ipratropium bromide. Group B = 0.2mg ipratropium bromide. All medications were by inhaler at a dose of two to three puffs administered three times a day. Duration: 2 weeks. | |
| Outcomes | Outcomes: FEV1, symptom scores, concomitant medication usage, subjective effectiveness, safety and tolerability, total airway resistance, specific airway resistance. | |
| Notes | Limited data obtained because of translation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agostini 1983 | Exercise‐induced asthma |
| Agostini 1983b | Exercise‐induced asthma (study same trial as above, published in different journal) |
| Alanko 1973 | Study group ‐ adults only. |
| Allen 1985 | Study involved 11 patients but ages were not specified. Abstract only obtained so far, authors have been written to requesting more information. Their response is awaited. |
| Bach‐Mortensen 1987 | Exercise‐induced asthma. |
| Bandouvakis 1981 | Study group ‐adults only. |
| Beck 1985 | Study on acute asthma only. |
| BenitoFernandez 2000 | Study in acute asthma only. |
| Boner 1984 | Exercise‐induced asthma. |
| Boner 1987 | Study on chronic asthma but a single‐dose study only. |
| Boner 1987b | Study on chronic asthma but a single‐dose study only. |
| Borut 1977 | Exercise‐induced asthma. |
| Caubet 1988 | Study on chronic asthma but a single‐dose study only. |
| Chen 1981 | Exercise‐induced asthma. |
| Chervinsky 1977 | Study group ‐ adults only. |
| Clarke 1982 | Study group of 12 patients. Three patients were aged 13, 17 and 18 years. The author was written to and a response was received. Unfortunately Dr Clarke died a few years ago and so no further information could be obtained. |
| Cook 1985 | Study in acute asthma only. |
| Coulthard 1985 | Published data obtained is in abstract form only. The authors have been contacted for more information. Their response is awaited. |
| Craven 2001 | Study in acute asthma only. |
| Crimi 1986 | Study in acute asthma only. |
| Crimi 1992 | Study group ‐ adults only. |
| Cropp 1975 | Study on chronic asthma but a single‐dose study only. |
| Davis 1984 | Study on chronic asthma but a single‐dose study only. |
| DeStefano 1990 | Study on chronic asthma but a single‐dose study only. |
| Ducharme 1998 | Study in acute asthma only. |
| Dutt 1990 | Acute study conducted over 300 minutes |
| Filiz 1994 | Study group ‐ adults only. |
| Friberg 1989 | Study on chronic asthma but a single‐dose study only. |
| Godfrey 1975 | Exercise‐induced asthma. |
| Godfrey 1976 | Exercise‐induced asthma, sama data as in Godfrey, 1975 but published in different journal. |
| Greenough 1993 | Study on chronic asthma but a single‐dose study only. |
| Groggins 1981 | Study on chronic asthma but a single‐dose study only. |
| Groot 1994 | Study group ‐ adults only. |
| Gross 1975 | Study group ‐ adults only. |
| Guill 1987 | Study on acute asthma only. |
| Hemstreet 1980 | Unclear if study was an RCT. Published data unclear so authors have been contacted. Their response is awaited. |
| Henry 1989 | Study excluded as comparison of preservative‐free IB versus preservative containing IB. Study did therefore not meet inclusion criteria. |
| Hodges 1981 | Study in "acute wheeze" only. Alsp unclear if study an RCT. |
| Jedrys 1994 | Unclear if study a RCT. Authors have been contacted. Their response is awaited. |
| Kishida 1992 | Study excluded as did not involve an anticholinergic drug. |
| Krasnowska 1994 | Study less than 7 days duration |
| Krasnowska 2004 | Study conducted in adults |
| Kreisman 1984 | Study in acute asthma only. |
| Larsson 1987 | Study group ‐ adults only. |
| Lefcoe 1982 | Study group ‐ adults only. |
| Lenney 1986 | Study on chronic asthma but a single‐dose study only. Unclear if study an RCT. |
| Lew 1990 | Study in acute asthma only. |
| Li 2003 | Study conducted in acute setting. |
| Lightbody 1978 | Study in adults only. |
| Lin 1978 | Not an RCT ‐ maintenance phase of trial was "open". |
| Lines 1983 | Published data obtained in abstract form only. The authors were contacted for more information. A response was received from Professor Lines but no more data was onbtained. |
| Mannino 1996 | Study group ‐ adults only. |
| Mazzei 1986 | Study group ‐ adults only. |
| McNeill 1966 | Exercise‐induced asthma. |
| Miraglia 1983 | Study not explicitly described as randomised. |
| Muranyi 1974 | Study not an RCT. Study used parenteral atropine only. |
| Neijens 1981 | Exercise‐induced asthma. |
| Neild 1985 | Study group ‐ adults only. |
| Pasterkamp 1985 | Exercise‐induced asthma. |
| Pauwels 1995 | Study less than 7 days duration. |
| Peters 2000 | Study group ‐ adults only. Study also in acute asthma although followed for 24 hours after admission to hospital. |
| Poppius 1973 | Exercise‐induced asthma; study group ‐ adults only. |
| Rachelefsky 1978 | Exercise‐induced asthma. |
| Rayner 1987 | Study in acute asthma only. |
| Ream 2001 | Study in acute asthma only. |
| Rebuck 1983 | Study group ‐ adults only. |
