Abstract
Heat stress (HS) can be detrimental to the gut health of swine. Many negative outcomes induced by HS are increasingly recognized as including modulation of intestinal microbiota. In turn, the intestinal microbiota is a unique ecosystem playing a critical role in mediating the host stress response. Therefore, we aimed to characterize gut microbiota of pigs’ exposure to short-term HS, to explore a possible link between the intestinal microbiota and HS-related changes, including serum cytokines, oxidation status, and intestinal epithelial barrier function. Our findings showed that HS led to intestinal morphological and integrity changes (villus height, serum diamine oxidase [DAO], serum D-lactate and the relative expressions of tight junction proteins), reduction of serum cytokines (interleukin [IL]-8, IL-12, interferon-gamma [IFN-γ]), and antioxidant activity (higher glutathione [GSH] and malondialdehyde [MDA] content, and lower superoxide dismutase [SOD]). Also, 16S rRNA sequencing analysis revealed that although there was no difference in microbial α-diversity, some HS-associated composition differences were revealed in the ileum and cecum, which partly led to an imbalance in the production of short-chain fatty acids including propionate acid and valerate acid. Relevance networks revealed that HS-derived changes in bacterial genera and microbial metabolites, such as Chlamydia, Lactobacillus, Succinivibrio, Bifidobacterium, Lachnoclostridium, and propionic acid, were correlated with oxidative stress, intestinal barrier dysfunction, and inflammation in pigs. Collectively, our observations suggest that intestinal damage induced by HS is probably partly related to the gut microbiota dysbiosis, though the underlying mechanism remains to be fully elucidated.
Keywords: Pig, Heat stress, Intestinal mucosal barrier, Bacterial signature, Microbe–host interaction
1. Introduction
Pigs are particularly vulnerable to heat stress (HS) because they lack functional sweat glands (Baumgard and Rhoads, 2013). Adverse effects of HS on swine production will intensify if climate warming continues as expected (Baumgard and Rhoads, 2013; Rhoads et al., 2013). In pigs, HS markedly increases respiration rates and body temperatures, significantly lessens feed intake, compromises gut health, and increases mortality and morbidity (Baumgard and Rhoads, 2013; Ross et al., 2015). Heat-stressed pigs increase vasoconstriction in the gastrointestinal tract to redistribute blood to the periphery, for the purpose of maximizing heat dissipation (Hales et al., 1979; Lambert, 2009). Accordingly, a decrease in nutrient and blood flow causes hypoxia, which ultimately induces oxidative stress and compromises intestinal integrity. Tight junction proteins are essential for healthy intestinal barrier function, and alteration in their expressions is associated with HS, which can lead to leaky gut (Yan et al., 2006; Montilla et al., 2014; Akbarian et al., 2016; Lauridsen, 2019). Recent studies have found that intestinal barrier function can be damaged during HS, which will trigger inflammation in animals by facilitating bacterial and endotoxin translocation from the intestine to the circulation (Pearce et al., 2012, 2013a, 2013b). Moreover, the intensity and duration of HS applied to pigs are known to produce stress responses that range from an increase in the animsal's immune response to a decrease in their immunity due to HS-induced antigen challenge (Star et al., 2007; Cui et al., 2016). In our previous study, we found that long-term HS reduced the levels of serum IL-12 and IFN-γ in growing pigs, which means that the immune response of growing pigs changes under long-term HS conditions (Wen et al., 2019).
Gut microbiota and its metabolites (short-chain fatty acids, SCFA) have attracted extensive attention for their usefulness in regulating immune system development and function, strengthening the gut barrier, metabolizing undigested nutrients and xenobiotics, mitigating oxidative stress, and eliminating pathogens (Rooks and Garrett, 2016; Macpherson et al., 2017). Many factors contribute to the regulation of the intestinal microbiota composition, including diet (Kolodziejczyk et al., 2019), host genetics (Woo and Alenghat, 2017), and environment (de Morais et al., 2017), but also stress (Mackos et al., 2017). Accumulating evidence linking HS to intestinal damage suggest that the gut microbiota could be an underappreciated mediator of HS responses and associated sequelae in animals (He et al., 2019; Xiong et al., 2020). In support, recent studies have begun to explore the effects of environmental HS on gut microbiota and their metabolites. For example, Le Sciellour recently reported that HS had decreased the relative abundance of Lactobacillus whereas Turicibacter–Sarcina–Clostridium sensu stricto dominated enterotype was better adapted to HS (Le Sciellour et al., 2019). HS causes profound changes in the gut microbiota structure of primiparous sows, especially in the abundance and diversity of some SCFA-producing species (Singh et al., 2019). Many studies conducted on other animals, including broiler chickens (Wang et al., 2018a), goats (Contreras-Jodar et al., 2019), ducks (He et al., 2019), and dairy cows (Chen et al., 2018), have also shown that HS affected the composition of the gut microbiota. However, the alteration in the gut microbiota composition and its association with physiological changes induced by HS in pigs has not been well investigated.
Our previous study has indicated that 72 h HS influenced the composition of second bile acids, which were produced by gut microbiota (Fang et al., 2020). Hence, we hypothesized that acute HS may cause potential changes in gut microbiota, and this would be related to HS-related physiological responses. In the present study, we investigated the effect of short-term HS on the levels of cytokines, oxidation status, intestinal morphology and permeability, and relative expressions of tight junction proteins in the ileum and cecum of pigs. Recently developed integrative analyses of the biochemical parameters, cytokines, intestinal morphometric indices, and specific bacteria or SCFA were performed to explore the role of gut microbiota in the physiological alteration in pigs caused by HS.
2. Materials and methods
All procedures involving animal handling and treatment were approved by the Animal Welfare Committee in the Institutes of Animal Sciences, Chinese Academy of Agricultural Sciences on July 10, 2018 (Ethics Code Permit IAS, 2018-6), and were compliant with the Regulations for the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of P. R. China, 1988).
2.1. Animal and experimental design
A detailed description of the methods has been previously published (Fang et al., 2020). Briefly, 24 crossbred boars (63.2 ± 9.5 kg body weight) were randomly assigned to 1 of 3 groups: 1) thermoneutral conditions (TN, 23 ± 1 °C) (n = 8), 2) exposed to heat stress conditions (HS, 33 ± 1 °C) (n = 8), 3) pair-fed (PF, 23 ± 1 °C) (n = 8). After 3 days of adjustment, pigs from the TN and HS groups were fed ad libitum, and the PF pigs received an amount of feed equal to the feed consumed by the HS group on the previous day. All pigs had free access to water. More specifically, the average feed intake of the HS group was measured every day, and the same amount was provided to the PF group on the following day. The PF group was conducted to understand the effects of HS on gut microbiota independent of reduced nutrient intake. The treatment lasted for 72 h. The formulation met the nutrient recommendations of National Research Council (2012), and is listed in Appendix Table 1.
After the 72 h treatment, serum samples were collected by centrifuging venous blood at 3,000 × g for 15 min, and the supernatants were stored at −80 °C until later assays. After pigs were humanely euthanized, a 3-cm section of ileum and cecum was separated and submersed in 4% paraformaldehyde for morphology analysis. After that, the luminal digesta from the ileum and cecum was collected and frozen in liquid nitrogen for SCFA determination. After collecting the luminal digesta, the ileum and cecum segments were rinsed with ice-cold phosphate buffered saline (PBS) solution. The mucosa samples were collected by scraping the intestinal wall with glass microscope slides, frozen in liquid nitrogen and stored at −80 °C for subsequent bacterial community and mRNA and protein expressions measurements.
2.2. Histomorphological analysis
The ileum and cecum samples were dehydrated, embedded in paraffin, and then sliced at 5 μm thickness. The samples were stained with hematoxylin and eosin (H&E) and observed by a Leica DM2000 light microscope (Leica Microsystems, Wetzlar, Germany). The images were analyzed with Image J v1.8.0 software. Eight replicates of complete villus and crypt from each histological section were selected for measurement, and at least 4 vision fields were chosen.
