Abstract
With advances in bone tissue engineering, various materials and methods have been explored to find a better scaffold that can help in improving bone growth and regeneration. Three-dimensional (3D) printing by fused deposition modeling can produce customized scaffolds from biodegradable polyesters such as polycaprolactone (PCL). Although the fabricated PCL scaffolds exhibited a lack of bioactivity and poor cell attachment on their surfaces, herein, using a simple postfabrication modification method with hydroxyapatite (HA) and bioglasses (BGs), we obtained better cell proliferation and attachment. Biological behavior and osteosupportive capacity of the 3D-printed scaffolds including PCL, PCL/HA, PCL/BG, and PCL/HA/BG were evaluated in this study, while human adipose tissue-derived mesenchymal stem cells (hADSCs) were cultured on the scaffolds. The cell morphology, attachment, and proliferation were investigated using scanning electron microscopy (SEM), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, and 4′,6-diamidino-2-phenylindole (DAPI) staining. In the next step, the ability of stem cells to differentiate into osteoblasts was evaluated by measuring alkaline phosphatase (ALP) activity, calcium deposition, and bone-related gene and protein expression. In the end, the expression levels of miR-20a, miR-125a, and their target genes were also investigated as positive and negative regulators in osteogenesis pathways. The results showed that the coated scaffolds with bioceramics present a more appropriate surface for cell adhesion and proliferation, as well as efficient potential in inducing osteoconduction and osteointegration compared to PCL alone and control. The PCL/HA/BG scaffold exhibited higher in vitro cell viability and bone formation compared to the other groups, which can be due to the synergistic effect of HA and BG. On the whole, this tricomponent 3D-printing scaffold has a promising prospect for bone tissue engineering applications.
Introduction
Bone tissue engineering has three components including the cell source, growth factor, and scaffold, among which scaffold significantly affects mass transport and supports cell proliferation, adhesion, and growth.1 Regular methods used to make three-dimensional (3D) porous scaffolds are limited in accurately controlling the architecture and internal connection of the pores.2,3 Advanced techniques such as three-dimensional (3D) printing can overcome many of the limitations of the traditional fabrication methods by precisely controlling the pore structure and architecture.4,5 3D printing techniques commonly used in medical applications include fused deposition modeling (FDM), stereolithography (SLA), selective laser sintering (SLS), particle binding (PB), inkjet printing (IP), and direct ink writing (DIW).6 Various synthetic and natural materials are used to make bone scaffolds, like polymers, ceramics, and even metallic foams.7 Synthetic materials are more suitable in terms of control over the micro and macrostructures of the scaffold, including porosity and material composition.8 The most common synthetic polymers in bone tissue engineering are poly α-hydroxy acids, which are prone to nonenzymatic degradation, due to their hydrolytically labile ester bond.9,10 A biocompatible polymer that has been approved by the Food and Drug Administration (FDA), is poly-ε-caprolactone (PCL).11 PCL has a low melting point and high crystallinity as well better workability and machinability at normal temperatures.12 But, the problem is that PCL is a hydrophobic material and does not have a tendency for cell attachment.13,14 The most basic and important criteria for any scaffold material are initial cell attachment and proliferation.15 From a cell biological point of view, primary cells mostly need a rigid surface for cell attachment and proliferation, which are anchorage-dependent.16 Bioactive ceramics such as β-tricalcium phosphate (b-TCP), hydroxyapatite (HA), bioactive glass (BG), and calcium silicate (CS), which are similar to the bone mineral phase, have received much clinical attention.17 Bioactive ceramic scaffolds can interact with physiological fluids due to the formation of HA-like layers of bones, resulting in strong chemical bonds with the bone tissue.18,19
Hydroxyapatite is a bioceramic and exhibits superior biological properties such as osteoconductivity, osteoinductivity, and excessive biocompatibility.20,21 HA has excellent biocompatibility due to its chemical composition, which allows the protein to attach on its surface with strong electrostatic interaction. Because of its high bioactivity, it reacts rapidly with organic molecules, proteins, and essential amino acids, and effectively repairs hard tissues such as bones and teeth.22,23 Bioactive glasses as inorganic bioactive materials are the most widely used synthetic materials and are used in bone tissue engineering applications due to their superior potential in bonding to bones and their stimulating effects on the new bone formation.24,25 But, they are less appropriate in load-bearing applications because of their brittleness and low flexibility and strength.26 Nevertheless, using them with polymers could increase the bioactivity and osteoconductivity of the structure, as well as the acidic byproducts of polymer degradation could be buffered and degradation rates could be proportional. The addition of bioactive glasses to PCL could compensate for its inherent hydrophobic nature and poor cell adhesion.27
Human adipose tissue-derived mesenchymal stem cells (hADSCs) seem to be the most promising for tissue engineering among adult stem cells.28 Osteogenic differentiation of hADSCs is a complex process, which is regulated by several factors, including microRNAs (miRNAs). Previous research has revealed that miRNAs regulate the stemness of cells and function negatively in the posttranscriptional regulation level of gene expression. A large number of microRNAs were upregulated or downregulated during osteogenic differentiation of human adipose mesenchymal stem cells (hAMSCs). Therefore, certain miRNAs as positive or negative regulators have been introduced in the osteogenic differentiation of hAMSCs.29
In this study, an easy postfabrication modification method is introduced to modify the 3D-printed scaffolds to make them more appropriate for biomedical applications. Four groups of scaffolds including PCL, PCL/HA, PCL/BG, and PCL/HA/BG were prepared. Then, after isolation and characterization of hADSCs in vitro, the effect of these scaffolds on the osteogenic differentiation potential of hADSCs was evaluated by measuring ALP activity, total calcium, and bone-related gene and protein expression. Furthermore, the effect of nanotomography was also determined on the expression of involved microRNA during osteogenesis expression.
Results and Discussion
Results
Morphological Evaluation
The surface morphologies of the PCL, PCL/HA, PCL/BG, and PCL/HA/BG scaffolds were characterized by field emission scanning electron microscopy (FeSEM). The only PCL scaffold filament shows a smooth surface, whereas, after treatment with nanoparticles, the nanoscale rough surface morphology was observed for the scaffolds. The roughness is induced by the nanoparticle treatment due to polymer degradation and erosion and may also contribute to the hydrophilicity of scaffolds.
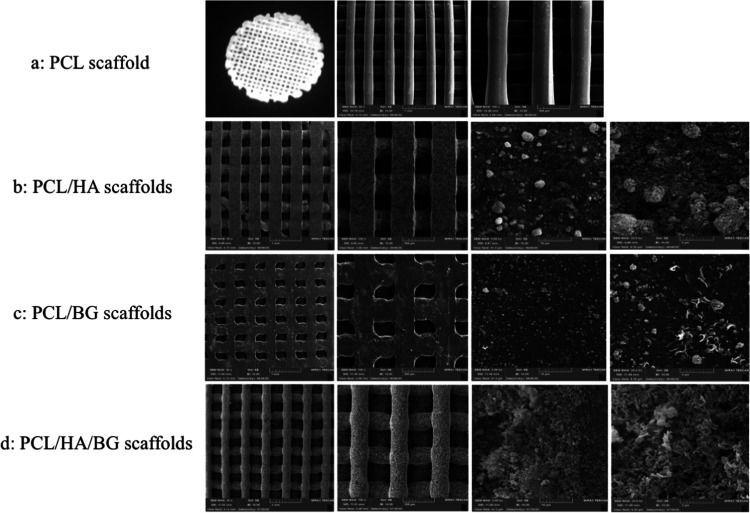
The surface morphology studied using SEM micrographs of PCL and surface-modified 3D-printed scaffolds at different magnifications are shown in Figure 1a–d. The well-aligned layers of the scaffolds can be seen at low magnification, which has not changed during postmodification. The nanoparticle incorporation did not cause the blocking of scaffold pores, which is desirable for biomedical applications. At high magnification, bioceramic particles can be seen on the surface of scaffolds. As seen from the micrographs, the pores are interconnected. The results obtained by Image J software are shown in Table 1.
Figure 1.
Morphology of 3D-printed scaffolds by scanning electron microscopy (FeSEM) at two magnifications (50×, 100×) and morphology of nanoparticles on the surface of scaffolds at two magnifications (5.00k×, 25.0k×). (a) PCL scaffolds, (b) PCL/HA scaffolds, (c) PCL/BG scaffolds, and (d) PCL/HA/BG scaffolds.
