Abstract
One of the most common causes of female infertility is polycystic ovarian syndrome, which affects 6–21% of the population. Regrettably, the currently available treatments are mostly symptomatic and ineffective. As a result, safer options are needed now more than ever. In a letrozole PCOS albino mouse model, the current study compares the therapeutic advantages of Turmeric extract (Curcuma longa) to metformin. Adiponectin is a circulating protein generated by adipocytes that has been linked to metabolic diseases (MDs) in an inverse relationship. The effects of Turmeric Extract (Curcuma Longa) in contrast to Metformin, as well as the involvement of adiponectin in endocrine-metabolic abnormalities in experimentally induced PCOS mice model, were studied in this study. Letrozole (6 mg/kg) was administered orally (p.o) for 21 days to induce PCOS, followed by a dose of Turmeric Extract (Curcuma longa) (175 mg/kg and p.o) and Metformin (150 mg/kg) for 30 days, both with normal saline water (0.9%) as the carrier. The findings revealed that LET-treated mice displayed PCOS-like characteristics, such as higher LH levels, increased body weight growth, and ovarian morphology with numerous cysts, increase in fasting blood glucose, lipid profile, plasma lipid peroxidation (MDA) and IL-6, as well as a decrease in serum Progesterone, Estrogen, FSH, SOD and GSH levels in the ovary. These changes were linked to lower levels of circulating adiponectin and were reversed when treated Turmeric extract. By altering circulating androgen-adiponectin balance, the data implies that Turmeric extract alleviates endocrine-metabolic abnormalities and inflammation-related comorbidities associated with LET-induced PCOS.
Keywords: Polycystic ovary syndrome, Adiponectin, Turmeric extract (Curcuma Longa), Metformin, Oxidative stress
1. Introduction
Polycystic Ovarian Syndrome (PCOS) is a prevalent complex and heterogeneous endocrine-metabolic illness in women of child-bearing age that affects 6–21% of women and accounting to 70–80% of infertility occurrences in women globally [[1], [2], [3]]. According to a recent study by Joham et al., 72% of women with PCOS are infertile, which is nearly 15 times greater than women without PCOS [4]. According to reports, PCOS is characterised by hyperandrogenism, persistent anovulation, multiple cysts in ovaries, and disrupted physiological functions, such as acne, hirsutism, changed basal metabolic index, insulin resistance, sleep disturbances, anxiety, and depression [3,5]. The etiopathology of this complex condition is mainly understood. However, because of the unknown aetiology, the diagnostic criteria are still inadequate, which explains why many women with PCOS go undiagnosed and untreated. According to epidemiological data, 75% of people with PCOS go to their doctors without being diagnosed [6,7]. As a result, a greater understanding of the pathophysiological mechanism could lead to a better diagnostic and therapeutic approach for PCOS management. In addition to endocrine disturbance, metabolic and associated illnesses, such as insulin resistance, obesity, type 2 diabetes, and cardiometabolic events, continue to play a role in PCOS aetiology [3,4,8]. Metabolic tissue, particularly adipose tissue, is an active endocrine organ that produces adiponectin and other proteins [9,10].
Adiponectin is an essential adipocytokine with insulin-sensitizing capabilities, as well as anti-inflammatory and anti-atherogenic effects [11,12]. It regulates energy, insulin, glucose, and lipids. However, metabolic problems have been linked to decreased secretion and, as a result, a drop in circulating levels [9,12]. Similarly, previous researchers has found an inverse relationship between circulating adiponectin and adipocyte mass and visceral adiposity, both of which are important factors in metabolic and related disorders [9,13,14], implying that an increase in adipocyte mass or visceral adiposity lowers circulating adiponectin. Despite decades of research, the precise role of adiponectin in metabolic disorders remains unknown; some researchers have proposed a causal involvement [12,15], while others have identified adiponectin as an insulin resistance biomarker [9,16]. Despite this, adiponectin is linked to metabolic dysfunction, including insulin resistance. Aside from that, some studies have shown that women with PCOS have lower levels of circulating adiponectin [17,18], but the precise role of this adipocytokine is unknown, though recent research suggests that it may be responsible for the metabolic disruptions that characterise obesity and obesity-related disorders, including PCOS [[19], [20], [21]]. As a result, looking into the role of adiponectin in PCOS-related endocrinemetabolic diseases would help with the diagnosis and treatment of this complex ovarian illness. Because of the disorder's complexity, conventionally available treatments for PCOS are now contentious. Normalizing ovulation, lowering testosterone levels, and reducing insulin resistance should be the goals for its treatment [22].
Metformin, an oral hypoglycemic medication that was first used to treat type 2 diabetes [23]. It has been recommended to use in PCOS by several studies, as it improves insulin sensitivity and restores a normal hormonal profile with enhanced menstrual cyclicity [24]. However, the growing body of evidence on metformin efficacy remains contentious [24]. The use of metformin is becoming increasingly accepted and widespread. Despite this increasing clinical use, the details of the mechanism of action of metformin are incomplete [25,26]. New developments in PCOS treatment are focusing on incorporating natural therapies into everyday diets [27]. Many methods are now being used to treat PCOS and stimulate ovulation. However, significant adverse effects have been documented, including arthritis, joint or muscular pain [28]. As a result, natural-source medicines with minimal or no adverse effects are becoming increasingly popular.
Curcumin, popularly known as turmeric, is a polyphenol produced from the curcumin longa plant and is used in Asian cuisine for centuries [29]. Curcumin has recently been examined as an adjunctive treatment for a wide range of diseases [30]. Curcumin shows anti-diabetic properties in the liver by increasing glycolysis and glycogen synthesis while decreasing gluconeogenesis, as well as in the skeletal muscle by elevating glucose uptake, glycolysis, and glycogen synthesis [31]. Curcumin has been known to have anti-inflammatory, antioxidant, hypoglycaemic, antihyperlipidemic properties, among others [32,33]. Curcumin inhibits proliferation and induces apoptosis in a variety of human cancer cell lines, including those generated from prostate, breast, and ovarian cancers [34,35]. Curcumin has been shown to protect Porcine Ovarian Granulosa cells in a recent study [36]. Curcumin has also been shown to have estrogenic effects in breast cancer cell lines [37]. Furthermore previous studies have also found curcumin as a safe phytochemical that can improve insulin resistance through increasing adiponectin circulation [38]. Because of the observed actions, we postulated that Curcumin could be useful in the treatment letrozole induced PCOS.
In rats, various experimental models for PCOS have been created, including the injection of Estradiol Valerate, DHEA, and an excess of pre pubertal androgen [39]. Despite the fact that these models generate PCOS, none of them are absolutely compelling and accurately reflect the symptoms of genuine PCOS. Letrozole, a non-steroidal aromatase inhibitor, causes a PCOS model that is similar to human PCOS in many aspects [40]. It inhibits the conversion of testosterone and androstenedione to estradiol and estrone, respectively, and mimics the symptoms of PCOS [41] by inducing hormonal imbalance, circulating hyperandrogenism, and intraovarian androgen excess, resulting in polycystic ovary formation. Letrozole induction has been linked to hyperglycemia, which has been linked to insulin resistance and hyperlipidemia, both of which have been linked to metabolic syndrome [42,43]. Accordingly, the current study looked at the potential therapeutic benefits of Turmeric Extract in treating PCOS abnormalities in a Letrozole-induced PCOS model, and its potential therapeutic efficacy was compared to that of the commonly used drug: metformin.
2. Materials and methods
2.1. Chemicals
Metformin and Letrozole were bought from a nearby pharmacy (Sun Pharma Company). Clementia Biotech Delhi provided LH (luteinizing hormone), FSH (follicle stimulating hormone), oestrogen, and progesterone measuring kits (Elisa kits). The Turmeric Extract was provided by Amruti Herbals Indore Madhya Pradesh, while the glucose, cholesterol, and triglycerides (TG) kits were acquired locally (Elab science and Meril diagnostics). The remaining chemicals that were used in this study were of analytical quality.
