Abstract
Early attachment shapes brain development underlying emotion regulation. Given that sensitivity to affective cues is heightened during adolescence and effective emotion regulation strategies continue to develop, it is imperative to examine the role of early attachment and parental influence on adolescent regulation. Fifty-one children (M age=32.61 months) participated in a modified Strange Situation with their mother and approximately 10 years later (M age =13.2 years) completed an fMRI scan during which they were presented with appetitive and aversive affective cues (images of adolescent interactions) during a Go-Nogo task. They completed the task alone and in the presence of a parent. Behavioral multilevel models and whole-brain analyses showed attachment-related patterns, such that affective cues elicited greater behavioral and neural dysregulation in insecure (versus secure) adolescents.Furthermore, parental presence buffered behavioral and neural dysregulation toward socially aversive cues for adolescents with early insecure attachment, underscoring the salience of caregivers across development in promoting regulation in their offspring
Keywords: Attachment, Regulation, Social buffering, Adolescence, FMRI
As adolescents explore their rapidly expanding social worlds, social cues become ever more salient as they navigate new friendships and romantic relationships and learn more complex social skills (Blakemore and Mills, 2014). Adolescents also undergo rapid neurobiological reorganization in ways that make them particularly sensitive to social cues from peers (Blakemore and Mills, 2014, Nelson et al., 2016). Such heightened social sensitivity, coupled with still developing emotion regulation, can place adolescents at risk for the development of internalizing and externalizing psychopathology (Aldao et al., 2016). Early attachment with caregivers can set the stage for successful development of emotional regulation skills (Booth-Laforce and Oxford, 2008), and parents continue to play an important role, buffering the negative effects of heightened social sensitivity during adolescence (Rogers et al., 2020). The current study investigated the role of early child-mother attachment security on adolescent behavioral and neurobiological regulation and whether parental presence during a social go-nogo task buffers adolescent regulation differentially for youth with early secure and insecure attachment.
Research across the globe shows that regulatory abilities continue to develop, reorganize, and fluctuate from preadolescence into middle adulthood (Steinberg et al., 2018), with adolescents between 12 and 15 exhibiting a significant increase in dysfunctional emotion regulation as compared with children and older adolescents (Cracco et al., 2017), and as compared with individuals in young and middle adulthood (Zimmermann and Iwanski, 2014). Indeed, compared to children and adults, adolescents show detriments in emotion regulation in the presence of appetitive (Perino et al., 2016) and aversive (Zimmermann and Iwanski, 2014) social cues. Developmental neuroscience frameworks propose that such emotion regulation detriments are due to changing neurobiological development (Somerville et al., 2010). In particular, the developing limbic system shows heightened sensitivity to socioemotional contexts during adolescence compared with childhood and adulthood, including activation in the amygdala (Hare et al., 2008, Stephanou et al., 2016), a region associated with affective processing, and the ventral striatum (VS; Somerville et al., 2011), a region implicated in reward processing. Of note, the VS and amygdala are suggested to supersede development of the prefrontal cortex (PFC), a region that encompasses subregions associated with modulating regulation through cognitive control and social cognitive processes (Ahmed et al., 2015, Heller and Casey, 2016, Somerville et al., 2010), such that heightened activation in the VS and amygdala may be associated with greater neural dysregulation during adolescence. Developmental neuroscience research shows that adolescents exhibit detriments in emotion regulation as evidenced by parallels between poor behavioral impulse control and positive activation in the VS and amygdala toward affective cues (Perino et al., 2016, Somerville et al., 2011). Further, reactivity in the amygdala during the use of affective regulatory strategies reflects neurobiological dysregulation (Diekhof et al, 2011), particularly in younger adolescents compared to older adolescents and adults (Silvers et al., 2015).
Attachment security with caregivers during infancy and toddlerhood lays the foundation for social expectations, autonomous behaviors, and regulatory competencies later in life (Thompson, 2006). These early attachment experiences inform the development of internal working models, which is how individuals mentally represent their expectations about social relationships, exploration, and safety (Bretherton, 1987). Children with secure internal working models of attachment tend to exhibit more successful social skills and effective emotion regulation across childhood (Booth-Laforce and Oxford, 2008), whereas insecure attachment can predispose individuals toward a multitude of difficulties in socioemotional processing into adolescence and adulthood (Mikulincer and Shaver, 2019). Attachment is inherently a biobehavioral process, whereby early caregiving shapes the developing brain in ways that can promote or hinder successful regulatory development (Callaghan and Tottenham, 2016, Morris et al., 2017, Vrtička, 2017). Indeed, brain regions associated with affective processing (i.e., amygdala), reward processing (i.e., VS), and decision-making (i.e., anterior cingulate cortex (ACC), prefrontal cortex (PFC)) exhibit differential activation as a function of attachment security during adolescent regulation (Vrtička et al., 2014). The amygdala appears to play a key role in attachment-based socioemotional functioning during development, such that early maternal deprivation is associated with reduced amygdala growth across childhood and adolescence (VanTieghem et al., 2021) and a lack of discrimination between attachment figures and strangers via amygdala activation during childhood and adolescence (Olsavsky et al., 2013). In addition, greater activation in brain regions associated with cognitive control, including the dorsolateral and medial PFC, and ACC, has been observed when increasing emotional responses to appetitive social cues for adults with early insecure attachment (Moutsiana et al., 2014). Despite accumulating evidence that attachment security formed during toddlerhood can shape neurobiological responses to social contexts, most prior work is cross-sectional or retrospective, highlighting the importance of using prospective longitudinal studies to examine the role early caregivers play on regulation across development.
It is noteworthy that the continuity of attachment security from infancy into adolescence is heavily contingent on the parent-child relationship, particularly the provision of warmth and responsiveness from parents to their children (Beijersbergen et al., 2012). Parents continue to influence their children’s emotion regulation into adolescence through social buffering, which occurs when parental physical presence or emotional responsiveness ameliorates the negative effects of the environment on offspring regulation and health (Gee et al., 2014). Social buffering of children’s emotion regulation is proposed to particularly occur through neurobiological processes involving the PFC and amygdala while processing socioemotional information (Callaghan and Tottenham, 2016, Gunnar et al., 2015, Hostinar and Gunnar, 2015). Unsurprisingly, parental buffering and attachment security are intimately linked. For instance, maternal physical presence improves child (4–10 years old) behavioral and neurobiological regulation in affective contexts, and this effect is stronger for parent-child relationships characterized by greater attachment security (Gee et al., 2014). However, most studies investigating parental buffering and attachment combine these constructs and thus do not examine their independent and interactive effects. Furthermore, previous research has primarily focused on childhood (e.g., Gee et al., 2014; Gunnar and Quevedo, 2006; Hostinar et al., 2015), despite recent work emphasizing the importance of parental buffering on adolescent health, behavior, and neurobiological processing (Farrell et al., 2016, Telzer et al., 2015, Guassi Moreira and Telzer, 2018, Rogers et al., 2020). This work has identified brain regions that exhibit modulation when parents are physically present, including heightened recruitment of the ventromedial PFC and dampened recruitment of the VS (Guassi Moreira and Telzer, 2018, Rogers et al., 2020, Telzer et al., 2015).
