Abstract
Background
Lung cancer with related pericardial effusion is not rare. Intervention is a crucial step for symptomatic effusion. It is unknown, however, whether the different invasive interventions for pericardial effusion result in different survival outcomes. This study analyzed the clinical characteristics and prognostic factors for patients with non-small-cell lung cancer (NSCLC) who have undergone different procedures.
Methods
From January 2006 to June 2018, we collected data from patients with NSCLC who have received invasive intervention for pericardial effusions. The patients were divided into three categories: simple pericardiocentesis, balloon pericardiotomy, and surgical pericardiectomy. Kaplan–Meier curve and log-rank test were used to analyze the pericardial effusion recurrence-free survival (RFS) and overall survival (OS).
Results
A total of 244 patients were enrolled. Adenocarcinoma (83.6%) was the major NSCLC subtype. Invasive intervention, including simple pericardiocentesis, balloon pericardiotomy, and surgical pericardiectomy, had been carried out on 52, 170, and 22 patients, respectively. The 1-year RFS rates in simple pericardiocentesis, balloon pericardiotomy, and surgical pericardiectomy were 19.2%, 31.2%, and 31.8%, respectively (P = 0.128), and the median RFS was 1.67, 5.03, and 8.32 months, respectively (P = 0.008). There was no significant difference in OS, however, with the median OS at 1.67, 6.43, and 8.32 months, respectively (P = 0.064). According to the multivariable analysis, the gravity in pericardial fluid analysis, receiving systemic therapy after pericardial effusion, and the time period from stage IV lung cancer to the presence of pericardial effusion were independent prognostic factors for pericardial effusion RFS and OS.
Conclusions
Patients who have undergone simple pericardiocentesis alone for the management of NSCLC-related pericardial effusion have lower 1-year RFS rates than those who have undergone balloon pericardiotomy and surgical pericardiectomy. Therefore, balloon pericardiotomy and surgical pericardiectomy should be carried out for patients with NSCLC-related pericardial effusion if tolerable.
Key words: lung adenocarcinoma, pericardial effusion, pericardiocentesis, pericardiotomy, pericardiectomy
Highlights
-
•
This is the first study to compare the three common procedures to manage NSCLC-related pericardial effusion.
-
•
Simple pericardiocentesis group had lower 1-year RFS rate than balloon pericardiotomy or surgical pericardiectomy group.
-
•
Surgical pericardiectomy as management demonstrated an improving OS trend.
Introduction
Malignancy-related pericardial effusion is not rare, occurring in about 10% of all cancer patients,1,2 and with lung cancer as the most common cause.3, 4, 5, 6, 7 The mechanism of malignancy-related pericardial effusion includes cancer metastasis, cancer direct invasion, and paramalignant causes. These paramalignant etiologies include pericardial effusion due to inflammation, lymphatic or venous drainage obstruction, or treatment-related effusion.8, 9, 10, 11, 12, 13
Intervention procedures are crucial steps for preventing death due to pericardial tamponade, and different procedures show varying success rates, safety, and pericardial effusion recurrence rates.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Nowadays, simple pericardiocentesis, balloon pericardiotomy, or surgical pericardiectomy are often carried out on patients harboring symptomatic pericardial effusion. Pericardiocentesis is often carried out by pigtail insertion to the pericardial space, and it can be echo-guided, computed tomography-guided, or fluoroscopy-guided. Pericardiocentesis can immediately relieve the symptomatic pericardial effusion.28 In malignancy-related pericardial effusion, some patients receive pericardial-pleural window creation to prevent the re-accumulation of pericardial effusion. Percutaneous balloon pericardiotomy is typically carried out in the catheterization room under fluoroscopy guidance, with the procedure replacing the pigtail catheter through an additional balloon-dilating catheter by guidewire.29 Pericardiectomy can be carried out by open surgery or thoracoscopic surgery, and the surgical pericardiotomy can often create a larger pericardial window size than pericardiotomy.15
It is unknown whether the different invasive interventions for pericardial effusion result in different survival outcomes in patients with non-small-cell lung cancer (NSCLC). This study aimed to analyze the clinical characteristics and prognostic factors for patients with NSCLC who have undergone simple pericardiocentesis, balloon pericardiotomy, or surgical pericardiectomy for symptomatic pericardial effusion.
Methods
Study design, setting, and participants
This investigation was approved by the National Taiwan University Hospital Research Ethics Committee (201907060RINB) which agreed waiving the informed consent. In this retrospective study, patients with NSCLC aged ≥20 years who have undergone simple pericardiocentesis, balloon pericardiotomy, or surgical pericardiectomy from 1 January 2006 to 31 June 2018 at our institution were identified. There was no formal protocol of managing NSCLC-related pericardial effusion in our hospital. The choice of treatment depended on the physicians’ decision. The exclusion criteria were as follows: patients of other cancers with lung metastasis, patients who underwent pericardiocentesis during cardiopulmonary resuscitation, patients with other clear etiology of pericardial effusion, patients who did not receive cancer treatment and instead received palliative care, patients who did not undergo invasive procedures, and patients with small-cell lung cancer.