2.2. Experimental animals
24 adult female Swiss Albino mice (weight: 22–25 g) were procured from Jeeva Life Sciences in Malkajgiri, Telangana, and divided into four groups of 6 mice each (Group 1: Normal Control, Group 2: PCOS untreated, Group 3: PCOS+ Ginger, and Group 4: PCOS+Metformin). Mice were given a week to adjust the laboratory settings (temperature: 22 ± 2 °C, relative humidity: 45 ± 5%, light/dark cycle: 12 h) before the experiment. All animal experimental protocols were approved by the Institutional Ethics Committee of Barkatullah University Bhopal (approval no. 1885/GO/Re/S/16/CPCSEA/IAEC/BU/21). In order to induce PCOS, mice were given Letrozole (6 mg/kg) bw orally for 21 days, after treatment with Letrozole (6 mg/kg we divided PCOS mice into three groups: PCOS untreated, PCOS+Turmeric Extract (175 mg/kg), and PCOS + Metformin (150 mg/kg). Turmeric Extract and Metformin were combined in a 0.9% NaCl solution and taken orally for 30 days. At 24 h after the final dosage of therapy, all mice were killed. After that, the tissues of interest were dissected out and fixed in Bouin's fixative for 24 h at room temperature, dehydrated in a graded ethanol series, and finally cleared in xylene. On paraffin slices cut at 5 m, hematoxylin and eosin staining were done.
2.3. Confirmation of PCOS induction
After the Letrozole administration, PCOS was confirmed by following Rotterdam criteria, 2003. Vaginal smears were taken using cotton buds dipped in saline and allowed to dry after rolling on a clean slide to confirm PCOS induction. According to, cells were fixed in methanol, allowed to dry, and stained with 0.76 g giemsa [44].
2.4. Biochemical analysis
2.4.1. Serum hormone analysis
Blood samples were obtained directly from the inferior vena cava with a 1 mL syringe at end of the trial. The serum was prepared by centrifuging it for 20 min at 1000 g and then keeping it at −80 °C until needed. LH, Estrogen, Progesterone, and follicular-stimulating hormone (FSH) levels in the blood were measured using ELISA kits (ELK Biotechnology Wuhan). When measuring hormone levels, the manufacturer's instructions were followed. The inter-assay and intra-assay precision were <8% and <10% respectively.
2.4.2. Serum fasting glucose
Using commercially available kits, serum fasting blood glucose (FBG) levels were determined calorimetrically (Elab science).
2.4.3. Serum lipid profile
Using commercially available kits, total cholesterol (TC) and triglycerids (TG) were measured calorimetrically (Meril Diagnostics).
2.4.4. Antioxidant assay
2.4.4.1. Lipid peroxidation assay
Lipid peroxidation (LPO) was measured in the Ovary and expressed as Malonaldehyde (MDA) content [45]. Using a glass homogenizer, the ovaries were removed and tissue homogenate (10%) was made in ice-cold normal saline. After that, the homogenate was centrifuged for 10 min at 3000 rpm. A millilitre of the supernatant was incubated for 2 h at 37 0.5C. Each sample received 1 mL of Tris hydrochloric acid (TCA) (10%), which was correctly mixed before centrifugation at 2000 rpm for 5 min at 4C. An equivalent volume of 0.67% of 2-thiobarbituric acid TBA was added to 1 ml of supernatant, stirred thoroughly, and maintained in a boiling water bath for 10 min. After cooling, the samples were diluted with 1 mL of distilled water. Using a Genesys-20 (Thermo Scientific, USA) spectrophotometer, the optical density (OD) was measured at 535 nm. The absolute MDA levels were measured in nanomoles per gram of moist tissue.
2.4.4.2. Reduced glutathione assay
5, 50-dithiobis-2-nitrobenzoic acid (DTNB) technique was used to evaluate tissue Glutathione (GSH) levels [46]. Tissues were homogenised in 0.02 M EDTA (5–8 ml) before being diluted in 2 ml ice-cold distilled water. After which, 1 mL of 50% TCA was added and gently mixed, following 15 min of centrifugation at 6000 rpm. One millilitre of the supernatant was combined with 2 mL of tris buffer (0.4 M, pH 8.9) and one hundred millilitres of DTNB (0.1 M). At 410 nm, the absorbance was measured and the findings were represented in mmol/gm moist tissue.
2.4.4.3. Superoxide dismutase
The activity of superoxide dismutase (SOD) was calculated using the Marklund and Marklund method [47]. The absorbance was measured at 420 nm at 1 min intervals for 3 min using 2.9 ml of tissue homogenate supernatant (10%) and 100 ml of pyrogallol (0.2 mM). The final result of SOD activity was measured in units per gram of moist tissue.
2.5. Plasma adiponectin
The quantity of adiponectin in the blood was determined using a quantitative standard sandwich ELISA technique using monoclonal antibodies specific for these parameters purchased from Elabscience Biotechnology Inc. having <10% of inter-assay and intra assay coefficient of variation.
2.6. Pro-inflammatory/inflammatory biomarkers (interleukin-6)
The plasma concentrations of IL-6 was assessed using a quantitative standard sandwich ELISA technique using mice kits supplied from Elabscience Biotechnology Inc., utilising a monoclonal antibody specific for these parameters. Both intra and inter-CV% are <10%.
2.7. Histological assessment of ovaries
For Histopathological studies the ovaries were fixed in 10% formolsaline overnight, dehydrated, embedded in paraffin, and sectioned at 5-m thickness for hematoxylin and eosin (H & E) stains. Using a Motic microscope, the slides were produced and examined.
2.8. Statistical analysis
The data was evaluated statistically using one-way ANOVA followed by Tukey's multiple comparison tests, and the results are expressed as mean and standard error of mean (SEM) for the different treatment groups. P value < 0.05 and < 0.0001 were treated as statistically significant and highly significant respectively. Graph pad prism was used for statistical analysis.
3. Results
3.1. Effects of different treatments on body weight
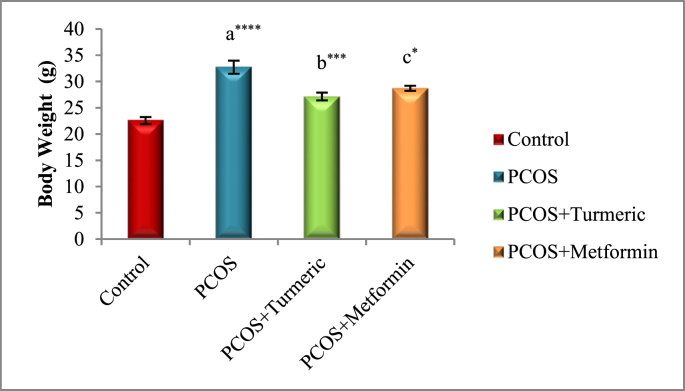
Fig. 1 illustrates Oral administration of letrozole for three weeks resulted in significant rise (p = 0.0001) in body weight in all groups when compared to the control group, indicating weight gain in comparison to the untreated PCOS group, However mice treated with Turmeric extract 175 mg/kg had a substantial drop in body weight (p = 0.0007) as compared to the untreated PCOS group (without therapy). Similarly treatment with Metformin resulted in significant drop (p = 0.01) in the body weights as compared to the PCOS untreated group. Furthermore we did not find any significant difference (p = 0.567) in the PCOS+Metformin and PCOS+Turmeric extract groups.
Fig. 1.
Control: Normal Saline, PCOS: Letrozole; PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; *P < 0.05, **P < 0.01, ****P < 0.0001, n = 6.
