Abstract
Short-chain fatty acids (SCFAs), the end products of fermentation carried out by the intestinal microbiota, were demonstrated to produce anti-oxidant and anti-inflammatory effects. Butyrate, part of the SCFAs, also shows the same effect. Renal ischemia/reperfusion (I/R) injury commonly occurs in renal transplantation and is often accompanied by oxidative stresses and inflammatory responses. In this study, we explore butyrate effect on renal I/R injury and SCFAs changes in renal transplant. Male Sprague-Dawley rats were pretreated with butyrate as research, and underwent the surgery of renal ischemia for 45 min followed by reperfusion. 90 rats were randomly divided into 3 groups (n=30 each group): (1) sham-operated group; (2) butyrate-treated group; (3) control group. The samples of blood and renal were collected immediately for further studies. Thirty-two patients were enrolled to investigate the levels of SCFAs after the renal transplantation. Rats model showed that butyrate treatments significantly enhanced the function and structure of kidney, as evidenced by the lower serum creatinine levels and less pathological damages of renal tissue. With the recovery of renal function after renal transplantation, SCFAs increased, which were negatively correlated with creatinine. Butyrate expressed like SCFAs. In this study, we demonstrated that butyrate increased with the recovery of renal function after renal transplantation. Most importantly, butyrate treatments alleviated the renal damages caused by I/R via the upregulation of intracellular oxidant stress and inflammations.
Keywords: kidney transplantation, reperfusion injury, fatty acids, unsaturated
Significance of this study.
What is already known about this subject?
Short-chain fatty acids (SCFAs), the end products of fermentation carried out by the intestinal microbiota, were demonstrated to produce anti-oxidant and anti-inflammatory effects.
Previous study has demonstrated that reactive oxygen species induces apoptosis, leading to cell death in kidney tissue after reperfusion of the ischemia.
Renal ischemia/reperfusion (I/R) injury commonly occurred in renal transplantation and is often accompanied by oxidative stresses and inflammatory responses.
What are the new findings?
With the recovery of renal function after renal transplantation, SCFAs increased, which were negatively correlated with creatinine.
Butyrate expressed like SCFAs.
Rats model showed that butyrate treatments significantly enhanced the function and structure of kidney, as evidenced by the lower serum creatinine levels and less pathological damages of renal tissue.
Butyrate-recipient rats demonstrated less I/R injury compared with saline-recipient rats.
How might these results change the focus of research or clinical practice?
Butyrate increased with the recovery of renal function after renal transplant.
Most importantly, butyrate treatments alleviated the renal damages caused by I/R via the upregulation of intracellular oxidant stress and inflammations.
Introduction
Renal transplantation is the effective treatment for patients with end-stage chronic kidney disease. However, this treatment comes with severe limitations due to the insufficient number of donors. Thus, cardiac death donors have become the most common source for donations. However, this treatment was accompanied by serious risks including primary graft non-function and acute rejection.1 Those risks are primarily caused by the prolonged ischemic damages caused by renal retrieval, preservation, engraftment and further serious injuries by caused reperfusion.2 Therefore, ischemia/reperfusion (I/R) injury reduction and donation after cardiac death (DCD) transplantation improvements are of critical importance in the clinical operations of renal transplantation. However, although the wide attentions have been payed to this research field, the detailed molecular mechanisms of I/R injuries are still not clear.
