Abstract
The gut microbiome affects many aspects of human health including aging and cancer. Recent evidence has demonstrated a causal relationship between the microbes in the gut and response to cancer treatment with immune checkpoint inhibitors (ICIs). Individuals whose cancer responds to ICIs can be distinguished from those who do not solely by the composition of their gut microbes at the start of treatment. Provocatively, preclinical models supplemented with a single microbial strain or microbially-derived metabolite can modify response to treatment. The microbiome therefore represents both a biomarker and therapeutic target for modifying and improving cancer care. However, as is often the case with emerging treatments, older adults are not strongly represented in the clinical trials leading to treatment approval. There are known shifts in the microbiome as one ages. The mechanism by which these shifts occur with age are important to consider considering efforts to modify the microbiome to promote response. Here we summarize the literature on the microbes related to aging and interpret them in the context of those associated with response to ICIs. We demonstrate that these age-related changes tend to shift the microbiome toward a non-responder-like composition, lacking microbes demonstrated to support treatment response, which may contribute to the decreased efficacy in this population.1 We review the potential mechanisms by which these effects occur and posit a model to interpret the broad-level changes observed. Finally, we discuss trials currently underway to target this novel treatment modality in the understudied and growing older adult population.
The microbiome changes with age
In 2007, The ELDERMET Study was the first major trial to focus on the microbiome of older adults by recruiting 400 participants >65 years old. Since then, similar studies have been performed in older adult populations from other European countries as well as China and Japan. 2,3 Each study found differences between younger and older adults, but a universal older adult microbiome was not observed across the geographically-distinct populations. For example, the ELDERMET study (Ireland) found increased relative abundance of Alistipes and Oscillibacter and decreased Prevotella and Ruminococcus 4. In Japan Firmicutes, Bacteroidetes and Proteobacteria were enriched3. Most consistently, the genus Bifidobacterium is decreased, which is notable as being the microbe most enriched in infants via the pre-biotic effects of breast milk. Bifidobacterium has been associated with health in a variety of settings, including response to immunotherapy5–8.
While Bifidobacterium is most consistently depleted in older adults, the diverse phylum Proteobacteria are consistently enriched. The Proteobacteria are more abundant in the environment than in the healthy gut, and a relatively high abundance (e.g. >~10%) is associated with diverse diseases 9. This has led to the speculation that a healthy gut is characterized by its ability to defend against constant incursions by Proteobacteria coming in from the environment.
Increased Proteobacteria in older adults could be driven by several changes including (1) reduced efficacy of the immune system leading to more frequent blooms of organisms encountered in the environment, (2) lower fiber diet, (3) decreased gut barrier function leading to a more aerobic gut and increased bacterial translocation across the gut barrier (Figure 1). Likely, these three are tightly connected, though the causal chain is unclear. Rather than a linear causal chain, a feedback loop may be more accurate, whereby each aspect can exacerbate, or conversely help to alleviate, the problem.
Figure 1. Summary of age-related effects on response to immunotherapy via the microbiome.
