Abstract
Objectives
The 2018 EAHP European Statements Survey focused on sections 1, 3 and 4 of the European Statements of Hospital Pharmacy. Statistical data on the level of implementation and on the main barriers to implementation of the Statements were collected. A further aim was to identify barriers in general, such as lack of awareness.
Methods
An online questionnaire was sent to all hospital pharmacies in EAHP member countries. Data were analysed at Keele University School of Pharmacy, UK. As with previous reports, the survey was divided into three sections: section A, asking general questions about the hospital pharmacy; Section B, addressing questions about the current activity of pharmacists around each statement from Sections 1, 3 and 4; and Section C, focusing on their ability to implement the statements.
Results
719 complete responses were obtained from a sample of 5164 hospital pharmacies, giving a response rate of 14% (719/5164). Section A results indicated that 45% (323/719) of responders worked in teaching hospitals, 79% (568/719) of hospital pharmacies had 10 or fewer pharmacists, and 48% (345/719) of hospital pharmacies served over 500 beds. Section B results found a high percentage of positive responses for activity in section 1 (introductory statements and governance) and section 3 (production and compounding). However, responses to questions in section 4 (clinical pharmacy services) were more variable, with 6 of the 15 questions being answered positively by less than half of respondents. The five questions that revealed the lowest implementation levels were then analysed in greater detail. These questions corresponded to Statements 4.4, 4.5, 4.8, 1.1, and 4.2, which need the greatest effort for implementation. The major identified barriers to implementation were 'lack of capacity' and that 'other health professionals in the hospital fulfil the tasks'.
Conclusions
This survey provides useful information on the implementation status (and the barriers to, and drivers of implementation) of sections 1, 3 and 4 of the Statements. This will allow the EAHP to plan its implementation support programme for its members. To increase the quality of data, as well as the feedback to hospital pharmacies, the EAHP is planning to combine the survey with the self-assessment tool of the European Statements of Hospital Pharmacy.
Keywords: clinical pharmacy, pharmacy management (personnel), health services administration & management, EAHP statements of hospital pharmacy, survey
Introduction
The European Statements of Hospital Pharmacy (‘Statements’)1 express the commonly agreed objectives that every European health system should aim for in the delivery of hospital pharmacy services. The Statements were formulated, via a methodological consultation process, by members of the European Association of Hospital Pharmacists (EAHP), together with patient and healthcare professional organisations.2 The EAHP survey has focused on measuring the implementation of the Statements, across European countries, since 2015. This new survey model was intended to support EAHP efforts in implementing the Statements. The EAHP Survey Group established a model with a ‘baseline survey’ and two ‘statements surveys’, rotating in two-year cycles and with each year covering three of the six sections of the Statements.3
The complete results are provided to all members of EAHP, and a detailed report with additional tables and figures is available.4 This article provides an overview of the most important results of the latest survey (in 2018) that covered sections 1, 3 and 4 of the Statements, and compares these data with the results of the 2016 survey (which focused on the same sections) and in some cases with the baseline survey.5
Methods
To be able to compare the results with previous statement surveys, the same questions were used as in the 2016 survey. The survey was conducted from October 2018 to November 2018, spanning 34 countries. In line with previous years, the survey consisted of three sections:
Section A: general questions about the participant’s hospital pharmacy, such as workforce skill-mix and number of beds served
Section B: questions about the current activity of pharmacists around each statement from Sections 1, 3 and 4 (see also online supplementary table 1)
Section C: questions about the hospital’s readiness and ability to implement the statements.
ejhpharm-2019-002028supp001.pdf (191.7KB, pdf)
The survey was created using the online survey software SurveyMonkey6 and distributed by email collector (a tool provided by SurveyMonkey) to one email address per hospital. National coordinators were provided with the list of emails for their country.
Results were exported from SurveyMonkey for further analysis and reporting. It was planned that responses would be analysed by the proportion of positive answers (regarding the implementation of a Statement overall) and also per country. Significance testing was performed to compare the results of some of the survey questions to the same questions asked in the 2016 EAHP Statements Survey.
For further information please see online supplementary additional material.
ejhpharm-2019-002028supp002.pdf (671.2KB, pdf)
Results
5164 hospital pharmacies were invited to complete the survey, and there were 719 complete responses (14% (719/5164)), a slight decrease compared with 2016 (16% (904/5711)). Both surveys had a similar completion rate: 82% (719/873) in 2018 and 81% (730/904) in 2016. Response rates varied widely across countries: the highest number came from Germany (with 99 responses), followed by Hungary (55 responses) and the Czech Republic (43 responses). Sixteen of the thirty-five countries had a response rate of over 30%, compared with twenty-one out of thirty-five in 2016. Table 1 shows the response rates broken down by country, and the response rates from the 2016 survey (for comparison).