Data collection
The medical records data were reviewed, including age at diagnosis, gender, smoking history, comorbidities, driver mutation type, intervention procedures type, procedure date, date stage IV NSCLC diagnosed, pericardial effusion recurrence date, date of mortality, NSCLC treatment regimens, initial drainage amount of pericardial effusion, cardiac echography signs (right atrium collapse and right ventricle collapse signs), pericardial fluid analysis (including gravity, total nucleated cell, red blood cell count, and cytology), and pericardial effusion culture. Chest computed tomography, brain imaging (computed tomography or magnetic resonance imaging), and whole body bone scans were carried out for lung cancer staging. The lung cancer staging followed the eight edition of the TNM (tumour–node–metastasis) stage classification for lung cancer.30
Survival analyses
Patients were enrolled for pericardial effusion-related recurrence-free survival (RFS) and overall survival (OS) analysis. Information on survival was obtained through active follow-up based on the verification of each patient’s vital status. Pericardial effusion-related RFS was defined as the duration from the date of first identification of pericardial effusion until the date of pericardial effusion recurrence or death. We used 1 year as the cut-off point for RFS. OS was defined as from the date of first identification of pericardial effusion until the date of death or the last follow-up.
Statistical analyses
Continuous variables were presented as median with ranges, and categorical variables were presented as percentages of the group from which they were derived. Continuous variables were compared using the Kruskal–Wallis test. Categorical variables were compared using the chi-square test or Fisher’s exact test. Kaplan–Meier curves were plotted for OS and RFS at 1 year, and the log-rank test was used to determine statistical significance. Cox proportional-hazards regression was used for covariate analysis to determine the hazard ratio of clinical factors and RFS and OS. Spearman’s correlation was used to analyze the correlation between continuous variables. A P value <0.05 was considered significant. All analyses were carried out using R version 4.0.3 (R core team 2020, Vienna, Austria).
Results
Participants’ characteristics
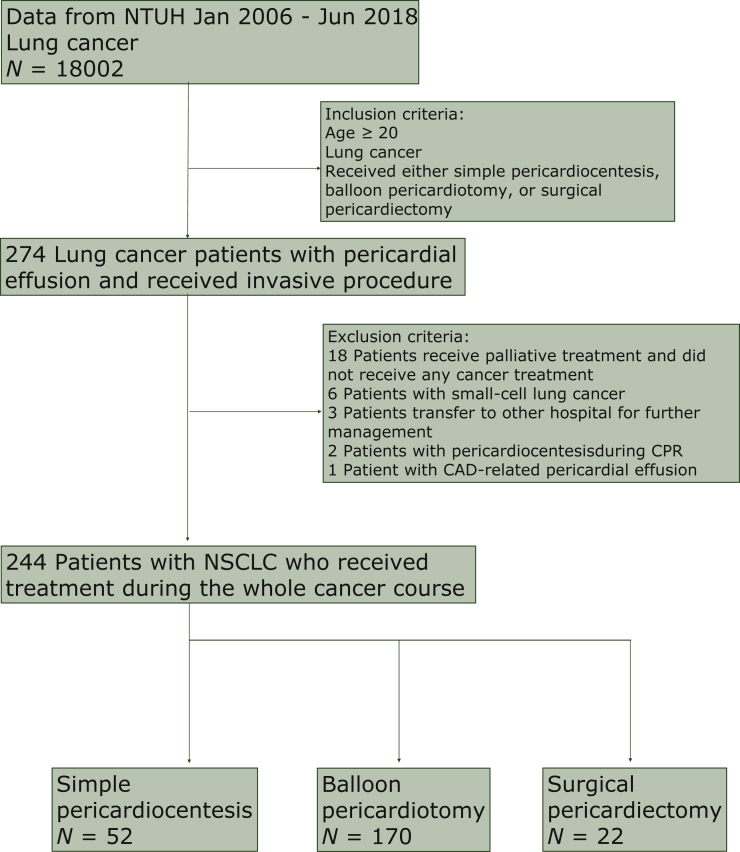
From 1 January 2006 to 31 June 2018, a total of 18 002 patients in our institution had the lung cancer code. A total of 274 patients met the inclusion criteria. After excluding those who met the exclusion criteria, a total of 244 patients were enrolled in the study for further analysis (Figure 1). Some 52 patients received simple pericardiocentesis treatment alone, 170 received balloon pericardiotomy treatment, and 22 received surgical pericardiectomy (Table 1). Among the surgical pericardiectomy: 9 patients received video-assisted thoracic surgery, 12 patients received thoracotomy, and 1 patient received the subxiphoid approach (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100354).
Figure 1.
Flowchart of the patient enrollment in this study.
CAD, coronary artery disease; CPR, cardiopulmonary resuscitation; NSCLC, non-small-cell lung cancer; NTUH, National Taiwan University Hospital.
Table 1.