I/R injury is caused by the interruption of blood flow for a certain period of time and aggravated by the ischemia of tissue. I/R injury predominately effects the clinical outcomes of renal transplantation. This injury is able to cause acute or chronic rejection after renal transplantation, especially when the source of donation comes from DCD donors. Because the organ is retrieved from donor without heart beats, it may lead to high morbidities and mortalities of the recipients after the transplantation.3
Although there are intensive studies in the cause and pathology of I/R, the underlying mechanisms of how I/R causes the damages in the organ are still no clear. I/R injury is initially caused by reactive oxygen species (ROS) and inflammatory reactions via the functions of chemokines and cytokines.4 Previous studies have demonstrated that ROS induces apoptosis, which will lead to cell death in kidney tissue after reperfusion of the ischemia.5
Short-chain fatty acids (SCFAs) generally refers to the fatty acids containing 2–4 carbon molecules and straight-chain backbones, such as acetate, propionate and butyrate. SCFAs are the products made by gut microbiota in the colon and cecum fermenting dietary fiber.6 Recent studies show that SCFAs produce potential anti-inflammatory and immunomodulatory functions.7 Butyrate, a part of SCFAs, has drawn worldwide attention in the studies of cancer treatments as a potential therapeutic agent, due to the inhibitory effects to histone deacetylase (HDAC).8 Recently, Kim et al found that the anti-inflammatory and neuroprotective influences of HDAC inhibitors in rat ischemic model of stroke.9 Therefore, it is of critical importance to understand the express of butyrate after renal transplantation and whether pretreatment of butyrate is able to prevent the I/R injury in the renal.
In this study, we examined the changes of SCFAs and the relation with renal function after renal transplantation. Most importantly, the potential effects of butyrate in preventing I/R-associated renal injury through its regulation in related to the oxidative stress and apoptosis was investigated.
Materials and methods
Animals
Sprague-Dawley (SD) rats (male, 200–250 g) were commercially purchased from the Department of Laboratory Animal Center of Soochow University. Rats were kept in a laminar flow atmosphere under specific pathogen-free (SPF) environment. Animal experiments were carried out according to the National Institutes of Health guideline and fully authorized by the Soochow University Laboratory Animal Center.
Renal I/R animal model experiments
Ninety rats were randomly divided into three groups (n=30 each group): (1) sham-operated group (laparotomy was carried out without renal ischemia); (2) butyrate-treated group (tail vein injection 30 min prior to the renal I/R operation); (3) control group (saline were administrated via tail vein injection). Sham group: after successful anesthesia, the right kidney was first removed and the left kidney and its pedicle were dissociated, but the pedicle was not clipped. The abdominal wall was closed by suture after surgery. I/R+butyrate group: the operation process is the same as that of sham group. After right kidney resection, the left kidney and its pedicle are fully exposed and dissociated, and the left kidney pedicle is clamped for 45 min, followed by opening and resuming perfusion, so as to construct an I/R model. The change of renal color from dark red to red indicates successful reperfusion. Butyrate (Sigma, 100 mg/kg) was delivered through tail vein injection 30 min before I/R. Control group: the surgical procedure was carried out the same as the I/R+butyrate group, with aliquot of saline (100 mg/kg) administration instead of butyrate. At 6 hours, 12 hours and 24 hours after the ischemia, 10 randomly chosen rats were euthanized in each group. Blood samples were collected by inferior vena cava puncture and kidney tissue were collected freshly. Serum was isolated from blood sample by centrifuging at 4500× g for 10 min, and was kept at −80°C. Whole kidney tissue for each rat was separated into two pieces and were fixed in 4% paraformaldehyde or stored at −80°C, respectively.
Kidney damage assessments
To assess renal function and cellular injury following renal ischemia, blood urea nitrogen (BUN) and serum creatinine (CR) levels in blood samples collected at 6 hours, 12 hours and 24 hours after the reperfusion were tested in order to evaluate the renal functional changes and cellular damages after renal ischemia. Assessments were carried out by standard automatic analyzer (type 7150, Hitachi).
Histopathology
Kidney tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Slices (4 μm) were stained with H&E. Markers for inflammation and tissue injuries were evaluated.
Myeloperoxidase activity and malondialdehyde assessments
Myeloperoxidase (MPO) activities in tissue served as an important maker for neutrophil infiltration, and the assessments were carried out as previously. Renal malondialdehyde (MDA) level was assessed by the evaluation of lipid peroxidation.10 Kidney tissue samples were prepared by homogenizing in 150 mM potassium chloride buffer on ice. MDA levels were assessed by spectrophotometric analysis. Results were demonstrated as nmol MDA/gram tissue.
TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) was carried out for the assessments of apoptosis in the kidney tissues. Paraffin slices of kidney tissues were processed by apoptosis detection commercial kit (Roche, Shanghai, China). The slices were counterstained with hematoxylin. Total nephrocytes and TUNEL-positive cells were measured and analyzed.