| Reisman 1988 | Study in acute asthma only. |
| Russo 1986 | Exercise‐induced asthma. Unclear if trial an RCT. |
| Sanguinetti 1986 | Exercise‐induced asthma. |
| Schlueter 1978 | Study group ‐ adults only. |
| Schuh 1995 | Study in acute asthma only. |
| Sienra Monge 2000 | Study in acute asthma only. |
| Silverman 1981 | Study excluded as did not involve an anticholinergic drug. |
| Simone 1986 | Study group ‐ adults only. |
| Simonsson 1979 | This paper was a review of other studies which have been retrieved. |
| Stokes 1983 | This paper was a review of other studies which have been retrieved. |
| Storms 1975 | Study group ‐ adults only. |
| Storms 1986 | Study group ‐ ? if in adults only. The author was contacted and a response was received. However, no further information about this could be found. |
| Storr 1986 | Study in acute asthma only. |
| Sur 1990 | Study group of 11 patients, 3 later excluded. Study group aged 10‐31 yrs ‐ 3 patients aged 13, 17 and 18 years. Authors contacted for data on children. Their response is awaited. |
| Svenonius 1988 | Exercise‐induced asthma. |
| Tasche 1997 | Study excluded as did not involve an anticholinergic drug. |
| Tashkin 1977 | Exercise‐induced asthma. |
| Taytard 1987 | Exercise‐induced asthma. |
| Thomson 1978 | Study group ‐ adults only. |
| Tinkelman 1976 | Exercise‐induced asthma. |
| Town 1988 | Study group ‐ adults only. |
| Tullett 1982 | Study group ‐ adults only. |
| Ulrik 1992 | Study did not meet inclusion criteria. |
| Vichyanond 1990 | Study on chronic asthma but a single‐dose study only. |
| Walker 1987 | Study group ‐ adults only. |
| Ward 1981 | Study group ‐ adults only. Study in acute asthma only. |
| Ward 1985 | Study group ‐ adults only. Study in acute asthma only. |
| Watson 1988 | Study in acute asthma only. |
| Watson 1994 | Study did not assess effects of drugs on asthma. |
| Wilson 1984 | Exercise‐induced asthma. |
| Wilson 1987 | Study included 10 patients, aged 9 ‐ 57 years. Authors were contacted for childrens' data, their response is awaited. |
| Wolkove 1981 | Study group ‐ adults only. |
| Yeung 1980 | Exercise‐induced asthma. |
| Yung 1998 | Study excluded as did not involve an anticholinergic drug. |
| Zaritsky 1999 | Study in acute asthma only. |
| Zorc 1999 | Study in acute asthma only. |
Contributions of authors
NM: Protocol initiation, assessed search results, data extraction, data analysis, interpretation AB: Protocol development, assessed search results, data extraction, data entry MM: Editorial guidance and support throughout
Sources of support
Internal sources
No sources of support supplied
External sources
Garfield Weston Foundation, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bratteby 1986 {published data only}
- Bratteby LE, Foucard T, Lonnerholm G. Combined treatment with ipratropium bromide and beta‐2‐adrenoceptor agonists in childhood asthma. European Journal of Respiratory Diseases 1986;68(4):239‐47. [PubMed] [Google Scholar]
Freeman 1989 {published data only}
- Freeman J, Landau LI. The effects of ipratropium bromide and fenoterol nebulizer solutions in children with asthma. Clinical Pediatrics 1989;28(12):556‐60. [DOI] [PubMed] [Google Scholar]
Hutchison 1980 {published data only}
- Hutchison AA, Olinsky A, Landau LI. Long term atropine in chronic severe childhood asthma. Australian Paediatric Journal 1980;16(4):267‐9. [DOI] [PubMed] [Google Scholar]
Mann 1982 {published data only}
- Mann NP, Hiller EJ. Ipratropium bromide in children with asthma. Thorax 1982;37(1):72‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raes 1989 {published data only}
- Raes M, Mulder P, Kerrebijn KF. Long‐term effect of ipratropium bromide and fenoterol on the bronchial hyperresponsiveness to histamine in children with asthma. Journal of Allergy & Clinical Immunology 1989;84(6 (Pt 1)):874‐9. [DOI] [PubMed] [Google Scholar]
Sly 1987 {published data only}
- Sly PD, Landau LI. Ipratropium bromide does not diminish the diurnal variation of asthma in children. Australian and New Zealand Journal of Medicine ‐ Supplement. 1985;15(4):496. [Google Scholar]
- Sly PD, Landau LI, Olinsky A. Failure of ipratropium bromide to modify the diurnal variation of asthma in asthmatic children. Thorax 1987;42(5):357‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vazquez 1984 {published data only}
- Vazquez C, Fidalgo I, Virto MC, Labayru MT, Casas C. Effectiveness of disodium cromoglycate, salbutamol, and ipratropium bromide in the inhibition of exercise‐induced bronchospasm. Anales Espanoles De Pediatria 1984;20(8):756‐62. [PubMed] [Google Scholar]
Zimmermann 1984 {published data only}
- Zimmermann T, Drexel W. Bronchial asthma in childhood. Therapy with fenoterol and ipratropium bromide powder. Monatsschr Kinderheilkd 1984;132(12):915‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Agostini 1983 {published data only}
