Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in a catastrophic pandemic and severely impacted people's livelihoods worldwide. In addition, the emergence of SARS-CoV-2 variants has posed a severe threat to humankind. Due to the dearth of therapeutic options during the commencement of the pandemic, convalescent plasma therapy (CPT) played a significant part in the management of patients with severe form of COVID-19. Several recent studies have proposed various protective effects of CPT, such as antiviral, anti-inflammatory, anti-thrombotic, and immunomodulatory actions, curtailing the devastating consequences of the SARS-CoV-2 infection. On the contrary, several clinical studies have raised some serious concerns about the effectiveness and reliability of CPT in the management of patients with COVID-19. The protective effects of CPT in severely ill patients are yet to be proved. Moreover, the emergence of SARS-CoV-2 variants has raised concerns about the effectiveness of CPT against COVID-19. Therefore, to establish concrete evidence of the efficacy of CPT and adjudicate its inclusion in the management of COVID-19, an updated review of present literature is required, which could help in the development of an efficient therapeutic regimen to treat COVID-19 amid the emergence of new viral variants.
Keywords: COVID-19, SARS-CoV-2, Convalescent plasma therapy, Variants, Effectiveness, Safety
1. Introduction
The COVID-19 (coronavirus disease 2019) pandemic has been caused by a novel coronavirus (nCoV) belonging to the sub-genus Sarbecovirus within the genus Betacoronavirus, known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The disease has spread throughout the globe resulting in more than 5.3 million fatalities in 223 nations [[1], [2], [3]]. This scenario has caused widespread panic, worldwide threats, financial losses, and a persistent escalation in daily COVID-19 cases [4,5]. Few vaccine candidates have recently been discovered, and immunization is underway to combat COVID-19 at the global level. However, many countries worldwide are still encountering drastic challenges in limiting the deleterious consequences of the pandemic due to the lack of appropriate therapeutic options [[6], [7], [8], [9]].
Numerous scientists and medical experts have suggested the use of existing antiviral medicines as repurposed drug options. Antiviral therapeutic options such as remdesivir, favipiravir, lopinavir, ritonavir, and arbidol have been identified as potential COVID-19 medication alternatives [[10], [11], [12], [13]]. These medicines have diverse mechanisms of action such as, blocking of the viral entry into host cells or virus multiplication within the host cells [[13], [14], [15]]. However, several newly proposed therapeutic options have shown disappointing clinical outcomes and harmful consequences on critically ill patients with SARS-CoV-2 infection.
There is an urgent need for the development of tailored treatments and vaccinations. Intensive efforts are being undertaken to find viable vaccines and treatment alternatives [11,16]. Rapid and significant research advancements have led to the characterization of SARS-CoV-2, understanding of the COVID-19 from various perspectives and efforts to eventually discover adequate vaccines, immunotherapeutics, and prophylactic and therapeutic strategies [17]. In addition to finding the vaccines and effective treatments, alternative and supportive techniques for mitigating the consequences of SARS-CoV-2 infections in humans and treating the COVID-19 patients are required [18,19].
Immunotherapeutic approaches are one of the therapeutic modalities that have received a lot of attention. Convalescent plasma therapy (CPT) or monoclonal antibodies (MABs) based therapy is an efficient and reliable immunotherapeutic approach that aims to boost patients’ immune systems and protect them from viral infection [20,21]. Among a plethora of therapeutic options, CPT has shown real potential in the treatment of patients with COVID-19. CPT relies on a high titer value of neutralizing antibodies (NABs) which are usually present in the plasma of recovered patients. NABs are transferred into recently infected patients with SARS-CoV-2, which helps in combating viral multiplication [[21], [22], [23]]. CPT has been employed in various viral outbreaks, namely SARS, MERS, Ebola, and H1N1 influenza [[24], [25], [26], [27], [28]]. Although the CPT was tested during the SARS, MERS, and Ebola epidemics, but there were no randomized control trials to back up its effectiveness. However, the recent clinical trials have produced encouraging outcomes, indicating the potentialities of this therapy in the management of COVID-19 hospitalized patients [22,29]. The Food and Drug Administration (FDA) has authorized CPT under the Emergency Use Authorization (EUA) for severely affected patients with SARS-CoV-2 infection [30,31].
Multiple trials examining the use of CPT in the treatment of COVID-19 have shown conflicting results, even though it seemed to be a promising therapeutic regimen against COVID-19. According to a recent clinical study based on the US national registry, the hospitalized COVID-19 patients who received a treatment of convalescent plasma (CP) with higher anti-SARS-CoV-2 IgG antibody levels had a lower risk of death than patients who received a transfusion of convalescent plasma with lower concentrations of antibodies [32]. In contrast to this, in other recent studies, patients treated with convalescent plasma vs. conventional treatment revealed no significant differences in clinical improvement or overall mortality [31,[33], [34], [35]]. Hence, this review article focuses on the therapeutic potentials of CPT and the challenges associated with this therapeutic regimen, with insights into the COVID-19 treatment prospects amid the emergence of new SARS-CoV-2 variants. Although, this review article is not a systemic one and may contain the personal biases of the authors.
2. Convalescent plasma therapy (CPT)
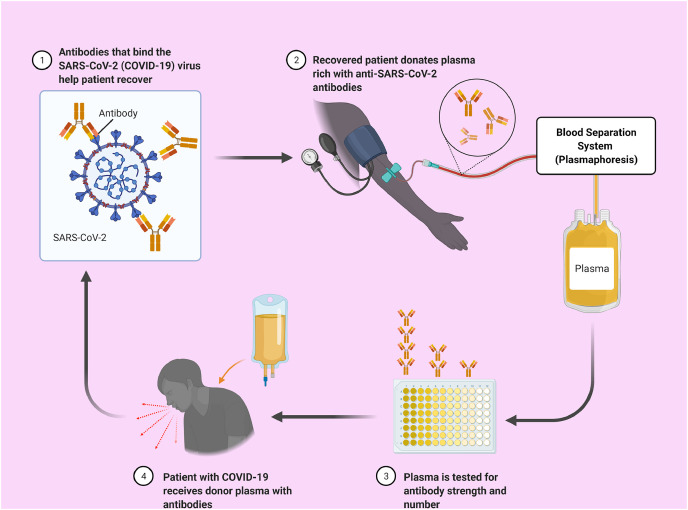
CPT has been utilized as a passive immunization technique to prevent and treat infectious illnesses since the 20th century [36]. CPT comprises of three basic steps: (1) whole blood extraction from the donor, (2) plasma separation from donor's blood, and (3) plasma transfer into the patient because the donor had previously recovered from a severe viral infection, thus having plasma which is predicted to contain a high concentration of antibodies that react specifically to the virus [37] (Fig. 1 ).
Fig. 1.
A schematic representation of convalescent plasma therapy (CPT). The convalescent plasma (CP) gets collected from the recovered patients and analyzed for the antibody's concentration and strength. Then plasma will be transferred to the patients with COVID-19 based on compatibility and other factors.
Convalescent plasma transfusions include the passive injection of large quantities of antibodies, providing the infected people with immediate immunity [29,38]. Management with convalescent plasma for treating the SARS patients resulted in reduced hospital stay and decreased mortality. Following the administration of this plasma, no acute side effects were detected [39]. Furthermore, the patients who received convalescent plasma before 14 days of sickness had a better prognosis [27].
Several previous reports have agreed that the transfusion of convalescent plasma also provides several other functional components viz., organic compounds, water, proteins such as albumin, globulins, coagulation and anti-thrombotic factors, complement and inorganic ions, which in turn enhance the positive outcomes of CPT in the treatment of patients with severe viral infection [40]. Previously, it has been found that the replenishing of coagulation factors by CPT for the treatment of hemorrhagic fevers in the case of Ebola showed positive outcomes. Modulation of the immune system via anti-inflammatory cytokines and antibodies present in convalescent plasma has been reported as a positive additive effect of CPT [41]. Another positive effect of CPT seems to the preservation of colloidal osmotic pressure in body fluid compartments, primarily by albumin [41,42]. Moreover, CPT has been shown to reduce the viral load in influenza patients [43]. Several studies have found that the patients treated with convalescent plasma or serum in severe forms of viral acute respiratory infections had a significantly lower death rate [41,42,44].