Enzyme-linked immunosorbent assay
Serum was isolated from blood sample. Tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were detected by ELISA according to the kit instructions (R&D, USA).
Clinical research objects
Thirty-two patients who underwent allograft renal transplantation from January to May 2020 were selected, including 20 males and 12 females, within an average age of 43±9.6 years. Inclusion criteria: (1) renal allograft recipients aged 18–60 years; (2) the donor and recipient have the same ABO blood type; (3) preoperative group reactive antibody <10%; (4) postoperative routine application of tacrolimus (FK506)+metmecolcophenol capsules+glucocorticoid triple immunosuppression regimen.
Clinical data
Sterile specimen tubes were used to collect fecal specimens from patients after renal transplantation on the first and seventh days, and 2 mL venous blood was collected from all participants. Determination of SCFAs content: 1 g stool sample was placed in a centrifuge tube and mixed with 5–10 times of double distilled water; 1 mL of the mixture was taken for the night, and then 0.2 mL of the mixed solution was added. The mixture was stored overnight at −20℃ in the refrigerator. After thawing, the supernatant was centrifuged at 12,000 rpm for 10 min, and the supernatant was centrifuged at 12,000 rpm for 10 min before the measurement. The relative correction factors of organic acids such as acetic acid, propionic acid and butyric acid can be calculated by the respective (or concentration) and peak area of the standard sample and the internal standard. Then the concentration of acetic acid, propionic acid and butyric acid in each sample can be calculated according to the weight (or concentration) of acetate, propionate and butyrate in direct proportion to their peak area. BUN and serum CR were detected by automatic analyzer (type 7150, Hitachi). The concentration of FK506 was measured in all patients.
Statistical analysis
Paired t-test, analysis of variance or Kaplan-Meier tests were applied for the statistical analysis to where it is appropriate. Statistical significance p<0.05 was set as significant. All data were shown as means±SD. Sample size for each group was marked in the figure legends.
Results
Butyrate pretreatment attenuate renal I/R injury
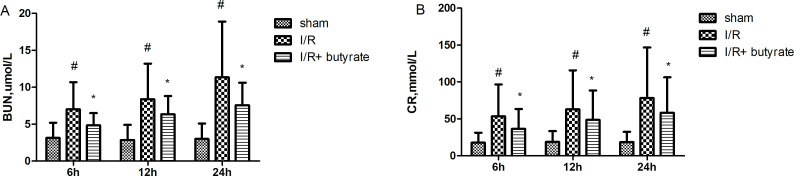
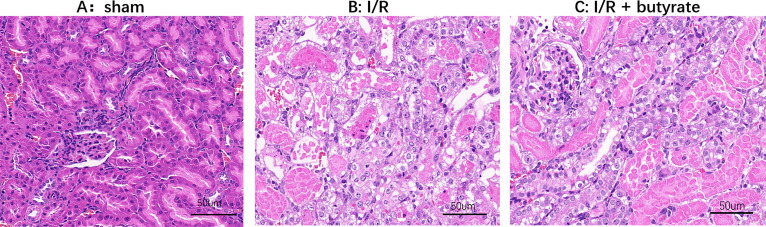
Serum BUN and CR levels were evaluated for the assessments of butyrate functions in renal I/R injury. Thirty minutes of partial renal ischemia were carried out after the rats were treated with sodium butyrate or saline. As a result, significant increase in the serum BUN and CR levels were demonstrated in saline-treated group 6 hours, 12 hours, 24 hours after I/R. In the butyrate-treated group, the serum levels of BUN and CR significantly decreased 6 hours, 12 hours, 24 hours after I/R, suggesting that the treatment of butyrate at the dose of 300 mg/kg in rats significantly rescued the renal injury caused by I/R (figure 1). The protective effects of butyrate were further demonstrated by the change in real pathological results after I/R (figure 2). In the vehicle group, the renal tubular epithelial cells were significantly swollen. Vacuolar degeneration, dilatation and necrotic exfoliated cells, interstitial congestion and edema, a large number of inflammatory cells infiltrated, perivascular dilatation and blood stasis. In contrast, butyrate treatments before I/R significantly rescued those changes in pathology. Renal tubular epithelial cell swelling and necrosis were observed in very limited areas of the renal sample, indicating actuated neutrophil infiltration in the butyrate-treated rats.