- Agostini M, Barlocco G, Mastella G. Protection against exercise induced asthma in children. Double blind controlled clinical trial with fenoterol spray, fenoterol plus ipratropium and oral clenbuterol. Rivista Italiana di Pediatrica 1983;9(4):347‐52. [PubMed] [Google Scholar]
Agostini 1983b {published data only}
- Agostini M, Barlocco G, Mastella G. Protective effect of fenoterol spray, ipratropium bromide plus fenoterol spray, and oral clenbuterol, on exercise‐induced asthma in children. Double blind controlled and randomized clinical trial. European Journal of Respiratory Diseases ‐ Supplement 1983;128(Pt 2):529‐32. [PubMed] [Google Scholar]
Alanko 1973 {published data only}
- Alanko K, Poppius H. Anticholinergic blocking of prostaglandin‐induced bronchoconstriction. British Medical Journal 1973;1(848):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1985 {published data only}
- Allen HD, Mellis CM. Reproducibility and blocking studies with metabisulphite. Australian and New Zealand Journal of Medicine ‐ Supplement 1985;15(4):497. [Google Scholar]
Bach‐Mortensen 1987 {published data only}
- Bach‐Mortensen N, Bundgaard A, Schmidt A. Ipratropium (Atrovent) treatment ‐ alone and in combination ‐ in children with exercise‐induced asthma. Postgraduate Medical Journal 1987;63(Suppl 1):11a. [Google Scholar]
Bandouvakis 1981 {published data only}
- Bandouvakis J, Cartier A, Roberts R, Ryan G, Hargreave FE. The effect of ipratropium and fenoterol on methacholine‐histamine‐induced bronchoconstriction. British Journal of Diseases of the Chest 1981;75(3):295‐305. [DOI] [PubMed] [Google Scholar]
Beck 1985 {published data only}
- Beck R, Robertson C, Galdes‐Sebaldt M, Levison H. Combined salbutamol and ipratropium bromide by inhalation in the treatment of severe acute asthma. Journal of Pediatrics 1985;107(4):605‐8. [DOI] [PubMed] [Google Scholar]
BenitoFernandez 2000 {published data only}
- Benito Fernandez J, Mintegui Raso S, Sanchez Echaniz J, Vazquez Ronco MA, Pijoan Zubizarreta JI. Efficacy of early administration of nebulized ipratropium bromide in children with acute asthma attack. Anales Espanoles de Pediatria 2002;53(3):217‐222. [PubMed] [Google Scholar]
Boner 1984 {published data only}
- Boner AL, Zamo CR, Marchiori MM, Biancotto R, Antolini I, Vallone G. Comparison of nebulized ipratropium, salbutamol and cromoglicate solutions, cromoglicate inhaled powder, theophylline elixir and placebo in exercise induced asthma of the children [Confronto fra nebulizzazione di ipratropio, salbutamolo e cromoglicato in soluzione, cromoglicato in polvere inalabile, teofillina elisir e placebo nell'asma da esercizio dei bambini]. Italian Journal of Chest Diseases (Giornale Italiano Malattie del Torace) 1984;38(6):395‐9. [Google Scholar]
Boner 1987 {published data only}
- Boner AL, Stefano G, Niero E, Vallone G, Gaburro D. Salbutamol and ipratropium bromide solution in the treatment of bronchospasm in asthmatic children. Annals of Allergy 1987;58(1):54‐8. [PubMed] [Google Scholar]
Boner 1987b {published data only}
- Boner AL, Antolini I, Andreoli A, Stefano G, Sette L. Comparison of the effects of inhaled calcium antagonist verapamil, sodium cromoglycate and ipratropium bromide on exercise‐induced bronchoconstriction in children with asthma. European Journal of Pediatrics 1987;146(4):408‐11. [DOI] [PubMed] [Google Scholar]
Borut 1977 {published data only}
- Borut TC, Tashkin DP, Fischer TJ, Katz R, Rachelefsky G, Siegel SC, Lee E, Harper C. Comparison of aerosolized atropine sulfate and SCH 1000 on exercise‐induced bronchospasm in children. Journal of Allergy and Clinical Immunology 1977;60(2):127‐33. [DOI] [PubMed] [Google Scholar]
Caubet 1988 {published data only}
- Caubet Y. Efficacy, safety and preference of bronchodilator aerosol‐dosers in older asthmatic children [Efficacite tolerance et preference chez le grand enfant des aerosols doseurs de bronchodilatateurs]. La Revue de Pediatrie 1988;24(5):215‐21. [Google Scholar]
Chen 1981 {published data only}
- Chen WY, Brenner AM, Weiser PC, Chai H. Atropine and exercise‐induced bronchoconstriction. Chest 1981;79(6):651‐6. [DOI] [PubMed] [Google Scholar]
Chervinsky 1977 {published data only}
- Chervinsky P. Double‐blind study of ipratropium bromide, a new anticholinergic bronchodilator. Journal of Allergy & Clinical Immunology 1977;59(1):22‐30. [DOI] [PubMed] [Google Scholar]
Clarke 1982 {published data only}
- Clarke PS, Jarrett RG, Hall GJ. The protective effect of ipratropium bromide aerosol against bronchospasm induced by hyperventilation and the inhalation of allergen, methacholine and histamine. Annals of Allergy 1982;48(3):180‐3. [PubMed] [Google Scholar]
Cook 1985 {published data only}
- Cook JJ, Fergusson DM, Dawson KP. Ipratropium and fenoterol in the treatment of acute asthma. Pharmatherapeutica 1985;4(6):383‐6. [PubMed] [Google Scholar]
Coulthard 1985 {published data only}
- Coulthard K, Lines D, Rossi S, Cressi N. Inhaled ipratropium bromide (atrovent) in asthmatic children. Australian Journal of Hospital Pharmacy 1985;Suppl:52. [Google Scholar]