The demographic data of patients in NSCLC with pericardial effusion, classified by different initial procedures for pericardial effusion
| Characteristic | Total N = 244 |
Simple pericardiocentesis N = 52 |
Balloon pericardiotomy N = 170 |
Surgical pericardiectomy N = 22 |
P value |
|---|---|---|---|---|---|
| Age, median (range), years | 58.9 (20-85.9) | 56.8 (27.2-76.8) | 59 (20 -85.9) | 59.9 (31.7-79.4) | 0.504 |
| Male gender, n (%) | 135 (55.3) | 31 (59.6) | 94 (55.3) | 10 (45.5) | 0.532 |
| Smoking, n (%) | 97 (40.9) | 21 (41.1) | 66 (40.2) | 10 (45.4) | 0.912 |
| Adenocarcinoma, n (%) | 204 (83.6) | 42 (80.8) | 142 (83.5) | 20 (90.9) | 0.642 |
| Driver mutation | 0.104 | ||||
| Wild type | 95 (49.5) | 17 (45.9) | 69 (51.1) | 9 (52.9) | |
| EGFR exon 21 L858R mutation | 46 (24.0) | 10 (29.4) | 34 (25.3) | 2 (11.8) | |
| EGFR exon 19 deletion | 32 (16.7) | 6 (16.2) | 23 (17.0) | 3 (17.6) | |
| EGFR exon 20 insertion | 2 (1.0) | 0 (0) | 0 (0) | 2 (11.8) | |
| Other uncommon mutation | 7 (3.6) | 2 (5.4) | 4 (3.0) | 1 (5.9) | |
| Anaplastic lymphoma kinase (ALK) mutation | 6 (3.1) | 1 (2.7) | 5 (3.7) | 0 (0) | |
| ROS1 mutation | 3 (1.6) | 0 | 2 | 1 (5.9) | |
| RET | 1 (0.5) | 1 | 0 | 0 (0) | |
| Lung cancer presentation with pericardial effusion initially, n (%) | 57 (23.4) | 7 (13.5) | 44 (25.9) | 6 (27.3) | 0.156 |
| Systemic therapy before pericardial effusion, n (%) | 172 (70.5) | 41 (78.8) | 117 (68.8) | 14 (63.6) | 0.297 |
| Chemotherapy, n (%) | 140 (57.3) | 34 (65.4) | 93 (54.7) | 13 (59.0) | 0.383 |
| TKI, n (%) | 100 (41.0) | 23 (44.2) | 69 (40.6) | 8 (36.4) | 0.813 |
| Thoracic RT, n (%) | 67 (27.5) | 20 (38.5) | 40 (23.5) | 7 (31.8) | 0.095 |
| Immunotherapy, n (%) | 4 (1.6) | 1 (1.9) | 3 (1.8) | 0 (0) | 1.000 |
| Anti-VEGF, n (%) | 6 (2.5) | 2 (3.8) | 3 (1.8) | 1 (4.5) | 0.290 |
| Treatment lines before pericardial effusion | 0.363 | ||||
| 0, n (%) | 72 (29.5) | 10 (19.2) | 54 (31.8) | 8 (36.4) | |
| 1, n (%) | 69 (28.3) | 15 (28.8) | 49 (28.8) | 5 (22.7) | |
| ≥2, n (%) | 103 (42.2) | 27 (51.9) | 67 (39.4) | 9 (40.9) | |
| Systemic therapy after pericardial effusion, n (%) | 175 (71.7) | 25 (48.1) | 134 (78.8) | 16 (72.7) | <0.001 |
| Chemotherapy, n (%) | 138 (56.6) | 20 (38.5) | 105 (61.8) | 13 (59.1) | 0.012 |
| TKI, n (%) | 103 (42.2) | 16 (30.8) | 76 (44.7) | 11 (50.0) | 0.145 |
| Thoracic RT, n (%) | 11 (4.5) | 0 (0) | 8 (4.7) | 3 (13.6) | 0.035 |
| Immunotherapy, n (%) | 11 (4.5) | 1 (1.9) | 9 (5.3) | 1 (4.5) | 0.602 |
| Anti-VEGF, n (%) | 13 (5.3) | 1 (1.9) | 11 (6.5) | 1 (4.5) | 0.412 |
| Treatment lines after pericardial effusion | <0.001 | ||||
| 0, n (%) | 69 (28.3) | 27 (51.9) | 36 (21.2) | 6 (27.3) | |
| 1, n (%) | 79 (32.4) | 10 (19.2) | 65 (38.2) | 4 (18.2) | |
| ≧2, n (%) | 96 (39.3) | 15 (28.8) | 69 (40.6) | 12 (54.5) | |
| Time from stage IV lung cancer to pericardial effusion, median (range), months | 4.1 (0-173.8) | 5.3 (0-106.4) | 3.9 (0-173.8) | 2.0 (0-89.9) | 0.279 |
Data are presented as median (range), number (%).
EGFR, epidermal growth factor receptor; RT, radiation therapy; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
The median age of the patients was 58.9 years. One hundred and nine patients were female (44.7%). Ninety-seven patients were smokers (40.9%). Two hundred and four patients had adenocarcinoma (83.6%). A total of 192 patients had undergone tests for driver mutation, and 95 patients exhibited the wild type (49.5%). Fifty-seven patients presented with symptomatic pericardial effusion during lung cancer diagnosis (23.4%). One hundred and seventy-two patients had received systemic cancer therapy before the pericardial effusion event (70.5%). A total of 69 patients received their first line of systemic treatment before the pericardial effusion event (28.3%), and 103 patients received more than two lines of systemic treatment before the pericardial effusion event (42.2%). One hundred and seventy-five patients received systemic therapy after the pericardial effusion event (71.7%).
There was no difference in age, gender, smoking status, lung cancer cell type, driver mutations, and systemic therapy before the precordial procedures among the three groups (Table 1). There was, however, a significant difference in the systemic therapy after pericardial procedures among the three groups (P < 0.001) (Table 1).
Procedure-related complications
Among the patients who received simple pericardiocentesis, there was no procedure-related complication. Among the patients who received balloon pericardiotomy, five patients (2.9%) encountered supraventricular tachycardia and two patients (1.2%) encountered pneumothorax after the procedure. Among the patients who received surgical pericardiectomy, one patient (4.5%) had surgical wound infection after the surgery; the infection improved after intravenous antibiotic treatment. There was no procedure-related mortality (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100354).
