Abstract
CDC25A phosphatase promotes cell cycle progression by activating G1 cyclin-dependent kinases and has been postulated to be an oncogene because of its ability to cooperate with RAS to transform rodent fibroblasts. In this study, we have identified apoptosis signal-regulating kinase 1 (ASK1) as a CDC25A-interacting protein by yeast two-hybrid screening. ASK1 activates the p38 mitogen-activated protein kinase (MAPK) and c-Jun NH2-terminal protein kinase–stress-activated protein kinase (JNK/SAPK) pathways upon various cellular stresses. Coimmunoprecipitation studies demonstrated that CDC25A physically associates with ASK1 in mammalian cells, and immunocytochemistry with confocal laser-scanning microscopy showed that these two proteins colocalize in the cytoplasm. The carboxyl terminus of CDC25A binds to a domain of ASK1 adjacent to its kinase domain and inhibits the kinase activity of ASK1, independent of and without effect on the phosphatase activity of CDC25A. This inhibitory action of CDC25A on ASK1 activity involves diminished homo-oligomerization of ASK1. Increased cellular expression of wild-type or phosphatase-inactive CDC25A from inducible transgenes suppresses oxidant-dependent activation of ASK1, p38, and JNK1 and reduces specific sensitivity to cell death triggered by oxidative stress, but not other apoptotic stimuli. Thus, increased expression of CDC25A, frequently observed in human cancers, could contribute to reduced cellular responsiveness to oxidative stress under mitogenic or oncogenic conditions, while it promotes cell cycle progression. These observations propose a mechanism of oncogenic transformation by the dual function of CDC25A on cell cycle progression and stress responses.
Cyclin-dependent kinases (CDKs) are the central machinery that promotes cell cycle progression (25, 50, 57, 65). Phosphorylation and dephosphorylation of CDK proteins, as well as association with cyclins, control protein kinase activity. CDC25 phosphatases remove inhibitory phosphates from specific tyrosine and threonine residues within the ATP-binding domain of the CDK proteins, thus activating these kinases (12). The cell cycle-dependent expression of three CDC25 proteins suggests that CDC25A activates cyclin E(A)-CDK2 during G1 to S transition, while CDC25B is involved in the regulation of cyclin A-CDK2 or cyclin A-CDK1 during S to G2 transition (15, 30). CDC25C activates cyclin B-CDK1 at the G2-M boundary (44, 51). Expression of CDC25A is controlled by proliferation regulatory signals involving E2F and other transcription factors (8, 61). Overexpression of CDC25A shortens the passage of serum-stimulated HeLa cells through G1 (4), while microinjection of anti-CDC25A antibody inhibits the initiation of the S phase in rat kidney epithelial cells (30). Thus, CDC25A participates in a rate-limiting mechanism for G1 progression and initiation of DNA replication. Intriguingly, CDC25A and CDC25B have been postulated to be oncogenes, overexpressed in various types of cancers (6, 18, 19, 47, 63). These CDC25 phosphatases can cooperate with Ha-RAS to transform rodent fibroblasts (18). These data suggest that overexpression of CDC25A or CDC25B plays a critical role in establishing transformed phenotypes, generally characterized by unrestricted cell cycle progression and/or suppressed cell death.
Abrupt changes in cellular homeostasis, such as alterations in the reduction-oxidation (redox) potential, DNA damage, and imbalance of proliferation, cause cellular stress (1). Cells have complex signaling mechanisms to trigger a variety of intracellular responses upon stress and undergo either cell death (apoptosis) or survival with rescue from stress, depending on the amplitude of stress and balance of death-inducing and -sparing genes. The stress-induced signaling pathways involve cascades of protein kinases that ultimately control expression of a number of stress-responsive genes (31). Apoptosis signal-regulating kinase 1 (ASK1) functions as an upstream component of the kinase cascades that interacts with a variety of stress-induced signals (26, 27, 62). ASK1 phosphorylates and activates MKK4/7, which then activates the c-Jun NH2-terminal protein kinases (JNKs), also known as the stress-activated protein kinases, or SAPKs (11, 24, 43, 54, 59, 64, 70). ASK1 also phosphorylates and activates MKK3 and MKK6, leading to activation of the p38 mitogen-activated protein kinases (MAPKs) (42, 49, 58). ASK1 is activated by oxidative stress (20, 53), genotoxic stress (9), and interaction with death receptor-associated proteins, such as TRAFs and Daxx (7, 45). Downstream activation of JNKs influences multiple proteins that control apoptosis, including c-Jun, p53, and Bax (2, 35). Activation of p38 kinases also affects a number of transcription factors, such as ATF2, Elk-1, and NF-κB (28, 46). Activation of JNKs and p38 kinases seems to play a role in induction of apoptosis (67), while it could also be involved in cell survival, depending on cell type or cellular context, e.g., the interaction with survival factors including NF-κB (36, 40).
Although complex regulatory cross talk exists between cell cycle progression and cellular response to stress, our knowledge of its molecular basis is still limited. In this study, we present evidence that CDC25A inhibits ASK1 by physical association, and increased expression of CDC25A inhibits oxidant-induced activation of ASK1 and the downstream JNK and p38 pathways, reducing the sensitivity of cells to oxidant-induced cell death. These findings provide a novel clue to the interaction between the cell cycle machinery and stress-responsive mechanisms.
MATERIALS AND METHODS
Yeast two-hybrid screening.
The entire coding region of human CDC25A cDNA was fused in frame to the GAL4 DNA-binding domain by using the pTBG2 vector (56) to produce a fusion protein with CDC25A as the amino terminus. A rat ovary cDNA library in pGAD-GH (Clontech) was screened with this bait vector in Y190 yeast (Saccharomyces cerevisiae) by standard two-hybrid procedures as described previously. Library plasmids recovered from the positive (His 3+ LacZ+) clones were reexamined by cotransformation of SFY526 yeast with the bait plasmid followed by a β-galactosidase assay.
Cell lines and transfection.
COS-7 cells and OVCAR-8 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). COS cells were transfected with 10 μg of plasmid by electroporation at the setting of 300 V, 950 μF. Human embryonic kidney 293 cells were cultured in Dulbecco's modified minimum essential medium (DMEM) supplemented with 10% FBS and transfected with Superfect (Qiagen) according to the manufacturer's instructions. To examine the effects of CDC25A on ASK1 homo-oligomerization, 293 cells were transfected with pcDNA3-HA-ASK1 and pcDNA3-Flag-ASK1 (1 μg of each) together with 3 μg of pcDNA3-CDC25A. 293 cells stably expressing the tetracycline (tet) repressor from the pcDNA6/TR plasmid (T-REx-293 cells) were obtained from Invitrogen. To obtain clones with tetracycline-inducible expression of CDC25A, T-Rex-293 cells in a 10-cm-diameter culture dish were transfected with 10 μg of the pcDNA4/TO plasmid (Invitrogen) carrying the coding region of human CDC25A cDNA, by using Superfect, and subjected to selection for stable transfectants with 0.4 mg of Zeocin per ml. Two weeks later, colonies were picked, expanded, and tested for induction of the transgene by doxycycline.
Antibodies.
Anti-CDC25A (DCS120 + 121) and anti-HA (12CA5) monoclonal antibodies were obtained from Neomarkers and Boehringer Mannheim, respectively. The production of anti-ASK1 peptide (DAV) antiserum was described previously (7). Antibodies against phosphorylated forms of MKK3/6, p38, and ATF2 were purchased from New England Biolabs. Anti-JNK1 antibody (C-17) and antihemagglutinin (HA) polyclonal antibody were purchased from Santa Cruz Biotechnology. Anti-Flag monoclonal antibody (M2) and Sepharose conjugated with the M2 antibody were obtained from Sigma.
Immunocytochemistry and confocal laser-scanning microscopy.