3. Mechanism of CPT
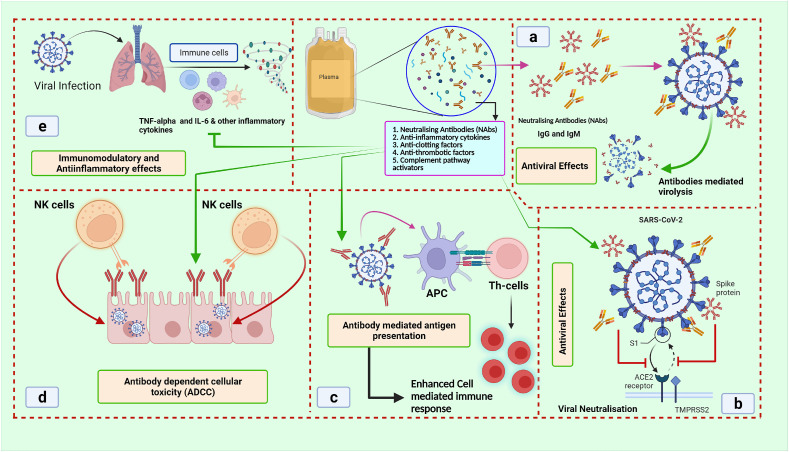
In previous studies of SARS and MERS, it has been found that the NAbs bind to spike proteins, limiting the viral entrance and amplification, which is considered as one of the possible explanations for the reported benefits of convalescent plasma treatment [45]. Additional constituents such as anti-inflammatory factors and other unknown proteins may attribute the additional benefits of plasma therapy. Hence the transfusion of convalescent plasma may also provide additional advantages to infected individuals, such as better immunological control through the reduction of a strong inflammatory response [46]. Prior to plasma donation, plasma donors must undertake a routine pre-donation evaluation. Subjects must be between the ages of 18 and 65, free of infectious symptoms, and have a test negative for COVID-19 after the 14th day of recovery. The test is performed 48 h later, as well as during the donation process [47]. The use of CP relies on the concept of passive immunization, where the recipients receive antibody-rich plasma from those individuals who had recovered from an illness. There are multiple proposed mechanisms by which CP can act a therapeutic option. CP contains IgM and IgG antibodies that may bind to a specific pathogen such as SARS, MERS-CoV and SARS-CoV-2 and act as neutralizing antibodies, thus inhibiting these viruses [48,49] (Fig. 2 ).
Fig. 2.
Various action mechanisms of convalescent plasma (CP) to reduce the devastating consequences of a viral infection such as SARS-CoV-2 infection. The figure depicting the a) antiviral effects of CP via antibody-mediated lysis of the viral particles b) blocking the entry of viral cells into the host cell leads to reduced viral load c) Antibody-dependent antigen presentation, which enhances the cellular immune response d) Antibody-dependent cell-mediated cytotoxicity (ADCC) which leads to lower viral load e) Immunomodulatory and anti-inflammatory actions of CP by blocking the inflammatory cytokines such as IL-6 and TNF-α. Abbreviations: SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; NK cells: natural killer cells; APC: antigen processing cells; Th cells: T helper cells; ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane protease serine 2.
The antiviral properties of CP may be one of the important mechanisms by which CPT protects against the viral infection. The use of CP produces neutralizing antibodies (NAbs) that suppress the infection by promoting viral clearance. In MERS and SARS-CoV models, NAbs bind to the spike-receptor binding protein, limiting the virus entry and replication. In addition, antibody-mediated cytotoxicity, complement activation, and phagocytosis increase the therapeutic potential of CP [48,50]. CPT-transfused antibodies directly neutralize the viral particles by attaching to their surface and phagocytosing. CPT also induces the complement activation and antibody-dependent cellular cytotoxicity [51] (Fig. 2), thus lowering the viral load. CP's antiviral properties can reduce the viral load [46,52], but the effect on patients with severe SARS-CoV-2 infection is still unknown. Apart from NAbs, Non-NAbs are also reported to be important in viral clearance. In vitro studies have shown that non-NAbs bind to viruses but have minimal antiviral activity because they do not interfere with viral replication. These are vital in patients' rehabilitation and prevention of infection. However, the NAbs as well as the Non-NAbs have antiviral activity against coronaviruses (CoVs). SARS-CoV and SARS-CoV-2 have been shown to produce viral-specific non-NAbs (IgM and IgG) that can be transfused with CP to stop the viral replication and multiplication [46,52].
Also, CPT's immunomodulatory properties have been reported as an important therapeutic strategy to reduce hyperinflammatory responses in SARS-CoV-2 patients. Intravenous immunoglobulin (IVIg) infusion has been shown to control the hyperinflammatory/autoimmune responses [46,52]. CP, like IVIg, blocks autoreactive antibodies with anti-idiotypic antibodies. Autoreactive antibody inhibition is critical in SARS-CoV-2 patients [46]. Controlling the autoimmune response may help prevent dangerous outcomes like thrombosis and disseminated intravascular coagulopathy. CPT neutralizes anti-β2-glycoprotein and anti-cardiolipin IgA antibodies, reducing thrombotic events [42,52]. Moreover, CPT has been shown to regulate hyperinflammatory responses such as cytokine and chemokine overproduction, protecting the lungs from SARS-CoV-2 infection. These immunoregulatory mechanisms have been linked to improved outcomes in COVID-19 patients treated with CP [42,46] (Fig. 2).
Several clinical trials have previously confirmed CPT's anti-inflammatory effects [53]. In addition to previous reports, recent research suggests that CP administration reduces circulating cytokines such as TNF-α (tumor necrosis factor-alpha) and IL-6 ((interleukin-6), which may reduce the risk of cytokine storm, which has been linked to increased mortality in COVID-19 patients [54,55]. Moreover, serine protease inhibitors like α-1 antitrypsin (AAT) have been linked to CPT's anti-inflammatory effects. Recent research suggests AAT inhibits neutrophil elastase, reducing pulmonary tissue damage and neutrophil extracellular trap formation. AAT is also antiviral and prevents viral entry into the host cell. AAT inhibits TMPRSS2 (transmembrane serine protease 2), which is required for SARS-CoV-2 entry into the host cell. Thus, AAT may be responsible for CPT's anti-inflammatory and antiviral effects [56]. Furthermore, recent research shows that CPT patients have lower D-dimer levels, a predictor of SARS-CoV-2 infection and thrombosis [57,58]. CPT is also a good source of antithrombin and albumin, two plasma proteins involved in hemostasis [59,60], which may help mitigate the effects of SARS-CoV-2 infection. However, future studies are needed to unravel the mechanisms involved in the anti-inflammatory and anti-thrombotic effects of CPT.
4. Dynamics of antibody profile of infected and recovered patients
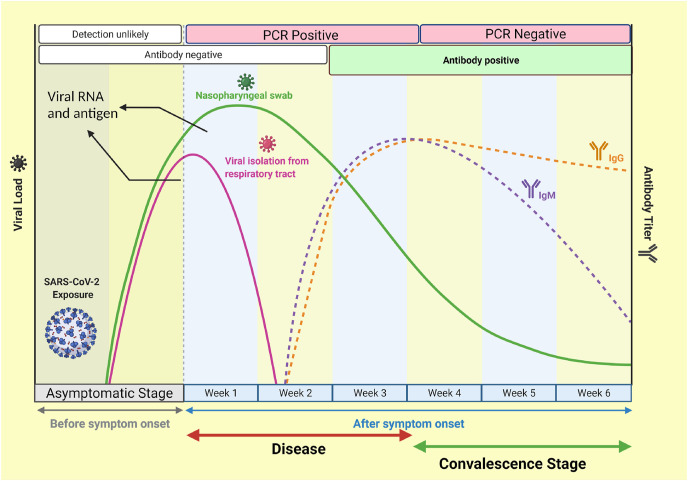
The creation of an effective CP-based therapy regimen could be aided by understanding the dynamics or changes in the antibody profile in recovered patients. In this context, semi-quantitative fluorescent immunoassay and ELISA techniques have been utilized to assess the generation of antibodies at the onset and rehabilitation stages of SARS-CoV-2 infection. Immunoreactivity to full-length Spike (S) proteins, the C-terminal domain (CTD), and the N-terminal domain (NTD) of S1 fragments have been evaluated. Both anti-S IgG and anti-S IgM antibodies have been shown to be absent at the beginning of the illness (Fig. 3 ). The increased anti-S IgG titers in the recovering COVID-19 patients might be valuable in determining the latently infected population via serological screening after the pandemic. The deduction of a high amount of anti-S IgG titres might justify the use of plasma transfusion for the treatment of severely affected COVID-19 patients [61].