Factors associated with RFS and OS
There were 23 patients (9.4%) with recurrent pericardial effusion requiring secondary intervention, 174 patients who encountered pericardial effusion recurrence or death (71.3%) at the 1-year follow-up period, and 236 patients who encountered pericardial effusion recurrence or death (96.7%) by the end of the follow-up period.
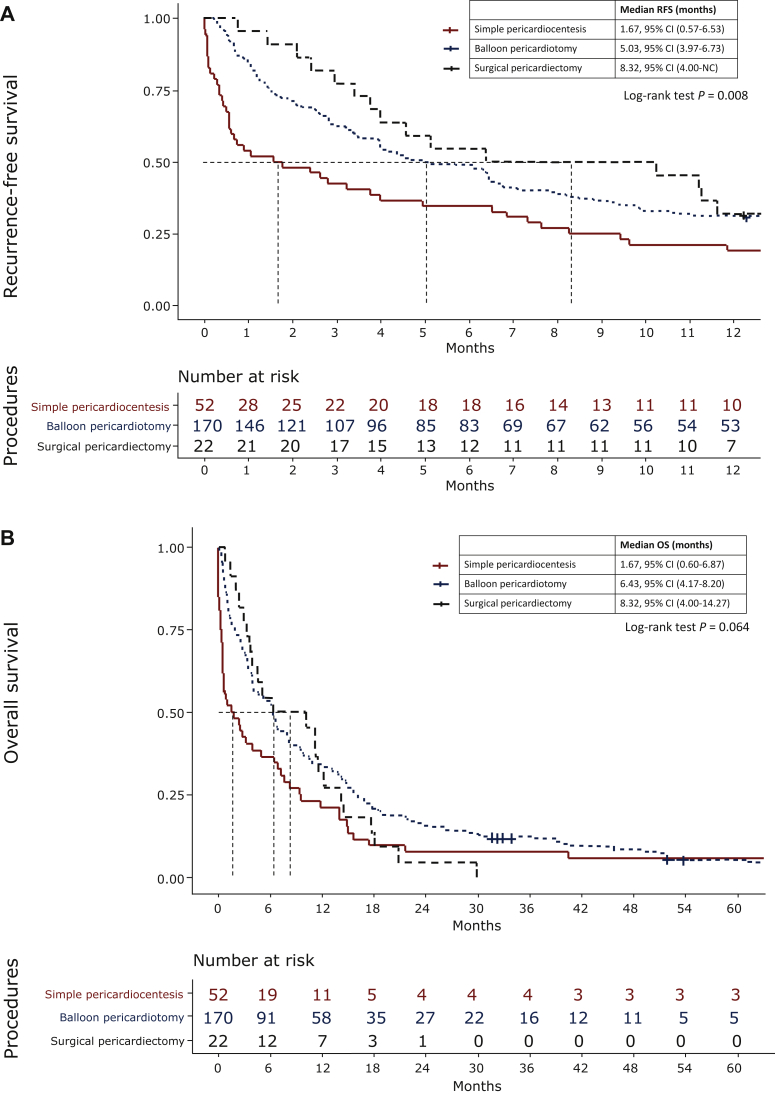
Patients who received simple pericardiocentesis had a higher 1-year RFS rate than those who received pericardial window creation either by balloon dilatation or surgical intervention. The 1-year RFS rates in simple pericardiocentesis, balloon pericardiotomy, and surgical pericardiectomy were 19.2%, 31.2%, and 31.8%, respectively (P = 0.128), whereas the median RFS was 1.67 months, 5.03 months, and 8.32 months, respectively (P = 0.008). There was no significant difference in OS, however, with the median OS at 1.67 months (95% CI 0.67-6.87 months), 6.43 months [95% confidence interval (CI) 4.17-8.20 months], and 8.32 months (95% CI 4.00-14.27 months), respectively (P = 0.064) (Figure 2).
Figure 2.
(A) Pericardial effusion recurrence-free survival of patients with non-small-cell lung cancer who have undergone simple pericardiocentesis, balloon pericardiotomy, and surgical pericardiectomy. (B) The overall survival of patients with non-small-cell lung cancer who have undergone simple pericardiocentesis, balloon pericardiotomy, and surgical pericardiectomy.
CI, confidence interval; NC, could not be calculated; OS, overall survival; RFS, recurrence-free survival.