Table 1. Topographical Feature Sizes.
| scaffold | min | max | average |
|---|---|---|---|
| (A) Diameter of scaffold filaments (mm) | |||
| PCL | 0.299 | 0.332 | 0.314 |
| PCL/HA | 0.355 | 0.392 | 0.376 |
| PCL/BG | 0.310 | 0.342 | 0.323 |
| PCL/HA/BG | 0.266 | 0.337 | 0.3 |
| (B) Pore size of scaffolds (mm) | |||
| PCL | 0.288 | 0.397 | 0.350 |
| PCL/HA | 0.263 | 0.274 | 0.270 |
| PCL/BG | 0.337 | 0.348 | 0.341 |
| PCL/HA/BG | 0.255 | 0.484 | 0.350 |
Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Analysis
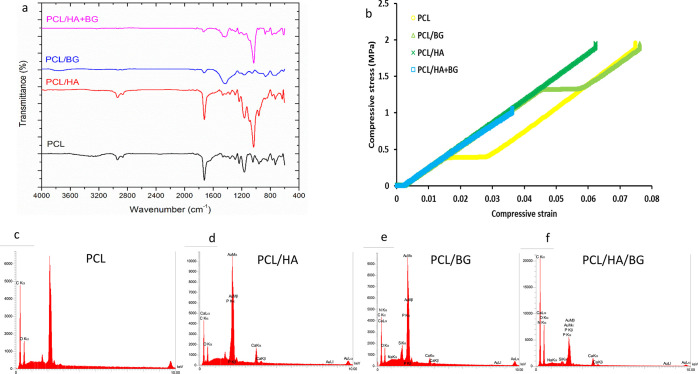
ATR-FTIR diagrams for PCL samples and PCL coated with hydroxyapatite and bioactive glasses (PCL/HA, PCL/BG), as well as PCL coated with both materials (PCL/HA + BG) are shown in Figure 2a. According to Figure 2a, the structural characteristics appearing in the infrared spectrum for the four groups are expressed as follows.
Figure 2.
(a) ATR-FTIR spectra of PCL, PCL/HA, PCL/BG, PCL/HA/BG scaffolds. (b) Stress–strain curves of 3D-printed scaffolds. (c–f) EDS analysis of PCL, PCL/HA, PCL/BG, and PCL/HA/BG scaffolds using FeSEM.
PCL Scaffold
The peaks at 2862 and 2938 cm–1 are attributed to symmetric and asymmetric stretchings of the −CH2 groups in the polymer chain, respectively. The prominent peak at 1724 cm–1 indicates the stretching vibrations of C=O groups. The peaks at 1294 and 1238 cm–1 show the asymmetric stretching vibrations of the C–O–C groups, and the absorption peak at 1166 cm–1 is due to its symmetric stretching vibrations. The broad peak at about 3300 cm–1 corresponds to the OH groups and the adsorbed moisture due to the H bonds in the system.
PCL/HA Scaffold
The absorption peaks in the wavenumber range of 1092 cm–1 and especially 1034 cm–1 are attributed to asymmetric stretching vibrations of P–O in phosphate groups. Also, the absorption peak at 960 cm–1 is due to their symmetrical stretching vibrations. The OH groups in the structure of HA also appear as absorption peaks at 634 cm–1 in the form of bending vibration.
PCL/BG Scaffold
The absorption peaks at 1052 and 1162 cm–1 are attributed to the asymmetric stretching of the Si–O–Si groups. Also, the absorption peak at 870 cm–1 and the shoulder at 940 cm–1 represent nonbridging oxygen in the Si–O- structure. In addition, an important absorption peak at 1428 cm–1 is attributed to the carbonate groups in the structure of BG. The wide peak at 3300 cm–1 can also be attributed to the OH groups and the adsorbed moisture due to the hydrogen bonds in the glass structure.
PCL/HA/BG Scaffold
By comparing the infrared spectrum of PCL coated with both HA and BG with the other three spectra, the presence of index absorption peaks of PCL attributed to carbonyl groups at about 1740 cm–1 and methylene groups in the range of 2800–3000 cm–1 was observed simultaneously, with the absorption peak at 1034 cm–1 attributed to the phosphate groups in the HA structure and the absorption peak due to the carbonate groups in the BG structure at a wavenumber of 1428 cm–1. Due to the simultaneous coating of PCL with both HA and BG, the size of the peaks attributed to PCL (1740 and 3000–2800 cm–1) decreased well compared to that of the uncoated state. Furthermore, the presence of HA, BG, and both on the surface of scaffolds is confirmed by ATR-FTIR spectroscopy.
Energy-Dispersive X-ray Spectroscopy (EDS) Analysis
The presence of P and Ca in PCL/HA scaffolds, Na, Si, Ca, and P in PCL/BG scaffolds, and also the presence of all of these elements in PCL/HA/BG scaffolds were confirmed using EDS. Meanwhile, their proper dispersion in the final structure was determined as shown in Figure 2c–f.
Compression Test
The stress–strain curves for the studied specimens are shown in Figure 2b, and the important characteristics of these diagrams are determined and presented in Table 2. As can be seen, the pure PCL sample has a yield stress of about 0.4 MPa, which indicates the mechanical change of the sample during loading. On the addition of BG to PCL, the mechanical behavior of the sample did not change, but its yield stress increased substantially by more than 300%, reaching 1.32 MPa. Moreover, the fracture toughness, which is the maximum mechanical energy that the material can withstand before failure and is calculated by the area below the strain–stress curve, and also the strain at break have increased. Meanwhile, the compressive modulus and the strength of the sample (the maximum compressive stress it can withstand before the material breaks) are reduced in comparison to the pure polymer. However, on the addition of HA to the pure polymer, it is observed that the behavior of the material has changed to reach full elasticity (absence of yield stress and plastic behavior). Also, the compressive modulus has improved significantly compared to the pure polymer and the compressive strength has remained almost unchanged. By simultaneously adding BG and HA to PCL, the modulus, strength, toughness, and strain at break are significantly reduced in comparison to the pure PCL, but the behavior of the material becomes quite elastic.
Table 2. Mechanical Characterization of 3D-Printed Scaffolds.
| sample | modulus (MPa) | yield stress (MPa) | strength (MPa) | strain at break (%) | toughness (MJ/m3) |
|---|---|---|---|---|---|
| PCL | 31.483 | 0.378 | 1.956 | 7.5 | 0.060 |
| PCL/BG | 29.688 | 1.320 | 1.951 | 7.6 | 0.074 |
| PCL/HA | 32.505 | 1.955 | 6.2 | 0.057 | |
| PCL/HA + BG | 30.696 | 1.062 | 3.6 | 0.017 |
Cell Adhesion and Proliferation on 3D-Printed Scaffolds
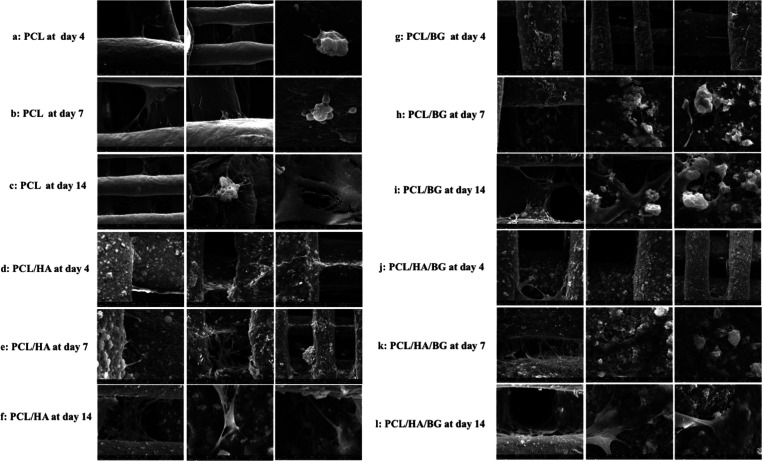
The morphology, distribution, and adhesion of hAMSCs seeded on PCL, PCL/HA, PCL/BG, and PCL/HA/BG scaffolds at days 4, 7, and 14 are presented by FeSEM images in Figure 3a–l. SEM analysis revealed that the four groups of 3D-printed scaffolds served as an excellent surface for the attachment and proliferation of hAMSCs. The cells expanded on all scaffolds to form a cell layer and penetrated into the pore of scaffolds.
Figure 3.
