Abstract
Aim:
To compare and evaluate the surface characteristics of different restorative materials used for restoration of cervical defects when subjected to periodontal prophylactic instrumentation techniques.
Materials and Methodology:
Sixty box-shaped cavities were prepared on the labial surface of decoronated permanent maxillary anterior teeth which were randomly divided into two groups (n = 30) based on the instrumentation technique Group I: Manual instrumentation using curettes and Group II: Ultrasonic instrumentation. The samples were further divided into three subgroups based on restorative material subgroups I V and II V-restored with Vitremer, subgroups I F and II F-restored with Filtek Z 250 XT and subgroups I D and II D-restored with Dyract flow, respectively. After finishing and polishing, the samples were subjected to surface profilometry analysis for determining the surface roughness values (Ra). Thereafter, the restored surfaces of all the samples in different subgroups were subjected to prophylactic instrumentation with Gracey's curettes (Group I) and ultrasonic scalers (Group II). Ra values were recorded again after prophylactic instrumentation and after polishing. The data thus obtained were subjected to the statistical analysis using the independent t-test and one-way ANOVA (p<0.05).
Results:
Ra values were significantly higher for both manual and ultrasonic prophylaxis compared to preprophylaxis and postpolishing in all the three restorative materials. Ultrasonic scaling produced significantly higher Ra for subgroup V as compared to subgroup F and subgroup D, whereas the difference between the materials was not significant for manual scaling.
Conclusion:
Manual prophylaxis resulted in significant reduction in surface roughness of all the three restorative materials while ultrasonic prophylaxis resulted in significant reduction for Vitremer only. Polishing after scaling significantly reduced the effect of both manual and ultrasonic prophylaxis on surface roughness.
Keywords: Dyract, filtek Z 250, manual prophylaxis, profilometry, surface roughness, ultrasonic prophylaxis, Vitremer
INTRODUCTION
Resin-based composite restorations were introduced in dentistry about half a century ago and since then they have been increasingly used in place of silver amalgam for the restoration of carious lesions. Among the commercially available composite resins, Filtek Z250XT is one of the most popular one with clinicians.
Dyract (DENTSPLY Sirona, USA) was introduced in 1993 as the first of a new class of materials combining some of the best properties of composites and glass ionomers. The material was immediately widely accepted and used because of its unique combination of ease of use, esthetics, physical properties, and fluoride release.[1]
The Vitremer™ Tri-Cure Glass Ionomer System (3M ESPE, USA) has the following three distinct curing reactions: (1) Acid–base glass-ionomer reaction (initiated when powder and liquid are mixed and can proceed without light curing); (2) Photo initiated free radical methacrylate cure (initiated when the powder/liquid mix is exposed to light and occurs only where light penetrates); and (3) Dark cure free radical methacrylate cure (initiated when powder and liquid are mixed and can proceed in the dark). This material offers the best features of conventional glass ionomers and light cure systems without the disadvantages of either.
The surface roughness of a restorative material is an important factor for the esthetic appearance and longevity of restorations. The presence of irregularities on the surface of the restorations may result in plaque and stain retention, gingival inflammation, and solubility of the organic matrix due to the formation of acids by plaque.[2,3,4]
Periodontal therapy and prophylaxis involve the removal of plaque, calculus, and endotoxin from teeth or exposed root surfaces with hand- or machine-driven instruments.[5] This procedure may be performed in patients both before and after restoration. As compared to manual prophylaxis, sonic and ultrasonic prophylaxis has become the most widely used methods among dental surgeons due to decreased time requirement and ease of application in comparison with hand instrumentation.[6]
Extent to which the surface of various restorative materials undergoes degradation or sustained damage during prophylaxis depends on the various factors including operator expertise, type of instrumentation technique, and instrument used. Another important influencing factor is type and surface hardness of restorative material which affects its vulnerability to abrasion.