Craven 2001 {published data only}
- Craven D, Kercsmar CM, Myers TR, O'Riordan MA, Golonka G, Moore S. Ipratropium bromide plus nebulized albuterol for the treatment of hospitalized children with acute asthma. Journal of Pediatrics 2001;138(1):51‐8. [DOI] [PubMed] [Google Scholar]
Crimi 1986 {published data only}
- Crimi N, Palermo F, Ciccarello C, Distefano SM, Vancheri C, Cacopardo B, Mistretta A. Effect of Duovent (ipratropium bromide and fenoterol) on non‐specific bronchial hyperreactivity. Respiration 1986;50(Suppl 2):206‐8. [DOI] [PubMed] [Google Scholar]
Crimi 1992 {published data only}
- Crimi N, Palermo F, Oliveri R, Polosa R, Settinieri I, Mistretta A. Protective effects of inhaled ipratropium bromide on bronchoconstriction induced by adenosine and methacholine in asthma. European Respiratory Journal 1992;5(5):560‐5. [PubMed] [Google Scholar]
Cropp 1975 {published data only}
- Cropp GJA. The role of the parasythmpathetic nervous system in the maintenance of chronic airway obstruction in asthmatic children. American Review of Respiratory Disease 1975;112(5):599‐605. [DOI] [PubMed] [Google Scholar]
Davis 1984 {published data only}
- Davis A, Vickerson F, Worsley G, Mindorff C, Kazim F, Levison H. Determination of dose‐response relationship for nebulized ipratropium in asthmatic children. Journal of Pediatrics 1984;105(6):1002‐5. [DOI] [PubMed] [Google Scholar]
DeStefano 1990 {published data only}
- DeStefano G, Bonetti S, Bonizzato C, Valletta EA, Piacentini GL, Boner AL. Additive effect of albuterol and ipratropium bromide in the treatment of bronchospasm in children. Annals of Allergy 1990;65(4):260‐2. [PubMed] [Google Scholar]
Ducharme 1998 {published data only}
- Ducharme FM, Davis GM. Randomized controlled trial of ipratropium bromide and frequent low doses of salbutamol in the management of mild and moderate acute pediatric asthma. Journal of Pediatrics 1998;133(4):479‐85. [DOI] [PubMed] [Google Scholar]
Dutt 1990 {published data only}
- Dutt NS, Reddy TC, Raghuveer TS, Yeshwanth M, Sunil Dutt N, Chinnapa Reddy T, et al. A comparative study of atropine sulphate, terbutaline and their combination in asthmatic children. Lung India 1990;8(2):73‐5. [Google Scholar]
Filiz 1994 {published data only}
- Filiz A, Ekinci E, Dikensoy O, Bulgur D, Oz M. Comparison of a single dose of aerosol salbutamol and fenoterol/ipratropium on bronchial asthmatic patients. Delaware Medical Journal 1994;66(10):549‐52. [PubMed] [Google Scholar]
Friberg 1989 {published data only}
- Friberg S, Graff‐Lonnevig V. Ipratropium bromide (Atrovent) in childhood asthma: a cumulative dose‐response study. Annals of Allergy 1989;62(2):131‐4. [PubMed] [Google Scholar]
Godfrey 1975 {published data only}
- Godfrey S, Konig P. Suppression of exercise‐induced asthma by salbutamol, theophylline, atropine, cromolyn, and placebo in a group of asthmatic children. Pediatrics 1975;56(5 pt‐2 Suppl):930‐4. [PubMed] [Google Scholar]
Godfrey 1976 {published data only}
- Godfrey S, Konig P. Inhibition of exercise induced asthma by different pharmacological pathways. Thorax 1976;31(2):137‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenough 1993 {published data only}
- Greenough A, Yuksel B, Everett L, Price JF. Inhaled ipratropium bromide and terbutaline in asthmatic children. Respiratory Medicine 1993;87(2):111‐4. [DOI] [PubMed] [Google Scholar]
Groggins 1981 {published data only}
- Groggins RC, Milner AD, Stokes GM. Bronchodilator effects of clemastine, ipratropium, bromide, and salbutamol in preschool children with asthma. Archives of Disease in Childhood 1981;56(5):342‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groot 1994 {published data only}
- Groot CA, Lammers JW, Festen J, Herwaarden CL. The protective effects of ipratropium bromide and terbutaline on distilled water‐induced bronchoconstriction. Pulmonary Pharmacology 1994;7(1):59‐63. [DOI] [PubMed] [Google Scholar]
Gross 1975 {published data only}
- Gross NJ. Sch 1000: a new anticholinergic bronchodilator. American Review of Respiratory Disease 1975;112(6):823‐8. [DOI] [PubMed] [Google Scholar]
Guill 1987 {published data only}
- Guill MF, Maloney MJ, DuRant RH. Comparison of inhaled metaproterenol, inhaled atropine sulfate, and their combination in treatment of children with acute asthma. Annals of Allergy 1987;59(5):367‐71. [PubMed] [Google Scholar]
Hemstreet 1980 {published data only}
- Hemstreet MP. Atropine nebulization‐‐simple and safe. Annals of Allergy 1980;44(3):138‐41. [PubMed] [Google Scholar]
Henry 1989 {published data only}
- Henry RL, Hankin RG, Abramson R. Comparison of preservative‐free and preservative‐containing ipratropium bromide. Australian Paediatric Journal 1989;25(2):86‐8. [DOI] [PubMed] [Google Scholar]
Hodges 1981 {published data only}
- Hodges IGC, Groggins RC, Milner AD, Stokes GM. Bronchodilator effect of inhaled ipratropium bromide in wheezy toddlers. Archives of Disease in Childhood 1981;56(9):729‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jedrys 1994 {published data only}