Given the potential for parents to provide a protective effect on adolescent behavioral and neural dysregulation, it would be fruitful to examine whether parents’ physical presence in the context of social cues may buffer their adolescent offspring from less optimal emotion-related responses and whether these benefits vary as a function of early attachment security. To this end, this study examined the longitudinal association between child-mother attachment security during toddlerhood and regulatory responses to social cues during adolescence using a social go-nogo task. We expected that adolescents who exhibited insecure compared with secure attachment in toddlerhood would show greater behavioral dysregulation, measured by higher false alarms, which in the context of this study represents more instances of the inability to withhold a behavioral response toward social cues. Furthermore, we expected that insecure compared with secure adolescents would also exhibit greater neurobiological dysregulation, as defined by greater activation in the amygdala and ventral striatum to affective cues relative to control cues. In addition, this study investigated whether parental physical presence serves as a potential social buffer against adolescent dysregulation. We hypothesized that parental presence would moderate emotion dysregulation toward affective cues displayed by secure versus insecure adolescents, such that parental presence would ameliorate false alarms and brain activation toward affective cues compared with parental absence in secure adolescents.
1. Methods
1.1. Participants
Toddlers (N = 128, 66 boys) and their mothers participated in a longitudinal study of socioemotional development (see McElwain, Holland, Engle, & Ogolsky, 2014; McElwain et al., 2012). At the initial time point, children ranged between 31 and 35 months of age (M = 32.7 months, SD =0.76). When children were approximately 13 years of age, families were contacted to participate in a follow-up study of family relationships and adolescent neural and behavioral regulation of stress. Sixty-seven families participated in the follow-up study, and 51 adolescents completed a functional magnetic resonance imaging (fMRI) scan (34 boys, M = 13.2 years, SD =0.56, range = 12.4–14.8 years). Reasons for adolescents not completing the fMRI scan included claustrophobia (n = 2), braces (n = 7), and declining to participate (n = 6). In addition, neuroimaging data from one adolescent were not usable due to malfunction of the computer delivering the task. Of the 67 families participating in the follow-up study, children were more likely to be male (χ2[1] = 11.43, p < .001) compared with those who did not participate in the follow-up. The two groups did not differ on toddler-mother attachment (secure-insecure classification), maternal education, age, or ethnicity. Of the 51 adolescents who had useable neuroimaging data compared with those who did not (but who participated in the follow-up study), no significant differences emerged on child-mother attachment or the demographic variables.
For this report, we focused on the sample of 51 adolescents with complete fMRI data. The adolescents completed a social go-nogo task during fMRI, both alone and in the presence of their parent (38 mothers returning from initial time point; 13 fathers). At the time of the scan session, the majority of adolescents identified as European-American (90%), with 2% identifying as African-American, and 8% as multi-ethnic. Parental education averaged 16.2 (SD = 1.8) years for mothers and 15.5 (SD = 2.3) years for fathers. The majority of parents were married (82.4%), 9.8% were divorced, 3.9% were separated, and 2% were single. Family annual income averaged between $60,000 and $90,000, with a range of families reporting under $15,000 and over $90,000. Informed consent/assent was obtained for all participants in accordance with the Institutional Review Board at the University of Illinois at Urbana-Champaign (protocols #05181 and #15435).
2. Early parent-child attachment security
A modified 17-minute Strange Situation procedure (Cassidy, Marvin, & the MacArthur Attachment Working Group, 1992) was utilized to assess child-mother attachment security at the initial time point. This procedure consists of five episodes: a warm-up (3 min), mother-child separation (3 min), mother-child reunion (3 min), second mother-child separation (5 min), and second mother-child reunion (3 min). During the separation episodes, no “stranger” was present, and the mother received no instructions about what to tell her child during the departure from the playroom.
Utilizing the Cassidy-Marvin (1992) coding system, children were classified as secure (n = 33), avoidant (n = 2), ambivalent (n = 8), or disorganized/insecure or other/controlling (n = 8) Two highly trained coders, certified by Jude Cassidy and blind to all other study information, coded all protocols. Twenty percent of the protocols were double-coded, and disagreements were resolved by consensus. Interobserver agreement (before consensus) was 88% (kappa =0.77) for the 4-way classification. Due to the small sizes of the insecure groups, we examined the binary secure-insecure classification. The Cassidy-Marvin system, which was designed specifically for children between 2.5 and 4.5 years of age, has established validity and is considered the measure of choice for assessing attachment security among preschool-aged children (see Solomon and George, 2008).
3. Social Go-NoGo task
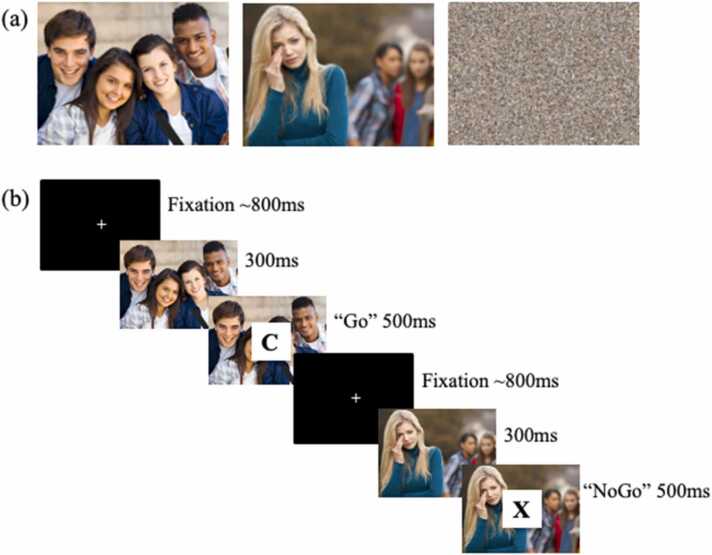
During an fMRI scan, adolescent participants completed a social go-nogo task, which couples salient socioemotional cues with a cognitive go-nogo task (Perino et al., 2016). Go-nogo tasks reliably measure cognitive control (i.e., disinhibition via false alarms), and coupled with affective cues, can provide information about emotion regulation as participants complete the task in socially appetitive and socially aversive contexts. This task included 9 randomized blocks of 27 trials each, such that 3 blocks included socially appetitive images (e.g., adolescent peers hanging out), 3 blocks included socially aversive images (e.g., peers rejecting an adolescent), and 3 blocks included control trials depicting scrambled images (Fig. 1a). Images were presented for 300 ms, followed by the appearance of a letter over the image for 500 ms (Fig. 1b). Participants were instructed to press a button as quickly as possible when a letter appeared (go trial; 66% of trials), but to withhold from pressing the button if an “X” appeared (nogo trial; 33% of trials). Jitters between trials averaged 800 ms. Behavioral dysregulation was measured by false alarms (i.e., pressing the button on nogo X trials), such that higher false alarms toward social images across the task reflected greater behavioral dysregulation.
