Abstract
Objectives
To evaluate the role of the clinical pharmacist in improving venous thromboembolism (VTE) prophylaxis prescription in patients with renal impairment (RI).
Methods
This was an interventional cross-sectional study conducted in a nephrology ward. Patients’ risk scores for VTE and bleeding during hospitalisation (evaluated by the Caprini Risk Assessment Model (RAM), Padua Prediction Score and IMPROVE Bleeding Risk Score, respectively), and the rate of VTE prophylaxis administration to patients, were evaluated before and after a clinical pharmacist’s intervention.
Results
In the pre-intervention phase, 34.8% of high-VTE-risk patients, of whom 12.5% were also at high risk of bleeding, received pharmacological prophylaxis. Moreover, 22.2% of low-VTE-risk patients received prophylaxis. In the intervention phase, prophylaxis was administered to all high-risk patients (mechanical prophylaxis in 7% of patients with a high risk of both VTE and bleeding, and heparin in the remainder) and to 3.3% of those at low risk of VTE.
Conclusions
The clinical pharmacist’s intervention using RAMs can improve the rate of thrombosis prophylaxis prescription in patients with RI who have a high risk of VTE.
Keywords: thromboembolism, hemorrhage, renal impairment, clinical pharmacist, risk assessment models
Introduction
The incidence of venous thromboembolism (VTE)—including deep vein thrombosis (DVT) and pulmonary embolism (PE)—in hospitalised medical patients not receiving appropriate prophylaxis is 5%–15%.1–3 Some medical patients, especially those with stroke, cancer, heart failure and renal impairment (RI),4 are at increased risk of developing thromboembolic events. It has been shown that a reduced glomerular filtration rate increases the risk of VTE.5 The incidence of PE among haemodialysis patients is two to three times more than that for patients without RI.6 In addition to the risk of VTE, patients with RI are at increased risk of bleeding.7 Therefore, a delicate balance between the risks of VTE and bleeding obligates physicians to choose a conservative and careful approach to the administration of anticoagulants for VTE prophylaxis in patients with RI. However, studies have shown that thromboprophylaxis is not optimal in renal-impaired patients, and 40%–70% of patients with renal impairment at moderate-to-high risk of VTE do not receive appropriate prophylaxis.8–10 On the other hand, VTE imposes a considerable economic burden on healthcare systems, and it causes short-term and long-term complications for patients.11 One of the responsibilities of clinical/hospital pharmacists is to evaluate and improve the prescription and administration of drugs in different patient populations. Therefore, we designed a cross-sectional intervention study to evaluate the role of clinical pharmacist intervention in the improvement of VTE prophylaxis administration to patients with RI.
Method
This was a prospective cross-sectional study composed of two 3 month phases. The study was conducted in a 20-bed nephrology ward of a tertiary teaching hospital affiliated to Kerman University of Medical Sciences (KUMS).
All patients admitted to the nephrology ward over the study period were considered for inclusion. Patients whose hospitalisation period was less than 24 hours, patients receiving anticoagulants for therapeutic indications and patients with any contraindication to anticoagulants (active bleeding and coagulopathy defined as a platelet count less than 50 000/µL, international normalised ratio (INR) >1.5 or partial thromboplastin time (PTT) greater than twice the upper limit of normal) were excluded.
In both phases of the study, the Padua Prediction Score (PPS) and the Caprini Risk Assessment Model (RAM) were used by pharmacists to classify the risk of VTE in patients. Risk of bleeding was also evaluated using the IMPROVE Bleeding Risk Score (IBRS).
In the pre-intervention phase, the risk of developing VTE and bleeding, and the physicians’ approaches to VTE prophylaxis, were evaluated.
During the intervention phase, three risk assessment tools were completely described to the nephrologists. A clinical pharmacist and a pharmacy student evaluated each patient’s risk of VTE and bleeding. Then, they participated in the physicians’ daily patient visits and made recommendations such as initiating VTE prophylaxis in those high-risk patients not receiving any prophylaxis, or the discontinuation of thrombosis prophylaxis in low-risk patients currently receiving prophylaxis.