Transfected 293 cells or OVCAR-8 cells without transfection were cultured on a four-well chamber slides (Nalge Nunc) for 16 h. Cells were fixed with 10% buffered-formalin, rinsed with phosphate-buffered saline (PBS) supplemented with 0.2% Triton X-100. Cells were incubated with primary antibodies in PBS containing 1% bovine serum albumin (BSA) at 25°C for 16 h. The following dilutions of the primary antibodies were used: anti-CDC25A monoclonal antibody (DCS120 + 121), 1:200 for 293 cells and 1:50 for OVCAR-8 cells; anti-HA polyclonal antibody, 1:200; anti-ASK1 polyclonal antibody (H-300), 1:50. These conditions were determined by several pilot experiments in which negligible nonspecific signals were observed in samples stained after preincubation of the antibodies with excess purified antigens. After being washed with PBS, cells were incubated with fluorescein-conjugated antimouse immunoglobulin G (IgG) antibody and Texas red-conjugated anti-rabbit IgG antibody (Vector Laboratories) in PBS containing 1% BSA at 25°C for 30 min. The slides were washed with PBS, and cover glasses were mounted with Vectashield with 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories). The stained samples were analyzed by an LSM510 laser-scanning confocal microscope (Carl Zeiss) equipped with a ×63 water immersion objective. Beams (488 nm for fluorescein, 568 nm for Texas red, and 351 and 364 nm for DAPI) from an argon krypton laser were used for excitation, and green, red, and blue fluorescence emissions were detected through LP505, LP560, and LP385, respectively. The collected images were processed with Adobe Photoshop, version 6.0. To better present nuclear structures in printed figure panels, the blue color was printed with a pseudo color (purple) in the panels showing nonmerged DAPI staining.
Protein expression and purification.
Site-directed mutagenesis of cDNAs was performed with the QuikChange kit (Stratagene). Recombinant baculoviruses for expression of ASK1, cyclin E, and CDK2(R169L) were constructed with the pBluebacHis-2 vector (Invitrogen). Extracts were prepared by sonication of virus-infected Sf9 cells in kinase buffer, which consisted of 50 mM HEPES-KOH (pH 7.5), 10 mM MgCl2, 1 mM dithiothreitol, and 10% glycerol. Glutathione S-transferase (GST) fusion proteins, including various forms of CDC25A and MKK6, were expressed in Escherichia coli BL21 transformed with the pGEX-3X vectors (Pharmacia) carrying the cDNAs, by growing bacteria with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 25°C for 8 h. Bacteria were then lysed by sonication in PBS, and Triton X-100 was added to a concentration of 1%. Lysates were incubated with glutathione-Sepharose 4B (Pharmacia) at 4°C for 1 h with rotation, and beads were then washed with PBS. Proteins were eluted in 50 mM Tris-HCl (pH 8) containing 20 mM reduced glutathione, followed by dialysis in kinase buffer.
Protein analyses.
Cells were lysed by sonication in lysis buffer including 50 mM HEPES-KOH (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 1 mM dithiothreitol, 10 mM β-glycerophosphate, 1 mM NaF, 0.1 mM sodium orthovanadate, 0.2 mM phenylmethylsulfonyl fluoride, 20 μg of aprotinin per ml, 20 μg of leupeptin per ml, 1 μg of pepstatin A per ml, 10 μg of soybean trypsin inhibitor per ml, 10% glycerol, and 1% NP-40 or Triton X-100. For ASK1 kinase activity, extracts of Sf9 insect cells infected with the ASK1 baculovirus were incubated with purified GST or GST-CDC25A in kinase buffer at 30°C for 30 min, and then [γ-32P]ATP (20 μM, 10 μCi per reaction) and 1 μg of purified GST-MKK6 were added to a final volume of 30 μl. GST-MKK6 used for the assay was a kinase-inactive KM mutant and did not display autophosphorylation (49). For some experiments, ASK1-expressing Sf9 extracts were immunoprecipitated with anti-ASK1 antibody (H-300) before incubation with purified GST or GST-CDC25A. After 30 min of further incubation, reaction was terminated by the addition of 4×-concentrated sodium dodecyl sulfate (SDS) sample buffer. ASK1 activity in tet-CDC25A-293 cells was measured by immunoprecipitation of lysates (200 μg of protein) with 2 μl of anti-ASK1(DAV) antiserum followed by in vitro kinase assay with GST-MKK6 as described above. p38 kinase activity was measured by immunoprecipitation of cell lysates with anti-p38 antibody followed by incubation with nonradioactive ATP and purified GST-ATF2 and immunoblotting with anti-phosphorylated ATF2 antibody. JNK1 activity was determined by immunoprecipitation of lysates with anti-JNK1 antibody followed by in vitro kinase assay with purified GST–c-Jun and [γ-32P]ATP. The ability of CDC25A to activate CDK2 was determined by incubating the sample with cyclin E-CDK2(R169L) complex immunoprecipitated from baculovirus-coinfected Sf9 cells, followed by histone H1 kinase assay as described previously (52). To examine ASK1 homodimerization, lysates (200-μg proteins) of 293 cells transfected with HA-ASK1 and Flag-ASK1 were incubated at 4°C for 60 min with Sepharose beads conjugated with anti-Flag antibody, followed by extensive washing with the lysis buffer and Western blotting with anti-HA antibody. Western blotting was performed with chemiluminescence reagents (Pierce). Data from autoradiography and chemiluminescence were quantitatively analyzed with the Molecular Imager system (Bio-Rad).
Cell death assays.
Cell death responses following treatment with H2O2 (0.5 to 4 mM), staurosporine (500 nM), actinomycin D (100 ng/ml), and cycloheximide (3 μg/ml) were assessed by a variety of criteria, as described previously (22). Apoptotic manifestations of cell death, which include shrinkage and cell rounding, were visualized by phase-contrast microscopy (Nikon Diaphot; Nikon, Garden City, N.Y). Condensation of chromatin was examined by epifluorescence, following incubation of cells with Hoechst 33342 (Sigma Chemical Co., St. Louis, Mo.). Cells that had lost adhesion and those maintaining adherence were isolated separately for quantification (Coulter Multisizer II; Beckman Coulter, Hialeah, Fla.). Nonadherent cells were recovered after gentle rinsing of plates twice with serum-free medium; adherent cells then were detached by trypsinization. The externalization of membrane phosphatidylserine was revealed by the binding of fluorescein isothiocyanate (FITC)-conjugated annexin V, and propidium iodide was employed to assess plasma membrane integrity. Mitochondrial membrane potential was revealed by staining cells with 100 nM tetramethyl rhodamine ethyl ester (TMRE; Molecular Probes, Eugene, Oreg.) at 37°C for 10 min.
RESULTS
Identification of ASK1 as a CDC25A-interacting protein.
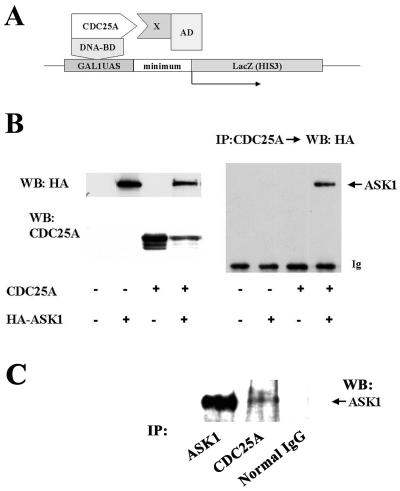
To investigate previously unidentified function or regulation of CDC25A, we performed a yeast two-hybrid screen for proteins that physically interact with CDC25A (Fig. 1A). The entire coding region of CDC25A was subcloned into the pTBG2 bait vector to express a CDC25A-Gal4-DNA binding domain fusion protein. A rat ovary cDNA library, constructed for expression of fusion proteins with the Gal4 activation domain, was screened with this bait plasmid by standard procedures to identify His 3+ LacZ+ colonies. Of 2 × 106 clones screened, nine positive clones were reexamined by retransformation with isolated library plasmids. Five of these clones consistently gave the two-hybrid signal (His 3+ LacZ+). One represented the rat 14–3-3ζ, which has been demonstrated to physically interact with CDC25A (10). Another clone was found to encode a sequence that is 95% identical to the amino acid residues 961 to 1020 of human ASK1. This domain of human ASK1 is located almost adjacent to the 3′ side of the kinase domain, residues 676 to 936. The sequences of the other three clones showed homology to mouse expressed sequence tag sequences, the identities of which are unknown at this moment.