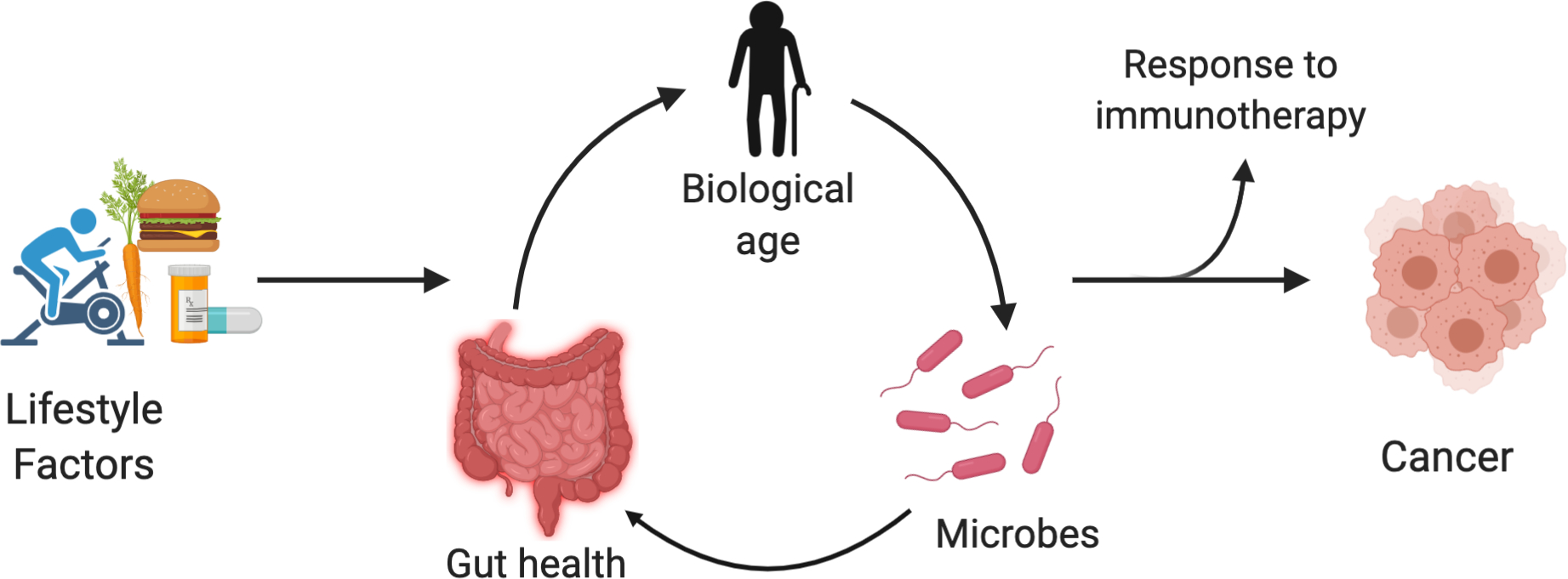
Lifestyle factors (e.g. diet, exercise, medications) affect the gut microbiota and particularly the fraction of Proteobacteria. This enters a cycle by which the microbes affect gut leakiness and systemic inflammation and thereby a variety of age-related illnesses, which then also affect the microbiome. Related diseases include cancer and particularly treatments that involve the immune system. Created with BioRender.com
A consistent feature across longitudinal studies of the microbiomes of older adults is higher intra-individual variability in older adults relative to younger. That is, the strains of microbes shift rapidly over time; in the context of common clustering approaches (e.g. principle components analysis) which demonstrates larger distances between points. The ELDERMET Study proposed diet to be the causal driver; individuals living in the community tended to have microbiomes more like healthy young controls, whereas individuals living in long-term care facilities showed reduced diversity and higher variability. Long term care was associated with lower-fiber diets, and the change in diet preceded a shift in the microbiome by roughly one year.
Regardless of the cause, the shift in microbiomes with age is a pressing concern when considering that age is a dominant risk factor for cancer and the microbiome plays a role in whether individuals will respond to ICIs. In many cancers ICIs are, or are predicted to soon be, the first-line treatment, making the link between the aging microbiome and ICI response a more pressing issue for more patients.
The microbiome and response to immune checkpoint inhibitors
Several recent papers suggested a critical role for the microbiome in response to ICIs. The first indications included retrospective analyses of patients who received microbiome-disrupting medications before the start of ICI treatment or shortly after1,10,11. Patients who received antibiotics showed shorter overall survival across many cancers when controlling for covariates that might represent differences between the retrospective cohorts, including the Charlson Comorbidity Index 10,12. Prospective studies and systematic reviews have validated these findings 13.
Direct measurements of the microbiome in patients receiving ICIs confirmed this epidemiological observation. Several groups demonstrated that the microbiomes of patients at the start of ICI treatment are distinct between patients who respond (R) and do not respond (NR)1,14,15. Moreover, the R phenotype could be transferred to mouse models using the patients’ stool1. Mice inoculated with a sarcoma cell line and treated with ICIs showed reduced tumor size when gavaged with R stool relative to NR stool. This suggests that the microbiome may be a biomarker for predicting response to ICIs.