Fig. 1.
Social Go-NoGo Task, Note. Panel (a) shows examples of the socially appetitive, socially aversive, and control trial cues. Panel (b) displays the sequence and timing of the social go-nogo task.
Participants completed two rounds of the task, including one alone condition and one controlled parental presence condition, which were counterbalanced across participants. In the alone condition, participants were told that “nobody will be watching you.” In the parental presence condition, participants were instructed that their parent was coming into the scan room to watch them play, which parallels procedures of other studies examining parental buffering (e.g., Telzer et al., 2015). The parent entered the scan room and read the following script verbatim into the microphone that connected to the scanner, “Hi [child’s name], I’m here and I just wanted to let you know that I’m looking at these pictures with you!” As such, parental presence was very controlled and minimal to ensure consistency in this condition across participants.
4. Behavioral analysis
Using Mplus 8.4 (Muthén and Muthén, 2017) we fitted two multilevel models—one for appetitive cues compared to control cues, and one for aversive cues compared to control cues—to examine the effect of attachment security, affect, parental presence, and their interactions, on false alarms during the task. Trials (Level 1) were nested within participants (Level 2), with the outcome variable defined as a false alarm on each “no-go” trial. Level 1 variables included the affective condition (1 =affective, 0 =control), parental presence condition (1 =parent present, −1 =alone), and the interaction between parental presence and affective conditions. Level 2 variables included attachment security (1 =secure, −1 =insecure) and cross-level interactions between attachment security and affective condition, parental presence condition, and the three-way interaction. In addition, adolescent age and gender were included as covariates to account for differences between older and younger adolescents, and girls and boys, in false alarm rates. The multilevel models estimated all variables of interest using the following equations:
Level-1 Equation.
Logit (FalseAlarmij) = β0j + β1j × (Affectij) + β2j × (ParentPresenceij) + β3j × (ParentPresence*Affectij) + rij.
Level-2 Equation.
β0j = γ00 + γ01 × (ChildGenderij) + γ02 × (ChildAgeij) + γ03 × (Attachmentj) + u0j.
β1j = γ10 + γ11 × (Attachmentj) + u1j.
β2j = γ20 + γ21 × (Attachmentj) + u2j.
β3j = γ30 + γ31 × (Attachmentj) + u3j.
5. fMRI acquisition
Imaging data were collected using a 3 Tesla Siemens Trio MRI scanner. The social go⎼nogo task included high resolution T2 * weighted echo-planar images (EPIs; TR = 2000 ms; TE = 25 ms; matrix = 92 × 92; FOV = 230 mm; slice thickness = 3 mm; 38 slices; voxel size = 2.5 × 2.5 × 3 mm3). Structural scans included a high resolution T2 * weighted matched⎼bandwidth anatomical scan (TR = 4000 ms; TE = 64 ms; matrix = 192 × 192; FOV = 230 mm; slice thickness = 3 mm; 192 slices; voxel size = 1.2 × 1.2 × 3 mm3) and a T1 * magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1900 ms; TE = 2.32 ms; matrix = 256 × 256; FOV = 230 mm; sagittal plane; slice thickness =0.9 mm; 192 slices; voxel size =0.9 ×.9 ×.9 mm3). The orientation for the EPI and T2 anatomical scans were oblique axial to maximize brain coverage and to reduce noise.
6. fMRI data preprocessing and analysis
FSL FMRIBs Software Library (FSL v6.0; https://fsl.fmrib.ox.ac.uk/fsl/) was used for preprocessing. These steps included skull stripping of images using BET, correcting for slice-to-slice head motion using MCFLIRT, and high-pass temporal filtering (128 s cutoff) to eliminate low-frequency drift across the time series. After resampling the functional images to a 2 × 2 × 2 mm space, the images were coregistered to the matched⎼bandwidth anatomical and MPRAGE images using FLIRT. Next, these images were normalized into standard sterotactic space as defined by the Montreal Neurological Institute (MNI) and the International Consortium for Brain Mapping. A FWHM 6 mm Gaussian kernel was applied for spatial smoothing to maximize signal-to-noise ratio. And last, individual-level independent component analysis (ICA), MELODIC, and an automated component classifier (Tohka et al., 2008; Neyman-Pearson threshold =0.3) was applied to filter noise signal such as physiological rhythms and motion.
At the individual level, a fixed-effects analysis was modeled for each of the conditions of interest, including the 3 affective conditions (appetitive, aversive, control) each separated by the 2 parental presence conditions (alone and parent present). The event-related design allowed each trial to be modeled individually (duration of 800 ms). The inter-trial jitter null events were not explicitly modeled and therefore served as an implicit baseline.
At the group level, two random effects whole-brain analyses were modeled based on the behavioral results to examine attachment security differences in neural activation in response to affective cues, one for appetitive social cues and one for aversive social cues. In addition, we examined attachment security differences in neural activation to parental presence (versus absence) in response to affective cues.
Monte Carlo simulations were conducted to correct for multiple comparisons using 3dClustSim in the AFNI software package (Ward, 2000; updated April 2016). First, individual-level acf parameters were estimated for each participant. Second, these individual-level acf values were used to compute the group-level acf parameters (0.504648103 5.266495344 14.0361904). Third, a group whole-brain mask was created using individual mask files, which yielded 120,793 voxels. And last, 3dclustsim was run using the group-level acf values, a voxel-wise threshold of p < .005, and a false wise error rate at.05. These calculations yielded a minimum cluster size of 239 voxels. We reported both unthresholded maps (p < .005 uncorrected) and corrected maps of whole-brain activation to improve the reproducibility of the results and to increase the utility of the data for assessing analytic variability (Botvinik-Nezer et al., 2020). All reported results are available on NeuroVault (Gorgolewski et al., 2015; see https://neurovault.org/collections/ZZNCRVVG/).
