Abstract
OBJECTIVE
To compare the timing of serum anti-drug antibodies in adult and pediatric age groups, males and females, treated for inflammatory bowel disease or arthritis with adalimumab or infliximab by retrospectively combining data collected during a 2-year therapeutic drug monitoring period.
METHODS
Four hundred thirty sera were divided in groups collected at 0, 3, 6, 12, and 24 months (T0, T3, T6, T12, and T24) after initiation of therapy and assayed for drug and relative anti-drug antibodies levels. At each time point, the percentage of sera presenting anti-drug antibodies, as well as the drug concentrations, were calculated and correlated with patient age and sex.
RESULTS
Anti-drug antibodies were present in 31.5% of sera and were significantly higher in the pediatric age group than in the adult age group, through all time points. The percentages of sera showing anti-drug antibodies were significantly different as early as 3 months and were sera from pediatric female group. The percentages of sera showing anti-drug antibodies reached the highest value at 6 months in the pediatric age group and at 12 months in the adult age group.
CONCLUSIONS
Sera from pediatric had an earlier presence of anti-drug antibodies than adults. In particular, pediatric females sera showed the fastest anti-drug antibodies development.
Keywords: adalimumab, anti-drug antibodies, autoimmune diseases, biologics, infliximab, therapeutic drug monitoring
Introduction
Infliximab (IFX) and adalimumab (ADA) are 2 biological agents (chimeric and humanized, respectively) blocking the activity of tumor necrosis factor alpha (TNFα). They are widely used in pediatric patients for treatment of rheumatological and gastrointestinal diseases at doses ranging from 3 to 5 mg/kg for IFX and 20 to 40 mg/kg for ADA. However, due to their side effects (i.e., blocked or runny nose, headaches, dizziness, flushing, a rash, stomach pain, indigestion or sickness, irregular heartbeat, infections), the formation of anti-drug antibodies and consequent reduction of their plasma levels, these drugs may lose their effectiveness over time.1–4 In fact, several studies have documented an ineffectiveness of both IFX or ADA treatments following an immunogenic response arising with a frequency from 6% to 16% for IFX and from 2.6% to 44% for ADA.5–10
Therapeutic drug monitoring (TDM) is a crucial tool to suggest an adjustment of the dose, or even the change to another class of drug.11,14 Although most retrospective studies analyzed the pharmacokinetics and serum concentrations of the 2 drugs in single pathologies,15–22 no analyses cross-correlate the serum drug concentrations and anti-drug antibodies levels with the time of their appearance in the serum by combining multiple diseases treated with the same drug. Similarly, there are no analyses that correlate the time of anti-drug antibodies onset with the sex and age of the patient.
The present retrospective study aims to clarify these points through analysis of data collected over a 2-year period using serum samples obtained from patients with inflammatory bowel disease (IBD) or arthritis who were being treated with IFX or ADA.
Materials and Methods
Data Collection. In this study, 430 sera from patients naïve to biological medications were included. These were routinely collected between June 2019 and January 2021 at the therapeutic drug monitoring unit of the University Polyclinic “Luigi Vanvitelli”; the sera were from the immunology-autoimmune diseases, gastroenterology, and pediatric rheumatology clinics. They were tested for ADA, IFX, anti-adalimumab antibody (ATA), and anti-infliximab antibody (ATI) levels. Sera were assayed blind of the pathology, treatment protocols, age, sex, and time point of treatment.
Criteria for Analysis. Exclusively for the purpose of this study, sera were retrospectively divided by treatment as 1) ADA and 2) IFX. In each of these, sera were grouped into 5 time points (months) according to the request made by the clinicians for therapeutic monitoring (T0, T3, T6, T12, T24). A further division was made by age (adults [A] 45 ± 16 years, 70 ± 6 kg; pediatrics [P] 13 ± 4 years, 45 ± 2 kg) and age combined with sex (males [M], females [F]).
Sera showing antibodies were cross-compared at each time point for the percentages of them showing ATAs or ATIs, for the levels achieved, for drug levels, and for patient's sex.
In order to avoid any misinterpretation of the final results, due to different treatment regimens with respect to those accepted by the international scientific community for each drug and pathology,23,24 the clinicians were asked to provide the protocol used. They declared that the samples were collected from patients treated in accordance with the consensus statements on the initiation and continuation of TNFα blocking therapy for IBD (Crohn disease, ulcerative colitis) and arthritis (ankylosing spondylitis, idiopathic juvenile arthritis, psoriatic arthritis, rheumatoid arthritis).25,26 Specifically, patients were treated as reported in the Table,27,28 and were naïve to biologic treatments.