Table 1.
Response rate per country for the 2018, 2016 and baseline surveys
| Country | Requests 2018 |
Requests 2016 |
Requests 2018 vs 2016 | Responses 2018 |
Responses 2016 |
Responses 2018 vs 2016 | Percentage 2018 |
Percentage 2016 |
Baseline 2015 |
Percentage 2018 vs 2016 |
| Austria | 45 | 48 | −3 | 32 | 27 | 5 | 71% | 56% | 47% | 15% |
| Belgium | 135 | 172 | −37 | 30 | 45 | −15 | 22% | 26% | 22% | −4% |
| Bosnia | 20 | 23 | −3 | 10 | 12 | -2 | 50% | 52% | 33% | −2% |
| Bulgaria | 66 | 73 | −7 | 12 | 17 | -5 | 18% | 23% | 14% | −5% |
| Croatia | 42 | 36 | 6 | 28 | 16 | 12 | 67% | 44% | 79% | 23% |
| Czech Republic | 92 | 104 | −12 | 43 | 42 | 1 | 47% | 40% | 63% | 7% |
| Denmark | 9 | 8 | 1 | 8 | 7 | 1 | 89% | 88% | 88% | 1% |
| Estonia | 24 | 25 | −1 | 5 | 10 | −5 | 21% | 40% | 64% | −19% |
| Finland | 62 | 82 | −20 | 12 | 16 | −4 | 19% | 20% | 17% | −1% |
| France | 1 560 | 1 835 | −275 | 23 | 50 | −27 | 1% | 3% | 7% | −2% |
| Germany | 342 | 383 | −41 | 99 | 82 | 17 | 29% | 21% | 22% | 8% |
| Greece | 119 | 106 | 13 | 33 | 32 | 1 | 28% | 30% | 31% | −2% |
| Hungary | 99 | 111 | −12 | 55 | 54 | 1 | 56% | 49% | 62% | 7% |
| Iceland | 2 | 2 | 0 | 1 | 2 | −1 | 50% | 100% | 100% | −50% |
| Ireland | 66 | 73 | −7 | 26 | 32 | −6 | 39% | 44% | 48% | −5% |
| Italy | 585 | 609 | −24 | 39 | 36 | 3 | 7% | 6% | 5% | 1% |
| Latvia | 37 | 45 | −8 | 1 | 6 | −5 | 3% | 13% | 11% | −10% |
| Lithuania | 38 | 39 | −1 | 6 | 9 | −3 | 16% | 23% | 7% | −7% |
| Luxembourg | 5 | 6 | −1 | 4 | 3 | 1 | 80% | 50% | 50% | 30% |
| Malta | 5 | 5 | 0 | 0 | 3 | −3 | 0% | 60% | 50% | −60% |
| Montenegro | 6 | 6 | 0 | 5 | 4 | 1 | 83% | 67% | N/A | 16% |
| Netherlands | 98 | 108 | −10 | 17 | 18 | −1 | 17% | 17% | 35% | 0% |
| North Macedonia | 29 | 31 | −2 | 8 | 13 | −5 | 28% | 42% | 58% | −14% |
| Norway | 31 | 32 | −1 | 12 | 20 | −8 | 39% | 63% | 56% | −24% |
| Poland | 81 | 38 | 43 | 19 | 21 | −2 | 23% | 55% | 7% | −32% |
| Portugal | 89 | 89 | 0 | 15 | 38 | −23 | 17% | 43% | 22% | −26% |
| Romania | 67 | 66 | 1 | 19 | 14 | 5 | 28% | 21% | 41% | 7% |
| Serbia | 63 | 65 | −2 | 28 | 45 | −17 | 44% | 69% | 78% | −25% |
| Slovakia | 71 | 76 | −5 | 31 | 33 | −2 | 44% | 43% | 52% | 1% |
| Slovenia | 29 | 31 | −2 | 19 | 22 | −3 | 66% | 71% | 57% | −5% |
| Spain | 250 | 250 | 0 | 6 | 39 | −33 | 2% | 16% | 17% | −14% |
| Sweden | 34 | 37 | −3 | 12 | 19 | −7 | 35% | 51% | 24% | −16% |
| Switzerland | 60 | 60 | 0 | 21 | 17 | 4 | 35% | 28% | 43% | 7% |
| Turkey | 696 | 821 | −125 | 21 | 70 | −49 | 3% | 9% | 6% | −6% |
| UK | 207 | 216 | −9 | 19 | 30 | −11 | 9% | 14% | 36% | −5% |
| Total | 5 164 | 5 711 | −547 | 719 | 904 | −185 | 14% | 16% | 17% | −2% |
Section A
The results showed that, overall, the participating sample of hospital pharmacies was comparable with those in the baseline and recent surveys.