7. Results
7.1. Behavioral results
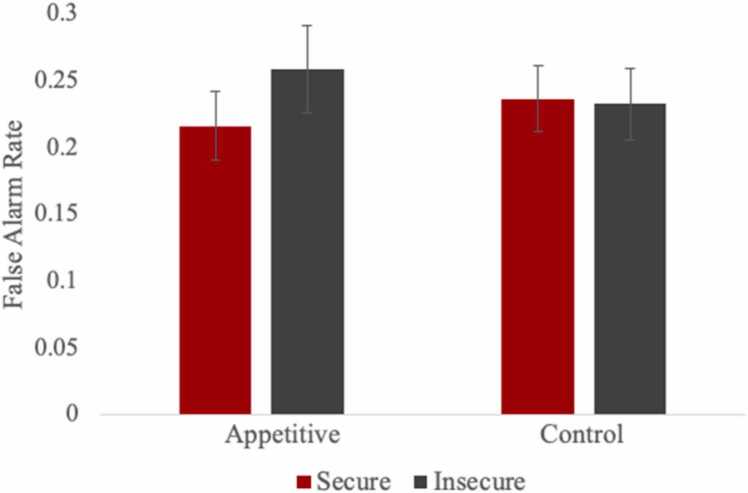
To test for attachment (secure versus insecure) and parent presence (versus alone) differences in false alarms toward affective cues, we fitted one multilevel model for appetitive cues and one for aversive cues (see Table 1). Specifically, these models allowed us to examine which of these factors may predict higher false alarms (i.e., inability to withhold a behavioral response) toward appetitive and aversive cues. For the appetitive model, the main effects of gender, age, attachment, affect, and parental presence were not significant. Of the two-way interactions, a significant cross-level interaction between attachment and affect was found (b = −0.15, SE =0.07, p = .038). As shown in Fig. 2, insecure adolescents displayed relatively more false alarms toward appetitive cues (versus control cues) compared with secure adolescents. Although false alarms for both appetitive cues (b = −0.18, SE =0.13, p = .165) and control cues (b = −0.02, SE =0.11, p = .835) were not significantly different between secure and insecure adolescents, the difference was more salient for appetitive cues than for control cues. In addition, there were no significant differences in false alarms between appetitive and control cues for adolescents with insecure (b =0.13, SE =0.10, p = .202) and secure (b = −0.18, SE =0.11, p = .100) attachment histories. The remaining two-way interactions (i.e., attachment × parental presence; affect × parental presence), as well as the three-way interaction between attachment, affect, and parental presence, were nonsignificant.
Table 1.
Within- and between-person associations between false alarms and variables of interest.
| Fixed Effects | b (SE) | p |
|---|---|---|
| Appetitive > Control | ||
| Adolescent Gender | -0.01 (0.11) | .935 |
| Adolescent Age | -0.07 (0.19) | .717 |
| Attachment | -0.02 (0.11) | .835 |
| Affect | -0.02 (0.07) | .751 |
| Parent | -0.001 (0.06) | .984 |
| Attachment × Affect | -0.15 (0.07) | .038 |
| Attachment × Parent | -0.06 (0.06) | .254 |
| Affect × Parent | -0.14 (0.08) | .072 |
| Attachment × Affect × Parent | .06 (0.06) | .329 |
| Aversive > Control | ||
| Adolescent Gender | -0.05 (0.11) | .658 |
| Adolescent Age | -0.10 (0.17) | .542 |
| Attachment | -0.03 (0.11) | .821 |
| Affect | -0.42 (0.08) | < 0.001 |
| Parent | -0.001 (0.06) | .992 |
| Attachment × Affect | -0.06 (0.05) | .460 |
| Attachment × Parent | -0.06 (0.05) | .249 |
| Affect × Parent | .00 (0.07) | .999 |
| Attachment × Affect × Parent | .20 (0.06) | .002 |
Note: Between-person variables included adolescent gender (female = 1, male = −1), adolescent age, and attachment (secure = 1, insecure = 0). Within-person variables included affect (affective trial = 1, control trial = 0) and parent (parent present =1, parent not present = −1). All coefficients with two-tailed significance at p < .05 are displayed in bold.
Fig. 2.
Adolescent False Alarm Rates Toward Appetitive and Control Cues by Early Attachment.
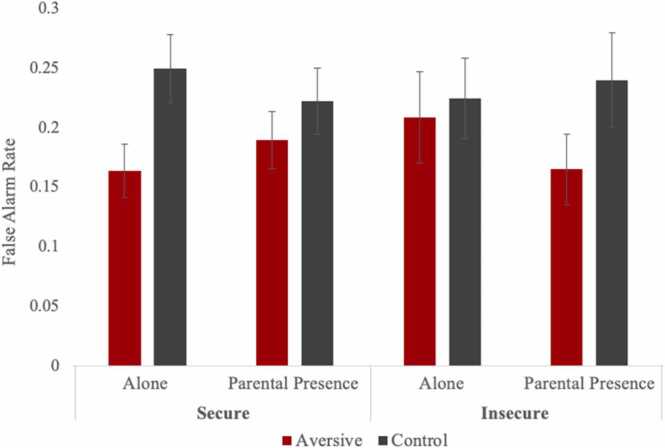
The aversive model showed a significant main effect of affect (b = −0.42, SE =0.08, p < .001), such that false alarm rates were higher in control trials compared with aversive trials, whereas the main effects of gender, age, attachment, and parental presence were nonsignificant. Although all two-way interactions were nonsignificant, there was a significant three-way cross-level interaction between attachment, affect, and parental presence (b =0.20, SE =0.06, p = .002). To probe the three-way interaction, we plotted affect and parental presence conditions for secure and insecure adolescents (see Fig. 3). In the alone condition, secure adolescents showed fewer false alarms to aversive cues than to control cues (b = −0.68, SE =0.11, p < .001), whereas this association was nonsignificant for insecure adolescents (b = −0.16, SE =0.19, p = .399), indicating that secure (but not insecure) adolescents demonstrated better behavioral regulation to aversive cues when alone. In the parental presence condition (see Fig. 3), insecure adolescents showed fewer false alarms to aversive cues than to control cues (b = −0.55, SE =0.14, p < .001), and this association was nonsignificant for secure adolescents (b = −0.28, SE =0.15, p = .065), indicating that parental presence may have promoted insecure children's regulation in response to aversive cues. Of note, false alarms to aversive cues did not significantly differ between parental presence and alone conditions for secure (b =0.13, SE =0.07, p = .057) or insecure adolescents (b = −0.14, SE =0.09, p = .121). Further, false alarms to control cues did not significantly differ between parental presence and alone conditions for secure (b = −0.06, SE =0.08, p = .405) or insecure adolescents (b =0.06, SE =0.08, p = .433).
Fig. 3.
Adolescent False Alarm Rates Toward Aversive and Control Cues by Early Attachment and Parental Presence.
8. Neuroimaging results
Whole-brain analyses were conducted for the appetitive and aversive conditions separately to parallel the behavioral results. First, the main effects of the task were examined for each model. Second, task effects were examined as a function of attachment history to reflect the research aims. Table 2 shows the unthresholded maps (p < .005 uncorrected) and corrected maps of whole-brain activation for all four models.
Table 2.
