Abstract
Prioritization of highly sensitized (HS) candidates under the kidney allocation system (KAS) and growth of large, multicenter kidney-paired donation (KPD) clearinghouses have broadened the transplant modalities available to HS candidates. To quantify temporal trends in utilization of these modalities, we used SRTR data from 2009 to 2017 to study 39 907 adult HS (cPRA ≥ 80%) waitlisted candidates and 19 003 recipients. We used competing risks regression to quantify temporal trends in likelihood of DDKT, KPD, and non-KPD LDKT for HS candidates (Era 1: January 1, 2009-December 31, 2011; Era 2: January 1, 2012-December 3, 2014; Era 3: December 4, 2014-December 31, 2017). Although the likelihood of DDKT and KPD increased over time for all HS candidates (adjusted subhazard ratio [aSHR] Era 3 vs 1 for DDKT: 1.741.851.97, P < .001 and for KPD: 1.702.202.84, P < .001), the likelihood of non-KPD LDKT decreased (aSHR: 0.690.820.97, P = .02). However, these changes affected HS recipients differently based on cPRA. Among recipients, more cPRA 98%−99.9% and 99.9%+ recipients underwent DDKT (96.2% in Era 3% vs 59.1% in Era 1 for cPRA 99.9%+), whereas fewer underwent non-KPD LDKT (1.9% vs 30.9%) or KPD (2.0% vs 10.0%). Although KAS increased DDKT likelihood for the most HS candidates, it also decreased the use of non-KPD LDKT to transplant cPRA 98%+ candidates.
Keywords: clinical research/practice, donors and donation: deceased, donors and donation: paired exchange, health services and outcomes research, kidney transplantation/nephrology, panel reactive antibody (PRA), registry/registry analysis
1 |. INTRODUCTION
Highly sensitized (HS) kidney transplant candidates have historically faced substantial difficulty achieving transplantation, with a much lower likelihood of deceased donor kidney transplantation (DDKT) and kidney-paired donation (KPD) compared to non-HS candidates.1–5 However, recent changes to the deceased donor allocation system, and the growth of large single- and multicenter KPD clearinghouses, have broadened the different transplant modalities available to HS candidates.6,7 The kidney allocation system (KAS), introduced in 2014, instituted a sliding scale system that gives increasing priority for DDKT candidates with higher levels of sensitization.8,9 Under KAS, candidates with a calculated panel reactive antibody (cPRA) ≥98% have seen an increase in DDKT rates (from a 1.77-fold increase for cPRA 98% candidates to an 11.58-fold increase for cPRA 99.9%+ candidates).10 Concomitantly, KPD use has increased over time. In a study of the largest KPD clearinghouse in the United States, the number of KPD transplants performed annually increased from 21 in 2006 to 399 in 2016.7
However, these studies focused on a single modality (either DDKT or KPD) and do not address the clinical reality that the modality chosen for a HS candidate depends on the relative likelihood of all available transplant options—DDKT, KPD, or non-KPD living donor kidney transplantation (LDKT). No studies have compared the joint effect of changes in the likelihood of all transplant modalities now available to the HS. Additionally, it is possible that the relative likelihood of each transplant modality varies across cPRA and that the net impact of these different likelihoods resulted in different patterns of modality usage across cPRA.10,11 For example, the substantial increase in DDKT rates for cPRA ≥ 98% candidates under KAS might have led to disproportionately more DDKT use, despite an overall increase in the use of KPD in this group. Conversely, decreased DDKT priority for cPRA 80%−89% candidates might have led to a substantial increase in KPD or non-KPD LDKT use in this group.10 Characterization of how these changes have acted together would allow for a better assessment of how DDKT policy change and clinical expansion of KPD have improved the ability of HS candidates to undergo transplant in the broader context of all available transplant modalities.
To better understand changes in how HS patients have been treated and transplanted over the last decade, we used national registry data to study utilization of different transplant modalities over time and how this varied across cPRA. The goals of our study were (1) to quantify temporal trends in likelihood of DDKT, KPD, and non-KPD LDKT for HS candidates; and (2) to understand how these changes affected the modalities ultimately used by HS recipients.
2 |. METHODS
2.1 |. Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.12 The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
2.2 |. Study population
We included all adult (age ≥18 years) HS (cPRA ≥ 80%) kidney-only waitlist candidates and transplant recipients between January 1, 2009 and December 31, 2017. The unit of analysis was the candidate, and only the first high-cPRA registration per candidate was included. This study was approved by the Johns Hopkins University Institutional Review Board.
2.3 |. Time periods
We categorized candidates by date of listing, and recipients by date of transplant, into 3 eras (Era 1: January 1, 2009-December 31, 2011, Era 2: January 1, 2012-December 3, 2014, and Era 3: December 4, 2014-December 31, 2017). We divided Era 2 and 3 on December 4, 2014 to reflect KAS implementation. These time periods were selected to best represent hypothesized changes over time in the use of both DDKT and KPD and not just one single modality. In order to understand how changes in transplant modality usage changed over time, we chose the earliest era (Era 1) as our reference era. As a sensitivity analysis, we divided Era 3 into two separate 18-month periods to determine whether a “bolus effect” was influencing trends in this era. Results of this were consistent with our main analysis.
2.4 |. cPRA ascertainment and categorization
cPRA values for candidates and recipients were obtained from the SRTR cPRA history dataset, which contains time-varying measurements of cPRA for every DDKT waitlist candidate. Some recipients underwent KPD or non-KPD LDKT directly and were never placed on the waitlist for DDKT and thus did not have a cPRA recorded in the cPRA history dataset. For these recipients, we obtained their cPRA from the candidate peak cPRA composite variable, which contains the peak cPRA recorded from one of several additional cPRA variables (eg, from the recipient histocompatibility form). We excluded recipients who did not have any cPRA recorded in any variable (n = 366, 0.09%).
We divided our study population into the following cPRA categories: 80%−89%, 90%−97%, 98%−99.9%, and 99.9%+. These categories were selected to best balance anticipated differences in likelihood of both DDKT and KPD across cPRA based on our prior work, while still maintaining sufficient group sizes for well-powered comparisons.10,11 As a sensitivity analysis, we also explored the following alternative cPRA categorization: 80%−89%, 90%−97%, 97.5%−98.49%, 98.5%−99.49%, and 99.5%+.
