Abstract
Background
Unresectable locally advanced pancreatic cancer (LAPC) is generally managed with chemotherapy or chemoradiotherapy, but prognosis is poor with a median survival of ∼13 months (or up to 19 months in some studies). We assessed a novel brachytherapy device, using phosphorous-32 (32P) microparticles, combined with standard-of-care chemotherapy.
Patients and methods
In this international, multicentre, single-arm, open-label pilot study, adult patients with histologically or cytologically proven unresectable LAPC received 32P microparticles, via endoscopic ultrasound-guided fine-needle implantation, planned for week 4 of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) or gemcitabine/nab-paclitaxel chemotherapy, per investigator’s choice. The primary endpoint was safety and tolerability measured using Common Terminology Criteria for Adverse Events version 4.0. The lead efficacy endpoint was local disease control rate at 16 weeks.
Results
Fifty patients were enrolled and received chemotherapy [intention-to-treat (ITT) population]. Forty-two patients received 32P microparticle implantation [per protocol (PP) population]. A total of 1102 treatment-emergent adverse events (TEAEs) were reported in the ITT/safety population (956 PP), of which 167 (139 PP) were grade ≥3. In the PP population, 41 TEAEs in 16 (38.1%) patients were possibly or probably related to 32P microparticles or implantation procedure, including 8 grade ≥3 in 3 (7.1%) patients, compared with 609 TEAEs in 42 (100%) patients attributed to chemotherapy, including 67 grade ≥3 in 28 patients (66.7%). The local disease control rate at 16 weeks was 82.0% (95% confidence interval: 68.6% to 90.9%) (ITT) and 90.5% (95% confidence interval: 77.4% to 97.3%) (PP). Tumour volume, carbohydrate antigen 19-9 levels, and metabolic tumour response at week 12 improved significantly. Ten patients (20.0% ITT; 23.8% PP) had surgical resection and median overall survival was 15.2 and 15.5 months for ITT and PP populations, respectively.
Conclusions
Endoscopic ultrasound-guided 32P microparticle implantation has an acceptable safety profile. This study also suggests clinically relevant benefits of combining 32P microparticles with standard-of-care systemic chemotherapy for patients with unresectable LAPC.
Key words: locally advanced pancreatic cancer, 32P microparticles, brachytherapy, safety profile, local disease control rate
Highlights
-
•
PanCO is the first prospective study of intratumoural 32P microparticles for locally advanced pancreatic cancer (LAPC).
-
•
This single-arm study assessed a novel brachytherapy (32P microparticles) combined with standard-of-care chemotherapy.
-
•
Treatment-emergent adverse events attributable to 32P microparticle implantation were relatively infrequent.
-
•
Local disease control rate at 16 weeks (82%) and resection rate (20%) suggest a clinical benefit of 32P microparticles.
-
•
The results suggest that 32P microparticles may address a significant unmet need in patients with unresectable LAPC.
Introduction
Locally advanced pancreatic cancer (LAPC) accounts for ∼30% of all pancreatic cancer presentations.1, 2, 3 By the time clinical symptoms are evident, most patients have tumour invasion that precludes resection with curative intent. Unresectable LAPC has a poor prognosis with a median survival of ∼13.3 months,4 with some reports of survival up to 19 months.2,5, 6, 7, 8
Guidelines recommend first-line chemotherapy or induction chemotherapy with consolidative chemoradiotherapy.5,9, 10, 11 Recommended combination regimens for patients with good performance status include 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX), gemcitabine/nab-paclitaxel, or other gemcitabine-based chemotherapy regimens. Combining systemic chemotherapy with radiotherapy for local disease control may improve time to progression of local disease, pain control, performance status, and quality of survival, and in a neoadjuvant setting may convert tumours to resectability.12,13 Improvements in overall survival (OS), however, have been elusive,6 and due to the location of the pancreas and risk of radiation damage to surrounding tissues and organs, locally-directed radiotherapy is needed.
Brachytherapy offers the option of delivering radiation directly to the tumour via endoscopic ultrasound (EUS)-guided fine-needle injection,14 potentially reducing the risk of collateral damage, and was shown to be clinically feasible in unresectable pancreatic cancer.15,16 Phosphorous-32 (32P) microparticles is a novel brachytherapy device, approved for use in unresectable LAPC, that implants the required activity of beta radiation-emitting 32P microparticles into pancreatic tumours via EUS guidance to deliver an absorbed dose of 100 Gy to the tumour. In combination with gemcitabine monotherapy in 23 patients with LAPC and metastatic disease, 32P microparticles showed acceptable tolerability and feasibility, and a case study from an ongoing study suggested positive efficacy outcomes.17,18 The PanCO study was initiated to assess the safety, efficacy, and feasibility of 32P microparticles in combination with current standard-of-care chemotherapy (FOLFIRINOX or gemcitabine/nab-paclitaxel) in patients with unresectable LAPC.19 Here we present the final results of the PanCO study.
Methods
The PanCO study was an international, multicentre, single-arm, open-label pilot study conducted at 10 centres in Australia, Belgium, and the UK (ClinicalTrial.gov ID: NCT03003078). The study was designed and conducted in accordance with ISO 14155, applicable local regulations (including European Directive 2001/20/EC), and with the ethical principles laid down in the Declaration of Helsinki. All appropriate ethics committee approvals were obtained, and all participants gave written informed consent.
Participants
Adult patients were included in the study if they had histologically or cytologically proven, unresectable LAPC, a target tumour diameter 2-6 cm, and Eastern Cooperative Oncology Group performance status 0-1. Included patients also had to have adequate renal, liver, and bone marrow function, life expectancy of at least 3 months at screening, and were not pregnant and using adequate birth control if of child-bearing potential. Exclusion criteria included: evidence of distant metastases based on computed tomography (CT) scan; evidence of radiographic invasion into the stomach, duodenum, or peritoneum; more than one primary lesion; any previous radiotherapy or chemotherapy for pancreatic cancer; use of any investigational agent within the last 30 days; unacceptable risks for EUS-directed implantation according to the investigator; history of malignancy in the last 5 years; or a known allergy to any of the components of the test device. A full list of inclusion and exclusion criteria is shown in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100356.