3.2. Serum hormonal levels
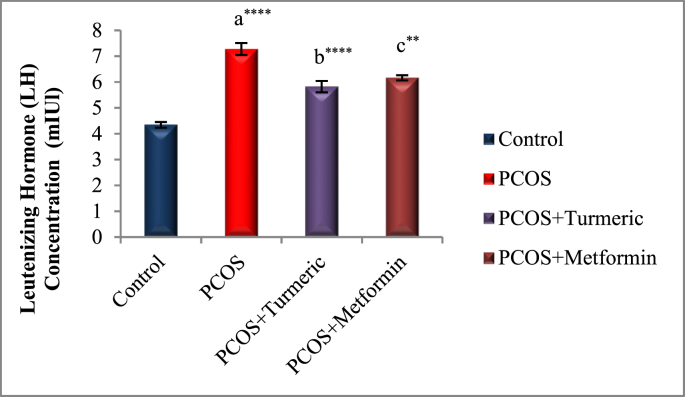
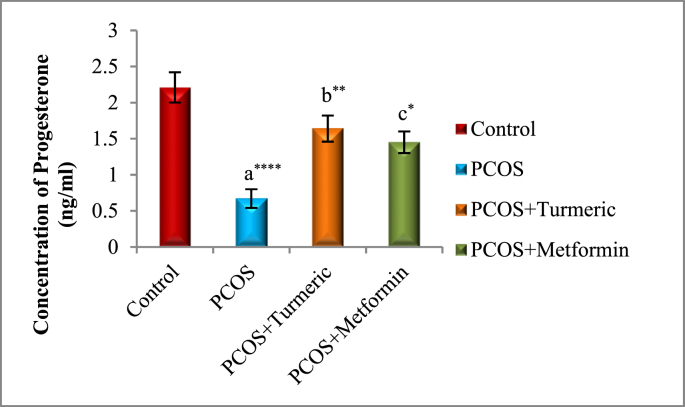
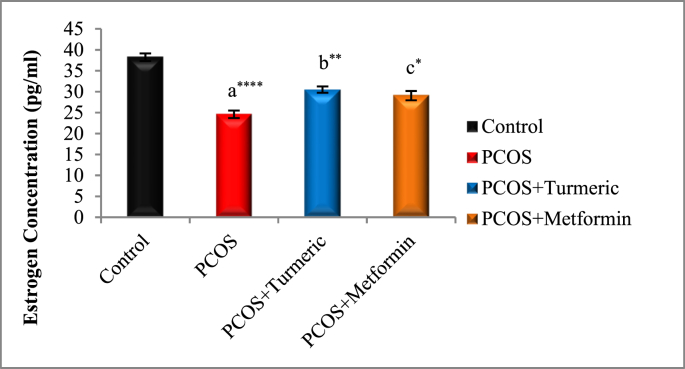
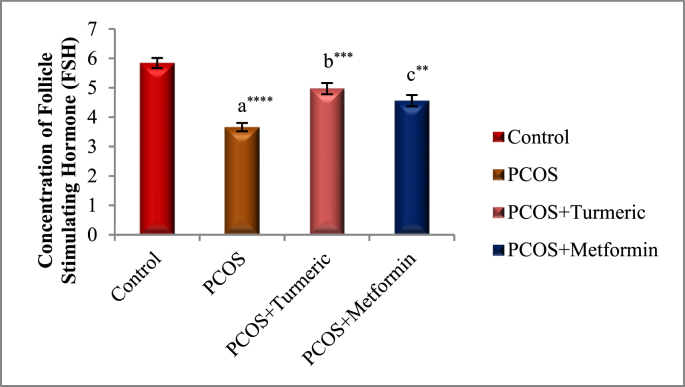
The effects of Turmeric Extract and Metformin on LH, Progesterone, Estrogen, and FSH levels are shown in Fig. 2A, Fig. 2B, Fig. 2C, Fig. 2DA–D). In Comparison to the normal control group, the serum levels of LH were significantly higher in the PCOS induced group (p = 0.001), whereas those of Progesterone, Estrogen, and FSH were significantly lower (p = 0.0001). LH levels fell significantly in the Turmeric (p = 0.0001) and Metformin-treated groups (P < 0.0013) compared to the PCOS-untreated group. However no significant difference were seen in the PCOS+Turmeric and PCOS+ Metformin treated groups (p = 0.521). In Turmeric Extract treatment group progesterone, oestrogen, and FSH levels increased significantly (p = 0.004), (p = 0.001) and (p = 0.0002) respectively. Similar effects were seen in Metformin treated group (p = 0.02, p = 0.01, p = 0.007). Interestingly we did not find any significant differences in these levels in Metformin and Turmeric Extract treated groups (p = 0.861, p = 0.699, p = 0.383).
Fig. 2A.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; **P < 0.01, ****P < 0.0001, (n = 6).
Fig. 2B.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; *P < 0.05, **P < 0.01, ****P < 0.0001, n = 6.
Fig. 2C.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; *P < 0.05, **P < 0.01, ****P < 0.0001, (n = 6).
Fig. 2D.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; **P < 0.01, ***P < 0.001, ****P < 0.0001, (n = 6).
3.3. Effect of Turmeric and Metformin treatment on glucose, cholesterol and triglycerides (TG) levels in PCOS mice
Table 1 illustrates the effect of Turmeric Extract and Metformin on Glucose, Cholesterol and Triglycerides (TG) levels. We Observed that in letrozole-induced PCOS mice, glucose, cholesterol, and tri glycerides (TG) levels were significantly higher (p = 0.0001) than in the control group (Fig. 3A, Fig. 3B, Fig. 3CA–C). When compared to the PCOS control group, we noticed a significant drop (p = 0.0001) in blood glucose levels in the Turmeric and metformin treated groups Similarly, when comparing the Turmeric and Metformin treated groups to the PCOS control group, we noticed a substantial drop (p = 0.0001) in cholesterol and TG levels. Further when comparing the Turmeric treatment group with the Metformin treated group we found a significant difference (p = 0.02) in the TG levels between these two treatment groups, while we did not find any significant difference in the levels of Glucose and Cholesterol in Metformin and Turmeric treated groups respectively (p = 0.229, p = 0.00.546).
Table 1.
Effect of various treatments on Lipid Profile, Glucose levels and Body weight.
| Groups | Total Cholesterol (TC) (mg/dl) | Triglycerides (TG) (mg/dl) | Blood Glucose (mg/dl |
|---|---|---|---|
| Control | 125.5 ± 1.52 | 123.86 ± 2.34 | 123.2 ± 1.65 |
| PCOS | 170.4 ± 2.32a**** | 168.73 ± 1.86a**** | 165.3 ± 2.02a**** |
| PCOS+Turmeric | 142.17 ± 2.08b**** | 134.82 ± 1.42b**** | 145.4 ± 1.69b**** |
| PCOS+Metformin | 150.0 ± 2.07c**** | 142.85 ± 0.9**** | 140.7 ± 1.31c**** |
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a = PCOS vs. control; b = PCOS+Turmeric VS PCOS; c = PCOS+Metformin vs. PCOS; ****P < 0.0001, (n = 6).
Fig. 3A.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; *P < 0.05, **P < 0.01, ****P < 0.0001, (n = 6).
Fig. 3B.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; *P < 0.05, **P < 0.01, ****P < 0.0001, (n = 6).
Fig. 3C.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a PCOS vs. control; b PCOS+Turmeric VS PCOS; c PCOS+Metformin vs PCOS; ****P < 0.0001, (n = 6).
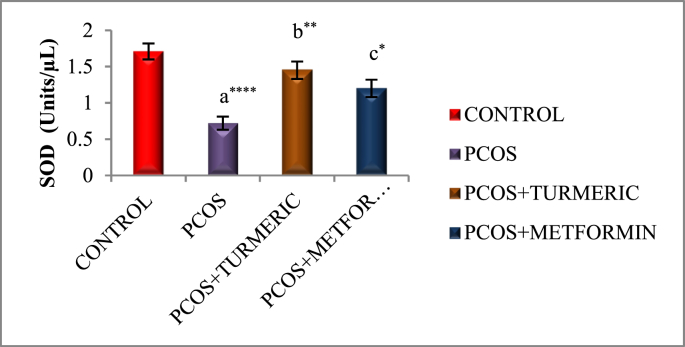
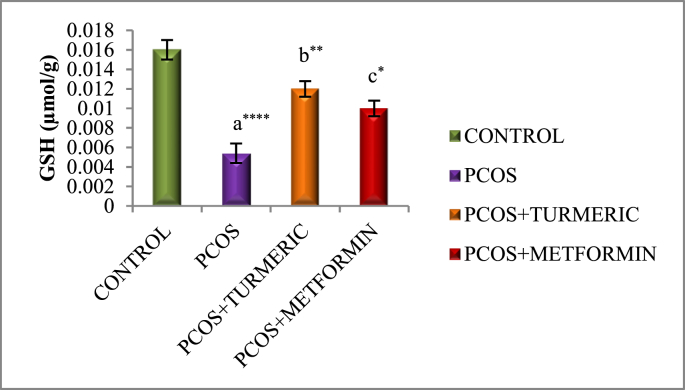
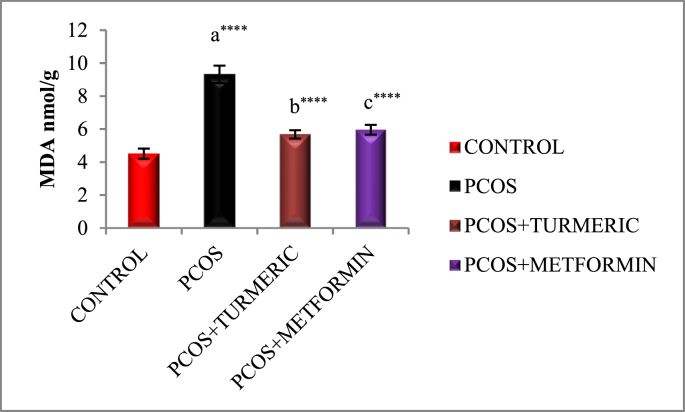
3.4. Effect of turmeric extract on antioxidant activity and lipid peroxidation
Fig. 3A, Fig. 3B, Fig. 3CA–C) illustrates effect of Turmeric Extract on antioxidant activity and lipid peroxidation. When compared to the normal control, the letrozole-induced PCOS group showed a substantial decrease in antioxidant enzyme activity SOD (p = 0.0001), GSH (p = 0.0001), and an increase in lipid peroxidation MDA (p = 0.0001). However, we discovered a substantial increase in SOD (p = 0.001 & p = 0.03) and GSH (p = 0.001 & p = 0.02) activity in PCOS groups treated with Turmeric Extract and Metformin, respectively. MDA is the final product of the LPO reaction, which is generated when Thiobarburic acid (TBA) reacts with it. In the current investigation, it was discovered that all Letrozole induced PCOS groups had higher MDA levels in ovarian tissues than controls, but those treated with Turmeric and Metformin had lower MDA levels (p = 0.001).