FIG. 1.
ASK1 is a CDC25A-interacting protein. (A) Yeast two-hybrid screening for CDC25A-interacting proteins. AD, activation domain; BD, binding domain; UAS, upstream activation sequence; minimum, TATA box minimum promoter; X, protein encoded in the library. (B) Complex formation of CDC25A and ASK1 in transfected COS cells. COS-7 cells were transfected with pcDNA3 expression vectors encoding the proteins shown in the panel. At 48 h posttransfection, extracts were prepared and analyzed by Western blotting (WB) with antibodies as shown (left panels). To detect complexes, extracts were immunoprecipitated (IP) with anti-CDC25A monoclonal antibody, followed by Western blotting with anti-HA monoclonal antibody (right panel). The arrow indicates HA-tagged ASK1 detected in CDC25A immunoprecipitation. Ig, IgG heavy chain in immunoprecipitation cross-reacted with the secondary antibody. (C) CDC25A-ASK1 complex in untransfected ovarian carcinoma cells. Extracts of exponentially growing OVCAR-8 cells were immunoprecipitated with anti-ASK1 polyclonal antibody, anti-CDC25A monoclonal antibody, or control mouse IgG, followed by Western blotting with the anti-ASK1 antibody.
To confirm that ASK1 is a CDC25A-interacting protein, COS-7 cells were transfected with expression vectors for human CDC25A and human ASK1 with an HA tag at the carboxyl terminus, and cell lysates were subjected to immunoprecipitation with anti-CDC25A monoclonal antibodies, followed by Western blotting with anti-HA antibody (Fig. 1B). With this assay, we observed complex formation between full-length ASK1 and CDC25A proteins. Furthermore, we attempted to detect endogenous CDC25A-ASK1 complexes in mammalian cells without transfection (Fig. 1C). In exponentially proliferating ovarian carcinoma OVCAR-8 cells, ASK1 in complex with CDC25A was detected by immunoprecipitation followed by Western blotting. These data indicate that ASK1 is a CDC25A-interacting protein in mammalian cells.
Subcellular localization of CDC25A and ASK1.
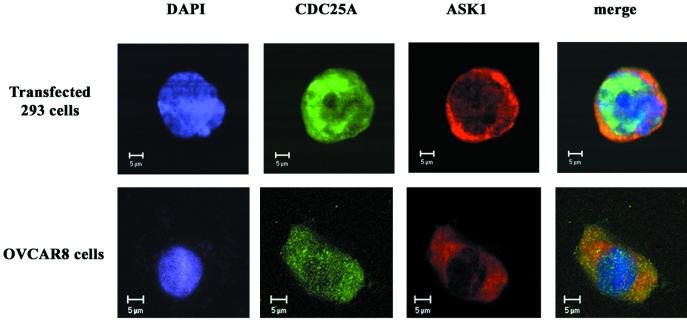
To extend our observation that CDC25A can physically associate with ASK1, we examined whether these proteins reside in the same cellular compartment. We transfected human embryonic kidney 293 cells for expression of CDC25A and HA-tagged ASK1. Immunocytochemistry followed by confocal laser-scanning microscopy showed that CDC25A was expressed both in the nucleus and in the cytoplasm (Fig. 2, upper panels). This expression pattern of CDC25A is consistent with previous studies with mouse 3T3 fibroblasts stably transfected with CDC25A (17). ASK1 was expressed mostly in the cytoplasm, partially colocalizing with CDC25A, as demonstrated by the yellow color in the merge panel. Under these conditions, only ectopically expressed CDC25A was detected, since the expression level of endogenous CDC25A was low in 293 cells and we used a low concentration of anti-CDC25A. HA-ASK1 was detected by anti-HA antibody. CDC25A functions in the nucleus as an activator of G1 CDKs. The colocalization of CDC25A and ASK1 in the cytoplasm observed in transfected cells might be due to forced overexpression of these proteins. To determine whether endogenous CDC25A and ASK1 colocalize in vivo, we performed immunocytochemistry for CDC25A and ASK1 in exponentially proliferating OVCAR-8 cells without transfection (Fig. 2, lower panels), in which CDC25A-ASK1 complex was detected by immunoprecipitation (Fig. 1C). Also in these cells, we detected both nuclear and cytoplasmic expression of CDC25A. Anti-ASK1 antibody demonstrated that ASK1 is predominantly expressed in the cytoplasm, and confocal analysis indicated that these two proteins partially colocalized in the cytoplasm. Background fluorescence was negligible when anti-CDC25A and anti-ASK1 antibodies were replaced with nonimmune mouse and rabbit IgG, and preincubation of the primary antibodies with purified antigens abolished the detected signals (data not shown). The clear cytoplasmic colocalization of CDC25A and ASK1 is consistent with the physical association of CDC25A and ASK1 demonstrated by the coimmunoprecipitation studies.
FIG. 2.
Subcellular localization of CDC25A and ASK1. Human embryonic kidney 293 cells, transfected with pcDNA3 expression vectors for CDC25A and HA-tagged ASK1, were stained with anti-CDC25A monoclonal antibody and anti-HA polyclonal antibody (upper panels). Human ovarian carcinoma OVCAR-8 cells, without transfection, were stained with anti-CDC25A monoclonal antibody and anti-ASK1 polyclonal antibody (lower panels). Signals were visualized by incubation with fluorescein-conjugated antimouse IgG antibody and Texas red-conjugated anti-rabbit IgG antibody, followed by analysis with a confocal laser-scanning microscope. These results are representative of three independent sets of experiments. Bar, 5 μm.
CDC25A inhibits ASK1 in a manner independent of the phosphatase activity.
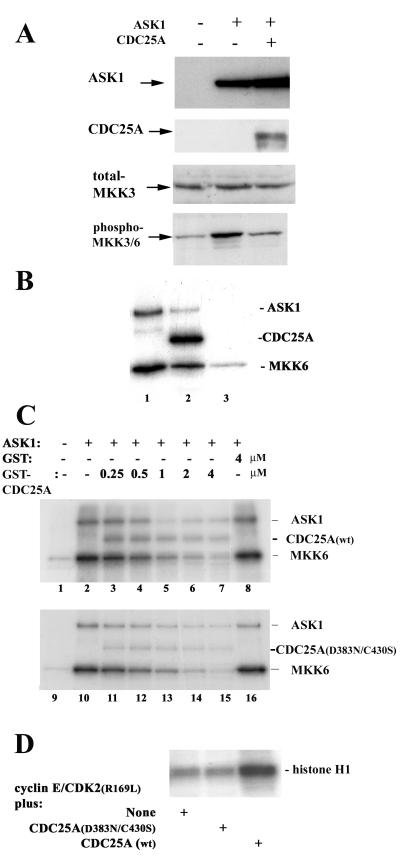
To address functional consequences of the CDC25A-ASK1 interaction, we compared phosphorylation of MKK3/6, physiological substrates of ASK1 kinase activity, in COS-7 cells transfected with ASK1 and ASK1 plus CDC25A (Fig. 3A). Immunoblotting of cell lysates with antibodies specific for the phosphorylated active forms of MKK3/6 demonstrated that transfection of ASK1 increased the activating phosphorylation of MKK3/6 relative to that of a mock transfection control. Cotransfection of CDC25A with ASK1 abrogated this ASK1-associated increase in MKK3/6 phosphorylation, whereas the expression of ASK1 or MKK3 was not affected. These data suggested that expression of CDC25A results in decreased activity of ASK1. To further investigate the interaction of ASK1 with CDC25A in vitro, we constructed a recombinant baculovirus encoding human ASK1. Extracts of Sf9 cells infected with the ASK1-encoding baculovirus contained large amounts (a range of 10 to 100 nM) of ASK1 protein (data not shown). Immunoprecipitates from the ASK1 virus-infected extracts with anti-ASK1 antibody (Fig. 3B, lane 1), as well as the total extracts (Fig. 3C, lane 2), were able to phosphorylate purified GST-tagged MKK6 protein in vitro with high activity. Autophosphorylation of ASK1 also was observed in this assay. In contrast, extracts of uninfected Sf9 cells had negligible activity to phosphorylate MKK6 (Fig. 3C, lane 1). Addition of purified GST-tagged CDC25A protein to immunoprecipitated ASK1 or ASK1-expressing Sf9 cell extracts resulted in inhibition of both MKK6 phosphorylation and ASK1 autophosphorylation (Fig. 3B, lane 2; and C, lanes 3 to 7). Interestingly, the incubation of GST-CDC25A with ASK1 led to phosphorylation of GST-CDC25A, which decreased in parallel with MKK6 phosphorylation and ASK1 autophosphorylation as higher doses of CDC25A were added. Incubation of immunoprecipitated ASK1 and GST-CDC25A without MKK6 also resulted in phosphorylation of GST-CDC25A, accompanied by a decrease in ASK1 autophosphorylation (data not shown). Extracts of Sf9 cells infected with a kinase-inactive mutant of ASK1, ASK1(K709R), phosphorylated neither MKK6 nor CDC25A (data not shown). These results suggest that CDC25A is both an inhibitor and a substrate of ASK1.