FeSEM images representing hADSCs cultured on 3D-printed scaffolds on days 4, 7, and 14. (a–c) hADSCs cultured on PCL scaffolds; (d–f) hADSCs cultured on PCL/HA scaffolds; (g–i) ADMSCs cultured on PCL/BG scaffolds; and (j–l) hADSCs cultured on PCL/HA/BG scaffolds.
Cytotoxicity Assay (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide (MTT))
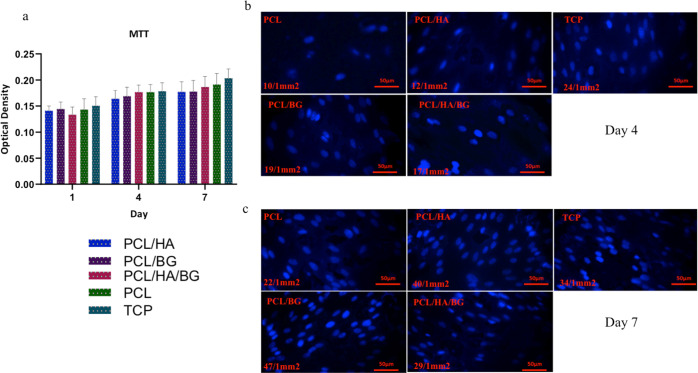
MTT assay was performed to evaluate the biocompatibility of scaffolds and viability of cells on the scaffolds. As shown in Figure 4a, an increasing pattern of the cell population is indicated during the period of time, for all groups and tissue culture polystyrene (TCPs). So, 3D-printed scaffolds did not show cytotoxicity on the proliferation level of stem cells. But, no significant difference was observed between different groups and TCPs as a control at the same period.
Figure 4.
(a) MTT assay of hADSCs on PCL, PCL/HA, PCL/BG, PCL/HA/BG, and TCP at days 1, 4, and 7 of the cell culture. (b) DAPI staining of the scaffolds on day 4 after the cell culture. (c) DAPI staining of the scaffolds on day 7 after the cell culture.
DAPI Staining
According to the DAPI staining results, which were performed on days 4 and 7 after the cell culture (Figure 4b,c), hAMSCs had an excellent cell attachment on the 3D-printed scaffolds. Images showed that a large population of hAMSCs adhered to the nanoparticle-coated PCL scaffolds than PCL alone. Therefore, the rate of cell attachment and viability on PCL/HA, PCL/BA, and PCL/HA/BG scaffolds were higher compared to those on the PCL scaffold.
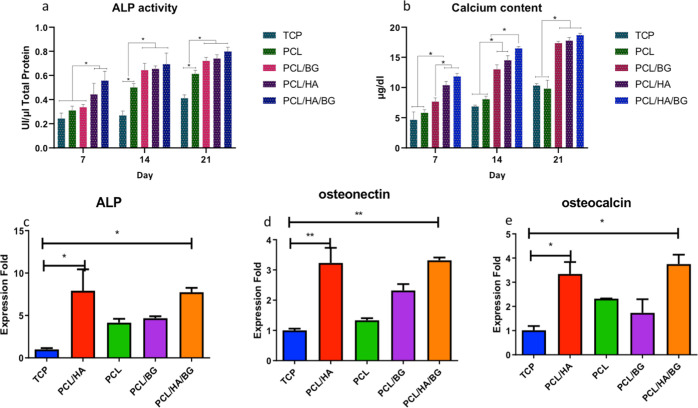
ALP Activity
As shown in Figure 5a, a significant increase in ALP activity was observed during the test period. On day 7, the two groups, PCL/HA/BG and PCL/HA, showed a significant increase compared to TCPs as control, and even PCL/HA/BG scaffolds had a significant increase compared to PCL and PCL/BG groups. On day 14, PCL scaffolds coated with nanoparticles indicated a significant increase in ALP activity compared to PCL alone and TCPs. According to the results, the highest level of the enzyme was related to day 21 of PCL/HA/BG scaffolds.
Figure 5.
(a) Alkaline phosphatase (ALP) activity of hAD-MSCs on coated and uncoated scaffolds, and TCPs after 7, 14, and 21 days of osteogenic differentiation. (b) Calcium content of hAD-MSCs on coated and uncoated scaffolds, and TCPs after 7, 14, and 21 days of osteogenic differentiation. (c–e) Relative expression of osteocalcin (OCN), osteonectin, and ALP genes after 21 days in hAD-MSCs on PCL, PCL/HA, PCL/BG, and PCL/HA/BG scaffolds, and TCPs during the osteogenic process.
Calcium Content and Mineralization
The calcium content was determined to evaluate the osteogenic differentiation in TCP, PCL, PCL/BG, PCL/HA, and PCL/HA/BG groups at days 7, 14, and 21 (see Figure 5b). A comparison of the four groups revealed a significant increase in mineralization in all groups during osteogenic differentiation. At the three time points, the highest calcium content was related to PCL/HA/BG, PCL/HA, and PCL/BG groups, respectively. There was no significant difference between PCL and TCP groups during the differentiation period. On day 7, PCL/HA/BG and PCL/HA scaffolds had a significant increase in the calcium content in comparison to PCL/BG scaffolds. In the second week, PCL/HA and PCL/BG groups had no significant difference, while the PCL/HA/BG group had a significant increase compared to PCL/HA and PCL/BG groups. On day 21, nanoparticle-coated scaffolds showed a very significant increase compared to PCL scaffolds and TCPs. However, there was no significant difference between them.
Gene Expression Analysis
A real-time PCR was performed to study the expression levels of osteoblast markers at mRNA levels. The relative expression levels of osteocalcin, osteonectin, and ALP genes were evaluated to clarify the osteogenic differentiation potential of hAMSCs on the scaffolds coated with HA and BG compared to PCL alone or the TCP group in the 21 day differentiation period. As shown in Figure 5c–e, there is a significant difference in bone genes expression, osteocalcin, osteonectin, and ALP between the 3D-printed scaffolds and TCPs. Evaluation of graphs related to all three genes indicated that PCL/HA/BG and PCL/HA scaffolds had a statistically significant increase compared to control. But, there was no significant difference between other groups. In addition, the expression of OCN in PCL/HA/BG and PCL/HA groups was significantly higher than that of PCL alone (p < 0.05).
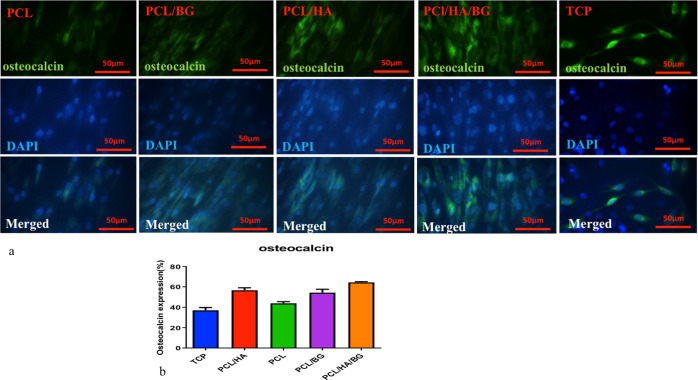
Immunocytochemical (ICC) Analysis
Immunocytochemistry was performed using the osteocalcin antibody, as an osteoblast cell marker. As shown in Figure 6a,b, the immunocytochemical staining revealed positive osteocalcin expression in all groups at day 21 but in groups where cells are seeded on 3D-printed scaffolds, we had higher expression compared to TCPs. According to Figure 6a,b, the highest levels of osteocalcin were seen in PCL/HA/BG, PCL/HA, PCL/BG, and PCL scaffolds.
Figure 6.
(a) Immunofluorescence staining of hAD-MSCs after 21 day induction in an osteoblast differentiation medium on 3D-printed scaffolds. (b) Diagram of immunofluorescence staining of hAD-MSCs after 21 day induction in an osteoblast differentiation medium.