Study interventions
Included patients received 32P microparticles (OncoSil Medical, Sydney, Australia) and chemotherapy with either FOLFIRINOX or gemcitabine/nab-paclitaxel. The chemotherapy regimen selection was at the investigators’ discretion and was administered according to the local guidelines. The selected chemotherapy regimen was started within 14 days of the screening visit, and implantation of the 32P microparticles was planned in week 4 of the chemotherapy cycles, allowing ≥48 h before and after chemotherapy administration and implantation of 32P microparticles. 32P activity was calculated from the patient's tumour volume to deliver a 100 Gy absorbed dose.20 The 32P microparticles were implanted directly into the pancreatic tumour via EUS guidance, using a fine-needle aspiration. Following 32P microparticle implantation, chemotherapy was continued according to local practice. 32P microparticle localization pattern following implantation was assessed by EUS and Bremsstrahlung single-photon emission CT (SPECT)/CT within 4 h and at 7 days post-implantation.
Assessments and outcome measures
Patient assessments took place at screening and on the day chemotherapy commenced, each week thereafter until 12 weeks after commencement, at week 16, and then every 8 weeks until progression of the target pancreatic tumour. After progression and the end-of-study visit, medical records of each patient were reviewed at 8-weekly intervals.
The primary endpoint was safety and tolerability as measured by the frequency of treatment-emergent adverse events (TEAEs), graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, from the date of chemotherapy commencement to end of study. TEAEs and laboratory values were assessed at each visit until end of study.
Blood and urine samples were collected on the day of 32P microparticle implantation (blood collected before and within 4 h of implantation; urine collected within 4 h of implantation) and at 1, 2, and 3 weeks post-implantation (and at 8 weeks post-implantation for blood sample) to assess non-target exposure to 32P in approximately 20 study participants. Radioactivity was measured in blood and urine samples using Wallac 1409 automatic liquid scintillation counters. Any free activity in blood and urine on the day samples were taken was expressed relative to implanted activity.
The lead secondary endpoint was local disease control rate (LDCR) at 16 weeks. LDCR was defined as stable disease, partial response, or complete response in the target tumour. Tumour response was measured by CT scan at screening and at 8-weekly intervals assessed independently by central reader analysis using RECIST version 1.1 and target tumour volume measurement.
Other secondary endpoints included: local progression-free survival (LPFS), defined as the time from enrolment to the date of death or the date of the scan used to determine local tumour progression; PFS at any site; and OS defined as the time from enrolment to death by any cause. LPFS and PFS were assessed with and without censoring resected patients at their last CT scan before surgery. Pain was measured using the 10-point Numerical Rating Scale (NRS). Changes in the level of the tumour marker carbohydrate antigen (CA) 19-9 from baseline were measured according to each site’s standard method. As an exploratory analysis, a ≥50% reduction from baseline values or normalization of CA 19-9 was considered clinically important if baseline levels were >35 U/ml.21 Additional exploratory analyses were conducted and included: metabolic tumour response according to [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography (18F-FDG-PET) measured at baseline and week 12, assessed by the independent central reader for total lesion glycolysis (TLG), and standardized uptake values, SUVMax and SULMax22; target tumour volume change assessed by central reader (using Voxels of Interest and eMass software [ERT; Brussels, Belgium]) from CT scans; and the number of patients undergoing potentially curative surgical resection. Quality of life was also assessed and will be the subject of a separate analysis.
Statistics
Assuming a probability of a device-related serious adverse event (SAE) of 0.05, 40 participants would provide a probability of observing at least one SAE of 0.87, which is an acceptable detection rate. For the LDCR at 16 weeks, assuming a null hypothesis of 55%, an alternative hypothesis of 75%, and a significance level of 0.05 with a two-sided test, the sample size required to achieve a power of 80% was 45. The target sample size was therefore set at 45.
Three populations were defined: the intention-to-treat (ITT) population (all enrolled patients); the safety population (all enrolled patients that received any study treatment); and the per protocol (PP) population (all enrolled patients that received 32P microparticles). The primary endpoint is presented for the safety and PP populations. Efficacy data are presented for the ITT and PP populations.
Descriptive statistics were used. Continuous variables are presented as mean, standard deviation (SD), median, minimum, and maximum. Categorical variables are presented as frequency counts and percentages. Significance of LDCR at 16 weeks was assessed using the Fisher’s exact test by comparing the binomial proportion to the null hypothesis proportion of 0.55. Change from baseline in tumour volume, 18F-FDG-PET values, and CA 19-9 was assessed using the paired t-test. OS, LPFS, and PFS were estimated using Kaplan–Meier survival analysis.
Results
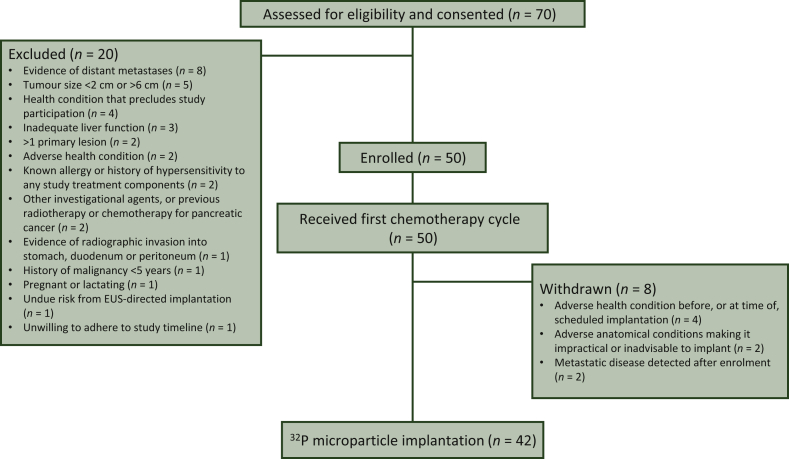
Between March 2017 and June 2018, 50 patients were enrolled (ITT population), 50 patients received any study treatment (safety population), and 42 patients subsequently received 32P microparticle implantation (PP population; Figure 1) at a median (range) of 31 (21-77) days from commencing chemotherapy. Median follow-up was 31.6 months [95% confidence interval (CI): 26.5-35.1 months]. Baseline characteristics of the ITT population are summarized in Table 1. Central review of the baseline PET scans identified seven patients (5 PP) with suspected liver metastases. One patient withdrew from the study following hospitalization for TEAEs which precluded 32P microparticle implantation and subsequently received 32P microparticles off-protocol (ITT population).