For further information please see online supplementary additional material.
Section B
The questions and the overall results are shown in online supplementary table 1.
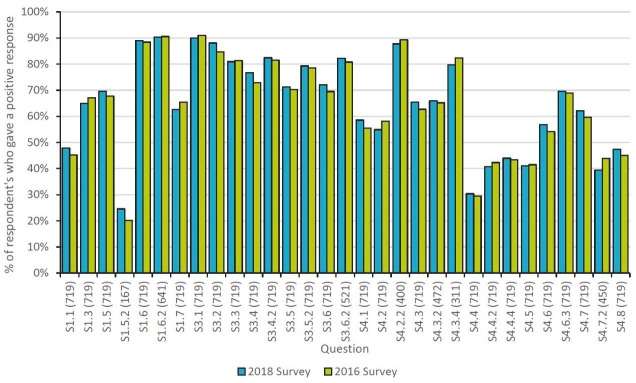
Figure 1 shows the results of the 2018 EAHP Statements Survey alongside the results of the 2016 survey. The numbers in brackets on the bottom axis are the number of responses by country for the 2018 survey. Most of the questions in section 1 (introductory statements and governance) and all of the questions in section 3 (production and compounding) produced a high percentage of positive responses. However, responses to questions in section 4 (clinical pharmacy services) produced more variable responses, with six of the fifteen questions being answered positively by less than half of respondents.
Figure 1.
Comparative data: overall percentage of positive responses from the 2018 EAHP statements survey and 2016 survey.
The five questions that received the fewest positive responses were:
S4.4: The pharmacists in our hospital enter all medicines used onto the patient’s medical record on admission (2018: 30% (218/719), 2016: 29% (214/730), baseline: 28% (306/1094)).
S4.5: The pharmacists in our hospital contribute to the transfer of information about medicines when patients move between and within healthcare settings (2018: 41% (295/719), 2016: 41% (302/730), baseline: 44% (481/1094)).
S4.8: Do you have an agreed strategic plan for the development of clinical pharmacy services in your hospital? (2018: 47% (340/719), 2016: 45% (329/730), baseline: no data).
S1.1: The pharmacists in our hospital work routinely as part of a multidisciplinary team (2018: 47% (340/719), 2016: 48% (349/730), baseline: 59% (645/1094).
S4.2: All prescriptions in our hospital are reviewed and validated as soon as possible by a pharmacist (2018: 55% (395/719), 2016: 58% (424/730), baseline: 63% (689/1094)).
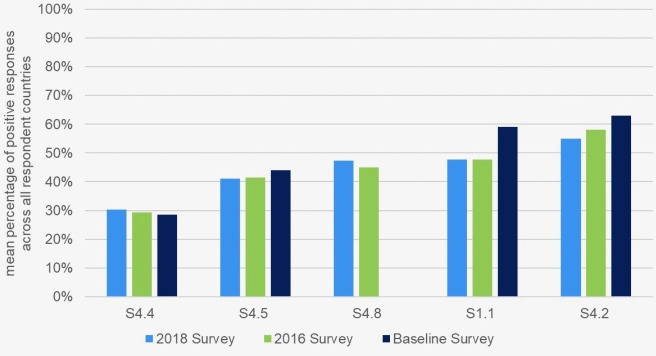
Figure 2 shows the mean percentage of positive responses to these five questions in 2018, across all respondent countries, compared with the results of the 2016 and baseline surveys. Further investigation resulted in more detailed information.
Figure 2.
The mean percentage, across all respondent countries, of the five questions that received the fewest positive responses in 2018, compared with the results of the 2016 and baseline surveys.
Question related to EAHP Statement 4.4
The pharmacists in our hospital enter all medicines used onto the patient’s medical record on admission.
