Abstract
Oesophageal carcinoma ranks the sixth leading cause of cancer death and affected 544,000 - 604,000 people in 2020. Patients often presented with a poor cancer prognosis with a low survival rate of 15–25%. Depending upon the cell type, oesophageal carcinoma is categorised into oesophageal squamous cell carcinoma (ESCC) and oesophageal adenocarcinoma (EAC). ESCC is predominantly reported in developing countries, while EAC is more common in developed countries. Aside from the presence of exogenous co-factors, such as cigarette smoking, alcohol consumption, obesity, gastroesophageal reflux disease (GERD); infection with oncogenic viruses is suspected to be one of the major factors contributing to EC development. Oncogenic viruses, including human papillomavirus (HPV), Epstein Barr virus (EBV), Cytomegalovirus (CMV) and Herpes Simplex Virus (HSV) have been detected in various proportions of EC samples. Nonetheless, their aetiological roles in EC remain debatable. In this review, we garnered previous studies that focus on the association between oncogenic viruses and EC. Among these oncogenic viruses, HPV appears to have a stronger association with EC than the others. In addition, we also discuss the pros and cons of the treatment regimens to treat EC patients, including immunotherapy, chemo- and chemoradiotherapy, and their efficacy.
Keywords: Oesophageal carcinoma, HPV, EBV, HSV, CMV
1. Introduction
Oesophageal cancer (EC) ranks the sixth leading cause of cancer death and seventh in incidence worldwide, with an estimation of 544,000 and 604,000 new cases in 2020 [1]. The incidence of EC varied geographically, with more cases reported in developing than developed countries. Countries that fall into the “Asian Oesophageal Cancer Belt”, including Northern Iran and East Turkey to East Asia (North and Central China) [2,3] exhibit the highest incidence of EC in both women and men, followed by South Africa, East Africa and Central Asia [1]. While the incidence of EC is relatively low in North America and Europe.
EC is classified into 2 common histological subtypes, namely oesophageal squamous cell carcinoma (ESCC) and oesophageal adenocarcinoma (EAC). Intriguingly, the prevalence of these 2 types of EC differ geographically, with EAC appearing to be the predominant type reported in developed countries, whilst ESCC is in developing countries [4]. The major risk factors that contribute to the development of EC in developed countries include cigarette smoking, alcohol consumption, obesity and Barrett’s esophagus caused by chronic gastroesophageal reflux disease (GERD) [5]. Chemical composition in tobacco and metabolite of alcohol can be carcinogenic and may regulate expression of human genes, including oncogenes [6,7]. On the other hand, other physiological conditions, including GERD and body mass index (BMI), also contribute to the likelihood of EC development. GERD is a chronic disorder in which gastric content flows back into the oesophagus, leading to symptoms like heartburn, taste of acid in the mouth and halitosis. Individuals affected by GERD have a higher risk to develop EC (odds ratio = 7.7, 95% CI 5.3–11.4) compared with those who do not suffer from such condition [8]. Similarly, high BMI (>30) has a higher chance of getting EAC (odds ratio = 3.17, 95% CI 1.43–7.04) than those of lower BMI [9]. Furthermore, infection by oncogenic viruses appears to be one of the important factors contributing to EC development [10]. The risk factors contributing to EC are summarized in Table 1.
Table 1.
The risk factors contributing to the development of oesophageal Cancer.
| The major risk factors |
|---|
|
| Other risk factors |
|
2. Association between oncoviruses and oesophageal cancer
Even though there are ample studies underlined microbial infection as one of the contributing elements to EC, this remains controversial. Some epidemiological findings revealed the involvement of oncogenic viruses, including Human Papillomavirus (HPV), Epstein Barr virus (EBV),Cytomegalovirus (CMV) and Herpes Simplex Virus (HSV) as potential contributors to EC development. Among these viruses, the majority of studies focused on the association between HPV infection and EC, whilst the association of EC with other DNA tumour viruses was relatively less studied. In the section below, we unveiled the positive association of these DNA viruses with EC and the contradicting findings. The prevalence of oncogenic viruses in EC was summarised in Table 2.
Table 2.
The summary of prevalence of oncoviruses in oesophageal carcinoma.
| Oncogenic virus | Prevalence in EC |
|
|---|---|---|
| ESCC | EAC | |
| HPV | 22.2% [15] a | 35.0–66.7% [15,20] a |
| EBV | 8.3–36% [50,51] | 36% [50] |
| HSV | 31.7% [48] | – |
EC = Oesophageal carcinoma; ESCC = Oesophageal squamous carcinoma cell; EAC = Oesophageal adenocarcinoma; HPV = Human Papillomavirus; HSV = Herpes simplex virus; EBV = Epstein Barr virus.
Total prevalence of HPV in ESCC is summarized and referenced from systematic review [15].