2.3. RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from the ileal and cecal mucosa using the RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The cDNA was transcribed using a Prime Script RT Kit (Takara, Kusatsu, Shiga, Japan). The qRT-PCR was performed according to the SYBR Premix Ex Taq II instructions (Takara, Kusatsu, Shiga, Japan), and conducted on a CFX96 Real-time PCR detection system (Bio-Rad, CA, USA). Cycling conditions were as follows: pre-denaturation at 95 °C for 30 s; followed by 40 cycles of denaturation at 95 °C for 5 s and annealing at 60 °C for 30 s; extension at 95 °C for 10 s and melt curve. All primer sequences are provided in Appendix Table 2. The geometric mean of house-keeping genes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH; β-actin) was used for normalization of the target gene Ct values (Metzler-Zebeli et al., 2018). The relative gene expression was calculated by the 2−ΔΔCt method based on the geometric mean of house-keeping genes (Livak and Schmittgen, 2001).
2.4. Measurement of serum parameters and cytokines
The activities of superoxide dismutase (SOD), glutathione (GSH), total glutathione (T-GSH), glutathione S-transferase (GST), and malondialdehyde (MDA) in serum were measured with commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. The diamine oxidase (DAO), as an indicator of intestinal permeability, was measured using commercial assay kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) according to the manufacturer's instructions. The content of D-lactate in serum was detected using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
The battery of cytokine concentrations in serum was assessed using the Luminex xMAP R technology, a multiplexed microsphere-based flow cytometric assay, as described in our previous article (Wen et al., 2019). Briefly, precoated Porcine Cytokine/Chemokine magnetic beads (Merck Millipore, Burlington, MA, USA) were used for the determination of interferon-gamma (IFN-γ), interleukin (IL)-1a, IL-1b, IL-1RA, IL-2, IL-4, IL- 6, IL-8, IL-10, IL-12, IL-18, and tumor necrosis factor-α (TNF-α). Data for cytokines were captured and analyzed using the Bio-Plex 200 system (BioRad, CA, USA).
2.5. Protein expression analysis by Western blotting
The ileal and cecal tissue lysates were extracted and homogenized using radio immunoprecipitation assay (RIPA) lysis and extraction buffer (Thermo Fisher Scientific Inc., MA, USA). Total proteins were quantified using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific Inc., MA, USA). Equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel and then blotted to polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% skim milk in tris-buffered saline-tween (TBST) buffer for 2 h and overnight incubated with the primary antibodies at 4 °C, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (ab6721, Abcam) in TBST buffer for 1 h at room temperature. The protein bands were developed with ECL kit (Bio-Rad, CA, USA) and visualized using Bio-Rad Chemi XRS imaging system (Bio-Rad). The antibodies used were purchased from the following suppliers: anti-Occludin (Thermo Fisher Scientific Inc., #40-4700), anti-Claudin-1 (Thermo Fisher Scientific Inc., #51-9000), and anti-β-actin (Proteintech, #20536-1-AP). Band density of the target protein was quantified after normalization to β-actin using image J v1.8.0 software.
2.6. Measurement of SCFA in ileal and cecal contents
Quantification of SCFA was analyzed using gas chromatography (GC) based on our previous studies (Wu et al., 2016). Briefly, the digesta were dissolved in and extracted with distilled water. The extracted samples were obtained by centrifuge at 9,000 × g. Metaphosphoric acid (25%, wt/vol) was added into the extracts at a ratio of 1:9. After centrifugation at 10,000 × g, the supernatant was subjected for SCFA analysis with Agilent 6890N GC (Palo Alto, CA).
2.7. Bacterial DNA extraction and 16S rRNA gene sequencing
DNA was extracted from the mucosa using the Qiagen DNA isolation kit (Qiagen, Hilden, Germany) and followed by the provided protocol. The V3–V5 hypervariable region of the 16S bacterial rRNA was amplified using primers 338F (5′- ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′- GGACTACHVGGGTWTCTAAT-3′). The amplicons were sequenced on the Illumina HiSeq sequencing platform, as previously described (Wu et al., 2020). Sequence data were analyzed with quantitative insights into microbial ecology (QIIME) package version 1.9.1. (Caporaso et al., 2010), using the Silva 123 reference database as a reference template. The low abundant operational taxonomic units (OTU), identified by filtering OTU that had <10% of samples below 10 read counts, were removed. Tax4fun was used to predict functional profiles of microbial communities (Asshauer et al., 2015).
2.8. Statistical analysis
The serum biochemical parameters, DAO and cytokines, intestinal morphometric indices, bacterial α-diversity, SCFA, and gene expressions, were analyzed by one-way ANOVA using JMP 13.0 software (SAS Institute, Inc., Cary, NC, USA). Statistical differences among the treatments were separated by least significant difference method. Data were shown as the means with a standard error of the mean or standard error. Statistical significance was declared at a P < 0.05 and trends at 0.05 < P ≤ 0.10.
All statistical analysis of OTU reads were performed using the R program (V3.6.1, https://www.r-project.org/). For β-diversity analysis, principal coordinates analysis (PCoA) based on the Bray–Curtis distance matrices was used to visualize among groups. A permutational multivariate analysis of variance (PERMANOVA) evaluated the microbial community structure comparison. Pairwise comparisons based on a negative binomial Wald test from the DESeq2 software package and sparse partial least squares discriminant analysis (sPLS-DA) from the mixOmics packages were used to measure the differences in individual OTU at different taxon levels (KA et al., 2011; Love et al., 2014; Mcmurdie and Holmes, 2014). A Benjamini & Hochberg-corrected P-value of 0.05 was considered statistically significant (Benjamini and Hochberg, 1995).
In order to identify critical features influencing the physiological responses to heat stress, sPLS regression and relevance network analysis were performed using the mixOmics package (Lê Cao et al., 2009a, 2009b) in the R program to integrate the data sets of relative abundances of genera, SCFA, serum biochemical parameters, cytokines, intestinal morphometric indices, and gene expressions. The threshold for absolute correlation was set to be at least 0.6.