FIG. 3.
CDC25A inhibits ASK1 in a manner independent of its phosphatase activity. (A) Decreased ASK1-dependent MKK phosphorylation by cotransfection with CDC25A. COS-7 cells were transfected with pcDNA3 vectors encoding the proteins shown in the top panel. Cell extracts were prepared at 48 h after transfection and analyzed by immunoblotting with antibodies for ASK1, CDC25A, MKK3, and phosphorylated active MKK3/6. (B) CDC25A is both an inhibitor and a substrate of ASK1. ASK1 was immunoprecipitated from Sf9 cells infected with a human ASK1-encoding recombinant baculovirus and incubated at 30°C for 30 min with purified GST (lane 1) or GST-CDC25A (lane 2) in the presence of [γ-32P]ATP and purified GST-MKK6 (kinase-inactive KM mutant form). Immunoprecipitates with normal IgG were used as a negative control (lane 3). Phosphorylation of the proteins was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiography. (C) Inhibition of ASK1 by CDC25A and CDC25A(D383N/C430S). Extracts of Sf9 cells infected with the ASK1-encoding baculovirus were preincubated for 30 min at 30°C with purified GST-CDC25A, GST-CDC25A(D383N/C430S), or GST at the indicated concentration, before the addition of [γ-32P]ATP and GST- MKK6(KM). After incubation for 30 more min, phosphorylation of the proteins was analyzed by SDS-PAGE and autoradiography. Extracts of uninfected Sf9 cells were used as negative controls (lanes 1 and 9). wt, wild type. (D) The CDC25A(D383N/C430S) double mutant is phosphatase inactive. The activity of CDC25A was assessed by its ability to activate cyclin E-CDK2 in vitro. Sf9 cells were coinfected with baculoviruses for the expression of human cyclin E and CDK2(R169L), a mutant CDK2 known to accumulate inhibitory phosphorylation at Tyr 15. Cyclin E-CDK2 complexes were immunoprecipitated with anti-CDK2 antibody and incubated at 30°C for 30 min with GST-CDC25A(D383N/C430S) or wild-type GST-CDC25A. CDK2 kinase activity then was measured with histone H1 as a substrate, and phosphorylation of histone H1 was analyzed by SDS-PAGE and autoradiography.
This inhibition of ASK1 by CDC25A could be a consequence of physical association of these two proteins, suggested by the two-hybrid and coimmunoprecipitation analyses. Alternatively, the phosphatase activity of CDC25A might play a direct or indirect role in the inhibition of ASK1. To examine whether the catalytic activity of CDC25A is involved in ASK1 inhibition, we constructed and purified mutant CDC25A proteins that are phosphatase inactive. The active catalytic site of CDC25A resides in the carboxyl terminus. The Cys 430 residue of CDC25A is critical in the formation of a phosphate binding loop, while the Asp 383 residue is structurally important in forming a conserved buried salt bridge that is essential for phosphate hydrolysis (13, 14) (also see Fig. 5A). Purified GST-CDC25A exhibited phosphatase activity in vitro, as measured by the hydrolysis of a simple paranitrophenylphosphate substrate (data not shown) and by the dephosphorylation-dependent activation of an authentic CDK2 substrate (52) (Fig. 3D). In contrast, GST-CDC25A(D383N,C430S) was completely inactive as a phosphatase (Fig. 3D). Strikingly, this CDC25A double mutant was as effective as the wild type in its ability to inhibit ASK1 (Fig. 3C, lanes 11 to 15). ASK1 activity also was inhibited similarly by purified GST-CDC25A(C430S), which exhibited minimum phosphatase activity (data not shown). These results indicate that the ability of CDC25A to inhibit ASK1 is independent of its activity to dephosphorylate and activate CDKs and imply that the physical interaction itself is important for the inhibition. This is in sharp contrast with previous observations that CDC25A can bind to and dephosphorylate Raf-1 in vitro, (17, 66). While Raf-1 appears to activate CDC25A phosphatase in vitro (17), ASK1 did not significantly affect the phosphatase activity of CDC25A (data not shown). Thus, CDC25A interacts with and inhibits ASK1 in a unique and specific manner.
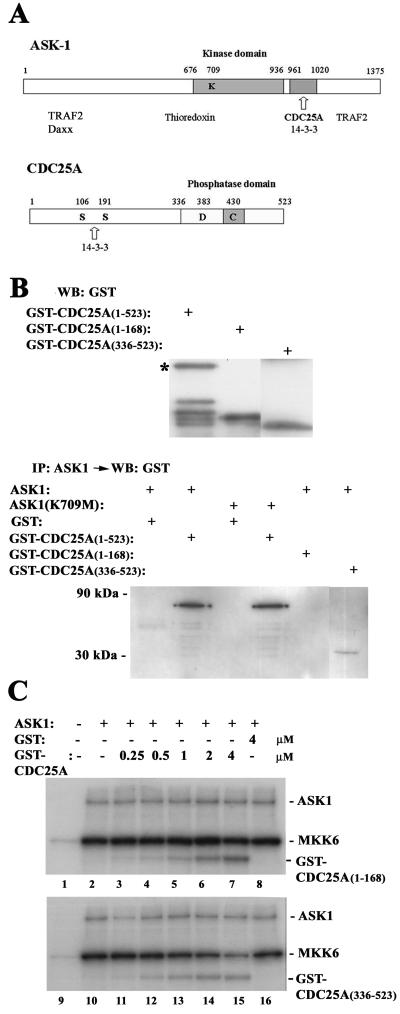
FIG. 5.
The carboxyl terminus of CDC25A is sufficient to bind and inhibit ASK1 in vitro. (A) Domain structures of ASK1 and CDC25A. The numbers shown represent amino acid residues. Names of proteins that bind to these proteins are listed below the putative binding domains. (B) The carboxyl terminus of CDC25A binds to ASK1 in vitro. The full-length and truncated fragments of CDC25A were purified from bacteria and analyzed by Western blotting (WB) with anti-GST antibody (upper panel). The asterisk indicates full-length GST-CDC25A(1–523), and other bands in the lane are degraded products in the preparation. ASK1 was immunoprecipitated (IP) from Sf9 cells infected with an ASK1-encoding baculovirus and then incubated at 30°C for 45 min with the GST-CDC25A fusion proteins. After extensive wash of the protein A-Sepharose beads, complexes were analyzed by Western blotting with anti-GST antibody (lower panel). (C) The carboxyl terminus of CDC25A can inhibit ASK1 in vitro. Extracts of Sf9 cells infected with the ASK1 baculovirus were incubated at 30°C for 30 min with purified GST or GST-tagged truncated CDC25A mutants at the indicated concentrations, before the addition of [γ-32P]ATP and GST-MKK6(KM). After incubation for 30 more min, phosphorylation of the proteins was analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. Extracts of uninfected Sf9 cells were used as negative controls (lanes 1 and 9).