2.5 |. Temporal trends in transplant likelihood for HS candidates
In order to understand how the likelihood of each transplant modality (DDKT, KPD, and non-KPD LDKT) changed over time for transplant candidates, we used competing risks regression with the method of Fine and Gray.13 The use of KPD was distinguished from non-KPD LDKT using the living donor relationship field as reported to the OPTN, and we considered KPD transplants to be those coded as “nonbiological, unrelated: paired donation” or “non-biological, unrelated: nondirected donation.” For each modality, and across cPRA groups, we constructed a separate model treating death or waitlist removal due to deteriorating medical condition, the remaining two modalities, and waitlist removal for any other reason as competing events (eg, if DDKT was the outcome, then KPD, non-KPD LDKT, death/waitlist removal due to deteriorating medical condition, and waitlist removal were all treated as competing events). Candidates entered the risk set on the day of listing if the candidate had a cPRA ≥ 80%, whereas candidates who entered the waitlist but had an initial cPRA < 80% were treated as late entries when their cPRA reached ≥80%. Candidates in the risk set whose cPRA dropped below 80% were censored, but their waitlist time up until that point was included in the analysis. Candidates remaining on the waitlist at era changes were censored and were otherwise followed until 3 years, or until administrative censorship on February 28, 2019. Analyses stratified by cPRA were adjusted for only candidate age, sex, and race due to a small number of events in certain subgroups.
2.6 |. Temporal trends in transplant modality used for HS recipients
To understand how the differing likelihood of each transplant modality for HS transplant candidates affected which transplant modalities were used by recipients, we compared the proportion of HS recipients who received each modality (DDKT, KPD, non-KPD) across cPRA groups and time eras using the chi-square test.
2.7 |. Use of non-KPD LDKT for HS recipients
In our initial analysis, we found that a significantly lower proportion of cPRA 98%+ recipients used non-KPD LDKT in Era 3 compared to Era 1. In order to understand whether this was due to a direct decrease in the transplant type (eg, non-KPD LDKT vs any other) used for HS recipients, we used logistic regression to predict the number of HS candidates expected to undergo non-KPD LDKT in each era. Our outcome was a binary variable for whether the candidate underwent non-KPD LDKT. We controlled for candidate age, sex, race, blood type, cause of end-stage renal disease (ESRD), previous transplant, time on dialysis, and waitlist time to reach cPRA ≥ 80%. The prediction model for receiving non-KPD LDKT was derived using candidates from Era 1. To obtain the cumulative number of expected events in each era, and for each cPRA group, we summed the individual predicted probabilities of non-KPD LDKT for each recipient in that era. We then divided the observed number of patients who actually received non-KPD LDKT by the cumulative number of expected events to yield an observed vs expected (O:E) ratio for each era/cPRA group. We calculated the 95% confidence intervals for the O:E ratio using the method outlined in the SRTR Technical Methods for the Program-Specific Report (https://www.srtr.org/about-the-data/technical-methods-for-the-program-specific-reports). Effectively, this model allowed us to distinguish between a direct decrease in the usage of non-KPD LDKT over time (O:E ratio <1), or an increase in the number of candidates undergoing DDKT (or KPD) who might not have otherwise been transplanted (O:E ratio ≥1).
2.8 |. Statistical analysis
Confidence intervals are reported as per the method of Louis and Zeger.14 All analyses were performed using Stata 16.0/IC for Windows (Stata Corp., College Station, TX).
3 |. RESULTS
3.1 |. Study population
3.1.1 |. HS candidates
We identified 39 907 HS candidates, of whom 14 595 (36.6%) were listed in Era 1, 13 487 (33.8%) were listed in Era 2, and 11 825 (29.6%) were listed in Era 3. Compared to candidates in Era 1, candidates in Era 3 were older (49.7 years vs 48.4 years, P < .001), less likely to be white (37.5% vs 41.2%, P < .001), more likely to have ESRD caused by glomerular disease (27.8% vs 28.4%, P < .001), more likely to have a higher median cPRA (94.0% vs 92.0%, P < .001), and had a higher estimated posttransplant survival score (49.7 vs 49.5, P < .001). Candidates in Era 3 spent less time on dialysis (1.8 years vs 2.5 years, P < .001) and were less likely to have had a prior transplant (44.0% vs 48.3%, P < .001) (Table 1).
TABLE 1.
Characteristics of highly sensitized candidates, by era of listing
| Characteristic | Era 1 (n = 14 595) | Era 2 (n = 13 487) | Era 3 (n = 11 825) | P value |
|---|---|---|---|---|
| Age (y), mean (SD) | 48.4 (13.0) | 49.4 (13.0) | 49.7 (12.9) | <.001 |
| Female sex, % | 36.3 | 35.8 | 34.0 | <.001 |
| Race, % | ||||
| White | 41.2 | 38.6 | 37.5 | <.001 |
| Black | 36.3 | 36.4 | 36.1 | |
| Other | 22.4 | 25 | 26.4 | |
| Cause of ESRD, % | ||||
| Glomerular diseases | 27.8 | 28.1 | 28.4 | <.001 |
| Diabetes | 23.5 | 25.3 | 26.6 | |
| Hypertension | 24 | 22.7 | 20.9 | |
| Polycystic kidney disease | 6.5 | 6.9 | 7.1 | |
| Other | 18.2 | 17.0 | 17.0 | |
| Blood type, % | ||||
| O | 49.4 | 48.7 | 48.7 | .41 |
| A | 32.1 | 32.2 | 32.7 | |
| B | 14.8 | 15.5 | 14.6 | |
| AB | 3.7 | 3.7 | 3.9 | |
| cPRA (%), median (IQR) | 92.0 (86.0, 98.0) | 93.0 (86.0, 99.0) | 94.0 (86.0, 99.0) | <.001 |
| Estimated posttransplant survival score, mean (SD) | 49.5 (28.8) | 50.9 (29.0) | 49.7 (28.8) | <.001 |
| Years on dialysis, median (IQR) | 2.5 (0.9, 5.2) | 2.2 (0.7, 4.9) | 1.8 (0.4, 4.2) | <.001 |
| History of prior transplant | 48.3 | 47.0 | 44.0 | <.001 |
| Prior living donor transplant, % of those with a prior transplant | 36.2 | 36.2 | 39.1 | |
Abbreviations: cPRA, calculated panel reactive antibody; ESRD, end-stage renal disease; IQR, interquartile range; SD, standard deviation.
3.1.2 |. HS recipients
We identified 19 003 HS recipients, of whom 5098 (26.8%) were transplanted in Era 1, 5531 (29.1%) were transplanted in Era 2, and 8374 (44.1%) were transplanted in Era 3. Compared to recipients in Era 1, recipients in Era 3 were more likely to be older (49.3 years vs 48.6 years, P = .02), less likely to be white (41.3% vs 48.5%, P < .001), more likely to be blood type O (51.1% vs 48.5%, P = .002), more likely to be cPRA 100% (13.1% vs 2.8%, P < .001), more likely to have spent longer on dialysis (median 3.9 years vs 3.5 years, P < .001) and more likely to have had a prior transplant (52.6% vs 46.9%, P < .001) (Table 2).