Brain regions that exhibited activation for appetitive and aversive models.
| Anatomical Region | x | y | z | t | k |
|---|---|---|---|---|---|
| Appetitive > Control | |||||
| Main Effects | |||||
| L Temporal parietal junction | -64 | -40 | 38 | 6.82 | 405 * |
| R Ventrolateral PFC | 50 | 50 | 4 | 5.18 | 359 * |
| Secure Versus Insecure Early Attachment | |||||
| R Temporal parietal junction | 66 | -42 | 30 | 4.18 | 119 |
| R Posterior superior temporal sulcus | 52 | -32 | 14 | 3.78 | 125 |
| R Supplemental motor area | 14 | -10 | 74 | 4.35 | 130 |
| L Supplemental motor area | -6 | 4 | 4 | 4.22 | 180 |
| R Cerebellum (VIII) | -10 | -74 | -32 | 3.32 | 191 |
|
Aversive: Parent > Alone Main Effects |
|||||
| L Medial temporal pole | -42 | 4 | -26 | 8.00 | 8480 * |
| R Dorsolateral PFC | 36 | 2 | 62 | 6.93 | 3382 * |
| L Orbitofrontal cortex | -48 | 30 | -14 | 5.36 | 648 * |
| L Postcentral gyrus | |||||
| L Cerebellum (VII) | -32 | -86 | -42 | 5.54 | 499 * |
| R Cerebellum (VII) | 38 | -78 | -48 | 5.39 | 758 * |
| L Postcentral gyrus | -46 | -24 | 62 | 4.03 | 110 |
| Secure Versus Insecure Early Attachment | |||||
| R Temporal parietal junction | 64 | -52 | 16 | 3.84 | 130 |
| ACC extending into dorsomedial PFC | -8 | 60 | 26 | 4.17 | 332 * |
| R Ventrolateral PFC | 30 | 62 | 2 | 3.75 | 168 |
| R Dorsolateral PFC | 40 | 16 | 48 | 3.57 | 256 * |
| L Inferior occipital gyrus | -30 | -90 | 0 | 4.60 | 377 * |
| L Superior occipital gyrus | -18 | -80 | 32 | 4.44 | 332 * |
| R Middle occipital gyrus | 32 | -90 | 10 | 4.02 | 464 * |
| R Interparietal sulcus | 32 | -66 | 32 | 3.90 | 231 |
| R Posterior cingulate | 2 | -50 | 26 | 3.90 | 178 |
| R Middle temporal gyrus | 42 | -56 | 16 | 3.66 | 116 |
| L Fusiform gyrus | -18 | -44 | -6 | 3.61 | 104 |
| R Fusiform gyrus | 38 | -76 | -8 | 3.91 | 28 |
| L Posterior superior temporal sulcus | -68 | -36 | -4 | 3.63 | 30 |
| Posterior cingulate | 0 | -58 | 16 | 3.59 | 57 |
| Medial PFC | 14 | 64 | 14 | 3.38 | 45 |
| Precuneus | 14 | -62 | 36 | 3.47 | 39 |
| R Caudate nucleus | 8 | 18 | 4 | 3.40 | 23 |
| L Ventromedial PFC | -4 | 26 | -12 | 3.28 | 26 |
| Posterior ACC | 8 | -10 | 32 | 3.17 | 32 |
| Temporal pole | 68 | -28 | -4 | 3.12 | 35 |
| R Linual gyrus | 26 | -58 | -4 | 3.96 | 94 |
| L Precentral gyrus | -36 | 0 | 38 | 3.85 | 41 |
| R Calcarine gyrus | 24 | -54 | 14 | 3.34 | 37 |
| Premotor cortex | 52 | -4 | 46 | 4.02 | 40 |
| R Inferior frontal gyrus (p. triangularis) | 54 | 22 | 34 | 3.75 | 27 |
| R Cerebellum (Crus 2) | 28 | -84 | -32 | 3.39 | 30 |
| R Cerebellum (VI) | 36 | -68 | -16 | 3.49 | 29 |
Note: x, y, and z, MNI coordinates; t, t-score at peak activation level; k, number of voxels in each significant cluster; L and R = left and right hemispheres; ACC = anterior cingulate cortex; PFC = medial prefrontal cortex. Brain regions were based on a whole-brain mask significant at p < .005.
*Regions meeting the multiple comparison threshold of a minimum cluster size of 239 voxels.
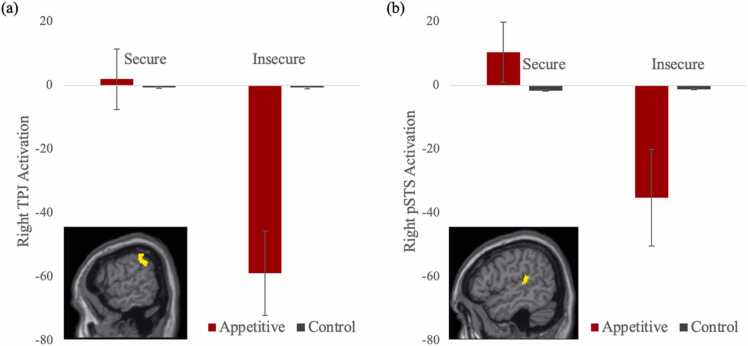
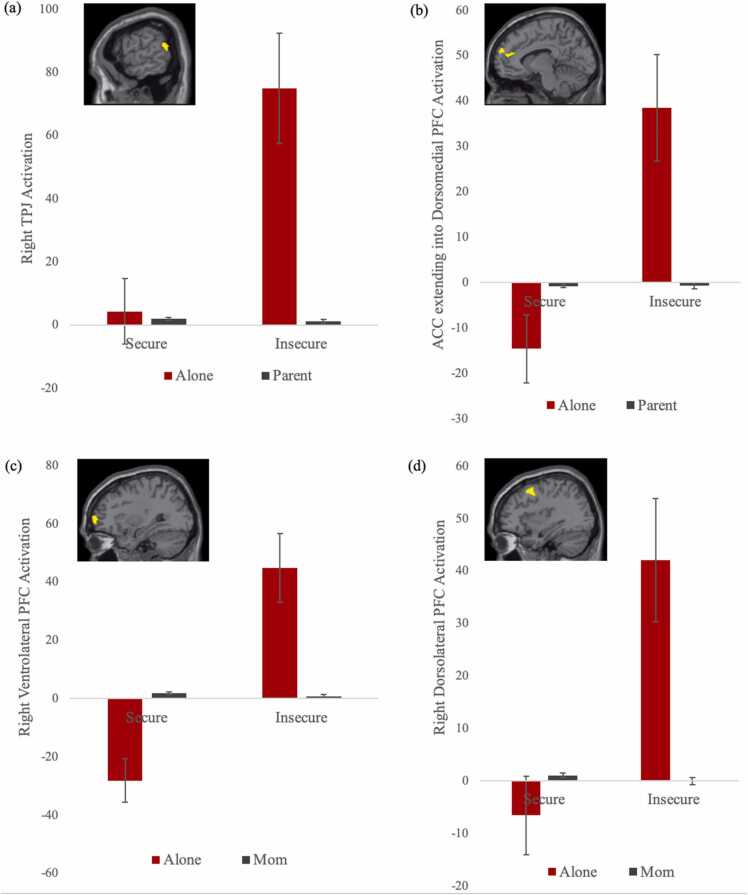
In the appetitive model, we estimated the main effect of the contrast appetitive > control. We collapsed across the alone and parental presence conditions given that they did not yield significant differences in behavior for appetitive trials. Adolescents showed significantly more activation in the left TPJ and right vlPFC when observing socially appetitive versus control cues. Next, attachment history (secure > insecure) was estimated as a regressor on the contrast of appetitive > control. Adolescents with an insecure compared with a secure attachment history exhibited less activation in the TPJ, pSTS, supplemental motor area, and cerebellum when viewing socially appetitive versus control cues. Of note, these regions did not survive the multiple correction threshold of a minimum cluster size of 239 voxels. For descriptive purposes, we extracted parameter estimates of signal intensity from the TPJ and pSTS and plotted activation for secure and insecure adolescents separately. As shown in Fig. 4, adolescents with early insecure attachment show hypoactivation toward appetitive social cues, whereas adolescents with early secure attachment do not display a difference in neural activation to appetitive social cues compared with control cues.
