Abstract
Group data means from individuals who self-assess as emotional eaters do not reliably show increased food intake in response to stress or negative emotions. This inconsistency in predictive validity of self-reported emotional eating (EE) could be attributable to unconsidered moderation of the relationship between self-reported EE and behavioral measures of EE. Greater emotional relief from stress by eating may provide enhanced negative reinforcement and promote future EE in response to stress as a form of self-medication. Thus, we predicted that greater emotional relief from stress by eating (decrease in negative affect from stress to post-eating) would moderate the extent to which heightened stress reactivity (measured by systolic blood pressure, SBP) moderates the relationship between self-reported EE and food intake post-stress. We also hypothesized that self-reported EE would not predict greater food consumption on the rest day. 43 undergraduate women completed online assessments of eating behaviors. Participants were given snacks to eat after a mental stress task (TSST) or rest period on separate days in counterbalanced order. Our prediction was supported, as the moderated moderation model (PROCESS model 3) was highly significant on the stress day. Self-reported EE predicted increased food intake post-stress only under conditions of high stress reactivity and high emotional relief. On the rest day, self-reported EE predicted greater snack food intake only when SBP was high. This conditional increased intake substantiates stress as a promoter of snack food consumption for women with greater EE. Overall, our findings identified factors that may distinguish the subset of self-reported emotional eaters who are more likely to display EE behaviors in a laboratory setting, yet further studies are needed to directly test whether negative reinforcement via emotional relief from stress by eating drives enhanced EE following stress.
Keywords: Emotional eating, Stress, Negative affect, Food intake, Emotional relief
1. Introduction
Emotional eating commonly refers to the act of eating that occurs in response to stress or negative emotions (i.e., anxiety, anger, depression, and loneliness) as an attempt to mitigate these undesired states [3, 28, 49, 60]. Emotional eating occurs mostly in women, including those who are normal weight, overweight, and underweight, and is observed in community, clinical, and non-clinical samples across the lifespan [21, 41, 61, 64]. Furthermore, emotional eating is associated with disordered eating [16, 46], increased body mass index, as well as poor weight loss outcomes [2, 62].
1.1. The relationship between self-reported emotional eating and behavioral measure of emotional eating
Assessment of emotional eating is typically via self-report questionnaires rather than via observation, and despite validation studies, self-assessment as an emotional eater may not consistently be associated with increased food intake in response to stress or negative emotions. Both laboratory and naturalistic study designs have been used to validate the measurement of self-reported emotional eating by assessing whether it is associated with behavioral measures of emotional eating [8, 20, 51]. In a naturalistic study, Reichenberger et al. [51] found that only those who reported high self-reported emotional eating (assessed via the Dutch Eating Behavior Questionnaire (DEBQ)) increased their food intake in response to negative emotions. Using the DEBQ, Dweck et al. [18] found that emotional eating was associated with greater food consumption in a stress and short sleep condition, a finding that suggests a combination of stressors are predictive of food intake in self-assessed emotional eaters, and Schnepper at al. (2020) reported that only self-reported emotional eaters rated food pictures as more pleasant and showed a more appetitive reaction in response to a negative mood induction compared to a neutral condition.
In contrast, in a comprehensive review on the predictive validity of a variety of self-report emotional eating questionnaires, Bongers and Jansen [8] found that, across both laboratory and naturalistic studies, people who assess themselves as emotional eaters do not consistently show increased food intake in response to stress or negative emotions. Evers et al. [20] corroborated these findings in a meta-analysis in which negative emotions did not affect eating behavior in self-reported emotional eaters, as did Braden and colleagues [11] who reported that self-reported emotional eating measured via the DEBQ and the Emotional Eating Scale was not associated with greater food intake in response to a negative mood induction procedure. Thus, in contrast to a widely held belief that negative emotions increase food intake in people who use eating as means to regulate their negative emotions (i.e. emotional eaters) [42], self-assessment as an emotional eater does not reliably result in greater eating in response to stress or negative mood induction.
1.2. Moderators of the relationship between self-reported emotional eating and food intake
This inconsistency in the predictive validity of self-reported emotional eating could be attributable to individual variation in factors that moderate the relationship between self-reported emotional eating and behavioral measures of emotional eating. Fig. 1 represents an emotion control system in which emotional eating is part of a feed-forward cycle. This emotional eating cycle provides a theoretical foundation that we will use to investigate moderators of the relationship between self-reported emotional eating and increased food consumption (Fig. 2). When components of the emotional eating cycle (e.g. trigger or relief) are heightened for a subset of self-reported emotional eaters, then those individuals may be more likely to display emotional eating behaviors due to the feed-forward nature of the cycle [34]. The primary aim of the current study was to investigate whether the trigger and relief components of the emotional eating cycle (Fig. 1) act as moderators of the relationship between self-reported emotional eating and food intake (Fig. 2) in order to distinguish the subset of self-reported emotional eaters who are more likely to display emotional eating behaviors in a laboratory setting.
Fig. 1.
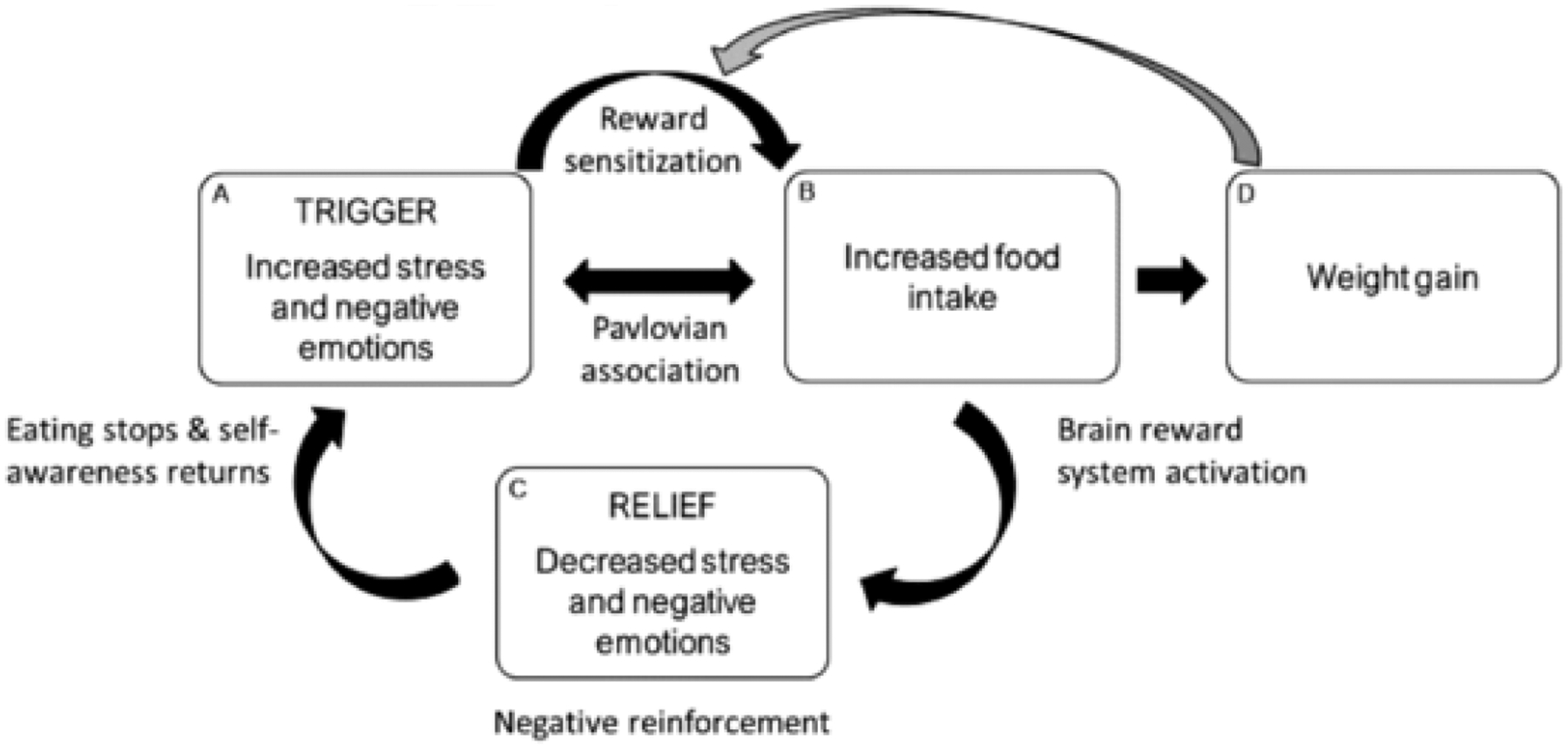
An emotion regulation model is presented in which emotional eating is part of a feed-forward cycle. Increased stress and negative emotions (i.e., trigger; box A) sensitize the brain reward system (pathway) and lead to increased food intake (box B) and weight gain (box D). Increased food intake (box B) causes further activation of the brain reward system and leads to decreased stress and negative emotions (i.e., relief; box C). However, this short-term emotional relief (i.e., negative reinforcement) is not sustained, as stress and negative emotions (box A) return upon the cessation of eating. Over time, increased stress and negative emotions (box A) are more likely to trigger food intake because of positive feedback from factors such as conditioning, brain reward processes, enhanced emotion regulation motives, and weight gain. The gray arrow indicates that weight gain (box D) enhances reward sensitization, which creates a positive feedback loop. Reproduced from [34].