TABLE 2.
Characteristics of highly sensitized recipients, by era of transplant
| Characteristic | Era 1 (n = 5098) | Era 2 (n = 5331) | Era 3 (n = 8374) | P value |
|---|---|---|---|---|
| Recipient | ||||
| Age (y), mean (SD) | 48.6 (13.0) | 49.2 (13.1) | 49.3 (12.8) | .02 |
| Female sex, % | 65.2 | 63.7 | 64.6 | .3 |
| Race, % | ||||
| White | 48.5 | 47.6 | 41.3 | <.001 |
| Black | 31.0 | 29.7 | 31.6 | |
| Other | 20.5 | 22.8 | 27.1 | |
| Cause of ESRD, % | ||||
| Glomerular diseases | 32.3 | 32.7 | 33.5 | .5 |
| Diabetes | 17.6 | 17.9 | 18.0 | |
| Hypertension | 21.2 | 20.7 | 21.2 | |
| Polycystic kidney disease | 8.1 | 8.5 | 8.0 | |
| Other | 20.8 | 20.3 | 19.3 | |
| Blood type, % | ||||
| O | 48.5 | 47.7 | 51.1 | .002 |
| A | 36.6 | 36.7 | 33.9 | |
| B | 11.1 | 11.4 | 11.2 | |
| AB | 3.8 | 4.2 | 3.9 | |
| cPRA (%) | ||||
| 80–89 | 41.0 | 39.1 | 17.6 | <.001 |
| 90–97 | 37.5 | 38.3 | 25.7 | |
| 98–99.9 | 18.7 | 19.6 | 43.6 | |
| 99.9%+ | 2.8 | 3.1 | 13.1 | |
| DDKT sharing, % | ||||
| Local | 61.6 | 64.3 | 39.0 | <.001 |
| Regional | 6.2 | 6.7 | 17.7 | |
| National | 32.2 | 29.0 | 43.3 | |
| Years on dialysis, median (IQR) | 3.5 (1.5, 6.5) | 3.6 (1.4, 6.7) | 3.9 (1.5, 7.3) | <.001 |
| History of a prior transplant, % | 46.9 | 50.0 | 52.6 | <.001 |
| Prior living donor transplant, % of those with a prior transplant | 39.0 | 42.3 | 41.3 |
Abbreviations: cPRA; calculated panel reactive antibody; DDKT, deceased donor kidney transplant; ESRD, end-stage renal disease; IQR, interquartile range; SD, standard deviation.
3.2 |. Temporal trends in transplant likelihood for HS candidates
3.2.1 |. Overall temporal trends
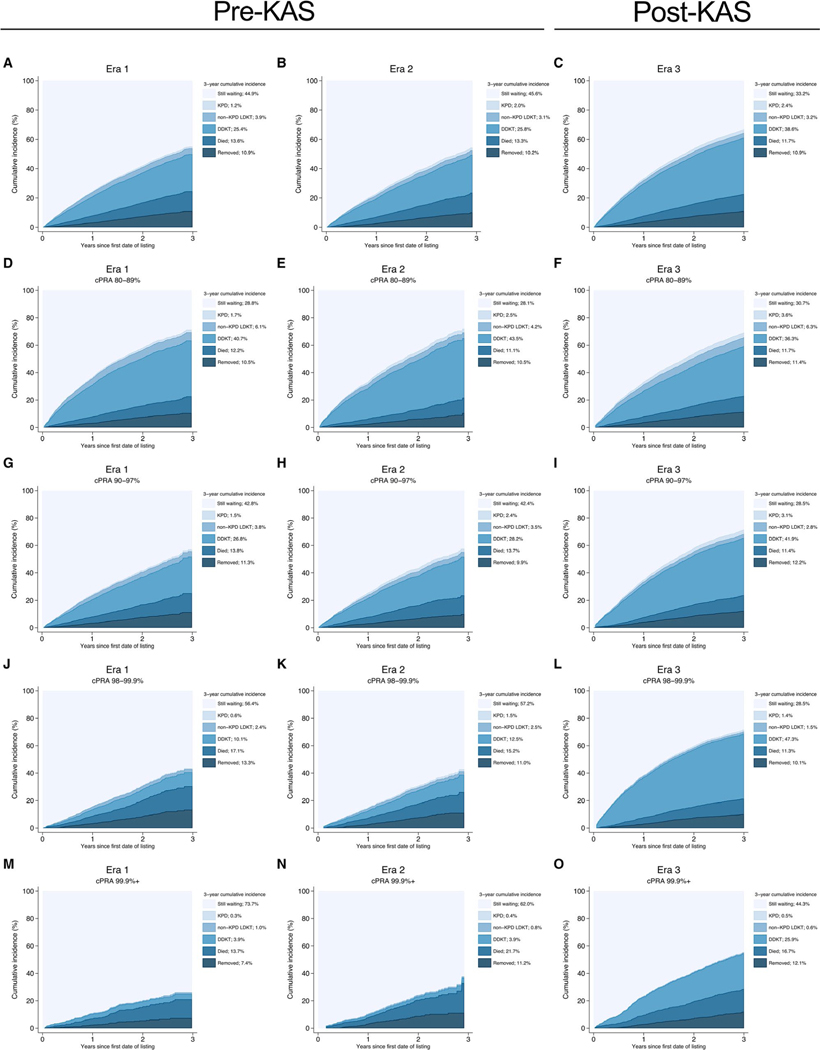
Compared to candidates in Era 1, candidates in Era 3 were more likely to receive DDKT (adjusted subhazard ratio [aSHR]: 1.741.851.97, P < .001). Although there were no statistically significant changes in the likelihood of receiving any LDKT across eras (aSHR for Era 2 vs 1:0.861.001.16, P = 1.0; for Era 3 vs 1:0.991.131.30, P = .08) (Table 3), there were differences between types of LDKT. Candidates in Era 2 and 3 were more likely to receive KPD (aSHR for Era 2 vs 1:1.311.732.29, P < .001; for Era 3 vs 1:1.702.202.84, P < .001), but candidates in Era 2 and 3 were less likely to receive non-KPD LDKT compared to candidates in Era 1 (aSHR for Era 2 vs 1:0.650.780.94, P = .008; for Era 3 vs 1:0.690.820.97, P = .02). These changes led to a 3-year cumulative incidence of 38.6% for DDKT in Era 3 (vs 25.4% in Era 1), 2.4% for KPD (vs 1.2%), and 3.2% for non-KPD LDKT (vs 3.9%) (Figure 1A–C).