Table.
Patients' Treatment
| Disease | ADA | IFX |
|---|---|---|
| Arthritis | ||
| Rheumatoid arthritis | Adults: 40 mg in a single administration every 2 wk subcutaneously. | Adults and pediatrics: an intravenous infusion of 3 mg/kg followed by additional infusions of 3 mg/kg at weeks 2 and 6 after the first infusion, then every 8 wk. |
| Ankylosing spondylitis | Adults: 40 mg in a single administration every 2 wk subcutaneously. | Adults and pediatrics: an intravenous infusion of 5 mg/kg followed by additional infusions of 5 mg/kg at weeks 2 and 6 from the first infusion, then repeated after a time that can vary from 6 to 8 wk. |
| Pediatrics: weighing 30 kg or more, 40 mg every 2 wk; weighing between 15 kg and < 30 kg, 20 mg every 2 wk. | ||
| Psoriatic arthritis | Adults: 40 mg in a single administration every 2 wk subcutaneously. | Adults and pediatrics: an intravenous infusion of 5 mg/kg followed by additional 5 mg/kg infusions at weeks 2 and 6 after the first infusion, then repeated every 8 wk. |
| Pediatrics: weighing 30 kg or more, 40 mg every 2 wk; weighing between 15 kg and < 30 kg, 20 mg every 2 wk. | ||
| Juvenile idiopathic arthritis | Pediatrics: weighing 30 kg or more, 40 mg every 2 wk; weighing between 10 kg and < 30 kg, 20 mg every 2 wk. | Pediatrics: an intravenous infusion of 3–4 mg/kg followed by additional 3–4 mg/kg infusions at weeks 2 and 6 after the first infusion, then repeated every 8 wk. |
| Inflammatory bowel disease | ||
| Crohn disease | Adults: 80 mg (via 2 injections in 1 day) followed by 40 mg every other week after 2 wk. | Adults and pediatrics: 5 mg/kg administered as an intravenous infusion followed by an additional 5 mg/kg infusion 2 wk after the first infusion. Maintenance: additional 5 mg/kg infusion at week 6 after the first dose, followed by repeated infusions every 8 wk. |
| Pediatrics: weighing 40 kg or more, initial dose of 80 mg followed by 40 mg every 2 wk; weighing < 40 kg, initial dose of 40 mg followed by 20 mg every 2 wk. | ||
| Ulcerative colitis | Adults: 160 mg (through 4 injections in 1 day or 2 injections per day for 2 consecutive days) at week 0, 80 mg (through 2 injections in 1 day) at week 2 and subsequently 40 mg every other week. | Adults and pediatrics: an intravenous infusion of 5 mg/kg followed by additional 5 mg/kg infusions at weeks 2 and 6 after the first infusion, then repeated every 8 wk. |
| Pediatrics: weighing 40 kg or more, 160 mg (through 4 injections in 1 day or 2 injections per day for 2 consecutive days) at week 0, 80 mg (through 2 injections in 1 day) at week 2 and subsequently 40 mg every other week; weighing between 20 and < 40 kg, 80 mg (through 4 injections in 1 day or 2 injections per day for 2 consecutive days) at week 0, 40 mg (through 2 injections in 1 day) at week 2 and subsequently 20 mg every other week. | ||
ADA, adalimumab; IFX, infliximab
Determination of Drug Trough Concentrations and Anti-Drug Antibodies. Adalimumab, IFX, ATA, and ATI serum levels were assayed in samples collected just prior to the administration of the subsequent dose. Triplicate measurements were performed for each sample. A commercial and validated enzyme-linked immunosorbent assay was used for the monitoring of drugs and anti-drug antibodies according to the manufacturer's instructions (R-BioPharm, Melegnano, Italy).29 The detection limit for ADA and IFX was 0.1 mcg/mL, whereas for ATAs and ATIs it was 0.06 ng/mL.
Statistical Analysis. All results were reported as mean ± SEM. Two-way analysis of variance followed by the Tukey comparison test was used for the statistical analysis and performed with Prism 6.0 (GraphPad, San Diego, CA, USA) . A p value < 0.05 was set as the level of significance.
Results
Demographics of Total Sera Collected. Fifteen percent of sera (65/430) were from patients starting anti-TNFα therapy (T0), whereas 75% of sera were from patients in maintenance regimen (365/430). The latter were further composed of 67.5% ADA (6.24 ± 1.0 mcg/mL) and 32.5% IFX (6.02 ± 0.7 mcg/mL).