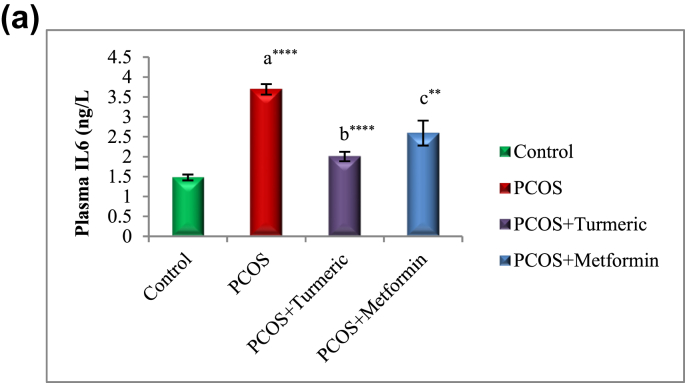
3.5. Turmeric extract treatment reduces plasma IL-6 in rats with letrozole induced PCOS
Fig. 4A illustrates the effect of Turmeric extract on IL6. There was a significant increase (p = 0.0001) in the concentration of plasma IL-6 in animals with LET-induced PCOS when compared with normal control group, and these alterations were reversed by Turmeric Extract treatment (p = 0.0001).
Fig. 4A.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a = PCOS vs. control; b = PCOS+Turmeric VS PCOS; c = PCOS+Metformin vs PCOS; *P < 0.05, **P < 0.01, ****P < 0.0001, n = 6.
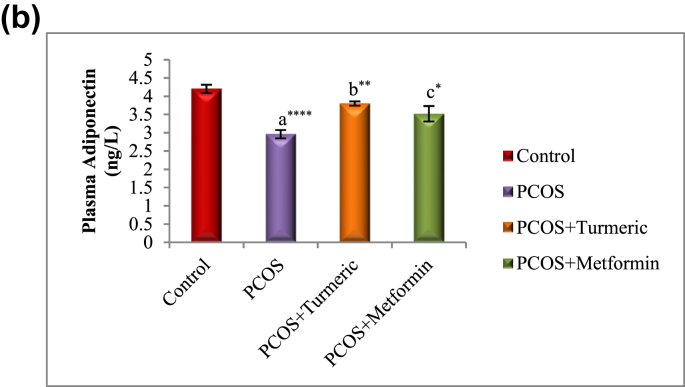
3.6. Turmeric extract treatment increases circulating adiponectin concentration in rats with letrozole induced PCOS
Fig. 4B illustrates the effect of Turmeric extract on adiponectin levels. Our results showed a significant decrease (p = 0.0001) in the concentration of plasma adiponectin in animals with LET-induced PCOS when compared with normal control group, and this was significantly increased (p = 0.01) when treated with Turmeric extract.
Fig. 4B.
Control: Normal Saline, PCOS: Letrozole (6 mg/kg); PCOS+ Turmeric: Turmeric (175 mg/kg), PCOS+Metformin: Metformin (150 mg/kg) a = PCOS vs. control; b = PCOS+Turmeric VS PCOS; c = PCOS+Metformin vs. PCOS; **P < 0.01, ****P < 0.0001, *p < 0.05; n = 6.
4. Discussion
Polycystic ovarian syndrome (PCOS) is a complicated, polygenic condition with larger personal and society economic consequences [48]. Clinical symptoms that are associated with Polycystic ovarian Syndrome (PCOS), include abdominal obesity, acne, scalp bolding, and hirsutism, as well as secondary effects such as infertility, cardiovascular problems, and insulin resistance, predispose women to anxiety and depression [49]. New pharmaceutical therapies with less adverse effects, on the other hand, should be pursued. PCOS treatment aims to improve ovulation in women who desire to become pregnant, as well as lower androgen levels, body weight, and long-term diabetes and cardiovascular disease risks [50]. In this study, Letrozole, an aromatase inhibitor, was used to induce Polycystic Ovary Syndrome in female albino mice. In many ways, the Letrozole-induced PCOS condition matches human PCOS, according to earlier study [41]. The model's efficacy was demonstrated by regular vaginal inspections. In this study the PCOS induction was confirmed by examining vaginal smears [44], were we demonstrated that letrozole groups had higher leukocytes, indicating a continuous diestrous phase. Letrozole, it should be mentioned, inhibits the conversion of androgens to estrogens, leading to hyperandrogenism [51], as well as disruption of the estrous cycle and increased body and reproductive organ weight [52].
Turmeric Extract showed promising results in alleviating endocrine-metabolic disturbances associated with Letrozole-induced PCOS by increasing circulating levels of adiponectin and reducing circulating LH, according to the results of this study. Our findings confirmed that letrozole causes PCOS in experimental mice, a condition marked by hyperandrogenism, obesity, ovarian abnormalities such as numerous cysts, hyperglycemia, and dyslipidaemia. In addition, the current findings demonstrated that Letrozole-induced PCOS is related with related increased lipid peroxidation, impaired cellular antioxidant capacity, proinflammation/inflammation, and lower levels circulating adiponectin. In almost half of PCOS patients, abdominal fat gain has been reported [53]. Obesity is a common symptom of PCOS, with 30–60% of patients being overweight at some point [54]. Abdominal fat accumulation has been linked to insulin resistance and an increased risk of cardiovascular disease. Because many PCOS patients have abdominal obesity, it's possible that it's the source of insulin resistance [55]. In this study, like in earlier investigations, an increase in external visceral fat was observed in PCOS rats (Fig. 5). Aromatase, a crucial regulatory enzyme for oestrogen metabolism, is modulated by IL-6 [56]. The release of IL-6 into the systemic circulation, as well as the fact that it is higher in obese people, suggests that IL-6 could play a new role as a systemic regulator of body weight and lipid metabolism. The presence of IL6 receptors in the hypothalamus supports the idea that this cytokine has direct central effects [57].In the present study it was found that the PCOS induced group had raised level of Luteinizing Hormone (LH), Turmeric extract normalised serum LH levels in the same way that Metformin does, while as the PCOS-induced group had lower levels of progesterone, estrogen, and Follicle Stimulating Hormone (FSH) in their blood. These findings are consistent with previous research [[58], [59], [60], [61], [62]]. Turmeric extract successfully restores progesterone levels to normal, which are also symptoms of anovulation [39]. Repetitive administration of turmeric extract significantly increased the decreased estrogen levels caused by aromatase inhibition in the PCOS-induced group, confirming its previously described phytoestrogen activity [37]. FSH levels were also found increased in the Turmeric and Metformin treated groups. PCOS is also a metabolic disease linked to type 2 diabetes mellitus [63] that begins with hyperglycemia and progresses to insulin resistance over time. In our research, PCOS-affected mice had a significant increase in fasting blood glucose in comparison to the normal control group. This is consistent with previous studies that showed hyperglycaemia in Letrozole-induced PCOS animals [43]. Turmeric supplementation significantly reduced the rise in fasting blood glucose levels, indicating that Curcumin has a favourable effect in reducing insulin resistance and diabetic complications. This conclusion is backed up by Ref. [64].
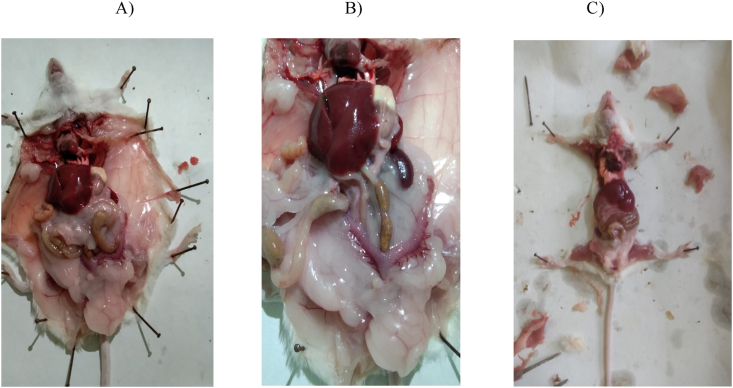
Fig. 5.