Furthermore, certain areas may be more vulnerable to the effect of prophylactic instrumentation, especially interproximal and cervical areas of the buccal and lingual aspects of anterior and posterior teeth. Hence, while choosing the material to be restored in these areas, it is important to consider their vulnerability to prophylactic instruments. Therefore, the present study was conducted to compare and evaluate the surface characteristics of different restorative materials used for the restoration of cervical defects when subjected to periodontal prophylactic instrumentation techniques.
MATERIALS AND METHODOLOGY
Sixty extracted human permanent maxillary anterior teeth with intact labial surfaces, (free of all defects) were selected and decoronated along the cementoenamel junction. The crowns were embedded in self-curing acrylic resin (Pyrax Polymers, Roorkee, India) using molds while ensuring the horizontal orientation of labial surface placing 2 mm of the sample above the rim of the mould. A cavity preparation (4 mm × 2 mm × 1.5 mm) was carried out with using high speed airotor under air water spray [Figure 1a].
Figure 1.
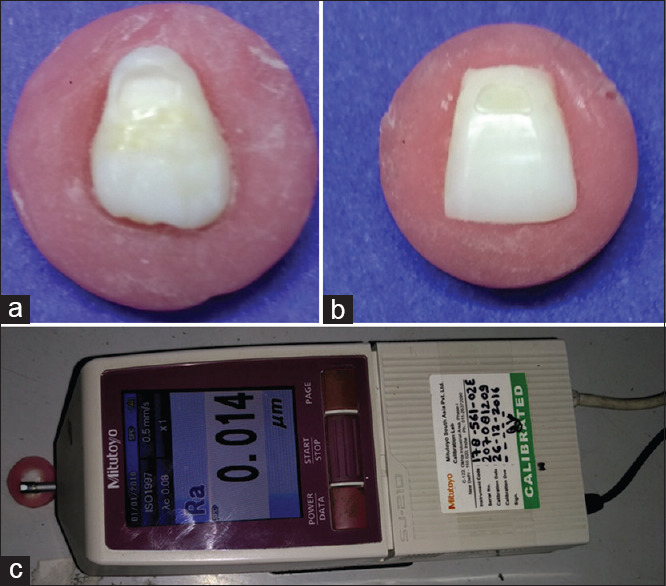
(a) Preparation of Class V cavity, (b) restoration of Class V cavity, (c) surface profilometry analysis
The samples were then randomly allotted into two groups based on the technique used for simulating prophylactic periodontal instrumentation on restoration surfaces. Group I: Manual instrumentation using Gracey curettes (n = 30) and Group II: Ultrasonic instrumentation (n = 30).
Samples in each group were further distributed randomly into three subgroups based on the restorative material used to restore the prepared cavities. Sub Group G (n = 10): (IG and IIG) Resin-modified glass ionomer cement (RMGIC) (Vitremer,). Sub Group N (n = 10): (IN and IIN) Nanohybrid composite resin (Filtek Z250XT, 3M ESPE) Sub Group C (n = 10): (IC and IIC) Compomer (Dyract flow).
The restorative material was placed in single increment with the cavities slightly overfilled and cured under Mylar strip using LED Light Curing Unit (Guillin, Woodpecker Medical Instrument Co. Ltd, China) for 40 s [Figure 1b].
The samples were finished and polished using the Shofu super snap finishing and polishing kit (Gmbh Ratingen, Germany). The Super Snap discs were used in the sequential manner in a decreasing order of their grit size at a speed of 20,000 rpm for 15 s. Both disc and the samples were washed thoroughly after each polishing step.
The restored samples were further stored in distilled water for a period of 30 days after which they were subjected to surface profilometry analysis [Figure 1c] for determining the surface roughness values (Ra). Thereafter, the samples were subjected to prophylactic instrumentation.
Prophylactic instrumentation
For Group I
(Manual instrumentation with Gracey's curette [Hu-Friedy]). The curette blade was kept approximated with restoration surface making an arbitrary angle of 45°–60° with the restored surface. The blade was then moved from cervical to incisal direction while engaging it against the surface. A total of 20 strokes in a time span of 15 s were made for each sample.