Figure 1.
Participant flow in the PanCO study.
EUS, endoscopic ultrasound.
Table 1.
Baseline characteristics of the ITT population
| Characteristic | n (%)a |
|---|---|
| Median (range) age, years | 65 (42-84) |
| Sex | |
| Male | 28 (56) |
| Female | 22 (44) |
| Race | |
| White/Caucasian | 40 (80) |
| Asian | 7 (14) |
| Black/African American | 3 (6) |
| ECOG performance status | |
| 0 | 26 (52) |
| 1 | 24 (48) |
| Pancreatic tumour location | |
| Head | 42 (84) |
| Body | 8 (16) |
| Median (range) target lesion longest diameter, cmb | 4.5 (2.6-7.1) |
| Median (range) tumour volume, ccb | 24.4 (7.9-68.7) |
| Median (range) CA 19-9 level, U/mlc | 163 (1-6576) |
CA, carbohydrate antigen; ECOG, Eastern Cooperative Oncology Group; ITT, intention-to-treat.
Unless otherwise stated in left-hand column.
By independent central reader analysis.
n = 49.
Treatment delivered
The chemotherapy received by patients in the ITT population was gemcitabine/nab-paclitaxel in 40 (80.0%) patients with a median of 4 (range, 1-25) 28-day cycles and was FOLFIRINOX in 10 (20.0%) patients with a median of 6 (range, 2-13) 14-day cycles. The median relative dose intensity was 47.0% and 41.1% in the groups receiving gemcitabine/nab-paclitaxel and FOLFIRINOX, respectively, for the first 6 or 12 cycles of chemotherapy. Chemotherapy dose reduction or delay ≥1 week occurred in 92.5% and 100.0% of participants, respectively. In the group receiving gemcitabine/nab-paclitaxel, 34 patients also received 32P microparticle implantation, and among these patients a pre-implantation chemotherapy dose reduction or delay of ≥1 week was observed in 26 (76.5%) patients. In the FOLFIRINOX group, eight patients also received 32P microparticle implantation, and among these patients a pre-implantation chemotherapy dose reduction or delay of ≥1 week was observed in seven (87.5%) patients.
Bremsstrahlung SPECT/CT imaging confirmed radiation localized to the implant site in 40 of the 42 implanted participants at 4 h post-implantation and in 36 participants at 7 days post-implantation.
Safety and tolerability
In the ITT population, 1102 TEAEs were reported, of which 167 were grade ≥3, with 956 TEAEs (139 grade ≥3) in the PP population (Table 2). There were no treatment-related grade 5 TEAEs. The most common grade ≥3 AEs were haematological (neutropenia and anaemia) and fatigue, most of which were considered to be related to chemotherapy by the treating physician. In the PP population, 289 (30.2%) TEAEs occurred before 32P microparticle implantation (median follow-up: 31 days), and 667 (69.8%) occurred after 32P microparticle implantation (median follow-up 31 months; Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100356). A higher proportion of the PP population receiving gemcitabine/nab-paclitaxel (29/34, 85.3%) had a grade ≥3 TEAE than patients receiving FOLFIRINOX (5/8, 62.5%; Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100356).
Table 2.
Number of patients with treatment-emergent adverse events in ≥10% of participants, by population and by attribution (possible or probable causality)
| Treatment-emergent adverse event | ITT population (n = 50) |
PP population (n = 42) |
TEAEs attributed to 32P device or implantation procedure (PP population; n = 42) |
TEAEs attributed to chemotherapy (PP population; n = 42) |
||||
|---|---|---|---|---|---|---|---|---|
| All grade n (%) | Grade ≥3 n (%) | All grade n (%) | Grade ≥3 n (%) | All grade n (%) | Grade ≥3 n (%) | All grade n (%) | Grade ≥3 n (%) | |
| Total events, n | 1102 | 167 | 956 | 139 | 41 | 8 | 609 | 67 |
| Total participants with ≥1 TEAE | 50 (100.0) | 41 (82.0) | 42 (100.0) | 34 (81.0) | 16 (38.1) | 3 (7.1) | 42 (100.0) | 28 (66.7) |
| Fatigue | 41 (82.0) | 7 (14.0) | 35 (83.3) | 6 (14.3) | 5 (11.9) | 1 (2.4) | 34 (81.0) | 5 (11.9) |
| Nausea | 30 (60.0) | 5 (10.0) | 25 (59.5) | 3 (7.1) | 3 (7.1) | — | 23 (54.8) | 2 (4.8) |
| Diarrhoea | 29 (58.0) | 1 (2.0) | 26 (61.9) | 1 (2.4) | — | — | 21 (50.0) | 1 (2.4) |
| Neutropeniaa | 28 (56.0) | 24 (48.0) | 22 (52.4) | 18 (42.9) | 2 (4.8) | 1 (2.4) | 20 (47.6) | 16 (38.1) |
| Abdominal paina | 26 (52.0) | 6 (12.0) | 22 (52.4) | 5 (11.9) | 3 (7.1) | 1 (2.4) | 5 (11.9) | 1 (2.4) |
| Constipation | 24 (48.0) | 1 (2.0) | 19 (45.2) | 1 (2.4) | — | — | 10 (23.8) | — |
| Alopecia | 21 (42.0) | — | 16 (38.1) | — | — | — | 16 (38.1) | — |
| Decreased appetite | 18 (36.0) | 1 (2.0) | 18 (42.9) | 1 (2.4) | — | — | 16 (38.1) | — |
| Vomiting | 18 (36.0) | 4 (8.0) | 14 (33.3) | 3 (7.1) | — | — | 10 (23.8) | 1 (2.4) |
| Pyrexia | 17 (34.0) | 3 (6.0) | 16 (38.1) | 3 (7.1) | — | — | 11 (26.2) | 2 (4.8) |
| Peripheral neuropathya | 17 (34.0) | 1 (2.0) | 15 (35.7) | 1 (2.4) | — | — | 15 (35.7) | 1 (2.4) |
| Thrombocytopeniaa | 17 (34.0) | 5 (10.0) | 14 (33.3) | 4 (9.5) | 1 (2.4) | 1 (2.4) | 13 (31.0) | 3 (7.1) |
| Anaemiaa | 15 (30.0) | 7 (14.0) | 14 (33.3) | 7 (16.7) | 1 (2.4) | — | 12 (28.6) | 5 (11.9) |