Figure 1.
Effects of butyrate on renal injury after renal ischemia/reperfusion (I/R). Blood urea nitrogen (BUN) (A) and creatinine (CR) (B) serum levels of samples collected 6 hours, 12 hours, 24 hours after I/R from the sham-operated, saline and butyrate-treated rats (n=10/group, one-way analysis of variance, *p<0.05 vs the sham group, #p<0.05 vs the vehicle group).
Figure 2.
H&E staining of renal slices of sham-operated, saline and ischemia/reperfusion (I/R)+butyrate-treated rats 24 hours after renal I/R (×50). Massive hemorrhages and necrosis with neutrophil infiltration were observed in the kidney of saline-treated rats, meanwhile, butyrate-treated rats demonstrated attenuated pathological damages induced by I/R. Limited areas and severity of renal tubular epithelial cell swelling and necrosis were demonstrated by the H&E staining, indicating less neutrophil infiltration.
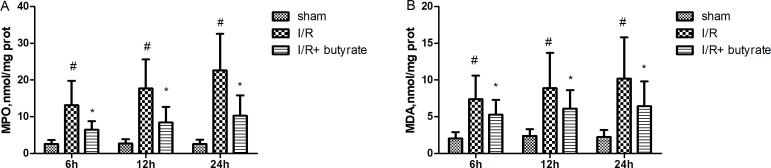
Butyrate treatments attenuate neutrophil infiltration and lipid peroxidation
Evidenced by the significantly increased MPO activities in the renal tissues of saline-treated group, neutrophil infiltration was induced by I/R. In butyrate-treated group, MPO activation were significantly reduced in 6 hours, 12 hours, 24 hours after I/R compared with saline-treated group (p<0.05, figure 3A), suggesting an inhibitory effect of butyrate treatment to neutrophil infiltration. Also, the renal MDA level, as an indicator for lipid peroxidation, significantly elevated in sham-operated groups in 6 hours, 12 hours, 24 hours after comparing with the saline-treated group (p<0.001). Butyrate (300 mg/kg) treatments significantly suppressed the MDA level after renal I/R (p<0.05) (figure 3B).
Figure 3.
Butyrate treatments affecting the myeloperoxidase (MPO) activities and malondialdehyde (MDA) levels after renal ischemia/reperfusion (I/R). Reduced MPO activities (A) and MDA levels (B) were observed in butyrate-treated rats (n=10/group, *p<0.05 vs the sham group, #p<0.05 vs the vehicle group). Data were shown as means±SD.
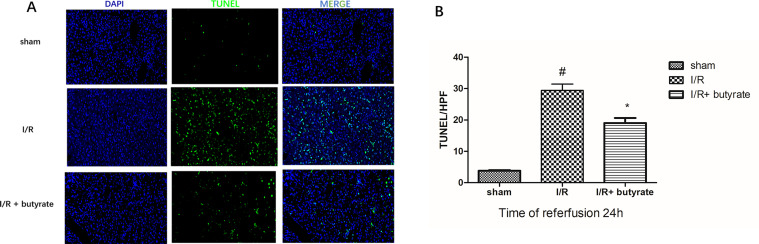
Butyrate inhibits renal tubular epithelial cell apoptosis induced by renal I/R
TUNEL assays were carried out in order to examine renal tubular epithelial cell apoptosis. In the results (figure 4A), I/R after saline treatments significantly elevated TUNEL-positive cell number compared with sham-operation. The TUNEL-positive cell was very limited in sham-operated rats, meanwhile significantly larger number of positive cells were observed in saline-treated rats. Importantly, significantly fewer TUNEL-positive renal tubular epithelial cells were observed in the renal tissue of butyrate-treated rats. The above observation consisted of microscopic TUNEL-positive cell counting (figure 4B).
Figure 4.