For Group II
(Ultrasonic scaling with DTE, D5 (Guilin, Woodpecker Medical Instrument Co. Ltd, China) Ultrasonic scaler was used on restored surface having GD3 insert/tip under copious water flow for 15 s at a power setting of 5 W and frequency of 28 kHz). The scaling tip was angled at approximately 0° to the restoration surface. The direction of scaling was maintained perpendicular to the long axis of the tooth in the horizontal plane while moving the scaler insert slowly from gingival to coronal third of the restoration.
After prophylactic instrumentation, the restorations in the samples of all the subgroups were again subjected to surface profilometry analysis to evaluate the effect of prophylactic instrumentation on surface roughness.
Thereafter, polishing of all the samples was performed in a similar manner as described above. Postpolishing surface profilometry analysis was carried out for restorations in samples of all the subgroups using the same protocol as before instrumentation.
Protocol for profilometeric analysis
The stylus (tip radius of 5 μm) of the contact Profilometer (Surftest SJ210, Mitutoyo, Japan) was moved on the restoration from mesial to distal for a distance of 2 mm at the speed of 0.25 mm/s and pressure of 4 mN. Three successive measurements were recorded for each sample at different locations, and the average surface roughness (Ra in μ) value was obtained. The observed Ra values were recorded, tabulated, and subjected to the statistical analysis using the independent t-test and one-way ANOVA.
RESULTS
Manual prophylaxis (Group I) caused significant increase in surface roughness of all the three restorative materials with no significant difference amongst the subgroups. (b) Ultrasonic scaling (Group II) produced a significantly rough surface for Vitremer (subgroup G) but had no significant difference on the surface roughness of Filtek Z 250 XT (subgroup N) and Dyract flow (subgroup C). (c) Polishing of all the three restorative materials (postmanual and ultrasonic scaling) decreased the surface roughness significantly with no significant difference observed between preinstrumentation and postpolishing groups.
On comparison of manual and ultrasonic scaling, (d) ultrasonic scaling resulted in significantly higher surface roughness for Vitremer (subgroup G) as compared to manual scaling. (e) Manual scaling resulted in significantly greater change in surface roughness values of Filtek Z 250 (subgroup N) and Dyract flow (subgroup C) as compared to ultrasonic scaling [Table 1].
Table 1.
Intergroup test of significance for resin-modified glass ionomer cement, nanohybrid and compomer using unpaired t-test at different intervals
| Group | Preinstrumentation |
Postinstrumentation |
Postpolishing |
|||
|---|---|---|---|---|---|---|
| Group I (manual scaling) | Group II (ultrasonic scaling) | Group I (manual scaling) | Group II (ultrasonic scaling) | Group I (manual scaling) | Group II (ultrasonic scaling) | |
| Resin modified GIC | ||||||
| Mean±SD | 0.11±0.05 | 0.14±0.09 | 0.18±0.05 | 0.49±0.20 | 0.12±0.04 | 0.16±0.09 |
| Mean difference | −0.02 | −0.31 | −0.04 | |||
| t-test | −0.721 | −4.665 | −1.344 | |||
| P | 0.480 | 0.001* | 0.196 | |||
| Nanohybrid composite | ||||||
| Mean±SD | 0.14±0.08 | 0.12±0.09 | 0.24±0.15 | 0.14±0.07 | 0.15±0.05 | 0.13±0.02 |
| Mean difference | −0.02 | −0.10 | −0.02 | |||
| t-test | 0.471 | 1.879 | 1.009 | |||
| P | 0.643 | 0.077 | 0.326 | |||
| Compomer | ||||||
| Mean±SD | 0.12±0.04 | 0.09±0.04 | 0.23±0.08 | 0.13±0.05 | 0.13±0.05 | 0.10±0.05 |
| Mean difference | −0.03 | −0.10 | −0.03 | |||
| t-test | 1.518 | 3.511 | 1.192 | |||
| P | 0.146 | 0.002* | 0.249 | |||
*A significant difference at 0.05 level of significance (P<0.05). SD: Standard deviation
DISCUSSION
Various methodologies have been used in the literature for the evaluation of surface characteristics which include profilometery,[7,8,9,10] scanning electron microscopy,[11] atomic force microscopy,[12] confocal microscopy,[13] interferometry, and focus variation.[14] Out of these, profilometry analysis is the most common surface measurement technique. It is an economical method and has higher lateral resolution than optical techniques, depending on the stylus tip radius chosen.[10]
According to the results of this study, manual prophylaxis resulted in significant surface deterioration of all the three materials while ultrasonic prophylaxis resulted in significant surface detoriation for Vitremer only. The mean Ra values after manual prophylaxis for subgroup V, subgroup F, and subgroup D were 0.182 μm, 0.240 μm, and 0.232 μm, respectively, which suggests that the surface roughness created was just above the threshold value for biofilm formation (0.2.μm). This suggests the need for polishing of these restorations after manual prophylaxis.