Shows ovary Morphology showing decrease in visceral fat in turmeric treated group compared with Letrozole induced PCOS group. Fat tissue in abdominal cavity particularly around the uterus and Ovary decreased in turmeric treated group: Picture A & B showing Visceral fat in PCOS groups While as Picture C represents the PCOS+Turmeric.
Dyslipidemia is one of the side effects of PCOS. Hyperandrogenism is blamed for lipid profile imbalances [65,66]. In terms of lipid profile, the current investigation found similar results. The Total cholesterol (TC) and Triglycerides (TG) levels in the PCOS-induced group were significantly higher. Turmeric extracts antihyperlipidemic activity was demonstrated by a significant reduction in serum TC and TG levels in comparison to the PCOS group (without therapy). These results are backed up by the previous findings [67]. Furthermore when comparing the turmeric treated group with the Metformin treated group we found significant increase in the TG levels of animals thereby indicating more efficacy of Turmeric in normalizing the Triglycerides.
Several investigations have shown oxidative stress as a pathogenic component in PCOS [68,69]. Higher oxidant levels in the ovaries may change the stereo diagnosis, resulting in increased androgen production and polycystic ovaries [68]. In this study, it was discovered that PCOS animals had higher levels of oxidative stress indicators and lower levels of endogenous antioxidants in their ovary. The PCOS group had considerably lower SOD and GSH activity, which was restored by concurrent therapy with Turmeric extract. This is consistent with Curcumin's previously documented antioxidant properties [70].
Lipid peroxidation is commonly used as a marker for oxidative tissue damage as it causes free radical damage to cell membrane components, resulting in cell necrosis and inflammation [71]. MDA is the final product of the LPO reaction, which is generated when TBA is added. MDA development was found to be considerably higher in Polycystic Ovaries in our study. Turmeric treatment brought things back to normal [72].
Furthermore, when compared to the control group, mice with LET-induced PCOS had higher plasma levels of the pro-inflammatory/inflammatory cytokine IL-6. Similarly, previous research has shown that, in addition to oxidative stress, hyperglycemia and hyperinsulinemia stimulate pro-inflammatory/inflammatory signalling[73], which could explain the higher IL-6 levels seen in PCOS mice in this work. As a result, the findings of this study show that oxidative stress and pro-inflammation/inflammation may have a role in the basic phenotypic expression of PCOS, such as ovarian cysts.
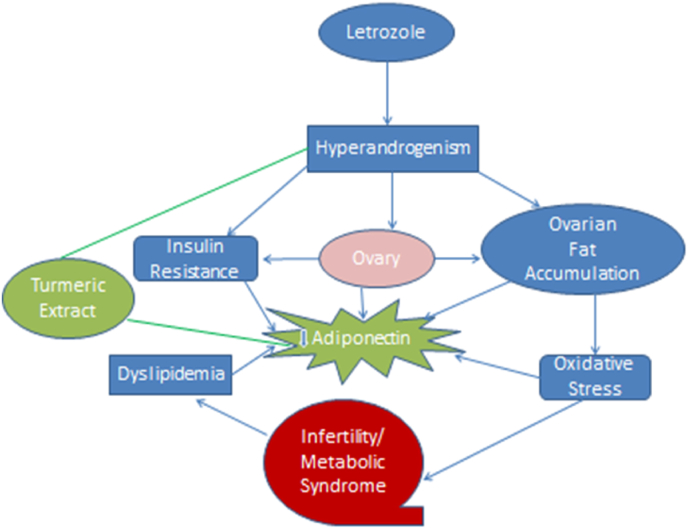
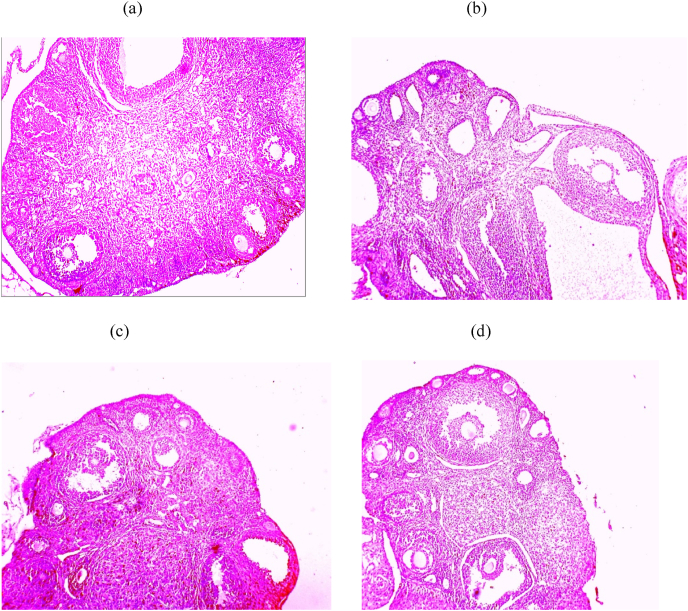
It's also worth noting that circulating adiponectin was significantly lower in animals with LET-induced PCOS compared to animals without PCOS, which corroborated prior findings [9,16] that adiponectin is a measure of insulin resistance in people with PCOS. Furthermore, the current study discovered that circulating LH, FSH, Estrogen, Progesterone, blood glucose, lipid regulation, oxidant-antioxidant status, ovarian tissue histology, and pro-inflammatory biomarkers are all associated with a significant decrease in circulating adiponectin in LET-induced PCOS animals (Fig. 7). Previous studies also seen the lower levels of adiponectin in metabolic diseases and associated syndromes in Refs. [11,12]. Adiponectin has been shown in several in vitro experiments to be a metabolic agent that modulates gonadal steroidogenesis and gametogenesis [74] and may influence GnRH production and gonadal function, in addition to its metabolic effects [75]. In rodents, the adiponectin transcript in the ovarian cells of the dominant follicle correlates with the concentration of follicular fluid estradiol, establishing a link between adiponectin and follicular dominance and oocyte competence [76]. Recombinant adiponectin at physiological concentrations (5–10 g/mL) has also been shown to boost the release of hormones, mainly oestrogen, in IGF1-stimulated cells in rodents and women [77]. As a result, the current findings imply that adiponectin may be a common feature of PCOS with metabolic and to some extent, endocrine variables, and that a decrease in circulating concentration may lead to infertility in metabolic illnesses such as PCOS. As a result, adiponectin overexpression could be used as a therapeutic approach to treat metabolic and possibly endocrine issues in people with PCOS. In contrast to untreated animals with LET-induced PCOS, administration of Turmeric Extract significantly increased circulating adiponectin, which is consistent with previous studies [77], with a corresponding decrease in LH, fasting blood glucose, and increase in estrogen, progesterone in animals with LET-induced PCOS. To our knowledge, this is the first study to show the beneficial benefits of Turmeric extract (Curcumin) on endocrine-metabolic problems associated with LET-induced PCOS in Swiss Albino mice, as well as the potential role of circulating adiponectin in comparison with the Metformin. The current study's histopathological findings revealed that letrozole caused ovarian dysfunction, with follicular cysts visible in the ovaries of PCOS mice (Fig. 6a&b). This could be due to a high level of luteinizing hormone (LH), which causes the ovary to release androgens [45]. Clearly, therapy Turmeric Extract restored the abnormalities caused by letrozole in the reproductive organs of female albino mice (Fig. 6c). The existence of different stages of mature follicles with corpus luteum indicating ovulation and a regular estrous cycle [78] supports these findings.
Fig. 7.
Schematic diagram showing possible effect of Turmeric Extract and involvement of Adiponectin in metabolic endocrine changes associated with PCOS.
Fig. 6.
Histopathological photomicrographs of each experimental group's ovaries (H&E, magnificent ×10): (A) control, (B) PCOS, (C) PCOS+Turmeric Extract, (D) PCOS+Metformin.
The study did not identify the molecular mechanism underlying the regulatory role of Turmeric Extract, and causative link between adiponectin and endocrine-metabolic abnormalities in LET-induce PCOS. Moreover, the findings of this study showed Turmeric extract as a traditional medicine and may have public health and clinical implications for the 6–21% of women worldwide who suffer with PCOS without any side effects.