Fig. 4.
Adolescent Neural Reactivity Toward Socially Appetitive Cues by Early Attachment, Note. Adolescents with early insecure attachment exhibited significantly less activation in the right TPJ and pSTS in response to appetitive cues (versus control) compared with adolescents with early secure attachment.
In the aversive model, we estimated the main effect of the contrast parental presence > alone for aversive trials only. Control trials were excluded to focus on affective regulation and to reduce multiple comparisons. Adolescents showed significantly more activation in the left temporal pole, left orbitofrontal cortex, and bilateral dlPFC when observing socially aversive cues in the presence of the parent compared with the alone condition. Next, attachment history (secure > insecure) was estimated as a regressor on the contrast of parental presence > alone for aversive trials only. Adolescents with an insecure attachment history showed significantly more activation in the dlPFC and ACC extending into dmPFC when viewing socially aversive cues alone, compared with adolescents with a secure attachment history. In addition, insecure adolescents exhibited more activation in the TPJ and vlPFC compared to secure adolescents, but these regions did not survive the multiple correction threshold. For descriptive purposes, we extracted parameter estimates of signal intensity from the TPJ, ACC extending into dmPFC, dlPFC, and vlPFC and plotted activation for secure and insecure adolescents separately. As shown in Fig. 5, adolescents with early insecure attachment did not display hyperactivation in these brain regions toward aversive social cues when parents were present, suggesting social buffering via neural modulation toward more effective regulation.
Fig. 5.
Adolescent Neural Reactivity Toward Socially Aversive Cues by Early Attachment and Parental Presence,Note. Insecure adolescents exhibited significantly more activation in the ACC extending into dorsomedial PFC and dorsolateral PFC in response to aversive cues compared with secure adolescents in the alone condition, but not during parental presence.
9. Discussion
Attachment security provides children with an internal working model to explore their world and regulate their emotions and behavior in novel environments (Thompson, 2006), yet little is known about how early attachment might modulate regulatory abilities during adolescence when sensitivity to affective cues are heightened, and whether parents might still play a role in buffering dysregulation during this developmental period. We examined these inquiries using a longitudinal multi-method approach and found behavioral and neurobiological differences in adolescent regulation based on early attachment history and parental presence. This study corroborates a growing literature indicating that early attachment security may lay the foundation for adolescents’ regulatory abilities and that parents can continue to buffer offspring from dysregulation during adolescence.
Our findings showcase that compared with adolescents with secure attachment histories, adolescents with insecure attachment histories struggle more with behavioral dysregulation in affective environments. Although prior work indicates that adolescents experience behavioral and neurobiological dysregulation toward appetitive social cues (Perino et al., 2016, Rogers et al., 2020), this work did not account for early attachment histories. Extending this prior work, we found that adolescents with an insecure attachment history experienced relatively more difficulty inhibiting their behavior toward socially appetitive contexts than those with a secure attachment history. Unexpectedly, when alone, secure (but not insecure) adolescents displayed better regulation toward socially aversive contexts, yet the presence of a parent promoted regulation for insecure adolescents. Given the socioemotional patterns that typically develop among individuals with insecure attachment styles, such as difficulties identifying or attending to emotional states and information (Mikulincer and Shaver, 2019, Stevens, 2014), the physical presence of parents may be especially beneficial to their insecure adolescent offspring during negative peer experiences, such as rejection, bullying, or conflict. Although Gee and colleagues (2014) reported that children (4–10 years old) with secure attachment benefited from viewing images of their mother compared with strangers, our finding indicates that parental physical presence served as a buffer for adolescents with insecure attachment. We speculate that developmental stage plays a key role in the discrepancy between our finding and Gee and colleagues’ (2014) study of children in preschool and elementary school. Whereas physical contact maintenance with a caregiver is a defining characteristic of the attachment behavioral system during infancy and early childhood, “psychological proximity” with the attachment figure is maintained across distance and becomes increasingly salient with development (see Marvin and Britner, 2008). Thus, our finding suggests that the attachment behavioral system of adolescents with an insecure attachment history may be less mature such that physical proximity continues to serve a “secure base” function. Adolescents with a secure history, in contrast, may have psychological resources that enable them to respond to moderately negative stimuli in a regulated manner and rely less on parents’ physical presence during such challenges. Together, these findings contribute to the accumulating evidence that parents can redirect their offspring toward more optimal behavior and socioemotional processing, even during adolescence (Guassi Moreira and Telzer, 2018, Rogers et al., 2020, Telzer et al., 2015).
Our findings also highlight differences in neurobiological reactivity toward affective environments between adolescents based on their history of attachment. Adolescents with early insecure attachment, compared to secure, showed hypoactivation and hyperactivation of social cognition brain regions (i.e., TPJ) in appetitive and aversive social environments, respectively. These exaggerated brain patterns exhibited by adolescents with early insecure attachment are apparent when considering that both secure and insecure adolescents showed activation in brain regions associated with social cognition (i.e., TPJ, temporal pole) toward appetitive and aversive social cues, correspondingly. Adolescents with an insecure attachment history may attend to and process positive social information less, and negative social information more, than adolescents with a secure attachment history. Importantly, these findings should be interpreted with caution given that these brain regions did not survive multiple correction, and as such, provide direction for future studies. It is noteworthy that these findings are consistent with prior work that examined peer interactions in the laboratory and found that insecure adolescents tend to reflect on these social exchanges as more negative and less positive than secure adolescents (Dykas et al., 2012). Thus, insecure adolescents’ hypo- and hyperactivation of social brain regions may underly socioemotional and regulatory patterns that were developed through early attachment security.