Fig. 2.
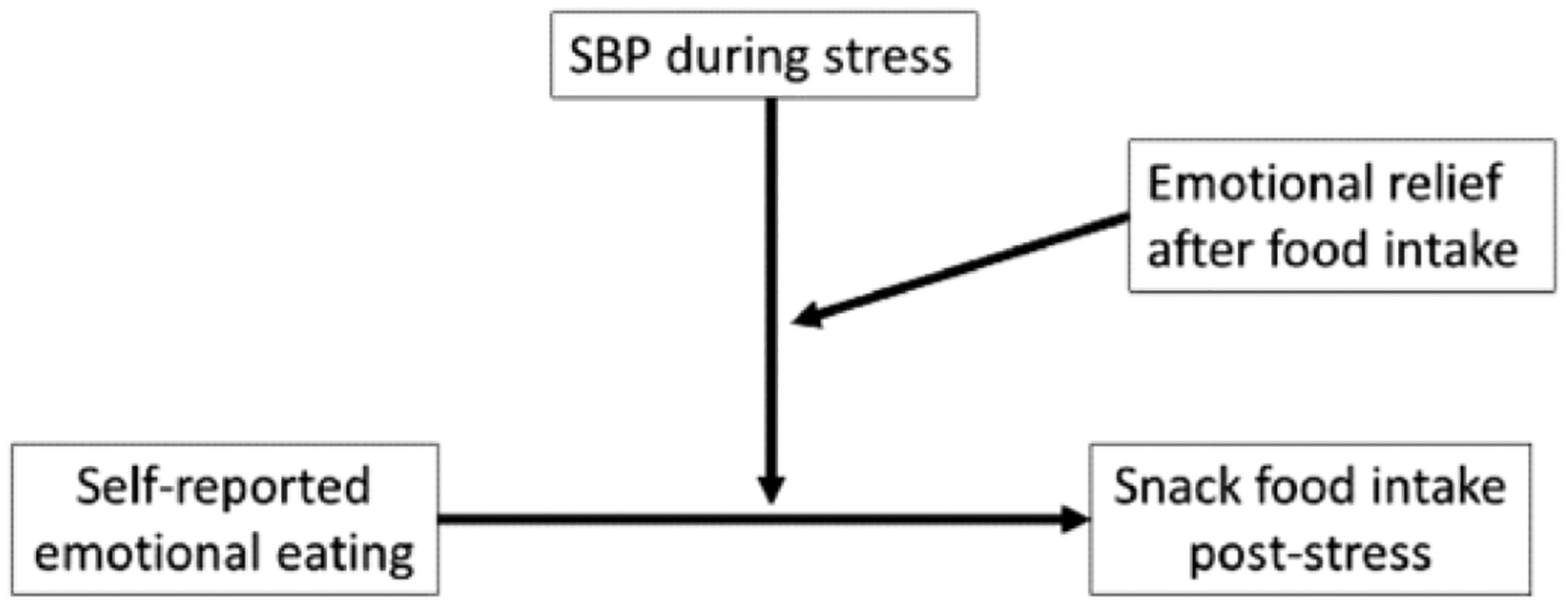
PROCESS theoretical model 3: moderated moderation (SBP = systolic blood pressure).
Stress activates reward pathways and enhances the salience of reward-related cues (e.g. palatable foods) to increase the desire to eat, as well as downregulates cognitive control centers of the brain, a combination of neural adaptations that often leads to greater intake of palatable foods (i.e., comfort foods that are high in fat and/or sugar) [1, 6, 19]. Thus, emotional eaters with heightened stress responsivity (i.e. trigger component, Fig. 1) may experience greater reward sensitization that promotes subsequent components of the emotional eating cycle in a feed-forward manner to increase food intake [19, 22, 50].
In contrast to these findings, increases in stress or negative emotions have been associated with decreased intake or are unrelated to eating in some groups [12, 20] and downregulation of the hypothalamic pituitary adrenal (HPA) axis has been associated with heightened emotional eating [14, 19]. van Strien et al. [63] reported that, for women with extremely high scores on an emotional eating questionnaire, those with a blunted cortisol stress response ate more food following stress than those with a heightened cortisol stress response. The inconsistencies in the literature regarding the relationship between stress and eating for self-reported emotional eaters warrant further investigation into potential moderators of this relationship. We propose that heightened emotional relief from stress upon eating (i.e. relief component, Fig. 1) may be such a moderator.
Emotional eaters are more receptive to the reinforcing value of food and therefore eating is more likely to serve as ‘self-medication’ and provide short-term relief from stress and negative emotions [60]. Eating palatable foods tends to make people feel better in the face of stress or negative emotions ([15, 40, 44]; but also see [24, 26, 56]) and triggers increased dopamine secretion in the mesolimbic pathway signaling pleasure and reward [54, 66]. Following a stressor, palatable food consumption reduces activity in the brain’s stress response network [19] and reduces behavioral and neuroendocrine responses to stress in rats [23, 37, 48]. Skinner’s reinforcement learning theory [53] posits that rewards such as relief from stress and negative affect are reinforcing consequences of eating and can promote future eating behaviors [69]. Therefore, emotional eating may be promoted by negative reinforcement, the relief from stress or seeking pleasure to reduce stress [19, 43].
Enhanced emotional relief (i.e., negative reinforcement in response to stress-induced palatable food intake) may promote future emotional eating in response to stress as a form of self-medication [19]. Thus, enhanced negative reinforcement should strengthen the trigger component of the emotional eating cycle (Fig. 1) and thereby increase the potency of stress as a trigger of food intake for self-reported emotional eaters. In contrast, the subset of self-reported emotional eaters who do not show enhanced emotional relief from stress upon eating may not experience the negative reinforcement learning necessary to increase the potency of stress as a trigger for increased food intake [34]. Inclusion of both those who do and do not experience emotional relief in samples may explain the low predictive validity of self-report questionnaires assessing emotional eating observed in the literature.
1.3. Proposed model
A solution to this problem may be to show that heightened stress reactivity and emotional relief from stress by eating, major components of the emotional eating cycle (Fig. 1), moderate the relationship between self-reported emotional eating and increased food intake. We predicted that greater emotional relief from stress by eating (decrease in negative affect from stress to post-eating) would moderate the degree to which heightened stress reactivity (measured by systolic blood pressure, SBP) moderates the relationship between self-reported emotional eating and food intake post-stress (see Fig. 2).