TABLE 3.
Likelihood of different transplant modalities among highly sensitized candidates by era and cPRA category
| aSHR | |||
|---|---|---|---|
|
|
|||
| Era 2 vs Era 11 | Era 3 vs Era 11 | Era 3 vs Era 21 | |
| Overall | |||
| DDKT | 0.971.041.12 | 1.74 1.85 1.97 | 1.67 1.77 1.89 |
| LDKT | 0.861.001.16 | 0.991.131.30 | 0.991.131.30 |
| Non-KPD | 0.65 0.78 0.94 | 0.69 0.82 0.97 | 0.871.041.25 |
| KPD | 1.31 1.73 2.29 | 1.70 2.20 2.84 | 1.02 1.27 1.57 |
| cPRA | |||
| 80%-89% | |||
| DDKT | 0.941.031.15 | 0.76 0.84 0.92 | 0.73 0.81 0.89 |
| LDKT | 0.710.891.11 | 1.05 1.27 1.53 | 1.16 1.43 1.76 |
| Non-KPD | 0.54 0.70 0.92 | 0.831.041.31 | 1.14 1.49 1.93 |
| KPD | 1.02 1.53 2.29 | 1.41 2.03 2.91 | 0.951.331.85 |
| 90%-97% | |||
| DDKT | 0.951.071.22 | 1.75 1.95 2.17 | 1.64 1.82 2.02 |
| LDKT | 0.921.181.52 | 0.981.241.58 | 0.841.051.32 |
| Non-KPD | 0.670.921.25 | 0.580.781.07 | 0.620.851.17 |
| KPD | 1.25 1.98 3.13 | 1.71 2.62 4.00 | 0.941.321.86 |
| 98%-99.9% | |||
| DDKT | 0.961.201.49 | 5.57 6.62 7.85 | 4.71 5.52 6.48 |
| LDKT | 0.801.151.65 | 0.630.911.31 | 0.560.791.13 |
| Non-KPD | 0.590.901.39 | 0.37 0.58 0.92 | 0.400.641.03 |
| KPD | 1.05 2.21 4.66 | 1.14 2.32 4.73 | 0.611.051.81 |
| 99.9%+ | |||
| DDKT | 0.380.701.31 | 4.23 6.49 9.96 | 5.73 9.24 14.90 |
| LDKT | 0.471.313.67 | 0.350.952.59 | 0.300.731.73 |
| Non-KPD | 0.361.284.54 | 0.210.772.79 | 0.190.601.85 |
| KPD | 0.241.377.99 | 0.271.336.68 | 0.240.973.93 |
Note: Bold indicates P < .05.
Abbreviations: aSHR, adjusted subhazard ratio; cPRA, calculated panel reactive antibody, DDKT, deceased donor kidney transplantation; KPD, kidney-paired donation; LDKT, living donor kidney transplantation.
Reference group.
FIGURE 1.
Outcomes of highly sensitized candidates by era and cPRA category. cPRA, calculated panel reactive antibody; DDKT, deceased donor kidney transplant; KPD, kidney-paired donation; LDKT, living donor kidney transplant; KAS, kidney allocation system. Era 1: January 1, 2009-December 31, 2011; Era 2: January 1, 2012-December 3, 2014; Era 3: December 4, 2014-December 31, 2017. Each figure shows the crude cumulative incidence of each potential waitlist outcome at 3-y after entering the DDKT waitlist by cPRA group (rows) and Era (columns) [Color figure can be viewed at wileyonlinelibrary.com]
3.2.2 |. Temporal trends by cPRA category
The trends highlighted here varied across cPRA. For candidates with cPRA 80%−89%, candidates in Era 3 were less likely to receive DDKT (aSHR: 0.760.840.92, P < .001) compared to candidates in Era 1 (Table 3). Although there were no statistically significant changes in the likelihood of LDKT in Era 2, candidates were more likely to undergo KPD (aSHR: 1.021.532.29, P = .04) but less likely to undergo non-KPD LDKT (aSHR: 0.540.700.92, P = .01) compared to candidates in Era 1. However, candidates in Era 3 were more likely to undergo LDKT compared to those in Era 1 (aSHR: 1.051.271.53, P = .01), which was driven by an increase in the likelihood of KPD (aSHR: 1.412.032.91, P < .001). There were no statistically significant changes in the likelihood of non-KPD LDKT across eras. These changes led to a 3-year cumulative incidence of 36.3% for DDKT in Era 3 (vs 40.7% in Era 1), 3.6% for KPD (vs 1.7%), and 6.3% for non-KPD LDKT (vs 6.1%) (Table 4, Figure 1D–F).
TABLE 4.
Three-year cumulative incidence of overall transplant for highly sensitized candidates by era and cPRA1
| cPRA | Era 1 | Era 2 | Era 3 |
|---|---|---|---|
| 80%-89% | 48.5 | 50.2 | 46.2 |
| 90%-97% | 32.0 | 34.1 | 47.9 |
| 98%-99.9% | 13.1 | 16.5 | 50.1 |
| 99.9%+ | 5.2 | 5.1 | 27.0 |
Note: Over time, there was a substantial increase in the percentage of cPRA 90%+ candidates receiving a transplant by 3 y after listing. For example, 50.1% of cPRA 98%−99.9% candidates received a transplant within 3 y in Era 3, compared to 13.1% in Era 1.
Abbreviation: cPRA, calculated panel reactive antibody.
1Includes all modalities (ie, deceased donor kidney transplantation and overall living donor kidney transplantation).
For candidates with cPRA 90%−97%, candidates in Era 3 were more likely to receive DDKT (aSHR: 1.751.952.17, P < .001) compared to candidates in Era 1 (Table 3). Although there were no statistically significant changes in the likelihood of LDKT across eras, there was an increase in the likelihood of KPD (aSHR for Era 2 vs 1:1.251.983.13, P = .004; Era 3 vs 1:1.712.624.00, P < .001). There were no statistically significant changes in the likelihood of non-KPD LDKT across eras. These changes led to a 3-year cumulative incidence of 41.9% for DDKT in Era 3 (vs 26.8% in Era 1), 3.1% for KPD (vs 1.5%), and 2.8% for non-KPD LDKT (vs 3.8%) (Table 4, Figure 1G–I).
For candidates with cPRA 98% to 99.9%, candidates in Era 3 were more likely to receive DDKT (aSHR: 5.576.627.85, P < .001) compared to candidates in Era 1 (Table 3). Although there were no statistically significant changes in the likelihood of LDKT, candidates in Era 3 were more likely to receive KPD (aSHR: 1.142.324.73, P = .02) but less likely to receive non-KPD LDKT than candidates in Era 1 (aSHR: 0.370.580.92, P = .02). These changes led to a 3-year cumulative incidence of 47.3% for DDKT in Era 3 (vs 10.1% in Era 1), 1.4% for KPD (vs 0.6%), and 1.5% for non-KPD LDKT (vs 2.4%) (Table 4, Figure 1J–L).