2.1. Human Papillomavirus (HPV) in EC
HPV is a small double-stranded circular DNA virus that belongs to the Papillomaviridae family. HPV infection is the most common viral sexually transmitted disease, and it was estimated that approximately 50% of both men and women have been exposed to HPV at least once in their lifetime [11]. To date, more than 200 HPV genotypes have been identified. Among them, 13 types are classified as the high-risk HPVs (hrHPVs) due to their high carcinogenic properties [12]. Most individuals with HPV infection presented with no symptom. However, persistent infection with hrHPVs may lead to anogenital warts, cervical cancers, head and neck cancer (HNC), EC, and other anogenital cancers [13].
2.1.1. HPV in ESCC
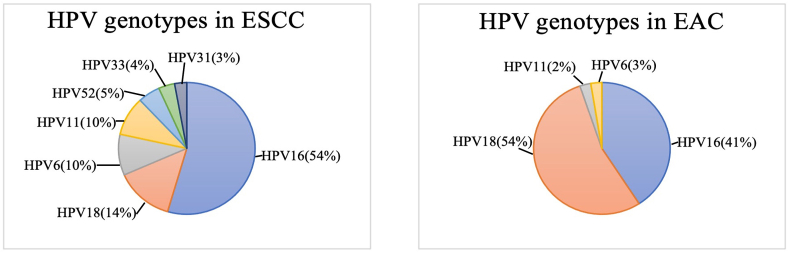
In 1982, HPV was first suggested as an aetiologic agent contributing to the development of ESCC [14]. Since then, more studies focused on the association of HPV and ESCC had been conducted. A meta-analysis revealed the overall prevalence of HPV infection in ESCC and EAC was 22.2% (95% CI, 18.3–26.7%) and 35.0% (95% CI, 13.2–65.7%), respectively. The most common HPV genotypes detected in ESCC were HPV16 (11.4%), HPV18 (2.9%), HPV6 (2.1%), HPV11 (2.0%), HPV52 (1.1%), HPV33 (0.8%) and HPV31 (0.6%) [15]. The prevalence of HPV genotypes in EC (ESCC and EAC) was summarised in Fig. 1a and b. Demographically, 20% of men and 18.4% of women were positive for HPV in ESCC. In terms of geography, HPV positive rate in ESCC in Asia (26.3%) is almost double that of in America and Europe (14.0%) [15].
Fig. 1.
The prevalence of HPV genotypes in two types of oesophageal Cancer: (a) Oesophageal squamous cell carcinoma (ESCC); and (b) Oesophageal adenocarcinoma (EAC).
We found that in ESCC, HPV18 oncoproteins do not exploit the same mechanism to promote cancer progression as known for cervical cancer. Instead of targeting pRB, E7 preferentially abrogates p130 in EC109 and EC9706, which are the HPV18 containing ESCC cell lines of Asian origin [16]. In addition to p53 and its downstream transcription transactivator [16], HPVE6 deregulates miR-125b, a microRNA that acts as a tumour suppressor [17]. The downregulation of miR-125b may subsequently lead to activation of the Wnt/β-catenin signaling pathway, which is a critical pathway involved in cell differentiation, migration, proliferation, and cell death. Dysregulation of this pathway has been implicated in human cancers, including cervical and HNC [18,19]. Although miR-125b is not a biomarker or therapeutic target, this finding suggested a possible mechanism on how HPV promotes ESCC development [20].
2.1.2. HPV and EAC
A strong association between hrHPV and EAC was first documented by Rajendra et al., in which HPV DNA was detected in 81 out of 261 oesophageal biopsies. Compare with controls (18%), HPV positivity was significantly more common in EAC (66.7%, IRR 2.87, 95% CI 1.69–4.86, p<0.001), with 93% were infected with hrHPV genotypes [21]. HPV infection can be a “hit-and-run” incident. The detection of HPV DNA may not accurately inform an active HPV infection. Hence, Rajendra and co-workers detected the HPV DNA and E6/E7 mRNA (HPV DNA+/RNA+) in formalin-fixed paraffin-embedded (FFPE) samples. They detected transcriptionally active HPV in 25.7% of EAC samples, and only 9.6% in dysplastic and adenocarcinoma samples [22]. Besides detecting HPV nucleic acids in tissue specimens, HPV cell-free circulating DNA (ctDNA) in plasma can be detected using an ultrasensitive droplet-digital polymerase chain reaction (ddPCR) [23].
However, the aetiological role of HPV in EAC can be inconclusive. For instance, in Australia, a study reported the detection of HPV16 and 18 E7 genes in 15% of EAC patients [24], while the other study did not find HPV DNA in their samples collected [25]. In another systematic review which gathered 19 studies, a pooled HPV prevalence of 12.5% (Cl:1.5–28.8%) was found in EAC samples. Meanwhile, a study conducted by The Cancer Genome Atlas (TCGA) Research Network found no aetiological role of HPV in EC [26].
The high degree of heterogeneity (I2:92.6%) is likely due to the variety of HPV detection methods used. The specificity of PCR primer pairs, the sensitivity of the tests, sample storing duration and condition may affect the sample integrity. In particular, archived samples collected for more than 10 years may contain DNA of poor quality as a portion of the DNA may have been degraded [27,28]. In order to provide a conclusive role of HPV in EAC, more studies are required. Ideally, the biopsy collection and processing method should follow the standard protocol outlined in the Human papillomavirus Laboratory Manual by the World Health Organisation (WHO) [29]. In addition, HPV DNA should also be tested using validated HPV DNA detection platforms rather than those yet-to-be validated assays [30].