1.4. Does self-reported emotional eating predict greater food intake only in the context of stress?
A further contribution to variability in the predictive validity of self-reported emotional eating may be the observation that emotional eating is indicative of overeating in general rather than specifically in response to stress or negative emotions [8]. Bongers et al. [7] measured food intake in response to a negative mood manipulation, positive mood manipulation, food cue exposure, and a control condition in emotional eaters and non-emotional eaters. Emotional eaters, identified based on food intake in response to a negative mood manipulation as well as via self-report, consumed more food across all conditions. Furthermore, Bongers and colleagues [10] found that emotional eaters increased food consumption after positive emotions and many researchers have reported positive correlations between emotional eating and external eating [29, 47]. Thus, increased food intake in emotional eaters may occur in response to a variety of external and internal food-related cues rather than to stress or negative emotions only. Other food-related cues may become associated with eating and elicit learned cue reactivity to increase food intake for self-reported emotional eaters more generally and in a variety of circumstances [7, 31].
A secondary aim of the present study is to investigate the specificity of emotional eating behaviors by examining if higher self-reported emotional eating predicts greater food consumption at rest, rather than solely in response to stress or negative emotions. Given that emotional eating commonly refers to eating that arises specifically in response to stress or negative emotions [3, 28, 49, 60], we predicted that the relationship between self-reported emotional eating and increased food intake would not be present under control conditions (i.e. rest vs. stress), which would provide evidence to refute the conceptualization of emotional eating as simply overeating in general.
2. Materials and methods
2.1. Participants
The current report represents a secondary analysis of data used in previous studies [27, 35]. Female undergraduate students (n = 43) between the ages of 18 and 22 years responded to an advertisement for research examining stress, and participants were told that the study was investigating stress physiology in college women. We specifically recruited women for the study because women report more emotional eating and greater food intake in response to stress than men, and because the relationship between stress and obesity is greater in women than men [36, 38, 58].
Participants were excluded from participating in the study if they were in treatment for eating or weight problems, regular smokers, currently taking blood pressure, stimulant, or psychoactive medications, or self-reported current or prior cardiovascular disease, diabetes, or hyper-tension. Eighteen of the participants were taking oral contraceptives (41.8%). The college’s Institutional Review Board approved the research and all participants earned partial course credit.
2.2. Procedure
The procedures are described in detail in Klatzkin et al. [35]. Women responding to our advertisements completed preliminary screening questions aimed at assessing the exclusionary criteria described above, as well as measures associated with eating: perceived life stress, depressive symptoms, uncontrolled eating, emotional eating, cognitive restraint, and body mass index [5, 17, 68]. In order to collect data for a supplemental study on the same cohort at the same time [27], participants also completed a computerized task in which they created portions of the food they would typically eat as well as portions of food they would eat in response to a recalled interpersonal life stressor. If inclusionary criteria were met, participants then scheduled their rest and stress laboratory testing days, irrespective of menstrual cycle phase.
Each laboratory testing session began between the hours of 4:00 pm and 5:30 pm. The order of rest and stress days was counterbalanced and the mean interval between them was 6.5 days (standard deviation = 2 days). The rest day was identical to the stress day, with the exception that the stress testing portion of the protocol was replaced with a rest period of the same length (Fig. 3). Participants did not exercise strenuously, wake from sleep less than two hours prior to the testing session, drink more than a single caffeinated beverage in the morning, eat or drink (except water) two hours prior to the study, consume any alcohol 12 h prior to the study, or take any antihistamines, psychotropic medications, and neural stimulants on the day of testing. Participants were also asked to arrive “not too hungry, but not too full” and to “make sure to eat some food at 2 h before the study visit to avoid excess hunger.”
Fig. 3.
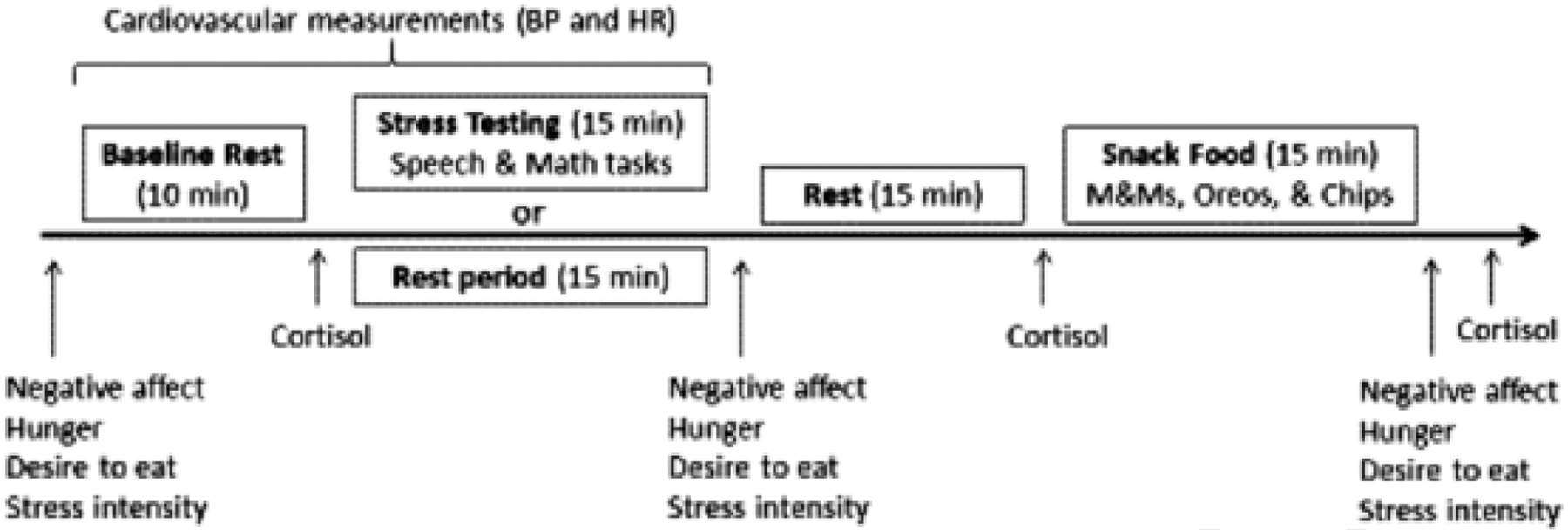
Laboratory protocol for stress and rest days.
2.3. Physiological measures
The Oscar 2 oscillometric ambulatory blood pressure monitor (Sun-Tech Medical Instruments, Inc., Raleigh, NC) provided automated measurement of SBP, DBP, and HR during the testing session while participants were seated comfortably. BP and HIR measures were taken at minutes 0, 5, and 10 of baseline, minutes 0, 2, and 4 of both the speech and serial subtraction periods during the TSST, and minutes 0, 5, and 10 of quiet rest during the rest day. The cardiovascular measures taken at minute 10 of baseline constituted the baseline value for each participant. For cardiovascular measures during stress, the peak value for each participant during each task constituted the speech and math stress values.
Saliva was collected in 1.5 mL Eppendorf tubes at the end of the baseline rest period, and 35 and 70 min following stress or rest induction. Participants passively drooled into the tube for a maximum of two minutes per sample. Saliva samples were frozen within 30 min of collection at −20°C until assayed. On the day of testing, all samples were centrifuged at 3000 rpm for 15 min to remove mucins and were analyzed in duplicate by enzyme immunoassay (Salimetrics, State College, PA). The mean intra-assay coefficient of variation was 8.03% and the inter-assay coefficient was 9.88%. Cortisol data was not included in this analysis because previously published results from the same dataset showed no significant increase in cortisol during stress testing [35].
2.4. Psychological measures - Preliminary Screening
Subjective eating measures: The Three Factor Eating Questionnaire (TFEQ-R18; [32]), which is a revised and shortened version of the original 51-item TFEQ [57] assessed uncontrolled eating (eating more than usual due to a loss of control over food intake accompanied by subjective feelings of hunger; range 3–12), emotional eating (inability to resist emotional cues; range 9–36), and restrained eating (conscious restriction of food intake in order to control body weight or to promote weight loss; range 6–24). Greater scores indicate greater eating-related psychopathology. Cronbach’s alpha for the 8 items on the uncontrolled eating subscale (α = 0.75), the 3 items on the emotional eating subscale (α = 0.82), and the 6 items on the restrained eating subscale (α = 0.80) of the TFEQ were satisfactory.