- Jedrys U, Kurzawa R, Haluszka J, Smieszek J, Doniec Z, Willim G. Evaluation of the bronchodilatory activity of berodual and its components: fenoterol and ipratropium bromide in children with bronchial asthma. Pneumonologia I Alergologia Polska 1994;62(11‐12):615‐22. [PubMed] [Google Scholar]
Kishida 1992 {published data only}
- Kishida M, Sasamoto A, Saito S, Iikura Y. Oxatomide modifies methacholine‐ and exercise‐induced bronchoconstriction in asthmatic children. Annals of Allergy 1992;69(5):455‐61. [PubMed] [Google Scholar]
Krasnowska 1994 {published data only}
- Krasnowska M, Jankowska R, Jagiello E. The effect of inhaling pirenzepine‐‐selective M1 receptor antagonist and ipratropium bromide on lung ventilation in patients with bronchial asthma. Pneumonologia i Alergologia Polska 1994;62(11‐12):609‐14. [PubMed] [Google Scholar]
Krasnowska 2004 {published data only}
- Krasnowska M, Fal AM, Jagiello E. Effect of inhaled pirenzepine and ipratropium bromide on ventilation parameters in patients with asthma. International Review of Allergology & Clinical Immunology 2004;10(1):18‐21. [Google Scholar]
Kreisman 1984 {published data only}
- Kreisman H, Cohen C, Ghezzo H, Vickerson F, Frank H, Wolkove N. Combined therapy with ipratropium and theophylline in asthma. Annals of Allergy 1984;52(2):90‐3. [PubMed] [Google Scholar]
Larsson 1987 {published data only}
- Larsson K. Ipratropium bromide: bronchodilator action and effect on methacholine‐induced bronchoconstriction. Journal of Asthma 1987;24(1):29‐35. [DOI] [PubMed] [Google Scholar]
Lefcoe 1982 {published data only}
- Lefcoe NM, Toogood JH, Blennerhassett G, Baskerville J, Paterson NA. The addition of an aerosol anticholinergic to an oral beta agonist plus theophylline in asthma and bronchitis. A double‐blind single dose study. Chest 1982;82(3):300‐5. [DOI] [PubMed] [Google Scholar]
Lenney 1986 {published data only}
- Lenney W, Evans NA. Nebulized salbutamol and ipratropium bromide in asthmatic children. British Journal of Diseases of the Chest 1986;80(1):59‐65. [DOI] [PubMed] [Google Scholar]
Lew 1990 {published data only}
- Lew DB, Herrod HG, Crawford LV. Combination of atropine and isoetharine aerosol therapy in pediatric acute asthma. Annals of Allergy 1990;64(2 Pt 2):195‐200. [PubMed] [Google Scholar]
Li 2003 {published data only}
- Li SF, Yao WJ. Combined application of salbutamol and ipratropium bromide in the treatment of bronchial asthma in children. Herald of Medicine 2003;22(2):96‐7. [Google Scholar]
Lightbody 1978 {published data only}
- Lightbody IM, Ingram CG, Legge JS, Johnston RN. Ipratropium bromide, salbutamol and prednisolone in bronchial asthma and chronic bronchitis. British Journal of Diseases of the Chest 1978;72(3):181‐6. [DOI] [PubMed] [Google Scholar]
Lin 1978 {published data only}
- Lin MT, Lee‐Hong E, Collins‐Williams C. A clinical trial of the bronchodilator effect of Sch 1000 aerosol in asthmatic children. Annals of Allergy 1978;40(5):326‐32. [PubMed] [Google Scholar]
Lines 1983 {published data only}
- Lines DR, Coulthard KP. Inhaled Ipratropium Bromide (atrovent) in asthmatic children. Australian Paediatric Journal 1983;19(2):128. [Google Scholar]
Mannino 1996 {published data only}
- Mannino F, Anticoli S, Luca A, Terzano C. Time course of methacholine induced bronchoconstriction during drugs and spontaneous resolution. Allergologia Et Immunopathologia 1996;24(2):70‐4. [PubMed] [Google Scholar]
Mazzei 1986 {published data only}
- Mazzei JA, Torres J. Blind randomized cross‐over comparative study of salbutamol and the combination fenoterol‐ipratropium IK6 in patients with bronchial asthma. Respiration 1986;50(Suppl 2):313‐7. [DOI] [PubMed] [Google Scholar]
McNeill 1966 {published data only}
- McNeill RS, Nairn JR, Millar JS, Ingram CG. Exercise‐induced asthma. Quarterly Journal of Medicine 1966;35(137):55‐67. [PubMed] [Google Scholar]
Miraglia 1983 {published data only}
- Miraglia Del Giudice M, Maiello N, Capristo AF, Caputo A, Pinto F. Short and medium‐term controlled study of the bronchodilating effects of a fenoterol‐ipratropium bromide combination in asthmatic children. Archivio Monaldi Per La Tisiologia e Le Malattie Dell'Apparato Respiratorio 1983;38(5‐6):279‐94. [PubMed] [Google Scholar]
Muranyi 1974 {published data only}
- Muranyi L, Szekeres I, Butor E. Pharmacocapnography, a clinical pharmacological method based on analysis of the CO2‐curve for the pharmacodynamic study of bronchial reactivity. Action of atropine on acetylcholine‐‐induced bronchoconstriction in asthmatic children. International Journal of Clinical Pharmacology, Therapy & Toxicology 1974;9(2):93‐7. [PubMed] [Google Scholar]
Neijens 1981 {published data only}
- Neijens HJ, Wesselius TR, Kerrebijn KF. Effect of drugs on effort dyspnoea in children with severe CARA. Nederlands Tijdschrift voor Geneeskunde 1981;125(32):1277‐81. [PubMed] [Google Scholar]