Effect of butyrate on renal tubular epithelial cell apoptosis and cell proliferation after renal ischemia/reperfusion (I/R). (A) Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) assay on renal slices of sham-operated, saline and butyrate-treated rats. High TUNEL-positive renal tubular epithelial cell numbers were observed in saline-treated rats after I/R, whereas only very limited TUNEL-positive cells were observed in butyrate-treated rats. Representative images of TUNEL-positive staining cells. (B) In saline-treated group, the number of positive cells were higher than in butyrate pretreatment group. Data were shown as means±SD, n=10/group. *P<0.05 vs the sham group, #p<0.05 vs the vehicle group.
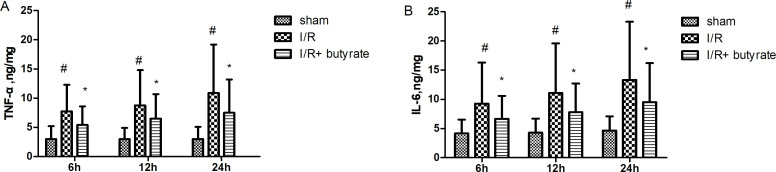
Butyrate inhibits renal I/R-induced inflammatory cytokine increase
It was previously demonstrated that various inflammatory cytokines, including TNF-α and IL-6, participated in the renal I/R injury.4 In our results, revealed by ELISA of the serum collected from rats after I/R, butyrate treatments demonstrated significantly lower level of TNF-α and IL-6 at 6 hours, 12 hours, 24 hours after I/R compared with saline treatments (figure 5).
Figure 5.
Effects of butyrate on inflammatory cytokine production. Serum tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) levels elevated significantly in saline-treated rats after ischemia/reperfusion (I/R), rescued by butyrate treatment prior to renal I/R. Data are shown as means±SD, n=10/group. *P<0.05 vs the sham group, #p<0.05 vs the vehicle group.
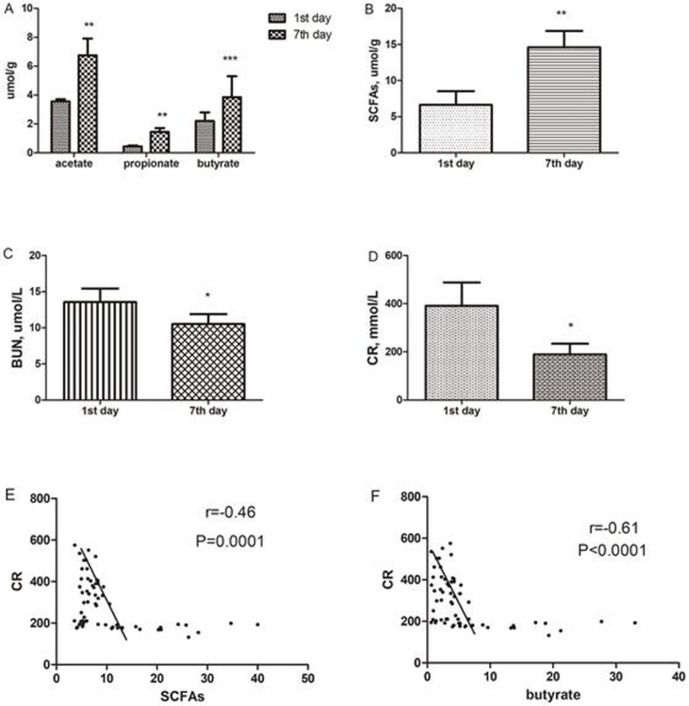
Expression of SCFAs, including butyrate, increases over time and was negatively correlated with creatinine in renal transplantation recipients
Serum BUN and CR levels were measured for the assessments of SCFAs functions in renal I/R injury. SCFAs levels on the seventh day after the renal transplant were significantly elevated compared with those on the first day after the surgeries (table 1). In contrast, BUN and CR levels on the seventh day after the renal transplantation were significantly lower than those on the first day after the surgeries (figure 6A–D). SCFAs were negatively correlated with creatinine. Butyrate was also negatively correlated with creatinine (figure 6E–F).