5. Conclusion
The current research shows that LET-induced PCOS is linked to endocrine and metabolic changes, which are caused by a decrease in circulating adiponectin and a concomitant increase in circulating Luteinizing hormone. In conclusion, Turmeric Extract showed promising effects in treating PCOS and inducing ovulation when compared to metformin. Turmeric Extract improved hormone and lipid profiles, antioxidant and glycemic status, and ovarian morphology in Letrozole-induced PCOS rats. These effects could be attributed to its multiple pharmacological actions, which include estrogenic, antihyperlipidemic, antioxidant, and hypoglycemic qualities, all of which could help regulate PCOS and prevent ovarian cell dysfunction, ovulation, and so improve fertility. Turmeric Extract is a promising drug for treating clinical and pathological disorders linked with PCOS due to its broad range of biological effects. Furthermore, adiponectin appears to be a common feature of PCOS with metabolic and endocrine aspects, at least in part. By altering circulating androgen-adiponectin balance, the data implies that Turmeric extract alleviates endocrine-metabolic abnormalities and inflammation-related comorbidities associated with LET-induced PCOS.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Mohd Zahoor ul Haq shah: Conceptualization, Writing – original draft, Formal analysis, Resources, Investigation. Vinoy Kumar Shrivastava: Supervision, Writing – review & editing.
Acknowledgement
Authors are highly thankful to Clementia Biotech New Delhi for their negotiation in providing the Elisa Kits at much affordable Cost and Amruti Herbals Indore for providing us the turmeric Extract for the research purpose.
Contributor Information
Mohd Zahoor ul Haq shah, Email: scholarendocrinology@gmail.com.
Vinoy Kumar Shrivastava, Email: vinoyks2001@yahoo.com.
References
- 1.Brakta S., Lizneva D., Mykhalchenko K., Imam A., Walker W., Diamond M.P., et al. Perspectives on Polycystic Ovary Syndrome: is Polycystic Ovary Syndrome research underfunded? J Clin Endocrinol Metab. 2017 Dec 1;102(12):4421–4427. doi: 10.1210/jc.2017-01415. PMID: 29092064. [DOI] [PubMed] [Google Scholar]
- 2.Melo A.S., Ferriani R.A., Navarro P.A. Treatment of infertility in women with Polycystic Ovary Syndrome: approach to clinical practice. Clinics. 2015 Nov;70(11):765–769. doi: 10.6061/clinics/2015(11)09. PMID: 26602525; PMCID: PMC4642490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zafar U., Memon Z., Moin K., Agha S., Hassan J.A., Zehra D. Prevalence of PCOS with associated symptoms and complications at tertiary care hospital of Karachi. J Adv Med Med Res. 2019;30(4):1–9. doi: 10.9734/jammr/2019/v30i430190. [DOI] [Google Scholar]
- 4.Joham A.E., Teede H.J., Ranasinha S., Zoungas S., Boyle J. Prevalence of infertility and use of fertility treatment in women with Polycystic Ovary Syndrome: data from a large community-based cohort study. J Womens Health (Larchmt) 2015 Apr;24(4):299–307. doi: 10.1089/jwh.2014.5000. Epub 2015 Feb 5. PMID: 25654626. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez R.C., Moore V.M., Van Ryswyk E.M., Varcoe T.J., Rodgers R.J., March W.A., et al. Sleep disturbances in women with Polycystic Ovary Syndrome: prevalence, pathophysiology, impact and management strategies. Nat Sci Sleep. 2018;10:45–64. doi: 10.2147/NSS.S127475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf W.M., Wattick R.A., Kinkade O.N., Olfert M.D. Geographical prevalence of Polycystic Ovary Syndrome as determined by region and race/ethnicity. Int J Environ Res Publ Health. 2018 Nov 20;15(11):2589. doi: 10.3390/ijerph15112589. PMID: 30463276; PMCID: PMC6266413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmina E. Diagnosis of Polycystic Ovary Syndrome: from NIH criteria to ESHRE-ASRM guidelines. Minerva Ginecol. 2004 Feb;56(1):1–6. PMID: 14973405. [PubMed] [Google Scholar]
- 8.Meyer C., McGrath B.P., Cameron J., Kotsopoulos D., Teede H.J. Vascular dysfunction and metabolic parameters in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2005 Aug;90(8):4630–4635. doi: 10.1210/jc.2004-1487. Epub 2005 May 3. PMID: 15870132. [DOI] [PubMed] [Google Scholar]
- 9.Groth S.W. Adiponectin and Polycystic Ovary Syndrome. Biol Res Nurs. 2010 Jul;12(1):62–72. doi: 10.1177/1099800410371824. Epub 2010 May 24. PMID: 20498127; PMCID: PMC3646519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magkos F., Sidossis L.S. Recent advances in the measurement of adiponectin isoform distribution. Curr Opin Clin Nutr Metab Care. 2007 Sep;10(5):571–575. doi: 10.1097/MCO.0b013e3282bf6ea8. PMID: 17693739. [DOI] [PubMed] [Google Scholar]
- 11.Ahima R.S. Metabolic actions of adipocyte hormones: focus on adiponectin. Obesity. 2006 Feb;14(1):9S–15S. doi: 10.1038/oby.2006.276. PMID: 16642957. [DOI] [PubMed] [Google Scholar]
- 12.von Frankenberg A.D., do Nascimento F.V., Gatelli L.E., Nedel B.L., Garcia S.P., et al. Major components of metabolic syndrome and adiponectin levels: a cross-sectional study. Diabetol Metab Syndrome. 2014;6(1):26. doi: 10.1186/1758-5996-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloomgarden Z.T. Insulin resistance: causes and consequences. Int Rev Neurobiol. 2005;65:1–24. doi: 10.1016/S0074-7742(04)65001-X. PMID: 16140051. [DOI] [PubMed] [Google Scholar]
- 14.Rasouli N., Kern P.A. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008 Nov;93(11 Suppl 1):S64–S73. doi: 10.1210/jc.2008-1613. PMID: 18987272; PMCID: PMC2585759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mente A., Meyre D., Lanktree M.B., Heydarpour M., Davis A.D., Miller R., et al. Causal relationship between adiponectin and metabolic traits: a Mendelian randomization study in a multiethnic population. PLoS One. 2013 Jun 24;8(6) doi: 10.1371/journal.pone.0066808. SHARE Investigators; SHARE-AP Investigators. PMID: 23826141; PMCID: PMC3691277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittas A.G., Joseph N.A., Greenberg A.S. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004 Feb;89(2):447–452. doi: 10.1210/jc.2003-031005. PMID: 14764746. [DOI] [PubMed] [Google Scholar]
- 17.Mirza S.S., Shafique K., Shaikh A.R., Khan N.A., Anwar Qureshi M. Association between circulating adiponectin levels and polycystic ovarian syndrome. J Ovarian Res. 2014 Feb 7;7:18. doi: 10.1186/1757-2215-7-18. PMID: 24502610; PMCID: PMC3928320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daan N.M., Koster M.P., de Wilde M.A., Dalmeijer G.W., Evelein A.M., Fauser B.C., et al. Biomarker profiles in women with PCOS and PCOS offspring; A pilot study. PLoS One. 2016 Nov 2;11(11) doi: 10.1371/journal.pone.0165033. PMID: 27806063; PMCID: PMC5091782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Chen Y., Li Y., Huang J., Zhao X., Chen X., Yang D. A case-control study of correlation between serum adiponectin levels and Polycystic Ovary Syndrome. Zhonghua Fu Chan Ke Za Zhi. 2015;50(11):814–818. [PubMed] [Google Scholar]
- 20.Benrick A., Chanclón B., Micallef P., Wu Y., Hadi L., Shelton J.M., et al. Adiponectin protects against development of metabolic disturbances in a PCOS mouse model. Proc Natl Acad Sci U S A. 2017 Aug 22;114(34):E7187–E7196. doi: 10.1073/pnas.1708854114. Epub 2017 Aug 8. PMID: 28790184; PMCID: PMC5576831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frithioff-Bøjsøe C., Lund M.A.V., Lausten-Thomsen U., Hedley P.L., Pedersen O., Christiansen M., et al. Leptin, adiponectin, and their ratio as markers of insulin resistance and cardiometabolic risk in childhood obesity. Pediatr Diabetes. 2020 Mar;21(2):194–202. doi: 10.1111/pedi.12964. Epub 2019 Dec 26. PMID: 31845423. [DOI] [PubMed] [Google Scholar]