In addition to a biomarker, the microbiome may be a therapeutic target. In preclinical studies, NR mice could be switched to an R state by supplementation with a single microbe that was enriched in the R stool: A. muciniphila. Similar findings have been reported when another microbe, an unnamed strain in the genus Ruminococcus, is enriched by feeding mice a pre-biotic16. Later work showed a similar increase in response to ICIs by giving a community of 11 microbes, lacking A. muciniphila but containing an unnamed strain in the Ruminococcus family17. Finally, response to ICIs was increased by mono-colonization with a strain of Bifidobacterium, and by a molecule produced by the microbe, inosine. This demonstrates that response to ICIs could be modified by enrichment of one or a few microbes, and possibly by supplementation with small molecules such as inosine.
A consensus set of organisms that are most important, and for whom, has not been defined. A. muciniphila associated with R-patients in only one study1. Matson et al found enrichment of Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium 14. Gopalakrishnan et al found that response correlated with higher alpha diversity and bacteria in the Ruminococcaceae family (which does not contain A. muciniphlia, nor any of the microbes found by Matson et al) 15. Chaput et al found enrichment of Faecalibacterium prausnitzii and Gemmiger formicilis 18. Potential sources of this variation could include geographic differences in the microbiomes of patients, and convergent evolution in terms of ecological roles of the microbes or the molecules they produce, or age differences in the cohorts of each study.
There’s a clear role for the microbiome in ICI response, leading to great hope for using it as a therapeutic target. However, more work is needed to define the microbes associated with the R and NR states and especially how best to modify them. It is prudent to use knowledge about healthy microbiomes to estimate which populations are likely to require microbiome modification.
Relating the microbes associated with response to ICIs and age