Table 1.
Clinical laboratory data of all participants
| First day | Seventh day | P value | |
| Acetate, μmol/g | 3.7±3.4 | 7.9±5.6 | 0.018** |
| Propionate, μmol/g | 0.5±0.4 | 1.7±1.2 | 0.004** |
| Butyrate, μmol/g | 2.8±1.6 | 5.3±2.4 | <0.001*** |
| SCFAs, μmol/g (acetate+propionate+butyrate) |
6.5±5.4 | 12.2±8.6 | 0.01** |
| BUN, μmol/L | 13.3±6.1 | 10.5±4.5 | 0.026* |
| CR, mmol/L | 391.7±318.6 | 189.9±145.2 | 0.032* |
Data are presented as mean±SD.
P value of each group was measured by paired t-test. *P<0.05; **p<0.01; ***p<0.001.
BUN, blood urea nitrogen; CR, creatinine; SCFA, short-chain fatty acid.
Figure 6.
Clinical laboratory data of all participants after renal transplantation on first and seventh days. (A and B) Acetate, propionate, butyrate and short-chain fatty acids (SCFAs) levels 7 days after the renal transplant were significantly elevated compared with those on 1 day. (C and D) By contrast, blood urea nitrogen (BUN) and creatinine (CR) levels 7 days after the renal transplant were significantly lower than those on 1 day after the surgeries. P value of each group was measured by paired t-test. *P<0.05; **p<0.01; ***p<0.001. The correlation analysis of SCFAs and butyrate with creatinine for all participants. (E) SCFAs were negatively correlated with creatinine. (F) Butyrate was also negatively correlated with creatinine.
Discussion
Renal I/R injury contributed to the most cases of renal dysfunction and rejection after renal transplantation in clinic. It was especially common happened in the cases where the sources of kidney were from non-heart-beating donors.11 It was of critical importance in clinical treatments to limit the I/R injury after renal transplantation in order to improve the outcomes of operations. Therefore, novel drugs for effective treatment and advanced technologies were urgently demined. The latest research suggested interaction between gut microbiota and kidney physiology, particularly focusing on SCFAs produced by gut microbiota.12 It was also found that the SCFAs levels significantly decreased in patients with chronic kidney disease (CKD), meanwhile butyrate supplementation produced potential effects in delaying the progression CKD.13 SCFA levels and effects were not clear after renal transplantation.
HDAC inhibitors, butyrate, has been widely studied for effective treatments for a variety of diseases. Butyrate was recently demonstrated presenting an anti-inflammatory effects through the inhibition of pro-inflammatory cytokine production.14 15 In previous studies, HDAC inhibitors were found presenting strong effects in neuroprotection in rat model of focal cerebral ischemia,9 and cardioprotection in heart ischemic injury.14 In this study, evidenced by improving renal function and tissue pathology through the assessments of renal tubular epithelial cell alleviated apoptosis and neutrophil infiltration, butyrate treatment demonstrated strong effects in attenuating renal I/R injury and the subsequent histopathological changes induced by I/R.
Butyrate demonstrated effects in anti-inflammation and anti-oxidation, thus contributed to the protection of kidneys.16 Combined with previous study, our results demonstrated the protective effects of butyrate through suppression of the MPO expression in renal tissue and inhibition of pro-inflammatory cytokines in the serum. MPO is an enzyme expressed in neutrophils, and has been used as an indicator for neutrophil infiltration.17 Butyrate treatments (300 mg/kg) before renal I/R remarkably suppressed the MPO activities in kidney tissue. In this study, we demonstrated the suppressive effects of butyrate against MPO activities in renal I/R, indicating butyrate attenuated the renal damages caused by inflammation through the inhibition of oxidative stress.
Renal MDA levels have been used as a biomarker for the assessment of oxidative damages.18 In this study, we demonstrated the significant elevation of renal MDA levels after renal I/R, meanwhile the treatment of butyrate before I/R significantly rescued this change caused by renal I/R, which proved the hypothesis that butyrate was able to attenuate the renal injury caused by I/R through the modulation in oxidative stress.