Similar to the behavioral findings, parents buffered against their insecure adolescents’ dysregulation in response to aversive social environments at the level of the brain. The presence of a parent abated offspring hyperactivation in regions associated with socioemotional processing emotion regulation (i.e., ACC extending into dmPFC) and cognitive control (i.e., dlPFC), which have each been identified in theories of social neuroscience on attachment (Vrtička, 2017). Importantly, both secure and insecure adolescents recruited brain regions associated with socioemotional processing (i.e., orbitofrontal cortex) and cognitive control (i.e., bilateral dlPFC) when viewing aversive social cues in the presence of their parent compared with alone, which emphasizes that adolescents with an insecure attachment history especially benefited from parental presence in reducing hyperactivation in aversive social contexts. These findings are consistent with previous work showing that parents can redirect adolescents toward more effective and mature neurobiological regulation (Guassi Moreira and Telzer, 2018, Rogers et al., 2020, Telzer et al., 2015), and in this case, play a significant and positive role through their physical presence in promoting regulatory abilities among adolescents with an insecure attachment history. Of note, these findings also emphasize that adolescents with a secure attachment history may demonstrate more mature regulation and require less parental scaffolding than their insecure adolescent counterparts, lending support to differences in neurobiological reactivity based on attachment (Vrtička, 2017).
Although this study contributes to our understanding of how early attachment and concurrent parental presence associates with adolescent behavioral and neurobiological regulation, several limitations should be considered. Due to our sample size, we relied on the binary attachment classification (secure vs insecure) rather than examining each type of insecure attachment separately. The sample was also rather homogenous as most participants identified as White and constituted higher socioeconomic status, which likely corresponded to a greater representation of children with secure attachment. Future work would benefit from obtaining a larger and more diverse sample to better understand variation within insecure attachment, such as differences between children with an avoidant versus ambivalent insecure attachment. Another limitation to bear in mind includes the threshold of the neuroimaging results, such that the effects that did not survive multiple correction should be interpreted with caution. Future work replicating and building upon this study will contribute to our understanding of which brain regions adolescents recruit during regulation as a function of their early attachment history.
Lastly, although we examined the role of parents using a well-established attachment paradigm and a rich fMRI task across time, two caveats should be considered. First, adolescents were exposed to a minimal level of parental presence under high experimental control (i.e., “Hi [child’s name], I’m here and I just wanted to let you know that I’m looking at these pictures with you!”), and it is uncertain whether the buffering effect of parental presence that emerged for insecure adolescents would remain under more naturalistic parent-adolescent interaction contexts. Second, although child-mother attachment was assessed at the first time point, it was not always logistically feasible for mothers to participate during the scan session at the adolescent timepoint. This concern is attenuated to some degree given that internal working models of attachment relationships become more global over the course of development as individuals integrate similar or disparate attachment experiences (Bretherton, 1987), and research indicates relatively strong concordance in attachment security across parents in low-risk community families (Kochanska and Kim, 2013). In this vein, we note that sensitivity analyses showed the same pattern of behavioral and neuroimaging findings for adolescents with mothers who participated at both time points compared to the full sample. Nonetheless, future work should strive to include consistent repeated measures of specific child-parent attachment relationships across time to evaluate stability and change in how parents influence their offspring’s regulatory abilities.
Despite these limitations, our findings underscore the importance of parental influence on offspring regulatory abilities from early childhood into adolescence. This study contributes to the accumulating literature that early attachment continues to impact adolescent behavior and neural reactivity during social exploration, and provides additional evidence that parents continue to matter into adolescence, particularly for children with a history of insecure attachment. Although this study informs our understanding of the behavioral and neurobiological risks of children who form insecure attachment during toddlerhood, it also lends support to programs that provide assistance and training to families in that parents can still make a difference in how their children behave and perceive their social world, even during adolescence.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to the families who participated in this research. We also thank Elissa Thomann Mitchell, Heather Ross, Jordan Bodway, Ethan McCormick, Michael Perino, and Tae-Ho Lee for assistance with data collection and Bonnie Conley and Susan Paris who coded the modified Strange Situation assessments.
Footnotes
This study was supported by grants from the National Science Foundation, USA, (BCS 1539651) to NLM and EHT, the USDA National Institute of Food and Agriculture, USA, (ILLU-793-362) to NLM, the University of Illinois Research Board, USA, to NLM, and the Jacobs Foundation, Switzerland, (2014-1095) to EHT. We are grateful to the families who participated in this research. We also thank Elissa Thomann Mitchell, Heather Ross, Jordan Bodway, Ethan McCormick, Michael Perino, and Tae-Ho Lee for assistance with data collection and Bonnie Conley and Susan Paris who coded the modified Strange Situation assessments.
Data Availability
Data will be made available on request.
References
- Ahmed S.P., Bittencourt-Hewitt A., Sebastian C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 2015;15:11–25. doi: 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A., Gee D.G., De Los Reyes A., Seager I. Emotion regulation as a transdiagnostic factor in the development of internalizing and externalizing psychopathology: current and future directions. Dev. Psychopathol. 2016;28:927–946. doi: 10.1017/S0954579416000638. [DOI] [PubMed] [Google Scholar]
- Beijersbergen M.D., Juffer F., Bakermans-Kranenburg M.J., van IJzendoorn M.H. Remaining or becoming secure: parental sensitive support predicts attachment continuity from infancy to adolescence in a longitudinal adoption study. Dev. Psychol. 2012;48(5):1277–1282. doi: 10.1037/a0027442. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. Is adolescence a sensitive period for sociocultural processing? Ann. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Booth-Laforce C., Oxford M.L. Trajectories of social withdrawal from grades 1 to 6: prediction from early parenting, attachment, and temperament. Dev. Psychol. 2008;44(5):1298–1313. doi: 10.1037/a0012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinik-Nezer R., Holzmeister F., Camerer C.F., Dreber A., Huber J., Johannesson M., Schonberg T. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–88. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretherton I. In: Handbook of Infant Development. second ed. Osofsky J.D., editor. Wiley; 1987. New perspectives on attachment relations: security, communication, and internal working models; pp. 1060–1099. [Google Scholar]
- Callaghan B.L., Tottenham N. January 1). The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology. 2016;Vol. 41:163–176. doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy, J. , Marvin, R.S. , & Group, with the M. A. W. (1992). Attachment organization in preschool children: Coding guidelines (4th ed.).