- 22.Ndefo U.A., Eaton A., Green M.R. Polycystic Ovary Syndrome: a review of treatment options with a focus on pharmacological approaches. P T. 2013 Jun;38(6):336–355. PMID: 23946629; PMCID: PMC3737989. [PMC free article] [PubMed] [Google Scholar]
- 23.Moon M.K., Hur K.Y., Ko S.H., Park S.O., Lee B.W., Kim J.H., et al. Committee of Clinical Practice Guidelines of the Korean Diabetes Association. Combination therapy of oral hypoglycemic agents in patients with type 2 diabetes mellitus. Korean J Intern Med. 2017 Nov;32(6):974–983. doi: 10.3904/kjim.2017.354. Epub 2017 Oct 27. PMID: 29096431; PMCID: PMC5668409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lashen H. Role of metformin in the management of Polycystic Ovary Syndrome. Ther Adv Endocrinol Metabol. 2010 Jun;1(3):117–128. doi: 10.1177/2042018810380215. PMID: 23148156; PMCID: PMC3475283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legro R.S., Zaino R.J., Demers L.M., Kunselman A.R., Gnatuk C.L., Williams N.I., et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in Polycystic Ovary Syndrome. Am J Obstet Gynecol. 2007 Apr;196(4) doi: 10.1016/j.ajog.2006.12.025. 402.e1-10; discussion 402.e10-1. PMID: 17403436. [DOI] [PubMed] [Google Scholar]
- 26.Diamanti-Kandarakis E., Christakou C., Kandarakis H. Polycystic ovarian syndrome: the commonest cause of hyperandrogenemia in women as a risk factor for metabolic syndrome. Minerva Endocrinol. 2007 Mar;32(1):35–47. PMID: 17353865. [PubMed] [Google Scholar]
- 27.Kolivand M., Kerama A., Khosravi A. The effect of herbal teas on management of Polycystic Ovary Syndrome: a systematic review. J Midwif Reprod Health. 2017;5(4):1098–1106. doi: 10.22038/jmrh.2017.9368. 2017. [DOI] [Google Scholar]
- 28.Badawy A., Elnashar A. Treatment options for Polycystic Ovary Syndrome. Int J Womens Health. 2011 Feb 8;3:25–35. doi: 10.2147/IJWH.S11304. PMID: 21339935; PMCID: PMC3039006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewlings S.J., Kalman D.S. vol. 6. 2017. p. 92. (Curcumin: a review of its effects on human health Foods). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nistico S., Tamburi F., Bennardo L., Dastoli S., Schipani G., Caro G., et al. Treatment of telogen effluvium using a dietary supplement containing Boswellia serrata, Curcuma longa, and Vitis vinifera: results of an observational study. Dermatol Ther. 2019 May;32(3) doi: 10.1111/dth.12842. Epub 2019 Feb 8. PMID: 30693615. [DOI] [PubMed] [Google Scholar]
- 31.Wojcik M., Krawczyk M., Wojcik P., Cypryk K., Wozniak L.A. Molecular mechanisms underlying curcumin-mediated therapeutic effects in type 2 diabetes and cancer. Oxid Med Cell Longev. 2018 Mar 20:9698258. doi: 10.1155/2018/9698258. 2018. PMID: 29743988; PMCID: PMC5884026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pari L., Murugan P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J Basic Clin Physiol Pharmacol. 2005;16(4):257–274. doi: 10.1515/jbcpp.2005.16.4.257. PMID: 16438392. [DOI] [PubMed] [Google Scholar]
- 33.Pari L., Murugan P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail. 2007;29(7):881–889. doi: 10.1080/08860220701540326. PMID: 17994458. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal B.B., Banerjee S., Bharadwaj U., Sung B., Shishodia S., Sethi G. Curcumin induces the degradation of cyclin E expression through ubiquitin-dependent pathway and up-regulates cyclin-dependent kinase inhibitors p21 and p27 in multiple human tumor cell lines. Biochem Pharmacol. 2007 Apr 1;73(7):1024–1032. doi: 10.1016/j.bcp.2006.12.010. Epub 2006 Dec 15. Retraction in: Biochem Pharmacol. 2016 Feb 15;102:147. PMID: 17240359. [DOI] [PubMed] [Google Scholar]
- 35.Watson J.L., Greenshields A., Hill R., Hilchie A., Lee P.W., Giacomantonio C.A., et al. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol Carcinog. 2010 Jan;49(1):13–24. doi: 10.1002/mc.20571. PMID: 19676105. [DOI] [PubMed] [Google Scholar]
- 36.Atilla Kadasi, Sirotkin Alexander V., Nora Maruniakova, Adriana Kolesarova, Jozef Bulla, Roland Grossmann. The effect of Curcumin on secretory activity, proliferation and apoptosis of the porcine ovarian granulose cells. JMBFS. 2012;2(1):349–357. [Google Scholar]
- 37.Bachmeier B.E., Mirisola V., Romeo F., Generoso L., Esposito A., Dell'eva R., et al. Reference profile correlation reveals estrogen-like transcriptional activity of Curcumin. Cell Physiol Biochem. 2010;26(3):471–482. doi: 10.1159/000320570. Epub 2010 Aug 24. PMID: 20798532. [DOI] [PubMed] [Google Scholar]
- 38.Panahi Y., Hosseini M.S., Khalili N., et al. Effects of supplementation with curcumin on serum adipokine concentrations: a randomized controlled trial. Nutrition. 2016;32(10):1116–1122. doi: 10.1016/j.nut.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava Raj Kamal, Krishna Amitabh. Pathophysiology of Polycystic Ovary Syndrome (PCOS): lessons from animal studies. Proc India Natl Sci Acad. 2006;B71:191–205. [Google Scholar]
- 40.Fisher C.R., Graves K.H., Parlow A.F., Simpson E.R. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6965–6970. doi: 10.1073/pnas.95.12.6965. PMID: 9618522; PMCID: PMC22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kafali H., Iriadam M., Ozardali I., Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004 Mar-Apr;35(2):103–108. doi: 10.1016/j.arcmed.2003.10.005. PMID: 15010188. [DOI] [PubMed] [Google Scholar]
- 42.Desai B.N., Maharjan R.H., Nampoothiri L.P. Aloe barbadensis Mill. formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacogn Res. 2012 Apr;4(2):109–115. doi: 10.4103/0974-8490.94736. PMID: 22518083; PMCID: PMC3326757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maharjan R., Nagar P.S., Nampoothiri L. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integr Med. 2010 Oct;1(4):273–279. doi: 10.4103/0975-9476.74090. PMID: 21731374; PMCID: PMC3117319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soumya V., Muzib Y.I., Venkatesh P. A novel method of extraction of bamboo seed oil (Bambusa bambos Druce) and its promising effect on metabolic symptoms of experimentally induced polycystic ovarian disease. Indian J Pharmacol. 2016 Mar-Apr;48(2):162–167. doi: 10.4103/0253-7613.178833. PMID: 27127318; PMCID: PMC4825433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman Shafiq-Ur. Lead-induced regional lipid peroxidation in brain. Toxicol Lett. 1984 Jun;21(3):333–337. doi: 10.1016/0378-4274(84)90093-6. PMID: 6740722. [DOI] [PubMed] [Google Scholar]
- 46.Rahman I., Kode A., Biswas S. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 47.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. PMID: 4215654. [DOI] [PubMed] [Google Scholar]
- 48.El-bahya A.A.Z., Radwanb R.A., Gadc M.Z., Abdel S.M. vol. 6. 2018. (A closer insight into the role of vitamin D in Polycystic Ovary Syndrome (Pcos)). 4. [Google Scholar]
- 49.Rodgers R.J., Suturina L., Lizneva D., Davies M.J., Hummitzsch K., Irving-Rodgers H.F., Robertson S.A. Is Polycystic Ovary Syndrome a 20th Century phenomenon? Med Hypotheses. 2019 Mar;124:31–34. doi: 10.1016/j.mehy.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Wang H.S. The role of metformin in the treatment of Polycystic Ovary Syndrome (PCOS) Chang Gung Med J. 2006 Sep-Oct;29(5):445–447. PMID: 17214387. [PubMed] [Google Scholar]