Apoptosis of renal tubular epithelial cell contributes to the renal injury caused by I/R. As we demonstrated, renal I/R caused the elevation in TUNEL-positive cell numbers in the kidney, while butyrate treatment before I/R recused this change potentially through the inhibition of apoptosis. Other factors, such as ROS accumulation, massive granulocyte infiltration and hepatocyte apoptosis, also participated in the I/R injury. Therefore, enhancement in the cleaning abilities in oxidant stress certainly was able to reduce the severity of I/R injury.19
Cytokines produce dominate effects in the responses to postoperative stress. Especially, cytokines such as TNF-α and IL-6 contribute to the subsequent injury through their pro-inflammatory effects.20 In this study, butyrate treatment before I/R inhibited the elevation of TNF-α and IL-6. This result is in accord with previous studies demonstrating the suppressive effects of butyrate in the regulation of inflammatory cytokines TNF-α and IL-6 expressions.21 Therefore, the renal protection of butyrate potentially achieved through its inhibitory effects to inflammation. This study demonstrated the suppressive effects of butyrate to I/R-induced TNF-α and IL-6 expression in renal tissues.
We also examined the expression of SCFAs in renal transplantation patients. In our study, the expression of SCFAs increased on the seventh day than the first day after renal transplantation, which was negatively correlated with creatinine. Butyrate, a component of SCFAs, gives the same result. BUN/CR after renal transplantation was affected by many factors, especially immunosuppressants. SCFAs were also significantly altered after renal transplantation, which may be attributed to oxidative stress levels and expression of inflammatory factors caused by I/R. SCFAs improve renal function after an injury through modulation of the inflammatory process was associated with levels of local and systemic inflammation, oxidative cellular stress, cell infiltration/activation and apoptosis.4 In this study, data were derived from an uncontrolled correlation between SCFA and BUN/CR in renal transplant patients. SCFAs play a protective role in acute kidney injury (AKI).22 Dietary manipulation of the gut microbiome protects against AKI, mediated by HDAC inhibition and SCFAs.23 Yamamura et al 24 founded serum SCFAs were positively associated with fecal SCFAs even after adjusting for age and sex. Furthermore, fecal acetate was likely to be positively associated with serum acetate. Intestinal microbiota control AKI severity by immune modulation. Targeting the intestinal microbiota might provide a novel therapeutic strategy in AKI.25 However, these data still need to be investigated in controlled studies for further validation.
Conclusions
As a conclusion, in this study, we demonstrated the renal protective effects of butyrate in I/R-induced injury through the suppression of oxidative stress, apoptosis and cell proliferation. In clinical application, whether it can achieve anti-oxidant and anti-inflammatory is affected by regulating gut microbiota and increasing SCFAs, especially butyrate, so as to better improve renal function. Further investigation in the potential application of butyrate is especially meaningful, thus large animal model in vivo studies and clinical trials are of critical clinical importance.
Acknowledgments
The authors would like to thank Dr Zheng for his assistance in producing the figures for this article.
Footnotes
Contributors: All authors participated in this study including data interpretation and manuscript preparation. CZ and YC performed the data management and statistical analyses. All authors took full responsibility for the manuscript.
Funding: The study was supported by Jiangsu Provincial 333 Department Support project (BRA2017116), Natural Science Foundation for Youths of Jiangsu Province (BK20200180) and Changzhou Municipal Science and Technology Bureau Support project (CE20175030, CJ20200089, CJ20200106).
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All study participants were approved by The Third Affiliated Hospital of Soochow University medical ethics committee (2020) no. 076.