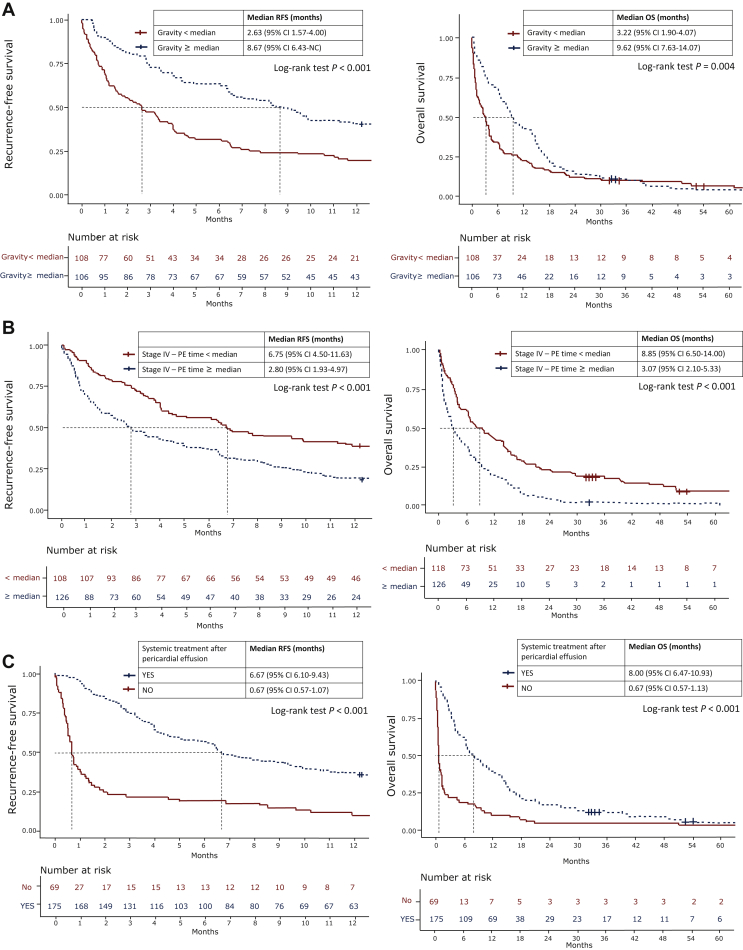
The analysis for the pericardial effusion RFS and OS using the Cox regression model is shown in Supplementary Tables S3 and S4, available at https://doi.org/10.1016/j.esmoop.2021.100354 and Figure 3. In the univariate analysis, systemic treatment before pericardial effusion, systemic therapy after pericardial effusion, the gravity of pericardial effusion, the total protein of pericardial effusion, the intervention procedures (simple pericardiocentesis, balloon pericardiotomy, or surgical pericardiectomy), and the period from stage IV lung cancer to the presence of pericardial effusion were associated with pericardial effusion RFS (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100354). According to the Kaplan–Meier method, the median pericardial effusion RFS in patients with higher gravity in the pericardial fluid analysis (≥median) and in patients with lower gravity in the pericardial fluid analysis (<median) was 8.67 and 2.63 months, respectively (P < 0.001) (Figure 3A); 6.75 and 2.80 months (P lt; 0.001) (Figure 3B) in patients with longer time from stage IV NSCLC to the detection of pericardial effusion (≥median) and patients with a shorter time from stage IV NSCLC to the detection of pericardial effusion (<median), respectively; and 6.67 and 0.67 months (P < 0.001) (Figure 3C) in patients who received systemic therapy and who did not receive systemic therapy after the detection of pericardial effusion, respectively.
Figure 3.
The median RFS and OS in: (A) patients who received and who did not receive systemic treatment after pericardial effusion; (B) patients with specific gravity in fluid analysis ≥ median and specific gravity < median; (C) patients with pericardial effusion time ≥ median and stage IV to pericardial effusion time < median.
CI, confidence interval; NC, could not be calculated; OS, overall survival; PE, pericardial effusion; RFS, recurrence-free survival.
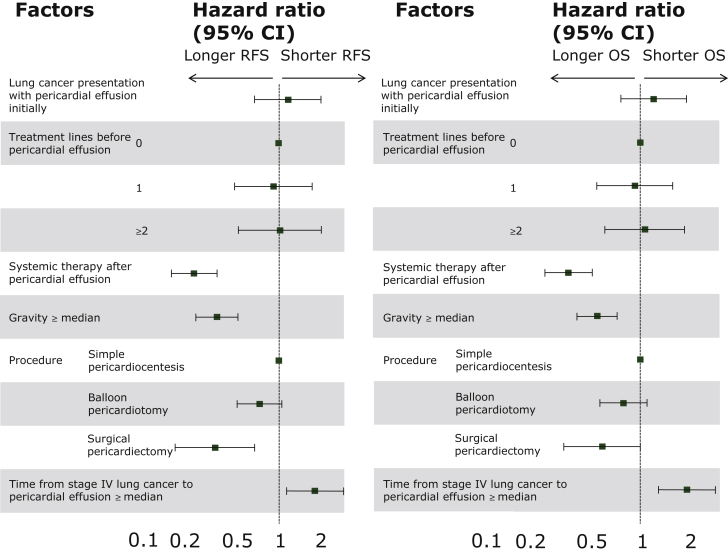
In the multivariable analysis using the Cox proportional hazard model, we found that those who received systemic therapy after pericardial effusion, the gravity in pericardial fluid, those patients who received surgical pericardiectomy, and the period from stage IV lung cancer to the detection of pericardial effusion were independent prognostic factors for the pericardial effusion RFS (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100354 and Figure 4).
Figure 4.
Multivariable analysis of pericardial effusion recurrence-free survival and overall survival, represented by a forest plot.
CI, confidence interval; OS, overall survival; RFS, recurrence-free survival.
In the univariate analysis for OS, presentation with pericardial effusion at the time of lung cancer diagnosis, systemic treatment before pericardial effusion, systemic therapy after pericardial effusion, the gravity of pericardial effusion, and the period from stage IV lung cancer to the detection of pericardial effusion were associated with pericardial effusion OS (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100354). The median OS in patients with higher gravity in the pericardial fluid analysis (≥median) and in patients with lower gravity in the pericardial fluid analysis (<median) was 9.62 and 3.22 months, respectively (P = 0.004) (Figure 3A); 8.85 and 3.07 months (P < 0.001) (Figure 3B) in patients with longer time from stage IV NSCLC to the detection of pericardial effusion (≥median) and patients with a shorter time from stage IV NSCLC to the presence of pericardial effusion (<median), respectively; and 8.00 and 0.67 months (P < 0.001) (Figure 3C) in patients who received systemic therapy and who did not receive systemic therapy after the detection of pericardial effusion, respectively.