Seventy-four percent of the total sera analyzed (318/430) were collected from A, whereas 26% (112/430) were collected from P. Among A, 80% were treated with ADA, whereas only 20% received IFX. On the contrary, IFX treatment was more frequent in P (55%) compared with ADA (45%) (Figure 1A). Considering the sex, samples consisted mainly of sera from adult females (AF) (48%), followed by adult males (AM) (26%), pediatric females (PF) (15%), and pediatric males (PM) (11%). Adalimumab was the prevalent drug administered in both AF (86%) and AM (70%), whereas IFX was more frequently administered in PF (57%) and PM (52%) (Figure 1B).
Figure 1.
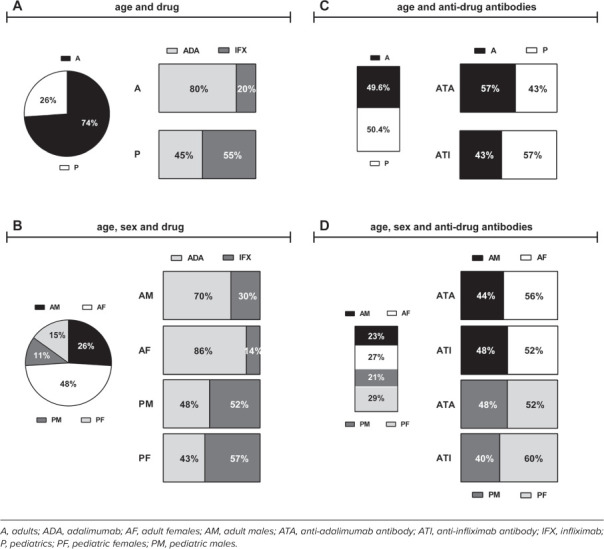
Demographics of total samples. Percentages of total samples: (A) by age and drug; (B) by age, sex, and drug; (C) by age and anti-drug antibodies; (D) by age, sex, and anti-drug antibodies.
Demographics of Sera in Maintenance Therapy Developing Anti-Drug Antibodies. Of the sera collected in maintenance therapy, 31.5% (115/365) were characterized by anti-drug antibodies. Of these, 49.6% (57/115) were collected from A and 50.4% were collected from P (58/115). ATAs were more present than ATIs in A samples (57% ATAs vs 43% ATIs). In contrast, P samples had 57% ATIs vs 43% ATAs (Figure 1C). Concerning the sex, anti-drug antibodies were more present in F (29% PF, 27% AF) than in M samples (23% AM, 21% PM). Adult females exhibited the highest percentage of ATAs (56% AF, 44% AM), whereas PF showed the highest percentage of ATIs (60% F, 40% M) (Figure 1D).
ATAs. Among A sera, the highest percentage showing ATAs was calculated in those collected at T12 (27.5% ± 3.4%), whereas it was calculated at T6 in P sera. At T6 there was a significant difference between the percentage of A sera and the percentage of P sera (e.g., A, 6.5% ± 1.2%; P, 27.5% ± 3.6%; p < 0.01) (Figure 2A). The same trend appeared by comparing sera from AM and PM (e.g., T6 AM, 7.0% ± 1.1%; PM, 28.0% ± 3.4%, p < 0.01) (Figure 2B). Interestingly, the comparison between AF and PF showed the highest percentage recorded at T3 in PF (AF, 7.0% ± 1.1%; PF, 28.0% ± 3.4%, p < 0.01) (Figure 2C). Pediatric females showed earlier presence of serum ATA levels than PM (T3, PM: 6.0% ± 1.4%; PF, 28.0% ± 3.4%, p < 0.01) (Figure 2D).
Figure 2.