Many of the microbes that have been shown to change with age have been implicated in response to ICIs. Three of the microbes that have been shown to improve response to ICIs in preclinical models (Akkermansia16, Bifidobacterium19, Ruminococcus16), are depleted in older adults (Figure 2). In addition, several microbes enriched in non-responders to ICIs are also enriched in older adults. This includes the Proteobacteria like Escherichia, but also several members of the Bacteroidetes phylum. A single genus enriched in NR have been associated with younger adults (Figure 2).
Figure 2. The gut microbes associated with aging and response to immune checkpoint inhibitors.
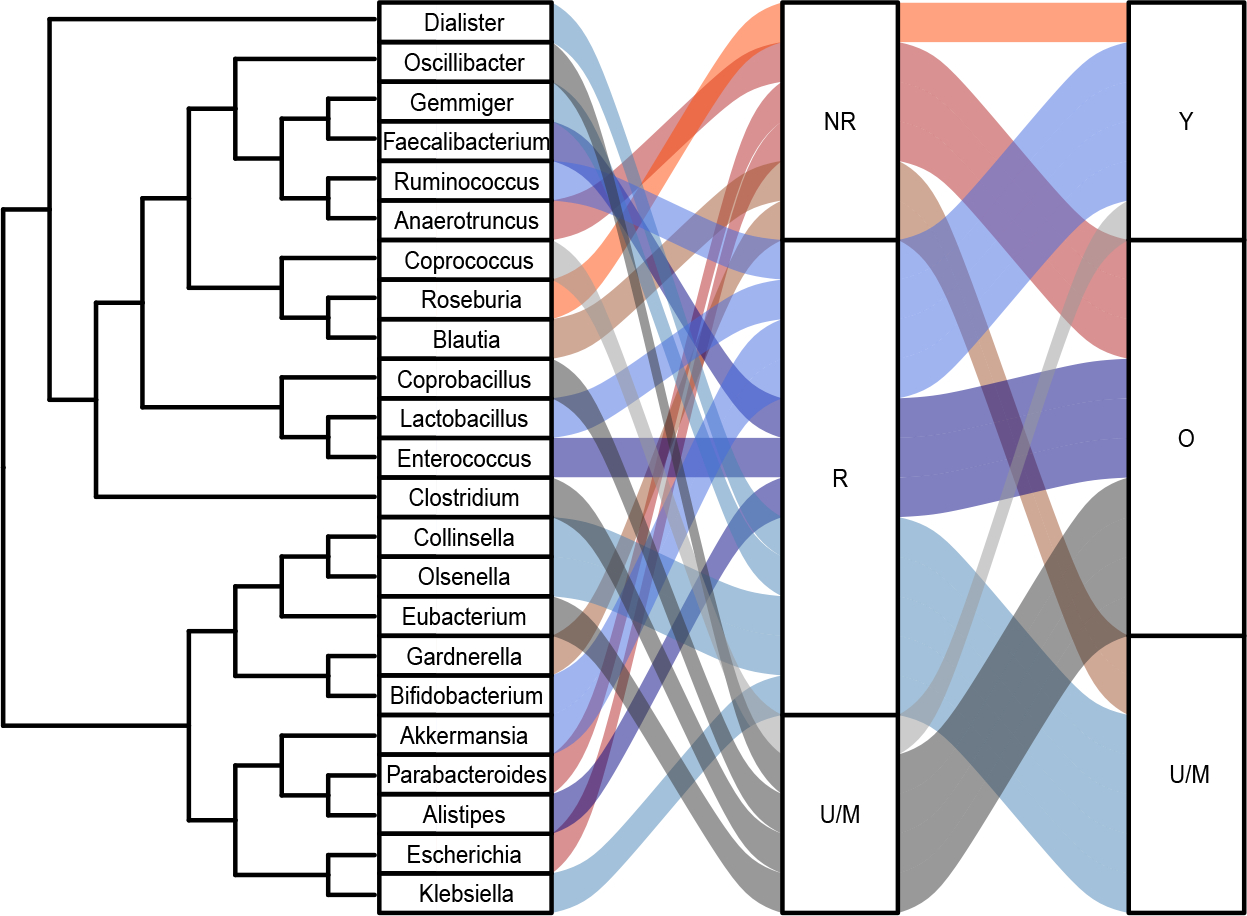
NR = non-responders, R = responders, U/M = unknown/mixed results, Y = young, O = old.
However, the current picture is somewhat mixed. Several genera associated with response have been shown to be enriched older adults (Faecalibacterium, Enterococcus, Alistipes) (Figure 2). Faecalibacterium, in particular, has been broadly associated with gut health and is marketed as a probiotic. While these microbes have not shown a causal relationship with response, such as for A. muciniphila described above, the possiblity remains that they will do so, perhaps in a way that is specific to older-adults Finally, the largest fraction of the genera associated with treatment response have either unknown or mixed associations with age, further highlighting the need for more study.
Underrepresentation of older adults in cancer and microbiome studies
The median age of a patient diagnosed with lung cancer is 70 years, and that statistic continues to rise. 20,21 As overall tolerance for ICIs is generally better than for chemotherapy 12, the risk-benefit balance of ICIs may be especially profitable in older patients. However, patients enrolled in clinical trials generally tend to be younger than those treated in clinical practice 13, possibly due to selection criteria that excludes based on performance status or the presence of comorbidities. Over the past two decades, <10% of older adults age 75+ years are included in cancer clinical trials and this value has remained static.22,23 The median age of a cancer diagnosis is higher than the median age of studies reporting on the association between the microbiome and response to ICIs and of trials that seek to modify the microbiome to improve cancer outcomes. As of August 2020, we found 25 trials that expressly intended to modify the microbiome to affect cancer outcomes. Four of these (16%) chose an age rage to focus on older adults (Table 1).
Table 1.