| Weight decreased | 15 (30.0) | 2 (4.0) | 13 (31.0) | 2 (4.8) | 1 (2.4) | — | 10 (23.8) | 1 (2.4) |
| Rash | 13 (26.0) | — | 12 (28.6) | — | — | — | 12 (28.6) | — |
| Peripheral oedemaa | 13 (26.0) | 1 (2.0) | 10 (23.8) | — | — | — | 8 (19.0) | — |
| Hypokalaemiaa | 10 (20.0) | 3 (6.0) | 8 (19.0) | 2 (4.8) | 1 (2.4) | 1 (2.4) | 4 (9.5) | 1 (2.4) |
| Dysgeusia | 8 (16.0) | — | 7 (16.7) | — | — | — | 7 (16.7) | — |
| Hypotension | 8 (16.0) | — | 7 (16.7) | — | 1 (2.4) | — | 1 (2.4) | — |
| Dyspnoea | 8 (16.0) | — | 7 (16.7) | — | — | — | 2 (4.8) | — |
| Pain | 8 (16.0) | 1 (2.0) | 5 (11.9) | 1 (2.4) | — | — | 1 (2.4) | — |
| Pruritus | 7 (14.0) | — | 7 (16.7) | — | — | — | 5 (11.9) | — |
| Pulmonary embolism | 7 (14.0) | 6 (12.0) | 6 (14.3) | 5 (11.9) | 1 (2.4) | 1 (2.4) | 3 (7.1) | 1 (2.4) |
| Mucosal inflammation | 7 (14.0) | 1 (2.0) | 6 (14.3) | 1 (2.4) | — | — | 6 (14.3) | 1 (2.4) |
| Cellulitis | 6 (12.0) | 1 (2.0) | 6 (14.3) | 1 (2.4) | — | — | 4 (9.5) | — |
| Back pain | 6 (12.0) | 1 (2.0) | 6 (14.3) | 1 (2.4) | — | — | — | — |
| Paraesthesia | 6 (12.0) | — | 6 (14.3) | — | — | — | 4 (9.5) | — |
| Hypomagnesemia | 6 (12.0) | — | 5 (11.9) | — | — | — | 4 (9.5) | — |
| Ascites | 6 (12.0) | 2 (4.0) | 4 (9.5) | 2 (4.8) | — | — | 1 (2.4) | 1 (2.4) |
| Device occlusion (stent) | 5 (10.0) | 3 (6.0) | 5 (11.9) | 3 (7.1) | — | — | — | — |
| Epistaxis | 5 (10.0) | 1 (2.0) | 5 (11.9) | 1 (2.4) | — | — | 4 (9.5) | — |
| Oral candidiasis | 5 (10.0) | — | 5 (11.9) | — | — | — | 4 (9.5) | — |
| Hypoalbuminemia | 5 (10.0) | 4 (8.0) | 4 (9.5) | 3 (7.1) | 1 (2.4) | 1 (2.4) | 3 (7.1) | 2 (4.8) |
| Dry mouth | 5 (10.0) | — | 4 (9.5) | — | — | — | 4 (9.5) | — |
| Dizziness | 5 (10.0) | — | 4 (9.5) | — | — | — | 1 (2.4) | — |
TEAEs in ≥10% of study participants at any grade (ITT or PP population). Multiple records from the same study participants are only counted once within the same category.
ITT, intention-to-treat; PP, per protocol (enrolled and implanted participants); TEAE, treatment-emergent adverse event; —, no TEAEs.
Combined records: abdominal pain includes TEAEs reported as abdominal pain irrespective of abdominal site of pain (lower, upper, or not otherwise specified); peripheral oedema includes TEAEs reported as oedema peripheral and/or peripheral swelling; neutropenia includes TEAEs reported as neutropenia, febrile neutropenia, neutropenic sepsis, and/or neutrophil count decreased; thrombocytopenia includes TEAEs reported as thrombocytopenia and/or platelet count decreased; anaemia includes TEAEs reported as anaemia and/or haemoglobin decreased; hypokalaemia includes TEAEs reported as hypokalaemia and/or blood potassium decreased; peripheral neuropathy includes TEAEs reported as peripheral neuropathy and/or peripheral sensory neuropathy.
Of the 41 TEAEs possibly or probably related to 32P microparticles or implantation procedure in 16 (38.1%) patients, including 8 grade ≥3 in 3 (7.1%) patients (Table 2), 27 (5 grade ≥3) were also attributed to chemotherapy. In the PP population, 609 TEAEs were considered possibly or probably related to chemotherapy in 42 (100%) patients, including 67 grade ≥3 in 28 (66.7%) patients. Most TEAEs possibly or probably related to 32P microparticles occurred within 30 days of the implantation procedure and none occurred >120 days after the implantation (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100356). No radiation-related treatment-emergent SAEs (TESAEs) were reported. There was no evidence that the incidence of grade ≥3 TEAEs per chemotherapy cycle changed after 32P microparticle implantation (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100356).
Three treatment-emergent severe adverse device-related events (TESADEs) were reported, of which two (abdominal pain and neutropenic sepsis) occurred in the same patient. Both these TESADEs resolved and were considered possibly related to the investigational device. The third TESADE involved intravasation of the 32P microparticle device resulting in shunting of the implanted activity to lung. Upon review, the independent study safety review committee advised that the patient should not have been enrolled due to the presence of intratumoural varices and the exclusion criteria were strengthened to reflect this. This patient experienced no respiratory sequelae over a follow-up period of >14 months.
Twenty-six study participants had blood and urine samples taken to assess non-target 32P relative activity (RA). Activity was detected in the blood of 4 (15.4%) and urine of 22 (84.6%) patients. The absolute activity was below the level of quantification ≤4 h post-implantation of the 32P microparticle device in both blood and urine. In urine, mean activity peaked at days 7-14 (RA, 0.12%), with decreased activity (RA, 0.07%) by day 21. Mean activity in blood peaked at day 21 (RA, 0.078%) and all sampled patients had activity below the level of quantification by day 56.