3. Results
3.1. Growth performance, intestinal barrier function after HS
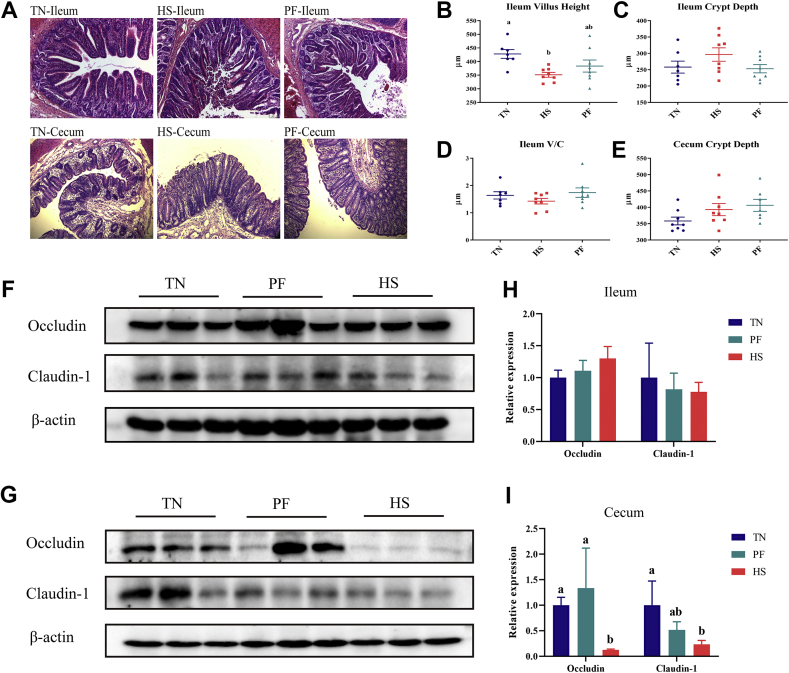
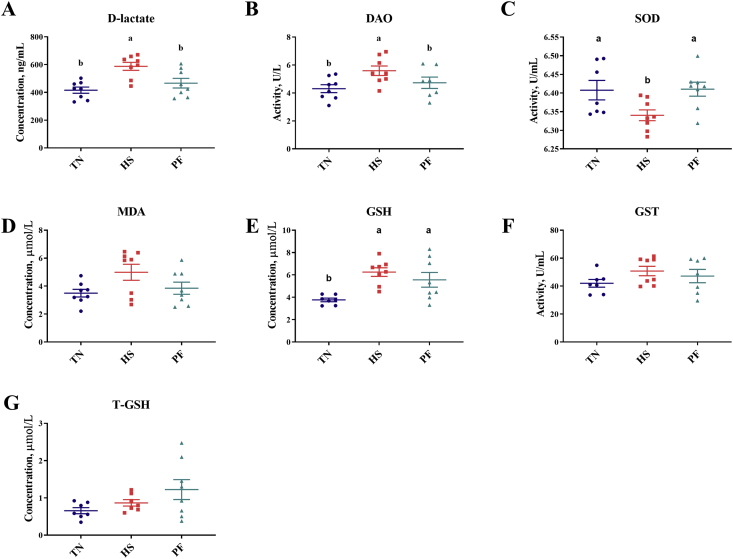
Performance responses of pigs in response to treatment have been previously described in detail (Fang et al., 2020). Briefly, pigs in the HS treatment had approximately 55% lower cumulative feed intake and lost body weight (−0.69 kg, P < 0.01) whereas TN and PF pigs gained 2.46 and 0.38 kg in body weight, respectively. All HS pigs were shown to have an accelerated respiratory rate and increased rectal temperature after exposure to 33 °C for 72 h. As shown in Fig. 1A, villus height was shorter in the ileum of HS pigs compared with TN (P = 0.016). No difference was found in crypt depth in the ileum and cecum among treatment groups. We found that HS significantly reduced the cecal Occludin (P = 0.033) and Claudin-1 mRNA levels (P = 0.047) compared with the TN groups (Table 1). Consistently, the protein expressions of Occludin and Claudin-1 were significantly lower due to HS in the cecum (Fig. 1G and H). As indicators of intestinal permeability, we found that the DAO activity (P = 0.04) and D-lactate content (P < 0.01) in serum significantly increased in the HS group compared with that of TN and PF pigs (Fig. 2A and B).
Fig. 1.
Effects of HS on the intestinal morphology and the expression of tight junction proteins. (A) Tissue sections of intestinal mucosa in ileum, and cecum in pigs (100×). (B to D) The villus height, crypt depth and villus height-to-crypt depth ratio (V/C) of ileum in pigs. (E) The crypt depth of cecum in pigs. (F and G) Representative images of immunoblotting in the ileum and cecum. (H and I) Protein expressions of Occludin and Claudin-1 in the ileum and cecum. TN = thermoneutral; HS = heat stress; PF = pair-fed. Values are means (n = 8/group), with their standard errors represented by vertical bars. a, b Mean values with different letters are statistically significant (P < 0.05).
Table 1.
Relative expressions of genes related to tight junction in the ileum and cecum of pigs after short term HS1.
| Genes of interest | TN | HS | PF | SEM | P-value |
|---|---|---|---|---|---|
| Ileum | |||||
| ZO-1 | 1.005 | 1.185 | 0.960 | 0.051 | 0.079 |
| Occludin | 1.058 | 1.280 | 1.475 | 0.181 | 0.312 |
| Claudin-1 | 1.022 | 0.939 | 0.753 | 0.133 | 0.390 |
| Claudin-3 | 1.090 | 1.182 | 1.456 | 0.269 | 0.632 |
| Claudin-4 | 1.046 | 1.856 | 1.949 | 0.174 | 0.845 |
| Cecum | |||||
| ZO-1 | 1.014 | 0.574 | 0.985 | 0.084 | 0.281 |
| Occludin | 1.010a | 0.653b | 0.901a | 0.062 | 0.033 |
| Claudin-1 | 1.032a | 0.634b | 0.896a | 0.015 | 0.047 |
| Claudin-3 | 1.110 | 1.009 | 1.678 | 0.314 | 0.555 |
| Claudin-4 | 1.096 | 1.258 | 0.912 | 0.151 | 0.309 |
TN = thermoneutral; HS = heat stress; PF = pair-fed; ZO-1 = zonula occludens 1.
a,b Mean values with unlike letters are statistically significant (P < 0·05).
Data were expressed as mean with their standard error of the mean; n = 8.
Fig. 2.
The levels of D-lactate (A), DAO (B), SOD (C), MDA (D), GSH (E), GST (F), T-GSH (G) in serum of pigs with/without HS. Values are means (n = 8/group), with their standard errors represented by vertical bars. a, b Mean values with unlike letters are statistically significant (P < 0.05). TN = thermoneutral; HS = heat stress; PF = pair-feeding; DAO = diamine oxidase; SOD = superoxide dismutase; GSH = glutathione; T-GSH = total glutathione; GST = glutathione S-transferase; MDA = malondialdehyde.
3.2. Effects of HS on serum biochemical parameters
To investigate whether heat exposure resulted in antioxidant system disorders, the levels of SOD, MDA, GSH, GST, and T-GSH in serum were evaluated. As illustrated in Fig. 2, compared to the TN pigs, the level of MDA was higher (P = 0.066), whereas the level of SOD was significantly lower (P = 0.032) in the HS pigs. The level of GSH was elevated in the HS and PF pigs compared with pigs under thermoneutral conditions (P = 0.005).
3.3. Intestinal cytokines variation after HS
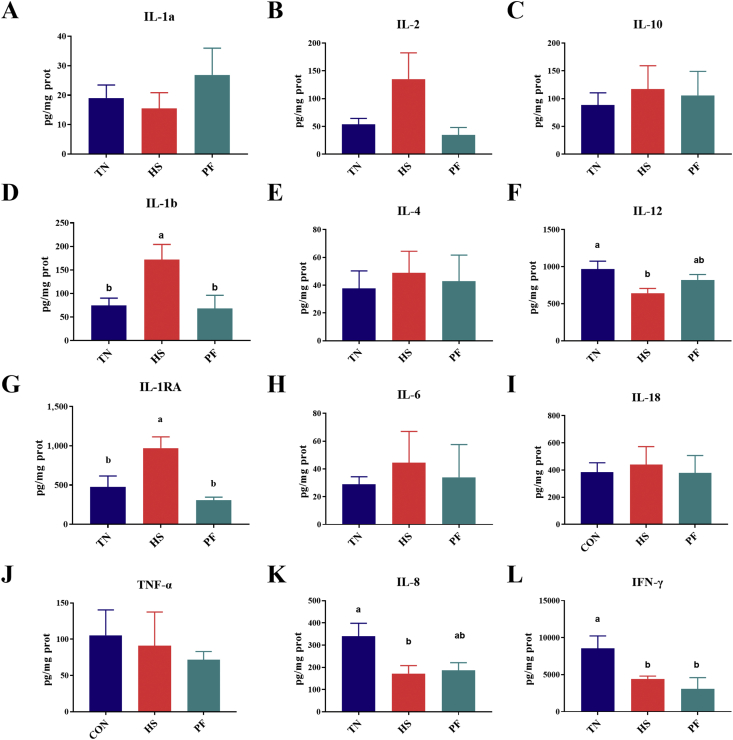
To examine potential modulatory properties of HS on immune status, concentrations of cytokines were measured by cytokine magnetic beads. We found that heat exposure significantly reduced IL-12 (P = 0.037) and IL-8 (P = 0.028) concentrations compared with TN groups. IFN-γ concentration was lower in HS and PF pigs (P = 0.021). On the other hand, heat exposure elevated the concentrations of IL-1RA (P = 0.003) and IL-1b (P = 0.017) and, as trends (P = 0.06), that of IL-2 compared with TN pigs (Fig. 3).