Clinical trials that aim to modify cancer outcomes via the microbiome
| NCT# | Age Range | Description | Location | Lead Investigator | Status | Time frame |
|---|---|---|---|---|---|---|
|
| ||||||
| NCT04267874 | 55 –77 A, OA | Black raspberry diet intervention for MB modification and LC prevention. | OSUCCC | D. Spakowicz | R | 10/19–12/22 |
| NCT04229381 | 60+ A, OA | Physical therapy and stress intervention to improve resiliency in LC patients. | OSUCCC | C. Presley | R | 1/20–12/21 |
| NCT02791737 | 60+ A, OA | Exercise intervention to improve physical activity in cancer patients. | OSUCCC | A. Rosko | N | 7/16–12/20 |
| NCT03686202 | 18+ A, OA | Assess efficacy of microbial ecosystem therapeutics in altering IO response. | Princess Margaret CC | L. Siu A. Spreafico | N | 11/18–12/23 |
| NCT03772899 | 19+ A, OA | Assess combination FMT and IO can enhance antitumor effects in melanoma. | LRCP | J. Lenehan | R | 3/19–12/23 |
| NCT03817125 | 18+ A, OA | Assess safety and tolerability of oral MB intervention in combination with PD-1 inhibitors in melanoma patients. | Angeles Clinic & Research Institute (& others) | R. Ibrahim (& others) | R | 1/19–2/22 |
| NCT04163289 | 18+ A, OA | Assess safety of FMT combination treatment in reducing the occurrence of immune-related toxicities. | LRCP | R. Fernandes S. Maleki | R | 1/20–11/28 |
| NCT04056026 | Y, A, OA | Enhance the MB via FMT to improve the efficacy PD-1 inhibitors. | ProgenaBiome | Progena Biome | C | 9/18–12/18 |
| NCT04130763 | 18–70 A, OA | Assess if FMT capsules improve anti-PD-1 response. | Beijing Cancer Hospital | L. Shen | R | 12/19–10/20 |
| NCT04116775 | 18+ A, OA | Assess effect of FMT from responders to PD-1 inhibitors into non-responders in PCA patients. | VA Portland Health Care System | J. N Graff | R | 10/19–10/23 |
| NCT03819296 | 18+ A, OA | Assess role of MB and FMT on medication colitis in cancer patients. | MD Anderson CC | Y. Wang | X | 2/20–7/22 |
| NCT03353402 | 18+ A, OA | Assess effect of FMT from responders to PD-1 inhibitors into non-responders in melanoma patients. | Sheba MC | G. Markel | R | 11/17–12/21 |
| NCT03341143 | 18+ A, OA | Assess if FMT improves the body’s ability to fight melanoma. | UPMC Hillman CC | D. Davar | S | 1/18–10/20 |
| NCT02843425 | 30+ A, OA | Assess if beans can increase healthy bacteria and reduce the effects of obesity on cancer risk. | MD Anderson CC | C. Daniel-MacDougall | N | 7/16–7/25 |
| NCT01929122 | 18+ A, OA | Assess effects of bean powder or rice bran on the MB and metabolome of CRC survivors and healthy adults. | Colorado State Poudre Valley Hospital | E. P Ryan | C | 8/10–12/14 |
| NCT04079270 | 18+ A, OA | Assess effect of diet intervention on breast cancer outcomes and biomarkers. | Sheba MC | E. Gal-Yam | R | 7/19–12/25 |
| NCT03782428 | 18+ A, OA | Assess the role of probiotics in reducing CRC related inflammatory markers. | National University of Malaysia | R. Affendi R. Ali | C | 8/16–11/18 |
| NCT03661047 | 18+ A, OA | Assess effects of omega-3 oil on tumor immune microenvironment in CCR. | Massachusetts General Hospital | M. Song | R | 11/19–9/23 |
| NCT03781778 | 18+ A, OA | Assess effect of resistant starch on inflammation and MB in CCR survivors. | Fred Hutch/UW Cancer Consortium | M. Neuhouser | S | 5/19–9/20 |
| NCT03448003 | 18 + A, OA | Assess if comprehensive lifestyle changes can prevent breast cancer. | MD Anderson CC | L. Cohen | R | 4/19–9/22 |
| NCT03358511 | 18 + A, OA | Assess effect of probiotics on breast cancer immune response. | Mayo Clinic | S. Chumsri | C | 10/17–5/20 |
| NCT03028831 | 40–65 A, OA | Fiber based intervention of the typical native Alaskan diet for MB modification and CRC reduction. | AK Native Tribal Health Consortium Pitt | G. Riscuta | R | 12/17–1/22 |