The results of this study showed that polishing of the instrumented restorations resulted in almost similar values of surface roughness as compared to preinstrumentation in both the manual and ultrasonic groups. This points out toward the need and importance of polishing after prophylactic instrumentation and correlates well with various other studies[15,16,17] in the literature.
After ultrasonic prophylaxis, the roughness values of Filtek Z 250 XT and Dyract flow (0.14 μm and 0.13 μm respectively) were less than the threshold value for plaque accumulation (0.2 μm) except for Vitremer which presented with a value of 0.49 μm. Ultrasonic tips presented with lesser surface roughness as compared to manual prophylaxis.
Ultrasonic scalers are power-driven scalers with frequencies in the range of 25000–42000 Hz and amplitude from 10 to 100 μm. The resultant vibration produces tip movement that is primarily linear in direction. A piezoelectric ultrasonic scaler may oscillate parallel to the tooth surface and gently remove calculus if the alignment is correct.[18] While using Gracey Curettes, the toe is directed interproximally and the terminal shank is parallel to the tooth. To remove deposits, the cutting edge is applied to the tooth surface, and the facial surface of the blade is tilted toward the tooth. Lateral pressure is applied against the tooth, and the curette is pulled upward while maintaining contact with the tooth. This motion used with manual scalers explains increased surface roughness observed with them. The application of lateral pressure against restoration surfaces in the absence of calculus might be a reason for poor surface roughness of all three materials seen with manual prophylaxis. No studies were found in the literature regarding the effect of manual scaling on surface roughness and behavior of the three restorative materials involved in the present study.
Ultrasonic instrumentation of Vitremer produced a significantly rough surface as compared to manual scaling, whereas no significant difference in the surface roughness was observed after ultrasonic instrumentation of Filtek Z 250 XT and Dyract flow.
This might be due to water diffusion during the storage of samples. According to Meyer,[19] the RMGIC (Vitremer) absorbs more water than compomers and nanohybrid composite resins. This absorbed water acts as plasticizer and reduces the surface hardness of the restorative materials. Poor surface roughness of RMGIC after ultrasonic instrumentation can be partly attributed to this fact.
Another possible reason for increased surface roughness of Vitremer can be that it is the only material among all the experimental materials that required manipulation and mixing as powder and liquid, the other two being directly dispensable single component syringe materials. Mixing of powder and liquid may have led to increased risk of air bubble incorporation and hence more porosity which got more enhanced after ultrasonic instrumentation leading to greater surface roughness.[20,21]
Another contributing factor can be that Vitremer has the weakest matrix phase and largest particle size (12.5 μm) among all the tested restorative materials.[15] Ultrasonic instrumentation of Vitremer may have resulted in the preferential removal of weaker matrix phase, leaving the harder unreacted glass or filler particles protruding out from the surface.[16,22]
In the present study, Filtek Z 250 XT demonstrated least change in surface roughness after ultrasonic instrumentation. Filtek Z 250XT has the smallest particle size of 100 nm (nano particles and nano clusters), wide distribution of different size particles and higher filler loading (82% by weight) resulting in high strength and high wear resistance, increased Vickers hardness number, better physical properties, and increased polish retention when compared to RMGIC and compomers.[16,22]
Dyract flow performed better than RMGIC and demonstrated better resistance against surface wear during ultrasonic scaling. Smaller filler particle size, syringeable consistency, and less water sorption might be the reasons for better surface roughness characteristics of Dyract flow as compared to RMGIC (with similar Vickers hardness number).[19]
The above discussion concludes that although ultrasonic prophylaxis might result in a significant rougher restorative surface for some materials as compared to manual scaling, the effect was reversed by postprophylaxis polishing of restorative surface.