References
- 1. Bell R, Farid S, Pandanaboyana S, et al. The evolution of donation after circulatory death renal transplantation: a decade of experience. Nephrol Dial Transplant 2019;34:1788–98. 10.1093/ndt/gfy160 [DOI] [PubMed] [Google Scholar]
- 2. Minami K, Bae S, Uehara H, et al. Targeting of intragraft reactive oxygen species by APP-103, a novel polymer product, mitigates ischemia/reperfusion injury and promotes the survival of renal transplants. Am J Transplant 2020;20:1527–37. 10.1111/ajt.15794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: pathogenesis and treatment. World J Transplant 2015;5:52–67. 10.5500/wjt.v5.i2.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrade-Oliveira V, Amano MT, Correa-Costa M, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 2015;26:1877–88. 10.1681/ASN.2014030288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan S, Jena G, butyrate S. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem Toxicol 2014;73:127–39. 10.1016/j.fct.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 6. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marzocco S, Fazeli G, Di Micco L, et al. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance—“Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins”—A Pilot Study (PLAN Study). J Clin Med 2018;7:315. 10.3390/jcm7100315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghorbani P, Santhakumar P, Hu Q, et al. Short-Chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J 2015;46:1033–45. 10.1183/09031936.00143614 [DOI] [PubMed] [Google Scholar]
- 9. Kim HJ, Rowe M, Ren M, et al. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 2007;321:892–901. 10.1124/jpet.107.120188 [DOI] [PubMed] [Google Scholar]
- 10. Lu Q, Yuan K, Li X, et al. Detecting migration and infiltration of neutrophils in mice. J Vis Exp 2020. 10.3791/60543. [Epub ahead of print: 06 02 2020]. [DOI] [PubMed] [Google Scholar]
- 11. Lia D, Singer P, Nair V, et al. DCD renal transplantation from donors with acute kidney injury. Transplantation 2021;105:886–90. 10.1097/TP.0000000000003317 [DOI] [PubMed] [Google Scholar]
- 12. Pluznick JL. Gut microbiota in renal physiology: focus on short-chain fatty acids and their receptors. Kidney Int 2016;90:1191–8. 10.1016/j.kint.2016.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez A, Krieg R, Massey HD, et al. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol Dial Transplant 2019;34:783–94. 10.1093/ndt/gfy238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun J, Wang F, Li H, et al. Neuroprotective effect of sodium butyrate against cerebral ischemia/reperfusion injury in mice. Biomed Res Int 2015;2015:1–8. 10.1155/2015/395895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rose S, Bennuri SC, Davis JE, et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl Psychiatry 2018;8:42. 10.1038/s41398-017-0089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang S, Xie S, Lv D, et al. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 2016;109:1389–96. 10.1007/s10482-016-0737-y [DOI] [PubMed] [Google Scholar]
- 17. Mikami D, Kobayashi M, Uwada J, et al. Short-Chain fatty acid mitigates adenine-induced chronic kidney disease via FFA2 and FFA3 pathways. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158666. 10.1016/j.bbalip.2020.158666 [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Zhang W, Nie J. Gut microbiota and renal injury. Adv Exp Med Biol 2020;1238:93–106. 10.1007/978-981-15-2385-4_7 [DOI] [PubMed] [Google Scholar]
- 19. Wang S, Lv D, Jiang S, et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci 2019;133:1857–70. 10.1042/CS20190171 [DOI] [PubMed] [Google Scholar]
- 20. Machado RA, Constantino LdeS, Tomasi CD, et al. Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transplant 2012;27:3136–40. 10.1093/ndt/gfr807 [DOI] [PubMed] [Google Scholar]
- 21. Vinolo MAR, Rodrigues HG, Nachbar RT, et al. Regulation of inflammation by short chain fatty acids. Nutrients 2011;3:858–76. 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carney EF. Acute kidney injury. protective role of gut microbial SCFAs. Nat Rev Nephrol 2015;11:127. 10.1038/nrneph.2015.10 [DOI] [PubMed] [Google Scholar]
- 23. Liu Y, Li YJ, Loh YW, et al. Fiber derived microbial metabolites prevent acute kidney injury through G-protein coupled receptors and HDAC inhibition. Front Cell Dev Biol 2021;9:648639. 10.3389/fcell.2021.648639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamamura R, Nakamura K, Kitada N, et al. Associations of gut microbiota, dietary intake, and serum short-chain fatty acids with fecal short-chain fatty acids. Biosci Microbiota Food Health 2020;39:11–17. 10.12938/bmfh.19-010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang J, Kim CJ, Go YS, et al. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int 2020;98:932–46. 10.1016/j.kint.2020.04.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.