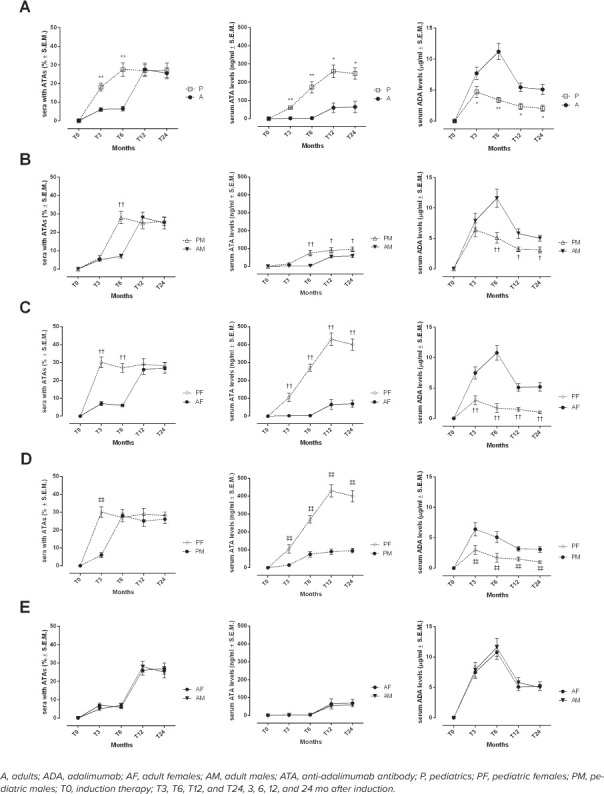
Development of serum ATA levels during time points of maintenance therapy. Number sera with ATAs (%), determination of serum ATA (ng/mL) and ADA (mcg/mL) levels in A and P populations (A), AM and PM (B), AF and PF (C), PM and PF (D), AM and AF (E) during different time points of maintenance regimen. Results are expressed as mean ± SEM. *p < 0.05 and **p < 0.01 vs A; †p < 0.05 and ††p < 0.01 vs A, same sex; ‡p < 0.05 and ‡‡p < 0.01 vs PM.
ATIs. The highest percentage of A sera showing ATIs was recorded at T12 (25.0% ± 3.4%), whereas for P sera it was at T6. At T6 there was a significant difference between the percentage of A and P sera (A, 3.5% ± 0.8%; P, 28.5% ± 3.4%, p < 0.01) (Figure 3A). The same trend was observed when considering AM and PM (at T6 AM, 5.0% ± 0.9%; PM, 27.0% ± 2.8%, p < 0.01) (Figure 3B). Moreover, the highest percentage of AF sera showing ATIs was calculated at T12 (27.0% ± 2.4%), whereas it was at T3 for PF sera (AF, 2.0% ± 0.5%; PF, 35.0% ± 3.6%, p < 0.01) (Figure 3C). Again, at T3 the percentage was significantly different in comparison to the percentage of PM sera (T3, PM, 4.0% ± 0.7%; PF, 35.0% ± 3.6%, p < 0.01) (Figure 3D).
Figure 3.
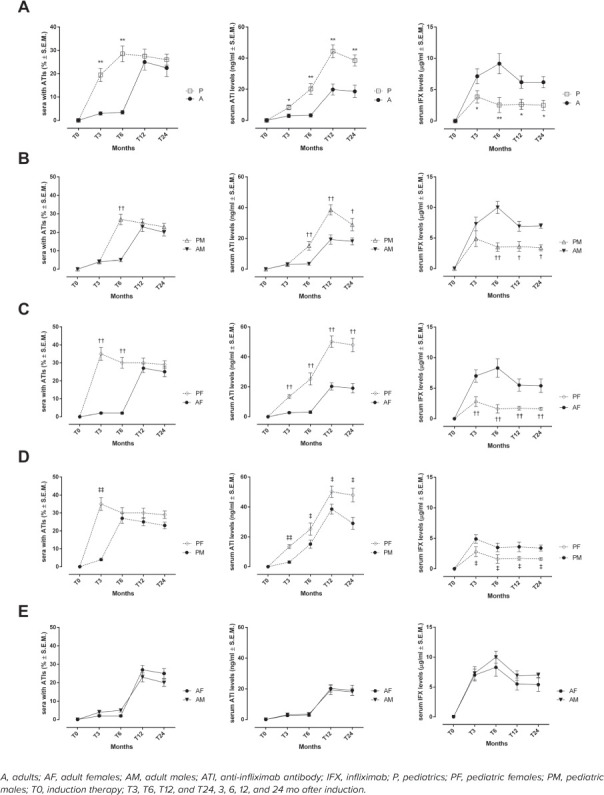
Development of serum ATI levels during time points maintenance therapy. Number of sera with ATIs (%), determination of serum ATIs (ng/mL) and IFX (mcg/mL) levels in A and P populations (A), AM and PM (B), AF and PF (C), PM and PF (D), AM and AF (E) during different time points of maintenance regimen. Results are expressed as mean ± SEM. *p < 0.05 and **p < 0.01 vs A; †p < 0.05 and ††p < 0.01 vs A, same sex; ‡p < 0.05 and ‡‡p < 0.01 vs PM.