2.4.1. Perceived life stress
Levels of perceived psychological stress were measured using the Perceived Stress Scale (PSS-10; [13]). The PSS measures “the degree to which situations in one’s life are appraised as stressful” ([13], p. 387). The ten items on the PSS assess how unpredictable, uncontrollable, and overloaded respondents view their lives, and directly inquire about levels of experienced stress in the past three months with answer choices ranging from 0 (Never) to 4 (Very Often). A sample item is: “How often have you felt nervous and stressed?” Scores range from 0 to 40 and higher scores indicate greater perceived life stress. Cronbach’s alpha for items on the PSS were satisfactory at α = 0.83.
2.4.2. Depressive symptoms
Depressive symptoms were assessed using the Beck Depression Inventory (BDI; [4]). The BDI assesses self-reported dysphoric symptoms, including affective, cognitive, somatic, overt behavioral, and interpersonal symptoms of depression. Each forced-choice question has a set of at least four possible answer choices, with increasing severity of depressive symptoms from 0 to 3. Cronbach’s alpha for items on the BDI were satisfactory at α = 0.89.
2.5. Subjective psychological measures - Baseline, post-stress/rest, and post-snack
2.5.1. Positive and negative affect
Affect was quantified with the Positive and Negative Affect Schedule (PANAS), a 20-item multiple-choice survey validated in a university population [67]. Participants had a choice of ratings from 1 (Very slightly or Not at all) to 5 (Extremely) for each word describing a different feeling or emotion felt at the present moment (e.g. distressed, hostile, nervous). The positive subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more positive affect. The negative subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more negative affect. Cronbach’s alpha for the 10 items on the positive affect subscale (α = 0.89) and the 10 items on the negative affect subscale (α = 0.77) of the PANAS were satisfactory.
2.5.2. Drive to eat
Current hunger and desire to eat were measured on separate visual analog scales from 0 (None) to 10 (Most imaginable).
2.5.3. Stress intensity
Current level of stress intensity was measured on a computerized sliding scale from 0 (None) to 100 (Strongest experienced).
2.6. Subjective psychological measure-Prior to debriefing on laboratory day 2
Motives for eating palatable foods: Reasons for eating tasty foods outside of hunger were measured by the Palatable Eating Motive Scale (PEMS). The PEMS is a multiple choice 20-item inventory with standard response choices (“Never/Almost never” = 1 through “Almost always/Always” = 5) to questions assessing the presence of four persistent motives for eating palatable foods: Coping, Reward Enhancement, Social, and Conformity. Scores range from 20 to 100 and greater scores indicate greater motives for eating tasty foods outside of hunger. Cronbach’s alpha for the 5 items on the coping subscale (α = 0.92), the 5 items on the reward enhancement subscale (α = 0.76), and the 5 items on the social subscale (α= 0.83) of the PEMS were satisfactory. Cronbach’s alpha for the 5 items on the conformity subscale (α= 0.45) was not satisfactory. Our a priori hypotheses and data analysis plan did not include the conformity subscale of the PEMS, and thus the low Cronbach’s alpha has no impact on the results reported here.
2.7. Laboratory protocol
For detailed information regarding the laboratory protocol, see Klatzkin et al. [35].
2.7.1. Baseline rest
Researcher placed an automated blood pressure cuff on the non-dominant arm of the participant and then participants completed questionnaires assessing their subjective well-being in the following order: stress intensity, hunger, desire to eat, and positive and negative affect. The cardiovascular measures assessed were systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR).
2.7.2. The trier social stress test (TSST)
The TSST [33] is a stress test that reliably induces large and consistent cardiovascular responses. During pre-task instructions, the researcher informed the participants that they would be giving a speech that will be audio- and video-recorded for later analysis and would be followed by a serial subtraction task. The researcher then asked participants to imagine that they were applying for their ideal job and to take 5 min to prepare their speech describing why they would be the ideal candidate for the position. Immediately following the preparation period, the selection committee returned to the testing room and asked the participants to deliver their speech for 5 min. Finally, the researcher asked the participants to perform mental math for 5 min by serially subtracting 7 from 2000 aloud as quickly and accurately as possible. During rest day, participants listened to classical music and were invited to read magazines about the city of Memphis while resting quietly. Cardiovascular and HPA axis activity were assessed throughout stress and rest periods.
Upon completion of the stress testing and rest periods, participants again completed assessments measuring stress intensity, hunger, desire to eat, and positive and negative affect. For the purposes of a supplemental study, participants also completed a computerized task in which they created food portions they wanted to eat at the present moment. Participants then rested quietly until the post-stress or post-rest salivary cortisol measures were taken.
2.7.3. Snack food
Forty minutes following the initiation of stress or rest, participants underwent a bogus taste test, a validated measure of food intake [52]. Participants were given a tray containing three plastic containers with a pourable lid. Each plastic container was the same size and filled to the top with either M&Ms (935 g, 19.5 servings, 4684 calories), mini golden Oreos (380 g, 13 servings, 1834 calories), or potato chips (110 g, 4 servings, 629 calories). Participants could serve themselves by pouring each of the three snack foods into three different bowls. Researchers asked participants to sample each snack food and then rate it on the taste dimensions of salty, sweet, and crunchy, as well as how much they liked the snack, and told participants that they could eat as much as they wanted. Participants were told that the purpose of this part of the study was to determine the effect of liking or disliking certain foods on salivary function and were then left alone for 15 min to portion, consume, and rate the snacks while free to move about the private testing room
2.7.4. Post-Snack
Following the snack period, participants again completed assessments measuring stress intensity, hunger, desire to eat, and positive and negative affect, hunger. During the second laboratory testing session, trained research assistants assessed height (cm) and weight (kg) calculate BMI (kg/m2) using a Seca 769 digital column scale and stadiometer and assessed waist circumference, a measure of central obesity, at the midway point between the lowest ribs and the iliac crest with an anthropometric tape measure. Finally, participants completed the Palatable Eating Motive Scale (PEMS).
2.8. Data analyses
The data were analyzed using IBM SPSS (version 23). After performing a multiple regression analysis to confirm acceptably low multi-collinearity (in this analysis all VIF values were less than 3), the moderated moderation analysis was performed using PROCESS model 3 (version 3.5.3; Hayes, 2018). The interaction was probed by use of the Johnson-Neyman test which allowed us to determine where in the distribution of the moderator the effect of EE on snack food intake is statistically significant.
After completing the stress day laboratory protocol, one participant did not return for their second laboratory visit (i.e. the rest day) which is when participants complete the PEMS questionnaire. Thus, for the analysis testing hypothesis #1, n = 42. For the analysis testing hypothesis #2, n = 40 because of the absence of this same participant as well as the removal of two outliers (see below).
Hypothesis #1: Greater emotional relief from stress by eating (decrease in negative affect from stress to post-eating) would moderate the degree to which heightened stress reactivity (SBP) moderates the relationship between self-reported emotional eating and food intake post-stress (Fig. 2).
To test hypothesis 1, PROCESS was used to examine whether the moderation of the association between emotional eating score and snack food intake by peak SBP during the speech stress task is itself moderated by the relief from stress experienced from eating; that is, the three-way interaction effect of emotional eating, SBP, and emotional relief from stress on the amount of snack food consumed (see Fig. 2).
We included the following variables as covariates in our analysis: uncontrolled eating score on the TFEQ, restrained eating score on the TFEQ, PEMS reward subscale score (motives to consume palatable food for reward enhancement), SBP at minute 10 of baseline rest, and post-stress measures of negative affect and desire to eat. We included the uncontrolled eating and the restrained eating scores on the TFEQ as covariates in our model because of their positive correlation with overeating behaviors such as emotional eating [30, 59, 65]. We controlled for baseline SBP because of its influence on a moderator in our analysis: the increase in SBP during stress testing. Similarly, we included post-stress measures of negative affect and desire to eat as covariates because these measures assessed during the peak of stress testing may have influenced both the amount of snack food eaten as well as ratings of emotional relief from stress by eating that followed (Fig. 1; [34]). Finally, given that emotional eaters are more receptive to the rewarding value of food [60] and that emotional eating may be promoted by negative reinforcement (i.e. rewarding feelings of stress reduction upon eating) [19, 43], we also controlled for the PEMS reward subscale score.