- Cracco E., Goossens L., Braet C. Emotion regulation across childhood and adolescence: evidence for a maladaptive shift in adolescence. Eur. Child Adolesc. Psychiatry. 2017;26(8):909–921. doi: 10.1007/s00787-017-0952-8. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Geier K., Falkai P., Gruber O. Fear is only as deep as the mind allows. A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Dykas M.J., Woodhouse S.S., Ehrlich K.B., Cassidy J. Attachment-related differences in perceptions of an initial peer interaction emerge over time: evidence of reconstructive memory processes in adolescents. Dev. Psychol. 2012;48(5):1381–1389. doi: 10.1037/a0027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A.K., Simpson J.A., Carlson E.A., Englund M.M., Sung S. The impact of stress at different life stages on physical health and the buffering effects of maternal sensitivity. Health Psychol. 2016;36:35–44. doi: 10.1037/hea0000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L., Telzer E.H., Humphreys K.L., Goff B., Shapiro M., Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014;25:2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., Schwarz Y., Ghosh S.S., Maumet C., Sochat V.V., Nichols T.E., Poldrack R.A., Poline J., Yarkoni T., Margulies D.S. NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics. 2015;9:1–9. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Telzer E.H. Mother still knows best: maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Dev. Sci. 2018;21:1–11. doi: 10.1111/desc.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M., Quevedo K. The neurobiology of stress and development. Ann. Rev. Psychol. 2006;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R., Hostinar C.E., Sanchez M.M., Tottenham N., Sullivan R.M. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Soc. Neurosci. 2015;10:474–478. doi: 10.1126/science.1249098.Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Casey B.J. The neurodynamics of emotion: Delineating typical and atypical emotional processes during adolescence. Dev. Sci. 2016;19(1):3–18. doi: 10.1111/desc.12373. [DOI] [PubMed] [Google Scholar]
- Hostinar C.E., Gunnar M.R. Social support can buffer against stress and shape brain activity. AJOB Neurosci. 2015;6:34–42. doi: 10.1080/21507740.2015.1047054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Johnson A.E., Gunnar M.R. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev. Sci. 2015;18:281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G., Kim S. Early attachment organization with both parents and future behavior problems: from infancy to middle childhood. Child Dev. 2013;84:283–296. doi: 10.1111/j.1467-8624.2012.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin R.S., Britner P.A. In: Handbook of Attachment: Theory, Research, and Clinical Applications. second ed. Cassidy J., Shaver P.R., editors. New York: Guilford Press; 2008. Normative development: the ontogeny of attachment; pp. 269–294. [Google Scholar]
- McElwain N.L., Holland A.S., Engle J.M., Ogolsky B.G. Getting acquainted: Actor and partner effects of attachment and temperament on young children’s peer behavior. Dev. Psychol. 2014;50:1757–1770. doi: 10.1037/a0036211. [DOI] [PubMed] [Google Scholar]
- McElwain N.L., Holland A.S., Engle J.M., Wong M.S. Child anger proneness moderates associations between child-mother attachment security and child behavior with mothers at 33 months. J. Family Psychol. 2012;26:76–86. doi: 10.1037/a0026454. [DOI] [PubMed] [Google Scholar]
- Mikulincer M., Shaver P.R. Attachment orientations and emotion regulation. Curr. Opin. Psychol. 2019;25:6–10. doi: 10.1016/j.copsyc.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Morris A.S., Criss M.M., Silk J.S., Houltberg B.J. The impact of parenting on emotion regulation during childhood and adolescence. Child Dev. Perspect. 2017;11(4):233–238. doi: 10.1111/cdep.12238. [DOI] [Google Scholar]
- Moutsiana C., Fearon P., Murray L., Cooper P., Goodyer I., Johnstone T., Halligan S. Making an effort to feel positive: Insecure attachment in infancy predicts the neural underpinnings of emotion regulation in adulthood. J. Child Psychol. Psychiatry Allied Discipl. 2014;55:999–1008. doi: 10.1111/jcpp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, L.K., & Muthén, B.O., 2017. Mplus User’s Guide. Los Angeles: Muthén & Muthén.
- Nelson E.E., Jarcho J.M., Guyer A.E. Social re-orientation and brain development: An expanded and updated view. Dev. Cogn. Neurosci. 2016;17:118–127. doi: 10.1016/j.dcn.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsavsky A.K., Telzer E.H., Shapiro M., Humphreys K.L., Flannery J., Goff B., Tottenham N. Indiscriminate amygdala response to mothers and strangers after early maternal deprivation. Biol. Psychiatry. 2013;74(11):853–860. doi: 10.1016/j.biopsych.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino M.T., Miernicki M.E., Telzer E.H. Letting the good times roll: adolescence as a period of reduced inhibition to appetitive social cues. Soc. Cogn. Affect. Neurosci. 2016;11:1762–1771. doi: 10.1093/scan/nsw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C.R., Perino M.T., Telzer E.H. Maternal buffering of adolescent dysregulation in socially appetitive contexts: from behavior to the brain. J. Res. Adolesc. 2020;30:41–52. doi: 10.1111/jora.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Shu J., Hubbard A.D., Weber J., Ochsner K.N. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev. Sci. 2015;18(5):771–784. doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J., George C. In: Handbook of attachment: Theory, Research, and Clinical Applications. second ed. Cassidy J., Shaver P.R., editors. New York: Guilford Press; 2008. The measurement of attachment security and related constructs in infancy and early childhood; pp. 383–416. [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure of appetitive cues in adolescence. J. Cogn. Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: behavioral and neural correlates of appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Icenogle G., Shulman E.P., Breiner K., Chein J., Bacchini D., Takash H.M.S. Around the world, adolescence is a time of heightened sensation seeking and immature self-regulation. Dev. Sci. 2018;21:1–13. doi: 10.1111/desc.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou K., Davey C.G., Kerestes R., Whittle S., Pujol J., Yücel M., Harrison B.J. Brain functional correlates of emotion regulation across adolescence and young adulthood. Human Brain Mapp. 2016;37:7–19. doi: 10.1002/hbm.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, F.L. , 2014. Affect regulation styles in avoidant and anxious attachment. Individual Differences Research, 12(3), 123–130. Retrieved from http://0-search.ebscohost.com.library.alliant.edu/login.aspx?direct=true&db=psyh&AN=2014–43846-004&site=ehost-live&scope=site%5Cnfstevens@wheelock.edu.
- Telzer E.H., Ichien N.T., Qu Y. Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Soc. Cogn. Affect. Neurosci. 2015;10:1383–1391. doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.A. In: Handbook of Child Psychology. sixth ed. Damon W., Lerner R.M., editors. John Wiley & Sons; 2006. The development of the person: social understanding, relationships, conscience, self; pp. 24–98. [Google Scholar]
- Tohka J., Foerde K., Aron A.R., Tom S.M., Toga A.W., Poldrack R.A. Automatic independent component labeling for artifact removal in fMRI. NeuroImage. 2008;39:1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem M., Korom M., Flannery J., Choy T., Caldera C., Humphreys K.L., Gabard-Durnam L., Goff B., Gee D.G., Telzer E.H., Shapiro M., Louie J.Y., Fareri D.S., Bolger N., Tottenham N. Longitudinal changes in amygdala, hippocampus and cortisol development following early caregiving adversity. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtička P. The social neuroscience of attachment. Neurosci. Soc. Sci. 2017:95–119. doi: 10.1007/978-3-319-68421-5. [DOI] [Google Scholar]
- Vrtička P., Sander D., Anderson B., Badoud D., Eliez S., Debbané M. Social feedback processing from early to late adolescence: influence of sex, age, and attachment style. Brain Behav. 2014;4(5):703–720. doi: 10.1002/brb3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Iwanski A. Emotion regulation from early adolescence to emerging adulthood and middle adulthood: age differences, gender differences, and emotion-specific developmental variations. Int. J. Behav. Dev. 2014;38(2):182–194. doi: 10.1177/0165025413515405. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.