2.2. Epstein Barr virus (EBV) in EC
EBV, or the human herpesvirus 4, is a double-stranded DNA virus that belongs to the Herpesviridae family [31]. EBV infects approximately 90% of the people in the world [32]. Indeed, EBV is associated with 1% of cancer globally, mainly associated to Burkitt’s lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma, gastric carcinoma and leiomyosarcoma [33]. Most acute EBV infections are asymptomatic. During the latency phase, EBV expresses Epstein-Barr nuclear 1 (EBNA1) which facilitates the viral replication and mitotic segregation of EBV episomes that persists for life [34,35].
To detect EBV in clinical samples, DNA extract or tissue sections were subjected to PCR, immunohistochemistry and in situ hybridization. Similar to HPV, the detection rate of EBV in EC varied greatly, ranging from 1.8 to 35.5% in different studies [[36], [37], [38], [39]]. The first EBV positive ESCC case was documented by Jenkins and colleagues in 1996, in which 8.33% ESCC tumour and 6.25% cell lines contained BamH1W fragment, a highly conserved EBV genome [40]. While EBV DNA was detected in 35–36% of samples in Taiwan [39] and Germany [41].
On the contrary, another study found no persistence of EBV in ESCC and EAC patients samples in Germany and Russia [42]. Similarly, studies from other regions, including Malaysia [38], China [43,44], Iran [45], Greek [46], Japan [47,48] also indicated no correlation between EBV and EC. Therefore, whether EBV is related to EC remains unclear. Even within the same country, controversial observations have been reported. A larger cohort study from different regions is required to straighten the controversial findings and unveil the correlation between EBV and EC.
3. Cytomegalovirus (CMV) and herpes simplex virus (HSV) in EC
CMV and Herpes Simplex Virus (HSV) belong to the Herpesviridae family. CMV has the largest genome among herpes viruses. CMV infections are acquired from perinatal periods or sexual contact during adulthood [49]. This virus can infect many organs including the eyes, gastrointestinal tract, colitis and oesophagus. CMV-associated oesophagitis was reported in EC patients [50]. However, the association between CMV-associated EC has not been delineated. Even in Shantou, a region in China with the highest incidence of EC, reported no CMV infection in EC patients [51].
HSV is divided into HSV-1 and HSV-2. Of these, HSV-1 infection usually causes herpes lesions in the head and neck region, especially in the oral cavity. Meanwhile, HSV-2 infection is frequently associated with genital lesions, and it can act as one of the co-factors for cervical cancer [52,53]. In Shantou, studies revealed the prevalence of HSV-associated EC stands at approximately 30%. Wu and colleagues investigated the prevalence of HSV and its aetiological roles in EC patients. They detected both DNA and protein of HSV-1 and -2 in 31.7% of the well-differentiated ESCC samples, suggesting a potential aetiological role of HSV in ESCC [52]. Moreover, mixed herpes viruses and human papillomavirus infection (HSV-1, HPV-16 and EBV) appear to be highly associated (71.4%) with the high grade of EC [51]. However, the mechanism of how these viruses perturb host cellular events during EC development remains to be further elucidated. Thus, it is crucial to detect the presence of various viruses during EC development and understand the virus-host cellular interaction that drives carcinogenesis.
4. The prognostic role of oncoviruses in oesophageal carcinoma
Albeit the controversial observations concerning the attribution of oncoviruses in EC, several studies were conducted to evaluate the prognostic significance of oncoviruses. The prognostic role of oncoviruses in EC remains inconclusive. Furihata et al. reported that HPV positivity and overexpression of p53 tumour suppressor contributed to a poorer survival rate than ESCC patients without HPV detected, nor p53 overexpression [54]. Whilst in EAC patients, finding from Rajendra et al. showed a favourable overall disease-free survival for patients with HPV and low level of retinoblastoma tumour suppressor expression [55]. These findings imply that HPV positivity and expression status of tumour suppressors possess important prognostic value. Nonetheless, another study may not agree to this notion. Dreilich and colleagues showed HPV status did not reflect the survival rate of EC patients, and does not affect the survival of patients who received chemotherapy or radiation therapy (P > 0.05) [56]. Asides of HPV, data on prognostic value of other oncoviruses in EC is lacking, perhaps due to the weak association of other oncoviruses with EC.
5. Therapeutics for oesophageal carcinoma
Currently, the treatment methods available for EC disregard whether the cancer is virally driven or not. Additionally, a specific antiviral agent for oncovirus-associated EC is lacking. Treatment options available for EC patients include surgery alone, immunotherapy, chemotherapy, and radiotherapy. Among these, surgical resection is the prime choice of treatment for locoregional EC [57]. Nonetheless, chemotherapy, radiotherapy and combined treatment modality have been shown to increase patient survival rate significantly as compared with surgical resection alone. The overall 5-year survival rate of EC patients ranged from 15% to 25% [58]. Similarly, immunotherapy can improve the survival of EC patients, with a one-year survival rate ranging from 23.4% [59] to 47% [60]. The 5-years survival rate for patients receiving chemotherapy followed by surgery increased by 10% to 20% [61,62], while neoadjuvant chemoradiotherapy increased to 39% [63].