CDC25A diminishes homo-oligomerization of ASK1.
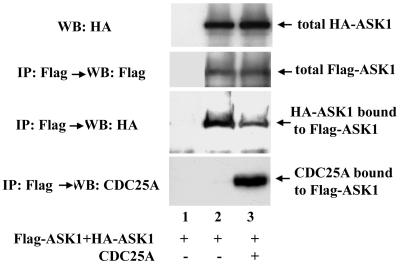
It has been demonstrated that the mechanism of ASK1 activation involves homo-oligomerization of this kinase (20, 37). ASK1 can form homo-oligomers in the cell, and coumermycin-dependent oligomerization of a gyrase B-ASK1 fusion protein results in activation of the kinase (20). TRAF2, an activator of ASK1, enhances oligomerization of ASK1 (37). To determine whether the inhibition of ASK1 by CDC25A involves an alteration in ASK1 oligomerization, HA- and Flag-tagged ASK1 proteins were expressed in 293 cells. Western blotting of anti-Flag immunoprecipitates with anti-HA antibody detected spontaneous oligomerization of ASK1 (Fig. 4). Coexpression of CDC25A resulted in a 60% decrease in the oligomerization, without affecting the levels of HA- and Flag-tagged ASK1 proteins. These observations suggest that physical association with CDC25A diminishes homo-oligomerization of ASK1, which is consistent with the noncatalytic inhibitory action of CDC25A.
FIG. 4.
Increased expression of CDC25A diminishes homo-oligomerization of ASK1. 293 cells were cotransfected with pcDNA3 expression vectors encoding the proteins shown in the panel. After 36 h, cell lysates were prepared and subjected to Western blotting (WB) or immunoprecipitation (IP) followed by Western blotting, with antibodies against the epitopes shown in the panel. To confirm the specificity of immunoprecipitation, normal mouse IgG-conjugated protein A-Sepharose was used in lane 1, instead of Sepharose conjugated with anti-Flag monoclonal antibody (lanes 2 and 3).
The carboxyl terminus of CDC25A contains residues important for ASK1 interaction.
We dissected further the region of CDC25A that interacts with ASK1. The amino-terminal portion of CDC25A, amino acid residues 1 to 336, contains 14–3-3 binding sites (68), while the carboxyl terminus has the catalytic domain with a phosphatase loop motif in residues 429 to 436 (14) (Fig. 5A). It is noteworthy that ASK1 also can bind 14–3-3 proteins via the consensus motif within residues 961 to 1020 (71), overlapping with the domain for association with CDC25A (Fig. 5A). Truncated forms of CDC25A, residues 1 to 386, 1 to 168, and 336 to 523, were produced as GST-fusion proteins and tested for their abilities to bind to ASK1 in vitro (Fig. 5B). Immunoprecipitation followed by immunoblotting demonstrated that the carboxyl-terminus form, CDC25A(336–523) could bind to ASK1, whereas neither CDC25A(1–168) nor CDC25A(1–386) showed detectable binding with ASK1 (Fig. 5B) (data not shown). In this assay, we also observed that CDC25A could bind to kinase-inactive ASK1(K709R), as well as wild-type ASK1. In vitro kinase assays showed that CDC25A(336–523) could inhibit ASK1 (Fig. 5C, lower panel), although the concentrations of this mutant required for ASK1 inhibition were higher than those of wild-type CDC25A. At the concentration of 4 μM, CDC25A(336–523) decreased the MKK6 kinase activity of ASK1 by 61% ± 12%, whereas wild-type CDC25A at the same concentration demonstrated 88% ± 9% inhibition (mean ± standard deviation; n = 3). These observations, as well as the weaker binding of CDC25A(336–523) with ASK1 compared to that of full-length CDC25A (Fig. 5B), imply that other unknown regions of CDC25A cooperate with the carboxyl terminus in the inhibitory interaction with ASK1. In contrast, CDC25A(1–168) did not inhibit ASK1 (Fig. 5C, upper panel). CDC25A(1–386) also showed minimum effects on ASK1 activity (data not shown). Interestingly, in these kinase assays, both CDC25A(336–523) and CDC25A(1–168) were phosphorylated by ASK1, suggesting that there are multiple ASK1-dependent phosphorylation sites in CDC25A. The kinase-inactive ASK1(K709R) was unable to cause phosphorylation of wild-type CDC25A or these truncated mutants (data not shown). These data suggest that the inhibitory interaction of CDC25A with ASK1 depends on the carboxyl terminus of CDC25A.
Increased expression of CDC25A suppresses oxidant-induced activation of ASK1 and downstream kinases.
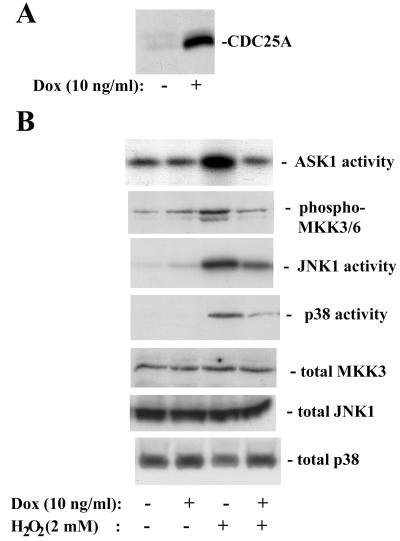
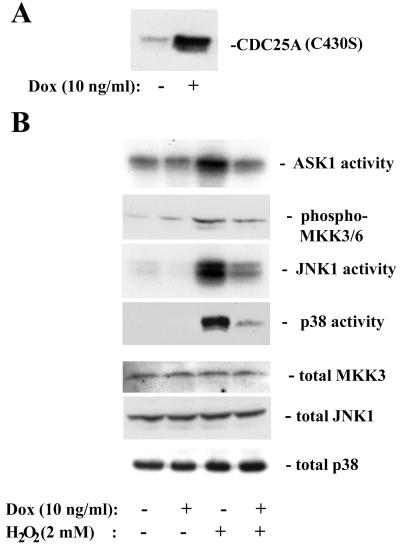
Our finding that CDC25A inhibits ASK1 prompted us to test whether increased expression of CDC25A interferes with stress-responsive pathways that involve ASK1 activation. To examine the effect of increased CDC25A expression on acute stress responses, we established clones of 293 cells with inducible expression of CDC25A, by using the tetracycline (tet)-regulatory system (69). Treatment of the clones with 10 ng of doxycycline per ml for 4 h increased CDC25A expression by five- to eightfold over the basal levels of endogenous CDC25A expression (Fig. 6A). We examined the effects of elevated CDC25A expression on stress kinase cascades by treating these tet-CDC25A-293 cells with H2O2 (Fig. 6B). The activities of JNK and p38, as well as that of ASK1, were increased within 20 min after treatment with 2 mM H2O2, indicating that these stress-responsive kinase cascades were rapidly activated by the oxidant, as previously reported (20). When cellular CDC25A expression was elevated by prior induction with doxycycline, the levels of activation of ASK1, JNK1, and p38 upon H2O2 treatment were diminished significantly. Activation of phosphorylation of MKK3/6 also was blocked by CDC25A upregulation. The oxidant treatment did not affect the expression of ASK1, MKK3, JNK1, or p38. We obtained similar results by using three independent tet-CDC25A-293 cell clones (data not shown). The induction of CDC25A over 4 h had no detectable effect on cell cycle progression according to flow cytometric analyses of cellular DNA content and incorporation of bromodeoxyuridine (data not shown). To further confirm that the diminished activation of the stress-responsive kinases is independent of the phosphatase activity of CDC25A, we created 293 cells with tet-inducible expression of phosphatase-inactive CDC25A(C430S) (Fig. 7A). Treatment of these cells with H2O2 demonstrated that induced expression of CDC25A(C430S) resulted in diminished activation of ASK1, MKK3/6, JNK1, and p38 in a similar fashion to the expression of wild-type CDC25A (Fig. 7B). These data indicate that elevated expression of CDC25A suppresses oxidative stress-dependent activation of the JNK and p38 kinase cascades and are consistent with the upstream inhibitory effect of CDC25A on ASK1.