Fig. 3.
A graphical representation of dynamics of antibody profile in the infected and recovered patients. IgM-mediated antibody response starts after the 5–7 days of infection and peaks by approximately week three and gradually declines over the six weeks. On the other hand, IgG antibody production starts after one week of the infection and peaks at the fourth week during the convalescence stage. The reasons behind the gradual decline of IgM concentration are still not clear.
Interestingly, a recent comparative analysis found that asymptomatic carriers had greater antiviral immunity to clear SARS-CoV-2 than symptomatic COVID-19 patients and that this antiviral immunity is due to innate and adaptive cellular immunity rather than humoral immunity [62]. Among asymptomatic carriers or those with greater innate and cellular immunity, antibody-mediated immune responses or humoral immunity against SARS-CoV-2 was not adequately activated [63,64]. This shows that symptomatic individuals are likely to be good candidates for plasma donation. It should be noted, however, that the antibody titre or antibody levels tend to drop with time. However, the mechanism by which the anti-SARS-CoV-2 immunoglobulins (Igs) quickly drop is unclear. Furthermore, repeated exposure to the same virus may result in increased humoral immunity [62].
In order to undermine the link between antibody titres with the time of sample collection, age, disease severity, and gender a cross-sectional, observational, non-intervention study was conducted with 102 CP donors. These factors can help in finding suitable donors for the CP [65]. However, it has been established that CP donation candidates with a prolonged time interval for the onset of the symptoms showed higher concentrations of IgM and IgG. Furthermore, males, those over the age of 35, and those who had been hospitalized showed higher levels of antibodies than the other categories. A total of 17 individuals had no reactive antibodies [65].
Some scientists have suggested that the people who are more seriously affected with COVID-19 might generate a significantly greater amount of the antibody-mediated immune response [66,67]. A recent study constituting 285 patients discovered that IgG levels were significantly lower in patients with less severity of the disease [63]. In a study of 113 individuals, the severity of illness has been found to be linked with greater immunoglobulin levels [66]. These findings have suggested that the antibody concentrations are significantly higher in symptomatic patients as compared to the asymptomatic group of the patients [66].
5. Therapeutic potential of CPT
The effectiveness of convalescent plasma therapy has been established in several clinical studies. Shen et al. [68] has reported clinical improvements in hospitalized patients treated with CPT. Some patients have been reported to be cured of acute respiratory distress syndrome after twelve days of treatment [68]. Another study of 10 people with severe COVID-19 revealed that the CPT dramatically raised and sustained NAb levels, resulting in a significant reduction of viral load after seven days of the treatment [69]. Over the course of 7 days, the imaging investigations have indicated various degrees of elimination of lung lesions. Employing CPT within 14 days of infection has showed a better result, similar to interferons therapy [69]. These findings indicate that convalescent plasma may be a viable treatment option for COVID-19 patients with severe symptoms. Notably, it appears that prompt treatment is a critical component. Because the viral load peaks in the first week, therapy at this point is likely to be more effective [27]. In Korea, two additional instances of COVID-19 with pneumonia and acute respiratory distress syndrome have been treated with plasma transfusions. After convalescent plasma treatment and systemic steroid therapy, all patients exhibited a favorable prognosis [70].
Many research groups anticipated that CPT might be useful in the treatment of severe acute respiratory infections with viral etiologies, such as SARS-COV-2 [71]. The Shanghai Public Health Clinical Center is enrolling patients for anti-SARS-CoV-2 CPT as part of an observational trial (NCT04292340) entitled “efficacy and safety of anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of Novel Coronavirus Pneumonia Patient (COVID-19)” [72]. Furthermore, at present, there are 56 clinical studies registered at www.clinicaltrials.gov that are evaluating the significance of CPT in COVID-19 [73].
NAbs have long been thought to be crucial for viral clearance and possibly protection against viral infection. Previously it has been found that the Nabs bind to the spike1-receptor-binding domain (S1-RBD) of SARS-CoV. The S1-N-terminal domain and the S2 hinder viral entrance and amplification. NAbs have been discovered to be important in employing passive immunity [45]. It has been reported that the SARS-CoV infection causes the development of IgG antibodies against nucleocapsid proteins (N), which may be identified on day four following the beginning of sickness and with seroconversion from day 14 [74]. Furthermore, recent studies found the maximum IgM concentration on the ninth day following the beginning of the illness, with the transition to IgG occurring in the second week [75]. The receivers of SARS-CoV-2 from donors had higher IgG and IgM titers after receiving convalescent plasma, and the existence of NAbs in the recipients played a significant role in limiting viral infection [73] and increases the survival rates of patients with SARS-CoV-2 infection.
Seriously ill patients with COVID-19 who received anti-SARS-CoV-2 antibody-containing CP coupled with antivirals were investigated subsequently in a recent clinical trial, and compared to the control group, the CP group had a shorter stay in the intensive care unit along with a lower rate of mechanical ventilation support and a lower rate of vasopressor support. In addition, the death rate was comparatively lower in the patients treated with CPT. Hence, CPT was suggested as a beneficial approach to managing severely ill patients with COVID-19 [76].
However, the point to be considered is that the use of CPT at earlier stages of viral infection, along with monitoring of patients given CPT, may help to avoid the emergence of hyperinflammatory immune responses [77]. The antibodies-based therapy such as CPT has recently been authorized by the FDA to relieve the symptoms of COVID-19 in individuals who have been diagnosed with SARS-CoV-2 infection. Apart from CP, other therapeutic regimens based on immunoglobulins/monoclonal antibodies (MABs) such as bamlanivimab have been approved by Eli Lilly and Regeneron Pharmaceuticals for the emergency use of severely infected patients with COVID-19 [78], which has encouraged the scientific community to explore the potentialities of CP in the treatment of patients with COVID-19.
According to a recent systematic review and meta-analysis, CPT may be a useful therapeutic option for COVID-19, with encouraging indications of safety and decreased mortality when used in conjunction with antiviral/antimicrobial medicines, steroids, and other supportive care [79]. Despite the fact that limited randomized trials have shown that CPT does not significantly reduce mortality, several non-RCTs and case reports (series) have indicated that CP can assist the patients in improving clinical symptoms, clearing the virus, and reducing death, particularly in COVID-19 patients who are sick within ten days. It has been postulated that CPT can be a viable therapeutic option to treat patients with SARS-CoV-2 infection if given earlier [35].
For the treatment of COVID-19, a recent development in CPT involving the use of extracellular vesicles such as exosomes. In the present situation, using recovered COVID-19 convalescent plasma-derived exosomes (CPExo) might speed up therapeutic methods. Exosome administration to the in vivo system has been demonstrated to be advantageous and can effectively and precisely target infections. In this concept, the researchers focus on CPExo rather than CP, maybe because exosomes are dispensed, and immune response conferred through antibodies is more efficiently obtained [80]. Exosomes may recognize antigens with a high degree of sensitivity and specificity. Many of these derivatives may cause immunological modulation in cells and function as an epigenetic inheritor response to infections through RNAs. COVID-19-activated plasma-derived exosomes have been reported for their actions beyond the immunological antibodies. Preselecting plasma-derived antibodies and RNA-merged exosomes, according to the presented theory, could be an optimal treatment strategy for COVID-19 patients. In addition, CPExo also exhibits multi-potential influences on treatment effectiveness by serving as an immunotherapeutic, drug carrier, and diagnostic target employing non-coding genetic elements as a biomarker [80].