6. Immunotherapy
Among the immunotherapeutics, immune checkpoint inhibitors (ICIs) are drugs that block the immune checkpoint proteins to allow the elimination of cancer cells more effectively. There are two immune checkpoint inhibitory receptors that have been investigated, namely programmed cell death protein 1 (PD1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA4). Under normal conditions, PD1 expresses on activated T-cells binds to its ligand, programmed death-ligand 1 (PD-L1) which expresses on macrophages, B- and T-cells, allowing T-cells to exert its cytotoxic and anti-tumour response [64]. PD-L1 is overexpressed on cancer cells, including oesophageal cancer. The binding of PD-1 to PD-L1 on cancer cells allows cancer cells to be recognized as “self”, hence, evade elimination. To counter this, PD-1 monoclonal antibody (mAB) that acts in stimulating the proliferation of tumour antigen-specific T-cells was developed and deemed effective in reducing tumour burden. Currently, the Food and Drug Administration (FDA) approves the use of nivolumab, an anti-PD-1 monoclonal antibody (mAb), which serves as a second-line treatment for advanced ESCC. Approximately 44% of ESCC expressed PD-L1, and its expression level is correlated negatively with the overall patients survival [65,66]. In a Phase II clinical trial, 42% of ESCC patients responded to the treatment with reduced tumour burden [67]. The mono-immunotherapy extends the overall survival from 8.4 months to 10.9 months in 419 advanced EC patients. The one-year survival rate was 47% (95% CI; 40-54) in the immunotherapy group as compared with 37% in the chemotherapy group [60]. As PD-L1 is suggested as one of the prognostic markers for human cancers [66], it is worthwhile to evaluate this in EC, with more samples included in the studies. This is important to clearly inform whether or not anti-PD1 antibodies can be included as a safe, effective and non-invasive treatment modality for PD-L1-expressing ESCC patients.
CTLA4 is expressed on regulatory T-cells (Tregs). It regulates the degree of T-cell activation at an early stage by inhibiting the activity of the T-cell co-stimulatory receptor, CD28. As CD28 can enhance the activation of the T-cell via TCR signalling, CTLA4 plays a crucial role in preventing T-cell hyperactivation [68]. Currently, the safety and efficacy of CTLA4 mAbs, including ipilimumab [69] and tremelimumab [70], were explored in clinical trials. Ipilimumab improved the median overall survival from 12.1 months (95% CI 9.3-not estimable) to 12.7 months (95% CI, 10.5–18.9) in a Phase II clinical trial [71]. The combined treatment with ipilimumab and nivolumab is also effective for elderly patients with ESCC [69]. For EAC patients treated with tremelimumab, their one-year survival rate was 33% (95% CI, 14-54%) [70]. Despite the high efficacy, side effects of these treatments are inevitable. Low-grade toxicities were reported for patients treated with nivolumab (5%) [72] and tremelimumab (33–50%) [70], while EC patients treated with ipilimumab (23%) presented in clinics with high-grade toxicities (grade 3 and 4) [71].
7. Chemotherapy and chemoradiotherapy
When a tumour has metastasised distantly and is irresectable, chemotherapy is a standard treatment method for EC patients. Chemotherapeutics commonly used to treat cancers, inclusive of EC, are 5-fluorouracil (5-FU), platinum agents (e.g. cisplatin) and taxanes (e.g. paclitaxel and docetaxel) [[73], [74], [75]]. In general, these drugs act directly or indirectly to induce DNA damage. For instance, 5-FU inhibits thymidylate synthetase and catalyses the rate-limiting step in DNA synthesis. Therefore, this anti-cancer agent works by restricting DNA biosynthesis in tumours cells [76].
Currently, the efficacy of combined chemotherapy and chemoradiotherapy followed by surgery has been studied extensively in clinical trials. Both of these approaches deem more effective in treating EC patients than surgery alone or mono-immunotherapy. When compared with patients who receive surgery only, the patients who received pre-operative cisplatin and 5-FU treatment had a higher survival rate. The 2-years survival rates of 5-FU/cisplatin and surgery alone in EC patients were 43% and 34%, respectively [61]. Similarly, the FLOT protocol (5-FU, leucovorin, oxaliplatin, docetaxel) and FOLFOX (5-FU, leucovorin and oxaliplatin) were also more effective than the standard mono-treatment [77], particularly in patients with locally advanced resectable EC [78] and irresectable advanced EC [79]. Furthermore, the combination of the neoadjuvant chemoradiotherapy (cisplatin, cisplatin combined with 5-FU, vinblastine, or bleomycin) with surgical resection also improved the patient’s survival significantly than definite chemoradiotherapy [80].