Those who received systemic therapy after pericardial effusion, the gravity in pericardial fluid analysis, and the period from stage IV lung cancer to the presence of pericardial effusion were independent prognostic factors for OS in multivariable analysis. Moreover, using surgical pericardiectomy for pericardial effusion in NSCLC demonstrated an improving OS trend (P = 0.05) (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100354, Figure 4).
We found that the specific gravity in pericardial effusion had a significant relationship with RFS and OS. Further analysis showed that the specific gravity in pericardial effusion showed a strong positive correlation with the total protein (r = 0705, P < 0.001) and very weak correlation with the total nucleated cell count (r = 0.254, P = 0.002) and the absolute lymphocyte cell count (r = 0.191, P = 0.048) in pericardial effusion. There was no correlation between the specific gravity and the total neutrophil count in pericardial effusion (Supplementary Figure S1 and Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2021.100354).
Discussion
In this study, we found that patients with NSCLC who have undergone simple pericardiocentesis had lower 1-year RFS rates. Surgical pericardiectomy as the management of malignancy-related pericardial effusion in NSCLC showed a trend of improving OS; however, further study is warranted to verify this result. To the best of our knowledge, the comparison of these three procedures has not been reported in a single study before, especially among patients with NSCLC.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Labbe et al.27 retrospectively compared pericardiotomy and pericardiocentesis, and a higher recurrence rate was noted in the pericardiocentesis group. The above study, however, included patients other than those with lung cancer, and it did not compare the recurrence rate with surgical pericardiectomy. Our results suggest not performing simple pericardiocentesis alone as the management of malignancy-related pericardial effusion in NSCLC. Patients who had undergone surgical pericardiectomy did not have any recurrent episodes. Moreover, our results demonstrated an improving OS trend for this group. Thus, we suggest that surgical pericardiectomy could be carried out for malignancy-related pericardial effusion among patients with NSCLC, if the patient’s clinical condition could tolerate the procedure. In particular, the thoracoscopic pericardial window procedure should be considered, because it is safe and less invasive.31 We show our suggested protocol for managing NSCLC-related pericardial effusion in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100354.
In our study, patients who have undergone lung cancer therapy before pericardial effusion exhibited a poorer outcome. Results from patients who received more lines of cancer therapies may also indicate that those patients had a longer period from lung cancer diagnosis to pericardial effusion development. This finding is similar to a study by Okamoto et al.,32 reporting that an interval from cancer diagnosis to pericardial effusion >200 days in patients with primary lung cancers was a poor prognosis factor (P = 0.005).
Our study showed that higher pericardial fluid specific gravity and protein concentration showed longer 1-year RFS; moreover, patients with higher pericardial effusion specific gravity had longer RFS and OS. The specific gravity was highly related with the protein level in pericardial effusion. Furthermore, the demographic data of the two groups divided by the pericardial fluid gravity showed nearly no difference except for tyrosine kinase inhibitor usage before the detection of pericardial effusion and the treatment lines after the detection of pericardial effusion (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2021.100354). A specific gravity of pericardial effusion >1.016 is generally attributed to an exudate with a sensitivity of 90%.33,34 In addition, cancer-related pericardial effusion, the gravity level, and protein level are usually elevated.34 No previous studies, however, have discussed the association between the gravity level and the increasing rate of pericardial effusion or mortality. Our study showed that the specific gravity has a weak association with the total nucleated cell count (Supplementary Figure S1B, available at https://doi.org/10.1016/j.esmoop.2021.100354) and the absolute lymphocyte cell count (Supplementary Figure S1C, available at https://doi.org/10.1016/j.esmoop.2021.100354), but not the absolute neutrophil count (Supplementary Figure S1D, available at https://doi.org/10.1016/j.esmoop.2021.100354). Oyakawa et al.35 also reported that lower neutrophil and higher lymphocyte counts in malignant pericardial effusions were associated with favorable effusion failure-free survival at 1 month. This result may be related to tumor-infiltrating lymphocytes.36, 37, 38 The increased specific gravity and total protein level may have resulted from more infiltrative lymphocytes in the malignancy-related pericardial effusion. Hence, the specific gravity of the pericardial fluid might be beneficial in the prediction of pericardial effusion RFS and OS in NSCLC. Nevertheless, further studies may be needed to confirm this hypothesis.
In East Asia, including Taiwan, the most commonly detected mutations are deletion in exon 19 (22.1%) and L858R point mutation in exon 21 (20.9%).39 In pericardial effusion, however, we found that the incidence of L858R point mutation in exon 21 (24%) was higher than the deletion in exon 19 (16.7%) (Table 1). This finding is consistent with the previous findings in pleural effusion among patients with NSCLC.40 The case numbers of anaplastic lymphoma kinase mutations and ROS1 mutations in our dataset were too small to analyze.