For candidates with cPRA 99.9%+, candidates in Era 3 were more likely to receive DDKT (aSHR: 4.236.499.96, P < .001) compared to candidates in Era 1 (Table 3). However, there were no statistically significant changes in the likelihood of LDKT, non-KPD LDKT, or KPD across eras. These changes led to a 3-year cumulative incidence of 25.9% for DDKT in Era 3 (vs 3.9% in Era 1), 0.5% for KPD (vs 0.3%), and 0.6% for non-KPD LDKT (vs 1.0%) (Table 4, Figure 1M–O).
3.3 |. Temporal trends in transplant modality used by HS recipients
3.3.1 |. Overall temporal trends
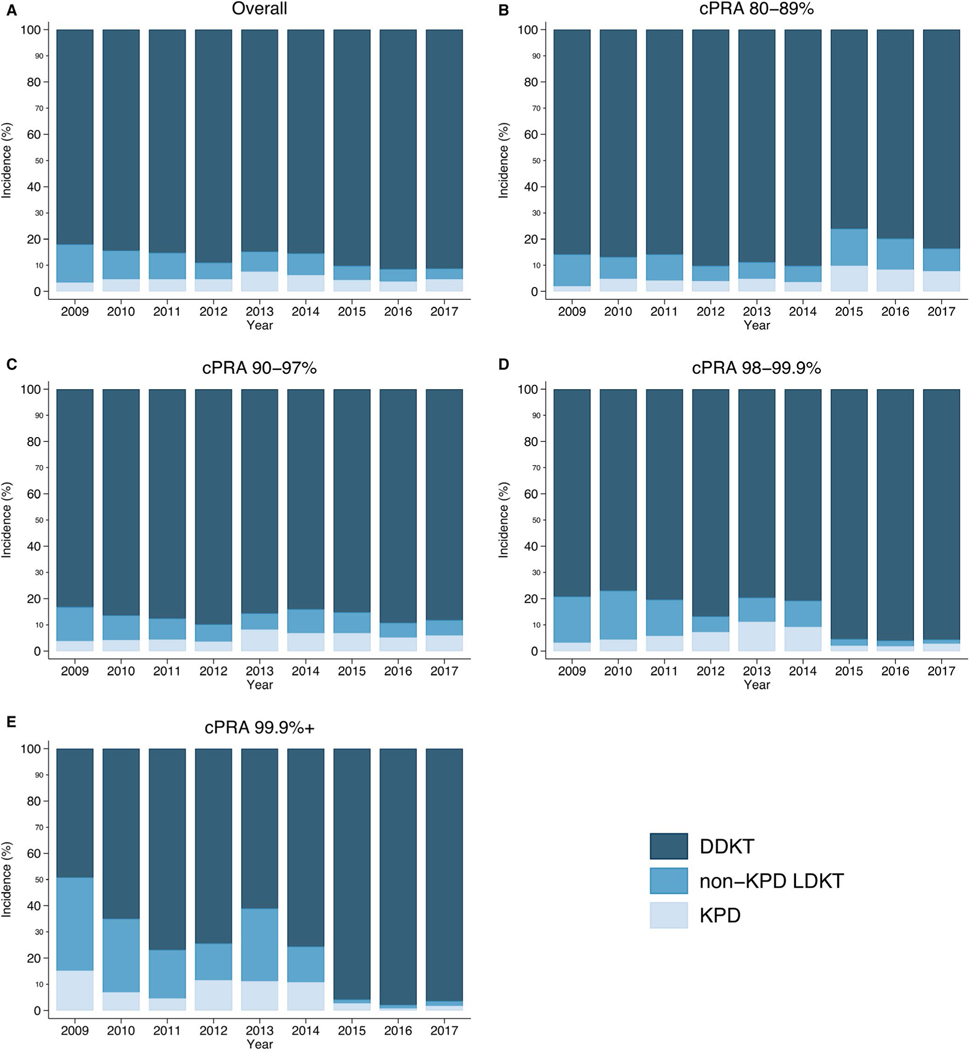
Over time, an increasing proportion of HS recipients were transplanted through DDKT (91.0% in Era 3% vs 84.1% in Era 1, P < .001) (Table 5, Figure 2). In contrast, a decreasing proportion of HS recipients were transplanted through non-KPD LDKT (4.8% vs 11.6%). There were no significant changes in the proportion of recipients who underwent KPD (4.2% vs 4.3%).
TABLE 5.
Temporal trends in transplant modalities received by highly sensitized recipients by era and cPRA
| Era 1 | Era 2 | Era 3 | P value | |
|---|---|---|---|---|
| Overall (%) | ||||
| DDKT | 84.1 | 86.4 | 91.0 | <.001 |
| LDKT | 15.9 | 13.6 | 9.0 | |
| Non-KPD | 11.6 | 7.5 | 4.8 | |
| KPD | 4.3 | 6.1 | 4.2 | |
| cPRA | ||||
| 80%-89% | ||||
| DDKT | 86.2 | 89.7 | 80.1 | <.001 |
| LDKT | 13.8 | 10.3 | 19.9 | |
| Non-KPD | 10.0 | 6.2 | 11.3 | |
| KPD | 3.8 | 4.1 | 8.6 | |
| 90%-97% | ||||
| DDKT | 86.0 | 86.5 | 87.7 | <.001 |
| LDKT | 14.0 | 13.5 | 12.3 | |
| Non-KPD | 9.8 | 7.3 | 6.4 | |
| KPD | 4.2 | 6.2 | 5.9 | |
| 98%-99.9% | ||||
| DDKT | 78.7 | 82.0 | 95.8 | <.001 |
| LDKT | 21.3 | 18.0 | 4.2 | |
| Non-KPD | 16.5 | 8.6 | 2.1 | |
| KPD | 4.8 | 9.4 | 2.1 | |
| 99.9%+ | ||||
| DDKT | 59.1 | 68.7 | 96.2 | <.001 |
| LDKT | 40.9 | 31.3 | 3.8 | |
| Non-KPD | 30.9 | 20.1 | 1.9 | |
| KPD | 10.0 | 11.2 | 2.0 |
Abbreviations: cPRA, calculated panel reactive antibody; DDKT, deceased donor kidney transplantation; KPD, kidney-paired donation; LDKT, living donor kidney transplantation.
FIGURE 2.