Fig. 3.
The concentrations of IL-1a (A), IL-2 (B), IL-10 (C), IL-1b (D), IL-4 (E), IL-12 (F), IL-1RA (G), IL-6 (H), IL-18 (I), TNF-a (J), IL-8 (K), and IFN-g (L) in serum of pigs with/without HS. Values are means (n = 8/group), with their standard errors represented by vertical bars. a, b Mean values with unlike letters are statistically significant (P < 0.05). TN = thermoneutral; HS = heat stress; PF = pair-fed; IL = interleukin; TNF-α = tumor necrosis factor-α; IFN-γ = interferon-gamma.
3.4. Heat stress alters the intestinal environment and predicted function in ileal and cecal mucosa
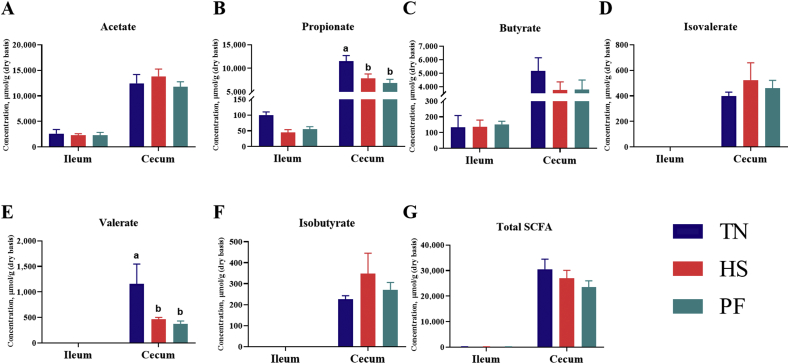
We further examined whether exposure to heat caused changes to the intestinal environment. Individual SCFA profiles showed significantly reduced propionate (P = 0.006) and valerate (P = 0.049) concentrations in the cecum of HS and PF groups compared with the TN group (Fig. 4).
Fig. 4.
Short-chain fatty acids (SCFA) concentrations (μmol/g, dry basis) in ileum and cecum of pigs with/without HS. The concentrations of acetate (A), propionate (B), butyrate (C), isovalerate (D), valerate (E), isobutyrate (F), and total SCFA (G) in the intestine. Total SCFA are the sum of the following SCFA: acetate, propionate, isobutyrate, butyrate, isovalerate, valerate. Values are means (n = 8/group), with their standard errors represented by vertical bars. a,b Mean values with unlike letters are statistically significant (P < 0.05). TN = thermoneutral; HS = heat stress; PF = pair-fed.
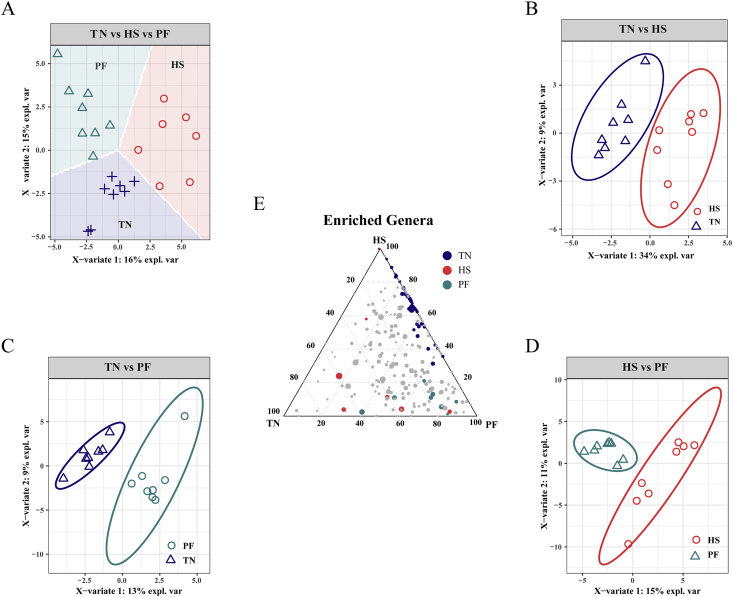
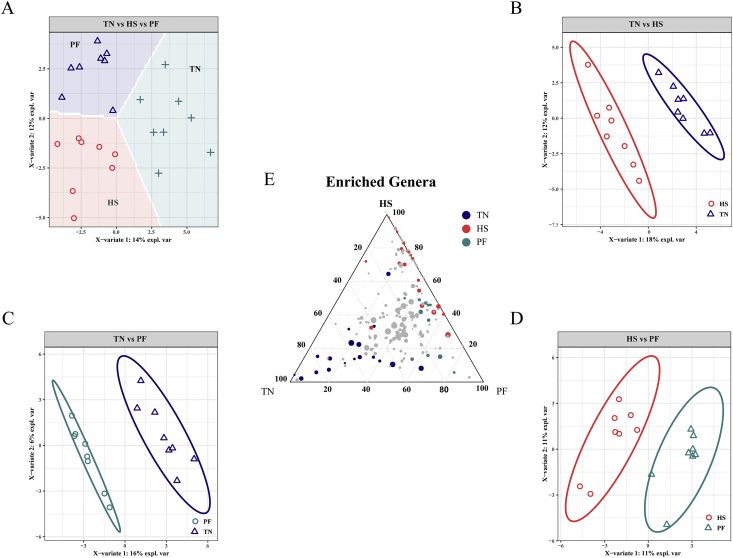
Before calculating α- and β-diversity, samples were rarefied to 36,014 reads to account for unequal numbers of sequences among samples. After filtration for the rare OTU, a total of 877 OTU remained in our data set. The species richness estimators (Chao1), evenness (Simpson), and diversity index (Shannon) were comparable among all treatments in the ileum (Appendix Fig. 1) and cecum (Appendix Fig. 2). Based on Bray–Curtis dissimilarity matrices at the genus level, the ileal samples in the HS group did not exhibit a distinct cluster that was clearly separated from those in the PF group but was basically separated from those in the TN group (R2 = 0.14, P < 0.05) (Appendix Fig. 1). Moreover, visualization of the cecal communities in the TN group using PCoA plots indicated that bacterial communities were distinct from those of the HS and PF groups (R2 = 0.14, P < 0.05) (Appendix Fig. 2).
The most abundant phylum was Firmicutes in the ileal and cecal mucosa of pigs, followed by Proteobacteria in the ileum and Bacteroidetes in the cecum. A core bacterial community was determined based on the DESeq2 method and sPLS-DA analysis implemented in R programming software. As indicated in Fig. 5, a total of 65 genera with significantly different abundance among the treatment groups were found in the ileum (Appendix Table 3). HS increased the relative abundance of 8 genera compared to the TN group, whereas it increased the relative abundance of 14 genera compared to the PF group. The relative abundances of 5 genera were significantly higher in the PF group compared to the TN group. Based on these comparisons, we found that the relative abundances of Flavonifractor, Thiomonas, and Bifidobacterium were lower in the ileum due to heat exposure, whereas the Lawsonia, Actinobacillus, Lachnospiraceae_UCG_001, Lachnoclostridium, Lachnospiraceae_UCG_004, and Chlamydia were more abundant, and this alteration was independent of reduced feed intake. The alteration of cecal microbiota is shown in Fig. 6, and the pairwise contrast highlighted 56 genera with significantly different abundance among the treatment groups (Appendix Table 4). HS increased the relative abundance of 22 genera compared to the TN group, whereas it increased the relative abundance of 10 genera compared to the PF group. The relative abundances of 17 genera were significantly higher in the PF group compared to the TN group. In the comparison of HS vs. TN, a few of the same genera were identified as different, as were in the PF vs. HS comparison. The relative abundances of Staphylococcus, Frischella, Bacteroides, Akkermansia, Lachnoclostridium, and Lachnospiraceae_UCG_004 were higher in the cecum due to HS, whereas the Lactobacillus, Anaerovibrio, Prevotella, and Succinivibrio were less abundant, and this change was not related to reduced feed intake. Interestingly, we observed that some genera belonging to Lachnospiraceae were consistently affected by HS in the 2 gut sites, including Lachnoclostridium, Lachnospiraceae_UCG_004, and their relative abundances were decreased with heat challenge.