Outcome measures
Unfortunately, there are no specific tools for assessing the risk of VTE and bleeding in renal-impaired patients. Therefore, we decided to use PPS12 and the Caprini RAM13 to evaluate the risk of VTE, and IBRS to classify the risk of bleeding. PPS consists of 11 risk factors, and assigns 1, 2 or three points to each factor. The final score is calculated by summing the score for all risk factors. Patients with a PPS score ≥4 are considered to be at high risk for VTE.12 It should be noted that the American College of Chest Physicians (ACCP) has suggested that PPS is the best available model for evaluating the risk of VTE in medical patients.14 However, since patients with creatinine clearance less than 30 mL/min were excluded from the largest study that evaluated the validity of PPS,12 we decided to use another RAM in addition to PPS. Recently, Stuck et al,15 in a review article, concluded that Caprini RAM and a full logistic model (FLM) might be more accurate than other VTE RAMs in recognising medical patients at high risk of VTE. The lower number of items in Caprini RAM (39 items) in comparison to FLM (86 items) makes it more applicable in a clinical setting. Another advantage of Caprini RAM is that it includes more of the risk factors for thrombosis that are prevalent in hospitalised patients with RI, such as having a central venous catheter, heart failure, planned surgery (eg, for placement of different dialysis access), respiratory failure, immobility, and so on. Therefore, we chose Caprini RAM as an additional tool for classifying the risk of VTE. In this model, each risk factor has a score of 1, 2, 3 or five, based on its contribution to the development of VTE. The final score is calculated by summing the scores for the risk factors. Total Caprini RAM scores of 0, 1, 2, 3–4 and ≥5 are representative of a low, moderate, high and super-high risk of VTE, respectively.13 It should be noted that in cases where one risk assessment tool assigned a patient to the high-risk group, but where the other assessment tool categorised the patient into a low-risk group, we assumed the patient to be at high risk of VTE.
We also used IBRS,16 which has been proposed as the best available RAM to predict the risk of bleeding in hospitalised medical patients.14 IBRS includes 13 risk factors, two of which are related to the severity of the RI. The individual scores of these risk factors were summed to calculate the total risk score. A score of ≥7 means that the patient has a high risk of bleeding.16
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Science (SPSS), version 19. Pearson’s Chi-square test was used to compare the results of pre-intervention and intervention phases, with a significance level of 0.05.
Results
Results of the pre-intervention phase
Of the 69 patients admitted to the nephrology ward during this phase, 64 patients (aged 56.9±17.2 years) were enrolled in the study (figure 1). Demographic characteristics and status of kidney function of the patients are presented in table 1.
Figure 1.
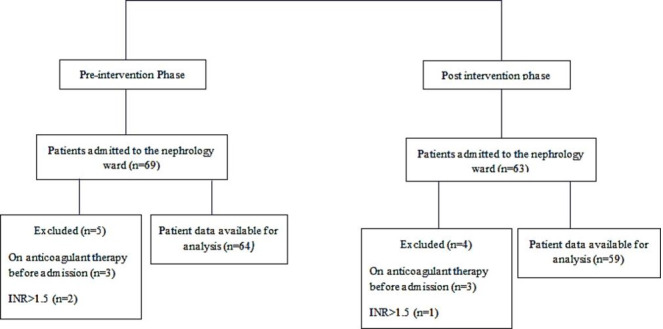
Flow diagram of participants in the study.
Table 1.
Demographic characteristics and status of kidney function of the patients at admission* of patients and main reasons for hospital admission
| Pre-intervention phase, n=64 | Intervention phase, n=59 | |
| Male | 30 (46.9) | 29 (49.2) |
| Age (year) | ||
| 16–40 | 14 (22.0) | 11 (18.6) |
| 41–60 | 19 (29.7) | 20 (33.9) |
| 61–74 | 23 (35.9) | 19 (32.2) |
| ≥75 | 8 (12.5) | 9 (15.3) |
| Kidney function status at admission | ||
| AKI | 15 (23.4) | 11 (18.6) |
| CKD | 15 (23.4) | 12 (20.3) |
| AKI superimposed on CKD | 3 (4.7) | 4 (6.8) |
| ESRD | 31 (48.4) | 32 (54.2) |
*All data are presented as numbers (%).
AKI, acute kidney injury; CKD, chronic kidney disease; ESRD, end stage renal disease.
In this phase, 46 patients (71.9%) were classified as a high-VTE-risk group, and 10 patients (15.6%) had an IBRS score of ≥7. All patients with high haemorrhagic risk were also at high risk of VTE. The most prevalent risk factors for VTE and bleeding are presented in table 2.