A recently conducted systematic review and meta-analysis comparing the efficacy of CP to conventional treatment/non-CP on clinical outcomes in patients with COVID-19 has revealed that the CP usage was related to a lower risk of all-cause fatality in COVID-19 patients who were critically infected [81]. In addition, a recent meta-analysis of systematic studies based on the study of the major endpoint mortality has also concluded that the clinical use of CP over conventional treatment when delivered at high titer and early in the course of COVID-19 is safe and effective [82]. However, it has been mentioned by Franchini et al. [82] that the research conducted by them has several limitations, and still, there is a dire need to conduct large-scale clinical trials in collaborations to decipher the true potentialities of CPT [82].
6. Challenges and risks associated with CPT
Screening, approving, collecting, and monitoring of donors, as well as access to adequate assay facilities are some of the administrative and technical challenges in handling CP. CPT usage in poor countries is limited mainly owing to systemic and transfusion-specific difficulties, including a lack of donor recruitment and collecting capability, as well as the lack of efficient healthcare systems [[83], [84], [85], [86], [87],[88], [89]]. Furthermore, donors consenting to give plasma must fulfill the exact requirements as the donors consenting to contribute blood; for example, for CPT, the donor can be harmful if not tested for the SARS-CoV-2. The donor should be free of any COVID-19 symptoms during the CP donation [42,90,91]. The production of CP for treating patients can be hindered by the lack of NAbs in the plasma of the donor, and these antibodies persist for a period of few weeks to months only. CPT also necessitates substantial infusion volumes (200–2400 mL) [92] and there is no standard CP transfusion dose; therefore, the amount is determined as per the patient. The time of administration has an impact on the outcome; for example, the greatest results have been obtained in recipients/patients who received CP transfusion before the onset of the humoral immune response [93]. Thus, there could be a significant disparity between the number of recovered and active/positive cases, and meeting the need for a big volume of plasma to treat the high number of infected patients could be difficult [42]. Viruses, especially coronaviruses, are prone to mutations, which might lead to a reduction in antibody production as well [35,92].
Additional concerns about CPT must be addressed besides regulation of the product's effectiveness and safety for the efficient treatment of patients with COVID-19 [95]. After the receiving CPT, modest complications like fever, skin erythema, and nausea may occur [69]. In the therapy with convalescent plasma, antibody-mediated proinflammatory or antibody-dependent exacerbation of infection consequences could arise. A recent study consisting of 5000 COVID-19 patients has reported only negligible occurrence of adverse effects in the patients. In the patients treated with CPT a mortality rate of approximately 0.3% has been found during the first 4 h following transfusions 0.3% [94,95].
The extending effects of CP transfusion have been demonstrated in another big safety research with 20,000 participants. The authors have documented 146 transfusion responses within 4 h of finishing convalescent plasma transfusion. Sixty-three fatalities (0.3%) were reported out of which 13 were potentially or certainly attributable to transfusion. There were 37 instances of transfusion-associated circulatory overload, 20 transfusion-associated acute lung damage, and 26 of severe allergic transfusion responses [96]. One thousand seven hundred eleven fatalities occurred within seven days following transfusion. Moreover, 1136 serious adverse events such as cardiac events and thromboembolic/thrombotic events have been reported [96]. However, it is important to consider that a number of adverse effects were not related to the transfusion of CP. Hence, the ambiguities with the risks associated with CP transfusion still exist.
A long-term antibody profile study has also been conducted on 115 successive plasma samples from 16 hospitalized COVID-19 patients treated with either CP or standard of care, and only half of them survived. Surprisingly, the presence of CP therapy had no impact on survival. Prior to death, the fatal patients’ neutralization titers dropped dramatically, and convalescent plasma therapy had no effect on this trend. Furthermore, regardless of CP therapy, the increased antibody affinity for the SARS-CoV-2 prefusion spike was linked to a better outcome in terms of survival [97]. It is worth noting that in these COVID-19 patients, a prolonged high IgA response was linked to a fatal event. Such data suggests that the convalescent plasma treatment for COVID-19 patients must be carefully addressed and that the treatment efficiency may be dependent on the physiological and serological condition of COVID-19 patients, as well as the concentration and quality of immunoglobulins in the CP [97].
The standard operating protocols for utilizing the CPT and convalescent plasma in patients with various illnesses have not been delineated yet. The current studies have shown that CPT is safe and effective in curing many illnesses, including COVID-19 [83,85,86], but there are still some ambiguities about the clinical utility of CPT in the treatment of COVID-19 [94] even though there is a hypothetical ground to believe that immunoglobulins in the plasma of people who have survived viral infections might lower viral levels in critically ill patients [2,98]. Moreover, the CPT might cause transfusion-related responses ranging from moderate to severe, including fever, allergies, life-threatening bronchospasm, acute lung damage, and circulatory overload in the elderly patients and in these having with renal and cardiovascular diseases [90,99].
7. SARS-CoV-2 variants and CPT
Ancestral SARS-CoV-2 genetic evolution was modest until the development of a worldwide dominant variant termed D614G, which enhanced transmissibility but not disease severity. Since then, other SARS-CoV-2 variants have been identified, some of which are designated variants of concerns (VOCs) due to their public health implications. VOCs can increase transmissibility or virulence, reduce neutralization by antibodies from natural infection or vaccination, evade detection, or reduce therapeutic or vaccine efficacy [100]. B.1.1.7, B.1.351; P.1; B.1.617.2; B.1.427; P.2; P.3; B.1.525; and C.37 are the major variants of SARS-CoV-2 discovered so far. There is a need to rediscover the potentialities of CPT against these variants as vast unvaccinated communities across the world still raise the possibilities of the emergence of variants [77].
Recently, it has been postulated that the SARS-CoV-2 variants such as B.1.1.7 don't confer increased resistance to plasma from individuals who have recovered from COVID-19 or sera from individuals who have been vaccinated against SARS-CoV-2. However, the B.1.351 variant has been found to be significantly resistant to neutralization by convalescent plasma of recovered individuals who have been vaccinated. It has been concluded that the B.1.351 and emerging variants with similar mutations in the spike protein present new challenges for therapeutic regimens such as CPT and MABs and threaten the protective efficacy of such therapy [98].
Convalescent plasma collected from the patients who had previously been infected with the ancestral SARS-CoV-2 strains has been tested in vitro and shown to have considerably decreased neutralization against the beta (B.1.351) subtype of the virus [101]. This is in corroboration with a recent in vitro study, in which the beta (B.1.351) variant of SARS-CoV-2 exhibited significantly greater resistance to neutralization by convalescent plasma compared to the alpha (B.1.1.7) variant [98]. These findings suggest that CPT may not be an effective therapeutic regimen against COVID-19 amid the emergence of new variants of SARS-CoV-2.
Furthermore, Vidal et al. [102] have correctly stated that broad SARS-CoV-2 immunity will be required to halt the COVID-19 pandemic. Natural infection and vaccinations both trigger NAb responses, which are important components of immunity. However, the SARS-CoV-2 variants are evolving with mutations in the spike gene that facilitate evasion of NAb responses. As a result, these mutations may prolong the vicious grip of the so-called COVID-19 pandemic. The two SARS-CoV-2 variants, B.1.1.7 and B.1.351, were significantly sensitive to NAb responses from early COVID-19 convalescent patients [102]. However, the B.1.351 strain, on the other hand, has a lower susceptibility to early pandemic NAb responses. It has been suggested that the additional virological, immunological, and clinical characterization of the emerging SARS-CoV-2 variants could aid in the development of effective therapeutic regimens [102].
8. Conclusion and future perspectives
Coronavirus illness is currently not treatable with any FDA-approved medicines (COVID-19). Because of the epidemic, the healthcare practitioners are under significant pressure to prescribe medications despite the scanty information regarding their safety and effectiveness. Due to this increased pressure, there is an even greater conflict between the value of practicing evidence-based medicine and the urgency of providing access to promising medicines before their safety and efficacy has been proved. Among a range of immunomodulatory therapeutic approaches, the convalescent plasma therapy (CPT) has emerged as an effective strategy to manage patients with COVID-19. The diverse range of activities possessed by CP the antiviral, immunomodulatory, anti-inflammatory, and anti-thrombotic properties makes it a suitable therapeutic regimen to explore for its effectiveness in the treatment of viral infections. Moreover, the CPT has resulted in a substantial reduction in fatality rates among SARS-CoV-2 patients.