Online supplementary figure 1 shows the proportion of respondents who gave a positive response when asked if pharmacists enter all medicines used onto the patient’s medical record on admission. Overall, only 30% (218/719) of responses were positive to this question, a similar result to that in the 2016 survey (in which 29% (214/730) of the total responses were positive). However, a paired samples t-test indicated that an increase in the mean percentage of positive responses for all countries between the 2016 survey (25.0%) and the 2018 survey (31.1%) was statistically significant (p=0.023).
ejhpharm-2019-002028supp003.pdf (246.7KB, pdf)
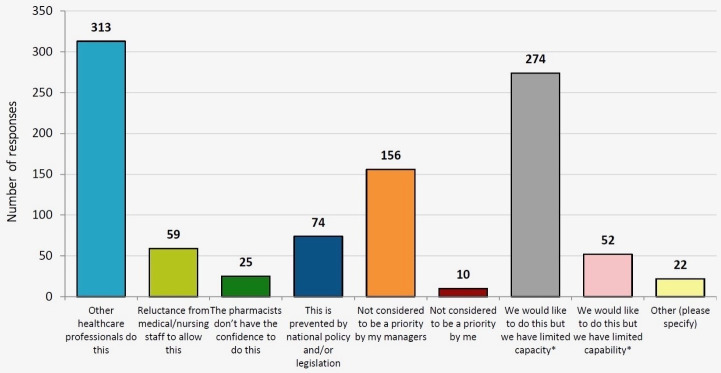
In every country surveyed (apart from the Netherlands, Spain, Turkey and the UK) less than half of respondents gave a positive response. When looking at the responses from individual countries that participated in both surveys, the proportion of positive responses increased in sixteen countries, decreased in fourteen countries and stayed the same in four countries. To further understand this, respondents who answered the question with a negative response were asked ‘What is preventing pharmacists from entering medicines onto patient’s medical records?’ The overall results are shown in figure 3. The most frequent overall response was that ‘other healthcare professionals do this’, with a total of 313 responses. This was also observed in previous EAHP surveys, where in many countries and hospitals the role of the hospital pharmacist is limited to the procurement of medicines, rather than engaging in clinical responsibilities. Another major barrier noted by respondents was that ‘we would like to do this but we have limited capacity’ (274 responses across all countries). ‘Not considered to be a priority by my managers’ was also identified as a barrier in 156 responses. These three options accounted for 75% (743/985) of all responses.
Figure 3.
Results from the question S4.4.1 ‘what is preventing pharmacists from entering medicines onto patient’s records on admission?’
Participants were also asked to respond to the statement ‘pharmacists in our hospital reconcile medicines on admission’. Overall, 41% (293/719) of responses were positive, a slight decrease from the 42% (309/730) observed in the 2016 survey. Of the thirty-four countries participating, twenty returned a more positive result compared with the 2016 survey. The largest increase was seen in Turkey, where the proportion of positive responses increased from 65% (24/37) to 95% (20/21).
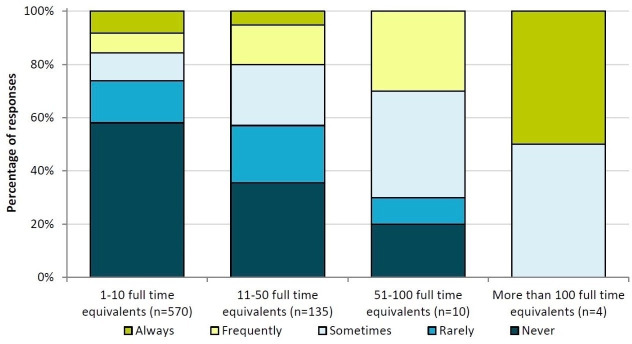
Figure 3 shows that ‘lack of capacity’ was cited as the second-largest barrier to pharmacists entering medicines used onto patients’ records on admission, so the relationship between this activity and the pharmacist workforce was investigated further. Responses to this question are shown in figure 4, where the results are grouped by the number of fully-qualified pharmacists employed by the hospital. The proportion of more negative responses (never or rarely) is much higher for the lowest staffing level (1–10 pharmacists), although it is important to note that the total number of responses for the higher staffing levels is fairly small (n=4).
Figure 4.
Overall results of responses to the statement “the pharmacists in our hospital enter all medicines used onto the patient’s medical record on admission” (grouped by number of fully-qualified pharmacists employed by the hospital).
A Kruskal-Wallis H Test showed that there was a statistically significant difference in responses regarding ‘pharmacists entering medicines used onto patients records on admission’ between the groupings of working pharmacist numbers (χ2(3)=30.0, p<0.01), with mean ranks of 342 for the ‘1 to 10 pharmacists’ group, 418 for the ‘11 to 50 pharmacists’ group, 492 for the ‘51 to 100 pharmacists’ group, and 621 for the ‘more than 100 pharmacists’ group. Hospitals employing a greater number of pharmacists were more likely to have pharmacists regularly entering medicines used onto patients’ medical records on admission.
Question related to EAHP Statement 4.5
The pharmacists in our hospital contribute to the transfer of information about medicines when patients move between and within healthcare settings.