Efficacy
LDCR at 16 weeks was 82.0% (95% CI: 68.6% to 91.4%) in the ITT population and 90.5% (95% CI: 77.4% to 97.3%) in the PP population (Table 3), which met the conditions for a priori performance criteria.
Table 3.
Efficacy analyses
| Efficacy measure | ITT population (N = 50) | PP population (N = 42) |
|---|---|---|
| Local disease controla,b | ||
| Patients with local disease control at 16 weeks, n (%) | 41 (82.0) | 38 (90.5) |
| Local disease control rate at 16 weeks (95% CI) | 82.0 (68.6-91.4) | 90.5 (77.4-97.3) |
| P valuec | 0.0001 | <0.0001 |
| Best tumour response by RECIST v1.1, n (%)a,b | ||
| Complete response (CR)d | 0 | 0 |
| Partial response (PR)d | 14 (29.8) | 13 (31.0) |
| Stable diseased | 31 (66.0) | 29 (69.0) |
| Progressive disease (PD)d | 2 (4.3) | 0 |
| Not evaluated | 3 | 0 |
| Objective response rate (CR + PR)e | 14 (28.0) | 13 (31.0) |
| Disease control rate (CR + PR + stable disease)e | 45 (90.0) | 42 (100.0) |
| Tumour volume response (CT scan)a,b | ||
| Median (range) maximal decrease from baseline, % | −51.6 (+72.2 to −89.9)g | −51.9 (+11.1 to −89.9) |
| Mean (SD) maximal decrease from baseline, % | −44.0 (34.8)g | −49.1 (26.4) |
| P valuef | <0.0001 | <0.0001 |
| Tumour response by 18F-FDG-PET at 12 weeksa,b | ||
| Patients with evaluable images, n | 42 | 39 |
| TLG: median (range) change from baseline, % | −60.5 (+319.2 to −100.0) | −60.5 (+319.2 to −100.0) |
| mean (SD) change from baseline, % | −37.1 (89.9) | −34.8 (92.8) |
| P valuef | 0.0001 | 0.0003 |
| SUVMax: median (range) change from baseline, % | −40.3 (+76.4 to −100.0) | −40.4 (+76.4 to −100.0) |
| mean (SD) change from baseline, % | −36.3 (43.1) | −35.8 (42.9) |
| P valuef | <0.0001 | <0.0001 |
| SULMax: median (range) change from baseline, % | −43.1 (+75.3 to −100.0) | −43.7 (+75.3 to −100.0) |
| mean (SD) change from baseline, % | −36.2 (46.3) | −35.9 (46.3) |
| P valuef | 0.0188 | 0.0232 |
| Surgical resection with curative intent, n (%) | 10 (20.0) | 10 (23.8) |
| R0 margin status, n (% of resections) | 8 (80.0) | 8 (80.0) |
| R1 margin status, n (% of resections) | 2 (20.0) | 2 (20.0) |
| CA 19-9 responseb | ||
| Assessable patients with baseline CA 19-9 ≥35 U/ml, n | 38 | 33 |
| Median (range) maximal decrease from baseline, % | −80.7 (+50.0 to −99.9) | −82.3 (+50.0 to −99.9) |
| Mean (SD) maximal decrease from baseline, % | −68.1 (35.4) | −70.9 (34.0) |
| P valuef | 0.0006 | 0.0024 |
| Local progression-free survival, monthsa | ||
| Median (95% CI), uncensored for resection | 9.9 (7.3-12.6) | 9.8 (7.3-12.6) |
| Median (95% CI), patients censored before resection | 9.5 (7.2-11.3) | 9.3 (7.2-11.3) |
| Progression-free survival, monthsa | ||
| Median (95% CI), uncensored for resection | 9.3 (5.7-11.3) | 9.3 (5.8-11.3) |
| Median (95% CI), patients censored before resection | 7.7 (5.7-9.9) | 7.7 (5.8-9.9) |
| Overall survival, months | ||
| Median (95% CI) | 15.2 (11.3-18.8) | 15.5 (11.4-20.1) |
CI, confidence interval; CT, computed tomography; 18F-FDG-PET, [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography; ITT, intention-to-treat population; PP, per protocol population; SD, standard deviation; SULMax, maximum standardized uptake value corrected for lean body mass; SUVMax, maximum standardized uptake value; TLG, total lesion glycolysis.
By central image reader analysis.
Response before surgical resection.
P values for Fisher’s Exact test, comparing the binomial proportion with the null hypothesis proportion of 0.55.
Percentages based on the number of assessable study participants.
Percentages based on the number of all study participants.
P value for paired t-test, percent change from baseline.
n = 47 Patients with evaluable post-baseline scans.
Other measures of target tumour response showed improvement (Table 3; Supplementary Figures S3-S5, available at https://doi.org/10.1016/j.esmoop.2021.100356). Total objective response rate was 28.0% and 31.0% in the ITT and PP populations, respectively. Target tumour volume was significantly reduced compared with baseline (Table 3; Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100356). 18F-FDG-PET imaging revealed significant improvements in TLG, SULMax, and SUVMax at 12 weeks, with a complete metabolic response in six patients (five in the PP population; Table 3; Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100356). CA 19-9 levels were also significantly reduced (Table 3; Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2021.100356). In the PP population, 26 (78.8%) patients had ≥50% maximal reduction in CA 19-9 and 15 (45.5%) had a normal nadir CA 19-9.
The mean pain score at study commencement was 2.0, suggesting relatively low and/or reasonably well-controlled pain at baseline (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2021.100356). Seventeen patients reported moderate-to-severe pain at baseline, defined as NRS ≥5. In this cohort, the mean change of NRS score from baseline was −3.3 before 32P microparticle implantation, compared with −4.7 following implantation through to end-of-study, resection, or local disease progression (median week 24).
Ten patients (all recipients of 32P microparticles; nine received gemcitabine/nab-paclitaxel and one received FOLFIRINOX) underwent surgical resection by Whipple procedure between 70 and 267 days after the 32P microparticle implantation (Table 3). Four additional patients treated with 32P microparticles were downstaged and technically considered for surgical resection, but could not undergo surgery due to distant metastases, comorbidities, and/or other considerations such as advanced age or patient choice. In the first 12 months following 32P microparticle implantation, 18 patients (36.0%) received second-line chemotherapy, 11 (22.0%) underwent surgery, and 13 (26.0%) had other treatments or procedures (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100356).