Fig. 5.
Score plot of 2-component sparse partial least square discriminant analysis models showing gut microbiota clustering according to the environment in ileum of pigs with/without HS (A to D) with percentage of variance captured for each principal component. The ternary analysis of the 3 treatment groups was shown in (E). TN = thermoneutral; HS = heat stress; PF = pair-fed.
Fig. 6.
Score plot of 2-component sparse partial least square discriminant analysis models showing gut microbiota clustering according to the environment in cecum of pigs with/without HS (A to D) with percentage of variance captured for each principal component. The ternary analysis of the 3 treatment groups was shown in (E). TN = thermoneutral; HS = heat stress; PF = pair-fed.
Predicted functional profiles were obtained through the Tax4Fun tools. Similar to the microbiota composition, the TN, HS, and PF groups displayed dissimilar predicted functional profiles in the ileum. The enriched pathways in HS pigs were related to infectious diseases: bacterial, signal transduction, biosynthesis of other secondary metabolites, etc. (Appendix Fig. 3A). In the cecum segment, metabolic pathways related to immune system, infectious diseases (viral, replication and repair), signal transduction, and bacterial infectious diseases, appear to be expanded in the pigs’ exposure to heat (Appendix Fig. 3B).
3.5. HS-related microbiota effects on the host
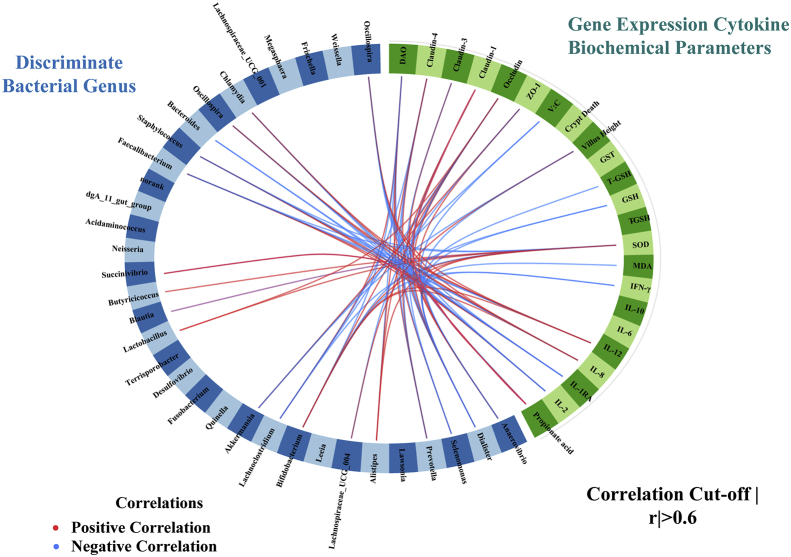
The sPLS regression offered us with a potentially effective model to classify the most discriminant bacterial genus and metabolites on intestinal morphology, serum cytokines, and biochemical parameters (Fig. 7). For example, Bacteroides, Chlamydia, Oscillospira, and Porphyromonas abundances were negatively associated with IL-8 and IL-12 concentrations. Acidibacter, Blautia, Butyricicoccus, and Bifidobacterium levels were positively correlated to the villus height and SOD activity whereas Actinobacillus, Chlamydia, and Lawsonia were negatively correlated. Moreover, the activity of DAO in serum was positively associated with 5 genera, including Metallibacterium, Alistipes, Akkermansia, Lachnoclostridium, and Acidithiobacillus. Lactobacillus, Lachnospiraceae_UCG_004, Prevotella, and Streptococcus were positively related to the levels of SOD and IFN-γ, whereas Staphylococcus, Akkermansia, and Lachnoclostridium were found to be positively related with the MDA, GSH, and IL-2 concentrations. In addition, the propionate acids and Lactobacillus, Succinivibrio, Prevotella, Selenomonas, Dialister, and Anaerovibrio were positively correlated with the expression of Occludin and Claudin-3.
Fig. 7.
Circos plots displaying correlations between the identified levels of the best discriminant genus and SCFA, cytokine, biochemical parameters, and expression levels of target genes. Positive and negative correlations (r > 0.6) were displayed by red and blue links, respectively. Relative abundance of genus = > 0.1%.
4. Discussion
Heat exposure is usually accompanied by health and physiological stress-related problems in humans and animals, and the gastrointestinal tract is one of the most affected organs (Baumgard and Rhoads, 2013; Pearce et al., 2013a, 2015). These losses are related to the adverse effects of HS on gut permeability and systemic inflammation. The microbiome is indispensable for maintaining intestinal homeostasis. There is a dearth of information about the relationship between mucosal microbiota and intestinal injuries under acute HS. In the present study, using a pair-fed group to eliminate the confounding effects of dissimilar nutrient intake, we demonstrated that HS induced the intestinal barrier dysfunction in the ileum and cecum independent of reduced feed intake. Furthermore, these results demonstrated the presence of specific microbiome signatures on the intestinal epithelium in the ileum and cecum. This can serve as evidence due to the identified associations between specific bacterial genera and changes with intestinal gene expressions, oxidative stress, and inflammatory response.
To explore a possible association between the intestinal damage and gut microbiota induced by HS, we investigated and compared the mucosal microbiota and microbial metabolites from specific intestinal sites. In contrast to the luminal microbial composition, bacterial community in the mucosa generally constitutes oxygen-tolerant populations and mucolytic species utilize host mucin as the substrate (Marteyn et al., 2011; Albenberg and Wu, 2014). These characteristics make mucosal adherent bacteria more sensitive to microenvironment changes induced by HS, such as oxygen and nutrients, and are more prone to reconstruction. We observed the separate clustering between HS and TN both in the ileum and cecum. Likewise, Xiong et al. found that HS significantly affected the fecal microbiome (Xiong et al., 2020). Diet and caloric restriction are considered to be the main factors driving gut microbiological alterations under HS (Zhang et al., 2013; Albenberg and Wu, 2014; Kolodziejczyk et al., 2019). Though there was no obvious difference between HS and caloric restriction under TN conditions from the PCoA plot in the ileum, the abundance of multiple specific bacterial groups based on sPLS-DA analysis was found to be significantly influenced by HS in both the ileum and cecum compared to the TN group, independent of reduced feed intake. In contrast, few genera were different due to dissimilar feed intake. HS-enriched bacteria in the mucosa are frequently described as potential opportunistic pathogens, which including Lawsonia, Chlamydia, Staphylococcus, and Bacteroides, have previously been related to inflammatory diarrhea and gut dysbiosis in pigs (Schautteet and Vanrompay, 2011; Leknoi et al., 2017; Wang et al., 2018b; Arnold et al., 2019). In favor of this, our predicted functional pathways analysis revealed that Toxoplasmosis, Bacterial invasion of epithelial cells, Staphylococcus aureus infection, and Vibrio cholerae infection are changed, which suggests that pathogen infection and subsequent intestinal disease are more likely to occur during acute HS. Well-known probiotics such as Lactobacillus, Bifidobacterium, Blautia, and Butyricicoccus contributing to gut health were reduced in the HS-challenge pigs (Gresse et al., 2017; Le Sciellour et al., 2019; Trachsel et al., 2018). The decrease of Lactobacillus and Bifidobacterium in HS conditions could be the first step of intestinal flora disturbance and subsequent gut inflammation. Our results indicated that a positive association between Lactobacillus, Bifidobacterium, and Blautia and intestinal barrier integrity may associate intestinal dysfunction with imbalanced gut microbiota induced by HS. From this, we can speculate that dietary regulation by adding probiotics could alleviate the damage caused by HS to the intestine of pigs. In broiler chickens, a symbiotic supplementation, including Lactobacillus and Bifidobacterium, inhibits the negative effects of HS on broiler health through the reduction of pathogens, regulation of stress reactions, and improvement of antioxidant status (Mohammed et al., 2019; Lee et al., 2005). In pigs, these probiotics are often used to improve gut health and growth performance but are yet to be tested in HS conditions (Gresse et al., 2017). Hence, future studies could be conducted on the effects of probiotics on mitigating the negative effects of HS on pigs.