Serum ATA and ATI Levels Through Time Points. ATAs. Serum ATA levels were significantly higher in P sera compared with A sera starting 3 months after initiation of therapy (A, 2.3 ± 1.0 ng/mL; P, 60.0 ± 2.3 ng/mL; p < 0.01) (Figure 2A). They were majorly present in PF sera. Indeed, PF sera showed significantly higher serum ATA levels at T3 than AF (AF, 2.1 ± 0.4 ng/mL; PF, 105.0 ± 24 ng/mL; p < 0.01) (Figure 2C), and compared with PM sera (PM, 15.0 ± 3.1 ng/mL; PF, 105.0 ± 24 ng/mL; p < 0.01) (Figure 2D). Anti-adalimumab antibodies were significantly higher at T6 in PM compared with AM (AM, 2.9 ± 0.3 ng/mL; PM, 75.0 ± 16.0 ng/mL; p < 0.01) (Figure 2B).
ATIs. Serum ATIs were significantly higher in P sera compared with A sera starting at T3 (A, 2.9 ± 1.2 ng/mL; P, 8.3 ± 1.5 ng/mL; p < 0.01) (Figure 3A). However, serum ATIs were significantly higher in PM compared with AM at T6 (AM, 3.5 ± 0.9 ng/mL; PM, 15.2 ± 2.8 ng/mL; p < 0.01) (Figure 3B). Interestingly, PF sera showed significantly higher ATI levels at T3 than AF (AF, 2.7 ± 0.6 ng/mL; PF, 13.5 ± 1.3 ng/mL; p < 0.01) (Figure 3C), and with respect to PM sera (PM, 3.1 ± 0.8 ng/mL; PF, 13.5 ± 1.3 ng/mL; p < 0.01) (Figure 3D).
All serum ATAs and ATIs levels were negatively correlated with serum ADA and IFX trough levels during time points of maintenance therapy (Figures 2 and 3). Their peaks raised at T12 for both A and P. In addition, 35 of the total 115 sera (30%) with anti-drug antibodies belonged to patients who had therapy changes. Of these, 24 were PF (70%), 4 were PM (13%) and 7 were AF (17%).
Discussion
Infliximab and ADA are 2 of the most effective and most commonly used drugs for the treatment of arthritis and IBD.30,31 Several studies have characterized the use of IFX and ADA in these autoimmune diseases, including kinetics, dynamics, and immunogenicity of the 2 drugs.5,32–42 However, some new elements have emerged in the present retrospective study that are worthy of attention. They are the timing of serum ATAs and ATIs in pediatric and adult patients and the relation they have with sex and age. It was established that each anti-TNF agent generated differences concerning the percentage of patients presenting antibodies and levels of the antibodies formed. Most importantly, differences in the timing of anti-drug antibodies appearance were noted between adults and pediatrics, and between pediatric females and males. Indeed, a higher percentage of sera from pediatric patients contained ATAs or ATIs and at high levels. This finding represents a novelty because no study has compared the 2 groups of patients time by time.
Interestingly, by comparing the timing of anti-drug antibodies appearance, this was shorter in pediatric sera compared with adult sera. A plausible hypothesis we formulated to explain this difference was that anti-TNFs induced an alteration of the children's immune system toward an easier onset hypersensitivity. This could have affected the immunogenicity of the 2 drugs IFX and ADA in pediatric patients. Again, this is a novelty because several studies have traced the possibility to generate antibodies during the therapy with anti-TNFs but no study compared the timing of their genesis in adult and pediatric patients. Moreover, anti-drug antibodies in P sera were always paralleled by lower drug trough levels of both ADA and IFX than A sera, regardless of sex. This represents a further novelty because no study has compared the 2 groups.
A further data analysis showed differences of antibody response and time of appearance as a function of sex in pediatric patients. PF sera showed the highest percentage of sera with ATAs or ATIs compared with PM. They always showed higher levels of ATIs or ATAs than males and the shortest onset time for a significant difference. This, underlines to our opinion that the immune system of PF may react differently from that of PM, possibly due to hormonal status. On another note, patient-related factors such as differences in human leukocyte antigen genotype and alleles (e.g., human leukocyte antigen-DRB1 alleles) may have influenced the formation of antibodies.43,44
In both adults and pediatrics, the majority of patients who underwent TDM were female regardless of the type of drug used. This means that females, especially adults, were more prone to undergo TDM with respect to males. Why this occurred needs clarification given the pure descriptive nature of the study. However, one would like to speculate that this happened because females respond poorly to therapy, as observed in studies done in both arthritis and IBD,20 or it may be related to the fact that they have biological cycles (pregnancy and lactation) that could have influenced the kinetics of anti-TNF drugs, unlike males.45
It should be noted that the total serum drug levels were overall in line with the evidence in the literature for the therapeutic success of arthritis and IBD with ADA or IFX.37,38,46–52 However, sera showing antibodies had total levels of ADA or IFX lower than those described earlier. In fact, it is well known that the concentration of ATIs is inversely proportional to the plasma concentration of IFX.1,6,42,53,54 Similarly, if initially the immunogenicity of ADA was thought to be an extremely rare event, being a fully humanized anti-TNFα, recent studies showed that drug concentrations are inversely correlated with ATAs.10 Particularly, serum drug levels were higher in A than in P. This is probably due to the high amount of antibodies formed in sera from pediatrics.