Table 2.
The most prevalent risk factors for VTE and bleeding in the study population
| Main risk factors for VTE (assigned point in the Caprini RAM or PPS) | Number (percent) of patients having the risk factor | Main risk factors for bleeding (assigned point in the IBRS) | Number (percent) of patients having the risk factor | ||
| Pre-intervention phase, n=64 | Intervention phase, n=59 | Pre-intervention phase, n=64 | Intervention phase, n=59 | ||
| Central venous access (2*) | 25 (39.0) | 19 (29.7) | Central venous catheter (2) | 25 (42.4) | 19 (32.2) |
| Age (year)* | Age (year) | ||||
| 41–60 (1)* | 19 (29.7) | 20 (33.9) | 40–84 (1.5) | 48 (75.0) | 46 (78.0) |
| 61–74 (2)* | 23 (35.9) | 19 (32.2) | ≥85 (3.5) | 2 (3.1) | 2 (3.4) |
| ≥75 (3)* | 8 (12.5) | 9 (15.3) | |||
| BMI>25 (kg/m2) (1*) | 19 (29.7) | 33 (51.6) | Male (1) | 30 (50.8) | 29 (49.2) |
| Reduced mobility†/ currently at bed rest (1)*‡ | 11 (17.2) | 13 (20.3) | Severe renal failure (GFR <30 mL/min) (2.5) | 51 (79.7) | 49 (83.0) |
| Swollen legs (1*) | 13 (20.3) | 11 (17.2) | Moderate renal failure (GFR 30–59 mL/min) (1) |
13 (20.3) | 10 (16.9) |
| Respiratory failure (1)‡ | 4 (6.3) | 6 (9.4) | Rheumatic disease (2) | 0 | 2 (3.4) |
| Rheumatologic disorder§ (1)‡ | 0 | 2 (3.4) | |||
*Assigned point in the Caprini RAM.
†Anticipated bed rest with bathroom privileges for at least 3 days.14
‡Assigned point in the PPS.
§Both patients had systemic lupus erythematosus, but no information about antiphospholipid antibodies (Ab) concentration was available (5 points are assigned for an elevated level of each Ab in the Caprini RAM). These patients receive 1 point in the PPS owing to the presence of a general rheumatologic disorder.
GFR, Glomerular filtration rate; IBRS, Improve bleeding risk score; PPS, Padua prediction score; RAM, Risk assessment model; VTE, venous thromboembolism.
Twenty patients (31.3%) received VTE prophylaxis, among whom four patients were classified as low risk for VTE, and two patients were at high risk of both VTE and bleeding. Of the 44 patients (68.8%) who did not receive any prophylaxis, 30 patients were at high risk of VTE. Collectively, 34.8% (16 from 46) of patients at high risk of VTE and 22.2% (4 from 18) of low-risk patients received pharmacologic prophylaxis (table 3). Moreover, 20% (2 from 10) of those at high risk of VTE and bleeding received anticoagulant drugs, while the remainder of patients did not receive any prophylaxis.
Table 3.
Physicians’ approaches regarding VTE prophylaxis prescription in the pre-intervention and intervention phases of the study
| Pre-intervention phase* | Intervention phase * | P† | |
| High VTE-risk patients, received prophylaxis | 16 (34.8) | 29 (100) | <0.001 |
| High VTE-risk patients, did not receive any prophylaxis | 30 (65.2) | 0 | <0.001 |
| Total high VTE-risk patients | 46 (100) | 29 (100) | |
| Low VTE-risk patients, received prophylaxis | 4 (22.2) | 1 (3.3%) | <0.001 |
| Low VTE-risk patients, did not receive any prophylaxis | 14 (77.8) | 29 (96.7) | <0.001 |
| Total low VTE-risk patients | 18 (100) | 30 (100) |
*All data are presented as numbers (%).
†Pearson Chi-square test.
Results of the intervention phase
Of the 63 patients admitted to the nephrology ward, 59 patients (aged 56.5±18.0 years) were enrolled in the study (figure 1)(table 1).