The responses to the statement ‘the pharmacists in our hospital contribute to the transfer of information about medicines when patients move between and within healthcare settings’ are shown in online supplementary figure 2. The overall response was only 41% (295/719) positive, showing that this statement is not currently implemented widely across European hospitals. The overall response observed in the 2016 survey was also 41% (302/730), indicating that progress on this issue might be minimal. The positive response rate between countries was varied: in twenty-one countries, less than half of the respondents gave a positive response, whereas five countries had (on average) more than three-quarters of respondents providing positive responses. This variation in responses between countries is similar to what was observed in online supplementary figure 1, which also described a more clinical role and suggests that the role of hospital pharmacists in some countries is less focused on clinical activities than in others. Although the mean proportion of positive responses for countries increased between the 2016 survey (38.3%) and the 2018 survey (42.5%), a t-test showed that this increase was not statistically significant (p=0.056).
ejhpharm-2019-002028supp004.pdf (285.6KB, pdf)
When asked 'what are the barriers to pharmacists contributing to the transfer of information about medicines when patients move between healthcare settings', the most frequent response was ‘other healthcare professionals do this' (249 responses), ‘limited capacity’ (213 responses) and ‘not considered to be a priority by my managers’ (132 responses). Nearly all countries identified ‘other healthcare professionals do this’ or ‘limited capacity’ as the biggest barrier to implementation. Most notably, North Macedonia, Bulgaria and Poland highlighted national policy/legislation as a barrier.
Question related to EAHP Statement 4.8
Do you have an agreed strategic plan for the development of clinical pharmacy services in your hospital?
Online supplementary figure 3 shows the percentage of respondents who gave a positive response when asked ‘Do you have an agreed strategic plan for the development of clinical pharmacy services in your hospital?’ The overall positive response rate for this question was 47% (340/719), up from 45% (329/730) in the 2016 survey. This question was not included in the original baseline survey. Of the countries who participated in both 2016 and 2018 surveys, twenty-one saw an increase in the percentage of positive responses, while eleven saw a decrease and two remained the same.
ejhpharm-2019-002028supp005.pdf (266.2KB, pdf)
A paired samples t-test indicated that the mean proportion of positive responses for countries in the 2016 survey (43.3%) was not significantly different when compared with the 2018 survey (49.2%) (p=0.062).
The main barriers to implementation regarding this statement were identified as ‘not considered to be a priority by my managers/clinicians’ (256 responses) and ‘limited capacity’ (219 responses). There were thirty-two free text responses from the ‘Other’ category, and many of these responses highlighted ‘capacity’ and ‘not being a priority’ to be the main barriers. All countries identified the biggest barrier as either ‘not being considered a priority by managers’ or ‘limited capacity’. There were very few responses for ‘not considered to be a priority by me’, suggesting that many pharmacists see the importance of a strategic plan.
The proportion of pharmacists responding that they ‘have an agreed strategic plan for the development of clinical pharmacy services in their hospital’ was then grouped by the number of fully-qualified pharmacists working at the hospital. The number of positive responses was much lower for the lowest grouping of working pharmacists (43% (247/570) for the 1–10 pharmacists group) compared with the groups with larger numbers of working pharmacists (ranging from 61%–80% (82/135 to 8/10)). An explanation for this could be that pharmacists working in hospitals that employ fewer pharmacists do not have time to spare for additional responsibilities such as this.
A Chi-square Test of independence was performed to examine the relationship between the number of pharmacists employed in a hospital and the number of pharmacists having an agreed strategic plan for the development of clinical pharmacy services in their hospital. The relationship between these variables was significant (χ2(3)=18.9, p<0.01). Hospitals employing fewer pharmacists were less likely to have an agreed strategic plan for the development of clinical pharmacy services in their hospital.
Question related to EAHP 1.1
The pharmacists in our hospital work routinely as part of a multidisciplinary team.
Online supplementary figure 4 shows the responses to the statement ‘The pharmacists in our hospital work routinely as part of a multidisciplinary team’. The overall positive response rate for this question was 48% (344/719), up from 46% (349/730) in the 2016 survey. Out of the thirty-four countries that participated in both the 2018 and 2016 surveys, eighteen countries increased their proportion of positive responses, fifteen showed a decrease, and one stayed the same. The mean proportion of positive responses for countries increased in the 2018 survey (51.0%) compared with the 2016 survey (44.7%), although a t-test showed that this result fell short of being statistically significant (p=0.051).
ejhpharm-2019-002028supp006.pdf (278.9KB, pdf)
Respondents who gave a positive response were also asked ‘What type of multidisciplinary activities are you involved with?’ Membership of multidisciplinary committees, specific therapeutic groups and educational activities all received a high number of responses (295, 305 and 275 respectively). Multidisciplinary ward rounds and consulting with patients about medicines received fewer responses (196 and 106 responses, respectively). A similar pattern of responses was observed in the 2016 survey.