This study has some limitations. First, it had the nature of a retrospective study design and potential bias. The study duration lasted for 13 years, and lung cancer treatments had a rapid evolution during these years. Second, we did not include patients who had not undergone any invasive procedures harboring lung cancer malignancy-related pericardial effusion. Third, there were no episodes of relapsed pericardial effusion in the surgical pericardiectomy group; thus, it was impossible to analyze the results by competing risk. Fourth, most of the patients died before recurrence, and this may also indicate that patients with pericardial effusion may have a poor survival time after the event. Fifth, most of the patients who have undergone surgical pericardiectomy have specific clinical considerations.
Conclusions
In NSCLC-related pericardial effusion, patients who have undergone simple pericardiocentesis alone have lower 1-year RFS rates than those who have undergone balloon pericardiotomy or surgical pericardiectomy. Surgical pericardiectomy as the management for pericardial effusion in NSCLC demonstrated an improving OS trend. Receiving systemic therapy after pericardial effusion and the higher gravity in pericardial fluid analysis were associated with favorable outcomes. A longer period from the diagnosis of stage IV NSCLC to the detection of pericardial effusion was related to unfavorable outcomes.
Acknowledgements
None.
Funding
None declared.
Disclosure
JYS has served as an advisory board member from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Chugai Pharma, Ono Pharmaceutical, Takeda, CStone Pharmaceuticals, Janssen, and Bristol Myers Squibb and received speaking honoraria from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Chugai Pharma, Ono Pharmaceutical, and Bristol Myers Squibb, as well as grant from Roche. SGW has received speaking honoraria from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Chugai, and Roche. All remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Maisch B., Ristic A., Pankuweit S. Evaluation and management of pericardial effusion in patients with neoplastic disease. Prog Cardiovasc Dis. 2010;53(2):157–163. doi: 10.1016/j.pcad.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A.K., Crake T., Manisty C., Westwood M. Pericardial disease in cancer patients. Curr Treat Options Cardiovasc Med. 2018;20(7):60. doi: 10.1007/s11936-018-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Enrique C., Nunez-Gil I.J., Viana-Tejedor A., et al. Cause and long-term outcome of cardiac tamponade. Am J Cardiol. 2016;117(4):664–669. doi: 10.1016/j.amjcard.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Cheong X.P., Law L.K.P., Seow S.C., et al. Causes and prognosis of symptomatic pericardial effusions treated by pericardiocentesis in an Asian Academic Medical Centre. Singapore Med J. 2020;61(3):137–141. doi: 10.11622/smedj.2019065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takayama T., Okura Y., Okada Y., et al. Characteristics of neoplastic cardiac tamponade and prognosis after pericardiocentesis: a single-center study of 113 consecutive cancer patients. Int J Clin Oncol. 2015;20(5):872–877. doi: 10.1007/s10147-015-0794-7. [DOI] [PubMed] [Google Scholar]
- 6.Cornily J.C., Pennec P.Y., Castellant P., et al. Cardiac tamponade in medical patients: a 10-year follow-up survey. Cardiology. 2008;111(3):197–201. doi: 10.1159/000121604. [DOI] [PubMed] [Google Scholar]
- 7.Lam K.Y., Dickens P., Chan A.C. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117(10):1027–1031. [PubMed] [Google Scholar]
- 8.Imazio M., Demichelis B., Parrini I., et al. Relation of acute pericardial disease to malignancy. Am J Cardiol. 2005;95(11):1393–1394. doi: 10.1016/j.amjcard.2005.01.094. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi K., Miyaoka E., Asamura H., et al. Modern surgical results of lung cancer involving neighboring structures: a retrospective analysis of 531 pT3 cases in a Japanese Lung Cancer Registry Study. J Thorac Cardiovasc Surg. 2012;144(2):431–437. doi: 10.1016/j.jtcvs.2012.05.069. [DOI] [PubMed] [Google Scholar]
- 10.Posner M.R., Cohen G.I., Skarin A.T. Pericardial disease in patients with cancer. The differentiation of malignant from idiopathic and radiation-induced pericarditis. Am J Med. 1981;71(3):407–413. doi: 10.1016/0002-9343(81)90168-6. [DOI] [PubMed] [Google Scholar]
- 11.Sakakura N., Mori S., Ishiguro F., et al. Subcategorization of resectable non-small cell lung cancer involving neighboring structures. Ann Thorac Surg. 2008;86(4):1076–1083. doi: 10.1016/j.athoracsur.2008.06.034. discussion 1083. [DOI] [PubMed] [Google Scholar]
- 12.Boerma M., Sridharan V., Mao X.W., et al. Effects of ionizing radiation on the heart. Mutat Res. 2016;770(Pt B):319–327. doi: 10.1016/j.mrrev.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ning M.S., Tang L., Gomez D.R., et al. Incidence and predictors of pericardial effusion after chemoradiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99(1):70–79. doi: 10.1016/j.ijrobp.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virk S.A., Chandrakumar D., Villanueva C., Wolfenden H., Liou K., Cao C. Systematic review of percutaneous interventions for malignant pericardial effusion. Heart. 2015;101(20):1619–1626. doi: 10.1136/heartjnl-2015-307907. [DOI] [PubMed] [Google Scholar]
- 15.Marcy P.Y., Bondiau P.Y., Brunner P. Percutaneous treatment in patients presenting with malignant cardiac tamponade. Eur Radiol. 2005;15(9):2000–2009. doi: 10.1007/s00330-004-2611-y. [DOI] [PubMed] [Google Scholar]