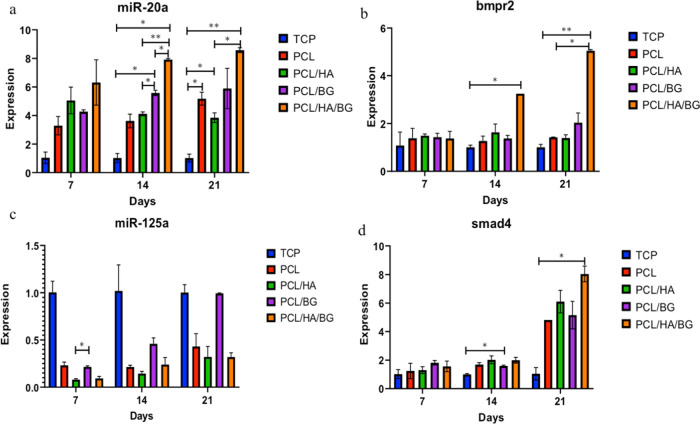
miRNA and Gene Expressions
Figure 7a–d shows that there are different expressions of microRNAs in the four groups of 3D-printed scaffolds relative to each other as well as to TCPs as a control. An increasing trend of expression of miR-20a as a positive regulator was clearly observed in the scaffolds compared to the control from days 7 to 21. The miR-20a expression levels were significantly upregulated during osteogenesis of hAMSCs on the PCL/HA/BG scaffolds, and there is a significant increase between the level of expression of miR-20a in PCL/HA/BG scaffolds compared to other groups of scaffolds and TCPs. miR-125a, as a negative regulator, is downregulated during osteogenesis. The greatest decrease in the expression level of this miR was observed in the PCL/HA/BG scaffold and subsequently in the PCL/HA scaffold on day 21. To determine the effect of miRs as regulators of BMP signaling, the expression levels of Smad4 and BMPR2 genes were evaluated in the osteogenesis period. BMPR2, as a positive regulator of BMP signaling, did not differ significantly in terms of the expression level on day 7 in the experimental groups, but there is a significant difference between the expression level of BMPR2 in PCL/HA/BG scaffolds compared to the control on days 14 and 21. The mRNA expression level of smad4 was upregulated after osteogenic differentiation compared with that on day 7. As shown in Figure 7d, the upregulation of smad4 in PCL/HA/BG scaffolds was significantly different compared to that of TCP on day 21.
Figure 7.
Relative expressions of (a) miR-20a, (b) BMPR2, (c) miR-125a, and (d) Smad4 on days 7, 14, and 21 in hAD-MSCs on PCL, PCL/HA, PCL/BG, and PCL/HA/BG scaffolds, and TCPs during the osteogenic process.
Discussion
Tissue engineering is a promising approach in the treatment of lost tissues of the human body, which are not treated by the current methods. In this regard, scaffold-based tissue engineering has been extensively investigated.30 Among the various scaffold fabrication methods, 3D printing is considered a useful method in fabricating tissue engineering scaffolds. Because of the advantages such as a simple workflow and low price, FDM that uses filaments or pellets made of PCL, poly (l-lactic acid) (PLLA), and other biodegradable and biocompatible polyesters is often used to produce the bone tissue engineering scaffold.31 By modifying the appropriate surface of the scaffold, biologically, cell molecules and cell-recognizable ligands can physically and chemically interact with the surface of the scaffold.30 Surface modification with ceramics like hydroxyapatite (HA), tri-calcium phosphate (TCP), bioactive glass (BG), and calcium silicate (CS) as biomaterials based on calcium, phosphate, and silicate are able to form direct bonds with living bones after implantation. By coating the bioceramics on the scaffold surface, this compound affects the cells and improves osteogenesis.32 Several research studies have been performed on the postfabrication modification of the scaffolds with bioceramics; for example, Bose et al., in 2018, have reported about the effect of surface modification of 3D-printed implants with calcium phosphate (CaP). Their findings showed that strontium oxide and silicon oxide doped calcium phosphate-coated titanium (Ti) porous cylinders with a nanotube film improved the early-stage osteointegration between the host bone and the implant compared to pure porous Ti rods.33 Also, the improving effects of interactions of BMP-2 and mineral-coated HAp microparticles on osteogenesis and chondrogenesis within hMSC aggregates were assessed by Dang et al.34 In addition to modifying the surface of scaffolds with bioceramics, a significant number of studies have already been performed in developing polymer–ceramic composites for clinical applications. Kalita and colleagues have fabricated TCP–polypropylene composites via FDM,35 and the scaffolds of PCL/HA/TCP composites were successfully manufactured using FDM for bone formation by Moukbil et al.36
The purpose of this study was to fabricate suitable 3D-printing scaffolds for bone repair and reconstruction. In this study, we selected PCL as the main material due to its mechanical strength and biocompatibility.37 Mondal et al., in 2020, studied the effects of printing at three orientations on the XY plane, 0, 45, and 90°, on the mechanical properties of PLA scaffolds. Their findings indicated that 3D printing at an orientation of 90° on the XY plane resulted in a scaffold with the highest compression strength and sufficient porosity.38 In this study, we fabricated circular four-layer scaffolds with a diameter of 1.5 cm, and the scaffolds were designed in a lay-down pattern of 0/90°, forming square pores. After that, a facile postfabrication modification was done on the 3D-printed PCL scaffold surface by coating HA, BG, and both nanoparticles. These bioceramics were selected due to their hydrophilicity, which could promote the hydrophilicity of the PCL matrix to improve the attachment of proteins and cells. After preparing the scaffolds, four scaffolds (PCL, PCL/HA, PCL/BG, PCL/HA/BG) were compared with each other in terms of different characteristics and with TCPs (cells seeded on the plastic area) as a control. Evaluation of 3D-printed scaffolds using FeSEM revealed the well-aligned layers and smooth surfaces of the PCL scaffolds and rough surfaces of scaffolds coated with nanoparticles. A porous network with sufficient porosity and interconnected pores was seen even after surface modification. Kumar et al. proved that 3D-printed scaffolds with an open porous structure are more suitable compared to other polymer scaffolds, such as foams and fibers, in facilitating uniform cell growth through the thickness of the scaffold.39 FTIR and EDX analysis confirmed the presence of HA, BG, and both on the surface of PCL/HA, PCL/BG, and PCL/HA/BG scaffolds, respectively.
The mesenchymal stem cells were isolated from human adipose tissues and characterized by flow cytometry (CD90+, CD105+, CD34–, CD45–). To our knowledge, there are some research studies that use bioceramics in scaffold fabrication but, in this study, we investigated the effect of surface modification of 3D-printed PCL scaffolds with only HA and BG and composites on the osteogenesis differentiation of hAD-MSCs. Therefore, in this research work, the effect of fabricated scaffolds on cell attachment, proliferation, and viability was examined at several time points after cell seeding by FeSEM, MTT, and DAPI staining assays. SEM analysis showed excellent attachment of cells to the surface of scaffolds. On days 7 and 14, the cells were seen as a layer on the surface of the scaffolds, and the newly proliferating cells communicated with each other, which were poured into the scaffold’s pores. In scaffolds coated with bioceramics, the interaction between the polymer, nanoparticles, and the cells is very interesting and the cell elongation on the nanoparticles is significant. In fact, the layer of HA and BG bioceramics improves cell attachment and proliferation; Mondal et al., in 2020, used human osteoblast-like MG-63 cells to investigate cell attachment and proliferation of synthesized and HAp-modified PLA scaffolds. Their result revealed that PLA-HAp scaffolds provided an excellent surface for cell proliferation and attachment.38 The nontoxicity of the scaffolds in cell survival was confirmed by the MTT assay. DAPI is a fluorescent stain that binds to A–T-rich regions in DNA molecules. Therefore, after staining, the nuclei of the cells can be seen using a fluorescence microscope. This dye can be used where the adhesion of cells to the scaffold is considered. Comparison of images from day 7 compared to day 4 after cell seeding showed better cell proliferation on scaffolds that were coated with bioceramics, which is due to their hydrophilic surfaces, compared to PCL alone. In bone tissue engineering, the measurement of the compressive strength of the scaffold is essential. Compressive strength is the ability of the scaffold to withstand pressure. Mechanical test analysis indicated that compressive moduli of scaffolds decreased with the addition of BG and increased with the addition of HA. The compressive strength and modulus of the PCL/HA scaffold are higher than those of the tricomponent PCL/HA/BG and PCL scaffolds. The values are in agreement with the mechanical properties (compressive strength: 0.8–11 MPa; modulus: 12–140 MPa) of the human cancellous bone.40 In our experiment, coating the scaffolds with BG caused a slight decrease in the mechanical strength, while Fathi and their groups, in 2020, showed the role of the BG in 3D-printed composite scaffolds. They printed PCL/multicomponent BG scaffolds via FDM and expressed that the addition of BG to PCL led to improved mechanical properties.41