- 51.Kauffman A.S., Thackray V.G., Ryan G.E., Tolson K.P., Glidewell-Kenney C.A., Semaan S.J., et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of Polycystic Ovary Syndrome in female mice. Biol Reprod. 2015 Sep;93(3):69. doi: 10.1095/biolreprod.115.131631. Epub 2015 Jul 22. PMID: 26203175; PMCID: PMC4710190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenfield R.L., Ehrmann D.A. The pathogenesis of Polycystic Ovary Syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016 Oct;37(5):467–520. doi: 10.1210/er.2015-1104. Epub 2016 Jul 26. PMID: 27459230; PMCID: PMC5045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faloia E., Canibus P., Gatti C., Frezza F., Santangelo M., Garrapa G.G., et al. Body composition, fat distribution and metabolic characteristics in lean and obese women with Polycystic Ovary Syndrome. J Endocrinol Invest. 2004 May;27(5):424–429. doi: 10.1007/BF03345285. PMID: 15279073. [DOI] [PubMed] [Google Scholar]
- 54.Dunaif A., Mandeli J., Fluhr H., Dobrjansky A. The impact of obesity and chronic hyperinsulinemia on gonadotropin release and gonadal steroid secretion in the Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 1988 Jan;66(1):131–139. doi: 10.1210/jcem-66-1-131. PMID: 2961783. [DOI] [PubMed] [Google Scholar]
- 55.Carmina E., Bucchieri S., Esposito A., Del Puente A., Mansueto P., Orio F., et al. Abdominal fat quantity and distribution in women with Polycystic Ovary Syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007 Jul;92(7):2500–2505. doi: 10.1210/jc.2006-2725. Epub 2007 Apr 3. PMID: 17405838. [DOI] [PubMed] [Google Scholar]
- 56.Purohit A., Ghilchik M.W., Duncan L., Wang D.Y., Singh A., Walker M.M., Reed M.J. Aromatase activity and interleukin-6 production by normal and malignant breast tissues. J Clin Endocrinol Metab. 1995 Oct;80(10):3052–3058. doi: 10.1210/jcem.80.10.7559896. PMID: 7559896. [DOI] [PubMed] [Google Scholar]
- 57.Mohamed-Ali V., Goodrick S., Rawesh A., Katz D.R., Miles J.M., Yudkin J.S., Klein S., Coppack S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997 Dec;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. PMID: 9398739. [DOI] [PubMed] [Google Scholar]
- 58.Gervásio C.G., Bernuci M.P., Silva-de-Sá M.F., Rosa-E-Silva A.C. The role of androgen hormones in early follicular development. ISRN Obstet Gynecol. 2014 Apr 10:818010. doi: 10.1155/2014/818010. 2014. PMID: 25006485; PMCID: PMC4003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baravalle C., Salvetti N.R., Mira G.A., Pezzone N., Ortega H.H. Microscopic characterization of follicular structures in letrozole induced polycystic ovarian syndrome in the rat. Arch Med Res. 2006;37:830–839. doi: 10.1016/j.arcmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Rezvanfar M.A., Ahmadi A., Shojaei-Saadi H.A., Baeeri M., Abdollahi M. Molecular mechanism of a novel selenium based complementary medicine which confers protection against hyperandrogenism induced polycystic ovary. Theriogenology. 2012;78:620–631. doi: 10.1016/j.theriogenology.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Sasikala S.L., Shamila S. Unique rat model exhibiting biochemical fluctuations of letrozole induced Polycystic Ovary Syndrome and subsequent treatment with allopathic and ayurvedic medicines. J Cell Tissue Res. 2009;9:2013–2017. [Google Scholar]
- 62.Khaled N., El-Bahy A.A., Radwan R., Handoussa H., AbdelMaksoud S. Ocimum kilimandscharicum L. restores ovarian functions in letrozole - induced Polycystic Ovary Syndrome (PCOS) in rats: comparison with metformin. Life Sci. 2019 Sep 1;232:116640. doi: 10.1016/j.lfs.2019.116640. Epub 2019 Jul 8. PMID: 31295470. [DOI] [PubMed] [Google Scholar]
- 63.Boudreaux M.Y., Talbott E.O., Kip K.E., Brooks M.M., Witchel S.F. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diabetes Rep. 2006 Feb;6(1):77–83. doi: 10.1007/s11892-006-0056-1. PMID: 16522285. [DOI] [PubMed] [Google Scholar]
- 64.Teich T., Pivovarov J.A., Porras D.P., Dunford E.C., Riddell M.C. Curcumin limits weight gain, adipose tissue growth, and glucose intolerance following the cessation of exercise and caloric restriction in rats. J Appl Physiol. 1985;123(6):1625–1634. doi: 10.1152/japplphysiol.01115.2016. 2017 Dec 1. Epub 2017 Aug 24. PMID: 28839007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Von Eckardstein A. In: Testosterone: action, deficiency, substitution. second ed. Nieschlag E., Behre H.M., editors. Springer; Berlin, Heidelberg, New York: 1998. Androgens, cardiovascular risk factors and atherosclerosis; pp. 229–258. [Google Scholar]
- 66.Croston G.E., Milan L.B., Marschke K.B., Reichman M., Briggs M.R. Androgen receptor-mediated antagonism of estrogen-dependent low density lipoprotein receptor transcription in cultured hepatocytes. Endocrinology. 1997;138:3779–3786. doi: 10.1210/endo.138.9.5404. [DOI] [PubMed] [Google Scholar]
- 67.Panahi Y., Ahmadi Y., Teymouri M., Johnston T.P., Sahebkar A. Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol. 2018 Jan;233(1):141–152. doi: 10.1002/jcp.25756. Epub 2017 Jun 6. PMID: 28012169. [DOI] [PubMed] [Google Scholar]
- 68.Sabuncu T., Vural H., Harma M., Harma M. Oxidative stress in Polycystic Ovary Syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001 Jul;34(5):407–413. doi: 10.1016/s0009-9120(01)00245-4. PMID: 11522279. [DOI] [PubMed] [Google Scholar]
- 69.Liu J., Zhang D. [The role of oxidative stress in the pathogenesis of Polycystic Ovary Syndrome] Sichuan Da Xue Xue Bao Yi Xue Ban. 2012 Mar;43(2):187–190. Chinese. PMID: 22650028. [PubMed] [Google Scholar]
- 70.Pari L., Murugan P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J Basic Clin Physiol Pharmacol. 2005;16(4):257–274. doi: 10.1515/jbcpp.2005.16.4.257. PMID: 16438392. [DOI] [PubMed] [Google Scholar]
- 71.Antonio Ayala, Munoz Mario F., Sandro Arguelles. Lipid peroxidation: production, metabolism and signalling mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev. 2014:360438. doi: 10.1155/2014/360438. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alizadeh M., Kheirouri S. Curcumin reduces malondialdehyde and improves antioxidants in humans with diseased conditions: a comprehensive meta-analysis of randomized controlled trials. Biomedicine. 2019 Dec;9(4):23. doi: 10.1051/bmdcn/2019090423. Epub 2019 Nov 14. PMID: 31724938; PMCID: PMC6855189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee Y., Fluckey J.D., Chakraborty S., Muthuchamy M. Hyperglycemia- and hyperinsulinemia-induced insulin resistance causes alterations in cellular bioenergetics and activation of inflammatory signaling in lymphatic muscle. Faseb J. 2017 Jul;31(7):2744–2759. doi: 10.1096/fj.201600887R. Epub 2017 Mar 15. PMID: 28298335; PMCID: PMC5471512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rak A., Mellouk N., Froment P., Dupont J. Adiponectin and resistin: potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction. 2017 Jun;153(6):R215–R226. doi: 10.1530/REP-17-0002. Epub 2017 Mar 22. PMID: 28330882. [DOI] [PubMed] [Google Scholar]
- 75.Yuan X., Hu T., Zhao H., Huang Y., Ye R., Lin J., et al. Brown adipose tissue transplantation ameliorates Polycystic Ovary Syndrome. Proc Natl Acad Sci U S A. 2016 Mar 8;113(10):2708–2713. doi: 10.1073/pnas.1523236113. Epub 2016 Feb 22. PMID: 26903641; PMCID: PMC4790997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chabrolle C., Tosca L., Ramé C., Lecomte P., Royère D., Dupont J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril. 2009 Dec;92(6):1988–1996. doi: 10.1016/j.fertnstert.2008.09.008. Epub 2008 Dec 10. PMID: 19081562. [DOI] [PubMed] [Google Scholar]
- 77.Panahi Y., Hosseini M.S., Khalili N., Naimi E., Soflaei S.S., Majeed M., Sahebkar A. Effects of supplementation with curcumin on serum adipokine concentrations: a randomized controlled trial. Nutrition. 2016 Oct;32(10):1116–1122. doi: 10.1016/j.nut.2016.03.018. Epub 2016 Mar 31. PMID: 27297718. [DOI] [PubMed] [Google Scholar]
- 78.Reddy P.S., Begum N., Mutha S., Bakshi V. Beneficial effect of curcumin in letrozole induced Polycystic Ovary Syndrome. Asian Pacific J Reprod. 2016;5(2):116–122. [Google Scholar]