The production of SCFA depends on the fermentation of diet by the microbiota. The concentrations of propionic acid and valeric acid in the TN group were significantly increased compared to both the HS and PF groups, but the levels in the HS and PF groups were comparable. This may be mainly attributed to reduced feed intake and some related producing bacteria, including Prevotella and Succinivibrio, consistent with previous studies on meat-type ducks and broiler chickens (Park and Kim, 2017; Xiao et al., 2017). Animal disease models provide incremental evidence that propionic acid has been reported to elicit particular health benefits for the host (Duscha et al., 2020). Our results imply that the deteriorative intestinal environment, characterized by a reduction in the relative abundance of SCFA producers induced by HS, may be associated with insufficient nutrient intake. The underlying mechanism is still unclear and requires further exploration. Furthermore, propionic acid is proposed to produce a significant increase in the gut-associated Treg cell population, subsequent decrease of systemic immune reaction, and accompanying disease amelioration (Duscha et al., 2020). Decreased beneficial bacteria community and metabolites in HS-challenged pigs will negatively affect the intestinal mucosal immune response as well as systemic cytokine levels (Brandsma et al., 2019; Mendes et al., 2019). Distinct cytokine patterns reflect the HS-related immunosuppression in pigs, which are responsible for Th1 and Th2 effector functions and their development (Bagath et al., 2019). Th1 cytokines (IL-12 and IFN-γ) were reduced in HS pigs, which was consistent with previous research by us and others (Zundler and Neurath, 2017). The ability to selectively produce Th1 cytokines and Th2 cytokines is an essential component in regulating the Th1: Th2 cytokine balance (Park et al., 2005), and the shift from Th1 to Th2 is a marker of immunosuppression (Elenkov et al., 2000). Hyperthermia is related with the down-regulation of Th1 cytokines, thus suppression of the cell-mediated immunity (Webster et al., 2002) and subsequently an increase in susceptibility to diseases (Elenkov et al., 2000; Eftychi et al., 2019). Furthermore, this immunosuppressive influence of HS is characterized by a significant down-regulation of pro-inflammatory IL-8, which is very important for the elimination of pathogens (Larsen, 2017). Similar results were also found in pigs' exposure to chronic social stress (Li et al., 2017). A few reports suggested that some specific microorganisms (e.g. Porphyromonas and Bacteroides) disturb or inhibit protective signaling normally induced by toll-like receptors (TLR), accompanied by lower levels of IL-8 (Coats et al., 2011; SenGupta et al., 2016). A possible explanation is that lipid A, as a toxic component of LPS, can be expressed in a variety of forms by these pathogens, allowing for evasion of the host's innate immune system and chronic infection (Coats et al., 2011; Paciello et al., 2013; SenGupta et al., 2016). Intriguingly, some potential links between these genera and IL-8 are further supported by our current findings, despite the potential mechanism needing to be further evaluated. Taken together, the data reinforce that HS influences the immune system by shifting the Th1-to-Th2 ratio and inducing immunosuppression, thereby destroying immune homeostasis. This disbalance in animals may result in them becoming immunocompromised and subsequently becoming more susceptible to diseases.
With an increase in heat-loads, blood expands through peripheral vasodilatation and gastrointestinal tract vasoconstriction from the splanchnic organs to the skin, orchestrating the distribution of blood away from the splanchnic bed to help dissipate the heat. Accordingly, the intestinal epithelium can gradually become hypoxic and hyperpermeable, and conclusively induce permeability, inflammation, and impairment (Hales et al., 1979; Lambert, 2009). DAO is one kind of endocellular enzyme that only exists in villus cytoplasm of the intestine, which is usually an extra supporting marker of intestinal permeability induced by HS (Zhang et al., 2015). We observed a higher serum DAO activity and D-lactate content in the HS group, which suggested the increased intestinal permeability in pigs. Furthermore, the damaged intestinal mucosa reduced expressions of tight junction proteins, and lower villus height were observed in pigs under HS. These findings indicated that heat stress impaired the intestinal physical barrier by affecting tight junction proteins and epithelial integrity in pigs (Pearce et al., 2012, 2013a, 2013b). Our results indicated lower Claudin-1 and Occludin mRNA levels in the cecum of HS pigs, which was not in line with previous findings, in which their mRNA levels did not change after 3d of heat exposure (Pearce et al., 2013b). This may be attributed to different breeds, physiological stages, and sampling locations. Based on the sPLS analysis, we found that Occludin level was significantly associated with propionate acid. Sprague–Dawley rats fed with propionic acid had higher levels of Occludin and Claudin-1, identified via an increase in the expression of phosphorylated extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) (Xia et al., 2017). This would support the present result. Furthermore, we and some others, have reported that some pathogen genera (e.g. Actinobacillus) have a strong relationship with reduced villus height (Fan et al., 2017; Lee et al., 2018). The tissue injury of the HS response results in the release of oxidative agents from the gastrointestinal tract into the circulation of the body (Akbarian et al., 2016). As a product of lipid oxidation, MDA significantly increased in crossbred gilts exposed to short-term heat stress (Akbarian et al., 2016). Lipid peroxidation could damage the intestinal epithelial cells and activate inflammatory mediators (Beltrán et al., 2010). The damaged antioxidant balance, immoderate oxygen free radicals and the substantial generation of reactive oxygen species (ROS) contribute to excessive oxidative stress (Pérez et al., 2017). Increased ROS production is one of the main consequences of the acute HS response in pigs (Lian et al., 2020). ROS can be generated by both mucosa and microbiota in the intestine (Montilla et al., 2014), and the decreased concentration of antioxidant enzyme SOD suggests that gut integrity may be compromised by heat-induced oxidative stress. Given the above, though they were clinically healthy, pigs in the HS group exhibited signs of reduced immune response and antioxidant capacity, and had higher abundances of potential bacterial pathogens in the intestinal mucosa. This means that the pigs’ exposure to short-term HS are more susceptible to gastrointestinal infection, and more likely to form “leaky gut”, which cause antigens, endotoxins, and bacteria to enter the blood and cause a systemic immune response. However, it is noteworthy to address that regression and network analysis cannot elucidate whether negative effects of HS on the structure of the intestine were achieved via an alteration in the microbiota community or via the changes in the concentrations of SCFA. Further studies are warranted to validate this finding and to confirm the role of gut microbes in the effects of HS on the gut.