| NCT03290651 | Y, A, OA | Probiotic intervention for displacement of cancer related inflammatory bacteria. | St. Joseph’s Health Care | G. Reid M. Brackstone | R | 7/19–12/21 |
| NCT03853928 | 18 + A, OA | Assess if probiotic intervention in patients with cirrhosis alters incidence of HCC. | Austral University (sponsor) | F. Piñero | X | 5/19–5/23 |
| NCT03268655 | 50–75 A, OA | Assess if ginger can create an anti-inflammatory, CRC-protective MB | Mayo Clinic CC (and others) | A. Prizment | C | 11/18–6/20 |
Abbreviations: OSUCCC = Ohio State University Comprehensive Cancer Center; CC = Cancer center; MC = Medical center; LRCP = London Regional Cancer Program; A = Adult; OA = Older adult; Y = Child; R = Recruiting; S = Suspended; N = Active not recruiting; C = Completed; X = Not yet recruiting; FMT = Fecal microbiota transplantation; IO = Immuno-oncology or immunotherapy; CRC = Colorectal cancer; HCC = Hepatocellular carcinoma; PCA = Prostate cancer; MB = Microbiome; LC = Lung cancer
There are several methods proposed to modify the microbiome including probiotic supplementation (24%), fecal microbiota transplantation (FMT) (32%) and interventions for the diet (32%) and lifestyle (12%). Each has potential benefits and pitfalls with regards to their safety, suspected efficacy, and speed of modification. FMTs have the strongest track record through successful clinical trials in the context of treatment for recurrent Clostridiodes difficile infections. However, they are challenged by demonstrating donor material is safe; on June 15, 2019, the FDA issued a safety alert requiring additional testing for clinical trials using FMT following a patient death24. Probiotics hold promise as most closely mirroring the experiments in which murine models were made to start responding to ICIs. However, probiotic supplementation has recently been shown to decrease gut diversity which has had negative effects on health such as increasing recovery time after antibiotic treatment25,26. Studies on response to ICIs found that the diversity of the gut microbiome, in addition to particular microbes such as A. muciniphila, was important for response15, though more recently a small consortium or even mono-colonization with Bifidobacterium was shown to modify response in murine models17,19. Further study is needed to determine if probiotic supplementation can improve response or decreases diversity in a way that is detrimental to cancer outcomes. Diet-based interventions have may also modify response through enriching for certain microbes, though this has not yet been demonstrated in humans16. Rational manipulation of the microbiome with diet has been complicated, with the same foods eliciting different responses in the microbiome, presumably based on the starting condition of the microbiome. Other longitudinal studies with dietary interventions have shown relatively minor changes, where individuals’ microbiomes clustered more closely with themselves at other time points than other individuals. Which method, or combination of methods, will effectively change a person’s microbiome to promote response to ICIs at a clinically relevant timescale may be highly individualized.
Conclusion
The microbiome is a promising way to monitor and modify the state of the immune system. Applying this to older adults is complicated by many factors, including age-related changes to the microbiome. Studies focused on older adults are needed to tailor interventions to this large and rapidly-growing demographic with cancer.
Supplementary Material
Table S1. Enrichment of microbes in young and old, and responders and non-responders to ICIs with references for each determination.