The ability of osteoconductivity of scaffolds was examined through biochemical tests. The ALP enzyme is an indicator of osteoblastic activity, which shows the commitment of stem cells to the osteoblastic phenotype. According to the achieved results, a significant increase in ALP activity was observed from the first to the third week. Two groups of scaffolds (PCL/HA/BG and PCL/HA) revealed a significant increase compared to the TCP in the first week. It is worth mentioning that the PCL/HA/BG scaffold showed a good increase compared to PCL and PCL/BG groups at that point in time. In the second week, PCL scaffolds coated with nanoparticles indicated a significant increase in ALP activity, compared to PCL alone and TCP. But, the highest level of ALP was related to day 21 and the PCL/HA/BG scaffolds. Karimi et al. have shown that the amount of ALP increased during osteogenic differentiation from day 1 to 14 and then it decreased, which means the highest amount of enzyme activity was found at mid-differentiation.32 But, our result indicated the highest amount of enzyme activity on day 21, which agrees with some previous studies. Ma et al., in 2019, fabricated the composite 3D-printed PCL scaffolds with polyvinylacetate (PVAc) and HA to promote cytocompatibility and osteogenesis. They also showed that the highest level of ALP activity was detected on day 21.42 Our results showed a significant enhancement in the biomineralization in all groups and TCPs during osteogenic differentiation. At all three time points, the highest calcium content was attributed to PCL/HA/BG, PCL/HA, and PCL/BG scaffolds. On day 21, the scaffolds coated with nanoparticles showed a very significant increasing trend compared to the PCL and TCP, while there was no significant difference between them. The results show that bioceramics nanoparticles improve the process of osteogenic differentiation. Some studies confirm this statement. For example, the role of CaP coating onto a PCL melt electrospun scaffold was examined and it was shown that CaP coating accelerated the osteogenic process.43 Also, the results of a study conducted in 2015 by Saito and their groups exhibited that the biomineral-coated scaffolds (PCL and PLLA) have significantly more bone in-growth in contrast to the uncoated scaffolds.44
Immunostaining was performed to detect the expression level of osteocalcin on the 3D-printed scaffolds and control on day 21. Osteocalcin is commonly produced by osteoblasts as a marker of osteoblastic transformation. The highest level of osteocalcin expression was determined in PCL/HA/BG, PCL/HA, PCL/BG, PCL scaffolds. A higher expression level of osteocalcin in the PCL/HA/BG scaffold illustrated that the tricomponent scaffold is more successful in osteogenesis. To take a closer look at the osteogenic behavior of hAMSCs, the expression of three important bone-related genes was also investigated in the TCP groups and 3D-printed scaffolds. Osteocalcin and osteonectin play an important role in the mineralization and initial nucleation of hydroxyapatite.45 Osteonectin is a glycoprotein that binds Ca2+ and regulates the initial stages of crystal growth.46 As demonstrated in this study, hydroxyapatite with bioactive glasses or only in PCL scaffolds stimulates the expression of osteocalcin and increased biomineralization. A similar result was obtained with the ICC assay. The expressions of osteonectin in PCL/HA/BG and PCL/HA groups were significantly higher than that in only PCL. ALP is a group of enzymes that hydrolyze a variety of monophosphate esters at a high pH level, and they have been considered as an early and medium marker of osteogenesis.47 In the case of ALP, the three-substance scaffolds (PCL/HA/BG) and two-substance scaffolds (PCL/HA) demonstrated better results in ALP expression. Meanwhile, the expression levels of all three osteogenic genes in the 3D-printed scaffolds were higher than in TCPs as a control. Dan et al. showed that a caP-PCL scaffold promotes bone formation within the periodontal defect.48
Furthermore, the nanotopography effect of 3D-printed scaffolds on related osteoblast microRNAs (miR-20a, miR-125a) and their effective genes (smad4, BMPR2) in BMP signaling was examined by qPCR. Zhang et al. showed that the expression of endogenous miR-20a was increased in the osteogenic differentiation period. Their results indicated that miR-20a promoted osteogenic differentiation by the upregulation of BMP/Runx2 signaling. Bioinformatics analysis, which they performed, predicted that PPARy, Bambi, and Crim1 would be targets of miR-20a. PPARy is a negative regulator for BMP/Runx2 signaling, and Bambi and Crim1 are antagonists for BMP signaling. Bambi is an inhibitor for BMP receptors and a target gene for miR-20a.49 Thus, inhibition of Bambi by miR-20a causes upregulation of BMP receptors. The results obtained in this study have shown increased expression levels of miR-20a and BMPR2 from the first week to the third week and the highest level related to the cells seeded on the PCL/HA/BG scaffolds. The miR-125 family includes miR-125a and miR-125b, which are significantly downregulated during osteogenic differentiation in human adipose-derived stem cells.50 Several studies revealed that miR-125a negatively regulates osteoblastic differentiation of hAMSCs by targeting smad4.51,52 So, we expected to see upregulation of smad4 and downregulation of miR-125a in the osteoblastic differentiation period. According to the diagram, the upregulation of smad4 in 3D-printing scaffolds, especially PCL/HA/BG scaffolds compared to control was shown, and also the downregulation of miR-125a was indicated in fabricated scaffolds compared to TCPs. In a similar study, the effect of nanotopography of electrospun PLLA on the noncoding RNA network to osteogenic differentiation was evaluated.53 The results of the nanotopography effect on the expression of positive and negative regulatory microRNAs on osteogenesis confirmed that the 3D-printed PCL/HA/BG scaffold is suitable for bone regeneration.
Conclusions
A suitable scaffold must be able to support excellent cell attachment and proliferation on its surface. We have successfully fabricated 3D-printed scaffolds (90° orientation) with adequate porosity. Furthermore, surface modification on the scaffolds was performed using HA and BG bioceramics. The analysis of the results revealed that surface modification 3D-printed scaffolds effectively improved proliferation and osteogenic differentiation of stem cells. The interaction of HA and BG and both nanoparticles on the PCL scaffold surface greatly affect cell attachment and facilitate cellular activity. The chemical, biological, and molecular experiments indicated that the presence of HA/BG, HA, and BG bioceramics, respectively, enhance the process of osteogenic differentiation on the 3D-printed scaffolds. Finally, it can be concluded that the competitive PCL/HA/BG scaffold can be a promising candidate for application in bone tissue engineering.
Materials and Methods
Materials
Polycaprolactone (PCL, Mn = 80 000, Sigma-Aldrich), hydroxyapatite (HA, NikCeram Razi, Iran), bioactive glass (BG, NikCeram Razi, Iran), and Dulbecco’s modified Eagle’s medium (DMEM, Gibco) were used in the experiments and obtained as indicated.
3D Printing
In this paper, a specific 3D printer (Omid Afarinan Mohandesi Ayande, BioFabX2, Iran) and its software (Repetier Host V2.1.3, 2011_2018) were used to fabricate 3D porous scaffolds by the FDM method. The 3D printer melts the polymer pellets to lay the polymer in a layer-by-layer fashion to fabricate the scaffold with controlled pore dimensions in the scaffold. Circular four-layer scaffolds with a diameter of 1.5 cm were designed. The scaffolds were designed in a lay-down pattern of 0/90°, forming square pores. All scaffolds were fabricated using the same parameters: 0.5 mm diameter nozzle, a layer thickness of 0.2 mm, a distance of 0.3 mm between the two strings, a temperature of 110, and a speed of 2 mm/s. Disc scaffolds of 15(D)_0.8(H) mm for in vitro cell cultures (Figure 1a) and column scaffolds of 6(D)_12(H) mm for mechanical tests were fabricated.
Postfabrication Modification
To increase the hydrophilicity of the surface of PCL scaffolds, plasma treatment was performed using a low-frequency plasma generator at 90 GHz, with a cylindrical quartz reactor (Diener Electronics, Nagold, Germany). Pure oxygen gas was conducted into the reaction chamber at 0.4 mbar pressure, followed by ignition of glow discharge for 3 min. After preparation of 1 mg/mL solution, HA and BG particles in deionized water were placed in an ultrasonic bath for 20 min at 37 °C for good dispersion of microparticles. Then, plasma-treated scaffolds were immersed in HA, BG, and HA/BG solutions to deposit microparticles on the surface of scaffolds overnight. The scaffolds were washed well with deionized water and dried under vacuum conditions. After surface modification, we prepared four groups of scaffolds: PCL, PCL/HA, PCL/BG, and PCL/HA/BG. The scaffolds were sterilized using UV and ethanol before using them for experiments.