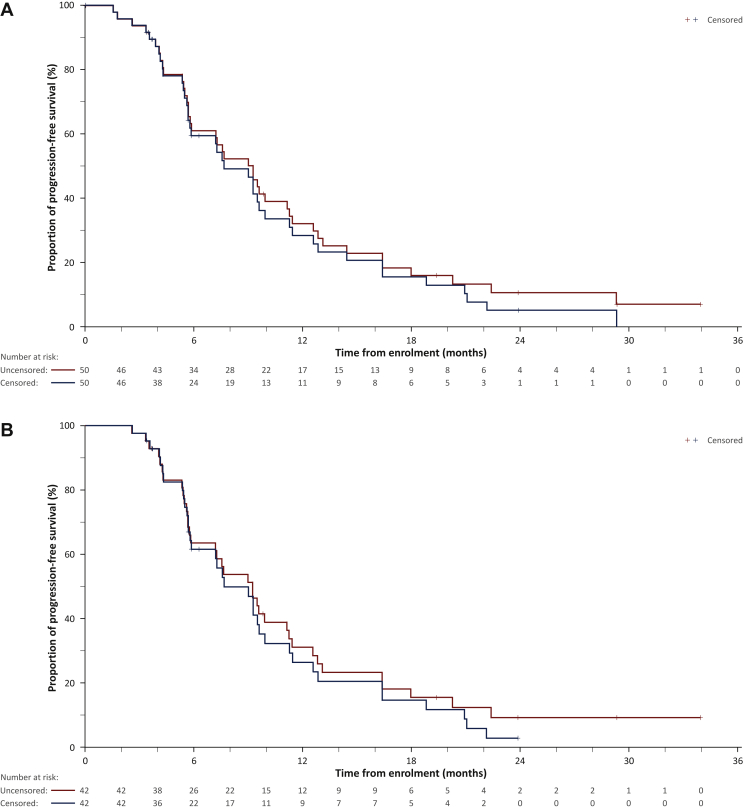
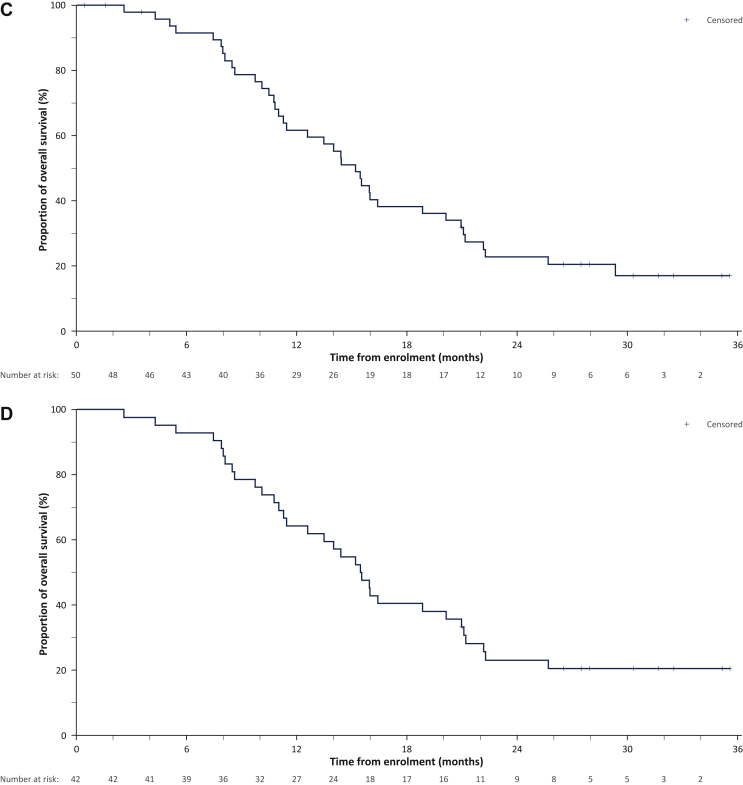
Median LPFS, PFS, and OS are summarized in Table 3 and Figure 2. Median OS was 15.2 months and 15.5 months in the ITT and PP populations, respectively.
Figure 2.
Kaplan–Meier analysis of progression-free survival at any site and overall survival.
(A) Progression-free survival in the ITT population, censored or uncensored before resection. (B) Progression-free survival in the PP population, censored or uncensored before resection. (C) Overall survival in the ITT population. (D) Overall survival in the PP population.
ITT, intention-to-treat; PP, per protocol (enrolled and implanted participants).
Discussion
The PanCO study shows that EUS-guided 32P microparticle implantation is feasible, with an acceptable safety profile, combined with first-line chemotherapy for LAPC over a prolonged study timeframe. This is the first published report of a prospective clinical study investigating the use of targeted intratumoural 32P microparticles to treat LAPC.
The overall safety profile was largely consistent with that expected in a population receiving standard-of-care chemotherapy, with no evidence suggesting significant additional risk (including the risk of radiation-related toxicity) when 32P microparticles were combined with contemporary systemic chemotherapy regimens.
This study also provides encouraging outcomes to suggest clinically relevant benefits for patients with unresectable LAPC treated with 32P microparticles combined with systemic chemotherapy. The lead secondary endpoint (LDCR at 16 weeks) was met in both the ITT and PP populations. This surrogate endpoint has been recommended in a recent consensus paper for trials of novel drug-radiotherapy combinations and may correlate with disease-free survival and OS.23 Even in the presence of micrometastatic disease, locoregional tumour control may translate into improvements in OS, symptom-free survival, and quality of life.24 Indeed, the median OS in the PP population of the PanCO study (15.5 months) compares favourably with a recent systematic literature review and meta-analysis of chemotherapy and chemoradiotherapy in LAPC (13.3 months).4 Individual studies (some included in the systematic review) have reported median OS in LAPC in the range of 15-19 months with induction chemotherapy using two or three agents followed by chemoradiation25,26 and with combination chemotherapy alone.8,27, 28, 29 Differences in selection criteria across studies, however, make cross-study comparisons unreliable. For example, in the PanCO study the chemotherapy regimen selection and dose reduction was at the investigators’ discretion, whereas in some other studies the delivery of the chemotherapy would be more strictly controlled and therefore the intensity of the chemotherapy regimen may vary between studies.