5. Conclusions
In the present study, we assessed the effects of heat exposure on gut mucosal barrier function and preliminarily explored whether HS-induced barrier dysfunction and antioxidant capacity are related to gut microbiota dysbiosis in pig. We observed apparent differences induced by HS in β-diversity and several specific genera in the ileum and cecum, accompanied by immunosuppression, impaired intestinal structure and permeability, and reduced antioxidant capacity. Moreover, relevance networks indicated a microbiome signature as HS-derived changes in bacterial genera and microbial metabolites, such as propionate and Lactobacillus, were associated with these parameters in pigs. Our data provided evidence that HS can lead to an altered gut microbiota, which can provide feedback and impact gastrointestinal function and stress-induced physiological responses. Future studies will need to be conducted to confirm the physiological role of gut microbiota in HS and develop targeted methods to mitigate adverse HS-related effects in swine.
Author contributions
Bing Xia: Conceptualization, Methodology, Writing - Original draft preparation. Weida Wu: Conceptualization, Software, Formal analysis, Visualization. Wei Fang: Methodology. Xiaobin Wen: Methodology. Jingjing Xie: Conceptualization, Writing – Review & Editing. Hongfu Zhang: Funding acquisition, Supervision, Writing – Review & Editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2016YFD0500501) and the National Science Foundation for Young Scientists of China (Grant NO. 31802072). We are grateful to thank members in Dr. Zhang's lab for their assistance in sample collections and Dr. Eric from Stanford University for revising the manuscript.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
The appendix to this article can be found online at https://doi.org/10.1016/j.aninu.2021.05.012.
Appendix.
The following is the supplementary data to this article:
References
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J Anim Sci Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albenberg L.G., Wu G.D. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146:1564–1572. doi: 10.1053/j.gastro.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M., Crienen A., Swam H., von Berg S., Jolie R., Nathues H. Prevalence of Lawsonia intracellularis in pig herds in different European countries. Porc Health Manag. 2019;5 doi: 10.1186/s40813-019-0137-6. 31-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asshauer K.P., Wemheuer B., Daniel R., Meinicke P. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 2015:btv287. doi: 10.1093/bioinformatics/btv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagath M., Krishnan G., Devaraj C., Rashamol V.P., Pragna P., Lees A.M., et al. The impact of heat stress on the immune system in dairy cattle: a review. Res Vet Sci. 2019;126:94–102. doi: 10.1016/j.rvsc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- Baumgard L.H., Rhoads R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci. 2013;1:311–337. doi: 10.1146/annurev-animal-031412-103644. [DOI] [PubMed] [Google Scholar]
- Beltrán B., Nos P., Dasí F., Iborra M., Bastida G., Martínez M., et al. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naive and treated Crohn's disease. Inflamm Bowel Dis. 2010;16:76–86. doi: 10.1002/ibd.21027. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Brandsma E., Kloosterhuis N.J., Koster M., Dekker D.C., Gijbels M.J.J., van der Velden S., et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res. 2019;124:94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang J., Peng D., Li G., Chen J., Gu X. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy cows. Sci Rep. 2018;8:14606. doi: 10.1038/s41598-018-32886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S.R., Berezow A.B., To T.T., Jain S., Bainbridge B.W., Banani K.P., et al. The lipid A phosphate position determines differential host Toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria. Infect Immun. 2011;79:203–210. doi: 10.1128/IAI.00937-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Jodar A., Nayan N.H., Hamzaoui S., Caja G., Salama A.A.K. Heat stress modifies the lactational performances and the urinary metabolomic profile related to gastrointestinal microbiota of dairy goats. PLoS One. 2019;14 doi: 10.1371/journal.pone.0202457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Hao Y., Li J., Bao W., Li G., Gao Y., et al. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: a proteomic approach. Int J Mol Sci. 2016;17:393. doi: 10.3390/ijms17050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morais M.B., Mello C.S., Carmo-Rodrigues M.S., Filho H.B.A., Melli L., Tahan S., et al. Microbiota, environment, and diet. J Pediatr Gastroenterol Nutr. 2017;65:e24. doi: 10.1097/MPG.0000000000001605. [DOI] [PubMed] [Google Scholar]
- Duscha A., Gisevius B., Hirschberg S., Yissachar N., Stangl G.I., Eilers E., et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180:1067–1080. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- Eftychi C., Schwarzer R., Vlantis K., Wachsmuth L., Basic M., Wagle P., et al. Temporally distinct functions of the cytokines IL-12 and IL-23 drive chronic colon inflammation in response to intestinal barrier impairment. Immunity. 2019;51:367–380.e4. doi: 10.1016/j.immuni.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J., Chrousos G.P., Wilder R.L. Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Ann N Y Acad Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- Fan P., Liu P., Song P., Chen X., Ma X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci Rep. 2017;7:43412. doi: 10.1038/srep43412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W., Wen X., Meng Q., Liu L., Xie J., Zhang H., et al. Running Head: heat affects cholesterol and bile acid alterations in cholesterol and bile acids metabolism in large white pigs during short-term heat exposure. Animals (Basel) 2020;10:359. doi: 10.3390/ani10020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Hales J.R., Rowell L.B., King R.B. Regional distribution of blood flow in awake heat-stressed baboons. Am J Physiol. 1979;237:H705–H712. doi: 10.1152/ajpheart.1979.237.6.H705. [DOI] [PubMed] [Google Scholar]
- He J., He Y., Pan D., Cao J., Sun Y., Zeng X. Associations of gut microbiota with heat stress-induced changes of growth, fat deposition, intestinal morphology, and antioxidant capacity in ducks. Front Microbiol. 2019;10:903. doi: 10.3389/fmicb.2019.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KA L.C., Boitard S., Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinf. 2011;12:253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk A.A., Zheng D., Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17:742–753. doi: 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- Lambert G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87:E101–E108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- Larsen J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen C. From oxidative stress to inflammation: redox balance and immune system. Poultry Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- Lê Cao K.-A., González I., Déjean S. integrOmics: an R package to unravel relationships between two omics datasets. Bioinformatics (Oxford, England) 2009;25:2855–2856. doi: 10.1093/bioinformatics/btp515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê Cao K.-A., Martin P.G.P., Robert-Granié C., Besse P. Sparse canonical methods for biological data integration: application to a cross-platform study. BMC Bioinf. 2009;10 doi: 10.1186/1471-2105-10-34. 34-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Sciellour M., Zemb O., Hochu I., Riquet J., Gilbert H., Giorgi M., et al. Effect of chronic and acute heat challenges on fecal microbiota composition, production, and thermoregulation traits in growing pigs. J Anim Sci. 2019;97:3845–3858. doi: 10.1093/jas/skz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hwang K.T., Heo M.S., Lee J.H., Park K.Y. Resistance of Lactobacillus plantarum KCTC 3099 from Kimchi to oxidative stress. J Med Food. 2005;8:299–304. doi: 10.1089/jmf.2005.8.299. [DOI] [PubMed] [Google Scholar]
- Lee S., Keirsey K.I., Kirkland R., Grunewald Z.I., Fischer J.G., de La Serre C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J Nutr. 2018;148:209–219. doi: 10.1093/jn/nxx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknoi Y., Mongkolsuk S., Sirikanchana K. Assessment of swine-specific bacteriophages of Bacteroides fragilis in swine farms with different antibiotic practices. J Water Health. 2017;15:251–261. doi: 10.2166/wh.2016.069. [DOI] [PubMed] [Google Scholar]