The present study has its own limitations in being in vitro and was carried out in controlled environment rather than the dynamic environment of the oral cavity where many other factors can affect the outcome. Similarly, operator experience in the selection and usage of prophylaxis instrument can have a bearing on surface roughness.
The effect of postprophylaxis surface roughness on the clinical outcomes such as plaque adherence, staining, and wear characteristics of restoration needs to be evaluated in future studies to add the clinical evidence to the outcome of this study.
Clinical significance
Ultrasonic prophylaxis should be the choice of prophylactic technique in patients having compomer and nanohybrid restorations since it leads to lesser surface deterioration as compared to manual scaling. However, with resin-modified glass ionomer cement (Vitremer), both manual and ultrasonic scaling can significantly decrease surface roughness. This article also emphasizes on the significance of polishing of restoration after scaling since the surfaces so obtained are significantly smoother and would lead to lesser plaque accumulation.
CONCLUSION
Manual prophylaxis resulted in significant reduction in surface roughness of all the three restorative materials while ultrasonic prophylaxis resulted in significant reduction for Vitremer only. Polishing after scaling significantly reduced the effect of both manual and ultrasonic prophylaxis on surface roughness.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Arabaci T, Ciçek Y, Ozgöz M, Canakçi V, Canakçi CF, Eltas A. The comparison of the effects of three types of piezoelectric ultrasonic tips and air polishing system on the filling materials: An in vitro study. Int J Dent Hyg. 2007;5:205–10. doi: 10.1111/j.1601-5037.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 2.Roeder LB, Tate WH, Powers JM. Effect of finishing and polishing procedures on the surface roughness of packable composites. Oper Dent. 2000;25:534–43. [PubMed] [Google Scholar]
- 3.Yap AU, Sau CW, Lye KW. Effects of finishing/polishing time on surface characteristics of tooth-coloured restoratives. J Oral Rehabil. 1998;25:456–61. doi: 10.1046/j.1365-2842.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 4.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 5.Axelsson P, Lindhe J. Effect of controlled oral hygiene procedures of caries and periodontal disease in adults. Results after 6 years. J Clin Periodontal. 1981;8:239–48. doi: 10.1111/j.1600-051x.1981.tb02035.x. [DOI] [PubMed] [Google Scholar]
- 6.Drisko CL, Cochran DL, Blieden T, Bouwsma OJ, Cohen RE, Damoulis P, et al. Position paper: Sonic and ultrasonic scalers in periodontics. Research, science and therapy committee of the American Academy of Periodontology. J Periodontol. 2000;71:1792–801. doi: 10.1902/jop.2000.71.11.1792. [DOI] [PubMed] [Google Scholar]
- 7.Bjornson, Elizabeth J, Edward CD, William EO. Surface alteration of composite resins after curette, ultrasonic, and sonic instrumentation: An in vitro study. Quintessence Int. 1990;21:381–9. [PubMed] [Google Scholar]
- 8.Grossman ES, Rosen M, Cleaton-Jones PE, Volchansky A. Scientific surface roughness values for resin based materials. SADJ. 2004;59:274, 276, 278–9. [PubMed] [Google Scholar]
- 9.Mahantesha, Shobha KS, Mani R, Deshpande A. Influence of scaler tip design on root surface roughness: An in vitro study. J. Int Oral Health. 2010;2:51–8. [Google Scholar]