FIG. 6.
Increased expression of CDC25A suppresses activation of the stress kinase cascades upon oxidative stress. (A) CDC25A expression after a 4-h induction with doxycycline (Dox; 10 ng/ml) in 293 cells carrying a tetracycline-inducible CDC25A transgene. Expression of CDC25A was measured by immunoblotting. (B) Induced CDC25A expression diminishes the activation of ASK1, JNK1, and p38 by H2O2 treatment. Cells were treated with doxycycline for 4 h, and then exposed to H2O2 (2 mM) for 20 min. For the activities of the stress-responsive kinases, cell extracts were immunoprecipitated with antibodies against ASK1, JNK1, or p38, and the activities were assayed as described in Materials and Methods. Phosphorylation of MKK3/6 was examined by Western blotting with an antibody specific for phosphorylated active forms. Total expression of MKK3, JNK1, and p38 was determined by Western blotting.
FIG. 7.
Ectopic expression of phosphatase-inactive CDC25A(C430S) suppresses activation of the stress kinase cascades upon oxidative stress. (A) The expression of CDC25A(C430S) after a 4-h induction with doxycycline (Dox; 10 ng/ml) in 293 cells carrying a tetracycline-inducible transgene. The sum of expression of CDC25A(C430S) and endogenous CDC25A was measured by immunoblotting. (B) Ectopic CDC25A(C430S) expression diminishes the activation of ASK1, JNK1 and p38 by H2O2 treatment. Cells were treated with doxycycline for 4 h and then exposed to H2O2 (2 mM) for 20 min. For the activities of the stress-responsive kinases, cell extracts were immunoprecipitated with antibodies against ASK1, JNK1, or p38, and the activities were assayed as described in Materials and Methods. Phosphorylation of MKK3/6 was examined by Western blotting with an antibody specific for phosphorylated active forms. Total expression of MKK3, JNK1, and p38 was determined by Western blotting.
Increased expression of CDC25A reduces the sensitivity of cells to oxidative stress-induced apoptosis.
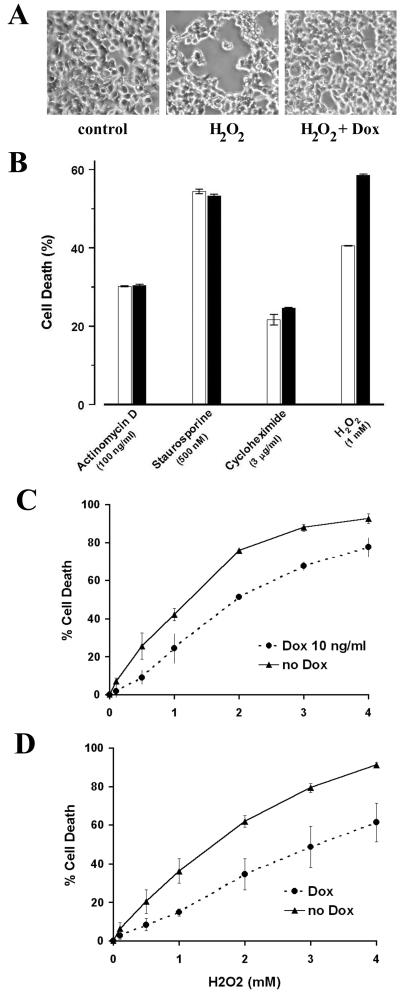
We examined whether CDC25A-mediated inhibition of oxidant-dependent stress kinase cascades altered the death response of cells subjected to oxidative stress. tet-CDC25A-293 cells underwent a rapid apoptotic response when treated with H2O2. Within 3 h of treatment with 2 mM H2O2, approximately 30% of cells already exhibited manifestations of apoptosis, including chromatin condensation, loss of mitochondrial membrane potential, membrane phosphatidylserine externalization, and alterations of cellular morphology and light scatter properties (Fig. 8A and data not shown). Preinduction of CDC25A in these cells by a 3-h preincubation with doxycycline consistently exerted a modest and significant reduction in this death response. This is evident qualitatively as a reduction in the extent of cellular condensation and loss of adhesion triggered by H2O2 (Fig. 8A, compare right and center panels) and quantitatively as the reduced percentages of cells that demonstrated the loss of mitochondrial membrane potential and diminution of cell size (Fig. 8B). The responses of these cells to other death stimuli, such as actinomycin D, staurosporine, and cycloheximide, were not altered by CDC25A upregulation (Fig. 8B). The data in Fig. 8C quantify the inhibitory effect of elevated CDC25A expression on this death response across a range of H2O2 concentrations. Cells with induced expression of phosphatase-inactive CDC25A(C430S) exhibited similar reductions in the death response to H2O2 (Fig. 8D), which is consistent with the inhibitory effects of this catalytically inactive CDC25A mutant on the stress kinase cascades shown in Fig. 7B. These observations suggest that the inhibitory effect of CDC25A expression on the initiation of oxidative stress-induced kinase cascades, which is independent of the phosphatase activity, is manifest as well by its suppression of oxidant-induced cell death.
FIG. 8.
Ectopic expression of wild-type or phosphatase-inactive CDC25A reduces the sensitivity of cells to oxidant-induced death specifically. (A) The morphology of cells after a 3-h exposure to H2O2 (2 mM) in 293 cells carrying a tetracycline-inducible CDC25A transgene (tet-CDC25A-293 cells). The Dox group was preincubated for 3 h with doxycycline (10 ng/ml) to increase cellular expression of CDC25A (Fig. 6A). Cells undergoing apoptosis exhibit round and condensed bodies with decreased adherence to the culture dish. (B) The extent of cell death was evaluated in cultures of tet-CDC25A-293 cells after treatment for 9 h with actinomycin (100 ng/ml), staurosporine (500 nM), cycloheximide (3 μg/ml), and H2O2 (1 mM). Death was assessed as the loss of mitochondrial membrane potential (diminished TMRE staining) as well as diminution of cell size (forward-angle light scatter). The open and solid columns represent cultures with and without a 3-h preincubation with doxycycline. Data from three independent experiments are presented as means ± standard errors. (C) Percentages of dead nonadherent cells in cultures of tet-CDC25A-293 cells after a 12-h exposure to H2O2 at the concentrations shown. The Dox group was preincubated for 3 h with doxycycline (10 ng/ml). Data from three independent experiments are shown as means ± standard errors. (D) Percentages of dead nonadherent cells in cultures of tet-CDC25A(C430S)-293 cells after a 12-h exposure to H2O2 at the concentrations shown. The Dox group was preincubated for 3 h with doxycycline (10 ng/ml). See Fig. 7A for induced expression of the phosphatase-inactive CDC25A(C430S). Data from three independent experiments are shown as means ± standard errors.