However, haematologic and non-haematologic toxicities were reported in the patients treated with chemotherapy and chemoradiotherapy. Despite the combined treatment with docetaxel and 5-FU/cisplatin appearing to be a good option for young EC patients, this Phase II clinical trial was terminated due to high toxicity. Grade 3 and 4 toxicities were exhibited in EC patients (71%) within the first 3 months of treatment, and 90% over the whole treatment course [81].
8. Anti-viral drugs and gene therapy
As mentioned, the availability of a specific antiviral agent to treat oncoviruses-associated EC patients is limited. Antivirals used to treat CMV-associated oesophagitis and carcinoma are ganciclovir (GCV) and its prodrug, valganciclovir (VGCV) [82]. A report suggested that GCV and VGCV can reduce the prolonged fever in CMV infected EC patients [83]. However, the application of these drugs has been limited due to its in vivo toxicity. To date, HSV-thymidine kinase (HSV-TK)/GCV-mediated gene therapy has been implemented as a treatment option for the EC patients [84]. The HSV-TK-mediated therapy, followed by the intravenous administration of GCV, displayed an enhanced efficacy in treating EC mouse model under radiofrequency hyperthermia [84]. However, the efficacy of this treatment modality has only been tested using murine preclinical model, its efficacy should be recapitulated using non-murine models prior to marching to clinical trial.
9. Conclusion
The aetiological role of oncogenic viruses in oesophageal carcinoma (EC) has been debatable for years. Studies from some regions reported no significant association, while some regions showed a high prevalence of oncogenic viruses in EC patients. This may depend upon the region where studies were conducted. For instance, in countries that fall into the “Asian Oesophageal Cancer Belt” where the incidence of EC is exceptionally higher than in other parts of the world, hence the higher prevalence of oncogenic viruses in EC was reported in these countries. Among the oncogenic viruses reported, the association of human papillomavirus (HPV) with EC appears to be stronger than herpesviruses (EBV, CMV, HSV-1 and -2). However, research teams across the globe may not come to a consensus that HPV is a promising aetiological factor of EC. Similarly, the prognostic value of oncoviruses in EC remains a debatable arena. Studies focusing on the mechanistic roles of oncoviruses in driving EC development and progression should be warranted and expanded to further elucidate their association with EC. While identifying the aetiology of EC is important, early detection and treatment are undoubtedly crucial to improve the survival rate of EC patients. Despite the efficacy of combinatorial therapies deem promising, the compromising element to deal with is the high toxicity. Certainly, a tailored antiviral agent for EC driven by oncoviruses remains to be a field that deserves more progressive studies. Ideally, these tailored therapeutics should target oncogenic virus-containing cancer cells, hence, reducing destruction to normal cells.
10. Methodology
Pubmed, MEDLINE (EBSCOhost), Scopus and Google Scholar were used as the search engine for research articles published in recent 10 years (2011–2021). Keywords used to search for the relevant articles includes: “oesophageal/oesophageal carcinoma”, “oesophageal/oesophageal cancer”, “ prevalence”, “epidemiology”, “ HPV”, “EBV”, “CMV”, “HSV”, “Human Papillomavirus”, “Epstein Barr virus”, “Cytomegalovirus”, “Herpes simplex virus”, “chemotherapy”, “immunotherapy”, “radiotherapy”, “ antiviral drug”.
Articles that deem eligible and appropriate for inclusion in this review include: (1) topics related to the prevalence of oesophageal carcinoma, the etiological factors of oesophageal carcinoma, the association between the oncoviruses and oesophageal cancer, and the treatment for oesophageal cancer; (2) articles were written in English; (3) full text of the articles were accessible. From a total of 5642 related articles, duplicated and irrelevant articles were excluded, and the remaining 84 articles were ultimately included in this review.