Beyond the local disease control, reduction in symptoms (i.e. pain) associated with tumour progression, and significant improvements from baseline in tumour volume and metabolic tumour response according to 18F-FDG-PET and CA 19-9, indicate a consistently encouraging response. There is no consensus on the optimum cut-off for clinically meaningful reductions in CA 19-9 in LAPC, with reductions of CA 19-9 from baseline ranging from >15% to >90% following treatment being proposed as predictors of prolonged survival in various studies.21,30, 31, 32, 33, 34, 35, 36 Even using the strictest of these cut-offs, an important proportion of those in the PP population of the PanCO study had significant reduction in CA 19-9 levels, and almost 50% of assessable patients had nadir CA 19-9 within the normal range.
Ten study participants had a surgical resection with curative intent following 32P microparticle implantation, with eight achieving an R0 resection. This represents a resection rate of 23.8% in the PP population of patients not considered surgical candidates at study enrolment. The aim of combining 32P-based brachytherapy with radiosensitising chemotherapy agents is to maximize the antitumoural effects in shrinking the pancreatic tumours, overall and away from the involved vessels, as well as sterilizing the surgical margins in order to increase the rate of surgical resection and the proportion with R0 margins, and minimize the risk of local recurrence. There is evidence in pancreatic cancer surgery that clear/R0 margins are associated with improved survival compared with R1 margins,37 and patients undergoing surgical resection had significantly better survival than patients who did not (median OS 35.3 months versus 16.3 months, respectively).38
Limitations include the small sample size of this pilot study and the absence of a comparator arm. Because the PanCO study was not designed to assess resection rate, the classification of patients as unresectable was left to the local multidisciplinary teams, which potentially could have led to inconsistencies among the recruited population in terms of their resectability status. The high resection rates may therefore be viewed with caution. Nevertheless, the centres involved in the study were highly experienced in managing patients with unresectable LAPC and the conversion of 23.8% of the PP population to resection does appear interesting.
The encouraging results of the PanCO study provide evidence that 32P microparticles can address a significant unmet clinical need in the unresectable LAPC population. Based on these results, further clinical studies are planned to assess the safety and efficacy of 32P microparticles in combination with standard-of-care chemotherapy regimens for LAPC, and the potential for converting unresectable patients to curative resection.
Acknowledgements
The authors thank all the investigators and patients involved in the PanCO study. We thank Dale Bailey at Royal North Shore Hospital, Sydney, Australia for his assistance in the dosimetry and activity calculations for the study. Martin Gilmour of Empowering Strategic Performance (ESP) Ltd, Crowthorne, UK provided medical writing and editorial support, which was sponsored by OncoSil Medical in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
This work was supported by OncoSil Medical Ltd, Sydney, Australia (no grant number). Nab-paclitaxel was supported by Specialised Therapeutics Australia Pty Ltd, Kew, Australia.
Disclosure
PJR: stock and other ownership interests: Perci Health Ltd. Honoraria: Sirtex Medical, Eisai, Servier, Pierre Fabre, Shire, Roche, AstraZeneca, Merck. Consulting or advisory role: Sirtex Medical, Eisai, Servier, Roche, AstraZeneca, Amgen. Speakers’ bureau: Amgen, Merck, Servier, Boston Scientific. Research funding: Sanofi, Bayer. Travel, accommodations, expenses: Roche, Ipsen. HSW: honoraria: BTG/Biocompatibles, Merck KGaA, and BMS. Consulting or advisory role: Incyte, Bayer, Roche/Genentech/ Foundation Medicine, Sirtex Medical, Celgene, OncoSil Medical, and Zymeworks. Research funding: Pfizer, Sirtex and Zymeworks. Travel, accommodations, expenses: BTG/Biocompatibles, Merck KGaA, and BMS. DC: stock and other ownership interests: MarginClear. Consulting or advisory role: Boston Scientific and OncoSil Medical. Research funding: Boston Scientific. MN: stock and other ownership interests: Pakinax Pty Ltd and Margin Clear Pty Ltd. DB: employment: SA Medical Imaging and SA Health. Research funding: Avener/Pankind and AstraZeneca. NEW: employment: OncoSil Medical Ltd. Stock and other ownership interests: OncoSil Medical Ltd. DMT: employment: OncoSil Medical Ltd. Stock and other ownership interests: OncoSil Medical Ltd. DCJ: employment: OncoSil Medical Ltd. Stock and other ownership interests: OncoSil Medical Ltd. EY: employment: Southern Star Research Pty Ltd (contractor to OncoSil Medical Ltd). All other authors have declared no conflicts of interest.
Data sharing
Data used in the current study are available from info@oncosil.com on reasonable request.