Characterization of Scaffolds
To characterize the surface morphology of scaffolds, in the first step, samples were visualized using a scanning electron microscope (FeSEM, Mira3, Tescan, Czech Republic) according to our previously reported method.54 The topographical feature size was measured using image analysis software (Image J, NIH). In the next step, to determine the surface chemical structure of the scaffolds and to ensure that the HA and BG nanoparticles are coated on the surface of the scaffolds, attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy (PerkinElmer) was performed. In addition, the mechanical properties of the fabricated scaffolds were determined using a universal testing machine (SANTAM, Iran) according to our previously reported method.55
Energy-Dispersive X-ray Spectroscopy (EDS)
Due to the fact that hydroxyapatite contains P and Ca and also bioactive glasses contain Si and Na, which are not present in PCL, elemental analyses were performed using energy-dispersive X-ray spectroscopy (EDS) to identify the presence of HA and BG on the surface of scaffolds. The samples were coated with gold and then analyzed with a scanning electron microscope (FeSEM, Mira3, Tescan, Czech Republic).
FeSEM: Cell Morphology and Adhesion
To study the attachment and behavior of 3D-printed scaffolds, isolated and characterized hAMSCs (see Supporting Information 1) were seeded with 104 cells/cm2. At 4, 7, and 14 days, the cell/scaffold constructs were fixed with 2.5% (w/v) glutaraldehyde for an hour and then rinsed thoroughly with phosphate buffered saline (PBS). Afterward, they were dehydrated through a series of increasing gradients of ethanol and dried under vacuum conditions. The constructs were coated with gold (20 A) and observed using a scanning electron microscope (FEI ESEM Quanta 200) at an accelerating voltage of 25 kV.
Scaffold Biocompatibility Assay
The cytotoxicity of the 3D-printed scaffolds on the seeded cells compared with tissue culture polystyrene (TCPs) as control was evaluated using MTT assay on days 1, 4, and 7. Sterilized scaffolds were placed in 24-well culture plates, seeded with 104 cells/cm2, incubated at 37 °C in 5% CO2. At required times, samples were incubated with MTT solution (5 mg/mL in DMEM) for 3 h. Afterward, the supernatant was removed and scaffolds were placed in 200 mL of dimethyl sulfoxide (Merck) and vortexed for 8 min to dissolve the dark-blue intracellular formazan crystals. In the end, the absorption of the purple solution was read via a spectrophotometer (Biotek Instrument) at a wavelength of 570 nm. Cell adhesion on the 3D-printed scaffolds was studied using DAPI staining. In this method, hAMSCs at a density of 104 cells/mL were seeded onto each scaffold in a 24-well plate, cultured in a medium (DMEM, 10% fetal bovine serum (FBS), %1 Pen/Sterp) and incubated at 37 °C and 5% CO2. After 4 and 7 days of incubation, the samples were washed two times with PBS and fixed with 4% paraformaldehyde (Sigma) for 45 min. Then, paraformaldehyde was removed, followed by washing with PBS. The samples were then stained with 50 μL of DAPI and incubated for 5 min at room temperature. To remove excess and unbound DAPI, they were washed with PBS. At the end, the cells were observed using a fluorescence microscope (LABOMED).
Osteogenic Differentiation
The osteosupportive capacity of the fabricated scaffolds was evaluated using the culture of hAMSCs with a cell density of 2 × 104 cells/cm2 in an osteogenic medium containing DMEM supplemented with 10% FBS, dexamethasone, β-glycerophosphate, and ascorbic acid for 21 days.
Alkaline Phosphatase Activity and Calcium Content Assay
Alkaline phosphatase (ALP) activity was investigated on days 7, 14, and 21 during osteogenic differentiation. Total protein was extracted from cells cultured on TCP, coated and uncoated scaffolds, using 200 μL of radioimmunoprecipitation assay (RIPA) lysis buffer. For sedimentation of cell debris, the lysate was shaken and centrifuged at 4 °C and 15 000g for 15 min. Then, the supernatant containing the total proteins was collected and ALP activity was assessed with the ALP assay kit (Pars Azmun, Iran). Eventually, the activity of the enzyme (IU/L) was normalized against the total protein (mg/dL). The amount of deposited calcium was measured during osteogenic induction on scaffolds and also TCPs as a control, using the cresolphthalein complexone method on days 7, 14, and 21. At first, for calcium extraction, all groups were homogenized in 0.6 N HCl (Merck, Germany) and shaken at 4 °C for 1 h. Next, the calcium content was measured using the calcium content kit protocol (Pars Azmoon, Iran), and the optical density (OD) of samples was measured at a wavelength of 570 nm in a microplate reader (Biotek Instruments). The calcium contents of samples were obtained from the standard curve of OD and a serial dilution of calcium concentrations.
Quantitative Real-Time RT-PCR
To detect the expression of bone-related genes in stem cells cultured on 3D-printed coated and noncoated scaffolds, compared to controls, a real-time PCR was carried out at day 21 postinduction. So, the expression of three genes, osteocalcin, osteonectin, and ALP, was investigated at the transcript level. Briefly, RNA was extracted from cell cultures using the extraction buffer RNA (Bonbiotech, Iran), according to the manufacturer’s instruction. Then, cDNA synthesis was performed using the cDNA synthesis kit (Bonbiotech, Iran). For quantitative real-time PCR, we used 6.5 μL of Syber Green qPCR Master Mix 2× (Bonbiotech, Iran), 1 μL of specific forward and backward primers, 1 μL of cDNA, and ROX Dye in accordance with the following program at 95 °C for 1 cycle and 2 min, 5 s at 95 °C for 40 cycles, and 30 s at 60 °C for 40 cycles. The specificity of the signals was confirmed by evaluating the melting curve of each gene. The relative expression of target genes were determined using the ΔΔCT method and REST 2009. Target genes were normalized against the B2-microglobulin gene as the internal control. The primer sequences used in qRT-PCR are presented in Table 3.
Table 3. Sequences of Primer Pairs Used for Quantitative Real-Time PCR.
| gene name | sequence |
|---|---|
| ALP | GCACCTGCCTTACTAACTC |
| AGACACCCATCCCATCTC | |
| osteocalcin (OSC) | GCAAAGGTGCAGCCTTTGTG |
| GGCTCCCAGCCATTGATACAG | |
| osteonectin (OSN) | AGGTATCTGTGGGAGCTAATC |
| ATTGCTGCACACCTTCTC | |
| β-2-microglobulin (β 2M) | TGGAAAGAAGATACCAAATATCGA |
| GATGATTCAGAGCTCCATAGAGCT |
Immunocytochemistry (ICC)
The immunocytochemistry (ICC) technique was performed to detect the expression levels of osteocalcin on the 3D-printed scaffolds. For this purpose, after being in osteogenic culture for 21 days, the cell suspension was cultured on sterile gelatin lamellae. After 24 h, it was washed with PBS and fixed at 4 °C for 20 min with 4% paraformaldehyde. Lamellae were incubated in 2 N HCL for 20 min at room temperature, after washing with PBS. Next, the lamellae were exposed to 0.3% Triton X-100 for 30 min (Triton-permeable cell membrane to antibodies). Afterward, 10% goat serum was added to the cells for half an hour (goat serum proteins cause nonspecific antigen sites to be coated and prevent a nonspecific reaction). Cells were then incubated overnight with primary antibodies; anti-osteocalcin (mouse anti-human, Santa Cruz) at a 1:100 (PBS) dilution at 4 °C. They were then washed twice with PBS and exposed to conjugated secondary antibodies (rabbit anti-mouse IgG-FITC, Santa Cruz) for 60 min at a 1:200 (PBS) dilution and 37 °C in the dark. After three times washing with PBS, DAPI was used to stain the nuclei and then examined with a fluorescence microscope (LABOMED).
MicroRNAs and Target Genes
In this section, based on previous studies, two microRNAs, miR-20a and miR-125a, and two related genes, Smad4 and BMPR2, which are effective in osteogenic differentiation pathways, were selected and their expression levels in the four groups of 3D printing scaffolds using quantitative real-time PCR in accordance with the above instruction were evaluated and compared at the days 7, 14, and 21 during osteogenic differentiation. The sequences of the primers are presented in Table 4, and U6 was used as an internal control.