Trends in highly sensitized (HS) recipient transplant modalities used by era and calculated panel reactive antibody (cPRA) category. Each figure (A-E) shows the annual percentage of HS recipients in each cPRA category that used each transplant modality. For example, 30.9% of cPRA 99.9%+ recipients underwent non-kidney-paired donation (KPD) living donor kidney transplant (LDKT) in Era 1, which decreased to 20.1% in Era 2, and then to 1.9% in Era 3. DDKT, deceased donor kidney transplant [Color figure can be viewed at wileyonlinelibrary.com]
3.3.2 |. Temporal trends by cPRA category
The trends described here varied across cPRA. A decreasing proportion of cPRA 80%−89% recipients were transplanted through DDKT over time (80.1% in Era 3% vs 86.2% in Era 1, P < .001) (Table 5, Figure 2). In contrast, an increasing proportion was transplanted through KPD (8.6% vs 3.8%). There were no substantial changes in the use of non-KPD LDKT (11.3% vs 10.0%).
In contrast, an increasing proportion of cPRA 90%−97% recipients was transplanted through DDKT (87.7% in Era 3% vs 86.0% in Era 1, P < .001) and KPD (5.9% vs 4.2%), but fewer were transplanted through non-KPD LDKT (6.4% vs 9.8%).
An increasing proportion of cPRA 98%−99.9% and cPRA 99.9%+ recipients was transplanted through DDKT (95.8% in Era 3% vs 78.7% in Era 1 for cPRA 98%−99.9% recipients; 96.2% vs 59.1% for cPRA 99.9%+ recipients, P < .001) (Table 5, Figure 2). However, a decreasing proportion was transplanted through non-KPD LDKT (2.1% vs 16.5% for cPRA 98%−99.9% recipients; 1.9% vs 30.9% for cPRA 99.9%+ recipients). Similarly, a decreasing proportion was transplanted through KPD (2.1% vs 4.8% for cPRA 98%−99.9% recipients; 2.0% vs 10.0% for cPRA 99.9%+ recipients).
3.4 |. Likelihood of receiving non-KPD LDKT by era
Among cPRA 98%−99.9% candidates, approximately one-third as many candidates underwent non-KPD LDKT in Era 3 as would have been expected given candidate characteristics (O:E: 0.460.670.94) (Table 6). cPRA 99.9%+ candidates had an O:E ratio consistent with no change in non-KPD LDKT usage (O:E: 0.441.202.61) although this comparison was limited by a small sample size (n = 6 recipients in Era 3).
TABLE 6.
Observed to expected ratios for highly sensitized recipients undergoing non-KPD LDKT, by era and cPRA
| Observed | Expected | O:E | 95% CI | |
|---|---|---|---|---|
| Overall | ||||
| Era 1 | 277 | 277 | Ref | - |
| Era 2 | 208 | 262 | 0.79 | 0.69–0.91 |
| Era 3 | 281 | 258 | 1.09 | 0.97–1.22 |
| cPRA | ||||
| 80%-89% | ||||
| Era 1 | 144 | 144 | Ref | - |
| Era 2 | 86 | 127 | 0.68 | 0.54–0.84 |
| Era 3 | 165 | 124 | 1.33 | 1.14–1.55 |
| 90%-97% | ||||
| Era 1 | 85 | 85 | Ref | - |
| Era 2 | 76 | 85 | 0.89 | 0.70–1.11 |
| Era 3 | 78 | 81 | 0.96 | 0.76–1.20 |
| 98%-99.9% | ||||
| Era 1 | 44 | 44 | Ref | - |
| Era 2 | 40 | 45 | 0.89 | 0.63–1.21 |
| Era 3 | 32 | 48 | 0.67 | 0.46–0.94 |
| 99.9%+ | ||||
| Era 1 | 4 | 4 | Ref | - |
| Era 2 | 6 | 5 | 1.20 | 0.44–2.61 |
| Era 3 | 6 | 5 | 1.20 | 0.44–2.61 |
Note: Although this analysis was motivated by initial results in the cPRA 98%−99.9% subpopulation, we have provided results for all cPRA groups. cPRA 98%−99.9% candidates were approximately one-third less likely to undergo non-KPD LDKT in Era 3 as would be expected given candidate characteristics.
Abbreviations: CI, confidence interval; cPRA, calculated panel reactive antibody; DDKT, deceased donor kidney transplantation; KPD, kidney-paired donation; LDKT, living donor kidney transplantation; O:E, observed to expected ratio.
3.5 |. Sensitivity analyses
We also explored alternative cPRA categorizations to understand whether our findings were sensitive to these categories. Our results were consistent with our main analyses (Table 7). An increasing proportion of cPRA 97.5%−98.49% recipients underwent DDKT over time (88.0% in Era 3% vs 78.2% in Era 1, P < .001), as did cPRA 98.5%−99.49% recipients (93.8% vs 80.3%, P < .001) and cPRA 99.5%+ recipients (97.9% vs 71.2%, P < .001). However, a decreasing proportion of these recipients underwent non-KPD LDKT (0.9% vs 22.3% for 99.5%+ recipients) and KPD (1.2% vs 6.5% for cPRA 99.5%+ recipients).
TABLE 7.
Temporal trends in transplant modalities received by highly sensitized recipients by era and cPRA using alternative cPRA categorization
| cPRA | Era 1 | Era 2 | Era 3 | P value |
|---|---|---|---|---|
| 80.00%-89.00% | ||||
| DDKT | 86.2 | 89.6 | 80.4 | <.001 |
| LDKT | 13.8 | 10.4 | 19.6 | |
| Non-KPD | 10.0 | 6.2 | 11.2 | |
| KPD | 3.8 | 4.2 | 8.4 | |
| 90.00%-97.49% | ||||
| DDKT | 86.0 | 86.4 | 87.8 | <.001 |
| LDKT | 14.0 | 13.6 | 12.2 | |
| Non-KPD | 9.8 | 7.4 | 6.2 | |
| KPD | 4.2 | 6.2 | 5.9 | |
| 97.50%-98.49% | ||||
| DDKT | 78.2 | 86.2 | 88.0 | <.001 |
| LDKT | 21.8 | 13.8 | 12.0 | |
| Non-KPD | 15.7 | 7.2 | 6.9 | |
| KPD | 6.1 | 6.6 | 5.1 | |
| 98.50%-99.49% | ||||
| DDKT | 80.3 | 79.3 | 93.8 | <.001 |
| LDKT | 19.7 | 20.7 | 6.2 | |
| Non-KPD | 16.2 | 9.3 | 3.0 | |
| KPD | 3.5 | 11.4 | 3.2 | |
| 99.50%+ | ||||
| DDKT | 71.2 | 77.4 | 97.9 | <.001 |
| LDKT | 28.8 | 22.6 | 2.1 | |
| Non-KPD | 22.3 | 12.8 | 0.9 | |
| KPD | 6.5 | 9.8 | 1.2 |
Note: This sensitivity analysis was consistent with our main analysis. A decreasing proportion of cPRA 98.5%+ recipients underwent non-KPD LDKT (0.9% in Era 3% vs 22.3% in Era 1 for 99.5%+ recipients) and KPD (1.2% vs 6.5% for cPRA 99.5%+ recipients).