Instead of the several upcoming pieces of evidence regarding the efficiency of CPT, many studies have reported the risks of unwanted reactions that have occurred in the patients treated with CP. Eventually, there has been an ongoing debate over its efficacy. The evidence-based information on the dose and therapeutical index could be beneficial in the clinical acceptance of CPT for routine medical usage in normal medical practice. CP administered early in the course of an infection and containing sufficient antibodies may prevent the development of unwanted immune responses and help in the treatment of patients with COVID-19. In addition, a clear portray of the antibody profile of naturally infected patients and vaccinated people would help in the selection of suitable donors for the plasma. However, the efficacy of CPT against the emerging variants of SARS-CoV-2 and its usefulness in the treatment of COVID-19 is a big question for the scientific community that needs has to be unveiled urgently for the development of safe and efficient therapeutic regimens. The SARS-CoV-2 virus has undergone multiple mutations since its emergence in 2019, resulting in changes in its virulence, thus impacting the disease severity globally. Furthermore, many research groups have found a decreased effectiveness of CP against some variants of SARS-CoV-2, such as B.1.351, raising serious concerns about the CPT. In addition, the emergence of more virulent variants of SARS-CoV-2 could make it more challenging to effectively control the COVID-19 pandemic. In this context, large-scale clinical trials are required to explore the effectiveness of several repurposed therapeutic regimens, including CPT. Moreover, it is essential to consider the prolonging effects of CPT. Hence, the patients receiving CP should be constantly monitored to ensure that there are no unforeseen side effects, which in turn could stipulate the in-field practicality and possible risks associated with CPT.
Ethical approval
This article does not require any human/animal subjects to acquire such approval.
Sources of funding
This compilation is a review article written by its authors and requires no substantial funding to be stated.
Author contribution
CRediT authorship contribution statement: Manish Dhawan: Conceptualization, Data Curation, Resources, Writing - Original Draft, Writing - Review & Editing. Priyanka: Writing - Review & Editing. Manisha Parmar: Conceptualization, Data Curation, Data Acquisition. Steffy Angural: Conceptualization, Data Curation, Data Acquisition, Formal Analysis, Interpretation. Om Prakash Choudhary: Supervision; Visualization; Writing - Original Draft, Writing - Review & Editing.
Research registration Unique Identifying number (UIN)
-
1.
Name of the registry: Not applicable.
-
2.
Unique Identifying number or registration ID: Not applicable.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Guarantor
Steffy Angural, Assistant Professor, Department of Medical Lab Technology, Faculty of Applied Health Sciences, GNA University, Phagwara-Hoshiarpur Road, Sri Hargobindgarh-144401, Punjab, India.
Om Prakash Choudhary, Assistant Professor, Department of Veterinary Anatomy and Histology, College of Veterinary Sciences and Animal Husbandry, Central Agricultural University (I), Selesih, Aizawl-796015, Mizoram, India.
Data statement
The data in this review is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.
Declaration of competing interest
All authors declare that there exist no commercial or financial relationships, that could, in any way, lead to a potential conflict of interest.
Acknowledgements
The figures have been created with BioRender (https://biorender.com/).
References
- 1.Choudhary O.P., Choudhary P., Singh I. India's COVID-19 vaccination drive: key challenges and resolutions. Lancet Infect. Dis. 2021;21(11):1483–1484. doi: 10.1016/S1473-3099(21)00567-3. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; 2015. Ethics of Using Convalescent Whole Blood and Convalescent Plasma during the Ebola Epidemic.https://apps.who.int/iris/handle/10665/161912 [Google Scholar]
- 3.Worldometer COVID live update: the coronavirus. 2021. https://www.worldometers.info/coronavirus/
- 4.Rodriguez-Morales A.J., Bonilla-Aldana D.K., Tiwari R., Sah R., Rabaan A.A., Dhama K. COVID-19, an emerging coronavirus infection: current scenario and recent developments-an overview. J. Pure Appl. Microbiol. 2020;14(1):5–12. doi: 10.22207/JPAM.14.1.02. [DOI] [Google Scholar]
- 5.Rabaan A.A., Al-Ahmed S.H., Al Mutair A., Alhumaid S., Sule A.A., Tirupathi R., Fawzy M., Muhammad J., Khan A., Hasan A., Shrestha D.B., Sah R., Dhawan M., Tiwari R., Bilal M., Ahmad T., Dhama K. Immunopathogenesis and immunobiology of SARS-CoV-2. Inf. Med. 2021;29(2):167–180. [PubMed] [Google Scholar]
- 6.Malik Y.S., Kumar N., Sircar S., Kaushik R., Bhat S., Dhama K., Gupta P., Goyal K., Singh M.P., Ghoshal U., El Zowalaty M.E., Vinodhkumar O.R., Yatoo M.I., Tiwari R., Pathak M., Patel S.K., Sah R., Rodriguez-Morales A.J., Ganesh B., Kumar P., Singh R.K. Coronavirus disease pandemic (COVID-19): challenges and a global perspective. Pathogens. 2020;9(7):519. doi: 10.3390/pathogens9070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhawan M., Angural S., Parmar M. Tuberculosis during the COVID-19: impact, challenges and management. J. Exp. Biol. Agric. Sci. 2020;8(Spl-1):79–86. [Google Scholar]
- 8.Zhang Y., Hu S., Wang J., Xue Z., Wang C., Wang N. Dexamethasone inhibits SARS-CoV-2 spike pseudotyped virus viropexis by binding to ACE2. Virology. 2021;554:83–88. doi: 10.1016/j.virol.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H., Zhu Q., Zhang C., Li J., Wei M., Qin Y., Chen G., Wang K., Yu J., Wu Z., Chen X. Tocilizumab combined with favipiravir in the treatment of COVID-19: a multicenter trial in a small sample size. Biomed. Pharmacother. 2021;133:110825. doi: 10.1016/j.biopha.2020.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan Z., Karataş Y., Rahman H. Anti-COVID-19 drugs: need for more clinical evidence and global action. Adv. Ther. 2020;37(6):2575–2579. doi: 10.1007/s12325-020-01351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawat K., Kumari P., Saha L. COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2021;892:173751. doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Ríos D., López-Agudelo V.A., Ramírez-Malule H. Repurposing antivirals as potential treatments for SARS-CoV-2: from SARS to COVID-19. J. Appl. Pharmaceut. Sci. 2020;10(5):1–9. doi: 10.7324/JAPS.2020.10501. [DOI] [Google Scholar]
- 13.Irshad K., Mudassir M., Irshad S., Irshad M. Recent advances in the knowledge of coronaviruses with special emphasis on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J. Appl. Biol. Biotechnol. 2021;9(1):96–103. doi: 10.7324/JABB.2021.9113. [DOI] [Google Scholar]
- 14.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2021;39(9):3409–3418. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera L. Immunoinformatics approach in designing SARS-CoV-2 vaccine from experimentally determined SARS-CoV T-cell epitopes. J. Appl. Pharmaceut. Sci. 2021;11(3):29–36. doi: 10.7324/JAPS.2021.110303. [DOI] [Google Scholar]
- 16.Izda V., Jeffries M.A., Sawalha A.H. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021;222:108634. doi: 10.1016/j.clim.2020.108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., Singh K.P., Chaicumpa W., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Coronavirus disease 2019-COVID-19. Clin. Microbiol. Rev. 2020;33(4):e00028. doi: 10.1128/CMR.00028-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhawan M., Parmar M., Sharun K., Tiwari R., Bilal M., Dhama K. Medicinal and therapeutic potential of withanolides from Withania somnifera against COVID-19. J. Appl. Pharmaceut. Sci. 2021;11(4):6–13. doi: 10.7324/JAPS.2021.110402. [DOI] [Google Scholar]