DISCUSSION
Reactive oxygen species (ROS), including peroxide, superoxide, singlet O2, and the highly reactive hydroxyl radical, cause oxidative damage and cellular stress. The balance between cellular life and death is, in part, a function of the ability of a cell to control oxidant insult (3). Cellular antioxidants and stress responses defend against the modification of macromolecular targets exerted by ROS (32). Accumulation of oxidative damage also has been implicated in the degenerative pathologies associated with organismal aging (41) and in the development of diseases such as atherosclerosis and cancer (reviewed in reference 21). The role of ROS as a trigger for apoptosis, in particular, has been suggested by a large body of work (reviewed in reference 29). Cytotoxic stresses often are accompanied by increases in intracellular ROS levels, and the generation of ROS within the cell may serve as a second messenger in the initiation of death responses triggered by other stimuli. Many antioxidants delay apoptotic responses triggered, for example, by anticancer drugs and gamma irradiation.
Activation of ASK1 is a critical cellular response to ROS, affecting the balance of death and survival (1). Our finding that CDC25A inhibits ASK1 suggests that CDC25A may play an important role in the cross talk between the cell cycle machinery and oxidative stress responsive pathways. That the inhibitory effect of CDC25A is manifest with respect to oxidant-triggered cell death, but not all death responses, is consistent with a specific role in the signaling phase (as distinct from the effector phase) of cell death. Significantly, this role of CDC25A is independent of its function as a CDK2 phosphatase in promoting cell cycle progression, suggesting that the physical association, but not the catalytic action, of CDC25A is important for the inhibition of ASK1. We have shown that overexpression of CDC25A diminishes homo-oligomerization of ASK1. The binding of CDC25A to ASK1 at the region adjacent to the kinase domain could inhibit oligomerization of ASK1. Homo-oligomerization is an important process for ASK1 activation (20, 37). ASK1 interacts physically with the reduced form of thioredoxin, a redox-sensitive protein, and is sequestered in an inactive form (53). Oxidation of thioredoxin by intracellular ROS results in the activation of ASK1, involving homo-oligomerization of ASK1 and its association with death receptor-associated proteins such as TRAF2 (20, 23, 37, 45). Thus, physical association of CDC25A is inhibitory to this series of events leading to full activation of ASK1.
Our data also imply that CDC25A is involved in the control of oxidative stress responses by mitogenic and oncogenic signals. The expression of CDC25A is regulated by mitogenic signals via the E2F transcription factors (61). A number of cancers, including breast and head or neck cancers, display overexpression of CDC25A (6, 18, 19, 47, 63). Increased expression of CDC25A under these mitogenic or oncogenic conditions may be related to reduced responsiveness of immortalized or transformed cells to oxidative stress (21, 60). Ectopic expression of CDC25A has been reported to trigger cell death under conditions of growth factor deprivation (16). However, our data, together with those from another work (34), suggest that CDC25A may not be involved in the induction of apoptosis generally. The transforming ability of CDC25A in rodent fibroblasts (18) rather implies that overexpression of CDC25A, together with that of Ras, facilitates survival of cells with unrestricted cell cycle progression. During oncogenic transformation, cells are thought to undergo various stresses. CDC25A could function simultaneously to inhibit stress-responsive pathways that normally lead to apoptosis, while it promotes cell cycle progression in mitogenically stimulated cells. The modest reduction in cellular susceptibility to oxidant-induced death afforded by upregulation of CDC25A may serve, under conditions of oncogenic transformation, to enhance the frequency of productively transformed cells that escape apoptosis. The expression of CDC25A also could affect the responsiveness of cancer cells to oxidative and/or genotoxic stresses caused by cancer therapies. In a recent study (6), overexpression of CDC25A was found in 47% of patients with small (<1 cm) breast carcinoma and was associated with poor prognosis. In addition, we have recently observed that ovarian cancer cell lines expressing high levels of CDC25A tend to display diminished activation of JNK1 and p38 in response to oxidative stress, compared with those lines expressing low levels of CDC25A (X. Zou and H. Kiyokawa, unpublished observations).
Persistent accumulation of intracellular ROS could result in DNA damage, which triggers checkpoint signals to inhibit cell cycle progression. The G1 checkpoint largely relies on the p53-mediated transcription of the CDK inhibitor p21 (5, 33), but also involves degradation of CDC25A protein triggered by the checkpoint kinase Chk1 (39, 48). When ROS accumulation causes DNA damage, activated Chk1 facilitates degradation of CDC25A, and ASK1 could activate the downstream stress kinases without the inhibitory interference from CDC25A. This presents a feedback loop from the cell cycle checkpoint to the stress-responsive pathways. Chk1 has been demonstrated to phosphorylate G2/M-regulatory CDC25C, facilitating cytoplasmic sequestration of CDC25C by some of the 14–3-3 proteins (38, 48, 55). Thus, CDC25 phosphatases are critical components of both G1 and G2 checkpoints. In this study, we have observed that CDC25A and ASK1 colocalize primarily in the cytoplasm, while CDC25A exists as well within the nucleus. Nuclear CDC25A presumably functions as a CDK activator. At present, it is unclear whether the subcellular localization of CDC25A is regulated by some kind of checkpoint mechanisms under various cellular conditions. We have observed no gross change in the localization of CDC25A and ASK1 in H2O2-treated OVCAR-8 cells or tet-CDC25A-293 cells (unpublished observations). It remains to be clarified whether other stressful conditions alter the subcellular localization of these two proteins.
An increase in CDC25A expression, associated with oncogenic transformation, could have diverse effects on the fine-tuned network of the cell cycle checkpoint and stress responses. Increased expression of CDC25A diminishes activation of the stress kinase cascades upon oxidative stress and suppresses the acute death response, as shown in this study. Disruption of the G1 checkpoint by transient overexpression of CDC25A has been shown to increase the amount of DNA breaks after UV irradiation (39). Many factors, including the extracellular environment, intracellular redox state, and expression of various genes, contribute to the determination of cell fate (i.e., cell death, survival with restored function, and transformation). We propose that the expression of CDC25A is one important factor that pertains particularly to the interplay of oxidative stresses, apoptotic stimuli, and mitogenic signaling. The detailed mechanism of the role of CDC25A at this interface awaits further investigation.
ACKNOWLEDGMENTS
We thank Dunya Lukovic and Keiichi Morita for providing preliminary data; Mei Ling Chen for assisting in confocal laser-scanning microscopy; Michele Pagano, Zhiyuan Shen, and Roger Davis for generous gifts of plasmids; and David Moons, Victor Levenson, William Beck, Ron Yu, Tony Kong, Xiaoping Du, Shahab Uddin, Leonidas Platanias, Jun-ya Kato, and Tatyana Voyno-Yasenetskaya for helpful suggestions and discussions.
This work was supported in part by funds provided to H.K. by the American Cancer Society (RPG-00–043-01-CCG), the American Cancer Society Illinois Division (98–41 and 99–54), the National Institutes of Health (R01HD38085), and the Cancer Center of the University of Illinois and funds provided to D.S.U. by the National Institutes of Health (R01GM38800). T.T. was supported by Osaka University.
Xianghong Zou and Tateki Tsutsui contributed equally to this work.