Hypothesis #2: Self-reported emotional eating will not predict greater snack food intake on the rest day
In order to test our second hypothesis and determine whether our proposed model is specific to the stress day or predicts food intake under control conditions, we performed the same analyses with rest day variables.
We examined the data for outliers on both stress and rest days and eliminated extreme scores (i.e. greater than the third quartile plus 1.5 times the interquartile range) from one participant who had extreme increases in negative affect ratings following food intake on the rest day and one participant who reported extremely high negative affect ratings following the rest period on rest day. Results reflect removal of these outliers.
3. Results
Hypothesis #1: Greater emotional relief from stress by eating (decrease in negative affect from stress to post-eating) will moderate the degree to which heightened stress reactivity (SBP) moderates the relationship between self-reported emotional eating and food intake post-stress (Fig. 2).
Our theoretical model (Fig. 2) was supported by our results. High emotional relief from stress by eating moderated the moderating effect of stress reactivity on the relationship between self-reported emotional eating and increased snack food intake (Fig. 3). The moderated moderation model was significant, F(13,28) = 4.68, p = .0003; R2 = 0.68. There was a significant conditional three-way interaction effect on snack food intake; self-reported emotional eating predicted increased food intake post-stress only under conditions of high SBP stress reactivity and high emotional relief from stress by eating (b = 0.20 g, SE = 0.09, p = .028; 95% CI: 0.02 – 0.39). The increase in R2 attributable to the three-way interaction was 0.06, F(1,28) = 5.4, p = .03. The interaction between emotional eating and SBP on snack food intake was significant at one SD above the mean of emotional relief from stress by eating (b = 1.20 g, F(1,28) = 5.19, p = .031), but not at mean (b = 0.23 g, F(1,28) = 1.33, p = .258) or mean minus 1 SD (b = −0.74 g, F(1,28) = 3.23, p = .067). SBP stress reactivity moderated the relationship between self-reported emotional eating and snack food intake post-stress with increasing emotional relief. The Johnson-Neyman test revealed that as emotional relief from stress by eating increased above 7.71, there was a significant increase in snack food intake for the emotional eating x SBP interaction; 26.19% of emotional relief scores were greater than 7.71. The test also showed that for those who experienced a reduction in emotional relief of 1, there was a reduction in eating, but only 4.76% of emotional relief scores were at that value and below.
Both emotional relief from stress by eating (b = 207.29 g, SE = 86.88, p = .024; 95% CI: 29.32 – 385.26) and emotional eating (b = 147.94 g, SE = 69.61, p = .043; 95% CI: 5.36 – 290.53) were significant predictors of snack food intake. The interaction between SBP stress reactivity and emotional relief from stress by eating on snack food intake was significant (b = −1.40 g, SE = 0.57, p = .020; 95% CI: −2.57 – −0.24) as was the interaction between emotional eating and emotional relief from stress by eating (b = −30.98 g, SE = 86.88, p = .029; 95%CI: −58.50 – −3.46). Neither the interaction between self-reported emotional eating and SBP stress reactivity (b = −0.94 g, SE = 0.47, p = .054; 95% CI: −1.90 – 0.02), nor the correlation between self-reported emotional eating and SBP stress reactivity (r = −0.12, p = .44) was significant.
Hypothesis #2: Self-reported emotional eating will not predict greater snack food intake on the rest day
On the rest day, the overall moderated moderation model was statistically significant, F(13,26) = 2.99, p = .008; R2 = 0.58. However, the conditional three-way interaction of SBP, emotional eating, and emotional relief from stress by eating on snack food intake was not statistically significant (b = −0.31 g, SE = 0.22, p = .177; 95%CI: −0.77 – 0.15). The three-way interaction did not produce a significant change in R2, F(1,26) = 1.92, p = .177; R2 change = 0.03.
In the resting condition, emotional relief from stress by eating did not significantly predict snack food intake (b = −281.93 g, SE = 195.53, p = .161; 95%CI: −683.86 – 120.00). Both emotional eating (b = −193.80 g, SE = 64.97, p = .006; 95%CI: −327.35 –−60.24) and SBP (b = −9.94 g, SE = 3.38, p = .007; 95%CI: −16.90 –−2.98) were significant predictors of snack food intake in the resting condition. Furthermore, the interaction between emotional eating and SBP on snack food intake (b = 1.63 g, SE = 0.52, p = .004; 95%CI: 0.57 – 2.70) was statistically significant, but the interaction between SBP and emotional relief from stress by eating on snack food intake (b = 2.54 g, SE = 1.61, p = .126; 95%CI: −0.76 – 5.84) and the interaction between emotional eating score and emotional relief from stress by eating on snack food intake (b = 33.51 g, SE = 27.71, p = .237; 95%CI = −23.44 – 90.47) were not significant.
3.1. Physiological stress measures
The speech task induced significant increases in SBP, F (1,42) = 278.7, p = .001, DBP, F(1,42) = 339.2, p = .001, and HR, F (1,42) = 164.7, p = .001. The math task also induced significant increases in SBP, F(1,42) = 228.7, p = .001, DBP, F(1,42) = 285.9, p = .001, and HR, F(1,42) = 94.4, p = .001. As reported in Klatzkin et al. [35], the stress tasks did not cause any overall changes in cortisol. Furthermore, cardiovascular measures did not change significantly over time on the rest day, but cortisol decreased from baseline to the end of the rest period [35].
3.2. Subjective measures
As reported in Klatzkin et al. [35], the stress task induced significant increases from baseline rest in subjective stress ratings and negative affect, but no changes in hunger or desire to eat. The rest period was associated with decreases from baseline rest in subjective stress ratings, and negative affect, and with increases in hunger, and desire to eat [35]. There was no difference in snack intake [35] or baseline hunger ratings, F(1, 41) = 0.14, p = .71, between rest and stress days.
4. Discussion
4.1. Stress reactivity and emotional relief moderate the relationship between self-reported emotional eating and food intake
The present study demonstrates that self-assessment of emotional eating in the aggregate has not been consistently associated with increased food intake in response to stress or negative emotions [8, 20] because the relationship between self-reported emotional eating and behavioral measures of emotional eating is moderated by the stress response and emotional relief of stress by eating. These moderators have not been measured previously (Hypothesis #1). Greater emotional eating predicted higher snack food intake post-stress only when stress reactivity and emotional relief were both high. Greater variance in post-stress food intake was explained by the addition of the second moderator, emotional relief. Thus, high emotional relief from stress by eating enhanced the moderating effect of stress reactivity on the relationship between self-reported emotional eating and increased snack food intake post-stress. We found that stress is a stronger predictor of increased snack food intake for women with higher self-reported emotional eating scores when emotional relief from stress by eating is heightened. Therefore, the trigger and relief components of the emotional eating cycle (Fig. 1) increase the likelihood of emotional eating behaviors for women with greater self-reported emotional eating.
Stress often leads to greater food consumption because it dampens activity in the prefrontal cortex to limit decision-making abilities and activates brain reward centers to enhance food “wanting” [19, 6]. Over time, repeated palatable food intake in response to stress causes elevated responsivity (i.e. dopamine release) of reward and attention regions to cues repeatedly associated with eating (e.g. stress) which facilitates overeating in the presence of these cues [6]. The stress response acts as a conditioned stimulus that elicits cue reactivity (i.e. eating initiation in response to stress and negative emotions) via Pavlovian learning [9].
Enhanced emotional relief after stress-induced consumption may promote emotional eating in response to stress as a way for emotional eaters to seek reward and reduce stress [19, 43]. Palatable food consumption mitigates aversive experiences of stress by increasing dopamine release in the mesolimbic pathway to signal pleasure [54, 66] and by reducing activity in the brain’s stress response network [19, 23, 37, 48]. This increased reward and diminished stress in response to eating provide emotional relief from stress and provides negative reinforcement that enhances stress as a facilitator of food intake, particularly for emotional eaters [19, 22, 50]. Therefore, the initiation of emotional eating may be a conditioned response to stress that is promoted by enhanced emotional relief from stress by eating. Although women with higher self-reported emotional eating underwent the stress test and consumed snack food before our measurement of emotional relief, they likely have prior experience with emotional relief from stress by eating that reflected their experience in the laboratory [60].