Table 4. Sequences of Primer Pairs Used for Quantitative Real-Time PCR.
| names | sequence |
|---|---|
| hsa-miR-20a-RT | GTC GTA TGC AGA GCA GGG TCC GAG GTA TTC GCA CTG CAT ACG ACC TAC CT |
| hsa-miR-20a-F | CAT GCC TAA AGT GCT TAT AGT G |
| hsa-miR-125aRT | GTC GTA TGC AGA GCA GGG TCC GAG GTA TTC GCA CTG CAT ACG ACT CAC AG |
| hsa-miR-125a-F | TCCCTGAGACCCTTTAAC |
| h-BMPR2-F | AGAGACCCAAGTTCCCAGAAGC |
| h-BMPR2-R | CCTTTCCTCAGCACACTGTGCA |
| h-SMAD4-F | TTGGATGGACGACTTCAGG |
| h-SMAD4-R | CACTAACATACTTGGAGCATTAC |
Statistical Analysis
Each experiment was conducted three times. The results were analyzed with GraphPad software. One-way analysis of variance (ANOVA) was selected to compare the results. A P-value < 0.05 was considered as the level of significance.
Acknowledgments
The authors thank all colleagues in the tissue engineering lab in the Biotechnology Department of the University of Tehran for their contributions to this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04015.
Isolation and culture of hAMSCs; characterization of isolated hAMSCs by flow cytometry; and flow cytometric analysis of ADMSCs for the evaluation of the expression of stem cell surface markers (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Boccaccini A. R.; Maquet V. Bioresorbable and bioactive polymer/Bioglass composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003, 63, 2417–2429. 10.1016/S0266-3538(03)00275-6. [DOI] [Google Scholar]
- Shaunak S.; S Dhinsa B.; S Khan W. The role of 3D modelling and printing in orthopaedic tissue engineering: a review of the current literature. Curr. Stem Cell Res. Ther. 2017, 12, 225–232. 10.2174/1574888X11666160429122238. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Zhang J.; Zhao S.; Zhu Y. Three-dimensional printing of cerium-incorporated mesoporous calcium-silicate scaffolds for bone repair. J. Mater. Sci. 2016, 51, 836–844. 10.1007/s10853-015-9406-1. [DOI] [Google Scholar]
- Tack P.; Victor J.; Gemmel P.; Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed. Eng. Online 2016, 15, 115 10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak A. M.; Unal S.; Sahin A.; Oktar F. N.; Sengor M.; Ekren N.; Gunduz O.; Kalaskar D. M. 3D printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymers 2020, 12, 1962 10.3390/polym12091962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvendiren M.; Molde J.; Soares R. M.; Kohn J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. 10.1021/acsbiomaterials.6b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin-Shamsabadi A.; Hashemi A.; Tahriri M.; Bastami F.; Salehi M.; Abbas F. M. Mechanical, material, and biological study of a PCL/bioactive glass bone scaffold: Importance of viscoelasticity. Mater. Sci. Eng., C 2018, 90, 280–288. 10.1016/j.msec.2018.04.080. [DOI] [PubMed] [Google Scholar]
- Mitragotri S.; Lahann J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Ma P. X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. 10.1023/B:ABME.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- Kim C. G.; Han K. S.; Lee S.; Kim M. C.; Kim S. Y.; Nah J. Fabrication of Biocompatible Polycaprolactone–Hydroxyapatite Composite Filaments for the FDM 3D Printing of Bone Scaffolds. Appl. Sci. 2021, 11, 6351 10.3390/app11146351. [DOI] [Google Scholar]
- Yao Q.; Wei B.; Guo Y.; Jin C.; Du X.; Yan C.; Yan J.; Hu W.; Xu Y.; Zhou Z. Design, construction and mechanical testing of digital 3D anatomical data-based PCL–HA bone tissue engineering scaffold. J. Mater. Sci.: Mater. Med. 2015, 26, 51 10.1007/s10856-014-5360-8. [DOI] [PubMed] [Google Scholar]
- Sousa I.; Mendes A.; Pereira R. F.; Bártolo P. J. Collagen surface modified poly (ε-caprolactone) scaffolds with improved hydrophilicity and cell adhesion properties. Mater. Lett. 2014, 134, 263–267. 10.1016/j.matlet.2014.06.132. [DOI] [Google Scholar]
- Dwivedi R.; Kumar S.; Pandey R.; Mahajan A.; Nandana D.; Katti D. S.; Mehrotra D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. 10.1016/j.jobcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerling A.; Yazdanpanah Z.; Cooper D. M.; Johnston J. D.; Chen X. 3D printing PCL/nHA bone scaffolds: exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 3 10.1186/s40824-021-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapourzadeh A.; Atyabi S.-M.; Irani S.; Bakhshi H. Osteoinductivity of polycaprolactone nanofibers grafted functionalized with carboxymethyl chitosan: Synergic effect of β-carotene and electromagnetic field. Int. J. Biol. Macromol. 2020, 150, 152–160. 10.1016/j.ijbiomac.2020.02.036. [DOI] [PubMed] [Google Scholar]
- Persson M.; Lorite G. S.; Kokkonen H. E.; Cho S.-W.; Lehenkari P. P.; Skrifvars M.; Tuukkanen J. Effect of bioactive extruded PLA/HA composite films on focal adhesion formation of preosteoblastic cells. Colloids Surf., B 2014, 121, 409–416. 10.1016/j.colsurfb.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Guarino V.; Causa F.; Ambrosio L. Bioactive scaffolds for bone and ligament tissue. Expert Rev. Med. Devices 2007, 4, 405–418. 10.1586/17434440.4.3.405. [DOI] [PubMed] [Google Scholar]
- Kokubo T.; Kim H.-M.; Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. 10.1016/S0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- Gerhardt L.-C.; Boccaccini A. R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Mondal S.; Jang B.; Manivasagan P.; Moorthy M. S.; Oh J. Biomimetic synthesis of metal–hydroxyapatite (Au-HAp, Ag-HAp, Au-Ag-HAp): Structural analysis, spectroscopic characterization and biomedical application. Ceram. Int. 2018, 44, 20490–20500. 10.1016/j.ceramint.2018.08.045. [DOI] [Google Scholar]
- Kim H.; Mondal S.; Bharathiraja S.; Manivasagan P.; Moorthy M. S.; Oh J. Optimized Zn-doped hydroxyapatite/doxorubicin bioceramics system for efficient drug delivery and tissue engineering application. Ceram. Int. 2018, 44, 6062–6071. 10.1016/j.ceramint.2017.12.235. [DOI] [Google Scholar]
- Vila M.; Sánchez-Salcedo S.; Vallet-Regí M. Hydroxyapatite foams for the immobilization of heavy metals: From waters to the human body. Inorg. Chim. Acta 2012, 393, 24–35. 10.1016/j.ica.2012.06.027. [DOI] [Google Scholar]
- Mondal S.; Mahata S.; Kundu S.; Mondal B. Processing of natural resourced hydroxyapatite ceramics from fish scale. Adv. Appl. Ceram. 2010, 109, 234–239. 10.1179/174367613X13789812714425. [DOI] [Google Scholar]
- Lee H.-H.; Yu H.-S.; Jang J.-H.; Kim H.-W. Bioactivity improvement of poly (ε-caprolactone) membrane with the addition of nanofibrous bioactive glass. Acta Biomater. 2008, 4, 622–629. 10.1016/j.actbio.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Baino F.; Fiorilli S.; Vitale-Brovarone C. Bioactive glass-based materials with hierarchical porosity for medical applications: review of recent advances. Acta Biomater. 2016, 42, 18–32. 10.1016/j.actbio.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Nichols H. L.; Zhang N.; Zhang J.; Shi D.; Bhaduri S.; Wen X. Coating nanothickness degradable films on nanocrystalline hydroxyapatite particles to improve the bonding strength between nanohydroxyapatite and degradable polymer matrix. J. Biomed. Mater. Res., Part A 2007, 82A, 373–382. 10.1002/jbm.a.31066. [DOI] [PubMed] [Google Scholar]