Respondents who gave a negative response to the initial question were then asked ‘What is preventing you or your pharmacists from routinely working as part of a multidisciplinary team?’ ‘Limited capacity’ was identified as the greatest barrier to implementation, with 267 responses – more than double the number of responses to the next most popular barrier (‘not considered to be a priority by my managers’, 122 responses). ‘Limited capacity’ was cited as a barrier in almost every country surveyed.
The overall results for this statement question, when grouped by the number of fully-qualified pharmacists working at the hospital, again show that the proportion of positive responses increases as the staffing levels increase (38% (218/570) for the 1–10 pharmacists group, increasing to 84% (113/135), 90% (9/10) and 100% (4/4) as the staffing-group level increased).
A Kruskal-Wallis H Test showed that there was a statistically significant difference in responses to ‘pharmacists working routinely as part of a multidisciplinary team’ between the groupings of working pharmacist numbers (χ2(3)=103.4, p<0.01), with mean ranks of 322 for the ‘1 to 10 pharmacists’ group, 501 for the ‘11 to 50 pharmacists’ group, 530 for the ‘51 to 100 pharmacists’ group, and 648 for the ‘more than 100 pharmacists’ group. Hospitals employing fewer pharmacists were less likely to have pharmacists working routinely as part of a multidisciplinary team.
Additionally, a Mann-Whitney U Test indicated that teaching/university hospitals reported more positive responses when asked ‘if pharmacists in the hospital routinely work as part of a multidisciplinary team’ than did non-teaching hospitals (p<0.01), with mean ranks of 390 for teaching/university hospitals and 335 for non-teaching hospitals.
Question related to EAHP Statement 4.2
All prescriptions in our hospital are reviewed and validated as soon as possible by a pharmacist.
When asked to respond to the statement ‘All prescriptions in our hospital are reviewed and validated as soon as possible by a pharmacist’, the overall positive response was 55% (395/719). This is a less positive response than in both the 2016 survey (58% (424/730)) and the baseline survey (63% (689/1094)). online supplementary figure 5 shows the results broken down by country, indicating that the response between countries was mixed, with a large range of results. In six countries, 100% of responses were positive, whilst many more countries gave a very low number of positive responses. When compared with the 2016 survey, fifteen countries increased their percentage of positive responses, fourteen countries saw a decrease, and five remained the same.
ejhpharm-2019-002028supp007.pdf (287.2KB, pdf)
A paired samples t-test indicated that the mean proportion of positive responses for countries was not significantly different for the 2016 survey (52.6%) compared with the 2018 survey (56.0%), (p=0.322).
Participants who gave a negative response to statement 4.2 were then asked what was preventing this. The most common response was ‘limited capacity‘, with 218 responses. ‘Not considered to be a priority by my managers’ had 154 responses. Additional barriers from the ‘Other’ category included ‘pharmacists not having access to patients’ records’.
Respondents who gave a positive response to the initial question were then asked ‘Does this review and validation by a pharmacist take place prior to the administration of medicines?’ The overall positive rate for the 2018 survey was 88% (633/719), similar to the 2016 survey (89% (650/730)). This question was not included in the baseline survey.
A Kruskal-Wallis H Test showed that the difference in responses to ‘pharmacists reviewing all prescriptions in the hospital’ between the groupings of working pharmacist numbers was not statistically significant (χ2(3)=5.5, p=0.137), with mean ranks of 354 for the ‘1 to 10 pharmacists’ group, 372 for the ‘11 to 50 pharmacists’ group, 460 for the ‘51 to 100 pharmacists’ group, and 510 for the ‘More than 100 pharmacists’ group.