4.2. Self-reported emotional eating and food intake: the importance of stress
Our rest day results supported out prediction regarding the specificity of emotional eating to stress contexts and further substantiate stress as a promoter of snack food consumption for women with higher self-reported emotional eating. Higher SBP on the rest day moderated the relationship between self-reported emotional eating and increased snack food intake, as self-reported emotional eating predicted greater snack food intake on the rest day only when SBP was high. Thus, greater indicators of cardiovascular stress enhance the relationship between emotional eating self-assessment and emotional eating behavior, even in the absence of acute stressors and emotional relief. Our rest day results refute previous findings that emotional eating is indicative of overeating in general and in a variety of circumstances [8] by showing that emotional eating may rely on factors associated with stress. Our rest day findings also indicate that experiencing emotional relief from stress by eating is not necessary for cardiovascular stress to moderate the relationship between self-reported emotional eating and food intake under rest conditions.
Emotions and stress are highly interdependent constructs and often accompany each other [39], yet Meule et al. [45] argued that although stress can overlap with negative emotions, there are also non-overlapping states in which one may feel stressed without experiencing specific emotions, such as shame, anger, or sadness. Despite these distinctions, scholars frequently use the terms “emotions” and “stress” interchangeably and inconsistently [20] and the term “emotional eating” is often used synonymously with “stress-eating” [25, 55]. Our results help to refine and standardize the conceptualization of emotional eating as eating that occurs in response to stress or negative emotions in order to mitigate these aversive states [3, 28, 49, 60].
4.3. Limitations
Lack of appropriate directionality between our variables limits our ability to interpret the results. Our measurement of emotional relief from stress by eating occurred at the end of the laboratory protocol, yet our results showed that this factor moderates other factors that were assessed earlier in the protocol (e.g. SBP stress reactivity). We have no reason to believe that the emotional relief from stress by eating that occurred during the laboratory visit would differ from previous experiences of emotional relief that occurred prior to the laboratory visit and likely enhanced stress as a trigger for emotional eating in the lab; however, this was not assessed in the current study. Future studies should eliminate this directionality issue by assessing emotional relief from stress by eating on a first laboratory visit prior to a second laboratory visit measuring food intake post-stress. This methodology would enable researchers to determine whether elevated emotional relief from stress by eating enhances negative reinforcement learning and thereby promotes stress as a trigger for increased food intake for those scoring higher on self-reported emotional eating scales.
Another limitation of the current study is that we cannot isolate our measurement of emotional relief from stress by eating from the confounding factors of increases in stress and negative affect during stress testing. Greater increases in stress (e.g. SBP) and negative affect in response to the mental stress tasks may promote greater emotional relief from stress by eating because they enhance the aversive state that eating can relieve. We attempted to mitigate this issue by controlling for post-stress ratings of negative affect in our analyses, but future experiments that independently control stress responses and emotional relief are needed to differentiate the effects of increases in stress and negative affect from the post-eating reductions in these variables.
4.4. Conclusions
Despite these limitations, our findings identified factors in the emotional eating cycle (Fig. 1) that may distinguish the subset of self-reported emotional eaters who are more likely to display emotional eating behaviors in a laboratory setting and support the conceptualization of emotional eating as eating that is specific to the context of stress rather than a general increase in eating in response to a variety of circumstances. Further investigation in this area is needed to directly test whether negative reinforcement via emotional relief from stress by eating drives enhanced emotional eating following stress.
Figure 4.
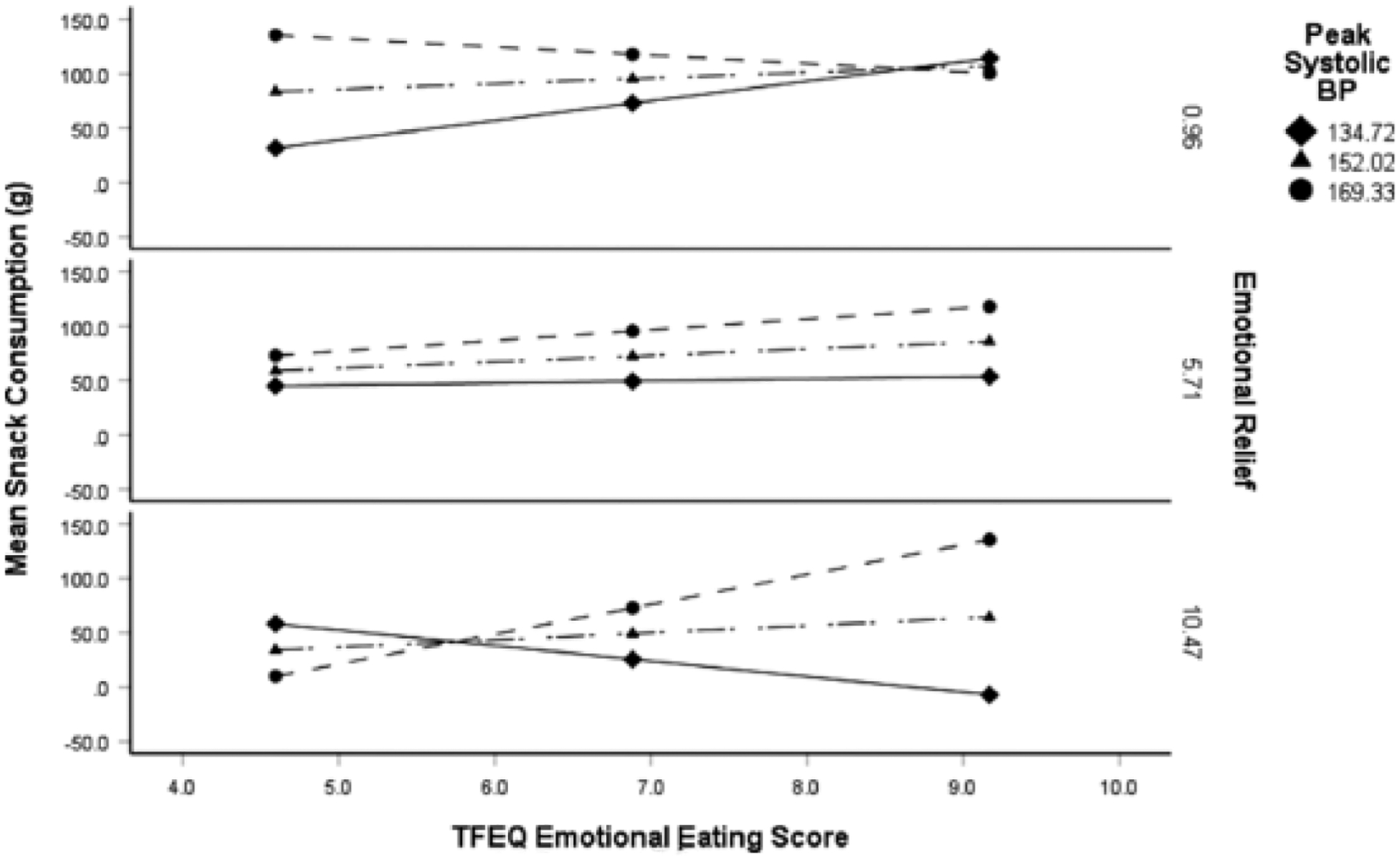
High emotional relief from stress by eating enhanced the moderating effect of stress reactivity on the relationship between self-reported emotional eating and increased snack food intake post-stress. The moderated moderation (i.e. three-way interaction) was highly significant, F(13,28) = 4.7, p = .0003; R2 = .68. Self-reported emotional eating predicted increased snack food intake post-stress only under conditions of high SBP stress re activity and high emotional relief from stress by eating (b = .20, SE = .09, p = .03; 95% CI: .02 – .39), F (1, 28) = 5.2, p = .03. SBP = systolicblood pressure.