The limitations of the study consist in the lack of disease activity data, as well as the lack of information concerning the whole therapy adopted and patients' lifestyle habits (e.g., smoking, sun exposure, and alcohol consumption) and the lack of compliance data due to the retrospective nature of the study.
Conclusion
In summary, the pediatric age group, particularly female, developed an earlier immunogenic response to IFX and ADA than the adult age group, and therefore great attention should be paid to this possibly. Translating these results in clinical relevance—70% of the patients with anti-drug antibodies underwent a change of therapy for another anti-TNFα drug and were female pediatric patients.
Acknowledgments
Drs Trotta and Alfano are co-first authors; Drs Giordano and D'Amico are co-last authors. They attest that they contributed equally to work. JPPT does not recognize co-first or co-senior authorship in reference lists. The authors acknowledge the support of Prof Strisciuglio Caterina of Vanvitelli Campania University and Prof Miele Erasmo of the Federico II University.
ABBREVIATIONS
- A
adult
- ADA
adalimumab
- AF
adult females
- AM
adult males
- ATA(s)
anti-adalimumab antibody(ies)
- ATI(s)
anti-infliximab antibody(ies)
- F
female
- IBD
inflammatory bowel disease
- IFX
infliximab
- M
male
- P
pediatric
- PF
pediatric females
- PM
pediatric males
- SEM
standard error of the mean
- TDM
therapeutic drug monitoring
- TNF
tumor necrosis factor
- TNFα
tumor necrosis factor alpha
Footnotes
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Dr Michele D'Amico had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent. This study was approved from Ethical Committee of University of Campania “Luigi Vanvitelli” (Number of Approval 0025474/i) and required a written informed consent.
References
- 1.Svenson M, Geborek P, Saxne T, Bendtzen K. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology . 2007;46(12):1828–1834. doi: 10.1093/rheumatology/kem261. [DOI] [PubMed] [Google Scholar]
- 2.Atzeni F, Talotta R, Salaffi F et al. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev . 2013;12(7):703–708. doi: 10.1016/j.autrev.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol . 2017;13(12):707–718. doi: 10.1038/nrrheum.2017.187. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Yoseph H, Levhar N, Selinger L et al. Early drug and anti-infliximab antibody levels for prediction of primary nonresponse to infliximab therapy. Aliment Pharmacol Ther . 2018;47(2):212–218. doi: 10.1111/apt.14410. [DOI] [PubMed] [Google Scholar]
- 5.Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn's disease. Am J Gastroenterol . 2008;103(4):944–948. doi: 10.1111/j.1572-0241.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- 6.Baert F, Noman M, Vermeire S et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med . 2003;348(7):301–308. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 7.Farrell RJ, Alsahli M, Jeen YT et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology . 2003;124(4):917–924. doi: 10.1053/gast.2003.50145. [DOI] [PubMed] [Google Scholar]
- 8.Hanauer SB, Wagner CL, Bala M et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol . 2004;2(7):542–553. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 9.Vermeire S, Noman M, Van Assche G et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut . 2007;56(9):1226–1231. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazor Y, Almog R, Kopylov U et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn's disease. Aliment Pharmacol Ther . 2014;40(6):620–628. doi: 10.1111/apt.12869. [DOI] [PubMed] [Google Scholar]