Abbreviations: cPRA, calculated panel reactive antibody; DDKT, deceased donor kidney transplantation; KPD, kidney-paired donation; LDKT, living donor kidney transplantation.
4 |. DISCUSSION
In this national study of 39 907 HS candidates and 19 003 recipients, we have shown that the likelihood of DDKT and KPD for HS candidates has increased by 1.85-fold and 2.20-fold, respectively, in Era 3 vs Era 1. Conversely, HS candidates had a 18% decreased likelihood of non-KPD LDKT in Era 3 vs Era 1. However, these changes varied across cPRA categories. The likelihood of KPD increased for cPRA 80%−89% (2.03-fold), cPRA 90%−97% candidates (2.62-fold), and cPRA 98%−99.9% candidates (2.32-fold) in Era 3 vs Era 1, but there were no statistically significant changes for cPRA 99.9%+ candidates. The net impact of these changes at the candidate level had different effects on HS recipients based on cPRA—such that DDKT was used less frequently over time for cPRA 80%−89% recipients (80.1% in Era 3% vs 86.2% in Era 1), but more frequently for cPRA 98%−99.9% (95.8% vs 78.7%) and cPRA 99.9%+ (96.2% vs 59.1%) recipients. In contrast, KPD was used more frequently over time for cPRA 80%−89% (8.6% in Era 3% vs 3.8% in Era 1) and 90%−97% recipients (5.9% vs 4.2%), but less frequently for cPRA 98%−99.9% (2.1% vs 4.8%) and cPRA 99.9%+ (2.0% vs 10.0%) recipients. Moreover, this decrease in non-KPD LDKT use in cPRA 98%+ recipients appears to be driven by a direct decrease in utilization of this modality, because one-third fewer recipients used non-KPD LDKT in Era 3 as would have been expected given candidate characteristics (O:E for cPRA 98%−99.9%+ candidates: 0.67). Although KAS has led to substantially higher DDKT rates for most HS candidates, this may have inadvertently led to decreased use of non-KPD LDKT to transplant cPRA 98%+ candidates.
Our findings of an increased likelihood of DDKT for cPRA 90%+ candidates and decreased likelihood for cPRA 80%−89% candidates are consistent with a number of studies that have described the impact of KAS on DDKT rates for the HS.10,15–18 However, we have extended this work by using national data to quantify changes in likelihood of KPD and non-KPD LDKT, which were not addressed in those studies. Our findings of increased likelihood of KPD for most HS candidates in the era of large, single- and multicenter KPD clearinghouses are also consistent with simulation data that showed more HS candidates would be able to find a KPD match with increasing registry size.19 However, we extended this work by studying trends over the last decade (of which KAS is just one era) and by also quantifying how the net impact of these changes resulted in different trends in transplant modalities being used by HS recipients based on cPRA. Whereas cPRA 80%−89% recipients were less likely to have received DDKT and more likely to have received LDKT, the opposite was true for cPRA 98%+ recipients, who were more likely to receive DDKT and less likely to receive LDKT (3.4% of cPRA 99.9% recipients underwent LDKT in Era 3, compared to 38.0% in Era 1). This appears to be driven by a direct decrease in utilization of this non-KPD LDKT, because one-third fewer recipients used non-KPD LDKT in Era 3 as would have been expected given candidate characteristics. Although KAS has undoubtedly helped some cPRA 98%+ candidates undergo DDKT who might not previously have been able to undergo transplant, our findings suggest that some cPRA 98%+ candidates are actually foregoing LDKT, which might require a complicated KPD match or desensitization to facilitate incompatible LDKT, in favor of DDKT, which has become substantially easier under KAS. Although not every cPRA 98%+ candidate has a potential living donor available, it is possible that some of these candidates might benefit from KPD or incompatible LDKT.
This decrease in non-KPD LDKT use by cPRA 98%+ recipients might be concerning for two reasons. First, mortality and graft loss are significantly lower after compatible LDKT than after DDKT, and thus these candidates might be better served attempting to find a compatible match, such as through KPD.20 Second, even if a candidate cannot find a compatible living donor, incompatible LDKT is associated with a survival benefit compared to waiting for DDKT.21 Moreover, some candidates are able to find a “less incompatible” match by combining incompatible LDKT with KPD.22,23 However, incompatible LDKT is possibly associated with higher costs and more complications than compatible LDKT, and it thus might not be appropriate for every cPRA 98%+ candidate with an incompatible living donor.24–27 Nevertheless, beyond a direct benefit to the cPRA 98% recipient, increasing LDKT utilization would allow candidates without a living donor to undergo DDKT, thus increasing the number of patients able to benefit from kidney transplantation. One potential strategy to mitigate this decrease in the use of non-KPD LDKT might be to delay the awarding of priority points for cPRA 98%+ candidates until, for example, 1 year after waitlist registration. This might encourage candidates and centers to aggressively search for a living donor and, if necessary, provide sufficient time to find a compatible or “less incompatible” match through KPD. Such a policy might resemble the recent policy change that created a phase-in period for exception points for liver transplant candidates with hepatocellular carcinoma, which has led to more balanced transplant rates between candidates with and without hepatocellular carcinoma, without an increase in waitlist mortality.28
Several limitations of our study are worth considering. First, not every HS DDKT candidate has a living donor available to them, and our estimates of likelihood of KPD and non-KPD LDKT cannot distinguish between candidates who had a potential living donor and those who did not. The OPTN does not collect data on whether transplant recipients had a potential living donor, nor on whether they received desensitization. However, the O:E ratios we calculated attempted to address this issue indirectly by determining how many candidates would be expected to undergo non-KPD LDKT in Era 3, if non-KPD LDKT was used for similar candidates in Era 3 as it had been for Era 1. Additionally, it is likely that there are between-center differences in how unacceptable antigens are determined and managed, details of which are not captured by the OPTN. Despite these limitations, the major strength of our study is our use of national registry data, which allow us to characterize trends in transplant modality usage at all centers in the country.