Neild 1985 {published data only}
- Neild JE, Cameron IR. Bronchoconstriction in response to suggestion: its prevention by an inhaled anticholinergic agent. British Medical Journal Clinical Research Edition 1985;290(6469):674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasterkamp 1985 {published data only}
- Pasterkamp H, Tal A, Leahy F, Fenton R, Chernick V. The effect of anticholinergic treatment on postexertional wheezing in asthma studied by phonopneumography and spirometry. American Review of Respiratory Disease 1985;132(1):16‐21. [DOI] [PubMed] [Google Scholar]
Pauwels 1995 {published data only}
- Pauwels JH, Bever HP, Desager KN, Creton W, Veken J. Dose‐response curve of oxitropium bromide (OB) in preschool asthmatics [abstract]. European Respiratory Journal 1995;19(Suppl 8):517s. [Google Scholar]
Peters 2000 {published data only}
- Peters JI, Shelledy DC, Jones AP Jr, Lawson RW, Davis CP, LeGrand TS. A randomized, placebo‐controlled study to evaluate the role of salmeterol in the in‐hospital management of asthma. Chest 2000;118(2):313‐20. [DOI] [PubMed] [Google Scholar]
Poppius 1973 {published data only}
- Poppius H, Salorinne Y. Comparative trial of salbutamol and an anticholinergic drug, SCH 1000, in prevention of exercise‐induced asthma. Scandinavian Journal of Respiratory Diseases 1973;54(3):142‐7. [PubMed] [Google Scholar]
Rachelefsky 1978 {published data only}
- Rachelefsky GS, Tashkin DP, Katz RM, Kershnar H, Siegel SC. A comparison of aerosolized atropine, isoproterenol, atropine plus isoproterenol, disodium cromoglycate and placebo in the prevention of exercise‐induced asthma. Chest 1978;73(6 Suppl):1017‐9. [DOI] [PubMed] [Google Scholar]
Rayner 1987 {published data only}
- Rayner RJ, Cartlidge PH, Upton CJ. Salbutamol and ipratropium in acute asthma. Archives of Disease in Childhood 1987;62(8):840‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ream 2001 {published data only}
- Ream RS, Loftis LL, Albers GM, Becker BA. Efficacy of IV theophylline in children with severe status asthmaticus. Chest 2001;119(5):1480‐8. [DOI] [PubMed] [Google Scholar]
Rebuck 1983 {published data only}
- Rebuck AS, Gent M, Chapman KR. Anticholinergic and sympathomimetic combination therapy of asthma. Journal of Allergy & Clinical Immunology 1983;71(3):317‐23. [DOI] [PubMed] [Google Scholar]
Reisman 1988 {published data only}
- Reisman J, Galdes‐Sebalt M, Kazim F, Canny G, Levison H. Frequent administration by inhalation of salbutamol and ipratropium bromide in the initial management of severe acute asthma in children. Journal of Allergy & Clinical Immunology 1988;81(1):16‐20. [DOI] [PubMed] [Google Scholar]
Russo 1986 {published data only}
- Russo GH, Bellia CA, Bodas AW. Exercise‐induced asthma (EIA): its prevention with the combined use of ipratropium bromide and fenoterol. Respiration 1986;50(Suppl 2):258‐61. [DOI] [PubMed] [Google Scholar]
Sanguinetti 1986 {published data only}
- Sanguinetti CM, Luca S, Gasparini S, Massei V. Evaluation of Duovent in the prevention of exercise‐induced asthma. Respiration 1986;50(Suppl 2):181‐5. [DOI] [PubMed] [Google Scholar]
Schlueter 1978 {published data only}
- Schlueter DP, Neumann JL. Double blind comparison of acute bronchial and ventilation‐perfusion changes to atrovent and isoproterenol. Chest 1978;73(6 Suppl):982‐3. [DOI] [PubMed] [Google Scholar]
Schuh 1995 {published data only}
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium bromide added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126(4):639‐45. [DOI] [PubMed] [Google Scholar]
Sienra Monge 2000 {published data only}
- Sienra Monge JJ, Bermejo Guevara MA, Rio Navarro BE, Rosas Vargas MA, Reyes Ruiz NI. Degree and duration of bronchodilatation after administration of beta 2 agonist alone or in combination with ipratropium bromide in children with acute asthma. Revista Alergia Mexico 2000;47(1):26‐9. [PubMed] [Google Scholar]
Silverman 1981 {published data only}
- Silverman M, Tooley M. Oxatomide and exercise‐induced asthma in children: the value of serial exercise tests. Clinical Allergy 1981;11(5):421‐8. [DOI] [PubMed] [Google Scholar]
Simone 1986 {published data only}
- Simone P, Robuschi M, Vaghi A, Fasano W, Bianco S. Prevention of fog‐induced bronchospasm by Duovent and its components (fenoterol, ipratropium bromide) in asthmatics. Respiration 1986;50(Suppl 2):192‐5. [DOI] [PubMed] [Google Scholar]
Simonsson 1979 {published data only}
- Simonsson BG. Acute effects of ipratropium bromide (ITBR, Sch 1000, Atrovent): a review of previous studies. Scandinavian Journal of Respiratory Diseases 1979;Suppl 103:130‐42. [PubMed] [Google Scholar]
Stokes 1983 {published data only}
- Stokes GM, Milner AD, Hodges IG, Henry RL. Nebulised ipratropium bromide in wheezy infants and young children. European Journal of Respiratory Diseases ‐ Supplement 1983;128(Pt 2):494‐8. [PubMed] [Google Scholar]
Storms 1975 {published data only}