- 11.Khanna R, Sattin BD, Afif W et al. Review article: a clinician's guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther . 2013;38(5):447–449. doi: 10.1111/apt.12407. [DOI] [PubMed] [Google Scholar]
- 12.Bortlik M, Duricova D, Malickova K et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohn's Colitis. 2013;7(9):736–743. doi: 10.1016/j.crohns.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Papamichael K, Chachu KA, Vajravelu RK et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol . 2017;15(10):1580–1588. doi: 10.1016/j.cgh.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesarini M, Katsanos K, Papamichael K et al. Dose optimization is effective in ulcerative colitis patients losing response to infliximab: a collaborative multicentre retrospective study. Dig Liver Dis . 2014;46(2):135–139. doi: 10.1016/j.dld.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Gremese E, Bernardi S, Bonazza S et al. Body weight, sex and response to TNF-α blockers in axial spondyloarthritis. Rheumatol (United Kingdom) . 2014;53(5):875–881. doi: 10.1093/rheumatology/ket433. [DOI] [PubMed] [Google Scholar]
- 16.Gremese E, Carletto A, Padovan M et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res . 2013;65(1):94–100. doi: 10.1002/acr.21768. [DOI] [PubMed] [Google Scholar]
- 17.Lopez A, Billioud V, Peyrin-Biroulet C, Peyrin-Biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis . 2013;19(7):1528–1533. doi: 10.1097/MIB.0b013e31828132cb. [DOI] [PubMed] [Google Scholar]
- 18.Rusman T, Ten Wolde S, Euser SM et al. Gender differences in retention rate of tumor necrosis factor alpha inhibitor treatment in ankylosing spondylitis: a retrospective cohort study in daily practice. Int J Rheum Dis . 2018;21(4):836–842. doi: 10.1111/1756-185X.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Højgaard P, Ballegaard C, Cordtz R et al. Gender differences in biologic treatment outcomes-a study of 1750 patients with psoriatic arthritis using Danish Health Care Registers. Rheumatol (United Kingdom) . 2018;57(9):1651–1660. doi: 10.1093/rheumatology/key140. [DOI] [PubMed] [Google Scholar]
- 20.Laganà B, Zullo A, Scribano ML et al. Gender differences in response to TNF-Inhibiting drugs in patients with spondyloarthropathies or inflammatory bowel diseases. Front Pharmacol . 2019;10(47) doi: 10.3389/fphar.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.dos Santos CHM. Women respond better to biological therapy in Crohn's Disease. Med Express . 2014;1(3):150–152. [Google Scholar]
- 22.Greuter T, Manser C, Pittet V et al. Gender differences in inflammatory bowel disease. Digestion . 2020;101(suppl 1):98–104. doi: 10.1159/000504701. [DOI] [PubMed] [Google Scholar]
- 23.Smolen JS, Landewé R, Bijlsma J et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis . 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 24.Aardoom MA, Veereman G, de Ridder L. A review on the use of anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci . 2019;20(10):2529. doi: 10.3390/ijms20102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh JA, Saag KG, Bridges SL et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) . 2016;68(1):1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 26.Aratari A, Bossa F, Cappello M et al. Safety of treatments for inflammatory bowel disease: clinical practice guidelines of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD) Dig Liver Dis . 2017;49(4):338–358. doi: 10.1016/j.dld.2017.01.141. [DOI] [PubMed] [Google Scholar]
- 27.Humira (adalimumab) [package insert] AbbVie Deutsch-land GmbH & Co. KG; 2019. Accessed January, 9, 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/humira.
- 28.Remicade (infliximab) [package insert]. Janssen Biologics B.V.; 2019. Accessed January, 9, 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/remicade .