- 19.Rabaan A.A., Al-Ahmed S.H., Muhammad J., Khan A., Sule A.A., Tirupathi R., Mutair A.A., Alhumaid S., Al-Omari A., Dhawan M., Tiwari R., Sharun K., Mohapatra R.K., Mitra S., Bilal M., Alyami S.A., Emran T.B., Moni M.A., Dhama K. Role of inflammatory cytokines in COVID-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines. 2021;9(5):436. doi: 10.3390/vaccines9050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keam S., Megawati D., Patel S.K., Tiwari R., Dhama K., Harapan H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev. Med. Virol. 2020;30(5):e2123. doi: 10.1002/rmv.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keikha M., Karbalaei M. Convalescent plasma therapy as a conventional trick for treating COVID-19: a systematic review and meta-analysis study. New Microbes New Infect. 2021;42:100901. doi: 10.1016/j.nmni.2021.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajendran K., Krishnasamy N., Rangarajan J., Rathinam J., Natarajan M., Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J. Med. Virol. 2020;92(9):1475–1483. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharun K., Tiwari R., Iqbal Yatoo M., Patel S.K., Natesan S., Dhama J., Malik Y.S., Harapan H., Singh R.K., Dhama K. Antibody-based immunotherapeutics and use of convalescent plasma to counter COVID-19: advances and prospects. Expet Opin. Biol. Ther. 2020;20(9):1033–1046. doi: 10.1080/14712598.2020.1796963. [DOI] [PubMed] [Google Scholar]
- 24.Dodd L.E., Follmann D., Proschan M., Wang J., Malvy D., van Griensven J., Ciglenecki I., Horby P.W., Ansumana R., Jiang J.F., Davey R.T., Lane H.C., Gouel-Cheron A. A meta-analysis of clinical studies conducted during the West Africa Ebola virus disease outbreak confirms the need for randomized control groups. Sci. Transl. Med. 2019;11(520) doi: 10.1126/scitranslmed.aaw1049. eaaw1049. [DOI] [PubMed] [Google Scholar]
- 25.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment. Ann. Intern. Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 26.Hung I.F., To K.K., Lee C.K., Lee K.L., Chan K., Yan W.W., Liu R., Watt C.L., Chan W.M., Lai K.Y., Koo C.K., Buckley T., Chow F.L., Wong K.K., Chan H.S., Ching C.K., Tang B.S., Lau C.C., Li I.W., Liu S.H., Chan K.H., Lin C.K., Yuen K.Y. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H., Chan P., Wong K.C., Leung C.B., Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko J.H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H., Kim Y.J., Park J.K., Chung C.R., Kang E.S., Cho D., Müller M.A., Drosten C., Kang C.I., Chung D.R., Song J.H., Peck K.R. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir. Ther. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 29.Aviani J.K., Halim D., Soeroto A.Y., Achmad T.H., Djuwantono T. Current views on the potentials of convalescent plasma therapy (CPT) as Coronavirus disease 2019 (COVID-19) treatment: a systematic review and meta-analysis based on recent studies and previous respiratory pandemics. Rev. Med. Virol. 2021;31(6):e2225. doi: 10.1002/rmv.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyner M.J., Bruno K.A., Klassen S.A., Kunze K.L., Johnson P.W., R Lesser E., Wiggins C.C., Senefeld J.W., Klompas A.M., Hodge D.O., Shepherd J.R.A., Rea R.F., Whelan E.R., Clayburn A.J., Spiegel M.R., Baker S.E., Larson K.F., Ripoll J.G., Andersen K.J., Buras M.R., Vogt M.N.P., Herasevich V., Dennis J.J., Regimbal R.J., Bauer P.R., Blair J.E., van Buskirk C.M., Winters J.L., Stubbs J.R., van Helmond N., Butterfield B.P., Sexton M.A., Diaz Soto J.C., Paneth N.S., Verdun N.C., Marks P., Casadevall A., Fairweather D., Carter R.E., Wright R.S. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., Gamarnik A.V., Ojeda D.S., Santoro D.M., Camino P.J., Antelo S., Rainero K., Vidiella G.P., Miyazaki E.A., Cornistein W., Trabadelo O.A., Ross F.M., Spotti M., Funtowicz G., Scordo W.E., Losso M.H., Ferniot I., Pardo P.E., Rodriguez E., Rucci P., Pasquali J., Fuentes N.A., Esperatti M., Speroni G.A., Nannini E.C., Matteaccio A., Michelangelo H.G., Follmann D., Lane H.C., Belloso W.H., PlasmAr Study Group A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N. Engl. J. Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyner M.J., Carter R.E., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., Theel E.S., Wiggins C.C., Bruno K.A., Klompas A.M., Lesser E.R., Kunze K.L., Sexton M.A., Diaz Soto J.C., Baker S.E., Shepherd J.R.A., van Helmond N., Verdun N.C., Marks P., van Buskirk C.M., Winters J.L., Stubbs J.R., Rea R.F., Hodge D.O., Herasevich V., Whelan E.R., Clayburn A.J., Larson K.F., Ripoll J.G., Andersen K.J., Buras M.R., Vogt M.N.P., Dennis J.J., Regimbal R.J., Bauer P.R., Blair J.E., Paneth N.S., Fairweather D., Wright R.S., Casadevall A. Convalescent plasma antibody levels and the risk of death from Covid-19. N. Engl. J. Med. 2021;384(11):1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H., Hu C., Tao C., Yang R., Wang J., Yu Y., Guo Y., Wu X., Xu Z., Zeng L., Xiong N., Chen L., Wang J., Man N., Liu Y., Xu H., Deng E., Zhang X., Li C., Wang C., Su S., Zhang L., Wang J., Wu Y., Liu Z. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m4232. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K.Y., Shah P. Pierce convalescent plasma for COVID-19 complicated by ARDS due to TRALI. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-239762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M., Grazzini G. Convalescent plasma: new evidence for an old therapeutic tool. Blood Transfus. 2016;14(2):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo H., Zhou L., Ma Z., Tian Z., Zhou F. Promising immunotherapies against COVID-19. Adv. Ther. 2021;16:2100044. doi: 10.1002/adtp.202100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soo Y.O., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K., Ng M.H., Chan P., Cheng G., Sung J.J. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin. Microbiol. Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu X., Wong G., Audet J., Bello A., Fernando L., Alimonti J.B., Fausther-Bovendo H., Wei H., Aviles J., Hiatt E. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Li D., Jin X., Huang Z. Fighting Ebola with ZMapp: spotlight on plant-made antibody. Sci. China Life Sci. 2014;57:987. doi: 10.1007/s11427-014-4746-7. [DOI] [PubMed] [Google Scholar]
- 42.Nagoba B., Gavkare A., Jamadar N., Mumbre S., Selkar S. Positive aspects, negative aspects and limitations of plasma therapy with special reference to COVID-19. J. Infect. Public Heal. 2020;13:1818–1822. doi: 10.1016/j.jiph.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyon G.M., Mehta A.K., Varkey J.B., Brantly K., Plyler L., McElroy A.K., Kraft C.S., Towner J.S., Spiropoulou C., Ströher U. Clinical care of two patients with Ebola virus disease in the United States. N. Engl. J. Med. 2014;371:2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 44.Flexner S., Lewis P.A. Experimental poliomyelitis in monkeys: seventh note: active immunization and passive serum protection. J. Am. Med. Assoc. 1910;54:1780–1782. doi: 10.1001/jama.1910.92550480001001i. [DOI] [Google Scholar]
- 45.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R., Mantilla R.D., Shoenfeld Y., Anaya J.M. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020;19(7):102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiberghien P., Xavier de L., Pascal M., Gallian P., Lacombe K., Yazdanpanah Y. Vox Sang.; 2020. Collecting and Evaluating Convalescent Plasma for COVID-19 Treatment: Why and How? [DOI] [PubMed] [Google Scholar]