- Storms WW, DoPico GA, Reed CE. Aerosol Sch 1000. An anticholinergic bronchodilator. American Review of Respiratory Disease 1975;111(4):419‐22. [DOI] [PubMed] [Google Scholar]
Storms 1986 {published data only}
- Storms WW, Bodman SF, Nathan RA, Busse WW, Bush RK, Falliers CJ, O'Hollaren JD, Weg JG. Use of ipratropium bromide in asthma. Results of a multi‐clinic study. American Journal of Medicine 1986;81(5A):61‐6. [DOI] [PubMed] [Google Scholar]
Storr 1986 {published data only}
- Storr J, Lenney W. Nebulised ipratropium and salbutamol in asthma. Archives of Disease in Childhood 1986;61(6):602‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sur 1990 {published data only}
- Sur S, Mohiuddin AA, Vichyanond P, Nelson HS. A random double‐blind trial of the combination of nebulized atropine methylnitrate and albuterol in nocturnal asthma. Annals of Allergy 1990;65(5):384‐8. [PubMed] [Google Scholar]
Svenonius 1988 {published data only}
- Svenonius E, Arborelius M Jr, Wiberg R, Ekberg P. Prevention of exercise‐induced asthma by drugs inhaled from metered aerosols. Allergy 1988;43(4):252‐7. [DOI] [PubMed] [Google Scholar]
Tasche 1997 {published data only}
- Tasche MJA, Wouden JC, Uijen JHJM, Ponsioen BP, Bernsen RMD, Suijlekom‐Smit LWA, Jongste JC. Randomised placebo‐controlled trial of inhaled sodium cromoglycate in 1‐4 year old children with moderate asthma. Lancet 1997;350(9084):1060‐64. [DOI] [PubMed] [Google Scholar]
Tashkin 1977 {published data only}
- Tashkin DP, Katz RM, Kerschnar H, Rachelefsky GS, Siegel SC. Comparison of aerosolized atropine, isoproterenol, atropine plus isoproterenol, disodium cromoglycate and placebo in the prevention of exercise‐induced asthma. Annals of Allergy 1977;39(5):311‐8. [PubMed] [Google Scholar]
Taytard 1987 {published data only}
- Taytard A, Vergeret J, Guenard H, Vaida P, Bellvert P, Freour P. Prevention of exercise‐induced asthma by oxitropium bromide. European Journal of Clinical Pharmacology 1987;33(5):455‐8. [DOI] [PubMed] [Google Scholar]
Thomson 1978 {published data only}
- Thomson NC, Patel KR, Kerr JW. Sodium cromoglycate and ipratropium bromide in exercise‐induced asthma. Thorax 1978;33(6):694‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinkelman 1976 {published data only}
- Tinkelman DG, Cavanaugh MJ. Inhibition of exercise‐induced bronchospasm by atropine. American Review of Respiratory Disease 1976;114(1):87‐94. [DOI] [PubMed] [Google Scholar]
Town 1988 {published data only}
- Town GI. Comparison between aerosol and powder delivery system of fenoterol plus ipratropium bromide ('Duovent') in patients with asthma and chronic bronchitis. Pharmatherapeutica 1988;5(4):246‐8. [PubMed] [Google Scholar]
Tullett 1982 {published data only}
- Tullett WM, Patel KR, Berkin KE, Kerr JW. Effect of lignocaine, sodium cromoglycate, and ipratropium bromide in exercise‐induced asthma. Thorax 1982;37(10):737‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulrik 1992 {published data only}
- Ulrik CS, Backer V, Bach‐Mortensen N. Bronchodilating effect of ipratropium bromide inhalation powder and aerosol in children and adolescents with stable bronchial asthma. Allergy 1992;47(2 Pt 2):133‐7. [DOI] [PubMed] [Google Scholar]
Vichyanond 1990 {published data only}
- Vichyanond P, Sladek WA, Sur S, Hill MR, Szefler SJ, Nelson HS. Efficacy of atropine methylnitrate alone and in combination with albuterol in children with asthma. Chest 1990;98(3):637‐42. [DOI] [PubMed] [Google Scholar]
Walker 1987 {published data only}
- Walker FB IV, Kaiser DL, Kowal MB, Suratt PM. Prolonged effect of inhaled glycopyrrolate in asthma. Chest 1987;91(1):49‐51. [DOI] [PubMed] [Google Scholar]
Ward 1981 {published data only}
- Ward MJ, Fentem PH, Smith WH, Davies D. Ipratropium bromide in acute asthma. British Medical Journal Clinical Research Edition 1981;282(6264):598‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 1985 {published data only}
- Ward MJ, Macfarlane JT, Davies D. A place for ipratropium bromide in the treatment of severe acute asthma. British Journal of Diseases of the Chest 1985;79(4):374‐8. [DOI] [PubMed] [Google Scholar]
Watson 1988 {published data only}
- Watson WTA, Becker AB, Simons FER. Comparison of ipratropium solution, fenoterol solution, and their combination administered by nebulizer and face mask to children with acute asthma. Journal of Allergy and Clinical Immunology 1988;82:1012‐8. [DOI] [PubMed] [Google Scholar]
Watson 1994 {published data only}
- Watson TA, Shuckett EP, Becker AB, Simons ER. Effect on nebulized ipratropium bromide on intraocular pressures in children. Chest 1994;105:1439‐41. [DOI] [PubMed] [Google Scholar]
Wilson 1984 {published data only}
- Wilson N, Dixon C, Silverman M. Bronchial responsiveness to hyperventilation in children with asthma: inhibition by ipratropium bromide. Thorax 1984;39(8):588‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1987 {published data only}