Supplementary data
References
- 1.Vincent A., Herman J., Schulick R., et al. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruarus A., Vroomen L., Puijk R., et al. Locally advanced pancreatic cancer: a review of local ablative therapies. Cancers (Basel) 2018;10:16. doi: 10.3390/cancers10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurusamy K.S., Kumar S., Davidson B.R., et al. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev. 2014:CD010244. doi: 10.1002/14651858.CD010244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang J.S., Chiu Y.F., Yu J.C., et al. The role of consolidation chemoradiotherapy in locally advanced pancreatic cancer receiving chemotherapy: an updated systematic review and meta-analysis. Cancer Res Treat. 2018;50:562–574. doi: 10.4143/crt.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducreux M., Cuhna A.S., Caramella C., et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 6.Hammel P., Huguet F., van Laethem J.L., et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. J Am Med Assoc. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 7.Quaresma M., Coleman M.P., Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet. 2015;385:1206–1218. doi: 10.1016/S0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- 8.Picozzi V., Alseidi A., Winter J., et al. Gemcitabine/nab-paclitaxel with pamrevlumab: a novel drug combination and trial design for the treatment of locally advanced pancreatic cancer. ESMO Open. 2020;5:e000668. doi: 10.1136/esmoopen-2019-000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaban E.P., Mangu P.B., Yee N.S. Locally advanced unresectable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2017;13:265–269. doi: 10.1200/JOP.2016.017376. [DOI] [PubMed] [Google Scholar]
- 10.Balaban E.P., Mangu P.B., Khorana A.A., et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:2654–2668. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 11.Tempero M.A., Malafa M.P., Chiorean E.G., et al. Pancreatic adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw. 2019;17:202–210. doi: 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
- 12.Li C.P., Chao Y., Chi K.H., et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104. doi: 10.1016/s0360-3016(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 13.Loehrer PJ Sr., Feng Y., Cardenes H., et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cazacu I.M., Singh B.S., Saftoiu A., et al. Endoscopic ultrasound-guided treatment of pancreatic cancer. Curr Gastroenterol Rep. 2020;22:27. doi: 10.1007/s11894-020-00767-1. [DOI] [PubMed] [Google Scholar]
- 15.Jin Z., Du Y., Li Z., et al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314–320. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 16.Sun S., Xu H., Xin J., et al. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399–403. doi: 10.1055/s-2006-925253. [DOI] [PubMed] [Google Scholar]
- 17.Bhutani M.S., Cazacu I.M., Luzuriaga Chavez A.A., et al. Novel EUS-guided brachytherapy treatment of pancreatic cancer with phosphorus-32 microparticles: first United States experience. VideoGIE. 2019;4:223–225. doi: 10.1016/j.vgie.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhutani M.S., Klapman J.B., Tuli R., et al. An open-label, single-arm pilot study of EUS-guided brachytherapy with phosphorus-32 microparticles in combination with gemcitabine +/- nab-paclitaxel in unresectable locally advanced pancreatic cancer (OncoPaC-1): Technical details and study protocol. Endosc Ultrasound. 2020;9:24–30. doi: 10.4103/eus.eus_44_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross P., Hendlisz A., Ajithkumar T., et al. PanCO: Updated results of an open-label, single-arm pilot study of OncoSil P-32 Microparticles in unresectable locally advanced pancreatic adenocarcinoma (LAPC) with gemcitabine + nab-paclitaxel or FOLFIRINOX chemotherapy. Ann Oncol. 2020;31(suppl 3):S232. [Google Scholar]
- 20.Gholami Y.H., Wilson N., James D., et al. Toward personalized dosimetry with (32)P microparticle therapy for advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2017;99:1029–1038. doi: 10.1016/j.ijrobp.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Ye C., Sadula A., Ren S., et al. The prognostic value of CA19-9 response after neoadjuvant therapy in patients with pancreatic cancer: a systematic review and pooled analysis. Cancer Chemother Pharmacol. 2020;86:731–740. doi: 10.1007/s00280-020-04165-2. [DOI] [PubMed] [Google Scholar]
- 22.Wahl R.L., Jacene H., Kasamon Y., et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad S.S., Crittenden M.R., Tran P.T., et al. Clinical development of novel drug-radiotherapy combinations. Clin Cancer Res. 2019;25:1455–1461. doi: 10.1158/1078-0432.CCR-18-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma R.A., Plummer R., Stock J.K., et al. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016;13:627–642. doi: 10.1038/nrclinonc.2016.79. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein D., Spry N., Cummins M.M., et al. The GOFURTGO Study: AGITG phase II study of fixed dose rate gemcitabine-oxaliplatin integrated with concomitant 5FU and 3-D conformal radiotherapy for the treatment of localised pancreatic cancer. Br J Cancer. 2012;106:61–69. doi: 10.1038/bjc.2011.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passardi A., Scarpi E., Neri E., et al. Chemoradiotherapy (Gemox plus helical tomotherapy) for unresectable locally advanced pancreatic cancer: a phase II study. Cancers (Basel) 2019;11:663. doi: 10.3390/cancers11050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conroy T., Paillot B., Francois E., et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23:1228–1236. doi: 10.1200/JCO.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K., Iwashita T., Uemura S., et al. A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget. 2017;8:111346–111355. doi: 10.18632/oncotarget.22795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philip P.A., Lacy J., Portales F., et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285–294. doi: 10.1016/S2468-1253(19)30327-9. [DOI] [PubMed] [Google Scholar]
- 30.Ballehaninna U.K., Chamberlain R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halm U., Schumann T., Schiefke I., et al. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer. 2000;82:1013–1016. doi: 10.1054/bjoc.1999.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stemmler J., Stieber P., Szymala A.M., et al. Are serial CA 19-9 kinetics helpful in predicting survival in patients with advanced or metastatic pancreatic cancer treated with gemcitabine and cisplatin? Onkologie. 2003;26:462–467. doi: 10.1159/000072980. [DOI] [PubMed] [Google Scholar]
- 33.Yang G.Y., Malik N.K., Chandrasekhar R., et al. Change in CA 19-9 levels after chemoradiotherapy predicts survival in patients with locally advanced unresectable pancreatic cancer. J Gastrointest Oncol. 2013;4:361–369. doi: 10.3978/j.issn.2078-6891.2013.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko A.H., Hwang J., Venook A.P., et al. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer. 2005;93:195–199. doi: 10.1038/sj.bjc.6602687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macedo F.I., Ryon E., Maithel S.K., et al. Survival outcomes associated with clinical and pathological response following neoadjuvant FOLFIRINOX or gemcitabine/nab-paclitaxel chemotherapy in resected pancreatic cancer. Ann Surg. 2019;270:400–413. doi: 10.1097/SLA.0000000000003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurent L., Sefrioui D., Bignon A.L., et al. CA19.9 decrease >15% is a predictor of favourable outcome in patients treated for advanced pancreatic carcinoma: analysis of two independent cohorts. HPB (Oxford) 2019;21:582–588. doi: 10.1016/j.hpb.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Demir I.E., Jager C., Schlitter A.M., et al. R0 versus R1 resection matters after pancreaticoduodenectomy, and less after distal or total pancreatectomy for pancreatic cancer. Ann Surg. 2018;268:1058–1068. doi: 10.1097/SLA.0000000000002345. [DOI] [PubMed] [Google Scholar]
- 38.Gemenetzis G., Groot V.P., Blair A.B., et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;270:340–347. doi: 10.1097/SLA.0000000000002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.