- 29.Neveu B, Kunst A, Prosser C, Robitaille R. An in vitro comparison of four different immunoassays for the monitoring of Infliximab biosimilars drug levels. Clin Biochem . 2020;78:58–62. doi: 10.1016/j.clinbiochem.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Monaco C, Nanchahal J, Taylor P, Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol . 2015;27(1):55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macaluso FS, Orlando A. Anti-TNF combination therapy in inflammatory bowel disease: de novo or selective? Minerva Gastroenterol Dietol . 2019;65(4):291–297. doi: 10.23736/S1121-421X.19.02617-5. [DOI] [PubMed] [Google Scholar]
- 32.Vande Casteele N, Ferrante M, Van Assche G et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology . 2015;148(7):1320–1329.e3. doi: 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Adedokun OJ, Sandborn WJ, Feagan BG et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology . 2014;147(6):1296–1307.e5. doi: 10.1053/j.gastro.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Cassinotti A, Ardizzone S, Porro GB. Adalimumab for the treatment of Crohn's disease. Biol Targets Ther . 2008;2(4):763–777. doi: 10.2147/btt.s3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mease P. Infliximab (Remicade) in the treatment of psoriatic arthritis. Ther Clin Risk Manag . 2006;2(4):389–400. doi: 10.2147/tcrm.2006.2.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grainger R, Harrison AA. Infliximab in the treatment of ankylosing spondylitis. Biol Targets Ther . 2007;1(2):163–171. [PMC free article] [PubMed] [Google Scholar]
- 37.Carlsen A, Omdal R, Karlsen L et al. Determination of lower cut-off levels of adalimumab associated with biochemical remission in Crohn's disease. JGH Open . 2020;4(3):410–416. doi: 10.1002/jgh3.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juncadella A, Papamichael K, Vaughn BP, Cheifetz AS. Maintenance adalimumab concentrations are associated with biochemical, endoscopic, and histologic remission in inflammatory bowel disease. Dig Dis Sci . 2018;63(11):3067–3073. doi: 10.1007/s10620-018-5202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulsen ASK, Thomsen SF, Vinkel C et al. Fluctuations in serum levels of adalimumab and infliximab in patients on stable treatment for psoriasis. Dermatol Ther . 2020;33(4):e13497. doi: 10.1111/dth.13497. [DOI] [PubMed] [Google Scholar]
- 40.Mease PJ. Adalimumab in the treatment of arthritis. Ther Clin Risk Manag . 2007;3(1):133–148. doi: 10.2147/tcrm.2007.3.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emi Aikawa N, De Carvalho JF, Artur Almeida Silva C, Bonfá E. Immunogenicity of anti-TNF-α agents in autoimmune diseases. Clin Rev Allergy Immunol . 2010;38(2–3):82–89. doi: 10.1007/s12016-009-8140-3. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Horin S, Yavzori M, Katz L et al. The immunogenic part of infliximab is the F(ab′)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut . 2011;60(1):41–48. doi: 10.1136/gut.2009.201533. [DOI] [PubMed] [Google Scholar]
- 43.Murdaca G, Spanò F, Contatore M et al. Immunogenicity of infliximab and adalimumab: what is its role in hypersensitivity and modulation of therapeutic efficacy and safety? Expert Opin Drug Saf . 2016;15(1):43–52. doi: 10.1517/14740338.2016.1112375. [DOI] [PubMed] [Google Scholar]
- 44.Billiet T, Vande Casteele N, Van Stappen T et al. Immunogenicity to infliximab is associated with HLA-DRB1. Gut . 2015;64(8):1344–1345. doi: 10.1136/gutjnl-2015-309698. [DOI] [PubMed] [Google Scholar]
- 45.Seow CH, Leung Y, Vande Casteele N et al. The effects of pregnancy on the pharmacokinetics of infliximab and adalimumab in inflammatory bowel disease. Aliment Pharmacol Ther . 2017;45(10):1329–1338. doi: 10.1111/apt.14040. [DOI] [PubMed] [Google Scholar]
- 46.Vande Casteele N, Jeyarajah J, Jairath V et al. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol . 2019;17(9):1814–1821. e1. doi: 10.1016/j.cgh.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 47.Papamichael K, Rakowsky S, Rivera C et al. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment Pharmacol Ther . 2018;47(4):478–484. doi: 10.1111/apt.14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vande Casteele N, Papamichael K, Jeyarajah J et al. DOP45 Adequate infliximab exposure during the induction phase is associated with early complete fistula response in patients with fistulizing Crohn's disease: a post-hoc analysis of the ACCENT-2 trial. J Crohn's Colitis . 2019;13(S1):S053–S054. [Google Scholar]
- 49.Papamichael K, Rakowsky S, Rivera C et al. Association between serum infliximab trough concentrations during maintenance therapy and biochemical, endoscopic, and histologic remission in Crohn's disease. Inflamm Bowel Dis . 2018;24(10):2266–2271. doi: 10.1093/ibd/izy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolbink GJ, Voskuyl AE, Lems WF et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis . 2005;64(5):704–707. doi: 10.1136/ard.2004.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulleman D, Chu Miow Lin D, Ducourau E et al. Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis. Ther Drug Monit . 2010;32(2):232–236. doi: 10.1097/FTD.0b013e3181cc6fef. [DOI] [PubMed] [Google Scholar]
- 52.Pouw MF, Krieckaert CL, Nurmohamed MT et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis . 2015;74(3):513–518. doi: 10.1136/annrheumdis-2013-204172. [DOI] [PubMed] [Google Scholar]
- 53.Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol . 2006;4(10):1248–1254. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 54.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol . 2013;108(1):40–47. doi: 10.1038/ajg.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]