Author statement
Sile Li: Methodology, Writing- Original draft preparation Ho Yin Luk: Writing- Original draft preparation Chichao Xia: Writing- Original draft preparation Zigui Chen: Writing- Reviewing and Editing Paul Kay Sheung Chan: Supervision, Writing- Reviewing and Editing, Siaw Shi Boon: Conceptualization, Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Huang J., et al. Global burden, risk factors, and trends of esophageal cancer: an analysis of cancer registries from 48 countries. Cancers. 2021;13(1):141. doi: 10.3390/cancers13010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pakzad R., et al. The incidence and mortality of esophageal cancer and their relationship to development in Asia. Ann. Transl. Med. 2016;4(2) doi: 10.3978/j.issn.2305-5839.2016.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edgren G., et al. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62(10):1406–1414. doi: 10.1136/gutjnl-2012-302412. [DOI] [PubMed] [Google Scholar]
- 5.Wise M.E., et al. National estimates of central line-associated bloodstream infections in critical care patients. Infect. Control Hosp. Epidemiol. 2013;34(6):547–554. doi: 10.1086/670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rassekh C. Tobacco cancer of the oral cavity and pharynx. W. Va. Med. J. 2001;97(1):8–12. [PubMed] [Google Scholar]
- 7.Seitz H.K., Homann N. Novartis Foundation Symposium. 2007. John Wiley; Chichester; New York: 1999. The role of acetaldehyde in alcohol-associated cancer of the gastrointestinal tract. [DOI] [PubMed] [Google Scholar]
- 8.Lagergren J., et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999;340(11):825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 9.Corley D.A., Kubo A., Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol. Epidemiol. Biomark. 2008;17(2):352–358. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Martel C., et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Global Health. 2020;8(2):e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 11.Brianti P., De Flammineis E., Mercuri S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80–85. [PubMed] [Google Scholar]
- 12.Farhadi M., et al. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J. Gastroenterol. 2005;11(8):1200–1203. doi: 10.3748/wjg.v11.i8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano B., et al. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:14–26. doi: 10.1016/j.bpobgyn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Syrjänen K., et al. Squamous cell papilloma of the esophagus: a tumour probably caused by human papilloma virus (HPV) Diagn. Histopathol. 1982;5(4):291–296. [PubMed] [Google Scholar]
- 15.Li X., et al. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment. Pharmacol. Ther. 2014;39(3):270–281. doi: 10.1111/apt.12574. [DOI] [PubMed] [Google Scholar]
- 16.Boon S.S., et al. Human papillomavirus type 18 oncoproteins exert their oncogenicity in esophageal and tongue squamous cell carcinoma cell lines distinctly. BMC Cancer. 2019;19(1):1–12. doi: 10.1186/s12885-019-6413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Zeng G., Jiang Y. The emerging roles of miR-125b in cancers. Cancer Manag. Res. 2020;12:1079–1088. doi: 10.2147/CMAR.S232388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B., et al. β-Catenin: oncogenic role and therapeutic target in cervical cancer. Biol. Res. 2020;53(1) doi: 10.1186/s40659-020-00301-7. 33-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alamoud K.A., Kukuruzinska M.A. Emerging insights into Wnt/β-catenin signaling in head and neck cancer. J. Dent. Res. 2018;97(6):665–673. doi: 10.1177/0022034518771923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zang B., et al. HPV-16 E6 promotes cell growth of esophageal cancer via downregulation of miR-125b and activation of Wnt/β-catenin signaling pathway. Int. J. Clin. Exp. Pathol. 2015;8(10):13687–13694. [PMC free article] [PubMed] [Google Scholar]
- 21.Rajendra S., et al. Transcriptionally active human papillomavirus is strongly associated with Barrett's dysplasia and esophageal adenocarcinoma. Am. J. Gastroenterol. 2013;108(7):1082–1093. doi: 10.1038/ajg.2013.94. [DOI] [PubMed] [Google Scholar]
- 22.Rajendra S., et al. Active human papillomavirus involvement in Barrett's dysplasia and oesophageal adenocarcinoma is characterized by wild‐type p53 and aberrations of the retinoblastoma protein pathway. Int. J. Cancer. 2017;141(10):2037–2049. doi: 10.1002/ijc.30896. [DOI] [PubMed] [Google Scholar]
- 23.Hanna G., et al. Plasma HPV cell-free DNA monitoring in advanced HPV-associated oropharyngeal cancer. Ann. Oncol. 2018;29(9):1980–1986. doi: 10.1093/annonc/mdy251. [DOI] [PubMed] [Google Scholar]
- 24.Parameshwaran K., et al. Circulating human papillomavirus DNA detection in Barrett's dysplasia and esophageal adenocarcinoma. Dis. Esophagus. 2019;32(12) doi: 10.1093/dote/doz064. [DOI] [PubMed] [Google Scholar]
- 25.Antonsson A., Knight L., Whiteman D.C. Human papillomavirus not detected in esophageal adenocarcinoma tumor specimens. Cancer Epidemiol. 2016;41:96–98. doi: 10.1016/j.canep.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Network C.G.A.R. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajendra S., et al. Human papillomavirus infection in esophageal squamous cell carcinoma and esophageal adenocarcinoma: a concise review. Ann. N. Y. Acad. Sci. 2020;1482(1):36–48. doi: 10.1111/nyas.14509. [DOI] [PubMed] [Google Scholar]