Among the 59 patients, 29 (49.2%) were at high risk of VTE, and two of these patients had an IBRS score of ≥7. Ultimately, after the intervention of pharmacists, all high-risk patients received VTE prophylaxis (anticoagulants in 27 cases and mechanical prophylaxis in two patients who were at high risk of thrombosis and bleeding). Our recommendation to discontinue prophylaxis in one patient, who was classified as having a low risk of VTE (based on both RAMs), was not accepted (Table 3).
It should be noted that, in both phases of the study, the administered anticoagulant regimen was a low dose of subcutaneous unfractionated heparin (UFH), which included 5000 IU, two-to-three times daily. Since the pharmacokinetic properties of UFH do not change significantly in renal impairment,7 no specific dosage adjustment is necessary for low doses of UFH. Therefore, the above-mentioned regimens, which are recommended for VTE prophylaxis in other patients,7 14 were also considered to be appropriate prophylactic interventions in our study.
Comparison of the results of the Caprini RAM and PPS
Of the 123 patients who enrolled in this study, 75 patients (61%) were classified in the high risk or super-high risk categories using Caprini RAM, while PPS categorised only 15 patients (12.2%) as high risk (p<0.001). Moreover, whilst the Caprini RAM categorised 36% of patients as high-risk, the calculated score for PPS was zero for these patients.
Discussion
The results of this study showed that VTE prophylaxis was administered to less than half the high-risk patients in the pre-intervention phase. Similarly, other studies have shown that only 40%–60% of renal-impaired patients at a high risk of VTE receive prophylaxis.8–10 The reasons for this under-utilisation might be related to the adverse effects of anticoagulant drugs (such as bleeding, which is especially important in patients with RI as they have an increased risk of haemorrhage), a lack of tools specifically designed to evaluate the risk of VTE in renal-impaired patients, or forgetfulness in evaluating VTE risk and prescribing prophylaxis (owing to the various complications that must be managed simultaneously in each patient).
The important finding of our study is that the recommendations of pharmacists, based on the applied RAMs, significantly increased the proportion of high-risk patients who received thromboprophylaxis, and reduced the proportion of low-risk patients receiving anticoagulant drugs. Therefore, these results might indicate that the fear of bleeding due to anticoagulant use is not a sufficient reason for their under-utilisation. In addition, these findings emphasise that the application of reliable RAMs and the use of a multidisciplinary team approach in the management of patients will significantly improve the rate of VTE prophylaxis prescription.
The applied intervention in our study was a type of active intervention. The results of several studies have shown that thromboprophylaxis is used in less than 40% of high-risk medical patients.17–19 Therefore, many strategies have been proposed to improve the rate of VTE prophylaxis prescription. These strategies are classified as either active or passive. Most active interventions include clinical audit and feedback, the improvement of healthcare providers’ education, the use of multidisciplinary team approaches,20 and the use of admonitory programmes that remind physicians to evaluate VTE risk and, if appropriate, to prescribe prophylaxis.21 Examples of passive interventions are educational mailing, passive dissemination of guidelines,21 22 computerised alert systems23–25 and sticker reminders.26 Studies have shown that increments in the prescription of appropriate prophylaxis for VTE following active interventions (18%–40%)27–29 are greater than those for passive interventions (<20%).26
Another finding from our study was the significant difference between Caprini RAM and PPS in the estimation of VTE risk in the enrolled patients. The number of patients who were recognised as being of high-risk or super-high risk for VTE using Caprini RAM was five times as much as was recognised using PPS. Although our study was not designed to examine the sensitivity or specificity of these risk assessment tools, our results are in accordance with the results of other studies. Two observational case-control studies30 31 compared the risk of VTE in medical patients diagnosed with VTE and in those without VTE, by using Caprini RAM and PPS. The results of those studies demonstrated that, whilst PPS is a more specific tool, Caprini RAM is a more sensitive scale for the prediction of VTE risk in hospitalised medical patients. We believe that, although Caprini RAM was primarily designed for the evaluation of VTE risk in surgical patients, the risk factors included in this RAM are also prevalent in medical patients, especially in those with RI. However, the role of each risk factor in contributing to the occurrence of thromboembolic events might differ between medical and surgical patients. Therefore, we suggest that both Caprini RAM and PPS should be used as a guide to develop specific tools for the assessment of VTE risk in renal-impaired patients.