- 48.Van Griensven J., De Weiggheleire A., Delamou A., Smith P.G., Edwards T., Vandekerckhove P., Bah E.I., Colebunders R., Herve I., Lazaygues C., Haba N. The use of Ebola convalescent plasma to treat Ebola virus disease in resource-constrained settings: a perspective from the field. Clin. Infect. Dis. 2016;62:69–74. doi: 10.1093/cid/civ680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu J., Zheng R., Qiu H. Convalescent plasma for coronavirus disease 2019: dose is the key. J. Transl. Int. Med. 2021;9(2):68–70. doi: 10.2478/jtim-2021-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimoni Z., Niven M.J., Pitlick S., Bulvik S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg. Infect. Dis. 2001;7(4):759. doi: 10.3201/eid0704.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders R.W., Derking R., Cupo A., Julien J.P., Yasmeen A., de Val N., Kim H.J., Blattner C., de la Peña A.T., Korzun J. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP. 664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garraud O., Heshmati F., Pozzetto B., Lefrere F., Girot R., Saillol A., Laperche S. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus. Clin. Biol. 2016;23(1):39–44. doi: 10.1016/j.tracli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acosta-Ampudia Y., Monsalve D.M., Rojas M., Rodríguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., Manrique R. COVID-19 convalescent plasma composition and immunological effects in severe patients. J. Autoimmun. 2021;118:102598. doi: 10.1016/j.jaut.2021.102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bandopadhyay P., D'Rozario R., Lahiri A., Sarif J., Ray Y., Paul S.R., Roy R., Maiti R., Chaudhuri K., Bagchi S., Maiti A., Perwez M.M., Sarkar B.S., Roy D., Chakraborty R., Vasudevan J.S., Sharma S., Biswas D., Maiti C., Saha B., Bhattacharya P., Pandey R., Chatterjee S., Paul S., Ganguly D. Nature and dimensions of systemic hyperinflammation and its attenuation by convalescent plasma in severe COVID-19. J. Infect. Dis. 2021;224(4):565–574. doi: 10.1093/infdis/jiab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang C., Chapman K.R., Wong A., Liu M. α1-Antitrypsin deficiency and the risk of COVID-19: an urgent call to action. Lancet Respir. Med. 2021;9(4):337–339. doi: 10.1016/S2213-2600(21)00018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olivares-Gazca J.C., Priesca-Marín J.M., Ojeda-Laguna M., Garces-Eisele J., Soto-Olvera S., Palacios-Alonso A., Izquierdo-Vega J., Chacon-Cano R., Arizpe-Bravo D., López-Trujillo M.A., Cantero-Fortiz Y., Fernandez-Lara D., Ruiz-Delgado G.J., Ruiz-Argüelles G.J. Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with covid-19: a pilot study. Rev. Invest. Clin. 2021;72(3):159–164. doi: 10.24875/RIC.20000237. [DOI] [PubMed] [Google Scholar]
- 58.Franchini M., Glingani C., Morandi M., Corghi G., Cerzosimo S., Beduzzi G., Storti A., Di Stasi V., Rastrelli G., Vignozzi L., Mengoli C., Garuti M., Beccaria M., Inglese F., Caruso B., Petilino R.A., Amato M., Nicchio M., Pagani M., Bellani A., Castelli G., Casari S., De Donno G. Safety and efficacy of convalescent plasma in elderly COVID-19 patients: the RESCUE Trial. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5(2):403–412. doi: 10.1016/j.mayocpiqo.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gazzaruso C., Valenti C., Coppola A., Gallotti P. Impact of convalescent and nonimmune plasma on mortality of patients with COVID-19: a potential role for antithrombin. Clin. Microbiol. Infect. 2021;27(4):637–638. doi: 10.1016/j.cmi.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Violi F., Cangemi R., Romiti G.F., Ceccarelli G., Oliva A., Alessandri F., Pirro M., Pignatelli P., Lichtner M., Carraro A., Cipollone F., D'ardes D., Pugliese F., Mastroianni C.M. Is albumin predictor of mortality in COVID-19. Antioxidants Redox Signal. 2021;35(2):139–142. doi: 10.1089/ars.2020.8142. [DOI] [PubMed] [Google Scholar]
- 61.Bao Y., Ling Y., Chen Y.Y., Tian D., Zhao G.P., Zhang X.H., Hang H., Li Y., Su B., Lu H.Z., Xu J., Wang Y. Dynamic anti-spike protein antibody profiles in COVID-19 patients. Int. J. Infect. Dis. 2021;103:540–548. doi: 10.1016/j.ijid.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y., Li P., Ding Y., Liu M., Liu L., Yi B., Wu T., Dong H., Lao X., Ding K., Wang H., Zhang D., Tan X., Wang Z., Xu G., Cao G. Epidemiological feature, viral shedding, and antibody seroconversion among asymptomatic SARS-CoV-2 carriers and symptomatic/presymptomatic COVID-19 patients. J. Infect. Public Health. 2021;14(7):845–851. doi: 10.1016/j.jiph.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., Wang D.Q., Hu Y., Ren J.H., Tang N., Xu Y.Y., Yu L.H., Mo Z., Gong F., Zhang X.L., Tian W.G., Hu L., X Zhang X., Xiang J.L., Du H.X., Liu H.W., Lang C.H., Luo X.H., Wu S.B., Cui X.P., Zhou Z., Zhu M.M., Wang J., Xue C.J., Li X.F., Wang L., Li Z.J., Wang K., Niu C.C., Yang Q.J., Tang X.J., Zhang Y., Liu X.M., Li J.J., Zhang D.C., Zhang F., Liu P., Yuan J., Li Q., Hu J.L., Chen J., Huang A.L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 64.Li W., Su Y.Y., Zhi S.S., Huang J., Zhuang C.L., Bai W.Z., Wan Y., Meng X.R., Zhang L., Zhou Y.B., Luo Y.Y., Ge S.X., Chen Y.K., Ma Y. Virus shedding dynamics in asymptomatic and mildly symptomatic patients infected with SARS-CoV-2. Clin. Microbiol. Infect. 2020;26(11):1556. doi: 10.1016/j.cmi.2020.07.008. e1-1556.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prudente T.P., Castro R.G., Candido M.A., Rodrigues R.L., de Souza L.M., Roberti M.D.R.F. Antibody response against SARS-CoV-2 in convalescent plasma donors: can we predict subjects' eligibility? Hematol. Transfus. Cell Ther. 2021 doi: 10.1016/j.htct.2021.07.008. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., Hauser B.M., Caradonna T.M., Clayton K.L., Nitido A.D., Murali M.R., Alter G., Charles R.C., Dighe A., Branda J.A., Lennerz J.K., Lingwood D., Schmidt A.G., Iafrate A.J., Balazs A.B. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2):476–488. doi: 10.1016/j.cell.2020.12.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ledford H. What the immune response to the coronavirus says about the prospects for a vaccine. Nature. 2020;585(7823):20–21. doi: 10.1038/d41586-020-02400-7. [DOI] [PubMed] [Google Scholar]
- 68.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Xu Y., Chen W., Peng Y., Hu Y., Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U.S.A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Nam S.K., Yeom J.S., Roh J., Ahn M.Y., Chin B.S., Young S.K., Hyukmin L., Dongeun Y., Hyun O.K., Kim S., Choi J.Y. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J. Kor. Med. Sci. 2020;35(14):e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo J.H. Convalescent plasma therapy for corona virus disease 2019: a long way to go but worth trying. J. Kor. Med. Sci. 2019;35(14) doi: 10.3346/jkms.2020.35.e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manuel R., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R., Shoenfeld Y., Anaya J.M. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020;(7):102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin. Microbiol. Infect. 2004;10(12):1062–1066. doi: 10.1111/j.1469-0691.2004.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev. Med. Virol. 2020;30(3) doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Altuntas F., Ata N., Yigenoglu T.N., Bascı S., Dal M.S., Korkmaz S., Namdaroglu S., Basturk A., Hacıbekiroglu T., Dogu M.H., Berber İ., Dal K., Kınık K., Haznedaroglu İ., Yılmaz F.M., Kılıç İ., Demircioğlu S., Yosunkaya A., Erkurt M.A., Turgut B., Caglayan M., Celik O. Convalescent plasma therapy in patients with COVID-19. Transfus. Apher. Sci. 2021;60(1):102955. doi: 10.1016/j.transci.2020.102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moubarak M., Kasozi K.I., Hetta H.F., Shaheen H.M., Rauf A., Al-kuraishy H.M., Qusti S., Alshammari E.M., Ayikobua E.T., Ssempijja F., Afodun A.M., Kenganzi R., Usman I.M., Ochieng J.J., Osuwat L.O., Matama K., Al-Gareeb A.I., Kairania E., Musenero M., Welburn S.C., Batiha G.E.S. The rise of SARS-CoV-2 variants and the role of convalescent plasma therapy for management of infections. Life. 2021;11(8):734. doi: 10.3390/life11080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reuters Italy approves GSK-Vir antibody to treat COVID-19. 2021. https://www.reuters.com/world/europe/italy-approves-gsk-vir-antibody-treat-covid-19-2021-07-13/ Published on 13 July 2021.