- 28.Kunzmann A.T., et al. The prevalence of viral agents in esophageal adenocarcinoma and Barrett's esophagus: a systematic review. Eur. J. Gastroenterol. Hepatol. 2017;29(7):817–825. doi: 10.1097/meg.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 29.Organization W.H. World Health Organization; 2010. Human Papillomavirus Laboratory Manual. [Google Scholar]
- 30.Xia C., et al. Current updates on cancer-causing types of human papillomaviruses (HPVs) in East, Southeast, and South Asia. Cancers. 2021;13(11):2691. doi: 10.3390/cancers13112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smatti M.K., et al. Epstein-barr virus epidemiology, Serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front. Oncol. 2018;8 doi: 10.3389/fonc.2018.00211. 211-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravorty S., et al. Integrated pan-cancer map of EBV-associated neoplasms reveals functional host-virus interactions. Cancer Res. 2019;79(23):6010–6023. doi: 10.1158/0008-5472.CAN-19-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakkalci D., et al. Risk factors for Epstein Barr virus-associated cancers: a systematic review, critical appraisal, and mapping of the epidemiological evidence. J. Global Health. 2020;10(1) doi: 10.7189/jogh.10.010405. 010405-010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan A.T., et al. Epstein-Barr virus latency type and spontaneous reactivation predict lytic induction levels. Biochem. Biophys. Res. Commun. 2016;474(1):71–75. doi: 10.1016/j.bbrc.2016.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frappier L. The epstein-Barr virus EBNA1 protein. Scientifica, 2012. 2012. 438204-438204. [DOI] [PMC free article] [PubMed]
- 36.Wu M.Y., Wu X.Y., Zhuang C.X. Detection of HSV and EBV in esophageal carcinomas from a high-incidence area in Shantou China. Dis. Esophagus. 2005;18(1):46–50. doi: 10.1111/j.1442-2050.2005.00423.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D.H., et al. Prevalence and association of human papillomavirus 16, Epstein-Barr virus, herpes simplex virus-1 and cytomegalovirus infection with human esophageal carcinoma: a case-control study. Oncol. Rep. 2011;25(6):1731–1738. doi: 10.3892/or.2011.1234. [DOI] [PubMed] [Google Scholar]
- 38.Sunpaweravong S., Mitarnun W., Puttawibul P. Absence of Epstein-Barr virus in esophageal squamous cell carcinoma. Dis. Esophagus. 2005;18(6):398–399. doi: 10.1111/j.1442-2050.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang L.-S., et al. Detection of Epstein-Barr virus in esophageal squamous cell carcinoma in Taiwan. Am. J. Gastroenterol. 1999;94(10):2834–2839. doi: 10.1111/j.1572-0241.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins T.D., Nakagawa H., Rustgi A.K. The association of Epstein-Barr virus DNA with esophageal squamous cell carcinoma. Oncogene. 1996;13(8):1809–1813. [PubMed] [Google Scholar]
- 41.Awerkiew S., et al. Esophageal cancer in Germany is associated with Epstein-Barr-virus but not with papillomaviruses. Med. Microbiol. Immunol. 2003;192(3):137–140. doi: 10.1007/s00430-002-0128-z. [DOI] [PubMed] [Google Scholar]
- 42.Awerkiew S., et al. Presence of Epstein-Barr virus in esophageal cancer is restricted to tumor infiltrating lymphocytes. Med. Microbiol. Immunol. 2005;194(4):187–191. doi: 10.1007/s00430-004-0233-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., et al. Esophageal squamous cell carcinomas arising in patients from a high-risk area of North China lack an association with Epstein-Barr virus. Cancer Epidemiol. Biomarkers Prev. 1999;8(12):1111–1114. [PubMed] [Google Scholar]
- 44.Chang F., et al. Evaluation of HPV, CMV, HSV and EBV in esophageal squamous cell carcinomas from a high-incidence area of China. Anticancer Res. 2000;20(5C):3935–3940. [PubMed] [Google Scholar]
- 45.Yahyapour Y., et al. Prevalence and association of human papillomavirus, Epstein-Barr virus and Merkel Cell polyomavirus with neoplastic esophageal lesions in northern Iran. Caspian. J. Intern. Med. 2018;9(4):353–360. doi: 10.22088/cjim.9.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyronis I.D., et al. Evaluation of the prevalence of human papillomavirus and Epstein-Barr virus in esophageal squamous cell carcinomas. Int. J. Biol. Markers. 2005;20(1):5–10. doi: 10.5301/jbm.2008.4549. [DOI] [PubMed] [Google Scholar]
- 47.Mizobuchi S., et al. Absence of human papillomavirus-16 and -18 DNA and Epstein-Barr virus DNA in esophageal squamous cell carcinoma. Jpn. J. Clin. Oncol. 1997;27(1):1–5. doi: 10.1093/jjco/27.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Yanai H., et al. Epstein-Barr virus association is rare in esophageal squamous cell carcinoma. Int. J. Gastrointest. Cancer. 2003;33(2-3):165–170. doi: 10.1385/ijgc:33:2–3:165. [DOI] [PubMed] [Google Scholar]
- 49.Goodgame R.W. Gastrointestinal cytomegalovirus disease. Ann. Intern. Med. 1993;119(9):924–935. doi: 10.7326/0003-4819-119-9-199311010-00010. [DOI] [PubMed] [Google Scholar]
- 50.Murakami D., et al. Cytomegalovirus‐associated esophagitis on early esophageal cancer in immunocompetent host: a case report. Gut Pathog. 2021;13(1):1–8. doi: 10.1186/s13099-021-00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang D.-H., et al. Prevalence and association of human papillomavirus 16, Epstein-Barr virus, herpes simplex virus-1 and cytomegalovirus infection with human esophageal carcinoma: a case-control study. Oncol. Rep. 2011;25(6):1731–1738. doi: 10.3892/or.2011.1234. [DOI] [PubMed] [Google Scholar]