- Rezaei A.; Mohammadi M. In vitro study of hydroxyapatite/polycaprolactone (HA/PCL) nanocomposite synthesized by an in situ sol–gel process. Mater. Sci. Eng., C 2013, 33, 390–396. 10.1016/j.msec.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Bacakova L.; Zarubova J.; Travnickova M.; Musilkova J.; Pajorova J.; Slepicka P.; Kasalkova N. S.; Svorcik V.; Kolska Z.; Motarjemi H.; et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells–a review. Biotechnol. Adv. 2018, 36, 1111–1126. 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Luo T.; Yang X.; Sun Y.; Huang X.; Zou L.; Liu J. Effect of MicroRNA-20a on Osteogenic Differentiation of Human Adipose Tissue-Derived Stem Cells. Cells Tissues Organs 2019, 208, 148–157. 10.1159/000506304. [DOI] [PubMed] [Google Scholar]
- Wang C.; Huang W.; Zhou Y.; He L.; He Z.; Chen Z.; He X.; Tian S.; Liao J.; Lu B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. 10.1016/j.bioactmat.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grémare A.; Guduric V.; Bareille R.; Heroguez V.; Latour S.; L’heureux N.; Fricain J. C.; Catros S.; Le Nihouannen D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res., Part A 2018, 106, 887–894. 10.1002/jbm.a.36289. [DOI] [PubMed] [Google Scholar]
- Karimi Z.; Seyedjafari E.; Mahdavi F. S.; Hashemi S. M.; Khojasteh A.; Kazemi B.; Mohammadi-Yeganeh S. Baghdadite nanoparticle-coated poly l-lactic acid (PLLA) ceramics scaffold improved osteogenic differentiation of adipose tissue-derived mesenchymal stem cells. J. Biomed. Mater. Res., Part A 2019, 107, 1284–1293. 10.1002/jbm.a.36638. [DOI] [PubMed] [Google Scholar]
- Bose S.; Banerjee D.; Shivaram A.; Tarafder S.; Bandyopadhyay A. Calcium phosphate coated 3D printed porous titanium with nanoscale surface modification for orthopedic and dental applications. Mater. Des. 2018, 151, 102–112. 10.1016/j.matdes.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang P. N.; Dwivedi N.; Yu X.; Phillips L.; Bowerman C.; Murphy W. L.; Alsberg E. Guiding chondrogenesis and osteogenesis with mineral-coated hydroxyapatite and BMP-2 incorporated within high-density hMSC aggregates for bone regeneration. ACS Biomater. Sci. Eng. 2016, 2, 30–42. 10.1021/acsbiomaterials.5b00277. [DOI] [PubMed] [Google Scholar]
- Kalita S. J.; Bose S.; Hosick H. L.; Bandyopadhyay A. Development of controlled porosity polymer-ceramic composite scaffolds via fused deposition modeling. Mater. Sci. Eng., C 2003, 23, 611–620. 10.1016/S0928-4931(03)00052-3. [DOI] [Google Scholar]
- Moukbil Y.; Isindag B.; Gayir V.; Ozbek B.; Haskoylu M. E.; Oner E. T.; Oktar F. N.; Ikram F.; Sengor M.; Gunduz O. 3D printed bioactive composite scaffolds for bone tissue engineering. Bioprinting 2020, 17, e00064 10.1016/j.bprint.2019.e00064. [DOI] [Google Scholar]
- Lee S. J.; Lee D.; Yoon T. R.; Kim H. K.; Jo H. H.; Park J. S.; Lee J. H.; Kim W. D.; Kwon I. K.; Park S. A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. 10.1016/j.actbio.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Mondal S.; Nguyen T. P.; Hoang G.; Manivasagan P.; Kim M. H.; Nam S. Y.; Oh J.; et al. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 2020, 46, 3443–3455. 10.1016/j.ceramint.2019.10.057. [DOI] [Google Scholar]
- Kumar G.; Tison C. K.; Chatterjee K.; Pine P. S.; McDaniel J. H.; Salit M. L.; Young M. F.; Simon C. G. Jr. The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials 2011, 32, 9188–9196. 10.1016/j.biomaterials.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr T.; Villars P.; Mitchell S.; Hsu H.-P.; Spector M. Compressive properties of cancellous bone defects in a rabbit model treated with particles of natural bone mineral and synthetic hydroxyapatite. Biomaterials 2001, 22, 1953–1959. 10.1016/S0142-9612(00)00370-7. [DOI] [PubMed] [Google Scholar]
- Fathi A.; Kermani F.; Behnamghader A.; Banijamali S.; Mozafari M.; Baino F.; Kargozar S. Three-dimensionally printed polycaprolactone/multicomponent bioactive glass scaffolds for potential application in bone tissue engineering. Biomed. Glasses 2020, 6, 57–69. 10.1515/bglass-2020-0006. [DOI] [Google Scholar]
- Ma J.; Lin L.; Zuo Y.; Zou Q.; Ren X.; Li J.; Li Y. Modification of 3D printed PCL scaffolds by PVAc and HA to enhance cytocompatibility and osteogenesis. RSC Adv. 2019, 9, 5338–5346. 10.1039/C8RA06652C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquette C.; Ivanovski S.; Hamlet S. M.; Hutmacher D. W. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 2013, 34, 5538–5551. 10.1016/j.biomaterials.2013.03.088. [DOI] [PubMed] [Google Scholar]
- Saito E.; Suarez-Gonzalez D.; Murphy W. L.; Hollister S. J. Biomineral Coating Increases Bone Formation by Ex Vivo BMP-7 Gene Therapy in Rapid Prototyped Poly (l-lactic acid) (PLLA) and Poly (ε-caprolactone) (PCL) Porous Scaffolds. Adv. Healthcare Mater. 2015, 4, 621–632. 10.1002/adhm.201400424. [DOI] [PubMed] [Google Scholar]
- Ji S.; Guvendiren M. 3D printed wavy scaffolds enhance mesenchymal stem cell osteogenesis. Micromachines 2020, 11, 31 10.3390/mi11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshirylajimi A.; Mossahebi-Mohammadi M.; Vakilian S.; Langroudi L.; Seyedjafari E.; Atashi A.; Soleimani M. Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres. Cell Prolif. 2015, 48, 47–58. 10.1111/cpr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedjafari E.; Soleimani M.; Ghaemi N.; Shabani I. Nanohydroxyapatite-coated electrospun poly (l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules 2010, 11, 3118–3125. 10.1021/bm1009238. [DOI] [PubMed] [Google Scholar]
- Dan H.; Vaquette C.; Fisher A. G.; Hamlet S. M.; Xiao Y.; Hutmacher D. W.; Ivanovski S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 2014, 35, 113–122. 10.1016/j.biomaterials.2013.09.074. [DOI] [PubMed] [Google Scholar]
- Zhang J.-f.; Fu W.-m.; He M.-l.; Xie W.-d.; Lv Q.; Wan G.; Li G.; Wang H.; Lu G.; Hu X.; et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 2011, 8, 829–838. 10.4161/rna.8.5.16043. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Zhang H.; Kang Y.; Sheng P.; Ma Y.; Yang Z.; Zhang Z.; Fu M.; He A.; Liao W. miRNA expression profile during osteogenic differentiation of human adipose-derived stem cells. J. Cell. Biochem. 2012, 113, 888–898. 10.1002/jcb.23418. [DOI] [PubMed] [Google Scholar]
- Gong Y.; Xu F.; Zhang L.; Qian Y.; Chen J.; Huang H.; Yu Y. MicroRNA expression signature for Satb2-induced osteogenic differentiation in bone marrow stromal cells. Mol. Cell. Biochem. 2014, 387, 227–239. 10.1007/s11010-013-1888-z. [DOI] [PubMed] [Google Scholar]
- Gu Z.; Long J.; Li Y.; Wang X.; Wang H. MiR-125a-3p negatively regulates osteoblastic differentiation of human adipose derived mesenchymal stem cells by targeting Smad4 and Jak1. Am. J. Transl. Res. 2019, 11, 2603–2615. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Izadpanahi M.; Seyedjafari E.; Arefian E.; Hamta A.; Hosseinzadeh S.; Kehtari M.; Soleimani M. Nanotopographical cues of electrospun PLLA efficiently modulate non-coding RNA network to osteogenic differentiation of mesenchymal stem cells during BMP signaling pathway. Mater. Sci. Eng., C 2018, 93, 686–703. 10.1016/j.msec.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Hosseini F. S.; Soleimanifar F.; Aidun A.; Enderami S. E.; Saburi E.; Marzouni H. Z.; Khani M. M.; Khojasteh A.; Ardeshirylajimi A. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) improved osteogenic differentiation of the human induced pluripotent stem cells while considered as an artificial extracellular matrix. J. Cell. Physiol. 2019, 234, 11537–11544. 10.1002/jcp.27807. [DOI] [PubMed] [Google Scholar]
- Golchin A.; Hosseinzadeh S.; Staji M.; Soleimani M.; Ardeshirylajimi A.; Khojasteh A. Biological behavior of the curcumin incorporated chitosan/poly (vinyl alcohol) nanofibers for biomedical applications. J. Cell. Biochem. 2019, 120, 15410–15421. 10.1002/jcb.28808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.