Limitations
The first limitation of this study is that the patients were not followed up for a specific period (eg, 30–90 days) to monitor the occurrence of thromboembolic and haemorrhagic events. Therefore, this study could not validate the results of Caprini RAM and PPS, nor could it evaluate the effects of our interventions on patient outcomes (occurrence of VTE and/or bleeding) or costs. The second limitation is the inclusion of a limited number of patients from only one medical centre. Third, patients with coagulopathy were excluded from the study, but coagulopathy may not be a protective factor against thromboembolic events in all of the hospitalised medical patients.
Conclusion
Our results are in line with previous findings that show that VTE prophylaxis is under-utilised in hospitalised patients with RI. For the first time, we showed that an active collaboration between pharmacists and the medical team increased the proportion of high-VTE-risk renal-impaired patients who received thromboprophylaxis. Similar to the results of other studies, Caprini RAM classified more patients into the high and super-high VTE risk groups than did PPS.
What this paper adds.
What is already known on this subject
Patients with renal impairment are at increased risk of thromboembolic and haemorrhagic events.
VTE prophylaxis administration is not optimal in patients with renal impairment.
There are no specific risk assessment tools to evaluate the risk of thromboembolic or bleeding events in medical patients with renal impairment.
What this study adds
Clinical pharmacist intervention improves thromboprophylaxis prescription to patients with renal impairment.
Caprini RAM classifies more renal-impaired patients into the high VTE risk group than the Padua prediction score.
Footnotes
Contributors: Conception and design of study: Naemeh Nikvarz; Acquisition of data: Naemeh Nikvarz, Zahra Seyedi; Analysis and interpretation of data: Naemeh Nikvarz; Drafting of the manuscript: Naemeh Nikvarz, Zahra Seyedi; Critical revision of the manuscript for important intellectual content: Naemeh Nikvarz; Statistical analysis: Naemeh Nikvarz; Obtained funding: Naemeh Nikvarz; Study supervision: Naemeh Nikvarz.
Funding: This study was supported by Kerman University of Medical Sciences (grant number: 95000267)
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol of the study was approved by the Ethics Committee of Kerman Universityof Medical Sciences (reference: IR.KMU.REC.1395.699).
References
- 1. Cohen AT, Alikhan R, Arcelus JI, et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost 2005;94:750–9. 10.1160/TH05-06-0385 [DOI] [PubMed] [Google Scholar]
- 2. Khalafallah AA, Kirkby BE, Wong S, et al. Venous thromboembolism in medical patients during hospitalisation and 3 months after hospitalisation: a prospective observational study. BMJ Open 2016;6:e012346. 10.1136/bmjopen-2016-012346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness. Arch Intern Med 2004;164:963–8. 10.1001/archinte.164.9.963 [DOI] [PubMed] [Google Scholar]
- 4. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest 2001;119:132S–75. 10.1378/chest.119.1_suppl.132S [DOI] [PubMed] [Google Scholar]
- 5. Wattanakit K, Cushman M, Stehman-Breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 2008;19:135–40. 10.1681/ASN.2007030308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang I-K, Shen T-C, Muo C-H, et al. Risk of pulmonary embolism in patients with end-stage renal disease receiving long-term dialysis. Nephrol Dial Transplant 2017;32:1386–93. 10.1093/ndt/gfw272 [DOI] [PubMed] [Google Scholar]
- 7. Dager WE, Kiser TH. Systemic anticoagulation considerations in chronic kidney disease. Adv Chronic Kidney Dis 2010;17:420–7. 10.1053/j.ackd.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 8. Hergenroeder GW, Levine RL, Miller CC. Thromboembolism prophylaxis in end-stage renal disease. Dial Transplant 2008;37:439–44. 10.1002/dat.20280 [DOI] [Google Scholar]
- 9. Daneschvar H, Seddighzadeh A, Piazza G, et al. Deep vein thrombosis in patients with chronic kidney disease. Thromb Haemost 2008;99:1035–9. 10.1160/TH08-02-0107 [DOI] [PubMed] [Google Scholar]
- 10. Dentali F, Riva N, Gianni M, et al. Prevalence of renal failure and use of antithrombotic prophylaxis among medical inpatients at increased risk of venous thromboembolic events. Thromb Res 2008;123:67–71. 10.1016/j.thromres.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 11. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy 2009;29:943–53. 10.1592/phco.29.8.943 [DOI] [PubMed] [Google Scholar]