- 52.Wu M.-y., Wu X.-y., Zhuang C.-x. Detection of HSV and EBV in esophageal carcinomas from a high-incidence area in Shantou China. Dis. Esophagus. 2005;18(1):46–50. doi: 10.1111/j.1442-2050.2005.00423.x. [DOI] [PubMed] [Google Scholar]
- 53.Han C.-P., et al. Human papillomavirus, cytomegalovirus and herpes simplex virus infections for cervical cancer in Taiwan. Cancer Lett. 1997;120(2):217–221. doi: 10.1016/s0304-3835(97)00312-1. [DOI] [PubMed] [Google Scholar]
- 54.Furihata M., et al. Prognostic significance of human papillomavirus genomes (type‐16,‐18) and aberrant expression of p53 protein in human esophageal cancer. Int. J. Cancer. 1993;54(2):226–230. doi: 10.1002/ijc.2910540211. [DOI] [PubMed] [Google Scholar]
- 55.Rajendra S., et al. Association of biomarkers for human papillomavirus with survival among adults with Barrett high-grade dysplasia and esophageal adenocarcinoma. JAMA Netw. Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2019.21189. e1921189-e1921189. [DOI] [PubMed] [Google Scholar]
- 56.Dreilich M., et al. High-risk human papilloma virus (HPV) and survival in patients with esophageal carcinoma: a pilot study. BMC Cancer. 2006;6(1):1–7. doi: 10.1186/1471-2407-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rustgi A.K., El-Serag H.B. Esophageal carcinoma. N. Engl. J. Med. 2014;371(26):2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 58.Pennathur A., et al. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs C.S., et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5) doi: 10.1001/jamaoncol.2018.0013. e180013-e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kato K., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 61.Working M.R.C.O.C. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359(9319):1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 62.Cunningham D., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 63.Tepper J., et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2008;26(7):1086. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel S.P., Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Therapeut. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 66.Ohigashi Y., et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 67.Kudo T., et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18(5):631–639. doi: 10.1016/S1470-2045(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 68.Schizas D., et al. Immunotherapy for esophageal cancer: a 2019 update. Immunotherapy. 2020;12(3):203–218. doi: 10.2217/imt-2019-0153. [DOI] [PubMed] [Google Scholar]
- 69.Hartel N., et al. Wolters Kluwer Health; 2021. Nivolumab and Ipilimumab for Second-Line Therapy in Elderly Patients with Advanced Esophageal Squamous Cell Cancer: Safety Interim Analysis of the RAMONA Trial. [Google Scholar]
- 70.Ralph C., et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin. Cancer Res. 2010;16(5):1662–1672. doi: 10.1158/1078-0432.CCR-09-2870. [DOI] [PubMed] [Google Scholar]
- 71.Bang Y.-J., et al. Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin. Cancer Res. 2017;23(19):5671–5678. doi: 10.1158/1078-0432.CCR-17-0025. [DOI] [PubMed] [Google Scholar]
- 72.Kang Y.-K., et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 73.Muro K., et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann. Oncol. 2004;15(6):955–959. doi: 10.1093/annonc/mdh231. [DOI] [PubMed] [Google Scholar]
- 74.Kato K., et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother. Pharmacol. 2011;67(6):1265–1272. doi: 10.1007/s00280-010-1422-x. [DOI] [PubMed] [Google Scholar]
- 75.Igaki H., et al. A randomized trial of postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus neoadjuvant chemotherapy for clinical stage II/III squamous cell carcinoma of the thoracic esophagus (JCOG 9907) J. Clin. Oncol. 2008;26(15_suppl) 4510-4510. [Google Scholar]
- 76.Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 77.Bollschweiler E., et al. Current and future treatment options for esophageal cancer in the elderly. Expet Opin. Pharmacother. 2017;18(10):1001–1010. doi: 10.1080/14656566.2017.1334764. [DOI] [PubMed] [Google Scholar]
- 78.Al-Batran S.-E., et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 79.Conroy T., et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15(3):305–314. doi: 10.1016/S1470-2045(14)70028-2. [DOI] [PubMed] [Google Scholar]
- 80.Kranzfelder M., et al. Meta‐analysis of neoadjuvant treatment modalities and definitive non‐surgical therapy for oesophageal squamous cell cancer. Br. J. Surg. 2011;98(6):768–783. doi: 10.1002/bjs.7455. [DOI] [PubMed] [Google Scholar]
- 81.Shah M.A., et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J. Clin. Oncol. 2015;33(33):3874–3879. doi: 10.1200/JCO.2015.60.7465. [DOI] [PubMed] [Google Scholar]
- 82.Emery V. Cytomegalovirus: recent progress in understanding pathogenesis and control. QJM: Int. J. Med. 2012;105(5):401–405. doi: 10.1093/qjmed/hcr262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Umemoto K., et al. Cytomegalovirus esophagitis developing during chemoradiotherapy for esophageal cancer: two case reports. J. Med. Case Rep. 2016;10(1):1–4. doi: 10.1186/s13256-016-0947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi Y., et al. Radiofrequency hyperthermia-enhanced herpes simplex virus-thymidine kinase/ganciclovir direct intratumoral gene therapy of esophageal squamous cancers. Am. Journal.Cancer Res. 2016;6(9):2054. [PMC free article] [PubMed] [Google Scholar]