- 10.Dahiya P, Kamal R, Gupta R, Pandit N. Comparative evaluation of hand and power-driven instruments on root surface characteristics: A scanning electron microscopy study. Contemp Clin Dent. 2011;2:79–83. doi: 10.4103/0976-237X.83065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arabaci T, Ciçek Y, Ozgöz M, Canakçi V, Canakçi CF, Eltas A. The comparison of the effects of three types of piezoelectric ultrasonic tips and air polishing system on the filling materials: An in vitro study. Int J Dent Hyg. 2007;5:205–10. doi: 10.1111/j.1601-5037.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 12.Mittal A, Nichani AS, Venugopal R, Rajani V. The effect of various ultrasonic and hand instruments on the root surfaces of human single rooted teeth: A planimetric and profilometric study. J Indian Soc Periodontol. 2014;18:710–7. doi: 10.4103/0972-124X.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gantes BG, Nilvéus R, Lie T, Knut NL. The effect of hygiene instruments on dentin surfaces: Scanning electron microscopic observations. J Periodontol. 1992;63:151–7. doi: 10.1902/jop.1992.63.3.151. [DOI] [PubMed] [Google Scholar]
- 14.Flemmig TF, Petersilka GJ, Mehl A, Hickel R, Klaiber B. Working parameters of a magnetostrictive ultrasonic scaler influencing root substance removal in vitro. J Periodontol. 1998;69:547–53. doi: 10.1902/jop.1998.69.5.547. [DOI] [PubMed] [Google Scholar]
- 15.Lee AR, Chung CH, Jung GU, Pang EK. The effect of copper alloy scaler tip on the surface roughness of dental implant and restorative materials. J Korean Acad Prosthodont. 2014;52:177–85. [Google Scholar]
- 16.Soares PB, Magalhães D, Fernandes Neto AJ, Castro CG, Santos Filho PC, Soares CJ. Effect of periodontal therapies on indirect restoration: A scanning electron microscopic analysis. Braz Dent J. 2010;21:130–6. doi: 10.1590/s0103-64402010000200007. [DOI] [PubMed] [Google Scholar]
- 17.Arabaci T, Cicek Y, Dilsiz A, Erdogan İY, Kose O, Kizildağ A. Influence of tip wear of piezoelectric ultrasonic scalers on root surface roughness at different working parameters. A profilometric and atomic force microscopy study. Int J Dent Hyg. 2013;11:69–74. doi: 10.1111/idh.12003. [DOI] [PubMed] [Google Scholar]
- 18.Buchgraber B, Kqiku L, Allmer N, Jakopic G, Städtler P. Surface roughness of one nanofill and one silorane composite after polishing. Coll Antropol. 2011;35:879–83. [PubMed] [Google Scholar]
- 19.Gladys S, Van Meerbeek B, Braem M, Lambrechts P, Vanherle G. Comparative physico-mechanical characterization of new hybrid restorative materials with conventional glass-ionomer and resin composite restorative materials. J Dent Res. 1997;76:883–94. doi: 10.1177/00220345970760041001. [DOI] [PubMed] [Google Scholar]
- 20.Jyothi K, Annapurna S, Kumar AS, Venugopal P, Jayashankara C. Clinical evaluation of giomer and resin modified glass ionomercement in Class V noncarious cervical lesions: An in vivo study. J Conserv Dent. 2011;14:409–13. doi: 10.4103/0972-0707.87214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filtek™ Z250×T Nano Hybrid Universal Restorative, Technical Data Sheet. [Last updated on 2014 Jan 12]. Available from Available from: http://solutions.3mae.ae/3MContentRetrievalAPI/BlobServlet?lmd=1316442495000&locale=en_EU&assetType=MMM_Image&assetId=1273695174257&blobAttribute=ImageFile .
- 22.Breininger DR, O'Leary TJ, Blumenshine RV. Comparative effectiveness of ultrasonic and hand scaling for the removal of subgingival plaque and calculus. J Periodontol. 1987;58:9–18. doi: 10.1902/jop.1987.58.1.9. [DOI] [PubMed] [Google Scholar]