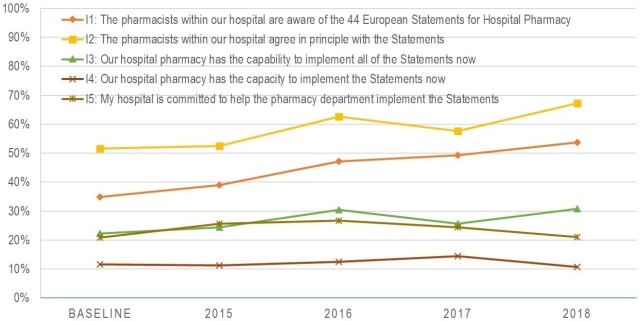
Section C
Results of the Implementation Questions
Figure 5 shows the main results of the implementation statements. Positive responses regarding ‘awareness of the Statements’ (I1, baseline: 35%; 2018: 54% (388/719)) and ‘agreement with the Statements’ (I2, baseline: 52% (569/1094); 2018: 67% (482/719)) have both been steadily increasing since the baseline survey. The proportion of respondents agreeing that their hospital pharmacy has the capability (I3, baseline: 22% (241/1094); 2018: 31% (223/719)) and the capacity (I4, baseline: 12% (131/1094); 2018: 11% (79/719)) to implement the statements, and that their hospital is committed to helping the pharmacy to implement the statements (I5, baseline: 21% (230/1094); 2018: 21% (151/719)) are relatively low and have seen no relevant changes since the baseline survey.
Figure 5.
Agreement with the implementation statements.
Discussion
The survey reflects the high professional standards of hospital pharmacy in Europe. Even though the results presented in this publication focus on the most challenging statements (with the lowest proportions of positive answers), the overall results show a high implementation rate for many of the Statements. In particular, the results for section 3 were largely positive (on average, within the range of 71% to 90% (512/719 to 647/719)) and produced a higher proportion of positive responses compared with 2016. This reflects the tremendous professional contribution of hospital pharmacists to ensuring that safe and high-quality medicines are prepared individually for patients or for patient groups in hospital pharmacies all over Europe.
Responses to questions in section 4 (clinical pharmacy services) were more variable, with six of the fifteen questions being answered positively by less than half of respondents. A possible broad explanation for the reduction in positive responses could be that the overall capacity of hospital pharmacists has been further stretched since the baseline survey. Another possible explanation for this increase in negative responses could be that some respondents might now be familiar enough with the EAHP Statements surveys to know that if they give a negative response to a question they are then offered the opportunity to provide further feedback on an issue, which they wish to do.
Examination of the five statements for which the barriers to implementation were greatest revealed that the barriers to implementing the statements, as reported in 2016, are still in place. A major barrier to implementation was a lack of capacity to implement the statements. Interestingly, this barrier is mostly independent (and not correlated with) the number of pharmacists working in a hospital or in the country. Another highly ranked reason was that other healthcare professionals are doing this at the moment. As the results from many projects have shown, hospital pharmacists are very well accepted if they provide services to patients;7 8 the professionals who carry out these services at the moment should be considered as partners for future change.
As seen from the results of section C and in figure 5, awareness of, and agreement with, the Statements by hospital pharmacists have both been steadily increasing. This is fundamental to implementing change. The slow (or minimal) changes seen with other implementation barriers supports the evidence that implementation is a gradual process, so any changes on a large scale happen slowly and are not yet reflected in the survey results. It should also be noted that this result measures the average change across all countries, so individual countries might have seen greater changes. The positive change in the reported level of awareness also reflects the activities of the EAHP Statement Implementation Ambassadors, suggesting that the implementation project should continue to be developed. Removing the main barriers (such as insufficient staffing) will take a long time, and increasing awareness is a necessary first step in this journey. EAHP provides a self-assessment tool9 to not only assess but also to benchmark the implementation of the Statements with other hospital pharmacies in Europe. This gives clearer, more individualised and more detailed information to hospital pharmacies than the overall results of the surveys can provide.
There are several limitations to this study. The first and most important limitation was that the number of responses from some member countries was very small, and hence did not allow a precise statistical evaluation at country level. The reason for this is that countries have a wide variation in their numbers of hospital pharmacies, which do not always correlate with the number of inhabitants. The second limitation was the necessity to find a balance between the length of the questionnaire (and the workload for responders) and the level of detail sought in identifying the main implementation barriers. Another limitation in comparing the 2018 results with those of 2016 is the small numbers. Therefore, it is not clear whether observed changes are the result of different respondents or whether they really indicate changes.
Despite these limitations, the survey results provide an up-to-date picture of the current state of hospital pharmacy in Europe (in relation to the Statements). There appear to be more barriers to hospital pharmacies engaging in more clinically and patient-focused activities—such as medication history reconciliation, direct patient information, or working in a multidisciplinary team. Lack of capacity, capability and support from managers are the commonly-cited reasons for this. Again, there was considerable variation across the different countries, reflecting the diversity of the situation in European countries. The role of the clinical pharmacist (where pharmacists are visible on the wards and in clinics)—while well established in some countries—is still a rarity in others. In these countries, many hospitals employ low numbers of staff for hospital pharmacies in relation to their numbers of beds, which supports the ‘lack of capacity’ responses. In addition, the capacity of hospital pharmacists is often negatively impacted by inevitable non-productive external causes, such as medicine shortages and the Falsified Medicines Directive (FMD).10
Conclusion
The main objective of the 2018 EAHP Statements Survey was to provide an assessment throughout European countries of the level of implementation of sections 1, 3, and 4. The main barriers to, and drivers of, implementation should be identified and possible progress in implementation should be investigated. This objective has been reached, thanks to the enormous efforts of national coordinators and all the hospital pharmacists who responded to the survey.