- 16.Galli M., Politi A., Pedretti F., Castiglioni B., Zerboni S. Percutaneous balloon pericardiotomy for malignant pericardial tamponade. Chest. 1995;108(6):1499–1501. doi: 10.1378/chest.108.6.1499. [DOI] [PubMed] [Google Scholar]
- 17.Vaitkus P.T., Herrmann H.C., LeWinter M.M. Treatment of malignant pericardial effusion. J Am Med Assoc. 1994;272(1):59–64. [PubMed] [Google Scholar]
- 18.Jeon H.W., Cho D.G., Park J.K., et al. Prognostic factors affecting survival of patients with cancer-related pericardial effusion managed by surgery. World J Surg Oncol. 2014;12:249. doi: 10.1186/1477-7819-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celik S., Lestuzzi C., Cervesato E., et al. Systemic chemotherapy in combination with pericardial window has better outcomes in malignant pericardial effusions. J Thorac Cardiovasc Surg. 2014;148(5):2288–2293. doi: 10.1016/j.jtcvs.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 20.McDonald J.M., Meyers B.F., Guthrie T.J., Battafarano R.J., Cooper J.D., Patterson G.A. Comparison of open subxiphoid pericardial drainage with percutaneous catheter drainage for symptomatic pericardial effusion. Ann Thorac Surg. 2003;76(3):811–815. doi: 10.1016/s0003-4975(03)00665-9. discussion 816. [DOI] [PubMed] [Google Scholar]
- 21.Patel N., Rafique A.M., Eshaghian S., et al. Retrospective comparison of outcomes, diagnostic value, and complications of percutaneous prolonged drainage versus surgical pericardiotomy of pericardial effusion associated with malignancy. Am J Cardiol. 2013;112(8):1235–1239. doi: 10.1016/j.amjcard.2013.05.066. [DOI] [PubMed] [Google Scholar]
- 22.Saltzman A.J., Paz Y.E., Rene A.G., et al. Comparison of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J Invasive Cardiol. 2012;24(11):590–593. [PMC free article] [PubMed] [Google Scholar]
- 23.El Haddad D., Iliescu C., Yusuf S.W., et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion. J Am Coll Cardiol. 2015;66(10):1119–1128. doi: 10.1016/j.jacc.2015.06.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petcu C.P., Droc I. The efficiency of surgical subxiphoid pericardial drainage and percutaneous pericardial drainage in pericardial effusions associated with cardiac tamponade. Chirurgia (Bucur) 2013;108(2):226–233. [PubMed] [Google Scholar]
- 25.Celik S., Celik M., Aydemir B., Tanrikulu H., Okay T., Tanrikulu N. Surgical properties and survival of a pericardial window via left minithoracotomy for benign and malignant pericardial tamponade in cancer patients. World J Surg Oncol. 2012;10:123. doi: 10.1186/1477-7819-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bischiniotis T.S., Lafaras C.T., Platogiannis D.N., Moldovan L., Barbetakis N.G., Katseas G.P. Intrapericardial cisplatin administration after pericardiocentesis in patients with lung adenocarcinoma and malignant cardiac tamponade. Hellenic J Cardiol. 2005;46(5):324–329. [PubMed] [Google Scholar]
- 27.Labbe C., Tremblay L., Lacasse Y. Pericardiocentesis versus pericardiotomy for malignant pericardial effusion: a retrospective comparison. Curr Oncol. 2015;22(6):412–416. doi: 10.3747/co.22.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler Y., Charron P., Imazio M., et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karam N., Patel P., deFilippi C. Diagnosis and management of chronic pericardial effusions. Am J Med Sci. 2001;322(2):79–87. doi: 10.1097/00000441-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Detterbeck F.C. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg. 2018;155(1):356–359. doi: 10.1016/j.jtcvs.2017.08.138. [DOI] [PubMed] [Google Scholar]
- 31.Sakanoue I., Hamakawa H., Okubo Y., et al. Efficacy and safety of thoracoscopic pericardial window in patients with pericardial effusions: a single-center case series. J Cardiothorac Surg. 2016;11(1):92. doi: 10.1186/s13019-016-0488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto H., Shinkai T., Yamakido M., Saijo N. Cardiac tamponade caused by primary lung cancer and the management of pericardial effusion. Cancer. 1993;71(1):93–98. doi: 10.1002/1097-0142(19930101)71:1<93::aid-cncr2820710115>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Meyers D.G., Meyers R.E., Prendergast T.W. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111(5):1213–1221. doi: 10.1378/chest.111.5.1213. [DOI] [PubMed] [Google Scholar]
- 34.Maisch B., Seferovic P.M., Ristic A.D., et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25(7):587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Oyakawa T., Muraoka N., Iida K., Kusuhara M., Naito T., Omae K. Characteristics of cellular composition in malignant pericardial effusion and its association with the clinical course of carcinomatous pericarditis. Jpn J Clin Oncol. 2018;48(3):291–294. doi: 10.1093/jjco/hyx187. [DOI] [PubMed] [Google Scholar]
- 36.Paijens S.T., Vledder A., de Bruyn M., Nijman H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18(4):842–859. doi: 10.1038/s41423-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu P., Fu Y.X. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86(3):231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 38.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y., Au J.S., Thongprasert S., et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9(2):154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S.G., Gow C.H., Yu C.J., et al. Frequent epidermal growth factor receptor gene mutations in malignant pleural effusion of lung adenocarcinoma. Eur Respir J. 2008;32(4):924–930. doi: 10.1183/09031936.00167407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.