Acknowledgements
We would like to thank Ellery Hayden, Cleo Nikodem, Catrina Cattaneo, Reedhi Dasani, McKay Warren, and Tzvi Nadel for their work on data collection. HR Kissileff was partially supported by NIH grant R01DK108643 Mechanisms Underlying Predictors of Success from Obesity Surgery.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
References
- [1].Adam TC, Epel ES, Stress, eating and the reward system, Physiol. Behav 91 (4) (2007) 449–458, 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- [2].Annesi JJ, Mareno N, McEwen K, Psychosocial predictors of emotional eating and their weight-loss treatment-induced changes in women with obesity, Eat. Weight Disord. - Stud. Anorexia Bulimia Obes 21 (2) (2016) 289–295, 10.1007/s40519-015-0209-9. [DOI] [PubMed] [Google Scholar]
- [3].Arnow B, Kenardy J, Agras WS, The emotional eating scale: the development of a measure to assess coping with negative affect by eating, Int. J. Eat. Disord 18 (1) (1995) 79–90, . [DOI] [PubMed] [Google Scholar]
- [4].Beck AT, Beamesderfer A, Assessment of depression: the depression inventory, Mod. Probl. Pharmacopsychiatry 7 (0) (1974) 151–169. [DOI] [PubMed] [Google Scholar]
- [5].Bekhbat M, Neigh GN, Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety, Brain Behav. Immun (2017), 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Berridge KC, Ho C−Y, Richard JM, DiFeliceantonio AG, The tempted brain eats: pleasure and desire circuits in obesity and eating disorders, Brain Res. 1350 (2010) 43–64, 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bongers P, de Graaff A, Jansen A, ‘Emotional’ does not even start to cover it: generalization of overeating in emotional eaters, Appetite 96 (2016) 611–616, 10.1016/j.appet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- [8].Bongers P, Jansen A, Emotional eating is not what you think it is and emotional eating scales do not measure what you think they measure, Front. Psychol 7 (2016), 10.3389/fpsyg.2016.01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bongers P, Jansen A, Emotional eating and Pavlovian learning: evidence for conditioned appetitive responding to negative emotional states, Cognit. Emot 31 (2) (2017) 284–297, 10.1080/02699931.2015.1108903. [DOI] [PubMed] [Google Scholar]
- [10].Bongers P, Jansen A, Havermans R, Roefs A, Nederkoorn C, Happy eating. The underestimated role of overeating in a positive mood, Appetite 67 (2013) 74–80, 10.1016/j.appet.2013.03.017. [DOI] [PubMed] [Google Scholar]
- [11].Braden A, Emley E, Watford T, Anderson L, Musher-Eizenman D, Self-reported emotional eating is not related to greater food intake: results from two laboratory studies, Psychol. Health 35 (4) (2020) 500–517, 10.1080/08870446.2019.1649406. [DOI] [PubMed] [Google Scholar]
- [12].Cardi V, Leppanen J, Treasure J, The effects of negative and positive mood induction on eating behaviour: a meta-analysis of laboratory studies in the healthy population and eating and weight disorders, Neurosci. Biobehav. Rev 57 (2015) 299–309, 10.1016/j.neubiorev.2015.08.011. [DOI] [PubMed] [Google Scholar]
- [13].Cohen S, Kamarck T, Mermelstein R, A global measure of perceived stress, J. Health Soc. Behav 24 (4) (1983) 385–396. [PubMed] [Google Scholar]
- [14].Dallman MF, Stress-induced obesity and the emotional nervous system, Trends Endocrinol. Metab 21 (3) (2010) 159–165, 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dallman MF, Pecoraro NC, la Fleur SE, Chronic stress and comfort foods: self-medication and abdominal obesity, Brain Behav. Immun 19 (4) (2005) 275–280, 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [16].Davis C, Levitan RD, Carter J, Kaplan AS, Reid C, Curtis C, Patte K, Kennedy JL, Personality and eating behaviors: a case-control study of binge eating disorder, Int. J. Eat. Disord 41 (3) (2008) 243–250, 10.1002/eat.20499. [DOI] [PubMed] [Google Scholar]
- [17].de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, Penninx BWJH, Depressive and anxiety disorders and the association with obesity, physical, and social activities, Depress Anxiety 27 (11) (2010) 1057–1065, 10.1002/da.20738. [DOI] [PubMed] [Google Scholar]
- [18].Dweck JS, Jenkins SM, Nolan LJ, The role of emotional eating and stress in the influence of short sleep on food consumption, Appetite 72 (2014) 106–113, 10.1016/j.appet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- [19].Epel ES, Tomiyama AJ, & Dallman MF (2012). Stress and reward: neural networks, eating, and obesity. In Food and Addiction: A Comprehensive Handbook (pp. 266–272). Oxford University Press. 10.1093/med:psych/9780199738168.003.0040. [DOI] [Google Scholar]
- [20].Evers C, Dingemans A, Junghans AF, Boevé A, Feeling bad or feeling good, does emotion affect your consumption of food? A meta-analysis of the experimental evidence, Neurosci. Biobehav. Rev 92 (2018) 195–208, 10.1016/j.neubiorev.2018.05.028. [DOI] [PubMed] [Google Scholar]
- [21].Evers C, Marijn Stok F, de Ridder DTD, Feeding your feelings: emotion regulation strategies and emotional eating, Pers. Soc. Psychol. Bull 36 (6) (2010) 792–804, 10.1177/0146167210371383. [DOI] [PubMed] [Google Scholar]
- [22].Fay SH, Finlayson G, Negative affect-induced food intake in non-dieting women is reward driven and associated with restrained-disinhibited eating subtype, Appetite 56 (3) (2011) 682–688, 10.1016/j.appet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- [23].Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF, Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint, Endocrinology 150 (5) (2009) 2325–2333, 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Franja S, Wahl DR, Elliston KG, Ferguson SG, Comfort eating: an observational study of affect in the hours immediately before, and after, snacking, Br. J. Health Psychol 26 (3) (2021) 825–838, 10.1111/bjhp.12505. [DOI] [PubMed] [Google Scholar]
- [25].Greeno CG, Wing RR, Stress-induced eating, Psychol. Bull 115 (3) (1994) 444–464. [DOI] [PubMed] [Google Scholar]
- [26].Haedt-Matt AA, Keel PK, Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment, Psychol. Bull 137 (4) (2011) 660–681, 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hamm JD, Klatzkin RR, Herzog M, Tamura S, Brunstrom JM, Kissileff HR, Recalled and momentary virtual portions created of snacks predict actual intake under laboratory stress condition, Physiol. Behav 238 (2021), 10.1016/j.physbeh.2021.113479 113479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heatherton TF, Baumeister RF, Binge eating as escape from self-awareness, Psychol. Bull 110 (1) (1991) 86–108, 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- [29].Heaven PC, Mulligan K, Merrilees R, Woods T, Fairooz Y, Neuroticism and conscientiousness as predictors of emotional, external, and restrained eating behaviors, Int. J. Eat. Disord 30 (2) (2001) 161–166, 10.1002/eat.1068. [DOI] [PubMed] [Google Scholar]
- [30].Herman CP, Polivy J, Anxiety, restraint, and eating behavior, J. Abnorm. Psychol 84 (6) (1975) 666–672, 10.1037/0021-843X.84.6.666. [DOI] [PubMed] [Google Scholar]
- [31].Jansen A, Schyns G, Bongers P, van den Akker K, From lab to clinic: extinction of cued cravings to reduce overeating, Physiol. Behav 162 (2016) 174–180, 10.1016/j.physbeh.2016.03.018. [DOI] [PubMed] [Google Scholar]
- [32].Karlsson J, Persson LO, Sjöström L, Sullivan M, Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study, Int. J. Obes. Relat. Metabol. Disord.: J. Int. Assoc. Stud. Obes 24 (12) (2000) 1715–1725. [DOI] [PubMed] [Google Scholar]
- [33].Kirschbaum C, Pirke KM, Hellhammer DH, The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting, Neuropsychobiology 28 (1–2) (1993) 76–81, https://doi.org/119004. [DOI] [PubMed] [Google Scholar]