- Li Y., Song Z., Kerr K.A., Moeser A. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian P., Braber S., Garssen J., Wichers H.J., Folkerts G., Fink-Gremmels J., et al. Beyond heat stress: intestinal integrity disruption and mechanism-based intervention strategies. Nutrients. 2020;12:734. doi: 10.3390/nu12030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15 doi: 10.1186/s13059-014-0550-8. 550-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackos A.R., Maltz R., Bailey M.T. The role of the commensal microbiota in adaptive and maladaptive stressor-induced immunomodulation. Horm Behav. 2017;88:70–78. doi: 10.1016/j.yhbeh.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A.J., de Agüero M.G., Ganal-Vonarburg S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 2017;17:508–517. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- Marteyn B., Scorza F.B., Sansonetti P.J., Tang C. Breathing life into pathogens: the influence of oxygen on bacterial virulence and host responses in the gastrointestinal tract. Cell Microbiol. 2011;13:171–176. doi: 10.1111/j.1462-5822.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- Mcmurdie P.J., Holmes S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes V., Galvão I., Vieira A.T. Mechanisms by which the gut microbiota influences cytokine production and modulates host inflammatory responses. J Interferon Cytokine Res. 2019;39:393–409. doi: 10.1089/jir.2019.0011. [DOI] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Newman M.A., Grüll D., Zebeli Q. Consumption of transglycosylated starch down-regulates expression of mucosal innate immune response genes in the large intestine using a pig model. Br J Nutr. 2018;119:1366–1377. doi: 10.1017/S0007114518001113. [DOI] [PubMed] [Google Scholar]
- Mohammed A.A., Jiang S., Jacobs J.A., Cheng H.W. Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poultry Sci. 2019;98:4408–4415. doi: 10.3382/ps/pez246. [DOI] [PubMed] [Google Scholar]
- Montilla S.I., Johnson T.P., Pearce S.C., Gardan-Salmon D., Gabler N.K., Ross J.W., et al. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature (Austin) 2014;1:42–50. doi: 10.4161/temp.28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 11th ed. National Academies Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Paciello I., Silipo A., Lembo-Fazio L., Curcurù L., Zumsteg A., Noël G., et al. Intracellular Shigella remodels its LPS to dampen the innate immune recognition and evade inflammasome activation. Proc Natl Acad Sci U S A. 2013;110:E4345–E4354. doi: 10.1073/pnas.1303641110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.G., Han S.I., Oh S.Y., Kang H.S. Cellular responses to mild heat stress. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PubMed] [Google Scholar]
- Park S., Kim W. Effects of betaine on biological functions in meat-type ducks exposed to heat stress. Poultry Sci. 2017;96:1212–1218. doi: 10.3382/ps/pew359. [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Lonergan S.M., Huff-Lonergan E., Baumgard L.H., Gabler N.K. Acute heat stress and reduced nutrient intake alter intestinal proteomic profile and gene expression in pigs. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S.C., Mani V., Boddicker R.L., Johnson J.S., Weber T.E., Ross J.W., et al. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J Anim Sci. 2012;90(Suppl 4):257–259. doi: 10.2527/jas.52339. [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Mani V., Boddicker R.L., Johnson J.S., Weber T.E., Ross J.W., et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S.C., Mani V., Weber T.E., Rhoads R.P., Patience J.F., Baumgard L.H., et al. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J Anim Sci. 2013;91:5183–5193. doi: 10.2527/jas.2013-6759. [DOI] [PubMed] [Google Scholar]
- Pérez S., Taléns-Visconti R., Rius-Pérez S., Finamor I., Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med. 2017;104:75–103. doi: 10.1016/j.freeradbiomed.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Rhoads R.P., Baumgard L.H., Suagee J.K., Sanders S.R. Nutritional interventions to alleviate the negative consequences of heat stress. Adv Nutr. 2013;4:267–276. doi: 10.3945/an.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.W., Hale B.J., Gabler N.K., Rhoads R.P., Keating A.F., Baumgard L.H. Physiological consequences of heat stress in pigs. Anim Prod Sci. 2015;55:1381–1390. [Google Scholar]
- Schautteet K., Vanrompay D. Chlamydiaceae infections in pig. Vet Res. 2011;42:29. doi: 10.1186/1297-9716-42-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta S., Hittle L.E., Ernst R.K., Uriarte S.M., Mitchell T.C. A Pseudomonas aeruginosa hepta-acylated lipid A variant associated with cystic fibrosis selectively activates human neutrophils. J Leukoc Biol. 2016;100:1047–1059. doi: 10.1189/jlb.4VMA0316-101R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Yeoh B.S., Walker R.E., Xiao X., Saha P., Golonka R.M., et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut. 2019;68:1801–1812. doi: 10.1136/gutjnl-2018-316250. [DOI] [PubMed] [Google Scholar]
- Star L., Nieuwland M.G., Kemp B., Parmentier H.K. Effect of single or combined climatic and hygienic stress on natural and specific humoral immune competence in four layer lines. Poultry Sci. 2007;86:1894–1903. doi: 10.1093/ps/86.9.1894. [DOI] [PubMed] [Google Scholar]
- Trachsel J., Humphrey S., Allen H.K. Butyricicoccus porcorum sp. nov., a butyrate-producing bacterium from swine intestinal tract. Int J Syst Evol Microbiol. 2018;68:1737–1742. doi: 10.1099/ijsem.0.002738. [DOI] [PubMed] [Google Scholar]
- Wang X.J., Feng J.H., Zhang M.H., Li X.M., Ma D.D., Chang S.S. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poultry Sci. 2018;97:2153–2158. doi: 10.3382/ps/pey032. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Sun L., Grenier D., Yi L. Streptococcus suis biofilm: regulation, drug-resistance mechanisms, and disinfection strategies. Appl Microbiol Biotechnol. 2018;102:9121–9129. doi: 10.1007/s00253-018-9356-z. [DOI] [PubMed] [Google Scholar]
- Webster J.I., Tonelli L., Sternberg E.M. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wen X., Wu W., Fang W., Tang S., Xin H., Xie J., et al. Effects of long-term heat exposure on cholesterol metabolism and immune responses in growing pigs. Livest Sci. 2019;230:103857. [Google Scholar]
- Woo V., Alenghat T. Host-microbiota interactions: epigenomic regulation. Curr Opin Immunol. 2017;44:52–60. doi: 10.1016/j.coi.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Xie J., Zhang H. Dietary fibers influence the intestinal SCFAs and plasma metabolites profiling in growing pigs. Food Funct. 2016;7:4644–4654. doi: 10.1039/c6fo01406b. [DOI] [PubMed] [Google Scholar]
- Wu W., Zhang L., Xia B., Tang S., Liu L., Xie J., et al. Bioregional alterations in gut microbiome contribute to the plasma metabolomic changes in pigs fed with inulin. Microorganisms. 2020;8:111. doi: 10.3390/microorganisms8010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Han Y., Wang K., Guo S., Wu D., Huang X., et al. Oral administration of propionic acid during lactation enhances the colonic barrier function. Lipids Health Dis. 2017;16:62. doi: 10.1186/s12944-017-0452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Ao D., Zhou B., Spears J.W., Lin X., Huang Y. Effects of supplemental chromium propionate on serum lipids, carcass traits, and meat quality of heat-stressed broilers. Biol Trace Elem Res. 2017;176:401–406. doi: 10.1007/s12011-016-0852-7. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yi H., Wu Q., Jiang Z., Wang L. Effects of acute heat stress on intestinal microbiota in grow-finishing pigs, and associations with feed intake and serum profile. J Appl Microbiol. 2020;128:840–852. doi: 10.1111/jam.14504. [DOI] [PubMed] [Google Scholar]
- Yan Y.E., Zhao Y.Q., Wang H., Fan M. Pathophysiological factors underlying heatstroke. Med Hypotheses. 2006;67:609–617. doi: 10.1016/j.mehy.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li S., Yang L., Huang P., Li W., Wang S., et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4:2163. doi: 10.1038/ncomms3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Fan X., Zhong Z., Xu G., Shen J. Association of plasma diamine oxidase and intestinal fatty acid-binding protein with severity of disease in patient with heat stroke. Am J Emerg Med. 2015;33:867–871. doi: 10.1016/j.ajem.2015.01.047. [DOI] [PubMed] [Google Scholar]
- Zundler S., Neurath M.F. Novel insights into the mechanisms of gut homing and antiadhesion therapies in inflammatory bowel diseases. Inflamm Bowel Dis. 2017;23:617–627. doi: 10.1097/MIB.0000000000001067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.