- 12. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua prediction score. J Thromb Haemost 2010;8:2450–7. 10.1111/j.1538-7836.2010.04044.x [DOI] [PubMed] [Google Scholar]
- 13. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon 2005;51:70–8. 10.1016/j.disamonth.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 14. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTe in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e195S–226. 10.1378/chest.11-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stuck A, Spirk D, Schaudt J, et al. Risk assessment models for venous thromboembolism in acutely ill medical patients. Thromb Haemost 2017;117:801–8. 10.1160/TH16-08-0631 [DOI] [PubMed] [Google Scholar]
- 16. Decousus H, Tapson VF, Bergmann J-F, et al. Factors at admission associated with bleeding risk in medical patients: findings from the improve Investigators. Chest 2011;139:69–79. 10.1378/chest.09-3081 [DOI] [PubMed] [Google Scholar]
- 17. Bergmann J-F, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in hospitalised medically ill patients. The ENDORSE global survey. Thromb Haemost 2010;103:736–48. 10.1160/TH09-09-0667 [DOI] [PubMed] [Google Scholar]
- 18. Kahn SR, Panju A, Geerts W, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res 2007;119:145–55. 10.1016/j.thromres.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 19. Ageno W, Squizzato A, Ambrosini F, et al. Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns. Haematologica 2002;87:746–50. [PubMed] [Google Scholar]
- 20. Gibbs H, Fletcher J, Blombery P, et al. Venous thromboembolism prophylaxis guideline implementation is improved by nurse directed feedback and audit. Thromb J 2011;9:7. 10.1186/1477-9560-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khorana AA. The NCCN clinical practice guidelines on venous thromboembolic disease: strategies for improving VTe prophylaxis in hospitalized cancer patients. Oncologist 2007;12:1361–70. 10.1634/theoncologist.12-11-1361 [DOI] [PubMed] [Google Scholar]
- 22. Cohn SL. Prophylaxis of venous thromboembolism in the US: improving Hospital performance. J Thromb Haemost 2009;7:1437–45. 10.1111/j.1538-7836.2009.03533.x [DOI] [PubMed] [Google Scholar]
- 23. Durieux P, Nizard R, Ravaud P, et al. A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior. JAMA 2000;283:2816–21. 10.1001/jama.283.21.2816 [DOI] [PubMed] [Google Scholar]
- 24. Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005;352:969–77. 10.1056/NEJMoa041533 [DOI] [PubMed] [Google Scholar]
- 25. Haut ER, Lau BD, Kraenzlin FS, et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg 2012;147:901–7. 10.1001/archsurg.2012.2024 [DOI] [PubMed] [Google Scholar]
- 26. Sharif-Kashani B, Raeissi S, Bikdeli B, et al. Sticker reminders improve thromboprophylaxis appropriateness in hospitalized patients. Thromb Res 2010;126:211–6. 10.1016/j.thromres.2010.05.020 [DOI] [PubMed] [Google Scholar]
- 27. Dobesh PP, Stacy ZA. Effect of a clinical pharmacy education program on improvement in the quantity and quality of venous thromboembolism prophylaxis for medically ill patients. J Manag Care Pharm 2005;11:755–62. 10.18553/jmcp.2005.11.9.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khalili H, Dashti-Khavidaki S, Talasaz AH, et al. Is deep vein thrombosis prophylaxis appropriate in the medical wards? A clinical pharmacists’ intervention study. Pharm World Sci 2010;32:594–600. 10.1007/s11096-010-9412-y [DOI] [PubMed] [Google Scholar]
- 29. Gladding P, Larsen F, Durrant H, et al. Education together with a preprinted Sticker improves the prescribing of prophylactic enoxaparin. N Z Med J 2007;120:U2461. [PubMed] [Google Scholar]
- 30. Liu X, Liu C, Chen X, et al. Comparison between Caprini and Padua risk assessment models for hospitalized medical patients at risk for venous thromboembolism: a retrospective study. Interact Cardiovasc Thorac Surg 2016;23:538–43. 10.1093/icvts/ivw158 [DOI] [PubMed] [Google Scholar]
- 31. Pop TR, Vesa SC, Trifa AP, et al. Pai-1 4G/5G and MTHFR C677T polymorphisms increased the accuracy of two prediction scores for the risk of acute lower extremity deep vein thrombosis. Rom J Morphol Embryol 2014;55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