REFERENCES
- 1.Adler V, Yin Z, Tew K D, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 2.Behrens A, Sibilia M, Wagner E F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- 3.Biguet C, Wakasugi N, Mishal Z, Holmgren A, Chouaib S, Tursz T, Wakasugi H. Thioredoxin increases the proliferation of human B-cell lines through a protein kinase C-dependent mechanism. J Biol Chem. 1994;269:28865–28870. [PubMed] [Google Scholar]
- 4.Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19:6183–6194. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 6.Cangi M G, Cukor B, Soung P, Signoretti S, Moreira G, Jr, Ranashinge M, Cady B, Pagano M, Loda M. Role of the Cdc25A phosphatase in human breast cancer. J Clin Investig. 2000;106:753–761. doi: 10.1172/JCI9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang H Y, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Prywes R. Serum-induced expression of the cdc25A gene by relief of E2F-mediated repression. Mol Cell Biol. 1999;19:4695–4702. doi: 10.1128/mcb.19.7.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- 10.Conklin D S, Galaktionov K, Beach D. 14–3-3 proteins associate with cdc25 phosphatases. Proc Natl Acad Sci USA. 1995;92:7892–7896. doi: 10.1073/pnas.92.17.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 12.Draetta G, Eckstein J. Cdc25 protein phosphatases in cell proliferation. Biochim Biophys Acta. 1997;1332:M53–M63. doi: 10.1016/s0304-419x(96)00049-2. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein J W, Beer-Romero P, Berdo I. Identification of an essential acidic residue in Cdc25 protein phosphatase and a general three-dimensional model for a core region in protein phosphatases. Protein Sci. 1996;5:5–12. doi: 10.1002/pro.5560050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauman E B, Cogswell J P, Lovejoy B, Rocque W J, Holmes W, Montana V G, Piwnica-Worms H, Rink M J, Saper M A. Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell. 1998;93:617–625. doi: 10.1016/s0092-8674(00)81190-3. [DOI] [PubMed] [Google Scholar]
- 15.Galaktionov K, Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991;67:1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- 16.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 17.Galaktionov K, Jessus C, Beach D. Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 1995;9:1046–1058. doi: 10.1101/gad.9.9.1046. [DOI] [PubMed] [Google Scholar]
- 18.Galaktionov K, Lee A K, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. CDC25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 19.Gasparotto D, Maestro R, Piccinin S, Vukosavljevic T, Barzan L, Sulfaro S, Boiocchi M. Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res. 1997;57:2366–2368. [PubMed] [Google Scholar]
- 20.Gotoh Y, Cooper J A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 21.Guyton K Z, Kensler T W. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49:523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- 22.Harvey K J, Lukovic D, Ucker D S. Caspase-dependent Cdk activity is a requisite effector of apoptotic death events. J Cell Biol. 2000;148:59–72. doi: 10.1083/jcb.148.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeflich K P, Yeh W C, Yao Z, Mak T W, Woodgett J R. Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1) Oncogene. 1999;18:5814–5820. doi: 10.1038/sj.onc.1202975. [DOI] [PubMed] [Google Scholar]
- 24.Holland P M, Suzanne M, Campbell J S, Noselli S, Cooper J A. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J Biol Chem. 1997;272:24994–24998. doi: 10.1074/jbc.272.40.24994. [DOI] [PubMed] [Google Scholar]
- 25.Hunter T, Pines J. Cyclins and cancer. II. Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 26.Ichijo H. From receptors to stress-activated MAP kinases. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- 27.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 28.Ihle J N. The challenges of translating knockout phenotypes into gene function. Cell. 2000;102:131–134. doi: 10.1016/s0092-8674(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson M D. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- 30.Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H, Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann N Y Acad Sci. 1998;851:139–146. doi: 10.1111/j.1749-6632.1998.tb08987.x. [DOI] [PubMed] [Google Scholar]
- 32.Klaunig J E, Xu Y, Isenberg J S, Bachowski S, Kolaja K L, Jiang J, Stevenson D E, Walborg E F., Jr The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect. 1998;106(Suppl. 1):289–295. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 34.Krupitza G, Grusch M, Braun K, Fuhrmann G, Steinbrugger R, Hulla W, Simonitsch I, Chott A, Hengstschlager M. TNFalpha-mediated cell death is independent of cdc25A. Cell Death Differ. 1998;5:758–764. doi: 10.1038/sj.cdd.4400417. [DOI] [PubMed] [Google Scholar]
- 35.Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- 37.Liu H, Nishitoh H, Ichijo H, Kyriakis J M. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14–3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 39.Mailand N, Falck J, Lukas C, Syljuasen R G, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 40.Mercurio F, Manning A M. Multiple signals converging on NF-kappaB. Curr Opin Cell Biol. 1999;11:226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 41.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi P P, Lanfrancone L, Pelicci P G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 42.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 43.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFalpha and cellular stresses. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagata A, Igarashi M, Jinno S, Suto K, Okayama H. An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol. 1991;3:959–968. [PubMed] [Google Scholar]
- 45.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 46.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 47.Parsons R. Phosphatases and tumorigenesis. Curr Opin Oncol. 1998;10:88–91. doi: 10.1097/00001622-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14–3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 49.Raingeaud J, Whitmarsh A J, Barrett T, Dérijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed S I. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 51.Sadhu K, Reed S I, Richardson H, Russell P. Human homolog of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc Natl Acad Sci USA. 1990;87:5139–5143. doi: 10.1073/pnas.87.13.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saha P, Eichbaum Q, Silberman E D, Mayer B J, Dutta A. p21CIP1 and Cdc25A: competition between an inhibitor and an activator of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4338–4345. doi: 10.1128/mcb.17.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 56.Shen Z, Peterson S R, Comeaux J C, Zastrow D, Moyzis R K, Bradbury E M, Chen D J. Self-association of human RAD52 protein. Mutat Res. 1996;364:81–89. doi: 10.1016/0921-8777(96)00025-0. [DOI] [PubMed] [Google Scholar]
- 57.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 58.Stein B, Brady H, Yang M X, Young D B, Barbosa M S. Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J Biol Chem. 1996;271:11427–11433. doi: 10.1074/jbc.271.19.11427. [DOI] [PubMed] [Google Scholar]
- 59.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toyokuni S. Oxidative stress and cancer: the role of redox regulation. Biotherapy. 1998;11:147–154. doi: 10.1023/a:1007934229968. [DOI] [PubMed] [Google Scholar]
- 61.Vigo E, Müller H, Prosperini E, Hateboer G, Cartwright P, Moroni M C, Helin K. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol. 1999;19:6379–6395. doi: 10.1128/mcb.19.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X S, Diener K, Jannuzzi D, Trollinger D, Tan T H, Lichenstein H, Zukowski M, Yao Z. Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J Biol Chem. 1996;271:31607–31611. doi: 10.1074/jbc.271.49.31607. [DOI] [PubMed] [Google Scholar]
- 63.Wu W, Fan Y H, Kemp B L, Walsh G, Mao L. Overexpression of cdc25A and cdc25B is frequent in primary non-small cell lung cancer but is not associated with overexpression of c-myc. Cancer Res. 1998;58:4082–4085. [PubMed] [Google Scholar]
- 64.Wu Z, Wu J, Jacinto E, Karin M. Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase. Mol Cell Biol. 1997;17:7407–7416. doi: 10.1128/mcb.17.12.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wuarin J, Nurse P. Regulating S phase: CDKs, licensing and proteolysis. Cell. 1996;85:785–787. doi: 10.1016/s0092-8674(00)81261-1. [DOI] [PubMed] [Google Scholar]
- 66.Xia K, Lee R S, Narsimhan R P, Mukhopadhyay N K, Neel B G, Roberts T M. Tyrosine phosphorylation of the proto-oncoprotein Raf-1 is regulated by Raf-1 itself and the phosphatase Cdc25A. Mol Cell Biol. 1999;19:4819–4824. doi: 10.1128/mcb.19.7.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 68.Yaffe M B, Rittinger K, Volinia S, Caron P R, Aitken A, Leffers H, Gamblin S J, Smerdon S J, Cantley L C. The structural basis for 14–3-3:phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 69.Yao F, Svensjo T, Winkler T, Lu M, Eriksson C, Eriksson E. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum Gene Ther. 1998;9:1939–1950. doi: 10.1089/hum.1998.9.13-1939. [DOI] [PubMed] [Google Scholar]
- 70.Yao Z, Diener K, Wang X S, Zukowski M, Matsumoto G, Zhou G, Mo R, Sasaki T, Nishina H, Hui C C, Tan T H, Woodgett J P, Penninger J M. Activation of stress-activated protein kinases/c-Jun N-terminal protein kinases (SAPKs/JNKs) by a novel mitogen-activated protein kinase kinase. J Biol Chem. 1997;272:32378–32383. doi: 10.1074/jbc.272.51.32378. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Chen J, Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc Natl Acad Sci USA. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]