- [34].Klatzkin R, Nolan L, Chaudhry R, Geliebter A, & Kissileff H (2021). Measures of emotions as influences on eating and weight control (pp. 871–906). 10.1016/B978-0-12-821124-3.00027-2. [DOI] [Google Scholar]
- [35].Klatzkin RR, Dasani R, Warren M, Cattaneo C, Nadel T, Nikodem C, Kissileff HR, Negative affect is associated with increased stress-eating for women with high perceived life stress, Physiol. Behav 210 (2019), 10.1016/j.physbeh.2019.112639 112639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Konttinen H, Männistö S, Sarlio-Lähteenkorva S, Silventoinen K, Haukkala A, Emotional eating, depressive symptoms and self-reported food consumption. A population-based study, Appetite 54 (3) (2010) 473–479, 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [37].la Fleur SE, Houshyar H, Roy M, Dallman MF, Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint, Endocrinology 146 (5) (2005) 2193–2199, 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- [38].Larsen JK, van Strien T, Eisinga R, Engels RCME, Gender differences in the association between alexithymia and emotional eating in obese individuals, J. Psychosom. Res 60 (3) (2006) 237–243, 10.1016/j.jpsychores.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [39].Lazarus RS (2006). Stress and Emotion: A New Synthesis. Springer Publishing Company. [Google Scholar]
- [40].Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, Giel KE, Emotion regulation model in binge eating disorder and obesity—A systematic review, Neurosci. Biobehav. Rev 49 (2015) 125–134, 10.1016/j.neubiorev.2014.12.008. [DOI] [PubMed] [Google Scholar]
- [41].Litwin R, Goldbacher E, Cardaciotto L, Gambrel L, Negative emotions and emotional eating: the mediating role of experiential avoidance, Eat. Weight Disord. - Stud. Anorexia Bulimia Obes 22 (2016), 10.1007/s40519-016-0301-9. [DOI] [PubMed] [Google Scholar]
- [42].Macht M, How emotions affect eating: a five-way model, Appetite 50 (1) (2008) 1–11, 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [43].Macht M, Haupt C, Ellgring H, The perceived function of eating is changed during examination stress: a field study, Eat. Behav 6 (2) (2005) 109–112, 10.1016/j.eatbeh.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [44].Macht M, Mueller J, Immediate effects of chocolate on experimentally induced mood states, Appetite 49 (3) (2007) 667–674, 10.1016/j.appet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- [45].Meule A, Reichenberger J, Blechert J, Development and Preliminary Validation of the Salzburg Emotional Eating Scale, Front. Psychol 9 (2018), 10.3389/fpsyg.2018.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nolan LJ, Geliebter A, Night eating is associated with emotional and external eating in college students, Eat. Behav 13 (3) (2012) 202–206, 10.1016/j.eatbeh.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ouwens MA, van Strien T, van Leeuwe JFJ, Possible pathways between depression, emotional and external eating. A structural equation model, Appetite 53 (2) (2009) 245–248, 10.1016/j.appet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- [48].Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF, Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress, Endocrinology 145 (8) (2004) 3754–3762, 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- [49].Polivy J, Herman CP, Dieting and binging. A causal analysis, Am. Psychol 40 (2) (1985) 193–201. [DOI] [PubMed] [Google Scholar]
- [50].Pool E, Brosch T, Delplanque S, Sander D, Stress increases cue-triggered “wanting” for sweet reward in humans, J. Exp. Psychol. Anim. Learn. Cogn 41 (2) (2015) 128–136, 10.1037/xan0000052. [DOI] [PubMed] [Google Scholar]
- [51].Reichenberger J, Kuppens P, Liedlgruber M, Wilhelm FH, Tiefengrabner M, Ginzinger S, Blechert J, No haste, more taste: an EMA study of the effects of stress, negative and positive emotions on eating behavior, Biol. Psychol 131 (2018) 54–62, 10.1016/j.biopsycho.2016.09.002. [DOI] [PubMed] [Google Scholar]
- [52].Robinson E, Haynes A, Hardman CA, Kemps E, Higgs S, Jones A, The bogus taste test: validity as a measure of laboratory food intake, Appetite 116 (2017) 223–231, 10.1016/j.appet.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Skinner BF, Operant behavior, Am. Psychol 18 (8) (1963) 503–515, 10.1037/h0045185. [DOI] [Google Scholar]
- [54].Small DM, Jones-Gotman M, Dagher A, Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers, Neuroimage 19 (4) (2003) 1709–1715, 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- [55].Stammers L, Wong L, Brown R, Price S, Ekinci E, Sumithran P, Identifying stress-related eating in behavioural research: a review, Horm. Behav 124 (2020), 10.1016/j.yhbeh.2020.104752 104752. [DOI] [PubMed] [Google Scholar]
- [56].Stevenson BL, Dvorak RD, Wonderlich SA, Crosby RD, Gordon KH, Emotions before and after loss of control eating, Eat. Disord 26 (6) (2018) 505–522, 10.1080/10640266.2018.1453634. [DOI] [PubMed] [Google Scholar]
- [57].Stunkard AJ, Messick S, The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger, J. Psychosom. Res 29 (1) (1985) 71–83. [DOI] [PubMed] [Google Scholar]
- [58].Udo T, Grilo CM, McKee SA, Gender differences in the impact of stressful life events on changes in body mass index, Prev. Med 69 (2014) 49–53, 10.1016/j.ypmed.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vainik U, Neseliler S, Konstabel K, Fellows LK, Dagher A, Eating traits questionnaires as a continuum of a single concept, Uncontrolled eating. Appetite. 90 (2015) 229–239, 10.1016/j.appet.2015.03.004. [DOI] [PubMed] [Google Scholar]
- [60].van Strien T, Causes of Emotional Eating and Matched Treatment of Obesity, Curr. Diab. Rep (6) (2018) 18, 10.1007/s11892-018-1000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Van Strien T, Frijters JER, Roosen RenéG. F.M., Knuiman-Hijl WJH, Defares PB, Eating behavior, personality traits and body mass in women, Addict. Behav 10 (4) (1985) 333–343, 10.1016/0306-4603(85)90029-2. [DOI] [PubMed] [Google Scholar]
- [62].van Strien T, Konttinen H, Homberg JR, Engels RCME, Winkens LHH, Emotional eating as a mediator between depression and weight gain, Appetite 100 (2016) 216–224, 10.1016/j.appet.2016.02.034. [DOI] [PubMed] [Google Scholar]
- [63].van Strien T, Roelofs K, de Weerth C, Cortisol reactivity and distress-induced emotional eating, Psychoneuroendocrinology 38 (5) (2013) 677–684, 10.1016/j.psyneuen.2012.08.008. [DOI] [PubMed] [Google Scholar]
- [64].Vandewalle J, Moens E, Beyers W, Braet C, Can we link emotional eating with the emotion regulation skills of adolescents? Psychol. Health 31 (7) (2016) 857–872, 10.1080/08870446.2016.1149586. [DOI] [PubMed] [Google Scholar]
- [65].Verzijl CL, Ahlich E, Schlauch RC, Rancourt D, The role of craving in emotional and uncontrolled eating, Appetite 123 (2018) 146–151, 10.1016/j.appet.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R, Food and drug reward: overlapping circuits in human obesity and addiction, Curr. Top. Behav. Neurosci 11 (2012) 1–24, 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- [67].Watson D, Clark LA, Tellegen A, Development and validation of brief measures of positive and negative affect: the PANAS scales, J. Pers. Soc. Psychol 54 (6) (1988) 1063–1070. [DOI] [PubMed] [Google Scholar]
- [68].Yau YHC, Potenza MN, Stress and eating behaviors, Minerva Endocrinol. 38 (3) (2013) 255–267. [PMC free article] [PubMed] [Google Scholar]
- [69].Yeomans MR, Blundell JE, Leshem M, Palatability: response to nutritional need or need-free stimulation of appetite? Br. J. Nutr 92 (1) (2004) S3–14, Suppl 10.1079/bjn20041134. [DOI] [PubMed] [Google Scholar]