ACKNOWLEDGEMENTS
This work was supported by Young Investigator Award in Immuno-Oncology (LCFA-BMS/ILC Foundation) and Pelotonia Young Investigator Award to DS. This work was supported by a Ohio State University Comprehensive Cancer Center Pelotonia Young Investigator Award (D.S.), a Lung Cancer Foundation of America & Bristol-Meyers Squibb Foundation & International Lung Cancer Foundation Award in Immuno-Oncology (D.S.), the National Institute of Aging (C.J.P., R03AG064374), and The Ohio State University K12 Training Grant for Clinical Faculty Investigators (C.J.P., K12CA133250).
DS+CP conceived of the study, MM+AB+NW+DS collected the data, RH+AB+MM generated the figures and tables, RH created the code repository, DS drafted the manuscript, all authors reviewed and approved the manuscript.
ABBREVIATIONS
- ICI
Immune Checkpoint Inhibitor
- R
responder to immune checkpoint inhibitor
- NR
non-responder to immune checkpoint inhibitor
- FMT
Fecal microbiota transplantation
Footnotes
DISCLOSURE DECLARATION
The authors declare no conflicts of interest.
REPRODUCIBILITY STATEMENT
Code to generate Figure 2 from Supplementary Table 1 are available at github.io/spakowiczlab/mageio.
REFERENCES
- 1.Routy B et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Mueller S et al. Differences in Fecal Microbiota in Different European Study Populations in Relation to Age, Gender, and Country: a Cross-Sectional Study. Appl. Environ. Microbiol. 72, 1027–1033 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claesson MJ et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. PNAS 108, 4586–4591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claesson MJ et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Lawson MAE et al. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. The ISME Journal 14, 635–648 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schell MA et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc Natl Acad Sci U S A 99, 14422–14427 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favier CF, Vaughan EE, De Vos WM & Akkermans ADL Molecular Monitoring of Succession of Bacterial Communities in Human Neonates. Appl Environ Microbiol 68, 219–226 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmsen HJM et al. Analysis of Intestinal Flora Development in Breast-Fed and Formula-Fed Infants by Using Molecular Identification and Detection Methods. Journal of Pediatric Gastroenterology and Nutrition 30, 61–67 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Shin N-R, Whon TW & Bae J-W Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends in Biotechnology 33, 496–503 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Spakowicz D et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: causal modeling, timing, and classes of concomitant medications. BMC Cancer 20, 383 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson BE, Routy B, Nagrial A & Chin VT The effect of antibiotics on clinical outcomes in immune-checkpoint blockade: a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother 69, 343–354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyd Rebecca, Muniak Mitchell & Spakowicz Dan. spakowiczlab/co-med-io: BMC Cancer & zenodo. (Zenodo, 2019). doi: 10.5281/zenodo.3530835. [DOI] [Google Scholar]
- 13.Pinato DJ et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol 5, 1774–1778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matson V et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopalakrishnan V et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messaoudene M et al. Abstract 5730: A new polyphenol prebiotic isolated from Myrciaria dubia improves gut microbiota composition and increases anti-PD-1 efficacy in murine cancer models. Cancer Res 80, 5730–5730 (2020). [Google Scholar]
- 17.Tanoue T et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Chaput N et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 28, 1368–1379 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Mager LF et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD & Jemal A Cancer statistics, 2019. CA Cancer J Clin 69, 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Smith BD, Smith GL, Hurria A, Hortobagyi GN & Buchholz TA Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. JCO 27, 2758–2765 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Liu J et al. Strategies to Improve Participation of Older Adults in Cancer Research. J Clin Med 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh H, Beaver JA, Kim G & Pazdur R Enrollment of older adults on oncology trials: An FDA perspective. J Geriatr Oncol 8, 149–150 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Commissioner O of the. FDA In Brief: FDA warns about potential risk of serious infections caused by multi-drug resistant organisms related to the investigational use of Fecal Microbiota for Transplantation. FDA (2019). [Google Scholar]
- 25.Suez J et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 174, 1406–1423.e16 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Zmora N et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 174, 1388–1405.e21 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Enrichment of microbes in young and old, and responders and non-responders to ICIs with references for each determination.