- Wilson NM, Green S, Coe C, Barnes PJ. Duration of protection by oxitropium bromide against cholinergic challenge. European Journal of Respiratory Diseases 1987;71(5):455‐8. [PubMed] [Google Scholar]
Wolkove 1981 {published data only}
- Wolkove N, Kreisman H, Frank HG. The effect of ipratropium on exercise‐induced bronchospasm. Annals of Allergy 1981;47(5 Pt 1):311‐5. [PubMed] [Google Scholar]
Yeung 1980 {published data only}
- Yeung R, Nolan GM, Levison H. Comparison of the effects of inhaled SCH 1000 and fenoterol on exercise‐induced bronchospasm in children. Pediatrics 1980;66(1):109‐14. [PubMed] [Google Scholar]
Yung 1998 {published data only}
- Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Archives of Disease in Childhood 1998;79(5):405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaritsky 1999 {published data only}
- Zaritsky A, Qureshi F. Ipratropium does indeed reduce admissions to hospital with severe asthma. British Medical Journal 1999;318(7185):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zorc 1999 {published data only}
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4):748‐52. [DOI] [PubMed] [Google Scholar]
Additional references
Brophy 1998
- Brophy C, Ahmed B, Bayston S, Arnold A, McGivern D, Greenstone M. How long should atrovent be given in acute asthma?. Thorax 1998;53(5):363‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1993
- British Thoracic Society, British Paediatric Association, Royal College of Physicians of London, National Asthma Campaign. Guidelines on the management of asthma. Thorax 1993;48:S1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society, British Paediatric Association, Royal College of Physicians of London, The King's Fund Centre, National Asthma Campaign. Asthma in adults and schoolchildren. Thorax 1997;52(Suppl 1):S2‐S8. [Google Scholar]
BTS 1997a
- British Thoracic Society, British Paediatric Association, Royal College of Physicians of London, The King's Fund Centre, National Asthma Campaign. Asthma in children under five years of age. Thorax 1997;52(Suppl 1):S9‐S21. [PubMed] [Google Scholar]
BTS 2003
- British Thoracic Society, SIGN. British Guideline on the Management of Asthma. Thorax 2003;58(Suppl 1):1‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1996
- Chapman KR. An international perspective on anticholinergic therapy. The American Journal of Medicine 1996;100(Suppl 1a):2s‐4s. [DOI] [PubMed] [Google Scholar]
Doull 1997
- Doull IJM, Lampe FC, Smith S, Schreiber J, Freezer NJ, Holgate ST. Effect of inhaled corticosteroids on episodes of wheezing associated with viral infection in school age children: randomised double blind placebo controlled trial. BMJ 1997;10(315):858‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Everard 2005
- Everard ML, Bara A, Kurian M, Elliott TM, Ducharme F, Mayowe V. Anticholinergics drugs for wheeze in children under the age of two years (Cochrane review). Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001279] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 1996
- Goodman, Gilman. Muscarinic Receptor Agonists and Antagonists. In: Hardman JG, Limbard LE editor(s). The Pharmacological Basis of Therapeutics. 9th Edition. McGraw‐Hill, 1996:154‐9. [Google Scholar]
Gross 1988
- Gross NJ. Ipratropium Bromide. The New England Journal of Medicine 1988;319(8):486‐93. [DOI] [PubMed] [Google Scholar]
Handbook 2004
- Deeks JJ, Higgins, JPT, Altman DG, editors. Analysing and presenting results. In: Alderson P, Green S, Higgins J, editor(s). Cochrane Reviewers’ Handbook 4.2.2 [updated December 2003]; Section 8, The Cochrane Library. Chichester: John Wiley & Sons Ltd, 2004. [Google Scholar]
Jadad 1995
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: is blinding necessary?. Controlled Clinical Trials 1995;143:1‐12. [DOI] [PubMed] [Google Scholar]
Keeley 1999
- Keeley DJ, Silverman M. Are we too ready to diagnose asthma in children?. Thorax 1999;54(7):625‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plotnick 2000
- Plotnick LH, Ducharme FM. Combined inhaled anticholinergics and beta 2‐agonists for initial treatment of acute asthma in children (Cochrane review). Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000060] [DOI] [PubMed] [Google Scholar]
Qureshi 1997
- Qureshi F, Zaritsky A, Lakkis H. Efficacy of nebulized ipratropium in severely asthmatic children. Annals of Emergency Medicine 1997;29(2):205‐11. [DOI] [PubMed] [Google Scholar]
Rubin 1996
- Rubin BK, Albers GM. Use of anticholinergic bronchodilatation in children. The American Journal of Medicine 1996;100(Suppl 1a):49s‐53s. [DOI] [PubMed] [Google Scholar]
Serafin 1996
- Serafin W. Drugs used in the treatment of asthma. In: Hardman JG, Limbard LE editor(s). The Pharmacological Basis of Therapeutics. 9th Edition. McGrath‐Hill, 1996:659‐65. [Google Scholar]
Silverman 1997
- Silverman M, Wilson N. Asthma ‐ time for a change of name?. Archives of Disease in Childhood 1997;77(7):62‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