The results enable EAHP to prioritise efforts in its implementation activities. The Statement Self-Assessment Tool (SAT) is already being used by many pharmacists and will be widely promoted over the next few months. The goal is to extract data from the SAT and to analyse it using a similar methodology to that used for the statement survey data, allowing the continued analysis of trends. This will increase the consistency of the data and will hopefully result in a wider response from European hospital pharmacies.
What this paper adds.
What is already known on this subject
The 2014/2015 European Association of Hospital Pharmacists (EAHP) baseline survey and the 2016 Statements Survey provided general knowledge of the baseline level of implementation of the Statements in sections 1, 3 and 4.
What this study adds
This paper updates our knowledge of the level of implementation of sections 1, 3 and 4 of the Statements, and identifies the main barriers to, and drivers of, implementation. The biggest implementation challenges in hospital pharmacies were identified for Statements 1.1., 4.2, 4.4, 4.5 and 4.8. Barriers to hospital pharmacies engaging in more clinically-focused activities seem to be greater than for more traditional areas, such as compounding. Lack of capacity, capability and support from managers are the commonly cited reasons for this, although there was considerable variation across the different countries.
Footnotes
Contributors: PH, JU and AB planned the study and designed the questionnaire. NG set up the online form, sent the questionnaire to responders and tracked responses. PH, AB, SA and NM communicated with EAHP members and raised awareness about the survey. NG, JU and SA conducted the survey, evaluated data and performed statistical analysis. SA, JU, NG, PH, AB and NM prepared the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Anon . The European statements of hospital pharmacy. Eur J Hosp Pharm 2014;21:256–8. 10.1136/ejhpharm-2014-000526 [DOI] [Google Scholar]
- 2. Maskrey N, Underhill J. The European statements of hospital pharmacy: achieving consensus using Delphi and world Café methodologies. Eur J Hosp Pharm 2014;21:264–6. 10.1136/ejhpharm-2014-000520 [DOI] [Google Scholar]
- 3. Horák P, Peppard J, Sýkora J, et al. EAHP survey and European statements of hospital pharmacy – can we achieve a perfect match? Eur J Hosp Pharm 2014;21:291–3. 10.1136/ejhpharm-2014-000541 [DOI] [Google Scholar]
- 4. EAHP . European statements of hospital pharmacy survey results 2018, statements sections 1, 3 and 4. Available: http://www.eahp.eu/sites/default/files/eahp_survey_report_2018-19.pdf
- 5. Horák P, Peppard J, Sýkora J, et al. EAHP European statements baseline survey 2015: results. Eur J Hosp Pharm 2016;23:69–75. 10.1136/ejhpharm-2016-000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. SurveyMonkey . SurveyMonkey and GDPR. Available: https://www.surveymonkey.com
- 7. Quintens C, De Rijdt T, Van Nieuwenhuyse T, et al. Development and implementation of "Check of Medication Appropriateness" (CMA): advanced pharmacotherapy-related clinical rules to support medication surveillance. BMC Med Inform Decis Mak 2019;19:29. 10.1186/s12911-019-0748-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phan H, Williams M, McElroy K, et al. Implementation of a student pharmacist-driven medication history service for ambulatory oncology patients in a large academic medical center. J Oncol Pharm Pract 2019;25:1419–24. 10.1177/1078155219831066 [DOI] [PubMed] [Google Scholar]
- 9. EAHP . Hospital self-assessment tool. Available: http://sat.eahp.eu/en/home
- 10. Frontini R. Falsified medicines directive: are we heading in the right direction? 2017. Available: 10.5301/maapoc.0000011 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2019-002028supp001.pdf (191.7KB, pdf)
ejhpharm-2019-002028supp002.pdf (671.2KB, pdf)
ejhpharm-2019-002028supp003.pdf (246.7KB, pdf)
ejhpharm-2019-002028supp004.pdf (285.6KB, pdf)
ejhpharm-2019-002028supp005.pdf (266.2KB, pdf)
ejhpharm-2019-002028supp006.pdf (278.9KB, pdf)
ejhpharm-2019-002028supp007.pdf (287.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.