- 79.Bansal V., Mahapure K.S., Mehra I., Bhurwal A., Tekin A., Singh R., Gupta I., Rathore S.S., Khan H., Deshpande S., Gulati S., Armaly P., Sheraton M., Kashyap R. Mortality benefit of convalescent plasma in COVID-19: a systematic review and Meta-Analysis. Front. Med. 2021;8:624924. doi: 10.3389/fmed.2021.624924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anand K., Vadivalagan C., Joseph J.S., Singh S.K., Gulati M., Shahbaaz M., Abdellattif M.H., Prasher P., Gupta G., Chellappan D.K., Dua K. A novel nano therapeutic using convalescent plasma derived exosomal (CPExo) for COVID-19: a combined hyperactive immune modulation and diagnostics. Chem. Biol. Interact. 2021;344 doi: 10.1016/j.cbi.2021.109497. 109497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kloypan C., Saesong M., Sangsuemoon J., Chantharit P., Mongkhon P. CONVALESCENT plasma for COVID-19: a meta-analysis of clinical trials and real-world evidence. Eur. J. Clin. Invest. 2021;51(11) doi: 10.1111/eci.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Franchini M., Corsini F., Focosi D., Cruciani M. Safety and efficacy of convalescent plasma in COVID-19: an overview of systematic reviews. Diagnostics. 2021;11(9):1663. doi: 10.3390/diagnostics11091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125(Suppl. 2):S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shankar-Hari M., Estcourt L., Harvala H., Roberts D., Menon D.K. Convalescent plasma to treat critically ill patients with COVID-19: framing the need for randomised clinical trials. Crit. Care. 2020;24:1–5. doi: 10.1186/s13054-020-03163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Oliveira F.A., Nucci M.P., Rego G.N.D.A., Alves A.D.H., Marti L.C., Nucci L.P., Mamani J.B., Gamarra L.F. Convalescent plasma therapy in COVID-19 critically ill patients during advanced phases of clinical trials and their preliminary results. Einstein. 2021;19 doi: 10.31744/einstein_journal/2021RW6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloch E.M., Goel R., Montemayor C., Cohn C., Tobian A.A. Promoting access to COVID-19 convalescent plasma in low- and middle-income countries. Transfus. Apher. Sci. 2021;60(1):102957. doi: 10.1016/j.transci.2020.102957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahmood A., Mahmood R., Khan M., Ali S., Khan N., Jaffar S.R. Convalescent plasma therapy for Covid 19-A perspective. Hematol. Transfus. Int. J. 2021;9:28–30. doi: 10.15406/htij.2021.09.00247. [DOI] [Google Scholar]
- 88.World Health Organization . World Health Organization; 2021. Middle East Respiratory Syndrome Coronavirus (MERS-CoV)https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov) [Google Scholar]
- 89.World Health Organization . World Health Organization; 2015. Ethics of Using Convalescent Whole Blood and Convalescent Plasma during the Ebola Epidemic.https://apps.who.int/iris/handle/10665/161912 [Google Scholar]
- 90.Zhao Q., He Y. Challenges of convalescent plasma therapy on COVID-19. J. Clin. Virol. 2020;127:104358. doi: 10.1016/j.jcv.2020.104358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rejeki M.S., Sarnadi N., Wihastuti R., Fazharyasti V., Samin W.Y., Yudhaputri F.A., Johar E., Nurainy N., Bachtiar N.S., Muljono D.H. Convalescent plasma therapy in patients with moderate-to-severe COVID-19: a study from Indonesia for clinical research in low- and middle-income countries. EClinicalMedicine. 2021;36:100931. doi: 10.1016/j.eclinm.2021.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moubarak M., Kasozi K.I., Hetta H.F., Shaheen H.M., Rauf A., Al-kuraishy H.M., Qusti S., Alshammari E.M., Ayikobua E.T., Ssempijja F., Afodun A.M., Kenganzi R., Usman I.M., Ochieng J.J., Osuwat L.O., Matama K., Al-Gareeb A.I., Kairania E., Musenero M., Welburn S.C., Batiha G.E.-S. The Rise of SARS-CoV-2 Variants and the role of convalescent plasma therapy for management of infections. Life. 2021;11(8):734. doi: 10.3390/life11080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piechotta V., Chai K.L., Valk S.J., Doree C., Monsef I., Wood E.M., Lamikanra A., Kimber C., McQuilten Z., So-Osman C., Estcourt L.J., Skoetz N. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst. Rev. 2020;7(7):CD013600. doi: 10.1002/14651858.CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A., Carter R.E., Klompas A.M., Wiggins C.C., Shepherd J.R., Rea R.F., Whelan E.R., Clayburn A.J., Spiegel M.R., Johnson P.W., Lesser E.R., Baker S.E., Larson K.F., Ripoll J.G., Andersen K.J., Hodge D.O., Kunze K.L., Buras M.R., Vogt M.N., Herasevich V., Dennis J.J., Regimbal R.J., Bauer P.R., Blair J.E., Van Buskirk C.M., Winters J.L., Stubbs J.R., Paneth N.S., Verdun N.C., Marks P., Casadevall A. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joyner M.J., Bruno K.A., Klassen S.A., Kunze K.L., Johnson P.W., Lesser E.R., Wiggins C.C., Senefeld J.W., Klompas A.M., Hodge D.O., Shepherd J.R.A., Rea R.F., Whelan E.R., Clayburn A.J., Spiegel M.R., Baker S.E., Larson K.F., Ripoll J.G., Andersen K.J., Buras M.R., Vogt M.N.P., Herasevich V., Dennis J.J., Regimbal R.J., Bauer P.R., Blair J.E., van Buskirk C.M., Winters J.L., Stubbs J.R., van Helmond N., Butterfield B.P., Sexton M.A., Diaz Soto J.C., Paneth N.S., Verdun N.C., Marks P., Casadevall A., Fairweather D., Carter R.E., Wright R.S. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang J., Grubbs G., Lee Y., Golding H., Khurana S. Impact of convalescent plasma therapy on SARS CoV-2 antibody profile in COVID-19 patients. Clin. Infect. Dis. 2021;16 doi: 10.1093/cid/ciab317. ciab317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Graham B.S., Mascola J.R., Chang J.Y., Yin M.T., Sobieszczyk M., Kyratsous C.A., Shapiro L., Sheng Z., Huang Y., Ho D.D. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y., Huo P., Dai R., Lv X., Yuan S., Zhang Y., Guo Y., Li R., Yu Q., Zhu K. Convalescent plasma may be a possible treatment for COVID-19: a systematic review. Int. Immunopharm. 2021;91:107262. doi: 10.1016/j.intimp.2020.107262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aleem A., Akbar Samad A.B., Slenker A.K. StatPearls [Internet]. Treasure Island (FL) StatPearls Publishing; 2021. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). 2021 July 18. [Google Scholar]
- 101.Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., Lambson B.E., de Oliveira T., Vermeulen M., van der Berg K., Rossouw T., Boswell M., Ueckermann V., Meiring S., von Gottberg A., Cohen C., Morris L., Bhiman J.N., Moore P.L. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27(4):622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 102.Vidal S.J., Collier A.Y., Yu J., McMahan K., Tostanoski L.H., Ventura J.D., Aid M., Peter L., Jacob-Dolan C., Anioke T., Chang A., Wan H., Aguayo R., Ngo D., Gerszten R.E., Seaman M.S., Barouch D.H. Correlates of neutralization against SARS-CoV-2 variants of concern by early pandemic sera. J. Virol. 2021;95(14) doi: 10.1128/JVI.00404-21. [DOI] [PMC free article] [PubMed] [Google Scholar]