In conclusion, we found that the likelihood of DDKT and KPD increased for HS candidates over time, although the likelihood of non-KPD LDKT decreased. Moreover, we found that the net impact of these changes varied across cPRA, such that DDKT was used less frequently over time for cPRA 80%−89% recipients, but more frequently for cPRA 98%+ recipients. In contrast, KPD was used more frequently over time for cPRA 80%−97% recipients, but less frequently for cPRA 98%+ recipients. Additionally, this decrease in non-KPD LDKT appears to be the result of these candidates undergoing other transplant types instead, because one-third fewer recipients underwent non-KPD LDKT in Era 3 as would have been expected given candidate characteristics. One possible strategy to mitigate this decline might be to delay the awarding of priority points for DDKT until a specific time point after waitlist registration, in order to encourage the identification and utilization of potential living donors.
ACKNOWLEDGMENTS
This work was supported by grant numbers F32DK113719 (Jackson), F32DK117563 (Kernodle), K01DK101677 (Massie), K23DK115908 (Garonzik-Wang), and K24DK101828 (Segev) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr Garonzik-Wang is supported by a Clinician Scientist Development Award from the Doris Duke Charitable Foundation. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Funding information
Doris Duke Charitable Foundation; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: F32DK113719, F32DK117563, K01DK101677, K23DK115908 and K24DK101828
Abbreviations:
- aSHR
adjusted subhazard ratio
- cPRA
calculated panel reactive antibody
- DDKT
deceased donor kidney transplant
- HS
highly sensitized
- KAS
kidney allocation system
- KPD
kidney-paired donation
- LDKT
living donor kidney transplant
- O:E
observed to expected
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data used in this study are available from the Scientific Registry of Transplant Recipients.
REFERENCES
- 1.Keith DS, Vranic GM. Approach to the highly sensitized kidney transplant candidate. Clin J Am Soc Nephrol. 2016;11(4):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan SC, Choi J, Vo A. Kidney transplantation in highly sensitized patients. Br Med Bull. 2015;114(1):113–125. [DOI] [PubMed] [Google Scholar]
- 3.Chang P, Gill J, Dong J, et al. Living donor age and kidney allograft half-life: implications for living donor paired exchange programs. Clin J Am Soc Nephrol. 2012;7(5):835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostock IC, Alberú J, Arvizu A, et al. Probability of deceased donor kidney transplantation based on % PRA. Transpl Immunol. 2013;28(4):154–158. [DOI] [PubMed] [Google Scholar]
- 5.Wallis CB, Samy KP, Roth AE, Rees MA. Kidney paired donation. Nephrol Dial Transplant. 2011;26(7):2091–2099. [DOI] [PubMed] [Google Scholar]
- 6.Bingaman AW, Wright FH Jr, Kapturczak M, Shen L, Vick S, Murphey CL. Single-center kidney paired donation: the Methodist San Antonio experience. Am J Transplant. 2012;12(8):2125–2132. [DOI] [PubMed] [Google Scholar]
- 7.Flechner SM, Thomas AG, Ronin M, et al. The first 9 years of kidney paired donation through the National Kidney Registry: characteristics of donors and recipients compared with National Live Donor Transplant Registries. Am J Transplant. 2018;18(11):2730–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedewald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin North Am. 2013;93(6):1395–1406. [DOI] [PubMed] [Google Scholar]
- 9.Stegall MD, Stock PG, Andreoni K, Friedewald JJ, Leichtman AB. Why do we have the kidney allocation system we have today? A history of the 2014 kidney allocation system. Hum Immunol. 2017;78(1):4–8. [DOI] [PubMed] [Google Scholar]
- 10.Jackson KR, Covarrubias K, Holscher C, et al. The national landscape of deceased donor kidney transplantation for highly sensitized candidates: transplant rates, waitlist mortality, and post-transplant survival under the Kidney Allocation System. Am J Transplant. 2018;19(4):1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holscher CM, Jackson K, Chow EKH, et al. Kidney exchange match rates in a large multicenter clearinghouse. Am J Transplant. 2018;18(6):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 14.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;1:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colovai AI, Ajaimy M, Kamal LG, et al. Increased access to transplantation of highly sensitized patients under the new kidney allocation system. A single center experience. Hum Immunol. 2017;78(3):257–262. [DOI] [PubMed] [Google Scholar]
- 16.Hickey MJ, Zheng Y, Valenzuela N, et al. New priorities: analysis of the new kidney allocation system on UCLA patients transplanted from the deceased donor waitlist. Hum Immunol. 2017;78(1):41–48. [DOI] [PubMed] [Google Scholar]
- 17.Parsons RF, Locke JE, Redfield RR 3rd, Roll GR, Levine MH. Kidney transplantation of highly sensitized recipients under the new kidney allocation system: a reflection from five different transplant centers across the United States. Hum Immunol. 2017;78(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16(6):1834–1847. [DOI] [PubMed] [Google Scholar]
- 19.Segev DL, Gentry SE, Melancon JK, Montgomery RA. Characterization of waiting times in a simulation of kidney paired donation. Am J Transplant. 2005;5(10):2448–2455. [DOI] [PubMed] [Google Scholar]
- 20.Davis CL, Delmonico FL. Living-donor kidney transplantation: a review of the current practices for the live donor. J Am Soc Nephrol. 2005;16(7):2098–2110. [DOI] [PubMed] [Google Scholar]
- 21.Orandi BJ, Luo X, Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374(10):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery RA, Lonze BE, Jackson AM. Using donor exchange paradigms with desensitization to enhance transplant rates among highly sensitized patients. Curr Opin Organ Transplant. 2011;16(4):439–443. [DOI] [PubMed] [Google Scholar]
- 23.Holscher CM, Jackson KR, Segev DL. Transplanting the untransplantable. Am J Kidney Dis. 2020;75(1):114–123. [DOI] [PubMed] [Google Scholar]
- 24.Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014;14(7):1573–1580. [DOI] [PubMed] [Google Scholar]
- 25.Orandi BJ, Luo X, King EA, et al. Hospital readmissions following HLA-incompatible live donor kidney transplantation: a multi-center study. Am J Transplant. 2018;18(3):650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentine KL, Axelrod D, Klein C, et al. Early clinical complications after ABO-incompatible live-donor kidney transplantation: a National Study of Medicare-insured recipients. Transplantation. 2014;98(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelrod D, Lentine KL, Schnitzler MA, et al. The incremental cost of incompatible living donor kidney transplantation: a National Cohort Analysis. Am J Transplant. 2017;17(12):3123–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishaque T, Massie AB, Bowring MG, et al. Liver transplantation and waitlist mortality for HCC and non-HCC candidates following the 2015 HCC exception policy change. Am J Transplant. 2019;19(2):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]